Abstract
Studies assessing the impact of sleep restriction (SR) on blood pressure (BP) are limited by short study length, extreme SR (<4 h/night), and lack of attention to psychological distress as a possible mediator. A community-based cohort was assembled with 237 women (age 34.1±13.5y; BMI 25.4±5.4kg/m2) and a randomized, crossover, intervention study was conducted in 41 women (24 completed: age 30.2±6.5y; BMI 24.3±2.8kg/m2) to determine the causal effect of SR on BP. Sleep was maintained as usual (HS) or reduced by 1.5h/night (SR) for 6wk. In the cohort, associations between sleep and psychosocial factors were evaluated using multivariable models adjusted for demographic and clinical confounders. In the intervention study, in-office BP was measured weekly; ambulatory BP was measured at endpoint. Psychological factors were assessed at baseline and endpoint. Mixed-model analyses with total sleep time (TST, main predictor), week and fraction of time spent in physical activity (covariates), and subject (random effect), were performed. Among the community cohort, higher perceived stress, stressful events and distress and lower resilience were associated with shorter sleep, worse sleep quality, and greater insomnia symptoms (P<0.05). In the intervention, systolic BP increased as TST decreased (TST x week interaction, [coefficient ± standard error] −0.0097±0.0046, P=0.036). Wake ambulatory diastolic (−0.059±0.022, P=0.021) and mean arterial pressure (−0.067±0.023, P=0.018) were higher after SR vs HS. Psychological distress variables were not affected by TST and did not mediate the effects of SR on BP. These results suggest that SR influences CVD risk in women via mechanisms independent of psychological stressors.
INTRODUCTION
Short sleep duration (SSD) is increasingly recognized as a risk factor for the development of cardiovascular disease (CVD). The American Heart Association has highlighted strong evidence from meta-analyses for associations between SSD and hypertension, stroke, coronary heart disease, and type 2 diabetes1. In particular, the relation between sleep duration and hypertension has been well-studied in epidemiological cohorts and it has been noted that SSD may be more strongly associated with hypertension in younger, versus older, individuals, ≥65 y of age, and women compared to men2. Women are especially susceptible to the effects of short sleep on CVD risk. Individuals reporting sleeping <6 h/night had increased risk of noncoronary plaque burden and this was mostly significant in women3. However, results from clinical intervention studies of sleep restriction (SR), where sleep duration is controlled, on blood pressure are conflicting. In one study of total sleep deprivation, systolic and diastolic blood pressure (SBP and DBP) were increased by 13 and 7 mmHg, respectively, after a single night without sleep compared to habitual sleep (HS)4 but in another study of 6 nights of SR (4 h time in bed), blood pressure was not altered relative to an equivalent period of HS under otherwise identical conditions5. Although these studies were conducted under short-term, severe SR, Robertson et al. also found no difference in blood pressure in men randomized to a reduction in sleep of 1.5 h/night for 3 wk compared to those randomized to maintain their HS schedule6. No study to date has been done to assess the longer-term effects of inadequate sleep on ambulatory blood pressure and none have been performed in younger women.
In addition to sleep, psychological factors including perceived stress, life stressors and symptoms of distress (i.e., depression, anxiety) have also been proposed as contributors to allostatic burden leading to an increased risk of CVD. Exposure to psychological stressors has been associated with cardiovascular and cerebrovascular events in patients at high-risk of the disease but have also been discussed as underlying contributors to lifestyle behaviors that may elevate CVD risk (e.g. smoking, excessive drinking, sedentary behavior, poor diet quality, obesity)7. Interestingly, SSD and psychological stress often co-exist. Vgontzas et al. have reported that stress significantly predicted sleep duration in adult men and women8 and lack of sleep increased the risk for psychological stress in a large population of Korean adults9. The odds of being in the high-risk group for psychological stress was 1.5 in those reporting <6 h of sleep/night compared to those reporting 6-9 h/night. Sleep deprivation has also been shown to affect biomarkers of the stress system (e.g., heart rate variability, cortisol), which suggests that increased stress may help to explain the adverse effects of sleep restriction on BP10,11. In general, however, there has been a lack of attention to the bi-directionality of the relation between sleep duration and psychological stress and how their interaction could influence hypertension and CVD risk. Individual differences in reactivity to stressful events exist, and those who possess a phenotype that causes a large change in blood pressure due to exposure to psychological stressors are at increased risk for CVD death12. Inadequate sleep may be an underlying factor that increases psychological stress and distress resulting in a rise in blood pressure.
The goal of the present randomized controlled SR intervention was to examine the impact of mild, long-term inadequate sleep, mimicking real-life conditions of short sleepers, on blood pressure and psychological outcomes in pre-menopausal women. We hypothesized that restricting sleep by 1.5 h/night for 6 wk would increase blood pressure and lead to adverse changes in psychological outcomes, and that psychological changes would be a mediator in the pathway from SR to elevated blood pressure. Given that the effects of diet on blood pressure can be achieved within 1 to 4 wk13, a 6-wk intervention period was considered adequate to exert changes via sleep restriction. Women in this trial were also part of a larger, community-based sample of diverse women across the life span, the American Heart Association Go Red for Women Strategically Focused Research Network community-based study at Columbia University Irving Medical Center (CUIMC), in which we further examined the relation between sleep and psychological factors.
METHODS
Women, age 20-79 years, were enrolled in the community-based study, an ongoing prospective cohort study established as part of the American Heart Association Go Red for Women Strategically Focused Research Network at CUIMC. Women were family members or friends of patients at New York-Presbyterian Hospital or nearby hospitals, living in the neighboring communities, and/or respondents to flyers or online recruitment postings. Bilingual staff members recruited both English- and Spanish-speaking women. The protocol was approved by the CUIMC Institutional Review Board and all women provided informed consent prior to enrolling in the study.
Women enrolled in the randomized, crossover, controlled trial at CUIMC were pre-menopausal women referred from the community-based cohort described above and also separately recruited from the surrounding community. Women from the community-based cohort were provided information about the clinical trial and contacted by the study coordinator if they expressed interest. For the clinical trial, all women were required to have a body mass index (BMI) between 25 and 29.9 kg/m2 or 20-24.9 kg/m2 if they had a first-degree family member with obesity, type 2 diabetes, CVD, or other risk factor for CVD. We chose this BMI range to include women who were at risk of CVD while minimizing the likelihood of current chronic disorders. Women were carefully screened for sleep disorders or depression using standardized questionnaires (low-risk of sleep apnea on the Berlin Sleep Apnea Questionnaire14; Pittsburgh Sleep Quality Index ≤515, Epworth Sleepiness Scale <1216, no sleep disorders17 or significant delayed or advanced sleep phase18, Beck Depression Inventory II score <1319). In addition, all women were required to undergo a 2-wk screening period with actigraphy (GT3X+, Actigraph Corp, Pensacola, FL) to verify adequate sleep duration. To be eligible, women had to sleep, on average, at least 7 h/night with 10/14 nights with sleep duration ≥7 h. The protocol was approved by CUIMC Institutional Review Board and all women provided informed consent prior to their enrollment in the study. This project is registered on clinicaltrials.gov (NCT02835261).
Upon successful screening into the study, women were randomized to either SR or HS for 6 wk. The alternate sleep duration phase was performed after a 6-wk washout period. Target bedtimes and wake times during HS corresponded to average bed and wake times during the screening period. For SR, the goal was to achieve a 1.5 h reduction in total sleep time by delaying bedtimes. Sleep was monitored using wrist actigraphy (Actigraph GT3X+), enhanced by daily sleep diaries, throughout each 6-wk period and verified weekly; adjustments were made to ensure adherence sleep duration targets.
Anthropometric and blood pressure measurements were taken weekly. Blood pressure was measured twice, on the left arm, after a 5-min resting period, in a seated position with legs uncrossed using an automated blood pressure monitor (OnTrak, Spacelabs Healthcare, Snoqualmie, WA). The two measures were averaged for data analyses. In addition, in the last week of each study phase, women were fitted with an ambulatory blood pressure monitor (model 90227, Spacelabs Healthcare) for 24 h. Only endpoint assessments were performed to reduce participant burden. The monitor was set to record every 30 min throughout the day and night. Erroneous or missed readings were repeated after a 2-min interval. Wake and sleep periods were defined based on each participant’s bedtime and wake time in each study phase. A successful wake blood pressure report was defined as having at least 10 successful readings during wake and 5 during night20. Some women (n=7) performed this test outside of the intervention period for SR and those data were not included in the analyses.
Psychological Measures
Psychological measures for both studies were secondary outcomes, which were added after the onset of each study. Participants in the community-based cohort completed a brief battery of 5 questionnaires assessing levels of perceived stress, stressful events, depressive symptoms, and psychological resilience. The Perceived Stress Scale (PSS-4) consists of 4 items assessing the degree to which situations in one’s life are appraised as stressful during the previous month21. The Global Perceived Stress Scale assesses chronic stress experienced throughout the previous year22. A 4-item subset of the 8-item original measure (GPSS-4) was used to assess chronic stress related to work, relationships, caregiving and meeting basic needs. The Life Events Checklist (LEC) addresses 8 stressful events that participants may have experienced after turning age 18 (e.g. divorce, victim of violence or abuse, major financial crisis)23,24. The two-item Patient Health Questionnaire (PHQ-2) is a self-administered version of the Primary Care Evaluation of Mental Disorders (PRIME-MD) and has been used to determine depression levels in the past two weeks25. The 6-item Brief Resilience Scale (BRS) assesses one’s ability to rebound from difficult situations using a 5-point scale26.
Women in the clinical trial completed the Brief Resilience Scale, the Patient Health Questionnaire 2, and the 10-item Perceived Stress Scale27 at baseline (n=22 for SR and 22 for HS) and endpoint (n=23 for HS and 24 for SR) of each study phase. They also completed the Hospital Anxiety and Depression Scale-Anxiety (HADS-A) subscale, which uses seven items to assess levels of anxiety symptoms28.
Statistical Analyses
For the community-based cohort data, psychological variables were used as continuous variables; sleep variables were dichotomized as follows: sleep duration (<7 vs ≥7 h/night)29, sleep quality (Pittsburgh Sleep Quality Index Score >5 vs ≤5)15, insomnia symptoms (Insomnia Severity Index score ≥8 vs <8)30, obstructive sleep apnea (Berlin Questionnaire score “high risk” vs “low risk”)14, and presence of snoring (yes vs no). Multivariable logistic regression models were used to evaluate associations between psychological and sleep variables; age, race/ethnicity, menopausal status, marital status, health insurance, BMI, and history of chronic illness were used as covariates in the model. Data were analyzed using R and SAS version 9.4.
Sleep during each intervention period was characterized using actigraphy data informed from nightly sleep diaries. Total sleep duration was the total amount of time that women spent asleep at night. Sleep onset latency (SOL) is the time taken to fall asleep (difference between time in bed from sleep diary data and actigraphy-assessed sleep [10 min of continuous detected sleep]). Wake after sleep onset (WASO) is the total amount of time, detected by actigraphy, that the participant is awake after the first 10-min period of continuous sleep. Sleep efficiency is the percent of time in bed that is spent sleeping.
Data from the clinical trial were analyzed using linear mixed-model analysis, with sleep (HS vs. SR) as the main predictor, and separately with total sleep time (TST) as the main predictor. Separate analyses were also done with and without adjusting for fraction of time spent in physical activity (activity counts divided by actigraph wear time) as a covariate. Subject was used as a random effect. Blood pressure and psychological variables were treated as continuous outcomes. Phase and week (for in-office BP measurements) were used as covariates. In each case, an initial analysis was performed to detect phase effect, carryover (phase x sleep), and any interaction of sleep x week, whenever data were recorded for multiple weeks. Other than sleep, week, and their interaction, terms that were not significant were removed from the final models. Baseline blood pressure values for phase 1 for one participant were considered outliers (>30% lower than values at week 1 and at baseline of phase 2) and were excluded from the analyses. For both SBP and DBP, partial-eta-squared for sleep (HS vs SR) was 0.02. Additionally, between-group Cohen’s d for 24-hour SBP and DBP were 0.260 and 0.269 respectively. One woman had 5 successful ABPM readings during the waking period. Removing her from our analyses did not change our interpretation and her data were kept in the analyses reported herein. Data reported in the text are from TST predictor analyses adjusted for physical activity. Data for psychological variables were not adjusted for physical activity because we could not include both week and physical activity in the models. Data in tables and figures are unadjusted raw means±SD by phase (HS vs SR). Results of all analyses are presented in Supplemental Table 1.
Mediation analysis was performed with Baron and Kenny’s method31 as well as with other methods. Each blood pressure and psychological outcome pair for which each measure was separately significantly affected by sleep, was considered for potential mediation of the effect of sleep on blood pressure by the psychological outcome. All analyses were done as linear mixed-effect models with subject as a random effect, and fraction of time in physical activity as a covariate. If any other independent variables (such as phase and carryover effects, interactions with covariates) were kept in the analyses reported above, they were also kept as independent variables for the mediation analyses.
In addition to Baron and Kenny’s method for mediation analyses, we have performed a variety of other methods to investigate mediation: Sobel Test, Path Analysis/Structural Equation and Bootstrapping using R. However, none of the psychological factors had a significant mediation effect between sleep and any of the BP measures (Supplemental table 2). Data reported below are from the Baron and Kenny method, as well as direct and indirect effects, proportion of effects mediated, goodness-of-fit chi-square, and p-value from path analysis.
We investigated the missingness pattern of the data, separately for each analysis, and concluded that most data are missing completely at random (MCAR). (That is, the missingness is not associated with any data related to the study.) For MCAR, the observed data are not biased due to missing values, and consequently, we have performed complete case analysis. That is, we have excluded the missing part for each analysis. Data were considered significant at a P-level <0.05 and are reported as raw means±SD. All analyses were done using R version 3.6.0, with packages Ime4, ImerTest, nlme, Pwr, sem and multilevel.
RESULTS
Study Participants
Our community-based cohort (n=237) was diverse, with ~70% of the participants identified as a racial and/or ethnic minority, and the mean age was 34.1±13.5 y (Table 1). A total of 33 pre-menopausal women were enrolled in the clinical trial and 24 completed both phases (Table 2). These women were part of the community-based cohort. Of the 9 women who failed to complete both phases of the study, 6 were excluded by the investigators for failing to adhere to the sleep schedule (SR, n=3; HS, n=3), starting shift work (exclusionary criterion for the study, n=1), becoming a caregiver (n=1) and vaso-vagal response lasting <2 s during a baseline procedure (n=1).
Table 1.
Characteristics of Participants in the Community-based Cohort (n=237)
| Characteristic | n | % | Mean | SD |
|---|---|---|---|---|
| Age, y | 34.1 | 13.5 | ||
| Race/ethnicity | ||||
| White | 122 | 51 | ||
| Minority race | 115 | 49 | ||
| Hispanic | 73 | 31 | ||
| Minority race and/or Hispanic ethnicity | 163 | 69 | ||
| Education less than college | 52 | 22 | ||
| Single/Widowed/Divorced/Separated | 176 | 74 | ||
| Postmenopausal | 55 | 23 | ||
| No health insurance | 70 | 30 | ||
| History of chronic illness | 62 | 26 | ||
| Body Mass Index, kg/m2 | 25.1 | 5.4 | ||
| Sleep Habits | ||||
| Pittsburgh Sleep Quality Index score(range=0-21) | 5.4 | 3.5 | ||
| Poor Pittsburgh Sleep Quality Index score (>5) | 85 | 36 | ||
| Snore (Yes) | 68 | 29 | ||
| Insomnia Severity Index Score (range=0-28) | 6.9 | 5.8 | ||
| Some/Moderate/Severe Insomnia (Insomnia Severity Index Score ≥8) | 88 | 37 | ||
| Obstructive sleep apnea score (Berlin Questionnaire, range=0-3) | 0.7 | 0.8 | ||
| High-risk obstructive sleep apnea (Berlin Questionnaire ≥2) | 37 | 16 | ||
| Sleep Duration (hours/night) | 6.79 | 1.18 | ||
| Short sleep duration (<7 hours/night) | 99 | 42 | ||
| Psychological Factors | ||||
| Perceived Stress Scale-4 | 5.14 | 2.96 | ||
| Global Perceived Stress Scale-4 | 3.84 | 2.37 | ||
| Patient Health Questionnaire-2 | 0.86 | 1.24 | ||
| Brief Resilience Scale | 15.73 | 4.67 | ||
| Life Events Checklist | 1.77 | 1.57 |
Table 2.
Screening characteristics of women enrolled in the clinical trial
| Characteristic | All enrolled (n=33) | Completers (n=24) | Completers ABPM (n=17) |
|---|---|---|---|
| Age, y | 30.1±7.3 | 30.2±6.5 | 30.7±7.4 |
| Race, Black/White/Asian/Other | 9/176/6/1 | 5/13/5/1 | 3/10/3/1 |
| Ethnicity, Hispanic/Non-Hispanic | 4/29 | 3/21 | 3/21 |
| Body mass index, kg/m2 | 24.4±2.4 | 24.3±2.8 | 24.7±2.9 |
| Total sleep time, h | 7.6±0.6 | 7.7±0.5 | 7.6±0.9 |
| Sleep onset latency, min | 4.5±3.0 | 4.7±3.2 | 5.7±5.0 |
| Wake after sleep onset, min | 44.1±15.2 | 41.6±15.1 | 34.9±21.5 |
| Sleep efficiency, % | 90.7±2.7 | 91.2±2.6 | 91.3±4.5 |
| Bedtime, h:min | 23:05±3:20 | 23:39±1:20 | 23:55±1:15 |
| Wake time, h:min | 07:58±0:48 | 07:54±0:52 | 07:28±1:19 |
Data are means±SD.
Sleep Duration
Per protocol, women in the clinical trial had excellent adherence to the sleep duration requirements in both HS and SR (Figure 1). Average sleep duration, measured with actigraphy, over each 6-wk phase was 7.58±0.53 h/night during HS and 6.17±0.52 h/night during SR (P< 0.0001). SOL was shorter during SR relative to HS (SOL: 4.21±4.12 vs 6.15±5.18 min, P=0.016) but there was no difference in WASO or sleep efficiency between phases (WASO: SR: 32.0±18.1 vs HS: 41.7±20.5 min; P=0.17; sleep efficiency: SR: 91.3±3.8% vs HS: 90.6±4.0%, P=0.90).
Figure 1.
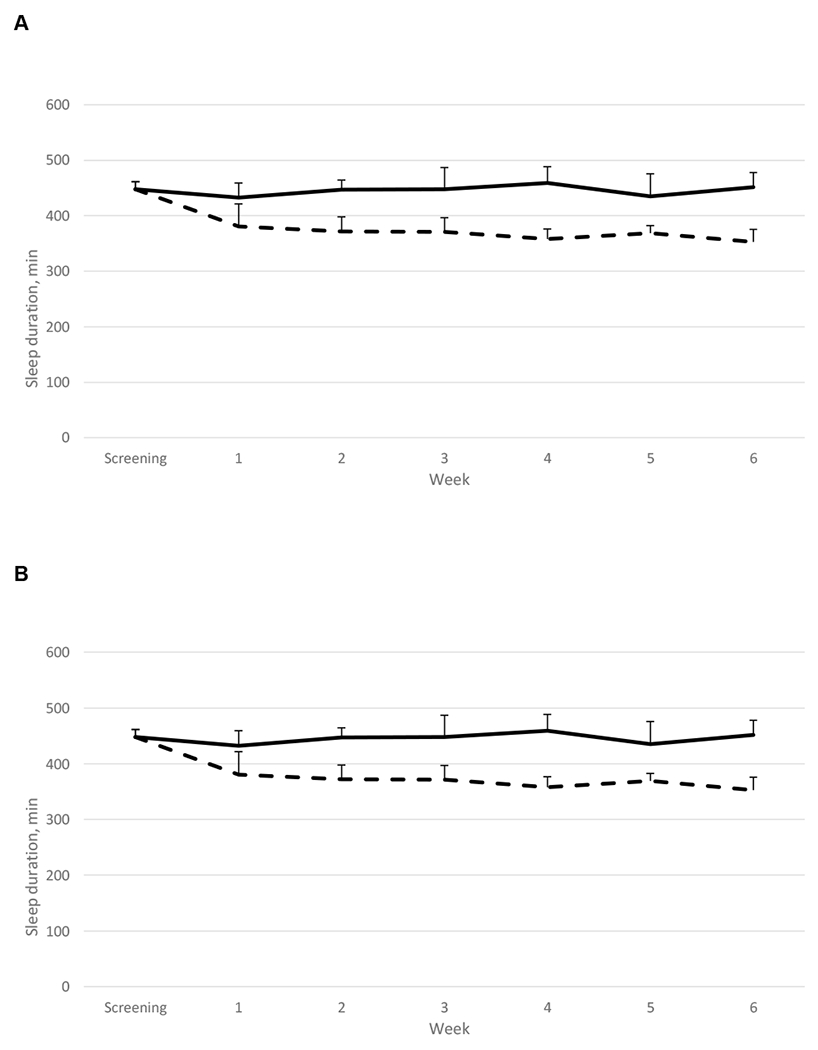
Weekly sleep duration measured by actigraphy during the 6-wk intervention periods (HS, solid line, and SR, hatched line). Panel A represents data from all women who completed the clinical trial (n=24); panel B represents data from women who have provided ambulatory blood pressure measurements (n=17). Week 0 sleep duration represents average sleep duration during the screening period. Data are weekly averages and SD.
Blood Pressure
Blood pressure data from the community-based cohort have been reported previously32. Briefly, higher sleep apnea risk score, assessed by the Berlin questionnaire, was associated with higher DBP after adjustment for confounders (β=2.31, P=0.01).
In the clinical trial, there was a significant sleep x week interaction on SBP (P=0.036), showing an increase over time with SR relative to HS. There was a trend for sleep x week interaction on weekly measures of DBP (P=0.11). (Figure 2, Table 3).
Figure 2.
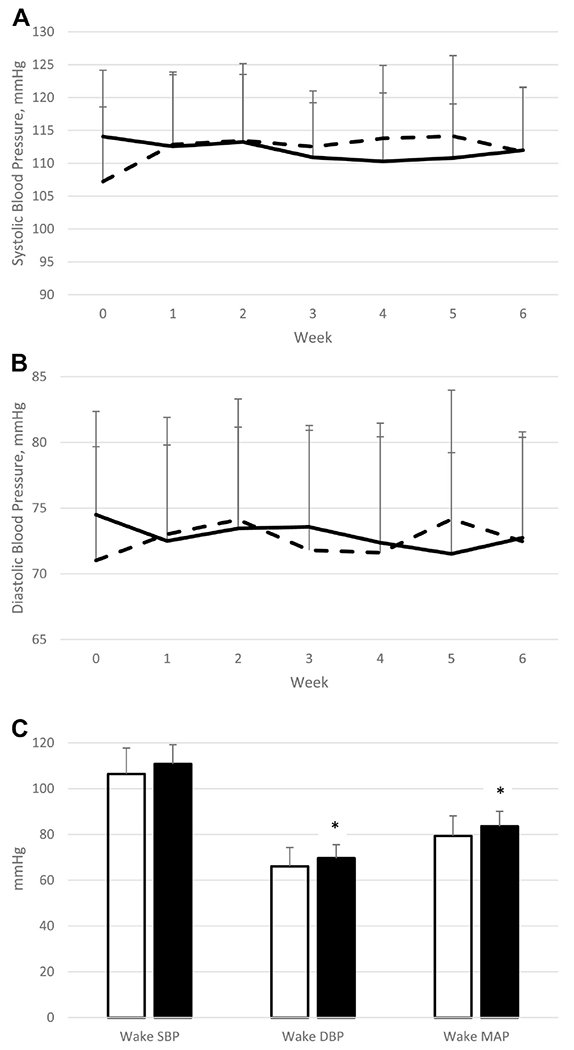
Weekly SBP (panel A) and DBP (panel B) during the 6-wk intervention periods (HS, solid line, and SR, hatched line). Data are unadjusted raw means±SD; n=24. There is a significant sleep x week interaction on SBP (P=0.0048) and trend for main effect of sleep (P=0.061). Twenty-four-hour wake SBP, DBP, and mean arterial pressure after 6 wk of HS (white bars) and SR (black bars) is shown in panel C. Data are unadjusted raw means±SD; n=17 for HS and 14 for SR. * Significantly higher than HS, P<0.05.
Table 3.
Clinic blood pressure and psychological outcomes at baseline and endpoint of each intervention phase during the clinical trial
| Sleep | Week | SBP* | DBP | BRS | HADS | PHQ-2 | PSS-10 |
|---|---|---|---|---|---|---|---|
| Habitual sleep | Baseline | 114.1±10.1 | 74.5±7.9 | 24.0±4.2 | 12.6±6.2 | 0.14±0.47 | 12.5±6.1 |
| Endpoint | 112.0±9.6 | 72.7±7.6 | 24.4±3.5 | 12.6±6.1 | 0.39±0.72 | 11.4±6.5 | |
| Sleep restriction | Baseline | 107.2±11.3 | 71.0±8.7 | 24.1±3.7 | 12.3±6.2 | 0.55±0.91 | 12.9±5.6 |
| Endpoint | 111.7±9.8 | 72.5±8.3 | 23.2±4.9 | 12.8±5.2 | 0.57±1.12 | 14.8±7.2 |
Data are means ± SD, n=24 for SBP and DBP data; sample sizes for psychological variables: n=22 for baseline (both sleep conditions); n=23 (habitual sleep) and 24 (sleep restriction) for endpoint.
Significant sleep x week interaction, P<0.05 (TST adjusted for physical activity).
Twenty-four-hour ambulatory DBP (P=0.024) was higher after 6 wk of SR compared to HS (Figure 2). This was due entirely to higher wake values during SR relative to HS (DBP and mean arterial pressure P=0.021 and 0.018, respectively; SBP P=0.421). No statistically significant difference was observed between SR and HS for sleeping SBP, DBP, and mean arterial pressure. Similarly, SBP and DBP dipping were not significantly different between SR and HS.
Psychological Outcomes in the Community-Based Cohort
The univariate and multivariate associations between psychological outcomes and sleep measures in the community-based cohort are presented in Supplemental Table 3 and in Table 4, respectively. Higher perceived stress (PSS-4) was significantly associated with sleeping <7h/night (OR, 1.21; 95% CI, 1.09-1.34), insomnia score ≥8 (OR, 1.26; 95% CI, 1.13-1.39), and poor sleep quality (PSQI >5) (OR, 1.16; 95% CI, 1.05-1.28), and high-risk for OSA (OR 1.18; 95% CI, 1.04-1.34). Higher chronic stress (GPSS-4) was significantly associated with sleeping <7 h/night (OR, 1.26; 95% CI, 1.11-1.44), insomnia severity index ≥8 (OR, 1.27; 95% CI, 1.12-1.44), and poor sleep quality (OR 1.17; 95% CI, 1.04-1.33). Higher depressive symptoms (PHQ-2) were significantly associated with sleeping <7 h/night (OR, 1.46; 95% CI, 1.14-1.86) and insomnia severity score ≥8 (OR, 1.31; 95% CI, 1.05-1.64). Lower psychological resilience (BRS) was associated with insomnia score ≥8, as well as poor sleep quality (OR, 0.90; 95% CI, 0.85-0.96 and OR, 0.94; 95% CI, 0.89-0.99, respectively). A greater number of stressful life events (LEC) was significantly associated with sleeping <7 h/night (OR, 1.37; 95% CI, 1.11-1.70), insomnia severity index score ≥8 (OR, 1.38; 95% CI, 1.12-1.70), poor sleep quality (OR 1.43; 95% CI, 1.16-1.77), and high risk for sleep apnea (OR 1.35; 95% CI, 1.05-1.74).
Table 4.
Multivariate Associations between Psychological Factors and Sleep in the Community-based Cohort (n=237)
| Sleep measures | Comparisons | PSS-4 | GPSS-4 | PHQ-2 | BRS | LEC | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | P-value | OR | P-value | OR | P-value | OR | P-value | OR | P-value | ||
| Sleep Duration | <7 vs ≥7 h/night | 1.21 | <0.001* | 1.26 | <0.001* | 1.46 | 0.003* | 0.97 | 0.250 | 1.37 | 0.004* |
| Insomnia | ≥8 vs <8 | 1.26 | <0.001* | 1.27 | <0.001* | 1.31 | 0.017* | 0.90 | 0.001* | 1.38 | 0.002* |
| Pittsburgh Sleep Quality Index | >5 vs ≤5 | 1.16 | 0.004* | 1.17 | 0.010* | 1.22 | 0.084 | 0.94 | 0.048* | 1.43 | 0.001* |
| Obstructive Sleep Apnea | High-risk vs low-risk | 1.18 | 0.011* | 1.34 | 0.097 | 1.08 | 0.597 | 0.98 | 0.523 | 1.35 | 0.020* |
| Snore | Yes vs no | 1.03 | 0.538 | 1.07 | 0.291 | 1.07 | 0.599 | 1.03 | 0.423 | 1.14 | 0.208 |
Abbreviations: BRS, Brief Resilience Scale; LEC, Life Events Checklist; GPSS-4, global perceived stress scale; PHQ-2, patient health questionnaire; PSS-4, perceived stress scale.
Models were adjusted for age, race/ethnicity, menopausal status, marital status, health insurance, BMI, and history of chronic illness
P<0.05
Psychological Outcomes in the Clinical Trial
There was no sleep x week interaction or main effect of TST on any of the psychological outcomes (Table 3).
Because there was a sleep x week interaction (P=0.017) on perceived stress (PSS-10) when data were analyzed using HS vs SR, we performed mediation analyses to examine whether perceived stress was a mediator in the sleep-BP outcomes. This was not significant.
DISCUSSION
This study provides, for the first time, solid evidence related to the causality of the short sleep-high blood pressure association. Indeed, this is the longest SR intervention conducted to date and our study shows that sustained, long-term SSD, similar to the sleep duration of ~38% of American adults age 25-44 y33, leads to increased office-based measurements of SBP as well as wake ambulatory DBP and mean arterial pressure. The greater ambulatory blood pressure observed a result of SR is similar to the impact of an 8-wk, 5% weight gain in normal weight, young adults34. The differences in wake ambulatory blood pressure between SR and HS are on par with results of other lifestyle interventions, such as the Dietary Approaches to Stop Hypertension with35 or without exercise36, for blood pressure management.
Increases of approximately 3.5 mmHg in 24-h SBP and DBP and closer to 4 mmHg in 24-h mean arterial pressure as a result of 6 wk of mild SR, leading to total sleep times of ~6 h/night, have meaningful health implications from a population perspective. Indeed, several studies have found that a 10-mmHg increase in SBP in midlife is linearly associated with increased risk of CVD37,38 and dementia39. In participants <50 y of age, a one SD increase in 24-h ambulatory DBP (~8.2 mmHg) was associated with hazard ratios for total mortality, cardiovascular mortality, and all cardiovascular events of 2.05, 4.07, and 1.74, respectively40. A 1 mmHg/y increase in brachial pulse pressure and yearly increase in mean arterial pressure have been associated with 61% and 68%, respectively, increased risk of mortality over 5.8 y of follow-up41.
The lack of effect of SR on blood pressure dipping was unexpected. Cross-sectional studies have linked poor sleep quality to reduced blood pressure dipping in adults42–44 and prior research has shown that repeated exposures to acute SSD of 4 h bedtimes/night for 3 nights followed by one night of 8 h bedtimes, repeated over 4 cycles, significantly attenuated blood pressure dipping in young, healthy men and women45. It is possible that mild reductions in sleep are not sufficient to induce changes in blood pressure dipping in young females. To our knowledge, no other intervention study has been done to assess the impact of SR on 24-h ambulatory blood pressure in adults. However, it is worth noting that non-dipping has not been related to cardiac structure abnormalities whereas wake and sleep SBP and DBP have 20. Therefore, our findings related to wake SBP and DBP are relevant for CVD risk.
Our cohort study also showed that worse scores on measures of psychological functioning were associated with poor sleep. These findings are consistent with previous studies linking psychological stress with inadequate sleep46–48. Resilience, a positive psychosocial factor which reflects the ability to bounce back from stress, was related to sleep quality and insomnia symptoms in our study. Resilience has previously been shown to buffer the negative impact of perceived stress on sleep disturbances49. The relation between sleep and resilience may also be bi-directional, as some studies suggest longer and more restorative sleep may boost levels of resilience50.
Given the association between sleep duration and psychological functioning, the causality of this relation has been understudied. Our clinical trial shows that reduced TST, in young healthy women, does not influence psychological stress and distress. However, assignment of SR, vs HS, was associated with greater stress and lower resilience, consistent with observational findings in our community-based study. Those markers of psychological functioning were not mediators in the SR-blood pressure pathway. The lack of a mediation effect may be due to the fact that participants were restricted on the total amount of sleep but did not have impairments in sleep quality as shown by optimal sleep efficiency and lack of negative effects on SOL and WASO. The body may be unable to maintain normal hypothalamic-pituitary-adrenal functioning in an acute psychological stress situation after a period of poor sleep quality; however, it may be able to retain its capacity to function during extended times of sleep deprivation51. Therefore, the biological mechanism of stress reactivity dysfunction after SR leading to increased blood pressure would not be applicable here and we propose that inadequate sleep duration can increase CVD risk via two separate pathways, one implicating increases in blood pressure and another implicating increases in psychological stress. Our separate analyses showing that objective TST does not influence psychological stress but assignment to the SR phase does, support this notion. The absence of a mediation effect could also be explained by a potential lack of association between self-reported and objective measures of stress. Because we did not assess biomarkers of stress system activation in this study, we cannot test these possible explanations for our findings.
Our clinical trial has a few limitations. First, all women were young and healthy. However, despite youth and general good health, we were able to exert increases in blood pressure from a 1.5 h reduction in sleep maintained for 6 wk. Second, participants were required to maintain this degree of SR daily for 6 wk. In free-living situations, sleeping in on weekends is available and may somewhat compensate for inadequate weekday SSD. However, population studies fail to report this behavior. In a large Swedish cohort of over 38,000 adults, approximately 56.5% remained in the same sleep category on weekdays and weekends; only 3.8% of short sleepers (≤5 h/night) reported medium (7 h) or long (≥9 h) sleep on weekends, a proportion slightly lower than that of weekday short sleepers who maintained short sleep on weekends (4.2%)52. Similarly, data from NHANES 2003-2006 showed that sleep in women from our age group was relatively stable across days of the week, with a difference between shortest and longest objectively-measured sleep duration of 32-47 min53. However, repetitive SR to 4 h sleep/night for 3 nights, followed by catch-up sleep of 8 h/night for one night, over 4 cycles, blunts blood pressure dipping relative to constant adequate sleep45. Other metabolic risk factors have not been shown to improve following weekend recovery sleep in participants subjected to short term severe SR54. Together, data do not suggest that catch-up sleep is an effective strategy to compensate for short-term pronounced inadequate sleep. Third, our participants were forced to restrict their sleep, which may not be truly representative of individuals who voluntarily curtail their sleep. Nonetheless, the end result remains the same: SSD of ~6 h/night maintained for 6 wk has detrimental blood pressure effects. Finally, our study used actigraphy to track nightly sleep duration rather than polysomnography. Polysomnography would have provided additional information on the influence of alterations of various sleep stages on our outcomes. However, repeated polysomnography assessments increase burden on participants. The community study results are limited by the subjectivity of self-reported measures and the prohibition of any inference regarding cause and effect. However, our clinical trial, which informs causality, indicates that SSD induces some degree of psychological distress. In addition, for both studies, psychological measures were added after the study onset. This likely has little impact since some data were missing in only 2 women in the clinical intervention.
Despite those limitations, our study has notable strengths. First, our clinical trial is the longest study to date to induce SR to provide a realistic representation of habitual short sleepers. Prior studies have been short-term, acute SR studies that necessitated in-patient settings and reduced the generalizability of the results to a free-living population. As such, this provides the best available evidence of a causal influence of mild, sustained inadequate sleep duration on CVD risk with objective measures of sleep duration over the entire duration of each intervention phase. Our participants were highly compliant with the sleep protocols for both HS and SR and our retention rate was outstanding. The use of ambulatory blood pressure monitoring is also superior to office measurements in predicting long-term CVD outcomes55. Our study is further strengthened by information from a community-based study of >230 women from diverse backgrounds which corroborate our findings. In addition, this is the only study, to our knowledge, that explores psychological factors as potential mediators of the SSD-blood pressure relation.
Finally, our study, representing the longest SR study to date, and inducing sleep durations representative of the general adult short sleeper, shows for the first time that SSD causes increases in blood pressure that are relevant for cardiovascular health. In normotensive populations, persistent reductions in average blood pressure similar to the differences observed between HS and SR herein, could avoid large numbers of premature deaths and strokes56. This knowledge, and information from this study, justify public health campaigns to promote adequate sleep in short sleepers and funding research to test the cardiovascular health effects of sleep extension in short sleepers.
Supplementary Material
Novelty and Significance.
1). What Is New?
This is the longest intervention study to date to test the impact of short sleep duration on blood pressure in healthy young women. Results from our randomized, controlled crossover study show that reducing sleep by 1.5 h/night for 6 wk leads to higher ambulatory blood pressure and is associated with no adverse changes in psychological functioning.
2). What Is Relevant?
A mild sleep reduction, equivalent to the sleep duration of habitual short sleepers, sustained over time, leads to similar increase in blood pressure as a 5% weight gain. Elevated blood pressure as a result of sustained short sleep duration can increase cardiovascular disease risk independently of stress and anxiety.
Summary:
Sustained mild sleep restriction, in healthy young pre-menopausal women, increases waking blood pressure. Our results contribute further evidence that insufficient sleep is a causal factor in the development of elevated blood pressure.
Acknowledgments
We would like to thank all of the women who have participated in this research as well as the graduate students who have contributed to its implementation.
Sources of Funding
Funding was provided by the American Heart Association Go Red for Women Strategically Focused Research Network 16SFRN27950012 (PI: St-Onge), 16SFRN28060011 (PI: Aggarwal), and 16SFRN28850003 (PI: Spruill). The clinical trial was also supported in part by an NIH Clinical and Translational Science Award (CTSA) through its Center for Advancing Translational Sciences, grant UL1TR001873.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration:Clinicaltrials.gov #NCT02835261 https://clinicaltrials.gov/ct2/show/NCT02835261?recrs=a&cond=sleep&cntry=US&state=US%3ANY&city=New+York&age=1&rank=9
Conflict of Interest
None of the authors have any conflicts of interest to declare. All authors have made substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of the data; drafted or revised the manuscript for important intellectual content; approved the final version and agree to be accountable for all aspects of the work reported herein. MPSO and BA had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.St-Onge MP, Grandner MA, Brown D, et al. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation. 2016;134(18):e367–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q, Xi B, Liu M, Zhang Y, Fu M. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res. 2012;35(10):1012–1018. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez F, Fuster V, Fernandez-Alvira JM, et al. Association of Sleep Duration and Quality With Subclinical Atherosclerosis. Journal of the American College of Cardiology. 2019;73(2): 134–144. [DOI] [PubMed] [Google Scholar]
- 4.Robillard R, Lanfranchi PA, Prince F, Filipini D, Carrier J. Sleep deprivation increases blood pressure in healthy normotensive elderly and attenuates the blood pressure response to orthostatic challenge. Sleep. 2011;34(3):335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Keeffe M, Roberts AL, Kelleman M, Roychoudhury A, St-Onge MP. No effects of short-term sleep restriction, in a controlled feeding setting, on lipid profiles in normal-weight adults. Journal of sleep research. 2013;22(6):717–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson MD, Russell-Jones D, Umpleby AM, Dijk DJ. Effects of three weeks of mild sleep restriction implemented in the home environment on multiple metabolic and endocrine markers in healthy young men. Metabolism: clinical and experimental. 2013;62(2):204–211. [DOI] [PubMed] [Google Scholar]
- 7.Kivimaki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. 2018;15(4):215–229. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Lin HM, Papaliaga M, et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. International journal of obesity. 2008;32(5):801–809. [DOI] [PubMed] [Google Scholar]
- 9.Nam JH, Lim MS, Choi HK, et al. Factors increasing the risk for psychosocial stress among Korean adults living in rural areas: using generalized estimating equations and mixed models. Ann Occup Environ Med. 2017;29:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morales J, Yanez A, Fernandez-Gonzalez L, et al. Stress and autonomic response to sleep deprivation in medical residents: A comparative cross-sectional study. PloS one. 2019;14(4):e0214858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep medicine reviews. 2008;12(3):197–210. [DOI] [PubMed] [Google Scholar]
- 12.Gianaros PJ, Sheu LK, Uyar F, et al. A Brain Phenotype for Stressor-Evoked Blood Pressure Reactivity. J Am Heart Assoc. 2017;6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juraschek SP, Woodward M, Sacks FM, Carey VJ, Miller ER 3rd, Appel LJ. Time Course of Change in Blood Pressure From Sodium Reduction and the DASH Diet. Hypertension. 2017;70(5):923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. [DOI] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 16.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 17.Douglass AB, Bornstein R, Nino-Murcia G, et al. The Sleep Disorders Questionnaire. I: Creation and multivariate structure of SDQ. Sleep. 1994;17(2):160–167. [DOI] [PubMed] [Google Scholar]
- 18.Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74(5):728–738. [DOI] [PubMed] [Google Scholar]
- 19.Arnau RC, Meagher MW, Norris MP, Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2001;20(2):112–119. [DOI] [PubMed] [Google Scholar]
- 20.Bello NA, Jaeger BC, Booth JN 3rd, et al. Associations of awake and asleep blood pressure and blood pressure dipping with abnormalities of cardiac structure: the Coronary Artery Risk Development in Young Adults study. J Hypertens. 2019;37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 22.Payne TJ, Wyatt SB, Mosley TH, et al. Sociocultural methods in the Jackson Heart Study: conceptual and descriptive overview. Ethn Dis. 2005;15(4 Suppl 6):S6-38–48. [PubMed] [Google Scholar]
- 23.Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. [DOI] [PubMed] [Google Scholar]
- 24.Gray MJ, Litz BT, Hsu JL, Lombardo TW. Psychometric properties of the life events checklist. Assessment. 2004;11(4):330–341. [DOI] [PubMed] [Google Scholar]
- 25.Muller-Tasch T, Lowe B, Lossnitzer N, et al. Anxiety and self-care behaviour in patients with chronic systolic heart failure: A multivariate model. Eur J Cardiovasc Nurs. 2017:1474515117722255. [DOI] [PubMed] [Google Scholar]
- 26.Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med. 2008;15(3):194–200. [DOI] [PubMed] [Google Scholar]
- 27.Lee EH. Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res (Korean Soc Nurs Sci). 2012;6(4):121–127. [DOI] [PubMed] [Google Scholar]
- 28.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 29.Watson NF, Badr MS, Belenky G, et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):843–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal B, Makarem N, Shah R, et al. Effects of Inadequate Sleep on Blood Pressure and Endothelial Inflammation in Women: Findings From the American Heart Association Go Red for Women Strategically Focused Research Network. J Am Heart Assoc. 2018;7(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of Healthy Sleep Duration among Adults--United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(6): 137–141. [DOI] [PubMed] [Google Scholar]
- 34.Covassin N, Sert-Kuniyoshi FH, Singh P, et al. Experimental Weight Gain Increases Ambulatory Blood Pressure in Healthy Subjects: Implications of Visceral Fat Accumulation. Mayo Clin Proc. 2018;93(5):618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CJ, Kim JY, Shim E, et al. The Effects of Diet Alone or in Combination with Exercise in Patients with Prehypertension and Hypertension: a Randomized Controlled Trial. Korean Circ J. 2018;48(7):637–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyson CC, Barnhart H, Sapp S, Poon V, Lin PH, Svetkey LP. Ambulatory blood pressure in the dash diet trial: Effects of race and albuminuria. J Clin Hypertens (Greenwich). 2018;20(2):308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacey B, Lewington S, Clarke R, et al. Age-specific association between blood pressure and vascular and non-vascular chronic diseases in 0.5 million adults in China: a prospective cohort study. Lancet Glob Health. 2018;6(6):e641–e649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei YC, George NI, Chang CW, Hicks KA. Assessing Sex Differences in the Risk of Cardiovascular Disease and Mortality per Increment in Systolic Blood Pressure: A Systematic Review and Meta-Analysis of Follow-Up Studies in the United States. PloS one. 2017;12(1):e0170218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGrath ER, Beiser AS, DeCarli C, et al. Blood pressure from mid- to late life and risk of incident dementia. Neurology. 2017;89(24):2447–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Wei FF, Thijs L, et al. Ambulatory hypertension subtypes and 24-hour systolic and diastolic blood pressure as distinct outcome predictors in 8341 untreated people recruited from 12 populations. Circulation. 2014;130(6):466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Protogerou AD, Vlachopoulos C, Thomas F, et al. Longitudinal Changes in Mean and Pulse Pressure, and All-Cause Mortality: Data From 71,629 Untreated Normotensive Individuals. Am J Hypertens. 2017;30(11):1093–1099. [DOI] [PubMed] [Google Scholar]
- 42.Loredo JS, Nelesen R, Ancoli-Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in normal adults. Sleep. 2004;27(6):1097–1103. [DOI] [PubMed] [Google Scholar]
- 43.Sherwood A, Routledge FS, Wohlgemuth WK, Hinderliter AL, Kuhn CM, Blumenthal JA. Blood pressure dipping: ethnicity, sleep quality, and sympathetic nervous system activity. Am J Hypertens. 2011;24(9):982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matthews KA, Kamarck TW, M HH, et al. Blood pressure dipping and sleep disturbance in African-American and Caucasian men and women. Am J Hypertens. 2008;21(7):826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang H, Haack M, Gautam S, Meier-Ewert HK, Mullington JM. Repetitive exposure to shortened sleep leads to blunted sleep-associated blood pressure dipping. J Hypertens. 2017;35(6):1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aanes MM, Hetland J, Pallesen S, Mittelmark MB. Does loneliness mediate the stress-sleep quality relation? The Hordaland Health Study. Int Psychogeriatr. 2011;23(6):994–1002. [DOI] [PubMed] [Google Scholar]
- 47.Alsaggaf MA, Wali SO, Merdad RA, Merdad LA. Sleep quantity, quality, and insomnia symptoms of medical students during clinical years. Relationship with stress and academic performance. Saudi Med J. 2016;37(2):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taouk L, Farrow VA, Schulkin J. Amount and quality of sleep: exploring the role of stress and work experience in a sample of obstetrician-gynecologists. J Psychosom Obstet Gynaecol. 2017:1–6. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, Liu C, Tian X, et al. Associations of Perceived Stress, Resilience and Social Support with Sleep Disturbance Among Community-dwelling Adults. Stress Health. 2016;32(5):578–586. [DOI] [PubMed] [Google Scholar]
- 50.Germain A, Dretsch M. Sleep and Resilience-A Call for Prevention and Intervention. Sleep. 2016;39(5):963–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bassett SM, Lupis SB, Gianferante D, Rohleder N, Wolf JM. Sleep quality but not sleep quantity effects on cortisol responses to acute psychosocial stress. Stress. 2015;18(6):638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akerstedt T, Ghilotti F, Grotta A, et al. Sleep duration and mortality - Does weekend sleep matter? Journal of sleep research. 2019;28(1):e12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urbanek JK, Spira AP, Di J, Leroux A, Crainiceanu C, Zipunnikov V. Epidemiology of objectively measured bedtime and chronotype in US adolescents and adults: NHANES 2003-2006. Chronobiol Int. 2018;35(3):416–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Depner CM, Melanson EL, Eckel RH, et al. Ad libitum Weekend Recovery Sleep Fails to Prevent Metabolic Dysregulation during a Repeating Pattern of Insufficient Sleep and Weekend Recovery Sleep. Curr Biol. 2019;29(6):957–967 e954. [DOI] [PubMed] [Google Scholar]
- 55.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2018;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
- 56.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


