Abstract
Background
Cystic fibrosis is a multi‐system disease characterised by the production of thick secretions causing recurrent pulmonary infection, often with unusual bacteria. This leads to lung destruction and eventually death through respiratory failure. There are no antibiotics in development that exert a new mode of action and many of the current antibiotics are ineffective in eradicating the bacteria once chronic infection is established. Antibiotic adjuvants ‐ therapies that act by rendering the organism more susceptible to attack by antibiotics or the host immune system, by rendering it less virulent or killing it by other means, would be a significant therapeutic advance. This is an update of a previously published review.
Objectives
To determine if antibiotic adjuvants improve clinical and microbiological outcome of pulmonary infection in people with cystic fibrosis.
Search methods
We searched the Cystic Fibrosis Trials Register which is compiled from database searches, hand searches of appropriate journals and conference proceedings.
Date of most recent search: 16 January 2020.
We also searched MEDLINE (all years) on 14 February 2019 and ongoing trials registers on 06 April 2020.
Selection criteria
Randomised controlled trials and quasi‐randomised controlled trials of a therapy exerting an antibiotic adjuvant mechanism of action compared to placebo or no therapy for people with cystic fibrosis.
Data collection and analysis
Two of the authors independently assessed and extracted data from identified trials.
Main results
We identified 42 trials of which eight (350 participants) that examined antibiotic adjuvant therapies are included. Two further trials are ongoing and five are awaiting classification. The included trials assessed β‐carotene (one trial, 24 participants), garlic (one trial, 34 participants), KB001‐A (a monoclonal antibody) (two trials, 196 participants), nitric oxide (two trials, 30 participants) and zinc supplementation (two trials, 66 participants). The zinc trials recruited children only, whereas the remaining trials recruited both adults and children. Three trials were located in Europe, one in Asia and four in the USA.
Three of the interventions measured our primary outcome of pulmonary exacerbations (β‐carotene, mean difference (MD) ‐8.00 (95% confidence interval (CI) ‐18.78 to 2.78); KB001‐A, risk ratio (RR) 0.25 (95% CI 0.03 to 2.40); zinc supplementation, RR 1.85 (95% CI 0.65 to 5.26). β‐carotene and KB001‐A may make little or no difference to the number of exacerbations experienced (low‐quality evidence); whereas, given the moderate‐quality evidence we found that zinc probably makes no difference to this outcome.
Respiratory function was measured in all of the included trials. β‐carotene and nitric oxide may make little or no difference to forced expiratory volume in one second (FEV1) (low‐quality evidence), whilst garlic probably makes little or no difference to FEV1 (moderate‐quality evidence). It is uncertain whether zinc or KB001‐A improve FEV1 as the certainty of this evidence is very low.
Few adverse events were seen across all of the different interventions and the adverse events that were reported were mild or not treatment‐related (quality of the evidence ranged from very low to moderate).
One of the trials (169 participants) comparing KB001‐A and placebo, reported on the time to the next course of antibiotics; results showed there is probably no difference between groups, HR 1.00 (95% CI 0.69 to 1.45) (moderate‐quality evidence). Quality of life was only reported in the two KB001‐A trials, which demonstrated that there may be little or no difference between KB001‐A and placebo (low‐quality evidence). Sputum microbiology was measured and reported for the trials of KB001‐A and nitric oxide (four trials). There was very low‐quality evidence of a numerical reduction in Pseudomonas aeruginosa density with KB001‐A, but it was not significant. The two trials looking at the effects of nitric oxide reported significant reductions in Staphylococcus aureus and near‐significant reductions in Pseudomonas aeruginosa, but the quality of this evidence is again very low.
Authors' conclusions
We could not identify an antibiotic adjuvant therapy that we could recommend for treating of lung infection in people with cystic fibrosis. The emergence of increasingly resistant bacteria makes the reliance on antibiotics alone challenging for cystic fibrosis teams. There is a need to explore alternative strategies, such as the use of adjuvant therapies. Further research is required to provide future therapeutic options.
Plain language summary
Antibiotic enhancing treatment for lung infections in cystic fibrosis
Review question
We reviewed the evidence about the use of agents to help antibiotics in treating lung infections in people with cystic fibrosis.
Background
People with cystic fibrosis suffer from infections in their lungs as they produce thick secretions which allow bacteria to grow in them. Often the infections are caused by unusual bacteria, including Pseudomonas aeruginosa, and these bacteria become resistant to treatment with antibiotics. Long‐term infection reduces a person's quality of life and their lung function. There are no new antibiotics currently being developed which use a new type of action. New agents ‐ antibiotic adjuvants ‐ are needed to work alongside antibiotics to make bacteria more sensitive to either antibiotics or to the body's own immune system, and to interfere with the formation of colonies of bacteria in the lungs.
Search date
The evidence is current to: 16 January 2020.
Trial characteristics
The review includes eight trials with 350 people with cystic fibrosis aged between five and 54 years of age. Trials compared different antibiotic adjuvants (beta‐carotene (one trial), garlic (one trial), a biological agent (two trials), nitric oxide (two trials) and zinc (two trials)) with placebo (a substance that contains no medication) and people were selected for one treatment or the other randomly. The trials lasted from two days to one year.
Key results
None of the treatments led to a longer time until the next flare up of lung disease compared to the people who received placebo. For the other measures we looked at (lung function, side effects, quality of life or number of infections) there were also no differences between the people given the adjuvant and the people given the placebo. All of the trials measured side effects of the treatment but these were mostly mild and happened in both the people receiving the treatment and those that did not.
None of the therapies for enhancing the actions of antibiotics which we found, showed a significant benefit for either lung function, rate of infection or quality of life. More randomised controlled trials are needed before we can recommend the routine use of any of these therapies.
Quality of the evidence
The quality of the evidence ranges from very low to moderate, but we judged it to be low overall. We made these judgements because of the small number of trials that looked at each of the different adjuvant treatments meaning we were unable to combine results. The quality was also affected by the small number of people who were included.
Summary of findings
Background
Description of the condition
Cystic fibrosis (CF) is a multi‐system disease characterised by the production of thick secretions causing recurrent pulmonary infection and pancreatic malabsorption. The altered lung environment in people with CF provides an ideal niche for bacteria such as Pseudomonas aeruginosa to flourish and chronic infection to develop. This results in damage to the airways leading to a decline in lung function that is largely responsible for the morbidity and mortality in CF. Over the last two decades more efficient treatment of pulmonary infections has contributed to a large increase in life‐expectancy (Dodge 2007). The mean life expectancy for a baby born in 2003 was 42 years for a boy and 36 years for a girl (Dodge 2007). However, the adaptive behaviour of P aeruginosa enables it to rapidly evolve in response to selective pressures exerted by the host environment and the use of bactericidal antibiotics. Thus, the increased use of broad spectrum antimicrobials has resulted in a reduction in their efficacy and an increase in bacterial resistance. In addition to P aeruginosa, other important, antibiotic‐resistant pathogens can be found in the lungs of people with CF such as Burkholderia cepacia and methicillin‐resistant Staphylococcus aureus (MRSA).
Description of the intervention
Novel agents are urgently needed, which act differently to antibiotics and can eradicate the organism without selecting for resistance and whilst re‐sensitising them to readily available antibiotics. Antibiotics commonly act by killing the organism or stopping its growth. Antibiotic adjuvants may exert their effect on the organism without killing it. Antibiotic adjuvant therapies are a diverse group of novel agents that are similar in that they act by interfering with a mechanism the organism uses to decrease its susceptibility to antibiotics; by reducing the organism's virulence; or by rendering the organism more susceptible to the host immune system. Such agents include: quorum sensing inhibitors (see below); agents that interfere with biofilm construction (the sugars fucose and galactose, and novel dendrimers acting on lectin blockade); efflux pump inhibitors that stop bacteria removing antibiotics from within the bacterial cell; glutamine as an amino acid supplement; and biological agents (such as bacteriophages) that infect bacteria causing their break‐down and demise. In the case of bacteriophages that may cause the death of the organism, they act by 'infecting' the bacteria and therefore act differently to, but alongside, conventional antibiotics.
Some of the agents that may be considered in the review are available direct to the consumer (e.g. garlic, zinc). It is therefore conceivable that the results of the trial may alter an individual's behaviour directly.
How the intervention might work
Antibiotic adjuvants are varied in their design. Many of the bacteria causing infections in CF communicate via quorum sensing, such that the bacteria only produce virulence factors when the population of bacteria has reached a critical size. The organism can therefore remain invisible to the host immune system until the population is in a position to withstand such host attack. Some antibiotic adjuvant therapies interfere with this bacterial communication, thus potentiating the efficacy of conventional antibiotics (von Bodman 2008). Some approaches attach to the genetic material of the organism, preventing the bacteria from becoming more resistant to antibiotics and some therapies target other mechanisms exploited by bacteria, such as the ability to form biofilms. Other antibiotic adjuvant therapies include bacteriophages or their products, viruses that directly target the bacterial cell, invade it and kill it (Borysowski 2006).
Why it is important to do this review
Worldwide, there is a burgeoning interest in agents that may potentiate, refine or replace the action of antibiotics without exerting selective pressure for antibiotic resistance. It is suggested that many of these agents are less susceptible to becoming inactive (due to the organism developing resistance toward them) because in many cases they do not directly influence the organisms' ability to reproduce. Currently both chronic pulmonary infections and acute infective exacerbations are difficult to treat and eradication of infection may be only temporary (Langton‐Hewer 2017; Lo 2018; Regan 2019). With research proceeding along diverse paths and individuals making decisions regarding supplementing conventional treatment with high‐street products, there is a need to evaluate current evidence to advise people with CF and their clinicians and to direct research targets.
This is an update of previous reviews (Hurley 2010; Hurley 2013).
Objectives
To determine if antibiotic adjuvant therapies improve clinical and microbiological outcome of pulmonary infection in people with CF.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs.
Types of participants
Adults and children with CF, diagnosed using the Cystic Fibrosis Foundation consensus statement (Rosenstein 1998). Therefore, a diagnosis of CF should be based on:
presence of one or more characteristic phenotypic features;
or a positive newborn screening test result;
or a history of CF in a sibling and laboratory evidence of an abnormality in the cystic fibrosis transmembrane regulator (CFTR) as documented by an elevated sweat chloride concentration or the identification of mutations in each CFTR gene known to cause CF or in vivo demonstration of characteristic abnormalities in ion transport across the nasal epithelium.
As no standardised, validated definitions of acute exacerbation or chronic infection exists, we have employed the definitions employed by the CF Trust Antibiotic Working Group (UK Cystic Fibrosis Trust Antibiotic Working Group) alongside those identified by Rosenfeld (Rosenfeld 2001). An acute exacerbation will be defined as at least four of the following (UK Cystic Fibrosis Trust Antibiotic Working Group):
increased productive cough or breathlessness;
changes in the appearance or volume of sputum;
new signs on auscultation;
new chest radiograph signs;
loss of appetite;
fall in respiratory function;
fever requiring treatment with intravenous antibiotics.
Alternatively, an acute exacerbation was defined by the following score meeting or exceeding 2.6 (Rosenfeld 2001).
| Feature | Score |
| Decreased exercise tolerance | 1.8 |
| Increased cough | 1.5 |
| Increased sputum or cough congestion | 1.5 |
| School or work absenteeism | 1.6 |
| Increased adventitial sounds on lung examination | 1.2 |
| Decreased appetite | 1.1 |
The bacterium targeted by such an approach will not be limited by species. A chronic P aeruginosa infection will be defined by more than 50% of months in a year when samples had been taken being P aeruginosa culture‐positive (Lee 2004). We previously had used the UK Cystic Fibrosis Trust definition of two or more occasions of P aeruginosa isolation over six months (UK Cystic Fibrosis Trust 2004); however, the definition cited above more closely replicates current clinical practice. A similar approach will be used for other infections, except for non‐tuberculous mycobacteria which will be considered chronic is infection persists despite a year of intensive eradication treatment.
In the case of trials including both clinical scenarios or less defined criteria, we shall aim to manage these separately using a pragmatic approach.
Types of interventions
Antibiotic adjuvant agents compared to conventional antibiotics (either alone or in combination), placebo or no therapy via any route of administration.
Antibiotic adjuvant agents are those which augment the host immune response or potentiate antibiotic action. The adjuvants themselves may exert a direct killing or bacteriostatic effect on the organism but their primary role is to augment the effect of the co‐administered antibiotics. Such adjuvants include those that exert an effect on bacterial susceptibility to antibiotics and include efflux pump inhibitors (agents that block the action of efflux pumps ‐ pumps on the cell membrane that remove toxic substances from within the bacterium) or quorum sensing inhibitors. Other adjuvants change the physical resistance of an infection (e.g. lectin inhibitors) or those that act at the genetic level of the organism to prevent the acquisition of antibiotic resistance (e.g. anti‐sense strategies).
Biological agents (such as bacteriophages) were eligible for inclusion in this review. Agents were considered which are intended to treat bacteria, fungi and viruses.
We excluded trials of agents that physically alter the host environment (e.g. gene therapy, immunotherapy); anti‐inflammatory agents (e.g. steroids, ibuprofen); agents that alter mucociliary clearance (e.g. mannitol, hypertonic saline and dornase alfa); physical interventions (such as physiotherapy and exercise); and environmental changes (such as an infection control policy).
Types of outcome measures
Primary outcomes
Pulmonary exacerbations (protocol defined)
-
Respiratory function
per cent (%) predicted forced expiratory volume in one second (FEV1) values for age, sex and height
% predicted forced vital capacity (FVC) values for age, sex and height
other validated measures of respiratory function
-
Adverse events (AEs)
mild (not requiring treatment)
moderate (requiring treatment or admission)
severe (life‐threatening)
Secondary outcomes
-
Need for antibiotics (oral, inhaled or intravenous (IV))
Time to next course of antibiotics (oral or IV)
Antibiotic consumption (days of antibiotic use)
-
Quality of life (QoL) using standardised and validated QoL scores (e.g. CFQ‐R (Quittner 2009)) and symptom scores (e.g. Respiratory and Systemic Symptoms Questionnaire (RSSQ), Respiratory Symptom Score (RSS) (Goss 2007))
time of work or school
treatment burden (e.g. challenges of living with CF evaluation)
-
Change in sputum microbiology:
emergence of new CF pathogens, including development of allergic bronchopulmonary aspergillosis (ABPA) (increase in serum IgE with clinical or radiological features)
quantitative microbiology (change in numbers of pathogens isolated from respiratory tract secretion culture)
-
Change in inflammatory markers
serum (C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR))
sputum (IL‐8, leukotrienes, cytokines)
Mortality
We assessed these outcomes for both chronic infection and acute infective exacerbations where data allowed. We assessed and analysed results separately for the two clinical scenarios at the time‐points detailed below (Data extraction and management).
Search methods for identification of studies
There are no restrictions regarding language or publication status.
Electronic searches
We searched for relevant trials from the Group's Cystic Fibrosis Trials Register using the terms 'pulmonary infection' AND 'non‐antibiotic'. The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective hand‐searching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cochrane Cystic Fibrosis and Genetic Disorders Group website.
Date of last search of the Cystic Fibrosis Trials Register: 16 January 2020.
We also searched MEDLINE separately; the search strategy is given in the appendices (Appendix 1; Appendix 2).
Date of last search of MEDLINE: 14 February 2019.
We also searched the ongoing trials registries as detailed in the appendices (Appendix 3); date of last search: 06 April 2020
Searching other resources
We hand‐searched reference lists of identified trials. We also contacted primary authors of identified trials and research institutions or biotech companies for unpublished trials for the original review. We have contacted authors for clarification and extra data for this update.
Data collection and analysis
We used methods described in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). We aimed to analyse acute infective exacerbations and chronic infection separately as defined previously in the section Types of participants. There were no interventions with outcomes in both acute and chronic conditions. We would aim to make this comparison in future updates of this review if there are suitable new trials.
Selection of studies
Originally two authors (MH and DF) independently reviewed all potential trials for inclusion in the original review. For the 2020 update MH and SS reviewed the potential trials. These two authors examined the title and abstract of potential publications to remove those that did not meet inclusion criteria (e.g. single case reports, reviews etc.). We then examined the full text publications of the remaining trials to determine if they met the eligibility criteria. In the case that we were unable to reach agreement regarding the determination of eligibility by discussion, we would have sought the opinion of the third independent author (AS); however, there were no such occurrences. Authors examined publications potentially eligible for inclusion for duplication by comparing author, institution, trial detail (intervention, dosing, timing etc.) and participant demographics.
Data extraction and management
For the 2020 update, two authors (MH and SS) independently extracted information from the eligible trials using a data collection form. We discussed the results from each data extraction form to ensure agreement of interpretation; if there had been an absence of agreement, we planned to seek the opinion from a third author (AS).
It is possible that during a trial a participant may experience more than one exacerbation; in this event the shortest time between exacerbations would have been used.
Where trials, particularly with regard to chronic infection, take multiple assessments of individuals over a protracted time period, we would have defined time‐frames of follow‐up to represent short‐, medium‐ and long‐term follow‐up. For the acute exacerbation trials we would have assessed data at time points of up to two weeks (standard duration of exacerbation therapy), over two weeks and up to six weeks (to assess efficacy of sustaining an effect). As previously discussed we would have assessed the outcome measure, time to next exacerbation as an indicator of long‐term effectiveness of antibiotic therapy. For the chronic infection trials, we would have taken assessment time points at one month, over one month and up to three months, over three months and up to six months, over six months and up to 12 months and annually thereafter. For future updates, if outcome data are recorded at other time points, we shall consider examining these as well.
Assessment of risk of bias in included studies
The authors assessed the risk of bias using the 'Risk of bias' assessment tool as documented in section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In particular, authors considered generation of allocation sequence and allocation concealment, blinding and incomplete outcome data. We also sought to identify selective outcome reporting by comparing those outcomes reported in the published paper to those considered in the protocol. For the 2020 update, MH and SS assessed the risk of bias for any new trials identified. We did not reassess the risk of bias for those trials already included in the review.
Measures of treatment effect
In identifying any treatment effect, we had planned to analyse the results of intervention measures separately for acute exacerbation and chronic infection. For dichotomous data we planned to analyse these by calculating the relative risk (RR) and its 95% confidence intervals (CIs) on an intention‐to‐treat basis (ITT). No trials reported mortality as an outcome measure, although we had planned to combine data in order to calculate the hazard ratio (HR) with 95% CIs, or if it is more appropriate, to describe the outcomes of mortality and adverse events descriptively.
For continuous outcome variables, we documented the mean difference (MD) of effect and standard deviation (SD) of each variable. We did not use a standardised scoring system; if trials had documented scores using the same scoring system, we had planned to calculate the MD and 95% CIs. If trials had used different validated scoring systems, we would have calculated the standardised mean difference (SMD) and 95% CIs.
No trial reported data for 'time to next exacerbation'. If in future updates, such data become available, we plan to calculate the HR and its 95% CIs (appropriate as the risk of an exacerbation is not dependent upon other variables and should be uniform throughout the time period).
We describe count data narratively.
Unit of analysis issues
Cross‐over trials are difficult to include as in many cases the duration of action of individual treatments may be prolonged. As a result we planned to only consider the first treatment phase of a cross‐over trial and would not include such trials in full until such time that duration of action detail is understood, a washout period provided, measures of treatment effect are available and the data are available from each treatment phase. When considering trials of long duration (and measures of number of exacerbations or requirement of antibiotics) repeated values should be available for each participant.
Dealing with missing data
In the case where it was obvious that any of the included publications missed important data from the review's primary or secondary outcomes, the authors approached the primary investigator for clarification or more detail. This was the case for the trial by Abdulhamid, who kindly provided the additional data (Abdulhamid 2008). If, in future, we do not receive any reply from primary investigators to possible enquires, we shall seek to account for all missing data by contact with the co‐investigators.
Assessment of heterogeneity
If we had identified data from multiple trials of a common therapy, we would have performed a meta‐analysis of these trials. To assess heterogeneity between trials in the meta‐analysis, we planned to use the I² statistic (Higgins 2003). We would have based our interpretation of this statistic on the guide given in the Cochrane Handbook of Systematic Reviews of Interventions such that we would regard an I² statistic below 40% as not important, between 30% and 60% as representing moderate heterogeneity, between 50% and 90% representing substantial and over 75% representing considerable heterogeneity (Higgins 2011). We would have interpreted this within the context of magnitude and direction of any effect and the strength of evidence for any heterogeneity, including a CI if possible for the I² result.
Assessment of reporting biases
We contacted the primary author of each included trial for the original iteration of the review to determine if they knew of other trials which have been completed but may not have been published. This process might have identified some small older trials and in this instance we could have determined the effects of these along with any reporting biases with the construction of funnel plots, accepting the multiple causes of asymmetry. When available, we made efforts to identify selective reporting of results by comparing the trial protocol (as published on a clinical trials register) with the final published results and by comparing the methods and results as detailed in the publication for any inconsistency in reporting.
Data synthesis
In the event of multiple trials we planned to analyse data using a fixed‐effects model unless the measures of heterogeneity were at least substantial (as defined above) in which case a random‐effects model would be most appropriate (RevMan 2011).
Subgroup analysis and investigation of heterogeneity
If we had identified a sufficient number of trials, we planned to undertake the following subgroup analyses for each class of agent:
adults (aged 18 years and over) versus children;
-
presence or absence of chronic infection (defined as three or more isolates in one year) with:
P aeruginosa;
B cepacia;
MRSA.
Sensitivity analysis
In the case that we had multiple analyses, we would have determined the effect our decisions made relating to arbitrary categorisations by repeating these analyses with different categorisations; for example, repeating the analyses of treatment effect with different measures of short, medium and long term. With regard to determining the effect of small trials on the end result, we would repeat the analyses without these small trials (e.g. participant numbers less than 20 in each group) to determine their effect.
Summary of findings tables
We have presented a summary of findings table for each separate comparison in the review:
β‐carotene versus placebo;
garlic versus placebo;
monoclonal antibody KB001‐A versus placebo;
inhaled nitric oxide versus placebo;
zinc versus placebo.
Within each table we present the following outcomes which we selected based on their clinical relevance and importance to the CF community:
pulmonary exacerbations;
respiratory function;
adverse events
need for antibiotics;
QoL;
sputum microbiology; and
inflammatory markers.
We determined the quality of the evidence using the GRADE approach and starting from a judgement of high quality, downgraded evidence in the presence of a high risk of bias in at least one trial, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, high probability of publication bias. We downgraded evidence by one level if we considered the limitation to be serious and by two levels if very serious.
Results
Description of studies
Results of the search
We have presented the results of the searches in a PRISMA chart (Figure 1).
1.
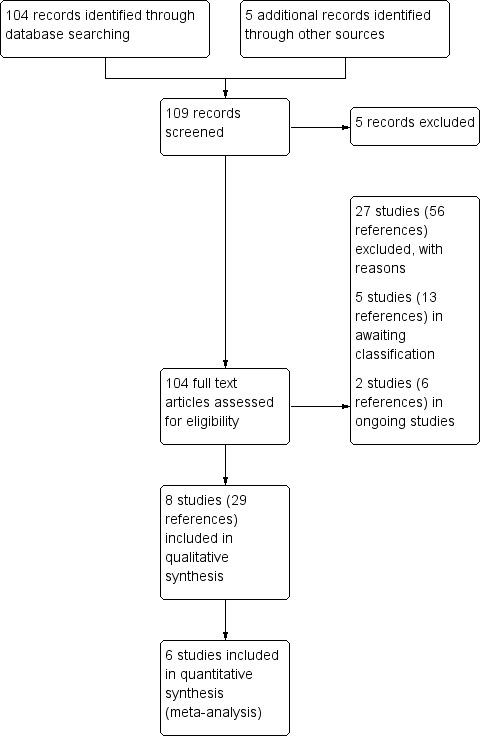
Study flow diagram.
The search strategy identified a total of 42 trials. Of these trials, eight investigating the use of antibiotic adjuvant therapies for chronic infection are included in the review (Abdulhamid 2008; Howlin 2017; Jain 2018; Milla 2013; Renner 2001; Sagel 2009; Sharma 2016; Smyth 2010) and 27 trials were excluded. Two trials are still ongoing and will be assessed for inclusion in the review when they are completed and results available (Pressler 2017; Zabner 2009a). Five trials are currently awaiting classification (CARE‐CF‐1; Khorasani 2009; Puvvadi 2019Rye 2015; Walshaw 2014). Two are cross‐over trials and we are awaiting first‐arm data (Rye 2015; Walshaw 2014); it is unclear at this stage whether one of the trials is randomised as it is only presented as an abstract (Khorasani 2009). The remaining two trials are only presented as conference abstracts (CARE‐CF‐1; Puvvadi 2019).
Included studies
Acute respiratory exacerbations
None of the included trials involved the treatment of acute infectious exacerbations.
Chronic infection
Eight trials with a total of 350 participants with chronic infection are included.
Trial design
All eight trials were randomised, double‐blind, placebo‐controlled trials of parallel design. Two of the trials were three‐armed trials, one comparing two different doses of KB001‐A to placebo (Milla 2013) and the other comparing high‐ and low‐dose nitric oxide (NO) with placebo (Sagel 2009). Two of the trials were multicentre and both were carried out in the USA (Jain 2018; Milla 2013). The remaining six trials were single centre; two were carried out in the USA (Abdulhamid 2008; Sagel 2009), two in the UK (Howlin 2017; Smyth 2010), one in Austria (Renner 2001) and one in Northern India (Sharma 2016). The duration of the trials ranged from a 48‐hour single‐dose trial (Sagel 2009) to one year (Abdulhamid 2008; Sharma 2016).
Participants
The number of participants in the included trials ranged from 12 (Howlin 2017) to 169 (Jain 2018). Six of the trials included both adults and children (age range 6.8 to 54 years) (Howlin 2017; Jain 2018; Milla 2013; Renner 2001; Sagel 2009; Smyth 2010) and the remaining two trials recruited children only (age range 5 to 18 years) (Abdulhamid 2008; Sharma 2016). Most of the trials required a confirmed diagnosis of CF for inclusion, but two of the trials did not make this explicit (Abdulhamid 2008; Howlin 2017).
Interventions
All eight trials compared the intervention to a placebo.
One trial (n = 24) reported on a high‐dose of β‐carotene supplementation at 1 mg/kg/day for three months followed by low‐dose regimen of 10 mg/day for three months (Renner 2001). One trial (n = 34) compared a garlic capsule once daily (656 mg of garlic oil macerate and 10 mg cardamom oil) to placebo (Smyth 2010). Two trials (n = 196) reported on a monoclonal antibody preparation KB001‐A, but at different doses and for different durations (Jain 2018; Milla 2013). Jain (n = 169) looked at the effect of up to five IV infusions of 10 mg/kg of KB001‐A once every four weeks during the 16‐week treatment period (Jain 2018), whilst Milla (n = 27) looked at the effect of a single IV infusion of either 3 mg/kg or 10 mg/kg given over one hour (Milla 2013). Two trials (n = 30) assessed inhaled NO, but again at different doses and duration (Howlin 2017; Sagel 2009). The Howlin trial (n = 12) administered 10 ppm NO delivered via nasal cannula for eight hours overnight for five to seven days of therapy (Howlin 2017). The second trial (n = 18) of inhaled NO studied a 'low' dose of 20 ppm via nasal cannula for 44 hours in one arm and a 'high' dose of 40 ppm via nasal cannula for 44 hours (Sagel 2009). The remaining two trials (n = 66) reported on zinc supplementation at a dose of 30 mg per day, both for a duration of 12 months (Abdulhamid 2008; Sharma 2016).
The dose regimens for each supplement are detailed in the tables (Characteristics of included studies).
Outcomes
Three trials reported our primary outcome of pulmonary exacerbations (Abdulhamid 2008; Renner 2001; Sharma 2016). Renner looked at the number of pulmonary exacerbations over six months during supplementation with β‐carotene (Renner 2001), whilst the two 12‐month zinc trials looked at respiratory tract infections against a defined criteria (Abdulhamid 2008) or the number of pulmonary exacerbations requiring antibiotics (Sharma 2016).
All eight trials reported some measure of respiratory function over different time periods; six of these specifically measured FEV1 (Howlin 2017; Jain 2018; Milla 2013; Sagel 2009; Sharma 2016; Smyth 2010), whilst Howlin and Milla also measured FVC (Howlin 2017; Milla 2013) and Milla also measured FEF25 - 75 (Milla 2013). Both of the trials looking at the effects of NO reported adverse events (Howlin 2017; Sagel 2009), as did one of the KB001‐A trials (Milla 2013) and one of the zinc trials (Sharma 2016).
Four trials reported on the first of our secondary outcomes, need for antibiotics (Abdulhamid 2008; Jain 2018; Sharma 2016; Smyth 2010). Only Jain reported on the time to a need for antibiotics (Jain 2018), whilst the remaining three trials reported the number of days or courses of antibiotics needed (Abdulhamid 2008; Sharma 2016; Smyth 2010). The two trials of KB001‐A measured QoL; one trial used the CFQ‐R (Milla 2013) and one trial measured the change in respiratory symptoms (Jain 2018). None of the trials looked at the emergence of new pathogens, but four trials looked at changes in quantitative microbiology (Howlin 2017; Jain 2018; Sagel 2009; Sharma 2016) and three trials reported on inflammatory markers (Abdulhamid 2008; Jain 2018; Milla 2013).
Excluded studies
Publications that were obviously not relevant to the review after interrogation of the abstracts were excluded and are not listed in the review; we have listed a total of 27 trials as excluded.
Nine trials were excluded for methodological reasons: seven trials were not RCTs or quasi‐RCTs (Durairaj 2007; Homnick 1995; Kollberg 2003; Lands 2010; Sagel 2011; Winklhofer‐Roob 1995); one was a single case report (Kutateladze 2008); and two were cross‐over trials which had no data from the first phase of the trial available for analysis; one assessed edetate sodium (EDTA) aerosolisation (Brown 1985) and one zinc supplementation (Safai‐Kutti 1991).
A further 16 trials were excluded as the interventions were not considered to be antibiotic adjuvant therapies. We excluded one trial investigating the effect of polyunsaturated fats (Panchaud 2006) and another trial of fatty acid supplements (Olveira 2010); these interventions appear to exert an independent anti‐inflammatory effect and so were considered not to meet our definition of an antibiotic adjuvant (as described in our exclusion criteria). A phase 2 trial of a CXCR2 antagonist was excluded as the mechanism of action is anti‐inflammatory (Moss 2013). One trial of magnesium (Gontijo‐Amaral 2012) and another of L‐arginine (Grasemann 2013) were excluded as an adjuvant effect does not appear to have been demonstrated in either trial. Two trials of immunotherapy agents for chronic infection were also excluded (Kollberg 2010; NCT01455675). Two trials of miglustat were excluded as this agent acts at the host genetic level and is not an adjuvant (NCT00742092; Leonard 2012). Two trials examined the effect of vitamin D supplementation, but not as an antibiotic adjuvant (Alvarez 2017; Tangpricha 2017) and one looked at a multivitamin with antioxidants but again, not as an antibiotic adjuvant (Sagel 2018). One trial looked at the distribution of nebulised solutions in the lungs, but not as an adjuvant therapy (Hodges 2014) and a further trial reported the effect of proton pump inhibition to prevent acid aspiration, not as an antibiotic adjuvant (DiMango 2014). One trial of glutamine supplementation ws excluded since direct bactericidal activity was expected (Forrester 2015). One trial was excluded because it looked at inhaled dry powder mannitol, but we deemed it to be an adjunct to airway clearance rather than as an antibiotic adjuvant (Middleton 2015).
Two trials in participants with an acute respiratory exacerbation were excluded (Hauber 2008; Winnie 1989). One trial randomised participants to receive either inhaled sugars (inhaled fucose and galactose) for treating their pulmonary exacerbation or inhaled sugars accompanied by IV antibiotics (Hauber 2008). While these agents are specific substrates for the galactophilic and fucophilic lectins (cell surface proteins involved in bacterial aggregation) which are known in vitro to inhibit aggregation and promote dispersal of P aeruginosa biofilms (Johansson 2008), these agents have a novel non‐osmotic mechanism of action. While the trial was not immediately excluded, further assessment ascertained that there was no true control group and it was therefore excluded (Hauber 2008). The second trial examined the effect of immunoglobulin on the treatment of pulmonary exacerbations and was excluded since it was a trial of immunotherapy (Winnie 1989).
Trials awaiting classification
Six trials are listed as 'Awaiting classification' (CARE‐CF‐1; Khorasani 2009; Puvvadi 2019; Rye 2015; Walshaw 2014; Zabner 2009).
Two cross‐over RCTs of OligoG have been completed but we are awaiting first‐arm data (Rye 2015; Walshaw 2014). OligoG is an oligosaccharide derived from alginate polysaccharide. It is suggested that this disrupts bacterial biofilm formation (a mode of bacterial growth associated with antibiotic tolerance and resistance). One RCT includes 12 participants with CF who also have chronic Burkholderia sp. infection; inhaled OligoG is given for 28 days to look at the effect on lung function, QoL, sputum rheology and microbiological outcomes (Rye 2015). The second RCT includes 26 participants aged 18 or over who have P aeruginosa in expectorated sputum. Inhaled OligoG was compared to saline to look at safety and tolerability (Walshaw 2014).
The third trial awaiting classification is looking at the effect of zinc on lung function, the rate of respiratory infections and the need for antibiotics; it is unclear at this stage whether it is randomised as it is only presented as an abstract. It includes 20 children with CF between seven and 18 years (Khorasani 2009).
A further trial awaiting assessment has six arms comparing five different doses of oral cysteamine (Lynovex®) to placebo over 14 days (CARE‐CF‐1). It is multicentre and double‐blind and has recruited 89 adults with CF experiencing an acute exacerbation. Investigators are assessing the change in sputum bacterial load, QoL, blood leukocyte count, adverse events and serious adverse events, the change from baseline in sputum neutrophil elastase and IL‐8 levels, in FEV1, in weight and in BMI. To date some information has been published in abstract form and we are awaiting the publication of the full trial before including or excluding the trial (CARE‐CF‐1).
One trial compared calcium ethylene diamine tetra‐acetate to saline in 24 people with CF with chronic P aeruginosa infection (Puvvadi 2019). For the initial two weeks, treatment was administered four times daily in conjunction with antibiotics; following this treatment was administered twice daily for a further two weeks and finally a four‐week safety follow‐up period. Investigators planned to assess the mean P aeruginosa sputum count, FEV1 % predicted and adverse events. Again, this trial has only been published in abstract form and we are awaiting the publication of the full trial before including or excluding the trial (Puvvadi 2019).
The final trial listed as 'Awaiting classification' is a double‐blind, parallel RCT in people with CF aged 16 or older (Zabner 2009). It compares hypertonic xylitol with hypertonic saline in terms of lung function, adverse events, respiratory symptoms, density of colonisation and time to exacerbations. The putative mechanism of action is a reduction in airway surface liquid salt concentration, thus facilitating lysozyme and β‐defensins' protective action. One of the trials excluded on the basis of non‐randomisation (Durairaj 2007) is a pre‐cursor to this trial; the trial has now been completed but the results are not yet available (Zabner 2009).
Ongoing trials
We will assess the ongoing trial for inclusion in the review when it is completed and results available (Pressler 2017). It is a double‐blind, cross‐over RCT looking at the effects of OligoG in 76 adults with CF and P aeruginosa in expectorated sputum. OligoG is given via inhalation compared to placebo and the outcomes include FEV1, mucociliary clearance, rheology, microbiology and QoL (Pressler 2017).
Risk of bias in included studies
We did not identify any trials undertaken during acute respiratory exacerbations; all the following information relates to trials in participants with chronic infection. The risk of bias summary figure summarises these judgements (Figure 2).
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Generation of sequence
We deemed four trials to be at low risk of bias for this domain (Howlin 2017; Jain 2018; Sharma 2016; Smyth 2010). In one of the NO trials the sequence was generated by an online randomisation service (Howlin 2017). One of the KB001‐A trials used an interactive web response system (Jain 2018) and one of the zinc trials used computer‐generated randomisation carried out by a person not involved in the trial (Sharma 2016). The garlic trial used a web‐based random allocation system provided by an external agency (Smyth 2010).
We judged four trials to have an unclear risk of bias (Abdulhamid 2008; Milla 2013; Renner 2001; Sagel 2009). The β‐carotene trial was described as 'randomised', unfortunately the randomisation method is not described (Renner 2001). In the second zinc trial, the assignment of participants to the treatment group was reported to have occurred 'randomly', but no methods are described (Abdulhamid 2008); this is also the case with the remaining KB001‐A trial (Milla 2013). The second NO trial claimed to be randomised but no description of the randomisation process was given (Sagel 2009).
Allocation concealment
We deemed four trials to be at low risk of bias for allocation concealment (Howlin 2017; Jain 2018; Sharma 2016; Smyth 2010). The Howlin NO trial stated that randomisation was undertaken via an online randomisation service to ensure concealment of treatment allocation (Howlin 2017). The Jain KB001‐A trial stated that the drug assignment was conducted via an interactive web response system (Jain 2018). In the Sharma zinc trial, the drugs were labelled sequentially and put into sealed opaque envelopes by a person not involved in the trial (Sharma 2016). The authors of the garlic trial confirmed that the garlic supplements were dispensed in coded opaque sealed containers (Smyth 2010).
The remaining four trials did not provide details on allocation concealment so we judged the risk of bias to be unclear; the β‐carotene trial (Renner 2001); one of the zinc trials (Abdulhamid 2008); one of the KB001‐A trials (Milla 2013); and one of the NO trials (Sagel 2009).
Blinding
We judged seven of our eight included trials to have a low risk of bias due to blinding (Howlin 2017; Jain 2018; Milla 2013; Renner 2001; Sagel 2009; Sharma 2016; Smyth 2010). Both NO trials were described as double‐blinded with NO and placebo being given via nasal cannulae so that participants were not able to differentiate between treatments (Howlin 2017; Sagel 2009). Similarly, both KB001‐A trials reported that they were double‐blind and IV infusion bags were labelled to maintain blinding (Jain 2018; Milla 2013). In the β‐carotene trial, the supplement was reported as being identical to a starch‐containing placebo (Renner 2001). The garlic trial detailed the flavour masking agent, described both active and placebo agents as identical and reported participant guesses of which treatment they thought they had received (no significant difference) (Smyth 2010). One of the zinc trials administered similar looking zinc or placebo tablets to the children (Sharma 2016).
The remaining zinc supplement trial detailed that the two groups were given either zinc gluconate or placebo preparations in capsules. The appearance of these capsules was unreported and so we judge the risk of bias to be unclear (Abdulhamid 2008).
Incomplete outcome data
We judged four trials to have a low risk of bias due to incomplete outcome data (Howlin 2017; Renner 2001; Sharma 2016; Smyth 2010). One NO trial reported that all 12 participants completed the trial and were included in the analysis (Howlin 2017). The β‐carotene trial reports end results for all 24 participants identified in the methods section (Renner 2001). One zinc trial carried out a ITT analysis as well as a per protocol analysis and only three participants withdrew consent (Sharma 2016). The garlic trial randomised 34 participants of whom eight withdrew: four in the garlic group (one received lung transplant, one forgot to take capsules, one withdrew due to indigestion and one could not attend the second visit); and four in the placebo group (two forgot to take capsules, one withdrew due to halitosis and one could not attend the second visit). Furthermore, data for the primary outcome (lung function) were missing for one participant in the placebo arm; analysis was per protocol (Smyth 2010).
We judged three trials to have an unclear risk of attrition bias (both KB001‐A trials and one NO trial) (Jain 2018; Milla 2013; Sagel 2009). Jain used a modified ITT analysis where any participant who had received three doses of the trial drug were included in the analysis; however, from the original 182 participants randomised, five withdrew from the placebo group before the start of treatment and eight from the KB001‐A group. A further 15 participants from the placebo group and 21 from the KB001‐A group withdrew before the end of the trial but no reasons were given (Jain 2018). Of the 27 participants randomised in the Milla trial, two participants discontinued the trial due to infusion‐related AEs (one from each of the KB001‐A groups) and a further three participants were excluded from the analysis as they received prohibited medications (two from the KB001‐A 3 mg/kg group and one from the placebo group). As the number of participants dropping out is more than 15%, we judged the risk of attrition bias to be unclear (Milla 2013). Although all 18 participants remained in the Sagel inhaled NO trial, one participant was given both NO and nitrogen and the authors state that they removed these data from their analysis. However, the data reported in the clinical trials registration document is for 18 participants, therefore it is not clear whether the data were removed for this participant or not; for this reason we judged the risk of attrition bias to be unclear (Sagel 2009).
The second zinc trial randomised 13 children to each group, but one participant in the treatment group withdrew from the trial and it is unclear whether this person was included in the final analysis (Abdulhamid 2008). We therefore judged there to be a high risk of bias since the incomplete outcome data were not addressed.
Selective reporting
Six trials were judged to have a low risk of selective reporting bias (Abdulhamid 2008; Jain 2018; Milla 2013; Renner 2001; Sharma 2016; Smyth 2010). We did not identify any evidence of selective outcome reporting in the Jain trial after comparing the protocol and the trial report (Jain 2018). We compared the published protocols of one of the KB001‐A trials and the garlic trial to the trial publications and no issues of selective reporting were identified (Milla 2013; Smyth 2010). In the Sharma trial, all outcomes stated in the methods section of the trial report are reported in the results section (Sharma 2016). The β‐carotene trial does not have a registration on a clinical trials registry making it difficult to determine the primary outcome measures; however, the impact of any bias due to this is likely to be minimal (Renner 2001). When considered in isolation, the individual publications of the β‐carotene trial each suffer from selective reporting as a result of duplicate publications with differing emphasis. The post hoc analysis describing an effect upon FEV1 in the 'younger' participants suffers from selective reporting as only data for the treatment group are described. However, with the post hoc analysis issue aside, when the individual reports are considered together, the results appear to not suffer from selective reporting (Renner 2001).
One of the zinc trials suffers considerably from selective reporting in the original paper (Abdulhamid 2008). The groups were originally assigned to one of the two trial arms 'randomly' without respect to their prior zinc status; however, the outcomes of these participants are reported in terms of prior zinc status. Investigators reported zinc status using a threshold of normal far below what is considered by their institutions' laboratory to be normal. Indeed, they later state that their groups had normal zinc levels within the clinically acceptable normal range and justify their use of a higher range of 'normal' zinc level by citing their own previous work which reports that those with lower zinc levels are more susceptible to infections. The protocol lists lung function as an outcome measure, but this is not reported in the original paper (Abdulhamid 2008). We therefore initially judged the risk of selective outcome reporting bias to be high; however, the authors have provided the raw data without reference to prior zinc status, which has reduced our interpretation of the bias introduced to low.
We deemed the remaining two trials of NO to have an unclear risk of bias for selective outcome reporting (Howlin 2017; Sagel 2009). In the Howlin trial, not all of the outcomes specified in the trial registration document and methods section of the paper were reported in the paper's results (Howlin 2017). In the second NO trial not all of the outcomes listed in the methods section of the paper were reported in the results (Sagel 2009).
Other potential sources of bias
We considered compliance to treatment, but in three trials we judged compliance not to be an issue (Howlin 2017; Milla 2013; Sagel 2009). In the safety trial of KB001‐A compliance is not an issue as this was a trial of a one‐dose infusion; this trial was sponsored by the pharmaceutical company developing KB001‐A (Milla 2013). Compliance was also not identified as a potential risk of bias in the NO trials as the intervention was administered in hospital (Howlin 2017; Sagel 2009).
We judged there to be a low risk of bias with regards to compliance in three trials (Jain 2018; Sharma 2016; Smyth 2010). We found no source of bias relating to compliance in the Jain trial (Jain 2018). One of the zinc trials measured adherence by capsule counting of returned medication and was deemed to be at a low risk of other potential bias (Sharma 2016). In the Smyth trial, compliance to the garlic protocol was assessed by participant report and capsule counting; this was reported as equal and so we judged the risk of bias to be low (Smyth 2010).
In the remaining two trials the risk of bias from compliance was judged to be unclear (Abdulhamid 2008; Renner 2001). Compliance to the zinc trial protocol for the was reported only in terms of prior zinc status and the per protocol analysis of compliance is not provided (Abdulhamid 2008). Compliance to the β‐carotene protocol was assessed by participant report and capsule counting; however, the result was not reported (Renner 2001).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings 1. Summary of findings: β‐carotene compared with placebo for chronic pulmonary infection in cystic fibrosis.
| β‐carotene compared with placebo for chronic pulmonary infection in cystic fibrosis | ||||||
|
Patient or population: children and adults with cystic fibrosis Settings: outpatients Intervention: oral β‐carotene supplementation Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | β‐carotene | |||||
|
Pulmonary exacerbations Days of antibiotic use Follow‐up: 3 months |
The mean number of days of antibiotics was 18.5 days in the control group. | The mean number of days of antibiotics in the intervention group was 8 days lower (18.8 days lower to 2.78 days higher). | MD ‐8.00 (‐18.78 to 2.78) | 24 (1) | ⊕⊕⊝⊝ lowa,b | The authors of this paper defined pulmonary exacerbations by the number of days of antibiotics. Pulmonary exacerbations were less frequent in the intervention group but this was not statistically significant (P = 0.15). |
|
Respiratory function absolute FEV1 % predicted Follow‐up: 3 months |
The mean FEV1 % predicted was 80.9% in the control group. | The mean FEV1 % predicted in the intervention group was 10.9% lower (32.2% lower to 10.4% higher). | MD ‐10.90 (‐32.23 to 10.43) | 24 (1) | ⊕⊕⊝⊝ lowa,b | The results favour the placebo for effect on FEV1 but this was not significant (P = 0.32). |
|
Adverse events Follow‐up: 6 months |
See comments. | 24 (1) | ⊕⊕⊝⊝ lowa,b | There were no adverse events reported during the trial. Some participants reported better tanning after exposure to sunlight. No further data were provided for adverse events. | ||
|
Need for antibiotics see pulmonary exacerbations |
See comments. | This outcome was reported by the authors as their definition of pulmonary exacerbations. The number of days of antibiotics was less in the β‐carotene group but not significantly so (P = 0.15). | ||||
| QoL | This outcome was not measured. | |||||
| Sputum microbiology | This outcome was not measured. | |||||
| Inflammatory markers | This outcome was not measured. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume in 1 second; MD: mean difference; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a Downgraded once due to risk of bias within the included trial, particularly from uncertainty of randomisation method and allocation concealment. b Downgraded once due to imprecision. The trial had a small number of participants which is well below the optimal information size and low event rates.
Summary of findings 2. Summary of findings: garlic supplementation compared with placebo for chronic pulmonary infection in cystic fibrosis.
| Garlic supplementation compared with placebo for chronic pulmonary infection in cystic fibrosis | ||||||
|
Patient or population: children aged over eight and adults with cystic fibrosis and chronic pulmonary infection with P aeruginosa Settings: outpatients Intervention: garlic capsule once daily Comparison: placebo capsule once daily | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Garlic supplement | |||||
| Pulmonary exacerbations | This outcome was not measured in this trial. | |||||
|
Respiratory function change from baseline in % predicted FEV1 Follow‐up: 8 weeks |
The mean change in FEV1 % predicted was ‐3.6 % in the control group. | The mean change in FEV1 % predicted in the intervention group was 1.59% higher (7.49% lower to 10.67% higher). | MD 1.59 (‐7.49 to 10.67) | 26 (1) | ⊕⊕⊕⊝ moderatea | There was no significant difference between the groups after 8 weeks of treatment (P = 0.73). |
|
Adverse events mild adverse events Follow‐up: 8 weeks |
See comments. | 26 (1) | ⊕⊕⊕⊝ moderatea | 5 participants in the garlic group reported minor adverse effects: diarrhoea: OR 5.87 (95% CI 0.25 to 135.15; P = 0.27); halitosis: OR 5.87 (95% CI 0.25 to 135.15; P = 0.27); abdominal pain: OR 3.24 (95% CI 0.12 to 87.13; P = 0.48); dysuria: OR 3.24 (95% CI 0.12 to 87.13; P = 0.48). 1 participant in each group reported minor haemoptysis, OR 1.00 (95% CI 0.06 to 17.90; P = 1.0). Overall there was no significant difference between garlic and placebo for any of the adverse effects. |
||
|
Need for antibiotics days of antibiotic use Follow‐up: 8 weeks |
This outcome was not measured in this trial. | Days of antibiotic use was not reported but 7 participants in the garlic group received intravenous antibiotics compared to 5 in the placebo group. | ||||
| QoL | This outcome was not measured in this trial. | |||||
| Sputum microbiology | This outcome was not measured in this trial | |||||
| Inflammatory markers | This outcome was not measured in this trial | |||||
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume in 1 second; MD: mean difference; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a Downgraded once due to imprecision from low number of participants which does not reach the optimal information size.
Summary of findings 3. Summary of findings: monoclonal antibody KB001‐A compared with placebo for chronic pulmonary infection in cystic fibrosis.
| Monoclonal antibody KB001‐A compared with placebo for chronic pulmonary infection in cystic fibrosis | ||||||
|
Patient or population: children (12 or over) and adults with cystic fibrosis and lung infection Settings: outpatients Intervention: monoclonal antibody KB001‐A Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | KB001‐A | |||||
|
Pulmonary exacerbations Follow‐up: 8 to 16 weeks |
Of the 2 trials reporting this outcome, the small trial reported no pulmonary exacerbations in the low‐dose group (10 mg/kg), 1 pulmonary exacerbation in the 3 mg/kg group and 2 pulmonary exacerbations in the placebo group (RR 0.25 (95% CI 0.03 to 2.40)). This showed a slight difference in favour of KB001‐A but this was non‐significant (Milla 2013). The larger Jain trial, did not report exacerbations as an outcome, but did report that exacerbations were the most commonly reported adverse event in both the KB001‐A and placebo group (57.8% of KB001‐A participants and 67.4% of placebo participants) (Jain 2018). |
196 (2) | ⊕⊕⊝⊝ lowa | The data from the 2 trials could not be combined as 1 was a single‐dose trial whilst the other trial administered KB001‐A via infusion every 4 weeks over 16 weeks in total. | ||
|
Respiratory function number of episodes of a drop in FEV1 (unit of measurement not given) Follow‐up: 8 to 16 weeks |
Six participants who received a single 3 mg/kg dose of KB001‐A experienced a decrease in FEV1, RR 4.74 (95% CI 0.28 to 79.44) compared to no drop in FEV1 in the placebo group (Milla 2013). At the end of the 16‐week trial of KB001‐A, the authors reported a 3 percentage point increase from baseline in % predicted FEV1, MD 3.2% (95% CI 1.12% predicted to 5.30% predicted; P = 0.003) in the KB001‐A arm versus placebo (Jain 2018). |
196 (2) | ⊕⊝⊝⊝ very lowa,b | |||
|
Adverse events number of participants experiencing adverse events Follow up: 56 days |
Milla (single‐dose trial) reported a slight non‐significant difference in how many participants experienced any adverse event favouring placebo, RR 1.30 (95% CI 0.90 to 1.87) and no significant difference between intervention and placebo for serious adverse events: bronchitis (1 participant in the KB001‐A group) RR 1.58 (95% CI 0.07 to 35.32); sinusitis (1 participant in the placebo group) RR 0.18 (95% CI 0.01 to 3.92) (Milla 2013). Jain reported no treatment‐emergent adverse events and similar numbers of participants with infusion reactions between intervention and placebo groups. |
196 (2) | ⊕⊝⊝⊝ very lowa,c | Neither of the trials reported significant differences in any adverse events, but event rates were very low. | ||
|
Need for antibiotics time to need for antibiotics Follow‐up: 16 weeks |
Jain found no difference in the time to need for antibiotics in the KB001‐A group versus placebo over the 16‐week trial period, HR 1.00 (95% CI 0.69 to 1.45). | 169 (1) | ⊕⊕⊕⊝ moderated | |||
|
QoL change in CFQ‐R score Follow‐up: 56 days |
Milla reported no difference in the change in CFQ‐R from baseline between the KB001‐A and placebo groups. | 27 (1) | ⊕⊕⊝⊝ lowe,f | Only the Milla study reported CFQ‐R (Milla 2013). Jain (n = 169) did not measure CFQ‐R, but did measure change in respiratory symptom scores from baseline using the CFRSD‐CRISS symptom severity score. There was no difference found between treatment groups (Jain 2018). |
||
|
Sputum microbiology Follow‐up: 28 days |
We were not able to enter the data into the analysis and have reported results narratively (Milla 2013). At day 28 there was a numerical reduction from baseline in median mucoid P aeruginosa density in the KB001‐A 10 mg/kg group (‐0.4 log10) compared to placebo (0.8 log10), but the changes were not significant. | 27 (1) | ⊕⊕⊝⊝ lowe,f | |||
|
Inflammatory markers Follow‐up: 28 to 56 days |
Milla reported no significant change from baseline in any biomarkers but there was a trend towards a KB001‐A dose‐dependent reduction in sputum MPO, IL‐8, IL‐1β and NE (Milla 2013). Jain reported a ‐0.24 log10 reduction in IL‐8 in the KB001‐A group compared to placebo at week 16 (P = 0.04) (Jain 2018). |
196 (2) |
⊕⊝⊝⊝ very lowa,c | The Jain trial also reported a non‐significant decrease in sputum NE, MD ‐0.27 (95% CI ‐0.58 to 0.04; P = 0.084) (Jain 2018). | ||
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFQ‐R: CF Questionnaire‐Revised; CFRSD‐CRISS: Cystic Fibrosis Respiratory Symptom Diary‐Chronic Respiratory Infection Symptom Score; CI: confidence interval; FEV1: forced expiratory volume in 1 second; HR: hazard ratio; IL‐1β: interleukin‐1β ;IL‐8: interleukin 8; MD: mean difference; MPO: myeloperoxidase; NE: neutrophil elastase; P aeruginosa: Pseudomonas aeruginosa; QoL: quality of life; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a. Dowgraded twice due to risk of bias within the trials contributing to this outcome. There was concern around randomisation and allocation concealment in 1 trial and a risk from attrition bias in the other included trial.
b. Downgraded due to inconsistency caused by heterogeneity between the 2 studies for his outcome.
c. Downgraded due to imprecision from low event rates.
d. Downgraded once because there was unclear risk of bias from attrition in the 1 included trial. Although an intention‐to‐treat analysis was used, there was a 27% drop‐out rate
e. Downgraded once due to risk of bias within the 1 included trial for this outcome. There was concern around randomisation and allocation concealment as this was not clearly stated.
f. Downgraded once due to imprecision caused by low participant numbers and low event rates.
Summary of findings 4. Summary of findings: nitric oxide compared with placebo for chronic pulmonary infection in cystic fibrosis.
| Nitric oxide compared with placebo for chronic pulmonary infection in cystic fibrosis | ||||||
|
Patient or population: children (12 or over) and young adults with cystic fibrosis Settings: unclear if inpatient or outpatient Intervention: inhaled NO (10 ppm inhaled overnight for 5 ‐ 7 days; 20 ppm or 40 ppm inhaled continuously for 44 hours) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | NO | |||||
| Pulmonary exacerbations | This outcome was not measured in this trial. | |||||
|
Respiratory function mean change in FEV1 % predicted from baseline Follow‐up: 20 days |
The mean change in FEV1 % predicted in the control group is 6.17%. | The mean FEV1 % predicted in the intervention group was 2% lower (10% lower to 6% higher). | MD ‐1.95 (‐9.94 to 6.04) | 12 (1) | ⊕⊕⊝⊝ lowa,b | There was no significant difference between the NO group and placebo (P = 0.63). |
|
Adverse events Follow‐up: 48 hours to 20 days |
In one trial 4 participants reported adverse events including epistaxis, cough and cold, increased cough and haemoptysis but all were considered mild and only possibly related to the trial treatment. In the placebo group only one adverse event was reported, which was epistaxis (Howlin 2017). Sagel reported no serious adverse events in either NO or placebo group at either the low dose or high dose of NO (Sagel 2009). |
30 (2) | ⊕⊝⊝⊝ very lowa,b | |||
| Need for antibiotics | This outcome was not measured. | |||||
| QoL | This outcome was not measured. | |||||
|
Sputum microbiology Follow‐up: 48 hours to 20 days |
One trial showed a significant reduction in S aureus colony counts in the high‐dose NO group relative to placebo (P = 0.03) and a near significant reduction in P aeruginosa colony counts in the high‐dose group relative to placebo (P = 0.06) (Sagel 2009). The second trial found a significant reduction in P aeruginosa biofilm aggregates compared with those receiving placebo with antibiotics over the 7‐day treatment period (P = 0.029) and there was less P aeruginosa biofilm in the NO group compared with placebo (Howlin 2017). |
30 (2) | ⊕⊝⊝⊝ very lowb,c | |||
| Inflammatory markers | This outcome was not measured. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume in 1 second; NO: nitric oxide; P aeruginosa: Pseudomonas aeruginosa; RR: risk ratio; S aureus: Staphylococcus aureus. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a Downgraded once from risk of bias because of uncertainty around selective reporting of outcomes. b Downgraded once due to imprecision from very low number of participants and low event rates. c Downgraded twice for risk of bias within the included trials. There were concerns around randomisation, allocation concealment, selective reporting and attrition bias.
Summary of findings 5. Summary of findings: Zinc supplementation compared with placebo for chronic pulmonary infection in cystic fibrosis.
| Zinc supplementation compared with placebo for chronic pulmonary infection in cystic fibrosis | ||||||
|
Patient or population: children with cystic fibrosis Settings: outpatients Intervention: zinc supplementation (30 mg orally once daily) Comparison: placebo (orally once daily) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Zinc supplement | |||||
|
Pulmonary exacerbations number of participants requiring IV antibiotics Follow‐up: 12 months |
211 per 1000. | 390 per 1000 (137 to 1000). | RR 1.85 (0.65 to 5.26) | 37 (1) | ⊕⊕⊕⊝ moderatea | Results favour placebo, but not significantly (P = 0.25) (Sharma 2016). |
|
Respiratory function mean FEV1 % predicted Follow‐up: 24 months |
One trial showed no difference between groups, MD ‐5.46 (95% CI ‐19.44 to 8.52) (Abdulhamid 2008). A further paper reported that the median (IQR) FEV1 % predicted value was 8.97% (‐18.23% to 0.33%) lower than baseline in the zinc group and 9.55% (‐9.59% to 12.88%) higher in the placebo group (P = 0.08) (Sharma 2016). |
62 (2) | ⊕⊝⊝⊝ very lowb,c,d | Results have been reported narratively as the outcome was reported differently in the 2 trials. | ||
|
Adverse events number of participants hospitalised during the trial Follow‐up: 12 months |
316 per 1000. | 389 per 1000 (161 to 939). | RR 1.23 (0.51 to 2.97) | 37 (1) | ⊕⊕⊕⊝ moderatea | All of the serious adverse events were related to exacerbations. Although no data were reported, the Abdulhamid trial reported there were no adverse events (Abdulhamid 2008). |
|
Need for antibiotics number of days on IV or oral antibiotics Follow‐up: 24 months |
Abdulhamid reported that significantly fewer oral antibiotics alone were needed by participants in the zinc group, MD ‐17.74 (95% CI ‐26.98 to ‐8.50); but there was no significant difference between groups in the need for IV antibiotics alone, MD 0.52 (95% CI ‐3.07 to 4.11) (Abdulhamid 2008). The Sharma trial found no significant difference in the number of days on IV or oral antibiotics (P = 0.76) (Sharma 2016). |
62 (2) | ⊕⊝⊝⊝ very lowb,c | |||
| QoL | This outcome was not reported. | |||||
| Sputum microbiology | This outcome was not reported. | |||||
| Inflammatory markers | This outcome was not reported. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume in 1 second; IV: intravenous; IQR: interquartile range; RR: risk ratio; MD: mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a Downgraded once due to imprecision caused by low event rates and a small participant numbers (n = 37). b Downgraded once due to risk of bias within 1 of the included trials; there were concerns across 5 out of the 6 domains for assessing risk of bias. c Downgraded once due to imprecision from small number of participants. d Downgraded once due to inconsistency caused by heterogeneity between the 2 trial results.
The effects of interventions are summarised in the summary of findings tables, the quality of the evidence has been graded for pre‐defined outcomes (see above) and definitions of these gradings provided (Table 1; Table 2; Table 3; Table 4; Table 5).
Acute respiratory exacerbations
There were no trials included which considered acute respiratory exacerbations.
Chronic infection
All eight included trials investigated antibiotic adjuvant therapies for chronic pulmonary infection (Abdulhamid 2008; Howlin 2017; Jain 2018; Milla 2013; Renner 2001; Sagel 2009; Sharma 2016; Smyth 2010). For simplicity we have reported below only the review's outcome measures that have been reported in the selected trials.
β‐carotene supplementation
One trial (n = 24) compared β‐carotene supplementation to placebo (Renner 2001).
Primary outcomes
1. Pulmonary exacerbations (protocol defined)
Investigators report a reduced rate of pulmonary exacerbations in the treatment arm, defined as the number of days of antibiotic consumption, although this is not statistically significant, MD ‐8.00 (95% CI ‐18.78 to 2.78) (Analysis 1.1). We judged the quality of evidence for this outcome to be low.
1.1. Analysis.

Comparison 1: Chronic infection: β‐carotene supplementation versus placebo, Outcome 1: Days of antibiotic consumption
2. Respiratory function
a. FEV1 % predicted
There appeared to be no effect upon lung function (FEV1 % predicted) between the two groups, MD ‐10.90% (95% CI ‐32.23% to 10.43%) (Analysis 1.2). The authors describe an apparently post hoc analysis of the 'younger patients' (undefined) and suggest their FEV1 'clearly improved'. Unfortunately only data for the treatment group are presented. We judged the quality of evidence for this outcome to be low.
1.2. Analysis.

Comparison 1: Chronic infection: β‐carotene supplementation versus placebo, Outcome 2: Respiratory function absolute FEV1 % predicted up to 3 months
b. FVC % predicted
This outcome was measured but not reported per protocol. The authors state that mean (SD) FVC did not change during the treatment (baseline 87.6% predicted (21.2%), week 12 85.1% predicted (24.6%) and week 24 85.6% predicted (25.1%)) (Renner 2001).
3. Adverse events
There were no adverse events reported during the trial, but some participants reported better tanning after exposure to sunlight (Renner 2001). We judged the quality of evidence for this outcome to be low.
Secondary outcomes
1. Antibiotic consumption
b. As above, the protocol definition of pulmonary exacerbation was the antibiotic consumption rate in days (Analysis 1.1) (low‐quality evidence).
5. Mortality
There were no dropouts during the trial and so we assume that no deaths occurred, although this was not explicitly discussed (Analysis 1.3).
1.3. Analysis.

Comparison 1: Chronic infection: β‐carotene supplementation versus placebo, Outcome 3: Mortality
Garlic supplementation
One included trial (n = 34) investigated garlic supplementation (Smyth 2010). There were no statistically significant changes in clinical score and lung function. It was also not possible to detect a difference in quorum sensing molecules in plasma or sputum.
Primary outcomes
2. Respiratory function
a. FEV1 % predicted
The percentage change in % predicted FEV1 between the treated and control groups did not reach significance, MD 1.59% predicted (95% CI ‐7.49% to 10.67%) (Analysis 2.1). We judged the quality of evidence to be moderate.
2.1. Analysis.

Comparison 2: Chronic infection: garlic supplementation versus placebo, Outcome 1: Respiratory function (% change FEV1)
3. Adverse events
a. mild (not requiring treatment)
Five participants in each group had abnormal liver function or triglyceride levels. Five participants in the garlic group reported minor adverse effects (diarrhoea (two participants), halitosis (two participants), abdominal pain (one participant), dysuria (one participant), minor haemoptysis (one participant)). One participant from the placebo group reported a minor haemoptysis (Analysis 2.3). We again judged the quality of evidence to be moderate.
2.3. Analysis.

Comparison 2: Chronic infection: garlic supplementation versus placebo, Outcome 3: Mild adverse events
Secondary outcomes
2. Antibiotic consumption (days of antibiotic use)
Seven participants in the garlic group received intravenous antibiotics compared to five in the placebo group; however, days of antibiotic use was not reported (Smyth 2010).
5. Mortality
There were no deaths during the trial period (Analysis 2.2).
2.2. Analysis.

Comparison 2: Chronic infection: garlic supplementation versus placebo, Outcome 2: Mortality
KB001‐A
Two trials compared KB001‐A to placebo (n = 196) (Jain 2018; Milla 2013). One trial was single‐dose trial (n = 27) with participants followed up for eight weeks (Milla 2013), whilst the second trial (n = 169) administered KB001‐A via infusion once every four weeks over a 16‐week period (Jain 2018). For this reason we could not combine the results in a meta‐analysis.
Primary outcomes
1. Pulmonary exacerbations (protocol defined)
In the single‐dose trial investigators reported the number of CF exacerbations (Milla 2013). No participants experienced a CF exacerbation after the 10 mg/kg dose of KB001‐A, one participant who received a 3 mg/kg dose of KB001‐A experienced a pulmonary exacerbation and there were two reports of pulmonary exacerbations in the placebo group, RR 0.25 (95 % CI 0.03 to 2.40) (Analysis 3.1).
3.1. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 1: Pulmonary exacerbations
Jain did not report pulmonary exacerbations as a primary outcome, but found that an infective pulmonary exacerbation was the most common adverse event in both groups (57.8% of KB001‐A participants and 67.4% of placebo participants) (Jain 2018).
The GRADE assessment for this outcome was judged to be low.
2. Respiratory function
a. FEV1 % predicted
Milla reports the number of episodes of a drop in lung function (FEV1, unit of measurement not given); however, the lung function data in terms of the size of decrease in FEV1 are not reported (Milla 2013). Six participants who received a 3 mg/kg dose of KB001‐A experienced a decrease in FEV1, RR 4.74 (95% CI 0.28 to 79.44) (Analysis 3.2).
3.2. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 2: Decrease in respiratory function (FEV1)
In the trial of KB001‐A given over a longer period, the authors reported a 3% increase in the absolute change from baseline in % predicted FEV1 at week 16 for the KB001‐A arm versus placebo, MD 3.2% (95% CI 1.12% to 5.30%) (P = 0.003) (Jain 2018).
We judged the GRADE assessment for this outcome to be very low.
b. FVC % predicted
Milla found no significant differences between active treatment and placebo groups for FVC (unit not stated) at any time point (Milla 2013).
c. Other validated measures of respiratory function
Milla also found no significant differences between active treatment and placebo groups for any other spirometry parameters (FEF25-75) at any time point (Milla 2013).
3. Adverse events
The primary outcome of the Milla trial was the number of participants experiencing adverse events. Seven of those who received placebo and all (n = 18) of those who received KB001‐A experienced an adverse event, RR 1.30 (95% CI 0.90 to 1.87) (Analysis 3.3).
3.3. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 3: Number of participants experiencing an adverse event
There is insufficient information to determine the severity of the events according to our classification of mild, moderate and severe, we have therefore used the classification reported in the trial ‐ 'serious' and 'other'. The trial reports the following serious adverse effects: a participant receiving a 3 mg/kg dose of KB001‐A developed bronchitis, RR 1.58 (95% CI 0.07 to 35.32); and a participant who received placebo developed sinusitis, RR 0.18 (95% CI 0.01 to 3.92) (Analysis 3.4). The 'other' events are described under group headings and presented in the analyses (Analysis 3.5; Analysis 3.6; Analysis 3.7; Analysis 3.8; Analysis 3.9; Analysis 3.10; Analysis 3.11; Analysis 3.12; Analysis 3.13; Analysis 3.14; Analysis 3.15).
3.4. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 4: Serious adverse events
3.5. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 5: Ear and labyrinth adverse effects
3.6. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 6: Gastrointestinal adverse events
3.7. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 7: General adverse events
3.8. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 8: Infections and infestations
3.9. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 9: Injury, poisoning and procedural complications
3.10. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 10: Investigative adverse events
3.11. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 11: Nervous system adverse events
3.12. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 12: Psychiatric adverse events
3.13. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 13: Respiratory, thoracic and mediastinal adverse events
3.14. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 14: Skin and subcutaneous tissue adverse events
3.15. Analysis.

Comparison 3: Chronic infection: KB001‐A versus placebo, Outcome 15: Vascular adverse events
Jain reported no treatment‐emergent adverse events and similar numbers of participants with infusion reactions between intervention and placebo. The number of hospitalised participants was higher in the KB001‐A group compared to placebo (28.9% versus 16.3%) (Jain 2018).
Again, we judged the GRADE assessment of quality to be very low.
Secondary outcomes
1. Need for antibiotics
a. Time to next course of antibiotics (oral, inhaled or IV)
The Jain trial found no difference in the time to need for antibiotics in the KB001‐A group versus placebo over the 16‐week period, HR 1.00 (95% CI 0.69 to 1.45) (result taken directly from the paper) (Jain 2018). We deemed the quality of this evidence to be moderate.
2. QoL
b. Treatment burden
Milla looked at change in CFQ‐R scores but found no significant differences between groups at any time point (data not given) (Milla 2013). Similarly, Jain measured change in respiratory symptom scores from baseline using the CFRSD‐CRISS symptom severity score, but found no difference between treatment groups (Jain 2018). We rated the quality of evidence as low for this outcome.
3. Change in sputum microbiology
b. Quantitative microbiology
At day 28 there was a numerical reduction from baseline in median mucoid P aeruginosa density in the KB001‐A 10 mg/kg group (‐0.4 log10) compared to placebo (0.8 log10) but the changes were not significant (Milla 2013). We deemed the quality of this evidence to be low.
4. Change in inflammatory markers
Milla reported no significant changes in biomarkers from baseline, but there was a trend towards a KB001‐A dose‐dependent reduction in sputum myeloperoxidase, IL‐8, IL‐1β and neutrophil elastase (Milla 2013). Jain reported a ‐0.24 log10 reduction in IL‐8 in the KB001‐A group compared to placebo at week 16 (P = 0.04), but the decrease in sputum neutrophil elastase (NE) was not significant, MD ‐0.27 (95% CI ‐0.58 to 0.04; P = 0.084) (Jain 2018). The quality of this evidence was found to be very low.
5. Mortality
Milla reported no deaths during the trial (Milla 2013).
Nitric oxide
Two trials (n = 30) reported on the effect of inhaled NO as an antibiotic adjuvant, but the data cannot be combined in a meta analysis because one trial administered NO at 10 ppm for eight hours overnight for five to seven days (Howlin 2017), whilst the second trial administered NO continuously for 44 hours at doses of either 20 or 40 ppm (Sagel 2009).
Primary outcomes
2. Respiratory function
a. FEV1 % predicted
Howlin reported a greater increase in FEV1 % predicted in the placebo group compared to the inhaled NO group but this was not significant after 20 days (Howlin 2017), MD ‐1.95 (95% CI ‐9.94 to 6.04) (P = 0.63) (Analysis 4.1). The Sagel trial only reported a value for baseline FEV1 (L) for each group and a value after 48 hours, but no statistics were given (Sagel 2009). We graded the quality of evidence as low.
4.1. Analysis.

Comparison 4: Chronic infection: nitric oxide versus placebo, Outcome 1: Change from baseline in FEV1 % predicted
b. FVC % predicted
This was only reported in the Howlin trial which found that after five to seven days of inhaled NO or placebo gas, there was a greater (non‐significant) change from baseline in FVC % predicted in the placebo group compared to the NO group, MD ‐8.03 (95 % CI ‐18.50 to 2.44) (P = 0.13) (Howlin 2017).
3. Adverse events
Both trials reported adverse events but numbers were low. Howlin reported that in the NO group four participants reported adverse events including epistaxis, cough and cold, increased cough and haemoptysis, but all events were considered mild and only possibly related to the trial treatment. In the placebo group only one adverse event was reported, which was epistaxis (Howlin 2017). Sagel reported no serious adverse events in either the NO group or the placebo group at either the low or high dose of NO (Sagel 2009). We rated the quality of evidence as very low for this outcome.
Secondary outcomes
3. Change in sputum microbiology
b. Quantitative microbiology
Howlin found that there was a significant reduction in P aeruginosa biofilm aggregates compared with those receiving placebo with antibiotics over the seven‐day treatment period (P = 0.029) and there was less P aeruginosa biofilm in the NO group compared with placebo (Howlin 2017). Sagel reported that there was a significant reduction in S aureus colony counts in the high‐dose NO group compared to placebo (P = 0.03) and a near significant reduction in P aeruginosa colony counts in the high‐dose group compared to placebo (P = 0.06) (Sagel 2009). Again, we rated the quality of this evidence as very low.
Zinc supplementation
Two trials (n = 66) compared zinc supplementation to placebo (Abdulhamid 2008; Sharma 2016).
Primary outcomes
1. Pulmonary exacerbations (protocol‐defined)
Investigators in the Abdulhamid trial do not report on their protocol definition of an exacerbation; they only report the number of episodes requiring IV and oral antibiotics (see results below) (Abdulhamid 2008). In the Sharma trial, the authors report on the number of participants requiring IV antibiotics during the treatment phase. Seven participants (38.9%) in the zinc group and four participants (20.1%) in the placebo group experienced exacerbations requiring IV antibiotics during the 12‐month trial, RR 1.85 (95% CI 0.65 to 5.26) (Analysis 5.1); this result is not statistically significant (Sharma 2016). We graded the quality of evidence as moderate.
5.1. Analysis.

Comparison 5: Chronic infection: zinc supplementation versus placebo, Outcome 1: Pulmonary exacerbations (number of participants requiring IV antibiotics)
2. Respiratory function
a. FEV1 % predicted Abdulhamid presents this measure in the baseline characteristics, but the outcome is not reported post‐treatment in the published paper (Abdulhamid 2008). After contacting the authors, we received the data to allow us to analyse this outcome in the graphs and these showed no difference between groups, MD ‐5.46 (95% CI ‐19.44 to 8.52) (Analysis 5.6).
5.6. Analysis.

Comparison 5: Chronic infection: zinc supplementation versus placebo, Outcome 6: Respiratory function (FEV1 % predicted)
Sharma measured the change in FEV1 % predicted and reported the median and interquartile range (IQR); thus we were not able to combine the results of the two trials. Sharma found that the median (IQR) FEV1 % predicted was 8.97% (‐18.23 to 0.33) lower than baseline in the zinc group and 9.55% (‐9.59 to 12.88) higher in the placebo group (P = 0.08).
We judged the quality of this evidence to be very low.
b. FVC % predicted Only the Abdulhamid trial reported this outcome; again, the measure was presented in the baseline characteristics, but not reported in the published paper (Abdulhamid 2008). Dr Abdulhamid supplied data for analysis and these showed a statistically non‐significant difference between groups, MD ‐1.75 (95% CI ‐13.09 to 9.59) (Analysis 5.2).
5.2. Analysis.

Comparison 5: Chronic infection: zinc supplementation versus placebo, Outcome 2: Respiratory function (FVC %predicted)
3. Adverse events
Abdulhamid reported no adverse events (Abdulhamid 2008). Sharma reported 15 serious adverse events or hospitalisations in 13 participants; all of the serious adverse events were related to exacerbations (Sharma 2016). Seven children were hospitalised in the zinc group compared to six in the placebo group, RR 1.23 (95% CI 0.51 to 2.97) (P = 0.64) (Analysis 5.7). We judged to quality of evidence for this outcome to be moderate.
5.7. Analysis.

Comparison 5: Chronic infection: zinc supplementation versus placebo, Outcome 7: Adverse events (number of participants hospitalised in the study period)
Secondary outcomes
2. Antibiotic consumption (days of antibiotic use)
Additional data for one trial (provided by the investigators) showed that zinc supplementation resulted in a statistically significant reduction in the number of days of oral antibiotics alone, MD ‐17.74 (95% CI ‐26.98 to ‐8.50) (P = 0.05) (Analysis 5.3) (Abdulhamid 2008). However, the difference between groups was not statistically significant for duration of IV antibiotics, MD 0.52 (95% CI ‐3.07 to 4.11) (Analysis 5.4). When the data for both oral and IV antibiotics combined, as supplied by Dr Abdulhamid, were analysed these showed a statistically significant difference between treatment and control groups, MD ‐17.22 (95% CI ‐27.06 to ‐7.38) (Analysis 5.5).
5.3. Analysis.

Comparison 5: Chronic infection: zinc supplementation versus placebo, Outcome 3: Antibiotic consumption (days oral antibiotics)
5.4. Analysis.

Comparison 5: Chronic infection: zinc supplementation versus placebo, Outcome 4: Antibiotic consumption (days IV antibiotics)
5.5. Analysis.

Comparison 5: Chronic infection: zinc supplementation versus placebo, Outcome 5: Antibiotic consumption (days of oral and IV antibiotics)
The Sharma trial reported the median (range) number of days of oral or IV antibiotics, but found no significant difference between groups. The median (range) in the zinc group was 42 days (0 to 238) and in the placebo group 38 days (0 to 224) (P = 0.76) (Sharma 2016).
We judged the quality of this outcome to be very low.
5. Mortality
Sharma reported that none of the participants died during the trial (Sharma 2016).
Discussion
Summary of main results
We identified eight RCTs with 350 participants from our systematic search of the literature (Abdulhamid 2008; Howlin 2017; Jain 2018; Milla 2013; Renner 2001; Sagel 2009; Sharma 2016; Smyth 2010). The trials looked at the effects of five different interventions compared with placebo: β‐carotene; garlic; monoclonal antibody KB001‐A; NO; and zinc supplementation. Neither the trial of β‐carotene supplementation or the trial of garlic supplementation detected a statistically significant difference between those in the treatment and control groups for any outcome measure (Renner 2001; Smyth 2010). Similarly, the trials of KB001‐A found no significant difference in exacerbations and we are uncertain as to the effect on FEV1; there is also probably no difference in time to need for antibiotics between KB001‐A and placebo (Jain 2018; Milla 2013). The earlier trial of KB001‐A was a safety trial with limited data published online (Milla 2013), but this led to the repeat‐dose RCT where therapeutic efficacy was the primary outcome (measured by time‐to‐next antibiotic treatment) with safety and tolerability being secondary outcome measures (Jain 2018). The NO trials did not report on exacerbations and there may be little or no difference between NO and placebo in the effect on FEV1 (Howlin 2017; Sagel 2009). The addition of two further trials of zinc supplementation since the last update offers no further evidence of a difference between zinc and placebo for our primary outcomes (Abdulhamid 2008; Sharma 2016). One of these trials found a reduction in the number of days of oral or IV antibiotics needed during the trial period, but the quality of evidence was very low and so we are uncertain if this is a genuine effect (Abdulhamid 2008). It is notable that the need for IV antibiotics did not differ between the two groups, an inconsistency that is difficult to explain.
Respiratory function was measured in all of the included trials and no difference between intervention and placebo was found for β‐carotene, garlic, NO or zinc (Abdulhamid 2008; Howlin 2017; Renner 2001; Sagel 2009; Sharma 2016; Smyth 2010). The earlier trial of single‐dose KB001‐A reported a non‐significant drop in FEV1 in the intervention group compared to placebo, where a slight increase in FEV1 was seen, RR 4.74 (95% CI 0.28 to 79.44) (Milla 2013). However, in the 16‐week trial of KB001‐A there was a 3% absolute increase from baseline in % predicted FEV1 at week 16 for the KB001‐A arm versus placebo, MD 3.2% (95% CI 1.12 % to 5.30 %) (P = 0.003) (Jain 2018). The quality of this evidence, however, was deemed to be very low.
Few adverse events were seen across all of the different interventions and the adverse events that were reported were mild or not treatment related. QoL was only reported in the trials of KB001‐A where no difference in CFQ‐R was seen between KB001‐A and placebo (Jain 2018; Milla 2013). Sputum microbiology was measured and reported for the trials of KB001‐A and NO. There was very low‐quality evidence of a numerical reduction in P aeruginosa density after KB001‐A, but it was non‐significant (Milla 2013). The trials looking at the effects of NO reported significant reductions in S aureus and near significant reductions in P aeruginosa but the quality of this evidence is very low (Howlin 2017; Sagel 2009).
Overall completeness and applicability of evidence
We are confident that we have identified all the trials that meet our inclusion criteria as our searches were thorough and we contacted authors for extra information where necessary. One of the trials included in the 2013 update was only in abstract form, but the full paper has now been published and included in the 2020 update (Jain 2018). Not all of the included trials measure our primary outcome (pulmonary exacerbations) and some of those that did used surrogate measures such as days on antibiotics. There is a preponderance to include participants in the younger age group ‐ children and adolescents. Potentially this group of younger individuals, prior to chronic colonisation with multi‐resistant bacteria, could have most to gain from an intervention that might act to reduce infection and hence spare lung function and QoL at an earlier age. However, the generalisability of this approach to the more unwell individuals, who tend not to be included in such trials, is questionable.
It should also be noted that since the β‐carotene trial was conducted, larger randomised trials with subsequent analyses have demonstrated an increased risk of development of certain malignancies (lung and stomach) in individuals with lung disease when given a daily 20 mg to 30 mg β‐carotene supplement (Druesne‐Pecollo 2010). The authors believe this precludes further investigation of the high‐dose supplementation regimen investigated in this review.
One trial is yet to be completed and published and we await the results of this (Pressler 2017). There are six trials which have been completed but which are listed as 'Awaiting assessment' until we have the results we can include and analyse (CARE‐CF‐1; Khorasani 2009; Puvvadi 2019; Rye 2015; Walshaw 2014; Zabner 2009a).
Quality of the evidence
The gradings for the quality of the evidence for each comparison are presented in the summary of findings tables (Table 1; Table 2; Table 3; Table 4; Table 5).
The quality of the evidence for this review is limited by the size and quality of the underlying trials and the fact that there were no data to combine in a meta‐analysis. Two of our five comparisons included only one trial (β‐carotene (Renner 2001); garlic (Smyth 2010)), the remaining three comparisons include two trials each (KB001‐A (Jain 2018; Milla 2013); NO (Howlin 2017; Sagel 2009); and zinc (Abdulhamid 2008; Sharma 2016)).
The quality of the evidence for β‐carotene was low because of concerns around randomisation and allocation concealment as well as the trial having a small sample size and low event rates. We are uncertain whether β‐carotene improves our primary outcomes (Renner 2001).
Although there was only one trial included in the comparison of garlic with placebo the quality of the trial was deemed to be moderate and only downgraded because of small sample size. However, the trial did not report on our primary outcome of pulmonary exacerbations and there is probably little or or no difference in respiratory function or adverse events (Smyth 2010).
There were two trials looking at the effect of KB001‐A, but they were heterogeneous in design, intervention and in the outcomes they reported (Jain 2018; Milla 2013). The quality of evidence for our primary outcomes was deemed to be very low and we are very uncertain as to whether KB001‐A improves pulmonary exacerbation rates (Milla 2013). The evidence for one of our secondary outcomes (need for antibiotics) was deemed to be moderate because the trial reporting on this outcome had a larger sample size and fewer concerns around trial design (Jain 2018).
The quality of evidence for NO was deemed to be low or very low due again to small sample sizes, low event rates and risk of bias within the two included trials. We are very uncertain as to the effect of NO on our primary and secondary outcomes (Howlin 2017; Sagel 2009).
The evidence for zinc supplementation was deemed moderate for pulmonary exacerbations and adverse events, but very low for the remaining outcomes reported. This is because we were unable to combine the data and so the quality reflects the underlying quality of the trials (Abdulhamid 2008; Sharma 2016).
Potential biases in the review process
One of the co‐authors of this review is lead investigator of one of the included trials (Smyth 2010).
Agreements and disagreements with other studies or reviews
There have been no previous systematic reviews of antibiotic adjuvant therapies for pulmonary infection in individuals with CF. Reviews of strategies in development stress the pre‐clinical nature of much of these approaches and unfortunately biotechnology companies developing new therapies may defer publication of commercially sensitive results.
Authors' conclusions
Implications for practice.
Various strategies have been investigated in an attempt to improve the current position where individuals are infected with resistant bacteria for which no successful treatment is available. Unfortunately, however, trials of these strategies have been of poor quality and often limited to observational or limited cohort studies. The more rigorous trials have suffered from either a lack of statistical power or robust outcome measures.
There continues to be no alternative to conventional antibiotics alone in the treatment of pulmonary infection in those with cystic fibrosis (CF).
Implications for research.
Antibiotics remain the mainstay of treatment for pulmonary infection in CF. The emergence of increasingly‐resistant bacteria makes the reliance on antibiotics alone challenging for CF teams. There is a need to explore alternative strategies, such as the use of adjuvant therapies, that may prove a beneficial alternative. Novel strategies should aim to be founded on robust in vitro pre‐clinical development work that includes clinical isolates of mixed phenotypes of bacteria in models of infection that include both planktonic and biofilm modes of growth. Well‐designed placebo‐controlled double‐blind randomised trials of sufficient power and duration are required to determine the efficacy of any new strategy in both children and adults with CF. Such trials need to use outcome measures that are both objective and important to people with CF, specifically lung function, pulmonary exacerbation frequency, hospital attendance and measures of quality of life.
What's new
| Date | Event | Description |
|---|---|---|
| 8 September 2020 | Amended | Contact details updated. |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 10, 2010
| Date | Event | Description |
|---|---|---|
| 7 July 2020 | New citation required but conclusions have not changed | Despite the inclusion of three new studies in this review, our conclusions have not changed. A new author, Sherie Smith, has joined the team. |
| 7 July 2020 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Genetic Disorders Review Group's Cystic Fibrosis Trials Register identified 60 new references potentially eligible for inclusion in the review. Five references were identified from trial registry searches. Extra Medline searches carried out January 2016 (n = 142), December 2016 (n = 182) and February 2019 (n = 16) did not identify any trials that had not already been identified. Eight references were found to three newly included trials (Howlin 2017; Sagel 2009; Sharma 2016). Two additional references to a trial previously listed as ongoing (Molfino 2012 unpublished) were identified and the trial has now been included (Jain 2018). There were seven additional references to three already included trials (Abdulhamid 2008; Milla 2013; Smyth 2010). 24 references to seven new trials were excluded (Alvarez 2017; DiMango 2014; Forrester 2015; Hodges 2014; Middleton 2015; Sagel 2018; Tangpricha 2017). There were eight additional references to six already excluded trials (Grasemann 2013; Hauber 2008; Kollberg 2010; Leonard 2012; Moss 2013; Panchaud 2006). 11 new references were identified to four trials listed as 'Awaiting classification' (CARE‐CF‐1; Khorasani 2009; Puvvadi 2019; Rye 2015;). One additional reference was identified to a trial previously listed as ongoing and now listed as 'Awaiting classification' (Walshaw 2014). A further trial previously listed as ongoing has been marked as completed on clinicaltrials.gov and moved to 'Awaiting assessment' pending publication of the trial results (Zabner 2009). Five references were identified to a new trial listed as ongoing (Pressler 2017). The outcome measures have been updated in line with current MECIR guidelines and summary of findings tables added. |
| 7 May 2013 | New citation required but conclusions have not changed | No new studies were identified for inclusion in the review; limited results from a safety study previously listed as ongoing have been presented, but our conclusions have not changed. |
| 7 May 2013 | New search has been performed | A search of the Cystic Fibrosis Trials Register identified three references to two separate studies which were potentially eligible for inclusion in the review. Two of these references relate to the ongoing study identified for the initial version of the review (NCT00633191) now listed as (Kollberg 2010) which we have excluded as it refers to an immunotherapy. The remaining reference was excluded for the same reason (Winnie 1989). A renewed search of MEDLINE revealed six more studies. Two of these were excluded due to adjuvant activity being unclear (Gontijo‐Amaral 2012; Grasemann 2013). One study of immunotherapy (NCT01455675) and one of anti‐inflammatory therapy (Moss 2013) were also excluded. Two studies were excluded as they were not randomised (Lands 2010; Sagel 2011). A search of ongoing trials registers identified two studies which are listed as ongoing and which will be assessed for inclusion when completed (Molfino 2012; Walshaw 2014a). Four studies, which were listed as ongoing in the previous version of the review, have now been excluded. Two studies of miglustat were excluded as this is not an adjuvant (NCT00742092; Leonard 2012); the full publication of one of these studies was identified in the MEDLINE search (Leonard 2012). A safety study of a biological agent is now included (Milla 2013). The remaining cross‐over study was excluded after the study investigators confirmed that there were no first‐arm data available for analysis (Brown 1985). A study previously listed as 'Awaiting classification' has been excluded as the order of treatment was not randomised (Safai‐Kutti 1991). |
Acknowledgements
We would like to thank Professor Kevin Southern and Nikki Jahnke (Managing Editor, Cochrane Cystic Fibrosis and Genetic Disorders Group, Institute of Child Health) for their constructive advice in the preparation of this review and Natalie Hall (Information Specialist) for her help in searching for trials.
Appendices
Appendix 1. MEDLINE search strategy (1950 to present ) searched 11January 2010
| 1. | exp Cystic Fibrosis/ |
| 2. | cystic fibrosis.tw. |
| 3. | fibrocystic near disease near pancreas.tw. |
| 4. | mucoviscidos$.tw. |
| 5. | (cystic$ adj10 fibros$).tw. |
| 6. | 1 or 2 or 3 or 4 or 5 |
| 7. | (immune adj4 (stimulant$ or augment$ or activat$ or potentiat$ or modulat$)).tw. |
| 8. | exp Adjuvants, Immunologic/ |
| 9. | exp quorum sensing/ |
| 10. | Plant Extracts/ or Dietary Supplements/ or Vitamins/ or Phytotherapy/ or nutriceutical.mp. or Antioxidants/ or Nutritional Physiological Phenomena/ |
| 11. | (quorum and (sensing or quenching$)).tw. |
| 12. | (garlic or ajoene).tw. |
| 13. | exp Glutamine/ |
| 14. | glutamine.tw. |
| 15. | exp Bacteriophages/ |
| 16. | bacteriophage$.tw. |
| 17. | alginate lyase.tw. |
| 18. | exp endolysin/ |
| 19. | endolysin$.tw. |
| 20. | (IgY or immunoglobulin Y).tw. |
| 21. | ((efflux pump or lectin) adj inhibitor$).tw. |
| 22. | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 |
| 23. | 6 and 22 |
| 24. | limit 23 to (humans and clinical trial, all) |
Appendix 2. Ovid MEDLINE search strategy (1946 to present) searched 16 February 2019
1. Cystic Fibrosis/
2. cystic fibrosis.tw.
3. (fibrocystic adj10 disease adj10 pancreas).tw.
4. mucoviscidos$.tw.
5. (cystic$ adj10 fibros$).tw.
6. or/1‐5
7. exp Adjuvants, Immunologic/
8. adjuvant$.tw.
9. (immune adj4 (stimulant$ or augment$ or activat$ or potentiat$ or modulat$)).tw.
10. exp Quorum Sensing/
11. (quorum and (sensing or quenching$)).tw.
12. Garlic/
13. (garlic or ajoene).tw.
14. glutamine.mp. or exp Glutamine/
15. exp Bacteriophages/
16. bacteriophage$.tw.
17. alginate lyase.tw.
18. (IgY or immunoglobin Y).tw.
19. ((efflux pump or lectin) adj inhibitor$).tw.
20. or/7‐19
21. 6 and 20
22. randomized controlled trial.pt.
23. controlled clinical trial.pt.
24. randomized.ab.
25. placebo.ab.
26. drug therapy.fs.
27. randomly.ab.
28. trial.ab.
29. groups.ab.
30. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
31. exp animals/ not humans.sh.
32. 30 not 31
33. 21 and 32
Lines 22 – 32 are the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format
Appendix 3. Online trial registry searches
| Database | Search terms | Date last searched |
| ClinicalTrials.gov (www.clinicaltrials.gov) |
'cystic fibrosis' and limited to phase III and IV clinical trials | 06 April 2020 |
| International Standard Randomised Controlled Trial Number Registry (isrctn.orgctn.org/) |
'cystic fibrosis' and limited to phase III and IV clinical trials | 06 April 2020 |
| WHO clinical trials platform (ICTRP) (apps.who.int/trialsearch/) |
'cystic fibrosis' and limited to phase III and IV clinical trials | 06 April 2020 |
Appendix 4. Glossary
| Medical term | Explanation |
| antibiotic adjuvant | an agent that acts alongside an antibiotic, but itself has little or no bactericidal activity; it acts by increasing the organisms' susceptibility to the co‐administered antibiotic |
| bacteriophage | a virus that infects bacteria |
| biofilm | a thin layer of micro‐organisms (e.g. bacteria) that form on and coat various surfaces. Various substances coat the biofilm and provide protection to those organisms living within the biofilm |
| dendrimer | a large and complex molecule with a very well‐defined chemical structure |
| dysuria | painful urination |
| efflux pump | a mechanism located in the cell wall allowing substances toxic to the cell to be removed |
| efflux pump inhibitor | a molecule that interferes with the process of removing toxic substances and antibiotics from the cell |
| haemoptysis | coughing up of blood or of blood‐stained sputum |
| halitosis | bad breath |
| lectin | a sugar‐binding protein located on the surface off cells to allow cells to link to each other |
| quorum sensing inhibitors | molecules that interrupt the pathway of communication bacteria use to regulate expression of virulence factors |
| resistance | refers to the pathogens ability to withstand exposure to toxic substances, including antibiotics. |
| virulence factor | a substance produced by a pathogen that promotes its ability to cause disease |
Data and analyses
Comparison 1. Chronic infection: β‐carotene supplementation versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Days of antibiotic consumption | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Over 1 month and up to 3 months | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐8.00 [‐18.78, 2.78] |
| 1.2 Respiratory function absolute FEV1 % predicted up to 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Over 1 month and up to 3 months | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐10.90 [‐32.23, 10.43] |
| 1.3 Mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 Over 1 month and up to 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Chronic infection: garlic supplementation versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Respiratory function (% change FEV1) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Over 1 month and up to 3 months | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 1.59 [‐7.49, 10.67] |
| 2.2 Mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2.1 Over 1 month and up to 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Mild adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Diarrhoea | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.87 [0.25, 135.15] |
| 2.3.2 Halitosis | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.87 [0.25, 135.15] |
| 2.3.3 Abdominal pain | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.12, 87.13] |
| 2.3.4 Dysuria | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.12, 87.13] |
| 2.3.5 Minor haemoptysis | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 17.90] |
Comparison 3. Chronic infection: KB001‐A versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Pulmonary exacerbations | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.40] |
| 3.2 Decrease in respiratory function (FEV1) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.3 Number of participants experiencing an adverse event | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.90, 1.87] |
| 3.4 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.4.1 Bronchitis | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.07, 35.32] |
| 3.4.2 Sinusitis | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.92] |
| 3.5 Ear and labyrinth adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.5.1 Tinnitus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6 Gastrointestinal adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6.1 Abdominal pain upper | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6.2 Cheilitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6.3 Pancreatitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6.4 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7 General adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7.1 Chest discomfort | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7.2 Chills | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7.3 Crepitations | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7.4 Fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7.5 Infusion‐related reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7.6 Infusion site discolouration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7.7 Infusion site rash | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7.8 Irritability | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7.9 Pyrexia (fever) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.8 Infections and infestations | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.8.1 Sinusitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.9 Injury, poisoning and procedural complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.9.1 Procedural pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.9.2 Sunburn | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.10 Investigative adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.10.1 Blood pressure increased | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.10.2 Abnormal breath sounds | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.10.3 Increased heart rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.11 Nervous system adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.11.1 Dizziness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.11.2 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.11.3 Sinus headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.12 Psychiatric adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.12.1 Anxiety | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.12.2 Depression | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.12.3 Stress | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13 Respiratory, thoracic and mediastinal adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.1 Asthma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.2 Cough | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.3 Dysphonia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.4 Dyspnoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.5 Haemoptysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.6 Nasal congestion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.7 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.8 Pharyngolaryngeal pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.9 Productive cough | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.10 Rales | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.11 Respiratory tract congestion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.12 Rhinorrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.13 Sinus congestion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.14 Throat irritation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.13.15 Wheezing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.14 Skin and subcutaneous tissue adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.14.1 Oral pruritus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.14.2 Pruritus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.14.3 Urticaria | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.15 Vascular adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.15.1 Flushing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. Chronic infection: nitric oxide versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Change from baseline in FEV1 % predicted | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐1.95 [‐9.94, 6.04] |
| 4.1.1 Up to 1 month | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐1.95 [‐9.94, 6.04] |
| 4.2 Change from baseline in FVC % predicted | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐8.03 [‐18.50, 2.44] |
| 4.2.1 Up to 1 month | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐8.03 [‐18.50, 2.44] |
4.2. Analysis.

Comparison 4: Chronic infection: nitric oxide versus placebo, Outcome 2: Change from baseline in FVC % predicted
Comparison 5. Chronic infection: zinc supplementation versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Pulmonary exacerbations (number of participants requiring IV antibiotics) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 Up to 12 months | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.65, 5.26] |
| 5.2 Respiratory function (FVC %predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.2.1 Up to 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3 Antibiotic consumption (days oral antibiotics) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3.1 Up to 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.4 Antibiotic consumption (days IV antibiotics) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.4.1 Up to 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.5 Antibiotic consumption (days of oral and IV antibiotics) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.5.1 Up to 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.6 Respiratory function (FEV1 % predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.6.1 Up to 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.7 Adverse events (number of participants hospitalised in the study period) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.51, 2.97] |
| 5.7.1 Up to 12 months | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.51, 2.97] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdulhamid 2008.
| Study characteristics | ||
| Methods | Double‐blind placebo‐controlled RCT. Parallel design. Location: Michigan, USA. Duration: 12 months. |
|
| Participants | 26 children (aged 7 ‐ 18 years) with CF and mild to moderate lung disease without a concurrent acute severe infection were recruited. | |
| Interventions |
Intervention: zinc gluconate supplementation (30 mg daily dose). Control: placebo. |
|
| Outcomes | Outcomes not stratified. At each visit, the participant's interval medical history, height and weight, number of hospitalizations, the use of oral and intravenous antibiotics, pulmonary function test, and physical examination findings were recorded. Respiratory tract infections were also recorded at each visit based on reported criteria. Primary outcome: number of days of oral antibiotics during active infection per year. Secondary outcomes: production of inflammatory cytokines and IL‐2. |
|
| Notes | Dr Abdulhamid has kindly provided a per‐protocol analysis of the data with analysis limited to results of the treatment group compared to that of the placebo group. No specific comment on P aeruginosa infection. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not described |
| Blinding (performance bias and detection bias) PUFA first | Unclear risk | Both groups (treatment and placebo) were given capsules although these were not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 child withdrew (no reason provided) and does not contribute to the data analysis |
| Selective reporting (reporting bias) | Low risk | In the published trial, there is considerable selective reporting with the outcomes reported as per a subgroup analysis comparing those that were previously 'zinc adequate' or 'zinc inadequate'. The thresholds used to define zinc adequacy varied considerably to the 'clinically acceptable normal range'. Dr Abdulhamid has kindly provided the per‐protocol data with groups defined by their treatment group (placebo or zinc supplementation). After the provision of this additional information we therefore judge the risk of selective reporting to be low. |
| Other bias | Unclear risk | The compliance with treatment is reported as per the subgroup analysis and not as per protocol which further introduces uncertainty. |
Howlin 2017.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled RCT. Parallel design. Location: single centre in the UK. Duration: 20 days. |
|
| Participants | 12 adolescents and young adults with CF. Age, mean (SD): NO group 30 (13.99) years; placebo group 29.3 (15.6) years. Gender split: not stated Baseline disease status: FEV1 % predicted, mean (SD): NO group 40.2% (20.14); placebo group 45.7% (18.28). FVC % predicted, mean (SD): NO group 54.4% (17.6); placebo group: 71.5 (21.11). |
|
| Interventions |
Intervention group: NO gas (10 ppm; INOmax, 400 ppm mol/mol inhalation gas; INO Therapeutics) delivered via INOvent (Ikaria, supplied by INO Therapeutics), inhaled via nasal cannula for 8 h overnight for 5 – 7 days of therapy. Placebo group: air or air and oxygen mix, inhaled via nasal cannula for 8 h overnight for 5 – 7 days of therapy. Participants were followed up for the 7 days of therapy and at day 20. |
|
| Outcomes | Change from baseline in Ln CFU, FEV1 (% predicted), FVC (% predicted). Adverse events. Change in Paerugoinosa biofilm aggregates. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation with block lengths 2 and 4 was undertaken via an online randomisation service in a 1:1 ratio. |
| Allocation concealment (selection bias) | Low risk | Randomisation was undertaken via an online randomisation service to ensure concealment of treatment allocation. |
| Blinding (performance bias and detection bias) PUFA first | Low risk | Double‐blind trial; participants and outcome assessors were blinded to allocation. The placebo was administered via nasal cannula in the same way as the NO so that participants did not know whether they were getting NO or placebo. They also used sham weaning procedures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 participants were randomised and all completed the trial and were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Not all of the outcomes specified in the trial registration document and methods were reported in the results. In the methods the authors state they will measure QoL via CFQ‐UK but there is no mention of this in the results or in any of the tables. Authors state that it is presented in the supplementary tables but we have not been able to locate these. |
| Other bias | Unclear risk | None identified. |
Jain 2018.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, repeat‐dose clinical RCT. Parallel design. Location: multicentre in USA (58 centres), Australia (3 centres), Israel (4 centres), New Zealand (2 centres). Duration: 16 weeks. |
|
| Participants | 169 children and adults with a confirmed diagnosis of CF based on a sweat chloride > 60 mEq or 2 identifiable mutations consistent with CF (or both) and 1 or more clinical feature of CF. Infected with P aeruginosa. Age, mean (SD), median: KB001‐A group 29.1 (9.7) years, 28 years; placebo group: 29.8 (11.1) years, 28.5 years. Gender males/females (%): KB001‐A group 41 males (49.4%) and 42 females (50.6%); placebo group 42 males (48.8%) and 44 females (51.2%). Baseline FEV1 % predicted, mean (SD): KB001‐A group: 60.7% (15); placebo group: 61.4% (13.5). Antibiotic regimen, n (%): KB001‐A group 33 (39.8%) continuous and 50 (60.2%) intermittent; placebo group38 (44.2%) continuous and 48 (55.8%) intermittent. Baseline CFRSD‐CRISS, mean (SD): KB001‐A group 36.5 (9.7); placebo group: 37.2 (10.3). |
|
| Interventions |
Intervention group: KB001‐A up to 5 IV infusions of 10 mg/kg once every 4 weeks during the 16‐week treatment period with an additional loading dose at week 2. Placebo group: up to 5 IV infusions of once every 4 weeks during the 16‐week treatment period with an additional loading dose at week 2. Details of placebo not given. Trial duration included a 2‐week screening period, 16‐week treatment period and 4‐week follow‐up period. |
|
| Outcomes |
Primary outcome: time to need for antibiotics. Secondary outcomes: change from baseline in respiratory symptoms, BMI, weight, % predicted FEV1, change from baseline in inflammatory markers, change in bacterial density. Adverse events: treatment adverse events and other adverse events. |
|
| Notes | All participants continued their regularly scheduled inhaled antibiotic regimen (concurrently with the trial drug) for the first 4 weeks of treatment. After 4 weeks this scheduled maintenance therapy was discontinued. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drug assignment was conducted via interactive web response system. Randomised 1:1. |
| Allocation concealment (selection bias) | Low risk | Drug assignment was conducted via interactive web response system. |
| Blinding (performance bias and detection bias) PUFA first | Low risk | This is a double‐blind trial in which participants, trial personnel and outcome assessors were blinded. No information given on blinding of the trial drug. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A modified ITT analysis was used where any participant who had received 3 doses of the trial drug were included in the analysis. However, from the original 182 randomised, 5 withdrew from the placebo group before the start of treatment and 8 from the KB001‐A group. A further 15 from the placebo group and 21 from the KB001‐A group withdrew before the end of the trial. No reasons were given. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting bias found. |
| Other bias | Unclear risk | None identified. |
Milla 2013.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled single‐dose RCT. Parallel design (3‐armed). Location: 10 CF centres in the USA. Duration: single‐dose given on day 0 and followed up until day 56. |
|
| Participants | 27 participants (aged over 12 years) with P aeruginosa infection. KB001‐A 3 mg/kg n = 10; KB001‐A 10 mg/kg n = 8; placebo n = 9. Age, mean (range): 3 mg/kg 29.9 (17 – 58) years; 10 mg/kg group 27.8 (20 – 37) years; placebo 33.4 (19 – 55) years. Gender split, n (%): 3 mg/kg group 5 males (50%); 10 mg/kg group 6 males (75%); placebo 7 males (78%). Baseline FEV1 % predicted, mean (SD): 3 mg/kg group 75.2% (21.4); 10 mg/kg group 69.6% (19.7); placebo 67.8% (13.0). Baseline CFQ‐R respiratory symptom score, mean (SD): 3 mg/kg group 66.7 (7.4); 10 mg/kg group 75.0 (14.6); placebo 71.6 (15.3). Baseline sputum P aeruginosa density log10 CFU/g, mean (SD): 3 mg/kg group 8.00 (1.20); 10 mg/kg group 7.80 (0.73); placebo 7.33 (2.07). |
|
| Interventions | KB001‐A is a monoclonal antibody that works by inactivating the infection mechanism of P aeruginosa. Intervention 1: KB001‐A (3 mg/kg), single IV infusion given over 1 hour. Intervention 2: KB001‐A (10 mg/kg), single IV infusion given over 1 hour. Control: placebo (0.9 % sodium chloride), single IV infusion given over 1 hour. |
|
| Outcomes | Safety outcomes, adverse events, inflammatory markers, CFQ‐R respiratory domain scores, spirometry (FEV1, FEF25-75, FVC) Outcomes were reported at baseline and day 28 and day 56. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomized" but no detail provided. |
| Allocation concealment (selection bias) | Unclear risk | No detail provided. |
| Blinding (performance bias and detection bias) PUFA first | Low risk | Research teams and participants were blinded. Unblinded pharmacists prepared the infusion bags but labelled bags to maintain blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 27 participants randomised. 2 participants discontinued the trial, 1 from each of the KB001‐A groups (due to infusion related AE). A further 2 from the KB001‐A 3 mg/kg group were excluded from the analysis (received prohibited medications) and 1 from the placebo group for the same reason. All participants were included in the safety analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol deposited on clinicaltrials.gov. Primary outcomes are safety and tolerability which are reported. |
| Other bias | Unclear risk | The trial was sponsored by the pharmaceutical company developing KB001‐A. |
Renner 2001.
| Study characteristics | ||
| Methods | Double‐blind placebo‐controlled RCT. Parallel design. Location: single centre in Vienna, Austria. Duration: 6 months. |
|
| Participants | 24 participants with CF from 1 unit in Vienna; 13 randomised to supplementation group and 11 to placebo group. Age, mean (range): supplementation group 12.8 years (6.8 to 27.7); placebo group 10.5 years (6.7 to 17.3). Gender split: 18/24 female, 9 in supplementation group and 9 in placebo group. |
|
| Interventions |
Intervention: 'high dose supplementation' of β‐carotene at 1 mg/kg/day for 3 months followed by low‐dose regimen of 10 mg/day for 3 months. Control: starch‐containing placebo. |
|
| Outcomes | Primary outcomes: normalised plasma concentration of β‐carotene; number of pulmonary exacerbations, lung function. | |
| Notes | No specific data regarding bacteria presented ‐ author contacted for more information 26 Jan 2010. The control group was on average 2.3 years younger than the intervention group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Capsules were of identical appearance. Procedure for dispensing preparation unclear. |
| Blinding (performance bias and detection bias) PUFA first | Low risk | Described as 'double‐blind' but method not described. Capsules were of identical appearance. Rust 2000: Described as double blind but no further detail. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts. Two publications for this trial appear to report separate outcome data. |
| Selective reporting (reporting bias) | Low risk | Selective reporting not identified other than the separate reporting of different outcome data. |
| Other bias | Unclear risk | Participant compliance not reported. |
Sagel 2009.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled RCT. Parallel 3‐arm trial. Location: single centre, USA. Duration: 48 hours. |
|
| Participants | 18 participants aged 12 or over. Clinically stable with confirmed CF diagnosis Age, mean (SD): total cohort 16.7 years (3.40); low‐dose NO 16.9 years (2.14); high‐dose NO 15.6 years (2.33); placebo 17.7 years (5.14). Gender split: total cohort 13 males and 5 females; low‐dose NO 4 males and 2 females; high‐dose NO 4 males and 2 females; placebo 5 males and 1 female. |
|
| Interventions |
Low‐dose group: inhaled NO at 20 ppm via nasal cannula for 44 hours. High‐dose group: inhaled NO at 40 ppm via nasal cannula for 44 hours. Placebo: nitrogen at 20 ppm or 40 ppm via nasal cannula. |
|
| Outcomes | Respiratory function (FEV1), quantitative microbiology, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised trial but method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation method not described. |
| Blinding (performance bias and detection bias) PUFA first | Low risk | The trial was quadruple blinded i.e. participants, care providers, investigator, outcome assessor. NO and placebo were administered via nasal cannulae. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although all participants remained in the trial, 1 participant was given both NO and nitrogen and the authors state that they removed the data from their analysis. The data reported in the clinical trials registration document is for 18 participants therefore it is not clear whether the data were removed for this participant or not. |
| Selective reporting (reporting bias) | Unclear risk | The sputum inflammatory markers were not reported, but all other outcomes listed were reported. |
| Other bias | Unclear risk | None identified. |
Sharma 2016.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled RCT. Parallel design. Location: single centre, Northern India. Duration: 12 months. |
|
| Participants | 40 children aged 5 – 15 years. CF diagnosed by 2 abnormal sweat chloride values in the presence of physical symptoms. Age, median (IQR): zinc group 127.5 (100 – 150.5) months; placebo group 147.5 (126.5 – 162) months; P = 0.34. Gender split, n (%): zinc group 13 boys (65%); placebo group 11 boys (55%); P = 0.52. FEV1 % predicted at baseline, median (IQR): zinc group 58.4% (37.5% – 81.75%); placebo group 48.1% (41.1% – 72.5%); P = 0.84. FVC % predicted at baseline, median (IQR): zinc group 65.4% (52.5% – 80.05%); placebo group 65.05% (53.33% – 73.75%); P = 0.84. BMI Z score at baseline, median (IQR): zinc group ‐2.4 (‐2.75 to ‐1.37); placebo group ‐1.75 (‐2.74 to ‐0.53). Participants with zinc deficiency at baseline n (%): zinc group 15 (75); placebo group 18 (90). |
|
| Interventions |
Intervention: 1.5x 20 mg zinc tablet (giving 30 mg zinc) in 5 – 10 mL of water given orally once a day for 12 months. Control: placebo tablet given in the same way as active intervention for 12 months. Zinc tablets supplied by Bharat Immunologicals and Biologicals (Bulandshahr, India) a Government of India undertaking. |
|
| Outcomes |
Primary outcome: reduction in the average days of systemic antibiotics. Secondary outcomes: change in FEV1, rate of colonisation with PA, mortality, adverse events, pulmonary exacerbations requiring antibiotics. Timepoints: baseline, 3, 6, 9 and 12 months. |
|
| Notes | Children who received zinc in the month prior to enrolment and those not willing to attend for regular follow‐up were excluded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation described and was carried out by a person not involved in the trial. Computer‐generated randomisation. Block randomisation. |
| Allocation concealment (selection bias) | Low risk | The drugs were labelled by a person not involved in the trial. Strips were labelled as per the randomisation list. Sequentially numbered, sealed, opaque envelopes. |
| Blinding (performance bias and detection bias) PUFA first | Low risk | Double‐blind trial. Similar looking zinc or placebo tablets were administered to the children. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data analysed as ITT and per protocol. 3 participants withdrew consent (1 from the placebo group and 2 from the zinc group). |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods were reported in the results. |
| Other bias | Low risk | No other risk of bias found. |
Smyth 2010.
| Study characteristics | ||
| Methods | Pilot double‐blinded, placebo‐controlled RCT. Parallel design. Location: Nottingham, UK. Duration: 8 weeks. |
|
| Participants | 34 participants over 8 years of age with CF (definition given) and chronic pulmonary infection with P aeruginosa and at the time of randomisation. Age, median (range): 18 years (11– 54 years). Adult/child split: garlic group 8 adults (62%) and 5 children (38%); placebo group 9 adults (69%) and 4 children (31%). Gender split: 14/26 (54%) were male. Baseline FEV1 (L), mean (SD): garlic group 1.84 L (0.79); placebo group 1.91 L (1.05) Baseline FEV1 % predicted, mean (SD): garlic group 62.0% (18.4); placebo group 57.4% (26.1). Weight (kg), mean (SD): garlic group 46.7 kg (14.6); placebo group 54.2 kg (14.1). Baseline clinical score, median (range): garlic group 2 (0 to 6) 3 (0 to 6); placebo group: 4 (0 to 6). |
|
| Interventions |
Intervention: garlic capsule once daily (656 mg of garlic oil macerate and 10 mg cardamom oil). Control: placebo capsule once daily (656 mg of olive oil and 10 mg cardamom oil). |
|
| Outcomes |
Primary outcome: FEV1. Secondary outcomes: weight, clinical score, quorum sensing signal molecule level, number of courses of IV antibiotics over the 8‐week trial period. Measured at baseline and 8 weeks. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Procedure for dispensing preparation unclear ‐ unpublished details demonstrate the supplements were dispensed in coded sealed opaque containers. |
| Blinding (performance bias and detection bias) PUFA first | Low risk | Double‐blind, identical placebo. The cardamom oil was present as an odour control agent. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 participants withdrew ‐ four in each group: Garlic ‐ 1 received lung transplant, 1 forgot to take capsules, 1 side effect of indigestion, 1 could not attend second visit; Placebo ‐ 2 forgot to take capsules, 1 side effect of halitosis, 1 could not attend visit 2. Analysis was per protocol. |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified. |
| Other bias | Low risk | No other bias identified. |
BMI: body mass index CF: cystic fibrosis CFQ‐R: cystic fibrosis questionnaire revised CFU: colony forming units FEF25-75: mid peak expiratory volume FEV1: forced expiratory volume at 1 second FVC: forced vital capacity ITT: intention‐to‐treat IV: intravenous
Ln: natural logarithm NO: nitric oxide P aeruginosa: Pseudomonas aeruginosa QoL: quality of life SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvarez 2017 | Not an antibiotic adjuvant ‐ vitamin D supplementation. |
| Brown 1985 | Cross‐over RCT but authors have confirmed that data from 1st phase are unavailable. |
| DiMango 2014 | Effect of proton pump inhibition to prevent acid aspiration ‐ not a trial of an adjuvant. |
| Durairaj 2007 | Safety study therefore not blinded, randomised or controlled. |
| Forrester 2015 | Not an antibiotic adjuvant ‐ direct bactericidal activity expected. |
| Gontijo‐Amaral 2012 | Examination of the effect of magnesium on respiratory muscle strength was the objective of the trial and so not an adjuvant. |
| Grasemann 2013 | Effect of arginine on smooth muscle tone (via nitric oxide) is the target of the trial and so not an adjuvant. |
| Hauber 2008 | Lacked a control group that did not receive examined intervention. |
| Hodges 2014 | Trial of the distribution of nebulised solutions in the lungs not as an adjuvant therapy. |
| Homnick 1995 | Non‐randomised study. |
| Kollberg 2003 | Non‐randomised study, immunotherapy. |
| Kollberg 2010 | Immunotherapy not an antibiotic adjuvant therapy. |
| Kutateladze 2008 | Single patient experience described. |
| Lands 2010 | Non‐randomised study. |
| Leonard 2012 | Trial of oral miglustat ‐ not an antibiotic adjuvant therapy. |
| Middleton 2015 | Trial of airway clearance adjunct, not an antibiotic adjuvant therapy. |
| Moss 2013 | Immunotherapy not an antibiotic adjuvant therapy. |
| NCT00742092 | Trial of nasal miglustat ‐ not an antibiotic adjuvant therapy. |
| NCT01455675 | Immunotherapy not an antibiotic adjuvant therapy. |
| Olveira 2010 | The fatty acid supplement exerts an anti‐inflammatory effect and does not meet the definition of antibiotic adjuvant. |
| Panchaud 2006 | Independent anti‐inflammatory effect ‐ does not meet the definition of antibiotic adjuvant. |
| Safai‐Kutti 1991 | Placebo‐controlled, double‐blind, cross‐over RCT of zinc supplementation, but no 1st arm data available. |
| Sagel 2011 | Non‐randomised, open‐label study. |
| Sagel 2018 | Not an antibiotic adjuvant ‐ multivitamin with antioxidant for antioxidant concentrations, inflammation and oxidative stress. |
| Tangpricha 2017 | Not an antibiotic adjuvant ‐ vitamin D for improving immune response. |
| Winklhofer‐Roob 1995 | Non‐randomised, healthy controls. |
| Winnie 1989 | Immunotherapy not an antibiotic adjuvant therapy. |
RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
CARE‐CF‐1.
| Methods | Multicentre, double‐blind, placebo‐controlled RCT. 6‐arm. |
| Participants | 89 adults with CF experiencing acute exacerbations. Age, mean (SD): 29.8 years (9.6). Gender split: 48% female. FEV1 % predicted, mean (SD): 43.3% (18.3) predicted. |
| Interventions | Oral cysteamine (Lynovex®) as an adjunct to conventional treatment over 14 days. Intervention arm 1: 450 mg 4x daily. Intervention arm 2: 150 mg 3x daily. Intervention arm 3: 450 mg 2x daily. Intervention arm 4: 300 mg 3x daily. Intervention arm 5: 450 mg 3x daily. Intervention arm 6: placebo. |
| Outcomes | Change from baseline in sputum gram‐negative bacterial load. Changes in patient‐reported outcome measures from baseline: assessment using the CF respiratory symptom diary; chronic respiratory infection symptom score (CFRSD‐CRSS); CFQ‐R; and Jarad and Sequeiros Symptom Score Questionnaire. Blood leukocyte count. Change from baseline in sputum neutrophil elastase and IL‐8 levels. Change in FEV1, weight and BMI. Adverse events and serious adverse events. |
| Notes | The trial looks at the safety and efficacy of oral cysteamine in addition to finding the most important patient‐reported outcomes. Awaiting full publication of efficacy trial. |
Khorasani 2009.
| Methods | Double‐blind placebo‐controlled trial. Unclear if participants were randomised. |
| Participants | 20 children aged between 7 and 18 years with CF. |
| Interventions | Daily 5 mg/kg (max 30 mg) elemental zinc for 1 year. |
| Outcomes | Plasma zinc, pulmonary function tests, rate of respiratory infections and use of antibiotics measured at baseline and end of the trial. |
| Notes | Unclear whether this trial was randomised as it is only an abstract and no detail is given. |
Puvvadi 2019.
| Methods | Placebo‐controlled RCT. |
| Participants | 24 people with CF with chronic P aeruginosa infection. |
| Interventions | Standard IV antibiotic treatment. Intervention: 5 mg CaEDTA (n = 12). Placebo: saline (n = 12). Both interventions given 4x daily for 2 weeks (while on IV antibiotics) and 2x daily for the following 4 weeks, with a 4‐week safety follow‐up period subsequently. |
| Outcomes | Mean P aeruginosa sputum count (log10 CFU/g). Lung function (FEV1 % predicted). Adverse events. |
| Notes | Randomisation process is unclear ‐ abstract only. |
Rye 2015.
| Methods | A double‐blind, placebo‐controlled cross‐over RCT. |
| Participants | 12 participants with CF with chronic Burkholderia sp. infection. |
| Interventions | 28 days treatment with OligoG as dry powder for inhalation. |
| Outcomes | Lung function, quality of life, sputum rheology and other microbiological outcome measures. |
| Notes | Presented in abstract form as a placeholder abstract without data. Cross‐over trial and so we had planned to only consider the first treatment phase of a cross‐over trial and did not include such trials in full until such time that duration of action detail is understood, a washout period is provided, measures of treatment effect are available and the data are available from each treatment phase ‐ awaiting classification until such time as individual‐patient data and measure of duration of treatment effect are provided. Email sent to AlgiPharma and PI 17/05/2017. |
Walshaw 2014.
| Methods | Double‐blind (participant, caregiver, investigator, outcomes assessor), randomised, placebo‐controlled, cross‐over trial. |
| Participants | 26 participants aged 18 years and older with a positive microbiological finding of P aeruginosa in expectorated sputum or cough swab documented within 24 months prior to screening. The FEV1 at screening was between 35% ‐ 80% of the predicted normal value following adjustment for age, gender, and height according to the Knudson equation. Those with ABPA, an isolate of Burkholderia species, in the last year, or those who had, or were considering transplantation were excluded. Also those who had commenced inhaled TOBI in the previous 4 months were excluded. |
| Interventions |
Intervention: inhaled 6% OligoG. Control: 0.9% saline. |
| Outcomes |
Primary outcome: safety and local tolerability of multiple dose administration of inhaled OligoG in people with CF. Secondary outcome: effect of multiple dose administration of inhaled OligoG on various efficacy variables. |
| Notes | Primary completion date: July 2012. Cross‐over trial and so we had planned to only consider the 1st treatment phase of a cross‐over trial and did not include such trials in full until such time that duration of action detail is understood, a washout period is provided, measures of treatment effect are available and the data are available from each treatment phase ‐ awaiting classification until such time as IPD and measure of duration of treatment effect are provided. Email sent to AlgiPharma and primary investigator July 2019. |
Zabner 2009.
| Methods | Double‐blind (participant, caregiver, investigator), parallel assignment, safety/efficacy RCT. |
| Participants | People with CF aged 16 years or older. |
| Interventions |
Intervention: hypertonic xylitol. Control: hypertonic saline. |
| Outcomes | Primary outcome measures: FEV1 change from baseline, adverse events and respiratory symptom score at 14 days. Secondary outcome measures: density of colonization/g of sputum, time to next exacerbation, sputum cytokines and revised CF QoL questionnaire at 14 days. |
| Notes | Marked as completed on clinicaltrials.gov. |
CaEDTA: calcium ethylene diamine tetra‐acetate CF: cystic fibrosis IPD: individual patient data QoL: quality of life RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Pressler 2017.
| Study name | A Phase IIb Study of OligoG in Subjects With Cystic Fibrosis (SMR‐2984). |
| Methods | Double‐blind, placebo‐controlled cross‐over RCT. |
| Participants | 76 adults with CF and P aeruginosa in expectorated sputum or cough swab within 24 months prior to screening. FEV1 between 40% ‐ 100% and no clinical or laboratory findings suggestive of significant pulmonary illness, other than CF. |
| Interventions |
Intervention 1: inhalation of a dry powder OligoG in the 1st treatment period, and of placebo in the 2nd period. Intervention 2: inhalation of placebo in the 1st treatment period, and of a dry powder OligoG in the 2nd period. |
| Outcomes | FEV1; mucociliary clearance, rheology, microbiology and QoL. |
| Starting date | October 2014. |
| Contact information | |
| Notes | Estimated trial completion date: April 2017. |
CF: cystic fibrosis FEV1: forced expiratory volume at 1 second P aeruginosa: Pseudomonas aeruginosa QoL: quality of life RCT: randomised controlled trial
Differences between protocol and review
We altered the title of the review to be more informative ‐ changing 'non‐antibiotic' terminology to a new term ‐ antibiotic adjuvants.
We have altered the definition of chronic infection to a definition that is most commonly used in routine clinical practice (Lee 2004) and have added requirement of antibiotics (days) as a secondary outcome measure.
We restated the outcome measures in line with current MECIR guidelines.
Contributions of authors
For the original review
MH, DF and AS conceived the title. MH wrote the protocol, completed a search of the literature, was an independent assessor of the trials identified and drafted the review. DF was the second independent assessor of the trials identified from the literature search and commented upon the drafts of the protocol and the substantive review. AS supervised, gave comments and advised on the protocol and review.
MH acts as guarantor of the review.
For the 2020 update
Selection of trials and data extraction were performed by MH and SS for newly identified references. SS created summary of findings tables, added in the extra data from the new trials and updated the text of the review.
MH acts as guarantor of the review.
Sources of support
Internal sources
-
Nottingham Respiratory Biomedical Research Unit, UK
MH and DF are funded by the Nottingham Respiratory BRU
External sources
-
National Institute for Health Research, UK
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Dr Matthew Hurley Dr Hurley has no conflicts of interest to declare.
Sherie Smith Sherie Smith has no conflicts of interest to declare.
Dr Doug Forrester Dr Doug Forrester declares he has received support from Wellcome Trust as a Wellcome Trust Clinical Research Training Fellow, travel support from Vertex Pharmaceuticals and GSK and consultancy fees from Mologic.
Professor Alan Smyth Professor Smyth is lead investigator on one of the trials included in the review (Smyth 2010). He further declares relevant activities of lectures paid for by Teva and Novartis. He is affiliated to a research group which holds a patent: "ALKYL QUINOLONES AS BIOMARKERS OF PSEUDOMONAS AERUGINOSA INFECTION AND USES THEREOF".
Edited (no change to conclusions)
References
References to studies included in this review
Abdulhamid 2008 {published and unpublished data}
- Abdulhamid I, Beck FW, Millard S, Chen X, Prasad A. Effect of zinc supplementation on respiratory tract infections in children with cystic fibrosis. Pediatric Pulmonology 2008;43(3):281-7. [CFGD REGISTER: GN111b] [DOI: 10.1002/ppul.20771] [DOI] [PubMed] [Google Scholar]
- Abdulhamid I, Millard S, Beck F, Chen X, Van Wagnen C, Prasad A. Effect of zinc supplementation on respiratory tract infections in children with cystic fibrosis. Pediatric Pulmonology 2005;40 Suppl 28:348. [DOI] [PubMed] [Google Scholar]
Howlin 2017 {published data only}
- Cathie K, Howlin R, Barraud N, Carroll M, Clarke S, Connett G, et al. Low dose nitric oxide as adjunctive therapy to reduce antimicrobial tolerance of pseudomonas aeruginosa biofilms in the treatment of patients with cystic fibrosis: Report of a proof of concept clinical trial. American Journal of Respiratory and Critical Care Medicine 2014;189:A2843. [Google Scholar]
- Cathie K, Howlin R, Carroll M, Clarke S, Connett G, Cornelius V, et al. RATNO-reducing antibiotic tolerance using nitric oxide in cystic fibrosis: Report of a proof of concept clinical trial. Archives of disease in childhood 2014;99:A159. [Google Scholar]
- Howlin RP, Cathie K, Hall-Stoodley L, Cornelius V, Duignan C, Allan RN, et al. Low-dose nitric oxide as targeted anti-biofilm adjunctive therapy to treat chronic Pseudomonas aeruginosa infection in cystic fibrosis. Molecular Therapy 2017;25(9):2104-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jain 2018 {published data only}
- Chmiel J, Hamblett NM, Geller D, Konstan M, VanDevanter D, Thompson V, et al. A phase 2, 16-week, randomized, double-blind, placebo-controlled study to assess safety, tolerability and efficacy of repeated doses of KB001-A in subjects infected with P. aeruginosa. Pediatric Pulmonology 2015;50 Suppl 41:272. [ABSTRACT NO.: 218] [CENTRAL: 1092183] [CFGD REGISTER: PI287] [Google Scholar]
- Jain R, Beckett VV, Konstan MW, Accurso FJ, Burns JL, Mayer-Hamblett N, et al. KB001-A, a novel anti-inflammatory, found to be safe and well-tolerated in cystic fibrosis patients infected with Pseudomonas aeruginosa. Journal of Cystic Fibrosis 2018;17(4):484-91. [DOI] [PubMed] [Google Scholar]
- NCT01695343. Study to evaluate the effect of KB001-A on time-to-need for antibiotic treatment (KB001-A). www.clinicaltrials.gov/ct2/show/NCT01695343 (first received 27 September 2012).
Milla 2013 {published and unpublished data}NCT00638365
- Milla C, Accurso F, Chmiel J, McCoy K, Billings J, Atkinson J, et al. A phase I/II study of the anti-PcrV antibody KB001 in cystic fibrosis patients with pseudomonas aeruginosa. American Journal of Respiratory and Critical Care Medicine 2009;179:A1190. [CFGD REGISTER: PI267d] [Google Scholar]
- Milla C, Chmiel J, McCoy KS, Accurso FJ, Billings J, Boyle MP, et al. A phase 1/2 randomized, double-blind, placebo-controlled, single-dose, dose escalation study of KB001 in cystic fibrosis patients infected with pseudomonas aeruginosa. Pediatric Pulmonology 2008;43 Suppl 31:341. [ABSTRACT NO.: 392] [CENTRAL: 921645] [CFGD REGISTER: PI267b ] [Google Scholar]
- Milla C, Chmiel JF, Accurso FJ, McCoy KS, Billings JL, Atkinson JJ, et al. Anti-inflammatory effect of KB001, an anti-PCRV antibody fragment, in CF patients chronically infected with pseudomonas aeruginosa. Pediatric Pulmonology 2009;44 Suppl 32:341. [ABSTRACT NO.: 369] [CENTRAL: 921646] [CFGD REGISTER: PI267c ] [Google Scholar]
- Milla CE, Accurso F J, Chmiel J, McCoy K, Billings J, Atkinson JJ, et al. Modulating Pseudomonas aeruginosa chronic Inflammation with the anti-PcrV antibody KB001: Results of a pilot clinical and pharmacodynamic study In subjects with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A1845. [CENTRAL: 758759] [CFGD REGISTER: PI267g] [Google Scholar]
- Milla CE, Chmiel JF, Accurso FJ, Vandevanter DR, Konstan MW, Yarranton G, et al. Anti-PcrV antibody in cystic fibrosis: a novel approach targeting Pseudomonas aeruginosa airway infection. Pediatric Pulmonology 2014;49(7):650-8. [CENTRAL: 995342] [CFGD REGISTER: PI267e] [EMBASE: 2014431697] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00638365. Dose escalation study of KB001 in cystic fibrosis patients infected with Pseudomonas aeruginosa [A phase I/II randomized, double-blind, placebo-controlled, single-dose, dose escalation study of KB001 in cystic fibrosis patients infected with Pseudomonas aeruginosa ]. clinicaltrials.gov/ct2/show/NCT00638365 (first received 19 March 2008). [CFGD REGISTER: PI267a]
Renner 2001 {published data only}
- Engl B, Rust P, Eichler I, Renner S, Elmadfa I. Bioavailability of therapeutic Beta-Carotine (BC) in patients with cystic fibrosis (CF) and effects on anthropometrical parameters over 6 months. Monatsschrift fur Kinderheilkunde 1997;145:S 134. [CFGD REGISTER: GN69g] [Google Scholar]
- Renner S, Rath R, Rust P, Lehr S, Frischer T, Elmadfa I, et al. Effects of beta-carotene supplementation for six months on clinical and laboratory parameters in patients with cystic fibrosis. Thorax 2001;56(1):48-52. [CFGD REGISTER: GN69f] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner S, Wojnarowski C, Koller DY, Rust P, Elmadfa I, Eichler I. Patients with cystic fibrosis (CF) benefit from β-Carotene supplementation for 6 months. In: 22nd European Cystic Fibrosis Conference; 1998 June 13-19; Berlin, Germany. 1998:97. [CFGD REGISTER: GN69b]
- Renner S, Wojnarowski C, Koller DY, Rust P, Elmadfa I, Eichler I. Patients with cystic fibrosis (CF) benefit from β-Carotene supplementation for 6 months. Pediatric Pulmonology 1997;24(Suppl 15):314. [CFGD REGISTER: GN69a] [Google Scholar]
- Rust P, Eichler I, Elmadfa I. Influence of an oral beta-carotene supplementation on the antioxidant status of patients with cystic fibrosis. Atemwegs und Lungenkrankheiten 1997;23:51. [CFGD REGISTER: GN69a] [Google Scholar]
- Rust P, Eichler I, Renner S, Elmadfa I. Effects of long-term oral beta-carotene supplementation on lipid peroxidation in patients with cystic fibrosis. International Journal for Vitamin and Nutrition Research 1998;68(2):83-7. [CFGD REGISTER: GN69b] [PubMed] [Google Scholar]
- Rust P, Eichler I, Renner S, Elmadfa I. Long term beta-carotene supplementation in patients with cystic fibrosis - effects on oxidative status and pulmonary function. Annals of Nutrition and Metabolism 2000;44(1):30-7. [CFGD REGISTER: GN69e] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sagel 2009 {published data only}
- NCT00570349. Safety and Tolerability of Inhaled Nitric Oxide in Patients with Cystic Fibrosis. www.clinicaltrials.gov/ct2/show/NCT00570349 (first received 10 December 2007).
- Sagel SD, Monchil L, Parker D, Emmett P, Wagner B, Abman S. Safety and antimicrobial effects of inhaled nitric oxide in CF: a pilot study. Pediatric Pulmonology 2009;44 Suppl 32:299. [ABSTRACT NO.: 251] [CENTRAL: 921940] [CFGD REGISTER: OV21] [Google Scholar]
Sharma 2016 {published data only}
- Kabra SK. Effect of zinc supplementation in children with cystic fibrosis. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=3508&EncHid=&userName=zinc (first received 14 December 2011).
- Sharma G, Lodha R, Shastri S, Saini S, Kapil A, Singla M, et al. Zinc supplementation for one year among children with cystic fibrosis does not decrease pulmonary infection. Respiratory Care 2016;61(1):78-84. [CFGD REGISTER: GN256] [PMID: ] [DOI] [PubMed] [Google Scholar]
Smyth 2010 {published data only}
- Smyth A, Cifelli P, Lewis S, Erskine P, Holland E, Willaims P, et al. Quorum sensing molecules in sputum and plasma as biomarkers in patients with CF and chronic pseudomonas aeruginosa. Pediatric Pulmonology 2008;43(Suppl 31):337. [CFGD REGISTER: PI238c] [Google Scholar]
- Smyth A, Cifelli P, Lewis S, Erskine P, Holland E, Williams P, et al. A randomized controlled trial of macerated garlic oil in patients with CF and chronic pseudomonas aeruginosa. Pediatric Pulmonology 2008;43(Suppl 31):336. [CFGD REGISTER: PI238b] [Google Scholar]
- Smyth A. The Garlic Against Pseudomonas (GAP) study [A randomised controlled trial (pilot study) of the use of macerated garlic in patients with cystic fibrosis who have pulmonary infection with Pseudomonas aeruginosa]. www.isrctn.com/ISRCTN21133397 (first received 01 April 2007). [CFGD REGISTER: PI238a] [DOI: 10.1186/ISRCTN21133397] [DOI]
- Smyth AR, Cifelli PM, Ortori CA, Righetti K, Lewis S, Erskine P, et al. Garlic as an inhibitor of Pseudomonas aeruginosa quorum sensing in cystic fibrosis – a pilot randomised controlled trial. Pediatric Pulmonology 2010;45(4):356-62. [CFGD REGISTER: PI238d] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alvarez 2017 {published data only}
- Alvarez JA, Chong EY, Walker DI, Chandler JD, Michalski ES, Grossmann RE, et al. Corrigendum to "Plasma metabolomics in adults with cystic fibrosis during a pulmonary exacerbation: a pilot randomized study of high-dose vitamin D3 administration". Metabolism: Clinical and Experimental 2017;74:41-2. [CFGD REGISTER: GN217h] [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Chong EY, Walker DI, Chandler JD, Michalski ES, Grossmann RE, et al. Plasma metabolomics in adults with cystic fibrosis during a pulmonary exacerbation: a pilot randomized study of high-dose vitamin D3 administration. Metabolism: Clinical and Experimental 2017;70:31-41. [CFGD REGISTER: GN217f] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JA, Smith EM, Jones DP, Grossman RE, Frediani JK, Uppal K, et al. Discovery-based, high resolution plasma metabolomics following a vitamin D3 intervention in adult patients with cystic fibrosis. Pediatric Pulmonology 2014;49 Suppl 38:418-9. [ABSTRACT NO.: 551] [CFGD REGISTER: GN217g] [Google Scholar]
- Grossmann RE, Zughaier S, Kumari M, Seydafkan S, Liu S, Lyles R, et al. Clinical responses to a novel vitamin D supplementation strategy in adult CF patients hospitalized for pulmonary exacerbations. Pediatric Pulmonology 2011;46 Suppl 34:404. [ABSTRACT NO.: 526] [CFGD REGISTER: GN217b] [Google Scholar]
- Grossmann RE, Zughaier SM, Kumari M, Seydafkan S, Lyles RH, Liu S, et al. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: a randomized, controlled trial. Dermato-endocrinology 2012;4(2):191-7. [CFGD REGISTER: GN217d] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann RE, Zughaier SM, Liu S, Lyles RH, Tangpricha V. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. European Journal of Clinical Nutrition 2012;66(9):1072-4. [CFGD REGISTER: GN217c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann RE, Zughaier SM, Lyles RH, Liu S, Sueblinvong V, Schechter MS, et al. High dose vitamin D is associated with decreased inflammatory markers and increased number of hospital-free days in adults with cystic fibrosis: A pilot study. FASEB journal 2012;26 Suppl:no pagination. [CFGD REGISTER: GN217e] [Google Scholar]
- Kumari M, Colman L, Grossmann R, Wolfenden LL, Tangpricha V. High dose vitamin D supplements in cystic fibrosis patients hospitalized for respiratory exacerbation. Pediatric Pulmonology 2009;44 Suppl 32:409. [ABSTRACT NO.: 549] [CFGD REGISTER: GN217a] [Google Scholar]
Brown 1985 {published data only}
- Brown J, Mellis CM, Wood RE. EDTA aerosol in pseudomonal lung infection. In: 9th International Cystic Fibrosis Congress; 1984 June 9-15; Brighton, England. 1984:4.10. [CFGD REGISTER: PI235a]
- Brown J, Mellis CM, Wood RE. Edetate sodium aerosol in Pseudomonas lung infection in cystic fibrosis. American Journal of Diseases of Children 1985;139(8):836-9. [CFGD REGISTER: PI235b] [DOI] [PubMed] [Google Scholar]
DiMango 2014 {published data only}
- DiMango E, Walker P, Keating C, Berdella M, Robinson B, Langfelder-Schwind E, et al. Effect of proton pump inhibitors on cystic fibrosis (CF) pulmonary exacerbations in adults [abstract]. Pediatric Pulmonology 2012;47 Suppl 35:353, Abstract no: 362. [CENTRAL: 962121] [CFGD REGISTER: GN235a] [Google Scholar]
- Dimango E, Walker P, Keating C, Berdella M, Robinson N, Langfelder-Schwind E, et al. Effect of esomeprazole versus placebo on pulmonary exacerbations in cystic fibrosis. BMC Pulmonary Medicine 2014;14:21. [CFGD REGISTER: GN235b] [CTG: ] [GR: P30ES009089/ES/NIEHS NIH HHS/United States] [JID: 100968563] [PMCID: PMC3931289] [PMID: ] [UL1: TR000040/TR/NCATS NIH HHS/United States] [DOI] [PMC free article] [PubMed] [Google Scholar]
Durairaj 2007 {published data only}
- Durairaj L, Launspach J, Watt JL, Mohamad Z, Kline J, Zabner J. Safety assessment of inhaled xylitol in subjects with cystic fibrosis. Journal of Cystic Fibrosis 2007 Jan;6(1):31-4. [DOI] [PubMed] [Google Scholar]
Forrester 2015 {published data only}ISRCTN22534872
- Forrester D, Knox A, Smyth A, Barr H, Simms R, Pacey S, et al. Glutamine supplementation for cystic fibrosis - a randomised controlled trial. Pediatric Pulmonology 2011;46 Suppl 34:288. [ABSTRACT NO.: 214] [CENTRAL: 867950] [CFGD REGISTER: GN231a] [DOI] [PubMed] [Google Scholar]
- Forrester DL, Knox AJ, Smyth AR, Barr HL, Simms R, Pacey SJ, et al. Glutamine supplementation in cystic fibrosis: A randomized placebo-controlled trial. Pediatric Pulmonology 2015;51:253-7. [CFGD REGISTER: GN231b] [DOI: 10.1002/ppul.23370] [DOI] [PubMed] [Google Scholar]
Gontijo‐Amaral 2012 {published data only}
- Gontijo-Amaral C, Guimarães EV, Camargos P. Oral magnesium supplementation in children with cystic fibrosis improves clinical and functional variables: a double-blind, randomized, placebo-controlled crossover trial. American Journal of Clinical Nutrition 2012;96(1):50-6. [CENTRAL: 880141] [CFGD REGISTER: GN241] [DOI: 10.3945/ajcn.112.034207] [PMID: ] [DOI] [PubMed] [Google Scholar]
Grasemann 2013 {published data only}
- Grasemann H, Tullis E, Ratjen F. A randomized placebo controlled study on the effects of L-arginine inhalation in patients with cystic fibrosis. Pediatric Pulmonology 2011;46 Suppl 34:293. [ABSTRACT NO.: 229] [CENTRAL: 921615] [CFGD REGISTER: GN233a] [DOI: 10.1016/j.jcf.2012.12.008] [DOI] [Google Scholar]
- Grasemann H, Tullis E, Ratjen F. Inhaled L-arginine in patients with cystic fibrosis - a randomized controlled trial. Journal of Cystic Fibrosis 2011;10 Suppl 1:S21. [ABSTRACT NO.: 81] [CENTRAL: 871494] [CFGD REGISTER: GN233b] [DOI] [PubMed] [Google Scholar]
Hauber 2008 {published data only}
- Hauber H, Schulz M, Pforte A, Mack D, Zabel P, Schumacher U. Inhalation with fucose and galactose for treatment of Pseudomonas aeruginosa in cystic fibrosis patients. International Journal of Medical Sciences 2008;5(6):371-6. [CFGD REGISTER: PI268a] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pforte A, Hauber H P, Mack D, Schumacher U. Inhalation with Fucose and Galactose a new therapeutic approach in adult cystic fibrosis patients with Pseudomonas aeruginosa-Infection [Inhalation mit Fucose und Galactose als neuer Therapieansatz bei erwachsenen Mukoviszidosepatienten mit Pseudomonas aeruginosa-Infektion]. Pneumologie (Stuttgart, Germany) 2001;55(SH1):S68. [CENTRAL: 402236] [CFGD REGISTER: PI268b] [Google Scholar]
Hodges 2014 {published data only}
- Hodges L, MacGregor G, Stevens H, Dessen A, Myrset A. An open label, randomised, two-way crossover scintigraphic study to investigate lung deposition of radiolabelled alginate oligosaccharide delivered as a dry powder and as a nebulised solution in cystic fibrosis patients. Pediatric Pulmonology 2014;49 Suppl 38:305. [ABSTRACT NO.: 251] [CENTRAL: 1012384] [CFGD REGISTER: BD215] [Google Scholar]
Homnick 1995 {published data only}
- Homnick DN, Spillers CR, Cox SR, Cox JH, Yelton LA, DeLoof MJ, et al. Single- and multiple-dose-response relationships of beta-carotene in cystic fibrosis [see comment]. Journal of Pediatrics 1995;127(3):491-4. [CFGD REGISTER: GN68] [DOI] [PubMed] [Google Scholar]
Kollberg 2003 {published data only}
- Kollberg H, Carlander D, Olesen H, Wejaker PE, Johannesson M, Larsson A. Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: a phase I feasibility study. Pediatric Pulmonology 2003;35(6):433-40. [DOI] [PubMed] [Google Scholar]
- Nilsson E, Kollberg H, Johannesson M, Wejaker PE, Carlander D, Larsson A. More than 10 years' continuous oral treatment with specific immunoglobulin Y for the prevention of Pseudomonas aeruginosa infections: a case report. Journal of Medicinal Food 2007;10(2):375-8. [DOI] [PubMed] [Google Scholar]
- Nilsson E, Larsson A, Olesen HV, Wejaker PE, Kollberg H. Good effect of IgY against Pseudomonas aeruginosa infections in cystic fibrosis patients. Pediatric Pulmonology 2008;43(9):892-9. [DOI] [PubMed] [Google Scholar]
Kollberg 2010 {published data only}
- Kollberg H, Larsson A, Nilsson E. Anti-pseudomonas IGY ready for phase III. Pediatric Pulmonology 2010;45(Suppl 33):343. [ABSTRACT NO.: 345] [CFGD REGISTER: PI252a] [Google Scholar]
- Larsson A. Phase III study (IMPACTT) on anti-pseudomonas IgY. Journal of Cystic Fibrosis 2011;10(Suppl 1):S24. [ABSTRACT NO.: 92] [CFGD REGISTER: PI252b] [Google Scholar]
- Schuster A, Bend J, Hoiby N, Verde PE, Rottmann A, Larsson A, et al. Clinical study to evaluate an anti-Pseudomonas aeruginosa IgY gargling solution (EUDRACT 2011-000801-39). Journal of Cystic Fibrosis 2019;18:S23. [ABSTRACT NO.: WS12-5] [CENTRAL: CN-01990653] [CFGD REGISTER: PI252d] [EMBASE: 2001976053] [Google Scholar]
Kutateladze 2008 {published data only}
- Kutateladze M, Adamia R. Phage therapy experience at the Eliava Institute. Medecine et Maladies Infectieuses 2008;38(8):426-30. [DOI] [PubMed] [Google Scholar]
Lands 2010 {published data only}
- Lands LC, Iskandar M, Beaudoin N, Meehan B, Dauletbaev N, Berthiuame Y. Dietary supplementation with pressurized whey in patients with cystic fibrosis. Journal of Medicinal Food 2010;13(1):77-82. [DOI: 10.1089/jmf.2008.0326] [DOI] [PubMed] [Google Scholar]
Leonard 2012 {published data only}NCT00945347
- Leonard A, Dingemanse J, Lebecque P, Leal T. Oral miglustat in homozygous F508del CF patients. Journal of Cystic Fibrosis 2010;9 Suppl 1:S20. [ABSTRACT NO.: 75] [CFGD REGISTER: BD193a] [Google Scholar]
- Leonard A, Lebecque P, Dingemanse J, Leal T. A randomized placebo-controlled trial of miglustat in cystic fibrosis based on nasal potential difference. Journal of Cystic Fibrosis 2012;11(3):231-6. [CFGD REGISTER: BD193b] [DOI: 10.1016/j.jcf.2011.12.004] [DOI] [PubMed] [Google Scholar]
Middleton 2015 {published data only}
- Middleton A, Robinson P, McKay K, Selvadurai H. Pilot study of inhaled dry powder mannitol in young people with CF hospitalised with pulmonary exacerbation. Pediatric Pulmonology 2012;47 Suppl 35:355. [ABSTRACT NO.: 368] [CENTRAL: 962113] [CFGD REGISTER: BD199a] [Google Scholar]
- Middleton A, Robinson P, McKay K, Selvadurai H. Pilot study of inhaled dry powder mannitol in young people with cystic fibrosis hospitalised with pulmonary exacerbation. Department of Respiratory Medicine, Children's Hospital at Westmead (www.thoracic.org.au) (accessed 21 Oct 2014) 2014. [CENTRAL: 1012538] [CFGD REGISTER: BD199b]
- Middleton A, Robinson PD, McKay K, Jaffe A, Selvadurai H. A pilot study of inhaled dry-powder mannitol during cystic fibrosis-related pulmonary exacerbation [letter]. European Respiratory Journal 2015;45(2):541-4. [CENTRAL: 1048444] [CFGD REGISTER: BD199c] [EMBASE: 2015713766] [DOI] [PubMed] [Google Scholar]
Moss 2013 {published data only}
- Moss RB, Mistry SJ, Konstan MW, Pilewski JM, Kerem E, Tal-Singer R, et al. Online Supplement to: "Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis". Journal of Cystic Fibrosis 2013;13(3):241-8. [CENTRAL: 1000130] [CFGD REGISTER: PI275b] [NCT00903201] [DOI] [PubMed] [Google Scholar]
- Moss RB, Mistry SJ, Konstan MW, Pilewski JM, Kerem E, Tal-Singer R, et al. Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis. Journal of Cystic Fibrosis 2013;12(3):241-8. [CENTRAL: 967627] [CFGD REGISTER: PI275a] [JID: 101128966] [PMID: ] [SI: ClinicalTrials.gov/NCT00903201] [DOI] [PubMed] [Google Scholar]
NCT00742092 {unpublished data only}
- NCT00742092. Single center, double-blind, randomized, placebo-controlled, two-period/two-treatment crossover study investigating the effect of miglustat on the nasal potential difference in patients with cystic fibrosis homozygous for the F508del mutation. clinicaltrials.gov/ct2/show/NCT00742092 (first received 27 August 2008).
NCT01455675 {published data only}NCT01455675
- NCT01455675. Phase III study to evaluate clinical efficacy and safety of avian polyclonal anti-Pseudomonas antibodies (IgY) in prevention of recurrence of Pseudomonas aeruginosa infection in cystic fibrosis patients. clinicaltrials.gov/ct2/show/NCT01455675 (first received 20 October 2011).
Olveira 2010 {published data only}
- Olveira G, Olveira C, Acosta E, Espíldora F, Garrido-Sánchez L, García-Escobar E. Fatty acid supplements improve respiratory, inflammatory and nutritional parameters in adults with cystic fibrosis. Archivos de Bronconeumologia 2010;46(2):70-7. [DOI] [PubMed] [Google Scholar]
Panchaud 2006 {published data only}
- Panchaud A, Sauty A, Kernen Y, Decosterd LA, Buclin T, Boulat O, et al. Biological effects of a dietary omega-3 polyunsaturated fatty acids supplementation in cystic fibrosis patients: a randomized, crossover placebo-controlled trial. Clinical Nutrition 2006;25(3):418-27. [CFGD REGISTER: GN109b] [DOI: 10.1016/j.clnu.2005.10.011] [DOI] [PubMed] [Google Scholar]
- Panchaud A, Sauty A, Kernen Y, Decosterd LA, Buclin T, Roule M. Dietary supplementation with omega-3 in cystic fibrosis (CF) patients. Journal of Cystic Fibrosis 2005;4 Suppl:S88. [CFGD REGISTER: GN109a] [Google Scholar]
Safai‐Kutti 1991 {published data only}
- Safai-Kutti S, Selin E, Larsson S, Jagenburg R, Sten G, Kjellmer I. Zinc therapy in children with cystic fibrosis. Beitraege zur Infusionstherapie 1991;27:104-14. [CFGD REGISTER: GN36] [PubMed] [Google Scholar]
Sagel 2011 {published data only}
- Sagel SD, Sontag MK, Anthony MM, Emmett P, Paps KA. Effect of an antioxidant-rich multivitamin supplement in cystic fibrosis. Journal of Cystic Fibrosis 2011;10(1):31-6. [DOI: 10.1016/j.jcf.2010.09.005] [DOI] [PubMed] [Google Scholar]
Sagel 2018 {published data only}
- Jain R, Khan U, Baines A, Sagel SD. Biomarkers of inflammation and oxidative stress in CF: implications for antiinflammatory drug development. Pediatric Pulmonology 2016;51 Suppl 45:262. [CFGD REGISTER: GN265b] [Google Scholar]
- Sagel SD, Baines A, Abdulhamid I, Borowitz D, Clancy JP, Daines C, et al. Effects of an antioxidant-enriched multivitamin supplement on inflammation and oxidative stress in CF. Pediatric Pulmonology 2016;51 Suppl 45:283. [CFGD REGISTER: GN265a] [Google Scholar]
- Sagel SD, Khan U, Jain R, Graff G, Daines CL, Dunitz JM, et al. Effects of an antioxidant-enriched multivitamin in cystic fibrosis: randomized, controlled, multicenter trial. American Journal of Respiratory and Critical Care Medicine 2018;198(5):639-47. [CFGD REGISTER: GN265c] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tangpricha 2017 {published data only}
- Lee MJ, Alvarez JA, Smith EM, Killilea DW, Chmiel JF, Joseph PM, et al. Changes in mineral micronutrient status during and after pulmonary exacerbation in adults with cystic fibrosis. Nutrition in Clinical Practice 2015;30(6):838-43. [CFGD REGISTER: GN262a] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Kearns MD, Smith EM, Hao L, Ziegler TR, Alvarez JA, et al. Free 25-hydroxyvitamin D concentrations in cystic fibrosis. American Journal of the Medical Sciences 2015;350(5):374-9. [CFGD REGISTER: GN262c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01426256. Vitamin D for enhancing the immune system in cystic fibrosis. clinicaltrials.gov/show/NCT01426256 (first received 31 August 2011). [CFGD REGISTER: GN262b]
- Tangpricha V, Michalski E, Lukemire J, Binongo J, Judd S, Ziegler T, et al. High-dose vitamin D3 during acute pulmonary exacerbation in cystic fibrosis: a multi-center, double-blind, placebo-controlled trial. Pediatric Pulmonology 2017;52 Suppl 47:471-2. [CFGD REGISTER: GN262e] [Google Scholar]
- Tangpricha V, Smith EM, Binongo J, Judd SE, Ziegler TR, Walker S, et al. The vitamin D for enhancing the immune system in cystic fibrosis (DISC) trial: rationale and design of a multi-center, double-blind, placebo-controlled trial of high dose bolus administration of vitamin D3 during acute pulmonary exacerbation of cystic fibrosis. Contemporary Clinical Trials Communications 2017;6:39-45. [CFGD REGISTER: GN262d] [DOI] [PMC free article] [PubMed] [Google Scholar]
Winklhofer‐Roob 1995 {published data only}
- Winklhofer-Roob BM, Puhl H, Khoschsorur G, van't Hof MA, Esterbauer H, Shmerling DH. Enhanced resistance to oxidation of low density lipoproteins and decreased lipid peroxide formation during beta-carotene supplementation in cystic fibrosis. Free Radical Biology & Medicine 1995;18(5):849-59. [DOI] [PubMed] [Google Scholar]
- Winklhofer-Roob BM, Schlegel-Haueter SE, Khoschsorur G, van't Hof MA, Suter S, Shmerling DH. Neutrophil elastase/alpha 1-proteinase inhibitor complex levels decrease in plasma of cystic fibrosis patients during long-term oral beta-carotene supplementation. Pediatric Research 1996;40(1):130-4. [DOI] [PubMed] [Google Scholar]
- Winklhofer-Roob BM, van't Hof MA, Shmerling DH. Response to oral beta-carotene supplementation in patients with cystic fibrosis: a 16-month follow-up study.[erratum appears in Acta Paediatr 1996 Jan;85(1):124]. Acta Paediatrica 1995 Oct;84(10):1132-6. [DOI] [PubMed] [Google Scholar]
Winnie 1989 {published data only}
- Winnie GB, Cowan RG, Wade NA. Intravenous immune globulin treatment of pulmonary exacerbations in cystic fibrosis. Journal of Pediatrics 1989;114(2):309-14. [CFGD REGISTER: PI224] [DOI: 10.1016/S0022-3476(89)80804-2] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
CARE‐CF‐1 {published data only}
- Devereux G, Bourke S, Daines C, Doe S, Dougherty R, Franco R, et al. Care-CF-1 trial: cysteamine, an oral adjunct to standard of care interventions in CF infectious exacerbations. Pediatric Pulmonology 2018;53(S2):260. [CENTRAL: CN-01785552] [CFGD REGISTER: PI309a] [EMBASE: 624049687] [Google Scholar]
- Devereux G, Bourke S, Daines C, Doe S, Dougherty R, Franco R, et al. Evaluating appropriate PROMs in CARE-CF-1 trial: lynovex (cysteamine) an oral adjunct to SOC interventions in cystic fibrosis infectious exacerbations. Journal of Cystic Fibrosis 2019;18(Supplement 1):S23-4. [CENTRAL: CN-02006157] [CFGD REGISTER: PI309b] [EMBASE: 2001976515] [Google Scholar]
Khorasani 2009 {published data only}
- Boskabady M, Nemat Khorasani E, Mansouri F, Soltanian E, Boskabady M. Effect of zinc supplementation on respiratory tract infections in children with cystic fibrosis. Journal of Research in Medical Sciences 2012;17(12):jrms.mui.ac.ir/index.php/jrms/article/view/8833. [Google Scholar]
- Khorasani EN, Mansouri F. Effect of zinc supplementation on respiratory infections in children with cystic fibrosis. In: Proceedings of European Respiratory Society Annual Congress; 2009; Sept 12-16; Vienna, Austria. 2009:722s. [ABSTRACT NO.: P4032] [CENTRAL: 793188] [CFGD REGISTER: GN255]
Puvvadi 2019 {published data only}
- Puvvadi R, Mikkelsen H, McCahon L, Ditcham W, Reid D, Lamont I, et al. EDTA combined with nebulised tobramycin improves bacterial clearance and lung function in cystic fibrosis patients with chronic pseudomonas aeruginosa infection. Pediatric Pulmonology 2019;54:299. [CENTRAL: CN-01990631] [CFGD REGISTER: PI319] [EMBASE: 629388437] [Google Scholar]
Rye 2015 {published data only}
- EUCTR2014-002125-35-DE. A phase IIb study of OligoG in subjects with cystic fibrosis colonized with Burkholderia spp. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014-002125-35-DE (first received 21 July 2014).
- NCT02453789. A study of oligog in cystic fibrosis subjects with burkholderia spp. infection. clinicaltrials.gov/show/NCT02453789 (first received 27 May 2015). [CFGD REGISTER: BD223e]
- Rye PD, Mahenthiralingam E, Weiser R, Marchesi JR, Opitz P, Trentmann M, et al. Microbiota analysis of samples from a randomized double-blind, placebo-controlled cross-over study of inhaled alginate oligosaccharide (OLIGOG) in cystic fibrosis individuals using aztreonam due to chronic colonization with burkholderia SPP. Pediatric Pulmonology 2015;50 Suppl 41:328. [ABSTRACT NO.: 363] [CENTRAL: 1092173] [CFGD REGISTER: BD223a] [Google Scholar]
- Weiser R, Mahenthiralingham E, Marchesi J, Opitz P, Trentmann M, Rye PD, et al. Standardised cultivation-independent methods to understand the effect of OligoG on Burkholderia and microbial diversity in the CF lung during a clinical trial. Journal of Cystic Fibrosis 2016;15 Suppl 1:S60, Abstract no: 35. [CENTRAL: 1157480] [CFGD REGISTER: BD223b] [Google Scholar]
- Weiser R, Marchesi J, Opitz P, Trentmann M, Rye PD, Onsoyen E, et al. Microbiota analysis of samples from a randomized double-blind, placebo-controlled cross-over study of inhaled alginate oligosaccharide (oligog) in cystic fibrosis individuals using aztreonam due to chronic colonization with burkholderia SPP. Pediatric pulmonology 2016;51:332. [CFGD REGISTER: BD223c] [Google Scholar]
- Weiser R, Smith A, Marchesi J, Rye PD, Dessen A, Hilde Myrset A, et al. Application of microbiota analysis to phase IIb trials of inhaled alginate oligosaccharide (Oligo G) in cystic fibrosis patients. Journal of Cystic Fibrosis 2017;16 Suppl 1:S79. [CFGD REGISTER: BD223d] [Google Scholar]
Walshaw 2014 {published and unpublished data}NCT01465529
- NCT01465529. A cross-over study of OligoG in subjects with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT01465529 (first posted 04 November 2011).
- Walshaw M, McElvaney G, Williams R, Morice A, Carroll M, Haworth C, et al. A first-in-patient clinical trial demonstrates that inhaled alginate oligosaccharide (OligoG) is well tolerated in cystic fibrosis (CF) patients [abstract]. Journal of Cystic Fibrosis 2014;13 Suppl 2:S58, Abstract no: 45. [CENTRAL: 996544] [CFGD REGISTER: BD202] [Google Scholar]
Zabner 2009 {unpublished data only}
- NCT00928135. Aerosolized hypertonic xylitol versus hypertonic saline in cystic fibrosis (CF) subjects. clinicaltrials.gov/ct2/show/NCT00928135 (first received 25 June 2009).
References to ongoing studies
Pressler 2017 {published data only}
- Davies J. Oligo G: a novel mucolytic from basic science to clinical trials. Pediatric Pulmonology 2017;52 Suppl 47:150-1. [CFGD REGISTER: BD235c] [Google Scholar]
- Donaldson SH, Bennett WD, Zeman K, Pressler T, Gow L, Conway J, et al. A first in class mucus conditioning drug-effect of an inhaled alginate oligosaccharide (oligo-g) on mucus clearance in subjects with cystic fibrosis. Pediatric Pulmonology 2017;52 Suppl 47:305. [CFGD REGISTER: BD235d] [Google Scholar]
- Pressler T, Donaldson S, Smerud KT, Myrset AH, OligoG Study Team. A double blind, randomised, placebo-controlled cross over study of inhaled alginate oligosaccharide (OligoG) administered for 28 days in subjects with cystic fibrosis. Journal of Cystic Fibrosis 2017;16 Suppl 1:S1. [CFDG REGISTER: BD235b] [Google Scholar]
- Pressler T, Donaldson SH, Davies JC, Hilde Myrset A. A double-blind, randomised, placebo-controlled cross-over study of inhaled alginate oligosaccharide (OLIGOG) administered for 28 days in subjects with cystic fibrosis. Pediatric Pulmonology 2016;51 Suppl:271. [CFGD REGISTER: BD235a] [Google Scholar]
- Pressler T. A Phase IIb Study of OligoG in Subjects With Cystic Fibrosis (SMR-2984). Clinicaltrials.gov August 2016.
Additional references
Borysowski 2006
- Borysowski J, Weber-Dbrowska B, Górski A. Bacteriophage endolysins as a novel class of antibacterial agents. Experimental Biology and Medicine 2006;231(4):366-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dodge 2007
- Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947–2003. European Respiratory Journal 2007;29(3):522-6. [DOI: 10.1183/09031936.00099506] [DOI] [PubMed] [Google Scholar]
Druesne‐Pecollo 2010
- Druesne-Pecollo N, Latino-Martel P, Norat T, Barrandon E, Bertrais S, Galan P, et al. Beta-carotene supplementation and cancer risk: a systematic review and meta-analysis of randomized controlled trials. International Journal of Cancer 2010;127(1):172-84. [DOI: 10.1002/ijc.25008] [DOI] [PubMed] [Google Scholar]
Goss 2007
- Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax 2007;62(4):360-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Johansson 2008
- Johansson EM, Crusz SA, Kolomiets E, Buts L, Kadam RU, Cacciarini M, et al. Inhibition and dispersion of Pseudomonas aeruginosa biofilms by glycopeptide dendrimers targeting the fucose-specific lectin LecB. Chemistry and Biology 2008;15(12):1249-57. [DOI: 10.1016/j.chembiol.2008.10.009] [DOI] [PubMed] [Google Scholar]
Langton‐Hewer 2017
- Langton-Hewer SC, Smyth AR. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD004197. [DOI: 10.1002/14651858.CD004197.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2004
- Lee TW, Brownlee KG, Denton M, Littlewood J, Conway SP. Reduction in prevalence of chronic Pseudomonas aeruginosa infection at a regional pediatric cystic fibrosis center. Pediatric Pulmonology 2004;37(2):104-10. [DOI: 10.1002/ppul.10401] [DOI] [PubMed] [Google Scholar]
Lo 2018
- Lo DKH, Muhlebach MS, Smyth AR. Interventions for the eradication of meticillin‐resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2018, Issue 7. Art. No: CD009650. [DOI: 10.1002/14651858.CD009650.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Regan 2019
- Regan KH, Bhatt J. Eradication therapy for Burkholderia cepacia complex in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2019, Issue 4. Art. No: CD009876. [DOI: 10.1002/14651858.CD009876.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rosenfeld 2001
- Rosenfeld M, Emerson J, Williams-Warren J, Pepe M, Smith A, Montgomery B, et al. Defining a pulmonary exacerbation in cystic fibrosis. Journal of Pediatrics 2001;139(3):359-65. [DOI: 10.1067/mpd.2001.117288] [DOI] [PubMed] [Google Scholar]
Rosenstein 1998
- Rosenstein BJ, Cutting GP for the Cystic Fibrosis Foundation consensus panel. The diagnosis of cystic fibrosis: A consensus statement. Journal of Pediatrics 1998;132(4):589-95. [DOI: 10.1016/S0022-3476(98)70344-0 ] [DOI] [PubMed] [Google Scholar]
UK Cystic Fibrosis Trust 2004
- UK Cystic Fibrosis Trust Infection Control Group. Pseudomonas aeruginosa infection in people with cystic fibrosis: Suggestions for prevention and infection control. Report of the UK Cystic Fibrosis Infection Control Group 2004. [ISBN: 0-9540536-9-9]
UK Cystic Fibrosis Trust Antibiotic Working Group
- UK Cystic FIbrosis Antibiotic Working Group. Antibiotic Treatment for Cystic Fibrosis - 3rd Edition. Cystic Fibrosis Trust 2009.
von Bodman 2008
- Bodman SB, Willey JM, Diggle SP. Cell-cell communication in bacteria: united we stand. Journal of Bacteriology 2008;190(13):4377-91. [DOI: 10.1128/JB.00486-08] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hurley 2010
- Hurley MN, Forrester DL, Smyth AR. Antibiotic adjuvant therapy for pulmonary infection in cystic fibrosis. Cochrane Database of Systematic Reviews 2010, Issue 10. Art. No: CD008037. [DOI: 10.1002/14651858.CD008037.pub2] [DOI] [PubMed] [Google Scholar]
Hurley 2013
- Hurley MN, Forrester DL, Smyth AR. Antibiotic adjuvant therapy for pulmonary infection in cystic fibrosis. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD008037. [DOI: 10.1002/14651858.CD008037.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


