Abstract
Background
Adrenergic drugs have been used for the treatment of urinary incontinence. However, they have generally been considered to be ineffective or to have side effects which may limit their clinical use.
Objectives
To determine the effectiveness of adrenergic agonists in the treatment of urinary incontinence in adults.
Search methods
We searched the Cochrane Incontinence Group Specialised Trials Register (searched 15 September 2010) and the reference lists of relevant articles.
Selection criteria
Randomised or quasi‐randomised controlled trials in adults with urinary incontinence which included an adrenergic agonist drug in at least one arm of the trial.
Data collection and analysis
Two reviewers independently assessed eligibility, trial quality and extracted data. Data were processed as described in the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
Twenty‐two eligible randomised trials were identified, of which 11 were crossover trials. The trials included 1099 women with 673 receiving an adrenergic drug (phenylpropanolamine in 11 trials, midodrine in two, norepinephrine in three, clenbuterol in another three, terbutaline in one, eskornade in one and Ro 115‐1240 in one). No trials included men.
The limited evidence suggested that an adrenergic agonist drug is better than placebo in reducing the number of pad changes and incontinence episodes, as well as improving subjective symptoms. In two small trials, the drugs also appeared to be better than pelvic floor muscle training, possibly reflecting relative acceptability of the treatments to women but perhaps due to differential withdrawal of women from the trial groups. There was not enough evidence to evaluate the use of higher compared to lower doses of adrenergic agonists nor the relative merits of an adrenergic agonist drug compared with oestrogen, whether used alone or in combination.
Over a quarter of women reported adverse effects. There were similar numbers of adverse effects with adrenergics, placebo or alternative drug treatment. However, when these were due to recognised adrenergic stimulation (insomnia, restlessness and vasomotor stimulation) they were only severe enough to stop treatment in 4% of women.
Authors' conclusions
There was weak evidence to suggest that use of an adrenergic agonist was better than placebo treatment. There was not enough evidence to assess the effects of adrenergic agonists when compared to or combined with other treatments. Further larger trials are needed to identify when adrenergics may be useful. Patients using adrenergic agonists may suffer from minor side effects, which sometimes cause them to stop treatment. Rare but serious side effects, such as cardiac arrhythmias and hypertension, have been reported.
Keywords: Adult; Female; Humans; Adrenergic Agonists; Adrenergic Agonists/therapeutic use; Clenbuterol; Clenbuterol/therapeutic use; Midodrine; Midodrine/therapeutic use; Phenylpropanolamine; Phenylpropanolamine/therapeutic use; Randomized Controlled Trials as Topic; Urinary Incontinence; Urinary Incontinence/drug therapy; Urinary Incontinence, Stress; Urinary Incontinence, Stress/drug therapy
Plain language summary
Adrenergic drugs for urinary incontinence in adults
Urinary incontinence is the leakage of urine, and when caused by coughing or exercising it is called stress incontinence. It may be caused by damage to muscles either holding up the bladder or holding the bladder neck closed. Adrenergic agonist drugs may help the bladder neck muscle to contract more strongly. This review of 22 trials involving 673 women and seven different adrenergic drugs found weak evidence that adrenergic agonists may help stress urinary incontinence. Side effects do occur but are usually minor. Rarely, more serious adverse effects such as high blood pressure can occur. More evidence is needed to compare adrenergic drugs with other drugs for stress incontinence and also with pelvic floor muscle exercises.
Background
Severe urinary incontinence is estimated to affect between 6% and 10% of the adult female population. Stress incontinence accounts for about half of all cases (Hunskaar 2002; Thomas 1980). The prevalence of any incontinence increases with age from around 20% to 30% during young adult life, through a broad peak around middle age (30% to 40%) and then a steady increase in the elderly (30% to 50%, Hunskaar 2002). The prevalence rates of urinary incontinence in men are reported to be about half of those in women (5% to 20%, Hunskaar 2002).
In men, urinary incontinence after prostatectomy is a recognised complication. It occurs in 5% to 45% after radical prostatectomy, albeit at varying times after the operation (Hunskaar 2002). Even after transurethral resection of the prostate, an estimated 11% men use pads initially (Emberton 1996).
Types of incontinence Current urodynamic theory suggests that continence is normally maintained by two mechanisms: 1) the simultaneous transmission of intra‐abdominal pressure to both the bladder and the proximal urethra; and 2) the integrity of the internal urethral sphincter mechanism.
Stress urinary incontinence (SUI) results from failure to maintain the normal retropubic position of the bladder neck and proximal urethra during increases in intra‐abdominal pressure and/or when the internal urethral sphincter mechanism is impaired. Urge urinary incontinence (UUI) results from involuntary bladder contractions during bladder filling. Mixed urinary incontinence (MUI) is diagnosed when SUI and UUI co‐exist.
Treatment Stress urinary incontinence is conventionally treated with conservative physical therapies and if this is unsuccessful, surgery. Surgical interventions have a cure rate of up to 90%, but on the other hand they also carry risks (Lapitan 2005). Such procedures may not be appropriate in all cases and failure rates tend to increase with time. Furthermore, Black et al suggested that the outcome of traditional surgical treatments may be less satisfactory in practice than previously thought, or anticipated, from the literature (Black 1996).
A variety of pharmacological agents have been investigated for the treatment of women with stress urinary incontinence though few, if any, have become accepted into mainstream practice. As with many other areas of incontinence research, data from randomised controlled trials are scarce. Drug treatment of stress incontinence is generally considered to be ineffective or their use severely limited by side effects such as hypertension, piloerection and central nervous system (CNS) stimulation. As a result, they are rarely used in clinical practice. Despite this pessimism, there is continuing clinical and pharmacological interest in their use. Furthermore, the introduction of a new drug class, serotonin reuptake inhibitors, may prove promising and is the subject of a new Cochrane review (Mariappan 2005).
There are two main types of adrenergic drugs, alpha and beta.
Alpha adrenergics The adrenoceptor subtype mediating contractile responses to noradrenaline (norepinephrine) in the human urethra and bladder neck has been identified pharmacologically as alpha 1A (Byland 1998). In vitro studies on segments of urethra and bladder neck show that alpha 1A‐adrenoceptors mediate the smooth muscle contractile response to noradrenaline and related sympathomimetic amines (Kava 1998). Thus, an alpha 1A‐adrenoceptor agonist would be expected to promote continence, particularly in mild cases of stress incontinence where residual sphincter function is still present.
The alpha 1A‐adrenoceptor is, however, not restricted to the lower urinary tract and is widespread in the cardiovascular system (Michel 1998). Clearly, activity at other alpha 1‐adrenoceptor subtypes, alpha 2‐ or beta‐adrenoceptors is unwanted, as is penetration into the CNS or release of endogenous noradrenaline from sympathetic nerves. To a greater or lesser degree, these unwanted actions are exhibited by all alpha adrenergic drugs, such as ephedrine, pseudoephedrine and phenylpropanolamine. It may prove impossible to divorce accompanying cardiovascular sequelae and piloerection from significant and clinically relevant elevations in urethral pressure with an alpha‐adrenoceptor selective agonist. Side effects may exceed the beneficial effects on increased urethral pressure and continence. Furthermore, the sympathetic nervous system plays a key homeostatic role in the maintenance of blood pressure, but it may be largely subservient to other factors, such as striated muscle tone, for the provision of urinary continence.
Beta adrenergics Beta adrenergic drugs have also been used to treat stress urinary incontinence. Experimental studies by Morita showed that, in contrast to the known muscle‐relaxing effect of beta adrenergic agonists on detrusor muscle, clenbuterol produced a concentration‐dependent relaxation of the detrusor muscle and contraction of urethral sphincter muscle. This suggested a role for clenbuterol in the management of urinary incontinence (Morita 1989).
The intrinsic urethral sphincter may not be the most important element in the continence mechanism; the equal transmission of abdominal pressure to the proximal urethra may be of greater consequence (Enhoring 1961). However, in women with prolapse and bladder neck hypermobility this pressure transmission is compromised and the intrinsic sphincter mechanism assumes greater importance (McGuire 1995). Conversely, in women with stress incontinence without significant bladder neck hypermobility, it is assumed that a deficiency in the intrinsic sphincter mechanism is present. Hypermobility may be successfully treated by a bladder neck repositioning procedure, but the outcome of surgical treatment when intrinsic sphincter deficiency exists is less predictable. Treatments which augment urethral sphincter function are, therefore, theoretically attractive therapeutic options. In practice, however, the two problems usually co‐exist and thus distinguishing between them may not be clinically helpful.
The efficacy of adrenergic agonists in urinary incontinence may vary for a number of reasons. Many of these agents have both peripheral and central effects, cause systemic release of endogenous catecholamines or are non‐selective in their receptor activity. Clearly, the issues of unwanted side effects and patient compliance must be addressed, as well as their effects on incontinence.
The wide variety of treatments for urinary incontinence indicates the lack of consensus as to which procedure is the best. Provided that a sufficient number of trials of adequate quality have been conducted, the most reliable evidence is likely to come from consideration of all well‐designed randomised controlled trials. Hence, there is a need for an easily accessible, periodically updated, comprehensive systematic review of such trials. This review will help to identify optimal practice and also highlight gaps in the evidence base. The present review assesses adrenergic drugs in the management of urinary incontinence.
Objectives
To determine the effects of adrenergic agonists in the treatment of stress urinary incontinence.
The following comparisons were addressed:
1. adrenergic agonists compared with placebo or no treatment; 2. adrenergic agonists compared with conservative non‐pharmacological therapies; 3. adrenergic agonist drug therapy compared with surgery; 4. a higher dose of an adrenergic agonist compared with a lower dose; 5. one adrenergic agonist compared with another adrenergic agonist; 6. an adrenergic agonist compared with alternative forms of pharmacotherapy; 7. adrenergic agonist drug therapy used in combination with another drug compared with the other drug treatment alone; and 8. adrenergic agonist drug therapy used in combination with another drug compared with adrenergic agonist treatment alone.
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi randomised controlled trials of adrenergic drugs for the treatment of urinary incontinence.
Types of participants
Adults with urinary incontinence.
Types of interventions
At least one trial group was treated with an adrenergic agonist drug. Comparison interventions included conservative treatment (for example pelvic floor muscle training), surgery, other classes of drugs and hormones such as oestrogen.
Types of outcome measures
Both subjective and objective outcome measures were included in this review.
A. Patient symptoms assessed by history and questionnaire, or the use of urinary diaries:
perception of cure or improvement;
incontinent episodes / unit time;
pad changes / unit time;
number of micturitions / unit time.
B. Patient findings measured by pad test or urodynamics:
urinary pad test ‐ measured loss;
urodynamic‐diagnosed detrusor overactivity (DO);
urodynamic stress incontinence (USI).
C. Adverse outcomes:
numbers of people experiencing adverse effects;
numbers of people withdrawing from treatment or trial arm;
numbers of people changing dose.
D. Health status measures:
quality of life questionnaires;
psychological measures;
general health status.
E. Economic measures.
F. Other outcomes:
non pre‐specified outcomes judged important when performing the review.
Search methods for identification of studies
This review has drawn on the search strategy developed for the Cochrane Incontinence Review Group. Relevant trials were identified from the Group's Specialised Register of controlled trials which is described under the Cochrane Incontinence Group's details in The Cochrane Library (For more details please see the ‘Specialized Register’ section of the Group’s module in The Cochrane Library). The register contains trials identified from MEDLINE, CINAHL, the Cochrane Central Register of Controlled Trials (CENTRAL) and handsearching of journals and conference proceedings. Date of the most recent search of the register for this review: 15 September 2010. The trials in the Cochrane Incontinence Group Specialised Register are also contained in CENTRAL. The terms used to search the Incontinence Group trials register are given below:
(TOPIC.URINE.INCON*) AND ({DESIGN.CCT*} OR {DESIGN.RCT*}) AND ({INTVENT.CHEM.DRUG.adrenergic*}) (All searches were of the keyword field of Reference Manager 9.5 N, ISI ResearchSoft).
The review authors also searched the reference lists of relevant articles. We did not impose any language or other restrictions on any of these searches.
Data collection and analysis
Reports of studies identified as possibly eligible for the review were evaluated for methodological quality and appropriateness for inclusion by the review authors working independently and without prior consideration of the results. At least two review authors assessed the methodological quality using the Incontinence Review Group assessment criteria: quality of random allocation and concealment; description of dropout and withdrawal; analysis by intention to treat; and 'blinding' at treatment and outcome assessment. Any disagreements were resolved by discussion with a third person.
Data extraction was undertaken independently by two review authors and cross‐checked. Where data may have been collected but were not reported, further clarification was sought from the researchers. Included trial data were processed as described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2006). Trial data were considered in relation to the eight main hypotheses. Sub‐categories were identified according to the type(s) of drugs being compared. Any difference of opinion related to the data extracted was discussed and resolved with a third person.
Analysis When appropriate, meta‐analysis was undertaken. For categorical outcomes we related the numbers reporting an outcome to the numbers at risk in each group to derive a relative risk. For continuous variables we used means and standard deviations to derive a weighted mean difference. A fixed effect model was used for calculation of summary statistics (pooled estimates) and 95% confidence intervals. Differences between trials were further investigated when significant heterogeneity was found at the 10% level, or from consideration of the I2 statistic (Higgins 2003), or appeared obvious from visual inspection of the results.
For crossover trials, and trials where continuous data were reported without measures of dispersion (eg standard deviations), the data were entered into Other Data Tables and comparisons made only on the direction of effect. Crossover trials were identified by the suffix '#'. We were unable to use the generic inverse variance for crossover trials as the necessary data were not reported in the trials.
Studies were excluded from the review if they were neither randomised nor quasi‐randomised trials.
Results
Description of studies
Thirty possibly eligible studies were identified. Eight were excluded (Amark 1992; Farghaly 1987; Fossberg 1981; Jackobsen 1989; Jorgensen 1991; Radley 2001; Woodhouse 1983; Zinner 2002). The reasons for exclusion are listed in the table 'Characteristics of Excluded Studies'.
Twenty two trials were included in the review. Eleven were crossover trials (Ahlstrom 1990 #; Ani 1987 #; Beisland 1984; Collste 1987 #; Ek 1978 #; Ek 1980 #; Fossberg 1983 #; Hilton 1982 #; Kinn 1985 #; Kromann 1991 #; Musselman 2004 #) and another eight were randomised placebo‐controlled, double‐blind, parallel group trials (Gnad 1984; Gruneberger 1984; Hilton 1990; Lehtonen 1986; Lose 1988; Walter 1990; Weil 1995; Yasuda 1993). Two others included a physical intervention (pelvic floor muscle training, PFMT) (Ishiko 2000; Wells 1991). The study design of one further trial was unclear and therefore the data were not useable (Gibson 1989). There were 1099 participants in the included trials, with 713 treated with an adrenergic agonist. All trials included only women with urodynamic stress incontinence (USI) except for two: one used a symptom diagnosis for stress urinary incontinence (Ishiko 2000) and the other included some women with mixed urinary incontinence (Wells 1991). The ages of the participants ranged from 18 to 90 years. Crossover trials Crossover trials are identified by the suffix '#'.
Three trials compared an alpha adrenergic agonist plus oestrogen in one arm versus oestrogen alone in the other arm: phenylpropanolamine (Ahlstrom 1990 #; Kinn 1985 #); and norepinephrine (Ek 1980 #). No wash out period was specified and no power calculations were made.
One trial compared an alpha adrenergic agonist (phenylpropanolamine) with oestrogen but again a wash out period was not specified (Beisland 1984): but because data from the first arm of the trial was available.
In six crossover trials, comparisons were made between an alpha adrenergic agonist and placebo: phenylpropanolamine, (Ani 1987 #; Collste 1987 #; Fossberg 1983 #; Hilton 1982 #); norepinephrine, (Ek 1978 #); and Ro 115‐1240, (Musselman 2004 #). In one of these there was a one week wash out period between the two active arms (Hilton 1982 #). There was no washout period in the others.
One trial compared a beta adrenergic drug with placebo: terbutaline (Kromann 1991 #).
Parallel group trials
In two trials, participants were randomised to an alpha adrenergic agonist plus oestrogen or oestrogen alone (Hilton 1990; Walter 1990). One trial (Hilton 1990) included other arms with placebo, oestrogen and the alpha adrenergic agonist alone. In that trial, data were not reported in detail within the text and had to be estimated from graphs.
Three further trials compared an alpha adrenergic agonist with placebo: midodrine (Gnad 1984); phenylpropanolamine (Lehtonen 1986); and norepinephrine (Lose 1988).
Another compared a beta adrenergic agonist with placebo: clenbuterol (Yasuda 1993).
Another parallel group trial (Weil 1995) was the only one to compare different doses of an alpha adrenergic drug (midodrine) versus placebo. However, there were no data presented on subjective assessment in this trial.
A beta adrenergic drug, clenbuterol (Ishiko 2000) and an alpha adrenergic drug, phenylpropanolamine (Wells 1991) were compared with a physical intervention (pelvic floor muscle training) in two trials.
Gruneberger 1984 compared a beta adrenergic agonist (clenbuterol) against an antispasmodic drug, flavoxate.
One further trial included eskornade (a mixture of alpha adrenergic drugs (phenylpropanolamine and diphenylpyramine) with mazindol, an appetite suppressant similar to dexamphetamine, but its study design was unclear and it did not provide useable data (Gibson 1989).
Risk of bias in included studies
The methodology of individual trials is summarised in the table of "Characteristics of Included Studies".
The group allocation methodology was not described in detail in all trials. One trial used an adequate method of concealment of allocation, which was sealed envelopes (Ishiko 2000). We have assumed that if the authors stated that a trial was randomised, double‐blind and placebo‐controlled, allocation to groups was adequately concealed, and classed this as A. All of the trials were described as such except for three. One of these (Beisland 1984) was described as a randomised, open‐comparative trial and the other two gave no details about the method of allocation (Gruneberger 1984; Wells 1991); all three were classed as B.
Descriptions of drop‐outs, withdrawals and adverse effects were reported in all included trials. However, dropouts and adverse effects were not always recorded according to allocation to treatment.
There were 14 trials which failed to provide standard deviations for continuous data (Ahlstrom 1990 #; Ani 1987 #; Beisland 1984; Ek 1978 #; Ek 1980 #; Gruneberger 1984; Hilton 1982 #; Hilton 1990; Kinn 1985 #; Kromann 1991 #; Lehtonen 1986; Lose 1988; Musselman 2004 #; Walter 1990) and only two trials did provide standard deviations (Wells 1991; Yasuda 1993).
Outcome measures Overall, there was consistency in reporting subjective outcome measures. However, objective measures, such as the number of pads used or pad test weights were lacking in all but five of the included trials (Ani 1987 #; Hilton 1990; Kinn 1985 #; Musselman 2004 #; Yasuda 1993) while the number of incontinence episodes was described in six included trials (Ani 1987 #; Collste 1987 #; Kinn 1985 #; Musselman 2004 #; Walter 1990; Wells 1991). Health status measures and economic measures were not provided in any of the trial reports.
Effects of interventions
The 22 trials included 1099 women, of whom 713 received an adrenergic drug.
COMPARISON 1: Adrenergic agonists compared with placebo or no treatment (Comparison 01; Other Data Tables 01) Fourteen eligible trials addressed this comparison, seven crossover trials (Ani 1987 #; Collste 1987 #; Ek 1978 #; Fossberg 1983 #; Hilton 1982 #; Kromann 1991 #; Musselman 2004 #) and seven parallel group trials (Gnad 1984; Hilton 1990; Lehtonen 1986; Lose 1988; Walter 1990; Weil 1995; Yasuda 1993).
Seven trials compared phenylpropanolamine with placebo. The remainder compared:
midodrine with placebo (Gnad 1984);
three doses of midodrine with placebo (Weil 1995);
clenbuterol with placebo (Yasuda 1993);
terbutaline with placebo (but provided no useable data, except on adverse events) (Kromann 1991 #); and
a new drug (Ro 115‐1240) with placebo (Musselman 2004 #).
Using subjective outcomes, cure rates tended to favour the adrenergic drugs over the placebo (Comparison 01.01) (Gnad 1984; Lehtonen 1986; Lose 1988; Yasuda 1993) although this was only statistically significant for midodrine (Gnad 1984) and clenbuterol (Yasuda 1993). Combined cure and improvement rates also favoured adrenergics over placebo, for example:
RR for phenylpropanolamine 1.58, 95% CI 0.87 to 2.85, Comparison 01.02.01, (Hilton 1990, Lehtonen 1986);
RR for midodrine 1.55, 95% CI 1.02 to 2.35, Comparison 01.02.02, (Gnad 1984);
RR for clenbuterol 1.96, 95% CI 1.26 to 3.05, Comparison 01.02.03, (Yasuda 1993);
RR for norepinephrine 1.57, 95% CI 0.76, 3.22, Comparison 01.02.04, (Lose 1988).
There were too few data with which to compare objective cure or improvement rates for norepinephrine (Comparisons 01.03.03, 01.04.03, Lose 1988).
For all other outcome measures for which data were available, the results favoured the adrenergic group in terms of:
numbers cured or improved in crossover trials (more women improved after phenylpropanolamine than after placebo in four trials, Other Data Tables 01.09.01) (Ani 1987 #; Collste 1987 #; Fossberg 1983 #; Hilton 1982 #) (norepinephrine better than placebo in one trial, Other Data Tables 01.09.02) (Ek 1978 #);
objective improvement (while there was no evidence of improvement with phenylpropanolamine in one trial (Other Data Tables 01.10.01) (Hilton 1982 #), more women improved with norepinephrine than with placebo in a small trial (Other Data Tables 01.10.02) (Ek 1978 #);
pad changes (fewer pad changes were reported during phenylpropanolamine than during placebo in one out of two trials (Other Data Tables 01.11.01) (Hilton 1982 #; Hilton 1990); and during Ro 115‐1240 than during placebo in one trial (Other Data Tables 01.11.02) (Musselman 2004 #);
incontinence episodes (fewer incontinence episodes during phenylpropanolamine than during placebo in two trials, Other Data Tables 01.12.01) (Ani 1987 #; Collste 1987 #); and during Ro 115‐1240 than placebo in one trial (Other Data Tables 01.12.02) (Musselman 2004 #);
pad weights (lower weights during phenylpropanolamine than during placebo in two trials, Other Data Tables 01.14.01) (Ani 1987 #; Hilton 1990) and lower weights during midodrine than during placebo at each of three doses (Other Data Tables 01.14.02 to 01.14.04) (Weil 1995).
More adverse effects were reported during active treatment than during placebo (see the table of Characteristics of Included Studies for details, and Comparison 01.08), but this did not reach statistical significance in any of the individual subcategories. However, they were severe enough to cause 23 of 604 (3.8%) women in eleven trials to drop out or discontinue treatment (Ani 1987 #; Ek 1978 #; Gnad 1984; Hilton 1982 #; Hilton 1990; Kromann 1991 #; Lehtonen 1986; Lose 1988; Musselman 2004 #; Weil 1995; Yasuda 1993, see Table of Included Studies for details). In 15 of the 23 women (2.5% of total), this could be ascribed to the known stimulant side effects of alpha adrenergic drugs (insomnia, restlessness and vasomotor stimulation).
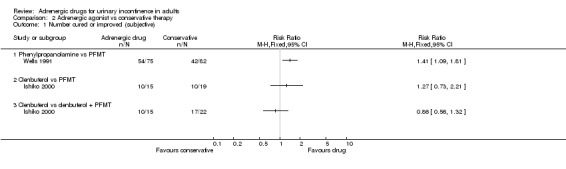
COMPARISON 2: Adrenergic agonists compared with conservative non‐pharmacological therapies (Comparison 02) In one trial, a beta‐adrenergic drug (clenbuterol) was compared with pelvic floor muscle training (PFMT) alone and with a combination of the two treatments (Ishiko 2000). In another trial an alpha‐adrenergic drug (phenylpropanolamine) was compared with PFMT alone (Wells 1991).
The trial involving clenbuterol was small and so provided only an imprecise estimate of differences in cure rates. The evidence suggested that the women preferred the drug alone (number satisfied with treatment 11of 13 (85%) versus 6 of 19 (32%), RR 2.68, 95% CI 1.33 to 5.40, Comparison 02.06.01) (Ishiko 2000). In the trial involving phenylpropanolamine, more women reported subjective cure or improvement while using the drug (54 of 75 (72%) versus 42 of 82 (51%) on PFMT, RR 1.41, 95% CI 1.09 to 1.81, Comparison 02.01.01) (Wells 1991); there was also a non‐significant trend for fewer incontinence episodes on the drug. More women dropped out from the PFMT group (described as 'failed to adhere to treatment' by the trialists), perhaps suggesting that they found the treatment onerous; they were counted as failures in assessing subjective cure or improvement rates (Wells 1991).
In a sensitivity analysis, varying the assumptions about the dropouts altered the findings: if they were treated as successes (RR 1.02, 95% CI 0.89 to 1.15), or by using data only from those remaining in the study (RR 1.08, 95% CI 0.91 to 1.30), there was no longer any evidence of difference between the groups. Objective outcomes (also available only for those remaining in the study) also did not show significant differences between the drug and PFMT.
COMPARISON 3: Adrenergic agonist drug therapy compared with surgery (Comparison 03) No eligible trials were found.
COMPARISON 4: A higher dose of an adrenergic agonist compared with a lower dose (Comparison 04, Other Data Tables 04) One eligible trial was found that compared different dosages of an alpha adrenergic agonist (midodrine) for urinary incontinence in women (Weil 1995). There were no clear differences between the doses in terms of pad weights (Other Data Tables 04.06) or adverse effects (Comparison 04.07), but the numbers were too small to draw definite conclusions.
COMPARISON 5: One adrenergic agonist compared with another adrenergic agonist (Comparison 05) No eligible trials were found.
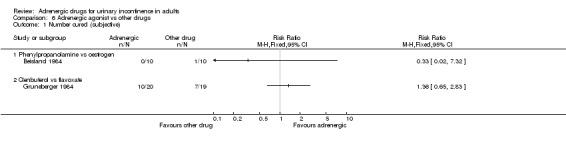
COMPARISON 6: An adrenergic agonist compared with alternative forms of pharmacotherapy (Comparison 06) Three eligible trials were found:
in one, an alpha adrenergic agonist drug (phenylpropanolamine) was compared with oestrogen (Beisland 1984);
in another trial, a beta‐adrenergic agonist (Clenbuterol) was compared with an antispasmodic / smooth muscle relaxant (Flavoxate) (Gruneberger 1984); and
in the third Eskornade was compared with Mazindol (Gibson 1989).
There were no clear differences between the two arms of two of the trials in terms of subjective cure or improvement (Comparison 06.01 and 06.02, Beisland 1984; Gruneberger 1984) or urgency with clenbuterol (Comparison 06.03.01, Gruneberger 1984). There were two adverse effects reported in one trial (Beisland 1984) but it was unclear whether these occurred during active treatment. The data were too few to comment on adverse effects due to clenbuterol and flavoxate (Comparison 06.04.01 and 06.05.01, Gruneberger 1984). It was not possible to interpret the data from the trial comparing eskornade with mazindol (Gibson 1989).
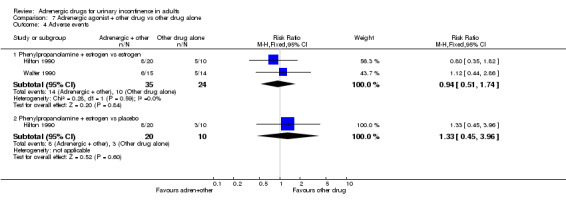
COMPARISON 7: adrenergic agonist drug therapy used in combination with another drug compared with the other drug treatment alone (Comparison 07, Other Data Table 07) Five eligible trials were identified. The comparisons were:
phenylpropanolamine plus oestrogen versus oestrogen alone (Ahlstrom 1990 #; Kinn 1985 #; Walter 1990);
phenylpropanolamine plus oestrogen versus placebo (Hilton 1990); and
norepinephrine plus oestrogen versus placebo plus oestrogen (Ek 1980 #).
There were no clear differences in terms of numbers cured or improved (Comparison 07.01 and 07.02), adverse effects (Comparison 07.04) or any other outcome for which data were available (Other Data Tables 07.06 to 07.12). Confidence intervals, where derivable, were all wide and did not rule out a clinically important difference.
COMPARISON 8: adrenergic agonist drug therapy used in combination with another drug compared with adrenergic agonist treatment alone (Comparison 08, Other Data Table 08) One eligible trial was identified (Hilton 1990) comparing phenylpropanolamine plus oestrogen in one arm versus phenylpropanolamine alone and versus placebo. The results for the combination were better than for the monotherapy or placebo in terms of numbers cured or improved (Comparison 08.01), number of pad changes (2 of 2 active treatment trial arms, Other Data Tables 08.02), and pad weights (1of 2 trial arms, Other Data Tables 08.03) but these differences did not reach statistical significance. There were no clear differences in the number of adverse effects (Comparison 08.04). Dropouts and adverse effects Only six of the trials failed to mention whether any participants dropped out (Ahlstrom 1990 #; Beisland 1984; Collste 1987 #; Fossberg 1983 #; Gibson 1989; Walter 1990). In the remainder, 118 of a total of 913 (12.9%) people dropped out, but this was attributed to adverse effects in only 38 of 118 (32.2%, or 4.2% of the total population). Adverse effects were not reported in two trials (Ek 1980 #; Wells 1991). In the remaining 20 trials, 281 adverse effects were reported in 1065 people (26.4%). Of these, 17 of 601 (28%) occurred while on adrenergic treatment, 72 of 305 (23.6%) while on placebo treatment and 39 of 140 (27.9%) in women using alternative drug treatment. No side effects were reported by 19 women undergoing PFMT (Ishiko 2000; Wells 1991) but the numbers who dropped out from PFMT treatment in one trial were high (28 of 82, 34%) (Wells 1991).
Discussion
The eligible trials in this review included comparisons of adrenergic agonists with placebo, oestrogens or pelvic muscle floor training or combinations of these treatments in women. No trials included men. Eleven of the trials tested phenylpropanolamine, two tested midodrine, three tested norepinephrine, three tested clenbuterol, one tested terbutaline and one trial tested a new selective adrenergic drug, Ro 115‐1240. The trials used adequate methods of concealment of allocation to groups (assuming that a double‐blind design was acceptable) except three which gave insufficient information for this to be assessed.
Of the 22 included trials, eleven were crossover trials, but the data were presented in the reports as if they were from parallel groups. They could not be analysed in RevMan as the appropriate statistics were not available in the trials. They are therefore reported as 'Other Data' only. An important criticism of the crossover trials was that only one trial used a washout period (Hilton 1982 #), although as the half‐life of adrenergic drugs is short this may not be so critical. All the trials were small, with an average of 42 participants per trial and 31 participants treated with an adrenergic drug. Of the nine trials which reported continuous data, only two provided standard deviations. All trials reported dropouts, withdrawals and adverse effects. In addition, most provided participant‐reported outcomes such as cure or improvement, but only five reported the objective outcome measure pad test, and only six reported incontinence episodes. None of the trials included measures of general health status or health economics. Dropouts were ignored when there were no systematic differences between the groups.
There was weak evidence to suggest that active treatment with an adrenergic agonist drug is better than placebo in reducing the number of pad changes and incontinence episodes, as well as improving subjective symptoms. However, most trials reported only that symptoms were improved; women rarely achieved complete continence, in contrast to the results of surgery for stress urinary incontinence. There was a lack of evidence to evaluate the effectiveness of dose escalation when treating people with alpha adrenergic agonists. The evidence currently available is insufficient to comment on the relative merits of an alpha adrenergic agonist compared with oestrogen therapy, whether used alone or in combination. There was no information about outcomes after treatment was finished. However, the drug would only be expected to be active during treatment. None of the trials reported on whether women who benefited from treatment continued to take an adrenergic agonist after the trial period: there is no biological reason to suppose that the benefits might persist after stopping treatment.
Although the effect of adrenergic agonists is at best moderate, they may be useful as an adjunct to other non‐pharmacological treatments such as pelvic floor muscle training as suggested by Lehtonen et al (Lehtonen 1986). In the direct comparisons of pelvic floor muscle training with adrenergic drugs, the drugs appeared to have better results than the physical exercises. This, however, depended on the assumption in one trial (Wells 1991) that the women who dropped out of the pelvic floor muscle training group had failed treatment: data for the women remaining in the trial were inconclusive, as was a sensitivity analysis assuming that the dropouts were cured. The duration of the treatments were also different: 4 weeks of drug treatment were being compared with 6 months of pelvic floor muscle training, which may explain some of the difference in compliance. The trial which compared drug treatment alone with combination treatment was too small to allow conclusions (Ishiko 2000).
Adverse effects All trials reported adverse effects, although not always according to treatment arm. While it was clear that more adverse effects occurred while participants were on the adrenergic treatment arm (28% in total), this did not reach statistical significance compared to placebo treatment. The majority of the adverse effects were minor and they also occurred during placebo (24%) or comparison (28%) treatment.
Although a total of 118 of 913 (13%) women dropped out, the side effects were characteristic of adrenergic stimulation (insomnia, restlessness and vasomotor symptoms) in only 38 of the 913 (4%). Thus, these symptoms rarely resulted in withdrawal from the trials, suggesting that side effects were not a major issue. However, rare but serious adverse effects have been reported in the literature for adrenergic drugs, including cardiac arrhythmias and severe hypertension (Clark 1983).
Dose Most of the trials of phenylpropanolamine used a dose of 50 mg twice a day. Beisland suggested that this was the minimum dose necessary to achieve a plasma level of 150 ng/ml (Beisland 1984). There may therefore be a need for a dose escalation study to assess the benefits of higher doses. The one trial which addressed this issue was too small to be reliable (Weil 1995).
Outcome measures Objective outcome measures such as the weighed pad test and urodynamic detection of urodynamic stress incontinence may reflect the effects these agents have on lower urinary tract physiology and give important information as to their likely clinical effects. However, although urodynamic investigations are undoubtedly useful diagnostic tests, urodynamic parameters per se can only be regarded as surrogate end‐points in the assessment of outcome because they have no proven correlation with clinical outcome (Meyer 1994; Swift 1995). This review, therefore, concentrated on the ultimate goal of curing or improving patients' symptoms and quality of life.
Urinary incontinence in men after prostate surgery Urinary incontinence is a recognised complication after prostate surgery (Hunskaar 2002). Treatment ranges from conservative measures such as pelvic floor muscle training (Hunter 2004), to minimally invasive urethral bulking agent injections (Pickard 2003), to more major surgery such as artificial urinary sphincter insertion. There were no controlled studies on the use of adrenergic agonist drugs for incontinence after prostatectomy, but they might have a place in management. This should be tested by randomised controlled trials.
Authors' conclusions
Implications for practice.
There is some weak evidence in support of adrenergic agonists as effective treatment for incontinence compared with placebo treatment. There was not enough evidence to assess their effectiveness in relation to other treatments. They do have minor side effects, which may sometimes result in stopping treatment. Rare but serious side effects such as cardiac arrhythmias and hypertension have also been reported in the literature.
Implications for research.
Larger trials would strengthen the evidence base for the use of adrenergic drugs for the treatment of stress urinary incontinence. In particular, their use needs to be evaluated in comparison with other effective treatments such as surgery and pelvic floor muscle training, and in combination with these alternatives, both in terms of continence and unwanted effects. A randomised controlled trial in men with post‐prostatectomy incontinence should be considered. Trials should use standardised outcomes including both subjective and objective measures of cure and improvement, general health status measures, and quality of life and health economic outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 10 November 2010 | Review declared as stable | drugs no longer used, no trials to add |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 13 August 2008 | Amended | Converted to new review format. |
| 25 May 2005 | New citation required and conclusions have changed | Substantive amendment |
Notes
Adrenergic drugs are no longer generally used for treatment of stress urinary incontinence in adults
Acknowledgements
SC Radley, U Azam, PG Collin, DH Richmond and CR Chapple wrote the original protocol for this review. We thank Dr. Bettina Wagner for translation of a German paper.
Data and analyses
Comparison 1. Adrenergic agonist vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number cured (subjective) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Phenylpropanolamine vs placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Midodrine vs placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Clenbuterol vs placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Norepinephrine vs placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number cured or improved (subjective) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Phenylpropanolamine vs placebo | 2 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.87, 2.85] |
| 2.2 Midodrine vs placebo | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.02, 2.35] |
| 2.3 Clenbuterol vs placebo | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.26, 3.05] |
| 2.4 Norepinephrine vs placebo | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.76, 3.22] |
| 3 Number cured (objective) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Phenylpropanolamine vs placebo | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Midodrine vs placebo | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Norepinephrine vs placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number improved (objective) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Phenylpropanolamine vs placebo | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Midodrine vs placebo | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Norepinephrine vs placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Incontinence episode/24 hrs | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Phenylpropanolamine vs placebo | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Midodrine vs placebo | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pad changes/24 hrs | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Phenylpropanolamine vs placebo | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Midodrine vs placebo | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Pad weight/24 hrs | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Phenylpropanolamine vs placebo | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Midodrine vs placebo | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Clenbuterol vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse events | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Phenylpropanolamine vs placebo | 2 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.40, 2.06] |
| 8.2 5 mg Midodrine vs placebo | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.57, 2.52] |
| 8.3 7.5 mg Midodrine vs placebo | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.75, 2.22] |
| 8.4 10 mg Midodrine vs placebo | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.97, 3.51] |
| 8.5 Clenbuterol vs placebo | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.59, 2.54] |
| 8.6 Norepinephrine vs placebo | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.57 [0.58, 35.96] |
| 9 Number cured or improved (subjective)‐ cross‐over trial # | Other data | No numeric data | ||
| 9.1 Phenylpropanolamine vs placebo | Other data | No numeric data | ||
| 9.2 Norepinephrine vs placebo | Other data | No numeric data | ||
| 10 Number improved (objective)‐ cross‐over trial # | Other data | No numeric data | ||
| 10.1 Phenylpropanolamine vs placebo | Other data | No numeric data | ||
| 10.2 Norepinephrine vs placebo | Other data | No numeric data | ||
| 11 Pad changes/24 hrs‐ cross‐over trial # or no SDs | Other data | No numeric data | ||
| 11.1 Phenylpropanolamine vs placebo | Other data | No numeric data | ||
| 11.2 Ro 115‐1240 vs placebo | Other data | No numeric data | ||
| 12 Incontinence episode/24 hrs‐ cross‐over trial # | Other data | No numeric data | ||
| 12.1 Phenylpropanolamine vs placebo | Other data | No numeric data | ||
| 12.2 Ro 115‐1240 vs placebo | Other data | No numeric data | ||
| 13 Adverse events ‐ cross‐over trial # | Other data | No numeric data | ||
| 13.1 Phenylpropanolamine vs placebo | Other data | No numeric data | ||
| 13.2 Norepinephrine vs placebo | Other data | No numeric data | ||
| 13.3 Ro 115‐1240 vs placebo | Other data | No numeric data | ||
| 13.4 Terbutaline vs placebo | Other data | No numeric data | ||
| 14 Pad weight/24 hrs‐ cross‐over trial # or no SDs | Other data | No numeric data | ||
| 14.1 Phenylpropanolamine vs placebo | Other data | No numeric data | ||
| 14.2 Midodrine 5 mg vs placebo | Other data | No numeric data | ||
| 14.3 Midodrine 7.5 mg vs placebo | Other data | No numeric data | ||
| 14.4 Midodrine 10 mg vs placebo | Other data | No numeric data |
1.1. Analysis.

Comparison 1 Adrenergic agonist vs placebo, Outcome 1 Number cured (subjective).
1.2. Analysis.

Comparison 1 Adrenergic agonist vs placebo, Outcome 2 Number cured or improved (subjective).
1.3. Analysis.

Comparison 1 Adrenergic agonist vs placebo, Outcome 3 Number cured (objective).
1.4. Analysis.

Comparison 1 Adrenergic agonist vs placebo, Outcome 4 Number improved (objective).
1.7. Analysis.

Comparison 1 Adrenergic agonist vs placebo, Outcome 7 Pad weight/24 hrs.
1.8. Analysis.

Comparison 1 Adrenergic agonist vs placebo, Outcome 8 Adverse events.
1.9. Analysis.
Comparison 1 Adrenergic agonist vs placebo, Outcome 9 Number cured or improved (subjective)‐ cross‐over trial #.
| Number cured or improved (subjective)‐ cross‐over trial # | ||
|---|---|---|
| Study | Adrenergic drug | Placebo |
| Phenylpropanolamine vs placebo | ||
| Ani 1987 # | 9/20 | 3/20 |
| Collste 1987 # | 14/24 | 4/24 |
| Fossberg 1983 # | 13/23 | 4/23 |
| Hilton 1982 # | 9/19 | 3/19 |
| Norepinephrine vs placebo | ||
| Ek 1978 # | 12/22 | 1/22 |
1.10. Analysis.
Comparison 1 Adrenergic agonist vs placebo, Outcome 10 Number improved (objective)‐ cross‐over trial #.
| Number improved (objective)‐ cross‐over trial # | ||
|---|---|---|
| Study | Adrenergic drug | Placebo |
| Phenylpropanolamine vs placebo | ||
| Hilton 1982 # | 0/22 | 0/22 |
| Norepinephrine vs placebo | ||
| Ek 1978 # | 4/22 | 1/22 |
1.11. Analysis.
Comparison 1 Adrenergic agonist vs placebo, Outcome 11 Pad changes/24 hrs‐ cross‐over trial # or no SDs.
| Pad changes/24 hrs‐ cross‐over trial # or no SDs | ||
|---|---|---|
| Study | Adrenergic drug | Placebo |
| Phenylpropanolamine vs placebo | ||
| Hilton 1982 # | mean=2.1 (SD 2.4), n=19 | mean=3.0 (SD 2.6), n=19 |
| Hilton 1990 | mean=2, n = 10 | mean=1.1, n=10 |
| Ro 115‐1240 vs placebo | ||
| Musselman 2004 # | mean=9.90 (SEM 0.61), n=34 | mean=11.69 (SEM 0.61), n=34 |
1.12. Analysis.
Comparison 1 Adrenergic agonist vs placebo, Outcome 12 Incontinence episode/24 hrs‐ cross‐over trial #.
| Incontinence episode/24 hrs‐ cross‐over trial # | ||
|---|---|---|
| Study | Adrenergic drug | Placebo |
| Phenylpropanolamine vs placebo | ||
| Ani 1987 # | 1.04 (SD 0.72), n=20 | 2.05 (1.46), n=20 |
| Collste 1987 # | 1 (SD 1.5), n=24 | 3 (3), n=24 |
| Ro 115‐1240 vs placebo | ||
| Musselman 2004 # | 7.16 (SEM 0.46), n=34 | 8.67 (SEM 0.46), n=34 |
1.13. Analysis.
Comparison 1 Adrenergic agonist vs placebo, Outcome 13 Adverse events ‐ cross‐over trial #.
| Adverse events ‐ cross‐over trial # | ||
|---|---|---|
| Study | Adrenergic drug | Placebo |
| Phenylpropanolamine vs placebo | ||
| Ani 1987 # | 4/22 (of whom 2 dropped out) | 0/20 |
| Collste 1987 # | 3/24 | 3/24 |
| Hilton 1982 # | 11/22 | 1/22 |
| Norepinephrine vs placebo | ||
| Ek 1978 # | 5/22 Withdrew: 2/25 | 0/22 Withdrew: 1/25 |
| Ro 115‐1240 vs placebo | ||
| Musselman 2004 # | 30/36 | 26/36 |
| Terbutaline vs placebo | ||
| Kromann 1991 # | 4/17 | 2/17 |
1.14. Analysis.
Comparison 1 Adrenergic agonist vs placebo, Outcome 14 Pad weight/24 hrs‐ cross‐over trial # or no SDs.
| Pad weight/24 hrs‐ cross‐over trial # or no SDs | ||
|---|---|---|
| Study | Adrenergic drug | Placebo |
| Phenylpropanolamine vs placebo | ||
| Ani 1987 # | mean urine loss on pad = 6.67 ml (SD 10.51), n=20 | mean urine loss on pad = 13.87 ml (SD 17.84), n=20 |
| Hilton 1990 | mean pad weight = 4 gms, n=10 | mean pad weight = 12, n=10 |
| Midodrine 5 mg vs placebo | ||
| Weil 1995 | n= 25, median change from baseline = ‐ 3.4 gms | n= 24, median change from baseline = + 0.5 gms |
| Midodrine 7.5 mg vs placebo | ||
| Weil 1995 | n= 30, median change from baseline = ‐ 0.5 gms | n= 24, median change from baseline = + 0.5 gms |
| Midodrine 10 mg vs placebo | ||
| Weil 1995 | n= 26, median change from baseline = ‐ 1.0 gms | n= 24, median change from baseline = + 0.5 gms |
Comparison 2. Adrenergic agonist vs conservative therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number cured or improved (subjective) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Phenylpropanolamine vs PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Clenbuterol vs PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Clenbuterol vs clenbuterol + PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number cured (objective) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Phenylpropanolamine vs PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number cured or improved (objective) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Phenylpropanolamine vs PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Incontinence episode/24 hrs | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Phenylpropanolamine vs PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Clenbuterol vs PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Clenbuterol vs clenbuterol + PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Satisfaction with treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Clenbuterol vs PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Clenbuterol vs clenbuterol + PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
Comparison 2 Adrenergic agonist vs conservative therapy, Outcome 1 Number cured or improved (subjective).
2.2. Analysis.

Comparison 2 Adrenergic agonist vs conservative therapy, Outcome 2 Number cured (objective).
2.3. Analysis.

Comparison 2 Adrenergic agonist vs conservative therapy, Outcome 3 Number cured or improved (objective).
2.4. Analysis.

Comparison 2 Adrenergic agonist vs conservative therapy, Outcome 4 Incontinence episode/24 hrs.
2.5. Analysis.

Comparison 2 Adrenergic agonist vs conservative therapy, Outcome 5 Adverse events.
2.6. Analysis.

Comparison 2 Adrenergic agonist vs conservative therapy, Outcome 6 Satisfaction with treatment.
Comparison 4. Lower dose of an adrenergic agonist vs higher dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number cured (subjective) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number improved (subjective) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Number cured (objective) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number improved (subjective) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Pad weight/24 hrs | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Pad weight/ 24 hrs‐ cross‐over trial | Other data | No numeric data | ||
| 6.1 Midodrine 5 mg vs midodrine 7.5 mg | Other data | No numeric data | ||
| 6.2 Midodrine 5 mg vs midodrine 10 mg | Other data | No numeric data | ||
| 6.3 Midodrine 7.5 mg vs midodrine 10 mg | Other data | No numeric data | ||
| 7 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.2 5 mg Midodrine vs 7.5 mg Midodrine | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 5 mg Midodrine vs 10 mg Midodrine | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 7.5 mg Midodrine vs 10 mg Midodrine | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.6. Analysis.
Comparison 4 Lower dose of an adrenergic agonist vs higher dose, Outcome 6 Pad weight/ 24 hrs‐ cross‐over trial.
| Pad weight/ 24 hrs‐ cross‐over trial | ||
|---|---|---|
| Study | Lower dose | Higher dose |
| Midodrine 5 mg vs midodrine 7.5 mg | ||
| Weil 1995 | n= 25, median change from baseline= ‐3.4 gms | n= 30, median change from baseline = ‐0.5 gms |
| Midodrine 5 mg vs midodrine 10 mg | ||
| Weil 1995 | n= 25, median change from baseline= ‐3.4 gms | n= 26, median change from baseline= ‐ 1.0 gms |
| Midodrine 7.5 mg vs midodrine 10 mg | ||
| Weil 1995 | n= 30, median change from baseline= ‐0.5 gms | n= 26, median change from baseline= ‐1.0 gms |
4.7. Analysis.

Comparison 4 Lower dose of an adrenergic agonist vs higher dose, Outcome 7 Adverse events.
Comparison 6. Adrenergic agonist vs other drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number cured (subjective) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Phenylpropanolamine vs oestrogen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Clenbuterol vs flavoxate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number cured or improved (subjective) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Phenylpropanolamine vs oestrogen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Clenbuterol vs flavoxate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number with urgency or urge incontinence improved | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Clenbuterol vs flavoxate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Phenylpropanolamine vs oestrogen | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Clenbuterol vs flavoxate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Withdrawal due to adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Phenylpropanolamine vs oestrogen | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Clenbuterol vs flavoxate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
Comparison 6 Adrenergic agonist vs other drugs, Outcome 1 Number cured (subjective).
6.2. Analysis.

Comparison 6 Adrenergic agonist vs other drugs, Outcome 2 Number cured or improved (subjective).
6.3. Analysis.

Comparison 6 Adrenergic agonist vs other drugs, Outcome 3 Number with urgency or urge incontinence improved.
6.4. Analysis.

Comparison 6 Adrenergic agonist vs other drugs, Outcome 4 Adverse events.
6.5. Analysis.

Comparison 6 Adrenergic agonist vs other drugs, Outcome 5 Withdrawal due to adverse effects.
Comparison 7. Adrenergic agonist + other drug vs other drug alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number cured (subjective) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Phenylpropanolamine + estrogen vs estrogen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number cured or improved (subjective) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Phenylpropanolamine + estrogen vs estrogen | 2 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.70, 2.95] |
| 2.2 Phenylpropanolamine + estrogen vs placebo | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.66, 9.20] |
| 3 Incontinence episode/24 hrs | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Phenylpropanolamine + estrogen vs estrogen | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Phenylpropanolamine + estrogen vs estrogen | 2 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.51, 1.74] |
| 4.2 Phenylpropanolamine + estrogen vs placebo | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.45, 3.96] |
| 6 Incontinence episodes/24 hrs ‐ (No SDs) | Other data | No numeric data | ||
| 6.1 Phenylpropanolamine + estrogen vs placebo | Other data | No numeric data | ||
| 6.2 Phenylpropanolamine + estrogen vs estrogen | Other data | No numeric data | ||
| 7 No of pads/24 hrs ‐ (No SDs) | Other data | No numeric data | ||
| 7.1 Phenylpropanolamine + estrogen vs placebo | Other data | No numeric data | ||
| 7.2 Phenylpropanolamine + estrogen vs estrogen | Other data | No numeric data | ||
| 8 Pad weight/24 hrs ‐ (No SDs) | Other data | No numeric data | ||
| 8.1 Phenylpropanolamine + estrogen vs placebo | Other data | No numeric data | ||
| 8.2 Phenylpropanolamine + estrogen vs estrogen | Other data | No numeric data | ||
| 9 Cured or improved (subjective) ‐ cross‐over trials # | Other data | No numeric data | ||
| 9.1 Phenylpropanolamine + estrogen vs estrogen | Other data | No numeric data | ||
| 9.2 Norepinephrine + oestrogen vs placebo + oestrogen | Other data | No numeric data | ||
| 10 Incontinence episode/24 hrs‐ cross‐over trial # | Other data | No numeric data | ||
| 10.1 Phenylpropanolamine + estrogen vs estrogen | Other data | No numeric data | ||
| 11 Pad weight/24 hrs‐ cross‐over trial # | Other data | No numeric data | ||
| 11.1 Phenylpropanolamine + estrogen vs estrogen | Other data | No numeric data | ||
| 12 Adverse events ‐ cross‐over trials # | Other data | No numeric data | ||
| 12.1 Phenylpropanolamine + estrogen vs estrogen | Other data | No numeric data |
7.1. Analysis.

Comparison 7 Adrenergic agonist + other drug vs other drug alone, Outcome 1 Number cured (subjective).
7.2. Analysis.

Comparison 7 Adrenergic agonist + other drug vs other drug alone, Outcome 2 Number cured or improved (subjective).
7.4. Analysis.
Comparison 7 Adrenergic agonist + other drug vs other drug alone, Outcome 4 Adverse events.
7.6. Analysis.
Comparison 7 Adrenergic agonist + other drug vs other drug alone, Outcome 6 Incontinence episodes/24 hrs ‐ (No SDs).
| Incontinence episodes/24 hrs ‐ (No SDs) | ||
|---|---|---|
| Study | Adrenergic + other | Other drug alone |
| Phenylpropanolamine + estrogen vs estrogen | ||
| Walter 1990 | mean=2, n=28 | mean=0.8, n=29 |
7.7. Analysis.
Comparison 7 Adrenergic agonist + other drug vs other drug alone, Outcome 7 No of pads/24 hrs ‐ (No SDs).
| No of pads/24 hrs ‐ (No SDs) | ||
|---|---|---|
| Study | Adrenergic + other | Other drug alone |
| Phenylpropanolamine + estrogen vs placebo | ||
| Hilton 1990 | n= 30, mean = 1.2 pad changes/day | n= 10, mean = 1.1 pad changes/day |
| Phenylpropanolamine + estrogen vs estrogen | ||
| Hilton 1990 | n= 30, mean = 1.2 pad changes/day | n= 20, mean = 0.8 pad changes/day |
7.8. Analysis.
Comparison 7 Adrenergic agonist + other drug vs other drug alone, Outcome 8 Pad weight/24 hrs ‐ (No SDs).
| Pad weight/24 hrs ‐ (No SDs) | ||
|---|---|---|
| Study | Adrenergic + other | Other drug alone |
| Phenylpropanolamine + estrogen vs placebo | ||
| Hilton 1990 | n= 30, mean change = ‐ 5.3 gms | n= 10, mean change = ‐ 12 gms |
| Phenylpropanolamine + estrogen vs estrogen | ||
| Hilton 1990 | n= 30, mean change = ‐ 5.3 gms | n= 20, mean change = ‐ 4 gms |
| Walter 1990 | n= 15, mean = 5 gms | n= 14, mean = 15 gms |
7.9. Analysis.
Comparison 7 Adrenergic agonist + other drug vs other drug alone, Outcome 9 Cured or improved (subjective) ‐ cross‐over trials #.
| Cured or improved (subjective) ‐ cross‐over trials # | ||
|---|---|---|
| Study | Adrenergic + other | Other drug alone |
| Phenylpropanolamine + estrogen vs estrogen | ||
| Ahlstrom 1990 # | 10/27 | 5/26 |
| Kinn 1985 # | 16/30 | 9/30 |
| Norepinephrine + oestrogen vs placebo + oestrogen | ||
| Ek 1980 # | 8/13 | 5/13 |
7.10. Analysis.
Comparison 7 Adrenergic agonist + other drug vs other drug alone, Outcome 10 Incontinence episode/24 hrs‐ cross‐over trial #.
| Incontinence episode/24 hrs‐ cross‐over trial # | ||
|---|---|---|
| Study | Adrenergic + other | Other drug alone |
| Phenylpropanolamine + estrogen vs estrogen | ||
| Kinn 1985 # | n=36, mean= 2.4 | n= 36, mean= 2.4 |
7.11. Analysis.
Comparison 7 Adrenergic agonist + other drug vs other drug alone, Outcome 11 Pad weight/24 hrs‐ cross‐over trial #.
| Pad weight/24 hrs‐ cross‐over trial # | ||
|---|---|---|
| Study | Adrenergic + other | Other drug alone |
| Phenylpropanolamine + estrogen vs estrogen | ||
| Kinn 1985 # | n= 36, mean= 24.9 | n= 36, mean= 34.9 |
7.12. Analysis.
Comparison 7 Adrenergic agonist + other drug vs other drug alone, Outcome 12 Adverse events ‐ cross‐over trials #.
| Adverse events ‐ cross‐over trials # | ||
|---|---|---|
| Study | Adrenergic + other | Other drug alone |
| Phenylpropanolamine + estrogen vs estrogen | ||
| Ahlstrom 1990 # | 3/29 | 7/29 |
Comparison 8. Adrenergic agonist + other drug vs adrenergic agonist alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number cured or improved (subjective) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Phenylpropanolamine + estrogen vs placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Phenylpropanolamine + estrogen vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 No of pads/24 hrs ‐ (No SDs) | Other data | No numeric data | ||
| 2.1 Phenylpropanolamine + estrogen vs placebo | Other data | No numeric data | ||
| 2.2 Phenylpropanolamine + estrogen vs PPA | Other data | No numeric data | ||
| 3 Pad weight/24 hrs ‐ (No SDs) | Other data | No numeric data | ||
| 3.1 Phenylpropanolamine + estrogen vs placebo | Other data | No numeric data | ||
| 3.2 Phenylpropanolamine + estrogen vs PPA | Other data | No numeric data | ||
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Phenylpropanolamine + estrogen vs placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Phenylpropanolamine + estrogen vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.

Comparison 8 Adrenergic agonist + other drug vs adrenergic agonist alone, Outcome 1 Number cured or improved (subjective).
8.2. Analysis.
Comparison 8 Adrenergic agonist + other drug vs adrenergic agonist alone, Outcome 2 No of pads/24 hrs ‐ (No SDs).
| No of pads/24 hrs ‐ (No SDs) | ||
|---|---|---|
| Study | Adrenergic + other | Adrenergic |
| Phenylpropanolamine + estrogen vs placebo | ||
| Hilton 1990 | mean=0.9. n=20 | mean=1.1, n=10 |
| Phenylpropanolamine + estrogen vs PPA | ||
| Hilton 1990 | mean=0.9, n=20 | mean=2, n=10 |
8.3. Analysis.
Comparison 8 Adrenergic agonist + other drug vs adrenergic agonist alone, Outcome 3 Pad weight/24 hrs ‐ (No SDs).
| Pad weight/24 hrs ‐ (No SDs) | ||
|---|---|---|
| Study | Adrenergic+ other | Adrenergic |
| Phenylpropanolamine + estrogen vs placebo | ||
| Hilton 1990 | mean=6, n=20 | mean=12, n=10 |
| Phenylpropanolamine + estrogen vs PPA | ||
| Hilton 1990 | mean=6, n=20 | mean=4, n=10 |
8.4. Analysis.

Comparison 8 Adrenergic agonist + other drug vs adrenergic agonist alone, Outcome 4 Adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahlstrom 1990 #.
| Methods | RCT (double blind cross‐over) ITT | |
| Participants | n= 29 women Inclusion: USI, postmenopausal Exclusion: Hypertension, significant bacteruria, previous breast or uterine cancer, residual urine, drugs (neuroleptics, sedatives, antihistamines, ephedrine, B‐blockers, gestagens, estrogens within previous 2 months Mean age 63 (R 51‐73) years Mean weight 71 (R 55‐90) kg | |
| Interventions | I (29): Estriol (4 mg) + PPA (50 mg twice a day) II (29): Estriol + placebo Oral tablets Duration of treatment: 6 weeks each arm | |
| Outcomes | Subjective cure rate: I: 4/27, II: 2/26 Subjective improved rate: I: 6/27, II: 3/26 Residual urine volume change: mean difference= ‐2.1 (SEM 7.5), n= 27, P= 0.127, Adverse events: I: 3/29, II: 8/29 | |
| Notes | No power calculation Wash out period not mentioned No SDs Comparisons: Alpha adrenergic + estrogen versus estrogen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ani 1987 #.
| Methods | RCT (double blind, placebo controlled cross‐over trial | |
| Participants | n= 20 women Dropouts: 2 further women stopped due to side effects (headache, skin rash) Inclusion: Genuine stress incontinence (now urodynamic stress incontinence, USI) Exclusion.: Urethral instability, previous incontinence surgery | |
| Interventions | I (20): PPA 50 mg 3 times /day II (20): Placebo Duration: 4 weeks | |
| Outcomes | Subjective improvement: I: 9/20, II: 3/20 Subjectively same or worse: I: 8/20, II: 8/20 Incontinent episodes /day N, mean (SD): I: 20, 1.04 (0.72), II: 20, 2.05 (1.46) Pad test (ml urine lost): I: 20,6.67 (10.51), II: 20, 13.87 (17.84) Adverse effects: I: 1/22 (headaches, dizziness, stopped trial), 1 (skin rash, stopped trial), 2/20 (mild insomnia) Bacteriuria (4) and cystitis (1) but not clear which arm of trial Urodynamic measures, BP and pulse rate also measured | |
| Notes | No power calculations Limited data Comparison: PPA versus placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Beisland 1984.
| Methods | RCT (cross‐over trial, but data available from first arm) | |
| Participants | n= 20 women Inclusion: Urinary incontinence Exclusion: Neurological disorders, gynaecological disorders, UTI, tumours, general conditions that contra‐indicate PPA or oestrogen Age mean (range): 69 years (49‐84) | |
| Interventions | I (10): Estriol (1 mg vaginal suppository) for 4 weeks followed by PPA (50 mg twice daily) in second arm II (10): PPA (50 mg twice daily) for 4 weeks followed by Estriol (1 mg vaginal suppository) in second arm Further non‐randomised period: I & II combined (20): Estriol (1 mg PV) + PPA (50 mg twice daily) for 4 weeks. | |
| Outcomes | Data from first arm of trial: Subjective cure: I: 1/10, II: 0/10 Subjective improvement: I: 3/10, II: 8/10 Subjectively worse: I: 6/10, II: 2/10 Adverse events: 1 complete insomnia on PPA but unclear which arm of trial | |
| Notes | No power calculation Washout period not mentioned No SDs Comparisons: Alpha adrenergic drug versus estrogen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Collste 1987 #.
| Methods | RCT (double blind cross‐over) | |
| Participants | n= 24 women Inclusion: USI Exclusion: Pelvic surgery, oestrogen, sympathomimetic drugs < 1 year, cardiovascular disease, UTI, non‐infectious irritative symptoms, DO Age mean (range): 47 years (36‐65) | |
| Interventions | I (24): PPA (50 mg twice daily) II (24): Placebo (twice daily) Duration of treatment: 14 days | |
| Outcomes | Subjective cure: I: 7/24, II: 0/24 Subjective improvement: I: 7/24, II: 4/24 Subjectively worse: I: 10/24, II: 20/24 Incontinence episode/day: I: 1+/‐ 1.5 (n=21), II: 3 +/‐ 3 (n=21) Number of voids/day: I: 9.5+/‐ 7.5, II: 9+/‐ 6.5 (n= 21) Adverse events: I: 3/24 (insomnia, nausea), II: 3/24 (insomnia, headache) | |
| Notes | No power calculation Washout period not mentioned Comparisons: Alpha adrenergic drug versus placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ek 1978 #.
| Methods | RCT (double blind cross‐over) | |
| Participants | n= 25 women Inclusion: SUI (slight 16, moderate 9) Exclusion: not specified Menopausal: 13/25 Parous: 21/25 Previous surgery: 9/25 Age range (mean): 37‐71 (54) years | |
| Interventions | I (25): Norepinephrine chloride 200 mg twice daily, orally, slow release tablets II (25): Placebo twice daily, orally Duration: 4 weeks | |
| Outcomes | Subjective cure: I: 2/22, II: 0/22 Subjective cure or improvement: I: 12/22, II: 1/22 Subjectively unchanged: I: 9/22, II: 9/22 Objective cure (cough test): I: 4/22, I: 1/22 Adverse events: I: 5/22, II: 0/22 (insomnia 1, exanthema 1, voiding difficulty 3) Numbers withdrawn: I: 2/22, II: 1/22 | |
| Notes | No power calculations No washout period 3 months after end of trial: 8/22 continued on unmodified norephedrine: 6/8 on continuous norephedrine, 2/8 on intermittent norepinephrine Comparison: Adrenergic (norepinephrine) versus placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ek 1980 #.
| Methods | RCT (double blind cross‐over) | |
| Participants | n= 13 women Dropouts: 3 other women (I: did not like urodynamics, II: gastritis 1, nausea and dryness 1) Inclusion: mild SUI 9, moderate SUI 7 Exclusion: not given Previous treatment: 9 women had been in previous trial (Ek 1978) Age: 61 years (range 38‐71) Parity (mean): 2 (0‐5) Menopause (mean): 11.5 years ago (0.5‐24) | |
| Interventions | I (13): Oestradiol + placebo II (13): Oestradiol + norepinephrine All women also treated with oestradiol valerate (2 mg /day for 3 weeks then 1 mg /day) Duration of treatment: 2 weeks in 5th and 7th week of oestradiol treatment | |
| Outcomes | Improved: I: 5/13, II: 8/13 | |
| Notes | No power calculations No washout period Comparison: Adrenergic (norepinephrine) + oestradiol versus oestradiol alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Fossberg 1983 #.
| Methods | RCT (randomised placebo‐controlled cross‐over) | |
| Participants | n= 23 women Inclusion: USI Exclusion: Recurrent UTI, uninhibited DO, neurological disease, severe cardiovascular disease Age mean (range): 53 years (36‐77) Previous anti‐incontinence operations = 8 Previous gynaecological operations = 1 | |
| Interventions | I (23): PPA (50 mg twice daily) II (23): Placebo (twice daily) Duration of treatment: 14 Days | |
| Outcomes | Subjective cure: I: 0/23, II: 0/23 Subjective improvement: I: 13/23, II: 4/23 Subjectively worse: I: 7/23, II: 16/23 Adverse events: 4/23 but not clear during which phase (rash, insomnia, itch, restlessness) | |
| Notes | No power calculation Washout period not mentioned Comparisons: Alpha adrenergic drug (PPA) versus placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gibson 1989.
| Methods | RCT (randomised double‐blind placebo controlled parallel) | |
| Participants | n= 60 women Inclusion: USI Exclusion: patients with medical indications Age range 18‐81 years | |
| Interventions | I (20): Eskornade vs placebo/Mazindol II (20): Mazindol vs Placebo/ Eskornade III (20): Eskornade/ placebo vs Mazindol/placebo | |
| Outcomes | Adverse events: (Placebo): dry mouth, blurred vision and drowsiness in 5 participants. (Mazondol): Dry mouth, nausea and insomnia in 7 participants. (Eskornade): dry mouth, nausea and headache in 5 participants. | |
| Notes | Data not useable (uninterpretable due to lack of information about study design and numbers in groups, ? may have been a cross‐over trial) Dosage of drugs not mentioned Eskornade is a mixture of diphenylpyramine and phenylpropanolamine Mazindol is an appetite suppressant, similar to dexamphetamine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gnad 1984.
| Methods | RCT (randomised placebo‐controlled) | |
| Participants | n= 48 women Inclusion: USI grade I and II Exclusion: Neurological disease, cardiovascular disease, metabolic disease, UTI, previous incontinence surgery Age range 29‐58 years | |
| Interventions | I (26): Midodrine (2.5 mg thrice daily) II (22): Placebo (Thrice daily) Duration of treatment: 30 days | |
| Outcomes | Subjective cure: I: 16/26, II: 6/22 Subjective improvement: I: 6/26, II: 6/22 Subjectively unchanged: I: 4/26, II: 10/22 Adverse events: I: 10/26, II: 5/22 (Sleep disturbances, tachycardia, pilo‐erection and abdominal pain) Drop‐out: I: 0/26, II: 1/22 (one woman discontinued during the placebo arm due to abdominal pain) | |
| Notes | No power calculation No SDs Comparisons: Alpha adrenergic (midodrine) versus placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gruneberger 1984.
| Methods | RCT (randomly assigned to parallel groups) | |
| Participants | n= 39 women Inclusion: motor urge incontinence (cause defined in 23 women: radiation, previous pelvic surgery, spinal disc operation, poliomyelitis Exclusion: urogenital inflammation, subvesical obstruction, gynaecological abnormalities, retention of residual urine (>30 ml) Age (years, mean, SD): I: 53 (11.8), II: 48 (10) | |
| Interventions | I (20): Clenbuterol (0.01 mg 3 times/day) II (19): Flavoxate hydrochloride (200 mg 3 times/day) Duration of treatment: 6 weeks | |
| Outcomes | Cured: I: 10/20, II: 7/19 Improved or cured: I: 15/20, II: 11/19 No change: I: 4/20, II: 3/19 Urge incontinence or urgency improved: I: 5/20, II: 3/19 Treatment abandoned: I: 1/20 (interaction with other drug), II: 5/19 (side effects) Side effects: I: trembling of fingers and tachycardia (4); nervousness (3); interaction with other drug, clonidine (1), II: Withdrew due to side effects (4 gastrointestinal, 1 neurosis); other transitory gastro‐intestinal effects (5) | |
| Notes | Groups comparable at baseline Comparison: Adrenergic (clenbuterol) versus antispasmodic / muscle relaxant (flavoxate) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hilton 1982 #.
| Methods | RCT (randomised double blind cross‐over) | |
| Participants | n= 22 women Dropouts: 3 (2 UTI, 1 sudomotor side‐effects) Inclusion: USI Exclusion: No mention of any exclusion criteria | |
| Interventions | I (19): PPA (5 mg thrice daily) II (19): Placebo (thrice daily) Duration of treatment: 6 weeks Washout period: 1 week | |
| Outcomes | Subjective improvement: I: 9/19, II: 3/19 Objective improvement: I: 0/19, II: 0/19 Number of pads/day: I: 2.1+/‐ 2.4, II: 3.0 +/‐ 2.6 Adverse events: I: 11/22, II: 1/22 (vasomotor, pilomotor or sudomotor functions) | |
| Notes | No power calculation No SDs Comparisons: Alpha adrenergic drug (PPA) versus placebo Two withdrawals due to UTI and another one due to severe sudomotor side effects. Complete data only available on 19 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hilton 1990.
| Methods | RCT (randomised double blind placebo‐controlled) | |
| Participants | n= 60 women Inclusion: USI, postmenopausal Exclusion: Undiagnosed vaginal bleeding, oestrogen therapy within preceding 6 months, malignancy of oestrogen‐dependent tissue, BP >160 mmHg systolic, >100 mmHg diastolic, previous thromboembolic disease, liver disease, patients withholding informed consent Age range 45‐70 years | |
| Interventions | I (10): Intravaginal oestrogen (2 gr nocturnal) + PPA (50 mg twice daily) II (10): Intravaginal oestrogen (2 gr nocturnal) + Placebo (twice daily) III (10): Oral oestrogen (1.25 mg daily) + PPA (50 mg twice daily) IV (10): Oral oestrogen (1.25 mg daily) + placebo (twice daily) V (10): PPA (50 mg twice daily) VI (10): Intravaginal placebo (nocturnal) + Placebo (twice daily) Duration of treatment: 4 weeks | |
| Outcomes | Subjective improvement: I: 9/10, II: 1/9, III: 4/10, IV: 2/10, V: 0/9, VI: 2/11 Mean number of pads/day: I: 0.9, II: 0.3, III: 0.9, IV: 1.3, V: 2, VI: 1.1 Mean pad weights: I: 8, II: 4, III: 4, IV: 4, V: 4, VI: 12 Adverse events: I: 3/10, II: 3/10, III: 5/10, IV: 5/10, V: 4/10, VI: 3/10 (headache, nausea, flushing, sweating, tingling, breast tenderness, ecchymoses) Side effects causing discontinuation: III: 1/10, V: 1/10 | |
| Notes | Data estimated from graphs No SDs Comparisons: Alpha adrenergic drug + estrogen (I & III) versus estrogen alone (II & IV) versus alpha adrenergic drug alone (V) versus placebo (VI) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ishiko 2000.
| Methods | RCT (by envelope method) Setting: 7 hospitals in Japan | |
| Participants | n= 61 women Dropouts: 5 left study I: 2, II: 1, III: 2 5 dropped out due to side effects: I: 2, II: 0, III: 3) Inclusion: stress urinary incontinence diagnosed by Japanese questionnaire (SUI); conventional investigation used whenever necessary to confirm diagnosis Age range 30‐75 years | |
| Interventions | I (13): Clenbuterol (20 µg twice daily, orally) II (19): PFMT (verbal instruction from gynecologic specialists, videotape demonstrating method, expected to perform PFMT for 10 minutes daily) III (19): Clenbuterol (20 µg twice daily orally) + PFMT (10 minutes daily) Duration of study: 8 weeks Follow up: none | |
| Outcomes | Subjective cure: I: 10/13, II: 10/19, III: 17/19 (P=0.036)
ITT including dropouts due to side effects as failures: I: 10/15, II: 10/19, III: 17/22
Side effects: I: 2/15 (headache 1, hepatopathy 1), II: 0/19, III: 3/22 (headache 1, rash 1, numbness 1)
5 other mild side effects, not causing dropout and not given by group (rash 1, constipation 1, numbness 1, tremor 1, mild AST and ALT elevation 1) Satisfaction with treatment: I: 11/13, II: 6/19, III: 13/19 (P=0.006) Frequency and urgency data also reported |
|
| Notes | No power calculations Groups comparable at baseline Comparisons: Beta adrenergic agonist (I) vs PFMT (II) vs combination (III) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kinn 1985 #.
| Methods | RCT (randomised double blind cross‐over) | |
| Participants | n= 39 women Dropouts: 3 (co‐existing diseases), 3 possible drug effects (arrhythmia, itch, depression) Inclusion: USI Exclusion: not mentioned Mean age 66 (range 49‐82) Urge incontinence n = 7, previous anti‐incontinence operation n = 8, previous gynaecological operations n = 5 | |
| Interventions | I (36): Estriol (2 mg daily orally) + placebo (twice daily orally) II (36): Estriol (2 mg daily orally) + PPA (50 mg twice daily) Duration of treatment: 4 weeks | |
| Outcomes | Subjective improvement: I: 9/30, II: 16/30 Leakage episode/day: I: 2.4, II: 2.4 Mean number of voids/day: I: 7.2, II: 6.9 Mean pad weight/day: I: 34.9, II: 24.9 Side effects: dryness of the mouth + arrhythmia, itch, depression; but not clear on which treatment | |
| Notes | No SDs Comparisons: Alpha adrenergic drug (PPA) + estrogen vs estrogen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kromann 1991 #.
| Methods | RCT (random double‐blind crossover trial, according to random number) | |
| Participants | n= 23 women Inclusion: urinary symptoms (frequency over 7/day; nocturia at least 2/night; urge or urge incontinence) Age: medain 92 years (range 35‐80) | |
| Interventions | 3 week run‐in period I (23): Terbutaline 7.5 mg 2 times/day II (23): Matching placebo Then women could continue with an increased dose to 3 times/day | |
| Outcomes | Side effects resulting in withdrawal: 3/23 Failed to complete study: 3/23 Frequency significantly improved: I vs II P<0.05 but numbers not given Cystometric parameters 'markedly improved' Subjective symptoms 'slightly improved' but not significant due to small numbers Side effects: I: 4/17; II: 2/17 (palpitations and trembling hands) | |
| Notes | No useable outcome data, apart from adverse events Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lehtonen 1986.
| Methods | RCT (randomised double blind placebo‐controlled) | |
| Participants | n= 43 women Inclusion: USI, treated UTI Exclusion: Intermittent or chronic medication with alpha agonist or alpha antagonist Mean height 162 cm (152‐173) Duration of symptoms 6.3 years Menopause 22/43 Estrogen 5/43 Previous anti‐incontinence operation n=3, previous gynaecological operations n=11, concomitant medication n= 18 | |
| Interventions | I (21): PPA (50 mg twice daily) II (22): Placebo (twice daily) Duration of treatment 2 weeks | |
| Outcomes | Subjective cure: I: 3/21, II: 1/22 Subjective improvement: I: 12/21, II: 7/22 Subjectively worse: I: 6/21, II: 14/22 Adverse events: I: 4/21, II: 6/22 (dryness of mouth, restlessness) 2 other withdrawals due to insomnia (I) and nausea (II) | |
| Notes | Comparisons: Alpha adrenergic drug (PPA) versus placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lose 1988.
| Methods | RCT (double blind, parallel groups, placebo controlled) ITT Setting: USA | |
| Participants | n= 47 women Dropouts: 3 other women excluded (failed inclusion criteria 1; personal reason 1; headache 1) Inclusion: USI Exclusion: severe genital prolapse, neurological disease, hypertension, heart disease, diabetes mellitus, organic bladder diseases, senility, drugs acting on lower urinary tract, DI, residual urine >50 ml on two occasions. Age range: 23‐73 years Menopausal: I:8, II: 7 | |
| Interventions | I (21): Placebo 3 times /day, oral II (23): Norepinephrine 5 mg 3 times /day, oral, sustained release Duration of study: 6 weeks, dose doubled after first 3 weeks if no effect | |
| Outcomes | Dose increased after first 3 weeks: I: 17/21, II: 18/21 Subjective cure: I: 3/21, II: 6/23 Subjective improvement: I: 7/21. II: 12/23 Objective cure (stress test): I: 7/21, II: 7/23 Objective improvement: I:5/21, II:11/23 Swapped to alternative arm after end of trial: I: 10/21, II: 10/23 Continence surgery: I: 5/21, II: 5/23 Long term cure after 1 year: I: 8/21, II: 6/23 (but not trial outcome) Adverse events: I: 1/21, II:5/23 (headache, dizziness, palpitations) | |
| Notes | Sample size calculation give Comparison: Adrenergic drug (norepinephrine) versus placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Musselman 2004 #.
| Methods | RCT (double blind, placebo controlled, cross‐over) ITT Setting: USA | |
| Participants | n= 37 women randomised Dropouts: I: 2/37 (withdrew consent 1, chest pain 1); II: 2/37 (received wrong drug 1, scalp tingling 1) Inclusion: Postmenopausal women, surgically sterile women, SUI (by symptoms and clinical examination), must have 7‐35 episodes of SUI/week during the lead‐in period and must not have changed by >10 SUI episodes from screening week. Exclusion: Predominant urge incontinence, >91 voids/wk, >21 episodes of nocturia/wk, 71‐91 voids/wk, >7 urge incontinence episodes/wk, 15‐21 episodes of nocturia/wk. postvoid residual urine >150 ml, previous pelvic surgery for incontinence, bladder pathology, cardiovascular disease, significant neurological disease, significant gastrointestinal disease, any medication that might interfere with the trial medicine. Age: 18‐75 years, mean (SD); 47.5 (8.1) | |
| Interventions | I (36): Placebo II (36): Ro 115‐1240 3 mg Duration of study: 6 weeks (after 3 weeks screening and 1 week placebo lead‐in, 2 weeks one randomised arm, crossed over to 4 weeks other arm) | |
| Outcomes | Voiding diaries Incontinence episodes/week during treatment, N, mean (SEM): I: 34, 8.67 (0.46), II: 34, 7.16 (0.46); mean difference (SEM) 1.51 (17.4) Number of pads changed/week during treatment: I: 34, 11.69 (0.61), II: 34, 9.90 (0.61); mean difference (SEM) 2.06 (17.2) Adverse events: I: 26/36, II: 30/36 (paraesthesia, headache, rigors, piloerection, pruritus) Paraesthesia (eg scalp tingling): I: 4/36, II: 21/36 Headache: I: 9/36, II: 11/36 Rigors (eg chills): I: 0/36, II: 10/36 Piloerection (eg goose bumps): I: 1/36, II 4/36 Itch: I: 1/36, II: 4/36 | |
| Notes | New drug, described as: 'highly selective alpha 1A/1L adrenoceptor agonist' No washout phase Comparison: Adrenergic drug (Ro 115‐1240) versus placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Walter 1990.
| Methods | RCT (randomised double blind placebo‐controlled) | |
| Participants | n= 29 women Inclusion: SUI by pad test, postmenopausal over 1 year, no estrogen therapy later within 2 months Exclusion: Neurological disease, senility, diabetes mellitus, liver dysfunction, previous breast cancer, previous uterine cancer, hypertension, drugs affecting lower urinary tract, not complying to protocol Concomitant medication n=29 | |
| Interventions | I (15): PPA (50 mg twice daily) + estriol (4 mg daily‐orally) II (14): Placebo (twice daily) + estriol (4 mg daily‐orally) Duration of treatment: 4 weeks | |
| Outcomes | Subjective cure: I: 2/15, II: 2/14 Subjective improvement: I: 2/;15, II: 4/14 Subjectively worse: I: 10/15, II: 8/14 Leakage episode/day: I: 2, II: 0.8 median pad weight/day: I: 5, II: 15 Side effects: I: 6/15, II: 5/14 (epigastric pain, depression, dizziness, blurred vision, dryness of the mouth, hot flushes, breast tenderness) | |
| Notes | No SDs Comparison: Alpha adrenergic drug (PPA) + estrogen versus estrogen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Weil 1995.
| Methods | RCT (randomised double blind placebo‐controlled), multicentre | |
| Participants | n= 105 women Inclusion: Mild to moderate USI, body weight not more than 45% from Broca index, normotensive, no UTI, negative urine cytology Exclusion: Bladder compliance <20 ml/cmH2O, DO, RU > 50 ml, cardiac disease, CVA, extensive pelvic surgery, phaeochromocytoma, surgery for urinary incontinence, thyrotoxicosis, diabetes mellitus, vaginal descent grade III, pelvic radiotherapy, pregnancy, breast feeding, diuretics, adrenergic agonist /antagonists, other drugs for incontinence, renal/hepatic/haematological diseases Age: range 18 to 70 years | |
| Interventions | I (24): Placebo (once daily) II (25): Midodrine (5 mg daily‐orally) III (30): Midodrine (7.5 mg daily‐orally) IV (26): Midodrine (10 mg daily‐orally) Duration of treatment: 6 weeks | |
| Outcomes | At 2 weeks group (IV) is better than placebo group (I) in patient's assessment. At 4 weeks, group (II) was better than placebo (I) in both patients and investigators assessment but no data provided Pad weight/day (median): I: +0.5, II: ‐3.4, III: ‐0.5, IV: ‐1.0 Adverse events: I: 8/24, II: 10/25, III: 12/30, IV: 16/26 (pilomotor eg goose bumps, itchy skin, piloerection; CNS eg headaches and dizziness) Side effects causing dropout in 3 women: oedema of the face (IV); depression, headache and vomiting (IV); feeling of fear (II) | |
| Notes | No SDs Midodrine = an alpha‐1 adrenergic receptor agonist Comparisons: Alpha adrenergic drug 5 mg versus 7.5 mg versus 10 mg versus placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wells 1991.
| Methods | RCT (randomly assigned) Recruitment: newspaper articles and other strategies | |
| Participants | n= 157 women Inclusion: USI and MUI, age 55 years and older, history of SI, living in community Exclusion: Hypertension, residential care Age: range 55‐90 years, mean age 66 (SD 8) | |
| Interventions | I (82): PFMT (1‐hour oral instruction session, written information + 6 months of active PFMT with monthly monitoring visits) II (75): PPA (50 mg daily for 2 weeks then increased up to 50 mg twice daily, orally for another 2 weeks) | |
| Outcomes | Subjective cure or improvement: I: 42/54, II: 54/64 Dropouts: I: 28, B: 11 ITT assuming dropouts are failures: I: 42/82, II: 54/75 Objective from diary: Cured/dry: I: 9/34, II: 2/52 Improved: I: 7/34, II: 20/52 Same: I: 7/34, II: 10/52 Worse: I: 9/34, II: 15/52 No. of incontinent episodes (n, mean (SD)): I: 51 0.75 (0.18, II: 62 0.67 (0.14) | |
| Notes | Differential dropout from groups, more from PFMT group, P<0.01) Comparisons: PFMT (I) versus PPA (II) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yasuda 1993.
| Methods | RCT (randomised double blind placebo‐controlled) | |
| Participants | n= 175 women Inclusion: USI, SI episode 1‐2/day, average SI episode for 3 days/week, 2 grams urine loss on pad test Exclusion: Urge incontinence, nervous disorder of lower urinary tract, vesicovaginal fistula, symptoms of SI due to neurogenic bladder Mean age (SD): I: 58 +/‐ 12.8, II: 58.1 +/‐ 13.5 years | |
| Interventions | I (82): Clenbuterol (20 mcg twice daily, orally) II (93): Placebo (twice daily, orally) Duration of study: 2 weeks | |
| Outcomes | Subjective cure: I: 8/77, II: 2/88 Subjective cure or improvement: I: 36/77, II: 21/88 For USI, subjective cure or improvement: I: 26/56, II: 16/62 For MUI, subjective cure or improvement: I: 10/21, II: 5/26 Mean pad weight: I: n=46, 6 gr (SD 12.3), II: n=54,12.6 (24.7) Adverse events: I: 13/82 (finger tremor 8; tachycardia 2), II: 12/93 (GI disturbance/nausea 7; dizziness 2) Withdrawals due to adverse effects: I: 5/13, II: 4/12 | |
| Notes | No power calculations Comparisons: Beta adrenergic agonist versus placebo. Adverse events: I: tremor, dizziness, urinary hesitancy, loss of appetite, II: dizziness, nausea. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
BP= Blood Pressure, CVA = cardiovascular accident, DO= Detrusor overactivity (previously detrusor instability, DI), ITT = Intention to treat, kg = kilogrammes, PFMT= pelvic floor muscle training, PPA = Phenylpropanolamine (alpha adrenergic), PV= Per Vagina, RCT = Randomised Controlled Trial, RU = Residual Urine, SEM = Standard Error of the Mean, SUI = Stress urinary incontinence, USI = Urodynamic stress incontinence (previously GSI, genuine stress incontinence)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amark 1992 | RCT Intervention: Phenylpropanolamine Population: Children with detrusor overactivity due to neurogenic bladder |
| Farghaly 1987 | RCT Intervention: Mazindol (not adrenergic drug) |
| Fossberg 1981 | Not RCT Intervention: Phenylpropanolamine vs placebo sequentially. |
| Jackobsen 1989 | Study population: after ileocaecal bladder replacement. Not native bladder. |
| Jorgensen 1991 | Not RCT Intervention: Norepinephrine in females with genuine stress incontinence |
| Radley 2001 | RCT Intervention: Methoxamine (alpha‐1‐adrenergic) Administered intravenously in laboratory setting, no clinical outcomes The study assessed only the threshold dose level for the onset of urethral versus cardiovascular actions of Methoxamine |
| Woodhouse 1983 | RCT Intervention: Mazindol (not adrenergic drug) |
| Zinner 2002 | RCT Intervention: Duloxetine (not adrenergic drug) |
Contributions of authors
Ammar Alhasso and Cathryn Glazener screened the studies and abstracted the data. All four authors contributed to the interpetation of the data and writing of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
NHS Executive Research and Development Programme, UK.
Declarations of interest
None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Ahlstrom 1990 # {published data only}
- Ahlstrom K, Sandahl B, Sjoberg B, Ulmsten U, Stormby N, Lindskog M. Effect of combined treatment with phenylpropanolamine and estriol, compared with estriol treatment alone, in postmenopausal women with stress urinary incontinence. Gynecologic & Obstetric Investigation 1990;30(1):37‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ani 1987 # {published data only}
- Ani IF. The place of alpha‐adrenergic agonist in the management of genuine stress incontinence of urine (Thesis). Master of Obstetrics and Gynaecology, University of Liverpool 1987.
Beisland 1984 {published data only}
- Beisland HO, Fossberg E, Moer A, Sander S. Urethral sphincteric insufficiency in postmenopausal females: treatment with phenylpropanolamine and estriol separately and in combination. A urodynamic and clinical evaluation. Urologia Internationalis 1984;39(4):211‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Collste 1987 # {published data only}
- Collste L, Lindskog M. Phenylpropanolamine in treatment of female stress urinary incontinence. Double‐blind placebo controlled study in 24 patients. Urology 1987;30(4):398‐403. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ek 1978 # {published data only}
- Ek A, Andersson KE, Gullerg B, Ulmsten U. The effect of long‐term treatment with norepinephrine on stress incontinence and urethral closure pressure profile. Scandinavian Journal of Urology and Nephrology 1978;12(2):105‐10. [DOI] [PubMed] [Google Scholar]
Ek 1980 # {published data only}
- Ek A, Andersson KE, Gullberg B, Ulmsten U. Effects of oestradiol and combined norephidrin and oestradiol treatment on female stress incontinence. Zentralblatt fur Gynakologie 1980;102(15):839‐44. [PubMed] [Google Scholar]
Fossberg 1983 # {published data only}
- Fossberg E, Beisland HO, Lundgren RA. Stress incontinence in females: treatment with phenylpropanolamine. A urodynamic and pharmacological evaluation. Urologia Internationalis 1983;38(5):293‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gibson 1989 {published data only}
- Gibson JS, Dwyer P, Anderson RS, Pardey J. A multicentre controlled double dummy parallel group study of the effectiveness of mazindol vs eskornade vs placebo in stress incontinence. Proceedings of the International Continence Society (ICS), 19th Annual Meeting; 1989 Sept 7‐9; Ljubljana, Slovenia. 1989:192‐3.
Gnad 1984 {published data only}
- Gnad H, Burmucic R, Petritsch P, Steindorfer P. Conservative therapy of female stress incontinence. Double‐blind study with the alpha‐sympathomimetic midodrin. [German]. Fortschritte der Medizin 1984;102(20):578‐80. [MEDLINE: ] [PubMed] [Google Scholar]
Gruneberger 1984 {published data only}
- Gruneberger A. Treatment of motor urge incontinence with clenbuterol and flavoxate hydrochloride. British Journal of Obstetrics and Gynaecology 1984;91(3):275‐8. [PubMed] [Google Scholar]
Hilton 1982 # {published data only}
- Hilton P, Stanton SL. The use of phenylpropanolamine in genuine stress incontinence. Proceedings of the International Continence Society (ICS), 12th Annual Meeting; Leiden, Germany. 1982:23‐4.
Hilton 1990 {published data only}
- Hilton P, Tweddell AL, Mayne C. Oral and intravaginal estrogens alone and in combination with alpha‐adrenergic stimulation in genuine stress incontinence. International Urogynaecology Journal 1990;1:80‐6. [PubMed] [Google Scholar]
Ishiko 2000 {published data only}
- Ishiko O, Ushiroyama T, Saji F, Mitsuhashi Y, Tamura T, Yamamoto K, et al. Beta(2)‐adrenergic agonists and pelvic floor exercises for female stress incontinence. International Journal of Gynaecology & Obstetrics 2000;71(1):39‐44. [DOI] [PubMed] [Google Scholar]
Kinn 1985 # {published data only}
- Kinn AC, Lindskog M. Estrogens and phenylpropanolamine as a combined treatment for stress incontinence in post menopausal women. Proceedings of the International Continence Society (ICS), 15th Annual Meeting; 1985 Sept 3‐6; London, UK. 1985:401‐2.
- Kinn AC, Lindskog M. Estrogens and phenylpropanolamine in combination for stress urinary incontinence in postmenopausal women. Urology 1988;32(3):273‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kromann 1991 # {published data only}
- Kromann B, Nielsen KK, Jakobsen H, Poulsen A, Nordling J, Ibsen T. Terbutaline (Bricanyl) in the treatment of frequency and urge incontinence, a placebo controlled study (Abstract). Proceedings of the International Continence Society (ICS), 21st Annual Meeting; 1991 Oct 10‐12; Hannover, Federal Republic of Germany. 1991:172‐3.
Lehtonen 1986 {published data only}
- Lehtonen T, Rannikko S, Lindell O, Talja M, Wuokko E, Lindskog M. The effect of phenylpropanolamine on female stress urinary incontinence. Annales Chirurgiae et Gynaecologiae 1986;75(4):236‐41. [MEDLINE: ] [PubMed] [Google Scholar]
Lose 1988 {published data only}
- Lose G, Diernaes E, Rix P. Does Medical therapy Cure Female Stress incontinence?. Urology International 1989;44:25‐7. [DOI] [PubMed] [Google Scholar]
- Lose G, Rix P, Diernaes E, Alexander N. Norfenefrine in the treatment of female stress incontinence. Urology International 1988;43(1):11‐5. [DOI] [PubMed] [Google Scholar]
Musselman 2004 # {published data only}
- Musselman DM, Ford APDW, Gennevois DJ, Harbison ML, Laurent AL, Stoltz RR. A randomised crossover study to evaluate Ro 115‐1240, a selective alpha1A/1L‐adrenoceptor partial agonist in women with stress urinary incontinence. BJU International 2004;93(1):78‐83. [DOI] [PubMed] [Google Scholar]
Walter 1990 {published data only}
- Walter S, Kjaergaard B, Lose G, Andersen JT, Heisterberg L, Jakobsen H, et al. Stress urinary incontinence in postmenopausal women treated with oral estrogen (estriol) and an alpha‐adrenoceptor‐stimulating agent (phenylpropanolamine): a randomised double‐blind placebo‐controlled study. International Urogynaecology Journal 1990;1:74‐9. [Google Scholar]
Weil 1995 {published data only}
- Weil E, Dijkman G, Ralph G, Feyereisl J, Vierhout E, Eerdmans P. The effect of the alpha‐1‐adrenoceptor selective agonist midodrine on mild to moderate female stress‐incontinence. Proceedings of the International Continence Society (ICS), 25th Annual Meeting; 1995 Oct 17‐20, Sydney, Australia. 1995:50‐1.
- Weil E, Eerdmans P, Waalwijk van Doorn E, Ralph G, Janknegt R. The effect of the alpha‐1‐adrenoceptor selective agonist midodrine on mild to moderate female stress‐incontinence. Neurourology & Urodynamics 1994;13(4):446‐7. [Google Scholar]
- Weil EH, Eerdmans PH, Dijkman GA, Tamussino K, Feyereisl J, Vierhout, ME, et al. Randomized double‐blind placebo‐controlled multicenter evaluation of efficacy and dose finding of midodrine hydrochloride in women with mild to moderate stress urinary incontinence: a phase II study. International Urogynecology Journal & Pelvic Floor Dysfunction 1998;9(3):145‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wells 1991 {published data only}
- Wells TJ, Brink CA, Diokno AC, Wolfe R, Gillis GL. Pelvic muscle exercise for stress urinary incontinence in elderly women. Journal of the American Geriatrics Society 1991;39(8):785‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yasuda 1993 {published data only}
- Yasuda K, Kawabe K, Takimoto Y, Kondo A, Takaki R, Imabayashi K, et al. A double‐blind clinical trial of a beta2‐adrenergic agonist in stress incontinence. International Urogynecology Journal 1993;4:146‐51. [Google Scholar]
- Yasuda K, Kawabe K, Takimoto Y, Shimazaki J. Effects of clenbuterol on stress incontinence. Neurourology & Urodynamics 1990;9(4):341‐2. [Google Scholar]
References to studies excluded from this review
Amark 1992 {published data only}
- Amark P, Beck O. Effect of phenylpropanolamine on incontinence in children with neurogenic bladders. A double‐blind crossover study. Acta Paediatrica 1992;81(4):345‐50. [DOI] [PubMed] [Google Scholar]
Farghaly 1987 {published data only}
- Farghaly S. Clinical trial of mazindol in the control of female stress urinary incontinence (Abstract). Proceedings of the American Urogynecology Society, 8th Annual Meeting; 1987 Sept 9‐13; San Francisco, California. 1987.
Fossberg 1981 {published data only}
- Fossberg E, Beisland HO, Sander S. Sensory urgency in females: treatment with phenylpropanolamine. European Urology 1981;7(3):157‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jackobsen 1989 {published data only}
- Jackobsen H, Steven K. Lack of effect of cholinergic blocking and alpha‐adrenergic stimulation on noctural incontinence after ileocaecal bladder replacement. British Journal of Urology 1989;63:379‐83. [DOI] [PubMed] [Google Scholar]
Jorgensen 1991 {published data only}
- Jorgensen L, Lose G, Alexander N. Acute effect of norfenefrine on the urethral pressure profile in females with genuine stress incontinence. Urologia Internationalis 1991;46(2):176‐9. [DOI] [PubMed] [Google Scholar]
Radley 2001 {published data only}
- Radley SC, Chapple CR, Bryan NP, Clarke DE, Craig DA. Effect of methoxamine on maximum urethral pressure in women with genuine stress incontinence: a placebo‐controlled, double‐blind crossover study. Neurourology & Urodynamics 2001;20(1):43‐52. [DOI] [PubMed] [Google Scholar]
- Radley SC, Chapple CR, Bryan NP, Craig D, Clarke DE. Effects of methoxamine on maximum urethral pressure in women with genuine stress incontinence: a placebo controlled, double blind crossover study. Proceedings of the 29th Annual Meeting of the International Continence Society (ICS); 1999 Aug 23‐26; Denver, Colorado. 1999:383. [DOI] [PubMed]
Woodhouse 1983 {published data only}
- Woodhouse CR, Tiptaft RC. Mazindol in the control of micturition. British Journal of Urology 1983;55(6):636‐8. [84081265] [DOI] [PubMed] [Google Scholar]
Zinner 2002 {published data only}
- Zinner N, Dmochowski R, Miklos J, Norton P, Yalcin I, Bump R. Duloxetine versus placebo in the treatment of stress urinary incontinence (SUI) (Abstract). Neurourology and Urodynamics 2002;21(4):383‐4. [Google Scholar]
Additional references
Black 1996
- Black NA, Downs SH. The effectiveness of surgery for stress incontinence in women: a systematic review. British Journal of Urology 1996;78(4):497‐510. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Byland 1998
- Byland DB, Bond RA, Clarke DE, Eikenberg DC, Heible JP, Langer SZ, et al. The IUPHAR Receptor Code. The IUPHAR Compendium of Receptor Characterisation and Classification. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification, 1998:59‐74. [Google Scholar]
Clark 1983
- Clark JE, Simon WA. Cardiac arrhythmias after phenylpropanolamine ingestion. Drug Intelligene & Clinical Pharmacy 1983;17(10):737‐8. [DOI] [PubMed] [Google Scholar]
Deeks 2006
- Deeks JJ, Higgins JPT, Altman DG, editors. Analysing and presenting results. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]; Section 8. Available from: www.cochrane.org.resources/handbook/hbook.htm (accessed 6th October 2006).
Emberton 1996
- Emberton M, Neal DE, Black N, Fordham M, Harrison M, McBrien MP, et al. The effect of prostatectomy on symptom severity and quality of life. British Journal of Urology 1996;77:233‐47. [DOI] [PubMed] [Google Scholar]
Enhoring 1961
- Enhoring E. Simultaneous recording of intravesical and intraurethral pressure. Acta Chirurgica Scandinavica ‐ Supplementum 1961;276:1‐68. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunskaar 2002
- Hunskaar S, Burgio KL, Diokno AC, Herzog AR, Hjalmas K, Lapitan MC. Epidemiology and natural history of urinary incontinence. Incontinence: 2nd International Consultation on Incontinence. Plymouth, UK: Health Publication Ltd, 2002:165‐202. [Google Scholar]
Hunter 2004
- Hunter KF, Glazener CMA, Moore KN. Conservative management for postprostatectomy urinary incontinence. Cochrane Database of Systematic Reviews 2007, Issue 2. [Art. No.: CD001843. DOI: 10.1002/14651858.CD001843.pub3] [DOI] [PubMed] [Google Scholar]
Kava 1998
- Kava MS, Blue DR Jr, Vimont RL, Clarke DE, Ford AP. Alpha 1L‐adrenoceptor mediation of smooth muscle contraction in rabbit bladder neck: a model for lower urinary tract tissues of man. British Journal of Pharmacology 1998;123(7):1359‐66. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lapitan 2005
- Lapitan MC, Cody DJ, Grant AM. Open retropubic colposuspension for urinary incontinence in women. Cochrane Database of Systematic Reviews 2005, Issue 3. [Art. No.: CD002912. DOI: 10.1002/14651858.CD002912.pub2] [DOI] [PubMed] [Google Scholar]
Mariappan 2005
- Mariappan P, Ballantyne Z, N'Dow JMO, Alhasso AA. Serotonin and noradrenaline reuptake inhibitors (SNRI) for stress urinary incontinence in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. [Art. No.: CD004742. DOI: 10.1002/14651858.CD004742.pub2] [DOI] [PubMed] [Google Scholar]
McGuire 1995
- McGuire EJ. Diagnosis and treatment of intrinsic sphincter deficiency. International Journal of Urology 1995;2(Suppl 1):7‐10, 16‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Meyer 1994
- Meyer S, Grandi P, Schmidt N, Sanzeni W, Spinosa JP. Urodynamic parameters in patients with slight and severe genuine stress incontinence: is the stress profile useful?. Neurourology & Urodynamics 1994;13(1):21‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Michel 1998
- Michel MC, Taguchi K, Schafers RS, Williams TJ, Clarke DE, Ford AP. Alpha 1‐adrenoceptor subtypes in the human cardiovascular and urogenital systems. Advances in Pharmacology 1998;42:394‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morita 1989
- Morita T. [Experimental studies for urinary incontinence. Effect of beta 2‐ agonists on the vesicourethral function]. Nippon Hinyokika Gakkai Zasshi ‐ Japanese Journal of Urology 1989;80(11):1597‐604. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pickard 2003
- Pickard R, Reaper J, Wyness L, Cody DJ, McClinton S, N'Dow J. Periurethral injection therapy for urinary incontinence in women. Cochrane Database of Systematic Reviews 2003, Issue 2. [Art. No.: CD003881. DOI: 10.1002/14651858.CD003881] [DOI] [PubMed] [Google Scholar]
Swift 1995
- Swift SE, Ostergard DR. Evaluation of current urodynamic testing methods in the diagnosis of genuine stress incontinence. Obstetrics & Gynecology 1995;86(1):85‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thomas 1980
- Thomas TM, Plymat KR, Blannin J, Meade TW. Prevalence of urinary incontinence. British Medical Journal 1980;281(6250):1243‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


