Abstract
BACKGROUND & AIMS:
The assessment of therapeutic response after neoadjuvant treatment and pancreatectomy for pancreatic ductal adenocarcinoma (PDAC) has been an ongoing challenge. Several limitations have been encountered when employing current grading systems for residual tumor. Considering endoscopic ultrasound (EUS) represents a sensitive imaging technique for PDAC, differences in tumor size between preoperative EUS and postoperative pathology after neoadjuvant therapy were hypothesized to represent an improved marker of treatment response.
METHODS:
For 340 treatment-naïve and 365 neoadjuvant-treated PDACs, EUS and pathologic findings were analyzed and correlated with patient overall survival (OS). A separate group of 200 neoadjuvant-treated PDACs served as a validation cohort for further analysis.
RESULTS:
Among treatment-naïve PDACs, there was a moderate concordance between EUS imaging and postoperative pathology for tumor size (r = 0.726, P < .001) and AJCC 8th edition T-stage (r = 0.586, P < .001). In the setting of neoadjuvant therapy, a decrease in T-stage correlated with improved 3-year OS rates (50% vs 31%, P < .001). Through recursive partitioning, a cutoff of ≥47% tumor size reduction was also found to be associated with improved OS (67% vs 32%, P < .001). Improved OS using a ≥47% threshold was validated using a separate cohort of neoadjuvant-treated PDACs (72% vs 36%, P < .001). By multivariate analysis, a reduction in tumor size by ≥47% was an independent prognostic factor for improved OS (P = .007).
CONCLUSIONS:
The difference in tumor size between preoperative EUS imaging and postoperative pathology among neoadjuvant-treated PDAC patients is an important prognostic indicator and may guide subsequent chemotherapeutic management.
Keywords: Pancreas, Prognosis, Cancer, Diagnosis, Survival, Size, Stage, Treatment
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer deaths in the United States and is associated with a dismal 5-year survival rate of only 9%.1 Currently, combined surgical intervention and adjuvant therapy offer potential for cure. However, only 10%–20% of patients have resectable disease at the time of diagnosis. In addition, nearly half of all patients undergoing curative-intent pancreatectomy fail to complete systemic treatment because of postoperative complications.2 This has led to an increased emphasis on the use of neoadjuvant therapy as a strategy to treat PDAC patients. Neoadjuvant therapy has multiple potential benefits including treatment of occult micrometastases, better compliance with chemotherapy, increased margin-negative resection rates, downstaging of nodal disease, and improved patient operability and performance status to receive adjuvant treatment.3–6 The assessment of responsiveness to chemotherapy can also aid in deciding a subsequent adjuvant treatment regimen. Nevertheless, determining therapeutic response after neoadjuvant treatment and pancreatectomy for PDAC has been an ongoing challenge.
In the past decade, several studies have attempted to define an optimal method of posttreatment evaluation. The fundamental premise has been to identify objective markers of response that correlate with an improvement in overall survival (OS). For example, an estimation of tumor burden from pretreatment and posttreatment abdominal imaging scans has been examined. However, the major obstacle with abdominal imaging such as multidetector computed tomography (MDCT) scans is the inability to accurately measure the size of PDAC.7,8 Furthermore, the diagnostic performance of MDCT is reported to be reduced after neoadjuvant therapy.9 Radiographic response data also correlate poorly with OS. In contrast, a reduction in serum tumor markers such as carbohydrate antigen 19-9 (CA19-9) has demonstrated significant promise. We have previously reported that a reduction in CA19-9 by ≥85% after neoadjuvant therapy is associated with 2-fold increase in patient survival.10 The limitations of serum CA19-9 are its elevation in benign pancreatobiliary diseases, such as cholangitis, obstructive jaundice, and pancreatitis, and normal levels in up to 10% of PDAC patients who are Lewis antigen negative.11 Finally, grading of pathologic treatment response by histologic examination is often considered subjective because the amount of tumor present before neoadjuvant therapy is uncertain.12 Thus, alternative indicators of neoadjuvant response are required to determine the effectiveness of treatment after surgery.
Endoscopic ultrasound (EUS) is one of the most sensitive imaging modalities of the pancreas and is considered to be more accurate than MDCT in the detection of PDAC and staging of local disease.13–16 In addition, combined with fine-needle aspiration (FNA), pancreatic EUS-FNA can obtain tissue samples for pathologic examination to confirm the presence of PDAC. Considering the high sensitivity of EUS in detecting PDAC, we hypothesized that tumor size measurements, specifically the greatest dimension, that are based on EUS findings closely approximated those obtained by pathology on treatment-naive postsurgical specimens, and that determining the difference in tumor size between preoperative EUS and postoperative pathology after neoadjuvant therapy may represent an improved method of evaluating treatment response. Therefore, the aims of this study were to (1) ascertain the concordance of unidimensional tumor size between preoperative EUS imaging and postoperative pathology in treatment-naive PDAC patients, (2) determine whether tumor size differences between preoperative EUS and postoperative pathology after neoadjuvant therapy correlate with OS, and (3) identify the prognostic significance of changes in tumor size after treatment in conjunction with other clinicopathologic variables.
Methods
Study Design and Case Selection
The study design is summarized in Figure 1 and was approved by the Institutional Review Board at the University of Pittsburgh (IRB# STUDY19070069). The study comprised 3 separate patient cohorts (Supplementary Material). First, the surgical pathology archives at the University of Pittsburgh Medical Center (UPMC) were queried for pancreatectomy specimens harboring PDAC between 2006 and 2016. Cases were cross-referenced with clinical, imaging, laboratory, treatment, and follow-up data obtained from patient paper and/or electronic medical records. Patient inclusion criteria included the following: pancreatectomy with curative intent, documentation of presence or absence of preoperative treatment, availability of preoperative EUS reports with corresponding images, postoperative gross pathology reports, availability of all H&E-stained slides for pathologic re-review, and follow-up OS data of at least 3 months. In total, 705 patients with a resected PDAC fulfilled the aforementioned criteria and consisted of 2 patient cohorts, 340 treatment-naive and 365 neoadjuvant-treated PDAC patients. For validation purposes, a separate cohort of PDAC patients was queried from the UPMC surgical pathology archives between 2017 and 2019. Inclusion criteria were the same as those mentioned previously; however, treatment-naive patients were excluded. Of note, in the 2-year timeframe, >90% of resected PDAC patients at UPMC received neoadjuvant therapy. This separate validation cohort consisted of 200 neoadjuvant-treated PDAC patients.
Figure 1.
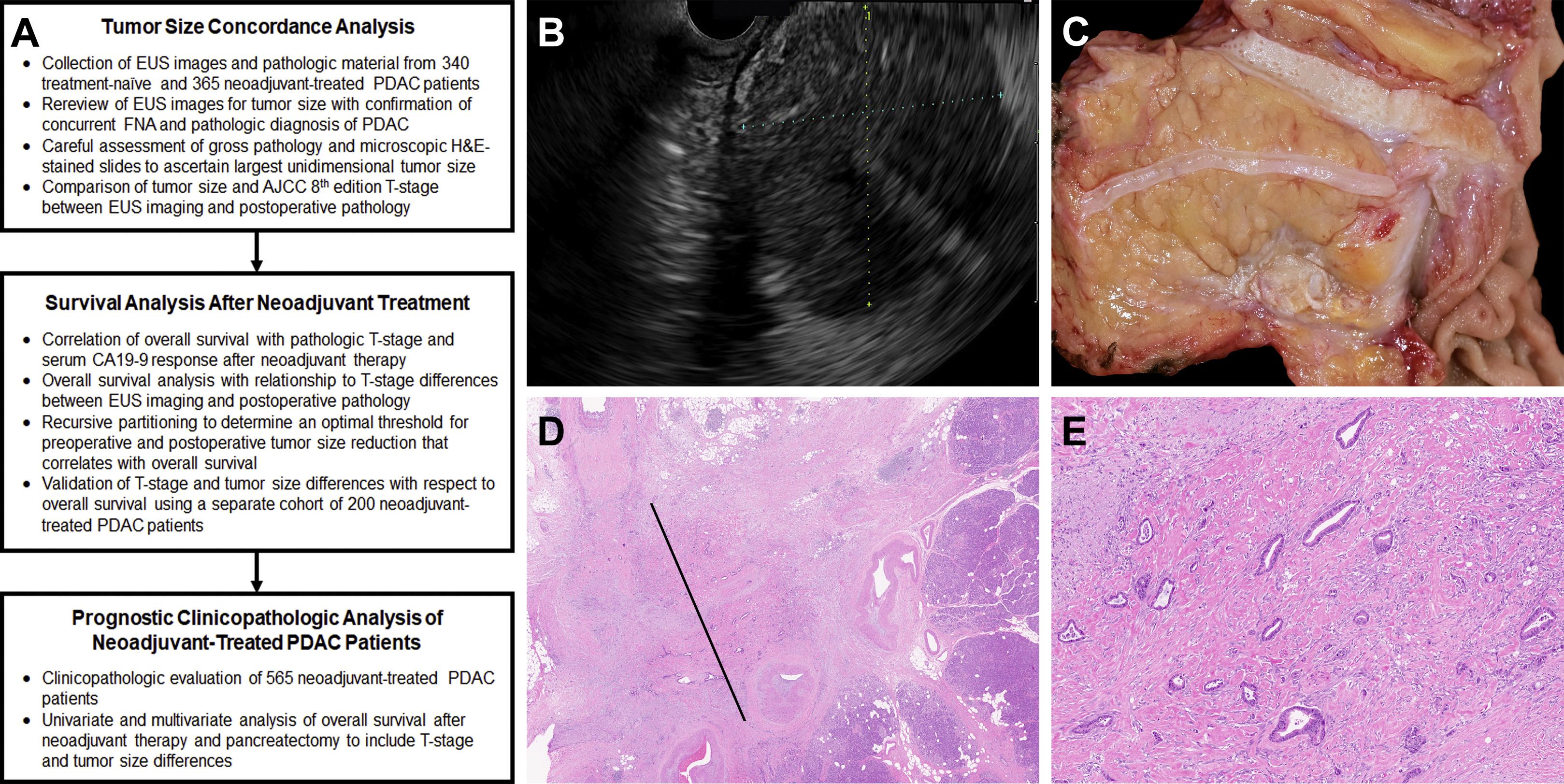
Summary of study design (A) and methodology used to evaluate 905 pancreatic ductal adenocarcinoma (PDAC) patients. (B) Within this example, a 54-year-old woman presented with a 2.6-cm pancreatic ductal adenocarcinoma by endoscopic ultrasound (EUS). (C and D) After neoadjuvant FOLFIRINOX and pancreatectomy, the adenocarcinoma grossly measured 1.2 cm; however, microscopically it measured 0.3 cm in greatest dimension. AJCC, American Joint Committee on Cancer; CA19-9, carbohydrate 19-9; FNA, fine-needle aspiration.
Individual patient medical records were reviewed to confirm that a pancreatic mass was identified by EUS (Figure 1A and B), a concurrent FNA was performed, and a diagnosis of PDAC was rendered by pathologic evaluation. The largest tumor dimension by EUS was recorded and designated as preoperative tumor size within this study. Both EUS images and reports were evaluated by at least 2 gastroenterologists. For postoperative pathologic specimens, gross pathology reports and corresponding H&E-stained slides were reviewed by at least 2 pathologists to document several pathologic features including the largest unidimensional tumor size. Of note, the UPMC Department of Pathology evaluates pancreatectomy specimens by using a standardized approach. Briefly, for specimens with a grossly identifiable mass (Figure 1C), macroscopic tumor size and other gross pathologic findings are correlated with histologic sections taken for microscopic assessment (Figure 1D and E). However, for cases with no grossly identifiable mass, the entire pancreatectomy specimen is systematically submitted for microscopic examination. Particular attention is made to carefully map sections taken of the pancreas such that the tumor size can be measured using corresponding H&E-stained slides. For PDAC cases that consist of a single microscopic focus of adenocarcinoma, the largest linear dimension of the tumor is measured from an H&E-stained slide. If the tumor consists of multiple microscopic foci (multifocal distribution), the largest linear dimension that is involved by adenocarcinoma to include intervening fibrotic stroma and/or uninvolved tissue is used as tumor size.17 This measurement excludes associated fibrosis/desmoplasia beyond the neoplastic cells.
In addition to tumor size, each pancreatectomy specimen was evaluated for tumor location, involvement of resection margins, lymphovascular invasion, perineural invasion, and regional lymph node metastases. Pathologic primary tumor classification (T-stage) and positive resection margins were determined according to the 8th edition of the American Joint Committee on Cancer (AJCC) Staging Manual.18 Demographic data; tumor anatomic type (resectability criteria) based on a multidisciplinary consensus conference/clinic as per Society of Surgical Oncology/Society for Surgery of the Alimentary Tract/Americas Hepato-Pancreato-Biliary Association guidelines; serum CA19-9 levels with total bilirubin; chemotherapeutic regimens; and OS were extracted from the patient’s medical record.19 Serum CA19-9 levels were excluded from assessment if pretreatment serum CA19-9 was normal (<37 IU/mL) or if total bilirubin was elevated (>2 mg/dL) because hyperbilirubinemia may falsely elevate serum CA19-9.20
Statistical Analysis
Chi-squared analysis or Fisher exact tests were used to compare categorical data, and Mann-Whitney U test was used to compare continuous variables. Tumor size and T-stage correlation between preoperative EUS imaging and postoperative pathology was calculated by Pearson correlation. Survival curves were constructed by using the Kaplan-Meier method, and differences between groups were evaluated by the log-rank test. OS was calculated from the date of surgery to the date of death and censored at the date of last follow-up. The prognostic significance of clinical and pathologic characteristics was determined by using univariate Cox regression analysis. Multivariate analyses of significant risk factors by univariate analysis were performed by using Cox proportional hazard regression to identify independent risk factors for OS. To determine the significance of confounding variables, multiple Cox proportional hazard regression models were evaluated by inclusion of non-confounding and confounding variables (Supplementary Table 2). All statistical analyses were performed by using the SPSS Statistical software, version 26 (IBM, Armonk, NY), and statistical significance was defined as P value <.05.
Results
Concordance Analysis of Unidimensional Tumor Size Between Preoperative Endoscopic Ultrasound Imaging and Postoperative Pathology
To determine the degree of concordance between preoperative EUS tumor size and postoperative pathologic tumor size, a cohort of 340 treatment-naive PDAC patients who underwent surgical resection with curative intent was evaluated (Table 1, Supplementary Material). EUS imaging revealed a tumor that measured 0.7–8.7 cm in greatest dimension (mean, 2.9 cm; median, 2.7 cm). In comparison, postoperative pathologic tumor size ranged from 1.0 to 13.0 cm (mean, 3.3 cm; median, 3.0 cm) and significantly correlated with EUS tumor size (r = 0.726, P < .001) (Figure 2). However, pathologic tumor size was larger by a median of 0.4 cm when compared with EUS imaging for the same patient. Overall, 241 tumors (71%) were larger on the basis of pathology than EUS. The difference between these 2 measurements was ≤0.5 cm for 170 cases (50%), ≤1.0 cm for 244 cases (72%), and ≤1.5 cm for 290 cases (85%). Of note, there was no difference in tumor size for 29 cases (9%). On the basis of the AJCC 8th edition T-staging system, which is dependent on tumor size, the Pearson correlation coefficient between EUS T-staging and pathologic T-staging was 0.586 (P < .001). A similar analysis was performed for MDCT but demonstrated a weaker correlation than EUS (Supplementary Figure 1). As expected, no correlation between preoperative EUS/MDCT and postoperative pathology was observed for 365 neoadjuvant-treated PDAC patients.
Table 1.
Clinicopathologic Features of 340 Treatment-Naive and 365 Neoadjuvant-Treated PDAC Patients
| Patient/tumor characteristics | Treatment-naive cohort (n = 340, 48%), n (%) | Neoadjuvant treated cohort (n = 365, 52%), n (%) | P value |
|---|---|---|---|
| Gender | |||
| Female | 167 (49) | 185 (51) | .706 |
| Male | 173 (51) | 180 (49) | |
| Median age (range), y | 70.5 (21–90) | 65.0 (34–89) | <.001 |
| Location | |||
| Head/neck/uncinate | 248 (73) | 276 (76) | .438 |
| Body/tail | 92 (27) | 89 (24) | |
| EUS median tumor size (range), cm | 2.5 (0.7–8.7) | 2.9 (1.0–8.0) | <.001 |
| Primary tumor (cT), by EUS | |||
| cT1 | 85 (25) | 50 (14) | .001 |
| cT2 | 228 (67) | 276 (76) | |
| cT3 | 27 (8) | 39 (11) | |
| Pathologic median tumor size (range), cm | 3.0 (1.0–13.0) | 2.5 (0–11.5) | <.001 |
| Primary tumor (pT), by pathology | |||
| (y)pT0 | 0 (0) | 5(1) | <.001 |
| (y)pT1 | 53 (15) | 125 (34) | |
| (y)pT2 | 230 (68) | 187 (51) | |
| (y)pT3 | 57 (17) | 48 (14) | |
| Histologic grade | |||
| Well/moderately differentiated | 240 (71) | 273 (75) | .236 |
| Poorly differentiated/undifferentiated | 100 (29) | 92 (25) | |
| Perineural invasion | 307 (90) | 288 (79) | <.001 |
| Lymphovascular invasion | 249 (73) | 244 (67) | .071 |
| Regional node (pN), by pathology | |||
| (y)pN0 | 110 (32) | 136 (37) | .061 |
| (y)pN1 | 117 (34) | 137 (38) | |
| (y)pN2 | 113 (34) | 92 (25) | |
| Positive margin(s) | 129 (38) | 135 (37) | .816 |
EUS, endoscopic ultrasound; PDAC, pancreatic ductal adenocarcinoma.
Figure 2.
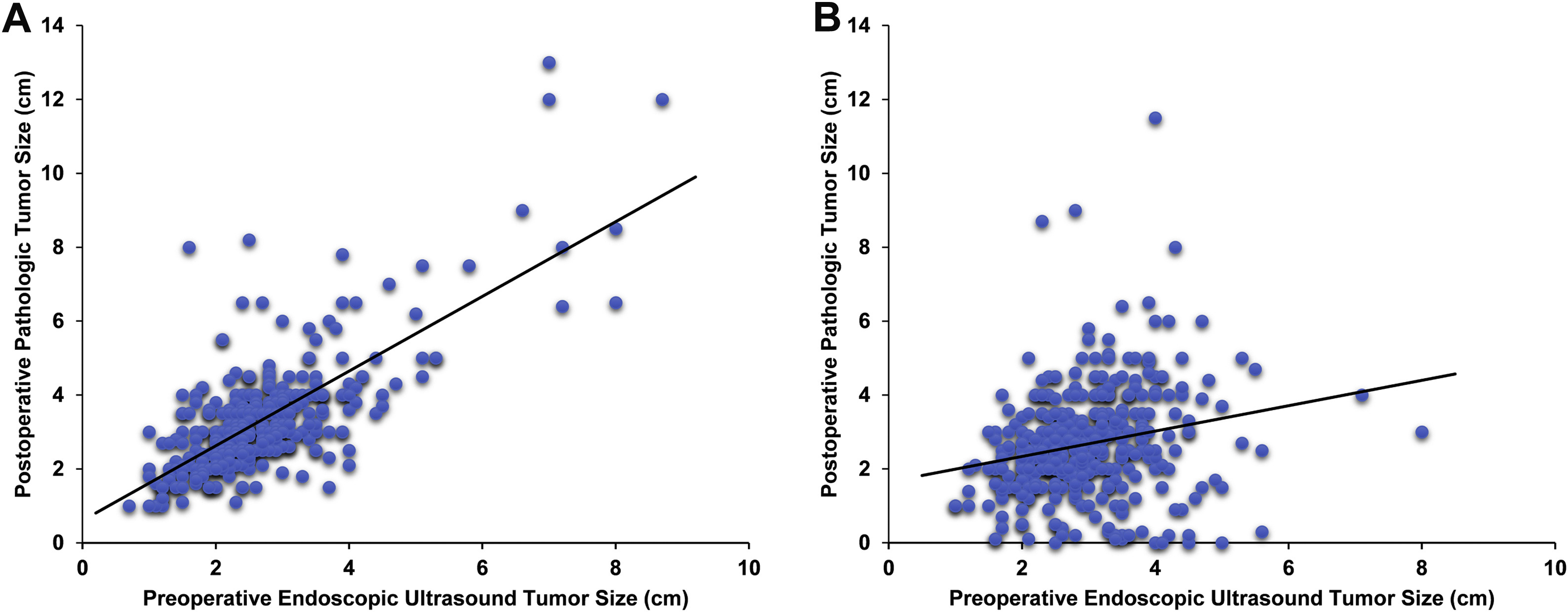
Tumor size concordance analysis between preoperative EUS imaging and postoperative pathology. (A) Among 340 treatment-naive PDACs, the Pearson correlation between preoperative EUS imaging and postoperative pathology was 0.726 (P < .001). (B) For 365 neoadjuvant-treated PDACs, the association between EUS imaging and pathology was weaker (r = 0.214, P < .001). Solid line indicates linear regression relationship. EUS, endoscopic ultrasound; PDAC, pancreatic ductal adenocarcinoma.
Survival Analysis Based on Unidimensional Tumor Size Differences Between Preoperative Endoscopic Ultrasound Imaging and Postoperative Pathology After Neoadjuvant Treatment
Among the 365 PDAC patients who received neoadjuvant therapy, the AJCC pathologic T-staging system was prognostically significant for OS (P < .001) (Figure 3A). Excluding 5 ypT0 patients as no deaths were reported during follow-up, the OS rates for ypT1, ypT2, and ypT3 patients at 3 years and 5 years were 46% and 31%, 38% and 16%, and 26% and 12%, respectively (P < .001). Pretreatment and posttreatment serum CA19-9 levels were available for 232 patients (64%), and 78 patients (21%) had ≥85% decrease in serum CA19-9. The 3-year and 5-year OS rates for patients with ≥85% CA19-9 reduction were 55% and 32%, respectively, as compared with 29% and 16%, respectively, for patients with <85% CA19-9 reduction (P < .001) (Figure 3B).
Figure 3.

Kaplan-Meier curves comparing OS for 365 PDAC patients after neoadjuvant treatment and pancreatectomy. Consistent with prior studies, (A) pathologic T-stage (American Joint Committee on Cancer 8th edition) and (B) ≥85% serum CA19-9 response (n = 232) were statistically significant (P < .001). (C) Similarly, evaluation of preoperative EUS imaging and postoperative pathology revealed a decrease in T-stage was associated with improved OS as compared with a T-stage that remained unchanged or increased (P < .001). (D) OS for patients with tumors that shrunk by ≥47% in tumor size was improved as compared with patients with tumors that shrunk by <47%, remained the same size, or increased in size (P < .001). CA19-9, carbohydrate 19-9.
On the basis of a comparison of preoperative EUS and postoperative pathology, 114 tumors (31%) decreased, 194 tumors (53%) remained unchanged, and 57 tumors (16%) increased in T-stage. The OS rates at 3 years and 5 years for patients that decreased, remained unchanged, and increased in T-staging after treatment were 50% and 31%, 37% and 19%, and 34% and 16%, respectively (P = .003). When combining patients with tumors that remained unchanged or increased T-stage after neoadjuvant therapy, a decrease in T-stage was associated with improved 3-year (50% vs 33%) and 5-year OS rates (31% vs 18%, P < .001) (Figure 3C). Recursive partitioning was used to determine an optimal threshold for tumor size reduction (Supplementary Table 1). In this analysis, a cutoff of ≥47% was identified, and within the neoadjuvant cohort, 64 patients (18%) had a PDAC that decreased in size by ≥47%. Overall survival rates for patients with tumors that shrunk by ≥47% were 67% and 47% at 3 years and 5 years as compared with 32% and 16%, respectively, for patients with tumors that shrunk by <47%, remained the same size, or increased in size (P < .001) (Figure 3D).
To validate the association between improved OS and either decreased T-stage or tumor size reduction by ≥47% after neoadjuvant therapy, a separate cohort of 200 neoadjuvant-treated PDAC patients was analyzed (Supplementary Material). Although patient follow-up for this validation cohort was insufficient to calculate 5-year OS rates, 3-year OS rates were longer for patients who experienced a decrease in T-stage after neoadjuvant therapy as compared with patients whose tumors remained unchanged or increased in T-staging (66% vs 35%, P = .009). Similarly, a reduction in tumor size by ≥47% after neoadjuvant therapy was associated with improved patient 3-year OS as compared with patients with tumors that shrunk by <47%, remained the same size, or increased in size (72% vs 36%, P < .001) (Supplementary Figure 2).
Clinicopathologic Features and Prognostic Significance of Differences in Unidimensional Tumor Size Between Preoperative Endoscopic Ultrasound Imaging and Postoperative Pathology After Neoadjuvant Treatment
By combining both neoadjuvant cohorts, a decrease in T-stage and tumor size reduction by ≥47% after neoadjuvant therapy were identified in 179 (32%) and 109 (19%) PDAC patients, respectively (Table 2). Statistically, patients with tumors that decreased in T-stage or shrunk by ≥47% were more likely to have received a 5-fluorouracil–based regimen and exhibit lower mean posttreatment serum CA19-9 levels (P < .05). Upon pathologic examination, they were found to harbor neoplasms that were smaller in size, lower T-stage, lower histologic grade, less frequent perineural and lymphovascular invasion, lower N-stage, and less often with positive margins (P < .001). Of note, a ≥47% decrease correlated with ≥85% serum CA19-9 response to neoadjuvant treatment (P = .014).
Table 2.
Clinicopathologic Features of 565 Neoadjuvant-Treated PDAC Patients With Respect to Changes in T-Stage and Tumor Size
| Patient/tumor characteristics | Unchanged/increased T-stage (n = 386, 68%), n (%) | Decreased T-stage (n = 179, 32%), n (%) | P value | <47% Tumor size reduction (n = 456, 81%), n (%) | ≥47% Tumor size reduction (n = 109, 19%), n (%) | P value |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | 186 (48) | 93 (52) | .417 | 221 (48) | 58 (53) | .395 |
| Male | 200 (52) | 86 (48) | 235 (52) | 51 (47) | ||
| Mean age (range), y | 65.1 (35–85) | 64.3 (34–85) | .534 | 65.4 (35–85) | 62.5 (34–85) | .006 |
| Location | ||||||
| Head/neck/uncinate | 287 (74) | 150 (84) | .013 | 350 (77) | 87 (80) | .527 |
| Body/tail | 99 (26) | 29 (16) | 106 (23) | 22 (20) | ||
| EUS mean tumor size (range), cm | 2.7 (1.0–5.5) | 3.2 (2.1–8.0) | <.001 | 2.8 (1.0–7.1) | 3.3 (1.4–8.0) | <.001 |
| Primary tumor (cT), by EUS | ||||||
| cT1 | 88 (23) | 0 (0) | <.001 | 74 (16) | 14 (13) | <.001 |
| cT2 | 282 (73) | 144 (80) | 354 (78) | 72 (66) | ||
| cT3 | 16(4) | 35 (20) | 28 (6) | 23 (21) | ||
| Neoadjuvant chemotherapya | ||||||
| Gemcitabine-based | 265 (69) | 103 (58) | .022 | 310 (68) | 58 (53) | .008 |
| 5-Fluorouracil based | 104 (27) | 69 (39) | 126 (28) | 47 (43) | ||
| Both | 17(4) | 7(3) | 20 (4) | 4(4) | ||
| Combined neoadjuvant chemoradiation | 57 (15) | 21 (12) | .361 | 63 (14) | 15 (14) | 1.000 |
| ≥85% CA19-9 responseb | 55 (29) | 38 (40) | .084 | 68 (29) | 25 (48) | .014 |
| Mean pathologic tumor size (range), cm | 3.1 (0.1–11.5) | 1.4 (0.0–4.0) | <.001 | 2.9 (0.8–11.5) | 0.9 (0.0–4.0) | <.001 |
| Primary tumor (pT), by pathology | ||||||
| ypT0 | 0(0) | 8(4) | <.001 | 0(0) | 8(7) | <.001 |
| ypT1 | 58 (15) | 154 (86) | 116 (26) | 96 (88) | ||
| ypT2 | 259 (67) | 17(10) | 271 (59) | 5(5) | ||
| ypT3 | 69 (18) | 0(0) | 69 (15) | 0(0) | ||
| Histologic grade | ||||||
| Well/moderately differentiated | 288 (75) | 151 (84) | .009 | 347 (76) | 92 (84) | .073 |
| Poorly differentiated/undifferentiated | 98 (25) | 28 (16) | 109 (24) | 17(16) | ||
| Perineural invasion | 343 (89) | 117 (65) | <.001 | 409 (90) | 51 (47) | <.001 |
| Lymphovascular invasion | 291 (75) | 89 (50) | <.001 | 336 (74) | 44 (40) | <.001 |
| Regional node (pN), by pathology | ||||||
| ypN0 | 114 (30) | 96 (54) | <.001 | 142 (31) | 68 (62) | <.001 |
| ypN1 | 152 (39) | 57 (32) | 176 (39) | 33 (30) | ||
| ypN2 | 120 (31) | 26 (14) | 138 (30) | 8(7) | ||
| Positive margin(s) | 182 (47) | 55 (31) | <.001 | 211 (46) | 26 (24) | <.001 |
| Received adjuvant therapyb | 264 (69) | 115 (65) | .329 | 313 (70) | 66 (61) | .107 |
CA19-9, carbohydrate antigen 19-9; EUS, endoscopic ultrasound; PDAC, pancreatic ductal adenocarcinoma.
Based on 557 patients (99%) with available adjuvant therapy data.
Based on 284 patients (50%) with available pretreatment and posttreatment CA19-9 serum levels.
Cox regression analysis for OS with respect to various clinicopathologic features is summarized in Table 3. By univariate analysis, shorter OS was associated with age, postoperative pathologic tumor size, ypT3 stage, poor/ undifferentiated histologic grade, perineural and lymphovascular invasion, metastasis in ≥4 regional lymph nodes (ypN2), and positive resection margin(s) (P < .01). In comparison, longer OS was associated with ≥85% CA19-9 response, decreased postoperative T-stage, ≥47% tumor size reduction, and receipt of adjuvant therapy (P < .01). Multivariate analysis was used to determine the prognostic significance of decreased T-stage and ≥47% shrinkage in tumor size after neoadjuvant therapy and included patient age, poor/undifferentiated histologic grade, perineural invasion, lymphovascular invasion, ypN2, positive margin(s), ≥85% CA19-9 response, and adjuvant treatment status. A reduction in tumor size by ≥47% was an independent prognostic factor for improved OS (P = .007), whereas a change in T-stage was not (P = .418) (Supplementary Table 2).
Table 3.
Univariate and Multivariate Cox Regression Analysis of Overall Survival for 565 Neoadjuvant-Treated PDAC Patients
| Patient/tumor characteristics | Univariate analysis |
Multivariate analysis model 1 |
Multivariate analysis model 2 |
|||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Gender, female (ref) vs male | 1.136 (0.924–1.396) | .225 | ||||
| Age, y | 1.016 (1.005–1.027) | .003 | 1.007 (0.995–1.020) | .264 | 1.005 (0.992–1.018) | .455 |
| Location, body/tail (ref) vs head/neck/uncinate | 1.209 (0.942–1.552) | .136 | ||||
| EUS tumor size, cm | 0.941 (0.842–1.052) | .287 | ||||
| EUS T-stage, cT1/cT2 (ref) vs cT3 | 0.675 (0.451–1.010) | .056 | ||||
| Neoadjuvant treatment,a Gem-based (ref) vs 5-FU based | 0.797 (0.627–1.013) | .064 | ||||
| Pathologic tumor size, cm | 1.229 (1.155–1.309) | <.001 | ||||
| Pathologic T-stage, ypT0/ypT1/ypT2 (ref) vs ypT3 | 1.633 (1.168–2.285) | .004 | ||||
| Histologic grade, well/moderate (ref) vs poor/undiff | 1.943 (1.541–2.451) | <.001 | 1.645 (1.216–2.225) | .001 | 1.668 (1.232–2.257) | .001 |
| Perineural invasion, absence (ref) vs presence | 2.238 (1.661–3.015) | <.001 | 1.790 (1.197–2.678) | .005 | 1.530 (1.013–2.310) | .043 |
| Lymphovascular invasion, absence (ref) vs presence | 1.747 (1.383–2.207) | <.001 | 1.134 (0.796–1.617) | .486 | 1.101 (0.774–1.567) | .591 |
| Lymph node metastasis, ypN0/ypN1 (ref) vs ypN2 | 1.764 (1.409–2.207) | <.001 | 1.637 (1.212–2.212) | .001 | 1.661 (1.231–2.242) | .001 |
| Resection margin(s), negative (ref) vs positive | 1.704 (1.385–2.097) | <.001 | 1.414 (1.069–1.871) | .015 | 1.482 (1.119–1.964) | .006 |
| CA19-9 response,b <85% (ref) vs ≥85% | 0.560 (0.412–0.762) | <.001 | 0.630 (0.460–0.864) | .004 | 0.639 (0.466–0.876) | .005 |
| T-stage, unchanged/increase (ref) vs decrease | 0.625 (0.496–0.789) | <.001 | 0.882 (0.651–1.195) | .418 | ||
| Decrease in tumor size, <47% (ref) vs ≥47% | 0.385 (0.280–0.529) | <.001 | 0.554 (0.359–0.853) | .007 | ||
| Adjuvant therapy,c did not receive (ref) vs did not receive | 0.575 (0.461–0.716) | <.001 | 0.397 (0.296–0.532) | <.001 | 0.410 (0.305–0.549) | <.001 |
5-FU, 5-fluorouracil; CI, confidence interval; EUS, endoscopic ultrasound; Gem, gemcitabine; PDAC, pancreatic ductal adenocarcinoma; ref, reference.
Excludes 24 patients (4%) who received both gemcitabine and 5-fluorouracil based neoadjuvant therapy.
Pretreatment and posttreatment serum CA19-9 levels were available for 284 patients (50%).
Adjuvant treatment data were available for 557 patients (99%).
Discussion
Updated guidelines from the National Comprehensive Cancer Network (NCCN) not only suggest neoadjuvant therapy for borderline resectable PDAC patients but also emphasize the consideration for neoadjuvant therapy in patients with resectable PDAC.21 Among resectable PDAC patients, barriers to complete neoadjuvant therapy are limited and include low patient compliance in accepting neoadjuvant therapy and an adverse drug reaction to neoadjuvant therapy. In addition, the NCCN guidelines recommend that patients who received neoadjuvant therapy should consider adjuvant treatment after pancreatectomy. As per the NCCN, “adjuvant treatment options are dependent on the response to neoadjuvant therapy and other clinical considerations.” Thus, determining the responsiveness of PDAC to neoadjuvant therapy can potentially aid in deciding whether the patient should continue with a similar or different adjuvant treatment regimen. Herein we found neoadjuvant downstaging of the primary PDAC based on a decrease in tumor size between preoperative EUS imaging and postoperative pathology correlated with improved patient OS. Through recursive partitioning, a tumor size reduction of 47% or greater was established as an optimal cutoff for median OS. This ≥47% threshold was further validated in a separate cohort of neoadjuvant-treated PDAC patients and identified to be an independent prognostic indicator for improved OS.
There are many advantages to using differences in tumor size as a marker of neoadjuvant response. The standardized approach to the preoperative and postoperative evaluation of PDAC is to report tumor size along the long axis of the neoplasm. In addition, the recently revised AJCC 8th edition T-staging system for the prognostic classification of pancreatic exocrine neoplasms is based on the greatest dimension of the tumor.18 Moreover, the general method of assessing tumor burden for solid neoplasms after therapy, especially for oncologic clinical trials, has been Response Evaluation Criteria in Solid Tumors (RECIST).22,23 The basic principle of RECIST is to determine the change in a measurable primary tumor using a transaxial image such as those obtained by MDCT. However, RECIST assumes that tumors are spherical objects. As shown by Rezai et al24 and Welsh et al,25 PDAC is not spherical in shape, and as a result, RECIST may significantly overestimate tumor burden. Katz et al9 applied RECIST criteria to MDCT images from 129 borderline resectable PDAC patients who received neoadjuvant therapy. The authors concluded that RECIST response did not associate with median OS and appeared to be of little clinical value. In comparison, high-resolution images by EUS are reported to be superior to MDCT in the detection of PDAC.26 Within a prospective, observational cohort study of 80 patients with proven PDAC, the sensitivity of EUS and MDCT for detecting a pancreatic mass was 98% and 86%, respectively.27 Furthermore, for tumors ≤2.0 cm, the sensitivity of MDCT is reported to decrease to between 68% and 77%, whereas the sensitivity of EUS remains at >95%.28 Although previous studies have not evaluated the accuracy of EUS in measuring tumor size, our findings demonstrate that the discrepancy between EUS and pathology was limited to ≤1.0 cm for most cases. Upon applying these measurements to the AJCC T-staging classification system, the Pearson correlation coefficient yielded a moderate association between preoperative EUS imaging and postoperative pathology for treatment-naive PDAC patients. In comparison, a weaker correlation was observed between MDCT and postoperative pathology. Hence, within this study, tumor size measurements based on EUS imaging rather than MDCT were used as a baseline to gauge neoadjuvant response.
Grading of response to treatment by using unidimensional differences in tumor size alone also has its disadvantages. PDAC is tridimensional and ill-defined and has irregular margins. Alternatively, volumetric and functional imaging studies have been proposed as methods of assessing treatment response. However, the preoperative volumetric quantification of tumor size can be difficult and time-consuming and is not widely available.24,29 Functional imaging studies, such as fluorodeoxyglucose positron emission tomographic techniques, can exhibit false-negative and false-positive results.30 For example, hyperglycemia and inflammation of the pancreatic gland itself can lead to reduced fluorodeoxyglucose uptake through competitive inhibition.31,32 False-negative cases may also occur in colloid carcinomas because of tissue hypocellularity and in necrotic neoplasms. In contrast, PDACs in proximity to areas of high physiological uptake such as the ampulla can lead to erroneous measurements.31
In addition, changes in tumor size do not account for reported pathologic features associated with treatment response, such as loss of tumor cells with retention of desmoplastic stroma, acellular mucin pools, and necrosis.33 However, the histologic distinction between desmoplasia and fibrosis as a result of obstructive chronic pancreatitis can be problematic. Similar difficulties exist in determining whether the presence of acellular mucin pools is indicative of treatment response or extrusion from an associated intraductal precursor neoplasm, such as an intraductal papillary mucinous neoplasm or pancreatic intraepithelial neoplasia. It should also be noted that current histologic grading schemes for treated PDAC are subjective for percentage of tumor destruction, residual viable tumor cells, and radiation-induced fibrosis and regressive changes.12,34–37 Moreover, applying these pathologic systems has not consistently demonstrated statistical significance with respect to OS.38
It is worth noting that there are several limitations to our study. Although it represents one of the largest series of neoadjuvant-treated PDAC to be prognostically analyzed for markers of therapeutic response, our study is retrospective in design and may suffer from surgical selection bias. In addition, not all patients were treated with the same neoadjuvant regimen or protocol, and no standardized approach was used to determine operability. Similarly, this study did not control for adjuvant therapy. The treatment of PDAC represents a formidable challenge, and a uniform method of management for all patients or at least a significant subset of patients has yet to be defined. Nevertheless, patients seen at our institution are routinely evaluated within a pancreatic cancer-focused clinic, and therapeutic decisions are based on a multidisciplinary approach to consider opinions from several specialties that include gastroenterology, radiology, oncology, radiation oncology, surgery, pathology, and others.39 Another issue with our study is the inherent operator dependence of EUS imaging and interobserver variability of pathologic evalution.40 To minimize the subjectivity of using both modalities, it would be ideal to use a single assay to determine differences in tumor size before and after treatment. However, repeat EUS imaging is not typically performed once a diagnosis of PDAC has been rendered, and to our knowledge, a comprehensive study evaluating the role of EUS imaging after neoadjuvant therapy has not been reported. Preoperative pathologic evaluation would certainly be the gold standard; however, this is not technically feasible. Therefore, a comparison of preoperative EUS imaging and postoperative pathology currently represents a widely available and generalizable option to assess therapeutic response among neoadjuvant-treated PDAC patients.
In summary, our study demonstrates that changes in unidimensional tumor size can be a useful marker in predicting patient OS after neoadjuvant therapy and pancreatectomy. Through recursive partitioning, an optimal cutoff of ≥47% tumor size reduction achieved the greatest difference in median OS and was validated in a separate cohort of neoadjuvant-treated PDAC patients. In addition, a decrease in tumor size by ≥47% after treatment correlated with several favorable prognostic findings, such as ≥85% serum CA19-9 response, and was an independent prognostic indicator for improved OS. Although additional studies are required, incorporating preoperative EUS and postoperative pathologic tumor size measurements into the standard evaluation of neoadjuvant-treated PDAC patients may guide subsequent management in the adjuvant setting.
Supplementary Material
What You Need to Know.
Background
The assessment of treatment response after neoadjuvant therapy and pancreatectomy for PDAC can be a useful indicator for prognosis and further management. However, several limitations to current grading systems necessitate the identification of additional markers of response.
Findings
Through systematic analysis of preoperative EUS imaging and postoperative pathologic specimens of 565 neoadjuvant-treated PDAC patients, tumor size reduction of ≥47% correlated with improved overall survival and was an independent, positive prognostic factor.
Implications for patient care
Incorporating tumor size differences between preoperative EUS and postoperative pathology into the standard evaluation of PDAC after neoadjuvant therapy and pancreatectomy can potentially improve the prognostic stratification and adjuvant treatment of patients.
Acknowledgments
The authors thank Mrs Lynn Wolkenstein for outstanding administrative assistance.
Funding
Supported in part by grants from National Pancreas Foundation, Sky Foundation, and the Pittsburgh Liver Research Center at the University of Pittsburgh (NIH/NIDDK P30DK120531) (to A. D. Singhi).
Abbreviations used in this paper:
- AJCC
American Joint Committee on Cancer
- CA19-9
carbohydrate 19-9
- EUS
endoscopic ultrasound
- FNA
fine-needle aspiration
- MDCT
multidetector computed tomography
- NCCN
National Comprehensive Cancer Network
- OS
overall survival
- PDAC
pancreatic ductal adenocarcinoma
- UPMC
University of Pittsburgh Medical Center
Footnotes
Conflicts of interest
This author discloses the following: Dr Singhi has received an honorarium from Foundation Medicine, Inc. The remaining authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2020.11.041.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Chawla A, Ferrone CR. Neoadjuvant therapy for resectable pancreatic cancer: an evolving paradigm shift. Front Oncol 2019;9:1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes CA, Chavez MI, Tsai S, et al. Survival of patients with borderline resectable pancreatic cancer who received neoadjuvant therapy and surgery. Surgery 2019;166:277–285. [DOI] [PubMed] [Google Scholar]
- 4.Zhan HX, Xu JW, Wu D, et al. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med 2017;6:1201–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhir M, Malhotra GK, Sohal DPS, et al. Neoadjuvant treatment of pancreatic adenocarcinoma: a systematic review and meta-analysis of 5520 patients. World J Surg Oncol 2017;15:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011–1024. [DOI] [PubMed] [Google Scholar]
- 7.Arvold ND, Niemierko A, Mamon HJ, et al. Pancreatic cancer tumor size on CT scan versus pathologic specimen: implications for radiation treatment planning. Int J Radiat Oncol Biol Phys 2011;80:1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu H, Wild AT, Wang H, et al. Comparison of conventional and 3-dimensional computed tomography against histopathologic examination in determining pancreatic adenocarcinoma tumor size: implications for radiation therapy planning. Radiother Oncol 2012;104:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118:5749–5756. [DOI] [PubMed] [Google Scholar]
- 10.Al Abbas AI, Zenati M, Reiser CJ, et al. Serum CA19-9 response to neoadjuvant therapy predicts tumor size reduction and survival in pancreatic adenocarcinoma. Ann Surg Oncol 2020; 27:2007–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tempero MA, Uchida E, Takasaki H, et al. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res 1987;47:5501–5503. [PubMed] [Google Scholar]
- 12.Katimuthu SN, Serra S, Dhani N, et al. Regression grading in neoadjuvant treated pancreatic cancer: an interobserver study. J Clin Pathol 2017;70:237–243. [DOI] [PubMed] [Google Scholar]
- 13.Kitano M, Yoshida T, Itonaga M, et al. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol 2019;54:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietryga JA, Morgan DE. Imaging preoperatively for pancreatic adenocarcinoma. J Gastrointest Oncol 2015;6:343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho S, Bonasera RJ, Pollack BJ, et al. A single-center experience of endoscopic ultrasonography for enlarged pancreas on computed tomography. Clin Gastroenterol Hepatol 2006; 4:98–103. [DOI] [PubMed] [Google Scholar]
- 16.Klapman JB, Chang KJ, Lee JG, et al. Negative predictive value of endoscopic ultrasound in a large series of patients with a clinical suspicion of pancreatic cancer. Am J Gastroenterol 2005;100:2658–2661. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee D, Katz MH, Foo WC, et al. Prognostic significance of new AJCC tumor stage in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant therapy. Am J Surg Pathol 2017;41:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg 2017;265:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009; 16:1727–1733. [DOI] [PubMed] [Google Scholar]
- 20.Marrelli D, Caruso S, Pedrazzani C, et al. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg 2009;198:333–339. [DOI] [PubMed] [Google Scholar]
- 21.Tempero MA, Malafa MP, Chiorean EG, et al. NCCN clinical practice guidelines in oncology (NCCN guidelines): pancreatic adenocarcinoma, version 1.2020. J Natl Compr Canc Netw 2019;17:202–210.30865919 [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–216. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 24.Rezai P, Mulcahy MF, Tochetto SM, et al. Morphological analysis of pancreatic adenocarcinoma on multidetector row computed tomography: implications for treatment response evaluation. Pancreas 2009;38:799–803. [DOI] [PubMed] [Google Scholar]
- 25.Welsh JL, Bodeker K, Fallon E, et al. Comparison of response evaluation criteria in solid tumors with volumetric measurements for estimation of tumor burden in pancreatic adenocarcinoma and hepatocellular carcinoma. Am J Surg 2012;204:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fusaroli P, Kypraios D, Caletti G, et al. Pancreatico-biliary endoscopic ultrasound: a systematic review of the levels of evidence, performance and outcomes. World J Gastroenterol 2012;18:4243–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeWitt J, Devereaux B, Chriswell M, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med 2004;141:753–763. [DOI] [PubMed] [Google Scholar]
- 28.Volmar KE, Vollmer RT, Jowell PS, et al. Pancreatic FNA in 1000 cases: a comparison of imaging modalities. Gastrointest Endosc 2005;61:854–861. [DOI] [PubMed] [Google Scholar]
- 29.Topkan E, Yavuz AA, Aydin M, et al. Comparison of CT and PET-CT based planning of radiation therapy in locally advanced pancreatic carcinoma. J Exp Clin Cancer Res 2008;27:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jha P, Bijan B. PET/CT for pancreatic malignancy: potential and pitfalls. J Nucl Med Technol 2015;43:92–97. [DOI] [PubMed] [Google Scholar]
- 31.Low G, Panu A, Millo N, et al. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics 2011;31:993–1015. [DOI] [PubMed] [Google Scholar]
- 32.Pery C, Meurette G, Ansquer C, et al. Role and limitations of 18F-FDG positron emission tomography (PET) in the management of patients with pancreatic lesions. Gastroenterol Clin Biol 2010;34:465–474. [DOI] [PubMed] [Google Scholar]
- 33.Kalimuthu SN, Serra S, Dhani N, et al. The spectrum of histopathological changes encountered in pancreatectomy specimens after neoadjuvant chemoradiation, including subtle and less-well-recognised changes. J Clin Pathol 2016; 69:463–471. [DOI] [PubMed] [Google Scholar]
- 34.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 1992;127:1335–1339. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa O, Ohhigashi H, Sasaki Y, et al. [The histopathological effect of preoperative irradiation in adenocarcinoma of the periampullary region]. Nihon Gan Chiryo Gakkai Shi 1988; 23:720–727. [PubMed] [Google Scholar]
- 36.White RR, Xie HB, Gottfried MR, et al. Significance of histological response to preoperative chemoradiotherapy for pancreatic cancer. Ann Surg Oncol 2005;12:214–221. [DOI] [PubMed] [Google Scholar]
- 37.Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer 2012;118:3182–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng JS, Wey J, Chalikonda S, et al. Pathologic tumor response to neoadjuvant therapy in borderline resectable pancreatic cancer. Hepatobiliary Pancreat Dis Int 2019;18:373–378. [DOI] [PubMed] [Google Scholar]
- 39.Schiffman SC, Abberbock S, Winters S, et al. A pancreatic cancer multidisciplinary clinic: insights and outcomes. J Surg Res 2016;202:246–252. [DOI] [PubMed] [Google Scholar]
- 40.Gress F, Schmitt C, Savides T, et al. Interobserver agreement for EUS in the evaluation and diagnosis of submucosal masses. Gastrointest Endosc 2001;53:71–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


