Abstract
Background
Primary postpartum haemorrhage (PPH) is commonly defined as bleeding from the genital tract of 500 mL or more within 24 hours of birth. It is one of the most common causes of maternal mortality worldwide and causes significant physical and psychological morbidity.
An earlier Cochrane Review considering any treatments for the management of primary PPH, has been split into separate reviews. This review considers treatment with mechanical and surgical interventions.
Objectives
To determine the effectiveness and safety of mechanical and surgical interventions used for the treatment of primary PPH.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (26 July 2019) and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCTs) of mechanical/surgical methods for the treatment of primary PPH compared with standard care or another mechanical/surgical method. Interventions could include uterine packing, intrauterine balloon insertion, artery ligation/embolism, or uterine compression (either with sutures or manually).
We included studies reported in abstract form if there was sufficient information to permit risk of bias assessment. Trials using a cluster‐RCT design were eligible for inclusion, but quasi‐RCTs or cross‐over studies were not.
Data collection and analysis
Two review authors independently assessed studies for inclusion and risk of bias, independently extracted data and checked data for accuracy. We used GRADE to assess the certainty of the evidence.
Main results
We included nine small trials (944 women) conducted in Pakistan, Turkey, Thailand, Egypt (four trials), Saudi Arabia, Benin and Mali. Overall, included trials were at an unclear risk of bias. Due to substantial differences between the studies, it was not possible to combine any trials in meta‐analysis. Many of this review's important outcomes were not reported. GRADE assessments ranged from very low to low, with the majority of outcome results rated as very low certainty. Downgrading decisions were mainly based on study design limitations and imprecision; one study was also downgraded for indirectness.
External uterine compressionversus normal care (1 trial, 64 women)
Very low‐certainty evidence means that we are unclear about the effect on blood transfusion (risk ratio (RR) 2.33, 95% confidence interval (CI) 0.66 to 8.23).
Uterine arterial embolisationversus surgical devascularisation plus B‐Lynch (1 trial, 23 women)
The available evidence for hysterectomy to control bleeding (RR 0.73, 95% CI 0.15 to 3.57) is unclear due to very low‐certainty evidence. The available evidence for intervention side effects is also unclear because the evidence was very low certainty (RR 1.09; 95% CI 0.08 to 15.41).
Intrauterine Tamponade
Studies included various methods of intrauterine tamponade: the commercial Bakri balloon, a fluid‐filled condom‐loaded latex catheter ('condom catheter'), an air‐filled latex balloon‐loaded catheter ('latex balloon catheter'), or traditional packing with gauze.
Balloon tamponade versus normal care (2 trials, 356 women)
One study(116 women) used the condom catheter. This study found that it may increase blood loss of 1000 mL or more (RR 1.52, 95% CI 1.15 to 2.00; 113 women), very low‐certainty evidence. For other outcomes the results are unclear and graded as very low‐certainty evidence: mortality due to bleeding (RR 6.21, 95% CI 0.77 to 49.98); hysterectomy to control bleeding (RR 4.14, 95% CI 0.48 to 35.93); total blood transfusion (RR 1.49, 95% CI 0.88 to 2.51); and side effects. A second study of 240 women used the latex balloon catheter together with cervical cerclage. Very low‐certainty evidence means we are unclear about the effect on hysterectomy (RR 0.14, 95% CI 0.01 to 2.74) and additional surgical interventions to control bleeding (RR 0.20, 95% CI 0.01 to 4.12).
Bakri balloon tamponade versus haemostatic square suturing of the uterus (1 trial, 13 women)
In this small trial there was no mortality due to bleeding, serious maternal morbidity or side effects of the intervention, and the results are unclear for blood transfusion (RR 0.57, 95% CI 0.14 to 2.36; very low certainty). Bakri balloon tamponade may reduce mean 'intraoperative' blood loss (mean difference (MD) ‐426 mL, 95% CI ‐631.28 to ‐220.72), very low‐certainty evidence.
Comparison of intrauterine tamponade methods (3 trials, 328 women)
One study (66 women) compared the Bakri balloon and the condom catheter, but it was uncertain whether the Bakri balloon reduces the risk of hysterectomy to control bleeding due to very low‐certainty evidence (RR 0.50, 95% CI 0.05 to 5.25). Very low‐certainty evidence also means we are unclear about the results for the risk of blood transfusion (RR 0.97, 95% CI 0.88 to 1.06).
A second study (50 women) compared Bakri balloon, with and without a traction stitch. Very low‐certainty evidence means we are unclear about the results for hysterectomy to control bleeding (RR 0.20, 95% CI 0.01 to 3.97).
A third study (212 women) compared the condom catheter to gauze packing and found that it may reduce fever (RR 0.47, 95% CI 0.38 to 0.59), but again the evidence was very low certainty.
Modified B‐Lynch compression sutureversus standard B‐Lynch compression suture (1 trial, 160 women)
Low‐certainty evidence suggests that a modified B‐Lynch compression suture may reduce the risk of hysterectomy to control bleeding (RR 0.33, 95% CI 0.11 to 0.99) and postoperative blood loss (MD ‐244.00 mL, 95% CI ‐295.25 to ‐192.75).
Authors' conclusions
There is currently insufficient evidence from RCTs to determine the relative effectiveness and safety of mechanical and surgical interventions for treating primary PPH. High‐quality randomised trials are urgently needed, and new emergency consent pathways should facilitate recruitment.
The finding that intrauterine tamponade may increase total blood loss > 1000 mL suggests that introducing condom‐balloon tamponade into low‐resource settings on its own without multi‐system quality improvement does not reduce PPH deaths or morbidity. The suggestion that modified B‐Lynch suture may be superior to the original requires further research before the revised technique is adopted. In high‐resource settings, uterine artery embolisation has become popular as the equipment and skills become more widely available. However, there is little randomised trial evidence regarding efficacy and this requires further research. We urge new trial authors to adopt PPH core outcomes to facilitate consistency between primary studies and subsequent meta‐analysis.
Plain language summary
Mechanical and surgical interventions for treating women with severe bleeding after childbirth
This review considers evidence from randomised controlled trials on using mechanical and surgical interventions for stopping severe bleeding after giving birth. Other Cochrane Reviews consider the use of drugs that promote blood clotting or contractions of the uterus.
What is the issue?
Primary postpartum haemorrhage (PPH) occurs when a mother has excessive vaginal bleeding within 24 hours of giving birth (typically > 500 mL or > 1000 mL). The most common cause of primary PPH is failure of the uterus to contract after birth, and usual care is based on using drugs to reverse this. Other causes include retained placenta, vaginal or cervical tears, and failure of the blood to clot. Surgical and mechanical methods apply direct pressure on blood vessels to reduce uterine blood flow. Pressure can be applied from inside the uterus by inflating a balloon within the uterus. Alternatively, pressure can be applied on the outside surface of the uterus. This can be directly (using a hand), or by passing a stitch through the front and back walls of the uterus to compress the uterine walls together. Blood flow can also be stopped by tying off or blocking the blood vessels that feed the uterus.
Why is this important?
PPH is a common cause of maternal death and illness worldwide. Nearly 300,000 pregnant women die annually across the world with approximately 25% of those deaths caused by haemorrhage. It can also lead to the mother having significant long‐term medical and psychological problems.
What evidence did we find?
We searched for evidence (July 2019) and included nine small randomised controlled trials (944 women). The studies were conducted in hospital settings in Pakistan, Turkey, Thailand, Egypt (four studies) and Saudi Arabia, and health facilities in Benin and Mali. Overall, the studies were very different, with various interventions being compared. The small number of women in each study, few or zero events, lack of data on important outcomes, and wide variation in results meant that few clear findings were obtained. It was not possible to pool the results from the studies. Our certainty assessments for the trials ranged from low to very low, with the majority rated as very low certainty. This means that we cannot be confident about the results.
Two studies (356 women) compared internal pressure using non‐commercial balloons (a water‐filled condom and a sterilised, air‐filled 'party' balloon) and normal care. The condom catheter may result in increased blood loss, but no other important effects were seen in either study. A third study found that the condom catheter may reduce postpartum fever compared to packing of the uterus with gauze, but no other effects.
Three studies used a commercially available balloon (Bakri). This was compared to external pressure with stitches in one study (13 women) and it was found that Bakri balloon may reduce blood loss but no other effects were seen. Another study (66 women) compared the Bakri balloon to a condom system and found little to no differences between groups. The third study (50 women) looked to see if using a stitch to tether the upper end of the balloon to the top of the uterus had any benefit, but found little to no effect.
One study (64 women) compared external compression of the uterus with normal care but with no clear findings. Another study of 160 women compared a standard compression suture with a modified version in which the uterus is not only compressed, but the main vessel supplying the uterus was tied off. The results suggested that the modified suture may reduce blood loss and the risk of hysterectomy.
One study (23 women) compared using imaging to block the blood vessels to the uterus (uterine artery embolisation) with surgical techniques to cut off the blood supply and compress the uterus but found little to no effect.
What does this mean?
We did not find sufficient high‐quality evidence to determine the effectiveness and safety of mechanical and surgical interventions for treating primary PPH. High‐quality randomised trials are urgently needed to test some of the findings of this review. We urge new trial authors to adopt standardised PPH core outcomes.
Summary of findings
Background
An earlier Cochrane Review (Mousa 2014c) considered various treatments for the management of primary postpartum haemorrhage (PPH). That review has now been split, with different treatment types considered in separate reviews.
Antifibrinolytic agents for treating primary PPH is covered by Shakur 2018.
Mechanical and surgical interventions are considered in this review.
Uterotonic agents for treating primary PPH are covered by a new review on First‐line uterotonics for treating postpartum haemorrhage: a systematic review and network meta‐analysis (Parry Smith 2017).
Description of the condition
Primary PPH is defined as bleeding from the genital tract of 500 mL or more in the first 24 hours following delivery of the baby (WHO 2012; WHO 2015) and severe PPH is defined as blood loss more than 1000 mL, regardless of the route of delivery (Hoveyda 2001; Sentilhes 2016). Secondary PPH occurs when women have abnormal or heavy vaginal bleeding between 24 hours and 12 weeks after the birth and it affects fewer than two in 100 women (WHO 2012).
Primary PPH, with a global prevalence rate of about 6%, is one of the most common causes of maternal morbidity and mortality worldwide (Carroli 2008). Nearly 300,000 women die annually across the world from causes related to pregnancy and childbirth (Alkema 2016; Lozano 2011), and approximately a quarter of these are due to primary PPH (WHO 2012). In high‐income countries there is an increasing rate of primary PPH (Knight 2009). In the Netherlands there was an increase from 4.1% in 2000 to 6.4% by 2013 (van Stralen 2016). Similar trends have been reported in the United States of America with a rise from 1.9% in 1999 to 4.2% in 2008 (Kramer 2013). In sub‐Saharan Africa the rate of primary PPH is reported as high at 10.5% (Carroli 2008). In the low‐ and middle‐income countries, primary PPH remains the leading cause of maternal death, accounting for one‐third of maternal deaths in Asia and Africa (Carroli 2008; Kassebaum 2014; Khan 2006).
Major primary PPH can lead to significant maternal morbidity including shock, renal failure, respiratory failure and/or liver failure (Bonnar 2000). Severe PPH can cause ischaemic pituitary necrosis (Sheehan’s syndrome) which can be life‐threatening (Matsuzaki 2017). Furthermore, PPH may have a long‐term psychological impact on women's health in the form of negative memories of childbirth and postpartum period with the main fear being of death (Sentilhes 2011a).
Visual assessment of blood loss is the most frequently used method of determining blood loss following childbirth (Hancock 2015). Unfortunately, this method often under‐estimates blood loss. Compared to measured blood loss following delivery, visual (clinical) assessment underestimates blood loss by 100 mL to 150 mL (Kerr 2016; Sloan 2010). For accurate measurement of blood loss, many clinicians use a combination of direct measurement and gravimetric methods.
Causes and risk factors
Uterine atony is considered the most common cause of primary PPH. Other aetiological factors include retained parts of the placenta, vaginal or cervical tears, and coagulation failure. Uterine rupture and uterine inversion are extremely rare but, when they occur, could result in heavy bleeding (WHO 2015). Investigators have identified risk factors for PPH as first pregnancy (Gilbert 1987), maternal obesity (Aisaka 1988), a large baby (Stones 1993), twin pregnancy (Combs 1991; Suzuki 2012), prolonged or augmented labour (Gilbert 1987), chorioamnionitis, pre‐eclampsia, maternal anaemia and placenta praevia and abruptio placenta (antepartum haemorrhage) (Kramer 2011; Wetta 2013). The largest bleeds occur usually in women with retained placenta, emergency caesarean section, and placental abruption and praevia (Green 2016). However women with no potential risk factors often unpredictably develop primary PPH (Mousa 2008; Weeks 2015a).
The morbidity and mortality due to PPH in low‐income countries is aggravated by poor nutritional status, lack of easy access to treatment, inadequate intensive care and blood bank services (El Ayadi 2013; Khan 2006).
Treatment approaches
Treatment for primary PPH requires a multidisciplinary team approach. The treatment involves resuscitation to manage obstetric haemorrhage, identification of the causes, and management. After exclusion of lower genital tract tears and coagulopathy, uterine atony is managed by using uterotonics that increase the efficiency of uterine contraction, and tranexamic acid to promote coagulation (WOMAN 2017). If uterine bleeding is ongoing despite these interventions, the use of haemostatic drugs with mechanical methods to compress the uterus is advisable. Several mechanical methods have been described to control the bleeding and these are described below under Description of the intervention.
The non‐pneumatic anti‐shock garment (NASG) has also been used in the management of primary PPH. It is used before transport of patients with shock due to primary PPH to stabilise them for referral to higher level hospitals (Miller 2013b). This technique will be the focus of another Cochrane Review.
Hysterectomy works by ligating the blood vessels to the uterus and removing the uterus which is the bleeding site. Peripartum hysterectomy is a major operation and is performed only in the event of life threatening haemorrhage during or immediately after abdominal or vaginal deliveries when all other options have been exhausted (Machado 2011). Indeed, the first caesarean hysterectomy ever described was conducted in order to prevent death from uterine haemorrhage (Porro 1876). In this review it is included as an outcome rather than an intervention, as it is the last resort to control intractable bleeding, and is therefore unlikely to be ever be an intervention tested in a randomised trial.
The choice of the type of mechanical intervention depends on several factors and, in particular, the experience of the surgeon.
Other relevant published Cochrane Reviews on management of PPH are Mousa 2014c which evaluated the comprehensive treatment of PPH, Begley 2019, which compares active with expectant third‐stage management; Liabsuetrakul 2018; McDonald 2004; Pantoja 2016; Tunçalp 2012 and Oladapo 2018, which considered the role of different prophylactic uterotonics in third‐stage management; Abdel‐Aleem 2015, Grillo‐Ardila 2014; Mori 2012, which examined the treatment of retained placenta; Oladapo 2012, which evaluated advance community distribution of misoprostol for preventing or treating PPH; Novikova 2015a, Shakur 2018, which evaluated the place of tranexamic acid for preventing PPH, and Alexander 2002, which examined drug treatment for secondary PPH. Other Cochrane Reviews have looked at the use of uterine massage (Hofmeyr 2013b) and breastfeeding or nipple stimulation (Abedi 2016) for preventing PPH.
Description of the intervention
In the event of failure of the uterotonics, PPH management turns to mechanical and surgical methods (WHO 2012). Whilst this review will consider mechanical and surgical methods, there is no clear distinction between the two approaches. Both approaches act through direct pressure on the placental bed, by reducing uterine blood flow through external pressure on the uterus, or through the interruption of vascular flow to the uterus.
Direct pressure on the placental bed can be achieved by internal balloon tamponade using the Sengstaken‐Blakemore tube (Chan 1997), the Rusch catheter (Johanson 2001), the Bakri balloon (Bakri 2001), or by packing the uterine cavity with several metres of gauze (Eastman 1950). The overall success rate for packing is reported to be around 80% (Doumouchtsis 2007; Georgiou 2009).
External pressure on the uterus can be achieved with uterine compression sutures (B‐Lynch 1997; Marasinghe 2011; Zheng 2011). B‐Lynch was the first to describe a suture that runs through the full thickness of both uterine walls (anterior and posterior; B‐Lynch 1997). When tied, the suture allows tight compression of the uterine walls and stops the bleeding (Mousa 2001). The modifications of this include the simple brace suture (Hayman 2002) or square sutures (Cho 2000). Uterine compression sutures have been found to be effective in stopping uterine bleeding (Cekmez 2015a), but complications of intrauterine synechiae (Poujade 2011; Rathat 2011) and/or infection (Ochoa 2002) can occur.
Vascular flow to the uterus can be interrupted by uterine devascularisation, ligation of the uterine or internal iliac arteries, embolisation or aortic compression. These are considered in selected cases where bleeding is persistent (Cekmez 2015a; Jouppila 1995). The success rate of devascularisation appears to be less than 50% (Clark 1985). A simple way of achieving temporary uterine devascularisation is through compression of the aorta. This techniquemay be especially useful for PPH with a retained placenta.
How the intervention might work
Compression of the aorta through the abdomen is an emergency manoeuvre proposed to reduce PPH and permit time for resuscitation and control of bleeding. This technique involves compression of the aorta by placing a fist or the heel of the hand onto the aorta through the lax postnatal abdominal wall. The femoral pulse is checked to ensure occlusion, and the compression can be maintained for up to 90 minutes (Pereira 2005). This cuts off the blood supply to the uterus and hence reduces uterine bleeding.
The packing of the uterus with gauze or Sengstaken‐Blakemore tube or the Rusch catheter balloon applies pressure on the placental site and this reduces uterine bleeding (Georgiou 2009).
The devascularisation or ligation of uterine blood vessels and/or occasionally internal iliac arteries that supply blood to the uterus cuts off the blood supply to the uterus and stops uterine bleeding (Cekmez 2015a). The B‐Lynch suture reinforces the contraction of the uterus by keeping the uterus contracted, thus reducing uterine bleeding (Cekmez 2015a; Mousa 2001). The Bakri balloon stops the bleeding by exerting pressure at the placental site (Cekmez 2015a).
Why it is important to do this review
This review is dedicated to assessing the effectiveness and safety of mechanical and surgical methods for the treatment of primary PPH and aims to inform local and national practices and guide clinicians and midwives on their role when managing PPH. Whilst first‐line treatment is usually pragmatic, using uterotonics, ongoing bleeding is managed with a combination of uterotonics, mechanical and surgical methods depending on the underlying cause of the bleeding (WHO 2012). These can be used successively or in combination. Much of our current practice is based on expert opinion as randomised trials in this area are difficult to carry out (Weeks 2015a).
An earlier Cochrane Review (Mousa 2014c), considered all of the various treatment modalities for the management of primary PPH; the review has now been split in order to facilitate a more detailed analysis of different types of interventions. The use of antifibrinolytic drugs for treating primary PPH is covered in a recent new Cochrane review (Shakur 2018). In contrast, our review will focus on the use of mechanical and surgical interventions for treating primary PPH. The topic of uterotonic agents for treating primary PPH will be covered by another review.
Objectives
To determine the effectiveness and safety of mechanical and surgical interventions used for the treatment of primary postpartum haemorrhage.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of mechanical and surgical management of postpartum haemorrhage (PPH). In future updates of this review, if identified, trials using a cluster‐RCT design will be included, and studies reported as abstracts will only be included if there is sufficient information to allow assessment of risk bias.
Quasi‐RCTs or cross‐over studies are not eligible for inclusion in this review.
Types of participants
Women after delivery following a pregnancy of at least 24 weeks’ gestation with a diagnosis of primary PPH, regardless of mode of delivery (vaginal or caesarean section) or other aspects of third‐stage management.
We did not include trials of 'all' women that also happened to include women with PPH. We also did not include trials of all women including those with PPH (where the trial authors did not provide subgrouped results for women with PPH).
Obtaining a blood loss measurement can be difficult, therefore trials may have different ways of defining PPH:
women with blood loss of 500 mL or more; and/or
women with primary PPH requiring blood transfusion and/or blood products; and/or
women with a clinical diagnosis of primary PPH (as defined by the trialists).
Exclusion criteria
Women with PPH with gestational age less than 24 weeks.
Women with heavy vaginal bleeding after 24 hours of birth (secondary PPH).
Types of interventions
Mechanical or surgical interventions such as uterine packing or intrauterine balloon insertion, artery ligation, uterine compression (sutures or manually).
We compared one mechanical/surgical method (or a combined methods) versus standard care or another mechanical/surgical method, or combined methods.
For example, the following interventions were eligible.
External uterine compression (all methods) versus normal care
External uterine compression (all methods) versus another mechanical/surgical method
One external uterine compression technique verus another external uterine compression technique
Uterine devascularisation (all methods) versus normal care
Uterine devascularisation (all methods) versus another mechanical/surgical method
One uterine devascularisation versus another uterine devascularisation technique
Intrauterine tamponade (all methods) versus normal care
Intrauterine tamponade (all methods) versus another mechanical/surgical method
One intrauterine tamponade versus another intrauterine tamponade technique
Uterine compression sutures (all methods) versus normal care
Uterine compression sutures (all methods) versus another mechanical/surgical method
One uterine compression suture technique versus another uterine compression suture technique
Use of non‐pneumatic antishock garment, or arterial embolisation were not considered in this review.
We present results stratified by the type of mechanical or surgical method.
Types of outcome measures
Types of outcome measures are shown below.
Primary outcomes
Mortality due to bleeding
Hysterectomy to control bleeding
Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure)
Secondary outcomes
All‐cause mortality*
Mortality from causes other than bleeding
Shock as defined by trialist*
Coagulopathy as defined by trialist*
Number of women with total blood loss 500 mL or more after randomisation*
Number of women with total blood loss 1000 mL or more after randomisation*
Mean blood loss (mL) (trialist defined)*
Blood transfusion (red cell or whole blood)*
Blood product transfusion*
Post‐randomisation additional uterotonic agent used to control bleeding*
Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy)*
Post‐randomisation additional non‐surgical intervention to control bleeding (uterine packing, bimanual uterine massage, tamponade, external aortic compression and compression garments)
Admission to higher level of care*
Side effects of the intervention (e.g. trauma, necrosis)
Days in hospital
Breastfeeding (defined as any breastfeeding at hospital discharge)*
Maternal satisfaction with therapy (trialist defined)*
Quality of life, including physiological activity and social and emotional changes (sense of well‐being) (trialist defined)*
*outcomes form part of a core outcome set that will be used in all PPH reviews.
NOTE: we anticipated that assessment of blood loss could vary between trials. We considered that measurement of blood and blood clots in jars and weighing of linen are likely to be more precise than clinical judgement. The latter is known to underestimate blood loss. The way of reporting the amount of loss as ’greater than’ or ’greater than or equal to’ a certain cut‐off level (e.g. greater than 500 mL or greater than or equal to 500 mL) may affect the total reported amount of blood loss.
Search methods for identification of studies
The following methods section of this protocol is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (26 July 2019).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (26 July 2019) using the methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Three review authors (AW/FK/JW) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion and had no need to consult with the other member of the review team.
We created a study flow diagram to map out the number of records identified, included and excluded.
Data extraction and management
We designed a form to extract data. Extracted data included trial dates, sources of trial funding and trial authors' declarations of interest. For eligible studies, at least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, where required, we consulted with a third member of the review team. We entered data into Review Manager software (RevMan 2014) and checked them for accuracy. When information regarding any of the above is unclear, we attempted to contact authors of the original reports to provide further details; where we contacted trial authors, information is listed in Characteristics of included studies.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We describe for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies are at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We describe for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes are measured in the same way between trials. In future updates, we will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐RCTs for inclusion in this review. In future updates, if we identify any cluster‐RCTs we will include them in our analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
A cross‐over trial is not a valid methodology for trials of PPH as it is an acute emergency.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if appropriate, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out our analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial is the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
In future updates, we will assess statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We will regard heterogeneity as substantial if the I² is greater than 30% and either the Tau² is greater than zero, or if there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We did not combine data in meta‐analysis due to clinical heterogeneity. In future updates, is appropriate, we will carry out statistical analysis using the Review Manager software (RevMan 2014). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average of the range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
Our planned subgroup analysis:
placenta praevia versus no placenta praevia;
mode of delivery (caesarean section versus vaginal birth)
Subgroup analysis will be restricted to the review’s primary outcomes.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
In future updates, if appropriate, we will undertake sensitivity analyses to explore the effect of risk of bias by temporarily excluding trials at high or unclear risk of bias (for selection bias or attrition bias) to see if it makes a difference in the overall result. We will also carry out sensitivity analysis to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect. We will also carry out sensitivity analysis to investigate the effect of the randomisation unit where we combine cluster‐RCTs and individually‐randomised trials in meta‐analysis. Sensitivity analysis will only be performed for the review's primary outcomes.
Summary of findings and assessment of the certainty of the evidence
The certainty of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the certainty of the body of evidence relating to the following outcomes for the main comparisons.
External uterine compression (all methods) versus normal care
External uterine compression (all methods) versus another surgical/mechanical method
One external uterine compression technique verus another external uterine compression technique
Uterine devascularisation (all methods) versus normal care
Uterine devascularisation (all methods) versus another surgical/mechanical method
One uterine devascularisation versus another uterine devascularisation technique
Intrauterine tamponade (all methods) versus normal care
Intrauterine tamponade (all methods) versus another surgical/mechanical method
One intrauterine tamponade versus another intrauterine tamponade technique
Uterine compression sutures (all methods) versus normal care
Uterine compression sutures (all methods) versus another mechanical or surgical method
One uterine compression suture technique versus another uterine compression suture technique
We assessed the following outcomes.
Mortality due to bleeding
Hysterectomy to control bleeding
Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure
Number of women with total blood loss 1000 mL or more after randomisation*
Mean blood loss (mL) (trialist defined)*
Blood transfusion (red cell or whole blood)*
Side effects of the intervention (e.g. trauma, necrosis)
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Studies contained within this review are described below.
Results of the search
See: Figure 1
1.
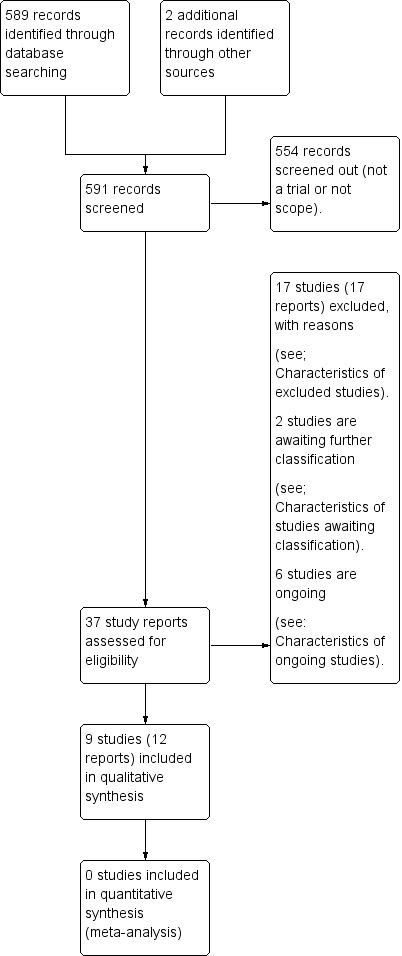
Study flow diagram.
We retrieved 35 study reports to assess. In addition, we identified two further trials (Soltan 2007; Ashraf 2018) from correspondence. We included nine trials (12 trial reports), excluded 17 trials, and six trials are ongoing (see Characteristics of ongoing studies). There are also two trials listed in Characteristics of studies awaiting classification ‐ these trials are listed in trials registries as 'completed' and we have contacted the authors for more information about whether the results are currently available.
Included studies
We included nine trials (involving a total of 944 women) (Ashraf 2018; Chantrapitak 2009; Darwish 2018; Dumont 2017; El‐Sokkary 2016; Farouk 2016; Kavak 2013; Khalil 2011; Soltan 2007).
Methods, trial dates and settings
Methods
Ashraf 2018, Chantrapitak 2009, Darwish 2018, El‐Sokkary 2016, Farouk 2016, Kavak 2013, Khalil 2011 and Soltan 2007 were randomised controlled trials (RCTs). Dumont 2017, was a multi‐centre RCT. Sample sizes ranged between 13 (Kavak 2013) and 240 (Soltan 2007).
Trial dates
The trials were conducted between 2003 and 2015:
the Ashraf 2018 trial report does not provide the study dates but states the study took place over one year;
Soltan 2007 2003 to 2004;
Khalil 2011 April 2004 until April 2009;
January to August 2008 Chantrapitak 2009;
Farouk 2016 May 2011 until May 2013;
August 2011 to August 2012 Kavak 2013;
January 2013 to October 2015 El‐Sokkary 2016;
May 2013 to December 2015 Dumont 2017;
Darwish 2018 October 2014 until December 2015.
Settings
Settings included a university hospital setting in Pakistan (Ashraf 2018), Turkey (Kavak 2013), hospital settings in Thailand (Chantrapitak 2009), Egypt (Darwish 2018; El‐Sokkary 2016; Farouk 2016; Soltan 2007), a security forces hospital in Saudi Arabia (Khalil 2011), and healthcare facilities in Benin and Mali (Dumont 2017).
Participants
All of the women in the included studies were reported as having primary postpartum haemorrhage (PPH). In five of the studies, PPH was due to uterine atony (Darwish 2018; Soltan 2007) or suspected uterine atony (Dumont 2017) following vaginal birth or either vaginal birth or and caesarean in Farouk 2016. Women in the Chantrapitak 2009 and Ashraf 2018 studies had a vaginal birth, but the precise cause of PPH was not reported by the trial authors. The women in Soltan 2007 had given birth either at home or in hospital.
In two trials, women had PPH due to atony following caesarean section (El‐Sokkary 2016; Khalil 2011). The women in Kavak 2013 had PPH due to complete placenta praevia and intractable bleeding following a caesarean section (Kavak 2013).
How was PPH defined in the trials?
We recorded the trial authors' definitions of PPH ‐ these are provided below.
Ashraf 2018 defined PPH as "excessive blood loss from genital tract occurring during third stage of labour and within first 24 hours after parturition" p 890.
Chantrapitak 2009 defined PPH as "blood loss > 500ml after delivery" p 601.
Darwish 2018 provided no definition of PPH, nor was there a systematic method of diagnosis.
Dumont 2017 defined PPH as, "visual estimation of excessive blood loss and patient status (blood pressure and cardiac frequency)" p 2.
El‐Sokkary 2016 did not define PPH.
Farouk 2016 defined PPH as blood loss more than 1000 mL within two hours of birth.
Kavak 2013 did not define PPH.
Khalil 2011 did not define PPH.
Soltan 2007 did not define PPH in the trial report, and there is no information on how blood loss was assessed.
Interventions and comparisons
External uterine compression versus usual care (comparison 1)
One trial (Chantrapitak 2009) compared external uterine compression versus usual care. The study compared external lower uterine compression with 'usual care'. External lower uterine compression (either by grasping the uterus through a lax abdominal wall or compressing the uterus against the sacrum and lower vertebrae) was applied for a duration of 10 minutes. The usual care group involved "massage, oxytocin (10‐20 units in 1000 mL of intravenous solution, 200 mL/min), intravenous ergometrine (Methergin®, 0.2 mg), placed cold pack on uterus, and urinary catheterisation" p 601.
One uterine devascularisation technique versus another uterine devascularisation technique
One trial compared one uterine devascularisation technique with another technique of uterine devascularisation (Farouk 2016).
Uterine arterial embolisation versus surgical devascularisation plus B‐Lynch (comparison 2)
Farouk 2016 compared uterine arterial embolisation with surgical devascularisation plus B‐Lynch compression sutures.
Intrauterine balloon tamponade versus normal care
Condom‐loaded Foley catheter plus normal care (misoprostol) versus normal care (misoprostol) (comparison 3)
Dumont 2017 compared uterine balloon tamponade (condom‐loaded Foley catheter) and usual care (misoprostol) with usual care (misoprostol) alone. All women received rectal misoprostol (1000 ug) or sublingually (600 ug) following randomisation. In the balloon tamponade group, the condom was inflated "by increments of 250 mL of solute" p2. Further increments were added (up to a maximum of 1000 mL) if bleeding was still evident five minutes after adding the solution.
Latex balloon (air filled) tamponade + cervical stitch and normal care (comparison 4)
Soltan 2007 compared uterine balloon tamponade the El‐Menia balloon tamponade versus normal care alone. The El‐Menia balloon tamponade is a standard latex party balloon (19 mm thick) inflated to 140 mmHg and attached to a Nelton catheter with suture silk. To keep the balloon in place, cervical cerclage was applied ("at 3 and 9 o'clock" p54). Women in the balloon tamponade group also received antibiotic prophylaxis immediately after balloon insertion and every eight hours thereafter for the subsequent three days. Both groups received normal care, which consisted of uterine massage and ecbolics as per the World Health Organization (WHO) protocol.
Intrauterine tamponade versus another mechanical/surgical method
Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (comparison 5)
The Kavak 2013 trial compared Bakri balloon tamponade with haemostatic square suturing to the lower segment of the uterus. The Bakri balloon tamponade was inflated with saline (100 mL to 200 mL) "according to the uterine size" p 706. Endouterine haemostatic square suturing (four to five sutures) was applied to the lower segment of the uterus.
All women were given prophylactic antibiotics.
One intrauterine tamponade technique versus another intrauterine tamponade technique
Two trials compared one intrauterine tamponade technique versus another intrauterine tamponade technique (Darwish 2018; Khalil 2011) ‐ it was not possible to combine these data due to the nature of the interventions under investigation.
Bakri balloon tamponade versus condom‐loaded Foley catheter (comparison 6)
Darwish 2018 compared Bakri balloon with a condom‐loaded Foley catheter. The Bakri balloon or condom‐loaded Foley catheter was positioned correctly and then inflated with saline (150 mL initially, and subsequently inflated to 400 mL to 500 mL) until there was a decrease in blood draining through the catheter.
Bakri balloon + traction stitch versus Bakri balloon without traction stitch (comparison 7)
Khalil 2011 compared the use of Bakri balloon with a traction stitch (to help hold it in place) with Bakri balloon without a traction stitch. The trial report did not provide information about how much saline was used to inflate the Bakri balloons. All women were given intravenous antibiotics for the first 48 hours and oxytocin for the first eight hours.
Condom‐loaded catheter versus uterovaginal packing (comparison 8)
Ashraf 2018 compared the use of a condom‐loaded catheter intrauterine balloon tamponade with the use of uterovaginal packing. Each intervention was left in place for 24 hours and all women were given prophylactic antibiotics (no further information provided in the trial report).
One uterine compression suture technique versus another uterine compression suture technique
Modified B‐Lynch compression suture versus standard B‐Lynch compression suture (comparison 9)
El‐Sokkary 2016 compared the standard B‐Lynch compression suture technique with a modified version of the technique in which the sutures are applied in a figure of eight in order to exert greater uterine compression than the standard B‐Lynch compression sutures.
Outcomes
Chantrapitak 2009 reported blood loss (mL) before treatment, mean blood loss and standard deviations, and median blood loss after treatment. Incidence of blood transfusion, and need for additional uterotonic agent to control bleeding (prostaglandin) were also reported.
Darwish 2018 reported the number of women who needed some sort of further surgical intervention (i.e. hysterectomy, B‐Lynch compression suture) to stop bleeding. Secondary outcomes included time (minutes) between intervention and cessation of bleeding, blood transfusion, referral to intensive care unit, development of disseminated intravascular coagulation (DIC), and post‐insertion fever.
Dumont 2017 employed a composite outcome as their primary outcome ‐ "the proportion of women with recourse to an invasive surgery (arterial ligatures, uterine compression sutures, hysterectomy) or who died before hospital discharge" (p3). Secondary outcomes included, arterial ligations, uterine compression sutures, hysterectomy to control bleeding, transfer to intensive care, total blood loss greater than 1000 mL, and maternal death.
The main outcome in El‐Sokkary 2016 was whether the intervention was successful: successful (cessation of bleeding and hysterectomy not needed), unsuccessful (bleeding continued and hysterectomy needed). Other outcomes reported include postoperative blood loss; hysterectomy; blood transfusion; hospital stay; duration of the procedure; pre‐ and postoperative haemoglobin (Hb); bleeding from multiple bites; haematoma; wound haematoma; wound infection; fever.
Farouk 2016 reported cessation of bleeding, hysterectomy to control bleeding, postpartum fever (temperature > 38.5 deg C), and complications.
Kavak 2013 reported mortality, hysterectomy, intraoperative blood loss and blood transfusion as well as postoperative blood loss. Postoperative Hb and haematocrit are also reported along with 'time of operation' (assumed to be duration). Adverse effects were also recorded along with postoperative fever. We tried to contact the authors to provide data on post‐randomisation blood loss only (rather than the quoted 'intrapartum blood loss') but we did not receive a response. We therefore used the data provided on 'total intrapartum blood loss' assuming that the randomisation process would equalise the pre‐randomisation blood loss between the two arms of the study.
Khalil 2011 reports the 'estimated blood loss', 'hysterectomy required', 'other surgeries required', along with three outcomes not in this review: 'displacement of the balloon', 'bleeding after displacement of the balloon', and 'bleeding after deflation of balloon'. The author provided data on the number of women with blood loss of 1000 mL and with blood loss over 1000 mL. The continuous variable for blood loss was provided as mean and range only (not mean and standard deviation), so it was not possible to use this outcome.
The primary outcome in the Soltan 2007 trial was maternal mortality. Secondary outcomes were treatment failure, abdominal hysterectomy, surgical operations, reinsertion of the balloon, balloon rupture, pyrexia, allergic reactions, The author provided data on units of syntocinon and ampoules of ergometrine, but these were provided as means and standard deviations and it is unclear whether these were 'additional' uterotonics or part of the normal care (WHO ecobolic protocol) that both groups received. Similarly, the author provided continuous data for number of units of blood transfused, and days stay in intensive care unit, but since these are in mean and standard deviation format it is not possible to identify the number of women in each group with these outcomes. Soltan 2007 also reported Hb and haematocrit on discharge, time to resuscitate, and time to regain normal uterine tone. Soltan 2007 reports that 19 women in the normal care group were given the intervention (El‐Menia balloon) because bleeding did not stop. The women remain in the 'control' group despite having received the intervention thus preserving intention‐to‐treat but since these data were not reported separately we are unaware of the impact that this may have on the outcome data for the control group.
The Ashraf 2018 reports fever (side effect of the intervention) relevant to the outcomes in this review. The study also reported on the following outcomes not featured in this review: Mean blood loss; efficacy (defined as blood loss stopped within 15 minutes of insertion and no recurrence of bleeding after removal of tamponade/packing); safety (no infection or fever; perforation (no further information given).
Sources of trial funding
Sources of trial funding were not mentioned in seven studies (Chantrapitak 2009; Darwish 2018; El‐Sokkary 2016; Farouk 2016; Kavak 2013, Khalil 2011; Soltan 2007). Dumont 2017, was funded by the Research Institute for Development (IRD) and United Nations Children's Fund (UNICEF). Ashraf 2018 reported that there were no sources of trial funding.
Trial authors' declarations of interest
Six trial authors reported that they had no conflicts of interest (Darwish 2018; Dumont 2017; El‐Sokkary 2016; Kavak 2013; Khalil 2011; Ashraf 2018). Trial authors' declarations were not mentioned in Chantrapitak 2009, Farouk 2016 or Soltan 2007.
Was informed consent obtained from the trial participants?
Five trials reported that consent was sought from the trial participants (Darwish 2018; Dumont 2017; Farouk 2016; Kavak 2013; Khalil 2011), and in one trial (Soltan 2007), informed consent was sought from the women's husbands. Informed consent was not mentioned in two trials (Chantrapitak 2009; El‐Sokkary 2016). Ashraf 2018 reports that informed consent was obtained from the women regarding "using their data for study purpose" p891, but it is not clear whether the women gave informed consent to receive the intervention.
Was ethical approval sought?
Seven trials reported having obtained ethical approval for the trial (Darwish 2018; Dumont 2017; El‐Sokkary 2016; Farouk 2016; Kavak 2013; Khalil 2011; Soltan 2007), but ethical approval was not mentioned in Chantrapitak 2009. In the Ashraf 2018 trial report, it states, "ethical approval: Given" p902 but not further detail is provided.
Excluded studies
We excluded 17 trials.
Two trials were quasi‐RCTs (Soltan 2009; Soltan 2010), and four were only available in abstract and had insufficient information to assess risk of bias (Gelany 2012; Khalil 2014; Mohamed 2014; von Beckerath 2016). One trial (Anger 2016) was a stepped‐wedge cluster‐RCT which randomised 18 hospitals to a policy of introducing uterine balloon tamponade (UBT) into standard care. The participants included all women (not just women with PPH) and sensitivity analysis for women with PPH was not provided. One trial (Letouzey 2013), comparing Bakri balloon versus routine care was terminated due to recruitment difficulties. One trial (Rahman 2015), comparing Tampostat (TM) balloon tamponade device versus a condom catheter tamponade was also terminated.
Six trials were prevention of PPH studies (Azmy 2016; Chen 2017; Farouk 2018; Nermeen 2015; Rezk 2016; Sallam 2019). One trial was not an RCT (Purwosonu 2015), and one other (Liu 2016), was a comparison of two different nursing methods for women who had all had uterine embolisation for PPH.
See Characteristics of excluded studies for further information.
Risk of bias in included studies
Overall, the included trials were at an unclear risk of bias. See Figure 2 and Figure 3.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
We assessed seven studies as low risk of bias for sequence generation (Ashraf 2018; Darwish 2018; Dumont 2017; El‐Sokkary 2016; Farouk 2016; Khalil 2011; Soltan 2007). Methods included computer‐generated random tables (Darwish 2018; Dumont 2017; El‐Sokkary 2016), a computer‐generated list (Farouk 2016), computer‐based randomisation (Khalil 2011;Soltan 2007,) or using the lottery method (Ashraf 2018).
In Chantrapitak 2009 and Kavak 2013, the methods of sequence generation were unclear. Chantrapitak 2009 reported that the two groups were 'equally divided' and were 'randomly assigned' to the treatment groups, and Kavak 2013 reported that the participants were 'randomly divided' ‐ but in both cases, no further information was provided.
Allocation concealment
We assessed two trials as being low risk of bias for allocation concealment. Darwish 2018 used serially numbered opaque sealed envelopes. Soltan 2007 used closed opaque envelopes.
We assessed the remaining seven studies as being at unclear risk of bias. No information pertaining to allocation concealment was mentioned in Ashraf 2018; Chantrapitak 2009; Kavak 2013; and Khalil 2011. Two trials (El‐Sokkary 2016; Farouk 2016) mentioned 'closed' or 'sealed' envelopes, but it was not clear whether these were sequentially numbered opaque sealed envelopes. In Dumont 2017 ‐ the randomisation code was known to four people (suggesting this was a revealed list) and the trial supervisor decided over the telephone whether to randomise or not.
Blinding
Blinding of participants and personnel (performance bias)
The studies of Darwish 2018 and Kavak 2013 were described as single‐blinded. In both Darwish 2018 and Kavak 2013, it is not specified who was blinded, but presumably it was the patients who were all under general anaesthetic. The surgeons could not have been blinded due to the nature of the intervention, and so the risk of bias is still judged to be 'high risk' (please refer to Figure 2. Chantrapitak 2009, Dumont 2017, El‐Sokkary 2016, Khalil 2011, Farouk 2016 and Soltan 2007 used closed opaque envelopes to conceal allocation, but there was no blinding reported. Blinding was not mentioned in Ashraf 2018. Blinding was difficult in all of these studies because the investigators were also the surgeons who were the involved in applying the surgical techniques ‐ it is therefore very difficult to blind them.
Blinding of outcome assessors (detection bias)
The outcome data were not reported to be blinded in any of the studies and we assessed seven of trials as 'unclear' risk of detection bias. We assessed two trials as being at a high risk of detection bias. In the Darwish 2018 trial, it is possible that non‐independent blinded providers have biased the outcomes assessments. In the Dumont 2017 it is possible that non‐independent blinded providers have biased the outcomes assessments (please refer to Figure 2).
Incomplete outcome data
We assessed one trial Chantrapitak 2009) to be at a high risk of attrition bias. In the trial report, there are two women with cervical and vaginal tears whose outcomes are not reported. It is unclear whether these were excluded before or after randomisation. However, cervical and vaginal tears are only usually found upon detailed examination after failure of primary treatment (in this case oxytocics plus uterine compression). It therefore seems likely that they were excluded after randomisation.
In the Darwish 2018 study, the study's main outcomes were reported for all 66 women recruited. However, treatment failed in eight women across the study and they went on to have either the insertion of a B‐Lynch compression suture (n = 5) or a hysterectomy (n = 3). The study's secondary outcome data on blood transfusion, intensive care unit referral, DIC and fever were not reported for these eight women and the data in the trial report relate to the remaining 58 (analysed 'per protocol'). The incomplete data (especially given that they were the ones who had the worst outcomes) led to a score of 'high risk' for attrition bias.
We assessed six trials as being at low risk of attrition bias (Ashraf 2018; Dumont 2017; Farouk 2016; Kavak 2013; Khalil 2011; Soltan 2007).
We assessed one trial El‐Sokkary 2016 as 'unclear' for this domain because, in table 1 of the trial report, the authors did not state the number of women. In the text, there was no mention of incomplete data but we cannot assume that data were complete.
Selective reporting
In six trials there was no mention of a protocol and we assessed these trials as having an unclear risk of reporting bias (Chantrapitak 2009; El‐Sokkary 2016; Farouk 2016; Kavak 2013; Khalil 2011; Soltan 2007). We assessed Darwish 2018 as unclear risk of bias ‐ whilst the trial was prospectively registered, there are outcomes in the trial report that were not mentioned in the protocol. Similarly, Dumont 2017 was prospectively registered but there were some minor differences between the outcomes in the protocol and the published trial report, but we assessed the trial as low risk of reporting bias. We assessed Ashraf 2018 as having a high risk of bias for selective reporting ‐ a protocol was not available for this study. We note that one outcome (perforation) not detailed in the methods, is reported in the results. Generally, there are very few outcomes reported, including none of the outcomes listed in the article background, and there are no escalation outcomes reported (e.g. hysterectomy due to bleeding). It is also unclear from the report whether the mean blood loss outcome is pre‐randomisation (i.e. a participant characteristic) or post‐randomisation.
Other potential sources of bias
We assessed six studies as low risk of other bias as no other potential sources of bias were identified. The remaining three trials were at unclear (Ashraf 2018) and high (Dumont 2017; Soltan 2007) risk of other bias.
Ashraf 2018 was assessed as having an unclear risk of other bias ‐ the trial methods contain discrepancies with the reported participant characteristics and there is no mention of whether the women also received usual care in addition to the interventions of interest and, if so, what that was comprised of. Soltan 2007 was assessed as having a high risk of other bias because 19 women in the control group also received the intervention as a second‐line treatment. This secondary use of UBT was not prespecified in the methods
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
Summary of findings 1. External uterine compression (all methods) plus normal care compared to normal care for treating primary postpartum haemorrhage.
| External uterine compression (all methods) compared to normal care for treating primary postpartum haemorrhage | ||||||
| Patient or population: women diagnosed with primary PPH following vaginal birth. PPH was defined as quote: "blood loss > 500mL after delivery" p 601 Setting: hospital setting in Bangkok, Thailand (Chantrapitak 2009) Intervention: external lower uterine compression (either by grasping the uterus through a lax abdominal wall or compressing the uterus against the sacrum and lower vertebrae) for a duration of 10 minutes (plus standard care) Comparison: normal care alone ‐ consisting of "massage, oxytocin (10‐20 units in 1,000 ml of intravenous solution, 200 ml/min), intravenous ergometrine (Methergin®, 0.2 mg), placed cold pack on uterus, and urinary catheterisation" p 601. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with normal care | Risk with external uterine compression (all methods) | |||||
| Mortality due to bleeding ‐ not reported | See comment | Outcome not reported by trial authors | ||||
| Hysterectomy to control bleeding ‐ not reported | See comment | Outcome not reported by trial authors | ||||
| Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure) ‐ not reported | See comment | Outcome not reported by trial authors | ||||
| Number of women with total blood loss 1000 mL or more after randomisation ‐ not reported | See comment | Outcome not reported by trial authors | ||||
| Mean blood loss (mL) (trialist defined) | See comment | Outcome not reported by trial authors | ||||
| Blood transfusion (red cell or whole blood) | Study population | RR 2.33 (0.66 to 8.23) | 64 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 94 per 1000 | 218 per 1000 (62 to 772) | |||||
| Side effects of the intervention (e.g. trauma, necrosis) ‐ not reported | See comment | Outcome not reported by trial authors | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (1) level for serious limitations in study design (risk of bias)
2 We downgraded (2) levels for very serious imprecision due to small sample size, few events and wide confidence interval crossing the line of no effect
Summary of findings 2. One uterine devascularisation technique (uterine arterial embolisation) versus another uterine devascularisation technique (surgical devascularisation plus B‐Lynch).
| One uterine devascularisation technique (uterine arterial embolisation) versus another uterine devascularisation technique (surgical devascularisation plus B‐Lynch) | ||||||
| Patient or population: treating primary postpartum haemorrhage Setting: Ain‐Shams University Maternity Hospital, Egypt (Farouk 2016) Intervention: uterine devascularisation (uterine arterial embolisation) Comparison: another uterine devascularisation technique (surgical devascularisation plus B‐Lynch compression sutures) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with another uterine devascularisation technique (surgical devascularisation plus B‐Lynch compression suture) | Risk with uterine arterial embolisation | |||||
| Mortality due to bleeding ‐ not reported | See comment | Outcome not reported by trial authors | ||||
| Hysterectomy to control bleeding | Study population | RR 0.73 (0.15 to 3.57) | 23 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 250 per 1000 | 183 per 1000 (38 to 893) | |||||
| Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure) | See comment | Outcome not reported by trial authors | ||||
| Number of women with total blood loss 1000 mL or more after randomisation | See comment | Outcome not reported by trial authors | ||||
| Mean blood loss (mL) (trialist defined) | See comment | Outcome not reported by trial authors | ||||
| Blood transfusion (red cell or whole blood) | See comment | Outcome not reported by trial authors | ||||
| Side effects of the intervention (e.g. trauma, necrosis) | Study population | RR 1.09 (0.08 to 15.41) | 23 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 83 per 1000 | 91 per 1000 (7 to 1000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (1) level for serious limitations in study design
2 We downgraded (2) levels for very serious imprecision due to small sample size with few events and wide confidence intervals crossing the line of no effect
Summary of findings 3. Intrauterine balloon tamponade plus normal care (misoprostol) compared to normal care (misoprostol) for treating primary postpartum haemorrhage.
| Intrauterine balloon tamponade plus normal care (misoprostol) compared to normal care (misoprostol) for treating primary postpartum haemorrhage | ||||||
|
Patient or population: women diagnosed with primary PPH following vaginal birth. Women were suspected to have PPH due to clinical atony and who were quote: "unresponsive to oxytocin and who needed additional uterotonics" p1. PPH was defined as quote: "visual estimation of excessive blood loss and patient status (blood pressure and cardiac frequency)" p 2 (Dumont 2017) Setting: 7 healthcare facilities in Benin and Mail Intervention: intrauterine balloon tamponade (plus misoprostol 'standard care') Comparison: standard care (misoprostol) alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with normal care | Risk with intrauterine balloon tamponade (all methods) | |||||
| Mortality due to bleeding | Study population | RR 6.21 (0.77 to 49.98) | 116 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 17 per 1000 | 105 per 1000 (13 to 847) | |||||
| Hysterectomy to control bleeding | Study population | RR 4.14 (0.48 to 35.93) | 116 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 17 per 1000 | 70 per 1000 (8 to 609) | |||||
| Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure) | Outcome not reported by trial authors | |||||
| Number of women with total blood loss 1000 mL or more after randomisation | Study population | RR 1.52 (1.15 to 2.00) | 113 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | ||
| 525 per 1000 | 799 per 1000 (604 to 1000) | |||||
| Mean blood loss (mL) (trialist defined) | See comment | Outcome not reported by trial authors | ||||
| Blood transfusion (red cell or whole blood) | Study population | RR 1.49 (0.88 to 2.51) | 116 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | ||
| 271 per 1000 | 404 per 1000 (239 to 681) | |||||
| Side effects of the intervention (e.g. trauma, necrosis) ‐ Severe shivering, diarrhoea, vomiting or high temperature | Study population | not estimable | 116 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 5 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (‐2) levels for very serious limitations in study design (risk of bias)
2 We downgraded (‐2) levels for very serious imprecision due to small sample size, few events and wide confidence interval crossing the line of no effect
3 We downgraded (‐1) level for serious imprecision due to a small sample size
4 We downgraded (‐2) levels for very serious imprecision due to small sample size, and wide confidence interval crossing the line of no effect
5 We downgraded (‐2) levels for very serious imprecision due to small sample size and zero events
Summary of findings 4. Intrauterine balloon tamponade (latex balloon inflated with air) plus cerclage and normal care compared to normal care for treating primary postpartum haemorrhage.
| Intrauterine balloon tamponade (latex balloon inflated with air) plus cerclage and normal care compared to normal care for treating primary postpartum haemorrhage | ||||||
|
Patient or population: women with PPH, due to atony, following vaginal birth at home or in hospital (Soltan 2007) Setting: Hospital (Egypt) Intervention: Latex balloon‐loaded Nelson catheter intrauterine tamponade (air filled) plus stitch and standard care (uterine massage and uterotonics) Comparison: standard care (uterine massage and uterotonics) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with normal care | Risk with intrauterine balloon tamponade (all methods) | |||||
| Mortality due to bleeding | Study population | Not estimable | 240 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | There were no maternal deaths due to bleeding | |
| 0 per 1,000 | 0 per 1,000 | |||||
| Hysterectomy to control bleeding | Study population | RR 0.14 (0.01 to 2.74) | 240 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 4 | ||
| 25 per 1,000 | 4 per 1,000 | |||||
| Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure) | See comment | Outcome not reported by trial authors | ||||
| Number of women with total blood loss 1000 mL or more after randomisation | See comment | Outcome not reported by trial authors | ||||
| Mean blood loss (mL) (trialist defined) | See comment | Outcome not reported by trial authors | ||||
| Blood transfusion (red cell or whole blood) | ||||||
| Side effects of the intervention (e.g. trauma, necrosis) ‐ trauma, pyrexia, allergic reaction | Study population | Not estimable | 240 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 1 2 3 | There were no side effects of the intervention (reported as trauma, pyrexia, or allergic reaction) | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (‐1) level for serious limitations in study design (risk of bias)
2 We downgraded (‐2) levels for very serious indirectness (the study population included all women with PPH (UBT used as a first‐line treatment), rather than only those who did not respond to treatment with uterotonics
3 We downgraded (‐2) levels for very serious imprecision (small sample size, no events, not estimable)
4 We downgraded (‐2) levels for very serious imprecision due to small sample size, few events and wide confidence interval including appreciable benefit and appreciable harm
Summary of findings 5. Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (intrauterine tamponade versus another mechanical/surgical method) for treating primary postpartum haemorrhage.
| Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (intrauterine tamponade versus another mechanical/surgical method) for treating primary postpartum haemorrhage | ||||||
| Patient or population: women with complete placenta praevia and intractable bleeding following caesarean section Setting: university hospital setting in Turkey (Kavak 2013) Intervention: intrauterine tamponade (Bakri balloon) Comparison: endouterine compression suturing to the lower segment of the uterus | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with another mechanical/surgical method (endouterine compression sutures) | Risk with Intrauterine Bakri balloon tamponade | |||||
| Mortality due to bleeding | Study population | not estimable | 13 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | There were zero events | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Hysterectomy to control bleeding | Study population | not estimable | 13 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | There were zero events | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure) | Study population | not estimable | 13 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | There were zero events | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Number of women with total blood loss 1000 mL or more after randomisation | See comment | Outcome not reported by trial authors | ||||
| Mean blood loss (mL) (trialist defined) | The mean blood loss in endouterine compression suture group was 1946 mL | The mean difference in blood loss (mL) in the intervention group was 426 mL lower (631.28 lower to 220.72 mL lower) | 13 (1 RCT) |
⊕⊝⊝⊝
VERY LOW 1 3 |
||
| Blood transfusion (red cell or whole blood) | Study population | RR 0.57 (0.14 to 2.36) | 13 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | ||
| 500 per 1000 | 285 per 1000 (70 to 1000) | |||||
| Side effects of the intervention (e.g. trauma, necrosis) | Study population | not estimable | 13 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (2) levels for very serious limitations in study design (risk of bias)
2 We downgraded (2) levels for very serious imprecision due to small sample size and zero events (not estimable)
3 We downgraded (1) level for serious imprecision due to a small sample size (continuous data)
4 We downgraded (2) levels for very serious imprecision due to small sample size, few events and wide confidence interval crossing the line of no effect
Summary of findings 6. Bakri balloon tamponade versus condom‐loaded Foley Catheter (one intrauterine tamponade compared to another intrauterine tamponade technique) for treating primary postpartum haemorrhage.
| Bakri balloon tamponade versus condom‐loaded Foley Catheter (one intrauterine tamponade compared to another intrauterine tamponade technique for treating primary postpartum haemorrhage) | ||||||
| Patient or population: women with primary atonic postpartum haemorrhage following vaginal delivery Setting: Assuit Women's Health Hospital in Egypt (Darwish 2018) Intervention: 1 intrauterine tamponade: Bakri balloon Comparison: another intrauterine tamponade technique: condom‐loaded Foley catheter | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with condom‐loaded Foley catheter | Risk with Bakri balloon tamponade | |||||
| Mortality due to bleeding | See comment | Outcome not reported by trial authors | ||||
| Hysterectomy to control bleeding | Study population | RR 0.50 (0.05 to 5.25) | 66 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | ||
| 61 per 1000 | 30 per 1000 (3 to 318) | |||||
| Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure) | See comment | Outcome not reported by trial authors | ||||
| Number of women with total blood loss 1000 mL or more after randomisation | See comment | Outcome not reported by trial authors | ||||
| Mean blood loss (mL) (trialist defined) | See comment | Outcome not reported by trial authors | ||||
| Blood transfusion (red cell or whole blood) | Study population | RR 0.97 (0.88 to 1.06) | 58 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3,4 | Note: the trial report states that analysis for this and other trial secondary outcomes excluded those women in whom there was treatment failure. | |
| 1000 per 1000 | 970 per 1000 (880 to 1000) | |||||
| Side effects of the intervention (e.g. trauma, necrosis) | See comment | Outcome not reported by trial authors | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (1) level for serious limitations in study design (risk of bias)
2 We downgraded (2) levels for very serious imprecision (small sample size, few events, and wide confidence interval including appreciable benefit and appreciable harm)
3 We downgraded (1) level for serious imprecision due to small sample size
4 We downgraded (2) levels for very serious limitations in study design (post‐randomisation exclusions ‐ analysis excluded those women in whom there was treatment failure)
Summary of findings 7. Bakri balloon tamponade+traction stitch versus Bakri balloon (one intrauterine tamponade compared to another intrauterine tamponade technique) for treating primary postpartum haemorrhage).
| Bakri balloon tamponade+traction stitch versus Bakri balloon (one intrauterine tamponade compared to another intrauterine tamponade technique) for treating primary postpartum haemorrhage | ||||||
| Patient or population: women with primary atonic postpartum haemorrhage Setting: a security forces hospital in Riyadh, Saudi Arabia (Khalil 2011) Intervention: intrauterine tamponade: Bakri balloon with traction stitch Comparison: another intrauterine tamponade technique: Bakri balloon without traction stitch | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Bakri balloon tamponade (without traction stitch) | Risk with Bakri balloon tamponade+traction stitch) | |||||
| Mortality due to bleeding | See comment | Outcome not reported by trial authors | ||||
| Hysterectomy to control bleeding | Study population | RR 0.20 (0.01 to 3.97) | 50 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 80 per 1000 | 16 per 1000 (1 to 318) | |||||
| Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure) | See comment | Outcome not reported by trial authors | ||||
| Number of women with total blood loss 1000 mL or more after randomisation | See comment | Outcome not reported by trial authors | ||||
| Mean blood loss (mL) (trialist defined) | See comment | Outcome not reported by trial authors | ||||
| Blood transfusion (red cell or whole blood) | See comment | Outcome not reported by trial authors | ||||
| Side effects of the intervention (e.g. trauma, necrosis) | See comment | Outcome not reported by trial authors | ||||
| Total blood loss >= 1000 mL (before and after randomisation, outcome not pre‐specified in our protocol) | Study population | RR 1.00 (0.93 to 1.08) | 50 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | ||
| 1000 per 1000 | 1000 per 1000 (930 to 1000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (1) level for serious limitations in study design (risk of bias)
2 We downgraded (2) levels for very serious imprecision due to small sample sizes, few events and wide confidence interval crossing the line of no effect
3 We downgraded (1) level for serious imprecision due to small sample size
Summary of findings 8. Intrauterine balloon tamponade (condom‐loaded catheter) versus uterovaginal packing (intrauterine tamponade versus another intrauterine tamponade method) for treating primary postpartum haemorrhage.
| Intrauterine balloon tamponade (condom‐loaded catheter) versus uterovaginal packing (intrauterine tamponade versus another intrauterine tamponade method) for treating primary postpartum haemorrhage | ||||||
|
Patient or population: Women (aged between 20 and 40) diagnosed with primary PPH following 'normal' vaginal birth and unresponsive to medical treatment (Ashraf 2018) Setting: Hospital setting in Pakistan Intervention: Condom‐loaded catheter intrauterine balloon tamponade Comparison: uterovaginal packing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with normal care | Risk with intrauterine balloon tamponade (all methods) | |||||
| Mortality due to bleeding | See comment | Outcome not reported by trial authors | ||||
| Hysterectomy to control bleeding | See comment | Outcome not reported by trial authors | ||||
| Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure) | See comment | Outcome not reported by trial authors | ||||
| Number of women with total blood loss 1000 mL or more after randomisation | See comment | Outcome not reported by trial authors | ||||
| Mean blood loss (mL) (trialist defined) | See comment | Mean blood loss is reported as 600.28 mL (+/‐ 25.33 mL) in the condom catheter group and 699 mL (+/‐ 70.176 mL) in the intrauterine packing group. However, it is unclear whether this is pre‐ or post‐randomisation, especially as this is also presented in a table detailing participant characteristics of each group (age, parity, gestational age, and blood loss). For this reason, we have not included these data in our data and analysis tables. | ||||
| Blood transfusion (red cell or whole blood) | See comment | Outcome not reported by trial authors | ||||
| Side effects of the intervention (e.g. trauma, necrosis) ‐ fever | Study population | RR 0.47 (0.38 to 0.59) | 212 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 925 per 1000 | 435 per 1000 (351 to 545) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (2) levels for very serious limitations in study design (risk of bias)
2 We downgraded (1) level for serious imprecision (small sample size)
Summary of findings 9. Modified B‐Lynch compression suture technique versus standard B‐Lynch compression suture (one uterine compression suture technique versus another uterine compression suture technique) for treating primary postpartum haemorrhage.
| Modified B‐Lynch compression suture technique versus standard B‐Lynch compression suture (one uterine compression suture technique versus another uterine compression suture technique) for treating primary postpartum haemorrhage | ||||||
|
Patient or population: women with uncontrolled atonic PPH following caesarean section, and not responding to standard care (uterine massage, ecbolics and bimanual compression). Setting: university hospital in Cairo, Egypt (El‐Sokkary 2016) Intervention: 1 uterine compression suture technique: modified B‐Lynch compression suture Comparison: another uterine compression suture technique: standard B‐Lynch compression suture | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard B‐Lynch uterine compression suture technique | Risk with modified B‐Lynch compression suture technique | |||||
| Mortality due to bleeding | See comment | Outcome not reported by trial authors | ||||
| Hysterectomy to control bleeding | Study population | RR 0.33 (0.11 to 0.99) | 160 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 150 per 1000 | 50 per 1000 (17 to 149) | |||||
| Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure) | See comment | Outcome not reported by trial authors | ||||
| Number of women with total blood loss 1000 mL or more after randomisation | See comment | Outcome not reported by trial authors | ||||
| Mean blood loss (mL) (trialist defined) | The mean blood loss in the classic B‐Lynch group was 568 mL | The mean difference in blood loss (mL) in the modified B‐Lynch group was 244 mL lower (295.25 mL lower to ‐ 192.75 mL lower) | ‐ | ⊕⊕⊝⊝ LOW 1 3 | ||
| Blood transfusion (red cell or whole blood) | Outcome not reported by trial authors | |||||
| Side effects of the intervention (e.g. trauma, necrosis) | See comment | Outcome not reported by trial authors | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded (1) level for serious limitations in study design (risk of bias)
2 We downgraded (1) level for serious imprecision due to small sample size and few events
3 We downgraded (1) level for serious imprecision due to small sample size
We included nine small studies, involving a total of 944 women with primary PPH following either caesarean section or vaginal birth. It was not possible to combine any of the studies in meta‐analysis due to differences in the interventions and comparisons under investigation. We therefore present outcome data for individual studies below. Outcomes that form part of a core outcome set that will be used in all PPH reviews are highlighted below with an asterisk.
External uterine compression versus normal care (external uterine compression versus normal care) (comparison 1)
One small study (Chantrapitak 2009) contributed data to this comparison. The study, which involved 64 women diagnosed with uncontrolled primary PPH following vaginal birth. The study was conducted in Thailand and examined the use of external lower uterine compression versus usual care for treating primary PPH.
Primary outcomes
Mortality due to bleeding
This outcome was not reported in the included study under this comparison.
Hysterectomy to control bleeding
This outcome was not reported in the included study under this comparison.
Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure)
This outcome was not reported in the included study under this comparison.
Secondary outcomes
Mean blood loss (mL) (trialist defined)*
Chantrapitak 2009 reported amount of blood loss (mL) after treatment (data were reported as medians with interquartile range ‐ external uterine compression:120 mL (∓ 211.0 mL); normal care: 225.0 mL (∓ 401.0 mL).
Blood transfusion (red cell or whole blood)*
Very low‐certainty evidence means that we are unclear about the evidence relating to the incidence of red cell or whole blood transfusion (risk ratio (RR) 2.33, 95% confidence interval (CI) 0.66 to 8.23; 64 women; 1 study; Analysis 1.1). There were 7/32 women with red cell or whole blood transfusion in the external uterine compression group and 3/32 women in the normal care control.
1.1. Analysis.

Comparison 1: External lower uterine compression versus normal care, Outcome 1: Blood transfusion (red cell or whole blood)
Post‐randomisation additional uterotonic agent used to control bleeding*
Two of the 32 women in the external uterine compression group and 3/32 women in the normal care group required post‐randomisation additional uterotonic agent to control bleeding (RR 0.67, 95% CI 0.12 to 3.73; 64 women, 1 study).
Secondary outcomes not reported by the study under this comparison
The following secondary outcomes of interest in this review were not reported in the one included study under this comparison.
All‐cause mortality*
Mortality from causes other than bleeding
Shock as defined by trialist*
Coagulopathy as defined by trialist*
Number of women with total blood loss 500 mL or more after randomisation*
Number of women with total blood loss 1000 mL or more after randomisation*
Blood product transfusion*
Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy)*
Post‐randomisation additional non‐surgical intervention to control bleeding (uterine packing, bimanual uterine massage, tamponade, external aortic compression and compression garments)
Admission to higher level of care*
Side effects of the intervention (e.g. trauma, necrosis)
Days in hospital
Breastfeeding (defined as any breastfeeding at hospital discharge)*
Maternal satisfaction with therapy (trialist defined)*
Quality of life, including physiological activity and social and emotional changes (sense of well‐being) (trialist defined)*
Uterine arterial embolisation versus surgical devascularisation plus B‐Lynch (One uterine devascularisation technique versus another uterine devascularisation technique) (comparison 2)
One small study (Farouk 2016) contributed data to this comparison. The study was conducted in Egypt and examined the use of uterine arterial embolisation (UAE) versus surgical devascularisation for treating primary PPH. The study involved 23 women with uncontrolled primary atonic PPH following vaginal delivery (9/11 in the UAE group and 8/12 in the surgical devascularisation+B‐Lynch group) or caesarean section (2/11 in the UAE group and 4/12 in the surgical devascularisation+B‐Lynch group).
Primary outcomes
Mortality due to bleeding
This outcome was not reported by Farouk 2016.
Hysterectomy to control bleeding
Very low‐certainty evidence means that we are unclear about the results for hysterectomy to control bleeding, with 2/11 women having hysterectomy to control bleeding in the uterine arterial embolisation group compared with 3/12 women in the surgical devascularisation+ B‐Lynch group (RR 0.73, 95% CI 0.15 to 3.57; 23 women, 1 study; Analysis 2.1).
2.1. Analysis.

Comparison 2: Uterine arterial embolisation versus surgical devascularisation plus B‐Lynch (one uterine devascularisation technique versus another uterine devascularisation technique), Outcome 1: Hysterectomy to control bleeding
Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure)
This outcome was not reported by Farouk 2016.
Secondary outcomes
Side effects of the intervention (e.g. trauma, necrosis)
Very low‐certainty evidence means we are unclear about the results for this outcome, with just one woman in each group reporting side effects (RR 1.09, 95% CI 0.08 to 15.41; 23 women, 1 study). One woman in the embolisation group reported gluteal pain and one women in the surgical group had bladder injury. There were other 'complications' in each group (sepsis, fever, renal failure) but we do not consider these to be side effects of the intervention. See Analysis 2.2.
2.2. Analysis.

Comparison 2: Uterine arterial embolisation versus surgical devascularisation plus B‐Lynch (one uterine devascularisation technique versus another uterine devascularisation technique), Outcome 2: Side effects of the intervention (e.g. trauma, necrosis)
Secondary outcomes not reported by the study under this comparison
All‐cause mortality*
Mortality from causes other than bleeding
Shock as defined by trialist*
Number of women with total blood loss 500 mL or more after randomisation*
Number of women with total blood loss 1000 mL or more after randomisation*
Mean blood loss (mL) (trialist defined)*
Coagulopathy as defined by trialist*
Blood transfusion (red cell or whole blood)*
Blood product transfusion*
Post‐randomisation additional uterotonic agent used to control bleeding*
Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy)*
Post‐randomisation additional non‐surgical intervention to control bleeding (uterine packing, bimanual uterine massage, tamponade, external aortic compression and compression garments)
Admission to higher level of care*
Days in hospital
Breastfeeding (defined as any breastfeeding at hospital discharge)*
Maternal satisfaction with therapy (trialist defined)*
Quality of life, including physiological activity and social and emotional changes (sense of well‐being) (trialist defined)*
Intrauterine balloon tamponade plus normal care (misoprostol) versus normal care (misoprostol) (intrauterine tamponade versus normal care) (comparison 3)
One small study (Dumont 2017) contributed data to this comparison. In this multi‐centre study, which involved 116 women with primary PPH (thought to be due to clinical atony) following vaginal birth. The study was conducted in seven different healthcare facilities in Benin and Mali and examined the use of an intrauterine balloon tamponade plus normal are (misoprostol) versus usual care (misoprostol) alone for treating primary PPH.
Primary outcomes
Mortality due to bleeding
Due to very low‐certainty evidence, we are unclear about the results for maternal mortality due to bleeding (RR 6.21, 95% CI 0.77 to 49.98; 116 women, 1 study; very low‐certainty evidence; Analysis 3.1). There were 6/57 maternal deaths due to bleeding in the intrauterine tamponade group compared to 1/59 in the normal care control.
3.1. Analysis.

Comparison 3: Intrauterine balloon tamponade plus normal care (misoprostol) versus normal care (misoprostol), Outcome 1: Mortality due to bleeding
Hysterectomy to control bleeding
Similarly, very low‐certainty evidence for the outcome 'hysterectomy to control bleeding' means that we are unclear about the results for this outcome (RR 4.14, 95% CI 0.48 to 35.93; 116 women, 1 study; very low‐certainty evidence; Analysis 3.2).
3.2. Analysis.

Comparison 3: Intrauterine balloon tamponade plus normal care (misoprostol) versus normal care (misoprostol), Outcome 2: Hysterectomy to control bleeding
Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure)
This outcome was not reported by Dumont 2017.
Secondary outcomes
All‐cause mortality*
There were 6/57 all cause maternal deaths in the intrauterine tamponade group compared to 1/59 in the normal care control (RR 6.21, 95% CI 0.77 to 49.98; 116 women, 1 study; Analysis 3.3).
3.3. Analysis.

Comparison 3: Intrauterine balloon tamponade plus normal care (misoprostol) versus normal care (misoprostol), Outcome 3: All cause mortality
Mortality from causes other than bleeding
There were no maternal deaths in either group resulting from causes other than bleeding. Analysis 3.4.
3.4. Analysis.

Comparison 3: Intrauterine balloon tamponade plus normal care (misoprostol) versus normal care (misoprostol), Outcome 4: Mortality from causes other than bleeding
Number of women with total blood loss 1000 mL or more after randomisation*
Balloon tamponade may increase the incidence of total blood loss 1000 mL or more after randomisation, but the evidence is of very low certainty (RR 1.52, 95% CI 1.15 to 2.00, 113 women, 1 study; low‐certainty evidence; Analysis 3.5). There were 43/54 women with this outcome in the intrauterine tamponade group compared to 31/59 women receiving standard care. The reported blood loss includes all blood lost postnatally, rather than that just following randomisation. However, we have assumed that the correct randomisation has equally distributed the pre‐randomisation blood loss between the two study arms.
3.5. Analysis.

Comparison 3: Intrauterine balloon tamponade plus normal care (misoprostol) versus normal care (misoprostol), Outcome 5: Number of women with total blood loss 1000 mL or more after randomisation
Blood transfusion (red cell or whole blood)*
We found very low‐certainty evidence relating to blood transfusion which means we are unclear about the results for this outcome (RR 1.49, 95% CI 0.88 to 2.51; 116 women, 1 study).
Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy)*
Additional surgical interventions to control bleeding included uterine compression sutures (2/57 in the intrauterine tamponade group and 0/59 in normal care group) and artery ligation (4/57 in the intrauterine tamponade group and 3/59 in the normal care group). See Analysis 3.7. Very low‐certainty evidence means we are uncertain about the results for this outcome.
3.7. Analysis.

Comparison 3: Intrauterine balloon tamponade plus normal care (misoprostol) versus normal care (misoprostol), Outcome 7: Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy)
Admission to higher level of care*
The number of women admitted to a higher level of care was (10/57) in the intrauterine tamponade group (10/57) and 8/59 in the standard care group (8/59) (RR 1.29, 95% CI 0.55 to 3.04; 116 women, 1 study; Analysis 3.8).Very low‐certainty evidence means the results are unclear for this outcome.
3.8. Analysis.

Comparison 3: Intrauterine balloon tamponade plus normal care (misoprostol) versus normal care (misoprostol), Outcome 8: Admission to higher level of care
Side effects of the intervention (e.g. trauma, necrosis)
We are uncertain about the results for this outcome due to very low‐certainty evidence. Dumont 2017 reported that none of the women in either group experienced side effects of the intervention (reported as severe shivering, diarrhoea, vomiting or high temperature). See Analysis 3.9.
3.9. Analysis.

Comparison 3: Intrauterine balloon tamponade plus normal care (misoprostol) versus normal care (misoprostol), Outcome 9: Side effects of the intervention (e.g. trauma, necrosis)
Secondary outcomes not reported by the study under this comparison
Shock as defined by trialist*
Coagulopathy as defined by trialist*
Number of women with total blood loss 500 mL or more after randomisation*
Mean blood loss (mL) (trialist defined)*
Blood product transfusion*
Post‐randomisation additional uterotonic agent used to control bleeding*
Post‐randomisation additional non‐surgical intervention to control bleeding (uterine packing, bimanual uterine massage, tamponade, external aortic compression and compression garments)
Days in hospital
Breastfeeding (defined as any breastfeeding at hospital discharge)*
Maternal satisfaction with therapy (trialist defined)*
Quality of life, including physiological activity and social and emotional changes (sense of well‐being) (trialist defined)*
Latex balloon (inflated with air) tamponade plus cerclage and normal care versus normal care (intrauterine tamponade versus normal care) (comparison 4)
One small study (Soltan 2007) contributed data to this comparison. The study, which involved 240 women with PPH due to uterine atony following vaginal birth. Women had given birth in either at home or in hospital. The study was conducted in Egypt and examined the use of a latex party balloon inflated with air and attached to a catheter with suture silk, the balloon was held in place with a cervical stitch. Women in the comparison group received standard care which comprised of uterine massage and ecbolics as per WHO protocol. Women in the intervention group also received antibiotics (metronidazole 500 mg, gentamycin 80 mg and ampicillin 500 mg after the balloon was inserted, and every eight hours for three days.
The trial protocol for Soltan 2007 stated that all of the women in the control group would receive standard care as the first‐line treatment. However, 19/120 women in the control group subsequently received the intervention as a second‐line treatment. The authors preserved intention‐to‐treat (the 19 women remained in the control group).
Primary outcomes
Mortality due to bleeding
There were no maternal deaths due to bleeding.
Hysterectomy to control bleeding
Very low‐certainty evidence for the outcome 'hysterectomy to control bleeding' means that we are unclear about the results for this outcome (RR 0.14, 95% CI 0.01 to 2.74; 240 women, one study; Analysis 4.2).
4.2. Analysis.

Comparison 4: Latex balloon (air filled) tamponade + stitch and normal care versus normal care (Intrauterine tamponade versus normal care), Outcome 2: Hysterectomy to control bleeding
Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure)
This outcome was not reported in the included study under this comparison.
Secondary outcomes
All‐cause mortality*
There were no mortalities reported in Soltan 2007.
Mortality from causes other than bleeding
There were no mortalities from causes other than bleeding reported in Soltan 2007.
Days in hospital
The length of time (days) that women spent in hospital appeared to be shorter in the intervention group compared to the normal care group but we are uncertain about this result because the evidence was very low certainty (MD ‐1.20 days, 95% CI ‐1.33 to ‐1.07; 240 women; one study; Analysis 4.5).
4.5. Analysis.

Comparison 4: Latex balloon (air filled) tamponade + stitch and normal care versus normal care (Intrauterine tamponade versus normal care), Outcome 5: Days in hospital
Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy)
Two women in the control group required additional post‐randomisation surgical interventions (B‐lynch suture and artery ligation) in order to control bleeding compared to the intervention group where no women required additional surgical interventions to control bleeding (RR 0.20, 95% CI 0.01 to 4.12; 240 women, one study; Analysis 4.6)
4.6. Analysis.

Comparison 4: Latex balloon (air filled) tamponade + stitch and normal care versus normal care (Intrauterine tamponade versus normal care), Outcome 6: Post‐randomisation additional surgical interventions used to control bleeding
*Post‐randomisation additional non‐surgical intervention to control bleeding (uterine packing, bimanual uterine massage, tamponade, external aortic compression and compression garments)
The Soltan 2007 trial methods specified that all women in the control group would receive standard care as the first‐line treatment. However, 19/120 women in the control group subsequently received the intervention as a second‐line treatment (RR 0.03, 95% CI 0.00 to 0.42; 240 women, one study; Analysis 4.7).
4.7. Analysis.

Comparison 4: Latex balloon (air filled) tamponade + stitch and normal care versus normal care (Intrauterine tamponade versus normal care), Outcome 7: Post‐randomisation additional non‐surgical interventions used to control bleeding
Side effects of the intervention (e.g. trauma, necrosis)
Soltan 2007 reported that there were no cases of pyrexia, or allergic reaction ‐ Analysis 4.8.
4.8. Analysis.

Comparison 4: Latex balloon (air filled) tamponade + stitch and normal care versus normal care (Intrauterine tamponade versus normal care), Outcome 8: Side effects of the intervention (e.g. trauma, necrosis)
The trial report also mentioned that quote: "undesired over inflation of the balloon, has results in three complications; two cases of cervical tear treated with surgical repair under general anaesthesia. The third one was a raise of uterine size above umbilicus; associated with tachycardia and hypotension, which had returned back to normal after deflation of the balloon bringing the uterine level just below the umbilicus" (Soltan 2007 page 59). However, it is unclear which group the three women were from (given that 19/120 women in the control group subsequently received the intervention as a second‐line treatment). We attempted to contact the author for clarification (20 January 2020) but received no response.
Secondary outcomes not reported by the study under this comparison
The following prespecified secondary outcomes in this review were not reported by the included study/studies under this comparison.
Shock as defined by trialist*
Coagulopathy as defined by trialist*
Number of women with total blood loss 500 mL or more after randomisation*
Number of women with total blood loss 1000 mL or more after randomisation*
Mean blood loss (mL) (trialist defined)*
Blood transfusion (red cell or whole blood)*
Blood product transfusion*
Post‐randomisation additional uterotonic agent used to control bleeding*
Admission to higher level of care*
Breastfeeding (defined as any breastfeeding at hospital discharge)*
Maternal satisfaction with therapy (trialist defined)*
Quality of life, including physiological activity and social and emotional changes (sense of well‐being) (trialist defined)*
Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (intrauterine tamponade versus another mechanical/surgical method) (comparison 5)
One very small study (Kavak 2013) contributed data to this comparison. The study, which involved 13 women with placenta praevia and primary PPH following caesarean section. The study was conducted in Turkey and examined the use of a Bakri balloon versus endouterine haemostatic square suturing to the lower segment of the uterus for treating primary PPH.
Primary outcomes
Mortality due to bleeding
The effects of intervention on maternal mortality due to bleeding are unclear due to very low‐certainty evidence. There were no maternal deaths in either group. See Analysis 5.1.
5.1. Analysis.

Comparison 5: Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (Intrauterine tamponade versus another mechanical/surgical method), Outcome 1: Mortality due to bleeding
Hysterectomy to control bleeding
The effects of intervention on hysterectomy to control bleeding are also unclear due to very low‐certainty evidence. There were no hysterectomies to control bleeding in either group. See Analysis 5.2.
5.2. Analysis.

Comparison 5: Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (Intrauterine tamponade versus another mechanical/surgical method), Outcome 2: Hysterectomy to control bleeding
Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure)
The effects of intervention on serious maternal morbidity are also unclear, again due to very low‐certainty evidence. There were no serious maternal morbidities in either group. See Analysis 5.3.
5.3. Analysis.

Comparison 5: Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (Intrauterine tamponade versus another mechanical/surgical method), Outcome 3: Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure)
Secondary outcomes
Mean blood loss (mL) (trialist defined)*
Bakri balloon tamponade may reduce mean 'intraoperative' blood loss (mL) compared to haemostatic square suturing (mean difference (MD) ‐426 mL, 95% CI ‐631.28 to ‐220.72; very low‐certainty evidence; Analysis 5.6)), but we are uncertain about this result because the evidence was very low certainty .
5.6. Analysis.

Comparison 5: Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (Intrauterine tamponade versus another mechanical/surgical method), Outcome 6: Mean blood loss (mL) (trialist defined)
Blood transfusion (red cell or whole blood)*
We found very low‐certainty evidence relating to blood transfusion (red cell or whole blood) which means we are uncertain about the results for this outcome. There were 2/7 events in the intrauterine tamponade group and 3/6 in the uterine compression (control) group (RR 0.57, 95% CI 0.14 to 2.36, 13 women, 1 study; very low‐certainty evidence; Analysis 5.7).
5.7. Analysis.

Comparison 5: Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (Intrauterine tamponade versus another mechanical/surgical method), Outcome 7: Blood transfusion (red cell or whole blood)
Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy)*
No women in either group required additional surgical interventions to control bleeding (13 women, one study). See Analysis 5.8.
5.8. Analysis.

Comparison 5: Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (Intrauterine tamponade versus another mechanical/surgical method), Outcome 8: Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy)
Side effects of the intervention (e.g. trauma, necrosis)
Kavak 2013 reported that there were no adverse effects requiring surgical intervention in either group, however there were two cases of fever in the control group. See Analysis 5.9. We identified very low‐certainty evidence for both of these outcomes which means we are uncertain about the results.
5.9. Analysis.

Comparison 5: Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (Intrauterine tamponade versus another mechanical/surgical method), Outcome 9: Side effects of the intervention (e.g. trauma, necrosis)
Postnatal blood loss (outcome not prespecified in our review protocol)
Kavak 2013 reported on postnatal blood loss associated with the use of intrauterine tamponade compared with another mechanical/surgical method (haemostatic sutures) (MD ‐231.00 mL, 95% CI ‐300.70 to ‐161.30; 13 women, 1 study; Analysis 5.10) but this is very low‐certainty evidence which means we are uncertain about the results for this outcome.
5.10. Analysis.

Comparison 5: Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (Intrauterine tamponade versus another mechanical/surgical method), Outcome 10: Postnatal blood loss (not pre‐specified)
Secondary outcomes not reported by the study under this comparison
All‐cause mortality*
Mortality from causes other than bleeding
Shock as defined by trialist*.
Coagulopathy as defined by trialist*
Number of women with total blood loss 500 mL or more after randomisation*
Number of women with total blood loss 1000 mL or more after randomisation*
Blood product transfusion*
Post‐randomisation additional uterotonic agent used to control bleeding*
Post‐randomisation additional non‐surgical intervention to control bleeding (uterine packing, bimanual uterine massage, tamponade, external aortic compression and compression garments)
Admission to higher level of care*
Days in hospital
Breastfeeding (defined as any breastfeeding at hospital discharge)*
Maternal satisfaction with therapy (trialist defined)*
Quality of life, including physiological activity and social and emotional changes (sense of well‐being) (trialist defined)*
Bakri balloon tamponade versus condom‐loaded Foley catheter (one intrauterine tamponade technique versus another intrauterine tamponade technique) (comparison 6)
One small study (Darwish 2018) contributed data to this comparison. The study, which involved 66 women with uncontrolled primary atonic PPH following vaginal birth. The study was conducted in Egypt and examined the use of a Bakri balloon versus a condom‐loaded Foley catheter for treating primary PPH. It is important to note that women in whom the treatment failed were excluded from the results for the trial's secondary outcomes, making it difficult to draw conclusions from the published results.
Primary outcomes
Mortality due to bleeding
This outcome was not reported in the included study under this comparison.
Hysterectomy to control bleeding
The certainty of the evidence is very low, which means that it is uncertain whether the Bakri balloon tamponade reduces the risk of hysterectomy to control bleeding: Bakri balloon group (1/33) versus condom‐loaded Foley catheter group (2/33) (RR 0.50, 95% CI 0.05 to 5.25; 66 women, 1 study; Analysis 6.1).
6.1. Analysis.

Comparison 6: Bakri balloon tamponade versus condom‐loaded Foley catheter (one intrauterine tamponade technique versus another intrauterine tamponade technique), Outcome 1: Hysterectomy to control bleeding
Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure)
This outcome was not reported in the included study under this comparison.
Secondary outcomes
Mean blood loss (mL) (trialist defined)*
Blood transfusion (red cell or whole blood)*
We are uncertain of the effects of intervention on the risk of blood transfusion (red cell or whole blood) between the Bakri balloon and condom‐loaded Foley catheter groups because the evidence is very low certainty. There were 29/30 events in the Bakri balloon group and 28/28 in the condom‐loaded Foley catheter group (RR 0.97, 95% CI 0.88 to 1.06, 58 women ‐ eight women with 'treatment failure' excluded from these data, 1 study; Analysis 6.3).
6.3. Analysis.

Comparison 6: Bakri balloon tamponade versus condom‐loaded Foley catheter (one intrauterine tamponade technique versus another intrauterine tamponade technique), Outcome 3: Blood transfusion (red cell or whole blood)
Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy)*
There were 2/33 women in the Bakri balloon group who needed additional surgical interventions (B‐Lynch compression sutures) to control bleeding and (3/33) in the condom‐loaded Foley catheter group (RR 0.67, 95% CI 0.12 to 3.73; 66 women; Analysis 6.4). These results are unclear due to very low‐certainty evidence.
6.4. Analysis.

Comparison 6: Bakri balloon tamponade versus condom‐loaded Foley catheter (one intrauterine tamponade technique versus another intrauterine tamponade technique), Outcome 4: Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy)
Admission to higher level of care*
The number of women who were admitted to a higher level of care was 2/30 in the Bakri balloon group (2/30) and 4/28 in the condom‐loaded Foley catheter group (RR 0.47, 95% CI 0.09 to 2.35; 66 women but with five women with 'treatment failure' excluded from this data, 1 study; Analysis 6.5). Very low‐certainty evidence means we are unclear about the results for this outcome.
6.5. Analysis.

Comparison 6: Bakri balloon tamponade versus condom‐loaded Foley catheter (one intrauterine tamponade technique versus another intrauterine tamponade technique), Outcome 5: Admission to higher level of care
Coagulopathy (as defined by trialist)
The number of women who developed coagulopathy (reported as 'Disseminated Intravascular Coagulopathy' or 'DIC') was 1/30 in the Bakri balloon group and 2/28 in the condom‐loaded Foley catheter group (RR 0.47, 95% CI 0.04 to 4.87; 58 women but five with 'treatment failure' excluded from this data, 1 study; Analysis 6.2). Very low‐certainty evidence means we are unclear about the results for this outcome.
6.2. Analysis.

Comparison 6: Bakri balloon tamponade versus condom‐loaded Foley catheter (one intrauterine tamponade technique versus another intrauterine tamponade technique), Outcome 2: Coagulopathy as defined by trialist
Secondary outcomes not reported by the study under this comparison
All‐cause mortality*
Mortality from causes other than bleeding
Shock as defined by trialist*
Number of women with total blood loss 500 mL or more after randomisation*
Number of women with total blood loss 1000 mL or more after randomisation*
Blood product transfusion*
Post‐randomisation additional uterotonic agent used to control bleeding*
Post‐randomisation additional non‐surgical intervention to control bleeding (uterine packing, bimanual uterine massage, tamponade, external aortic compression and compression garments)
Side effects of the intervention (e.g. trauma, necrosis)
Days in hospital
Breastfeeding (defined as any breastfeeding at hospital discharge)*
Maternal satisfaction with therapy (trialist defined)*
Quality of life, including physiological activity and social and emotional changes (sense of well‐being) (trialist defined)*
Bakri balloon + traction stitch versus Bakri balloon without traction stitch (one intrauterine tamponade versus another intrauterine tamponade technique (comparison 7)
One small study (Khalil 2011) contributed data to this comparison. The study, which involved 50 women with uncontrolled primary atonic PPH following caesarean section. The study was conducted in Saudi Arabia and examined the use of a Bakri balloon held in place with a traction stitch versus Bakri balloon without traction stitch for treating primary PPH.
Primary outcomes
Mortality due to bleeding
This outcome was not reported by Khalil 2011.
Hysterectomy to control bleeding
We are uncertain about the results for hysterectomy to control bleeding because the evidence was very low certainty. There were 0/25 women who had hysterectomy to control bleeding in the group of women who received Bakri balloon+traction stitch and 2/25 women who had hysterectomy to control bleeding in the group who received Bakri balloon without traction stitch (RR 0.20, 95% CI 0.01 to 3.97; 50 women, 1 study; Analysis 7.1).
7.1. Analysis.

Comparison 7: Bakri balloon + stitch versus Bakri balloon without stitch (one intrauterine tamponade versus another intrauterine tamponade technique), Outcome 1: Hysterectomy to control bleeding
Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure)
This outcome was not reported by Khalil 2011.
Secondary outcomes
Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy)*
The incidence of post‐randomisation additional non‐surgical interventions to control bleeding was 1/25 in the Bakri+traction stitch group, where the woman in the intervention group received uterine artery embolisation) and 3/25 in the Bakri balloon without traction stitch group, where al three women received uterine artery ligation and iliac artery ligation (RR 0.33, 95% CI 0.04 to 2.99, 50 women, 1 study; Analysis 7.2). Very low‐certainty evidence means we are unclear about the results for this outcome.
7.2. Analysis.

Comparison 7: Bakri balloon + stitch versus Bakri balloon without stitch (one intrauterine tamponade versus another intrauterine tamponade technique), Outcome 2: Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy)
Total blood loss >/= 1000 mL before and after randomisation (outcome not prespecified in our review protocol)
Khalil 2011 reported 100% of women in both groups had a total blood loss of greater than or equal to 1000 mL (RR 1.00, 95% CI 0.93 to 1.08; 50 women, 1 study; low‐certainty evidence; Analysis 7.3).
7.3. Analysis.

Comparison 7: Bakri balloon + stitch versus Bakri balloon without stitch (one intrauterine tamponade versus another intrauterine tamponade technique), Outcome 3: Total blood loss >= 1000 mL (before and after randomisation, not pre‐specified)
Secondary outcomes not reported by the study under this comparison
The following prespecified secondary outcomes in this review were not reported by the included study/studies under this comparison.
All‐cause mortality*
Mortality from causes other than bleeding
Shock as defined by trialist*
Coagulopathy as defined by trialist*
Number of women with total blood loss 500 mL or more after randomisation*
Number of women with total blood loss 1000 mL or more after randomisation*
Mean blood loss (mL) (trialist defined)*
Blood product transfusion*
Blood transfusion (red cell or whole blood)*
Post‐randomisation additional uterotonic agent used to control bleeding*
Post‐randomisation additional non‐surgical intervention to control bleeding (uterine packing, bimanual uterine massage, tamponade, external aortic compression and compression garments)
Admission to higher level of care*
Side effects of the intervention (e.g. trauma, necrosis)
Days in hospital
Breastfeeding (defined as any breastfeeding at hospital discharge)*
Maternal satisfaction with therapy (trialist defined)*
Quality of life, including physiological activity and social and emotional changes (sense of well‐being) (trialist defined)*
Intrauterine balloon tamponade (condom‐loaded catheter) versus uterovaginal packing (intrauterine tamponade versus another intrauterine tamponade method) (comparison 8)
One small study (Ashraf 2018) contributed data to this comparison. The study, which involved 212 women with uncontrolled primary PPH following vaginal birth. The study was conducted in Pakistan and examined the use of modified condom catheter balloon tamponade compared with uterovaginal packing for treating primary PPH.
Primary outcomes
Mortality due to bleeding
This outcome was not reported in the included study under this comparison.
Hysterectomy to control bleeding
This outcome was not reported in the included study under this comparison.
Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure)
This outcome was not reported in the included study under this comparison.
Secondary outcomes
Mean blood loss (mL) (trialist defined)*
In the results section of Ashraf 2018, mean blood loss is reported as 600.28 mL (+/‐ 25.33 mL) in the condom catheter group and 699 mL (+/‐ 70.176 mL) in the intrauterine packing group. However, it is unclear whether this is pre‐ or post‐randomisation, especially as this is also presented in a table detailing participant characteristics of each group (age, parity, gestational age, and blood loss). For this reason, we have not included these data in our data and analysis tables.
Side effects of the intervention (e.g. trauma, necrosis)
Fever
Ashraf 2018 reported that the incidence of fever may be reduced (46/106 in the condom catheter group versus 98/106 in the intrauterine packing group) (RR 0.47, 95% CI 0.38 to 0.59, 212 women, one study, very low certainty) (Analysis 8.1), but we are uncertain about this result because the evidence was very low certainty .
8.1. Analysis.

Comparison 8: Intrauterine balloon tamponade (condom catheter) versus uterovaginal packing (Intrauterine tamponade versus another mechanical/surgical method), Outcome 1: Side effects of the intervention
Perforation
Ashraf 2018 reported that 30 of the 106 women in the condom catheter group and 46 out of 106 women in the intrauterine packing group had 'perforation' but no definition or details were provided. We have not included these data in our data and analysis table.
Secondary outcomes not reported by the study under this comparison
The following prespecified secondary outcomes in this review were not reported by the included study/studies under this comparison.
All‐cause mortality*
Mortality from causes other than bleeding
Shock as defined by trialist*
Coagulopathy as defined by trialist*
Number of women with total blood loss 500 mL or more after randomisation*
Number of women with total blood loss 1000 mL or more after randomisation*
Blood transfusion (red cell or whole blood)*
Blood product transfusion*
Post‐randomisation additional uterotonic agent used to control bleeding*
Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy)*
Post‐randomisation additional non‐surgical intervention to control bleeding (uterine packing, bimanual uterine massage, tamponade, external aortic compression and compression garments)
Admission to higher level of care*
Days in hospital
Breastfeeding (defined as any breastfeeding at hospital discharge)*
Maternal satisfaction with therapy (trialist defined)*
Quality of life, including physiological activity and social and emotional changes (sense of well‐being) (trialist defined)*
Modified B‐Lynch compression suture versus standard B‐Lynch compression suture (one uterine compression suture technique versus another uterine compression suture technique) (comparison 9)
One small study (El‐Sokkary 2016) contributed data to this comparison. The study, which involved 160 women with uncontrolled PPH following caesarean section. The study was conducted in Egypt and examined the use of modified B‐Lynch compression sutures compared with standard B‐Lynch compression sutures for treating primary PPH.
Primary outcomes
Mortality due to bleeding
This outcome was not reported in the included study under this comparison.
Hysterectomy to control bleeding
There is low‐certainty evidence to suggest that one type of compression suture technique (modified B‐Lynch) may reduce the incidence of hysterectomy to control bleeding (four out of 80 women) compared to another type of compression suture technique (the classic B‐Lynch technique) (12 out of 80 women) (RR 0.33, 95% CI 0.11 to 0.99; 160 women, 1 study; low‐certainty evidence; Analysis 9.1).
9.1. Analysis.

Comparison 9: Modified B‐Lynch compression suture versus standard B‐Lynch compression suture (one uterine compression suture technique versus another uterine compression suture technique), Outcome 1: Hysterectomy to control bleeding
Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure)
This outcome was not reported in the included study under this comparison.
Secondary outcomes
Mean blood loss (mL) (trialist defined as postoperative blood loss)*
There is low‐certainty evidence to suggest that the modified B‐Lynch suture technique may be associated with a reduction in mean postoperative blood loss compared to the classic B‐Lynch technique (MD ‐244.00 mL, 95% CI ‐295.25 to ‐192.75; 160 women, 1 study; low‐certainty evidence; Analysis 9.2).
9.2. Analysis.

Comparison 9: Modified B‐Lynch compression suture versus standard B‐Lynch compression suture (one uterine compression suture technique versus another uterine compression suture technique), Outcome 2: Mean blood loss (mL) (trialist defined)
Days in hospital
The length of time (days) that women spent in hospital was similar between the two types of uterine compression suturing technique (MD 0.10, 95% CI ‐0.38 to 0.58; 160 women, 1 study;low‐certainty evidence; Analysis 9.3).
9.3. Analysis.

Comparison 9: Modified B‐Lynch compression suture versus standard B‐Lynch compression suture (one uterine compression suture technique versus another uterine compression suture technique), Outcome 3: Days in hospital
Secondary outcomes not reported by the study under this comparison
The following prespecified secondary outcomes in this review were not reported by the included study/studies under this comparison.
All‐cause mortality*
Mortality from causes other than bleeding
Shock as defined by trialist*
Coagulopathy as defined by trialist*
Number of women with total blood loss 500 mL or more after randomisation*
Number of women with total blood loss 1000 mL or more after randomisation*
Blood transfusion (red cell or whole blood)*
Blood product transfusion*
Post‐randomisation additional uterotonic agent used to control bleeding*
Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy)*
Post‐randomisation additional non‐surgical intervention to control bleeding (uterine packing, bimanual uterine massage, tamponade, external aortic compression and compression garments)
Admission to higher level of care*
Side effects of the intervention (e.g. trauma, necrosis)
Breastfeeding (defined as any breastfeeding at hospital discharge)*
Maternal satisfaction with therapy (trialist defined)*
Quality of life, including physiological activity and social and emotional changes (sense of well‐being) (trialist defined)*
Discussion
Summary of main results
We identified nine trials involving a total of 944 women with primary postpartum haemorrhage (PPH) for inclusion in this review. The trials were all small (with sample sizes ranging from 13 to 240 women) and no two studies compared the same interventions meaning that no data meta‐analysis was possible. The trials studied a wide range of interventions including external uterine compression (Chantrapitak 2009), arterial embolisation versus stepwise surgical devascularisation (Farouk 2016), Bakri balloon tamponade versus square sutures for placenta praevia (Kavak 2013), Bakri balloon tamponade versus condom catheter tamponade (Darwish 2018), Bakri balloon, with our without traction suture (Khalil 2011), condom‐loaded catheter tamponade versus uterovaginal packing (Ashraf 2018), latex air‐inflated balloon tamponade plus cerclage versus normal care (Soltan 2007), two types of B‐Lynch suture (El‐Sokkary 2016), and the use of a condom‐loaded Foley catheter versus normal care (Dumont 2017). There are currently six ongoing studies; the results of these studies will be added to this review in future updates. It is difficult to draw any definitive conclusions. Most of the included studies had small sample sizes and substantial differences between studies meant it was not possible to combine any trials in meta‐analysis. GRADE assessments ranged from very low to low certainty, with the majority of results rated as very low certainty. Downgrading decisions were mainly based on study design limitations and imprecision. This means that we cannot be confident about the main findings. However, in the randomised trial by Dumont 2017, use of the condom catheter for intrauterine tamponade in health centres in Mali and Benin was associated with increased blood loss (very low‐certainty evidence). In the Kavak 2013 study, those treated with the Bakri balloon had lower mean blood losses both during and after surgery than those who had uterine square sutures, and in the Ashraf 2018 study the condom catheter resulted in less postpartum fever than uterine packing with gauze (very low‐certainty evidence). Finally, in the El‐Sokkary 2016 study, women treated with a modified B‐Lynch suture (where the B‐Lynch suture is crossed, and then also wrapped around the proximal end of the uterus to close off the uterine arteries) required fewer hysterectomies to control the bleeding and had a lower mean blood loss than those with the standard suture (low‐certainty evidence).
Overall completeness and applicability of evidence
The nine studies included in this review are of insufficient size and/or quality to have a major effect on clinical practice. Most are small, and conducted by those who have invented a new technique, making them prone to bias. Furthermore, with only one study of each technique, none of the trial results have been replicated and so the results must all be treated with caution. Additionally, the use of non‐standardised outcomes in the trials also makes it difficult to compare studies. The scattergun nature of the studies reflects the overall lack of a co‐ordinated research strategy in this area. It is to be hoped that as the global postpartum haemorrhage focus moves away from uterotonics and towards quality of care, more high‐quality studies will be forthcoming.
The lack of data is not surprising as randomised trials of both surgical techniques and devices are uncommon generally, and especially for emergency situations where the rarity and urgency of the situation make it logistically very difficult to conduct high‐quality research. It is especially complex to obtain informed consent. Recently, however, emergency intrapartum studies have started using emergency consent procedures (COPE 2019; CORD 2018; WOMAN 2017) meaning that an abbreviated, short oral or no consent is required before study entry. This should expand greatly the number of high‐quality randomised controlled trials conducted in this area. There is evidence from the studies reviewed here that will help to direct clinical care. First, the small study by Kavak 2013 suggests that use of the Bakri intrauterine balloon tamponade could be more effective than haemostatic square sutures for those with bleeding placenta praevia. Both the intrauterine balloon tamponade and external uterine square sutures were originally designed to treat the atonic uterus rather than placenta praevia. In placenta praevia, the bleeding vessels arise from the lower segment and can been accessed directly at the time of caesarean section. The tendency has therefore been for clinicians to use surgical sutures to compress the bleeding vessels, rather than the more complex act of placing a balloon vaginally during the caesarean section, inflating it within the lower segment and closing the uterus without bursting or trapping the balloon. Whilst the study was too small to assess the risk of uterine scar breakdown due to balloon pressure, it is encouraging that the authors found it effective and usable in practice.
For those with a true atonic uterus at caesarean section, the study by El‐Sokkary 2016 suggests that there may be benefits to using a modification of the B‐Lynch suture in which (a) the sutures are crossed from left to right and vice versa as they cross the fundus, and (b) at the end of the procedure, the ends of the suture are passed back through the broad ligament bilaterally, around the back of the uterus before being tied anteriorly so as to obliterate the uterine arteries. Whilst this provides only low‐certainty evidence from a single‐centre study, it does provide an intriguing alternative for care and deserves to be tested in a further randomised trial. Finally, the Dumont 2017 study provides surprising evidence that the use of a condom catheter might not improve care in low‐ and middle‐income countries (LMICs) settings in comparison to normal care – indeed the study found more blood loss in the condom group. The results are very much at odds with the clinical experience of most clinicians and implementation studies where the roll‐out of condom catheters has been associated with impressive improvements in severe PPH outcomes (Burke 2016). The reasons for this discrepancy are not clear. It may be that the condom catheter produces insufficient intrauterine pressure (Antony 2017), or that the time taken to set up the catheter delays care as it allows ongoing blood loss. The increased blood loss could also have been caused by the requirement to manually explore the uterus prior to insertion, or by delays meaning that clotting disorders had already commenced. However, a step‐wedge cluster‐randomised trial which was not eligible to be included in this review (Anger 2016) supports the findings of Dumont 2017. Further studies are now planned to determine whether it is the setting or the balloon type that is at fault.
Quality of the evidence
Overall, the trials were of a mixed methodological quality (risk of bias). Whilst most of the trials (7/9) used adequate methods of sequence generation, nearly all trials (6/9) were unclear in terms of allocation concealment. All of the trials (9/9) were at a high risk of performance bias and detection bias was unclear in 7/9 trials and high risk in the remaining 2/9 trials. Attrition bias was suspected in two trials (Chantrapitak 2009; Darwish 2018), unclear in one trial (El‐Sokkary 2016) and low risk in the remaining six trials. Reporting bias was low risk in one trial, unclear in seven trials, and high risk in one trial (Ashraf 2018). No sources of other bias was identified in the majority of trials (6/9), with one trial at unclear risk of other bias and two trials assessed as being high risk of other bias (Dumont 2017; Soltan 2007). Our GRADE assessments of the certainty of the evidence ranged from very low to low, with the majority of outcomes rated as very low quality. Downgrading decisions were based on study limitations, and imprecision (small sample sizes, few or zero events, wide confidence intervals crossing the line of no effect). For one study (Soltan 2007), outcomes were also downgraded for indirectness.
Potential biases in the review process
The strength of this review is that it only contains randomised trials. This is particularly important in studies of postpartum haemorrhage as spontaneous resolution of bleeding is very common. Thus, observational studies almost always show very high success rates, and it is not until the publication of randomised trials that the true efficacy is revealed. This was seen in the randomised trials of misoprostol for PPH treatment (Geller 2006; Mousa 2014c), and is suggested in the contrast between the cohort studies of intrauterine balloon tamponade (Tindell 2013) and those found in the Dumont 2017 study. The presence of randomised trials in this difficult area (and the future ones in progress) are therefore very much welcomed. This review also has weaknesses. Whilst the review processes were robust and according to current Cochrane methodology, the study reports did not always provide the full dataset required, and not all authors responded to requests for further data. The review authors have therefore had to make some assumptions about the studies' methodologies (e.g. unclear ratings for 'Risk of bias' assessments when no information was provided).
Agreements and disagreements with other studies or reviews
To our knowledge, there are no systematic reviews of randomised trials of balloon tamponade. Tindell 2013, however, reviewed the evidence from case series and cohort studies and found a 96% success rate with the condom catheter in LMICs. This contrasts greatly with the findings from the Dumont 2017 study as discussed above. However, a large step‐wedged, cluster‐randomised trial of condom‐balloon tamponade versus normal care was conducted by Anger 2016. In this study, all women in the participating health units were included, irrespective of whether they had PPH or not. This meant that it did not meet the inclusion criteria for the review. It does, however, provide strong evidence that supports the surprising findings of Dumont 2017, the only other randomised trial of condom‐balloon tamponade. In the Anger 2016 study, the technique was introduced in a randomised fashion into 18 secondary‐level hospitals over 18 months. After the introduction, there was a significant increase in the incidence of the composite outcome of 'PPH surgery or maternal death' from 6.7 to 11.6 per 10,000 births, an adjusted incidence ratio of 4.08 (95% confidence interval 1.07 to 15.58). The results are unlikely to be due to the failure of the technique as most deaths occurred in women in whom it was not used, but it does emphasise that PPH is a multi‐system problem and that the condom catheter it not enough to reduce maternal deaths from PPH on its own (Weeks 2019). Shahin 2018 conducted a systematic review of endovascular interventional modalities for haemorrhage control in abnormal placentation deliveries. They included cohort and case series in their review and most of the studies placed the devices prophylactically prior to surgery in case of bleeding at the time of surgery. They found that the highest success rate was with prophylactic balloon occlusion of the abdominal aorta. These findings were echoed by the findings of the systematic review of Manzando‐Nunez 2018 who also found the endovascular balloon occlusion of the aorta to be highly effective. This method was not however addressed by any of the studies in this review. Soro 2017 conducted a systematic review of outcomes following arterial embolisation for PPH treatment. They found high success rates, and no effect on future menstrual cycle, or fertility. There was, however higher rates than expected of abnormal placentation in future pregnancies.
Authors' conclusions
Implications for practice.
The evidence presented in this review is generally not of high enough quality to have major effects on clinical practice. The available evidence is restricted to small, single‐centre studies providing low‐grade certainty evidence. However, two studies in this review are important and could have implications for practice. First, the study by El‐Sokkary 2016 provides low‐certainty evidence that use of the modified B‐Lynch suture (where the suture is combined with uterine artery obliteration) may be superior to that originally described. However, it was only conducted in a single centre and without long‐term follow‐up to assess effects on future fertility. This study deserves to be repeated. The other study of note is that of Dumont 2017, where the use of a condom‐loaded Foley catheter in two low‐income African settings led to increased rates of blood loss (very low‐certainty evidence). The findings are supported by the Anger 2016 step‐wedge cluster‐randomised trial. The reason for the failures are unclear, but do suggest that the global roll‐out of condom catheters alone will not reduce mortality or morbidity from primary postpartum haemorrhage (PPH) in these settings. The reasons for the surprising findings are explored in Weeks 2019. They are not thought to be necessarily a failure of the device itself, but more due to the setting in which the technique was introduced. It is suggested that in these settings, balloon tamponade is only introduced alongside multi‐system improvements in PPH care, including quality improvements in blood transfusion, operating theatres and staffing.
Implications for research.
High‐quality randomised trials into mechanical and surgical methods for the treatment of PPH are urgently needed. Given the large number of deaths from PPH seen each year globally, and that all PPH treatment pathways end with either mechanical or surgical interventions, it is disappointing that there is so little research in this area. The new emergency consent pathways will facilitate recruitment, and research networks in all income settings should consider large‐scale randomised trials in which women are initially recruited with minimal or no consent so as to compare techniques. The findings of the Dumont 2017 are very surprising, but supported by a large step‐wedge cluster‐randomised trial (Anger 2016) (excluded from this review as it included both women with and without PPH). Whilst the use of the condom‐loaded Foley used in Dumont 2017 and Anger 2016 is an attractive low‐cost option, it may be that it is too pliable or that it takes too long to make up to be effective in poorer settings where surgeons commonly act alone or with little support. Future studies could explore whether its failure in these studies were due to the setting, the type of balloon, or whether balloon tamponade is in fact an ineffective intervention. A wide array of uterine compression sutures have been described and, whilst appearing to be effective in the short term, they have been associated with future infertility and placental implantation abnormalities. Although the study of Kavak 2013 only recruited 13 women, it shows that research is possible in this area, and that an intrauterine balloon may be as effective but less invasive mode of treatment even for women with uterine bleeding during caesarean section. The study by El‐Sokkary 2016 provides evidence that use of the modified B‐Lynch suture may be superior to that originally described, and with minimal adverse effects. However, it is low‐certainty evidence and the study should be repeated before clinicians adopt the revised technique. In high‐resource settings, uterine artery embolisation has become popular as the equipment and skills required have become more widely available. There is little randomised trial evidence for its efficacy, however and its comparative clinical and cost‐benefits compared with surgical devascularisation, compression sutures or balloon tamponade have not been established. Given its high costs and clinical importance, this will be an important area for new research. Future studies could ensure that they collect minimum data for the PPH Core Outcome set (Meher 2019) so as to facilitate future meta‐analysis.
History
Review first published: Issue 7, 2020
Acknowledgements
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
This review is supported by funding from the World Health Organization (WHO) and the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP) to Cochrane Pregnancy and Childbirth (University of Liverpool). HRP supports and co‐ordinates research on a global scale, synthesises research through systematic reviews of literature, builds research capacity in low‐income countries and develops dissemination tools to make efficient use of ever‐increasing research information. In addition to its co‐sponsors, the International Planned Parenthood Federation (IPPF) and UNAIDS are both members of HRP’s governing body.
We are grateful to: Mohamed Khalil for providing us with further information relating to Khalil 2011; and Mohamed Rezk for providing us with further information about the Rezk 2016 study.
We would like to thank Myfanwy Williams for her assistance with checking risk of bias, 'Summary of findings' tables and for carrying out second checks of the data extraction for two studies (Ashraf 2018; Soltan 2007).
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team) and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Edwin Chandraharan, Lead Consultant, Labour Ward, St George's University Hospital's NHS Trust, London; Shireen Meher, Birmingham Women's and Children's Foundation Trust, Birmingham, UK.
Appendices
Appendix 1. ICTRP and ClnicalTrials.gov ‐ search methods
ICTRP
postpartum hemorrhage
obstetric hemorrhage
ClinicalTrials.gov
Advanced search
Interventional studies | postpartum hemorrhage
Data and analyses
Comparison 1. External lower uterine compression versus normal care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Blood transfusion (red cell or whole blood) | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.66, 8.23] |
| 1.2 Post‐randomisation additional uterotonic agent used to control bleeding | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.73] |
1.2. Analysis.

Comparison 1: External lower uterine compression versus normal care, Outcome 2: Post‐randomisation additional uterotonic agent used to control bleeding
Comparison 2. Uterine arterial embolisation versus surgical devascularisation plus B‐Lynch (one uterine devascularisation technique versus another uterine devascularisation technique).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Hysterectomy to control bleeding | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.15, 3.57] |
| 2.2 Side effects of the intervention (e.g. trauma, necrosis) | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.08, 15.41] |
Comparison 3. Intrauterine balloon tamponade plus normal care (misoprostol) versus normal care (misoprostol).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mortality due to bleeding | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.21 [0.77, 49.98] |
| 3.2 Hysterectomy to control bleeding | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [0.48, 35.93] |
| 3.3 All cause mortality | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.21 [0.77, 49.98] |
| 3.4 Mortality from causes other than bleeding | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.5 Number of women with total blood loss 1000 mL or more after randomisation | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.15, 2.00] |
| 3.6 Blood transfusion (red cell or whole blood) | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.88, 2.51] |
| 3.7 Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.7.1 Uterine compression sutures | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.17 [0.25, 105.44] |
| 3.7.2 Artery ligation | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.32, 5.90] |
| 3.8 Admission to higher level of care | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.55, 3.04] |
| 3.9 Side effects of the intervention (e.g. trauma, necrosis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.9.1 Severe shivering, diarrhoea, vomiting or high temperature | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
3.6. Analysis.

Comparison 3: Intrauterine balloon tamponade plus normal care (misoprostol) versus normal care (misoprostol), Outcome 6: Blood transfusion (red cell or whole blood)
Comparison 4. Latex balloon (air filled) tamponade + stitch and normal care versus normal care (Intrauterine tamponade versus normal care).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Mortality due to bleeding | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.2 Hysterectomy to control bleeding | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.74] |
| 4.3 All‐case mortality | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.4 Mortality from causes other than bleeding | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.5 Days in hospital | 1 | 240 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.33, ‐1.07] |
| 4.6 Post‐randomisation additional surgical interventions used to control bleeding | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.12] |
| 4.7 Post‐randomisation additional non‐surgical interventions used to control bleeding | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.42] |
| 4.8 Side effects of the intervention (e.g. trauma, necrosis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.8.1 Pyrexia | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.8.2 Allergic reaction | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
4.1. Analysis.

Comparison 4: Latex balloon (air filled) tamponade + stitch and normal care versus normal care (Intrauterine tamponade versus normal care), Outcome 1: Mortality due to bleeding
4.3. Analysis.

Comparison 4: Latex balloon (air filled) tamponade + stitch and normal care versus normal care (Intrauterine tamponade versus normal care), Outcome 3: All‐case mortality
4.4. Analysis.

Comparison 4: Latex balloon (air filled) tamponade + stitch and normal care versus normal care (Intrauterine tamponade versus normal care), Outcome 4: Mortality from causes other than bleeding
Comparison 5. Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (Intrauterine tamponade versus another mechanical/surgical method).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Mortality due to bleeding | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.2 Hysterectomy to control bleeding | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.3 Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple organ failure) | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.4 All cause mortality | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.5 Mortality from causes other than bleeding | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.6 Mean blood loss (mL) (trialist defined) | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐426.00 [‐631.28, ‐220.72] |
| 5.7 Blood transfusion (red cell or whole blood) | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.14, 2.36] |
| 5.8 Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy) | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.9 Side effects of the intervention (e.g. trauma, necrosis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.9.1 Adverse effects requiring surgical intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.9.2 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.10 Postnatal blood loss (not pre‐specified) | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐231.00 [‐300.70, ‐161.30] |
5.4. Analysis.

Comparison 5: Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (Intrauterine tamponade versus another mechanical/surgical method), Outcome 4: All cause mortality
5.5. Analysis.

Comparison 5: Bakri balloon tamponade versus haemostatic square suturing to the lower segment of the uterus (Intrauterine tamponade versus another mechanical/surgical method), Outcome 5: Mortality from causes other than bleeding
Comparison 6. Bakri balloon tamponade versus condom‐loaded Foley catheter (one intrauterine tamponade technique versus another intrauterine tamponade technique).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Hysterectomy to control bleeding | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.25] |
| 6.2 Coagulopathy as defined by trialist | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.04, 4.87] |
| 6.3 Blood transfusion (red cell or whole blood) | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.06] |
| 6.4 Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy) | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.73] |
| 6.5 Admission to higher level of care | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.09, 2.35] |
| 6.6 Side effects of the intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.6.1 Fever ≥ 38ºC in first 24 hours postpartum | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.18, 19.47] |
| 6.6.2 Endometritis | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 6.6.3 Uterine perforation | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
6.6. Analysis.

Comparison 6: Bakri balloon tamponade versus condom‐loaded Foley catheter (one intrauterine tamponade technique versus another intrauterine tamponade technique), Outcome 6: Side effects of the intervention
Comparison 7. Bakri balloon + stitch versus Bakri balloon without stitch (one intrauterine tamponade versus another intrauterine tamponade technique).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Hysterectomy to control bleeding | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 3.97] |
| 7.2 Post‐randomisation additional surgical interventions used to control bleeding (arterial ligation, compressive, uterine sutures, arterial embolisation, laparotomy) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.99] |
| 7.3 Total blood loss >= 1000 mL (before and after randomisation, not pre‐specified) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.93, 1.08] |
Comparison 8. Intrauterine balloon tamponade (condom catheter) versus uterovaginal packing (Intrauterine tamponade versus another mechanical/surgical method).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Side effects of the intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1.1 Fever | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.38, 0.59] |
Comparison 9. Modified B‐Lynch compression suture versus standard B‐Lynch compression suture (one uterine compression suture technique versus another uterine compression suture technique).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Hysterectomy to control bleeding | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.11, 0.99] |
| 9.2 Mean blood loss (mL) (trialist defined) | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐244.00 [‐295.25, ‐192.75] |
| 9.3 Days in hospital | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.38, 0.58] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ashraf 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial Setting: university hospital setting in Lahore, Pakistan |
|
| Participants | Women (aged between 20 and 40) diagnosed with primary PPH following 'normal' vaginal birth and unresponsive to medical treatment. There were 212 women in this study. PPH defined as....."excessive blood loss from genital tract occurring during third stage of labour and within first 24 hours after parturition" p 890 Gestational age >37 weeks Women were aged 20 to 40 Exclusion criteria included PPH due to perineal, cervical or vaginal tear or episiotomy; PPH due to retained placenta; vaginal birth following previous caesarean section; coagulation disorder; secondary PPH. |
|
| Interventions |
Experimental: balloon (condom) tamponade ‐ left in place for 24 hours ‐ 106 women Control: uterine packing with roll gauze and vaginal packing with epipad ‐ left in place for 24 hours ‐ 106 women All women received prophylactic antibiotics (no further information given) |
|
| Outcomes |
|
|
| Notes |
Trial authors' declarations of interest: "none" p892 Sources of trial funding: "none" p892 Trial dates: dates not mentioned but report states that the trial lasted for one year Informed consent obtained?: "informed consent was obtained from each female using their data for study purpose" p 891 (there was no mention of the women consenting to having treatment) Ethics approval obtained?: "Given" p892 Did we attempt to contact the trial authors?: no It is unclear what the initial 'medical treatment' was. Similarly, there is no mention of whether the women also received usual care in addition to the interventions and, if so, what that was comprised of. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "females were randomly divided in to two groups by using lottery method" p891 |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment provided in the trial report |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. Assume neither clinicians nor participants blinded because the intervention would look and feel different from the control group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. Blood loss assessed by "counting saturated pads or by weighing sponges used to absorb blood 1mL blood weights‐approx [sic] 1 blood clots removed from uterine cavity kept in kidney tray which is, full kidney tray‐approx 500 mL blood drop in hematocrit patient" p890 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data apparent |
| Selective reporting (reporting bias) | High risk | Protocol not available. Insufficient information to assess fully. However, one outcome not detailed in Methods is reported in the Results (perforation). There are very few outcomes reported, including none of those listed as important in the article background, and there is also no escalation outcomes reported e.g. hysterectomy due to bleeding. It is also unclear from the trial report whether the mean blood loss outcome (in table 1 on p891) is pre‐randomisation (participant characteristics) or post‐randomisation. |
| Other bias | Unclear risk | In the methods, it states gestational age >37 weeks and that women were aged 20 to 40. However, in the results, it states that the minimum age was 15, and that the minimum gestational age was 36 weeks. There is no mention of whether the women also received usual care in addition to the interventions and, if so, what that was comprised of. No other sources of bias were apparent |
Chantrapitak 2009.
| Study characteristics | ||
| Methods | A parallel group randomised trial. Setting: hospital setting in Bangkok hospital, Thailand. |
|
| Participants | Women diagnosed with primary PPH following vaginal birth. PPH was defined as "blood loss > 500ml after delivery" p 601 Gestational age 28 to 42 weeks 66 women were randomised but 2 excluded "due to cervical tear and extensive birth canal tear" p 601. Therefore 64 women were then included in the study (32 in each group). |
|
| Interventions |
Experiemtal: external lower uterine compression (either by grasping the uterus through a lax abdominal wall or compressing the uterus against the sacrum and lower vertebrae) for a duration of 10 minutes Control: usual care consisting of "massage, oxytocin (10‐20 units in 1000 ml of intravenous solution, 200 ml/min), intravenous ergometrine (Methergin®, 0.2 mg), placed cold pack on uterus, and urinary catheterisation" p 601. |
|
| Outcomes |
|
|
| Notes |
Trial authors' declarations of interest: not mentioned Sources of trial funding: not mentioned Trial dates: January to August 2008 Informed consent obtained?: not mentioned in the trial report Ethics approval obtained?: not mentioned in the trial report Did we attempt to contact the trial authors?: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States that participants were "equally divided into two groups and the treatment method was randomly assigned to each patients" but no further information given |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment provided in the trial report |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned but it would not be possible to blind this intervention. The paper suggests that all (n = 10) blood transfusions were administered based on haematocrit measurement, i.e. based on objective measure that would probably not be affected by lack of blinding in personnel, "Ten received blood transfusion because of underlying anemia (Hct less than 33%)" (p 602). This paper only reports 2 primary outcomes, volume of blood lost and blood transfusion and it is possible that a lack of blinding of either participants or personnel could have affected these outcomes and decision‐making around some co‐interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Well trained nurses were assigned to record the results in the record form", p 601. Insufficient information to assess whether outcome assessors were blinded. "All soaking drapes and blood in basket were weighed", p 601. Blood loss assessed by weighing, although there could have been room for outcome assessors to influence this measurement. A lack of blinding could have influenced outcome assessment for blood loss (because measurement of the assessment of amount of blood lost is not completely objective) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There are 2 women with cervical and vaginal tears whose outcomes are not reported. It is unclear whether these were excluded before or after randomisation. However, cervical and vaginal tears are only usually found upon detailed examination after failure of primary treatment (in this case oxytocics plus uterine compression). It seems likely therefore that they were excluded AFTER randomisation. Otherwise the outcomes for all the other 64 participants were reported |
| Selective reporting (reporting bias) | Unclear risk | No mention of a protocol but outcome reporting bias is not apparent |
| Other bias | Low risk | No other sources of bias were apparent |
Darwish 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial (described as single‐blinded) Setting: Assuit Women's Health Hospital, Egypt |
|
| Participants | Women with primary atonic PPH following vaginal delivery and not responding to standard treatment protocol. PPH was not defined, and nor was there a systematic method of diagnosis (the trial report states that, "Failure to use an accurate measure of blood loss estimation in patients and relying on indirect methods of estimation like HB before and after the intervention is a weak point of this study." p757, Darwish 2018). Exclusions: traumatic PPH, caesarean section, placental abruption, placenta praevia, chorioamnionitis, pregnancy complications (e.g. pre‐eclampsia, diabetes, anaemia, rheumatic heart disease) or women known to have coagulation problems. (Women were put under general anaesthetic before recruitment into the trial, "Under general anesthesia, traumatic lesions and placental remnants were properly excluded." p748, Darwish 2018). 100 potentially eligible women were identified but only 66 were randomised (34 refused to participate). Mean age 28 years, mean body mass index was 27.35. Mean parity was 3.0. |
|
| Interventions |
Intervention: Bakri balloon (33 women) "Bakri balloon is connected to a 24 French, 54 cm long silicone catheter. The Bakri balloon was well‐inserted inside the uterine cavity. After proper positioning, the balloon was initially inflated with 150 mL of sterile normal saline. Then the surgeon put his thumb and index finger around the cervix to keep the partially inflated balloon above the cervix. Bakri balloon was further inflated up to 400‐500 mL until the blood draining through the catheter is considerably decreased" p 748 Control: condom‐loaded Foley catheter (33 women) inflated in the same way as the Bakri balloon. All women in both groups also received IV cephradine 1 g every 12 hours after balloon insertion. |
|
| Outcomes |
Primary outcome: number of women requiring surgical intervention to stop bleeding Secondary outcomes
The following outcomes were reported but were not prespecified in the registration (NCT02430155) protocol:
* These outcomes are only reported for those women who were successfully treated by balloon tamponade, and did not include women who had treatment failure. |
|
| Notes |
Trial authors' declarations of interest: "The authors report no conflicts of interest" p 752. Sources of trial funding: not mentioned. Trial dates: October 2014 until December 2015. Informed consent obtained?: Ethics approval obtained?: yes, from the ethical board of the Faculty of Medicine of the Assiut University. Did we attempt to contact the trial authors?: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done using a computer‐generated random table" p 748 |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was done using serially numbered closed opaque envelopes" p 748 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial reported as single‐blind but unclear who was blinded. Women were under general anaesthesia so presumably effectively blinded whilst being operated on. Given the description in the study report, it is likely that personnel were not blinded, because the interventions would clearly look different when handled by clinicians. On this basis we assess this as high risk of performance bias due to likely lack of clinician blinding which could have affected clinical decision‐making |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial reported as single‐blind but unclear who was blinded; no mention of blinding of outcome assessors in trial report. It is possible that non‐independent blinded providers have biased the outcomes assessments |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on use of blood transfusion, intensive care unit referral and coagulopathy were not reported for those who had 'treatment failures' in each group. Rather than the denominator of 66 women recruited, the denominator for these outcomes is 58 |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered NCT02430155 but checking the outcomes in the protocol against the trial report there are some differences. We note that the following reported outcomes were not listed in the study protocol: referral to intensive care unit, successful procedure, pulse rate, systolic and diastolic blood pressure, urine output, haemoglobin and haematocrit levels (pre/post intervention) |
| Other bias | Low risk | No sources of other bias identified |
Dumont 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial (multi‐centre, 2 parallel groups) Setting: 7 healthcare facilities in Cotonou, Benin and Bamko, Mail. |
|
| Participants | 116 women diagnosed with primary PPH following vaginal birth. Women were suspected to have PPH due to clinical atony and who were quote: "unresponsive to oxytocin and who needed additional uterotonics" p 1 PPH was defined as quote: "visual estimation of excessive blood loss and patient status (blood pressure and cardiac frequency)" p 2 Exclusions: uterine rupture or placenta accreta |
|
| Interventions |
Intervention: uterine balloon tamponade plus normal care (misoprostol) (57 women). In the balloon tamponade group, a condom‐loaded Foley catheter was inflated "by increments of 250 mL of solute" p 2. Further Increments were added (up to a maximum of 1000 mL) if bleeding was still evident 5 minutes after adding the solution. Control: normal care (misoprostol) (59 women) All women received rectal misoprostol (1000 ug) or sublingually (600 ug) following randomisation. Although the report states in the methods p2 that, "In all cases, a single dose of cefazolin or ampicillin was administered as an antibiotic prophylaxis", in the results it reports only 15/57 vs 15/59 also received antibiotics (table 2, drug and dose not described). |
|
| Outcomes | Composite main outcome of recourse to invasive surgery (arterial ligatures, uterine compression sutures, hysterectomy) or death before hospital discharge Secondary outcomes
|
|
| Notes |
Trials registration: ISRCT Registry Number 01202389 post‐results Sources of trial funding: Research Institute for Development (IRD) and United Nations Children's Fund (UNICEF) Trial authors' declarations of interest: all authors quote: "declare no relationships or activities that could appear to have influenced the submitted work" p 8 Trial dates: May 2013 to December 2015 Informed consent obtained?: trial report states "obtained" (p 8) Ethics approval obtained?: Yes ‐ "Ethics and Research Committee of the Institute of the Biomedical Applied Sciences of Benin; Ethic Committee of the Research Institute for Development of France" p 8 Did we attempt to contact the trial authors?: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomisation sequence was generated by the principal investigator (AD) and stratified by health centre. Within the strata, women with PPH which was not controlled by the first‐line therapy were individually allocated by blocks randomisation (varying blocks of four and stratified by healthcare centre)" Judgement comment: "Computer generated random sequence generation... stratified by health centre... block randomisation" p 3 |
| Allocation concealment (selection bias) | High risk | Judgement comment: there is no evidence that the allocation was concealed to the trial supervisor ‐ and it was he/she that decided over the phone whether to randomise or not. The fact that there was a 'randomisation code' known to 4 people suggests that this was a revealed list. This trial also randomised in blocks of 4, stratified by centre. If any staff in local centres were aware of the method used, they would have bene able to anticipate the allocation for one in four women. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned but assume that blinding not possible because only 1 group received mechanical intervention. The role of visual estimation suggests a lack of personnel blinding could have affected clinical decision‐making based on diagnosis of volume of blood lost |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of the outcome assessors is not mentioned. It is possible that non‐independent blinded providers have biased the outcomes assessments. Report states that "Since the use of collection bag is not common practice in Benin and Mali, PPH was clinically assessed by the caregivers (midwife or doctor) according to the visual estimation of blood loss and patient status (blood pressure and cardiac frequency)" p 2 Dumont 2017. It is not clear whether these particular caregivers were blinded although given the nature of the intervention it seems unlikely. It is also unclear whether any attempt was made to blind assessors for most other outcomes or clinical decisions (except maternal death: "Each maternal death was audited by two independent experts in order to assess if the event was possibly due to the experimental treatment or not" p 3 Dumont 2017). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All those excluded were excluded prior to randomisation, and data reporting appears to be otherwise complete |
| Selective reporting (reporting bias) | Low risk |
Quote: "SRCT Registry Number 01202389 Quote: "The primary outcome is a composite outcome. It corresponds to the proportion of women with recourse to an invasive surgery (arterial ligatures, uterine compressive sutures, hysterectomy) or who died before hospital discharge. The secondary outcomes were each component of the composite outcome and also total blood loss more than 1000 mL, blood transfusion and transfer to intensive care unit." Judgement comment: the ISRCTN Entry states: the primary outcome is a composite outcome: individual recourse to an invasive surgery (arterial ligatures, uterine compressive sutures, hysterectomy of haemostasis) and/or maternal death before the hospital release. Secondary outcome measures: each element of the composite primary outcome is related to the point 1 and 2 only. So the secondary outcomes are formed by the 2 elements of the primary outcome measured separately and we will also measure 3 other outcomes: bleedings > 1000 mL, necessity of a transfusion, necessity of a transfer. 1. Invasive intervention rate (arterial ligatures, uterine compressive sutures or hysterectomy of haemostasis): number of women having received an invasive intervention divided by the number of women included 2. Hospital maternal mortality rate (number of women included in the study and died before the hospital release divided by the number of inclusive women) 3. Bleeding > 1000 mL. 4. Necessity of a transfusion 5. Necessity of a transfer The only differences are that 'hysterectomy for haemostasis' in the protocol has been changed to 'hysterectomy'; and the outcome of 'transfer' has been changed to 'transfer to intensive care unit'. We think that these are very minor and so classify this as 'low risk' of bias. |
| Other bias | High risk | Many women did not receive treatment per trial protocol, either due to delays in the steps of the diagnosis or administration of treatment, or not receiving the allocated intervention at all. Prior to randomisation, 25% of women in intervention group and 21% in control did not receive oxytocin within 10 minutes of diagnosis of PPH. Two of the women who died in the tamponade arm did not receive the device because they died before the procedure. Two other women did not receive the device because staff decided to postpone using it for unknown reasons. 42% of women in the intervention group received the UBT more than 30 min after PPH diagnosis. Table 2 in Dumont 2017 states that all women did in fact receive misoprostol as standard second‐line care, but it was administered late (more than 30 minutes after PPH diagnosis) to 54% in intervention group and 37% in the control group. We do however note that on p6 of this paper, the trial authors state that one women died (case 5) before misoprostol was administered. No other sources of bias were apparent. |
El‐Sokkary 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial Setting: Department of Obstetrics and Gynecology, Ain Shams University Hospital, Cairo, Egypt. |
|
| Participants | 160 women with uncontrolled atonic PPH following caesarean section, and not responding to standard care (uterine massage, uterotonics and bimanual compression). Exclusions: traumatic PPH, disseminated intravascular coagulopathy, bleeding diathesis, retained placenta, uterine anomalies. PPH not defined |
|
| Interventions |
Intervention: modified B‐Lynch compression suture (80 women) using a technique whereby the sutures are placed in the shape of the figure 8, and then threaded through the broad ligament and wrapped around the lower part of the uterus to obliterate the uterine arteries bilaterally. Control: standard B‐Lynch compression suture (80 women) |
|
| Outcomes |
|
|
| Notes |
Trial authors' declarations of interest: the authors reported that they had no completing interests Sources of trial funding: none declared Trial dates: January 2013 to October 2015 Informed consent obtained?: "Consent for publication = not applicable" ‐ no mention of informed consent for participants. Ethics approval obtained?: "The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Ain Shams Mater University" p 5 Did we attempt to contact the trial authors?: email to authors on 25 Aug 2018 requesting further information on blood transfusion and sequential numbering of trial envelopes ‐ response from El‐Sokkary on 25 Aug 2018 requesting joint authorship of the Cochrane Review in order to provide this information. Explained that this was not possible. No further response received as of 07 Sep 2018. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated list of random numbers was kept in Ain Shams Maternity Hospital computer and with research supervisors" (p 2) |
| Allocation concealment (selection bias) | Unclear risk | "Computer‐generated randomised series were kept in sealed envelopes" Comment: does not state whether the envelopes were consecutively numbered or opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. Women were under general anaesthesia during intervention. Assume blinding of surgical personnel not possible due to difference in intervention techniques between groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Table 1 (data) does not state number of women. No incomplete data were mentioned in the text but 1 cannot assume that outcome data are complete |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available ‐ insufficient information to permit assessment |
| Other bias | Low risk | No other sources of bias were apparent |
Farouk 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial Setting: Ain‐Shams University Maternity Hospital, Egypt |
|
| Participants | 24 women with primary PPH (blood loss more than 1000 mL) within 2 hours of birth and not responding to first‐line treatment. The majority of women (more than 70% in both groups) had primary atonic PPH, the remainder of the women had PPH due to lacerations or bleeding at the placental site. Exclusion criteria: "history of coagulopathy, thrombocytopenia or anticoagulant therapy, HELLP syndrome or eclampsia, impaired serum creatinine, and mental conditions rendering the patient unable to understand the nature, scope and possible consequences of the study" p 818 |
|
| Interventions |
Intervention: uterine arterial embolisation (11 women ‐ 1 other women refused to have UAE). Of the 11 randomised, 9 had vaginal delivery and 2 had caesarean section. "The procedures were done under fluoroscopic control using monoplane cath‐laboratory unit (Toshiba‐Japan) with a 5Fsheath (TERUMO) and a 5F Cobra2 catheter (Cordis) with a 0.35F hydrophilic guide wire (TERUMO). The sheaths were left in place for 24 hours and the patients were transferred to the ICU." p 818 Control: "emergency laparotomy for stepwise devascularisation and compression sutures" p 818 (12 women). Of the 12 women, 8 had vaginal delivery and 4 had caesarean section. |
|
| Outcomes |
|
|
| Notes |
Trial authors' declarations of interest: "we have no conflicts of interest to declare" p 823 Sources of trial funding: not mentioned Trial dates: May 2011 until May 2013 Informed consent obtained?: "Approval was obtained from the ethical committee of the department of Obstetrics and Gynecology, Ain‐Shams University" p 818 Ethics approval obtained?: yes ‐ "oral consent was obtained from each participant before proceeding to either of the options" p818 Did we attempt to contact the trial authors?: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomized using a computer‐generated list (MedCalc version 13.2.2, Acacialaan 22, Ostend, Belgium) in a 1:1 ratio into 2 groups" (p 818) |
| Allocation concealment (selection bias) | Unclear risk | "The randomization protocol was also concealed using closed envelopes so that each envelope contained the name of one of the 2 options" (p 818) Comment: does not state whether envelopes were opaque or consecutively numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. Assume blinding of personnel not possible due to difference in intervention techniques between groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 women in the intervention group refused to give consent. All other women were accounted for |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | No other sources of bias were apparent |
Kavak 2013.
| Study characteristics | ||
| Methods | "Randomised prospective single blind trial" (p 706) Setting: university hospital setting in Turkey |
|
| Participants | Women with complete placenta praevia and intractable bleeding (n = 13) following caesarean section.
PPH was not defined. Exclusions: serious medical, haematological or surgical diseases; placental implantation anomalies such as placenta accreta/increta/percreta; history of thromboembolism; emergency CS; macrosomia; poly ‐hydramnios; preeclampsia; gestational diabetes; intrauterine growth retardation; and presence of multiple gestations. |
|
| Interventions |
Intervention: Bakri balloon tamponade intraoperatively through uterine incision (7 women) ‐ was inflated with saline (100 mL to 200 mL) "according to the uterine size" p 706 Control: Endouterine haemostatic square suturing to the lower segment of the uterus (6 women). 4‐5 sutures were applied. All women were given prophylactic antibiotics. |
|
| Outcomes |
|
|
| Notes |
Trial authors' declarations of interest: the authors state that they have no conflicts of interest. Sources of trial funding: not reported. Trial dates: August 2011 to August 2012 Informed consent obtained? yes Ethics approval obtained? yes, from the local research ethics committee Did we attempt to contact the trial authors?: email to Dr Kavak on 25 Aug 2018 requesting data on blood loss following transfusion only (not total blood loss). No response as of 1 March 2019. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial report states "randomly divided" ‐ insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported to be a single‐blind trial but no further information explicitly provided, although women were under general anaesthesia so presumably effectively blinded whilst undergoing intervention. Assume blinding of surgical personnel not possible due to difference in intervention techniques between groups. (Blood loss during the operation calculated by the anaesthetist (evaluation of blood collected via suction plus weighing of absorbant pads) ‐ reasonably objective. During first 24 hours, postoperative blood loss measured by weighing pads worn by patients ‐ again, this is reasonably objective so we question the extent to which lack of blinding would matter ‐ it may have had lots of small impacts on clinical decision‐making) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to assess ‐ reported to be a single‐blind trial but no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other sources of bias identified |
Khalil 2011.
| Study characteristics | ||
| Methods | Randomised controlled trial Setting: security forces hospital, Riyadh, Saudi Arabia |
|
| Participants | Women with uncontrolled primary atonic PPH during caesarean section and not responding to standard treatment (standard treatment not described). PPH was not defined. Exclusions: < 28 weeks' gestation Traumatic bleeding or placenta praevia |
|
| Interventions |
Intervention: Bakri balloon inserted intraoperatively through uterine incision, then secured with traction stitch (25 women). "Bakri balloon was fixed with nylon loop stitching through the hole of its proximal shaft, and the needle was then passed through the uterine cavity and the anterior abdominal wall. The thread was fixed to the skin to keep the balloon within the uterine cavity without any additional packing or the insertion of a balloon vaginally" p 198. Control: Bakri balloon intraoperatively through uterine incision, without traction stitch (25 women) Both groups of women received intravenous antibiotics for the first 48 hours and oxytocin infusion for the first 8 hours. It is not clear how much saline was used to inflate the balloons...the trial report states that, "the balloon was inflated until it conformed to the contour of the uterus in order to provide a symmetric tamponade effect" p 198 |
|
| Outcomes |
|
|
| Notes | The study was stopped after 50 cases because there was a high rate of Bakri balloon displacement in the control group. Trial authors' declarations of interest: "the authors have no conflicts of interest" p 199 Sources of trial funding: not reported Trial dates: 1 April 2004 and 30 April 2009 Ethical approval? "the hospital ethics committee approved the study" p198 Informed consent? yes, "informed consent was obtained from all participants" p 198 Contacted trial authors?: yes, email to Dr Khalil on 25 Aug 2018 requesting data on dichotomous blood loss outcomes. Data sent to ADW on 06 Sep 2018. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐based random sampling" p 198 |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned. Assume blinding of personnel not possible due to difference in intervention techniques between groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Insufficient information to make an assessment of reporting bias |
| Other bias | Low risk | No other sources of bias were apparent |
Soltan 2007.
| Study characteristics | ||
| Methods | Randomised controlled trial The trial report says it is a "Randomized clinical trial/cross over study" (in the abstract only). Although some members of the control group do receive the intervention, the methods described in the report are not those of a cross‐over trial; the fact that the women in the control group received the intervention was not 'designed in': according to their methods it does not seem to be pre‐specified in the trial design. Setting: Beni‐Mazar District Hospital, El‐Menia, Egypt |
|
| Participants | 240 women with PPH, due to atony, following vaginal birth at home or in hospital PPH is not defined in the trial report, and there is no information on how blood loss was assessed. Exclusions: "cases of PPH due to traumatic, retained placental tissues, other cause and after cesarean delivery" p54 |
|
| Interventions |
Intervention: woman received the 'El‐Menia' balloon plus cervical stitch to "prevent herniation of the inflated balloon through the dilated cervix" (p54), plus standard care (see below). The El‐Menia balloon tamponade consists of an orange latex party balloon (0.19 mm thick) attached to a 15 cm long Nelton catheter. The balloon was inflated with air using a sphygmomanometer to 140 mmHg and then attached to the catheter using silk suture "tied over tightly several times... to prevent air escape" P54. To keep the balloon in place, cervical cerclage was applied ("at 3 and 9 o'clock" p54). Women in this group also received antibiotic prophylaxis: gentamycin 80 mg, metronidazole 500 mg, and ampicillin 500 mg, immediately after the balloon was inserted and every 8 hours thereafter for the subsequent three days. (120 women) Control: standard care ‐ "women were treated with uterine massage and ecbolics according to WHO protocol" p 54 (120 women) Women receiving the intervention were also given metronidazole 500 mg, gentamycin 80 mg, and ampicillin 500 mg after insertion of the balloon, every 8 hours for 3 days. |
|
| Outcomes |
|
|
| Notes |
Trial authors' declarations of interest: not reported Sources of trial funding: not reported Trial dates: 2003 to 2004 (precise date not given) Ethical approval? yes "the study design was approved by El‐Menia Faculty of medicine ethical committee" p54 Informed consent? Women were admitted to the study after their husbands gave informed consent Contacted trial authors? Yes. We attempted to email Professor Soltan on 20 January 2020 but did not receive a reply. The trial methods specified that all women in the control group would receive standard care as the first‐line treatment. However, 19/120 women in the control group subsequently received UBT as a second‐line treatment. The authors preserved intention‐to‐treat (those 19 women remained in the control group). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation (no further details provided) |
| Allocation concealment (selection bias) | Low risk | Closed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned. Assume blinding of personnel not possible due to difference in intervention techniques between groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data apparent |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Insufficient information to make an assessment of reporting bias |
| Other bias | High risk | 19 women in the control group also received the intervention as a second‐line treatment. This secondary use of UBT was not prespecified in the methods. No other sources of bias were apparent |
Hb: haemoglobin; ICU: intensive care unit; PPH: postpartum haemorrhage; UBT: uterine balloon tamponade.
Characteristics of excluded studies [ordered by year]
| Study | Reason for exclusion |
|---|---|
| Soltan 2009 | Quasi‐RCT (alternate allocation) |
| Soltan 2010 | Quasi‐RCT (alternate allocation) |
| Gelany 2012 | This is an abstract for a PPH treatment study but there is insufficient information to permit 'Risk of bias' assessment. |
| Letouzey 2013 | Study terminated due to recruitment difficulties. |
| Mohamed 2014 | Abstract with insufficient information to assess risk of bias. |
| Khalil 2014 | Abstract with insufficient information to assess risk of bias. Query whether this is report of Khalil 2011 but the limited outcome data do not match. Contacted trial author by email ‐ he replied confirming that this study is separate to Khalil 2011 trial. |
| Nermeen 2015 | Prevention of PPH study |
| Rahman 2015 | This was a trial of TempostatTM ‐ a self‐regulating tamponade device for management of postpartum haemorrhage (see NCT02416089). The trial was terminated. |
| Purwosonu 2015 | Not an RCT. This is an open‐label non‐randomised trial |
| Rezk 2016 | Prevention of PPH study |
| Anger 2016 | This step‐wedge cluster‐RCT looked at a policy of introducing UBT into routine practice. 18 hospitals were randomised and the participants included women with PPH and without PPH. We have excluded this study because (1) it does not meet the inclusion criteria; and (2) does not give a sensitivity analysis of PPH women that could have been included. |
| von Beckerath 2016 | Abstract with insufficient information to permit 'Risk of bias' assessment. We query whether this is part of Ali 2015a (ongoing) ‐ we have contacted the authors for clarification. |
| Liu 2016 | Not a PPH treatment RCT ‐ comparison of 2 different nursing methods for women who had all had uterine embolisation for PPH |
| Azmy 2016 | Prevention of PPH study |
| Chen 2017 | Prevention of PPH study |
| Sallam 2019 | Prevention of PPH study |
| Farouk 2018 | Prevention of PPH study |
PPH: postpartum haemorrhage;RCT: randomised controlled trial; UBT: uterine balloon tamponade.
Characteristics of studies awaiting classification [ordered by study ID]
Ali 2015.
| Methods | Randomised trial ‐ open‐label ‐ 2 arms |
| Participants | 66 women enrolled. Women (aged 20 to 40) with primary PPH (due to atony) Exclusions: traumatic PPH |
| Interventions | Celox haemostatic dressings versus Bakri balloon |
| Outcomes | Blood loss (mL) |
| Notes | Completion date listed as October 2015. Status listed as 'completed'. NCT0256857 Mohammed Khairy Ali, Assuit University No email contact listed in trial registration but we found the trial author's contact details from the host institution's website. We emailed Mohammed Khairy Ali on 7 November 2019, awaiting a reply. |
Elkateeb 2016.
| Methods | Prospective randomised trial ‐ 3 arms ‐ open‐label |
| Participants | 150 women with PPH (due to atony) but "not indicated for surgical intervention" Ages 18 to 45, vaginal birth or caesarean section Exclusions: genital tract laceration, uterine rupture, retained placenta, known blood coagulation issues. |
| Interventions | Bakri Balloon alone Bakri balloon and intrauterine misoprostol wash Control group = a further group 'treated conservatively' ‐ ecobolic |
| Outcomes |
Primary
Secondary
|
| Notes | PACTR201601001435165 Funding source: Minia Maternity Hospital, Egypt Recruitment status: completed Sources of trial funding: trial sponsored by Minia Maternity Hospital, and Faculty of Medicine, Minia (both in Egypt) Ethical approval obtained? No (was due to be submitted for ethical approval on 1 Feb 2016, date of approval is blank) Contacts: principal investigator: Reham Elkateeb (rehamelkhateeb78@yahoo.com); public enquiries: Hossam Shawki (hossam200002003@yahoo.com); scientific enquiries to Ahmad Mahran (ezzeldin_ahmad@yahoo.com) Emailed study authors on 7 November 2019, awaiting a reply. |
Hb: haemoglobin;ICU: intensive care unit; PPH: postpartum haemorrhage.
Characteristics of ongoing studies [ordered by study ID]
Abbas 2016.
| Study name | Bakri balloon with or without abdominal traction stitch in management of uterine bleeding in cases of placenta praevia |
| Methods | RCT ‐ single blind ‐ 2 arms |
| Participants | Women with primary PPH (atony) Exclusions: traumatic PPH, uterine infection (suspected or confirmed); pre‐eclampsia, gestational diabetes; deep vein thrombosis or other thromboembolic complications; history of rheumatic heart disease; coagulopathy. |
| Interventions | Intervention:Bakri balloon with abdominal traction stitch Control: Bakri balloon without abdominal traction stitch |
| Outcomes | Failure of Bakri balloon (balloon rupture or slippage [24‐hour timeframe]) |
| Starting date | March 2016 but as at 7 November 2019 the study is listed 'not yet recruiting' |
| Contact information | Ahmed Mohamed Abbas, Assuit University, Egypt No email address listed |
| Notes |
NCT02694341 Funding source: not mentioned Recruitment status: not yet recruiting Ethical approval: not mentioned |
Hofmeyr 2019.
| Study name | STUT study Uterine vacuum tamponade for treatment of postpartum haemorrhage: a randomized study |
| Methods | RCT |
| Participants | Women (over 18 years of age) with PPH Exclusions: PPH due to cervical/vaginal tears Plan to include 84 participants |
| Interventions | Suction tube uterine tamponade Standard care control |
| Outcomes |
Primary
Secondary
|
| Starting date | 1 August 2019 (planned) |
| Contact information | Justus Hofmeyr (justhof@gmail.com) ‐ Principal investigator |
| Notes | PACTR201907769424884 Funding source: trial sponsored by the University of Witwatersrand, South Africa Recruitment status: not yet recruiting Ethical approval: Yes (approved by the University of Witwatersrand Human Research Ethics Committee) |
Hu 2015.
| Study name | The comparative study to evaluate the success rate of the intrauterine tamponade balloon and gauze packing the event of uncontrollable haemorrhage due to placenta praevia in caesarean section cases: a randomised prospective controlled multicenter trial |
| Methods | Prospective RCT ‐ 2 arms |
| Participants | 204 women with PPH following caesarean section and placenta praevia. Term gestation >/= 28 weeks). Exclusion: praevia accreta; uterine infection; 'others'? Age between 18 and 50 |
| Interventions | Intrauterine inflated balloon Gauze packing |
| Outcomes |
Primary
Secondary
|
| Starting date | Study dates were planned to be 1 June 2015 until 31 December 2017 |
| Contact information | Jing Wei qlm_weijin@163.com (applicant) Yali Hu glyyhuyali@163.com (study leader) |
| Notes | ChiCTR‐ICR‐15006467 Ethics? Yes Status: recruiting IPD data will be made available |
Joshipura 2016.
| Study name | Does cold saline used to inflate a balloon tamponade catheter more significantly reduce blood loss from postpartum haemorrhage than room temperature saline? |
| Methods | Randomised trial ‐ open‐label ‐ 2 arms |
| Participants | 50 women aged 18 to 45 with PPH following vaginal birth Exclusions: women outside age range |
| Interventions | Cold Bakri balloon (filled with saline at 32 deg F) Room temperature Bakri balloon (filled with saline at room temperature) |
| Outcomes |
Primary Total blood loss |
| Starting date | |
| Contact information | Swati B Joshipura swati.joshipura@bswhealth.org Jack Stecher jack.stecher@bswhealth.org |
| Notes |
NCT02735733 Protocol ‐ status as at 7 November is 'not yet recruiting' But estimated completion time was June 2016 Sponsor: Baylor Research Institute |
Maged 2019.
| Study name | A comparative study between Bakri balloon and B‐Lynch suture used to control primary postpartum haemorrhage after caesarean section |
| Methods | RCT ‐ parallel ‐ open‐label |
| Participants | Women aged between 18 to 40 years of age undergoing elective caesarean section and having atonic PPH Exclusions: PPH following vaginal delivery; secondary PPH; PPH due to causes other than atony; antepartum haemorrhage; tendency towards bleeding; other caesarean section complication (e.g. bladder injury, DIC). Anticipated number of women recruited = 100 |
| Interventions | B‐Lynch suture Bakri balloon (control) |
| Outcomes |
Primary outcome
|
| Starting date | March 2019 |
| Contact information | Ahmed Maged ‐ profahmedmaged@gmail.com ‐ principal investigator Cairo University |
| Notes |
NCT03891082 Expected completion: December 2019 Status listed as 'not yet recruiting' as at 7 November 2019 Trial sponsored by Cairo University |
Rozenberg 2014.
| Study name | Assessment of the efficacy of early intrauterine tamponade with a belfort‐dildy balloon obstetric tamponande system in the treatment of immediate postpartum haemorrhage |
| Methods | RCT ‐ open‐label |
| Participants | Belfort‐Dildy balloon device plus intravenous sulprostone infusion intravenous sulprostone infusion Estimate 430 women |
| Interventions | Blood loss in first 24 hours greater than or equal to 1500 mL Blood transfusion Blood products transfusion Other markers of severe haemorrhage (Hg change; haematocrit; etc) Genital tract infection Fever Endometritis during postpartum hospitalisation Maternal death postpartum |
| Outcomes | Need for invasive procedure (embolisation/surgery) due to PPH |
| Starting date | April 2016 |
| Contact information | Patrick Rozenberg prozenberg@chi‐poissy‐st‐germain.fr Laurence Lecomte laurence.lecomte@nck.aphp.fr |
| Notes |
NCT02226731 Sponsor: Assistance Publique ‐ Hopital de Paris Status: recruiting (as at 7 November 2019) |
DIC: disseminated intravascular coagulation;Hb: haemoglobin; ICU: intensive care unit; IPD: individual patient data; PPH: postpartum haemorrhage; RCT: randomised controlled trial.
Differences between protocol and review
The protocol for this review was published in PROSPERO on 21 June 2018 ‐ see https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=65107.
In the full review, we have clarified that we did not include trials of 'all' women including those with PPH (where the trial authors do not provide subgrouped results for women with PPH).
For comparison 6 (data from Darwish 2018), we included an additional outcome, not prespecified in our published protocol: total blood loss >= 1000mL (before and after randomisation).
We edited our methods to remove our intention to carry out both subgroup and sensitivity analyses to investigate heterogeneity ‐ we will restrict our investigation to subgroup analysis.
Contributions of authors
Julius Wandabwa (JW), Andrew Weeks (AW), Hatem Mousa (HM) and Frances Kellie (FK) all contributed to design and write‐up of the protocol. FK is the contact person and guarantor for this review.
For the full review, AW and FK independently assessed trials for inclusion. JW, AW and FK independently assessed risk of bias. JW and AW independently extracted data, which were checked by a member of the PCG Editorial Team. AW added the data to the RevMan file. FK drafted pre‐GRADE documents and these were checked by a member of the PCG Editorial Team (see acknowledgements), FK also drafted the results, abstract and Plain language summary. The discussion, conclusion and Plain language summary were written by AW.
Sources of support
Internal sources
No sources of support supplied
External sources
-
WellBeing of Women/FIGO, UK
JJW and ADW were the recipient of a Wellbeing of Women/FIGO Academic Scholarship (Award Ref FIGO2) of which this systematic review was a part.
-
World Health Organization (WHO) and the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Switzerland
This review is supported by funding to Cochrane Pregnancy and Childbirth (University of Liverpool)
Declarations of interest
JW: has received FIGO/Wellbeing fellowship. This was a training fellowship and included facilitation of this review. The award also supported travel and attendance at training workshops in systematic review methodologies in Liverpool and at other UK institutions.
ADW: has been involved in misoprostol research for many years and runs www.misoprostol.org, which is a website that seeks to provide independent information on misoprostol doses. He has been an investigator on a team investigating the use of misoprostol for postpartum haemorrhage prophylaxis in rural Uganda. He is also one of two inventors of the PPH Butterfly ‐ a device to facilitate bimanual compression of the uterus. The patent is held by his employer (the University of Liverpool), but ADW would receive royalties from any future sales of the device.
HM: none known.
FK: is the Managing Editor of Cochrane Pregnancy and Childbirth and employed by the University of Liverpool via NIHR Cochrane Infrastructure funding paid to the host institution. Cochrane Pregnancy and Childbirth also received project funding from WHO to prepare Cochrane evidence to inform prioritised WHO recommendations for updating. FK is a member of the Executive Guideline Steering Group for Updating WHO Maternal and Perinatal Health Recommendations (2017 to 2019) and she has received travel and per diem expenses from WHO within the last three years in relation to attendance at WHO technical and prioritisation meetings. As a member of Cochrane Pregnancy and Childbirth's editorial team, Frances has not been involved in the editorial processing or any editorial decisions relating to the protocol, or the subsequent review.
New
References
References to studies included in this review
Ashraf 2018 {published data only}
- Ashraf N, Ashraf A, Khursheed K. Efficacy and safety of intrauterine balloon tamponade versus ueterovaginal roll gauze packing in patient presenting with primary postpartum haemorrhage after normal vaginal delivery. Annals of King Edward Medical University 2018;24(Special Issue):889-92. [Google Scholar]
Chantrapitak 2009 {published data only}
- Chantrapitak W, Srijanteok K, Puangsa-art S. Lower uterine segment compression for management of early postpartum hemorrhage after vaginal delivery at Charoenkrung Pracharak Hospital. Journal of the Medical Association of Thailand 2009;92(5):600-5. [PMID: ] [PubMed] [Google Scholar]
Darwish 2018 {published data only}
- Darwish AM, Abdallah MM, Shaaban OM, Ali MK, Khalaf M, Sabra AM. Bakri balloon versus condom-loaded foley's catheter for treatment of atonic postpartum hemorrhage secondary to vaginal delivery: a randomized controlled trial. Journal of Maternal-Fetal & Neonatal Medicine 2018;31(6):747-53. [DOI] [PubMed] [Google Scholar]
- NCT02430155. Comparison of condom-loaded foley's catheter versus bakri balloon for treatment of primary postpartum hemorrhage: a randomized controlled trial. clinicaltrials.gov/show/NCT02430155 (first received 30 April 2015).
Dumont 2017 {published data only}ISRCTN01202389
- Dumont A, Bodin C, Hounkpatin B, Popowski T, Traore M, Perrin R, et al. Uterine balloon tamponade as an adjunct to misoprostol for the treatment of uncontrolled postpartum haemorrhage: a randomised controlled trial in benin and mali. BMJ Open 2017;7(9):e016590. [DOI] [PMC free article] [PubMed]
- ISRCTN01202389. Evaluation of intrauterine balloon tamponade efficacy with condom catheter and misoprostol (usual treatment) compared to misoprostol alone in the severe postpartum management hemorrhage management in Benin and Mali: a randomized controlled trial. www.isrctn.com/ISRCTN01202389 (first received 9 September 2013).
- Tort J, Hounkpatin B, Popowski T, Traore M, Bodin C, Perrin R, et al. A randomized controlled trial to test the effectiveness of intrauterine balloon tamponade with condom catheter in severe postpartum hemorrhage management: a feasibility study in Benin. Journal of Women's Health Care 2013;2(4):135. [Google Scholar]
El‐Sokkary 2016 {published data only}
- El-Sokkary M, Wahba K, El-Shahawy Y. Uterine salvage management for atonic postpartum hemorrhage using "modified lynch suture". BMC Pregnancy and Childbirth 2016;16:251. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Farouk 2016 {published data only}
- Farouk O, Elbasuony W, Elbohouty A. Uterine artery embolization versus surgical management in primary atonic postpartum hemorrhage: A randomized clinical trial. Egyptian Journal of Radiology and Nuclear Medicine 2016;47(3):817-23. [Google Scholar]
Kavak 2013 {published data only}
- Kavak SB, Atilgan R, Demirel I, Celik E, Ilhan R, Sapmaz E. Endouterine hemostatic square suture vs. Bakri balloon tamponade for intractable hemorrhage due to complete placenta previa. Journal of Perinatal Medicine 2013;41(6):705-9. [DOI] [PubMed] [Google Scholar]
Khalil 2011 {published data only}
- Khalil MI, Al-Dohami H, Aldahish MM. A method to improve the effectiveness of the Bakri balloon for management of postpartum hemorrhage at cesarean. International Journal of Gynecology & Obstetrics 2011;115(2):198-200. [DOI] [PubMed] [Google Scholar]
Soltan 2007 {published data only}
- Soltan MH, Mohamed A, Ibrahim E, Gohar A, Ragab H. El-Menia air inflated balloon in controlling atonic post partum hemorrhage. International Journal of Health Sciences, Qassim University 2007;1(1):53-9. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Anger 2016 {published data only}
- NCT02910310. Introduction of a uterine balloon tamponade for postpartum hemorrhage in three low income countries: a stepped wedge cluster randomized trial. clinicaltrials.gov/show/NCT02910310 (first received 22 September 2016).
Azmy 2016 {published data only}
- NCT02660567. The impacts of using amr's maneuver (cervical traction) on atonic postpartum hemorrhage; multi-centre randomized controlled study. clinicaltrials.gov/show/NCT02660567 (first received 21 January 2016).
Chen 2017 {published data only}
- ChiCTR-IOR-17012244. Bilateral internal iliac artery balloon occlusion for the management of pernicious placenta previa and accreta. www.chictr.org.cn/showproj.aspx?proj=20741 (first received 4 August 2017).
Farouk 2018 {published data only}
- Farouk H, NCT03570723. Glove-loaded foley's catheter tamponade to reducing blood loss during cesarean delivery for complete placenta previa: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT03570723 (first received 27 June 2018).
Gelany 2012 {published data only}
- Gelany SA, Soltan MH. External aortic compression device, manual aortic compression & El Minya air inflated balloon: simple, cost-effective, and saving many lives in low resource settings. International Journal of Gynaecology and Obstetrics 2012;119:S335.
Khalil 2014 {published data only}
- Khalil M. Upper segment caesarean section, bilateral uterine artery ligation, with Khalil, s traction stitch for Bakri balloon tamponade to control postpartum hemorrhage from morbid adherent placenta previa. Journal of Maternal-Fetal & Neonatal Medicine 2014;27(Suppl 1):40. [Google Scholar]
Letouzey 2013 {published data only}
- Letouzey V, NCT01980173. Medico-economic comparison of postpartum hemorrhage management using the bakri balloon and standard care. https://clinicaltrials.gov/show/NCT01980173 (first received 8 Novemmber 2013).
Liu 2016 {published data only}
- Liu M, Li D. Effect of overall care for postpartum major hemorrhage patients with uterine artery embolization. Chinese Journal of Interventional Imaging and Therapy 2016;13(6):338-41. [Google Scholar]
Mohamed 2014 {published data only}
- Mohamed AM, Agawany AS, Abd Rabbo S. Stepwise devascularization versus B-Lynch for massive postpartum haemorrhage. BJOG: an international journal of obstetrics and gynaecology 2014;121(Suppl 2):120. [Google Scholar]
Nermeen 2015 {published data only}
- Nermeen SE, Aly K, Sameh SE, Amr AE. Uterine compression sutures and the amount of post partum blood loss in high risk cases of atonic post partum hemorrhage. Journal of Perinatal Medicine 2015;43(Suppl 1):Abstract no: O-0006. [Google Scholar]
Purwosonu 2015 {published data only}
- Purwosonu Y, Widyastuti. Control of post-partum hemorrhage by vacuum induced uterine tamponade. Journal of Obstetrics and Gynaecology Research 2015;41(Suppl S1):47, Abstract no: FC 9.01. [Google Scholar]
Rahman 2015 {published data only}
- NCT02416089. Tampostat: a low-cost, self-regulating tamponade for management of postpartum hemorrhage in Bangladesh. clinicaltrials.gov/show/NCT02416089 (first received 14 April 2015).
Rezk 2016 {published data only}
- PACTR201610001803327. Bilateral uterine artery ligation and square sutures versus a novel combined suture for controlling bleeding from the placental bed in placenta previa centralis at cesarean section: a randomized clinical trial. pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201610001803327 (first received 3 October 2016).
Sallam 2019 {published data only}
Soltan 2009 {published data only}
- Soltan MH, Faragallah MF, Mosabah MH, Al-Adawy AR. External aortic compression device: the first aid for postpartum hemorrhage control. Journal of Obstetrics and Gynaecology Research 2009;35(3):453-8. [DOI] [PubMed] [Google Scholar]
Soltan 2010 {published data only}
- Soltan MH, Imam HH, Zahran KA, Atallah SM. Assessing changes in flow velocimetry and clinical outcome following use of an external aortic compression device in women with postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2010;110(3):257-61. [DOI] [PubMed] [Google Scholar]
von Beckerath 2016 {published data only}
- Beckerath AK, Maul H, Elmohandes AM, Shaaban M, Habib DM, Nasr A, et al. Comparison of Celox and Bakri balloon in management of primary atonic postpartum hemorrhage. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S335, Abstract no: 628. [Google Scholar]
References to studies awaiting assessment
Ali 2015 {published data only}
- NCT02568657. Comparison between celox versus bakri balloon for treatment of primary atonic postpartum hemorrhage. clinicaltrials.gov/ct2/show/record/NCT02568657 (first received 6 October 2015).
Elkateeb 2016 {published data only}
- Elkateeb R, PACTR201601001435165. Using of bakri balloon cathter with intrauterine washing with misoprostol in cases of atonic postpartum hemorrhage. pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201601001435165 (first received 23 January 2016).
References to ongoing studies
Abbas 2016 {published data only}
- Abbas MA, NCT02694341. Bakri balloon with or without abdominal traction stitch in management of uterine bleeding in cases of placenta previa. clinicaltrials.gov/ct2/show/NCT02694341 (first received 29 February 2016).
Hofmeyr 2019 {published data only}
- Hofmeyr GJ, PACTR201907769424884. Uterine vacuum tamponade for treatment of postpartum haemorrhage: a randomized study. https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=8223 (first received 30 June 2019).
Hu 2015 {published data only}
- ChiCTR-ICR-15006467. The comparative study to evaluate the success rate of the intrauterine tamponade balloon and gauze packing the event of uncontrollable hemorrhage due to placenta previa in cesarean section cases: a randomized perspective controlled multicenter trial. chictr.org.cn/showproj.aspx?proj=11074 (first received 29 June 2015).
Joshipura 2016 {published data only}
- NCT02735733. Does cold saline used to inflate a balloon tamponade catheter more significantly reduce blood loss from postpartum hemorrhage than room temperature saline? clinicaltrials.gov/ct2/show/record/NCT02735733 (first received 16 April 2016).
Maged 2019 {published data only}
- Maged A, NCT03891082. A comparative study between Bakri Balloon and B lynch suture used to control primary postpartum hemorrhage after cesarean section. https://clinicaltrials.gov/ct2/show/NCT03891082 (first received 26 March 2019).
Rozenberg 2014 {published data only}
- NCT02226731. Assessment of the efficacy of early intrauterine tamponade with a belfort-dildy balloon obstetric tamponade system in the treatment of immediate postpartum hemorrhage. clinicaltrials.gov/show/NCT02226731 (first received 27 August 2014).
Additional references
Abdel‐Aleem 2015
- Abdel-Aleem H, Abdel-Aleem MA, Shaaban OM. Nitroglycerin for management of retained placenta. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD007708.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abedi 2016
- Abedi P, Jahanfar S, Namvar F, Lee J. Breastfeeding or nipple stimulation for reducing postpartum haemorrhage in the third stage of labour. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD010845.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aisaka 1988
- Aisaka K, Ando S, Kokuho K, Tawada T, Kaneda S, Yoshimatsu J. Effects of obesity and weight gain during pregnancy on obstetrical factors. Acta Obstetrica et Gynaecologica Japonica 1988;63:1851–8. [PubMed] [Google Scholar]
Alexander 2002
- Alexander J, Thomas PW, Sanghera J. Treatments for secondary postpartum haemorrhage. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD002867] [DOI] [PubMed] [Google Scholar]
Alkema 2016
- Alkema L, Chou D, Hogan D, Zhang S, Moller AB, Gemmill A, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet 2016;387(10017):462-74. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Antony 2017
- Antony KM, Racusin DA, Belfort MA, Dildy GA 3rd. Under pressure: intraluminal filling pressures of postpartum hemorrhage tamponade balloons. AJP Reports 2017;7(2):e86-e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
B‐Lynch 1997
- B-Lynch C, Coker A, Lawal AH, Abu J, Cowen MJ. The B-Lynch surgical technique for the control of massive postpartum haemorrhage: an alternative to hysterectomy? Five cases reported. British Journal of Obstetrics and Gynaecology 1997;104(3):372–5. [DOI] [PubMed] [Google Scholar]
Bakri 2001
- Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. International Journal of Gynaecology and Obstetrics 2001;74(2):139-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Begley 2019
- Begley CM, Gyte GM, Devane D, McGuire W, Weeks A, Biesty LM. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2019, Issue 2. [DOI: 10.1002/14651858.CD007412.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonnar 2000
- Bonnar J. Massive obstetric haemorrhage. Baillieres Best Practice and Research in Clinical Obstetrics and Gynaecology 2000;14(1):1-18. [DOI] [PubMed] [Google Scholar]
Burke 2016
- Burke TF, Ahn R, Nelson BD, Hines R, Kamara J, Oguttu M, et al. A postpartum haemorrhage package with condom uterine balloon tamponade: a prospective multi-centre case series in Kenya, Sierra Leone, Senegal, and Nepal. BJOG: an international journal of obstetrics and gynaecology 2016;123(9):1532-40. [DOI] [PubMed] [Google Scholar]
Carroli 2008
- Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum hemorrhage: a systematic review. Best Practice and Research. Clinical Obstetrics and Gynaecology 2008;22(6):999–1020. [DOI: 10.1016/j.bpobgyn.2008.08.004 ] [DOI] [PubMed] [Google Scholar]
Cekmez 2015a
- Cekmez Y, Ozkaya E, Ocal FD, Kucukozkan T. Experience with different techniques for the management of postpartum hemorrhage due to uterine atony: compression sutures, artery ligation and Bakri balloon. Irish Journal of Medical Science 2015;184(2):399-402. [DOI: 10.1007/s11845-014-1130-3] [DOI] [PubMed] [Google Scholar]
Chan 1997
- Chan C, Razvi K, Tham KF, Arulkumaran S. The use of a Sengstaken-Blakemore tube to control post-partum hemorrhage. International Journal of Gynaecology and Obstetrics 1997;58(2):251-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cho 2000
- Cho JH, Jun HS, Lee CN. Hemostatic suturing technique for uterine bleeding during cesarean delivery. Obstetrics & Gynecology 2000;96(1):129-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Clark 1985
- Clark SL, Phelan JP, Yeh SY, Bruce SR, Paul RH. Hypogastric artery ligation for obstetric hemorrhage. Obstetrics & Gynecology 1985;66(3):353-6. [PMID: 3875064] [PubMed] [Google Scholar]
Combs 1991
- Combs CA, Murphy EL, Laros RK Jr. Factors associated with postpartum haemorrhage with vaginal birth. Obstetrics & Gynecology 1991;77(1):69-76. [PMID: ] [PubMed] [Google Scholar]
COPE 2019
- The carboprost or oxytocin postpartum haemorrhage effectiveness study. https://doi.org/10.1186/ISRCTN16416766.
CORD 2018
- Duley L, Dorling J, Pushpa-Rajah A, Oddie SJ, Yoxall CW, Schoonakker B, et al. Randomised trial of cord clamping and initial stabilisation at very preterm birth. Archives of Disease in Childhood. Fetal and Neonatal Edition 2018;103(1):F6-F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doumouchtsis 2007
- Doumouchtsis SK, Papageorghiou AT, Arulkumaran S. Systematic review of conservative management of postpartum hemorrhage: what to do when medical treatment fails. Obstetrical and Gynecological Survey 2007;62(8):540-7. [DOI: 10.1097/01.ogx.0000271137.81361.93] [DOI] [PubMed] [Google Scholar]
Eastman 1950
- Eastman NJ. Anomalies of the third stage of labor. In: William’s Obstetrics. 10th edition. New York: Appleton-Century Crofts, 1950:917–9. [Google Scholar]
El Ayadi 2013
- El Ayadi A, Raifman S, Jega F, Butrick E, Ojo Y, Geller S, et al. Comorbidities and lack of blood transfusion may negatively affect maternal outcomes of women with obstetric hemorrhage treated with NASG. PLOS One 2013;8(8):e70446. [DOI: 10.1371/journal.pone.0070446] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geller 2006
- Geller SE, Adams MG, Kelly PJ, Kodkany BS, Derman RJ. Postpartum hemorrhage in resource-poor settings. International Journal of Gynaecology and Obstetrics 2006;92(3):202-11. [DOI] [PubMed] [Google Scholar]
Georgiou 2009
- Georgiou C. Balloon tamponade in the management of postpartum haemorrhage: a review. BJOG: an international journal of obstetrics and gynaecology 2009;116(6):748-57. [DOI: 10.1111/j.1471-0528.2009.02113.x] [DOI] [PubMed] [Google Scholar]
Gilbert 1987
- Gilbert L, Porter W, Brown VA. Postpartum haemorrhage: a continuing problem. British Journal of Obstetrics and Gynaecology 1987;94(1):67-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Green 2016
- Green L, Knight M, Seeney FM, Hopkinson C, Collins PW, Collis RE, et al. The epidemiology and outcomes of women with postpartum haemorrhage requiring massive transfusion with eight or more units of red cells: a national cross-sectional study. BJOG: an international journal of obstetrics and gynaecology 2016;123(13):2164-70. [DOI: 10.1111/1471-0528.13831] [DOI] [PubMed] [Google Scholar]
Grillo‐Ardila 2014
- Grillo-Ardila CF, Ruiz-Parra AI, Gaitán HG, Rodriguez-Malagon N. Prostaglandins for management of retained placenta. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD010312.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hancock 2015
- Hancock A, Weeks AD, Lavender DT. Is accurate and reliable blood loss estimation the 'crucial step' in early detection of postpartum haemorrhage: an integrative review of the literature. BMC Pregnancy and Childbirth 2015;15:230. [DOI: 10.1186/s12884-015-0653-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayman 2002
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstetrics & Gynecology 2002;99(3):502-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hofmeyr 2013b
- Hofmeyr GJ, Gulmezoglu AM, Novikova N, Lawrie TA. Postpartum misoprostol for preventing maternal mortality and morbidity. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD008982.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoveyda 2001
- Hoveyda F, MacKenzie IZ. Secondary postpartum haemorrhage: incidence, morbidity and current management. BJOG: an international journal of obstetrics and gynaecology 2001;108(9):927-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Johanson 2001
- Johanson R, Kumar M, Obhrai M, Young P. Management of massive postpartum haemorrhage: use of a hydrostatic balloon catheter to avoid laparotomy. BJOG: an international journal of obstetrics and gynaecology 2001;108(4):420-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jouppila 1995
- Jouppila P. Postpartum haemorrhage. Current Opinion in Obstetrics and Gynecology 1995;7(6):446–50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kassebaum 2014
- Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, et al. Global, regional, and national levels and causes of maternal mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384(9947):980-1004. [DOI: 10.1016/S0140-6736(14)60696-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerr 2016
- Kerr R, Eckert LO, Winikoff B, Durocher J, Meher S, Fawcus S, et al. Postpartum haemorrhage: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2016;34(49):6102-9. [DOI: 10.1016/j.vaccine.2016.03.039] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2006
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367(9516):1066–74. [DOI: 10.1016/S0140-6736(06)68397-9] [DOI] [PubMed] [Google Scholar]
Knight 2009
- Knight M, Callaghan WM, Berg C, Alexander S, Bouvier-Colle MH, Ford JB, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy and Childbirth 2009;9:55. [DOI: 10.1186/1471-2393-9-55] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 2011
- Kramer MS, Dahhou M, Vallerand D, Liston R, Joseph KS. Risk factors for postpartum hemorrhage: can we explain the recent temporal increase? Journal of Obstetrics and Gynaecology Canada 2011;33(8):810-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kramer 2013
- Kramer MS, Berg C, Abenhaim H, Dahhou M, Rouleau J, Mehrabadi A, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. American Journal of Obstetrics and Gynecology 2013;209(5):449.e1-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liabsuetrakul 2018
- Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam QM. Prophylactic use of ergot alkaloids in the third stage of labour. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD005456.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lozano 2011
- Lozano R, Wang H, Foreman KJ, Rajaratnam JK, Naghavi M, Marcus JR, et al. Progress towards Millennium Development Goals 4 and 5 on maternal and child mortality: an updated systematic analysis. Lancet 2011;378(9797):1139-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Machado 2011
- Machado LS. Emergency peripartum hysterectomy: Incidence, indications, risk factors and outcome. North American Journal of Medical Sciences 2011;3(8):358-61. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manzando‐Nunez 2018
- Manzano-Nunez R, Escobar-Vidarte MF, Naranjo MP, Rodriguez F, Ferrada P, Casallas JD, et al. Expanding the field of acute care surgery: a systematic review of the use of resuscitative endovascular balloon occlusion of the aorta (REBOA) in cases of morbidly adherent placenta. European Journal of Trauma and Emergency Surgery 2018;44(4):519-26. [DOI] [PubMed] [Google Scholar]
Marasinghe 2011
- Marasinghe JP, Condous G, Seneviratne HR, Marasinghe U. Modified anchored B-Lynch uterine compression suture for post partum bleeding with uterine atony. Acta Obstetricia et Gynecologica Scandinavica 2011;90(3):280-3. [DOI: 10.1111/j.1600-0412.2010.01048.x] [DOI] [PubMed] [Google Scholar]
Matsuzaki 2017
- Matsuzaki S, Endo M, Ueda Y, Mimura K, Kakigano A, Egawa-Takata T, et al. A case of acute Sheehan's syndrome and literature review: a rare but life-threatening complication of postpartum hemorrhage. BMC Pregnancy and Childbirth 2017;17(1):188. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2004
- McDonald SJ. Prophylactic ergometrine-oxytocin versus oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000201.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meher 2019
- Meher S, Cuthbert A, Kirkham JJ, Williamson P, Abalos E, Aflaifel N, et al. Core outcome sets for prevention and treatment of postpartum haemorrhage: an international Delphi consensus study. BJOG: an international journal of obstetrics and gynaecology 2019;126(1):83-93. [DOI] [PubMed] [Google Scholar]
Miller 2013b
- Miller S, Bergel EF, El Ayadi AM, Gibbons L, Butrick EA, Magwali T, et al. Non-pneumatic anti-shock garment (NASG), a first-aid device to decrease maternal mortality from obstetric hemorrhage: a cluster randomized trial. PLOS One 2013;8(10):e76477. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mori 2012
- Mori R, Nardin JM, Yamamoto N, Carroli G, Weeks A. Umbilical vein injection for the routine management of third stage of labour. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD006176.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mousa 2001
- Mousa HA, Walkinshaw S. Major postpartum haemorrhage. Current Opinion in Obstetrics and Gynecology 2001;13(6):595-603. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mousa 2008
- Mousa HA, Cording V, Alfirevic Z. Risk factors and interventions associated with major primary postpartum hemorrhage unresponsive to first-line conventional therapy. Acta Obstetricia et Gynecologica Scandinavica 2008;87(6):652-61. [PMID: 10.1080/00016340802087660] [DOI] [PubMed] [Google Scholar]
Mousa 2014c
- Mousa HA, Blum J, Abou El Senoun G, Shakur H, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD003249.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Novikova 2015a
- Novikova N, Hofmeyr GJ, Cluver C. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD007872.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ochoa 2002
- Ochoa M, Allaire AD, Stitely ML. Pyometria after hemostatic square suture technique. Obstetrics and Gynecology 2002;99(3):506-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Oladapo 2012
- Oladapo OT, Fawole B, Blum J, Abalos E. Advance misoprostol distribution for preventing and treating postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009336.pub2] [DOI] [PubMed] [Google Scholar]
Oladapo 2018
- Oladapo OT, Okusanya BO, Abalos E. Intramuscular versus intravenous prophylactic oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2018, Issue 9. [DOI: 10.1002/14651858.CD009332.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pantoja 2016
- Pantoja T, Abalos E, Chapman E, Vera C, Serrano VP. Oxytocin for preventing postpartum haemorrhage (PPH) in non-facility birth settings. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011491.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parry Smith 2017
- Parry Smith WR, Gallos ID, Williams HM, Widmer M, Angolkar M, Tobias A, et al. First-line uterotonics for treating postpartum haemorrhage: a systematic review and network meta-analysis. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD012754] [DOI] [Google Scholar]
Pereira 2005
- Pereira A, Nunes F, Pedroso S, Saraiva J, Retto H, Meirinho M. Compressive uterine sutures to treat postpartum bleeding secondary to uterine atony. Obstetrics and Gynecology 2005;106(3):569-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Porro 1876
- Porro E. Della amputazione utero-ovarica comecomplemento di taglio cesareo (Italian). https://catalog.hathitrust.org/Record/009895279 (accessed July 2018) 1876;237.
Poujade 2011
- Poujade O, Grossetti A, Mougel L, Ceccaldi PF, Ducarme G, Luton D. Risk of synechiae following uterine compression sutures in the management of major postpartum haemorrhage. BJOG: an international journal of obstetrics and gynaecology 2011;118(4):433-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rathat 2011
- Rathat G, Do Trinh P, Mercier G, Reyftmann L, Dechanet C, Boulot P, et al. Synechia after uterine compression sutures. Fertility and Sterility 2011;95(1):405-9. [DOI: 10.1016/j.fertnstert.2010.08.055] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sentilhes 2011a
- Sentilhes L, Gromez A, Clavier E, Resch B, Descamps P, Marpeau L. Long-term psychological impact of severe postpartum hemorrhage. Acta Obstetricia et Gynecologica Scandinavica 2011;90(6):615-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sentilhes 2016
- Sentilhes L, Vayssiere C, Deneux-Tharaux C, Aya AG, Bayoumeu F, Bonnet MP, et al. Postpartum hemorrhage: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians (CNGOF): in collaboration with the French Society of Anesthesiology and Intensive Care (SFAR). European Journal of Obstetrics, Gynecology, and Reproductive Biology 2016;198:12-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shahin 2018
- Shahin Y, Pang CL. Endovascular interventional modalities for haemorrhage control in abnormal placental implantation deliveries: a systematic review and meta-analysis. European Radiology 2018;28(7):2713-26. [DOI] [PubMed] [Google Scholar]
Shakur 2018
- Shakur H, Beaumont D, Pavord S, Gayet-Ageron A, Ker K, Mousa HA. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database of Systematic Reviews 2018, Issue 2. [DOI: 10.1002/14651858.CD012964] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sloan 2010
- Sloan NL, Durocher J, Aldrich T, Blum J, Winikoff B. What measured blood loss tells us about postpartum bleeding: a systematic review. BJOG: an international journal of obstetrics and gynaecology 2010;117(7):788-800. [DOI: 10.1111/j.1471-0528.2010.02567.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soro 2017
- Soro MP, Denys A, Rham M, Baud D. Short & long term adverse outcomes after arterial embolisation for the treatment of postpartum haemorrhage: a systematic review. European Radiology 2017;27(2):749-62. [DOI] [PubMed] [Google Scholar]
Stones 1993
- Stones RW, Paterson CM, Saunders NJ. Risk factors for major obstetric haemorrhage. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;48(1):15-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Suzuki 2012
- Suzuki S, Hiraizumi Y, Miyake H. Risk factors for postpartum hemorrhage requiring transfusion in cesarean deliveries for Japanese twins: comparison with those for singletons. Archives of Gynecology and Obstetrics 2012;286(6):1363-7. [DOI: 10.1007/s00404-012-2461-9] [DOI] [PubMed] [Google Scholar]
Tindell 2013
- Tindell K, Garfinkel R, Abu-Haydar E, Ahn R, Burke TF, Conn K, et al. Uterine balloon tamponade for the treatment of postpartum haemorrhage in resource-poor settings: a systematic review. BJOG: an international journal of obstetrics and gynaecology 2013;120(1):5-14. [DOI] [PubMed] [Google Scholar]
Tunçalp 2012
- Tunçalp Ö, Hofmeyr GJ, Gülmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD009336.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Stralen 2016
- Stralen G, Schmidt Auf Altenstadt JF, Bloemenkamp KW, Roosmalen J, Hukkelhoven CW. Increasing incidence of postpartum hemorrhage: the Dutch piece of the puzzle. Acta Obstetricia et Gynecologica Scandinavica 2016;95(10):1104-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weeks 2015a
- Weeks A. The prevention and treatment of postpartum haemorrhage: what do we know, and where do we go to next? BJOG: an international journal of obstetrics and gynaecology 2015;122(2):202-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weeks 2019
- Weeks AD. Does balloon tamponade really make postpartum haemorrhage worse? BJOG 2019;126:1622. [DOI: 10.1111/1471-0528.15948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetta 2013
- Wetta LA, Szychowski JM, Seals S, Mancuso MS, Biggio JR, Tita AT. Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery. American Journal of Obstetrics and Gynecology 2013;209(1):51.e1–51.e6. [DOI: 10.1016/j.ajog.2013.03.011] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2012
- WHO. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. World Health Organization, 2012. [PubMed] [Google Scholar]
WHO 2015
- WHO. WHO Guidelines for the Management of Postpartum Haemorrhage and Retained Placenta. World Health Organization, 2009. [PubMed] [Google Scholar]
WOMAN 2017
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389(10084):2105-16. [DOI: 10.1016/S0140-6736(17)30638-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2011
- Zheng J, Xiong X, Ma Q, Zhang X, Li M. A new uterine compression suture for postpartum haemorrhage with atony. BJOG: an international journal of obstetrics and gynaecology 2011;118(3):370-4. [DOI: 10.1111/j.1471-0528.2010.02809.x] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wandabwa 2018
- Wandabwa J, Mousa H, Kellie F, Weeks A. Mechanical and surgical interventions for treating primary postpartum haemorrhage. PROSPERO 2018 CRD42018065107. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018065107. [DOI] [PMC free article] [PubMed]


