Abstract
Background
Heart failure is associated with high mortality and hospital readmissions. Beta‐adrenergic blocking agents, angiotensin converting enzyme inhibitors (ACEIs), and angiotensin receptor blockers (ARBs) can improve survival and reduce hospital readmissions and are recommended as first‐line therapy in the treatment of heart failure. Evidence has also shown that there is a dose‐dependent relationship of these medications with patient outcomes. Despite this evidence, primary care physicians are reluctant to up‐titrate these medications. New strategies aimed at facilitating this up‐titration are warranted. Nurse‐led titration (NLT) is one such strategy.
Objectives
To assess the effects of NLT of beta‐adrenergic blocking agents, ACEIs, and ARBs in patients with heart failure with reduced ejection fraction (HFrEF) in terms of safety and patient outcomes.
Search methods
We searched the Cochrane Central Register of Controlled Trials in the Cochrane Library (CENTRAL Issue 11 of 12, 19/12/2014), MEDLINE OVID (1946 to November week 3 2014), and EMBASE Classic and EMBASE OVID (1947 to 2014 week 50). We also searched reference lists of relevant primary studies, systematic reviews, clinical trial registries, and unpublished theses sources. We used no language restrictions.
Selection criteria
Randomised controlled trials (RCTs) comparing NLT of beta‐adrenergic blocking agents, ACEIs, and/or ARBs comparing the optimisation of these medications by a nurse to optimisation by another health professional in patients with HFrEF.
Data collection and analysis
Two review authors (AD & JC) independently assessed studies for eligibility and risk of bias. We contacted primary authors if we required additional information. We examined quality of evidence using the GRADE rating tool for RCTs. We analysed extracted data by risk ratio (RR) with 95% confidence interval (CI) for dichotomous data to measure effect sizes of intervention group compared with usual‐care group. Meta‐analyses used the fixed‐effect Mantel‐Haenszel method. We assessed heterogeneity between studies by Chi2 and I2.
Main results
We included seven studies (1684 participants) in the review. One study enrolled participants from a residential care facility, and the other six studies from primary care and outpatient clinics. All‐cause hospital admission data was available in four studies (556 participants). Participants in the NLT group experienced a lower rate of all‐cause hospital admissions (RR 0.80, 95% CI 0.72 to 0.88, high‐quality evidence) and fewer hospital admissions related to heart failure (RR 0.51, 95% CI 0.36 to 0.72, moderate‐quality evidence) compared to the usual‐care group. Six studies (902 participants) examined all‐cause mortality. All‐cause mortality was also lower in the NLT group (RR 0.66, 95% CI 0.48 to 0.92, moderate‐quality evidence) compared to usual care. Approximately 27 deaths could be avoided for every 1000 people receiving NLT of beta‐adrenergic blocking agents, ACEIs, and ARBs. Only three studies (370 participants) reported outcomes on all‐cause and heart failure‐related event‐free survival. Participants in the NLT group were more likely to remain event free compared to participants in the usual‐care group (RR 0.60, 95% CI 0.46 to 0.77, moderate‐quality evidence). Five studies (966 participants) reported on the number of participants reaching target dose of beta‐adrenergic blocking agents. This was also higher in the NLT group compared to usual care (RR 1.99, 95% CI 1.61 to 2.47, low‐quality evidence). However, there was a substantial degree of heterogeneity in this pooled analysis. We rated the risk of bias in these studies as high mainly due to a lack of clarity regarding incomplete outcome data, lack of reporting on adverse events associated with the intervention, and the inability to blind participants and personnel. Participants in the NLT group reached maximal dose of beta‐adrenergic blocking agents in half the time compared with participants in usual care. Two studies reported on adverse events; one of these studies stated there were no adverse events, and the other study found one adverse event but did not specify the type or severity of the adverse event.
Authors' conclusions
Participants in the NLT group experienced fewer hospital admissions for any cause and an increase in survival and number of participants reaching target dose within a shorter time period. However, the quality of evidence regarding the proportion of participants reaching target dose was low and should be interpreted with caution. We found high‐quality evidence supporting NLT as one strategy that may improve the optimisation of beta‐adrenergic blocking agents resulting in a reduction in hospital admissions. Despite evidence of a dose‐dependent relationship of beta‐adrenergic blocking agents, ACEIs, and ARBs with improving outcomes in patients with HFrEF, the translation of this evidence into clinical practice is poor. NLT is one strategy that facilitates the implementation of this evidence into practice.
Keywords: Aged; Aged, 80 and over; Female; Humans; Male; Middle Aged; Practice Patterns, Nurses'; Adrenergic beta‐Antagonists; Adrenergic beta‐Antagonists/administration & dosage; Angiotensin Receptor Antagonists; Angiotensin Receptor Antagonists/administration & dosage; Angiotensin‐Converting Enzyme Inhibitors; Angiotensin‐Converting Enzyme Inhibitors/administration & dosage; Cause of Death; Dose‐Response Relationship, Drug; Drug Monitoring; Drug Monitoring/nursing; Heart Failure; Heart Failure/drug therapy; Heart Failure/mortality; Heart Failure/nursing; Heart Failure/physiopathology; Hospitalization; Hospitalization/statistics & numerical data; Randomized Controlled Trials as Topic; Stroke Volume; Time Factors
Plain language summary
Nurse‐led optimisation of medications in heart failure
Review question
To assess the effects of nurse‐led titration (NLT) of beta‐adrenergic blocking agents, angiotensin converting enzyme inhibitors (ACEIs), and angiotensin receptor blockers (ARBs) in patients with heart failure in terms of safety and patient outcomes.
Background
Heart failure has a high rate of hospitalisations and mortality. Large clinical trials have shown that the presence of beta‐adrenergic blocking agents, ACEIs, and ARBs will improve these outcomes. Also, there is a dose response, so the higher the dose of these medications, the greater the improvement in patient outcomes. However, primary care physicians are often reluctant to up‐titrate these medications. New ways of up‐titration of these medications is needed. Optimisation of these medications can be done by nurse practitioners or advanced practice nurses under medical supervision.
Study characteristics
We conducted a review of seven randomised controlled trials (1684 participants) comparing nurse titration of beta‐adrenergic blocking agents, ACEIs, and ARBs with titration of these medications by a primary care physician. The demographic characteristics of participants within each study were similar. There was an equal number of men and women in four of the studies. The mean age of participants ranged from 59 to 81 years of age. The evidence is current up to December 2014.
Key results
The review found that participants undergoing titration of these medications were less likely to experience a hospital admission or to die, and more participants reached the maximum dose compared to those participants having these medications titrated by their primary care physician. Approximately 27 deaths could be avoided for every 1000 patients undergoing titration of these medications by nurses under medical supervision or nurse practitioners. There was very little reported data on the titration of ACEIs and ARBs. Two studies reported on adverse events; one of these studies stated there were no adverse events, and the other study found one adverse event but did not specify the type or severity of the adverse event.
In conclusion, titration of these medications by nurses under medical supervision or nurse practitioners may improve their up‐titration, which may result in an improvement in patient outcomes.
Quality of the evidence
We rated the quality of evidence regarding the proportion of participants that reached optimal dose of these medications as low. This indicates uncertainty as to whether the number of participants reaching optimal dose of beta‐adrenergic blocking agents was different due to NLT or usual care. We found high‐quality evidence that NLT reduced hospitalisations for any cause compared to usual care. This indicates that we are confident that the reduction in all‐cause hospitalisations was due to NLT, and further research is unlikely to change this finding.
Summary of findings
Summary of findings for the main comparison. Nurse‐led titration versus usual care for people with heart failure with reduced ejection fraction.
| Nurse‐led titration versus usual care for people with heart failure with reduced ejection fraction | ||||||
| Patient or population: people with heart failure with reduced ejection fraction Settings: outpatient clinic, primary care clinic, residential care facility, telephone follow‐up Intervention: Nurse‐led titration versus usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Nurse‐led titration versus usual care | |||||
| All‐cause hospital admissions Follow‐up: median 12 months | Study population | RR 0.80 (0.72 to 0.88) | 560 (4 studies) | ⊕⊕⊕⊕ high | ||
| 763 per 1000 | 610 per 1000 (549 to 671) | |||||
| Moderate | ||||||
| 437 per 1000 | 350 per 1000 (315 to 385) | |||||
| Heart failure‐related hospital admissions Follow‐up: median 12 months | Study population | RR 0.51 (0.36 to 0.72) | 642 (4 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 248 per 1000 | 126 per 1000 (89 to 178) | |||||
| Moderate | ||||||
| 182 per 1000 | 93 per 1000 (66 to 131) | |||||
| All‐cause mortality Follow‐up: median 12 months | Study population | RR 0.66 (0.48 to 0.92) | 902 (6 studies) | ⊕⊕⊕⊝ moderate2,3 | ||
| 166 per 1000 | 110 per 1000 (80 to 153) | |||||
| Moderate | ||||||
| 163 per 1000 | 108 per 1000 (78 to 150) | |||||
| All‐cause event‐free survival Follow‐up: median 12 months | Study population | RR 0.60 (0.46 to 0.77) | 370 (3 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 487 per 1000 | 292 per 1000 (224 to 375) | |||||
| Moderate | ||||||
| 385 per 1000 | 231 per 1000 (177 to 296) | |||||
| Proportion reaching target dose of medications Follow‐up: median 12 months | Study population | RR 1.99 (1.61 to 2.47) | 966 (5 studies) | ⊕⊕⊝⊝ low1,2,3 | ||
| 171 per 1000 | 340 per 1000 (275 to 422) | |||||
| Moderate | ||||||
| 182 per 1000 | 362 per 1000 (293 to 450) | |||||
| *The assumed risk is based on the observed incidence across the pooled control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1,2 I = 68% and P = 0.03 with a high Chi2 in relation to degrees of freedom. 2Two studies had a total sample size of < 25 resulting in wide confidence intervals. 3At least two studies with a high risk of reporting bias.
Background
Heart failure with reduced ejection fraction (HFrEF) is associated with a high morbidity and mortality (Levy 2002; Najafi 2007). It is the most frequent cause of hospitalisation in people aged 65 years or older. Approximately 50% of people with severe heart failure die within five years of diagnosis (Levy 2002; Najafi 2007; Roger 2004). It has been well established that pharmacotherapy involving beta‐adrenergic blocking agents, angiotensin converting enzyme inhibitors (ACEIs), and angiotensin receptor blockers (ARBs) can improve morbidity and survival (CIBIS II Investigators and Committees 2003; Cohn 2001; Dulin 2005; Freemantle 1999; Garg 1995; MERIT‐HF Study Group 1999; Packer 1996). These medications are recommended as first‐line therapy in Australian, National Heart Foundation & CSANZ 2011, and international, McMurray 2012 and Yancy 2013, guidelines for the management of patients with chronic heart failure. Studies have also found that there is a dose‐dependent relationship, with an improvement in left ventricular function as the dose increases (Bristow 1996; Simon 2003; Wikstrand 2002). Despite this evidence, many people diagnosed with heart failure receive suboptimal doses, and so are denied full benefits of therapy (Krum 2001; Phillips 2004). In light of the poor uptake of expert guidelines and the reluctance of primary care physicians to up‐titrate these medications (Phillips 2004), new strategies are required to fill this treatment gap.
Description of the condition
Heart failure is a clinical syndrome that arises due to the heart’s inability to pump an adequate volume of blood around the body to meet the metabolic demand of the tissues either at rest or during exercise (Francis 2001). The inadequate volume of blood is due to poor cardiac filling and/or impaired contractility and emptying. Compensatory mechanisms attempt to increase or maintain cardiac output through increasing blood volume (through redistribution of blood flow, heart rate, cardiac contractility, and cardiac muscle mass) (Francis 2001). After a period of time, despite the compensatory mechanisms, the heart begins to fail and the ability of the myocardium to contract and relax deteriorates, resulting in worsening of heart failure. Heart failure is a potential complication of nearly all types of heart disease (Givertz 2001). Classical symptomatology includes dyspnoea, fatigue, and exercise intolerance because of left ventricular dysfunction (Francis 2001; Laurent‐Bopp 2000).
There are two broad types of heart failure: heart with reduced ejection fraction (HFrEF), and heart failure with preserved ejection function (HFpEF). HFrEF refers to the inability of the ventricle to contract adequately to eject a volume of blood that meets the body’s metabolic demands (National Heart Foundation & CSANZ 2011). This is the most common form of heart failure. People with HFrEF have reduced ejection fraction and may be symptomatic (overt heart failure) or asymptomatic (covert heart failure) (Francis 2001). HFrEF is also often referred to as chronic heart failure, which describes the long‐term inability of the heart to meet metabolic demands. This is opposed to acute heart failure, which refers to exacerbations of chronic heart failure but also includes the initial hospitalisation for the diagnosis of heart failure (Francis 2001). Both forms of heart failure are a debilitating condition with a poor prognosis and are associated with a high hospital readmission and mortality rate.
Many people diagnosed with heart failure may be asymptomatic for a number of years. However, as their condition worsens, they may experience exertional dyspnoea, lethargy, dizziness, palpitations, ascities, peripheral oedema, and/or orthopnoea. Unfortunately, the lifetime risk of developing heart failure at 40 years of age is one in five for both men and women, and at 80 years remains at 20% despite the shorter life expectancy (Lloyd‐Jones 2002).
Description of the intervention
In the outpatient setting, optimisation of ACEIs, beta‐adrenergic blocking agents, and/or ARBs is usually done by cardiologists or the patient's primary care physician. However, this has resulted in prolonged and extracted time delays in patients reaching their optimal dose. This is mainly due to appointments in the cardiologist clinic being quarterly. Also, primary care physicians have been reluctant to up‐titrate these medications (Phillips 2004). Several strategies have been implemented to increase the uptake of evidence‐based pharmacotherapy for the management of HFrEF, in particular the titration of ACEIs, beta‐adrenergic blocking agents, and ARBs. However, this continues to be problematic. Several studies have investigated the utility of nurse‐led titration (NLT) of beta‐adrenergic blocking agents and ACEIs in hospital‐based clinics and reported an increase in utilisation rates of key therapeutic agents and in the number of patients receiving target doses (Gustafsson 2007; Jain 2005; Phillips 2005; Ryder 2003; Stromberg 2003).
How the intervention might work
NLT of ACEIs, beta‐adrenergic blocking agents, and ARBs can occur in a hospital‐based outpatient clinic or in the community when the heart failure nurse visits the patient at home. In the outpatient clinic, the patient attends the clinic to visit the heart failure nurse. The nurse assesses the patient, reviews blood test results, and educates the patient and carer about heart failure. Based on the findings from the clinical assessment and blood test results, the heart failure nurse or nurse practitioner will titrate the beta‐adrenergic blocking agents or ACEI according to a predetermined protocol. Depending on hospital policy, the heart failure nurse may or may not consult with a cardiologist prior to titration of these medications. In the home visit setting, similar processes are undertaken as in the outpatient clinic but the heart failure nurse may or may not consult with a cardiologist over the telephone. Nurses that undertake optimisation of beta‐adrenergic blocking agents, ACEIs, and/or ARBs are advanced practice nurses and employed as a nurse practitioner or senior cardiac nurse. They must have institutional approval to titrate these medications. None of the studies described the training undertaken by the heart failure nurses. In clinical practice, provided the nurses are employed in an advanced practice role, no additional training is required.
Stromberg 2003 investigated the titration of medications in a nurse‐led heart failure clinic. They randomly assigned 106 participants to follow‐up in the nurse‐led heart failure clinic or to usual care, which was follow‐up in primary health care. The intervention consisted of an appointment in the nurse‐led heart failure clinic two to three weeks postdischarge. The clinic was staffed by specially educated and experienced cardiac nurses who were able to make protocol‐led changes to medications. The majority of participants had three to eight visits postdischarge. During each visit the nurse examined the participant to assess their heart failure status and treatment. The participant and their carer received education about heart failure and social support. At 12 months, the intervention group had fewer hospital admissions (33 versus 56, P = 0.047) and deaths (7 versus 20, P = 0.005) compared to the usual‐care group (Stromberg 2003). Another study by Driscoll 2011 examined the effect of NLT of beta‐adrenergic blocking agents in the community. Thirty‐three heart failure home visit nurses recruited 484 consecutive patients diagnosed with HFrEF. In this study, the heart failure nurses visited the participant in their home, assessed the participant for heart failure status and response to treatment, delivered education about heart failure, and provided social support to the participant and their carer (Driscoll 2011). In programs that enabled the heart failure nurse to titrate medications (14 programs and 229 participants), beta‐adrenergic blocking agents were adjusted according to a predetermined protocol. In the usual‐care programs (19 programs and 255 participants), all participants were visited by the heart failure nurse at home, but titration of medications was done by the primary care physician. Driscoll and colleagues (2011) found that patients participating in programs that allowed NLT were more likely to reach target dose (48% versus 36%, P = 0.05) and had lower all‐cause hospitalisations and mortality (hazard ratio 0.58, 95% confidence interval 0.42 to 0.81, P = 0.001) at six months compared to usual‐care group.
The adverse events associated with NLT of ACEIs, beta‐adrenergic blocking agents, and ARBs are primarily due to inappropriate titration of these medications. The main symptoms are dizziness, bradycardia, deterioration in renal function, and abnormal serum electrolyte results. Two studies reported adverse events of NLT of ACEIs, beta‐adrenergic blocking agents, and ARBs.
Why it is important to do this review
The optimisation of beta‐adrenergic blocking agents, ACEIs, and ARBs should result in a further improvement in patient outcomes. Observational studies have found an increase in utilisation rates and titration to optimal doses (Driscoll 2011; Jain 2005; Ryder 2003). However, no meta‐analysis or systematic review of randomised control trials has been done to investigate the utility of NLT to date. This review collated evidence of the benefits of NLT as an effective and safe strategy to ensure that patients receive the optimal benefits of beta‐adrenergic blocking agents, ACEIs, and ARBs through dose titration.
Objectives
We systematically reviewed the evidence from randomised controlled trials (RCTs) of NLT of ACEIs, beta‐adrenergic blocking agents, and ARBs in people with HFrEF for efficacy and risk of hospitalisations and mortality.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs that compared NLT of beta‐adrenergic blocking agents, ACEIs, and/or ARBs with optimisation by another health professional in people with HFrEF. We considered parallel or cross‐over trials. We excluded uncontrolled and non‐randomised studies.
Types of participants
People aged 18 years or older diagnosed with symptomatic HFrEF and prescribed beta‐adrenergic blocking agents, ACEIs, and/or ARBs.
Types of interventions
NLT of ACEIs, beta‐adrenergic blocking agents, and/or ARBs. NLT refers to heart failure nurses or nurse practitioners, or both visiting the patient at home or in an outpatient clinic. The heart failure nurses have been delegated the responsibility for making protocol‐led changes in the dosage of beta‐adrenergic blocking agents, ACEIs, and ARBs. The nurse practitioners are able to titrate the medications as part of their scope of practice.
Comparison: Usual care, in which participants are under the management of a primary care physician who is responsible for titration of ACEIs, ARBs, and/or beta‐adrenergic blocking agents. We also included studies where the participant was under the management of a heart failure nurse who did not alter medication, but analysed these studies as a subgroup.
Types of outcome measures
Primary outcomes
All‐cause hospital admissions
Heart failure‐related hospital admissions
All‐cause mortality
All‐cause event‐free survival
Secondary outcomes
Time to maximum dose
Adverse events associated with titration of ACEIs, beta‐adrenergic blocking agents, and/or ARBs
Proportion reaching target dose of medications
Change in quality‐of‐life scores
Cost‐effectiveness
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for RCTs of nurse‐led titration of beta‐adrenergic blocking agents, ACEIs, and ARBs in people with heart failure:
The Cochrane Central Register of Controlled Trials (CENTRAL, Issue 11 of 12, searched 19 December 2014, results: 120)
MEDLINE (Ovid, 1946 to November week 3 2014, searched 19 December 2014, results: 317)
EMBASE (Ovid, 1947 to 2014 week 50, searched 19 December 2014, results: 277)
We used the Cochrane Highly Sensitive Search Strategy for MEDLINE and an adaptation of it for EMBASE (Lefebvre 2011).
See Appendix 1 for details of the search strategies. We applied no date or language restrictions to any of the searches.
Searching other resources
We searched the following clinical trial registries: WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch) and ClinicalTrials.gov (www.clinicaltrials.gov) (searched 30 July 2015).
We searched full reference lists of all eligible papers and review articles to identify potential papers. We also searched the grey literature for unpublished theses and abstracts. We searched reference lists of heart failure guidelines (national and professional) and other relevant systematic review articles. We used Science Citation Index to forward search citations of key papers. We also contacted primary authors for additional information if required.
Data collection and analysis
Selection of studies
Using predetermined criteria outlined in this protocol, two review authors (AD & JC) independently assessed all titles and abstracts for eligibility. Both review authors are experts in NLT. If the title and abstract contained sufficient information to determine exclusion, the article was rejected.
Where an intervention or study type was not clear from the title and abstract, we obtained the full text of the paper. Two review authors (AD & JC) independently assessed the full text of all eligible papers. We reviewed any potential papers identified from reference lists of eligible papers and personal communication. We documented a list of rejected papers and reasons for rejection. Disagreements were resolved by discussion between the two review authors (AD & JC).
Data extraction and management
Two review authors (AD & JC) independently extracted data from all eligible studies using a predesigned data extraction form. The review authors (AD & JC) are experts in NLT and were not blinded to study authors or journals. Any disagreements were resolved by discussion between the two review authors (AD & JC).
We extracted information about participants (demographic data and severity of heart failure), sample size at baseline and follow‐up, medications (beta‐adrenergic blocking agents, ACEIs, and ARBs), method of titration (outpatient clinic, in‐hospital, community setting), comparison group (no intervention, usual care), length of follow‐up, descriptive statistics and inferential statistics of primary and secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (AD & JC) independently assessed the risk of bias using The Cochrane Collaboration's tool for 'Risk of bias' assessment (Higgins 2011). We assessed each study in terms of: selection bias (systematic differences between groups), performance bias (systematic differences in the care provided apart from the intervention being studied), attrition bias (systematic differences in withdrawals), and detection bias (systematic differences in outcome assessment). We judged the risk of bias in all studies (low risk of bias, high risk of bias, or unclear risk of bias). We presented this in a ‘Risk of bias' summary figure. In the NLT intervention, blinding of heart failure nurses and participants was not feasible and was not evaluated. However, blinding of the outcome assessor was evaluated.
Study quality was not a reason for exclusion from the whole review.
Measures of treatment effect
The measures of treatment effect for continuous variables were standardised mean difference, risk ratios for dichotomous variables with a 95% confidence interval, and hazard ratios for survival data. The number needed to treat (NNT) to benefit was also calculated.
Unit of analysis issues
The review included all RCTs where the participant was the unit of analysis and they were randomised to either the treatment or the control group. The number of observations matched the number of units randomised.
Dealing with missing data
Where data were missing from included studies, the review authors contacted the study authors to request the missing data. This included authors of published RCTs and/or unpublished studies with a published abstract. We contacted two authors of published abstracts (Doyon 2010; Guder 2015). One author provided us with the in‐press article, which has since been published (Guder 2015). We have included this in the review. The other author has not published her results, and we were unable to obtain a copy of her PhD thesis (Doyon 2010). We have excluded this abstract from the review due to the large amount of missing data.
Assessment of heterogeneity
We tested heterogeneity for statistical significance using the Q‐statistics with a 95% confidence interval and forest plots. We calculated the I2 statistic to determine the proportion of variability in the results due to heterogeneity. We performed Chi2 test and considered heterogeneity to be significant if P < 0.1. Where there was significant between‐study heterogeneity, we further interrogated the data for possible explanations.
Assessment of reporting biases
We included seven studies from the literature review in the analysis. Due to the limited number of included studies (less than 10), we did not use funnel plots to determine the risk of reporting bias.
Data synthesis
We pooled the outcome of homogenous studies in a meta‐analysis. We used a fixed‐effect model for the studies. We presented the overall effect sizes in a forest plot. We also performed sensitivity analyses in studies considered at risk of introducing bias; in trials where no intention‐to‐treat analysis was conducted; and in studies with a high rate of missing data and participant attrition.
In heterogeneous studies where I2 > 40%, we used a random‐effects model to determine if the conclusions were different. For time‐to‐event data, hazard ratios and 95% confidence intervals were converted to log rank observed minus expected events and variance of the log rank.
Subgroup analysis and investigation of heterogeneity
No subgroup analysis was undertaken. There were no RCTs where the patient was managed by a heart failure nurse but the titration of medications was done by another health professional.
Sensitivity analysis
We performed sensitivity analysis to examine factors in the included studies that may lead to potential bias. We defined a good quality study as one with a low risk of bias in: adequate allocation concealment, blinding of outcome assessment, and data analysis based on intention‐to‐treat.We did not create funnel plots due to the low number of included studies.
Results
Description of studies
Results of the search
We identified a total of 1016 studies from the literature search. After reviewing the titles, we retrieved 100 abstracts for possible inclusion. Of these, we identified 18 full‐text articles for retrieval. From the full‐text articles we excluded 11 studies. We excluded one additional study late in the review as only the abstract was available, which did not include absolute numbers. We included seven articles in the final analysis.
The PRISMA flow diagram in Figure 1 summarises the selection of RCTs.
1.
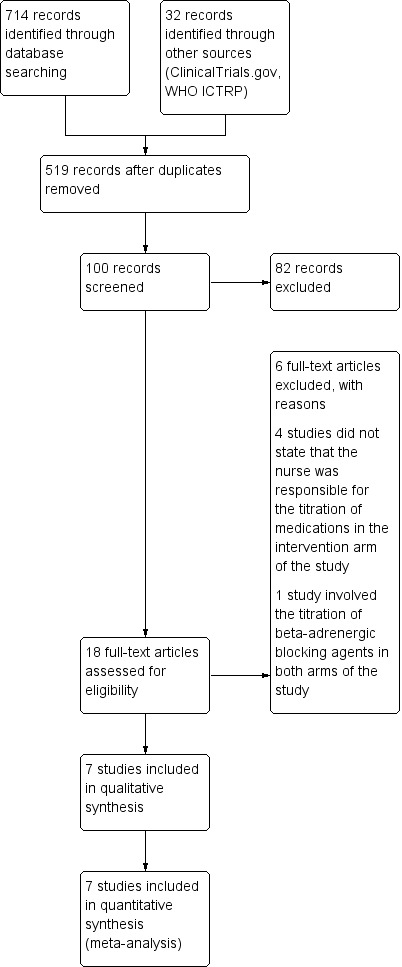
Study flow diagram.
Included studies
We included seven studies (1684 participants) comparing NLT of beta‐adrenergic blocking agents, ACEIs, and/or ARBs with usual care. All of the included studies were RCTs. One study randomised health professionals into three arms comparing education about titration of beta‐blockers, NLT of beta‐blockers, and a computer‐generated alert about potential patients for titration of beta‐blockers (Ansari 2003). The other studies compared two arms of usual care and NLT of medications (Bruggink‐Andre de la Porte 2007; Driscoll 2014; Guder 2015; Hancock 2012; Sisk 2006; Stromberg 2003).
In one study, Doyon 2010, we retrieved information from the abstract. However, we have not included this study in the analysis as the abstract did not contain absolute values, only P values. This abstract was sourced from a conference abstract publication. We contacted the author to ascertain if the full study had been published; to date the original study has not been published, and we were unable to access the thesis.
Definition of heart failure
Inclusion criteria for all of the included studies included a definition of heart failure. However, the definition of heart failure varied between the studies. One study defined HFrEF as a left ventricular ejection fraction (LVEF) less than 45% and meeting the Framingham criteria for heart failure (Ansari 2003). Two studies defined HFrEF as a LVEF less than 40% (Driscoll 2014; Guder 2015). Three studies did not stipulate a numerical limit for LVEF (Hancock 2012; Sisk 2006; Stromberg 2003).
Study setting
The setting and type of nurse‐led medication titration service differed between the studies. Four studies were implemented in an outpatient clinic environment in a tertiary hospital (Bruggink‐Andre de la Porte 2007; Driscoll 2014; Guder 2015; Stromberg 2003). Ansari and colleagues (2003) implemented a NLT clinic in primary care (Ansari 2003). In one study optimisation of medications was done via telephone follow‐up according to a pre‐approved titration protocol (Sisk 2006). Hancock and authors developed a NLT service in a residential care facility (Hancock 2012).
Intervention
All of the included studies involved the optimisation of key medications for the treatment of heart failure. However, there was heterogeneity in the medication titrated. Three studies optimised beta‐adrenergic blockers (Ansari 2003; Driscoll 2014; Sisk 2006), and one of these studies also titrated diuretics and hydralazine (Sisk 2006). Two studies titrated beta‐adrenergic blockers and ACEIs (Hancock 2012; Stromberg 2003). Only two studies titrated all three medications: beta‐adrenergic blockers, ACEIs, and ARBs (Bruggink‐Andre de la Porte 2007; Guder 2015). Bruggink‐Andre De La and colleagues (2007) also titrated spironolactone.
Two studies stipulated specific beta‐adrenergic blockers, ACEIs, or ARBs to be titrated (Ansari 2003; Hancock 2012). Hancock and colleagues (2012) investigated the titration of ramipril and bisoprolol, which they stated was mainly due to cost and ease of titration. In this study a heart failure nurse specialist visited the residential care facility to optimise medications according to a pre‐approved protocol. In another study, carvedilol and metoprolol tartrate were the only medications titrated (Ansari 2003). In this study a nurse practitioner optimised the medications; no pre‐approved protocol was followed.
In two other studies (Driscoll 2014; Sisk 2006), the titration of medication was based on a key therapeutic group such as beta‐adrenergic blocking agents, ACEIs, or ARBs, rather than a specific medication. Optimisation of medications in Sisk 2006 was done by a registered nurse according to a pre‐approved protocol over the telephone. In other studies (Bruggink‐Andre de la Porte 2007; Driscoll 2014; Stromberg 2003), the titration of medications was done by a heart failure nurse specialist in an outpatient setting and according to a pre‐approved protocol.
Outcomes reported
Reported outcomes varied across the seven included studies. The length of follow‐up varied between studies. Two studies had a six‐month follow‐up (Driscoll 2014; Hancock 2012). Four studies had a 12‐month follow‐up (Ansari 2003; Bruggink‐Andre de la Porte 2007; Sisk 2006; Stromberg 2003), and Guder and colleagues (2015) followed up participants for 18 months (Guder 2015).
Five studies reported all‐cause hospitalisation (Ansari 2003; Driscoll 2014; Hancock 2012; Sisk 2006; Stromberg 2003), but of these, only four studies reported heart failure hospitalisation (Ansari 2003; Hancock 2012; Sisk 2006; Stromberg 2003).
All of the included studies reported all‐cause mortality except the study by Guder 2015. Four studies reported the outcome of heart failure‐related hospitalisation (Ansari 2003; Hancock 2012; Sisk 2006; Stromberg 2003). Only three studies reported all‐cause hospitalisation or mortality, or both (Bruggink‐Andre de la Porte 2007; Driscoll 2014; Stromberg 2003).
Regarding titration of beta‐adrenergic blocking agents, ACEIs, and ARBs, six studies reported on the percentage of participants who were prescribed these medications. Of these, three studies focused on only the titration of beta‐adrenergic blocking agents (Ansari 2003; Driscoll 2014; Sisk 2006). Four studies reported the proportion of participants reaching target dose of beta‐adrenergic blocking agents (Ansari 2003; Driscoll 2014; Hancock 2012; Stromberg 2003), with three studies reporting on their time to optimal dose (Ansari 2003; Driscoll 2014; Hancock 2012). One study reported on the number of participants receiving optimal dose of ACEIs (Hancock 2012), and no studies reported on optimal dose of ARBs.
Two studies reported adverse events associated with NLT of beta‐adrenergic blocking agents, ACEIs, and/or ARBs (Driscoll 2014; Hancock 2012). One of these studies stated there were no adverse events (Driscoll 2014), and the other study found one adverse event but did not specify the type or severity of adverse event (Hancock 2012). Only two studies discussed the safety of NLT under the supervision of a cardiologist (Ansari 2003; Driscoll 2014). However, Ansari and colleagues did not report whether they had an adverse event related to the NLT intervention (Ansari 2003). Driscoll and colleagues reported that there were no safety issues related to the NLT intervention (Driscoll 2014).
One study reported on the maximal dose titrated of beta‐adrenergic blocking agents, ACEIs, and ARBs (Bruggink‐Andre de la Porte 2007). However, the authors did not comment on how this figure was calculated and whether it was a mean dose or median dose.
Two studies reported quality‐of‐life scores (Bruggink‐Andre de la Porte 2007; Sisk 2006), and both studies used the Minnesota Living with Heart Failure questionnaire.
No studies reported on the cost‐effectiveness of the NLT intervention.
More details of the included studies are described in Characteristics of included studies.
Excluded studies
We excluded two studies (Lowery 2012; Spaeder 2006). One study was a RCT, however both arms involved titration of beta‐adrenergic blocking agents (Spaeder 2006). The other study had a quasi‐experimental design (Lowery 2012).
Risk of bias in included studies
Figure 2 and Figure 3 display the review authors' judgements about the risk of bias across all of the included studies.
2.
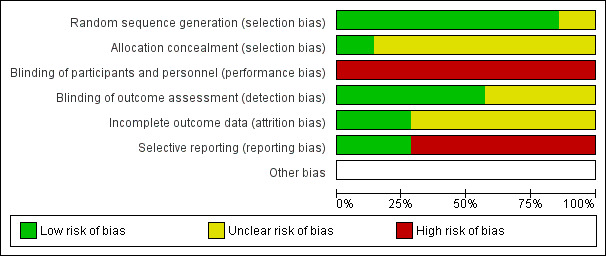
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
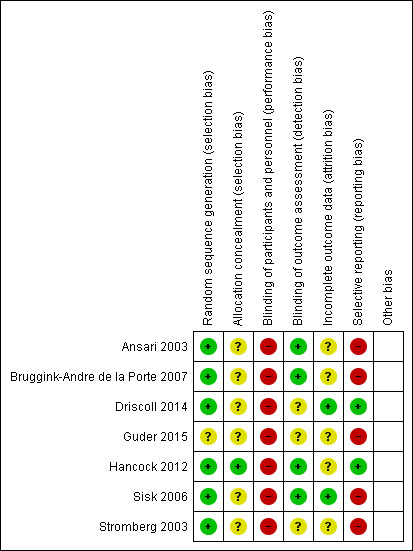
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We judged all of the studies to be at high risk of performance bias due to the inability to blind participants and personnel to group allocation.
Regarding random sequence generation, we judged six studies to be at low risk of bias as they discussed their randomisation process (Ansari 2003; Bruggink‐Andre de la Porte 2007; Driscoll 2014; Hancock 2012; Sisk 2006; Stromberg 2003). All of the studies except Guder 2015 reported on all‐cause hospitalisations or mortality, or both, so there was a low risk of reporting bias for these outcomes. However, only two studies reported on adverse events associated with the intervention, so there was a high risk of reporting bias for this outcome.
Allocation
The risk of bias for allocation concealment was unclear in six studies as there was insufficient information to make a judgement (Ansari 2003; Bruggink‐Andre de la Porte 2007; Driscoll 2014; Guder 2015; Sisk 2006; Stromberg 2003).
Blinding
In all of the studies the risk of bias for blinding of participants and health professionals was high as this was not possible due to the nature of the intervention.
In three studies, the risk of bias of blinding of outcome assessment was unclear as there was insufficient information provided (Driscoll 2014; Guder 2015; Stromberg 2003). The other four studies all provided information about a researcher/cardiologist who was blinded to treatment allocation assessing outcomes (Ansari 2003; Bruggink‐Andre de la Porte 2007; Hancock 2012; Sisk 2006).
Incomplete outcome data
We assessed five studies as at unclear risk of bias in incomplete outcome reporting as no information was provided about incomplete outcome data (Ansari 2003; Driscoll 2014; Guder 2015; Hancock 2012; Stromberg 2003). We assessed two studies as at low risk as the authors discussed the management of missing data (Driscoll 2014; Sisk 2006).
Selective reporting
We assessed two studies as at low risk of bias of selective outcome reporting as all outcomes were reported.
We assessed five studies as at high risk of bias as adverse events associated with the intervention were not reported (Ansari 2003; Bruggink‐Andre de la Porte 2007; Guder 2015; Sisk 2006; Stromberg 2003).
Effects of interventions
See: Table 1
The baseline characteristics of participants enrolled in the usual‐care and NLT groups were comparable in all of the studies (Ansari 2003; Bruggink‐Andre de la Porte 2007; Driscoll 2014; Guder 2015; Hancock 2012; Sisk 2006; Stromberg 2003). A summary of the main results of primary and secondary outcomes is described in the Table 1.
Primary outcomes
All‐cause hospital admissions
Four studies reported on all‐cause hospitalisation (Ansari 2003; Driscoll 2014; Hancock 2012; Sisk 2006). The four studies were homogenous (I2 = 0%), with two of the studies showing wide confidence intervals due to small sample sizes (Driscoll 2014; Hancock 2012). Pooled analysis suggested that participants randomised to the NLT group had a 21% reduction in all‐cause hospitalisation (risk ratio (RR) 0.79, 95% confidence interval (CI) 0.71 to 0.88) (Analysis 1.1). However, these results should be interpreted with caution as the pooled analysis was mainly influenced by one large study (weight 85%), Sisk 2006, due to the small sample sizes in the other studies.
1.1. Analysis.
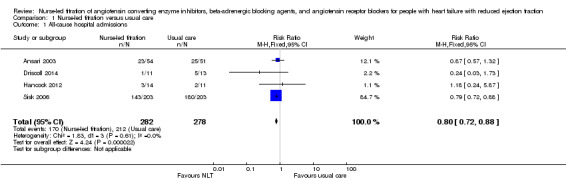
Comparison 1 Nurse‐led titration versus usual care, Outcome 1 All‐cause hospital admissions.
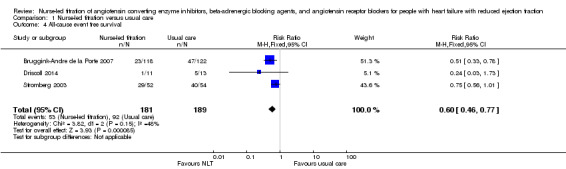
Heart failure‐related hospital admissions
We included four studies in the pooled analysis (Ansari 2003; Hancock 2012; Sisk 2006; Stromberg 2003), which suggested that participants receiving NLT were 39% less likely to experience a heart failure‐related hospital admission (RR 0.51, 95% CI 0.36 to 0.72) (Table 1). One study reported that no participants experienced a heart failure‐related hospital admission (Hancock 2012).
All‐cause mortality
Six of the seven included studies reported on all‐cause mortality. Pooled analysis suggested that participants in the NLT were 34% less likely to die (RR 0.66, 95% CI 0.48 to 0.92) (Analysis 1.3). However, one small study suggested that participants in the NLT group were more likely to die (Driscoll 2014). Driscoll and colleagues (2014) reported one death in the NLT group and none in the usual‐care group. Due to the size of this study (n = 24) and wide confidence interval (0.16 to 18.19), it should be interpreted with caution. As I2 was 15%, we did not consider the level of heterogeneity to be important; also, Chi2 and degrees of freedom were similar despite a P value of 0.31.
1.3. Analysis.
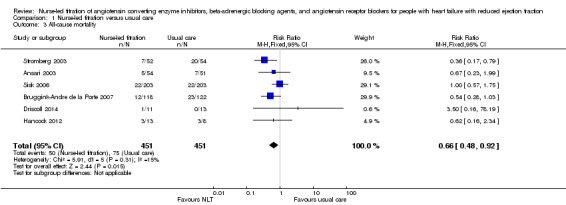
Comparison 1 Nurse‐led titration versus usual care, Outcome 3 All‐cause mortality.
All‐cause event‐free survival
Three studies reported on event‐free survival (Bruggink‐Andre de la Porte 2007; Driscoll 2014; Stromberg 2003). Participants in the NLT group were 40% more likely to remain event‐free compared to participants in usual care (RR 0.60, 95% CI 0.46 to 0.77).
Secondary outcomes
Time to maximum dose
Three studies reported on the time to maximal dose (Ansari 2003; Driscoll 2014; Hancock 2012). However, in one study (Hancock 2012), the time to maximal dose was reported for the NLT group and not for the usual‐care group. In the other two studies, the time to maximal dose was reduced in the NLT group compared to usual care. We did not undertake pooled analysis because in one study no standard deviation was reported (Ansari 2003). Driscoll 2014 was the only study to report the mean and standard deviation for both groups.
Adverse events associated with titration of ACEIs, beta‐adrenergic blocking agents, and/or ARBs
Two of the included studies reported on adverse events associated with NLT of beta‐adrenergic blocking agents, ACEIs, and ARBs (Driscoll 2014; Hancock 2012). One study reported no adverse events (Driscoll 2014). The other study reported an adverse event in the NLT group (Hancock 2012), but they did not specify what type or severity of adverse event.
Proportion reaching target dose of medications
Five studies comparing NLT with usual care reported on the proportion of participants reaching maximal dose of beta‐adrenergic blocking agents (Ansari 2003; Driscoll 2014; Guder 2015; Hancock 2012; Stromberg 2003). Pooled analysis suggested a 99% improvement in participants reaching maximal dose (RR 1.99, 95% CI 1.61 to 2.47) (Analysis 1.5). We used a random‐effects model as there was a substantial degree of heterogeneity in the pooled analysis (I2 = 72%). Also, three of the studies had wide confidence intervals.
1.5. Analysis.
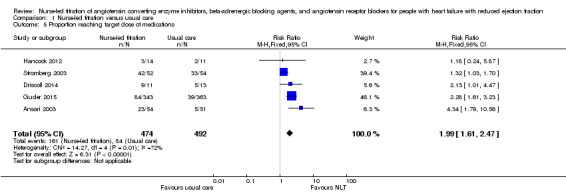
Comparison 1 Nurse‐led titration versus usual care, Outcome 5 Proportion reaching target dose of medications.
We planned a subgroup analysis of the proportion of participants receiving optimal dose of ACEIs and ARBs. One study reported on maximal dose of ACEIs and found that it was achieved in 100% of participants in the NLT group and 75% in the usual‐care group (Hancock 2012). Another study reported on the combined dose of ACEIs/ARBs (Guder 2015), finding the maximal dose of ACEI/ARBs in 39% of participants in the NLT group compared with 9% of participants in the usual‐care group.
Change in quality‐of‐life scores
Three studies reported on quality‐of‐life scores (Bruggink‐Andre de la Porte 2007; Driscoll 2014; Sisk 2006). All studies suggested an improvement in quality of life in participants in the NLT group.
Cost‐effectiveness
No studies reported on the cost‐effectiveness of NLT.
Discussion
Summary of main results
We included seven studies in the review. All of the included studies randomised participants diagnosed with HFrEF to NLT of beta‐adrenergic blocking agents, ACEIs, and/or ARBs or to usual care, which was medication titration by their primary care physician. The meta‐analysis found evidence suggesting that NLT of beta‐adrenergic blocking agents, ACEIs, and/or ARBs may result in a significant reduction in hospital admissions, improvement in survival, increase in number of participants reaching optimal dose, and reduction in time to maximal dose (Table 1). This will have an impact on clinical practice and NLT is one strategy that may be of benefit to patients diagnosed with heart failure.
Fewer participants in the NLT group experienced a hospital admission for any cause (RR 0.80, 95% CI 0.72 to 0.88) or heart failure‐related (RR 0.51, 95% CI 0.36 to 0.72) (Table 1). The meta‐analysis on all‐cause hospitalisation included four studies of 560 participants, and we rated the quality of the evidence as high. There was a good overlap of confidence intervals in each of the studies, with 0% heterogeneity between studies. However, the results should be interpreted with caution, as 85% of the effect was due to a large study of 400 participants (Sisk 2006).
The pooled analysis of heart failure‐related hospitalisations should be interpreted with caution. We downgraded the quality of the evidence for heart failure‐related hospital admissions to moderate due to the overall large effect size and low event rate. We detected no heterogeneity between the studies.
Pooled analysis suggested that participants in the NLT group had a 34% reduction in mortality. If this estimate is correct, approximately 56 deaths could be avoided for every 1000 patients receiving NLT of key therapeutic agents. This was based on the assumed risk minus corresponding risk in the Summary of Findings table, in 902 participants from six studies. All of the studies were homogenous.
Participants in the NLT group experienced fewer hospital admissions and/or deaths compared to participants in the usual‐care group (RR 0.60, 95% CI 0.46 to 0.77). Despite the homogenous nature of the studies, we downgraded the quality of the evidence to moderate due to the large effect size and low event rate.
Participants in the NLT group were more likely to reach target dose of beta‐adrenergic blocking agents (RR 1.99, 95% CI 1.61 to 2.47) and in a shorter period of time compared to the usual‐care group. Pooled analysis of the number of participants reaching maximal dose of beta‐adrenergic blocking agents showed a substantial degree of heterogeneity, so we used a random‐effects model. One study was not statistically significant (Hancock 2012). This study (Hancock 2012) was conducted in a residential care facility. Prescribing guidelines recommend that caution be used when prescribing and titration of beta‐adrenergic blocking agents in the elderly. This may have impacted on the low number of participants reaching maximal dose. The age of participants in this study was higher than the ages of participants in the other three studies (Ansari 2003; Driscoll 2014; Guder 2015) (mean age 81 ± 7.1 years compared to a mean age of 70 years respectively).
We did not undertake a pooled analysis of time to maximal dose due to incomplete data.
Only two studies (Driscoll 2014; Hancock 2012) reported that there were no adverse events related to NLT. The other five studies did not report on adverse events.
Overall completeness and applicability of evidence
We included all eligible RCTs up to 19 December 2014. The demographic characteristics of participants within each study were comparable. Four of the included studies enrolled equal numbers of men and women compared to one study that enrolled 79% women. The mean age of participants ranged from 59 to 81 years, with five of the seven included studies enrolling participants with a mean age of 70 years. The majority of the participants were white. In one study (Sisk 2006), the majority of participants were Hispanic (78%).
Two of the studies had small sample sizes with wide confidence intervals so we could not be confident that there was not a type II error. A type II error occurs when one believes that no relationship exists when in fact there is a relationship. Larger sample sizes would reduce the risk of a type II error.
There was a large degree of heterogeneity in the pooled analysis of participants reaching target dose of beta‐adrenergic blocking agents. In this analysis, one study had a small sample size with a wide confidence interval (RR 1.23, 95% CI 0.29 to 5.25) (Driscoll 2014), and another small study had low event rates and was conducted in a residential care facility (Hancock 2012).
We could not conduct a pooled analysis of time to maximal dose due to incomplete data in two of the three studies. Two studies did report a significant reduction in time to maximal dose in the NLT group compared to the usual‐care group. However, this should be interpreted with caution as one study had a small sample and the other study did not report standard deviations.
Quality of the evidence
The findings of our review are limited by the quality of the included studies. Our review followed a peer‐reviewed published Cochrane protocol and the GRADE methodology to explore the quality of evidence. The initial literature search identified 714 studies, of which only seven were included in the meta‐analysis. We rated our primary outcome of all‐cause hospital admissions as a high quality of evidence. This was mainly due to the small confidence intervals, and further research is unlikely to have an effect on our confidence in the estimate of effect. There was a low quality of evidence in our third secondary outcome of the proportion of participants reaching maximal dose. This was attributable to a high risk of methodological bias, and two of these studies had a total sample size of less than 25 participants, resulting in wide confidence intervals. Further research involving large RCTs is warranted and will have an impact on our confidence in the estimate of effect and is likely to change our estimate.
Potential biases in the review process
We judged all of the included studies to be at high risk of performance bias. Unfortunately, with NLT it is not feasible to blind participants and study personnel to group allocation.
In six of the seven included studies selection bias was unclear as allocation concealment was not reported. We rated reporting bias as high as only two studies reported on adverse events associated with the intervention. We excluded one potential study as we were unable to access their thesis, so it is possible that we excluded an important study. We did not create funnel plots due to the low number of included studies (less than 10).
Agreements and disagreements with other studies or reviews
A systematic review or meta‐analysis on NLT in people diagnosed with HFrEF has not been previously undertaken.
Observational studies investigating NLT of beta‐adrenergic blocking agents, ACEIs, and ARBs have found results similar to this review. Several observational studies reporting on the feasibility of NLT clinics suggest an increase in utilisation and dosage of these key therapeutic medications (Gustafsson 2007; Jain 2005; Phillips 2005; Ryder 2003). One study investigated the feasibility of NLT in the community, such as during a home visit (Driscoll 2011). They found NLT to be a safe and cost‐effective strategy to increase utilisation and dosage in maximising the best benefit‐risk ratio for people with heart failure.
A quasi‐experimental study (Lowery 2012), excluded from our review, reported similar results. They suggested a reduction in all‐cause and heart failure‐related hospital admissions and mortality and an increase in prescribing of beta‐adrenergic blocking agents by a nurse practitioner.
Authors' conclusions
Implications for practice.
This review has suggested that NLT of beta‐adrenergic blocking agents may be effective in reducing hospital admissions, improving survival, increasing the number of participants reaching optimal dose, and reducing the time to optimal dose safely. Despite many of these studies having been published several years ago, the translation of evidence into practice has been poor, with a paucity of NLT clinics being implemented. A meta‐analysis of NLT of beta‐adrenergic blocking agents, ACEIs, and ARBs has not been undertaken previously. It is hoped that the evidence from this review will provide further compelling evidence regarding the efficacy of NLT.
HFrEF is associated with high mortality and hospital readmission, even within the first 12 months of diagnosis, therefore it is of paramount importance that efficacious doses of these medications are reached. Primary care physicians are often reluctant to up‐titrate these medications, resulting in under‐utilisation and patients being denied the full benefits of these medications. The usual‐care component of the RCTs included in this review was titration of medications by participants' primary care physician. The meta‐analysis suggests that NLT was more effective at facilitating the up‐titration of beta‐adrenergic blocking agents than primary care physicians, resulting in improved survival and fewer hospital admissions.
The evidence supporting the efficacy of beta‐adrenergic blocking agents, ACEIs, and ARBs in improving survival and reducing hospital admissions in people diagnosed with HFrEF is overwhelming. However, application of this evidence into practice has been slow. Additional strategies to increase the translation of this evidence into practice is urgently needed. There is a dose‐dependent relationship between beta‐adrenergic blocking agents, ACEIs, and ARBs and patient outcomes. It is vital that the benefit‐risk ratio of these medications is optimised within the most appropriate flexible care system to support it. This review suggests that NLT is one such strategy.
In addition to improving patient outcomes, NLT also facilitates patient and carer education and self management of heart failure at home. During each visit, the heart failure nurse performs a clinical assessment of the patient, reviews all of their medication, provides education about heart failure and strategies to self manage their heart failure at home, and facilitates a rapid clinical review with a cardiologist, if warranted. The review and optimisation of key therapeutic medications is only a small component of each clinical visit, with education about self management being the main focus. These benefits play a vital role in optimising the patient's heart failure and further improvement of outcomes.
Implications for research.
Existing studies in this area are mainly observational. Of the RCTs that we included in the meta‐analysis, two studies had a small sample size, and three had sample sizes that were nearly 10‐fold larger than the small studies. Further research involving high‐quality, large, multi‐centre RCTs is warranted, particularly concerning the titration of ACEIs and ARBs.
In addition, RCTs investigating other strategies aimed at improving the titration of beta‐adrenergic blocking agents, ACEIs, and/or ARBs in people with HFrEF are warranted. The evidence regarding the efficacy of these key therapeutic medications is overwhelming, but the translation into practice is suboptimal.
What's new
| Date | Event | Description |
|---|---|---|
| 19 January 2016 | Amended | Minor corrections made to SOF all‐cause hospital admissions, in discussion and summary of results. |
Acknowledgements
None
Appendices
Appendix 1. Search strategies
CENTRAL
#1MeSH descriptor Ambulatory Care Facilities, this term only #2 (outpatient next (clinic* or department*)) #3MeSH descriptor House Calls, this term only #4 (house call* or home visit*) #5 "nurse specialist clinic*" #6("titration clinic*") #7"nurse‐led clinic*" #8MeSH descriptor Nurse Practitioners, this term only #9(titration) #10(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9) #11MeSH descriptor Heart Failure explode all trees #12((cardi* or heart* or myocard*) near/2 (failure* or incompet* or insufficien* or shock or arrest*)) #13MeSH descriptor Ventricular Dysfunction, Left, this term only #14(ventricular dysfunction) #15(#11 OR #12 OR #13 OR #14) #16MeSH descriptor Adrenergic beta‐Antagonists explode all trees #17(beta near/2 (block* or agent* or antagonist*)) #18(acebutolol) or (atenolol) or (alprenolol) or (betaxolol) or (bupranolol) #19 bisoprolol or (butoxamine) or (carteolol) or (carvedilol) or (celiprolol) #20(co‐tenidone) or (esmolol) or (dihydroalprenolol) or (labetalol) or (levobunolol) #21(metipranolol) or (metroprolol) or (nadolol) or (nebivolol) or (oxprenolol) #22(penbutolol) or (pindolol) or (practolol) or (propranolol) or (sotalol) #23(timolol) or (iodocyanopindolol) or (angilol) or (syprol) or (sectral) #24(tenormin) or (atenix) or (cardicor) or (emcor) or (eucardic) #25(celectol) or (tenoret) or (tenoretic) or (brevibloc) or (trandate) #26(betaloc) or (lopresor) or (corgard) or (nebilet) or (trasicor) #27(visken) or (beta‐cardone) or (sotacor) or (betim) #28(#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27) #29MeSH descriptor Angiotensin‐Converting Enzyme Inhibitors explode all trees #30MeSH descriptor Angiotensin Receptor Antagonists explode all trees #31(candesartan) or (eprosartan) or (irbesartan) or (losartan) or (olmesartan) #32(telmisartan) or (valsartan) #33(#29 OR #30 OR #31 OR #32) #34(#28 OR #33) #35(#10 AND #15 AND #34)
MEDLINE OVID
1 Ambulatory Care Facilities/ 2 (outpatient adj (clinic$ or department$)).tw. 3 House Calls/ 4 (house call$ or home visit$).tw. 5 "nurse specialist clinic$".tw. 6 titration clinic$.tw. 7 "nurse‐led clinic$".tw. 8 Nurse Practitioners/ 9 titration.tw. 10 or/1‐9 11 exp Heart Failure/ 12 ((cardi* or heart* or myocard*) adj2 (failure* or incompet* or insufficien* or shock or arrest*)).tw. 13 Ventricular Dysfunction, Left/ 14 ventricular dysfunction.tw. 15 or/11‐14 16 exp Adrenergic beta‐Antagonists/ 17 (beta adj2 (block* or agent* or antagonist*)).tw. 18 acebutolol.tw. 19 atenolol.tw. 20 alprenolol.tw. 21 betaxolol.tw. 22 bupranolol.tw. 23 bisoprolol.tw. 24 butoxamine.tw. 25 carteolol.tw. 26 carvedilol.tw. 27 celiprolol.tw. 28 co‐tenidone.tw. 29 esmolol.tw. 30 dihydroalprenolol.tw. 31 labetalol.tw. 32 levobunolol.tw. 33 metipranolol.tw. 34 metroprolol.tw. 35 nadolol.tw. 36 nebivolol.tw. 37 oxprenolol.tw. 38 penbutolol.tw. 39 pindolol.tw. 40 practolol.tw. 41 propranolol.tw. 42 sotalol.tw. 43 timolol.tw. 44 iodocyanopindolol.tw. 45 angilol.tw. 46 syprol.tw. 47 sectral.tw. 48 tenormin.tw. 49 atenix.tw. 50 cardicor.tw. 51 emcor.tw. 52 eucardic.tw. 53 celectol.tw. 54 tenoret.tw. 55 tenoretic.tw. 56 brevibloc.tw. 57 trandate.tw. 58 betaloc.tw. 59 lopresor.tw. 60 corgard.tw. 61 nebilet.tw. 62 trasicor.tw. 63 visken.tw. 64 beta‐cardone.tw. 65 sotacor.tw. 66 betim.tw. 67 or/16‐66 68 exp angiotensin‐converting enzyme inhibitors/ or 1‐sarcosine‐8‐isoleucine angiotensin ii/ or captopril/ or cilazapril/ or enalapril/ or enalaprilat/ or fosinopril/ or lisinopril/ or perindopril/ or ramipril/ or saralasin/ or teprotide/ 69 exp Angiotensin Receptor Antagonists/ 70 candesartan.tw. 71 eprosartan.tw. 72 irbesartan.tw. 73 losartan.tw. 74 olmesartan.tw. 75 telmisartan.tw. 76 valsartan.tw. 77 or/69‐76 78 67 or 68 or 77 79 10 and 15 and 78 80 randomized controlled trial.pt. 81 controlled clinical trial.pt. 82 randomized.ab. 83 placebo.ab. 84 drug therapy.fs. 85 randomly.ab. 86 trial.ab. 87 groups.ab. 88 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 89 exp animals/ not humans.sh. 90 88 not 89 91 79 and 90
EMBASE OVID
1 Ambulatory Care Facilities/ 2 (outpatient adj (clinic$ or department$)).tw. 3 House Calls/ 4 (house call$ or home visit$).tw. 5 "nurse specialist clinic$".tw. 6 titration clinic$.tw. 7 "nurse‐led clinic$".tw. 8 Nurse Practitioners/ 9 titration.tw. 10 or/1‐9 11 exp Heart Failure/ 12 ((cardi* or heart* or myocard*) adj2 (failure* or incompet* or insufficien* or shock or arrest*)).tw. 13 Ventricular Dysfunction, Left/ 14 ventricular dysfunction.tw. 15 or/11‐14 16 exp Adrenergic beta‐Antagonists/ 17 (beta adj2 (block* or agent* or antagonist*)).tw. 18 acebutolol.tw. 19 atenolol.tw. 20 alprenolol.tw. 21 betaxolol.tw. 22 bupranolol.tw. 23 bisoprolol.tw. 24 butoxamine.tw. 25 carteolol.tw. 26 carvedilol.tw. 27 celiprolol.tw. 28 co‐tenidone.tw. 29 esmolol.tw. 30 dihydroalprenolol.tw. 31 labetalol.tw. 32 levobunolol.tw. 33 metipranolol.tw. 34 metroprolol.tw. 35 nadolol.tw. 36 nebivolol.tw. 37 oxprenolol.tw. 38 penbutolol.tw. 39 pindolol.tw. 40 practolol.tw. 41 propranolol.tw. 42 sotalol.tw. 43 timolol.tw. 44 iodocyanopindolol.tw. 45 angilol.tw. 46 syprol.tw. 47 sectral.tw. 48 tenormin.tw. 49 atenix.tw. 50 cardicor.tw. 51 emcor.tw. 52 eucardic.tw. 53 celectol.tw. 54 tenoret.tw. 55 tenoretic.tw. 56 brevibloc.tw. 57 trandate.tw. 58 betaloc.tw. 59 lopresor.tw. 60 corgard.tw. 61 nebilet.tw. 62 trasicor.tw. 63 visken.tw. 64 beta‐cardone.tw. 65 sotacor.tw. 66 betim.tw. 67 or/16‐66 68 exp dipeptidyl carboxypeptidase inhibitor/ 69 exp Angiotensin Receptor Antagonists/ 70 candesartan.tw. 71 eprosartan.tw. 72 irbesartan.tw. 73 losartan.tw. 74 olmesartan.tw. 75 telmisartan.tw. 76 valsartan.tw. 77 or/69‐76 78 67 or 68 or 77 79 10 and 15 and 78 80 random$.tw. 81 factorial$.tw. 82 crossover$.tw. 83 cross over$.tw. 84 cross‐over$.tw. 85 placebo$.tw. 86 (doubl$ adj blind$).tw. 87 (singl$ adj blind$).tw. 88 assign$.tw. 89 allocat$.tw. 90 volunteer$.tw. 91 crossover procedure/ 92 double blind procedure/ 93 randomized controlled trial/ 94 single blind procedure/ 95 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 96 (animal/ or nonhuman/) not human/ 97 95 not 96 98 79 and 97
Data and analyses
Comparison 1. Nurse‐led titration versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause hospital admissions | 4 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.72, 0.88] |
| 2 Heart failure‐related hospital admissions | 4 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.36, 0.72] |
| 3 All‐cause mortality | 6 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.48, 0.92] |
| 4 All‐cause event free survival | 3 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.46, 0.77] |
| 5 Proportion reaching target dose of medications | 5 | 966 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.61, 2.47] |
1.2. Analysis.
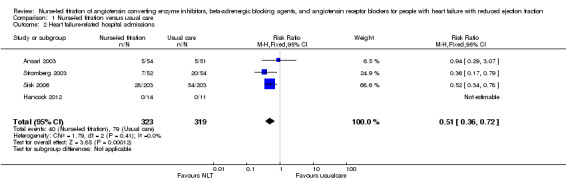
Comparison 1 Nurse‐led titration versus usual care, Outcome 2 Heart failure‐related hospital admissions.
1.4. Analysis.
Comparison 1 Nurse‐led titration versus usual care, Outcome 4 All‐cause event free survival.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ansari 2003.
| Methods | Randomised controlled trial | |
| Participants | 74 health professionals recruited 169 patients diagnosed with heart failure that met the Framingham criteria and a LVEF ≤ 45% or moderate or severe left ventricular systolic dysfunction on their "latest evaluation" | |
| Interventions | Health professionals were randomised to 1 of 3 groups Group 1: Health professionals were provided with education on the initiation and up‐titration of beta‐adrenergic blocking agents Group 2: Nurse facilitator group: The study nurse practitioner, supervised by 2 cardiologists, was responsible for initiating, titration, and stabilising heart failure patients on beta‐adrenergic blocking agents. Once the patient reached maximum tolerated dose of beta‐adrenergic blocking agents, they were referred back to the primary care physician Group 3: Provider and patient notification: Health professionals were given a list of their patients who were potential candidates for beta‐adrenergic blocking agents. Computer alerts were activated when the provider accessed their patient's electronic medical record for the first 2 visits post‐randomisation. All patients in this group were mailed a letter about beta‐adrenergic blocking agents for them to discuss with their health professional at their next visit |
|
| Outcomes | Primary outcome: Number of patients initiated, up‐titrated, and maintained on beta‐adrenergic blocking agents Secondary outcome: Proportion of patients reaching target doses of beta‐adrenergic blocking agents |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A stratified randomisation using computer‐generated, random numbers" Comment: Randomisation occurred at the health professional level |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: All patients and health professionals were aware of the group allocation. There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent research assistant assessed the use of beta‐blocker therapy" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: There was no report about incomplete outcome data |
| Selective reporting (reporting bias) | High risk | Comment: All outcomes except adverse events associated with the intervention were reported |
Bruggink‐Andre de la Porte 2007.
| Methods | Parallel‐group randomised controlled trial | |
| Participants | 240 people diagnosed with heart failure and NYHA class lll‐lV. Diagnosis was based on symptoms and echocardiographic or radionuclide ventriculography tests. LVEF ≤ 45% | |
| Interventions | Group 1: Control group comprised of usual care and follow‐up with a cardiologist Group 2: The intervention was comprised of an intensive follow‐up for 12 months at an outpatient clinic led by a cardiologist and cardiovascular nurse. Participants' first visit was in week 1 postdischarge or referral from an outpatient clinic. At the first and second visit to the heart failure clinic, the participant was provided with education about heart failure, fluid management, early warning signs of heart failure and when to call for medical assistance, exercise, medication, importance of adherence, and possible adverse events. All participants saw a dietician who provided information about a low‐salt diet, fluid restriction, and weight reduction. At each clinic visit, the nurse performed a physical assessment, reviewed laboratory results, and proposed a treatment plan to the physician. The physician then reviewed the participant in conjunction with the nurse's assessment. At subsequent follow‐up visits at weeks 5 and 7 and months 3, 6, 9, and 12, participants were assessed by the nurse and education was reinforced. At 6 of the 9 visits the physician also assessed the participant and optimised their medical management in conjunction with the nurse |
|
| Outcomes | Primary endpoint: composite of incidence of hospitalisation for worsening heart failure and/or all‐cause mortality Secondary endpoints: effect on LVEF, NYHA class, quality of life, NT‐proBNP, time to death, utilisation of heart failure medications and self care behaviour |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomised by computer‐generated allocation" Comment: Randomisation occurred at the level of the participant |
| Allocation concealment (selection bias) | Unclear risk | Comment: The concealment of group allocation was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: There was no blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An external clinical endpoint committee consisting of three experienced cardiologists and blinded to the allocation status of the patient, judged all causes of hospitalisation and death" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Incomplete outcome data was not reported |
| Selective reporting (reporting bias) | High risk | Comment: All outcomes except adverse events associated with the intervention were reported |
Driscoll 2014.
| Methods | Randomised controlled trial | |
| Participants | 28 stable CHF patients either with beta‐adrenergic blocking agent therapy newly initiated or with current beta‐adrenergic blocking agent therapy at less than half the recommended target dose Impaired left ventricular systolic dysfunction as documented by gated blood pool scanning or echocardiography within 6 months of enrolment into the study |
|
| Interventions | Group 1: Usual care: Participants were referred to their primary physician for titration of beta‐adrenergic blocking agents. Participants randomised to the usual‐care group underwent assessment by a cardiologist at the heart failure clinic. Information outlining beta‐adrenergic blocking agent up‐titration was communicated in writing to both the participant and the primary care physician. The participants were not reviewed again in the heart failure clinic until their scheduled cardiologist visits at both 3 and 6 months after randomisation Group 2: Nurse‐led titration: Participants in the intervention group were reviewed by the heart failure nurse in the clinic weekly, fortnightly, or monthly until they reached the maximum‐possible dose of beta‐adrenergic blocking agents and had attended for the 6‐month intervention period. At each visit the heart failure nurse undertook a clinical examination of the participant; determined appropriate medication changes, tests and referrals; and educated the participant concerning medication changes. The referring cardiologist also reviewed the participant and approved proposed changes and completed medication prescriptions and referral forms. Each participant received a printed list of current medications including the new titrated dose of medications. It is important to note that, whilst the titration clinic was run by the heart failure nurse, a cardiologist was available to briefly see each participant and, especially in participants who had significant comorbidities and up‐titration difficulties, guide the nurse in the up‐titration process |
|
| Outcomes | Primary endpoint was the difference in time taken to reach the optimal tolerated dose of beta‐adrenergic blocking agent Secondary endpoints were the likelihood of reaching maximal dose of beta‐adrenergic blocking agents by 6 months and the mean dose of beta‐adrenergic blocking agent at 6 months after entering the study. Tertiary endpoints of interest were all‐cause and heart failure hospital admissions, all‐cause and heart failure emergency department attendances, changes in general quality of life, and depression score |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was according to computer generated random numbers held in opaque, sealed envelopes by a third party" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...random numbers held in opaque, sealed envelopes by a third party" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants and nurses were aware of group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Blindng of outcomes assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Incomplete outcome data were not reported |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes were reported |
Guder 2015.
| Methods | Randomised controlled trial | |
| Participants | 706 people with systolic heart failure | |
| Interventions | Group 1: Telephone and nurse‐led intervention (HeartNetCare) (343 participants). As inpatients, participants were educated by a heart failure specialist nurse in self management of blood pressure, heart rate and rhythm, weight, and recognition of worsening signs and symptoms of heart failure. Telephone follow‐up calls commenced in week 1 postdischarge and occurred weekly for the first month Group 2: Usual care (363 participants). Standard follow‐up by primary care physican All participants were followed up for 18 months |
|
| Outcomes | Type and dosage of heart failure medication (beta‐adrenergic blocking agent, ACEI, ARB, and MRA), LVEF as determined on echocardiography, NYHA class, and quality of life | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: All participants and health professionals were aware of the group allocation. There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Blinding of outcomes was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: There was no report about incomplete outcome data |
| Selective reporting (reporting bias) | High risk | Comment: All outcomes except adverse events associated with the intervention were reported |
Hancock 2012.
| Methods | Randomised controlled trial | |
| Participants | A total of 28 residents from 33 long‐term aged care facilities and diagnosed with left ventricular systolic dysfunction | |
| Interventions | Group 1: Usual‐care group were referred to their primary care physician. The team cardiologist sent a letter to the primary care physician outlining the participant's management plan Group 2: Intervention group consisted of an initial visit with a cardiologist who implemented a management plan. The heart failure nurse then followed up the participant at the aged care facility once or twice a week. The heart failure nurse implemented the management plan including blood tests, clinical assessment, patient and carer education, and titration of medication All participants were followed up for 6 months |
|
| Outcomes | Primary outcome: proportion of participants receiving optimum dose of ACEIs and beta‐adrenergic blocking agents at 6 months Secondary outcomes: percentage of participants prescribed ACEI or beta‐adrenergic blocking agents or both, heart failure‐related mortality, heart failure‐related hospitalisation, and changes in functional capacity and quality of life |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomisation used stratified blocks according to NYHA classification" Quote: "Randomisation occurred patient level." |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation was concealed" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: All participants and health professionals were aware of the group allocation. There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...a blinded assessor reviewed medical notes for changes in prescribing and heart failure events" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: There was no report about incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes were reported |
Sisk 2006.
| Methods | Randomised controlled trial | |
| Participants | 406 people with documented left ventricular systolic dysfunction | |
| Interventions | Group 1: Usual care: Participants received information about how to manage their heart failure Group 2: Intervention group: The first visit with the heart failure nurse consisted of education about heart failure, self management strategies, lifestyle modifications, and medication adherence. All participants received printed information about heart failure. Nurses organised initiation and titration of medications. Subsequent visits were comprised of telephone follow‐up every 3 months for 12 months |
|
| Outcomes | Primary endpoint: all‐cause hospitalisation Secondary endpoints: emergency department presentations, heart failure hospitalisations, and medications prescribed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated, random number sequence without blocking or stratification to centrally determine randomisation assignments" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...concealed treatment group assignments in sealed, opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants and nurses were aware of group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...interviewers who were blinded to treatment assignments asked patients about hospitalisations at nonparticipating hospitals" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "...we conducted tests for missing data bias suggested by Hogan and colleagues and these tests gave little evidence of informative missingness. We also used linear mixed models, which are robust to data missing at random, to estimate treatment effectiveness" |
| Selective reporting (reporting bias) | High risk | Comment: All outcomes except adverse events associated with the intervention were reported |
Stromberg 2003.
| Methods | Randomised controlled trial | |
| Participants | 106 people diagnosed with heart failure based on symptoms or diagnostic tests | |
| Interventions | Group 1: Usual care: Participants were followed up by their primary physician. Group 2: Intervention group: All participants in this group were followed up in a nurse‐led heart failure outpatient clinic 2 to 3 weeks postdischarge from a heart failure hospital admission. The clinic was staffed by heart failure nurse specialists who were responsible for making protocol‐led changes in medication. All visits consisted of: education about heart failure, advanced patient assessment, titration of medication according to a predetermined protocol, lifestyle modifications, and self management strategies |
|
| Outcomes | Primary endpoint: composite of all‐cause mortality and/or all‐cause hospital admission at 12 months Secondary endpoint: mortality, number of all‐cause hospital readmissions, and self care behaviour |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was blinded with the use of a computer‐generated list of random numbers" Comment: Randomisation was at the participant level |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...random numbers and sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Incomplete outcome data were not reported |
| Selective reporting (reporting bias) | High risk | Comment: All outcomes except adverse events associated with the intervention were reported |
ACEI: angiotensin converting enzyme inhibitor ARB: angiotensin receptor blocker CHF: congestive heart failure LVEF: left ventricular ejection fraction MRA: mineralocorticoid receptor antagonist NT‐proBNP: N‐terminal pro‐brain natriuretic peptide NYHA: New York Heart Association
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blue 2001 | There was no outcome data about the titration of medications by the heart failure nurses |
| Doyon 2010 | We were unable to obtain a copy of the dissertation, and the results had not been published in an article |
| Kasper 2002 | There was no outcome data about the titration of medications by the heart failure nurses |
| Lowery 2012 | Quasi‐experimental design |
| Spaeder 2006 | Both arms titrated medications |
| Thompson 2005 | There was no outcome data about the titration of medications by the heart failure nurses |
Differences between protocol and review
We changed the term 'left ventricular systolic dysfunction' to 'heart failure with reduced ejection fraction (HFrEF)'.
We included all‐cause mortality as a primary outcome.
We modified the primary outcome of all‐cause or heart failure related event free survival to all‐cause event‐free survival.
We modified the secondary outcome of 'achieved dose as a percentage of target dose' to 'proportion reaching target dose of medications'.
We have reworded the Types of studies section to accurately reflect with what the intervention was compared.
We did not search CINAHL as planned due to access problems.
We did not create funnel plots due to the low number of included studies.
We included a 'Summary of findings' table in the review.
We have listed all‐cause hospital admissions and heart failure‐related hospital admissions as separate outcomes.
We did not undertake a subgroup analysis of the proportion of participants receiving optimal dose of ACEIs and ARBs due to a lack of studies reporting on this outcome.
We did not undertake a subgroup analysis of patients managed by a heart failure nurse but titration of medications by other health professionals as there were no RCTS investigating this intervention.
Contributions of authors
All review authors contributed to the conception and design of the protocol. All review authors provided input into the draft of the review and approved the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NHMRC, Australia.
NHMRC postdoctoral fellowship
-
Cochrane Heart Group, UK, Not specified.
Search strategy and primary eletronic searching of databases
Declarations of interest
Andrea Driscoll, the lead author of this review, was also the lead author of the included study Driscoll 2014. Andrea Driscoll was not involved in the screening and assessment of risk of bias and GRADE for this study.
Edited (no change to conclusions)
References
References to studies included in this review
Ansari 2003 {published data only}
- Ansari M, Shipak MG, Heidenreich PA, Ostaeyen DV, Pohl EC, Browner WS, et al. Improving guideline adherence: A randomised trial evaluating strategies to increase b‐blocker use in heart failure. Circulation 2003;107:2799‐804. [DOI: 10.1161/01.CIR.0000070952.08969.5B] [DOI] [PubMed] [Google Scholar]
Bruggink‐Andre de la Porte 2007 {published data only}
- Bruggink‐Andre de la Porte PWF, Lok DJA, Veldhuissen DJ, Wijngaarden J, Cornel JH, Zuithoff NPA, et al. Added value of a physician‐and‐nurse‐directed heart failure clinic: results from the Deventer‐Alkmaar heart failure study. Heart 2006;93:819‐25. [DOI: 10.1136/hrt.2006.095810] [DOI] [PMC free article] [PubMed] [Google Scholar]
Driscoll 2014 {published data only}
- Driscoll A, Srivastava P, Toia D, Gibcus J, Hare DL. Effectiveness of a specialist nurse‐led titration clinic on beta‐blocker therapy in patients with CHF: The MAXIMISE study. BMC: Research Notes 2014;7:668‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guder 2015 {published data only}
- Guder G, Stork S, Gelbrich G, Brenner S, Deubner N, Morbach C, et al. Nurse‐coordinated collaborative disease management improves the quality of guideline‐recommended heart failure therapy, patient‐reported outcomes, and left ventricular remodelling. European Journal of Heart Failure 2015 Mar 2 [Epub ahead of print]. [DOI: 10.1002/ejhf.252] [DOI] [PubMed]
Hancock 2012 {published data only}
- Hancock HC, Close H, Mason JM, Murphy JJ, Fuat A, Belder M, et al. Feasibility of evidence‐based diagnosis and management of heart failure in older people in care: a pilot randomised controlled trial. BMC Geriatrics 2012;12:70‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sisk 2006 {published data only}
- Sisk JE, Hebert PL, Horowitz CR, McLaughlin MA, Wang JJ, Chassin MR. Effects of nurse management on the quality of heart failure care in minority communities. Annals of Internal Medicine 2006;145:273‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stromberg 2003 {published data only}
- Stromberg A, Martensson J, Fridlund B, Levin L‐A, Karlsson JE, Dahlstrom U. Nurse‐led heart failure clinics improve survival and self‐care behaviour in patients with heart failure: results from a prospective, randomised trial. European Heart Journal 2003;24:1014‐23. [DOI: 10.1016/SO195-668X(03)00112-X] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Blue 2001 {published data only}
- Blue L, Lang E, McMurray JJV, Davie AP, McDonagh TA, Murdoch DR, et al. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ 2001;323(7315):715‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyon 2010 {published data only}
- Doyon O, Lemaire J, Rouleau J, Raymond I, Ducharme A, Brophy J, et al. Effect of a nurse‐led multidisciplinary heart failure clinic on professional practice: a randomised trial. Canadian Journal of Cardiology 2010;26(Supp D):150D. [Google Scholar]
Kasper 2002 {published data only}
- Kasper EK, Gerstenblith G, Hefter G, Anden E, Brinker JA, Thiemann DR, et al. A randomised trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. Journal of the American College of Cardiology 2002;39:471‐80. [DOI] [PubMed] [Google Scholar]
Lowery 2012 {published data only}
- Lowery J, Hopp F, Subramanian U, Wiitala W, Welsh DE, Larkin A, et al. Evaluation of a nurse practitioner disease management model for chronic heart failure: a multi‐site implementation study. Congestive Heart Failure 2012;18(1):64‐71. [DOI] [PubMed] [Google Scholar]
Spaeder 2006 {published data only}
- Spaeder JA, Najjar SS, Gerstenblith G, Hefter G, Kern L, Palmer JG, et al. Rapid titration of carvedilol in patients with congestive heart failure: A randomised trial of automated telemedicine versus frequent outpatient clinic visits. American Heart Journal 2006;151:1‐10. [DOI] [PubMed] [Google Scholar]
Thompson 2005 {published data only}
- Thompson DR, Roebuck A, Stewart S. Effects of a nurse‐led clinic and home‐based intervention on recurrent hospital use in chronic heart failure. European Journal of Heart Failure 2005;7:377‐84. [DOI] [PubMed] [Google Scholar]
Additional references
Bristow 1996
- Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, et al. Carvedilol produces dose‐related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation 1996;94:2807‐16. [DOI] [PubMed] [Google Scholar]
CIBIS II Investigators and Committees 2003
- CIBIS‐II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS II: a randomized trial). The Lancet 1999;353:9‐13. [PubMed] [Google Scholar]
Cohn 2001
- Cohn JN, Togoni G on behalf of Valsartan Heart Failure Trial Investigators. A randomised trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. The New England Journal of Medicine 2001;345(23):1667‐75. [DOI] [PubMed] [Google Scholar]
Driscoll 2011
- Driscoll A, Krum H, Wolfe R, Tonkin A. Nurse‐led titration of B‐adrenoreceptor blocking agents in chronic heart failure patients in the community. Journal of Cardiac Failure 2011;17:224‐30. [DOI] [PubMed] [Google Scholar]
Dulin 2005
- Dulin BR, Haas SJ, Abraham WT, Krum H. Do elderly systolic heart failure patients benefit from beta‐adrenoreceptor blocking agents to the same extent as the non‐elderly? Meta‐analysis of > 12,000 patients in large scale clinical trials. American Journal of Cardiology 2005;95:896‐8. [DOI] [PubMed] [Google Scholar]
Francis 2001
- Francis GS, Gassler JP, Sonnenblick EH. Pathophysiology and diagnosis of heart failure. In: Fuster V, Alexander RW, O’Rouke RA, Roberts R, King SB, Wellens HJJ editor(s). Hurst’s The Heart: Volume 1. 10th Edition. New York: McGraw‐Hill, 2001. [Google Scholar]
Freemantle 1999
- Freemantle N, Cleland JPF, Young P. Beta‐blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 1999;318:1730‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garg 1995
- Garg R, Yusuf S, Bussmann WD, Sleight P, Uprichard A, Massie B, et al. Overview of randomized trials of angiotensin‐converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 1995;273(18):1450‐6. [PubMed] [Google Scholar]
Givertz 2001
- Givertz MM, Colucci WS, Braunwald E. Clinical aspects of heart failure: high‐output failure; pulmonary oedema. In: Braunwald E, Zipes DP, Libby P editor(s). Heart Disease: A Textbook of Cardiovascular Medicine. 16th Edition. Philadelphia: W.B. Saunders Company, 2001. [Google Scholar]
Gustafsson 2007
- Gustafsson F, Schou M, Videbaek L, Nielsen T, Ulriksen H, Markenvard J, et al. Treatment with beta‐adrenoreceptor blocking agents in nurse‐led heart failure clinics: Titration efficacy and predictors of failure. European Journal of Heart Failure 2007;9:910‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jain 2005
- Jain A, Mills P, Nunn LM, Butler J, Luddington L, Ross V, et al. Success of a multidisciplinary heart failure clinic for initiation and up‐titration of key therapeutic agents. European Journal of Heart Failure 2005;7:405‐10. [DOI] [PubMed] [Google Scholar]
Krum 2001
- Krum H, Tonkin AM, Currie R, Djundjek R, Johnston CI. Chronic heart failure in Australian general practice: The Cardiac Awareness Survey and Evaluation (CASE) Study. The Medical Journal of Australia 2001;174:439‐44. [DOI] [PubMed] [Google Scholar]
Laurent‐Bopp 2000
- Laurent‐Bopp D. Heart failure. In: Woods SL, Sivarajan Froelicher ES, Underhill Motzer S editor(s). Cardiac Nursing. 4th Edition. Philadelphia: Lippincott, 2000. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Levy 2002
- Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, et al. Long‐term trends in the incidence of and survival with heart failure. The New England Journal of Medicine 2002;347:1397‐402. [DOI] [PubMed] [Google Scholar]
Lloyd‐Jones 2002
- Lloyd‐Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002;106:3068 –72. [DOI] [PubMed] [Google Scholar]
McMurray 2012
- McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal 2012;33:1787–847. [DOI] [PubMed] [Google Scholar]
MERIT‐HF Study Group 1999
- MERIT‐HF Study Group. Effect of Metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). The Lancet 1999;353:2001‐7. [PubMed] [Google Scholar]
Najafi 2007
- Najafi F, Dobson AJ, Jamrozik K. Recent changes in heart failure hospitalizations in Australia. European Journal of Heart Failure 2007;9:228‐33. [DOI] [PubMed] [Google Scholar]
National Heart Foundation & CSANZ 2011
- National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand (Chronic Heart Failure Guidelines Expert Writing Panel). Guidelines for the Prevention, Detection and Management of People With Chronic Heart Failure in Australia. Updated October 2011. Melbourne: National Heart Foundation of Australia, 2011. [Google Scholar]
Packer 1996
- Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. The New England Journal of Medicine 1996;334:1349‐55. [DOI] [PubMed] [Google Scholar]
Phillips 2004
- Phillips SM, Marton RL, Tofler GH. Barriers to diagnosing and managing heart failure in primary care. The Medical Journal of Australia 2004;181(2):78‐81. [DOI] [PubMed] [Google Scholar]
Phillips 2005
- Phillips CO, Singa RM, Rubin HR, Jaarsma T. Complexity of program and clinical outcomes of heart failure disease management incorporating specialist nurse‐led HF clinics. A meta‐regression analysis. European Journal of Heart Failure 2005;7:333‐41. [DOI] [PubMed] [Google Scholar]
Roger 2004
- Roger VL, Weston SA, Redfield MM, Hellermann‐Homan JP, Killian J, Yawn BP, et al. Trends in heart failure incidence and survival in a community‐based population. JAMA 2004;292(3):344‐50. [DOI] [PubMed] [Google Scholar]
Ryder 2003
- Ryder M, Travers B, Timmons L, Ledwidge M, McDonald K. Specialist nurse supervised in‐hospital titration to target dose ACE inhibitor‐is it safe and feasible in a community heart failure population?. European Journal of Cardiovascular Nursing 2003;2:183‐8. [DOI] [PubMed] [Google Scholar]
Simon 2003
- Simon T, Mary‐Kause M, Funck‐Brentano C, Lechat P, Jaillon P. Bisoprolol dose‐response relationship in patients with CHRONIC HF: a subgroup analysis in the Cardiac Insufficiency Bisoprolol Study (CIBIS II). European Heart Journal 2003;24:552‐9. [DOI] [PubMed] [Google Scholar]
Wikstrand 2002
- Wikstrand J, Hjalmarson A, Waagstein F, Fagerberg B, Goldstein S, Kjekshus J, et al. Dose of metoprolol CR/XL and clinical outcomes in patients with heart failure: analysis of the experience in metoprolol CR/XL randomized intervention trial in chronic heart failure (MERIT/HF). Journal of the American College of Cardiology 2002;40(3):491‐8. [DOI] [PubMed] [Google Scholar]
Yancy 2013
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:1‐375. [DOI: 10.1161/CIR.0b013e31829e8776] [DOI] [PubMed] [Google Scholar]


