Abstract
Microinjection of the restriction endonuclease HaeIII, which causes DNA double-strand breaks with blunt ends, induces nuclear accumulation of p53 protein in normal and xeroderma pigmentosum (XP) primary fibroblasts. In contrast, this induction of p53 accumulation is not observed in ataxia telangiectasia (AT) fibroblasts. HaeIII-induced p53 protein in normal fibroblasts is phosphorylated at serine 15, as determined by immunostaining with an antibody specific for phosphorylated serine 15 of p53. This phosphorylation correlates well with p53 accumulation. Treatment with lactacystin (an inhibitor of the proteasome) or heat shock leads to similar levels of p53 accumulation in normal and AT fibroblasts, but the p53 protein lacks a phosphorylated serine 15. Following microinjection of HaeIII into lactacystin-treated normal fibroblasts, lactacystin-induced p53 protein is phosphorylated at serine 15 and stabilized even in the presence of cycloheximide. However, neither stabilization nor phosphorylation at serine 15 is observed in AT fibroblasts under the same conditions. These results indicate the significance of serine 15 phosphorylation for p53 stabilization after DNA double-strand breaks and an absolute requirement for ATM in this phosphorylation process.
Upon various types of cellular stresses, protein levels of the tumor suppressor p53 increase rapidly, mainly by posttranslational mechanisms. The elevation of p53 in turn induces inhibition of cell cycle progression and/or apoptosis (17). This system is important for the maintenance of the integrity of genetic information and for elimination of abnormal cells. Defects in this system result in a high incidence of tumor progression (6) or in abnormal development (25). The stresses which induce the accumulation of p53 include genotoxic radiation (X ray, γ ray, UV, etc.), genotoxic drugs (8), inhibitors of DNA replication and transcription (32), and cellular stresses (9, 30) (heat shock, osmotic shock, hypoxia, etc.). How these stresses are sensed by cells and how the signals are transduced to effector molecules in cells are subjects of great interest.
Ataxia telangiectasia (AT) is an autosomal recessive disorder which is characterized by radiosensitivity of the affected individual. Patients suffering from AT often develop neoplasia, and thus a link between radiosensitivity and predisposition to develop cancer is suspected. AT cells are sensitive to ionizing radiation (IR) and show radioresistant DNA synthesis after IR. The gene responsible for AT has recently been identified (ATM), but its role and function in radiosensitivity and predisposition to develop cancer are not known. The AT gene encodes a protein whose homology suggest it to be a member of the phosphatidylinositol 3-kinase family (27). In AT cells, or cells derived from ATM gene knockout mice, p53 accumulation after IR is blunted or delayed compared with that of normal cells (15). However, the p53 responses of AT cells against other genotoxic agents such as UV are normal (4). For this reason, ATM has been suggested to be involved in the specific signaling pathway evoked by X-ray-type DNA-damaging agents.
p53 is degraded rapidly by the proteasome through a ubiquitination-dependent pathway. In this process, Mdm2 plays a crucial role. Mdm2 was found as a protein associating with p53. Expression of the mdm2 gene is controlled by p53 protein levels. Interestingly, Mdm2 regulates both the transactivator activity (23) and stability (18) of p53 by direct association. As a consequence of the Mdm2-p53 interaction, intracellular p53 levels are maintained at a low level throughout the cell cycle (10). It is reported that Mdm2 has a ubiquitin ligase activity for p53 via the ubiquitin-conjugating enzyme E2 (11). Treatment of normal cells with specific inhibitors of the proteasome results in the accumulation of p53 protein (21), indicating the importance of complex formation between p53 and Mdm2 for p53 turnover. It is reported that phosphorylation of serine 15 of p53 is detected with a phosphoserine-specific antibody in vivo after genotoxic treatments including γ or UV irradiation and that phosphorylation at this site results in inhibition of p53-Mdm2 complex formation (28).
DNA-specific protein kinase (DNA-PK), another member of the phosphatidylinositol 3-kinase family, has been reported to phosphorylate p53 at serines 15 and 37 in vitro (19). Its activity is regulated by the Ku heterodimer protein complex when it is bound to the ends of severed DNA strands. Thus, this kinase is a candidate for sensing DNA strand breaks. Recently, it is also reported that the phosphorylation of serine 15 of p53 is impaired in AT cells after γ irradiation (29). These results suggest the importance of ATM in the phosphorylation of this site, but it is uncertain whether DNA-PK is also involved in the phosphorylation process following DNA double-strand breaks and whether serine 15 phosphorylation is also involved in the accumulation of p53 protein after other cellular stresses.
To answer these questions, we have established an assay system to assess the contribution of ATM to the p53 response induced by DNA double-strand breaks. In this system, we microinjected restriction endonucleases into normal and AT cells to introduce DNA double-strand breaks and determined phosphorylation of p53 by immunostaining with phosphoserine-specific antibodies. We found that phosphorylation at serine 15 of p53 correlated with p53 stabilization after DNA double-strand breaks and that ATM was essential for the process.
MATERIALS AND METHODS
Cells and treatment.
Mori (12) and Turu are primary normal human skin fibroblasts. TKO2 and TKB2 are primary fibroblasts of xeroderma pigmentosum (XP) complementation groups F and C, respectively. All of these cells were diagnosed and established in this laboratory. AT2KY (7) and AT10S are primary AT fibroblasts purchased from the Japan Health Science Foundation (Osaka, Japan). The cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, penicillin G (100 U/ml), and streptomycin (100 μg/ml) in a humidified 5% CO2 incubator.
For X irradiation, cells were cultured in a plastic dish (30 mm in diameter). Just before irradiation, the medium volume was adjusted to 1.0 ml, and the dish was with X irradiated (Hitachi MBR-1520R) at a fluence of 0.22 Gy/s with a plastic dish cover. For UV (254 nm) irradiation, cells were washed once with phosphate-buffered saline (PBS) and irradiated at a fluence of 0.65 J/m2/s. For lactacystin (Kyowa, Japan) treatment, the drug was added to the culture medium at a concentration of 50 μM. For heat shock treatment, cells were cultured in a plastic dish (30-mm diameter). Just before heat shock, the medium volume was adjusted to 1.0 ml, and cells were incubated for 45 min at 43°C in a humidified CO2 incubator. Then the cells were cultured in a humidified CO2 incubator set at 37°C.
Immunostaining.
For immunostaining, fibroblasts were cultured on glass coverslips for at least 2 days and treated when in logarithmic growth. After treatment with various kinds of p53 inducers, cells were fixed for 10 min with 80% methanol at −10°C. p53 staining was performed as previously described (32). Briefly, fixed cells were stained for 30 min at room temperature with an anti-p53 monoclonal antibody (PAb 1801; 1:200 dilution; Calbiochem), washed with PBS, and then stained for 30 min at room temperature with fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin G (IgG; 1:100 dilution, Cappel). For staining of phosphorylated serine 15 or 37 of p53, affinity-purified rabbit polyclonal antibody (1:100 dilution of the affinity-purified IgG fraction [0.3 mg/ml]) was used. Preparation and purification of these phosphoserine-specific antibodies are described elsewhere (16). Briefly, phosphoserine 15- and phosphoserine 37-specific antisera were raised against chemically synthesized, keyhole limpet hemocyanin-conjugated phosphopeptides SVEPPLS(PO3)QETFSDC (amino acids 9 to 21) and VLSPLPS(PO3)QAMDDLC (amino acids 31 to 43), respectively. The antisera were affinity purified through columns conjugated with phosphorylated peptides and unphosphorylated peptides consecutively. These peptides were also used for antibody blocking experiments in Fig. 4. Fixed cells were stained for 30 min at room temperature with phosphoserine-specific antibodies, washed with PBS, and stained for 30 min at room temperature with FITC-conjugated anti-rabbit IgG (goat; 1:100 dilution; Cappel). To enhance the intensity of fluorescence, cells were further stained for 30 min at room temperature with FITC-conjugated anti-goat IgG (rabbit; 1:100 dilution; Chemicon International Inc.). For double staining for p53 and phosphorylated serine 15, fixed cells were first stained with an anti-phosphoserine 15 antibody (rabbit), washed, and then stained with a mixture of anti-p53 monoclonal antibody (mouse) and FITC-conjugated anti-rabbit IgG (goat). After a wash with PBS, the cells were further stained with a mixture of FITC-conjugated anti-goat IgG (rabbit) and rhodamine-conjugated anti-mouse IgG (rabbit; Cappel).
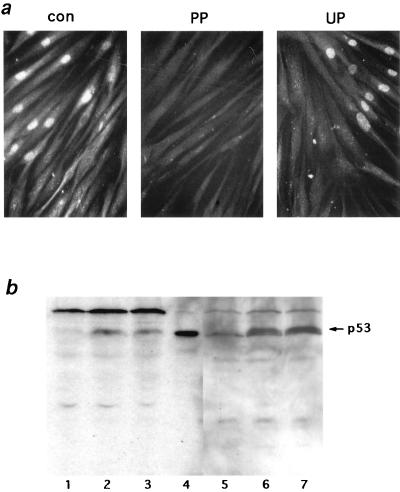
FIG. 4.
Specificity of the anti-phosphoserine 15 antibody. (a) Blocking with phosphorylated peptides. The rabbit antibody against phosphorylated serine 15 of p53 protein was preincubated for 30 min with phosphorylated serine peptides (PP), unphosphorylated peptides (UP), or H2O alone (con) at a peptide/antibody molar ratio 50. Mori cells which had been microinjected with HaeIII (1 U/μl) and cultured for 3 h were stained with the peptide-antibody mixtures as shown in Fig. 3. (b) Western blot with the phosphoserine 15 antibody. Cell extracts prepared from untreated control (lanes 1 and 5), UV-irradiated (18 J/m2, 5 h) (lanes 2 and 6), or X-irradiated (20 Gy, 3 h) (lanes 3 and 7) Mori cells were analyzed with either anti-p53 monoclonal antibody PAb 1801 (lanes 1 to 4) or an anti-phosphoserine 15 rabbit antibody (lanes 5 to 7). Lane 4 is a positive control cell extract prepared from simian virus 40-transformed human fibroblasts (XP3BRSV). p53 protein bands are indicated with an arrow.
Microinjection.
Microinjections with glass needles were performed as described elsewhere (33). To identify microinjected cells, cells were plated on glass coverslips on which small circles were engraved with a diamond knife. Usually, 50 to 100 cells in the small circle were microinjected for analysis. Since we found in preliminary experiments that both nuclear and cytoplasmic microinjection of restriction endonucleases induced nuclear accumulation of p53 protein in normal fibroblasts at similar levels, endonucleases were microinjected into the cytoplasm throughout this study. Viability of the microinjected cells was found to be more than 95%. Endonuclease HaeIII was purchased from Takara (Tokyo, Japan) in a high-concentration (50-U/μl) solution. Just before microinjection, endonucleases were diluted with an injection buffer (3 mM NaCl, 137 mM KCl, 8 mM NaH2PO4, 1 mM KH2PO4, 0.2 mg of bovine serum albumin per ml, 0.02% Triton X-100).
Western blot analysis.
Human fibroblasts were harvested in trypsin-EDTA, washed with PBS, and then lysed in lysis buffer (1.7% sodium dodecyl sulfate, 17% glycerol, 0.1 M dithiothreitol, 0.083 M Tris [pH 6.8]) at a cell concentration of 2.5 × 104 cells/μl. Cell extracts were boiled for 15 min and stored at −80°C before use. Samples (2.5 × 105 cells per lane) were analyzed by electrophoresis on a sodium dodecyl sulfate–12.5% polyacrylamide gel and transferred to polyvinylidene difluoride membranes (Millipore) by a semidry electroblotter (Bio-Rad). Membranes were immersed in blotting buffer consisting of 3% nonfat dry milk (Difco) and 3% fetal calf serum in Tris-buffered saline (TBS; 0.02 M Tris, 0.1 M NaCl [pH 7.5]), gently shaken for 30 min at room temperature, and subsequently immunoblotted with a monoclonal antibody against p53 (PAb 1801; Calbiochem) at a 1:50 dilution or with a rabbit polyclonal antibody against phosphoserine 15 of p53 at a 1:500 dilution. The membranes were washed three times with TTBS (0.05% Tween 20 in TBS) and then reacted with horseradish peroxidase-conjugated anti-mouse (sheep) or anti-rabbit (donkey) IgG (Amersham). Membranes were washed repeatedly with TTBS, and the signal was visualized by an enhanced chemiluminescence system (Amersham) according to the manufacturer’s protocol.
RESULTS
Defects in p53 protein accumulation induced by DNA double-strand breaks with restriction endonuclease in AT cells.
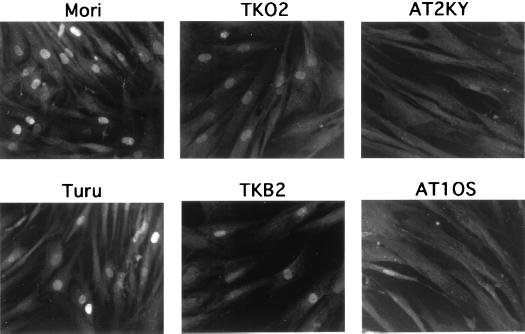
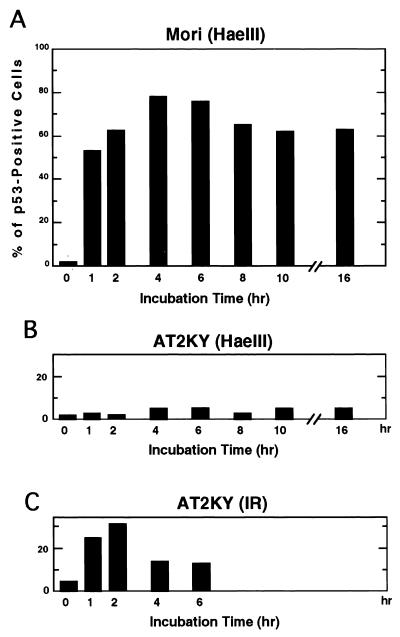
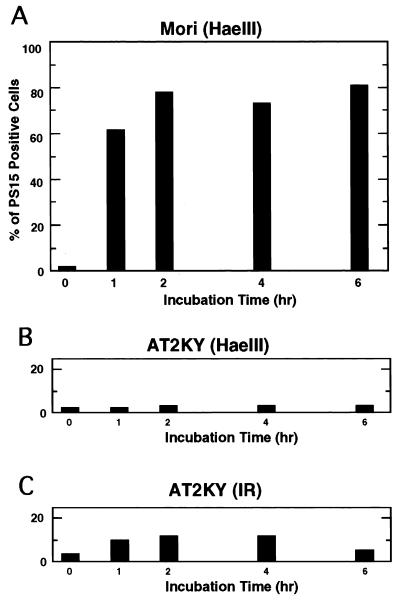
In normal cells, p53 protein levels are enhanced by treatment with IR. However, in addition to DNA strand breaks, IR causes various types of DNA damage, some of which are not identified, and cellular intoxication due to the generation of oxygen radicals. Such broad action of IR sometimes causes unexpected effects in irradiated cells, and makes interpretation of results difficult. We have systematically examined enzymatic inducers which incise DNA strands and evoke elevation of p53 protein levels after introduction. These included DNase I, DNase II, and various restriction endonucleases (EcoRI, PvuII, ScaI, and HaeIII). Introduction of restriction endonucleases into cells via electroporation has been shown to induce chromosome damage (2) and subsequent p53 accumulation (20, 22). Among the nucleases tested, we found that restriction endonuclease HaeIII, which causes blunt-ended DNA double-strand breaks at GGCC, was the strongest inducer. Microinjection of HaeIII enhanced p53 protein levels in normal and nucleotide excision repair-defective XP fibroblasts (complementation groups C and F) as determined by immunostaining (Fig. 1). Since no accumulation of p53 protein was observed with microinjection of the buffer solution for HaeIII, p53 accumulation by HaeIII was not induced nonspecifically through the microinjection procedure. p53 accumulation was detected with HaeIII concentrations ranging from 0.1 to 5 U/μl. Optimal induction was obtained at a concentration around 1 U/μl. At concentrations above 5 U/μl, the intensity of fluorescence and frequency of p53-stained cells decreased, possibly due to a toxic effect. Elevation of p53 protein levels were detectable as early as 1 h after microinjection. Maximal levels were observed first around 2 h and detected even 16 h after microinjection (Fig. 2A). In sharp contrast, the two AT cell strains examined in this study showed no p53 protein accumulation even when incubation times after microinjection of HaeIII were extended to 16 h (Fig. 2B). However, consistent with earlier studies (4, 15, 29), substantial numbers (∼30%) of these AT cells became p53 positive following X-ray treatment (Fig. 2C).
FIG. 1.
Defective accumulation of p53 protein in AT cells following DNA double-strand breaks. p53 protein accumulation was induced by microinjection of restriction endonuclease HaeIII (1 U/μl) in the indicated primary fibroblasts (see text for descriptions). After microinjection, cells were cultured for 3 h and stained for p53 protein with anti-p53 monoclonal antibody PAb 1801.
FIG. 2.
Time course of p53 accumulation after microinjection of HaeIII into normal and AT fibroblasts. HaeIII (1 U/μl) was microinjected into either normal (Mori; A) or AT (AT2KY; B) fibroblasts. (C) AT fibroblasts treated with X-rays (20 Gy). The cells were cultured for the indicated periods and stained for p53 protein. The frequency of positive cells was determined as the percentage of cells showing positive staining. Two independently performed experiments gave similar results; representative data from one experiment are shown.
p53 accumulation induced by HaeIII correlates with phosphorylation of p53 protein at serine 15.
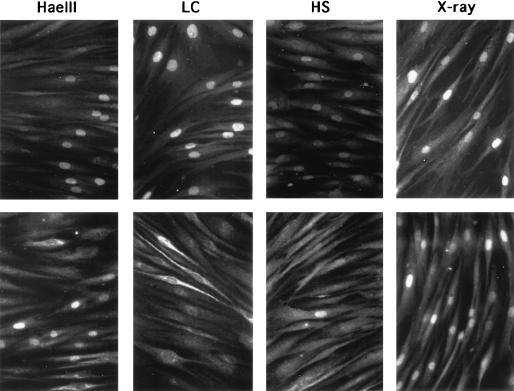
After treatment with γ rays or UV irradiation, p53 protein in established human cell lines with wild-type p53 alleles is phosphorylated at serine 15 (28, 29). Phosphorylation of p53 at its N-terminal domain is suspected to cause a structural change which hampers its interaction with Mdm2 and thus leads to its escape from degradation (28). We examined histochemically whether phosphorylation at serine 15 took place in normal fibroblasts after microinjection of HaeIII. A rabbit polyclonal antibody which specifically recognizes phosphorylated serine 15 of p53 protein was used to assess the levels of p53 phosphorylation. This antibody has been used for Western blotting analysis elsewhere (28, 29) and found to be specific for phosphorylated serine 15 of p53. As shown in Fig. 3, when normal fibroblasts were incubated for 3 h after microinjection of HaeIII, nuclei of these cells were stained positively with both an anti-p53 monoclonal antibody and a rabbit antibody specific for phosphoserine 15 of p53. Similar positive staining of nuclei for p53 and phosphoserine 15 was observed in normal cells irradiated with X rays (20 Gy, 3 h). Although more than 90% of the normal cells treated with lactacystin (50 μM, 3 h), a specific inhibitor of the proteasome, showed elevated levels of p53, no positive signals for phosphorylation at serine 15 of p53 were observed (Fig. 3). Similar staining patterns were seen in heat shock-treated (43°C for 45 min, 37°C for 3 h) cells except that less than 2% of cells were positive for the phosphorylation (Fig. 3). These results indicate that phosphorylation at serine 15 is a specific event after DNA double-strand breakage. To confirm the specificity of the staining for phosphorylated serine 15 of p53, we carried out two experiments. First, we tested whether the staining was blocked with the phosphorylated peptides originally used as antigens to generate the antibodies. The rabbit antibody was preincubated for 30 min with either phosphorylated or nonphosphorylated peptides, and normal fibroblasts microinjected with HaeIII were stained with these antigen-antibody mixtures. As shown in Fig. 4a, preincubation with phosphorylated peptides completely blocked the staining of HaeIII-treated cells, whereas with preincubation with nonphosphorylated peptides, phosphorylated serine 15 was stained at a positive control level. Blockage of staining with phosphorylated antigen peptides was observed with a peptide/antibody molar ratio of greater than 10. Second, we confirmed the specificity of the rabbit antibody with Western blotting. Normal fibroblasts used in this study were irradiated with either X rays or UV. Cell extracts prepared from the treated cells were electrophoresed, transferred to membranes, and probed with the serine 15-specific antibody. As shown in Fig. 4b, p53 was detected in both X- and UV-irradiated cells, and both p53 bands were phosphorylated at serine 15. This phosphorylated band was the only one which was induced by X irradiation. This finding excludes the possibility that the observed nuclear staining with the phosphorylated serine 15 antibody was due to cross-reaction with some other proteins which were induced following DNA double-strand breaks.
FIG. 3.
Phosphorylation at serine 15 of p53 following various types of treatments. Normal fibroblasts (Mori cells) were treated with X rays (20 Gy, 3 h), heat shock (HS; 43°C for 45 min, 37°C for 3 h), or lactacystin (LC; 50 μM, 3 h). Cells microinjected with HaeIII (1 U/μl) were cultured for 3 h after injection. Under these conditions, maximal levels of p53 protein were induced in normal fibroblasts. Cells were stained for p53 protein with monoclonal antibody PAb 1801 (top row) or for phosphorylated serine 15 with a rabbit polyclonal antibody (bottom row).
To determine the time course of phosphorylation of p53 at serine 15, normal fibroblasts were microinjected with HaeIII, incubated for various times, and stained with the antibody. Phosphorylation occurred as early as 1 h after microinjection, and high levels of phosphorylation continued at least for 6 h (Fig. 5A). This time course pattern of serine 15 phosphorylation is quite similar to that of p53 accumulation (Fig. 2A). When AT cells were treated with X-rays, small but significant fractions were positively stained for phosphorylated serine 15 (Fig. 5C). In contrast, when HaeIII was microinjected into AT cells, the frequency of cells showing phosphorylated serine 15 did not increase and remained at a basal level for up to 6 h after microinjection (Fig. 5B).
FIG. 5.
Time course of phosphorylation at serine 15 of p53 after microinjection of HaeIII into normal and AT fibroblasts. HaeIII (1 U/μl) was microinjected into either normal (Mori; A) or AT (AT2KY; B) fibroblasts. (C) AT fibroblasts treated with X rays (20 Gy). The cells were cultured for the indicated times and stained for phosphorylated serine 15. The frequency of phosphoserine 15-positive cells is shown as the percentage of treated cells showing positive staining. Two independently performed experiments gave similar results; data from one experiment are shown.
Requirement of ATM for phosphorylation of p53 at serine 15.
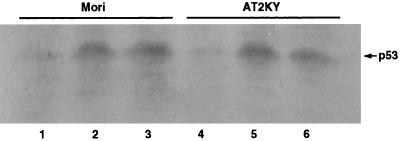
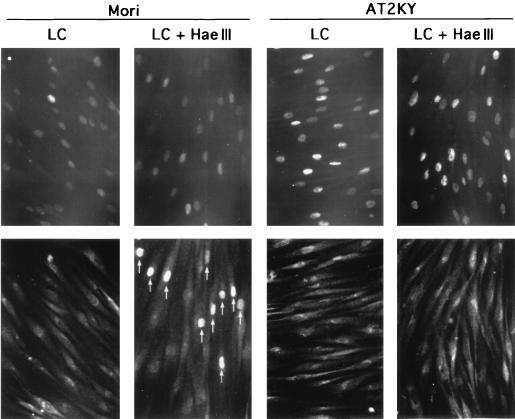
Neither p53 accumulation nor phosphorylation at serine 15 of p53 was observed in AT cells following microinjection of HaeIII (Fig. 2B and 5B). To determine whether p53 accumulation was required for phosphorylation at serine 15 or vice versa, we microinjected HaeIII into normal or AT cells where high levels of p53 protein had been induced by treatment with lactacystin for 3 h. As shown in Fig. 6 and 7, p53 protein levels were enhanced to similar levels in normal and AT cells as determined by Western blotting and immunostaining, respectively. But in both cell types, phosphorylation at serine 15 was not observed in the accumulated p53 as determined by double staining (Fig. 7). High levels of p53 protein were maintained even when these cells were cultured for another 3 h in the presence of cycloheximide (Fig. 6). When HaeIII was microinjected into lactacystin-treated normal cells, the up-regulated p53 protein was phosphorylated at serine 15 within 3 h in the presence of cycloheximide. In these cells, enhanced levels of p53 protein phosphorylated at serine 15 were sustained for at least 12 h even in the presence of cycloheximide, while levels of p53 protein without the phosphorylation in nonmicroinjected cells returned nearly to the basal level (data not shown). In contrast, even when HaeIII was microinjected into lactacystin-treated AT cells, no phosphorylation at serine 15 was observed (Fig. 7). These results indicate the requirement of ATM for the phosphorylation at serine 15, which results in stabilization of p53.
FIG. 6.
Accumulation of p53 protein in normal (Mori) and AT (AT2KY) fibroblasts induced by treatment with a proteasome inhibitor. Normal (lane 2) and AT (lane 5) fibroblasts were treated with lactacystin (50 μM) for 3 h. Some cells were treated with lactacystin (50 μM) for another 3 h in the presence of cycloheximide (20 μM) (lanes 3 and 6). p53 protein levels were determined by Western blotting with anti-p53 monoclonal antibody PAb 1801. Lanes 1 and 4 are extracts prepared from untreated cells. p53 bands are indicated with an arrow.
FIG. 7.
Phosphorylation at serine 15 of lactacystin-induced p53 protein following DNA double-strand breaks. Normal (Mori) and AT (AT2KY) fibroblasts were cultured for 3 h in the presence of lactacystin (50 μM) (LC), HaeIII (1 U/μl) was microinjected, and the cells were cultured for another 3 h in the presence of cycloheximide (20 μM) and lactacystin (50 μM) (LC + HaeIII). Fixed cells were double stained for p53 (top row) and phosphorylated serine 15 (bottom row); the same fields are shown. Perinuclear fluorescence was nonspecific staining due to the double staining. Arrows indicate microinjected cells containing p53 phosphorylated at serine 15. Note that lactacystin-induced p53 was phosphorylated at serine 15 following DNA double-strand breaks in normal cells, whereas p53 protein accumulated in AT cells was not phosphorylated after the same treatment.
No phosphorylation is observed at serine 37 of p53 after DNA damage.
Phosphorylation of serine 37 is also thought to contribute to p53 stabilization (28). We used a phosphorylated serine 37-specific antibody to determine whether phosphorylation occurred at this site on p53 in normal or AT cells after various cellular stresses. Although this antibody reacted specifically with the phosphorylated peptide antigen immobilized on a slide glass, we could not detect any positive staining in normal or AT cells treated with HaeIII, X rays, or UV. Furthermore, even when normal cells containing high levels of p53 protein induced by treatment with lactacystin were X irradiated or microinjected with HaeIII, no positive staining was observed (data not shown).
DISCUSSION
Using two novel experimental approaches, we have demonstrated that DNA double-strand breaks lead to the ATM-dependent phosphorylation of p53 protein at serine 15. First, instead of IR, we microinjected restriction endonucleases into cells to introduce DNA double-strand breaks. Unlike high-energy irradiation, which causes various types of cell damage due to activated oxygen radicals, the only known action of restriction endonucleases is to cut DNA at specific nucleotide sequences. Second, instead of Western blotting, phosphorylation of p53 protein was detected by immunostaining with phosphoserine-specific antibodies. The staining in this study was judged to be specific for phosphorylated p53 for the following reasons: (i) positive staining was blocked with phosphorylated peptides corresponding to the p53 phosphoserine epitope; (ii) no inducible protein bands other than p53 were detected after X irradiation using Western blotting, and (iii) positive staining was observed after the introduction of DNA double-strand breaks in normal and XP cells but not in AT cells. Generally, staining methods have advantages over biochemical methods in morphological studies. Using double staining for p53 protein and phosphorylated serines of p53 protein, we can determine whether accumulated p53 protein is phosphorylated at specific sites in a single cell. Thus, it was revealed that only p53 protein phosphorylated at serine 15, as a result of HaeIII treatment, was stabilized even after long culture periods in the presence of cycloheximide (Fig. 7). It is reported that p53 accumulation (4, 15), and phosphorylation (29) after IR in AT cells is reduced and/or delayed compared with that in normal cells (14). We confirmed these earlier observations in the present study using our AT cells and methods (Fig. 2C and 5C). We did not, however, observe such a delayed type of p53 accumulation in AT cells after microinjection of HaeIII. The complete absence of a p53 response in HaeIII-treated AT cells strongly suggests that the observed p53 accumulation after IR treatment may be caused by some unknown effects other than DNA double-strand breaks.
Phosphorylation at serine 15 was absolutely required for stabilization of p53 protein after DNA double-strand breaks. However, the results in the present study do not exclude the possibility that additional phosphorylation of p53 protein or its association with some other protein(s) is required for the stabilization. In this regard, it was of interest to determine whether serine 37 was also phosphorylated after DNA double-strand breaks, because this serine is phosphorylated by DNA-PK in vitro (19). However, we did not detect phosphorylation at this site after various kinds of DNA damage in our assay system. Given that nonphysiological phosphorylation is often seen in vitro, it is likely that this site may not be phosphorylated in an intact cell after DNA double-strand breaks. Alternatively, though serine 37 is phosphorylated, our antibody may not react with the phosphorylated serine due to steric hindrance caused by phosphorylation at serine 15. Such a situation is known to occur at the C-terminal domain of p53. When serine 376 is phosphorylated, an antibody against phosphorylated serine 378 cannot bind to the phosphorylated serine (31).
We could not detect phosphorylation at serine 15 in AT cells following DNA double-strand breaks. Furthermore, because cells from scid mice, which are defective in DNA-PK, show a normal IR-induced p53 response (26), DNA-PK does not appear to be involved in the phosphorylation of this site following DNA double-strand breaks. Nijmegen breakage syndrome is another radiosensitive disorder whose cells manifest similar characteristics to those of AT cells, including an abnormal IR-induced p53 response (13). Nijmegen breakage syndrome protein, named nibrin, complexes with Mre11 and Rad50. The Mre11-Rad50 protein complex can bind to the ends of severed DNA strands (5). Mec1, the Saccharomyces cerevisiae homologue of ATM, interacts with a protein complex including Ddc1 and Mec3 (24). It is tempting to speculate that the nibrin complex acts as a sensor of DNA double-strand breaks, with the signal being transduced directly or indirectly to the Ddc1 complex, so that ATM protein is activated to some phosphorylate specific site(s) of p53 protein for its stabilization and activation as a transactivator. Phosphorylation at serine 15 took place even in the presence of cycloheximide (Fig. 7), suggesting that the signal arising from double-strand breakage does not require newly synthesized protein(s). As reported by Siliciano et al. (29), following UV irradiation, phosphorylation at serine 15 was observed in not only normal fibroblasts (Fig. 4b) but also AT fibroblasts (data not shown). These results indicate that there is an ATM-independent signaling pathway for the phosphorylation at serine 15. Furthermore, since phosphorylation at serine 15 was observed in only a minor fraction of normal cells treated with heat shock (Fig. 3), the signaling pathway evoked by heat shock is different from that which is activated by DNA double-strand breaks.
During the preparation of this report, Canman et al. (3) and Banin et al. (1) demonstrated independently that ATM is activated following IR and directly involved in the phosphorylation of p53 protein at serine 15. Our present data are consistent with their results.
ACKNOWLEDGMENTS
We are grateful to Michael Kastan and Carol Prives for helpful discussions.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, and Culture of Japan (08280101). Y.T. is supported by a grant from the Ministry of Health and Welfare of Japan for the second-term comprehensive 10-year strategy for cancer control.
REFERENCES
- 1.Banin S, Moyal L, Shieh S-Y, Taya Y, Anderson C W, Chessa L, Smorodinsky N I, Prives C, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 2.Bryant P E. Induction of chromosomal damage by restriction endonuclease in CHO cells porated with streptolysin O. Mutat Res. 1992;268:27–34. doi: 10.1016/0027-5107(92)90079-h. [DOI] [PubMed] [Google Scholar]
- 3.Canman C E, Lim D-S, Cimprich K A, Taya Y, Tamai K, Sakaguchi K, Apella E, Kastan M B, Siliciano J D. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 4.Canman C E, Wolff A C, Chen C Y, Fornace A J, Jr, Kastan M B. The p53-dependent G1 cell cycle checkpoint pathway and ataxia-telangiectasia. Cancer Res. 1994;54:5054–5058. [PubMed] [Google Scholar]
- 5.Carney J P, Maser R S, Olivares H, Davis E M, Beau M L, Yates J R, Hays L, Morgan W F, Petrini J H J. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell. 1998;93:477–486. doi: 10.1016/s0092-8674(00)81175-7. [DOI] [PubMed] [Google Scholar]
- 6.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 7.Ejima Y, Oshimura M, Sasaki M S. Determination of the chromosomal site for the human radiosensitive ataxia telangiectasia gene by chromosome transfer. Mutat Res. 1991;250:337–343. doi: 10.1016/0027-5107(91)90190-y. [DOI] [PubMed] [Google Scholar]
- 8.Fritsche M, Haessler C, Brandner G. Induction of nuclear accumulation of the tumor-suppressor protein p53 by DNA-damaging agents. Oncogene. 1993;8:307–318. [PubMed] [Google Scholar]
- 9.Graeber T G, Peterson J F, Tsai M, Monica K, Fornace A J, Jr, Giaccia A J. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol Cell Biol. 1994;14:6264–6277. doi: 10.1128/mcb.14.9.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gudas J M, Oka M, Diella F, Trepel J, Cowan K H. Expression of wild-type p53 during the cell cycle in normal human mammary epithelial cells. Cell Growth Differ. 1994;5:295–304. [PubMed] [Google Scholar]
- 11.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 12.Itoh T, Fujiwara Y, Ono T, Yamaizumi M. UVs syndrome, a new general category of photosensitive disorder with defective DNA repair, is distinct from xeroderma pigmentosum variant and rodent complementation group I. Am J Hum Genet. 1995;56:1267–1276. [PMC free article] [PubMed] [Google Scholar]
- 13.Jongmans W, Vuillaume M, Chrzanowska K, Smeets D, Sperling K, Hall J. Nijmegen breakage syndrome cells fail to induce the p53-mediated DNA damage response following exposure to ionizing radiation. Mol Cell Biol. 1997;17:5016–5022. doi: 10.1128/mcb.17.9.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kastan M B, Zhan Q, el-Deiry W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 15.Khanna K K, Lavin M F. Ionizing radiation and UV induction of p53 protein by different pathways in ataxia-telangiectasia cells. Oncogene. 1993;8:3307–3312. [PubMed] [Google Scholar]
- 16.Kitagawa M, Higashi H, Jung H K, Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E, Nishimura S, Taya Y. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- 17.Ko L J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 18.Kubbutat M H, Jones S N, Vousden K H. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 19.Lees-Miller S P, Sakaguchi K, Ullrich S J, Appella E, Anderson C W. Human DNA-activated protein kinase phosphorylates serines 15 and 37 in the amino-terminal transactivation domain of human p53. Mol Cell Biol. 1992;12:5041–5049. doi: 10.1128/mcb.12.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X, Lane D P. Differential induction of transcriptionally active p53 following UV or ionizing radiation: defects in chromosome instability syndromes? Cell. 1993;75:765–778. doi: 10.1016/0092-8674(93)90496-d. [DOI] [PubMed] [Google Scholar]
- 21.Maki C G, Huibregtse J M, Howley P M. In vivo ubiquitination and proteasome-mediated degradation of p53. Cancer Res. 1996;56:2649–2654. [PubMed] [Google Scholar]
- 22.Nelson W G, Kastan M B. DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol. 1994;14:1815–1823. doi: 10.1128/mcb.14.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliner J D, Pietenpol J A, Thiagalingam S, Gyuris J, Kinzler K W, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 24.Paciotti V, Lucchini G, Plevani P, Longhese M. Mec1p is essential for phosphorylation of the yeast DNA damage checkpoint protein Ddc1p, which physically interacts with Mec3p. EMBO J. 1998;17:4199–4209. doi: 10.1093/emboj/17.14.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radinsky R, Fidler I J, Price J E, Esumi N, Tsan R, Petty C M, Bucana C D, Bar-Eli M. Terminal differentiation and apoptosis in experimental lung metastases of human osteogenic sarcoma cells by wild type p53. Oncogene. 1994;9:1877–1883. [PubMed] [Google Scholar]
- 26.Rathmell W K, Kaufmann W K, Hurt J C, Byrd L L, Chu G. DNA-dependent protein kinase is not required for accumulation of p53 or cell cycle arrest after DNA damage. Cancer Res. 1997;57:68–74. [PubMed] [Google Scholar]
- 27.Savitsky K, Sfez S, Tagle D A, Ziv Y, Sartiel A, Collins F S, Shiloh Y, Rotman G. The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species. Hum Mol Genet. 1995;4:2025–2032. doi: 10.1093/hmg/4.11.2025. [DOI] [PubMed] [Google Scholar]
- 28.Shieh S Y, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 29.Siliciano J D, Canman C E, Taya Y, Sakaguchi K, Appella E, Kastan M B. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugano T, Nitta M, Ohmori H, Yamaizumi M. Nuclear accumulation of p53 in normal human fibroblasts is induced by various cellular stresses which evoke the heat shock response, independently of the cell cycle. Jpn J Cancer Res. 1995;86:415–418. doi: 10.1111/j.1349-7006.1995.tb03072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waterman M J F, Stavridi E S, Waterman J L F, Halazonetis T D. ATM-dependent activation of p53 involves dephosphorylation and association with 14-3-3 proteins. Nat Genet. 1998;19:175–178. doi: 10.1038/542. [DOI] [PubMed] [Google Scholar]
- 32.Yamaizumi M, Sugano T. UV-induced nuclear accumulation of p53 is evoked through DNA damage of actively transcribed genes independent of the cell cycle. Oncogene. 1994;9:2775–2784. [PubMed] [Google Scholar]
- 33.Yamaizumi M, Sugano T, Asahina H, Okada Y, Uchida T. Microinjection of partially purified protein factor restores DNA damage specifically in group A of xeroderma pigmentosum cells. Proc Natl Acad Sci USA. 1986;83:1476–1479. doi: 10.1073/pnas.83.5.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]