Abstract
Background
The clinical implications of SARS‐CoV‐2 infection are highly variable. Some people with SARS‐CoV‐2 infection remain asymptomatic, whilst the infection can cause mild to moderate COVID‐19 and COVID‐19 pneumonia in others. This can lead to some people requiring intensive care support and, in some cases, to death, especially in older adults. Symptoms such as fever, cough, or loss of smell or taste, and signs such as oxygen saturation are the first and most readily available diagnostic information. Such information could be used to either rule out COVID‐19, or select patients for further testing. This is an update of this review, the first version of which published in July 2020.
Objectives
To assess the diagnostic accuracy of signs and symptoms to determine if a person presenting in primary care or to hospital outpatient settings, such as the emergency department or dedicated COVID‐19 clinics, has COVID‐19.
Search methods
For this review iteration we undertook electronic searches up to 15 July 2020 in the Cochrane COVID‐19 Study Register and the University of Bern living search database. In addition, we checked repositories of COVID‐19 publications. We did not apply any language restrictions.
Selection criteria
Studies were eligible if they included patients with clinically suspected COVID‐19, or if they recruited known cases with COVID‐19 and controls without COVID‐19. Studies were eligible when they recruited patients presenting to primary care or hospital outpatient settings. Studies in hospitalised patients were only included if symptoms and signs were recorded on admission or at presentation. Studies including patients who contracted SARS‐CoV‐2 infection while admitted to hospital were not eligible. The minimum eligible sample size of studies was 10 participants. All signs and symptoms were eligible for this review, including individual signs and symptoms or combinations. We accepted a range of reference standards.
Data collection and analysis
Pairs of review authors independently selected all studies, at both title and abstract stage and full‐text stage. They resolved any disagreements by discussion with a third review author. Two review authors independently extracted data and resolved disagreements by discussion with a third review author. Two review authors independently assessed risk of bias using the Quality Assessment tool for Diagnostic Accuracy Studies (QUADAS‐2) checklist. We presented sensitivity and specificity in paired forest plots, in receiver operating characteristic space and in dumbbell plots. We estimated summary parameters using a bivariate random‐effects meta‐analysis whenever five or more primary studies were available, and whenever heterogeneity across studies was deemed acceptable.
Main results
We identified 44 studies including 26,884 participants in total. Prevalence of COVID‐19 varied from 3% to 71% with a median of 21%. There were three studies from primary care settings (1824 participants), nine studies from outpatient testing centres (10,717 participants), 12 studies performed in hospital outpatient wards (5061 participants), seven studies in hospitalised patients (1048 participants), 10 studies in the emergency department (3173 participants), and three studies in which the setting was not specified (5061 participants). The studies did not clearly distinguish mild from severe COVID‐19, so we present the results for all disease severities together.
Fifteen studies had a high risk of bias for selection of participants because inclusion in the studies depended on the applicable testing and referral protocols, which included many of the signs and symptoms under study in this review. This may have especially influenced the sensitivity of those features used in referral protocols, such as fever and cough. Five studies only included participants with pneumonia on imaging, suggesting that this is a highly selected population. In an additional 12 studies, we were unable to assess the risk for selection bias. This makes it very difficult to judge the validity of the diagnostic accuracy of the signs and symptoms from these included studies.
The applicability of the results of this review update improved in comparison with the original review. A greater proportion of studies included participants who presented to outpatient settings, which is where the majority of clinical assessments for COVID‐19 take place. However, still none of the studies presented any data on children separately, and only one focused specifically on older adults.
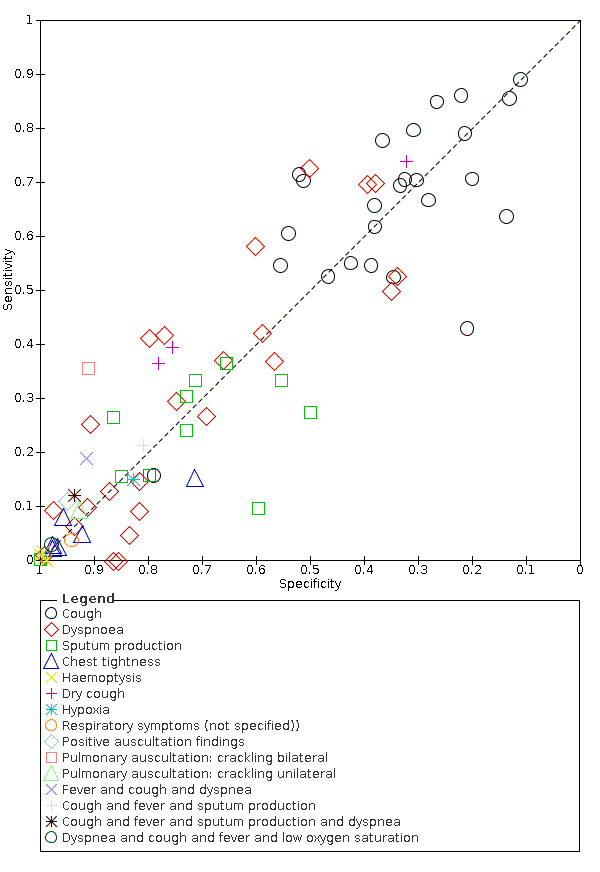
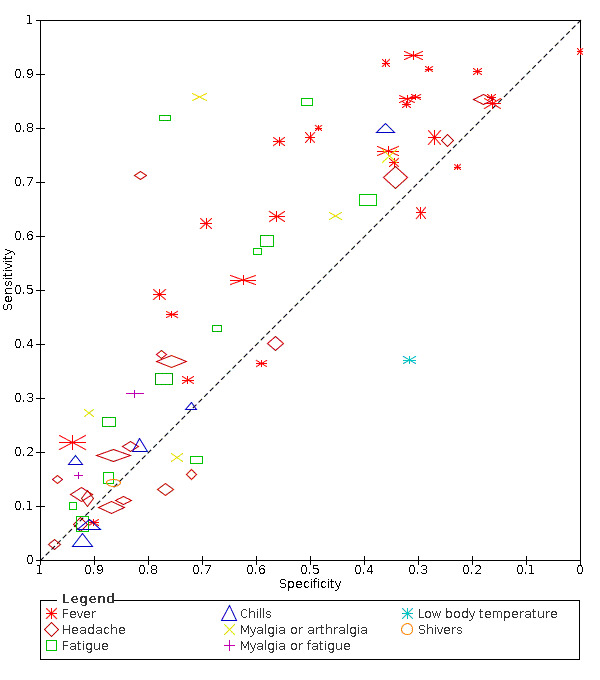
We found data on 84 signs and symptoms. Results were highly variable across studies. Most had very low sensitivity and high specificity. Only cough (25 studies) and fever (7 studies) had a pooled sensitivity of at least 50% but specificities were moderate to low. Cough had a sensitivity of 67.4% (95% confidence interval (CI) 59.8% to 74.1%) and specificity of 35.0% (95% CI 28.7% to 41.9%). Fever had a sensitivity of 53.8% (95% CI 35.0% to 71.7%) and a specificity of 67.4% (95% CI 53.3% to 78.9%). The pooled positive likelihood ratio of cough was only 1.04 (95% CI 0.97 to 1.11) and that of fever 1.65 (95% CI 1.41 to 1.93).
Anosmia alone (11 studies), ageusia alone (6 studies), and anosmia or ageusia (6 studies) had sensitivities below 50% but specificities over 90%. Anosmia had a pooled sensitivity of 28.0% (95% CI 17.7% to 41.3%) and a specificity of 93.4% (95% CI 88.3% to 96.4%). Ageusia had a pooled sensitivity of 24.8% (95% CI 12.4% to 43.5%) and a specificity of 91.4% (95% CI 81.3% to 96.3%). Anosmia or ageusia had a pooled sensitivity of 41.0% (95% CI 27.0% to 56.6%) and a specificity of 90.5% (95% CI 81.2% to 95.4%). The pooled positive likelihood ratios of anosmia alone and anosmia or ageusia were 4.25 (95% CI 3.17 to 5.71) and 4.31 (95% CI 3.00 to 6.18) respectively, which is just below our arbitrary definition of a 'red flag', that is, a positive likelihood ratio of at least 5. The pooled positive likelihood ratio of ageusia alone was only 2.88 (95% CI 2.02 to 4.09).
Only two studies assessed combinations of different signs and symptoms, mostly combining fever and cough with other symptoms. These combinations had a specificity above 80%, but at the cost of very low sensitivity (< 30%).
Authors' conclusions
The majority of individual signs and symptoms included in this review appear to have very poor diagnostic accuracy, although this should be interpreted in the context of selection bias and heterogeneity between studies. Based on currently available data, neither absence nor presence of signs or symptoms are accurate enough to rule in or rule out COVID‐19. The presence of anosmia or ageusia may be useful as a red flag for COVID‐19. The presence of fever or cough, given their high sensitivities, may also be useful to identify people for further testing.
Prospective studies in an unselected population presenting to primary care or hospital outpatient settings, examining combinations of signs and symptoms to evaluate the syndromic presentation of COVID‐19, are still urgently needed. Results from such studies could inform subsequent management decisions.
Plain language summary
Can symptoms and medical examination accurately diagnose COVID‐19?
COVID‐19 affects many organs of the body, so people with COVID‐19 may have a wide spectrum of symptoms. Symptoms and signs of the illness may be important to help them and the healthcare staff they come into contact with know whether they have the disease.
Symptoms: people with mild COVID‐19 might experience cough, sore throat, high temperature, diarrhoea, headache, muscle or joint pain, fatigue, and loss or disturbance of sense of smell and taste.
Signs are obtained by clinical examination. Signs of COVID‐19 examined in this review include lung sounds, blood pressure, blood oxygen level and heart rate.
Often, people with mild symptoms consult their doctor (general practitioner). People with more severe symptoms might visit a hospital outpatient or emergency department. Depending on the results of a clinical examination, patients may be sent home to isolate, may receive further tests or be hospitalised.
Why is accurate diagnosis important?
Accurate diagnosis ensures that people take measures to avoid transmitting the disease and receive appropriate care. This is important for individuals as it reduces harm and it saves time and resources.
What did we want to find out?
We wanted to know how accurate diagnosis of COVID‐19 is in a primary care or hospital setting, based on symptoms and signs from medical examination.
What did we do?
We searched for studies that assessed the accuracy of symptoms and signs to diagnose COVID‐19. Studies had to be conducted in primary care or hospital outpatient settings only. Studies of people in hospital were only included if symptoms and signs were recorded when they were admitted to the hospital.
The included studies
We found 44 relevant studies with 26,884 participants. The studies assessed 84 separate signs and symptoms, and some assessed combinations of signs and symptoms. Three studies were conducted in primary care (1824 participants), nine in specialist COVID‐19 testing clinics (10,717 participants), 12 studies in hospital outpatient settings (5061 participants), seven studies in hospitalised patients (1048 participants), 10 studies in the emergency department (3173 participants), and in three studies the setting was not specified (5061 participants). No studies focused specifically on children, and only one focused on older adults.
Main results
The studies did not clearly distinguish between mild and severe COVID‐19, so we present the results for mild, moderate and severe disease together.
The symptoms most frequently studied were cough and fever. In our studies, on average 21% of the participants had COVID‐19, which means in a group of 1000 people, around 210 would have COVID‐19.
According to the studies in our review, in the same 1000 people, around 655 people would have a cough. Of these, 142 would actually have COVID‐19. Of the 345 who do not have a cough, 68 would have COVID‐19.
In the same 1000 people, around 371 people would have a fever. Of these, 113 would actually have COVID‐19. Of the 629 patients without fever, 97 would have COVID‐19.
The loss of sense of smell or taste also substantially increase the likelihood of COVID‐19 when they are present. For example, in a population where 2% of the people have COVID‐19, having either loss of smell or loss of taste would increase a persons’ likelihood of having COVID‐19 to 8%.
How reliable are the results?
The accuracy of individual symptoms and signs varied widely across studies. Moreover, the studies selected participants in a way that meant the accuracy of tests based on symptoms and signs may be uncertain.
Conclusions
Most studies were conducted in hospital settings, so the results may not be entirely representative of primary care settings. The results do not apply to children or older adults specifically, and do not clearly differentiate between disease severities.
The results suggest that a single symptom or sign included in this review cannot accurately diagnose COVID‐19. However, the presence of loss of taste or smell may serve as a red flag for the presence of the disease. The presence of high temperature or cough may also be useful to identify people who might have COVID‐19. These symptoms may be useful to prompt further testing when they are present.
Further research is needed to investigate combinations of symptoms and signs; and testing unselected populations, in primary care settings and in children and older adults.
How up to date is this review?
For this update of the review, the authors searched for studies published from January to July 2020.
Summary of findings
Summary of findings 1. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient setting has COVID‐19.
| Sign or symptom | Study design | Setting | Number of studies/number of participants | Sensitivity (ranges) | Specificity (ranges) |
Strength of evidence Number of studies with high risk of bias per QUADAS‐2 domain: participant selection/index test/reference standard/flow and timing |
|
Patient or population: people with COVID‐19 symptoms Setting: primary care or hospital outpatient departments Index test(s): signs and symptoms of COVID‐19 Target condition: SARS‐CoV‐2 infection (symptomatic of any severity); mild or moderate COVID‐19; severe or critical COVID‐19 Reference standard: RT‐PCR Only signs and symptoms for which at least one cross‐sectional study observed a sensitivity of at least 50% are included. Pooled sensitivity and specificity were estimated for cross‐sectional studies only. | ||||||
| Cough | Cross‐sectional | Primary care | 2/968 | 52% to 70% | 30% to 47% | 1/1/1/1 |
| Outpatient clinics/ED | 19/13,061 | 16% to 89% | 11% to 79% | 5/19/1/2 | ||
| Hospital inpatients | 2/158 | 52% to 55% | 35% to 42% | 1/2/0/1 | ||
| Unclear | 2/1272 | 78% to 85% | 13% to 37% | 0/2/0/0 | ||
| All settings | 25/15,459 | 67% (pooled summary estimate) | 35% (pooled summary estimate) | |||
| Case‐control | Primary care | ‐ | ‐ | ‐ | ||
| Outpatient clinics/ED | 4/803 | 36% to 88% | 6% to 58% | 2/4/0/2 | ||
| Hospital inpatients | 3/294 | 47% to 80% | 15% to 20% | 3/2/0/0 | ||
| Unclear | ‐ | ‐ | ‐ | |||
| Fever | Cross‐sectional | Primary care | 2/968 | 33% to 49% | 73% to 78% | 1/1/1/1 |
| Outpatient clinics/ED | 19/11691 | 7% to 94% | 0% to 90% | 4/19/1/2 | ||
| Hospital inpatients | 3/633 | 64% to 90% | 19% to 48% | 1/3/0/1 | ||
| Unclear | 3/4656 | 22% to 85% | 32% to 94% | 0/2/0/0 | ||
| All settings (studies with prospective data collection only) | 7/5548 | 54% (pooled summary estimate) | 67% (pooled summary estimate) | |||
| Case‐control | Primary care | ‐ | ‐ | ‐ | ||
| Outpatient clinics/ED | 4/803 | 37% to 75% | 15% to 85% | 2/4/0/2 | ||
| Hospital inpatients | 2/158 | 76% to 79% | 7% to 7% | 2/2/0/0 | ||
| Unclear | ‐ | ‐ | ‐ | |||
| Anosmia | Cross‐sectional | Primary care | 3/1784 | 26% to 43% | 84% to 93% | 1/2/1/1 |
| Outpatient clinics/ED | 8/7768 | 10% to 65% | 70% to 98% | 1/7/0/1 | ||
| Hospital inpatients | ‐ | ‐ | ‐ | |||
| Unclear | ‐ | ‐ | ‐ | |||
| All settings | 11/9552 | 28% (pooled summary estimate) | 93% (pooled summary estimate) | |||
| Case‐control | Primary care | ‐ | ‐ | ‐ | ||
| Outpatient clinics/ED | 3/657 | 22% to 51% | 96% to 97% | 1/3/0/2 | ||
| Hospital inpatients | 1/124 | 53% | 83% | 1/1/0/0 | ||
| Unclear | ‐ | ‐ | ‐ | |||
| Ageusia | Cross‐sectional | Primary care | 2/1450 | 44% to 46% | 84% to 85% | 0/1/1/1 |
| Outpatient clinics/ED | 4/5929 | 10% to 55% | 70% to 100% | 1/4/0/1 | ||
| Hospital inpatients | ‐ | ‐ | ‐ | |||
| Unclear | ‐ | ‐ | ‐ | |||
| All settings | 6/7393 | 25% (pooled summary estimate) | 91% (pooled summary estimate) | |||
| Case‐control | Primary care | ‐ | ‐ | ‐ | ||
| Outpatient clinics/ED | 1/262 | 20% | 95% | 0/1/0/0 | ||
| Hospital inpatients | ‐ | ‐ | ‐ | |||
| Unclear | ‐ | ‐ | ‐ | |||
| Anosmia or ageusia | Cross‐sectional | Primary care | 1/816 | 59% | 80% | 0/1/0/0 |
| Outpatient clinics/ED | 4/6590 | 16% to 49% | 85% to 99% | 0/4/0/0 | ||
| Hospital inpatients | ‐ | ‐ | ‐ | |||
| Unclear | 1/736 | 73% | 75% | 0/1/0/0 | ||
| All settings | 6/8142 | 41% (pooled summary estimate) | 91% (pooled summary estimate) | |||
| Case‐control | Primary care | ‐ | ‐ | ‐ | ||
| Outpatient clinics/ED | ‐ | ‐ | ‐ | |||
| Hospital inpatients | ‐ | ‐ | ‐ | |||
| Unclear | ‐ | ‐ | ‐ | |||
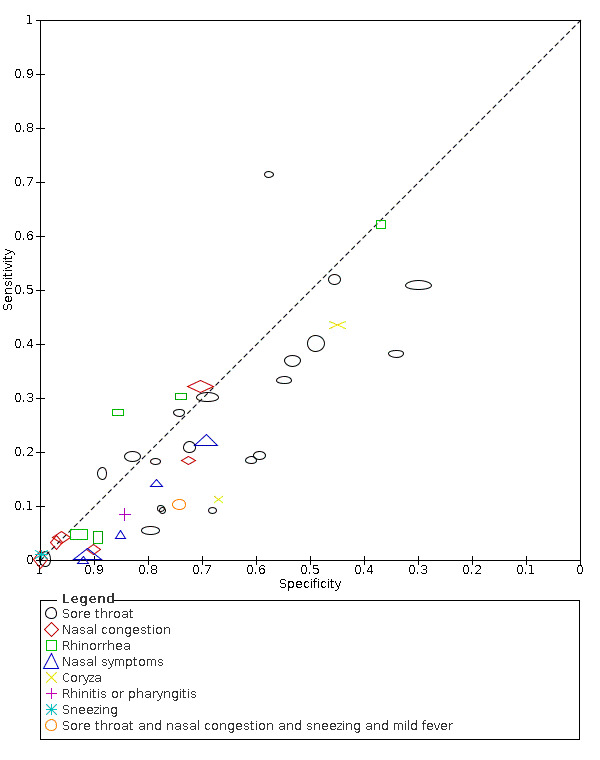
| Sore throat | Cross‐sectional | Primary care | 2/968 | 19% to 21% | 61% to 72% | 1/1/1/1 |
| Outpatient clinics/ED | 15/13,161 | 0% to 71% | 30% to 99% | 5/15/1/2 | ||
| Hospital inpatients | 1/475 | 16% | 88% | 0/1/0/0 | ||
| Unclear | 2/1272 | 38% to 52% | 34% to 45% | 0/2/0/0 | ||
| All settings | 20/15,876 | 21% (pooled summary estimate) | 70% (pooled summary estimate) | |||
| Case‐control | Primary care | ‐ | ‐ | ‐ | ||
| Outpatient clinics/ED | 3/657 | 17% to 45% | 37% to 55% | 1/3/0/2 | ||
| Hospital inpatients | 3/295 | 13% to 21% | 55% to 91% | 3/2/0/0 | ||
| Unclear | ‐ | ‐ | ‐ | |||
| Myalgia | Cross‐sectional | Primary care | 1/334 | 26% | 81% | 1/1/0/0 |
| Outpatient clinics/ED | 9/6455 | 1% to 61% | 53% to 99% | 2/9/0/0 | ||
| Hospital inpatients | 2/580 | 5% to 12% | 90% to 93% | 0/2/0/1 | ||
| Unclear | 1/736 | 65% | 33% | |||
| All settings | 13/8105 | 27% (pooled summary estimate) | 83% (pooled summary estimate) | |||
| Case‐control | Primary care | ‐ | ‐ | ‐ | ||
| Outpatient clinics/ED | 1/268 | 57% | 78% | 1/1/0/1 | ||
| Hospital inpatients | 1/124 | 59% | 30% | 1/1/0/0 | ||
| Unclear | ‐ | ‐ | ‐ | |||
| Fatigue | Cross‐sectional | Primary care | 2/968 | 19% to 59% | 58% to 71% | 1/1/1/1 |
| Outpatient clinics/ED | 9/4632 | 7% to 85% | 39% to 94% | 3/9/1/2 | ||
| Hospital inpatients | 1/53 | 10% | 94% | 1/1/0/0 | ||
| Unclear | ‐ | ‐ | ‐ | |||
| All settings | 12/5553 | 36% (pooled summary estimate) | 75% (pooled summary estimate) | |||
| Case‐control | Primary care | ‐ | ‐ | ‐ | ||
| Outpatient clinics/ED | 2/389 | 7% to 42% | 69% to 85% | 0/2/0/1 | ||
| Hospital inpatients | 3/294 | 11% to 93% | 13% to 100% | 3/2/0/0 | ||
| Unclear | ‐ | ‐ | ‐ | |||
| Headache | Cross‐sectional | Primary care | 2/968 | 11% to 40% | 56% to 85% | 1/1/1/1 |
| Outpatient clinics/ED | 13/10941 | 3% to 78% | 25% to 98% | 3/13/1/2 | ||
| Hospital inpatients | 2/528 | 12% to 15% | 91% to 97% | 1/2/0/0 | ||
| Unclear | 1/736 | 85% | 18% | 0/1/0/0 | ||
| All settings (studies with prospective data collection only | 6/6171 | 22% (pooled summary estimate) | 80% (pooled summary estimate) | |||
| Case‐control | Primary care | ‐ | ‐ | ‐ | ||
| Outpatient clinics/ED | 3/657 | 18% to 65% | 54% to 94% | 1/3/0/2 | ||
| Hospital inpatients | 2/158 | 11% to 73% | 43% to 100% | 2/2/0/0 | ||
| Unclear | ‐ | ‐ | ‐ | |||
| Dyspnoea | Cross‐sectional | Primary care | 2/968 | 15% to 30% | 75% to 82% | 1/1/1/1 |
| Outpatient clinics/ED | 19/12,198 | 0% to 73% | 35% to 99% | 5/19/1/2 | ||
| Hospital inpatients | 1/475 | 10% | 91% | 0/1/0/0 | ||
| Unclear | 2/1272 | 37% to 53% | 34% to 66% | 0/2/0/0 | ||
| All settings | 24/14,913 | 25% (pooled summary estimate) | 77% (pooled summary estimate) | |||
| Case‐control | Primary care | ‐ | ‐ | ‐ | ||
| Outpatient clinics/ED | 3/657 | 12% to 42% | 63% to 77% | 1/3/0/2 | ||
| Hospital inpatients | 1/124 | 34% | 41% | 1/1/0/0 | ||
| Unclear | ‐ | ‐ | ‐ | |||
| Diarrhoea | Cross‐sectional | Primary care | 2/968 | 04% to 36% | 72% to 93% | 1/1/1/1 |
| Outpatient clinics/ED | 14/10704 | 0% to 64% | 74% to 99% | 2/14/1/2 | ||
| Hospital inpatients | 3/633 | 5% to 15% | 88% to 97% | 1/3/0/1 | ||
| Unclear | 1/736 | 53% | 62% | 0/1/0/0 | ||
| All settings | 20/13,016 | 12% (pooled summary estimate) | 91% (pooled summary estimate) | |||
| Case‐control | Primary care | ‐ | ‐ | ‐ | ||
| Outpatient clinics/ED | 4/1173 | 8% to 45% | 77% to 92% | 1/4/0/2 | ||
| Hospital inpatients | 2/158 | 5% to 40% | 80% to 93% | 2/2/0/0 | ||
| Unclear | ‐ | ‐ | ‐ | |||
| Anosmia or dysgeusia | Cross‐sectional | Primary care | ‐ | ‐ | ‐ | |
| Outpatient clinics/ED | 2/457 | 9% to 74% | 78% to 97% | 0/2/0/0 | ||
| Hospital inpatients | ‐ | ‐ | ‐ | |||
| Unclear | ‐ | ‐ | ‐ | |||
| Case‐control | Primary care | ‐ | ‐ | ‐ | ||
| Outpatient clinics/ED | 1/268 | 65% | 92% | 1/1/0/1 | ||
| Hospital inpatients | ‐ | ‐ | ‐ | |||
| Unclear | ‐ | ‐ | ‐ | |||
| Myalgia or arthralgia | Cross‐sectional | Primary care | ‐ | ‐ | ‐ | |
| Outpatient clinics/ED | 5/556 | 19% to 86% | 35% to 91% | 2/5/1/2 | ||
| Hospital inpatients | ‐ | ‐ | ‐ | |||
| Unclear | ‐ | ‐ | ‐ | |||
| Case‐control | Primary care | ‐ | ‐ | ‐ | ||
| Outpatient clinics/ED | 1/262 | 34% | 81% | 0/1/0/0 | ||
| Hospital inpatients | ‐ | ‐ | ‐ | |||
| Unclear | ‐ | ‐ | ‐ | |||
| Rhinorrhoea | Cross‐sectional | Primary care | ‐ | ‐ | ‐ | |
| Outpatient clinics/ED | 4/1777 | 5% to 62% | 37% to 93% | 1/4/0/0 | ||
| Hospital inpatients | 1/475 | 4% | 89% | 0/1/0/0 | ||
| Unclear | ‐ | ‐ | ‐ | |||
| Case‐control | Primary care | ‐ | ‐ | ‐ | ||
| Outpatient clinics/ED | 3/657 | 10% to 45% | 46% to 80% | 1/3/0/2 | ||
| Hospital inpatients | 2/260 | 4% to 49% | 44% to 95% | 2/1/0/0 | ||
| Unclear | ‐ | ‐ | ‐ | |||
| ED: emergency department; RT‐PCR: reverse transcription polymerase chain reaction | ||||||
Background
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus and resulting COVID‐19 pandemic present important diagnostic evaluation challenges. These range from, on the one hand, understanding the value of signs and symptoms in predicting possible infection, assessing whether existing biochemical and imaging tests can identify infection and recognise patients needing critical care, and on the other hand, evaluating whether new diagnostic tests can allow accurate rapid and point‐of‐care testing. Also, the diagnostic aims are diverse, including identifying current infection, ruling out infection, identifying people in need of care escalation, or testing for past infection and immunity.
This review is part of a suite of reviews on the diagnosis of SARS‐CoV‐2 infection and COVID‐19 disease, and deals solely with the diagnostic accuracy of presenting clinical signs and symptoms.
Target condition being diagnosed
COVID‐19 is the disease caused by infection with the SARS‐CoV‐2 virus. The key target conditions for this suite of reviews are current SARS‐CoV‐2 infection, current COVID‐19, and past SARS‐CoV‐2 infection.
For current infection, the severity of the disease is important. SARS‐CoV‐2 infection can be asymptomatic (no symptoms); mild or moderate (symptoms such as fever, cough, aches, lethargy but without difficulty breathing at rest); severe (symptoms with breathlessness and increased respiratory rate indicative of pneumonia and oxygen need); or critical (requiring intensive support due to severe acute respiratory syndrome (SARS) or acute respiratory distress syndrome (ARDS), shock or other organ dysfunction). People with severe or critical disease require different patient management, which makes it important to distinguish between them.
Thus, there are three target conditions for current infection:
SARS‐CoV‐2 infection (asymptomatic or symptomatic of any severity);
mild or moderate COVID‐19;
severe or critical COVID‐19.
In planning review updates, we will consider the potential addition of another grouping (which is a subset of the above):
whether tests exist that identify people requiring respiratory support (SARS or ARDS) or intensive care.
Here we summarise the evidence on signs and symptoms; as a result asymptomatic SARS‐CoV‐2 and past SARS‐CoV‐2 infection are out of scope for this review.
Index test(s)
Signs and symptoms
Signs and symptoms are used in the initial diagnosis of suspected COVID‐19, and to identify people with COVID‐19 pneumonia. Symptoms are what is experienced by patients, for example, cough or nausea. Signs are what can be evaluated by clinical assessment, for example, lung auscultation findings, blood pressure or heart rate.
Key symptoms that have been associated with mild to moderate COVID‐19 include: troublesome dry cough (for example, coughing more than usual over a one‐hour period, or three or more coughing episodes in 24 hours), fever greater than 37.8 °C, diarrhoea, headache, breathlessness on light exertion, muscle pain, fatigue, and loss of sense of smell and taste. Red flags indicating possible severe disease or pneumonia include breathlessness at rest, loss of appetite, confusion, pain or pressure in the chest, and temperature above 38 °C.
Clinical pathway
Important in the context of COVID‐19 is that the pathway is multifaceted because it is designed to care for the diseased individual and to protect the community from further spread. Decisions about patient and isolation pathways for COVID‐19 vary according to health services and settings, available resources, and stages of the epidemic. They will change over time, if and when effective treatments and vaccines are identified. The decision points between these pathways vary, but all include points at which knowledge of the accuracy of diagnostic information is needed to be able to inform rational decision making.
Prior test(s)
In this review on signs and symptoms, no prior tests are required because signs and symptoms are used in the initial diagnosis of suspected COVID‐19. Patients can, however, self‐assess before presenting to healthcare services based on their symptoms. This is in contrast to contact tracing, in which patients or participants are tested based on a documented contact with a SARS‐CoV‐2‐positive person and may themselves be asymptomatic.
Role of index test(s)
Signs and symptoms are used as triage tests, that is, to rule out COVID‐19, but also to identify patients with possible COVID‐19 who may require further testing, care escalation or isolation.
Alternative test(s)
Other Cochrane diagnostic test accuracy (DTA) reviews in the suite of reviews are addressing the following tests.
Chest imaging (computed tomography (CT), chest X‐ray and ultrasound; Islam 2020)
Routine laboratory testing, such as for C‐reactive protein (CRP) and procalcitonin (PCT) (Stegeman 2020)
Antibody tests (Deeks 2020a)
Laboratory‐independent point‐of‐care and near‐patient molecular and antigen tests (Dinnes 2020)
Molecular laboratory tests (in preparation)
Rationale
It is essential to understand the accuracy of diagnostic tests including signs and symptoms to identify the best way they can be used in different settings to develop effective diagnostic and management pathways. We are producing a suite of Cochrane 'living systematic reviews', which will summarise evidence on the clinical accuracy of different tests and diagnostic features, grouped according to present research questions and settings, in the diagnosis of SARS‐CoV‐2 infection and COVID‐19 disease. Summary estimates of accuracy from these reviews will help inform diagnostic, screening, isolation, and patient management decisions.
New tests are being developed and evidence is emerging at an unprecedented rate during the COVID‐19 pandemic. We will aim to update these reviews as often as is feasible to ensure that they provide the most up‐to‐date evidence about test accuracy.
These reviews are being produced rapidly to assist in providing a central resource of evidence to assist in the COVID‐19 pandemic, summarising available evidence on the accuracy of the tests and presenting characteristics.
Objectives
To assess the diagnostic accuracy of signs and symptoms to determine if a person presenting in primary care or to hospital outpatient settings, such as the emergency department or dedicated COVID‐19 clinics, has COVID‐19.
Secondary objectives
Where data are available, we will investigate diagnostic accuracy (either by stratified analysis or meta‐regression) according to:
days since symptom onset;
population (children; older adults);
reference standard;
study design; and
setting.
Summary of previous review
In our initial review, we found 16 relevant studies with 7706 participants. The median number of participants was 134. Prevalence of the target disease varied from 5% to 38% with a median of 17%.
The studies assessed 27 separate signs and symptoms, but none assessed combinations of signs and symptoms. Seven were set in hospital outpatient clinics (2172 participants), four in emergency departments (1401 participants), but none in primary care settings. No studies included children, and only one focused on older adults. All the studies confirmed COVID‐19 diagnosis by the most accurate test available, which was reverse transcription polymerase chain reaction (RT‐PCR).
The studies did not clearly distinguish mild to moderate COVID‐19 from severe to critical COVID‐19, so we presented the results for all severities together. The results indicated that at least half of participants with COVID‐19 had a cough, sore throat, high temperature, muscle or joint pain, fatigue, or headache. However, cough and sore throat were also common in people without COVID‐19, so these symptoms alone are less helpful for diagnosing COVID‐19. High temperature, muscle or joint pain, fatigue, and headache substantially increase the likelihood of COVID‐19 when they are present.
Signs and symptoms for which sensitivity was reported above 50% in at least one study were the following:
Cough: sensitivity between 43% to 71%, specificity between 14% to 54%
Fever: sensitivity between 7% to 91%, specificity between 16% to 94%
Sore throat: sensitivity between 5% to 71%, specificity between 55% to 80%
Myalgia or arthralgia: sensitivity between 19% to 86%, specificity between 45% to 91%
Fatigue: sensitivity between 10% to 57%, specificity between 60% to 94%
Headache: sensitivity between 3% to 71%, specificity between 78% to 98%
All other signs and symptoms appeared to have very low sensitivities but high specificities, making them unsuitable for diagnosis individually.
We concluded that the diagnostic accuracy, especially the sensitivity, of individual signs and symptoms is low. In addition, results were highly variable across studies, making it difficult to draw firm conclusions.
New evidence since previous review
We retrieved 28 more studies on signs and symptoms in suspected COVID‐19 patients, allowing pooling of the data for some features and estimation of summary measures of diagnostic accuracy. Moreover, this update contains new studies on the diagnostic value of olfactory symptoms, and includes a limited number of studies on combinations of symptoms.
Limitations of previous review
The main weakness of the initial review was the high risk of selection bias; many studies included patients who had already been admitted to hospital or who presented to hospital settings to seek treatment.
The lack of data on combinations of signs and symptoms was an important evidence gap. Consequently, there was no evidence on syndromic presentation and the value of composite signs and symptoms on the diagnostic accuracy measures.
Our search did not find any articles providing data on children. Children have been disproportionally underrepresented in the studies on diagnosing SARS‐CoV‐2 infection. Their absence seems related to the general mild presentation of the disease in the paediatric population and even more frequently the complete asymptomatic course. The full scope of disease presentation in children is however not known. Misclassification of children both at their presentation to the healthcare system and in the near future, where children will be asked to remain in quarantine when they present with predefined, but not yet evidence‐based symptoms needs to be avoided to decrease the possible damage done to children’s health.
Another important patient group is older adults. They are most at risk of a negative outcome of SARS‐CoV‐2 infection, especially mortality but also intensive care support. In the initial version of the review, only one study focused on adults aged 55 to 75 years. All other studies included adults of all ages and did not present results separately for the older age groups. The lack of a solid evidence base for the diagnosis of COVID‐19 in older adults adds to the difficulty in diagnosing serious infections in this age group, as other serious infections such as bacterial pneumonia or urinary sepsis also tend to lead to aspecific presentations.
Methods
Criteria for considering studies for this review
Types of studies
We included studies of all designs that produce estimates of test accuracy or provide data from which estimates can be computed.
We included both single‐gate (studies that recruit from a patient pathway before disease status has been ascertained, cross‐sectional studies) and multi‐gate (where people with and without the target condition are recruited separately) designs.
When interpreting the results we made sure that we carefully considered the limitations of different study designs, using quality assessment and analysis.
Studies had to have a sample size of a minimum of 10 participants.
Participants
Studies recruiting people presenting with a clinical suspicion of SARS‐CoV‐2 infection, based on a symptomatic presentation, were eligible. At least 50% of the study population had to present with COVID‐19‐compatible symptoms.
We kept the eligibility criteria purposely broad to include all patient groups and all variations of a test at this initial stage of reviewing the evidence (that is, if the patient population was unclear, we included the study).
Index tests
-
All signs and symptoms, including:
signs such as oxygen saturation, measured by oximetry and blood pressure;
symptoms, such as fever or cough.
We included combinations of signs and symptoms, but not when they were combined with laboratory, imaging, or other types of index tests as these will be covered in the other reviews.
Target conditions
To be eligible studies had to identify at least one of:
mild or moderate COVID‐19;
severe or critical COVID‐19 (including COVID‐19 pneumonia).
Asymptomatic infection with SARS‐CoV‐2 is out of scope for this review, considering it is by definition not possible to detect this based on signs and symptoms.
Reference standards
We anticipated that studies would use a range of reference standards. Although RT‐PCR is considered the best available test, due to rapidly evolving knowledge about the target conditions, multiple reference standards on their own as well as in combination have emerged.
We expected to encounter cases defined by:
RT‐PCR alone;
RT‐PCR, clinical expertise, and imaging (for example, CT thorax);
repeated RT‐PCR several days apart or from different samples;
plaque reduction neutralisation test (PRNT) or enzyme‐linked immunosorbent assay(ELISA) tests;
information available at a subsequent time point;
World Health Organization (WHO) and other case definitions (see Appendix 1).
This list is not exhaustive, and we recorded all reference standards encountered. With a group of methodological and clinical experts, we are producing a ranking of reference standards according to their ability to correctly classify participants using a consensus process.
Search methods for identification of studies
The final search date for this version of the review is 15 July 2020.
Electronic searches
We conducted a single literature search to cover our suite of Cochrane COVID‐19 DTA reviews (Deeks 2020b; McInnes 2020).
We used three different sources for our electronic searches to 15 July 2020, which were devised with the help of an experienced Cochrane Information Specialist with DTA expertise (RS). These searches aimed to identify all articles related to COVID‐19 and SARS‐CoV‐2 and were not restricted to those evaluating symptoms and signs. Thus, the searches used no terms that specifically focused on an index test, diagnostic accuracy or study methodology.
Due to the increased volume of published and preprint articles, we used artificial intelligence text analysis from 25 May 2020 and onwards to conduct an initial classification of documents, based on their title and abstract information, for relevant and irrelevant documents. See Appendix 2.
Cochrane COVID‐19 Study Register searches
We also included searches undertaken by Cochrane to develop the Cochrane COVID‐19 Study Register (covid-19.cochrane.org). These include searches of trials registers at US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch), as well as PubMed.
Search strategies were designed for maximum sensitivity, to retrieve all human studies on COVID‐19 and with no language limits. See Appendix 3.
COVID‐19 Living Evidence Database from the University of Bern
From 28 March 2020, we used the COVID‐19 Living Evidence database from the Institute of Social and Preventive Medicine (ISPM) at the University of Bern (www.ispm.unibe.ch), as the primary source of records for the Cochrane COVID‐19 DTA reviews. This search includes PubMed, Embase, and preprints indexed in bioRxiv and medRxiv databases. The strategies as described on the ISPM website are described here (ispmbern.github.io/covid-19/). See Appendix 4.
The decision to focus primarily on the 'Bern' feed was due to the exceptionally large numbers of COVID‐19 studies available only as preprints. The Cochrane COVID‐19 Study Register has undergone a number of iterations since the end of March 2020 and we anticipate moving back to the Cochrane COVID‐19 Study Register as the primary source of records for subsequent review updates.
The Stephen B. Thacker CDC Library, COVID‐19 Research Articles Downloadable Database
We included Embase records within the CDC library on COVID‐19 Research Articles Database (see Appendix 5 for details), and deduplicated these against the Cochrane COVID‐19 Study Register.
Searching other resources
We also checked our search results against two additional repositories of COVID‐19 publications including:
the Evidence for Policy and Practice Information and Co‐ordinating Centre (EPPI‐Centre) 'COVID‐19: Living map of the evidence' (eppi.ioe.ac.uk/COVID19_MAP/covid_map_v4.html);
the Norwegian Institute of Public Health 'NIPH systematic and living map on COVID‐19 evidence' (www.nornesk.no/forskningskart/NIPH_diagnosisMap.html)
Both of these repositories allow their contents to be filtered according to studies potentially relating to diagnosis, and both have agreed to provide us with updates of new diagnosis studies added. For this iteration of the review, we examined all diagnosis studies from both sources up to 15 July 2020.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Pairs of review authors independently screened studies. We resolved disagreements by discussion with a third, experienced review author for initial title and abstract screening, and through discussion between three review authors for eligibility assessments.
Data extraction and management
Pairs of review authors independently performed data extraction. We resolved disagreements by discussion between three review authors.
We contacted study authors where we needed to clarify details or obtain missing information.
Assessment of methodological quality
Pairs of review authors independently assessed risk of bias and applicability concerns using the QUADAS‐2 (Quality Assessment tool for Diagnostic Accuracy Studies) checklist, which was common to the suite of reviews but tailored to each particular review (Whiting 2011; Table 2). For this review, we excluded the questions on the nature of the samples as these were not relevant, and we added a question on who assessed the signs. We resolved disagreements by discussion between three review authors.
1. QUADAS‐2 checklist.
| Index test(s) | Signs and symptoms |
| Patients (setting, intended use of index test, presentation, prior testing) | Primary care, hospital outpatient settings including emergency departments Inpatients presenting with suspected COVID‐19 No prior testing Signs and symptoms often used for triage or referral |
| Reference standard and target condition | The focus will be on the diagnosis of COVID‐19 disease and COVID‐19 pneumonia. For this review, the focus will not be on prognosis. |
| Participant selection | |
| Was a consecutive or random sample of patients enrolled? | This will be similar for all index tests, target conditions, and populations. YES: if a study explicitly stated that all participants within a certain time frame were included; that this was done consecutively; or that a random selection was done. NO: if it was clear that a different selection procedure was employed; for example, selection based on clinician's preference, or based on institutions. UNCLEAR: if the selection procedure was not clear or not reported. |
| Was a case‐control design avoided? | This will be similar for all index tests, target conditions, and populations. YES: if a study explicitly stated that all participants came from the same group of (suspected) patients. NO: if it was clear that a different selection procedure was employed for the participants depending on their COVID‐19 (pneumonia) status or SARS‐CoV‐2 infection status. UNCLEAR: if the selection procedure was not clear or not reported. |
| Did the study avoid inappropriate exclusions? | Studies may have excluded participants, or selected participants in such a way that they avoided including those who were difficult to diagnose or likely to be borderline. Although the inclusion and exclusion criteria will be different for the different index tests, inappropriate exclusions and inclusions will be similar for all index tests: for example, only elderly patients excluded, or children (as sampling may be more difficult). This needs to be addressed on a case‐by‐case basis. YES: if a high proportion of eligible patients was included without clear selection. NO: if a high proportion of eligible patients was excluded without providing a reason; if, in a retrospective study, participants without index test or reference standard results were excluded; if exclusion was based on severity assessment post‐factum or comorbidities (cardiovascular disease, diabetes, immunosuppression). UNCLEAR: if the exclusion criteria were not reported. |
| Did the study avoid inappropriate inclusions? | YES: if samples included were likely to be representative of the spectrum of disease. NO: if the study oversampled patients with particular characteristics likely to affect estimates of accuracy. UNCLEAR: if the exclusion criteria were not reported. |
| Could the selection of patients have introduced bias? | HIGH: if one or more signalling questions were answered with NO, as any deviation from the selection process may lead to bias. LOW: if all signalling questions were answered with YES. UNCLEAR: all other instances. |
| Is there concern that the included patients do not match the review question? | HIGH: if accuracy of signs and symptoms were assessed in a case‐control design, or in an already highly selected group of participants, or the study was able to only estimate sensitivity or specificity. LOW: any situation where signs and symptoms were the first assessment/test to be done on the included participants. UNCLEAR: if a description about the participants was lacking. |
| Index tests | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | This will be similar for all index tests, target conditions, and populations. YES: if blinding was explicitly stated or index test was recorded before the results from the reference standard were available. NO: if it was explicitly stated that the index test results were interpreted with knowledge of the results of the reference standard. UNCLEAR: if blinding was unclearly reported. |
| If a threshold was used, was it prespecified? | This will be similar for all index tests, target conditions, and populations. YES: if the test was dichotomous by nature, or if the threshold was stated in the methods section, or if authors stated that the threshold as recommended by the manufacturer was used. NO: if a receiver operating characteristic curve was drawn or multiple threshold reported in the results section; and the final result was based on one of these thresholds; if fever was not defined beforehand. UNCLEAR: if threshold selection was not clearly reported. |
| Could the conduct or interpretation of the index test have introduced bias? | HIGH: if one or more signalling questions were answered with NO, as even in a laboratory situation knowledge of the reference standard may lead to bias. LOW: if all signalling questions were answered with YES. UNCLEAR: all other instances. |
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | This will probably be answered 'LOW' in all cases except when assessments were made in a different setting, or using personnel not available in practice. |
| Reference standard | |
| Is the reference standard likely to correctly classify the target condition? | We will define acceptable reference standards using a consensus process once the list of reference standards that have been used has been obtained from the eligible studies. For severe pneumonia, we will consider how well processes adhered to the WHO case definition in Appendix 1. |
| Were the reference standard results interpreted without knowledge of the results of the index test? | YES: if it was explicitly stated that the reference standard results were interpreted without knowledge of the results of the index test, or if the result of the index test was obtained after the reference standard. NO: if it was explicitly stated that the reference standard results were interpreted with knowledge of the results of the index test or if the index test was used to make the final diagnosis. UNCLEAR: if blinding was unclearly reported. |
| Did the definition of the reference standard incorporate results from the index test(s)? | YES: if results from the index test were a component of the reference standard definition. NO: if the reference standard did not incorporate the index standard test. UNCLEAR: if it was unclear whether the results of the index test formed part of the reference standard. |
| Could the conduct or interpretation of the reference standard have introduced bias? | HIGH: if one or more signalling questions were answered with NO. LOW: if all signalling questions were answered with YES. UNCLEAR: all other instances. |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | HIGH: if the target condition was COVID‐19 pneumonia, but only RT‐PCR was used; if alternative diagnosis was highly likely and not excluded (will happen in paediatric cases, where exclusion of other respiratory pathogens is also necessary); if tests used to follow up viral load in known test‐positives. LOW: if above situations were not present. UNCLEAR: if intention for testing was not reported in the study. |
| Flow and timing | |
| Was there an appropriate interval between index test(s) and reference standard? | YES: this will be similar for all index tests, populations for the current infection target conditions: as the situation of a patient, including clinical presentation and disease progress, evolves rapidly and new/ongoing exposure can result in case status change, an appropriate time interval will be within 24 hours. NO: if there was more than 24 hours between the index test and the reference standard or if participants were otherwise reported to be assessed with the index versus reference standard test at moments of different severity. UNCLEAR: if the time interval was not reported. |
| Did all patients receive a reference standard? | YES: if all participants received a reference standard (clearly no partial verification). NO: if only (part of) the index test‐positives or index test‐negatives received the complete reference standard. UNCLEAR: if it was not reported. |
| Did all patients receive the same reference standard? | YES: if all participants received the same reference standard (clearly no differential verification). NO: if (part of) the index test‐positives or index test‐negatives received a different reference standard. UNCLEAR: if it was not reported. |
| Were all patients included in the analysis? | YES: if all included participants were included in the analyses. NO: if after the inclusion/exclusion process, participants were removed from the analyses for different reasons: no reference standard done, no index test done, intermediate results of both index test or reference standard, indeterminate results of both index test or reference standard, samples unusable. UNCLEAR: if this was not clear from the reported numbers. |
| Could the patient flow have introduced bias? | HIGH: if one or more signalling questions were answered with NO. LOW: if all signalling questions were answered with YES. UNCLEAR: all other instances. |
| ICU: intensive care unit; RT‐PCR: reverse transcription polymerase chain reaction; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; WHO: World Health Organization | |
Statistical analysis and data synthesis
We present results of estimated sensitivity and specificity using paired forest plots and summarised them in tables as appropriate.
We estimated summary sensitivity and specificity using a bivariate random‐effects meta‐analysis (Macaskill 2013), whenever five or more primary studies were available, and whenever heterogeneity across studies was deemed acceptable on visual inspection of the forest‐ and receiver operating characteristic (ROC) plots. We performed these analyses using data from studies with a cross‐sectional design only.
We presented results of estimated sensitivity and specificity using paired forest plots in Review Manager 5 (Review Manager 2020), and tables as appropriate.
We considered tests to be useful in ruling out a serious infection in ambulatory care if their negative likelihood ratio (LR‐) was lower than 0.20; conversely we considered diagnostic tests to be useful as 'red flags' for infections when their positive likelihood ratio (LR+) was 5.0 or higher (Jaeschke 1994, Van den Bruel 2010).
We disaggregated data by study design, reporting results from cross‐sectional studies separately from studies that used a multi‐gate or other design that were assessed as prone to high risk of bias.
We undertook meta‐analyses in R version 3.5.1 (lme4 package; R 2020).
Investigations of heterogeneity
We have listed sources of heterogeneity that we investigated if adequate data were available in the Secondary objectives. In this version of the review, we used stratification to investigate heterogeneity as we considered it was inappropriate to combine studies. In future updates, if meta‐analysis becomes possible, we will investigate heterogeneity through meta‐regression.
In this version of the review we have stratified by study design only, as stratification by reference standard was not yet possible.
Sensitivity analyses
We aimed to undertake sensitivity analyses considering the impact of unpublished studies. However, this was not possible in this version of the review. We performed sensitivity analyses to investigate the impact of prospective versus retrospective data collection.
Assessment of reporting bias
We aimed to publish lists of studies that we know exist but for which we have not managed to locate reports, and request information to include in updates of these reviews. However, at the time of writing this version of the review, we are unaware of unpublished studies.
Summary of findings
We have listed our key findings in a 'Summary of findings' table to determine the strength of evidence for each test and findings, and to highlight important gaps in the evidence.
Updating
We will undertake monthly searches of published literature and preprints and, dependent on the number of new and important studies that we find, we will consider updating each review with each search if resources allow.
Results
Results of the search
The first selection resulted in 7394 potentially eligible articles. This included the 658 articles that we screened in our initial review. After screening on title and abstract, we excluded 7092 articles, leaving 302 full‐text articles to be assessed. We included 44 articles in this version of the review, 16 of which were included in the initial review. The reasons for excluding 258 articles are listed in the flow chart (Figure 1; Moher 2009).
1.
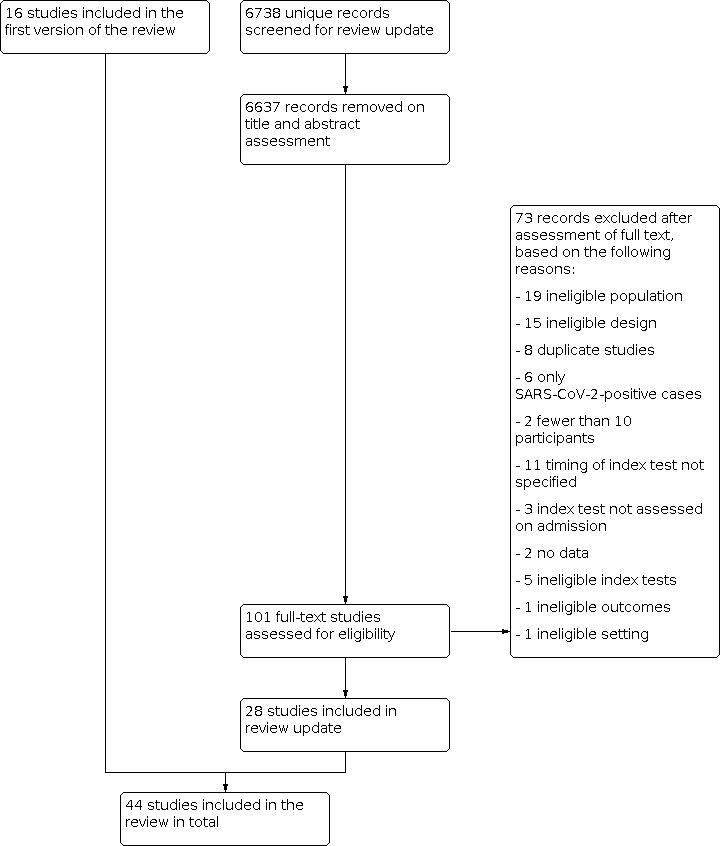
Flow diagram.
Two articles reported on the same cases (Chen 2020; Yang 2020), while using a different control group. Chen 2020 used a concurrent control group of pneumonia cases negative for SARS‐CoV‐2 on PCR testing but Yang 2020 used a historic control group of influenza pneumonia patients. For this reason we only included the Chen 2020 results in the analyses.
One study (Song 2020a), reported a study that included a derivation and validation part for the development of a prediction rule. The two parts are identical in set‐up and only differ in respect to the time of data collection, that is, the derivation part recruited patients up to 5 February 2020 and the validation part recruited patients from 6 February 2020 onwards. As a result, we consider this to be one study and have entered all data on signs and symptoms as such.
A summary of the main study characteristics can be found in Table 3.
2. Summary of study characteristics.
| Study ID | Sample size | Prevalence | Setting | Population | Design | Reference standard |
| Ahmed 2020 | 2043 | 7% | Primarily outpatient settings | All patients tested for SARS‐CoV‐2 in the UHealth system | Single‐gate (cross‐sectional), retrospective | Not specified |
| Ai 2020 | 53 | 38% | Hospital inpatients | Patients hospitalised with pneumonia diagnosed by imaging | Single‐gate (cross‐sectional), prospective | PCR on nasopharyngeal swabs |
| Brotons 2020 | 634 | 39% | Primary care | Patients who had a face‐to‐face or phone consultation with their GP | Single‐gate (cross‐sectional), prospective | Positive serology for SARS‐CoV‐2 (IgM and/or IgG) |
| Carignan 2020 | 268 | Not applicable | Hospital outpatients | Patients who underwent testing for SARS‐CoV‐2 at a hospital | Case‐control | PCR, samples not specified |
| Challener 2020 | 146 | Not applicable | Outpatients (drive‐through specimen collection site) | Patients screened for SARS‐CoV‐2 (suspicion based on presenting symptoms) | Case‐control | PCR, samples not specified |
| Cheng 2020 | 33 | 33% | Hospital outpatients | Patients presenting to a fever observation department | Single‐gate (cross‐sectional), retrospective | PCR on throat swab |
| Chen 2020 | 136 | Not applicable | Hospital inpatients | Patients admitted with pneumonia | Case‐control | PCR, samples not specified |
| Clemency 2020 | 961 | 23% | Outpatient settings | Healthcare workers triaged by phone, tested at drive‐through site | Single‐gate (cross‐sectional), prospective | PCR on nasopharyngeal or oropharyngeal swabs |
| Feng 2020 | 132 | 5% | Emergency department | Patients presenting to fever clinic of ED | Single‐gate (cross‐sectional), retrospective | PCR on throat swabs |
| Gilbert 2020 | 598 | 29% | Outpatient settings | Suspected patients sent to testing centres close to ED | Single‐gate (cross‐sectional), prospective | PCR on nasopharyngeal swabs |
| Haehner 2020 | 500 | 7% | Outpatient settings | Patients presenting with symptoms of a common cold to a COVID testing centre | Single‐gate (cross‐sectional), prospective | PCR on throat swabs |
| Huang 2020 | 475 | 71% | Hospital inpatients | Patients admitted into one of 26 COVID‐19‐designated hospitals | Single‐gate (cross‐sectional), retrospective | PCR, samples not specified |
| Just 2020 | 374 | 11% | Primary care | Convenience sample of patients who were tested in GP’s practices | Single‐gate (cross‐sectional), prospective | PCR, samples not specified |
| Chua 2020 | 688 | 3% | Emergency department | Patients with acute respiratory symptoms, tested at ED | Single‐gate (cross‐sectional), retrospective | PCR on oropharyngeal swabs |
| Leal 2020 | 1583 | 28% | Outpatient settings | Patients meeting the suspected COVID‐19 case definition (tested after initial screening questionnaire) | Single‐gate (cross‐sectional), prospective | PCR, samples not specified |
| Lee 2020 | 127 | Not applicable | Outpatient settings | Patients tested at ambulatory assessment centre | Nested case‐control | PCR on nasopharyngeal swabs |
| Liang 2020 | 88 | 24% | Hospital outpatients | Patients with pneumonia and presenting to fever clinic | Single‐gate (cross‐sectional), retrospective | PCR, sample not specified; conducted after panel discussion |
| Mao 2020 | 1004 | 19% | Hospital outpatients | Patients visiting the fever clinics (with fever or pulmonary symptoms) | Single‐gate (cross‐sectional), retrospective | PCR, sample not specified |
| Nobel 2020 | 516 | Not applicable | Hospital outpatients | Patients who underwent SARS‐CoV‐2 testing seeking hospital treatment or in essential personnel | Case‐control | PCR on nasopharyngeal swabs |
| O'Reilly 2020 | 240 | 5% | Emergency department | Patients who met the testing criteria for COVID‐19 and who presented at the ED | Single‐gate (cross‐sectional), prospective | PCR, sample not specified |
| Peng 2020 | 86 | 13% | Hospital outpatients | Patients clinically suspected and referred for testing | Single‐gate (cross‐sectional), retrospective | PCR on nasopharyngeal swabs |
| Peyrony 2020 | 391 | 58% | Emergency department | Patients tested at ED, decision to test based on clinician’s discretion | Single‐gate (cross‐sectional), prospective | PCR on nasal swabs |
| Pisapia 2020 | 37 | 46% | Emergency department/ lab |
Patients admitted in selected medical wards (ED + lab) of a mono‐specialist infectious diseases referral centre because of clinical suspicion | Single‐gate (cross‐sectional), retrospective | PCR, different tests used (commercial kits used during study changed), negatives re‐tested after 24 h, nasopharyngeal swab |
| Rentsch 2020 | 3789 | 15% | Unclear | Patients tested for SARS‐CoV‐2 in the Veterans Affairs Cohort born between 1945 and 1965 | Single‐gate (cross‐sectional), retrospective | PCR on nasopharyngeal swabs |
| Salmon 2020 | 1824 | 47% | Outpatient setting | Patients suspected of SARS‐CoV‐2 infection, tested at screening centre | Single‐gate (cross‐sectional), prospective | PCR on nasopharyngeal swabs |
| Shah 2020 | 316 | 10% | Emergency department | Patients presenting at an ED with an acute respiratory illness | Single‐gate (cross‐sectional), retrospective | PCR test on oropharyngeal and/or nasopharyngeal swabs |
| Song 2020a | 399 | 7% | Hospital outpatients | Patients tested for SARS‐CoV‐2 | Single‐gate (cross‐sectional), retrospective | PCR on sputum samples |
| Sun 2020 | 788 | Not applicable | Hospital outpatients | Patients presenting to testing centre, either self‐referred, referred from primary care or at‐risk cases identified by national contact tracing | Single‐gate (cross‐sectional), retrospective | PCR on sputum, endotracheal aspirate, nasopharyngeal swab or throat swab |
| Tolia 2020 | 283 | 10% | Emergency department | Patients presenting with symptoms, travel history, risk factors or healthcare workers | Single‐gate (cross‐sectional), retrospective | PCR on nasopharyngeal swabs |
| Tordjman 2020 | 100 | Not applicable | Emergency department | Patients with both RT‐PCR and CT‐scan results available with a 1:1 patient:control inclusion ratio from ED | Single‐gate (cross‐sectional), retrospective | PCR (specimen not specified) or CT‐scan lungs |
| Trubiano 2020 | 2935 | 4% | Outpatient setting | Patients presenting at a COVID‐19 rapid assessment screening clinic, meeting DHHS screening criteria | Single‐gate (cross‐sectional), prospective | PCR on nasopharyngeal swabs |
| Tudrej 2020 | 816 | 24% | Primary care/ outpatient setting | Patients referred by GPs for PCR testing at lab | Single‐gate (cross‐sectional), prospective | PCR on nasopharyngeal swabs |
| Wee 2020 | 870 | 18% | Emergency Department | Patients presenting with respiratory symptoms or travel history | Single‐gate (cross‐sectional), prospective | PCR on oropharyngeal swabs |
| Wei 2020 | 936 | 67% | Hospital outpatient | Febrile patients visiting a fever clinic | Single‐gate (cross‐sectional), retrospective | PCR on throat‐swab specimens |
| Xie 2020 | 105 | 20% | Hospital inpatients | Patients in whom PCR test was performed at two Shangai hospitals | Single‐gate (cross‐sectional), retrospective | PCR testing on throat swab and sputum specimens, patients pre‐selected on the presence of pneumonia (radiological findings) |
| Yan 2020 | 262 | 23% | Hospital outpatient | Patients presenting at hospital for SARS‐CoV‐2 testing, not otherwise specified | Other | PCR, samples not specified |
| Yang 2020 | 121 | Not applicable | Hospital inpatients | Patient with pneumonia from SARS‐CoV‐2 and patients with pneumonia from influenza in 2015‐2019 | Case‐control | PCR, samples not specified |
| Yombi 2020 | 536 | 33% | Unclear (healthcare workers working at tertiary hospital) | Healthcare workers were tested if they had respiratory symptoms with or without fever | Single‐gate (cross‐sectional), unclear retro‐or prospective |
PCR, samples not specified |
| Zavascki 2020 | 464 | 21% | Hospital outpatients | Patients attending a screening clinic, suspicion based on fever or any respiratory symptom | Cross‐sectional, retrospective | PCR, samples not specified |
| Zayet 2020a | 124 | 56% | Hospital inpatients + outpatients | Patients with confirmed COVID‐ 19 or confirmed influenza A/B who consulted or were hospitalised in the hospital | Case‐control | PCR on nasopharyngeal swabs, sputum, bronchial aspirates or bronchoalveolar lavage fluids |
| Zayet 2020b | 217 | 44% | Hospital outpatients | Patients presenting with possible COVID‐19 at the outpatient department | Single‐gate (cross‐sectional), retrospective | PCR on nasopharyngeal swabs |
| Zhao 2020 | 34 | Not applicable | Hospital inpatients | Patients with pneumonia and admitted to hospital | Case‐control | PCR on throat or sputum swabs |
| Zhu 2020 | 116 | 28% | Emergency department | Patients suspected of SARS‐CoV‐2 and presenting to the ED | Single‐gate (cross‐sectional), retrospective | PCR, samples not specified |
| Zimmerman 2020 | 736 | 7% | Unclear | Not specified | Not specified | PCR, samples not specified |
| CT: computed tomography; DHHS: Department of Health and Human Services; ED: emergency department; GP: general practitioner; PCR: polymerase chain reaction; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2 | ||||||
Methodological quality of included studies
The results of the quality assessment are summarised in Figure 2 and Figure 3. Of the 44 studies included in this review, six studies did not use a cross‐sectional design. Four studies were case‐control studies (Carignan 2020; Nobel 2020; Yang 2020; Zhao 2020), one study selected cases cross‐sectionally in five hospitals but only selected controls in one hospital (Chen 2020), and one study emailed patients who had undergone testing for SARS‐CoV‐2 about olfactory symptoms prior to the SARS‐CoV‐2 test, with a response rate of 58% in SARS‐CoV‐2 positive cases and 15% in negative cases (Yan 2020).
2.
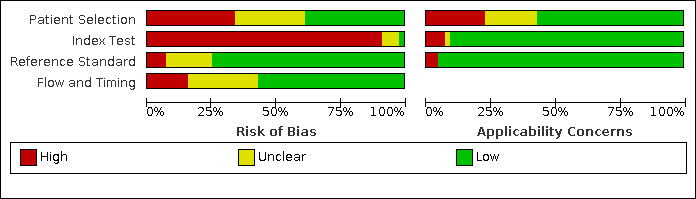
'Risk of bias' and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
3.
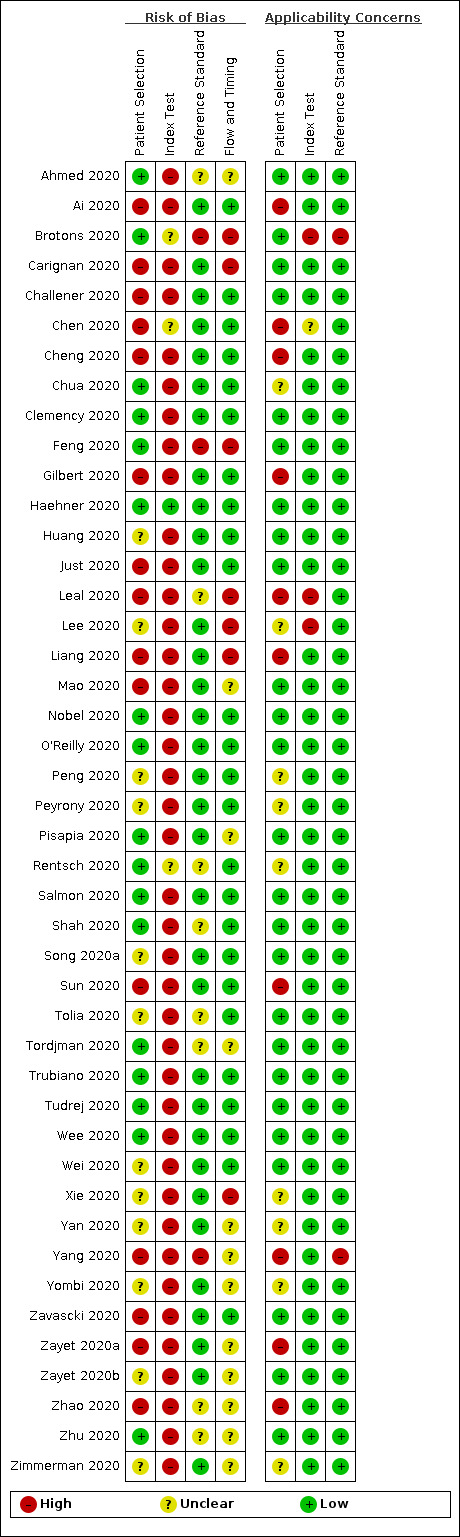
'Risk of bias' and applicability concerns summary: review authors' judgements about each domain for each included study
We rated patient selection as high risk of bias in 15 out of 44 studies. In five studies (Ai 2020; Chen 2020; Cheng 2020; Liang 2020; Yang 2020) this was because a CT scan or other imaging was used to diagnose patients with pneumonia prior to inclusion in the study. RT‐PCR results were then used to distinguish between COVID‐19 pneumonia and pneumonia from other causes. For all studies, testing was highly dependent on the local case definition and testing criteria that was in effect at the time of the study, meaning all patients that were included in studies had already gone through a referral or selection filter. The most extreme example of this is Liang 2020, in which patients with radiological evidence of pneumonia and a clinical presentation compatible with COVID‐19 were only tested for SARS‐CoV‐2 after a panel discussion.
We rated all studies except four as high risk of bias for the index tests because there was little to no detail on how, by whom and when the signs and symptoms were measured. Table 4 describes how studies measured olfactory symptoms. Studies collected information about symptoms in different ways: interviews by telephone or in person using standardised questionnaires, online surveys, self‐reporting at presentation, or systematic assessment by staff at enrolment without standardisation. Unfortunately, the standardised questionnaires themselves are rarely reported, and are often newly developed by each research team.
3. Study characteristics of papers investigating olfactory symptoms.
| Study | Recruitment | Prevalence of COVID‐19 | Setting + season | Measurement of symptoms |
| Brotons 2020 | Mild or moderate symptoms without confirmed diagnosis (observational study) | 634/742 underwent testing 244 were seropositive for IgM and/or IgG (38%) | Primary care Spring |
Standardised questionnaire A team of trained GPs, nurses, and medical students carried out the survey |
| Carignan 2020 | All patients who underwent testing for SARS‐CoV‐2 Adults who tested positive for SARS‐CoV‐2 were used to compare to control group |
134/2883 (4.6%) | Hospital outpatients Winter‐spring |
All participants were interviewed via telephone by trained interviewers using a standardised questionnaire. Questions were adapted from the self‐reported Mini Olfactory Questionnaire (validated questionnaire) |
| Clemency 2020 | HCWs with symptoms concerning COVID‐191 | 225 of 961 HCW (23%) tested positive | Outpatient settings Spring |
HCW were evaluated for potential testing through a centralised nurse call centre. A standardised list of symptoms was developed and utilised as part of usual care by the health system’s COVID‐19 call centre. |
| Haehner 2020 | Symptoms of a common cold + fulfilled COVID testing criteria | 34 of 500 (6.8%) patients | Outpatient settings Spring |
All patients who presented to the testing centre received a standardised questionnaire, which included the patients' main symptoms, time course and an additional self‐assessment of the patients' current smell, taste function and nasal breathing compared to the level before onset of symptoms. The patients had indicate whether they experienced loss of smell and/or taste (yes vs no) and quantify this on a scale of 0‐10 (0 = no function, 10 = best function) |
| Just 2020 | Patients who received a PCR test Comparison of patients with positive and negative test results | 40/347 tested positive for COVID‐19 (12%) | Convenience sample of patients who were tested in GP’s practices Spring |
Data were collected based on a uniform quality standard in the documentation of COVID‐19 suspect cases |
| Chua 2020 | Acute respiratory symptoms Fulfilled suspect or surveillance case definition | 31/717 tested positive for COVID‐19 (4.3%) | Emergency department Spring |
Self‐reported olfactory ability. ED started actively inquiring about olfactory loss in all patients who were included. |
| Leal 2020 | Suspected COVID‐19 symptoms | 2073 suspected cases: 1583 were tested.
444 were positive. (28%) 604/1136 PCR‐negative patients underwent serology. 52 tested positive. (8.6%) |
Outpatient settings Autumn |
Residents of the municipality of São Caetano do Sul aged ≥ 12 years with suspected COVID‐19 symptoms were encouraged to contact a dedicated platform, where they were invited to complete a screening questionnaire that included socio‐demographic data; information on symptoms type, onset and duration; and recent contacts. |
| Lee 2020 | Adults who underwent PCR test (reason not specified) |
102/1345 patients tested positive. (7.6%) 56/102 positive patients and 72 negative patients completed the survey |
Outpatient settings Spring |
Online survey. Baseline characteristics were collected and included. Smell and taste‐specific questions included the presence of smell or taste loss around the onset of COVID‐19 like symptoms, as well the current ability to smell. |
| O'Reilly 2020 | Fulfilled testing criteria Cases not feasible to obtain a history in order to exclude COVID‐19 | 240/1508 patients met inclusion criteria. 11 had a positive test result (4.6%) |
Emergency department Autumn |
Dedicated form embedded in the hospital’s electronic medical record |
| Peyrony 2020 | Symptomatic patients Patients with comorbidities that put them at risk of severe infection. No suspicion of COVID‐19 but needing hospitalization | 225/391 had positive test result for SARS‐CoV‐2 (58%) | Emergency department Winter‐spring |
Patient‐reported symptoms, physical examination by emergency physicians |
| Salmon 2020 | All consecutive patients who were tested for SARS‐CoV‐2 by RT‐PCR during the same period | 849 of 1824 (47%) tested positive | Outpatient setting Winter‐spring |
Patients were systematically assessed during the usual medical symptom’s screening about their olfactory and gustatory dysfunction |
| Trubiano 2020 | Patients that met DHHS criteria for SARS‐CoV‐2 testing | 4226 patients, 2976 were tested (41 excluded) 108/2935 tested positive (3.8%) |
Outpatient setting Autumn |
Data systematically gathered of patients presenting to the clinic by medical staff |
| Tudrej 2020 | Primary care patients with suspicion of COVID‐19 based on symptoms | 198/816 tested positive (24%) | Primary care/ outpatient setting Spring |
Self‐reported pre‐formatted questionnaire about their symptoms |
| Wee 2020 | New‐onset olfactory or taste disorders Suspected COVID‐19 case |
155 of 870 (18%) patients tested positive | Emergency department Spring |
Self‐reported, a questionnaire including respiratory symptoms, self‐reported OTD, and travel and epidemiological risk factors was administered at ED triage to risk‐stratify admissions |
| Zayet 2020a | Adult patients with confirmed COVID‐19 or confirmed influenza A/B | 124 patients 70 COVID + (56%) 54 Influenza A/B + |
Hospital inpatients + outpatients Winter |
Standardised questionnaire for each patient with suspected COVID‐19 (also suspected influenza) to help screen their functional symptoms and the onset and duration of their symptoms. |
| Zayet 2020b | Possible COVID‐19 based on symptoms |
95/217 had a positive PCR (44%) 122 had a negative PCR |
Hospital outpatients Spring |
Standardised questionnaire was designed to specify the symptoms in patients consulting for COVID‐19 suspicion. |
| Zimmerman 2020 | Suspected cases of COVID‐19 based on symptoms | 55/736 tested positive (7.4%) | Unclear Spring |
Symptoms reported at enrolment |
| ED: emergency department; GP: general practitioner; HCW: healthcare workers; OTD: olfactory and taste disorder; PCR: polymerase chain reaction; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2 | ||||
In addition, there was considerable uncertainty around the reference standard, with some studies providing little detail on the RT‐PCR tests that were used or lack of clarity on blinding.
Patient flow was unclear in 12 studies (Ahmed 2020; Mao 2020; Pisapia 2020; Tordjman 2020; Yan 2020; Yang 2020; Yombi 2020; Zayet 2020a; Zayet 2020b; Zhao 2020; Zhu 2020; Zimmerman 2020), either because the timing of recording signs and symptoms and conduct of the reference standard was unclear, or because some patients received a second or third reference standard at unclear time points during hospital admission, or because participant records were deleted when they contained missing data.
Findings
The main characteristics of all included studies are listed in Table 3.
There were seven studies in hospital inpatients (Ai 2020; Chen 2020; Huang 2020; Xie 2020; Yang 2020; Zayet 2020a; Zhao 2020), twelve studies in hospital outpatients (Carignan 2020; Cheng 2020; Liang 2020; Mao 2020; Nobel 2020; Peng 2020; Song 2020a; Sun 2020; Wei 2020; Yan 2020; Zavascki 2020; Zayet 2020b), ten studies in emergency departments (EDs) (Feng 2020; Chua 2020; O'Reilly 2020; Peyrony 2020; Pisapia 2020; Shah 2020; Tolia 2020; Tordjman 2020; Wee 2020; Zhu 2020), three studies in primary care settings (Brotons 2020; Just 2020; Tudrej 2020), and nine studies in other outpatient settings such as drive‐through testing sites (Ahmed 2020; Challener 2020; Clemency 2020; Gilbert 2020; Haehner 2020; Haehner 2020; Lee 2020; Salmon 2020; Trubiano 2020). Three studies did not specify setting (Rentsch 2020; Yombi 2020; Zimmerman 2020).
Nine studies assessed accuracy of signs and symptoms for the diagnosis of COVID‐19 pneumonia (Ai 2020; Chen 2020; Cheng 2020; Feng 2020; Liang 2020; Tordjman 2020; Xie 2020; Yang 2020; Zhao 2020), the remaining studies had SARS‐CoV‐2 infection as the target condition. The distinction between these two target conditions was not always very clear though, and a degree of overlap is to be assumed. All but one study used RT‐PCR testing as reference standard (Brotons 2020), with some variation in the samples that were used. Brotons 2020 used positive serology for SARS‐CoV‐2 (IgM and/or IgG) at the time of presentation and presence of symptoms and signs in the previous month as a reference standard.
There were 26,884 participants included in all studies, the median number of participants was 345. Prevalence varied from 3% to 71% with a median of 21% (cross‐sectional studies).
We found data on 84 signs and symptoms, which fall into six different categories, that is, upper respiratory, lower respiratory, systemic, gastro‐intestinal, cardiovascular and olfactory signs and symptoms. Results for the singe‐gate (cross‐sectional) studies are presented in forest plots (Figure 4; Figure 5 ; Figure 6; Figure 7; Figure 8; Figure 9), and are plotted in ROC space (Figure 10; Figure 11; Figure 12; Figure 13; Figure 14; Figure 15; Figure 16; Figure 17; Figure 18; Figure 19; Figure 20; Figure 21; Figure 22). Results of multi‐gate (non‐cross‐sectional studies) are presented in forest plots only (Figure 23; Figure 24; Figure 25; Figure 26; Figure 27).
4.
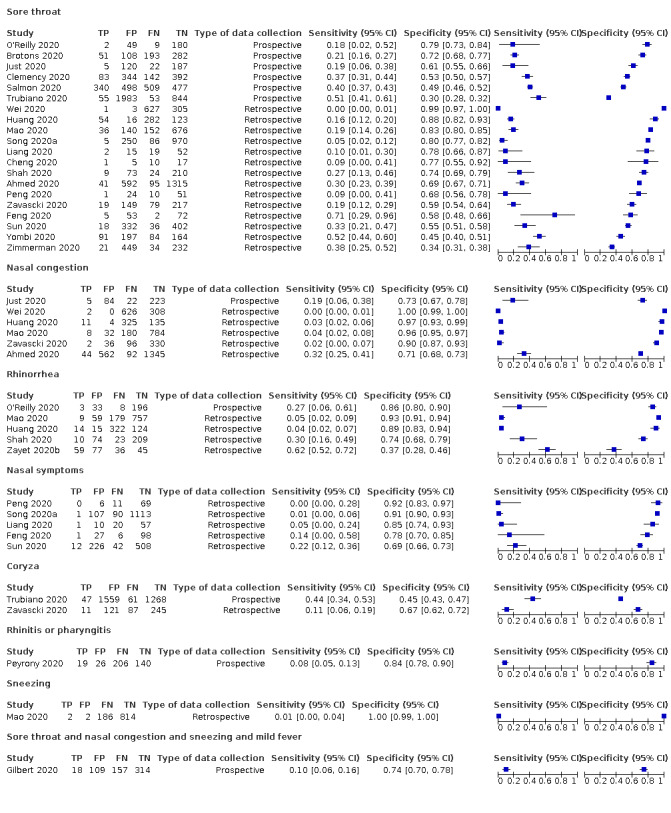
Forest plot of upper respiratory tract symptoms (cross‐sectional studies)
5.
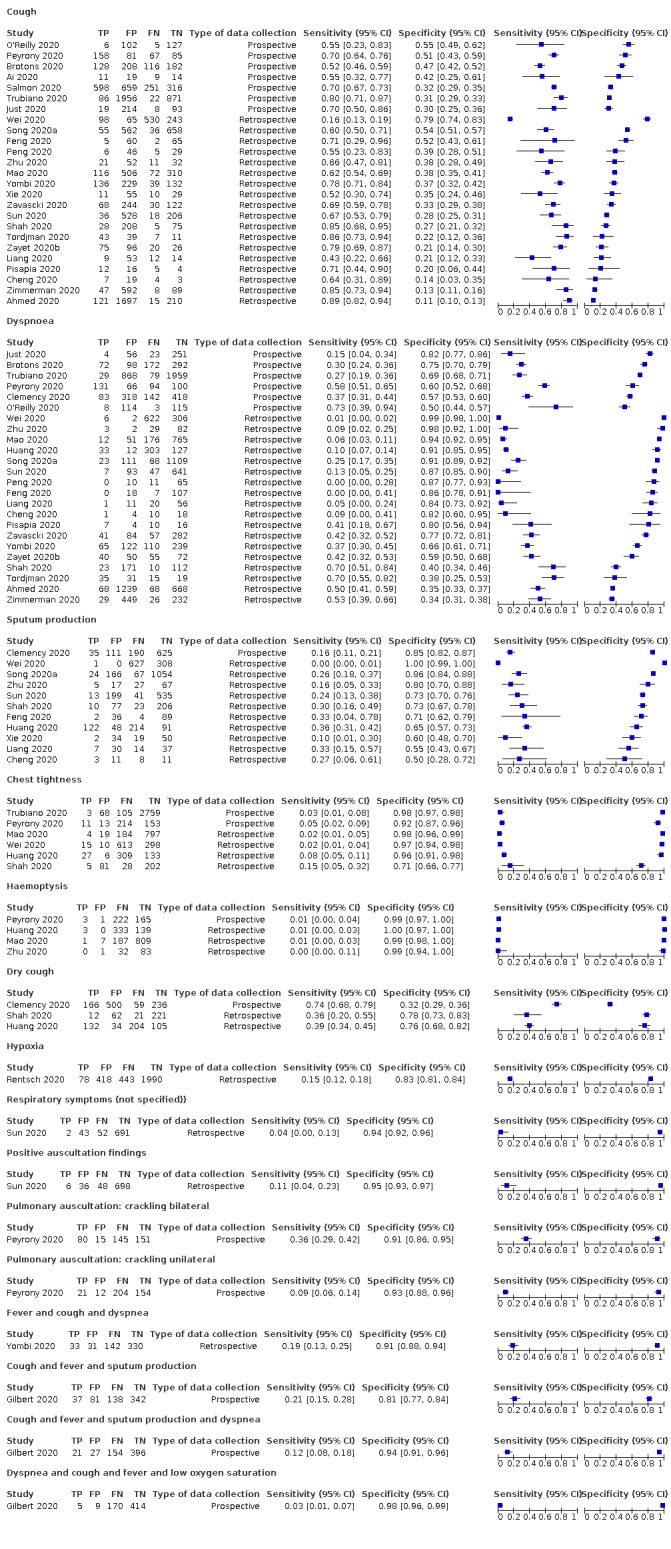
Forest plot of lower respiratory tract symptoms (cross‐sectional studies)
6.
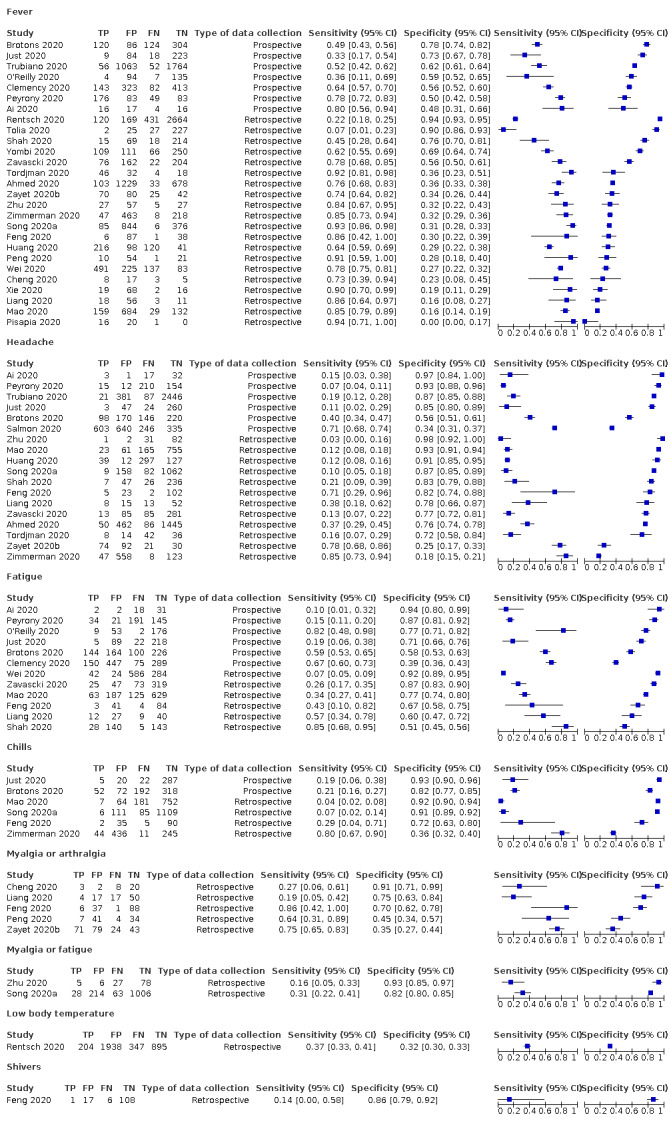
Forest plot of systemic signs and symptoms (cross‐sectional studies)
7.
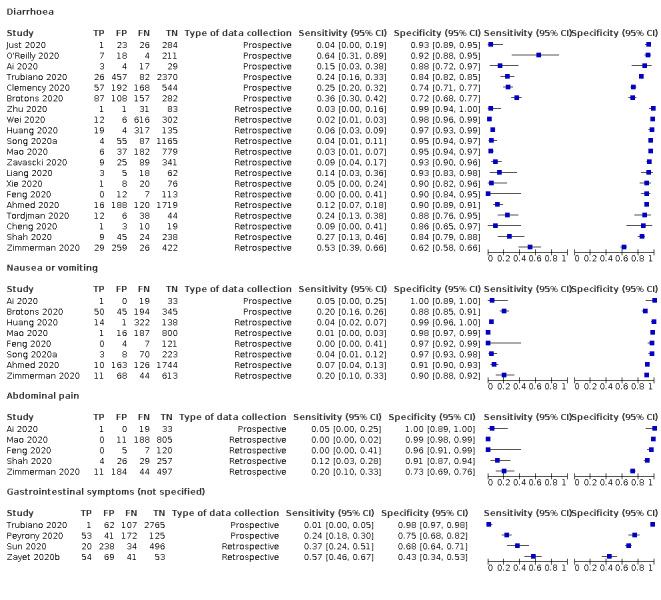
Forest plot of gastrointestinal signs and symptoms (cross‐sectional studies)
8.
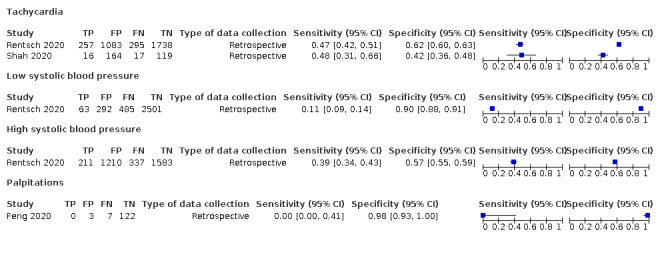
Forest plot of cardiovascular signs and symptoms (cross‐sectional studies)
9.
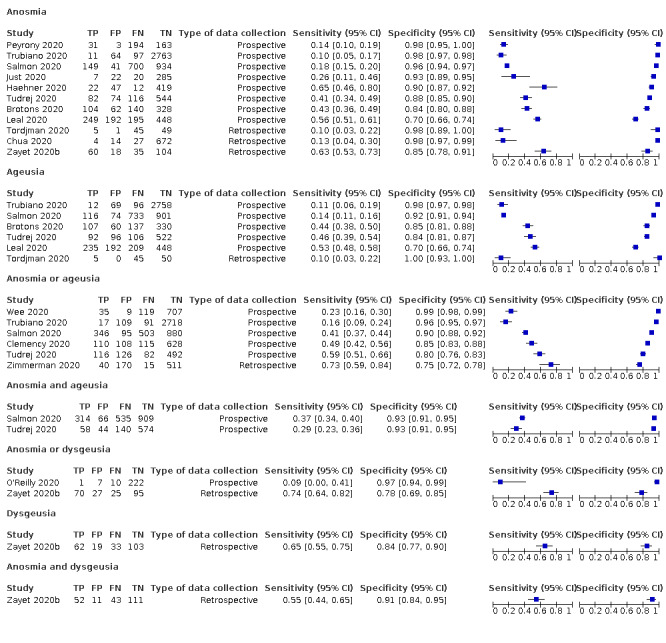
Forest plot of olfactory symptoms (cross‐sectional studies)
10.
Summary ROC plot of upper respiratory tract symptoms (cross‐sectional studies)
11.
Summary ROC plot of lower respiratory tract symptoms (cross‐sectional studies)
12.
Summary ROC plot of systemic signs and symptoms (cross‐sectional studies)
13.
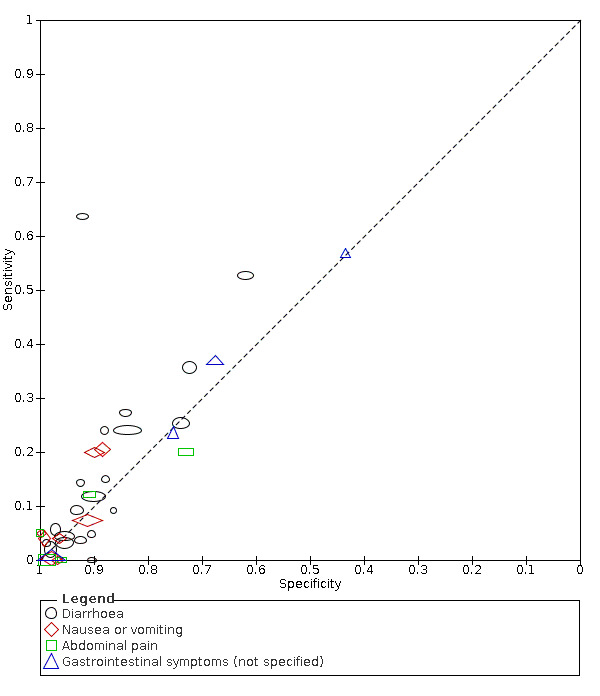
Summary ROC plot of gastrointestinal signs and symptoms (cross‐sectional studies)
14.
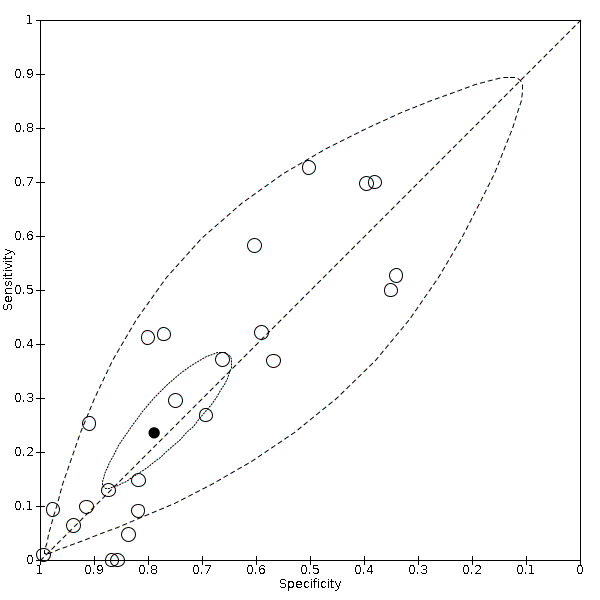
Summary ROC plot of dyspnoea
15.
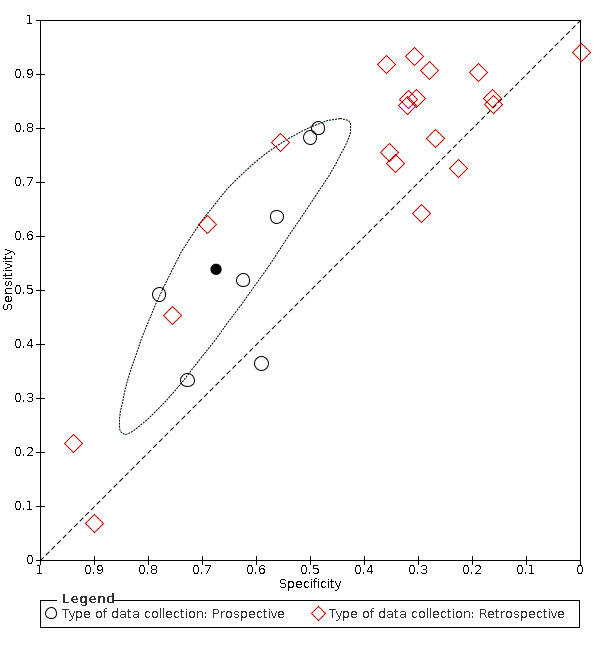
Summary ROC plot of fever. Summary point and 95% confidence region for prospective studies only
16.
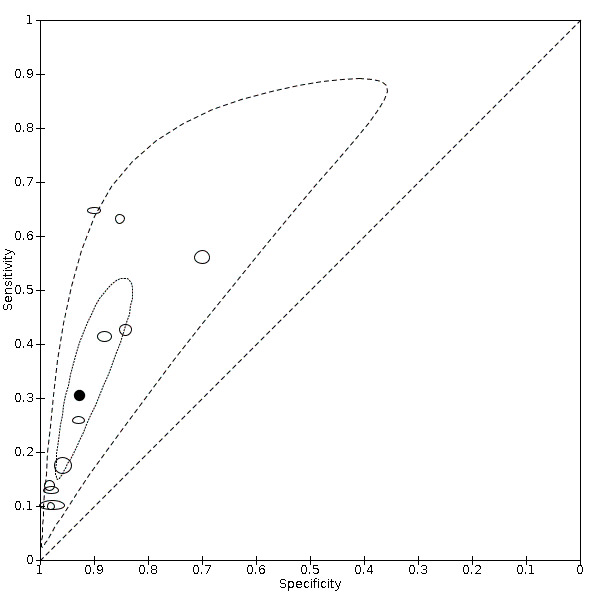
Summary ROC plot of anosmia
17.
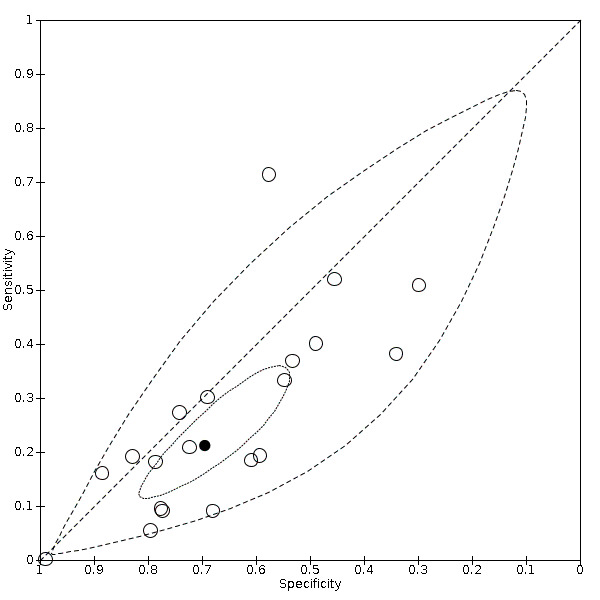
Summary ROC plot of sore throat (cross‐sectional studies)
18.

Summary ROC plot of ageusia
19.
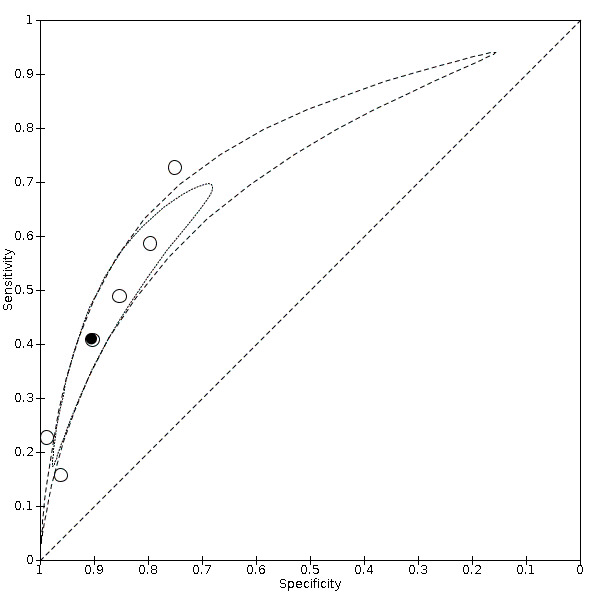
Summary ROC plot of anosmia or ageusia
20.
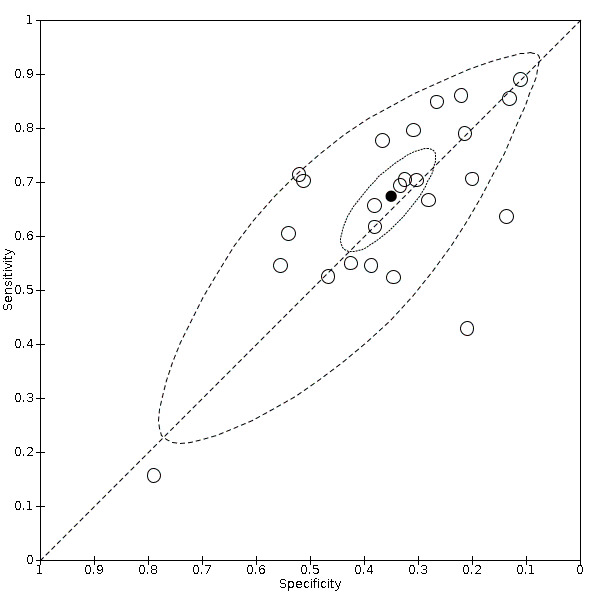
Summary ROC plot of cough (cross‐sectional studies)
21.
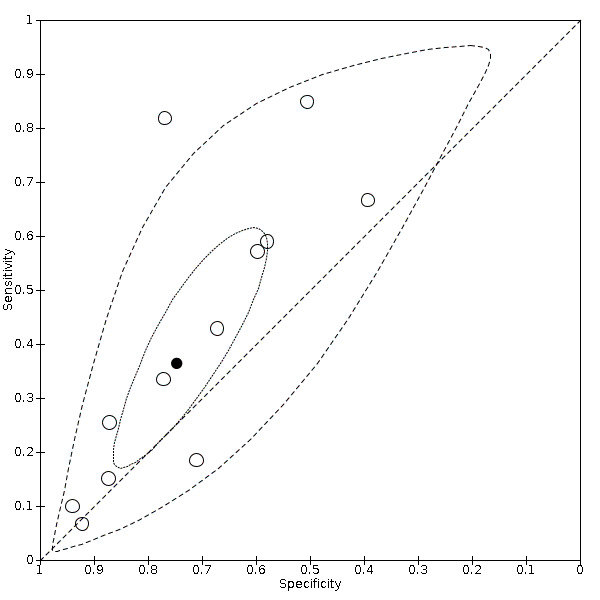
Summary ROC Plot of fatigue
22.
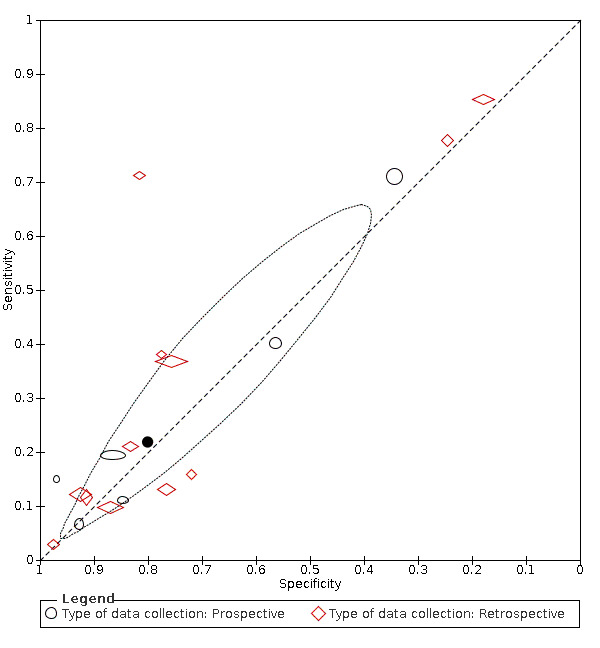
Summary ROC plot of headache. Summary point only estimable in prospective studies
23.
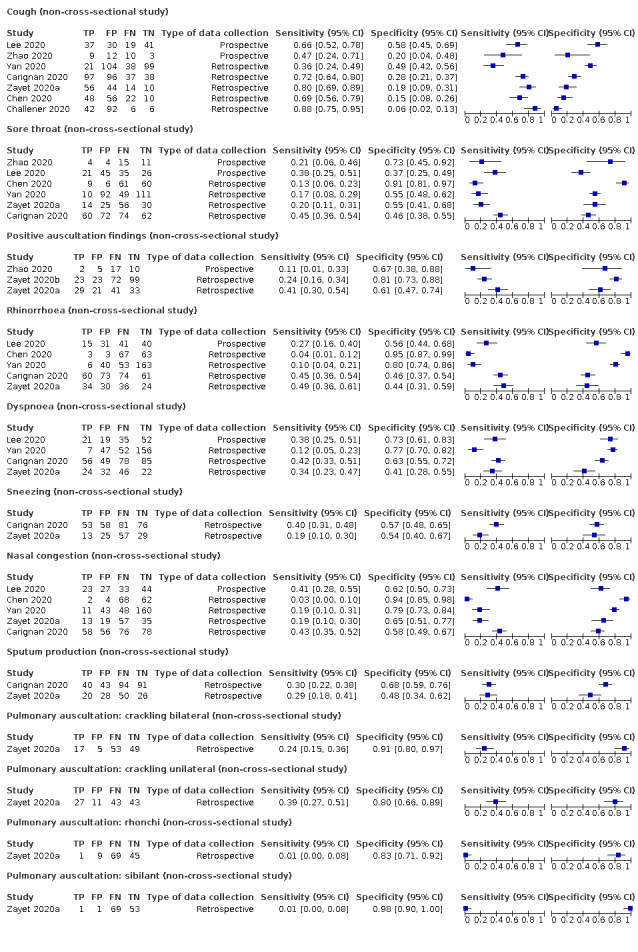
Forest plot of tests: cough (non‐cross‐sectional study), sore throat (non‐cross‐sectional study), positive auscultation findings (non‐cross‐sectional study), rhinorrhoea (non‐cross‐sectional study), dyspnoea (non‐cross‐sectional study), sneezing (non‐cross‐sectional study), nasal congestion (non‐cross‐sectional study), sputum production (non‐cross‐sectional study), pulmonary auscultation (crackling) bilateral (non‐cross‐sectional study), pulmonary auscultation (crackling unilateral; non‐cross‐sectional study), pulmonary auscultation (rhonchi; non‐cross‐sectional study), pulmonary auscultation: sibilant (non‐cross‐sectional study)
24.
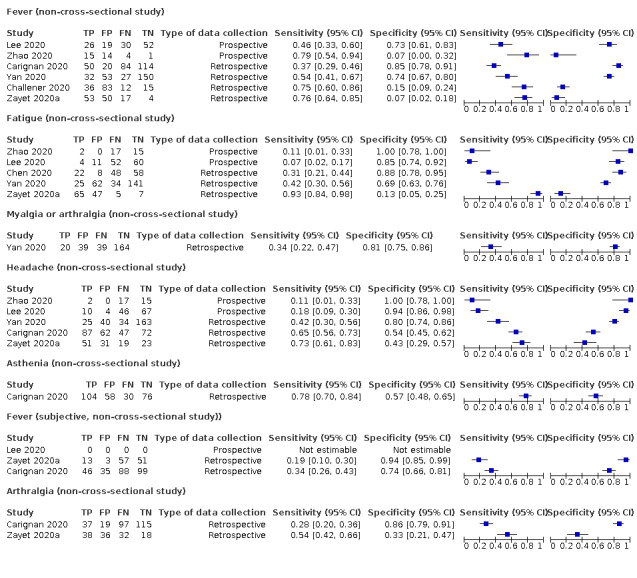
Forest plot of tests: fever (non‐cross‐sectional study), fatigue (non‐cross‐sectional study), myalgia or arthralgia (non‐cross‐sectional study), headache (non‐cross‐sectional study), asthenia (non‐cross‐sectional study), fever (subjective, non‐cross‐sectional study)), arthralgia (non‐cross‐sectional study)
25.
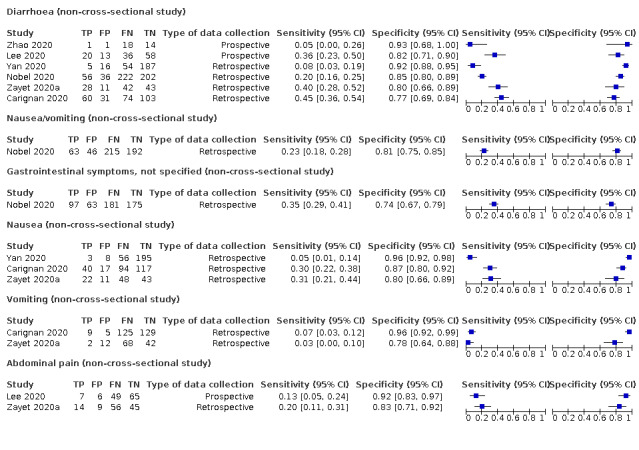
Forest plot of tests: diarrhoea (non‐cross‐sectional study), nausea/vomiting (non‐cross‐sectional study), gastrointestinal symptoms (not specified; non‐cross‐sectional study), nausea (non‐cross‐sectional study), vomiting (non‐cross‐sectional study), abdominal pain (non‐cross‐sectional study)
26.

Forest plot of chest tightness (non‐cross‐sectional study)
27.
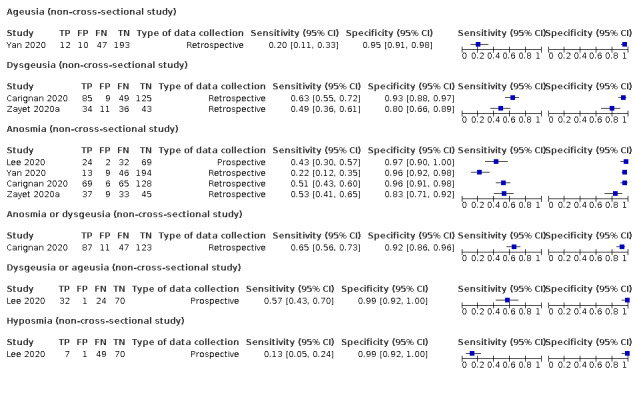
Forest plot of tests: ageusia (non‐cross‐sectional study), dysgeusia (non‐cross‐sectional study), anosmia (non‐cross‐sectional study), anosmia or dysgeusia (non‐cross‐sectional study), dysgeusia or ageusia (non‐cross‐sectional study), hyposmia (non‐cross‐sectional study)
Only two studies (Gilbert 2020; Yombi 2020), assessed combinations of different signs and symptoms. Gilbert 2020 investigated six combinations of two to four symptoms and signs each, while Yombi 2020 investigated three combinations of two to three symptoms each. Most of the combinations included fever and cough, on which both studies had preselected their participants. These combinations led to specificities above 80%, but at the cost of low sensitivities (< 30%).
Positivity rates of symptoms and signs depend on prevalence and population characteristics, especially pre‐selection. As a result, positivity rates were highly variable. In studies with prevalence less than 5%, suggesting little pre‐selection had taken place, positivity rates for fever (presence of the symptom in the study population) were between 9% and 41% (11.7% average), for cough between 45% and 70% (68% average), for anosmia between 2.5% and 2.6% (2.5% average), for ageusia (1 study) 2.8%, and for anosmia or ageusia (1 study) 4.3%.
Signs and symptoms for which sensitivity was reported above 50% in at least one cross‐sectional study are summarised below.
Symptoms and signs for which we performed pooling
We were able to conduct meta‐analyses for 14 signs or symptoms (cough, fever, anosmia, ageusia, anosmia or ageusia, sore throat, myalgia, fatigue, headache, dyspnoea, diarrhoea, sputum production, nausea or vomiting, chest tightness) based on clinically acceptable heterogeneity, the scatter of studies on visual inspection of the forest plots, and for which at least five studies were available. The analyses were restricted to cross‐sectional studies only. The ranges and summary estimates of the sensitivity and specificity of the 14 index tests are listed below. Additional summary point statistics are listed in additional Table 5.
4. Summary point statistics of selected index tests, including 95% confidence intervals (bivariate meta‐analysis, analyses restricted to cross‐sectional studies).
| Index test | Number of studies |
Number of COVID‐19 positives/ Total number of participants n/N (%) |
Sensitivity (95% CI) |
Specificity (95% CI) |
LR+ (95% CI) |
LR‐ (95% CI) |
DOR (95% CI) |
| A. All cross‐sectional studies | |||||||
| Cough | 25 | 3207/15,459 (20.7%) | 67.4% (59.8% to 74.1%) |
35.0% (28.7% to 41.9%) |
1.036 (0.969 to 1.107) |
0.933 (0.816 to 1.067) |
1.110 (0.909 to 1.356) |
| Anosmia | 11 | 2305/9552 (24.1%) | 28.0% (17.7% to 41.3%) |
93.4% (88.3% to 96.4%) |
4.254 (3.172 to 5.705) |
0.771 (0.676 to 0.879) |
5.549 (4.089 to 7.532) |
| Ageusia | 6 | 1893/7393 (25.6%) | 24.8% (12.4% to 43.5%) |
91.4% (81.3% to 96.3%) |
2.876 (2.021 to 4.092) |
0.823 (0.712 to 0.951) |
3.495 (2.408 to 5.072) |
| Anosmia or ageusia | 6 | 1589/8142 (19.5%) | 41.0% (27.0% to 56.6%) |
90.5% (81.2% to 95.4%) |
4.306 (3.002 to 6.177) |
0.652 (0.542 to 0.785) |
6.602 (5.271 to 8.270) |
| Sore throat | 20 | 3308/15,876 (20.8%) | 21.2% (13.5% to 31.6%) |
69.5% (58.1% to 78.9%) |
0.694 (0.565 to 0.853) |
1.134 (1.053 to 1.222) |
0.612 (0.473 to 0.793) |
| Myalgia | 13 | 2033/8105 (25.1%) | 26.6% (15.3% to 42.2%) |
83.1% (70.6% to 90.9%) |
1.575 (1.260 to 1.968) |
0.883 (0.810 to 0.962) |
1.783 (1.367 to 2.327) |
| Fatigue | 12 | 1727/5553 (31.1%) | 36.4 % (22.1% to 53.6%) |
74.7% (63.6% to 83.3%) |
1.438 (1.142 to 1.811) |
0.851 (0.727 to 0.997) |
1.689 (1.166 to 2.2447) |
| Dyspnoea | 24 | 2878/14,913 (19.3%) | 24.9% (16.6% to 35.5%) |
77.1% (66.8% to 84.8%) |
1.084 (0.906 to 1.299) |
0.975 (0.921 to 1.032) |
1.112 (0.878 to 1.409) |
| Diarrhoea | 20 | 2342/13,016 (18.0%) | 11.6% (7.6% to 17.4%) |
90.6% (86.6% to 93.5%) |
1.232 (1.006 to 1.509) |
0.976 (0.948 to 1.004) |
1.263 (1.004 to 1.588) |
| Anosmia or ageusia | 6 | 1589/8142 (19.5%) | 41.0% (27.0% to 56.6%) |
90.5% (81.2% to 95.4%) |
4.306 (3.002 to 6.177) |
0.652 (0.542 to 0.785) |
6.602 (5.271 to 8.270) |
| Sputum production | 10 | 1426/5144 (27.7%) | 18.9% (8.1% to 38.1%) |
81.3% (57.9% to 93.2%) |
1.009 (0.680 to 1.497) |
0.998 (0.912 to 1.092) |
1.011 (0.622 to 1.642) |
| Nausea or vomiting | 8 | 1059/5381 (19.7%) | 5.4% (2.4% to 11.5%) |
95.3% (92.0% to 97.3%) |
1.146 (0.676 to 1.942) |
0.993 (0.963 to 1.024) |
1.154 (0.660 to 2.017) |
| Chest tightness | 6 | 1518/6057 (25.1%) | 4.7% (2.5% to 8.9%) |
94.6% (88.6% to 97.6%) |
0.876 (0.568 to 1.349) |
1.007 (0.982 to 1.033) |
0.870 (0.550 to 1.373) |
| B. Sensitivity analysis: cross‐sectional studies with a prospective data‐collection only | |||||||
| Fever | 7 | 860/5548 (15.5%) | 53.8% (35.0% to 71.7%) |
67.4% (53.3% to 78.9%) |
1.651 (1.413 to 1.930) |
0.685 (0.534 to 0.879) |
2.411 (1.745 to 3.331) |
| Cough | 7 | 1484/6411 (23.1%) | 66.3% (57.8% to 73.8%) |
40.7% (33.6% to 48.3%) |
1.118 (1.005 to 1.243) |
0.829 (0.686 to 1.001) |
1.349 (1.008 to 1.805) |
| Headache | 6 | 1473/6171 (23.9%) | 21.9% (9.2% to 43.5%) |
80.1% (60.2% to 91.4%) |
1.097 (0.872 to 1.379) |
0.976 (0.914 to 1.043) |
1.124 (0.839 to 1.504) |
| Dyspnoea | 6 | 840/5495 (15.3%) | 37.0% (23.3% to 53.1%) |
66.0% (56.3% to 74.6%) |
1.089 (0.852 to 1.391) |
0.954 (0.821 to 1.110) |
1.140 (0.768 to 1.693) |
| Sore throat | 6 | 1464/6928 (21.1%) | 32.2% (23.0% to 43.1%) |
57.9% (43.9% to 70.8%) |
0.766 (0.690 to 0.849) |
1.170 (1.052 to 1.302) |
0.654 (0.540 to 0.793) |
| Diarrhoea | 6 | 635/5157 (12.3%) | 23.8% (13.8% to 37.8%) |
85.1% (77.2% to 90.6%) |
1.597 (0.903 to 2.826) |
0.895 (0.767 to 1.046) |
1.784 (0.869 to 3.660) |
| Myalgia | 4 | 488/1926 (25.3%) | NA | NA | NA | NA | NA |
| Fatigue | 6 | 752/2613 (28.8%) | 35.7% (17.2% to 59.7%) |
74.0% (56.1% to 86.4%) |
1.373 (0.901 to 2.094) |
0.869 (0.688 to 1.098) |
1.581 (0.837 to 2.984) |
| Sputum production | 1 | 225/961 (23.4%) | NA | NA | NA | NA | NA |
| Nausea or vomiting | 2 | 264/687 (38.4%) | NA | NA | NA | NA | NA |
| Chest tightness | 2 | 333/3326 (10.0%) | NA | NA | NA | NA | NA |
| Anosmia | 8 | 2129/8518 (25.0%) | 29.1% (18.9% to 42.1%) |
92.3% (85.8% to 95.9%) |
3.765 (2.783 to 5.092) |
0.768 (0.682 to 0.866) |
4.900 (3.717 to 6.460) |
| Ageusia | 5 | 1843/7293 (25.3%) | 29.4% (15.1% to 49.5%) |
89.0% (77.6% to 94.9%) |
2.667 (1.957 to 3.636) |
0.793 (0.669 to 0.941) |
3.362 (2.382 to 4.746) |
| Anosmia or ageusia | 5 | 1534/7406 (20.7%) | 36.5% (24.0% to 51.2%) |
92.4% (84.1% to 96.5%) |
4.782 (3.182 to 7.185) |
0.687 (0.586 to 0.806) |
6.955 (5.195 to 9.312) |
| CI: confidence interval; DOR: diagnostic odds ratio; LR+: positive likelihood ratio; LR‐: negative likelihood ratio; NA: not applicable, number of studies too small to perform meta‐analysis | |||||||
Cough
Sensitivity ranged from 16% to 89%; specificity from 11% to 79%
Pooled sensitivity 67.4% (95% confidence interval (CI) 59.8% to 74.1%); pooled specificity 35.0% (95% CI 28.7% to 41.9%); 25 studies, 15,459 participants
Anosmia
Sensitivity ranged from 10% to 65%; specificity from 70% to 98%
Pooled sensitivity 28.0% (95% CI 17.7% to 41.3%); pooled specificity 93.4% (95% CI 88.3% to 96.4%); 11 studies, 9552 participants
Ageusia
Sensitivity ranged from 10% to 55%; specificity from 70% to 100%
Pooled sensitivity 24.8% (95% CI 12.4% to 43.5%) pooled specificity 91.4% (95% CI 81.3% to 96.3%); 6 studies, 7393 participants
Anosmia or ageusia
Sensitivity ranged from 16% to 73%; specificity from 75% to 99%
Pooled sensitivity 41.0% (95% CI 27.0% to 56.6%); pooled specificity 90.5% (95% CI 81.2% to 95.4%); 6 studies, 8142 participants
Sore throat
Sensitivity ranged from 0% to 71%; specificity from 30% to 99%
Pooled sensitivity 21.2% (95% CI 13.5% to 31.6%); pooled specificity 69.5% (95% CI 58.1% to 78.9%); 20 studies, 15,876 participants
Myalgia
Sensitivity ranged from 1% to 65%; specificity from 33% to 99%
Pooled sensitivity 26.6% (95% CI 15.3% to 42.2%); pooled specificity 83.1% (95% CI 70.6% to 90.9%);13 studies, 8105 participants
Fatigue
Sensitivity ranged from 7% to 85%; specificity from 39% to 94%
Pooled sensitivity 36.4% (95% CI 22.1% to 53.6%); pooled specificity 74.7% (95% CI 63.6% to 83.3%); 12 studies, 5653 participants
Dyspnoea
Sensitivity ranged from 0% to 73%; specificity from 34% to 99%
Pooled sensitivity 24.9% (95% CI 16.6% to 35.5%); pooled specificity 77.1% (95% CI 66.8% to 84.8%); 24 studies, 14,913 participants
Diarrhoea
Sensitivity ranged from 0% to 64%; specificity from 62% to 99%
Pooled sensitivity 11.6% (95% CI 7.6% to 17.4%); pooled specificity 90.6% (95% CI 86.6% to 93.5%); 20 studies, 13,016 participants
Sputum production
Sensitivity ranged from 0% to 36%; specificity from 50% to 100%
Pooled sensitivity 18.9% (95% CI 8.1% to 38.1%); pooled specificity 81.3% (95% CI 57.9% to 93.2%); 10 studies, 5144 participants
Nausea or vomiting
Sensitivity ranged from 0% to 20%; specificity from 88% to 100%
Pooled sensitivity 5.4% (95% CI 2.4% to 11.5%); pooled specificity 95.3% (95% CI 92.0% to 97.3%); 8 studies, 5381 participants
Chest tightness
Sensitivity ranged from 2% to 15%; specificity from 71% to 98%
Pooled sensitivity 4.7% (95% CI 2.5% to 8.9%); pooled specificity 94.6% (95% CI 88.6% to 97.6%); 6 studies, 6057 participants
We performed sensitivity analyses to investigate the impact of prospective versus retrospective data collection:
Fever
Sensitivity analysis (prospective data collection only): sensitivity ranged from 7% to 94%; specificity from 0% to 94%
Pooled sensitivity 53.8% (95% CI 35.0% to 71.7%); pooled specificity 67.4% (95% CI 53.3% to 78.9%); 7 studies, 5548 participants
Headache
Sensitivity analysis (prospective data collection only): sensitivity ranged from 3% to 85%; specificity from 18% to 98%
Pooled sensitivity 21.9% (95% CI 9.2% to 43.5%); pooled specificity 80.1% (95% CI 60.2% to 91.4%); 6 studies, 6171 participants
Cough and fever (see sensitivity analyses) were the only index tests with a pooled sensitivity above 50% but their pooled specificity was only 35.5% and 67.4% respectively (Figure 20; Figure 15). Pooled specificity was above 90% for diarrhoea, nausea or vomiting, chest tightness, anosmia, ageusia, and for the presence of anosmia or ageusia (Figure 16; Figure 19). However, their pooled sensitivity was very low (maximum 11.6% for diarrhoea), except for anosmia (28.0%) and anosmia or ageusia (41.0%).
The only tests exceeding a pooled diagnostic odds ratio (DOR) of 5 were anosmia as a single test or in combination with ageusia (anosmia or ageusia). Yet, their pooled positive likelihood ratio (LR+) was below our predefined cut‐off of 5 for a useful red flag (4.25 (95% CI 3.17 to 5.71) and 4.31 (95% CI 3.00 to 6.18), respectively). The pooled negative likelihood ratios (LRs‐) were too high to make any of the reported tests useful to rule out the presence of COVID‐19 disease. In other words, the absence of the above mentioned index tests does not necessarily imply the absence of COVID‐19 disease.
Symptoms and signs for which we did not perform pooling
Rhinorrhoea (5 studies, 2252 participants): sensitivity between 4% to 62%, specificity between 37% to 93%
Chills (6 studies, 4151 participants): sensitivity between 4% to 80%, specificity between 36% to 93%
Myalgia or arthralgia (5 studies, 556 participants): sensitivity between 19% to 86%, specificity between 35% to 91%
Anosmia or dysgeusia (2 studies, 457 participants): sensitivity between 9% to 74%, specificity between 78% to 97%
Sensitivity analyses
In sensitivity analyses, we excluded studies that did not use a prospective study design (20 out of 32 cross‐sectional studies excluded). The results show that the pooled diagnostic accuracy estimates were not substantially different from the overall result (Table 5). In these sensitivity analyses, the scatter of studies on visual inspection of the forest plots appeared to decrease for fever and we decided to add a meta‐analysis for fever using prospective studies only. The pooled sensitivity and specificity of fever in prospective studies was 53.8% and 67.4% respectively Figure 15. This is the highest observed combination of both sensitivity and specificity for a symptom or sign, but the LR+ is still only 1.65 (95% CI 1.41 to 1.93).
To further illustrate a test's ability to either rule in or rule out COVID‐19, we constructed dumbbell plots showing pre‐ and post‐test probabilities for each olfactory symptom, fever and cough in each cross‐sectional study (Figure 28; Figure 29; Figure 30). For each test, we have plotted the pre‐test probability, which is the prevalence of COVID‐19 in the study (blue dot). The probability of having COVID‐19 after testing (post‐test probability) then changes depending on a positive test result (red dot marked +) or a negative test result (green dot marked ‐). The plot shows that the presence of anosmia, for example, increases the probability of COVID‐19 in all 11 studies. Its absence clearly decreases the probability of COVID‐19 in four studies (Brotons 2020; Leal 2020; Tudrej 2020; Zayet 2020b), and in the seven other studies there is not much difference between pre‐ and post‐test probability (Chua 2020; Haehner 2020; Just 2020; Peyrony 2020; Salmon 2020; Tordjman 2020; Trubiano 2020).
28.

Dumbbell plot: olfactory symptoms (cross‐sectional studies only). This plot shows how disease probability changes after a positive test result (red dot with plus sign) or after a negative test (green dot with minus sign). Pre‐test probability or prevalence is the blue dot
29.

Dumbbell plot: fever. This plot shows how disease probability changes after a positive test result (red dot with plus sign) or after a negative test (green dot with minus sign). Pre‐test probability or prevalence is the blue dot
30.

Dumbbell plot: cough. This plot shows how disease probability changes after a positive test result (red dot with plus sign) or after a negative test (green dot with minus sign). Pre‐test probability or prevalence is the blue dot
Discussion
Summary of main results
The majority of individual signs and symptoms included in this review appear to have very poor diagnostic accuracy, although this should be interpreted in the context of selection bias and heterogeneity between studies.
Based on currently available data, neither absence nor presence of a single sign or symptom are accurate enough to rule in or rule out COVID‐19. However, some combinations of signs and symptoms may be useful as a tool to triage patients for further testing. For example, combining the tests with the highest positive likelihood ratios in a hypothetical cohort with a disease prevalence (pre‐test probability) of 2%, the presence of either anosmia or ageusia would increase the post‐test probability of the presence of COVID‐19 to 8%. The presence of fever together with myalgia and anosmia would increase the post‐test probability to 17.8%.
We did not identify a useful combination of signs or symptoms that can safely rule out COVID‐19. For example, in the same hypothetical cohort with 2% disease prevalence, the absence of fever and anosmia would only lower the probability to 1% for the presence of COVID‐19. These results should be interpreted with caution as in reality these tests are correlated making it highly likely they would result in smaller changes in probability if they were tested in actual studies.
The seemingly better sensitivity for fever (and slightly lower specificity) compared to other index tests is unsurprising considering fever was a key feature of COVID‐19 that was used in selecting patients for further testing in included studies. As a result, most participants in these studies would have fever, both cases and non‐cases. The same applies to olfactory symptoms; only two studies did not select in any way for the presence of olfactory symptoms (Chua 2020; Peyrony 2020), whereas Leal 2020 selected their study participants on the presence of either fever, cough, sore throat, coryza or anosmia. In the studies with no prior selection, less than 10% of the study population presented with anosmia (2.5% in Chua 2020, 9.5% in Peyrony 2020), whereas the study with prior selection reported that 41% had anosmia. Without selection, sensitivity is low and specificity is high (13% to 14% sensitivity and 98% specificity); with prior selection, sensitivity is higher and specificity is lower (56% sensitivity and 70% specificity).
Selection bias is present when selective and non‐random inclusion and exclusion of participants applies and the resulting association between exposure and outcome (here the accuracy of the test) differs in the selected study population compared to the eligible study population, and it has been shown that this may decrease estimates of diagnostic accuracy (Rutjes 2006). For the diagnosis of COVID‐19, rapidly and constantly changing, and widely variable test criteria have influenced who was referred for testing and who was not. Inclusion in the study of only a fraction of eligible patients can give a biased estimate of the real accuracy of the index test when measured against the reference standard and real disease status. Griffith 2020 have reported on the problematic presence of collider stratification bias in the published studies on COVID‐19. Appropriate sampling strategies need to be applied to avoid conclusions of spurious relationships, more specifically in our case, the biased accuracy estimates of signs and symptoms for the diagnosis of COVID‐19. Selection of participants based on the presence of specific pre‐set symptoms, such as fever and cough, leads to biased associations between these symptoms and disease, and sensitivity and specificity estimates that differ from their true values. The example of collider bias for cough is illustrated in Figure 31. Grouping studies by diagnostic criteria for selection might clarify this issue, but studies do not clearly describe them, with study authors referring to the guidelines in general that were applicable at the time.
31.
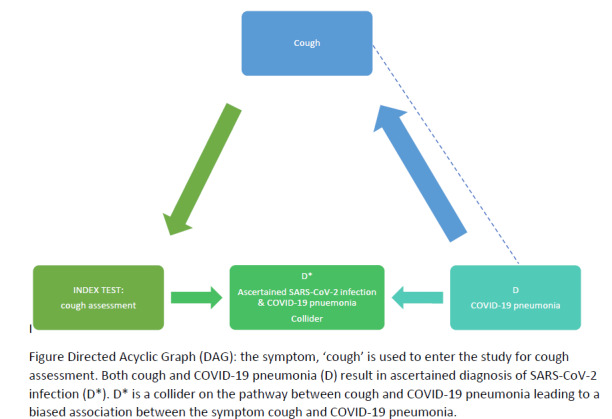
Directed acyclic graph on cough
Another form of selection bias is spectrum bias, where the patients included in the studies do not reflect the patient spectrum to which the index test will be applied. The inclusion of hospitalised patients can lead to such a bias, when in these patients both the distribution of signs and symptoms differ and assessment with the reference standard is differential. In addition, the distribution and severity of alternative diagnoses may be different in hospitalised populations than in patients presenting to ambulatory care settings.
Strengths and weaknesses of the review
Strengths of our review are the systematic and broad search performed to include all possible studies, including those prior to peer‐review, to gather the largest number of studies available at this point. Exclusion of cases‐only studies, the largest number of the published cohorts of patients with COVID‐19, limits the available data, however improves the quality of the evidence and the possibility to present both sensitivity and specificity (cases only cannot provide both accuracy measures). Because this is a living systematic review, this update offered the possibility of pooling estimates of diagnostic accuracy, which was not yet possible in our first review. Future updates will further increase the possibilities of analysing the data in more detail, and focusing the analyses on cross‐sectional data that were gathered prospectively.
The largest weakness of the review is the high risk of selection bias, as discussed above, with many studies including patients that had already been admitted to hospital or who presented to hospital settings seeking treatment.
The lack of data on combinations of signs and symptoms is an important evidence gap. Only two studies presented data on such combinations. The few composite signs and symptoms that were presented in those studies had little added diagnostic value compared to single tests. Combinations of tests increased the specificity, but at a large cost in sensitivity, because all signs and symptoms in the composite test had to be present to lead to a positive result. At this point, it is hard to assess the diagnostic value of combinations of signs and symptoms as the existing evidence is too scarce.
We need to assess multiple variables for their possible confounding effect on the summary estimates. Possible confounders include the presence of other respiratory pathogens (seasonality), the phase of the epidemic, exposure to high‐ versus low‐prevalence setting, high or low exposure risk, comorbidity of the participants, or time since infection. Seasonality may influence specificity, because alternative diagnoses such as influenza or other respiratory viruses are more prevalent in winter, leading to more non‐COVID‐19 patients displaying symptoms such as cough or fever, decreasing specificity. In this version of the review, all studies were conducted in winter or early spring, suggesting this may still have been at play. However, social distancing policies have shortened this year's influenza season in several countries (who.int/influenza/surveillance_monitoring/updates), which may have led to higher specificity for signs and symptoms than what we may expect in the next influenza season. In future updates of the review, we will explore seasonality effects if data allow. As for time since onset, given that the moment of infection is more likely than not an unrecognisable and unmeasurable variable, time since onset of symptoms can be used as a proxy. Reporting of studies, with presentation of the 2x2 table stratified by time since onset of disease, is informative and might have the potential to increase accuracy of the signs and symptoms and their diagnostic differential potential.
Applicability of findings to the review question
The high risk of selection bias, with many studies including patients who had already been admitted to hospital or who presented to hospital settings seeking treatment, leads to findings that are less applicable to people presenting in primary care, who on average experience a shorter illness duration, less severe symptoms and have a lower probability of the target condition.
Our search did not find any articles providing data on children. Children have been disproportionally underrepresented in the studies on diagnosing SARS‐CoV‐2 infection. Their absence seems related to the general mild presentation of the disease in the paediatric population and even more frequently the completely asymptomatic course. The full scope of disease presentation in children is, however, not known. It is important to identify signs and symptoms that can be used to assess children with suspected SARS‐CoV‐2 infection clinically, especially because non‐specific presentations and fever without a source are already common in this age group. Children present as a heterogeneous group; having separate data for neonates, young infants, toddlers, school aged children and adolescents is of value. Misclassification of children both at their presentation to the healthcare system and in the short term, where children will be asked to remain in quarantine when they present with predefined, but not yet evidence‐based symptoms needs to be avoided to decrease the possible damage done to children’s health.
Another important patient group is older adults. They are most at risk of a negative outcome of SARS‐CoV‐2 infection, especially mortality but also intensive care support. In this version of the review, only one study focused on adults aged 55 to 75 years. All other studies included adults of all ages and did not present results separately for the older age groups. The lack of a solid evidence base for the diagnosis of COVID‐19 in older adults adds to the difficulty in diagnosing serious infections in this age group, as other serious infections such as bacterial pneumonia or urinary sepsis also tend to lead to non‐specific presentations.
Studies that focus specifically on older adults or children may also enable us to estimate the diagnostic accuracy of signs and symptoms within these age groups. Given the distinct biological characteristics of children versus younger and versus older adults, these accuracy estimates are likely to be different in different age groups. The current presentation of overall pooled estimates may therefore prove too simplistic.
Authors' conclusions
Implications for practice.
Until results of further studies become available, broad investigation of people with suspected SARS‐CoV‐2 infection remains necessary. Neither absence nor presence of individual signs are accurate enough to rule in or rule out disease. Within the context of selection bias of all the studies in this review, the presence of fever, cough, or 'anosmia or ageusia' may be useful to identify people for further testing for COVID‐19.
Implications for research.
Our review update still reflects the need for improved study methodology and reporting in COVID‐19 diagnostic accuracy research.
Appropriate patient sampling strategies; prospective cross‐sectional design; investigating the presence or absence of clinical signs and symptoms in anyone with suspected COVID‐19
Improved reporting, with studies describing assessment of signs and symptoms (providing clearer definitions), and clear reporting of reference standards. Studies should report the definition of signs and symptoms more clearly, how they were measured, by whom and when. The measurement of key symptoms such as anosmia and ageusia could benefit from standardisation, including the severity and nature of the loss of smell or taste. Yet such standardisation should not be overly complicated, as signs and symptoms will typically be used by frontline clinicians who will incorporate these in their more holistic assessment of the patient which includes more than just COVID‐19.
Inclusion of a broader spectrum of patients, with studies in the primary healthcare setting to properly evaluate the diagnostic accuracy of signs and symptoms in this setting; inclusion of studies on patients with the aim of screening for infection (loosening up quarantine measurements may lead to an increased need for this); data on specific patient groups with comorbidities at higher risk of complications or severe disease and higher impact of missing diagnosis of SARS‐CoV‐2 infection at an early stage; addition of the paediatric population.
Prospective studies in an unselected population presenting to primary care or hospital outpatient settings, examining combinations of signs and symptoms to evaluate the syndromic presentation of COVID‐19, are needed. Results from such studies could inform subsequent management decisions such as self‐isolation or selecting patients for further diagnostic testing.
We would like to recommend that authors adhere to the STARD guidelines when reporting new studies on this topic (Bossuyt 2015).
What's new
| Date | Event | Description |
|---|---|---|
| 4 March 2021 | Amended | Corrected peer reviewer's name in Acknowledgements section |
History
Review first published: Issue 7, 2020
| Date | Event | Description |
|---|---|---|
| 11 February 2021 | New citation required and conclusions have changed | Review updated: We retrieved 28 more studies on signs and symptoms in suspected COVID‐19 patients, allowing pooling of the data for some features and estimation of summary measures of diagnostic accuracy. Moreover, this update contains new studies on the diagnostic value of olfactory symptoms, and includes a limited number of studies on combinations of symptoms. |
| 8 December 2020 | New search has been performed | Review updated |
| 7 July 2020 | Amended | Resolution of two figures improved |
Acknowledgements
Members of the Cochrane COVID‐19 Diagnostic Test Accuracy Review Group include:
the project team (Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MMG, Spijker R, Hooft L, Van den Bruel A, McInnes MDF, Emperador D, Dittrich S);
-
the systematic review teams for each review:
Molecular, antigen, and antibody tests (Adriano A, Beese S, Dretzke J, Ferrante di Ruffano L, Harris I, Price M, Taylor‐Phillips S)
Signs and symptoms (Stuyf T, Domen J, Horn S)
Routine laboratory markers (Yang B, Langendam M, Ochodo E, Guleid F, Holtman G, Verbakel J, Wang J, Stegeman I)
Imaging tests (Salameh JP, McGrath TA, van der Pol CB, Frank RA, Prager R, Hare SS, Dennie C, Jenniskens K, Korevaar DA, Cohen JF, van de Wijgert J, Damen JAAG, Wang J)
the wider team of systematic reviewers from University of Birmingham, UK who assisted with title and abstract screening across the entire suite of reviews for the diagnosis of COVID‐19 (Agarwal R, Baldwin S, Berhane S, Herd C, Kristunas C, Quinn L, Scholefield B).
We thank Dr Jane Cunningham (World Health Organization) for participation in technical discussions and comments on the manuscript.
The editorial process for this review was managed by Cochrane's Central Editorial Service in collaboration with Cochrane Infectious Diseases. We thank Helen Wakeford, Anne‐Marie Stephani and Deirdre Walshe for their comments and editorial management. We thank Robin Featherstone for comments on the search and Mike Brown and Paul Garner for sign‐off comments. We thank Denise Mitchell for her efforts in copy‐editing this review.
Thank you also to peer referees Alfonso Luca Pendolino, Trish Greenhalgh, Robert Walton, Chris Cates and Lynda Ware, consumer referee Jenny Negus, and methodological referees Gianni Virgili and Marta Roqué, for their insights.
The editorial base of Cochrane Infectious Diseases is funded by UK aid from the UK Government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK Government’s official policies.
Jonathan Deeks is a UK National Institute for Health Research (NIHR) Senior Investigator Emeritus. Yemisi Takwoingi is supported by a NIHR Postdoctoral Fellowship. Jonathan Deeks, Jacqueline Dinnes, Yemisi Takwoingi, Clare Davenport and Malcolm Price are supported by the NIHR Birmingham Biomedical Research Centre. This paper presents independent research supported by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Appendices
Appendix 1. World Health Organization case definitions
Severe pneumonia
Adolescent or adult: fever or suspected respiratory infection, plus one of the following: respiratory rate higher than 30 breaths/minute; severe respiratory distress; or oxygen saturation (SpO2) 93% or less on room air. Child with cough or difficulty in breathing, plus at least one of the following: central cyanosis or SpO2 less than 90%; severe respiratory distress (for example, grunting, very severe chest indrawing); signs of pneumonia with a general danger sign: inability to breastfeed or drink, lethargy or unconsciousness, or convulsions.
Other signs of pneumonia may be present: chest indrawing, fast breathing (in breaths/minute): aged under 2 months: 60 or higher; aged 2 to 11 months: 50 or higher; aged 1 to 5 years: 40 or higher. While the diagnosis is made on clinical grounds; chest imaging may identify or exclude some pulmonary complications.
Acute respiratory distress syndrome (ARDS)
Onset within one week of a known clinical insult or new or worsening respiratory symptoms.
Chest imaging (that is, X‐ray, computed tomography (CT) scan, or lung ultrasound): bilateral opacities, not fully explained by volume overload, lobar or lung collapse, or nodules.
Origin of pulmonary infiltrates: respiratory failure not fully explained by cardiac failure or fluid overload. Need objective assessment (for example, echocardiography) to exclude hydrostatic cause of infiltrates/oedema if no risk factor present.
Oxygenation impairment in adults:
mild ARDS: 200 mmHg less than ratio of arterial oxygen partial pressure/fractional inspired oxygen (PaO2/FiO2) 300 mmHg or less (with positive end‐expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) 5 cmH2O, or more, or non‐ventilated);
moderate ARDS: 100 mmHg < PaO2/FiO2 ≤ 200 mmHg (with PEEP ≥ 5 cmH2O, or non‐ventilated);
severe ARDS: PaO2/FiO2 ≤ 100 mmHg (with PEEP ≥ 5 cmH2O, or non‐ventilated);
when PaO2 is not available, SpO2/FiO2 ≤ 315 mmHg suggests ARDS (including in non‐ventilated patients).
Oxygenation impairment in children: note OI = Oxygenation Index and OSI = Oxygenation Index using SpO2. Use PaO2‐based metric when available. If PaO2 not available, wean FiO2 to maintain SpO2 ≤ 97% to calculate OSI or SpO2/FiO2 ratio:
bilevel (non‐invasive ventilation or CPAP) ≥ 5 cmH2O via full‐face mask: PaO2/FiO2 ≤ 300 mmHg or SpO2/FiO2 ≤ 264;
mild ARDS (invasively ventilated): 4 ≤ OI < 8 or 5 ≤ OSI < 7.5;
moderate ARDS (invasively ventilated): 8 ≤ OI < 16 or 7.5 ≤ OSI < 12.3;
severe ARDS (invasively ventilated): OI ≥ 16 or OSI ≥ 12.3.
Appendix 2. Search classification model
We needed a more efficient approach to keep up with the rapidly increasing volume of COVID‐19 literature. A classification model for COVID‐19 diagnostic studies was built with the model building function within Eppi Reviewer, which uses the standard SGCClassifier in Scikit‐learn on word trigrams. As outputs, new documents receive a percentage (from the predict_proba function) where scores close to 100 indicate a high probability of belonging to the class ‘relevant document’ and scores close to 0 indicate a low probability of belonging to the class ‘relevant document’. We used three iterations of manual screening (title and abstract screening, followed by full‐text review) to build and test classifiers. The final included studies were used as relevant documents, while the remainder of the COVID‐19 studies were used as irrelevant documents. The classifier was trained on the first round of selected articles, and tested and retrained on the second round of selected articles. Testing on the second round of selected articles revealed poor positive predictive value but 100% sensitivity at a cut‐off of 10. The poor positive predictive value is mainly due to the broad scope of our topic (all diagnostic studies in COVID‐19), poor reporting in abstracts, and a small set of included documents. The model was retrained using the articles selected of the second and third rounds of screening, which added a considerable number of additional documents. This led to a large increase in positive predictive value, at the cost of a lower sensitivity, which led us to reduce the cut‐off to 5. The largest proportion of documents had a score between 0‐5. This set did not contain any of the relevant documents. This version of the classifier with a cut‐off 5 was used in subsequent rounds and accounted for approximately 80% of the screening burden.
Appendix 3. Cochrane COVID‐19 Study Register searches
| Source | Strategy |
| ClinicalTrials.gov | COVID‐19 OR 2019‐nCoV OR SARS‐CoV‐2 OR 2019 novel coronavirus OR severe acute respiratory syndrome coronavirus 2 OR Wuhan coronavirus |
| WHO ICTRP | We screened the entire COVID‐19.csv file available from https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019 |
| PubMed | ("2019 nCoV"[tiab] OR 2019nCoV[tiab] OR "2019 novel coronavirus"[tiab] OR ((coronavirus[tiab] OR "corona virus"[tiab]) AND (Huanan[tiab] OR Hubei[tiab] OR Wuhan[tiab])) OR "coronavirus‐19"[tiab] OR "coronavirus disease‐19"[tiab] OR "coronavirus disease‐2019"[tiab] OR "COVID 19"[tiab] OR COVID19[tiab] OR "nCov 2019"[tiab] OR "new coronavirus"[tiab] OR "new coronaviruses"[tiab] OR "novel coronavirus"[tiab] OR "novel coronaviruses"[tiab] OR "novel corona virus"[tiab] OR "SARS‐CoV2"[tiab] OR "SARS CoV‐2"[tiab] OR SARSCoV2[tiab] OR "SARSCoV‐2"[tiab] OR "SARS‐coronavirus‐2"[tiab] OR "SARS‐like coronavirus"[tiab] OR "Severe Acute Respiratory Syndrome Coronavirus‐2"[tiab] OR "COVID‐19"[nm] OR "COVID‐19 drug treatment"[nm] OR "COVID‐19 diagnostic testing"[nm] OR "COVID‐19 serotherapy"[nm] OR "COVID‐19 vaccine"[nm] OR "LAMP assay"[nm] OR "severe acute respiratory syndrome coronavirus 2"[nm] OR "spike protein, SARS‐CoV‐2"[nm]) NOT ("animals"[mh] NOT "humans"[mh]) NOT (editorial[pt] OR newspaper article[pt]) |
Appendix 4. Living search from the University of Bern
We took the following information from the university of Bern website (see: ispmbern.github.io/covid-19/living-review/collectingdata.html).
The register is updated daily and CSV file downloads are made available.
1 April 2020
From 1 April 2020, we will retriev the curated BioRxiv/MedRxiv dataset (connect.medrxiv.org/relate/content/181).
26 to 31 March 2020
MEDLINE: (\"Wuhan coronavirus\" [Supplementary Concept] OR \"COVID‐19\" OR \"2019 ncov\"[tiab] OR ((\"novel coronavirus\"[tiab] OR \"new coronavirus\"[tiab]) AND (wuhan[tiab] OR 2019[tiab])) OR 2019‐nCoV[All Fields] OR (wuhan[tiab] AND coronavirus[tiab])))))
Embase: (nCoV or 2019‐nCoV or ((new or novel or wuhan) adj3 coronavirus) or covid19 or covid‐19 or SARS‐CoV‐2).mp.
BioRxiv/MedRxiv: ncov or corona or wuhan or COVID or SARS‐CoV‐2
With the kind support of the Public Health & Primary Care Library PHC (www.unibe.ch/university/services/university_library/faculty_libraries/medicine/public_health_amp_primary_care_library_phc/index_eng.html), and following guidance of the Medical Library Association (www.mlanet.org/p/cm/ld/fid=1713).
1 January 2020 to 25 March 2020
MEDLINE: ("Wuhan coronavirus" [Supplementary Concept] OR "COVID‐19" OR "2019 ncov"[tiab] OR (("novel coronavirus"[tiab] OR "new coronavirus"[tiab]) AND (wuhan[tiab] OR 2019[tiab])) OR 2019‐nCoV[All Fields] OR (wuhan[tiab] AND coronavirus[tiab])))))
Embase: ncov OR (wuhan AND corona) OR COVID
BioRxiv/MedRxiv: ncov or corona or wuhan or COVID
Appendix 5. CDC Library, COVID‐19 Research Articles Downloadable Database
Embase records from the Stephen B. Thacker CDC Library, COVID‐19 Research Articles Downloadable Database.
Records were obtained by the CDC library by searching Embase through Ovid using the following search strategy.
| Source | Strategy |
| Embase | (coronavir* OR corona virus* OR betacoronavir* OR covid19 OR covid 19 OR nCoV OR novel CoV OR CoV 2 OR CoV2 OR sarscov2 OR 2019nCoV OR wuhan virus*).mp. OR ((wuhan OR hubei OR huanan) AND (severe acute respiratory OR pneumonia*) AND outbreak*).mp. OR Coronavirus infection/ OR coronavirinae/ OR exp betacoronavirus/ Limits: 2020‐ OR (novel coronavir* OR novel corona virus* OR covid19 OR covid 19 OR nCoV OR novel CoV OR CoV 2 OR CoV2 OR sarscov2 OR 2019nCoV OR wuhan virus*).mp. OR ((wuhan OR hubei OR huanan) AND (severe acute respiratory OR pneumonia*) AND outbreak*).mp. OR ((wuhan OR hubei OR huanan) AND (coronavir* OR betacoronavir*)).mp. Limits: 2019‐ |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
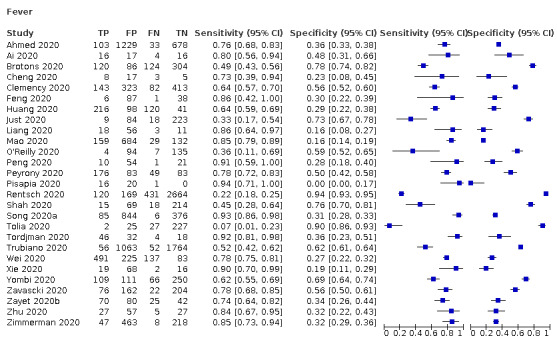
Fever
2. Test.
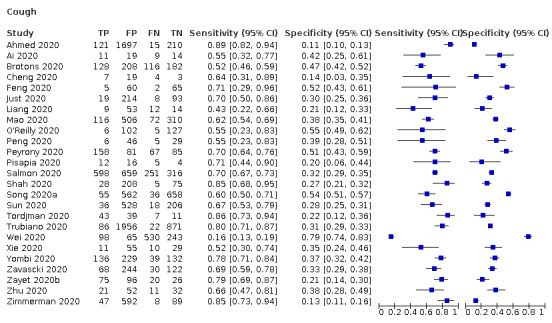
Cough
3. Test.
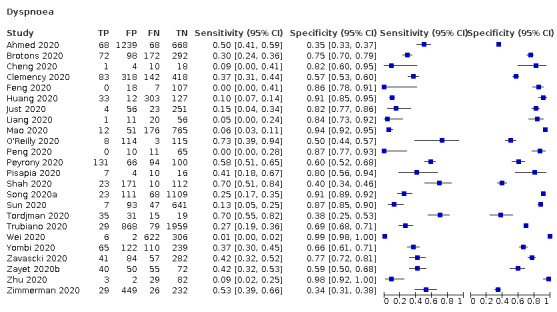
Dyspnoea
4. Test.

Sore throat
5. Test.
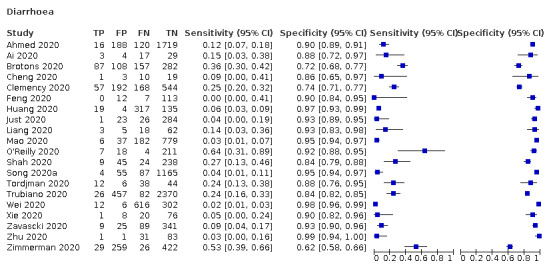
Diarrhoea
6. Test.
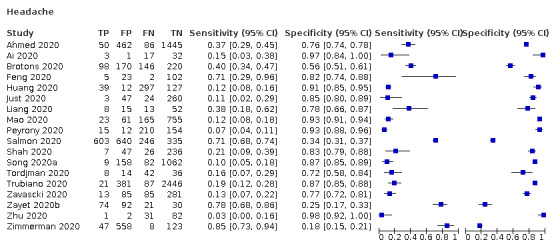
Headache
7. Test.
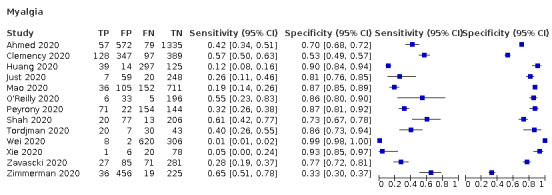
Myalgia
8. Test.
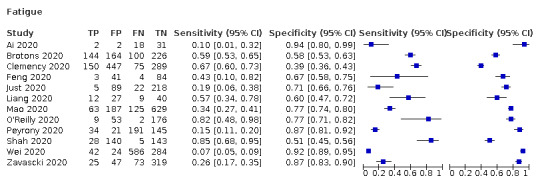
Fatigue
9. Test.
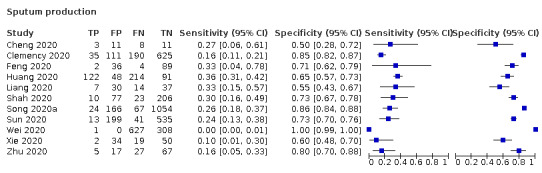
Sputum production
10. Test.
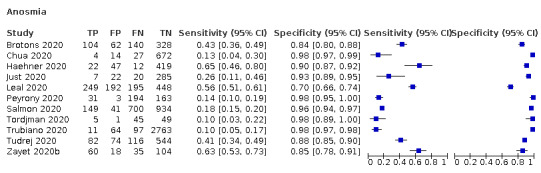
Anosmia
11. Test.
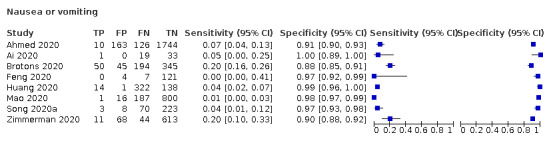
Nausea or vomiting
12. Test.
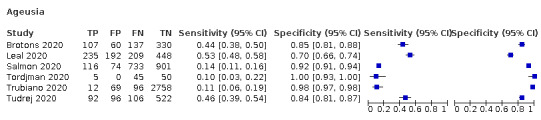
Ageusia
13. Test.
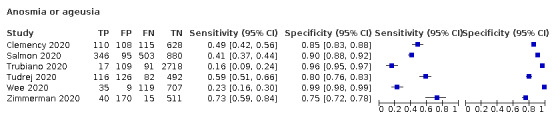
Anosmia or ageusia
14. Test.
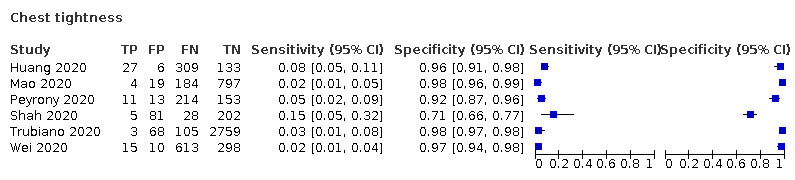
Chest tightness
15. Test.
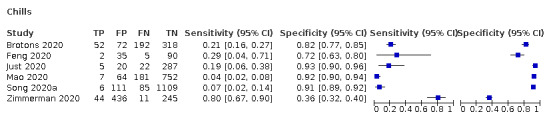
Chills
16. Test.
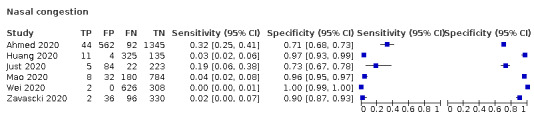
Nasal congestion
17. Test.
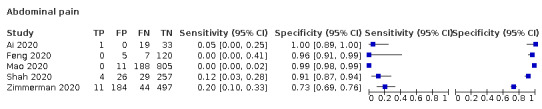
Abdominal pain
18. Test.
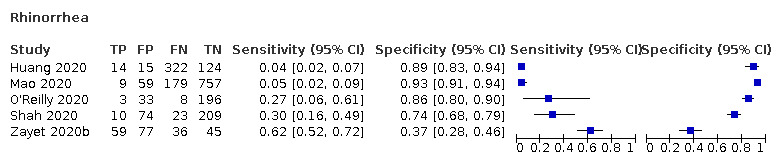
Rhinorrhea
19. Test.
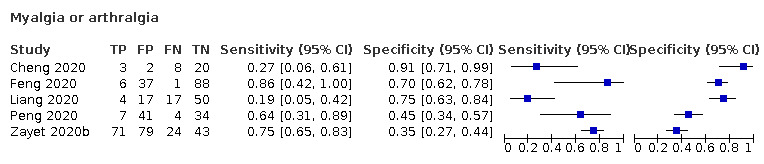
Myalgia or arthralgia
20. Test.
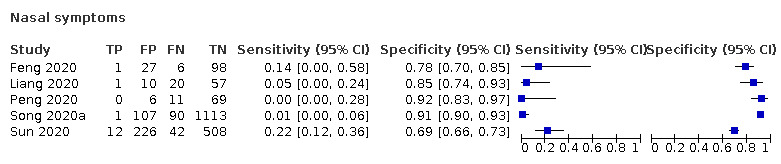
Nasal symptoms
21. Test.
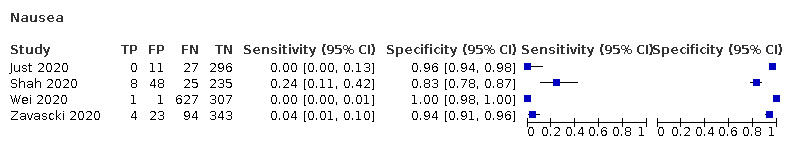
Nausea
22. Test.

Haemoptysis
23. Test.
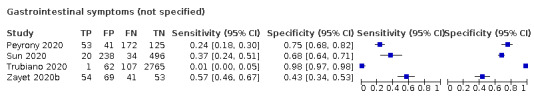
Gastrointestinal symptoms (not specified)
24. Test.
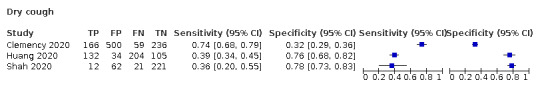
Dry cough
25. Test.

Vomiting
26. Test.

Skin lesions
27. Test.

Anosmia and ageusia
28. Test.

Anosmia or dysgeusia
29. Test.

Anorexia
30. Test.
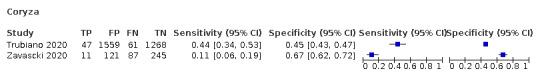
Coryza
31. Test.

Wheeze
32. Test.
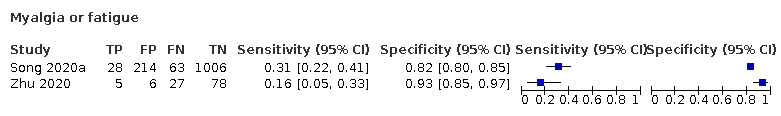
Myalgia or fatigue
33. Test.

Fever (subjective)
34. Test.
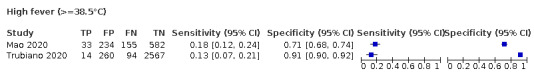
High fever (>=38.5°C)
35. Test.

Altered mentation
36. Test.

Weakness or fatigue
37. Test.
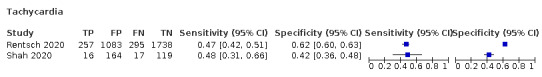
Tachycardia
38. Test.

Loss of appetite
39. Test.

Hypoxia
41. Test.

Respiratory symptoms (not specified))
42. Test.

Rhinitis or pharyngitis
43. Test.

Sinusitis
44. Test.

Isolated fever
45. Test.

Low body temperature
46. Test.

Shivers
47. Test.

Arthralgia
48. Test.

Systemic soreness (malaise/myalgia/arthralgia)
49. Test.

Abdominal distension
50. Test.

Low systolic blood pressure
51. Test.

High systolic blood pressure
52. Test.

Palpitations
53. Test.

Tachypnea
54. Test.

Lethargy
55. Test.

Hyposmia
56. Test.

Dysgeusia
57. Test.

Anosmia and dysgeusia
58. Test.

Rash
59. Test.

Isolated headache
60. Test.

Diarrhea and nausea
61. Test.

Dizziness or syncope
62. Test.

Earache
63. Test.

Enlargement of lymph nodes
64. Test.

Stomachache
65. Test.

Arthralgia
66. Test.

Unconsciousness
67. Test.

Aversion to cold
68. Test.

Xerostomia
69. Test.

Hypersomnia
70. Test.

Sneezing
71. Test.

Change to chronic cough
72. Test.

Dizziness
73. Test.

Positive auscultation findings
74. Test.

Pulmonary auscultation: crackling bilateral
75. Test.

Pulmonary auscultation: crackling unilateral
76. Test.

Conjunctivitis
77. Test.

Myalgia and asthenia and fever
78. Test.

Fever and cough
79. Test.

Fever and cough and sore throat
80. Test.

Fever and cough and dyspnea
81. Test.

Cough and fever and sputum production
82. Test.

Cough and fever and sputum production and dyspnea
83. Test.

Sore throat and nasal congestion and sneezing and mild fever
84. Test.

Dyspnea and cough and fever and low oxygen saturation
85. Test.
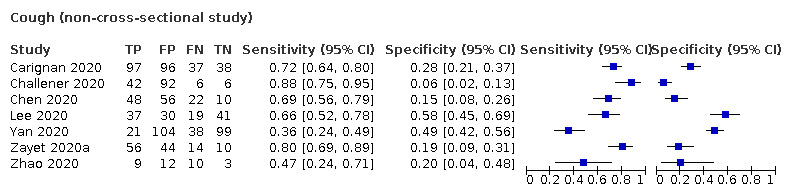
Cough (non‐cross‐sectional study)
86. Test.
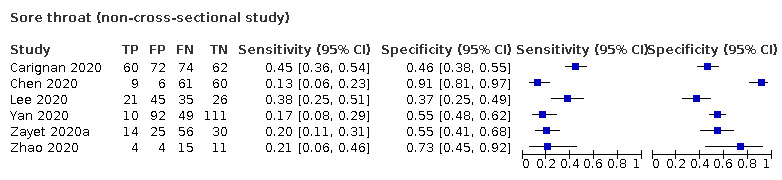
Sore throat (non‐cross‐sectional study)
87. Test.
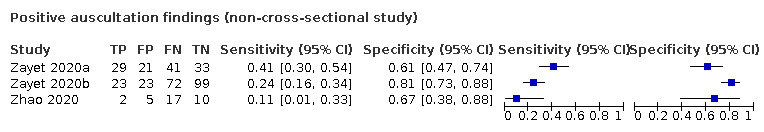
Positive auscultation findings (non‐cross‐sectional study)
88. Test.
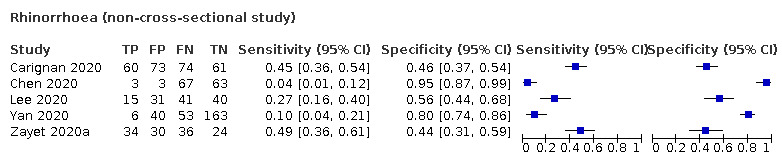
Rhinorrhoea (non‐cross‐sectional study)
89. Test.
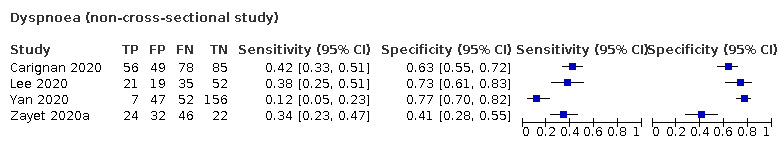
Dyspnoea (non‐cross‐sectional study)
90. Test.

Ageusia (non‐cross‐sectional study)
91. Test.

Chest tightness (non‐cross‐sectional study)
92. Test.
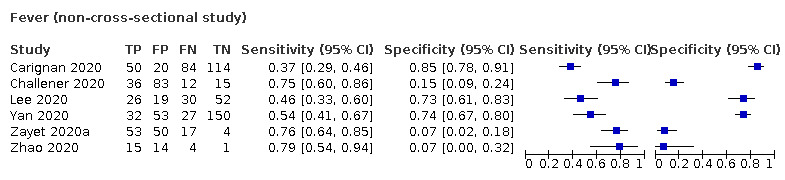
Fever (non‐cross‐sectional study)
93. Test.
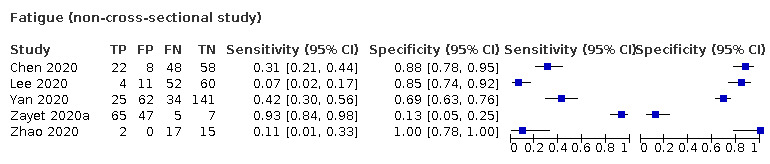
Fatigue (non‐cross‐sectional study)
94. Test.

Myalgia or arthralgia (non‐cross‐sectional study)
95. Test.
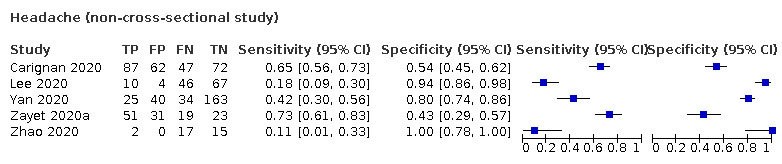
Headache (non‐cross‐sectional study)
96. Test.
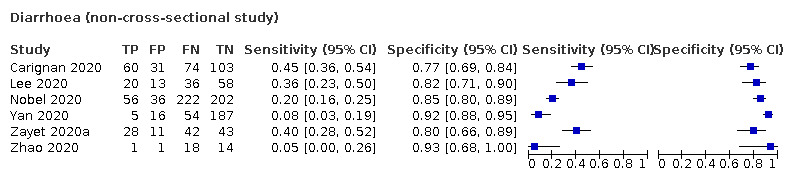
Diarrhoea (non‐cross‐sectional study)
97. Test.

Nausea/vomiting (non‐cross‐sectional study)
98. Test.

Red eyes (non‐cross‐sectional study)
99. Test.

Gastrointestinal symptoms, not specified (non‐cross‐sectional study)
TST-100. Test.

Asthenia (non‐cross‐sectional study)
TST-101. Test.

Fever (subjective, non‐cross‐sectional study))
TST-102. Test.
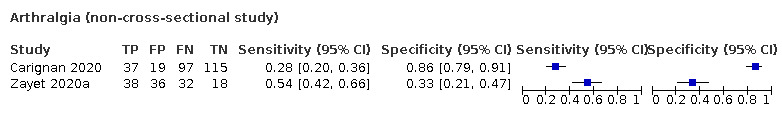
Arthralgia (non‐cross‐sectional study)
TST-103. Test.

Sneezing (non‐cross‐sectional study)
TST-104. Test.

Rash (non‐cross‐sectional study)
TST-105. Test.

Loss of temp. sens. in face (non‐cross‐sectional study)
TST-106. Test.

Vertigo or dizziness (non‐cross‐sectional study)
TST-107. Test.

Blurred vision (non‐cross‐sectional study)
TST-108. Test.

Nasal congestion (non‐cross‐sectional study)
TST-109. Test.

Dysgeusia (non‐cross‐sectional study)
TST-110. Test.

Anosmia (non‐cross‐sectional study)
TST-111. Test.

Loss of appetite (non‐cross‐sectional study)
TST-112. Test.

Myalgia (non‐cross‐sectional study)
TST-113. Test.

Anosmia or dysgeusia (non‐cross‐sectional study)
TST-114. Test.

Sputum production (non‐cross‐sectional study)
TST-115. Test.

Chills (non‐cross‐sectional study)
TST-116. Test.

Nausea (non‐cross‐sectional study)
TST-117. Test.

Vomiting (non‐cross‐sectional study)
TST-119. Test.

Abdominal pain (non‐cross‐sectional study)
TST-120. Test.

Conjunctival hyperemia (non‐cross‐sectional study)
TST-121. Test.

Diffuse headache (non‐cross‐sectional study)
TST-122. Test.

Frontal headache (non‐cross‐sectional study)
TST-123. Test.

Epistaxis (non‐cross‐sectional study)
TST-124. Test.

Dry eyes (non‐cross‐sectional study)
TST-125. Test.

Haemoptysis (non‐cross‐sectional study)
TST-126. Test.

Hearing loss (non‐cross‐sectional study)
TST-127. Test.

Pulmonary auscultation: crackling bilateral (non‐cross‐sectional study)
TST-128. Test.

Pulmonary auscultation: crackling unilateral (non‐cross‐sectional study)
TST-129. Test.

Pulmonary auscultation: rhonchi (non‐cross‐sectional study)
TST-130. Test.

Pulmonary auscultation: sibilant (non‐cross‐sectional study)
TST-131. Test.

Tachypnea (non‐cross‐sectional study)
TST-132. Test.

Tinnitus (non‐cross‐sectional study)
TST-133. Test.

Tearing (non‐cross‐sectional study)
TST-134. Test.

Dysgeusia or ageusia (non‐cross‐sectional study)
TST-135. Test.

Hyposmia (non‐cross‐sectional study)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmed 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐Cov‐2 infection (mild COVID‐19 disease) Design: retrospective, registry‐based study Recruitment: random subset of manually extracted charts of all patients tested for SARS‐CoV‐2 in the UHealth system Sample size: n = 2043 (136 cases) Inclusion criteria: manual extraction for a random subset of patients tested before 31 March 2020 of all patients having a SARS‐CoV‐2 test result in the UHealth system. Testing was performed in patients having at least one symptom (cough, fever, or shortness of breath). Exclusion criteria: none |
||
| Patient characteristics and setting |
Facility cases: positive SARS‐CoV‐2 test (specimen and test‐type unspecified). Population‐level testing. Primarily outpatient settings Facility controls: negative SARS‐CoV‐2 test (specimen and test‐type unspecified). Population‐level testing. Primarily outpatient settings Country: Utah, USA Dates: 10 March 2020‐31 March 2020 Symptoms and severity: random subset of all tested patients included. Tested if at least one symptom (cough, fever or shortness of breath). Population primarily comprised of mild and moderate infections. Demographics: median age cases: 38.4 years controls: 39.2 years. Gender: % female cases: 44%, controls: 56% (entire cohort) Exposure history: % prior exposure: cases: 57%, controls: 29% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval not specified | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Ai 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 pneumonia Design: cross‐sectional multicentre prospective study Recruitment: hospitalised pneumonia patients Sample size: n = 53 (20 cases) Inclusion criteria: suspected SARS‐CoV‐2 pneumonia patients, defined as having pneumonia after chest CT (with 1 of the 2 following criteria met: fever or respiratory symptoms, normal or decreased WBC counts/decreased) Exclusion criteria: not defined |
||
| Patient characteristics and setting |
Facility cases: confirmed case: a positive SARS‐CoV‐2 nucleotides result either by metagenomic sequencing or RT‐PCR assay for nasopharyngeal swab specimens Facility controls: pneumonia patients confirmed not to be infected by SARS‐CoV‐2 (2 PCR tests, 2 days in between) Country: China Dates: 22 January 2020‐19 February 2020 Symptoms and severity: suspected SARS‐CoV‐2 pneumonia (NCP): having pneumonia after chest CT with 1 of the 2 following criteria met: fever or respiratory symptoms, normal or decreased WBC counts/decreased lymphocyte counts, and a travel history or contact with patients with fever or respiratory symptoms from Hubei Province or confirmed cases within 2 weeks Demographics: median age cases 37 years, controls 39 years, gender distribution cases (M/F: 50/50), controls (M/F: 48.5/51.5) Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval not specified. Reference standard at day 0 and day 2, index tests from electronic medical records but stated at pneumonia onset | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Brotons 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to measure the seroprevalence of antibodies against SARS‐CoV‐2 infection in a community sample of asymptomatic and symptomatic patients. Design: multicenter prospective cohort Recruitment: patients with mild or moderate COVID‐19 symptoms who had a face‐to‐face or phone consultation with their GP between 2 March and 24 April 2020 Sample size: n = 634 (244 cases) Inclusion criteria: all patients aged ≥ 1 year consulting the primary care physician either face‐to‐face or by phone with mild or moderate symptoms (without a confirmed diagnosis) during the COVID‐19 pandemic from 2 March‐24 April 2020 Exclusion criteria: none |
||
| Patient characteristics and setting |
Facility cases: Facility controls: Country: Spain Dates: 2 March 2020‐24 April 2020 Symptoms and severity: mild to moderate symptoms Demographics: mean age: 46.97 years. Gender: % female cases: 55.3% cases, 59.23% controls Exposure history: contact: cases 50.82%, controls 38.97% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Reported on the same day, patients were sick between 10 days‐40 days before (recall bias risk) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Carignan 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to assess whether anosmia and dysgeusia are specific symptoms for SARS‐CoV‐2 Design: case−control study Recruitment: all adult patients who underwent testing for SARS‐CoV‐2 at the CHUS (Centre Hospitalier de Sherbrooke), cases: all positives, controls: random sample Sample size: n = 268 (134 cases) Inclusion criteria: the criteria for SARS‐CoV‐ 2 testing included symptomatic (fever, cough or dyspnea) travellers and contacts of confirmed COVID‐19 cases. All adult patients (≥ 18 years) who underwent testing were included. Exclusion criteria: patients with multiple tests during the study period |
||
| Patient characteristics and setting |
Facility cases: all adult (age ≥ 18 years) patients testing positive for SARS‐CoV‐2 by means of RT‐PCR Facility controls: matched (1:1) according to 5‐year age groups selected by means of a pseudorandom number generator from all patients who tested negative for SARS‐CoV‐2 at the CHUS during the same period Country: Quebec, Canada Dates: 10 March 2020‐23 March 2020 Symptoms and severity: mild to moderate severity Demographics: median age: cases: 57.1 years, controls: 57.2 years gender: % female cases: 52.2%, controls: 60.4% Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests within 72 h before or after SARS‐CoV‐2 testing (in reality: 3‐15 days) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Challener 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to determine predictors of a positive test for COVID‐19 Design: case‐control Recruitment: retrospective review of medical records of patients with the first 48 positive tests and a matched random selection of 98 patients with negative tests Sample size: n = 146 (48 cases) Inclusion criteria: all consecutive patients screened for SARS‐CoV‐2 (suspicion based on presenting symptoms, > 80% of cases and controls had fever and/or cough) Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: the first 48 patients with a RT‐PCR‐positive test for SARS‐CoV‐2 Facility controls: SARS‐CoV‐2‐negative patients that were selected randomly and matched by age (+/‐ 5 years), sex, collection date, and testing location (Minnesota, Wisconsin, or Arizona) with the positive patients Country: Minnesota, USA Dates: 12 March 2020‐26 March 2020 Symptoms and severity: mild to moderate severity, few co‐morbidities Demographics: mean age: cases: 45.9 years, controls: 46.0 years. Gender: % female cases: 46.0%, controls: 38.0% Exposure history: close exposure to lab‐confirmed case of COVID‐19: cases: 29.5%, controls: 5.6% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Reference standard immediately after index tests | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Chen 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of COVID‐19 pneumonia ‐ to identify differences in CT imaging and clinical manifestations between pneumonia patients with and without COVID‐19, and to develop and validate a diagnostic model for COVID‐19 based on radiological semantic and clinical features Design: cross‐sectional, multicentre, retrospective study Recruitment: cases: consecutive patients with COVID‐19 admitted in 5 independent hospitals; controls: at the same period, another 66 consecutive pneumonia patients without COVID‐19 from Meizhou People’s Hospital Sample size: n = 136 (cases = 70) Inclusion criteria: patients admitted with COVID‐19 pneumonia (cases) and patients admitted with non‐COVID‐19 pneumonia (controls) Exclusion criteria: not specified for cases except those from 1 hospital (Meizhou), for cases and controls in Meizhou: after chest CT neoplasm, tuberculosis, pulmonary oedema, pulmonary contusion, aspiration pneumonia, bronchitis, any local or systemic treatment before CT scan, normal CT image without epidemiological history |
||
| Patient characteristics and setting |
Facility cases: pneumonia patients with positive SARS‐CoV‐2 test Facility controls: CT pneumonia patients with consecutive negative RT‐PCR Country: China Dates: 1 January 2020‐8 February 2020 Symptoms and severity: pneumonia patients for cases and control; unclear severity of cases Demographics: M/F: cases 41/29, controls 43/23 mean age: cases 42.9 range, 16‐69 years, controls 46.7 range, 0.3‐93 years Exposure history: data about exposure to epidemic centres collected, but no results in the study nor in appendices |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval not specified | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Cheng 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: to identify the clinical features and CT manifestations of COVID‐19 and compare them with those of pneumonia occurring in patients who do not have COVID‐19 Design: cross‐sectional, single‐centre, retrospective study Recruitment: pneumonia patients who presented at a fever observation department in Shanghai Sample size: n = 33 (11 cases) Inclusion criteria: patients with clinical and radiological features of pneumonia, and a normal or reduced total leukocyte count or total lymphocyte count, plus an epidemiologic history that included travel or a history of residence in Hubei Province or other areas where continuous transmission of local cases occurred within 14 days before onset of symptoms, a history of contact with patients who had fever or respiratory symptoms and were from Hubei Province or other areas with continuous transmission of local cases within 14 days before onset of the disease, or clustering or epidemiologic association with the new coronavirus infection Exclusion criteria: not defined |
||
| Patient characteristics and setting |
Facility cases: confirmed case: positive RT‐PCR test result obtained by a throat swab. Test was repeated when the first test was negative Facility controls: pneumonia patients confirmed not to be infected by SARS‐CoV‐2 (2 PCR tests) Country: China Dates: 19 January 2020‐6 February 2020 Symptoms and severity: pneumonia was defined as patients with at least 1 clinical symptom (i.e. cough, sputum, fever, dyspnoea, or pleuritic chest pain), a finding of either coarse crackles on auscultation or elevated inflammatory biomarkers, and observation of a new pulmonary opacification on chest CT Demographics: median age ± SD cases 50.36 ± 15.5, controls 43.59 ± 16.02, gender distribution cases (M/F: 8/3), controls (M/F: 7/15) Exposure history: cases 8/11, controls 7/22 (in the last 14 days with patients with fever or respiratory symptoms or with known cases) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
Tests were repeated if the first test was negative |
||
| Flow and timing | Time interval not specified, reference test at day 0 (or later when the first test was negative), index tests were questionnaired at day 0 for the presence of symptoms in the past period of time | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Chua 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to evaluate the utility of acute olfactory loss as a risk‐ stratifying tool for COVID‐19 Design: retrospective cohort study Recruitment: chart review was performed for all patients who presented with acute respiratory symptoms, and in those who fulfilled the prevailing Ministry of Health suspect or surveillance case definition, at ED of tertiary hospital Sample size: n = 688 (24 cases) Inclusion criteria: all patients with suspected SARS‐CoV‐2 infection (suspicion based on presence of acute respiratory symptoms, and fulfilling the prevailing Ministry of Health suspect or surveillance case definition) Exclusion criteria: patients with pre‐existing olfactory loss, and those who were unable to give a history of olfactory loss reliably (e.g. those with cognitive impairment) |
||
| Patient characteristics and setting |
Facility cases: suspected patients with a positive PCR test Facility controls: suspected patients with a negative PCR test Country: Singapore Dates: 23 March 2020‐04 April 2020 Symptoms and severity: not specified Demographics: age: not specified gender: not specified Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken at presentation | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Clemency 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to develop symptom‐based criteria for screening of HCW for SARS‐CoV‐2 Design: prospective observational cohort Recruitment: HCW with symptoms concerning for COVID‐19 infection were evaluated for potential testing through a centralised nurse call center and referred to outpatient drive‐through testing sites if any suspicion of infection Sample size: n = 961 (225 cases) Inclusion criteria: all HCW tested for SARS‐CoV‐2, based on symptom‐based triage ("symptoms concerning for COVID‐19 infection" Exclusion criteria: none specified (141 excluded because symptoms were not documented, 12 excluded because test results not available) |
||
| Patient characteristics and setting |
Facility cases: all consecutive HCW with a single positive RT‐PCR test for SARS‐CoV‐2 Facility controls: all consecutive HCW with a single negative RT‐PCR test for SARS‐CoV‐2 Country: New York, USA Dates: 26 March 2020‐16 April 2020 Symptoms and severity: mild to moderate severity, inclusion based on presenting symptoms Demographics: mean age: not presented gender: not presented Exposure history: not presented (likely a high rate of exposure, because HCW) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | HCW referred for reference test after index test, but exact time interval not specified | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Feng 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of COVID‐19 pneumonia Design: cross‐sectional, retrospective, single‐centre study Recruitment: patients admitted to ED with history of exposure to COVID‐19 Sample size: n = 132 (cases = 7) inclusion criteria: all patients admitted to the fever clinic of the ED of the First Medical Center, Chinese People's Liberation Army General Hospital (PLAGH) in Beijing with the epidemiological history of exposure to COVID‐19 according to WHO interim guidance Exclusion criteria: < 14 years old, no other criteria specified |
||
| Patient characteristics and setting |
Facility cases: among clinically suspected patients: those with a positive RT‐PCR Facility controls: clinically non‐suspected patients + suspected patients with negative RT‐PCR Country: China Dates: 14 January 2020‐9 February 2020 Symptoms and severity: all patients admitted, with exposure history to COVID‐19, so all levels of severity; days from illness onset until admission (median, IQR): 2.0 (1.0‐5.0); patient population with general mild disease and limited presence of comorbidities (range 0%‐2.3% (COPD)) Demographics: age: controls median 40.0 years (IQR 32.5‐54.5), cases median 39.0 years (IQR 37.0‐41.5) M%/F%: cases 71.4/28.6, controls 63.2/36.8 Exposure history: epidemiological history of exposure to COVID‐19 (as per WHO guidance) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index test and RS both taken on admission | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Gilbert 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease) Design: prospective cohort, including consecutive patients with suspected SARS‐CoV‐2 infection Recruitment: all patients presenting to the ED triage center with symptoms suggestive of COVID‐19 Sample size: n = 598 (175 cases) Inclusion criteria: all consecutive patients suspected of SARS‐CoV‐2 infection and directed to the triage centres located close to the EDs and subjected to SARS‐CoV‐2 testing; suspicion = respiratory symptoms and/or fever in a healthcare provider, an immunosuppressed patient or a nursing home resident, and all patients who required an admission to the hospital Exclusion criteria: none |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR‐positive patients Facility controls: RT‐PCR‐negative patients Country: Belgium Dates: 02 March 2020‐23 March 2020 Symptoms and severity: consecutive patients (selection based on PCR testing), mild to moderate severity (83% sent home for self‐isolation, 1.9% ICU, 15% hospital admission) Demographics: mean age (all): 41.1 years gender: % female (all): 59.0% Exposure history: travel to endemic country: cases 5.1%, controls 12.5% contact with positive patients: cases: 10.9%, controls 9.0% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests followed by reference standard | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Haehner 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to investigate the frequency of olfactory loss in an outpatient population who presented to a coronavirus testing center. To evaluate the diagnostic value of the symptom "sudden smell loss" for screening procedures. Design: cross‐sectional cohort study (prospective data collection) Recruitment: patients who presented with symptoms of a common cold to a coronavirus testing centre and fulfilled coronavirus testing criteria. Sample size: n = 500 (cases 34) Inclusion criteria: patients with common cold complaints who met the criteria for SARS‐CoV‐2 testing to WHO recommendations Exclusion criteria: none |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR for SARS‐CoV‐2 positive Facility controls: RT‐PCR for SARS‐CoV‐2 negative Country: Germany Dates: not specified Symptoms and severity: olfactory loss Demographics: mean age: 41.3 years gender % female: 54.6% Exposure history: not specified |
||
| Index tests | Olfactory loss | ||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index test taken on the same day | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Huang 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to explore a novel risk score to predict diagnosis with COVID‐19 among all suspected patients at admission Design: retrospective, multicentre, observational study Recruitment: retrospective chart review of patients admitted into 26 COVID‐19 designated hospitals in Sichuan Province, China Sample size: n = 475 (336 cases) Inclusion criteria: patients with suspected COVID‐19 (suspected case is defined as having exposure history and 2 clinical manifestations. Patients without epidemiological exposure histories could also be seen as 'suspected COVID‐19' only if 3 clinical manifestations were present. Exclusion criteria: none |
||
| Patient characteristics and setting |
Facility cases: suspected patients with a positive RT‐PCR test Facility controls: suspected patients with a negative RT‐PCR test. If the first test was negative, at least a second test was done, 24 h apart. Country: China Dates: 21 January 2020‐07 February 2020 Symptoms and severity: mild to moderate severity, all suspected patients included Demographics: mean age: cases: 43 years, controls: 34 years gender: % female cases: 45.8%, controls: 41.0% Exposure history: epidemiological exposure history: cases: 69.6%, controls 12.9% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken on admission | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Just 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to identify predictive risk factors for a positive SARS‐CoV‐2 RT‐PCR result in a primary care setting Design: multicentre, cross‐sectional cohort study Recruitment: 26 office‐based specialists for internal and/or general medicine with a full primary care mandate from 14 different locations participated in the study. Suspected COVID‐19 patients for which a PCR was taken were included. Sample size: n = 374 (40 cases) Inclusion criteria: convenience sample of patients who received PCR in the participating GP’s practices within the study period Exclusion criteria: patients whose tests had been carried out for procedural reasons and did not correspond to a specific clinical indication were excluded (e.g. testing of recovered patients after end of quarantine). There were no other exclusion criteria. |
||
| Patient characteristics and setting |
Facility cases: suspected patients with a positive PCR test Facility controls: suspected patients with a negative PCR test Country: Germany Dates: 24 March 2020‐17 April 2020 Symptoms and severity: mild to moderate severity Demographics: median age: cases: 52.0 years, controls: 43.5 years gender: % female cases: 65.0%, controls: 57.2% Exposure history: first grade contact (with symptoms): cases: 35.0%, controls 17.4% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken on admission | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Leal 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to describe the clinical features predictive for SARS‐CoV‐2 infection in primary care Design: prospective population‐based cohort Recruitment: residents of the municipality aged ≥ 12 years with suspected COVID‐19 symptoms were encouraged to contact the dedicated platform via the website or phone. They were invited to complete an initial screening questionnaire. Sample size: n = 1583 (444 cases (only the PCR‐positive patients) Inclusion criteria: patients meeting the suspected COVID‐19 case definition (having at least 2 of the following symptoms: fever, cough, sore throat, coryza or change in/loss of smell (anosmia); or 1 of these symptoms plus at least 2 other symptoms consistent with COVID‐19 Exclusion criteria: all pregnant women, and patients meeting pre‐defined triage criteria for severe disease |
||
| Patient characteristics and setting |
Facility cases: patients with suspected COVID‐19 who tested positive (RT‐PCR, testing at home) Facility controls: patients with suspected COVID‐19 who tested negative (RT‐PCR, testing at home) Country: Brazil Dates: 13 April 2020‐13 May 2020 Symptoms and severity: mild to moderate severity, severe cases were excluded Demographics: all age groups represented from ≥ 10 years. Gender: % female cases: 55.0%, controls: 66.5% Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Swabs were taken within 5 days of symptom onset | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Lee 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to identify symptoms that are specific for SARS‐CoV‐2 infection Design: nested case‐control study (from cross‐sectional cohort study, random sampling 1:3) Recruitment: all adults (> 18 years) who underwent COVID‐19 tests at an ambulatory assessment centre Sample size: n = 127 (56 cases) Inclusion criteria: adults (≥ 18 years) who had undergone PCR testing and had confirmed results Exclusion criteria: none |
||
| Patient characteristics and setting |
Facility cases: tested adults with a positive PCR Facility controls: tested adults with a negative PCR Country: Canada Dates: 16 March 2020‐15 April 2020 Symptoms and severity: mild to moderate severity Demographics: median age: cases: 38.0 years, controls: 43.0 years gender: % female cases: 58.9%, controls: 62.0% Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests after RT‐PCR (index tests: questions about the presence of smell or taste loss around onset of COVID‐19‐like symptoms); index tests > 4 weeks since the diagnosis for 67.6% of controls versus 30.4% for cases | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Liang 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: to estimate the prevalence of COVID‐19 in pneumonias during this period and to find the unique features of COVID‐19 as compared to pneumonias caused by other agents Design: cross‐sectional, single‐centre, retrospective study Recruitment: 342 cases of pneumonia were diagnosed in Fever Clinic in Peking University Third Hospital. From these patients, 88 were reviewed by panel discussion as possible or probable cases of COVID‐19, and received 2019‐nCoV detection by RT‐PCR Sample size: n = 88 (21 cases) Inclusion criteria: patients visiting the Fever Clinic at Peking University Third Hospital. Based on epidemiological history, epidemiological evidence, fever and/or respiratory symptoms, chest radiological findings and WBC results, cases with possible or probable COVID‐19 were sent for panel discussion and then for 2019‐nCoV detection by RT‐PCR Exclusion criteria: COVID‐19 unlikely by panel discussion; lack of CT scan or no signs of pneumonia on CT scan; paediatric patients |
||
| Patient characteristics and setting |
Facility cases: 2019‐nCoV real‐time PCR testing, which was positive in 19 cases (confirmed cases). In another 2 patients, though PCR testing was negative, a clinical diagnosis was made according to
epidemiological evidence, consistent clinical and CT findings (clinical cases) Facility controls: for the cases with negative viral detection, the diagnosis of COVID‐19 was excluded based on inconsistent epidemiological, clinical or radiological data Country: China Dates: 21 January 2020‐15 February 2020 Symptoms
Severity of COVID‐19
Demographics: COVID‐group only: median age was 42.0 years (25th‐75th percentile, 34.5‐66.0 years). Range 24‐85. Male/female: 11 (52.4%)/10 (47.6%) Exposure history: 19/21 (90.5%) had a clear epidemiological history of COVID‐19. 7 patients, from 5 family clusters, had close contact with their family members |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval not specified | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Mao 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to ascertain the effectiveness of the screening strategy and provide insight for early diagnosis of COVID‐19 Design: multicentre, retrospective, observational cohort study Recruitment: all patients visiting the fever clinics within the study period Sample size: n = 1004 (cases = 188) Inclusion criteria: all patients visiting the fever clinics within the study period. Patients with fever (body temperature > 37.5° C), or patients with pulmonary symptoms and epidemiological exposure history were requested to visit the fever clinics. All patients visiting the fever clinics during the study period were included. Exclusion criteria: patients with missing data |
||
| Patient characteristics and setting |
Facility cases: RT‐PCR‐positive patients Facility controls: RT‐PCR‐negative patients Country: China Dates: 17 January 2020‐16 February 2020 Symptoms and severity: not specified Demographics: median age: cases 46 years, controls 39 years female; gender %: cases 50%, controls 47% Exposure history: recent visit to epidemic region: cases 51%, controls 28%; contact with infected person: cases 34%, controls 13% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests taken on the same day | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Nobel 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: assess GI symptoms in COVID‐19 and their association with short‐term outcomes Design: diagnostic case‐control, retrospective study Recruitment: adults who underwent nasopharyngeal swab testing for SARS‐CoV‐2 at outpatient settings: clinics or the ED, of New York‐Presbyterian‐Columbia or the medical centre's affiliates in New York Sample size: 516 (278 cases) Inclusion criteria: adults ≥ 18 years of age who underwent nasopharyngeal swab testing for SARS‐CoV‐2. Indications for testing during this period were respiratory symptoms (cough, fever, shortness of breath) with intent to hospitalise or the same symptoms in essential personnel. Exclusion criteria: if insufficient data were available in the electronic medical record or if testing was performed during a pre‐existing inpatient admission |
||
| Patient characteristics and setting |
Facility cases: SARS‐CoV‐2 PCR test result positive (1 test) Facility controls: SARS‐CoV‐2 PCR test result negative Country: USA Dates: 10 March 2020‐21 March 2020 Symptoms and severity: respiratory symptoms (cough, fever, shortness of breath) with intent to hospitalise or in essential workers Demographics: median age: 51‐70 years (cases and controls), gender distribution: cases (M/F(%): 52/48), controls (M/F(%): 45/55) Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval: both taken at intake | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
O'Reilly 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to determine the clinical and epidemiological predictors of a positive SARS‐CoV‐2 test result and the requirement for intensive respiratory support Design: prospective cohort study Recruitment: adult patients who meet testing criteria for COVID‐19 and have a SARS‐CoV‐2 PCR test requested in the ED Sample size: n = 240 (cases = 11) Inclusion criteria: all adults who met the testing criteria for COVID‐19 and who presented at the ED Exclusion criteria: patients who attended the screening clinic and did not present for medical assessment in the ED (no clinical data available) |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: Australia Dates: 01 April 2020‐14 April 2020 Symptoms and severity: moderate to severe Demographics: mean age: cases 51, controls 61 female gender %: cases 28%, controls 45% Exposure history: contact with infected person: cases 56%, controls 7% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests taken on the same day | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Peng 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: analyse the clinical features and imaging manifestations of COVID‐19 Design: cross‐sectional, single‐centre, retrospective study Recruitment: clinically suspected cases who were sent to hospital for screening Sample size: n = 86 (n = 11) Inclusion criteria: clinically suspected patients Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR via nasopharyngeal swab Facility controls: negative RT‐PCR via nasopharyngeal swab (once) Country: China Dates: 23 January 2020‐16 February 2020 Symptoms and severity: fever, cough, dyspnoea, sore throat, fatigue, systemic soreness, runny nose Demographics: M/F: total 39/47, cases: 5/6, controls 34/40 Case group: mean age 40.73 ± 11.32 years, 5 men. Control group: mean age 39.67 ± 13.90 years, 34 men Exposure history: 7/11 COVID‐19 patients (63.6%) had a history of travel to Hubei (5 Wuhan, 1 Huanggang, 1 Xiaogan), 2 patients had close contact with the COVID‐19 patients, and 2 taxi drivers |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval not specified | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Peyrony 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to assess utility of clinical parameters, physician clinical judgment, and lung ultrasonography to accurately identify SARS‐CoV‐2 infected patients at ED presentation Design: prospective cohort study Recruitment: cohort of all adult (≥ 18 years) patients with suspected COVID‐19 who were tested for SARS‐CoV‐2 prospectively enrolled at university ED (not every patient was tested for SARS‐CoV‐2: testing was left to the clinician’s discretion) Sample size: n = 391 (225 cases) Inclusion criteria: no predefined inclusion criteria. Testing was mostly performed in patients who had severe symptoms such as dyspnoea, reported shortness of breath, presented with comorbidities, or were > 70 years. Some patients without COVID‐19 symptoms were also tested when they needed admission to hospital. Exclusion criteria: patients who attended the ED more than once (only the last visit was included). There were no other exclusion criteria. |
||
| Patient characteristics and setting |
Facility cases: all patients who tested positive for SARS‐CoV‐2 by RT‐PCR Facility controls: all patients who tested negative for SARS‐CoV‐2 by RT‐PCR Country: France Dates: 09 March 2020‐04 April 2020 Symptoms and severity: moderate to mild severity, inclusion based on signs and symptoms suggestive of SARS‐CoV‐2 infection, 82% of included patients with comorbidities; not all included patients had COVID‐19 symptoms Demographics: all included patients (pos + neg): median age: 62 years % female: 38.4% Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken at presentation | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | No | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Pisapia 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to compare the characteristics at hospital admission of confirmed and not‐confirmed COVID‐19 patients, in the early phase of the epidemic Design: retrospective cohort study Recruitment: all patients consecutively admitted in selected medical wards (ED + lab) of the mono‐specialist infectious diseases referral centre because of clinical suspicion of COVID‐19 Sample size: n = 37 (17 cases) Inclusion criteria: all patients consecutively admitted in the selected medical wards because of clinical suspicion of COVID‐19. No specification of 'suspicion' Exclusion criteria: none |
||
| Patient characteristics and setting |
Facility cases: suspected cases with a positive RT‐PCR (second test after 24 h if first negative) Facility controls: suspected cases with a negative RT‐PCR (2 negative tests) Country: Italy Dates: 10 February 2020‐10 March 2020 Symptoms and severity: mild to moderate severity Demographics: median age cases: 49 years controls: 29 years. Gender: % female cases: 35%, controls: 35% Exposure history: travel to affected area: cases 35%, controls 95% contact with a confirmed case: cases 47%, controls: 0% contact with persons from affected area: cases: 12% controls: 0% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken on admission | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Rentsch 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis SARS‐CoV‐2 test positives Design: cross‐sectional, retrospective study Recruitment: electronic health record data from the national Veterans Affairs Healthcare System ‐ national Corporate Data Warehouse (USA) Sample size: 3789 (585 cases) Inclusion criteria: all patients in the Veterans Affairs cohort, born between 1945 and 1965 and active in care, tested for COVID‐19 between 8 February and 30 March 2020 Exclusion criteria: patients for whom results were pending (n = 93) or inconclusive (n = 33) were excluded |
||
| Patient characteristics and setting |
Facility cases: tested positive for SARS‐CoV‐2 Facility controls: tested negative for SARS‐CoV‐2 Country: USA Dates: 8 February 2020‐30 March 2020 Symptoms and severity: all patients who were tested were included Demographics: median age overall: 65.7 years (IQR 60.5‐70.7) (cases: 66.1 years, controls: 65.6 years); gender overall (M%/F%): 90.2/9.8, cases 95.4/4.6, controls 89.2/10.8 Exposure history: not specified (all over USA) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval maximum 2 days | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Salmon 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); second part of the study: to assess the diagnostic accuracy of olfactory/gustatory dysfunction for SARS‐CoV‐2 infection in the overall population tested for SARS‐CoV‐2 Design: prospective cohort study Recruitment: all consecutive patients who were tested for SARS‐CoV‐2 in the Paris‐based screening centre for COVID‐19 Sample size: n = 1824 (849 cases) Inclusion criteria: (second part of the study): all consecutive patients with a suspicion of SARS‐CoV‐2 infection, independent of loss of smell no specification of 'suspicion' Exclusion criteria: (second part of the study): none |
||
| Patient characteristics and setting |
Facility cases: all suspected patients with a positive RT‐PCR Facility controls: all suspected patients with a negative RT‐PCR Country: France Dates: 17 March 2020‐25 March 2020 Symptoms and severity: mild to moderate severity Demographics: not specified for second part of this study Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken at presentation | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Shah 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to describe characteristics, diagnostics and outcomes of patients with respiratory illness, comparing patients with and without COVID‐19 disease Design: retrospective cohort Recruitment: all patients presenting to an ED with an acute respiratory illness and tested for SARS‐CoV‐2 Sample size: n = 316 (33 cases) Inclusion criteria: all patients ≥ 18 years who underwent testing for COVID‐19 within 24 h of presentation to the ED. Patients with acute respiratory symptoms, influenza‐like illness Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for SARS‐CoV‐2 Facility controls: negative RT‐PCR for SARS‐CoV‐2 Country: California, USA Dates: 03 February 2020‐31 March 2020 Symptoms and severity: not specified Demographics: median age: cases 63, controls 62. % female: cases 36%, controls 50% Exposure history: travel in last 21 days or known COVID exposure: cases 46%, controls 11% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS performed maximum 24 h later than index tests | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Song 2020a.
| Study characteristics | |||
| Patient Sampling |
Purpose: to develop a tool for early diagnosis of SARS‐CoV‐2‐infected patients Design: cross‐sectional, retrospective, single‐centre (2 time frame study: training ‐ validation data set) Recruitment: 1311 patients who presented to the First Affiliated Hospital, School of Medicine, Zhejiang University with at least 1 SARS‐CoV‐2 RT‐PCR test Sample size: n = 304 (73 cases) (= subset of the study including training dataset only) n = 95 (18 cases) (= validation dataset) Inclusion criteria
Exclusion criteria
|
||
| Patient characteristics and setting |
Facility cases: positive SARS‐CoV‐2 Facility controls: negative SARS‐CoV‐2 Country: China Dates: 20 January 2020‐05 February 2020 Symptoms and severity: in positives: non‐severe (n = 31), including mild or moderate patients to severe (n = 42) including severe or critical patients
Demographics: M/F: cases 46/27, controls 104/127 median age: cases 53.0 years (43.5‐62.0) controls 34 years (29‐49) Exposure history: Wuhan‐related exposure and or close contact to confirmed COVID‐19 case: cases 40.7%, controls 57.5% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Within 3 h for RS, first in‐hospital stay for index tests | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sun 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: algorithm development for estimating risk of COVID‐19 Design: cross‐sectional, retrospective study Recruitment: patients presenting at the designated national outbreak screening centre and tertiary care hospital in Singapore for SARS‐CoV‐2 testing. Patients were either self‐referred, referred from primary care facilities, or were at‐risk cases identified by national contact tracing efforts (recruited n = 991) Sample size: n = 788 (n = 54) Inclusion criteria: patients presenting to the centre:
Exclusion criteria: PCR results not available at time of data collection ‐ no electronic medical records ‐ unavailable vital sign records |
||
| Patient characteristics and setting |
Facility cases: positive SARS‐CoV‐2 RT‐PCR test Facility controls: all SARS‐CoV‐2 RT‐PCR results were negative (minimum 2 test negatives in high‐risk patients, minimum 1 test low‐risk patients) Country: Singapore Dates: 26 January 2020‐16 February 2020 Symptoms and severity: 252 (33.2%) symptoms > 5 days at presentation, 75 (9.5%) any comorbidity
Demographics: median age 34 years (range 7 years‐98 years, IQR 27‐45) (cases median 42 years, range 16‐79; controls 34 years (range 7‐98); M/F: 48.3%/51.7% F (cases M: 88 (88.9%)) Exposure history: contact with a known COVID‐19 case (20.1% (32/54 cases (59.3%)); 126/734 controls (17.2%), contact with travellers from China (22.1%, 15/54 cases (27.8%); 42/734 controls (5.7%)), recent travel history, and visit to hospital in China within 14 days prior to symptom onset (0.8%) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval not specified | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Tolia 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of acute SARS‐CoV‐2 infection Design: cross‐sectional, retrospective study Recruitment: all patients presenting to 1 of 2 EDs, located at an urban teaching hospital, and academic quaternary medical centre, within the same healthcare system who had targeted testing based on clinician's decision during the initial 10 days of test availability Sample size: n = 283 (29 cases) Inclusion criteria:
Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: positive SARS‐CoV‐2 test Facility controls: negative SARS‐CoV‐2 test, visiting the same EDs and being tested Country: USA (San Diego, CA) Dates: 10 March 2020‐19 March 2020 Symptoms and severity:
Demographics: age (< 18 years: 0.7%, 18‐64 years: 83.4%, > 65 years: 15.9%); gender: cases M/F%: 55.2/44.8; controls M/F%: 52.8/47.2; all M/F%: 53.0/47.0 Exposure history: recent travel (5.5%), 90.6% symptom‐based criteria for testing, no known exposure history based |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Probably no time interval between index test and RS, but not specified | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Tordjman 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of COVID‐19 pneumonia; to determine the independent variables associated with SARS‐CoV‐2 infection Design: retrospective observational study Recruitment: a retrospective cohort of 100 patients with both RT‐PCR and CT‐scan results available with a 1:1 patient:control inclusion ratio from ED at Cochin Hospital (Paris, France) with a suspicion of SARS‐CoV‐2 infection: 50 consecutive infected patients and 50 consecutive controls (+ validation cohort) Sample size: n = 100 (50 cases) (no clinical data available from validation cohort) Inclusion criteria: suspicion of SARS‐CoV‐2 infection, and both RT‐PCR and CT‐scan available 'suspicion' not defined Exclusion criteria: absence of confirmed diagnosis (diagnosis still under investigation; N = 4); lack of blood test including complete white blood cell count and serum electrolytes (N = 6); absence of reported clinical characteristics (N = 2) |
||
| Patient characteristics and setting |
Facility cases: suspected patients with a positive RT‐PCR or positive CT‐scan (positive signs of COVID‐19 pneumonia: usually bilateral and peripheral ground‐glass and consolidated pulmonary opacities) Facility controls: suspected patients with a negative RT‐PCR and negative findings on CT‐scan Country: France Dates: 15 March 2020‐05 April 2020 Symptoms and severity: not specified Demographics: median age: cases 60.8 years, controls 54.1 years. Female %: cases 40%, controls 50% Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken at first presentation | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Trubiano 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease) Design: prospective cohort study Recruitment: data on all patients presenting at a COVID‐19 rapid assessment screening clinic were prospectively collected in an electronic database. Only those patients that met the DHHS (Victorian Department of Health and Human Services) criteria for SARS‐CoV‐2 testing had nasopharyngeal swab collected for SARS‐CoV‐2 nucleic acid detection by PCR Sample size: n = 2935 (108 cases) Inclusion criteria: all people meeting DHHS criteria for testing: Fever or chills in the absence of an alternative diagnosis that explains the clinical presentation or acute respiratory infection symptoms (e.g. cough, sore throat, shortness of breath, runny nose, loss of smell or loss of taste) Exclusion criteria: pending or intermediate results |
||
| Patient characteristics and setting |
Facility cases: patients with suspected COVID‐19 with a positive RT‐PCR for SARS‐CoV‐2 Facility controls: suspected patients with a negative RT‐PCR for SARS‐CoV‐2 Country: Australia Dates: 11 March 2020‐22 April 2020 Symptoms and severity: mild to moderate severity Demographics: median age: cases 51 years, controls 38 years. Female%: cases 49.1%, controls 64.1% Exposure history: overseas health facility exposure: cases 1.9%, controls 4.0%. Australian health facility exposure: cases 11.1%, controls 31.5%. Contact with known COVID‐19‐positive patient: cases 57.4%, controls 15.8% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken at presentation | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Tudrej 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to diagnose SARS‐CoV‐2 infection in primary care settings based on signs and symptoms Design: cross‐sectional prospective cohort study Recruitment: recruitment in 2 clinical laboratories in Lyon (France) to which GPs refer patients with suspected COVID–19 for a nasopharyngeal smear (RT‐PCR) Sample size: n = 816 (198 cases) Inclusion criteria: all consecutive patients referred by GPs for PCR testing Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: all suspected patients with a positive RT‐PCR Facility controls: all suspected patients with a negative RT‐PCR Country: France Dates: 24 March 2020‐14 April 2020 Symptoms and severity: not specified Demographics: all included patients: median age: 45 years, % female: 65% Exposure history: not specified, 37% of participants were healthcare professionals |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS specimen taken right after index tests, at presentation | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Wee 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: to analyse OTDs as a diagnostic criterion for COVID‐19 Design: cross‐sectional, prospective single‐centre study Recruitment: all suspected cases presenting to the ED Sample size: n = 870 (cases = 154) Inclusion criteria:
Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for 2019‐nCov Facility controls: negative RT‐PCR for 2019‐nCov Country: Singapore Dates: 26 March 2020‐10 April 2020 Symptoms and severity: loss of sense of smell/taste Demographics: not specified Exposure history: close contact of a confirmed COVID‐19 case: cases 42/112, controls 37/679 |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval: same day | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Wei 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); diagnosis of SARS‐CoV‐2 in outpatients visiting a fever clinic Design: retrospective cohort study Recruitment: all febrile patients visiting the fever clinic of Tongji Hospital Sample size: n = 936 (628 cases) Inclusion criteria: all febrile patients visiting the fever clinic Exclusion criteria: none specified |
||
| Patient characteristics and setting |
Facility cases: all febrile patients with a positive RT‐PCR for SARS‐CoV‐2 (tested twice in 24 h) Facility controls: all febrile patients with a negative RT‐PCR for SARS‐CoV‐2 (tested twice in 24 h) Country: China Dates: 30 January 2020‐04 February 2020 Symptoms and severity: cases: 88.1% mild, 11.5% severe, 0.5% critical; controls: 90.3% mild, 9.1% severe, 0.7% critical Demographics: median age: cases: 53 years, controls: 49 years. Gender: % female cases: 52.9%, controls: 53.9% Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken at presentation | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Xie 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of COVID‐19 pneumonia; to compare the epidemiological, clinical, laboratory and radiological characteristics, treatment and outcomes between patients with confirmed COVID‐19 pneumonia and those with suspected COVID‐19 infection (71% of SARS‐CoV‐2‐positive patients had CT‐confirmed pneumonia) Design: retrospective 2‐centre cohort Recruitment: patients in whom a RT‐PCR test was performed at 2 Shangai hospitals Sample size: n = 105 (21 cases) Inclusion criteria: not specified Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: patients with a positive RT‐PCR test for SARS‐CoV‐2 Facility controls: patients with a negative RT‐PCR test for SARS‐CoV‐2 Country: China Dates: 01 January 2020‐15 February 2020 Symptoms and severity: 72% of all participants were hospitalised, 71% of the cases had pneumonia, 88% of controls had pneumonia ("clinical symptoms usually mild") Demographics: mean age: cases: 54.0 years, controls: 41.6 years. Gender: % female cases: 38.1%, controls: 51.2% Exposure history: recently been to Wuhan: cases: 42.9%, controls: 17.9%. Contact with people from Wuhan: cases: 14.3%, controls: 0%. Recently been to supermarkets and groceries: cases: 28.6%, controls: 34.5%. Recently travelled: cases: 14.3%, controls: 47.6% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index tests both taken at admission | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Yan 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: to evaluate association of patient‐reported symptoms with a focus on sense of smell and taste and SARS‐CoV‐2 infection Design: internet survey of patients after presentation to a single centre Recruitment: email invitation with 1 phone call follow‐up to everyone who was tested for COVID‐19 between 3 March 2020 and 29 March 2020 Sample size: n = 262 (cases: 59) Inclusion criteria:
Exclusion criteria: |
||
| Patient characteristics and setting |
Facility cases: SARS‐CoV‐2‐positive Facility controls: SARS‐CoV‐2‐negative Country: USA, San Diego Dates: 3 March 2020‐29 March 2020 Symptoms and severity:
Demographics: adults only, M/F: cases 29/29, controls 69/132 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | PCR taken at presentation, not specified when the questionnaire was sent. Patients had to list their symptoms at presentation. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Yang 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: to identify differences in CT imaging and clinical features between COVID‐19 and influenza pneumonia in the early stage, and to identify the most valuable features in the differential diagnosis Design: diagnostic case‐control study, retrospective, multicentre with historic control group Recruitment: cases: confirmed SARS‐CoV‐2 patients; controls: influenza pneumonia patients (1 January 2015‐30 September 2019 from 2 hospitals) Sample size: n = 121 (73 cases) Inclusion criteria: patients confirmed with SARS‐CoV‐2; controls: patients who had 9 respiratory pathogen IgM antibody tested from January 2015‐September 2019 Exclusion criteria: cases: not specified controls:
No CT date, no clinical date |
||
| Patient characteristics and setting |
Facility cases: positive RT‐PCR for 2019‐nCov
Facility controls: influenza pneumonia
Country: China Dates: 1 January 2020‐15 February 2020 Symptoms and severity: all patients in early stages of COVID‐19 or influenza pneumonia Demographics: M/F: cases 41/32, controls 30/18 mean age: cases 41.9, controls 40.4 Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval unclear | ||
| Comparative | |||
| Notes | Overlaps with Chen 2020 | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Yombi 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); diagnosis of SARS‐CoV‐2 infection, using clinical signs in HCWs Design: cross‐sectional cohort study (unclear whether retrospective/prospective data collection) Recruitment: period 1: (before 30 March 2020) HCWs were tested only if they had fever and respiratory symptoms (some physicians were tested without fever); period 2 (after 30 March 2020), HCWs were tested if they had respiratory symptoms with or without fever Sample size: n = 536 (175 cases) Inclusion criteria: not specified (all suspected HCWs) Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: all suspected HCWs with a positive RT‐PCR Facility controls: all suspected HCWs with a negative RT‐PCR Country: Belgium Dates: 16 March 2020‐24 April 2020 Symptoms and severity: not specified (from tables: mild to moderate severity) Demographics: % age < 45 years: cases: 56.6%, controls: 62.3% gender: % female cases: 67.4%, controls: 73.1% Exposure history: not specified (all HCWs) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Not specified | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Zavascki 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); development of a predictive score for SARS‐CoV‐2 infection based on demographics and symptoms in patients who attended at a dedicated screening unit. Design: retrospective cohort study Recruitment: all patients with suspected COVID‐19 visiting a dedicated screening centre of a private tertiary‐care hospital in the study period were eligible. Suspicion = fever or any respiratory symptom and have returned from countries with confirmed COVID‐19 cases in the last 14 days (after 14 March, travel history was not necessary) Sample size: n = 464 (98 cases) Inclusion criteria: consecutive patients attending the screening clinic Exclusion criteria: health‐care professionals, < 18 years old, asymptomatic patients |
||
| Patient characteristics and setting |
Facility cases: patients with suspected COVID‐19 with 1 positive RT‐PCR Facility controls: patients with suspected COVID‐19 with ≥ 1 negative RT‐PCR Country: Brazil Dates: 28 January 2020‐13 April 2020 Symptoms and severity: mild to moderate severity Demographics: mean age: cases: 59.1 years, controls: 45.4 years % ≥ 60 years: cases: 55.1%, controls: 21.0% gender: % female cases: 37.8%, controls: 57.1% Exposure history: not specified |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | RS and index test both on the day of presentation | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Zayet 2020a.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to compare the clinical features of COVID‐19 and influenza Design: case‐control study (COVID cases vs influenza cases) Recruitment: all adult patients (> 18 years) with confirmed COVID‐ 19 or confirmed influenza A/B who consulted or were hospitalised in the hospital Sample size: n = 124 (70 cases) Inclusion criteria: all adult patients with symptoms (suspicion of SARS‐CoV‐2 or Influenza) with either confirmed SARS‐CoV‐2 infection or confirmed influenza A/B infection 'suspicion' not defined Exclusion criteria: pregnant women, children (< 18 years) and patients with dementia (unable to report functional symptoms) + not specified but following from inclusion criteria: patients testing negative for both SARS‐CoV‐2 and influenza A/B |
||
| Patient characteristics and setting |
Facility cases: patients with suspected COVID‐19 with a positive RT‐PCR for SARS‐CoV‐2 Facility controls: patients with suspected COVID‐19 with a positive RT‐PCR for influenza A/B Country: France Dates: 26 February 2020‐14 March 2020 Symptoms and severity: mild to moderate severity, 33 patients (47%) were hospitalised for a mean duration of 7 days (±6). During hospitalisation, 23 patients (33%) required oxygen therapy and 11 patients (16%) were admitted to ICU for acute respiratory failure and needed artificial ventilation for 8 days (± 7) Demographics: mean age: cases: 56.7 years, controls: 61.3 years. Gender: % female cases: 58.6%, controls: 68.5% Exposure history: not specified (31.4% of cases were HCWs versus 5.6% of controls) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Not specified | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Zayet 2020b.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to compare the symptoms of patients with positive and negative SARS‐CoV‐2 RT‐PCR results and to determine the sensitivity, specificity, positive predictive value and negative predictive value for each of these symptoms in regard to SARS‐CoV‐2 RT‐PCR Design: retrospective cohort study Recruitment: all adult patients (≥ 18 years) who presented for possible COVID‐19 at the outpatient department Sample size: n = 217 (95 cases) Inclusion criteria: all adult patients (≥ 18 years) who presented for possible COVID‐19 at the outpatient department Exclusion criteria: pregnant women, children (< 18 years) and patients with dementia (unable to report functional symptoms) |
||
| Patient characteristics and setting |
Facility cases: patients with suspected COVID‐19 with a positive RT‐PCR Facility controls: patients with suspected COVID‐19 with a negative RT‐PCR Country: France Dates: 30 March 2020‐03 April 2020 Symptoms and severity: mild to moderate severity Demographics: mean age: cases: 39.8 years, controls: 39.6 years. Gender: % female cases: 83.2%, controls: 86.9% Exposure history: not specified (mostly HCWs) |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Not specified | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Zhao 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: to compare and assess the clinical features of COVID‐19 pneumonia with features in non‐COVID‐19 pneumonia patients Design: diagnostic case control, retrospective study Recruitment: patients with similar duration between symptom onset to admission were selected as controls Sample size: n = 34 (n = 15) Inclusion criteria: admitted pneumonia cases with a history of travel to Hubei or exposure to a PCR SARS‐CoV‐2‐confirmed‐positive patient Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: single sputum or throat swab test RT‐PCR‐positive pneumonia Facility controls: for non‐COVID‐19 confirmation: 3 consecutive negative throat swabs or sputum sampling every other day during first 7 days of admission Country: China, Anhui Dates: 23 January 2020‐5 February 2020 Symptoms and severity:
Demographics: mean age (cases/controls): 48 (IQR 27~56)/35 (IQR 27~46) in COVID‐19 and non‐COVID‐19 patients, respectively; F/M (cases/controls): 8 (42.11%) Exposure history: all patients had a history of exposure to confirmed cases of 2019‐nCoV or travel to Hubei before illness. Investigators interviewed each patient and their relatives, where necessary, to determine exposure or close contact histories during the 2 weeks before the illness onset |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Time interval not specified | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Zhu 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: description of initial clinical features in patients with suspected and confirmed SARS‐CoV‐2 infection Design: cross‐sectional, retrospective study Recruitment: all patients with suspected COVID‐19 who presented to the ED of the First Affiliated Hospital of USTC and the Infectious Hospital of the First Affiliated Hospital of USTC for the first time Sample size: n = 116 (32 cases) Inclusion criteria:
Exclusion criteria: transfer from another hospital or previous visit to our hospital and previous diagnosis of COVID‐19 |
||
| Patient characteristics and setting |
Facility cases: positive nucleic acid amplification test on admission or 24 h later Facility controls: SARS‐CoV‐2 PCR test negative Country: China, Anhui Dates: 24 January 2020‐20 February 2020 Symptoms and severity: all suspected COVID‐19 patients included; days since onset of symptoms median 5 (IQR 2‐7) Demographics: median age: all: 40 years (IQR 27‐53), cases: 46 years (IQR 35‐52), controls: 35 years (IQR 27‐53); gender distribution M%/F%: all 46/54, cases 47/53, controls 46/54 Exposure history: no specific exposure history common to all patients with suspected disease: 8 (25%) diagnosed patients had visited Wuhan in the previous 2 weeks and 12 (38%) had been exposed to patients with infection in the previous 2 weeks |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Index tests and RS both taken on admission or after 24 h | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Zimmerman 2020.
| Study characteristics | |||
| Patient Sampling |
Purpose: diagnosis of SARS‐CoV‐2 infection (mild COVID‐19 disease); to develop a data‐driven set of clinical indicators for COVID‐19 that would help to identify outpatient symptoms and those who most benefit from limited testing availability Design: not specified Recruitment: not specified Sample size: n = 736 (55 cases) Inclusion criteria: not specified Exclusion criteria: not specified |
||
| Patient characteristics and setting |
Facility cases: adult patients testing positive for SARS‐CoV‐2 infection Facility controls: adult patients testing negative for SARS‐CoV‐2 infection Country: Pennsylvania, USA Dates: 29 March 2020‐26 April 2020 Symptoms and severity: mild to moderate severity Demographics: not specified Exposure history: contact with COVID‐19 case: cases: 70%, controls: 21% |
||
| Index tests |
|
||
| Target condition and reference standard(s) |
|
||
| Flow and timing | Not specified | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
BP: blood pressure; COPD: constructive obstructive pulmonary disease; COVID‐19: coronavirus disease 2019; CT: computed tomography; ED: emergency department; F: female; FiO2: fraction of inspired oxygen; GI: gastrointestinal; GP: general practitioner; HCW: healthcare workers; ICU: intensive care unit; IgM: immunoglobulin M;IQR: interquartile range; M: male; NCP: novel coronavirus pneumonia; OTD: olfactory and taste disorder; PaO2: partial pressure of oxygen; RS: reference standard; RT‐PCR: reverse transcription polymerase chain reaction; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; SD: standard deviation;SpO2: oxygen saturation; TC: target condition; WBC: blood white blood cell; WHO: World Health Organization; 2019‐nCoV: 2019 novel coronavirus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Guan 2020 | SARS‐CoV‐2‐positive cases only |
| Soares 2020 | No data |
| Song 2020b | SARS‐CoV‐2‐positive cases only |
| Wang 2020 | No data |
Differences between protocol and review
Clarification regarding inclusion criteria: suspicion of infection was interpreted as: clinical suspicion of SARS‐CoV‐2 infection based on a symptomatic presentation. At least 50% of the study population had to present with COVID‐19 compatible symptoms.
We performed sensitivity analyses to investigate the impact of prospective versus retrospective data collection in cross‐sectional studies.
Contributions of authors
JD, JDi, YT, CD, ML, RS, LH, AVdB, and DE, contributed clinical, methodological and/or technical expertise to drafting the protocol. JD co‐ordinated contributions from all co‐authors and drafted the protocol. ML drafted the QUADAS‐2 criteria. AVdB oversaw the overall progress of this review, participated in the selection process, data extraction and drafting of the manuscript. TS analyzed the data, drafted the manuscript and participated in the selection and data extraction. JD and BH participated in the data extraction, interpretation of the findings and commented on the manuscript.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK
University of Birmingham, UK
External sources
-
Department for International Development, UK
Project number: 300342‐104
National Institute for Health Research (NIHR), UK
NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham, UK
Declarations of interest
Thomas Struyf: none known
Jonathan J Deeks: none known
Jacqueline Dinnes: none known
Yemisi Takwoingi: none known
Clare Davenport: none known
Mariska MG Leeflang: none known
René Spijker: the Dutch Cochrane Centre (DCC) has received grants for performing commissioned systematic reviews. In no situation did the commissioner have any influence on the results of the work.
Lotty Hooft: none known
Devy Emperador: is employed by FIND. FIND is a global non‐for profit product development partnership and WHO Diagnostic Collaboration Centre. It is FIND’s role to accelerate access to high quality diagnostic tools for low resource settings and this is achieved by supporting both R&D and access activities for a wide range of diseases, including COVID‐19. FIND has several clinical research projects to evaluate multiple new diagnostic tests against published Target Product Profiles that have been defined through consensus processes. These studies are for diagnostic products developed by private sector companies who provide access to know‐how, equipment/reagents, and contribute through unrestricted donations as per FIND policy and external SAC review.
Julie Domen: none known
Sebastiaan Horn: none known
Ann Van den Bruel: none known
Edited (no change to conclusions)
References
References to studies included in this review
Ahmed 2020 {published data only}
- Ahmed SM, Shah RU, Bale M, Peacock JB, Berger B, Brown A, et al. Comprehensive testing highlights racial, ethnic, and age disparities in the COVID-19 outbreak. medRxiv [Preprint] 2020. [DOI: ] [Google Scholar]
Ai 2020 {published data only}
- Ai JW, Zhang HC, Xu T, Wu J, Zhu M, Yu YQ, et al. Optimizing diagnostic strategy for novel coronavirus pneumonia, a multi-center study in Eastern China. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.02.13.20022673] [DOI] [Google Scholar]
Brotons 2020 {published data only}
- Brotons C, Serrano J, Fernandez D, Garcia-Ramos C, Ichazo B, Lemaire J, et al. Seroprevalence against COVID-19 and follow-up of suspected cases in primary health care in Spain. medRxiv [Preprint] 2020. [DOI: ] [Google Scholar]
Carignan 2020 {published data only}
- Carignan A, Valiquette L, Grenier C, Musonera JB, Nkengurutse D, Marcil-Héguy A, et al. Anosmia and dysgeusia associated with SARS-CoV-2 infection: an age-matched case-control study. CMAJ : Canadian Medical Association Journal 2020;192(26):E702-e707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Challener 2020 {published data only}
- Challener DW, Challener GJ, Gow-Lee VJ, Fida M, Shah AS, O'Horo JC. Screening for COVID-19: patient factors predicting positive PCR test. Infection Control and Hospital Epidemiology 2020;41(8):968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2020 {published data only}
- Chen X, Tang Y, Mo Y, Li S, Lin D, Yang Z, et al. A diagnostic model for coronavirus disease 2019 (COVID-19) based on radiological semantic and clinical features: a multi-center study. European Radiology 2020;30(9):4893-902. [DOI: 10.1007/s00330-020-06829-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2020 {published data only}
- Cheng Z, Lu Y, Cao Q, Qin L, Pan Z, Yan F, et al. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. American Journal of Roentgenology 2020;215(1):121-6. [DOI: 10.2214/AJR.20.22959] [DOI] [PubMed] [Google Scholar]
Chua 2020 {published data only}
- Chua AJ, Chan EC, Loh J, Choong Charn T. Acute olfactory loss is specific for COVID-19 at the emergency department. Annals of Emergency Medicine 2020;76(4):10.1016/j.annemergmed.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clemency 2020 {published data only}
- Clemency BM, Varughese R, Scheafer DK, Ludwig B, Welch JV, McCormack RF, et al. Symptom criteria for COVID-19 testing of health care workers. Academic Emergency Medicine 2020;27(6):469-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feng 2020 {published data only}
- Feng C, Huang Z, Wang L, Chen X, Zhai Y, Zhu F, et al. A novel triage tool of artificial intelligence assisted diagnosis aid system for suspected COVID-19 pneumonia in fever clinics. Annals of Translational Medicine 2020;9(3):201. [DOI: 10.1101/2020.03.19.20039099] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbert 2020 {published data only}
- Gilbert A, Brasseur E, Petit M, Donneau AF, Diep AN, Hetzel Campbell S, et al. Immersion in an emergency department triage center during the COVID-19 outbreak: first report of the Liège University hospital experience. Acta Clinica Belgica 2020;Jun(12):1-7. [DOI] [PubMed] [Google Scholar]
Haehner 2020 {published data only}
- Haehner A, Draf J, Draeger S, With K, Hummel T. Predictive value of sudden olfactory loss in the diagnosis of COVID-19. Karger 2020;82(4):175-80. [DOI: 10.1159/000509143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2020 {published data only}
- Huang D, Wang T, Chen Z, Yang H, Yao R, Liang Z. A novel risk score to predict diagnosis with coronavirus disease 2019 (COVID-19) in suspected patients: a retrospective, multicenter, and observational study. Journal of Medical Virology 2020;92(11):2709-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Just 2020 {published data only}
- Just J, Puth M-T, Regenold F, Weckbecker K, Bleckwenn M. Distinguishing between COVID-19 and the common cold in a primary care setting - comparison of patients with positive and negative SARS-CoV-2 PCR results. BMC family practice 2020;21(1):251. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leal 2020 {published data only}
- Leal FE, Mendes-Correa MC, Buss LF, Costa SF, Bizario JC, Souza SR, et al. Clinical features and natural history of the first 2073 suspected COVID-19 cases in the Corona São Caetano primary care programme: a prospective cohort study. BMJ Open 14/01/2021;11(1):e042745. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2020 {published data only}
- Lee DJ, Lockwood J, Das P, Wang R, Grinspun E, Lee JM. Self-reported anosmia and dysgeusia as key symptoms of coronavirus disease 2019. CJEM 2020;22(5):595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liang 2020 {published data only}
- Liang Y, Liang J, Zhou Q, Li X, Lin F, Deng Z, et al. Prevalence and clinical features of 2019 novel coronavirus disease (COVID-19) in the Fever Clinic of a teaching hospital in Beijing: a single-center, retrospective study. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.02.25.20027763] [DOI] [Google Scholar]
Mao 2020 {published data only}
- Mao B, Liu Y, Chai YH, Jin XY, Lu HW, Yang JW, et al. Assessing risk factors for SARS-CoV-2 infection in patients presenting with symptoms in Shanghai, China: a multicentre, observational cohort study. Lancet Digital Health 2020;2(6):e323-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nobel 2020 {published data only}
- Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, et al. Gastrointestinal symptoms and COVID-19: case-control study from the United States. Gastroenterology 2020;159(1):373-5. [DOI: 10.1053/j.gastro.2020.04.017] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Reilly 2020 {published data only}
- O'Reilly GM, Mitchell RD, Rajiv P, Wu J, Brennecke H, Brichko L, et al. Epidemiology and clinical features of emergency department patients with suspected COVID-19: initial results from the COVID-19 Emergency Department Quality Improvement Project (COVED-1). Emergency Medicine Australasia 2020;32(4):638-45. [DOI] [PubMed] [Google Scholar]
Peng 2020 {published data only}
- Peng L, Liu KY, Xue F, Miao YF, Tu PA, Zhou C. Improved early recognition of coronavirus disease-2019 (COVID-19): single-center data from a Shanghai screening hospital. Archives of Iranian Medicine 2020;23(4):272-6. [DOI] [PubMed] [Google Scholar]
Peyrony 2020 {published data only}
- Peyrony O, Marbeuf-Gueye C, Truong V, Giroud M, Rivière C, Khenissi K, et al. Accuracy of emergency department clinical findings for diagnosis of coronavirus disease 2019. Annals of Emergency Medicine 2020;76(4):405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pisapia 2020 {published data only}
- Pisapia R, Pisaturo M, Fusco FM, Parrella G, Iodice V, Tambaro O, et al. Differences among confirmed and not-confirmed COVID-19 patients at "D.Cotugno" hospital, Naples (Italy): what we learned from first suspected cases? Infezioni in Medicina 2020;28 Suppl 1:84-8. [PubMed] [Google Scholar]
Rentsch 2020 {published data only}
- Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT, Skanderson M, et al. Covid-19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54-75 years. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.04.09.20059964] [DOI] [Google Scholar]
Salmon 2020 {published data only}
- Salmon Ceron D, Bartier S, Hautefort C, Nguyen Y, Nevoux J, Hamel AL, et al. Self-reported loss of smell without nasal obstruction to identify COVID-19. The multicenter Coranosmia cohort study. Journal of Infection 2020;81(4):614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2020 {published data only}
- Shah SJ, Barish PN, Prasad PA, Kistler AL, Neff N, Kamm J, et al. Clinical features, diagnostics, and outcomes of patients presenting with acute respiratory illness: A retrospective cohort study of patients with and without COVID-19. EClinicalMedicine 2020;27. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2020a {published data only}
- Song CY, Xu J, He JQ, Lu YQ. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.03.05.20031906] [DOI] [Google Scholar]
Sun 2020 {published data only}
- Sun Y, Koh V, Marimuthu K, Ng OT, Young B, Vasoo S, et al. Epidemiological and clinical predictors of COVID-19. Clinical Infectious Diseases 2020;71(15):786-92. [DOI: 10.1093/cid/ciaa322] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tolia 2020 {published data only}
- Tolia VM, Chan TC, Castillo EM. Preliminary results of initial testing for coronavirus (COVID-19) in the emergency department. Western Journal of Emergency Medicine 2020;21(3):503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tordjman 2020 {published data only}
- Tordjman M, Mekki A, Mali RD, Saab I, Chassagnon G, Guillo E, et al. Pre-test probability for SARS-Cov-2-related infection score: the PARIS score. PLOS ONE 2020;15(12):e0243342. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trubiano 2020 {published data only}
- Trubiano JA, Vogrin S, Smibert OC, Marhoon N, Alexander AA, Chua KY, et al. COVID-MATCH65 - a prospectively derived clinical decision rule for severe acute respiratory syndrome coronavirus 2. PLoS One 10/12/2020;15(12):e0243414. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tudrej 2020 {published data only}
- Tudrej B, Sebo P, Lourdaux J, Cuzin C, Floquet M, Haller DM, et al. Self- reported loss of smell and taste in SARS-CoV-2 patients: primary care data to guide future early detection strategies. Journal of General Internal Medicine 2020;35(8):2502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wee 2020 {published data only}
- Wee LE, Chan YZ, Teo NY, Cherng BZ, Thien SY, Wong M, et al. The role of self-reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID-19. European Archives of Oto-Rhino-Laryngology 2020;277(8):2389-90. [DOI: 10.1007/s00405-020-05999-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2020 {published data only}
- Wei Y, Lu Y, Xia L, Yuan X, Li G, Li X, et al. Analysis of 2019 novel coronavirus infection and clinical characteristics of outpatients: an epidemiological study from a fever clinic in Wuhan, China. Journal of Medical Virology 2020;92:2758-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xie 2020 {published data only}
- Xie S, Zhang G, Yu H, Wang J, Wang S, Tang G, et al. The epidemiologic and clinical features of suspected and confirmed cases of imported 2019 novel coronavirus pneumonia in north Shanghai, China. Annals of Translational Medicine 2020;8(10):637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2020 {published data only}
- Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. International Forum of Allergy & Rhinology 2020;10(7):806-13. [DOI: 10.1002/alr.22579] [DOI] [PMC free article] [PubMed]
Yang 2020 {unpublished data only}
- Yang Z, Lin D, Chen X, Qiu J, Li S, Huang R, et al. Distinguishing COVID-19 from influenza pneumonia in the early stage through CT imaging and clinical features. medRxiv [Preprint] 2020. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yombi 2020 {published data only}
- Yombi JC, De Greef J, Marsin A-S, Simon A, Rodriguez-Villalobos H, Penaloza A, et al. Symptom-based screening for COVID-19 in health care workers: the importance of fever. Journal of Hospital Infection 2020;105(3):428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zavascki 2020 {published data only}
- Zavascki AP, Gazzana MB, Bidart JP, Fernandes PS, Galiotto A, Kawski CT, et al. Development of a predictive score for COVID-19 diagnosis based on demographics and symptoms in patients attended at a dedicated screening unit. medRxiv [Preprint] 2020. [DOI: ] [Google Scholar]
Zayet 2020a {published data only}
- Zayet S, Kadiane-Oussou NJ, Lepiller Q, Zahra H, Royer PY, Toko L, et al. Clinical features of COVID-19 and influenza: a comparative study on Nord Franche-Comte cluster. Microbes and Infection 2020;22(9):481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zayet 2020b {published data only}
- Zayet S, Klopfenstein T, Mercier J, Kadiane-Oussou NJ, Lan Cheong Wah L, Royer PY, et al. Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients. Infection 2020;14:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2020 {published data only}
- Zhao D, Yao F, Wang L, Zheng L, Gao Y, Ye J, et al. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clinical Infectious Diseases 2020;71(15):756-61. [DOI: 10.1093/cid/ciaa247] [DOI] [PMC free article] [PubMed]
Zhu 2020 {published data only}
- Zhu W, Xie K, Lu H, Xu L, Zhou S, Fang S. Initial clinical features of suspected coronavirus disease 2019 in two emergency departments outside of Hubei, China. Journal of Medical Virology 2020;92(9):1525-32. [DOI: 10.1002/jmv.25763 ] [DOI] [PMC free article] [PubMed]
Zimmerman 2020 {published data only}
- Zimmerman RK, Nowalk MP, Bear T, Taber R, Sax TM, Eng H, et al. Proposed clinical indicators for efficient screening and testing for COVID-19 infection using Classification and Regression Trees (CART) analysis. Hum Vaccin Immunother 2020:1-4. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Guan 2020 {published data only}
- Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.02.06.20020974] [DOI] [Google Scholar]
Soares 2020 {published data only}
- Soares F, Villavicencio A, Anzanello MJ, Fogliatto FS, Idiart M, Stevenson M. A novel high specificity COVID-19 screening method based on simple blood exams and artificial intelligence. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.04.10.20061036] [DOI] [Google Scholar]
Song 2020b {published data only}
- Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, et al. Emerging coronavirus 2019-nCoV pneumonia. Radiology 2020;295(1):200274. [DOI: 10.1148/radiol.2020200274] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2020 {published data only}
- Wang Y, Kang H, Liu X, Tong Z. Combination of RT-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-CoV-2 outbreak. Journal of Medical Virology 2020;92(6). [DOI: 10.1002/jmv.25721 10.1002/jmv.25721] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR2000029462 {published data only}
- ChiCTR2000029462. Study for clinical characteristics and distribution of TCM syndrome of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=48922 (first received 27 April 2020).
ChiCTR2000029734 {published data only}
- ChiCTR2000029734. Epidemiological investigation and clinical characteristics analysis of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=48868 (first received 27 April 2020).
ChiCTR2000029770 {published data only}
- ChiCTR2000029770. Study for epidemiology, diagnosis and treatment of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/hvshowproject.aspx?id=23744 (first received 27 April 2020).
ChiCTR2000029839 {published data only}
- ChiCTR2000029839. An observational study on the clinical characteristics, treatment and outcome of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=49439 (first received 27 April 2020).
ChiCTR2000029865 {published data only}
- ChiCTR2000029865. Descriptive study on the clinical characteristics and outcomes of novel coronavirus pneumonia (COVID-19) in cardiovascular patients. www.chictr.org.cn/showproj.aspx?proj=49545 (first received 27 April 2020).
ChiCTR2000029866 {published data only}
- ChiCTR2000029866. Early warning prediction of patients with severe novel coronavirus pneumonia (COVID-19) based on multiomics. www.chictr.org.cn/showproj.aspx?proj=49519 (first received 27 April 2020).
ChiCTR2000029959 {published data only}
- ChiCTR2000029959. Clinical observation and research of severe acute respiratory syndrome coronavirus 2(COVID-19) infection in perinatal newborns. www.chictr.org.cn/showproj.aspx?proj=49636 (first received 27 April 2020).
ChiCTR 2000030096 {published data only}
- ChiCTR 2000030096. Study for establishment of correlation between virological dynamics and clinical features in novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showprojen.aspx?proj=49794 (first received 27 April 2020).
ChiCTR2000030256 {published data only}
- ChiCTR2000030256. Epidemiological and clinical characteristics of COVID-19: a large-scale investigation in epicenter Wuhan, China. www.chictr.org.cn/showproj.aspx?proj=50078 (first received 27 April 2020).
ChiCTR2000030327 {published data only}
- ChiCTR2000030327. Analysis of clinical characteristics of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50214 (first received 27 April 2020).
ChiCTR2000030363 {published data only}
- ChiCTR2000030363. Novel coronavirus infected disease (COVID-19) in children: epidemiology, clinical features and treatment outcome. www.chictr.org.cn/showproj.aspx?proj=49984 (first received 27 April 2020).
ChiCTR2000030387 {published data only}
- ChiCTR2000030387. Clinical observation and research of multiple organs injury in severe patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50329 (first received 27 April 2020).
ChiCTR2000030464 {published data only}
- ChiCTR2000030464. Study for the clinical characteristics of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50382 (first received 27 April 2020).
ChiCTR2000030491 {published data only}
- ChiCTR2000030491. A medical records based study for comparing differences of clinical features and outcomes of novel coronavirus pneumonia (COVID-19) patients between Sichuan Province and Wuhan City. www.chictr.org.cn/hvshowproject.aspx?id=23102 (first received 27 April 2020).
ChiCTR2000030519 {published data only}
- ChiCTR2000030519. Study for the clinical characteristics and digestive system damage of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50604 (first received 27 April 2020).
ChiCTR2000030544 {published data only}
- ChiCTR2000030544. Study for the risk factors of critically ill patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50134 (first received 27 April 2020).
ChiCTR 2000030679 {published data only}
- ChiCTR 2000030679. Cohort study of novel coronavirus infected diseases (COVID-19) in children. www.chictr.org.cn/hvshowproject.aspx?id=23417 (first received 27 April 2020).
ChiCTR2000030707 {published data only}
- ChiCTR2000030707. Retrospective study on novel coronavirus pneumonia (COVID-19) in Tibetan Plateau. www.chictr.org.cn/showproj.aspx?proj=50160 (first received 27 April 2020).
ChiCTR2000030722 {published data only}
- ChiCTR2000030722. Auscultatory characteristics of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50338 (first received 27 April 2020).
ChiCTR2000030739 {published data only}
- ChiCTR2000030739. Exploration of the clinical characteristics of patients with novel coronavirus pneumonia (COVID-19) and its differences from patients with severe influenza A and MERS. www.chictr.org.cn/showproj.aspx?proj=50896 (first received 27 April 2020).
ChiCTR2000030755 {published data only}
- ChiCTR2000030755. A medical records based study for characteristics, prognosis of elderly patients with novel coronavirus pneumonia (COVID-19) in Wuhan area. www.chictr.org.cn/hvshowproject.aspx?id=23554 (first received 27 April 2020).
ChiCTR2000030778 {published data only}
- ChiCTR2000030778. A medical records based study for epidemic and clinical features of novel coronavirus pneumonia (COVID-19) in Ningbo First Hospital. www.chictr.org.cn/hvshowproject.aspx?id=23642 (first received 27 April 2020).
ChiCTR2000030784 {published data only}
- ChiCTR2000030784. A study for clinical characteristics of novel coronavirus pneumonia (COVID-19) patients follow-up in Guangxi. www.chictr.org.cn/showproj.aspx?proj=50307 (first received 27 April 2020).
ChiCTR2000030796 {published data only}
- ChiCTR2000030796. Clinical characteristics and treatment of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50991 (first received 27 April 2020).
ChiCTR 2000030798 {published data only}
- ChiCTR 2000030798. A medical records based study for clinical characteristics of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/hvshowproject.aspx?id=23687 (first received 27 April 2020).
ChiCTR2000030803 {published data only}
- ChiCTR2000030803. Collection and analysis of clinical data in severe and critically ill patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=51007 (first received 27 April 2020).
ChiCTR2000030807 {published data only}
- ChiCTR2000030807. Clinical characteristics and prognosis of cancer patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=51019 (first received 27 April 2020).
ChiCTR2000030818 {unpublished data only}
- ChiCTR2000030818. A medical records based study for the value of Lymphocyte subsets in the diagnose and treatment. www.chictr.org.cn/hvshowproject.aspx?id=23742 (first received 27 April 2020).
ChiCTR2000030819 {published data only (unpublished sought but not used)}
- ChiCTR2000030819. Retrospective analysis of digestive system symptoms in 600 cases of novel coronavirus pneumonia (COVID-19) in Guanggu district, Wuhan. www.chictr.org.cn/showproj.aspx?proj=51039 2020.
ChiCTR2000030834 {published data only}
- ChiCTR2000030834. Epidemiological characteristics and antibody levels of novel coronavirus pneumonia (COVID-19) of pediatric medical staff working in quarantine area. www.chictr.org.cn/showproj.aspx?proj=51047 (frist received 27 April 2020).
ChiCTR2000030854 {published data only}
- ChiCTR2000030854. A clinical multicenter study for the occurrence, development and prognosis of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showprojen.aspx?proj=51083 (first received 27 April 2020).
ChiCTR2000030858 {published data only}
- ChiCTR2000030858. Clinical characteristics and outcomes of 483 mild patients with novel coronavirus pneumonia (COVID-19) in Wuhan, China during the outbreak: a single-center, retrospective study from the mobile cabin hospital. www.chictr.org.cn/showproj.aspx?proj=51097 (first received 27 April 2020).
ChiCTR2000030863 {published data only}
- ChiCTR2000030863. Clinical and CT imaging characteristics of novel coronavirus pneumonia (COVID-19): an multicenter cohort study. www.chictr.org.cn/showproj.aspx?proj=50767 (first received 27 April 2020).
NCT04270383 {published data only}
- NCT04270383. Clinical characteristics and long-term prognosis of 2019-nCoV infection in children. clinicaltrials.gov/ct2/show/NCT04270383 (first received 17 February 2020).
NCT04279782 {published data only}
- NCT04279782. Clinical features of suspected and confirmed patients of 2019 novel coronavirus infection. www.clinicaltrials.gov/ct2/show/NCT04279782 (first received 27 April 2020).
NCT04279899 {published data only}
- NCT04279899. The investigation of the neonates with or with risk of COVID-19. clinicaltrials.gov/ct2/show/NCT04279899 (first received 27 April 2020).
NCT04285801 {published data only}
- NCT04285801. Critically ill patients with COVID-19 in Hong Kong: a multicentre observational cohort study. clinicaltrials.gov/ct2/show/NCT04285801 (first received 27 April 2020).
NCT04292327 {published data only}
- NCT04292327. Clinical progressive characteristics and treatment effects of 2019-novel coronavirus. clinicaltrials.gov/ct2/show/NCT04292327 (first received 27 April 2020).
NCT04292964 {published data only}
- NCT04292964. Prognostic factors of patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04292964 (first received 27 April 2020).
NCT04315870 {published data only}
- NCT04315870. Clinical characteristics of coronavirus disease 2019 (COVID-19) in pregnancy: the Italian Registry on coronavirus in pregnancy. clinicaltrials.gov/ct2/show/NCT04315870 (first received 20 March 2020).
Additional references
Bossuyt 2015
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [DOI: 10.1136/bmj.h5527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2020a
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, et al, Cochrane COVID-19 Diagnostic Test Accuracy Group. Antibody tests for identification of current and past infection with SARS‐CoV‐2. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD013652. [DOI: 10.1002/14651858.CD013652] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dinnes 2020
- Dinnes J, Deeks JJ, Adriano A, Berhane S, Davenport C, Dittrich S, et al, Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point‐of‐care antigen and molecular‐based tests for diagnosis of SARS‐CoV‐2 infection. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD013705. [DOI: 10.1002/14651858.CD013705] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffith 2020
- Griffith G, Morris TT, Tudball M, Herbert A, Mancano G, Pike L, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nature Communications 2020;11(5749). [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Islam 2020
- Islam N, Salameh J-P, Leeflang MM, Hooft L, McGrath TA, Pol CB, et al, Cochrane COVID-19 Diagnostic Test Accuracy Group. Thoracic imaging tests for the diagnosis of COVID‐19. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD013639. [DOI: 10.1002/14651858.CD013639.pub3] [DOI] [PubMed] [Google Scholar]
Jaeschke 1994
- Jaeschke R, Guyatt GH, Sackett DL. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA 1994;271(9):703-7. [DOI] [PubMed] [Google Scholar]
Macaskill 2013
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
McInnes 2020
- McInnes M, Leeflang MM, Salameh J-P, McGrath T, Van der Pol CB, Frank RA, et al. Imaging tests for the diagnosis of COVID-19. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD013639. [DOI: 10.1002/14651858.CD013639] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
R 2020 [Computer program]
- R core team R Foundation for Statistical Computing. Version 3.5.1. Vienna, Austria: R core team, 2020.
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rutjes 2006
- Rutjes AW, Reitsma JB, Di Nisio M, Smidt N, Van Rijn JC, Bossuyt PM. Evidence of bias and variation in diagnostic accuracy studies. Canadian Medical Association Journal 2006;174(4):469476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stegeman 2020
- Stegeman I, Ochodo EA, Guleid F, Holtman GA, Yang B, Davenport C, et al. Routine laboratory testing to determine if a patient has COVID‐19. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD013787. [DOI: 10.1002/14651858.CD013787] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van den Bruel 2010
- Van den Bruel A, Haj-Hassan T, Thompson M, Buntinx F, Mant D. Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: a systematic review. Lancet 2010;375(9717):834-45. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Deeks 2020b
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, Spijker R, et al. Diagnosis of SARS-CoV-2 infection and COVID-19: accuracy of signs and symptoms; molecular, antigen, and antibody tests; and routine laboratory markers. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013596. [DOI: 10.1002/14651858.CD013596] [DOI] [Google Scholar]
Struyf 2020
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang M, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID‐19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] [DOI] [PMC free article] [PubMed] [Google Scholar]


