Abstract
Background
There is presently no certainty about the ideal feeding intervals for preterm infants. Shorter feeding intervals of, for example, two hours, have the theoretical advantage of allowing smaller volumes of milk. This may have the potential to reduce the incidence and severity of gastro‐oesophageal reflux. Longer feeding intervals have the theoretical advantage of allowing more gastric emptying between two feeds. This potentially provides periods of rest (and thus less hyperaemia) for an immature digestive tract.
Objectives
To determine the safety of shorter feeding intervals (two hours or shorter) versus longer feeding intervals (three hours or more) and to compare the effects in terms of days taken to regain birth weight and to achieve full feeding.
Search methods
We used the standard search strategy of Cochrane Neonatal to run comprehensive searches in CENTRAL (2020, Issue 6) and Ovid MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions, and CINAHL on 25 June 2020. We searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi‐RCTs.
Selection criteria
We included RCTs and quasi‐RCTs comparing short (e.g. one or two hours) versus long (e.g. three or four hours) feeding intervals in preterm infants of any birth weight, all or most of whom were less than 32 weeks' gestation. Infants could be of any postnatal age at trial entry, but eligible infants should not have received feeds before study entry, with the exception of minimal enteral feeding. We included studies of nasogastric or orogastric bolus feeding, breast milk or formula, in which the feeding interval is the intervention.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We used the GRADE approach to assess the certainty of evidence. Our primary outcomes were days taken to achieve full enteral feeding and days to regain birth weight. Our other outcomes were duration of hospital stay, episodes of necrotising enterocolitis (NEC) and growth during hospital stay (weight, length and head circumference).
Main results
We included four RCTs, involving 417 infants in the review. One study involving 350 infants is awaiting classification. All studies compared two‐hourly versus three‐hourly feeding interval. The risk of bias of the included studies was generally low, but all studies had high risk of performance bias due to lack of blinding of the intervention.
Three studies were included in meta‐analysis for the number of days taken to achieve full enteral feeding (351 participants). The mean days to achieve full feeds was between eight and 11 days. There was little or no difference in days taken to achieve full enteral feeding between two‐hourly and three‐hourly feeding, but this finding was of low certainty (mean difference (MD) ‒0.62, 95% confidence interval (CI) ‒1.60 to 0.36).
There was low‐certainty evidence that the days taken to regain birth weight may be slightly longer in infants receiving two‐hourly feeding than in those receiving three‐hourly feeding (MD 1.15, 95% CI 0.11 to 2.20; 3 studies, 350 participants).
We are uncertain whether shorter feeding intervals have any effect on any of our secondary outcomes including the duration of hospital stay (MD ‒3.36, 95% CI ‒9.18 to 2.46; 2 studies, 207 participants; very low‐certainty evidence) and the risk of NEC (typical risk ratio 1.07, 95% CI 0.54 to 2.11; 4 studies, 417 participants; low‐certainty evidence).
No study reported growth during hospital stay.
Authors' conclusions
The low‐certainty evidence we found in this review suggests that there may be no clinically important differences between two‐ and three‐hourly feeding intervals. There is insufficient information about potential feeding complications and in particular NEC. No studies have looked at the effect of other feeding intervals and there is no long‐term data on neurodevelopment or growth.
Plain language summary
Short versus long feeding interval for bolus feedings in very preterm infants
Review question
Are short feeding intervals (for example, two hours or shorter) better tolerated than long feeding intervals (three hours or longer) for regular milk feeds in very preterm infants?
Background
Feeding very preterm infants, less than 32 weeks' gestation at birth, is a major challenge. They have an immature gut which may lead to problems, varying from mild (feeding intolerance) to moderate (regurgitation of milk from the stomach) to severe (such as necrotising enterocolitis; NEC). NEC is an infectious complication leading to irreparable loss of parts of the bowel. The feeding interval, that is, the time interval between each feed, might matter but determining the appropriate feeding interval is a major challenge. Both short intervals, typically less than three hours, and longer intervals of three or more hours have their own risks. When the interval is short, a smaller volume of milk can be given more frequently. The infant might tolerate smaller volumes better, but their gut might not have sufficient time to rest between each feeding.
Study characteristics
We searched medical databases up 25 June 2020. We found four studies (involving 417 infants), all conducted in middle‐income countries. All four studies compared two‐hourly with three‐hourly feeding interval. All studies involved very low birth weight infants with a gestational age (time since the beginning of the woman's last menstrual period) range from 29 weeks to 35 weeks.
Key results
When we combined the results of the studies, they suggested little or no difference between the two‐ and three‐hourly feeding intervals. We are uncertain whether there is any difference between two‐ and three‐hourly feeding in terms of days to achieve full enteral (tube) feeding because the results were imprecise and there were biases in the studies. Days taken to regain the birth weight, after the usual initial drop in weight during the first days after birth, may be slightly longer in infants receiving two‐hourly feeds, but we are uncertain of the importance of this. We did not have enough data to determine whether there was any difference in any of the adverse outcomes that the studies reported. However, because only a small number of infants experienced NEC, we are uncertain whether there is any difference between the groups. There was no information on the effect of feeding interval on death, infant growth during hospital stay and neurodevelopmental outcome (brain development in childhood), and there was no information about other feeding intervals such as one‐hourly or four‐hourly feeds. There is one study awaiting classification as we need more information.
Quality of evidence
The quality of the evidence in this review was low. Therefore, there may be no clinically important differences between two‐ and three‐hourly feeding intervals.
Summary of findings
Summary of findings 1. Short feeding interval compared to long feeding interval for bolus feeding in very preterm infants.
| Short feeding interval compared to long feeding interval for bolus feeding in very preterm infants | ||||||
| Patient or population: preterm infants < 1500 g Setting: NICU in North India and East Peninsular Malaysia Intervention: short feeding interval (2 hours) Comparison: long feeding interval (3 hours) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with long feeding interval | Risk with short feeding interval | |||||
| Days taken to achieve full feeding | The mean days taken to achieve full feeding ranged from 8 to11 days | MD 0.62 days lower (1.60 lower to 0.36 higher) | — | 351 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | — |
| Days to regain birth weight | The mean days to regain birth weight ranged from 11 to 13 days | MD 1.15 days higher (0.11 higher to 2.20 higher) | — | 350 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | — |
| Duration of hospital stay (days) | The mean duration of hospital stay ranged from 31 to 46 days | MD 3.36 days lower (9.18 lower to 2.46 higher) | — | 207 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,c | — |
| Necrotising enterocolitis | Study population | RR 1.07 (0.54 to 2.11) | 417 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | — | |
| 72 per 1000 | 77 per 1000 (39 to 151) | |||||
| Growth during hospital stay (weight gain in g/kg/day) | — | — | — | — | — | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference;NICU: neonatal intensive care unit; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious concerns about lack of blinding. Both participants and medical personnel could not be blinded because the nature of the study. bDowngraded one level for imprecision. Very wide confidence interval. cDowngraded two levels for very wide confidence interval of more than 11 days and small number of participants.
Background
Description of the condition
Feeding preterm infants represents a major challenge. Besides a small gastric capacity, preterm infants have immature gastrointestinal function. This includes an immature gastro‐oesophageal sphincter and poorly co‐ordinated intestinal motility and sucking and swallowing, which, in turn, are poorly co‐ordinated with breathing. Peristalsis begins at 28 to 30 weeks, and co‐ordination of sucking, swallowing and breathing begins at 32 to 34 weeks. Mature gastrointestinal motility co‐ordinated with feeding and breathing develops between 33 weeks and term (Ayede 2011). Thus, feeding problems such as gastro‐oesophageal reflux, delayed gastric emptying and necrotising enterocolitis (NEC) are more common in preterm infants.
Because of these facts, most preterm infants initially receive parenteral nutrition (PN), and enteral feeding is introduced when the infant is judged ready to tolerate feeds. However, PN has some complications, for example, animal studies show that total PN is associated with gastrointestinal atrophy (Morgan 1987).
Feeding practices for preterm infants vary widely both within and across neonatal intensive care unit (NICU) settings (Klingenberg 2011), because many issues must be considered. These include the appropriate time to start feeding, the type of feed given, the initial feed volume, the rate of advancement of feeding and the feeding interval.
Formulae of both human milk and cow milk base are fed to preterm infants. The preferred human milk is the mother's own milk, but donated banked human milk can be used and is thought to be preferable to formula milk (Gartner 2005). Human milk feeding results in a reduced incidence of sepsis (Murphy 1983; Patel 2007), and NEC (Patel 2007), in the short term, and possibly better neurodevelopmental outcomes in the longer term (Gibertoni 2015; Horwood 2001; Lucas 1992).
Early initiation of a small feed volume compared with delayed feeding was thought to improve the maturation of gut motility, while preventing gut atrophy and cholestatic jaundice, shortening hospital stay, promoting postnatal growth and reducing the incidence of sepsis (Berseth 1992; Dunn 1988; Slagle 1988). Thus, the concept of trophic feeding was introduced (McClure 2001). Trophic feeding, also known as minimal enteral feeding (MEF), is defined as early initiation of a small milk volume (between 12 mL/kg/day and 24 mL/kg/day) without advancing feed volumes during the first postnatal week. However, one Cochrane Review found no strong evidence indicating that early trophic feeding offered any additional benefit to very low birth weight (VLBW) infants when compared with enteral fasting (Morgan 2013).
The timing of commencement of feeding has been extensively studied, and it has been postulated that this and rate of advancement are factors affecting the incidence of NEC (Henderson 2009). Earlier studies suggested that delaying the introduction of progressive enteral feeding would reduce the risk of NEC (Patole 2005). However, one Cochrane Review, using data from randomised controlled trials (RCTs), found no evidence that delaying the introduction of progressive enteral feeds reduces risk of NEC, mortality and other morbidities in VLBW infants (Morgan 2014).
An important question to be answered pertains to rate of advancement of the feeding volume. It was thought that slow increments in feed volume would reduce the risk of NEC. However, one Cochrane Review found that slow advancement resulted in a delay in the establishment of full feeding and the time to regain birth weight and increases in invasive infections (Oddie 2017).
Description of the intervention
When an infant is not capable of co‐ordinating sucking, swallowing and breathing, feed is provided through an orogastric or nasogastric tube. Oral insertion of the feeding tube is preferred over nasal insertion because infants are nasal breathers. Feeding can be given as a continuous infusion or intermittently (bolus feeding). Intermittent feeding seems more physiological, as it allows for cyclical surges of gastrointestinal hormones, as occur in the mature gut (Aynsley‐Green 1982; Lucas 1986).
Bolus enteral feeding may be given by push or using gravity. In push feeding, milk is administered via a syringe that is connected to the feeding tube, and the milk is gently pushed into the infant's stomach. In gravity feeding, milk is poured into a syringe that is attached to the feeding tube and is allowed to drip slowly under the effect of gravity. No evidence supports a preference for either of these methods. For very preterm infants, boluses are usually given at scheduled intervals in prescribed volumes; these intervals vary between one and two hours (shorter interval) and three and four hours (longer interval) (Siddell 1994).
How the intervention might work
Different feeding intervals may offer their own advantages and disadvantages. A shorter interval, such as one or two hours, delivers a smaller volume per feed, which may be more easily tolerated and may cause less gastric distension and pressure on the lower oesophageal sphincter, possibly reducing the incidence of significant reflux while delivering a higher feed volume per day (owing to more frequent feeds). However, frequent feeding may result in a higher preprandial blood flow velocity or persistent superior mesenteric artery hyperaemia, which might be less physiological (Lane 1998).
A longer feeding interval, such as three to four hours, results in a higher volume per feed, but complete gastric emptying may take longer. Higher feed volume might be less tolerable in small infants. However, feeding intervals of three or more hours may cause a higher maximum postprandial blood flow velocity, which could improve gut motility (Lane 1998).
One observational study reported that two‐hourly compared with three‐hourly feeding resulted in earlier achievement of full feeding, fewer episodes of feeding intolerance and a shorter duration of PN. However, the feeding interval had no influence on weight gain or on time to full enteral feeding (DeMauro 2011).
Why it is important to do this review
We believe this review is needed because there is wide variation in feeding practices in preterm infants. Clinical advantages may be associated with the use of one feeding interval rather than another. Furthermore, one technique might be safer than another. A critical review of the literature might allow a recommendation to be made on this issue, or may reveal the need for additional research.
Objectives
To determine the safety of shorter feeding intervals (two hours or shorter) versus longer feeding intervals (three hours or more) and to compare the effects in terms of days taken to regain birth weight and to achieve full feeding.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs, quasi‐RCTs and cluster trials of bolus feeding comparing two feeding intervals that fulfilled criteria as short and long interval.
Types of participants
We included preterm infants of any birth weight, all or most of whom were less than 32 weeks' gestation. Infants could be of any postnatal age at trial entry, but eligible infants should not have received feeds before study entry, with the exception of MEF. MEF given before study entry was included in the subgroup analyses.
Types of interventions
We included studies of bolus feeding of breast milk or formula in which the feeding interval was the intervention. We classified feeding intervals into two categories: short (such as less than three hours) and long (such as three hours or longer). A single comparison examined short versus long feeding intervals. However, we planned to conduct a subgroup analysis to look at different durations of intervals used in the comparison such as one or two hours for the short interval and three or four hours for the long interval. Some overlap might be noted between short and long feeding intervals. Infants in both groups were started at the same total daily volume, but the rate of advancement might vary owing to better tolerance of one feeding regimen over another. The decision to withhold feeding or to advance feeding volume was dependent on the feeding protocol of the individual study, provided both groups were managed similarly.
We only considered nasogastric or orogastric tube feeding. We excluded infants receiving gastrostomy, jejunostomy and transpyloric feeding, as these infants usually have pre‐existing feeding problems.
For assessment of the effect of feeding interval on our prespecified outcomes, the minimum duration of the intervention must have been two weeks. If the feeding interval had been increased slowly, for example, feeding initially at one‐hour intervals, then spaced to two‐hour intervals, and so forth, we analysed the feeding interval that had been practised for at least two weeks when assessing outcomes of interest.
Types of outcome measures
Primary outcomes
Days taken to achieve full enteral feeding (defined as able to tolerate enteral feeding without the need for supplemental intravenous fluid for at least 24 hours).
Days to regain birth weight.
Secondary outcomes
All causes of neonatal mortality (at less than 28 days)
Mortality before discharge.
One or more episodes of culture‐positive sepsis detected and treated with antibiotics before 28 days' postnatal age.
Duration of hospital stay (days).
Bronchopulmonary dysplasia (BPD) (defined as the need for supplemental oxygen at 36 weeks' postmenstrual age).
Necrotising enterocolitis (NEC) (Bell's stage 2 or higher) (Bell 1978).
Neurodevelopmental disability assessed at 12 to 24 months of age (defined as a Bayley or Griffith score two or more standard deviations (SDs) below the mean).
Growth during hospital stay such as weight (grams/kilogram/day), length (cm/day) and head circumference (cm/day).
Below the 10th percentile for weight at discharge and between six and 12 months of age (on any growth chart selected by study investigators).
Feeding intolerance as defined by the authors of the included studies.
Hypoglycaemia as defined by the authors of the individual studies.
Feeding intolerance and hypoglycaemia are post hoc outcomes that we added after examining the included studies. We considered these outcomes because all the included studies reported them suggesting they were important.
Search methods for identification of studies
We used the standard search strategy of Cochrane Neonatal, as documented in the Cochrane Library. See the Cochrane Neonatal search strategy at neonatal.cochrane.org/resources-review-authors.
Electronic searches
We conducted a comprehensive update search in June 2020 including: Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 6) in the Cochrane Library; Ovid MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions (1 January 2016 to 25 June 2020); and CINAHL EBSCOhost (1 January 2016 to 25 June 2020). We have included the search strategies for each database in Appendix 1. We applied no language restrictions.
We searched clinical trial registries for ongoing or recently completed trials. We searched The World Health Organization's International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/), and the US National Library of Medicine’s ClinicalTrials.gov (clinicaltrials.gov), via Cochrane CENTRAL. Additionally, we searched the www.isrctn.com/ for any unique trials not found through the Cochrane CENTRAL search.
Our previous search details (Ibrahim 2016), are listed in Appendix 2.
Searching other resources
We communicated with experts and searched the reference lists of any identified reviews and included trials for references to other trials. We searched previous reviews including cross‐references, abstracts, and conferences and symposia proceedings of the Perinatal Societies and Paediatric Academic Societies. We searched the clinical trial registry to identify any completed but unpublished trial. If we identified any unpublished trial, we contacted the corresponding investigator for information. We considered unpublished studies and studies reported only as abstracts as eligible for review. We also contacted the corresponding of identified RCTs for additional information about studies when required.
Data collection and analysis
We used the standard methods for conducting a systemic review presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020), and Cochrane Neonatal.
Selection of studies
We used Cochrane's Screen4Me workflow to help assess the search results. Screen4Me comprises three components: known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as 'an RCT' or as 'Not an RCT'; and the RCT classifier – a machine learning model that distinguishes RCTs from non‐RCTs, and if appropriate, Cochrane Crowd – Cochrane's citizen science platform where the Crowd help to identify and describe health evidence.
For more information about Screen4Me, see community.cochrane.org/organizational-info/resources/resources-groups/information-specialists-portal/crs-videos-and-quick-reference-guides#Screen4Me. Detailed information regarding evaluations of the Screen4Me components can be found in Marshall 2018; Noel‐Storr 2020; Noel‐Storr 2021; and Thomas 2020.
The lead review author performed the search for trials with the assistance of Cochrane Neonatal. Two review authors (NRI, AN) independently assessed for inclusion all potential studies to create a pool of eligible studies. We examined the title and abstract of each retrieved study. We reviewed the full articles of the included studies and scrutinised them for relevance using a standardised eligibility form with predefined inclusion criteria. A third review author (JJH) resolved any disagreements. There was one included study that belonged to three review authors (NRI, HVR, AN; Ibrahim 2017). The fourth review author (JJH) who was not involved in that study took the responsibility to review that study to reduce the bias.
Data extraction and management
For included studies, we extracted data concerning study identity (title, authors, reference), design, methods, eligibility, risk of bias, characteristic of participants, interventions and outcomes, and treatment effects, using a specially designed data extraction form. For studies that we initially considered eligible for inclusion but then excluded after reading the full report, we documented the reason of exclusion.
Two review authors (NRI, HVR) independently extracted and compared all data; they resolved any discrepancies by discussion or by consultation with a third review author (JJH). Since three of the four review authors (NRI, HVR, AN) were authors of one of the included studies (Ibrahim 2017), we invited Dr Mohd Alwi Mohd Helmi (MAMH) to extract the study data and assess risk of bias for that study along with JJH (see Acknowledgements).
Assessment of risk of bias in included studies
Three review authors (NRI, HVR, AN) independently assessed the risk of bias (low, high or unclear) of all included trials using the Cochrane risk of bias tool, for the following domains (Higgins 2011).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved any disagreements by discussion or with a fourth review author (JJH). See Appendix 3 for a more detailed description of risk of bias for each domain.
MAMH (see Acknowledgements), and one review author (JJH) assessed the risk of bias for the included study authored by three of the four review authors (NRI, AN, HVR; Ibrahim 2017).
Measures of treatment effect
We carried out data analysis using Review Manager 5 software (Review Manager 2020).
For dichotomous data, we presented the results as risk ratios (RRs) and risk differences (RDs), each with the 95% confidence interval (CI). We planned to calculate the number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) if we observed a statistically significant reduction in RD.
For continuous data, we reported the mean differences (MDs) with 95% CIs as all studies used the same scale for the outcome measured,
Unit of analysis issues
A cross‐over study design is not possible for the outcomes measured. We did not anticipate finding cluster‐RCTs, but if we had encountered such studies, we would have adjusted for cluster size using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020), using an estimate of the intracluster correlation co‐efficient (ICC) derived from the included studies (if possible) or from a study of a similar population in the literature. If we had used ICCs from other sources, we would have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. If we had identified both cluster‐RCTs and individually randomised trials, we planned to synthesise the relevant information. We would have considered it reasonable to combine results from both if we observed little heterogeneity in study designs, and if interaction between the effect of the intervention and the choice of randomisation unit was unlikely. We would have also acknowledged heterogeneity in the randomisation unit and performed a subgroup analysis to investigate effects of the randomisation unit.
Dealing with missing data
If we had encountered missing data, we would have performed sensitivity analyses to explore the impact of including, in the overall assessment of treatment, the effect of including studies with high levels of missing data. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis (i.e. we attempted to include in the analyses all participants randomised to each group, and analyse all participants in the group to which they were allocated, regardless of whether they received the allocated intervention). The denominator for each outcome in each trial is the number randomised minus any participants whose outcome data are known to be missing. If we considered the missing data to be critical to the final estimates in our meta‐analysis, we planned to contact the authors of individual studies to request additional data.
Assessment of heterogeneity
We estimated the amount of heterogeneity of treatment effects across trials using the I² statistic. We used the following cutoffs and labels:
less than 25% (no heterogeneity);
25% to 49% (low heterogeneity);
50% to 74% (moderate heterogeneity);
75% or higher (substantial heterogeneity).
If we detected moderate or substantial heterogeneity, we explored its possible causes (e.g. differences in study quality, participants, intervention regimens or outcome assessments) by completing subgroup and sensitivity analyses.
Assessment of reporting biases
If we obtained sufficient studies, we would have investigated reporting biases by constructing funnel plots. We would have assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we would have performed exploratory analyses to investigate this.
Data synthesis
We carried out statistical analysis using Review Manager 5 (Review Manager 2020). We used the fixed‐effect model for meta‐analysis in combining data when trials had similar characteristics (examining the same intervention, trial population and methods).
Subgroup analysis and investigation of heterogeneity
We had planned subgroup analyses according to:
birth weight: less than 1000 g; 1000 g to 1499 g; 1500 g and greater;
gestational age: less than 28 weeks; 28 weeks to 31 weeks;
type of milk: human milk, preterm formula;
feeding interval: different definitions of short and long intervals such as one or two hours for short interval, and two, three or four hours for the long interval;
prior MEF versus no prior MEF;
low increment volume (15 mL/kg/day or less) and high increment volume (greater than 15 mL/kg/day).
Sensitivity analysis
We planned to use sensitivity analysis to explore the effects of methodological quality if trials of different quality were included in the review. However, we did not proceed with the sensitivity analysis as all studies had no major methodological differences.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence of the following (clinically relevant) outcomes: days taken to achieve full enteral feeding, days to regain birth weight, duration of hospital stay, NEC (Bell's stage 2 or higher), and growth during hospital stay (weight gain).
Three review authors (NRI, HVR, JJH) independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro GDT to create Table 1 to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect and further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
We included four RCTs involving 417 infants in our review (Anushree 2018; Dhingra 2009; Ibrahim 2017; Tali 2016).
Results of the search
The search identified 4755 records. In assessing the studies, we used Cochrane's Screen4Me workflow to help identify potential reports of randomised trials. The results of the Screen4Me assessment process is shown in Figure 1. After removing duplicates, we assessed the remaining 2838 records left in after Screen4Me and excluded 2826 as irrelevant. We retrieved 12 full‐text articles and included four studies in the review. One article is awaiting a response from its author, and seven articles were excluded with reasons. We included four trials.
1.
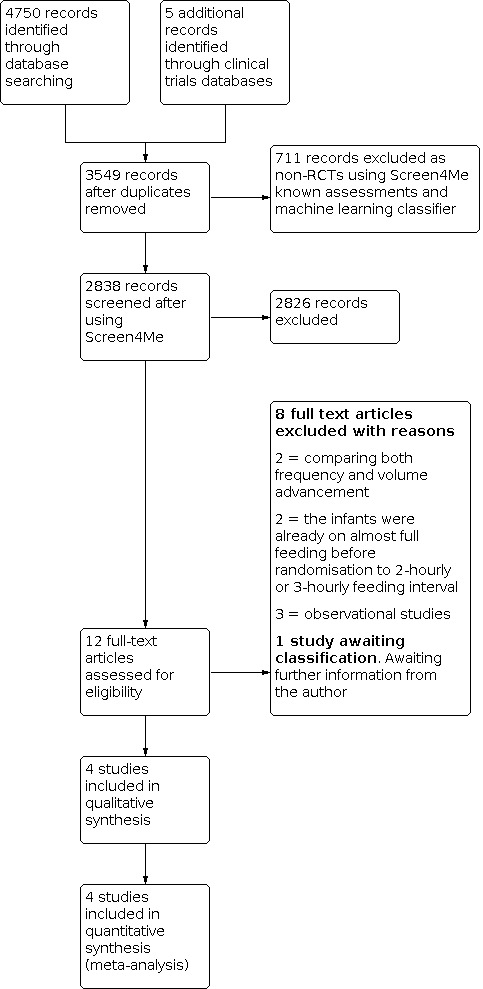
Study flow diagram. RCT: randomised controlled trial.
(See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of studies awaiting classification tables.)
Included studies
We included four RCTs (involving 417 infants) in this review (Anushree 2018; Dhingra 2009; Ibrahim 2017; Tali 2016). The RCTs had a parallel design and were performed in middle‐income countries. Details of the included studies are shown in the Characteristics of included studies table.
Anushree 2018 included 60 infants with a birth weight of less than 1500 g, stable for starting enteral feeding. The primary outcome was incidence of feeding intolerance. The secondary outcomes were episodes of hypoglycaemia, apnoea, NEC (Bell's stage IIA and beyond) and days to achieve full feeding (when infants tolerated 150 mL/kg/day).
Dhingra 2009 included medically fit infants with a birth weight of less than 1750 g who were able to start on increasing feeds, three‐hourly or two‐hourly. Feeding protocols were developed for feeding schedules and management of feeding intolerance. The primary outcome was incidence of feeding intolerance. Secondary outcomes included incidence of apnoea and hypoglycaemia. Other important outcomes reported were days to reach full feeds (enteral feeding of 150 mL/kg/day), duration of hospital stay, day on which birth weight was regained, duration of intravenous (IV) fluids in days, day of maximum weight loss, discharge weight in grams and total nursing time spent on feeding per day in minutes.
Ibrahim 2017 included 150 preterm infants with a gestational age less than 35 weeks and a birth weight between 1000 g and 1500 g in whom enteral feeding was started within 96 hours after birth. The primary outcome was days to achieve full feeding. Secondary outcomes were days to regain birth weight, incidence of NEC based on modified Bell's staging, feeding intolerance, nosocomial sepsis and gastro‐oesophageal reflux. Other outcomes reported were episodes of hypoglycaemia after PN had been stopped, episodes of ketonuria, the highest bilirubin documented, duration of phototherapy and death before discharge.
Tali 2016 included 120 preterm infants with a birth weight of 501 g to 1500 g. Three‐hourly or two‐hourly feeding were started when infants were haemodynamically stable. The primary outcome was days to reach full feeds (defined as tolerance of 150 mL/kg/day of feeds for at least 48 hours). Secondary outcomes were days to attain birth weight, time to discharge, growth parameters (weight, length and head circumference) at discharge, incidence of feeding intolerance, NEC, intraventricular haemorrhage, clinical sepsis, culture‐positive sepsis, hypoglycaemia, apnoea, jaundice, retinopathy of prematurity, duration of total PN, nursing time for feeding and death.
Participants
All four studies included preterm VLBW infants who were considered sufficiently clinically stable to start incremental enteral feeding. None of the studies specified the gestational age at recruitment except Ibrahim 2017, where they specified that only infants less than 35 weeks' gestation were included in the study. The four studies included 417 preterm infants with the birth weight ranging from 501 g to 1750 g, with the gestation age at birth ranging between 27 weeks and 36 weeks. All participants were started on total PN within 24 hours after delivery.
Interventions
All studies compared two‐hourly versus three‐hourly feeding. Feeding was given via indwelling orogastric or nasogastric tube. Expressed breast milk (EBM) was the milk of choice but was supplemented with low birth weight or preterm formula if EBM was inadequately available. The feeding increment followed a standard feeding protocol in each study. The decision to withhold or advance the feedings was made by the clinician in charge.
Outcomes
All four studies reported the primary outcome of days to achieve full enteral feeding; however, they applied different definitions of full enteral feeding. Two studies defined full enteral feeding as tolerating enteral feeds of 150 mL/kg/day (Anushree 2018; Dhingra 2009). Ibrahim 2017 defined full enteral feeding as total milk intake enterally of 100 mL/kg/day for at least 48 hours without PN and hypoglycaemia. Whereas Tali 2016 defined full enteral feeding as tolerance of 150 mL/kg/day of feeds for at least 48 hours.
Three studies reported days to regain birth weight (Dhingra 2009; Ibrahim 2017; Tali 2016).
For the secondary outcomes, all four studies reported incidence of NEC. Anushree 2018 reported only NEC stage IIA and beyond. Tali 2016 reported all stages of NEC. The remaining two studies reported the incidence of NEC without classifying the stages (Dhingra 2009; Ibrahim 2017). Two studies reported the duration of hospital stay (Dhingra 2009; Tali 2016). Two studies reported mortality (Ibrahim 2017; Tali 2016). Ibrahim 2017 reported eight deaths (four in each group). There were no deaths in Tali 2016. Two studies reported culture‐positive sepsis (Ibrahim 2017; Tali 2016). However, the age of neonates at the time of infection was not documented. Tali 2016 reported both culture‐positive sepsis and clinical sepsis. Ibrahim 2017 only included culture‐positive sepsis.
None of the studies reported BPD or neurodevelopmental disability at one and two years of age. Tali 2016 and Dhingra 2009 reported growth parameters at discharge. Dhingra 2009 documented the maximum percentage weight loss and when it occurred.
All four studies reported feeding intolerance, but there was some variability in the definitions used. All four studies reported episodes of hypoglycaemia.
Excluded studies
We excluded seven studies (see Characteristics of excluded studies for further details).
Two RCTs assessed the outcome of a combined intervention involving both frequency advancement (interval) and volume advancement (Hussain 2018; Zubani 2016).
Two RCTs enrolled participants who were on almost full gavage feeding before randomisation to two‐hourly or three‐hourly feeding interval (Gray 2017; Unal 2019).
Three were observational studies (Chu 2019; DeMauro 2011; Rüdiger 2008).
Studies awaiting classification
One study is awaiting classification (Yadav 2021). The study included infants meeting our inclusion criteria as well as some that did not. We contacted the authors regarding the possibility of obtaining data for those infants that met our inclusion criteria (see Characteristics of studies awaiting classification table for further details).
Risk of bias in included studies
The details of the quality assessment can be reviewed in the risk of bias table, the Characteristics of included studies table; Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All four trials described randomisation procedures that were likely to result in satisfactory randomisation and allocation concealment (low risk of selection bias).
Blinding
Due to nature of the intervention, none of the studies blinded participants, caregivers and medical personnel. The primary outcomes would both have been judged by the clinical team and hence they would have been aware of the intervention group, and we judged that knowledge of the study group could have impacted on their measurement of the outcome. Thus, we assessed the risk of performance bias as being high. However, most of the outcomes of the studies were well‐defined or measured, even though the clinical assessors were not blinded, we assessed the risk of detection bias as unclear,
Incomplete outcome data
We judged all included studies at low risk of attrition bias because the dropout rate or non‐reported data were less than 10% and were balanced across the groups and, where data were missing, it did not appear to be related to the outcome.
Selective reporting
We detected no selective reporting. All the studies reported their feeding protocol in their methodology and all the outcomes mentioned were reported. Therefore, we judged them at low risk for reporting bias.
Other potential sources of bias
We did not detect any other potential sources of bias in any of the studies.
Effects of interventions
See: Table 1
Comparison 1. Short versus long feeding interval
See Table 1.
Primary outcomes
1. Days to achieve full enteral feeding
All four studies reported days to achieve full enteral feeding. Anushree 2018 reported this outcome as median with interquartile range (IQR) and hence was not included in the meta‐analysis. The reported median days to attain full enteral feeding was similar in both groups (10 days, IQR 7 to 10) (Anushree 2018).
For the three included studies that reported means, the overall mean days to achieve full feeds across the three studies was between 8 and 11 days.
The meta‐analysis found short feeding interval made little or no difference to the days to achieve full feeds (MD −0.62, 95% CI −1.60 to 0.36; 3 studies, 351 participants; low‐certainty evidence; Analysis 1.1). We downgraded for risk of bias and imprecision.
1.1. Analysis.

Comparison 1: Short versus long feeding interval, Outcome 1: Days to achieve full enteral feeding
Using the two studies that provided sufficient data, we performed a subgroup analysis according to the birth weight less than 1000 g and 1000 g or greater (Ibrahim 2017; Tali 2016). The study by Tali 2016 presented data for both birth weight subgroups. Ibrahim 2017 only included infants in the 1000 g or greater birth weight group. The birth weight less than 1000 g group included 36 infants whereas for the birth weight 1000 g or greater included 228 infants. When the two studies were included in a subgroup analysis, we were uncertain whether there is a difference between the birth weight groups (P = 0.07, I² = 69%; 228 participants). We were unable to perform any other prespecified subgroup analysis due to few data.
2. Days to regain birth weight
Three studies reported days to regain birth weight (Dhingra 2009; Ibrahim 2017; Tali 2016). The mean days to regain birth weight was between 10 and 13 days. We found low‐certainty evidence that two‐hourly feeding may slightly increase the days to regain birth weight (MD 1.15, 95% CI 0.11 to 2.20; I² = 0%; 3 studies, 350 participants; Analysis 1.2). We downgraded for risk of bias and imprecision. We were unable to perform a subgroup analysis because data were not available.
1.2. Analysis.

Comparison 1: Short versus long feeding interval, Outcome 2: Days to regain birth weight
Secondary outcomes
1. Mortality (all cause and before discharge)
Two studies included mortality during hospital stay. Ibrahim 2017 reported four deaths in each group and Tali 2016 reported no deaths (Analysis 1.3).
1.3. Analysis.

Comparison 1: Short versus long feeding interval, Outcome 3: Mortality
2. Culture‐positive sepsis
Two studies reported culture‐positive sepsis (Ibrahim 2017; Tali 2016).
We are unsure whether short feeding intervals have any effect on culture‐positive sepsis (RR 0.73, 95% CI 0.30 to 1.74; I² = 0%; 2 studies, 270 participants; low‐certainty evidence; Analysis 1.4). We downgraded for risk of bias and imprecision.
1.4. Analysis.

Comparison 1: Short versus long feeding interval, Outcome 4: Culture‐positive sepsis
3. Duration of hospital stay
Two studies reported duration of hospital stay (Dhingra 2009; Tali 2016). The mean duration was 36 and 46 days.
There was very low‐certainty evidence that the duration of hospital stay in days was shorter with short feeding intervals (MD −3.36, 95% CI −9.18 to 2.46; I² = 0%; 2 studies, 207 participants; Analysis 1.5). We downgraded for concerns about study design and very serious concerns about imprecision.
1.5. Analysis.

Comparison 1: Short versus long feeding interval, Outcome 5: Duration of hospital stay
4. Bronchopulmonary dysplasia
No studies reported BPD.
5. Necrotising enterocolitis
All four studies reported NEC. It is uncertain if feeding interval has any effect on NEC (typical RR 1.07, 95% CI 0.54 to 2.11; I² = 0%; 4 studies, 417 participants; low‐certainty evidence; Analysis 1.6). We downgraded for risk of bias and imprecision.
1.6. Analysis.

Comparison 1: Short versus long feeding interval, Outcome 6: Necrotising enterocolitis
6. Neurodevelopmental disability assessed at 12 to 24 months of age
No studies reported neurodevelopmental disability.
7. Growth during hospital stay
No studies reported growth during hospital stay.
8. Below the 10th percentile for weight at discharge and between six and 12 months of age
No studies reported below the 10th percentile for weight.
9. Feeding intolerance
Four studies report this post hoc outcome. For two studies feeding intolerance was the primary outcome (Anushree 2018; Dhingra 2009). Authors used different definitions of feeding intolerance in their studies (see Characteristics of included studies table).
There may be little or no difference in feeding intolerance between feeding intervals (typical RR 1.11, 95% CI 0.82 to 1.50; I² = 0; 4 studies, 417 participants; Analysis 1.7).
1.7. Analysis.

Comparison 1: Short versus long feeding interval, Outcome 7: Feeding intolerance
10. Hypoglycaemia
Four studies reported hypoglycaemia, but there were slight differences in the values used to define hypoglycaemia. Tali 2016 used less than 40 mg/dL (2.2 mmol/L) while Anushree 2018 and Ibrahim 2017 used less than 45 mg/dL (2.6 mmol/L). Dhingra 2009 did not define hypoglycaemia.
Meta‐analysis showed little or no difference between feeding intervals (RR 0.88, 95% CI 0.45 to 1.75; I² = 0%; 4 studies, 412 participants; Analysis 1.8).
1.8. Analysis.

Comparison 1: Short versus long feeding interval, Outcome 8: Hypoglycaemia
Discussion
Summary of main results
From the four included studies, involving 417 preterm infants, we are uncertain if there are any clinically meaningful differences between two‐ and three‐hourly feeding intervals.
Our primary outcome, days taken to achieve full feeding, found little or no difference between two‐hourly and three‐hourly feeding, but this finding was of low certainty. However, we found low‐certainty evidence that a shorter feeding interval of two hours may result in a slightly longer time to regain birth weight. It is unlikely that this small difference of about one day is clinically meaningful.
We are uncertain whether shorter feeding intervals have any effect on any of our secondary outcomes including duration of hospitalisation (very low‐certainty evidence) and NEC (low certainty), feeding intolerance (low certainty) and hypoglycaemia (low certainty).
There were insufficient data available to perform a meta‐analysis on important secondary outcomes such as all‐cause mortality, mortality before discharge, growth during hospital stay and incidence of BPD. No studies reported long‐term growth or neurodevelopment.
Overall completeness and applicability of evidence
Most participants in the four included studies belonged to the group of moderately severe preterm infants, the mean gestational age ranging from 30 to 33 weeks, with birth weights of 1000 to 1500 g. Only one study included extremely low birth weight infants (Tali 2016).
The four included studies compared only intervals of two‐ and three‐hourly and for these two intervals we were unable to identify any clinically important differences. This could be because the data were derived from four small studies. There is no information from RCTs about other feeding intervals used in clinical practice such as one‐hourly or four‐hourly. There is insufficient information about the effect of the intervention on mortality, feeding complications and other adverse events. No study reported neurodevelopmental outcomes in childhood.
The findings of this review may not apply to infants with risk factors for NEC, such as extremely preterm, small‐for‐gestational age and ill infants requiring antibiotics and inotropic support as the studies excluded these infants.
The findings are therefore applicable to infants with birth weight above 1000 g who are stable. Although most studies were performed in middle‐income settings, we see no reason why the findings might not apply in other settings. The characteristics of included participants may reflect the case mix seen in either middle‐ or high‐income nations.
Even though rare complications may not be adequately addressed by the studies included in this review, the evidence from this review suggests that there may be no clinically important differences in the outcomes of infants receiving two‐hourly compared with three‐hourly enteral feeding. We did not include any data on ergonomic or cost advantages but in the absence of a firm conclusion these could be considered. Logically, three‐hourly feeding would reduce the time spent in preparing and administering feedings, and since fewer feeds need to be prepared it might also reduce the risk of errors in preparation or administration perhaps by over 30%, as well as the costs of syringes and other immediate costs related to providing gastric feeding to VLBW infants. These reductions could be significant, especially in low‐resource situations.
Non‐nutritive sucking and early examination for feeding cues are increasingly used in clinical practice resulting in the implementation of demand feeding at earlier gestations. For some preterm infants, particularly those of higher gestation, the feeding interval may become less relevant in the future.
Quality of the evidence
We used the GRADE approach to assess the certainty of evidence.
We considered the evidence to be of low certainty for almost all the outcomes. We downgraded the evidence for all outcomes because of risk of bias due to lack of blinding. The nature of the intervention is such that blinding is not possible for study personnel or outcome assessors. We considered the main outcomes to be sufficiently subjective that lack of blinding might affect them. We also downgraded for imprecision (because of wide CIs, small numbers of the reported events or participant numbers did not reach the optimal information size). For one outcome, duration of hospitalisation, we considered imprecision to be a very serious limitation because the CI was more than 11 days, which could be clinically extremely important.
Potential biases in the review process
One possible source of bias in this review is that three of the review authors also authored one of the included studies (Ibrahim 2017). However, the data extraction and risk of bias of this study were assessed by two people not involved in the study (author JJH and MAMH; see Acknowledgements). We included days to regain birth weight as a primary outcome, and it showed low‐certainty evidence for a slight increase in days to regain birth weight, and this was in fact the only potential difference between the two groups. However, in the process of conducting the review we came to consider this outcome to be less clinically important than our other primary outcome, days to achieve full feeds and so query the need to have this as a primary outcome. However, as there is some evidence that in the future it might be shown to be important, we have not changed it. Slower rate to regain birth weight has been reported as a negative predictor for growth velocity in neonates (Gao 2020). In addition, there is some evidence that higher growth velocity is associated with better neurodevelopmental outcomes (Ehrenkranz 2006; Simon 2019), and we do not yet have data on long‐term neurodevelopment. Hence, it may yet prove to be an important outcome.
We added feeding intolerance and hypoglycaemia as additional post hoc secondary outcomes as these outcomes were reported in all the included studies and could be considered important outcomes. Since feeding intolerance is the common problem encountered by premature infants, we considered that readers will be interested to know about this. However, since its definition varies widely, we urge caution in interpretation of the findings.
Agreements and disagreements with other studies or reviews
We found two other systematic reviews examining feeding interval for preterm infants (Binchy 2018; Razak 2019). Binchy and colleagues included 10 studies consisting of seven RCTs and three retrospective cohort studies. Six of the seven RCTs compared continuous feeding versus three‐hourly bolus feeding. Only one RCT comparing two‐hourly versus three‐hourly feeding was included (Binchy 2018). Razak and colleagues' systematic review included seven studies examining feeding intervals, four RCTs and three retrospective cohort studies (Razak 2019). The included RCTs were the same four that we included. The outcomes studied were days to achieve full enteral feeding and days to regain birth weight, incidence of NEC, feeding intolerance and hypoglycaemia. For the included RCTs, Razak and colleagues reported no significant differences for any outcomes in their pooled analyses (which included medians and IQRs), except the days to regain birth weight.
Authors' conclusions
Implications for practice.
The low‐certainty evidence we found in this review suggests that there may be no clinically important differences between two‐hourly and three‐hourly feeding intervals. There is insufficient information about potential feeding complications and in particular necrotising enterocolitis (NEC). No studies have looked at the effect of other feeding intervals and there are no long‐term data on neurodevelopment or growth. Hence, we cannot advocate which feeding interval approach is better between the two.
Implications for research.
Further large studies, involving a variety of settings including both low‐ and high‐income settings with adequate sample size for the main primary outcomes are needed not only to compare two‐hourly and three‐hourly feeding intervals but to also investigate one‐hourly and four‐hourly intervals. Extremely preterm infants should be included since there is an even greater need to understand the risks and benefits of feeding intervals in this high‐risk group. Such studies should be adequately powered to ensure that any difference in the primary outcome is determined. We suggest that the primary outcome be days to achieve full enteral feeding. The presence of risk factors for feeding intolerance and NEC should be described. Because lack of blinding cannot be avoided with this type of intervention, adequate description of feeding protocols is needed and ways of documenting non‐adherence would be useful. In particular, feeding protocols should be clear on how decisions are made regarding volume increments. Further studies should preferably include long‐term neurodevelopmental and growth outcomes and more data are needed on the effect of short‐ and long‐feeding intervals on mortality. Outcome assessors of long‐term outcomes should be blinded to the intervention.
History
Protocol first published: Issue 8, 2016
Acknowledgements
We thank Dr Megan Gray for replying to our enquiries regarding her study (Gray 2017).
We thank Dr Mohd Alwi Mohd Helmi for his contribution (data extraction for one of the included studies authored by three of the four review authors).
We would like to thank Cochrane Neonatal: Colleen Ovelman, former Managing Editor, Jane Cracknell, Managing Editor, Roger Soll, Co‐coordinating editor and Bill McGuire, Co‐coordinating Editor, who provided editorial and administrative support. Carol Friesen, former Information Specialist, designed and ran the updated literature searches, and Colleen Ovelman peer reviewed the Ovid MEDLINE search strategy.
Prakeshkumar Shah, Eugene Dempsey, William McGuire and Sarah Hodgkinson peer reviewed and offered feedback for this review.
Appendices
Appendix 1. 2020 Search methods
The RCT filters have been created using Cochrane's highly sensitive search strategies for identifying randomised trials (Higgins 2020). The neonatal filters were created and tested by the Cochrane Neonatal Information Specialist.
CENTRAL via CRS Web
Date ranges: 01 January 2016 to 25 June 2020 Terms: 1 MESH DESCRIPTOR Enteral Nutrition EXPLODE ALL AND CENTRAL:TARGET 2 MESH DESCRIPTOR Feeding Methods EXPLODE ALL AND CENTRAL:TARGET 3 (enteral* or feed* or fed) AND CENTRAL:TARGET 4 #1 OR #2 OR #3 5 MESH DESCRIPTOR Time Factors EXPLODE ALL AND CENTRAL:TARGET 6 (schedul* or interval* or hour* or bolus* or intermittent* or time* or ad libitum or continuous*) AND CENTRAL:TARGET 7 #6 OR #5 8 MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET 9 infant or infants or infant's or "infant s" or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET 10 #9 OR #8 AND CENTRAL:TARGET 11 #4 AND #7 AND #10 12 2016 TO 2020:YR AND CENTRAL:TARGET 13 #12 AND #11
MEDLINE via Ovid
Date ranges: 01 January 2016 to 25 June 2020 Terms: 1. exp Enteral Nutrition/ 2. exp Feeding Methods/ 3. (enteral* or feed* or fed).mp. 4. or/1‐3 5. exp Time Factors/ 6. (schedul* or interval* or hour* or bolus* or intermittent* or time* or ad libitum or continuous*).mp. 7. 5 or 6 8. exp infant, newborn/ 9. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or 'infant s' or infant's or infantile or infancy or neonat*).ti,ab. 10. 8 or 9 11. randomized controlled trial.pt. 12. controlled clinical trial.pt. 13. randomized.ab. 14. placebo.ab. 15. drug therapy.fs. 16. randomly.ab. 17. trial.ab. 18. groups.ab. 19. or/11‐18 20. exp animals/ not humans.sh. 21. 19 not 20 22. 10 and 21 23. randomi?ed.ti,ab. 24. randomly.ti,ab. 25. trial.ti,ab. 26. groups.ti,ab. 27. ((single or doubl* or tripl* or treb*) and (blind* or mask*)).ti,ab. 28. placebo*.ti,ab. 29. 23 or 24 or 25 or 26 or 27 or 28 30. 9 and 29 31. limit 30 to yr="2018 ‐Current" 32. 22 or 31 33. 4 and 7 and 32 34. limit 33 to yr="2016 ‐Current"
CINAHL via EBSCOhost
Date ranges: 01 January 2016 to 25 June 2020 Terms: (enteral* or feed* or fed) AND (schedul* or interval* or hour* or bolus* or intermittent* or time* or ad libitum or continuous*) AND (infant or infants or infant’s or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Limiters ‐ Published Date: 20160101‐20201231
ISRCTN
Date ranges: 2016 to 2020 Terms: bolus feeding within Participant age range: Neonate enteral feeding AND bolus within Participant age range: Neonate enteral AND feeding AND schedule within Participant age range: Neonate enteral AND feeding AND intermittent within Participant age range: Neonate enteral AND feeding AND interval within Participant age range: Neonate enteral AND feeding AND continuous within Participant age range: Neonate
Appendix 2. Previous search methods
We initially conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 9) in The Cochrane Library; MEDLINE via PubMed (1966 to October 5, 2016); Embase (1980 to October 5, 2016); and CINAHL (1982 to October 5, 2016) using the following search terms: (Enteral Feeding OR Enteral Nutrition OR Intermittent Feeding OR Bolus Feeding OR Scheduled Feeding OR Ad libitum Feeding OR Continuous Feeding), plus database‐specific limiters for RCTs and neonates. We did not apply language restrictions.
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Appendix 3. Risk of bias tool
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
Selective reporting bias. Were reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified outcomes of interest and were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Short versus long feeding interval.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Days to achieve full enteral feeding | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 All infants | 3 | 351 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐1.60, 0.36] |
| 1.1.2 Weight < 1000 g | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐5.56, ‐0.24] |
| 1.1.3 Weight > 1000 g | 2 | 228 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐1.47, 0.82] |
| 1.2 Days to regain birth weight | 3 | 350 | Mean Difference (IV, Fixed, 95% CI) | 1.15 [0.11, 2.20] |
| 1.3 Mortality | 2 | 270 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.24, 4.16] |
| 1.4 Culture‐positive sepsis | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.30, 1.74] |
| 1.5 Duration of hospital stay | 2 | 207 | Mean Difference (IV, Fixed, 95% CI) | ‐3.36 [‐9.18, 2.46] |
| 1.6 Necrotising enterocolitis | 4 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.54, 2.11] |
| 1.7 Feeding intolerance | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 All Infants | 4 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.82, 1.50] |
| 1.7.2 Birth weight < 1000 g | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.40, 1.19] |
| 1.7.3 Birth weight ≥ 1000 g | 2 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.82, 1.85] |
| 1.8 Hypoglycaemia | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 All infants | 4 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.45, 1.75] |
| 1.8.2 Birth weight < 1000 g | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.07, 14.79] |
| 1.8.3 Birth weight ≥ 1000 g | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.62] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anushree 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 60 infants Setting: NICU of a teaching hospital in North India Dates: October 2012 to March 2014 Inclusion criteria
In 2‐hourly group: 28/30 infants had BW 1000–1499 g, 22/30 infants categorised as gestational age 30–33 weeks, 4/30 infants < 30 weeks and 4/30 infants > 33 weeks In 3‐hourly group: 28/30 infants had BW 1000–1499 g, 22/30 infants categorised as gestational age 30–33 weeks, 4/30 infants < 30 weeks and 4/30 infants > 33 weeks Exclusion criteria
|
|
| Interventions |
3‐hourly vs 2‐hourly feeding schedule Infants were fed 2‐hourly or 3‐hourly by indwelling orogastric tube using the gravity method. EBM was the milk of choice, but low BW formula was given if EBM was inadequately available. Feeds were gradually advanced by 20 mL/kg/day until full feeds of 150 mL/kg/day based on clinical judgement of managing doctors The infants were started on trophic feeding (minimal enteral feeding) before being assessed for increment of feeding and being randomised to the study |
|
| Outcomes |
Primary outcome
Other outcomes
|
|
| Notes | Infants started on trophic feeding (minimal volumes) before being assessed for increment of feeding and being randomised to the study. No funding received and the researchers declared there were no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using computer‐generated random sequence generation." |
| Allocation concealment (selection bias) | Low risk | Quote: "… was ensured by serially numbered, sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the participants and medical personnel was not possible due to nature of the intervention. The type of intervention may have influenced the management of the healthcare providers. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Clinicians were not blinded; however, the outcomes were objectively planned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and were analysed. |
| Selective reporting (reporting bias) | Low risk | Feeding protocol was explained in the methodology. All outcomes outlined were reported. |
| Other bias | Low risk | No other sources of significant bias identified. |
Dhingra 2009.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 92 preterm infants; 87 infants were analysed Setting: NICU in North India Dates: February 2005 to September 2005 Inclusion criteria
In 3‐hourly group: mean gestational age 30.9 weeks (SD 2.1), mean BW 1210 g (SD 249), 16% SGA In 2‐hourly group: mean gestational age 31.6 weeks (SD 2.3), mean BW 1249 g (SD 250), 16% SGA Exclusion criteria
|
|
| Interventions |
3‐hourly vs 2‐hourly feeding schedule Feeds administered by an indwelling orogastric tube. EBM was the milk of choice; however, if EBM was inadequate, low BW formula was used. The feed increments were made as per standard protocol and clinical judgement by the treating physician. Neonates were followed up until discharge The infants were started on trophic feeding (minimal enteral feeding) before being assessed for increment of feeding and being randomised to the study |
|
| Outcomes |
Primary outcome
Other outcomes
|
|
| Notes |
No funding stated, but the researchers declared there were no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated random numbers." |
| Allocation concealment (selection bias) | Low risk | Use of (quote:) "group allocation was concealed in consecutively numbered sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and medical personnel was not possible due to the nature of the study. The type of intervention may have influenced the management of the healthcare providers. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The clinicians were not blinded, but some of the outcomes were objectively defined (feeding intolerance) or objectively measured such as hypoglycaemia. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
|
| Selective reporting (reporting bias) | Low risk | Study protocol and flow chart of study included, and all outcomes reported. |
| Other bias | Low risk | No other sources of significant biases identified. |
Ibrahim 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 150 preterm infants; 144 infants analysed Setting: NICU of tertiary teaching hospital, Hospital USM and NICU in Hospital Sultanah NurZahirah Dates: 1 June 2011 to 30 September 2012 Inclusion criteria
In 2‐hourly group: mean BW 1.27 kg (SD 0.15), mean gestational age 30.4 weeks (SD 2.3), 22.7% SGA In 3‐hourly group: mean BW 1.3 kg (SD 0.13), mean gestational age 30.9 week (SD 2.2), 26.7% SGA Exclusion criteria
|
|
| Interventions |
3‐hourly vs 2‐hourly feeding schedule Feeding started at 3‐hourly or 2‐hourly intervals with EBM at 10–20 mL/kg/day via orogastric tube. The feeds were given over 15–30 minutes using the gravity method. Preterm formula was given if EBM was not sufficiently available. Feeds were increased by 10–20 mL/kg every day based on clinical judgement of managing doctors. Infants were followed until they regained their BW and had reached full enteral feeding, defined as able to tolerate oral feeding of 100 mL/kg/day for at least 48 hours without parenteral nutrition or hypoglycaemia |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Analysed different numbers of participants for each of outcome Days to achieve enteral feeding: 144/150 infants analysed (6 died) Days to regain BW: 143/150 infants analysed (7 died) NEC: 150/150 analysed Feeding intolerance: 150/150 analysed Hypoglycaemia: 145/150 infants analysed (5 had no documented blood sugar in the patients' monitoring chart) Infants were considered to have completed the study once they achieved full enteral feeding and regained their BW. This study was funded by a short‐term grant from Universiti Sains Malaysia. All the researchers declared there were no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote. "Computer generated random numbers." |
| Allocation concealment (selection bias) | Low risk | Quote. "Group allocation was concealed consecutively numbered sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and medical personnel was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors knew the group assignment; however, the outcomes were predefined objectively. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 infants died before achieving the outcomes. incomplete data 4.6% |
| Selective reporting (reporting bias) | Low risk | Study protocol available. All outcomes were reported |
| Other bias | Low risk | No other risk of bias identified. |
Tali 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 120 infants Setting: NICU in Mumbai, India Inclusion criteria
In 2‐hourly group: mean gestational age 30.1 weeks (SD 2.84), mean BW 1176 g (SD 249), 20% SGA In 3‐hourly group: mean gestational age 30.5 weeks (SD 2.68), mean BW 1139 g (SD 225), 14% SGA Exclusion criteria
|
|
| Interventions |
3‐hourly vs 2‐hourly feeding schedule Initial feeding volume 20 mL/kg/day, which was gradually advanced by 20 mL/kg/day until full feeds were reached |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Data were available for BW subgroups. No date of when the study was conducted. The study was not funded and the researchers declared there were no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated random sequence. Stratified for BW 501‐1000g and 1001 to 1500g." |
| Allocation concealment (selection bias) | Low risk | Quote: "… consecutively numbered sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open labelled, no blinding because of the nature of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported; however, the outcomes were clearly defined. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other sources of risk of biases identified. |
BW: birth weight; EBM: expressed breast milk; IVH: intraventricular haemorrhage; NEC: necrotising enterocolitis; NICU: neonatal intensive care unit; SD: standard deviation: SGA: small for gestational age.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chu 2019 | Retrospective study. |
| DeMauro 2011 | Retrospective study. |
| Gray 2017 | We contacted the authors for further information about the study before deciding to exclude the study from our review. The study assessed the transition from gavage feeding to oral feeding by introducing a 3‐hourly or 6‐hourly oral feeding interval. The infants received 120 mL/kg/day of gavage feeding at the time of randomisation, which was considered as full gavage feeding. Therefore, participants did not fulfil our inclusion criteria. |
| Hussain 2018 | Study compared the frequency advancement (starting at 8‐hourly and increasing to 2‐hourly with feed volume of 10 mL/kg/day) vs volume advancement (20 mL/kg/day 3 hourly). |
| Rüdiger 2008 | Retrospective study. |
| Unal 2019 | Randomisation occurred when the infants weighed 1500 g and had already reached full enteral gavage feeding. |
| Zubani 2016 | Compared the frequency advancement (feeding started at 8 hourly then increased to 3 hourly with volume increments of 10 ml/kg/day) vs volume advancement (feeding started at 2 hourly with an increment of volume by 20–25 mL/kg/day). |
Characteristics of studies awaiting classification [ordered by study ID]
Yadav 2021.
| Methods | Randomised controlled trial |
| Participants | 350 preterm infants Setting: NICU in Delhi, India Dates: January 2018 to March 2019 Inclusion criteria
(The authors also included heavier infants who were not receiving tube feeding but were receiving direct breastfeeding, cup feeding or paladai feeding. These infants do not meet our inclusion criteria.) Exclusion criteria
|
| Interventions |
3‐hourly vs 2‐hourly feeding schedule
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | We contacted authors for further details about the study. This study included infants receiving tube feeding who could be included in this review but also an undefined proportion of infants receiving oral feeding (direct breastfeeding or using cup or paladai) who, if a substantial proportion, would be excluded from the review. We have requested the data for the smaller infants who were receiving tube feeding and are awaiting a response. The study was not funded and there were no conflicts of interest among the researchers. |
NEC: necrotising enterocolitis; NICU: neonatal intensive care unit.
Differences between protocol and review
2020
We made the following changes to the published protocol (Ibrahim 2016). We updated the 'Certainty of evidence' and 'Risk of bias' sections.
As of July 2019, Cochrane Neonatal no longer searches Embase for its reviews. Randomised controlled trials (RCTs) and controlled clinical trials (CCTs) from Embase are added to the Cochrane Central Register of Controlled Trials (CENTRAL) via a robust process (see How CENTRAL is created). Cochrane Neonatal has validated their searches to ensure that relevant Embase records are found while searching CENTRAL.
Also starting in July 2019, Cochrane Neonatal no longer searches for RCTs and CCTs on the following platforms: ClinicalTrials.gov or from The World Health Organisation's International Clinical Trials Registry Platform (ICTRP), as records from both platforms are added to CENTRAL on a monthly basis (see How CENTRAL is created). Comprehensive search strategies are executed in CENTRAL to retrieve relevant records. The ISRCTN Registry (www.isrctn.com/, formerly Controlled‐trials.com), is searched separately.
In 2020, we ran searches in the following databases: CENTRAL via CRS Web, MEDLINE via Ovid and CINAHL via EBSCOhost. The search strategies are available in Appendix 1. The previous search methods are available in Appendix 2. We used Cochrane's Screen4Me workflow to help assess the search results.
We standardised the objectives.
During the process of conducting the review we added two additional outcomes, feeding intolerance and hypoglycaemia. This was because all the included studies had reported these two outcomes and since we judged them to be clinically important, we have included them as a post hoc decision.
Contributions of authors
NRI: completed subsequent drafts with substantial input from JJH.
HVR: conceived the project and wrote the first draft of the Background and part of the Methods.
JJH extracted the data from the included study authored by NRI, HVR and AN (Ibrahim 2017).
AN: completed the Methods section part of the discussion.
All review authors commented on the final version of the review.
Sources of support
Internal sources
School of Medical Sciences, Universiti Sains Malaysia, Malaysia
RCSI & UCD Malaysia Campus (formerly Penang Medical College), Malaysia
External sources
-
South East Asia – Optimising Reproductive and Child Health in Developing Countries (SEA‐ORCHID project), Malaysia
The Wellcome Trust‐funded project provided support for initiation of this review
-
National Institute for Health Research, UK
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR, or the UK Department of Health.
-
The Gerber Foundation, USA
Editorial support for this review, as part of a suite of preterm nutrition reviews, has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private, 501(c)(3) foundation not related to Gerber Products Company in any way.
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
NRI was the corresponding author of a study that is included in this review (Ibrahim 2017). This study was funded by a short‐term Grant from School of Medical Sciences (PPSP), Universiti Sains Malaysia (USM). NRI is a General Paediatrician interested in neonatology at USM.
HVR was the author of a study that is included in this review (Ibrahim 2017). This study was funded by a short‐term Grant from School of Medical Sciences (PPSP), Universiti Sains Malaysia (USM).
JJH has no interest to declare. JJH extracted the data from Ibrahim 2017.
AN was the author of a study that is included in this review (Ibrahim 2017). This study was funded by a short‐term Grant from School of Medical Sciences (PPSP), Universiti Sains Malaysia (USM). AN is a General Paediatrician at USM.
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent from the Gerber Products Company. The grantor has no input on the content of the review or the editorial process (Sources of support).
New
References
References to studies included in this review
Anushree 2018 {published data only}
- Anushree N, Shaw S, Negi V. 2 hourly versus 3 hourly feeding schedule in very low birth weight preterm neonates. Journal of Marine Medical Society 2018;20(2):96-9. [DOI: 10.4103/jmms.jmms_18_18] [DOI] [Google Scholar]
Dhingra 2009 {published data only}
- Dhingra A, Agrawal SK, Kumar P, Narang A. A randomised controlled trial of two feeding schedules in neonates weighing ≤1750 g. Journal of Maternal-fetal & Neonatal Medicine 2009;22(3):198-203. [DOI: 10.1080/14767050802385749] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ibrahim 2017 {published data only}
- Ibrahim NR, Kheng TH, Nasir A, Ramli N, Foo JL, Syed Alwi SH, et al. Two-hourly versus 3-hourly feeding for very low birthweight infants: a randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2017;102(3):F225-9. [DOI: 10.1136/archdischild-2015-310246] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tali 2016 {published data only}
- Tali SH, Kabra NS, Ahmed J, Dash SK, Balasubramanian H, Avasthi BS, et al. Effect of feeding schedule on time to reach full feeds in ELBW and VLBW neonates: a randomised trial. Perinatology 2016;17(3):95-102. [Google Scholar]
References to studies excluded from this review
Chu 2019 {published data only}
- Chu E, Freck S, Zhang L, Bhakta KY, Mikhael M. Three-hourly feeding intervals are associated with faster advancement in very preterm infants. Early Human Development 2019;131:1-5. [DOI: 10.1016/j.earlhumdev.2019.01.021] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
DeMauro 2011 {published data only}
- DeMauro SB, Abbasi S, Lorch S. The impact of feeding interval on feeding outcomes in very low birth-weight infants. Journal of Perinatology 2011;31(7):481-6. [DOI: 10.1038/jp.2010.153] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gray 2017 {published data only}
- Gray MM, Medoff-Cooper B, Enlow EM, Mukhopadhyay S, DeMauro SB. Every three-hour versus every six-hour oral feeding in preterm infants: a randomised clinical trial. Acta Paediatrica 2017;106(2):236-41. [DOI: 10.1111/apa.13658] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hussain 2018 {published data only}
- Hussain A, Rehman A, Fatima N. Comparison of volume and frequency advancement feeding protocols in very low birth weight neonates. Pakistan Journal of Medical Sciences 2018;34(1):78-81. [DOI: 10.12669/pjms.341.14092] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rüdiger 2008 {published data only}
- Rüdiger M, Herrmann S, Schmalisch G, Wauer RR, Hammer H, Tschirch E. Comparison of 2-h versus 3-h enteral feeding in extremely low birth weight infants, commencing after birth. Acta Paediatrica 2008;97(6):764-9. [DOI: 10.1111/j.1651-2227.2008.00774.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Unal 2019 {published data only}
- Unal S, Demirel N, Bas AY, Arifoğlu İ, Erol S, Ulubas Isik D. Impact of feeding interval on time to achieve full oral feeding in preterm infants: a randomized trial. Nutrition in Clinical Practice 2019;34(5):783-8. [DOI: 10.1002/ncp.10244] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zubani 2016 {published data only}
- Zubani A, Mersal A, AlSaed S, AlAhmadi K, AlDeek A, Sadiq Bb. Comparison of volume and frequency based feeding protocols in very low birth weight infants: a prospective randomized study. Journal of Clinical Neonatology 2016;5(4):233-7. [Google Scholar]
References to studies awaiting assessment
Yadav 2021 {published data only}
- Yadav A, Siddiqui N, Debata PK. Two-hourly versus three-hourly feeding in very low birthweight neonates: a randomized controlled trial. Indian Pediatrics 2021;58(4):320-4. [CTRI/2018/01/016630] [S097475591600274] [PMID: ] [PubMed] [Google Scholar]
Additional references
Ayede 2011
- Ayede AI. Achieving optimal feeds for preterm babies, recommendations and realities in practice: Nigerian perspective. Annals of Ibadan Postgraduate Medicine 2011;9(1):1-7. [DOI: 10.4314/aipm.v9i1.72427] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aynsley‐Green 1982
- Aynsley-Green A, Adrian TE, Bloom SR. Feeding and the development of enteroinsular hormone secretion in the preterm infant: effects of continuous gastric infusions of human milk compared with intermittent boluses. Acta Paediatrica Scandinavica 1982;71(3):379-83. [DOI: 10.1111/j.1651-2227.1982.tb09438.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1-7. [DOI: 10.1097/00000658-197801000-00001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berseth 1992
- Berseth CL. Effect of early feeding on maturation of the preterm infant’s small intestine. Journal of Pediatrics 1992;120(6):947-53. [DOI: 10.1016/s0022-3476(05)81969-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Binchy 2018
- Binchy Á, Moore Z, Patton D. Feeding intervals in premature infants ≤ 1750g: an integrative review. Advances in Neonatal Care 2018;18:168-78. [DOI: 10.1097/AN.0000000000000486] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dunn 1988
- Dunn L, Hulman S, Weiner J, Kleigman R. Beneficial effects of early hypocaloric enteral feeding on neonatal gastrointestinal function: preliminary report of a randomized trial. Journal of Pediatrics 1988;112(4):622-9. [DOI: 10.1016/s0022-3476(88)80185-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ehrenkranz 2006
- Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight. Pediatrics 2006;117(4):1253-61. [DOI: 10.1542/peds.2005-1368] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gao 2020
- Gao C, Ehsan L, Jones M, Khan M, Middleton J, Vergales B, et al. Time to regain birth weight predicts neonatal growth velocity: a single-center experience. Clinical Nutrition ESPEN 2020;38:165-71. [DOI: 10.1016/j.clnesp.2020.05.010] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gartner 2005
- Gartner LM, Morton J, Lawrence RA, Naylor AJ, O'Hare D, Schanler RJ, et al, American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2005;115(2):496-506. [DOI: 10.1542/peds.2004-2491] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gibertoni 2015
- Gibertoni D, Corvaglia L, Vandini S, Rucci P, Savini S, Alessandroni R, et al. Positive effect of human milk feeding during NICU hospitalization on 24 month neurodevelopment of very low birth weight infants: an Italian cohort study. PloS One 2015;10(1):e0116552. [DOI: 10.1371/journal.pone.0116552] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version 29 December 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Henderson 2009
- Henderson G, Craig S, Brocklehurst P, McGuire W. Enteral feeding regimens and necrotising enterocolitis in preterm infants: a multicentre case-control study. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(2):F120-3. [DOI: 10.1136/adc.2007.119560] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Horwood 2001
- Horwood LJ, Darlow BA, Mogridge N. Breast milk feeding and cognitive ability at 7–8 years. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;84:F23-7. [DOI: 10.1136/fn.84.1.f23] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klingenberg 2011
- Klingenberg C, Embleton ND, Jacobs SE, O'Connell LA, Kuschel CA. Enteral feeding practices in very preterm infants: an international survey. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;97(1):F56-61. [DOI: 10.1136/adc.2010.204123] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lane 1998
- Lane AJ, Coombs RC, Evans DH, Levin RJ. Effect of feed interval and feed type on splanchnic haemodynamics. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;79(1):F49-53. [DOI: 10.1136/fn.79.1.f49] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lucas 1986
- Lucas A, Bloom SR, Aynsley-Green A. Gut hormones in minimal enteral feeding. Acta Paediatrica 1986;75(5):719-23. [DOI: 10.1111/j.1651-2227.1986.tb10280.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lucas 1992
- Lucas A, Morley R, Cole TJ, Lister G, Leeson-Payne C. Breast milk and subsequent intelligence quotient in children born preterm. Lancet 1992;339(8788):261-4. [DOI: 10.1016/0140-6736(92)91329-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner’s guide. Research Synthesis Methods 2018;9(4):602-14. [DOI: 10.1002/jrsm.1287] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McClure 2001
- McClure RJ. Trophic feeding of the preterm infant. Acta Paediatrica Supplement 2001;90(436):19-21. [DOI: 10.1111/j.1651-2227.2001.tb01623.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morgan 1987
- Morgan W 3rd, Yardley J, Luk G, Niemiec P, Dudgeon D. Total parenteral nutrition and intestinal development: a neonatal model. Journal of Pedaitric Surgery 1987;22(6):541-5. [DOI: 10.1016/s0022-3468(87)80217-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morgan 2013
- Morgan J, Bombell S, McGuire W. Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD000504. [DOI: 10.1002/14651858.CD000504.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2014
- Morgan J, Young L, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD001970. [DOI: 10.1002/14651858.CD001970.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 1983
- Murphy JF, Neale ML, Matthews N. Antimicrobial properties of preterm breast milk cells. Archives of Disease in Childhood 1983;58(3):198-200. [DOI: 10.1136/adc.58.3.198] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noel‐Storr 2020
- Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane Centralised Search Service showed high sensitivity identifying randomised controlled trials: a retrospective analysis. Journal of Clinical Epidemiology 2020;127:142-50. [DOI: 10.1016/j.jclinepi.2020.08.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2021
- Noel-Storr AH, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowd sourcing produced accurate results in identifying randomised trials. Journal of Clinical Epidemiology 2021;S0895-4356(21):00008-1. [DOI: 10.1016/j.jclinepi.2021.01.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Oddie 2017
- Oddie SJ, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD001241. [DOI: 10.1002/14651858.CD001241.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2007
- Patel AB, Shaikh S. Efficacy of breast milk gastric lavage in preterm neonates. Indian Pediatrics 2007;44(3):199-203. [PMID: ] [PubMed] [Google Scholar]
Patole 2005
- Patole SK, Klerk N. Impact of standardised feeding regimens on incidence of neonatal necrotising enterocolitis: a systematic review and meta-analysis of observational studies. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(2):147-51. [DOI: 10.1136/adc.2004.059741] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Razak 2019
- Razak A. Two-hourly versus three-hourly feeding in very low-birth-weight infants: a systematic review and meta-analysis. American Journal of Perinatology 2019;37(9):1-9. [DOI: 10.1055/s-0039-1691767] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager (RevMan). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Siddell 1994
- Siddell EP, Froman RD. A national survey of neonatal intensive-care units: criteria used to determine readiness for oral feedings. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1994;23(9):783-9. [DOI: 10.1111/j.1552-6909.1994.tb01953.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Simon 2019
- Simon L, Hanf M, Frondas-Chauty A, Darmaun D, Rouger V, Gascoin G, et al. Neonatal growth velocity of preterm infants: the weight Z-score change versus Patel exponential model. PloS One 2019;14(6):e46. [DOI: 10.1371/journal.pone.0218746] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Slagle 1988
- Slagle TA, Gross SJ. Effect of early low volume enteral substrate on subsequent feeding tolerance in low birth weight infants. Journal of Pediatrics 1988;113(3):526-31. [DOI: 10.1016/s0022-3476(88)80646-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thomas 2020
- Thomas J, McDonald S, Noel-Storr AH, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduces workload with minimal risk of missing studies: development and evaluation of an RCT classifier for Cochrane Reviews. Journal of Clinical Epidemiology 2020;S0895-4356(20):31172-0. [DOI: 10.1016/j.jclinepi.2020.11.003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ibrahim 2016
- Ibrahim NR, Van Rostenberghe H, Ho JJ. Short versus long feeding interval for bolus feedings in very preterm infants. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD012322. [DOI: 10.1002/14651858.CD012322] [DOI] [PMC free article] [PubMed] [Google Scholar]


