Abstract
Background
Early enteral feeding practices are potentially modifiable risk factors for necrotising enterocolitis (NEC) in very preterm or very low birth weight (VLBW) infants. Observational studies suggest that conservative feeding regimens, including slowly advancing enteral feed volumes, reduce the risk of NEC. However, it is unclear whether slow feed advancement may delay establishment of full enteral feeding, and if it could be associated with infectious morbidities secondary to prolonged exposure to parenteral nutrition.
Objectives
To determine the effects of slow rates of enteral feed advancement on the risk of NEC, mortality, and other morbidities in very preterm or VLBW infants.
Search methods
We searched CENTRAL (2020, Issue 10), Ovid MEDLINE (1946 to October 2020), Embase via Ovid (1974 to October 2020), Maternity and Infant Care database (MIDIRS) (1971 to October 2020), CINAHL (1982 to October 2020), and clinical trials databases and reference lists of retrieved articles for eligible trials.
Selection criteria
We included randomised or quasi‐randomised controlled trials that assessed effects of slow (up to 24 mL/kg/d) versus faster rates of advancement of enteral feed volumes on the risk of NEC in very preterm or VLBW infants.
Data collection and analysis
Two review authors separately evaluated trial risk of bias, extracted data, and synthesised effect estimates using risk ratio (RR), risk difference (RD), and mean difference. We used the GRADE approach to assess the certainty of evidence. Outcomes of interest were NEC, all‐cause mortality, feed intolerance, and invasive infection.
Main results
We included 14 trials involving a total of 4033 infants (2804 infants participated in one large trial). None of the trials masked parents, caregivers, or investigators. Risk of bias was otherwise low. Most infants were stable very preterm or VLBW infants of birth weight appropriate for gestation. About one‐third of all infants were extremely preterm or extremely low birth weight (ELBW), and about one‐fifth were small for gestational age, growth‐restricted, or compromised as indicated by absent or reversed end‐diastolic flow velocity in the foetal umbilical artery. Trials typically defined slow advancement as daily increments of 15 to 24 mL/kg, and faster advancement as daily increments of 30 to 40 mL/kg.
Meta‐analyses showed that slow advancement of enteral feed volumes probably has little or no effect on the risk of NEC (RR 1.06, 95% confidence interval (CI) 0.83 to 1.37; RD 0.00, 95% CI −0.01 to 0.02; 14 trials, 4026 infants; moderate‐certainty evidence) or all‐cause mortality prior to hospital discharge (RR 1.13, 95% CI 0.91 to 1.39; RD 0.01, 95% CI −0.01 to 0.02; 13 trials, 3860 infants; moderate‐certainty evidence). Meta‐analyses suggested that slow advancement may slightly increase feed intolerance (RR 1.18, 95% CI 0.95 to 1.46; RD 0.05, 95% CI −0.02 to 0.12; 9 trials, 719 infants; low‐certainty evidence) and may slightly increase the risk of invasive infection (RR 1.14, 95% CI 0.99 to 1.31; RD 0.02, 95% CI −0.00 to 0.05; 11 trials, 3583 infants; low‐certainty evidence).
Authors' conclusions
The available trial data indicate that advancing enteral feed volumes slowly (daily increments up to 24 mL/kg) compared with faster rates probably does not reduce the risk of NEC, death, or feed intolerance in very preterm or VLBW infants. Advancing the volume of enteral feeds at a slow rate may slightly increase the risk of invasive infection.
Plain language summary
Slowly advancing milk feeds to prevent necrotising enterocolitis in very low birth weight infants
Review question
Does limiting the rate of increase in milk feeds that very low birth weight infants receive each day during the first few weeks after birth reduce the risk of severe bowel problems?
Background
Very preterm (born more than eight weeks early) or very low birth weight (weighing < 1500 grams at birth) newborn babies are at risk of developing a severe bowel disorder called necrotising enterocolitis (where the bowel becomes inflamed and dies). It is thought that one way to prevent this condition may be to limit the milk feeds that infants receive each day for the first few weeks after birth.
Study characteristics
We searched for randomised controlled trials (a type of study where participants are randomly assigned to one of two or more treatment groups) comparing slow versus faster rates of increase in the amount of milk fed to newborn infants who were very preterm or very low birth weight. We included 14 trials involving a total of 4033 infants (2804 infants participated in one large trial). The search is up‐to‐date as of October 2020.
Key results
Combined analysis of the included trials showed that slow advancement of enteral feed volumes probably does not affect the risk of necrotising enterocolitis or death (moderate‐certainty evidence).
Conclusions and certainty of evidence
Slowly advancing enteral feed volumes probably does not reduce the risk of necrotising enterocolitis or death before hospital discharge for very preterm or very low birth weight infants.
Summary of findings
Summary of findings 1. Slow compared with faster rates of enteral feed advancement for preventing necrotising enterocolitis in very preterm or very low birth weight infants.
| Slow compared with faster rates of enteral feed advancement for preventing necrotising enterocolitis in very preterm or very low birth weight infants | |||||
| Patient or population: very preterm or very low birth weight infants Setting: neonatal care facilities in North America, Colombia, Bangladesh, Iran, India, Turkey, South Africa, Ireland, and the UK Intervention: slow rates of enteral feed advancement Comparison: faster rates of enteral feed advancement | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with faster rates of enteral feed advancement | Risk with slow rates of enteral feed enhancement | ||||
| Necrotising enterocolitis before hospital discharge | 54 per 1000 | 57 per 1000 (45 to 77) | RR 1.06 (0.83 to 1.37) | 4026 (14 trials) | ⊕⊕⊕⊝ MODERATEa |
| Mortality before hospital discharge | 71 per 1000 | 80 per 1000 (64 to 98) | RR 1.13 (0.91 to 1.39) | 3860 (13 trials) | ⊕⊕⊕⊝ MODERATEa |
| Feed intolerance before hospital discharge | 282 per 1000 | 333 per 1000 (268 to 412) | RR 1.18 (0.95 to 1.46 | 719 (9 trials) | ⊕⊕⊝⊝ LOWa, b |
| Invasive infection before hospital discharge | 170 per 1000 | 194 per 1000 (168 to 223) | RR 1.14 (0.99 to 1.31) | 3583 (11 trials) | ⊕⊕⊝⊝ LOWa, b |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level for serious study limitations (risk of bias due to lack of masking of clinicians, caregivers, and investigators in trials). bDowngraded one level for serious imprecision of effect estimate (95% CI around estimate consistent with substantial harm or no effect).
Background
Description of the condition
Necrotising enterocolitis (NEC), a syndrome of acute intestinal necrosis of unknown aetiology, affects about 5% of very preterm (< 32 weeks) or very low birth weight (VLBW) (< 1500 grams) infants (Horbar 2012). Infants who develop NEC experience more infections, have lower levels of nutrient intake, grow more slowly, and have longer durations of intensive care and hospital stay than gestation‐comparable infants who do not develop NEC (Battersby 2018; Berrington 2012). The associated mortality rate is greater than 20%, and infants who develop NEC, especially if associated with bloodstream infections, have a higher risk of neurodevelopmental problems and disability compared with their peers (Hickey 2018; Martin 2010; Shah 2012).
In addition to low gestational age at birth, the other major perinatal risk factor for developing NEC is intrauterine growth restriction, especially if it is associated with absent or reversed end‐diastolic flow velocities in Doppler studies of the foetal aorta or umbilical artery (Bernstein 2000; Dorling 2005; Garite 2004; Luig 2005; Samuels 2017). Most very preterm or VLBW infants who develop NEC have received enteral milk feeds. Feeding with human milk rather than cow's milk formula reduces the risk of NEC (Quigley 2019). Other differences in enteral feeding regimens, such as the timing of introduction of feeds and the size of daily volume increments, may also contribute to inter‐unit variation in the incidence of NEC (Walsh 2019). Observational studies have suggested that delaying the introduction of enteral feeds beyond the first few days after birth, or increasing the volume of feeds by less than about 24 mL/kg body weight each day, is associated with a lower risk of developing NEC in very preterm or VLBW infants (Henderson 2009; Patole 2005).
Description of the intervention
Approaches to early enteral feeding vary by gestational age and clinical condition of the very preterm or VLBW infant (Hay 2018). Oral feeding is not usually possible because of neurological immaturity or respiratory distress challenging breathing, suck, and swallow co‐ordination. Newborn infants, particularly extremely preterm (< 28 weeks) or extremely low birth weight (ELBW: < 1000 grams) infants, may not be able to tolerate enteral milk feed volumes required to match nutritional requirements because of delayed gastric emptying and immature intestinal peristalsis.
Substantial variation in practice exists with regard to early enteral feeding strategies for very preterm or VLBW infants (Klingenberg 2012). In many high‐income countries, policy and practice has tended to favour the conservative approach to introducing and advancing enteral feeds for these infants because of concerns that early full enteral feeding might increase the risk of feed intolerance, gastro‐oesophageal reflux and aspiration of stomach contents, and necrotising enterocolitis in very preterm or VLBW infants (de Waard 2018; Leaf 2013; Maas 2018). In low‐ and middle‐income countries with fewer resources for neonatal care, practice is more pragmatic and tends to favour early introduction and advancement of enteral feeds (sometimes facilitated by 'kangaroo' mother care) for stable infants born at 28 weeks' gestation or greater, or with a birth weight of 1000 grams or more (Conde‐Agudelo 2016; Sankar 2008).
How the intervention might work
Slow advancement of enteral milk feed volumes aims to reduce the risk of feed intolerance and NEC by limiting the physiological and metabolic stresses on the immature gastrointestinal tract during the weeks after birth. There are, however, potential disadvantages associated with conservative enteral feeding regimens (Flidel‐Rimon 2004). Because gastrointestinal hormone secretion and motility are stimulated by milk feeds, slow enteral feed volume advancement may delay the functional adaptation of the gastrointestinal tract and disrupt the patterns of microbial colonisation (Embleton 2017). Intestinal dysmotility and dysbiosis might exacerbate feed intolerance and delay the establishment of enteral feeding independently of parenteral nutrition (Pammi 2017). Prolonging the duration of parenteral nutrition is associated with infectious and metabolic complications that increase mortality and morbidity, prolong hospital stay, and adversely affect growth and development (Embleton 2013). It has been argued that the risk of NEC should not be considered in isolation from these other potential clinical outcomes in the determination of feeding policies and practices for very preterm or VLBW infants (Flidel‐Rimon 2006; Härtel 2009).
Why it is important to do this review
Given the potential for the rate of advancement of enteral feed volumes to affect important outcomes for very preterm or VLBW infants, it is important to identify, appraise, and synthesise the available evidence from randomised controlled trials (RCTs) to inform practice and research. This review focuses on the question of whether advancing feed volumes at slow rates compared with faster rates affects the risk of NEC, mortality, and other morbidities. Other Cochrane Reviews have addressed the questions of whether delaying the introduction of any enteral milk feeding or restricting feed volumes to trophic levels (minimal enteral nutrition) affects the risk of NEC in very preterm or VLBW infants (Morgan 2013a; Morgan 2014a; Walsh 2020).
Objectives
To determine the effects of slow rates of enteral feed advancement on the risk of NEC, mortality, and other morbidities in very preterm or VLBW infants.
Methods
Criteria for considering studies for this review
Types of studies
We included controlled trials utilising random or quasi‐random participant allocation.
Types of participants
We included enterally fed very preterm (< 32 weeks) or VLBW (< 1500 grams) newborn infants.
Types of interventions
Advancement of enteral feeds at no more than 24 mL/kg (birth weight or current body weight) per day versus faster rates of feed advancement. All infants should have received the same type of milk, and in both groups advancement of feed volume should have commenced within five days of introduction of enteral feeds.
Types of outcome measures
Infant‐ and parent‐important outcomes likely to affect survival and quality of life.
Primary outcomes
-
NEC confirmed at surgery or autopsy or using standardised clinical and radiological criteria (Walsh 1986):
at least one of: bilious gastric aspirate or emesis; or abdominal distention; or blood in stool; and
at least one of: abdominal radiograph showing pneumatosis intestinalis; or gas in the portal venous system; or free air in the abdomen.
All‐cause mortality before discharge from hospital.
Secondary outcomes
-
Growth:
Time to regain birth weight and rates of weight gain, linear growth, head growth, or skinfold thickness growth up to six months (corrected for preterm birth).
Long‐term growth: weight, height, or head circumference (or proportion of infants who remained below the 10th percentile for the index population's distribution) assessed at intervals from six months of age.
Neurodevelopmental disability, defined as moderate or severe developmental delay (> two standard deviations (SD) below the mean of standardised infant developmental assessment aged > 18 months), and classifications of disability, including non‐ambulant cerebral palsy and auditory or visual impairment.
Time to establish full enteral feeding independently of parenteral nutrition.
Time to establish oral feeding (independently of parenteral nutrition or enteral tube feeding, or both).
Feed intolerance (requirement to cease enteral feeds > four hours).
Invasive infection confirmed by culture of bacteria or fungus from blood, cerebrospinal fluid, or another normally sterile body space.
Duration of hospital stay (days).
Search methods for identification of studies
We used the standard methods of Cochrane and Cochrane Neonatal.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 10; 19 October 2020) in the Cochrane Library, Ovid MEDLINE (1946 to October 2020), Embase via Ovid (1974 to 19 October 2020), Maternity and Infant Care database (MIDIRS; 1971 to 19 October 2020), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 19 October 2020) using the search terms described in Appendix 1. We did not apply language restrictions. We searched ClinicalTrials.gov (clinicaltrials.gov) for ongoing or recently completed trials.
This search updates the searches conducted for previous versions of this review (Appendix 2).
Searching other resources
We searched the reference lists of the included studies to identify any additional relevant articles.
Data collection and analysis
We used the standard methods of Cochrane Neonatal (neonatal.cochrane.org/).
Selection of studies
One review author (WM) screened the titles and abstracts of all records identified by the search, coding the records as 'order' or 'exclude'. A second review author (LY or SO) assessed all records coded as 'order' and made the final decision as to which records should be ordered as full‐text articles. Two review authors read the full texts and used a checklist to assess each article's eligibility for inclusion based on the prespecified inclusion and exclusion criteria.
Data extraction and management
Two review authors (WM and LY) independently extracted data using a data collection form to aid extraction of information on design, methods, participants, interventions, outcomes, and treatment effects from each included study. We discussed disagreements until we reached consensus. If data from trial reports were insufficient, we contacted trialists to seek further information.
Assessment of risk of bias in included studies
Two review authors (WM and SO) independently assessed risk of bias (low, high, or unclear) of all the included trials using the Cochrane risk of bias tool (Higgins 2011), for the following domains (Appendix 3).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved disagreements by discussion or by consultation with a third review author.
Measures of treatment effect
We calculated risk ratio (RR) and risk difference (RD) for dichotomous data, and mean difference (MD) for continuous data, with respective 95% confidence intervals (CIs). When we deemed it appropriate to combine two or more study arms, we obtained treatment effects from combined data using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We determined the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) for a statistically significant difference in RD.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials. Had we identified any cluster‐randomised trials, we would have undertaken analyses at the level of the individual whilst accounting for clustering in the data using the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Dealing with missing data
We requested additional data from trial investigators when data on important outcomes were missing or required clarification. When data remained missing, we examined the impact on effect size estimates by performing sensitivity analyses.
Assessment of heterogeneity
We examined the treatment effects in individual trials and heterogeneity between trial results by inspecting forest plots if more than one trial was included in a meta‐analysis. We calculated the I² statistic for each analysis to quantify inconsistency across studies and to describe the percentage of variability in effect estimates that may be due to heterogeneity rather than to sampling error. If we detected moderate or high levels of heterogeneity (I² > 50%), we explored possible causes by performing subgroup analyses.
Assessment of reporting biases
We assessed reporting bias by comparing the stated primary outcomes and secondary outcomes with the reported outcomes. Where study protocols were available, we compared these to the full publications to determine the likelihood of reporting bias. We documented studies using the interventions in a potentially eligible infant population but not reporting on any of our primary and secondary outcomes in the Characteristics of included studies tables. We used funnel plots to screen for publication bias where there were a sufficient number of studies (> 10) reporting the same outcome. If publication bias was suggested by a significant asymmetry of the funnel plot on visual assessment, we incorporated this into our assessment of the certainty of the evidence.
Data synthesis
We used a fixed‐effect model inverse‐variance meta‐analysis for combining data where trials examined the same intervention, and the populations and methods of the trials were judged to be similar.
Subgroup analysis and investigation of heterogeneity
We prespecified subgroup analyses for our primary outcomes to compare the effects in trials in which most infants were exclusively formula‐fed versus trials in which most infants were at least partially fed with human milk.
Sensitivity analysis
We undertook sensitivity analyses to explore effects on primary outcomes in:
trials in which most participants were ELBW or extremely preterm;
trials in which participants were infants with intrauterine growth restriction;
infants with absent or reversed end‐diastolic flow velocities (AREDFV) detected on antenatal Doppler studies of the foetal aorta or umbilical artery.
We planned to perform sensitivity analyses if:
there was unexplained moderate to high heterogeneity (explored by removing the outlying trial or trials);
a trial with high risk of bias (including high level of missing outcome data) was included in the meta‐analysis of an outcome where the other studies had low risk of bias (removed the study with high risk of bias).
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence for the following outcomes: NEC, mortality, feed intolerance (causing interruption of enteral feeding), invasive infection.
Two review authors (WM and LY) independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from RCTs as high certainty, downgrading the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias (Walsh 2021). We used GRADEpro GDT to create Table 1 to report the certainty of the evidence (GRADEpro GDT).
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
See Figure 1.
1.
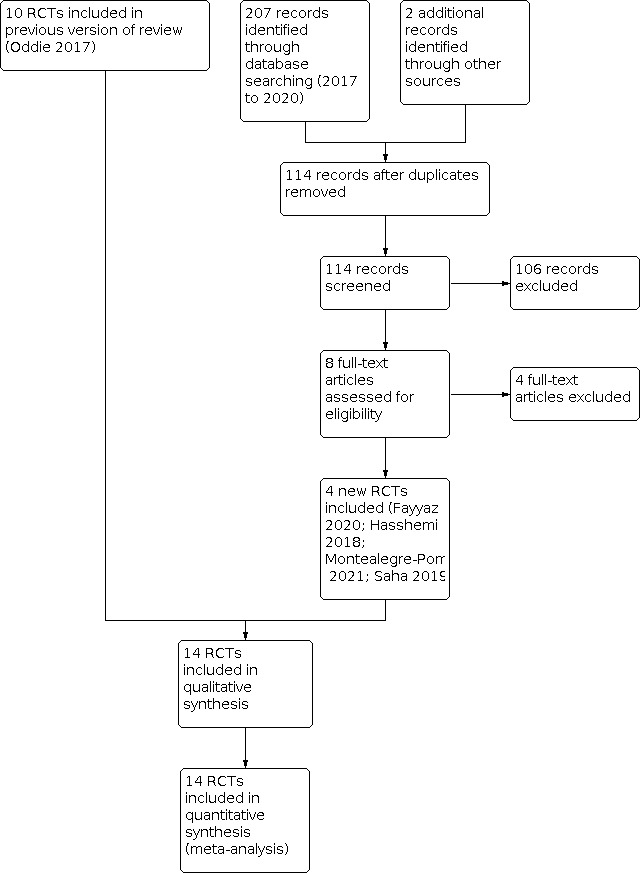
Study flow diagram: review update.
Results of the search
After removal of duplicates, we screened 114 titles and abstracts, which included forward and backward citation searches, clinical trials registers, and grey literature. We re‐evaluated previously included studies alongside eight articles sourced as full‐text reports, therefore assessing the eligibility of 18 articles.
Included studies
We included 14 RCTs in this updated review (see Characteristics of included studies) (Caple 2004; Fayyaz 2020; Hasshemi 2018; Jain 2016; Karagol 2013; Krishnamurthy 2010; Modi 2015; Montealegre‐Pomar 2021; Raban 2014a; Raban 2014b; Rayyis 1999; Saha 2019; Salhotra 2004; SIFT 2016).
Population
A total of 4033 infants participated in the 14 included trials. Almost 70% of infants were participants in a recent large, multicentre trial (SIFT 2016). Trials were undertaken at neonatal care centres in North America (Caple 2004; Rayyis 1999), Colombia (Montealegre‐Pomar 2021), Bangladesh (Saha 2019), Iran (Hasshemi 2018); India (Fayyaz 2020; Jain 2016; Krishnamurthy 2010; Modi 2015; Salhotra 2004), Turkey (Karagol 2013), South Africa (Raban 2014a; Raban 2014b), and the UK and Ireland (SIFT 2016).
All but one of the trials specified participant birth weight eligibility criteria (Hasshemi 2018 specified gestational age at birth criteria).
Caple 2004: 1000 to 2000 grams
Fayyaz 2020: < 1500 grams
Hasshemi 2018: 30 to 34 weeks
Jain 2016: 1000 to 1249 grams
Karagol 2013: 750 to 1250 grams
Krishnamurthy 2010: 1000 to 1500 grams
Modi 2015: 750 to 1250 grams
Montealegre‐Pomar 2021: < 1500 grams
Raban 2014a: < 1001 grams
Raban 2014b: < 1001 grams
Rayyis 1999: < 1500 grams
Saha 2019: < 1500 grams
Salhotra 2004: < 1250 grams
SIFT 2016: < 1500 grams
Most of the participants in Caple 2004, Hasshemi 2018, and Jain 2016 were of birth weight less than 1500 grams or gestational age less than 32 weeks, therefore we made a consensus decision to include these trials. Infants born 'small for gestational age' (birth weight < 10th percentile of the index population distribution) were not eligible to participate in Caple 2004 or Saha 2019, but were included in the other trials. More than 95% of participants in Salhotra 2004 were small for gestational age, and all participants in Jain 2016 had antenatal evidence of absent or reversed end‐diastolic flow.
Interventions and comparisons
All trials commenced interval bolus intragastric feeding within the first seven days after birth. Infants were randomly allocated to one of two rates of daily increments in enteral feed volume, as follows.
Caple 2004: 20 versus 35 mL/kg
Fayyaz 2020: 15 to 20 versus 25 to 30 mL/kg
Hasshemi 2018: 20 to 24 versus 30 mL/kg
Jain 2016: 20 versus 30 mL/kg
Krishnamurthy 2010: 20 versus 30 mL/kg
Karagol 2013: 20 versus 30 mL/kg
Modi 2015: 15 to 20 versus 30 to 40 mL/kg
Montealegre‐Pomar 2021: 20 versus 30 mL/kg
Raban 2014a: 24 versus 36 mL/kg
Raban 2014b: 24 versus 36 mL/kg
Rayyis 1999: 15 versus 35 mL/kg
Saha 2019: 20 versus 30 mL/kg
Salhotra 2004: 15 versus 30 mL/kg
SIFT 2016: 18 versus 30 mL/kg
In one trial, only formula‐fed infants were eligible to participate (Rayyis 1999). Infants in eight trials received expressed breast milk or formula, or a combination of both (Caple 2004; Fayyaz 2020; Jain 2016; Karagol 2013; Krishnamurthy 2010; Modi 2015; Montealegre‐Pomar 2021; SIFT 2016). In five trials, participating infants were fed exclusively with expressed human milk (Hasshemi 2018; Raban 2014a; Raban 2014b; Saha 2019; Salhotra 2004). Most trial protocols specified indications for interrupting or ceasing enteral feeding, such as residual gastric contents of more than about one‐third of the previous feed volume, frequent vomiting, abdominal distension, or detection of blood in the stools (including occult blood). SIFT 2016 did not prespecify these criteria but allowed clinicians and caregivers to apply unit‐specific policies and practices.
Outcomes
All 14 included trials reported NEC confirmed radiologically or at surgery or at autopsy. Other reported outcomes included all‐cause mortality, time to regain birth weight, time to establish full enteral feeding, duration of hospital stay, and rates of invasive infection.
Excluded studies
We excluded four reports after full‐text review, resulting in a total fo 10 excluded reports (Ahmed 2020; Berseth 2003; Book 1976; Gray 2017; Ibrahim 2017; Jayaraman 2017; Kadam 2016; Nangia 2019; Tewari 2018; Viswanathan 2017; see Characteristics of excluded studies).
Risk of bias in included studies
The methodological quality of the included trials was generally high, but the nature of the intervention meant that parents, caregivers, or clinical investigators were aware of each infant's allocated feeding group (Figure 2).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Trials employed adequate methods to generate random sequences (typically computer‐generated) and to ensure adequate allocation concealment (typically using sealed, opaque envelopes). We assessed no trial as at high risk of selection bias.
Blinding
None of the included trials was able to mask feeding strategies from parents, caregivers, or clinical investigators (although there was some masked assessment of abdominal radiographs for diagnosis of NEC). We assessed all of the included trials as at high risk of performance and detection bias.
Incomplete outcome data
All trials reported complete or near‐complete assessments of primary outcomes (low risk of attrition bias).
Selective reporting
Although trial protocols were not available for most trials, we did not consider selective reporting bias to be a major threat given that all relevant clinical outcomes were reported.
Other potential sources of bias
We did not find evidence of important between‐group baseline differences in participant characteristics or demographics in any of the included trials.
Effects of interventions
See: Table 1
See Table 1.
Primary outcomes
Necrotising enterocolitis
Meta‐analysis of data from 14 trials (4026 infants) showed that slow advancement probably does not reduce the risk of NEC (risk ratio (RR) 1.06, 95% confidence interval (CI) 0.83 to 1.37; risk difference (RD) 0.00, 95% CI −0.01, to 0.02; Analysis 1.1). The funnel plot was not markedly asymmetrical. We assessed the certainty of evidence as moderate using GRADE methods, downgrading for serious study design limitations (lack of masking).
1.1. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 1: Necrotising enterocolitis ‐ all infants
One trial (185 infants) restricted participation to exclusively formula‐fed infants (Rayyis 1999). In the other 13 trials (3841 infants), participating infants were at least partially fed with human milk. Subgroup analysis did not show evidence of differences in effect (Figure 3).
3.

Forest plot of comparison: 1 Slow versus faster rates of feed advancement, outcome: 1.1 Necrotising enterocolitis ‐ all infants.
Sensitivity analyses did not show an effect in:
extremely preterm or ELBW infants: RR 1.01 (95% CI 0.74 to 1.38); five trials, 1299 infants (Analysis 1.2);
infants small for gestation or with intrauterine growth restriction: RR 1.26 (95% CI 0.67 to 2.37); two trials, 639 infants (Analysis 1.3);
infants with evidence of AREDFV: RR 1.59 (95% CI 0.74 to 3.40); two trials, 465 infants (Analysis 1.4).
1.2. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 2: Necrotising enterocolitis ‐ ELBW/extremely preterm infants
1.3. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 3: Necrotising enterocolitis ‐ small for gestational age or growth restricted
1.4. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 4: Necrotising enterocolitis ‐ AREDFV
Mortality
Meta‐analysis of data from 13 trials (3860 infants) showed that slow advancement probably does not reduce the risk of mortality (RR 1.13, 95% CI 0.91 to 1.39; RD 0.01, 95% CI −0.01 to 0.02; Analysis 1.5). The funnel plot was not markedly asymmetrical. We assessed the certainty of evidence as moderate, downgrading for serious study design limitations (lack of masking).
1.5. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 5: Mortality ‐ all infants
One trial (185 infants) restricted participation to exclusively formula‐fed infants (Rayyis 1999). In the other 12 trials (3644 infants), participating infants were at least partially fed with human milk. Subgroup analysis did not show evidence of differences in effect (Figure 4).
4.

Forest plot of comparison: 1 Slow versus faster rates of feed advancement, outcome: 1.5 Mortality ‐ all infants.
Sensitivity analyses did not show an effect in:
extremely preterm or ELBW infants: RR 0.83 (95% CI 0.55 to 1.25); two trials, 200 infants (Analysis 1.6);*
infants small for gestation or with intrauterine growth restriction: RR 1.78 (95% CI 0.83 to 3.81); one trial (Salhotra 2004), 53 infants (Analysis 1.7);*
infants with evidence of AREDFV: RR 7.00 (95% CI 0.39 to 124.83); one trial (Jain 2016), 30 infants (Analysis 1.8).*
1.6. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 6: Mortality ‐ ELBW/extremely preterm infants
1.7. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 7: Mortality ‐ small for gestational age or growth restricted
1.8. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 8: Mortality ‐ AREDFV
(*Subgroup data not available for SIFT 2016.)
Secondary outcomes
Growth
Ten trials reported that infants in the slow‐rate‐of‐advancement group took a longer time to regain birth weight (meta‐analysis not possible).
Caple 2004: mean difference (MD) 2 days (95% CI 1 to 3)
Hasshemi 2018: data not available
Karagol 2013: MD 3.8 days (CI not given)
Krishnamurthy 2010: median difference 6 days
Montealegre‐Pomar 2021: median difference 0.5 days
Raban 2014a: data not available
Raban 2014b: data not available
Rayyis 1999: median difference 2 days
Saha 2019: MD 4.8 days (CI not given)
Salhotra 2004: median difference 5 days
Three trials did not report growth parameters (Fayyaz 2020; Jain 2016; Modi 2015).
SIFT 2016 did not show effects on weight (MD 0.00, 95% CI −0.08 to 0.08; 2602 infants; Analysis 1.9) or head circumference (MD 0.00, 95% CI −0.13 to 0.13; 2286 infants; Analysis 1.10), z‐scores at hospital discharge.
1.9. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 9: Weight z‐score at hospital discharge
1.10. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 10: Head circumference z‐score at hospital discharge
None of the included trials reported post‐hospital discharge growth parameters.
Neurodevelopment
One trial reported neurodevelopmental outcomes assessed in children aged 18 to 24 months (SIFT 2016). Analysis suggested that slow advancement of enteral feed volumes probably does not affect the risk of moderate or severe disability (motor, visual, or hearing impairment, or cognitive or language delay) (RR 0.90, 95% CI 0.79 to 1.02; 2325 infants; Analysis 1.11). Analyses of individual domains suggest little or no effect on visual or hearing impairment, or cognitive or language delay, but suggest that slow advancement of enteral feed volumes may reduce the risk of cerebral palsy slightly:
1.11. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 11: Moderate or severe disability
cerebral palsy (clinician diagnosed): RR 0.60, 95% CI 0.39 to 0.90; 2183 infants; RD −0.02, 95% CI −0.04 to −0.00 (Analysis 1.12);
visual impairment: RR 0.75, 95% CI 0.39 to 1.43; 2327 infants (Analysis 1.13);
hearing impairment: RR 0.69, 95% CI 0.47 to 1.02; 2315 infants (Analysis 1.14);
cognitive or language delay (assessed using the Parent Report of Children’s Abilities – Revised): RR 0.93, 95% CI 0.81 to 1.07; 2326 infants (Analysis 1.15).
1.12. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 12: Cerebral palsy
1.13. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 13: Visual impairment
1.14. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 14: Hearing impairment
1.15. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 15: Cognitive impairment
Time to establish full enteral feeding
Eleven trials reported that it took longer to establish full enteral feeds in infants in the slow‐rate‐of‐advancement group (meta‐analysis not possible).
Caple 2004: MD 3 days (95% CI 2 to 3)
Hasshemi 2018: data not available
Jain 2016: MD 0.6 days (CI not given)
Karagol 2013: MD 3.2 days (CI not given)
Krishnamurthy 2010: median difference 2 days
Modi 2015: MD 4 days (CI not given)
Montealegre‐Pomar 2021: median difference 1.5 days
Rayyis 1999: median difference 4 days
Saha 2019: MD 5.3 days (CI not given)
Salhotra 2004: MD 4.8 days (CI not given)
SIFT 2016: median difference 3 days
Three trials did not report this outcome (Fayyaz 2020; Raban 2014a; Raban 2014b).
Time to establish oral feeding
None of the trials reported time to establish full oral feeding.
Feed intolerance
Meta‐analysis of data from nine trials (719 infants) showed that slow advancement of enteral feed volumes may slightly increase the risk of feed intolerance (RR 1.18, 95% CI 0.95 to 1.46; RD 0.05, 95% CI −0.02 to 0.12) (Analysis 1.16). We assessed the certainty of evidence as low, downgrading for serious study design limitations (lack of masking) and imprecision.
1.16. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 16: Feed intolerance (causing interruption of enteral feeding)
Invasive infection
Meta‐analysis of data from 11 trials (3583 infants) showed that slow advancement of enteral feed volumes may slightly increase the risk of invasive infection (RR 1.14, 95% CI 0.99 to 1.31; RD 0.02, 95% CI −0.00 to 0.05) (Analysis 1.17). We assessed the certainty of evidence as low, downgrading for serious study design limitations and imprecision.
1.17. Analysis.

Comparison 1: Slow versus faster rates of feed advancement, Outcome 17: Invasive infection
Duration of hospital stay
Six trials reported no difference in duration of hospital stay between groups.
Caple 2004: MD 5 days (95% CI −1 to 8)
Hasshemi 2018: data not available
Raban 2014a: data not available
Raban 2014b: data not available
Rayyis 1999: median difference 4 days
SIFT 2016: median difference 0 days (54 versus 54 days)
Four trials reported that duration of hospital stay was longer amongst infants in the slow‐rate‐of‐advancement group.
Fayyaz 2020: MD 4 days (CI not given)
Karagol 2013: MD 6 days (CI not given)
Krishnamurthy 2010: median difference 1.5 days
Saha 2019: MD 4 days (CI not given)
Meta‐analysis was not feasible.
The remaining four trials did not report on duration of hospital stay (Jain 2016; Modi 2015; Montealegre‐Pomar 2021; Salhotra 2004).
Discussion
Summary of main results
The trial data included in this review provide moderate‐certainty evidence that advancing enteral feed volumes at slow rates (up to 24 mL/kg/d) compared with faster rates probably does not reduce the risk of NEC in very preterm or VLBW infants. The boundaries of the 95% CI for the estimate of effect are consistent with either two extra or one fewer cases of NEC in every 100 infants who have slow rates of feed advancement. Meta‐analysis of data from these trials did not show evidence of an effect on all‐cause mortality, and prespecified analyses did not show effects on risk of NEC or death amongst ELBW or extremely preterm infants, nor amongst infants with growth restriction or evidence of absent or reversed end‐diastolic flow velocity. Meta‐analysis of data from 11 trials showed a slightly higher risk of late‐onset infection amongst infants who had slow advancement of enteral feeds. The point estimate suggests that an extra episode of late‐onset infection occurs for every 50 infants who have slow rather than faster feed volume advancement, but the lower bound of the 95% CI was consistent with no effect.
Although meta‐analysis suggests that slow advancement of enteral feeds may slightly increase feed intolerance, and infants who had slow advancement established full enteral feeding and regained birth weight several days later than infants who had faster rates of advancement, there was no evidence of an effect on duration of hospital stay. The clinical importance of these effects is unclear, since long‐term growth outcomes were not assessed.
One large trial has now reported neurodevelopmental outcomes in participants assessed at about two years post‐term (SIFT 2016). Analysis did not show an effect on the risk of moderate or severe neurodevelopmental disability. Similarly, there was no evidence of an effect on visual or hearing impairment, or on cognitive or language delay. Slow advancement of feed volumes was associated with a lower risk of cerebral palsy (clinician diagnosed). The 95% CI for the estimate was consistent with either no effect or with four fewer cases of cerebral palsy in every 100 infants who have slow rather than faster feed volume advancement. This estimate should be interpreted with caution due to possible attrition bias: complete assessment was possible for less that 80% of trial participants (similar across study groups).
Overall completeness and applicability of evidence
Most participants in the included trials were stable very preterm or VLBW infants of birth weight appropriate for gestational age. About one‐third of all participants were extremely preterm or ELBW, and about one‐fifth were small for gestational age, growth‐restricted, or compromised in utero, as indicated by absent or reversed end‐diastolic flow velocity in the umbilical artery. Infants who had severe respiratory distress requiring oxygen supplementation or ventilatory support were eligible to participate in all but three of the trials (Karagol 2013; Krishnamurthy 2010; Salhotra 2004). The findings of this review should therefore be applicable across these populations, who are at highest risk of developing feed intolerance or NEC (Luig 2005).
Most participating infants were at least partially fed with breast milk. Evidence indicates that artificial formula feeding increases risks of feed intolerance and NEC (Quigley 2019). The risk‐benefit balance of enteral feeding strategies may differ between human milk‐fed and formula‐fed very preterm or VLBW infants, but available data were insufficient to show effects of different rates of feed advancement on important outcomes for infants fed exclusively with artificial formula. It is also unclear whether the review findings can be applied to infants who receive continuous infusion of intragastric feeds, as a vast majority of the infants in the included trials received enteral feeds as interval boluses. Randomised controlled trials have reported conflicting findings about the effect of continuous enteral infusion on feed tolerance in very preterm or VLBW infants (Premji 2011).
Although the finding that slow enteral feed volume advancement delays establishment of full enteral feeds may seem intuitive, it is plausible that advancing feed volumes faster could have resulted in more feed intolerance and therefore a delay in the establishment of full enteral feeding. The included trials prespecified definitions of feed intolerance that mandated interrupting or ceasing feed volume advancement, principally detection of pre‐feed 'gastric residuals' (gastric content aspirated before a planned gastric tube feed) and abdominal distension. However, trial reports presented only limited data on the frequency of these outcomes. Furthermore, limited evidence suggests that the volume or colour of gastric residuals is predictive of risk of NEC for infants whose feed volumes are advanced conservatively (Bertino 2009; Cobb 2004; Mihatsch 2002). Similarly, the clinical importance of abdominal distension or bowel loops visible through the abdominal wall (without other features of intra‐abdominal pathology) is unclear, especially in the modern era, when early and prolonged use of continuous positive airway pressure results in intestinal gaseous distension.
Quality of the evidence
Our GRADE assessment of the certainty of evidence for primary outcomes was moderate, downgraded from high due to lack of masking in the included trials (Table 1). Although the included trials were generally of good methodological quality, in common with other trials of feeding interventions in this population, it was not possible to mask parents, caregivers, and clinical assessors to the nature of the intervention (Figure 2). Lack of masking may have resulted in surveillance and ascertainment biases. It is more likely, however, to have caused an overestimation of feed intolerance and NEC amongst infants whose feed volumes were advanced faster. Assessment of abdominal radiographs for signs of NEC was masked in most trials to ensure that the diagnosis of severe NEC (confirmed by radiological detection of gas in the bowel wall or portal tract) was not prone to bias. However, as microbial generation of gas in the bowel wall is substrate‐dependent, infants who received more enteral milk (substrate) may have been more likely to demonstrate this radiological sign than infants with equally severe bowel disease who had less intraluminal substrate. This 'substrate effect' is also more likely to cause over‐ascertainment of NEC amongst infants who had faster rates of feed volume advancement (Tyson 2007).
Potential biases in the review process
The main concern with the review process is the possibility that findings are subject to publication and other reporting biases. We attempted to minimise this threat by screening the reference lists of included trials and related reviews and searching the proceedings of major international perinatal conferences to identify trial reports that were not published in full form in academic journals. Inspection of funnel plots of meta‐analyses that included at least 10 data points did not show sufficient asymmetry to raise concerns about possible publication or small‐study bias.
Agreements and disagreements with other studies or reviews
This review focused specifically on the comparison of slow versus faster rates of feed volume advancement. Other Cochrane Reviews have assessed how (i) enteral fasting with trophic feeding (minimal enteral nutrition), (ii) delayed versus early introduction of progressive enteral feeds, and (iii) early full enteral feeding versus gradual introduction of feeds affects important outcomes in very preterm or VLBW infants (Morgan 2013a; Morgan 2014a; Walsh 2020). These reviews, consistent with the findings of this review, found evidence that conservative feeding strategies probably do not reduce the risk of NEC, mortality, or associated morbidity.
Authors' conclusions
Implications for practice.
Advancing enteral feed volumes at slow rates (up to 24 mL/kg/d) probably does not reduce the risk of necrotising enterocolitis, death, or feed intolerance in very preterm or very low birth weight infants (moderate‐certainty evidence), including extremely preterm or extremely low birth weight infants, or in infants who are growth‐restricted or compromised in utero. Advancing the volume of enteral feeds at faster rates (typically 30 to 40 mL/kg/d) shortens by several days the time taken to regain birth weight and establish full enteral feeds, and may slightly reduce the risk of late‐onset invasive infection (low‐certainty evidence). Clinicians, policymakers, and guideline‐producers can consider how to apply this evidence to practice in their local context (Soll 2019).
Implications for research.
Additional trials are unlikely to alter these effect estimates for necrotising enterocolitis or death. With regard to very preterm or very low birth weight infants who are clinically stable after birth (typically infants with birth weight more than 1000 grams or gestational age more than 27 weeks), the key research question is now whether exclusive enteral feeding from birth is better than slow introduction and advancement (Walsh 2020).
What's new
| Date | Event | Description |
|---|---|---|
| 20 October 2020 | New search has been performed | This updates the review 'Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants' (Oddie 2017). |
| 20 October 2020 | New citation required but conclusions have not changed | The updated search identified four new trials for inclusion (Fayyaz 2020; Hasshemi 2018; Montealegre‐Pomar 2021; Saha 2019). |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 4, 1998
| Date | Event | Description |
|---|---|---|
| 11 January 2011 | New citation required and conclusions have changed | New data and an increased total number of participating infants (to 496) narrowed confidence intervals for the estimates of effect and modified the implications for practice and research. |
| 15 December 2010 | New search has been performed | This updates the review 'Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants', which was published in the Cochrane Database of Systematic Reviews, Issue 2, 2008 (McGuire 2008). We updated the search in December 2010 and included one new trial (Krishnamurthy 2010). We included the following new co‐authors on the review team: Jessie Morgan and Lauren Young. |
| 13 February 2008 | New citation required but conclusions have not changed | We added the following new review authors: Sarah Bombell and William McGuire. |
| 2 February 2008 | New search has been performed | This updates the review 'Rapid versus slow rate of advancement of feedings for promoting growth and preventing necrotizing enterocolitis in parenterally fed low‐birth‐weight infants', by Kennedy and Tyson, which was published in the Cochrane Database of Systematic Reviews, Issue 2, 2000 (Kennedy 2000). We modified the title to read 'Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants', and added the following new review authors: Sarah Bombell and William McGuire. We made the following changes to the original protocol.
We updated the search in December 2007. We included one new trial (Salhotra 2004), and excluded one previously included trial (Book 1976). The findings and implications for practice and research of this review have not changed overall. |
| 11 January 2008 | Amended | We converted the review to new review format. |
Acknowledgements
We thank Cochrane Neonatal: Colleen Ovelman, former Managing Editor, Jane Cracknell, Managing Editor and Roger Soll, Co‐ordinating Editor, who provided editorial and administrative support, and Melissa Harden, Information Specialist, University of York, UK, who designed and ran the literature searches.
Roger Soll and Sarah Hodgkinson peer reviewed and offered feedback for this updated review.
We are grateful to Drs Namasivayam Ambalavanan, Siddarth Ramji, Manoj Modi, and Kanya Mukhopadhyay for providing details and data (Jain 2016; Modi 2015; Rayyis 1999; Salhotra 2004).
Appendices
Appendix 1. 2020 search methods
Databases searches: CENTRAL (via Cochrane Library); CINAHL; Embase, Maternity & Infant Care (MIDIRS); MEDLINE; ClinicalTrials.gov (WHO ICTRP – currently not available)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
CENTRAL via Cochrane Library
Search date 19th October 2020
#1 MeSH descriptor: [Infant, Newborn] explode all trees
#2 MeSH descriptor: [Premature Birth] explode all trees
#3 ((neonat* or neo nat*)):ti,ab,kw OR ((newborn* or new born* or newly born*)):ti,ab,kw OR ((preterm or preterms or pre term or pre terms)):ti,ab,kw OR ((preemie* or premie or premies)):ti,ab,kw OR ((prematur* NEAR/3 (birth* or born or deliver*))):ti,ab,kw (Word variations have been searched)
#4 ((low NEAR/3 (birthweight* or birth weight*))):ti,ab,kw OR ((lbw or vlbw or elbw)):ti,ab,kw OR (infan*):ti,ab,kw OR ((baby or babies)):ti,ab,kw (Word variations have been searched)
#5 #1 OR #2 OR #3 OR #4
#6 MeSH descriptor: [Enteral Nutrition] explode all trees
#7 (((enteral or enteric) NEAR/2 (nutrition or feed*))):ti,ab,kw OR (((oral or sip or tube) NEAR/2 feeding*)):ti,ab,kw OR (((nasogastric or gastrostomy or jejunostomy) NEAR/2 tube*)):ti,ab,kw OR (((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteral feed*)):ti,ab,kw OR (((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteric feed*)):ti,ab,kw (Word variations have been searched)
#8 (((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteral intake*)):ti,ab,kw OR (((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteric intake*)):ti,ab,kw OR (((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteral nutrition)):ti,ab,kw OR (((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) NEAR/3 enteric nutrition)):ti,ab,kw OR (((aggressive* or fast or rapid* or slow* or speed*) NEAR/3 feed*)):ti,ab,kw (Word variations have been searched)
#9 (((aggressive* or fast or rapid* or slow* or speed*) NEAR/3 volume*)):ti,ab,kw OR (trophic feeding*):ti,ab,kw OR (((gut or gastrointestinal) NEAR/2 priming)):ti,ab,kw (Word variations have been searched)
#10 #6 or #7 or #8 or #9
#11 #5 and #10
#12 MeSH descriptor: [Parenteral Nutrition] explode all trees and with qualifier(s): [adverse effects ‐ AE]
#13 MeSH descriptor: [Enterocolitis, Necrotizing] explode all trees and with qualifier(s): [etiology ‐ ET, epidemiology ‐ EP, prevention & control ‐ PC]
#14 MeSH descriptor: [Infections] 1 tree(s) exploded and with qualifier(s): [epidemiology ‐ EP]
#15 (((prevent* or risk*) NEAR/3 necrotising enterocolitis)):ti,ab,kw OR (((prevent* or risk*) NEAR/3 necrotizing enterocolitis)):ti,ab,kw (Word variations have been searched)
#16 #12 or #13 or #14 or #15
#17 #5 and #16
#18 #11 or #17
CINAHL via EBSCO
Search date 19th October 2020
1062 records identified
S1 (MH "Infant, Newborn+")
S2 (MH "Childbirth, Premature")
S3 TI ( (neonat* or neo nat*) ) OR AB ( (neonat* or neo nat*) ) OR TI ( (newborn* or new born* or newly born*) ) OR AB ( (newborn* or new born* or newly born*) ) OR TI ( (preterm or preterms or pre term or pre terms) ) OR AB ( (preterm or preterms or pre term or pre terms) )
S4 TI ( (preemie* or premie or premies) ) OR AB ( (preemie* or premie or premies) ) OR TI ( (prematur* N3 (birth* or born or deliver*)) ) OR AB ( (prematur* N3 (birth* or born or deliver*)) ) OR TI ( (low N3 (birthweight* or birth weight*)) ) OR AB ( (low N3 (birthweight* or birth weight*)) )
S5 TI ( (lbw or vlbw or elbw) ) OR AB ( (lbw or vlbw or elbw) ) OR TI infan* OR AB infan* OR TI ( (baby or babies) ) OR AB ( (baby or babies) )
S6 S1 OR S2 OR S3 OR S4 OR S5
S7 (MH "Enteral Nutrition")
S8 TI ( ((enteral or enteric) N2 (nutrition or feed*)) ) OR AB ( ((enteral or enteric) N2 (nutrition or feed*)) ) OR TI ( ((oral or sip or tube) N2 feeding*) ) OR AB ( ((oral or sip or tube) N2 feeding*) ) OR TI ( ((nasogastric or gastrostomy or jejunostomy) N2 tube*) ) OR AB ( ((nasogastric or gastrostomy or jejunostomy) N2 tube*) )
S9 TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral feed*) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral feed*) ) OR TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric feed*) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric feed*) ) OR TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral intake*) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral intake*) )
S10 TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric intake*) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric intake*) ) OR TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral nutrition) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteral nutrition) ) OR TI ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric nutrition) ) OR AB ( ((advanc* or aggressive* or delay* or early or fast or full or increas* or minimal or progress* or prolonged or rapid* or routine* or speed* or slow* or volume*) N3 enteric nutrition) )
S11 TI ( ((aggressive* or fast or rapid* or slow* or speed*) N3 feed*) ) OR AB ( ((aggressive* or fast or rapid* or slow* or speed*) N3 feed*) ) OR TI ( ((aggressive* or fast or rapid* or slow* or speed*) N3 volume*) ) OR AB ( ((aggressive* or fast or rapid* or slow* or speed*) N3 volume*) ) OR TI trophic feeding OR AB trophic feeding* OR TI ( ((gut or gastrointestinal) N2 priming) ) OR AB ( ((gut or gastrointestinal) N2 priming) )
S12 S7 OR S8 OR S9 OR S10 OR S11
S13 S6 AND S12
S14 (MH "Double‐Blind Studies")
S15 (MH "Single‐Blind Studies")
S16 (MH "Random Assignment")
S17 (MH "Pretest‐Posttest Design")
S18 (MH "Cluster Sample")
S19 TI ( randomized or randomised ) OR AB random* OR TI trial
S20 MH "sample size" AND AB ( (assigned or allocated or control) )
S21 MH placebos
S22 PT randomised controlled trial OR AB control W5 group OR MH "crossover design" OR MH "comparative studies" OR AB cluster W3 RCT
S23 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22
S24 S13 AND S23
S25 (MH "Parenteral Nutrition/AE")
S26 (MH "Enterocolitis, Necrotizing/CO/ET/EP/PC")
S27 (MH "Infection/EP")
S28 TI ( ((prevent* or risk*) N3 necrotising enterocolitis) ) OR AB ( ((prevent* or risk*) N3 necrotising enterocolitis) ) OR TI ( ((prevent* or risk*) N3 necrotizing enterocolitis) ) OR AB ( ((prevent* or risk*) N3 necrotizing enterocolitis) )
S29 S25 OR S26 OR S27 OR S28
S30 S6 AND S23 AND S29
S31 S24 OR S30
View Results (1,062)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Embase via OVID
Search date 19th October 2020
1 Newborn/ (533014)
2 Prematurity/ (103974)
3 (neonat$ or neo nat$).ti,ab. (347799)
4 (newborn$ or new born$ or newly born$).ti,ab. (196226)
5 (preterm or preterms or pre term or pre terms).ti,ab. (107483)
6 (preemie$ or premie or premies).ti,ab. (272)
7 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (21963)
8 (low adj3 (birthweight$ or birth weight$)).ti,ab. (44434)
9 (lbw or vlbw or elbw).ti,ab. (11748)
10 infan$.ti,ab. (505921)
11 (baby or babies).ti,ab. (98472)
12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (1150651)
13 Enteric Nutrition/ (32699)
14 ((enteral or enteric) adj2 (nutrition or feed$)).ti,ab. (23164)
15 ((oral or sip or tube) adj2 feeding$).ti,ab. (15554)
16 ((nasogastric or gastrostomy or jejunostomy) adj2 tube$).ti,ab. (13591)
17 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral feed$).ti,ab. (1697)
18 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric feed$).ti,ab. (10)
19 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral nutrition).ti,ab. (2109)
20 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric nutrition).ti,ab. (7)
21 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral intake).ti,ab. (40)
22 ((advanc$ or aggressive$ or delay$ or early or fast or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric intake).ti,ab. (0)
23 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 feed$).ti,ab. (3431)
24 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 volume$).ti,ab. (5320)
25 trophic feeding$.ti,ab. (113)
26 ((gut or gastrointestinal) adj2 priming).ti,ab. (41)
27 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 (67117)
28 12 and 27 (10261)
29 Randomized controlled trial/ (626485)
30 Controlled clinical study/ (465422)
31 Random$.ti,ab. (1589685)
32 randomization/ (88577)
33 intermethod comparison/ (266507)
34 placebo.ti,ab. (313152)
35 (compare or compared or comparison).ti. (521918)
36 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (2190407)
37 (open adj label).ti,ab. (82341)
38 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. (237163)
39 double blind procedure/ (177360)
40 parallel group$1.ti,ab. (26293)
41 (crossover or cross over).ti,ab. (107555)
42 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. (339974)
43 (assigned or allocated).ti,ab. (400614)
44 (controlled adj7 (study or design or trial)).ti,ab. (360663)
45 (volunteer or volunteers).ti,ab. (252353)
46 human experiment/ (519930)
47 trial.ti. (311954)
48 or/29‐47 (5173763)
49 (random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) (8271)
50 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) (249866)
51 (((case adj control$) and random$) not randomi?ed controlled).ti,ab. (17728)
52 (Systematic review not (trial or study)).ti. (155685)
53 (nonrandom$ not random$).ti,ab. (16497)
54 "Random field$".ti,ab. (2428)
55 (random cluster adj3 sampl$).ti,ab. (1312)
56 (review.ab. and review.pt.) not trial.ti. (836864)
57 "we searched".ab. and (review.ti. or review.pt.) (33674)
58 "update review".ab. (110)
59 (databases adj4 searched).ab. (38341)
60 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ (1084361)
61 Animal experiment/ not (human experiment/ or human/) (2283901)
62 or/49‐61 (3568186)
63 48 not 62 (4602096)
64 28 and 63 (2237)
65 Parenteral Nutrition/ (29655)
66 complication/ (221706)
67 safety/ or patient safety/ (371167)
68 (adverse$ adj2 (effect$ or event$ or impact$ or outcome$)).ti,ab. (650603)
69 (complication$ or risk$ or safe or safely or safer or safety or sequaela or side effect$ or tolerated or toxicities or toxicity).ti,ab. (5830131)
70 65 and (66 or 67 or 68 or 69) (10653)
71 Necrotizing Enterocolitis/co, ep, et, pc [Complication, Epidemiology, Etiology, Prevention] (2682)
72 ((prevent$ or risk$) adj3 necrotising enterocolitis).ti,ab. (204)
73 ((prevent$ or risk$) adj3 necrotizing enterocolitis).ti,ab. (800)
74 Infection/ep [Epidemiology] (4639)
75 70 or 71 or 72 or 73 or 74 (18499)
76 12 and 63 and 75 (1149)
77 64 or 76 (3050)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Maternity & Infant Care Via OVID
Search date 19th October 2020
1 (neonat$ or neo nat$).ti,ab. (48117)
2 (newborn$ or new born$ or newly born$).ti,ab. (21553)
3 (preterm or preterms or pre term or pre terms).ti,ab. (28518)
4 (preemie$ or premie or premies).ti,ab. (57)
5 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (4262)
6 (low adj3 (birthweight$ or birth weight$)).ti,ab. (11416)
7 (lbw or vlbw or elbw).ti,ab. (3290)
8 infan$.ti,ab. (68527)
9 (baby or babies).ti,ab. (30608)
10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (127398)
11 (Infant ‐ premature or Infant ‐ very low birth weight or Infant ‐ newborn).de. (30913)
12 10 or 11 (130585)
13 Enteral nutrition.de. (287)
14 ((enteral or enteric) adj2 (nutrition or feed$)).ti,ab. (779)
15 ((oral or sip or tube) adj2 feeding$).ti,ab. (480)
16 ((nasogastric or gastrostomy or jejunostomy) adj2 tube$).ti,ab. (157)
17 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral feed$).ti,ab. (324)
18 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric feed$).ti,ab. (1)
19 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral intake$).ti,ab. (9)
20 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric intake$).ti,ab. (0)
21 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral nutrition).ti,ab. (71)
22 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric nutrition).ti,ab. (0)
23 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 feed$).ti,ab. (76)
24 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 volume$).ti,ab. (31)
25 trophic feeding$.ti,ab. (23)
26 ((gut or gastrointestinal) adj2 priming).ti,ab. (5)
27 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 (1471)
28 12 and 27 (1365)
29 limit 28 to randomised controlled trial (103)
30 Parenteral nutrition.de. (206)
31 Enterocolitis ‐ necrotizing.de. (1)
32 (adverse$ adj2 (effect$ or event$ or impact$ or outcome$)).ti,ab. (13711)
33 (complication$ or risk$ or safe or safely or safer or safety or sequaela or side effect$ or tolerated or toxicities or toxicity).ti,ab. (94379)
34 Complications.de. (199)
35 safety.de. (1953)
36 (30 or 31) and (32 or 33 or 34 or 35) (96)
37 ((prevent$ or risk$) adj3 necrotising enterocolitis).ti,ab. (71)
38 ((prevent$ or risk$) adj3 necrotizing enterocolitis).ti,ab. (231)
39 36 or 37 or 38 (392)
40 limit 39 to randomised controlled trial (17)
41 29 or 40 (110)
MEDLINE via OVID
Search date 19th October 2020 [Ovid MEDLINE(R) ALL <1946 to October 16, 2020>]
1 exp Infant, Newborn/ (611659)
2 Premature Birth/ (14154)
3 (neonat$ or neo nat$).ti,ab. (267306)
4 (newborn$ or new born$ or newly born$).ti,ab. (167592)
5 (preterm or preterms or pre term or pre terms).ti,ab. (76438)
6 (preemie$ or premie or premies).ti,ab. (174)
7 (prematur$ adj3 (birth$ or born or deliver$)).ti,ab. (15880)
8 (low adj3 (birthweight$ or birth weight$)).ti,ab. (35103)
9 (lbw or vlbw or elbw).ti,ab. (8568)
10 infan$.ti,ab. (441307)
11 (baby or babies).ti,ab. (70876)
12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (1067192)
13 Enteral Nutrition/ (19843)
14 ((enteral or enteric) adj2 (nutrition or feed$)).ti,ab. (14402)
15 ((oral or sip or tube) adj2 feeding$).ti,ab. (10325)
16 ((nasogastric or gastrostomy or jejunostomy) adj2 tube$).ti,ab. (8813)
17 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral feed$).ti,ab. (1664)
18 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric feed$).ti,ab. (14)
19 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral intake$).ti,ab. (41)
20 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric intake$).ti,ab. (0)
21 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteral nutrition).ti,ab. (1450)
22 ((advanc$ or aggressive$ or delay$ or early or fast or full or increas$ or minimal or progress$ or prolonged or rapid$ or routine$ or speed$ or slow$ or volume$) adj3 enteric nutrition).ti,ab. (5)
23 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 feed$).ti,ab. (2933)
24 ((aggressive$ or fast or rapid$ or slow$ or speed$) adj3 volume$).ti,ab. (3947)
25 trophic feeding$.ti,ab. (89)
26 ((gut or gastrointestinal) adj2 priming).ti,ab. (33)
27 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 (43937)
28 12 and 27 (7156)
29 Parenteral Nutrition/ae [Adverse Effects] (2782)
30 Enterocolitis, Necrotizing/ep, et, pc [Epidemiology, Etiology, Prevention & Control] (1662)
31 ((prevent$ or risk$) adj3 necrotising enterocolitis).ti,ab. (143)
32 ((prevent$ or risk$) adj3 necrotizing enterocolitis).ti,ab. (610)
33 Infection/ep [Epidemiology] (4070)
34 29 or 30 or 31 or 32 or 33 (8854)
35 12 and 34 (3319)
36 28 or 35 (9891)
37 randomized controlled trial.pt. (515341)
38 controlled clinical trial.pt. (93888)
39 randomized.ab. (495059)
40 placebo.ab. (211516)
41 drug therapy.fs. (2243399)
42 randomly.ab. (342541)
43 trial.ab. (523032)
44 groups.ab. (2102194)
45 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 (4813149)
46 exp animals/ not humans.sh. (4744580)
47 45 not 46 (4179397)
48 36 and 47 (2836)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Appendix 2. Previous search methods
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 5), MEDLINE via PubMed (2015 to June 2017), Embase (2015 to June 2017), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 2015 to June 2017) using search terms adapted for individual databases: ("Infant‐Nutrition"/all subheadings OR Infant Formula OR milk OR formula OR trophic feeding OR minimal enteral nutrition OR gut priming), plus database‐specific limiters for randomised controlled trials (RCTs) and neonates (see Appendix 1). We did not apply language restrictions. We searched clinical trials registries for ongoing or recently completed trials (ClinicalTrials.gov (clinicaltrials.gov); the World Health Organization International Clinical Trials Registry Platform (www.who.int/clinical-trials-registry-platform/); and the ISRCTN registry (www.isrctn.com/)).
Searching other resources
We searched abstracts from annual meetings of the Pediatric Academic Societies (1993 to 2017), the European Society for Paediatric Research (1995 to 2016), the UK Royal College of Paediatrics and Child Health (2000 to 2017), and the Perinatal Society of Australia and New Zealand (2000 to 2016). Trials reported only as abstracts were eligible if sufficient information was available from the report or through contact with study authors to fulfil the inclusion criteria.
Standard search methods
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 3. Risk of bias tool
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. We assessed blinding separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data, including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported; the numbers included in the analysis at each stage (compared with the total randomised participants); reasons for attrition or exclusion where reported; and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies for which study protocols were published in advance, we compared the prespecified outcomes versus outcomes reported in the published results. If the study protocol was not published in advance, we contacted the study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; the study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design, or if the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If necessary, we explored the impact of the level of bias through the undertaking of sensitivity analyses.
Data and analyses
Comparison 1. Slow versus faster rates of feed advancement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Necrotising enterocolitis ‐ all infants | 14 | 4026 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
| 1.1.1 Infants at least partially fed with human milk | 13 | 3841 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
| 1.1.2 Infants exclusively formula fed | 1 | 185 | Risk Difference (M‐H, Fixed, 95% CI) | 0.04 [‐0.05, 0.13] |
| 1.2 Necrotising enterocolitis ‐ ELBW/extremely preterm infants | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3 Necrotising enterocolitis ‐ small for gestational age or growth restricted | 2 | 639 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.67, 2.37] |
| 1.4 Necrotising enterocolitis ‐ AREDFV | 2 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.74, 3.40] |
| 1.5 Mortality ‐ all infants | 13 | 3860 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.01, 0.02] |
| 1.5.1 Infants at least partially fed with human milk | 12 | 3675 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
| 1.5.2 Infants exclusively formula fed | 1 | 185 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.02, 0.02] |
| 1.6 Mortality ‐ ELBW/extremely preterm infants | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7 Mortality ‐ small for gestational age or growth restricted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.8 Mortality ‐ AREDFV | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.9 Weight z‐score at hospital discharge | 1 | 2602 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.08, 0.08] |
| 1.10 Head circumference z‐score at hospital discharge | 1 | 2286 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.13, 0.13] |
| 1.11 Moderate or severe disability | 1 | 2325 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.79, 1.02] |
| 1.12 Cerebral palsy | 1 | 2183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.39, 0.90] |
| 1.13 Visual impairment | 1 | 2327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.39, 1.43] |
| 1.14 Hearing impairment | 1 | 2315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.47, 1.02] |
| 1.15 Cognitive impairment | 1 | 2326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.07] |
| 1.16 Feed intolerance (causing interruption of enteral feeding) | 9 | 719 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.95, 1.46] |
| 1.17 Invasive infection | 11 | 3583 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.99, 1.31] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Caple 2004.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants of birth weight 1000 to 2000 grams (appropriate birth weight for gestational age) and of gestational age < 35 weeks at birth, who were starting formula feeds Setting: Neonatal Unit, Department of Pediatrics, University of Texas Medical School, Houston, Texas, USA (1999 to 2002) |
|
| Interventions | Feed (formula only) advancement at 20 mL/kg/d (n = 84) vs 30 mL/kg/d (n = 74) | |
| Outcomes |
|
|
| Notes | Feeds were ceased if the residual gastric aspirate was more than one‐third of the previous feed volume, or if frequent vomiting, abdominal distention, or bloody stools (including occult blood) were noted. We were unable to obtain data on all‐cause mortality from the principal investigators. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequence |
| Allocation concealment (selection bias) | Low risk | Blinded draw from envelope by caregivers not involved in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked (radiologists may have been masked) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 infants excluded after enrolment because of protocol violations were included in this review and meta‐analysis. 2 infants (1 in each group) were excluded because they were determined ineligible for enrolment as the result of an in utero gastrointestinal perforation and foetal alcohol syndrome; these infants were not included in the meta‐analysis. |
| Selective reporting (reporting bias) | Unclear risk | Mortality not reported. |
| Other bias | Low risk | No evidence of baseline imbalance |
Fayyaz 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants of birth weight < 1500 grams (gestational age 30 to 36 weeks) Setting: Neonatal Unit, Izzat Ali Shah Hospital, Wah Cantt, India (2019 to 2020) |
|
| Interventions | Feed advancement at 15 to 20 mL/kg/d (n = 16) vs 25 to 30 mL/kg/d (n = 15) | |
| Outcomes |
|
|
| Notes | Information on randomisation methods and data on NEC and infection courtesy of Dr Shahzad Haider (March 2021) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for primary outcomes |
| Selective reporting (reporting bias) | Unclear risk | Mortality, NEC, and other clinical outcomes not reported. |
| Other bias | Unclear risk | Baseline data not reported. |
Hasshemi 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants of birth gestational age 30 to 34 weeks Setting: Neonatal Unit, Tabriz Alzahra Teaching Hospital, Tabriz, Iran (2011 to 2012) |
|
| Interventions | Feed advancement at (i) 20 mL/kg/d (n = 60) vs (ii) 24 mL/kg/d (n = 40) vs (iii) 30 mL/kg/d (n = 40) (groups (i) and (ii) combined for analysis in this review) |
|
| Outcomes |
|
|
| Notes | Information on randomisation methods and data on NEC, mortality, and infection courtesy of Dr Manizheh Mostafa Gharehbaghhi (April 2021) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Jain 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants (birth weight 1000 to 1249 grams and gestational age > 30 weeks at birth) who have antenatal evidence of absent end‐diastolic flow velocities (presumed in umbilical artery) Setting: Department of Paediatrics, Postgraduate Institute of Medical Education & Research, Chandigarh, India (2013 to 2014) |
|
| Interventions | Feed advancement at 20 mL/kg/d (n = 15) vs 30 mL/kg/d (n = 15) | |
| Outcomes |
|
|
| Notes | Prespecified subgroup of a larger trial that enrolled infants with birth weight > 1250 grams and compared feed advancement at 30 mL/kg/d vs 40 mL/kg/d Additional data courtesy of Dr Mukhopadhyay (September 2014) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for primary outcomes |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Karagol 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants < 32 weeks' gestation with birth weight of 750 to 1250 grams 32% of infants weighed < 1000 grams. Exclusion criteria included major congenital malformations, severe respiratory distress, presence of umbilical vessel catheters, contraindications to enteral feeding, perinatal asphyxia, and cardiovascular compromise. Setting: Division of Neonatology, Dr Sami Ulus Maternity, Children's Education and Research Hospital, Ankara, Turkey (2011 to 2012) |
|
| Interventions | Slow advancement at 20 mL/kg/d (n = 46) vs rapid advancement at 30 mL/kg/d (n = 46) | |
| Outcomes |
|
|
| Notes | Feeds were ceased if any of the following occurred: gastric residuals > 5 mL/kg or > 50% of feed volume, vomiting > 3 times in 24 hours, increase in abdominal girth > 2 cm between feeds, abdominal tenderness or erythema, reduced bowel sounds, blood in the stools, or recurrent apnoea. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Krishnamurthy 2010.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants (birth weight 1000 to 1499 grams) and gestational age < 34 weeks at birth Exclusion criteria included respiratory distress, mechanical ventilation, inotrope support, and umbilical arterial or venous catheterisation. Setting: Department of Paediatrics, University College of Medical Sciences, Delhi, India (2007 to 2009) |
|
| Interventions | Feed advancement at 20 mL/kg/d (n = 50) vs 30 mL/kg/d (n = 50) | |
| Outcomes |
|
|
| Notes | All feeds were delivered by gavage via nasogastric tube at 2‐hour intervals. Feeds were ceased if any of the following occurred: residual gastric contents > 50% of previous feed volume (delayed if volume was 25% to 50%), > 3 episodes of apnoea in the preceding hour, abdominal distension or tenderness, or bloody stools (including occult blood). Parenteral nutrition was not available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Modi 2015.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Newborn infants with birth weight of 750 to 1250 grams who commenced enteral feeds within 4 days after birth. Mean gestational age of participants was 31 weeks. Exclusion criteria were (quote: "gross congenital malformation and anomalies of gastrointestinal tract"). Setting: Department of Neonatology, Maulana Azad Medical College, New Delhi, India (2012 to 2014) |
|
| Interventions | Feed advancement at 15 to 20 mL/kg/d (n = 65) vs 30 to 40 mL/kg/d (n = 66) | |
| Outcomes |
|
|
| Notes | Published as abstract only Further information available from www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=5289 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified block randomisation (computer‐generated) |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Montealegre‐Pomar 2021.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants of gestational age < 34 weeks at birth and birth weight 1000 to 1499 grams Setting: Ignacio University Hospital, Bogata, Colombia (2014 to 2015) |
|
| Interventions | Feed advancement at 20 mL/kg/d (n = 12) vs 30 mL/kg/d (n = 8) | |
| Outcomes |
|
|
| Notes | Additional information courtesy of Dr Montealegre‐Pomar | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation (computer‐generated) |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near complete (1 study withdrawal postrandomisation) |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Raban 2014a.
| Study characteristics | ||
| Methods | Randomised controlled trial (2 × 2 factorial design with Raban 2014b) | |
| Participants | ELBW infants Setting: Groote Schuur Hospital, Cape Town, South Africa (2011 to 2013) |
|
| Interventions | Feed advancement (from 12 mL/kg/d on day 2) in daily increments of 24 mL/kg (n = 51) vs 36 mL/kg (n = 47) until enteral feeds of 200 mL/kg/d were attained | |
| Outcomes |
|
|
| Notes | Factorial design also randomised to commencing feeds on day 1 (24 mL/kg) or day 2 (12 mL/kg). Infants received maternal‐expressed breast milk or donor breast milk. Trial registration: ISRCTN96923718 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for primary outcomes |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Raban 2014b.
| Study characteristics | ||
| Methods | Randomised controlled trial (2 × 2 factorial design with Raban 2014a) | |
| Participants | ELBW infants Setting: Groote Schuur Hospital, Cape Town, South Africa (2011 to 2013) |
|
| Interventions | Feed advancement (from 24 mL/kg/d on day 1) in daily increments of 24 mL/kg (n = 52) vs 36 mL/kg (n = 50) until enteral feeds of 200 mL/kg/d were attained | |
| Outcomes |
|
|
| Notes | Factorial design also randomised to commencing feeds on day 1 (24 mL/kg) or day 2 (12 mL/kg). Infants received maternal‐expressed breast milk or donor breast milk. Trial registration: ISRCTN96923718 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for primary outcomes |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Rayyis 1999.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | VLBW infants of gestational age < 34 weeks at birth Setting: Neonatal Unit, Department of Pediatrics, University of Alabama, Birmingham, Alabama, USA (dates not stated) |
|
| Interventions | Feed advancement at 15 mL/kg/d (n = 98) vs 35 mL/kg/d (n = 87) | |
| Outcomes |
|
|
| Notes | Infants for whom full or partial feeding with expressed breast milk was planned were not eligible to participate. Feeds were ceased if: residual gastric contents > 30% of previous feed volume, abdominal distension or tenderness, or bloody stools (including occult blood). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 protocol violations occurred after enrolment, but all infants were included in the final data analysis. |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
Saha 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants of birth weight 1200 to 1500 grams (appropriate for gestational age) Exclusion criteria included recurrent apnoea, respiratory distress requiring supplemental oxygen, and receipt of inotrope support. Setting: Neonatal Unit, Dhaka Shishu Hospital, Dhaka, Bangladesh (2017 to 2018) |
|
| Interventions | Feed (expressed human milk only) advancement at 20 mL/kg/d (n = 55) vs 30 mL/kg/d (n = 38) | |
| Outcomes |
|
|
| Notes | Further information sought from Dr Saha and colleagues. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete until hospital discharge |
| Selective reporting (reporting bias) | Unclear risk | Invasive infection not reported. |
| Other bias | Unclear risk | No evidence of baseline imbalance |
Salhotra 2004.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Preterm infants of birth weight < 1250 grams (> 95% of participants were "small for gestational age") Exclusion criteria included recurrent apnoea, respiratory distress requiring supplemental oxygen, and receipt of inotrope support. Setting: Neonatal Unit, Maulana Azad Medical College (tertiary‐level teaching hospital), New Delhi, India (2001 to 2003) |
|
| Interventions | Feed advancement at 15 mL/kg/d (n = 26) vs 30 mL/kg/d (n = 27) | |
| Outcomes |
|
|
| Notes | Feeds were ceased if residual gastric content was > 30% of previous feed volume or if abdominal distension was noted. Mortality data courtesy of Dr Namasivayam Ambalavanan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Unlikely |
| Other bias | Low risk | No evidence of baseline imbalance |
SIFT 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Very preterm or VLBW infants | |
| Interventions | Feeds advancement at 18 mL/kg/d (n = 1404) vs 30 mL/kg/d (n = 1400) (June 2013 to June 2015) | |
| Outcomes |
|
|
| Notes | See: Declarations of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Computer‐based random allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked (radiologists may have been masked) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 99% outcome data for in‐hospital outcomes > 87% outcome data for neurodevelopmental outcomes at 18 to 24 months |
| Selective reporting (reporting bias) | Low risk | Adhered to protocol |
| Other bias | Low risk | No evidence of baseline imbalance |
ELBW: extremely low birth weight; n: number of infants; NEC: necrotising enterocolitis; VLBW: very low birth weight
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmed 2020 | RCT of early (< 48 h) vs later initiation of progressive enteral feeding in preterm infants |
| Berseth 2003 | Infants were randomly allocated to a stable (not progressively increased) trophic feeding volume or to feed volume advancement at 20 mL/kg/d. |
| Book 1976 | Enteral feeding volumes were advanced at 10 mL/kg/d vs 20 mL/kg/d (both groups received 'slow' advancement of feed volumes). |
| Gray 2017 | RCT of different feeding intervals (not different rates of feed volume advancement) in very preterm infants |
| Ibrahim 2017 | RCT of different feeding intervals (not different rates of feed volume advancement) in very preterm infants |
| Jayaraman 2017 | RCT examining the effect on breast milk feeding of early vs delayed kangaroo mother care in low birth weight infants (no intention to advance enteral feed volumes at different rates) |
| Kadam 2016 | Retrospective cohort study |
| Nangia 2019 | RCT of early full enteral feeding (not progressive advancement at different rates) |
| Tewari 2018 | RCT of early vs delayed initiation of progressive enteral feeding in very preterm infants (feeds were advanced at 10 to 15 mL/kg/d in both groups) |
| Viswanathan 2017 | Retrospective cohort study |
RCT: randomised controlled trial
Differences between protocol and review
We made the following changes to the previous publication of the review (Oddie 2017).
We updated the certainty of evidence and the risk of bias tool in Appendix 2.
-
We revised the text in the following sections:
How the intervention might work
Assessment of reporting biases
Sensitivity analysis
Allocation (selection bias)
Blinding (performance bias and detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other potential sources of bias
We added new external sources of support.
We developed a new search strategy. The previous search methods are shown in Appendix 2.
Contributions of authors
Drs Oddie, Young, and McGuire updated the search, independently determined the eligibility of identified studies, assessed the methodological quality of the included trials, and extracted relevant information and data.
Sources of support
Internal sources
-
Centre for Reviews and Dissemination, UK
Logistical
-
Bradford Neonatal, Bradford Royal Infirmary, UK
Logistical
External sources
-
National Institute for Health Research (NIHR), UK
This report is independent research funded by a UK NIHR Evidence Synthesis Programme Grant. The views expressed in this publication are those of the review authors and are not necessarily those of the National Health Service, the NIHR, or the UK Department of Health and Social Care.
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
The Gerber Foundation, USA
Editorial support for this review, as part of a suite of preterm nutrition reviews, has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private, 501(c)(3) foundation not related to Gerber Products Company in any way.
Declarations of interest
SO has no conflict of interest to declare.
LY has no conflict of interest to declare.
WM was a clinical investigator for SIFT 2016 (LY and SO extracted data for this study). SIFT 2016 was funded by the National Institute for Health Research (NIHR) (UK).
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent from the Gerber Products Company. The grantor has no input on the content of the review or the editorial process (see Sources of support).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Caple 2004 {published data only}
- Caple J, Armentrout D, Huseby V, Halbardier B, Garcia J, Sparks JW, et al. Randomized, controlled trial of slow versus rapid feeding volume advancement in preterm infants. Pediatrics 2004;114(6):1597-600. [DOI: 10.1542/peds.2004-1232] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fayyaz 2020 {published data only}
- Fayyaz M, Haider S, Khan AH, Nazir S, Hassan A, Hussain S. Comparison of slow versus rapid feeding regimen in preterm neonates in the reduction of hospital stay. Journal of Rawalpindi Medical College 2020;24(3):249-53. [DOI: 10.37939/jrmc.v24i3.1384] [DOI] [Google Scholar]
Hasshemi 2018 {published data only}
- Hasshemi F, Mostafa Gharehbaghi M, Ghojazadeh M, Sanaie G. Comparing outcomes of different feeding volumes in preterm infants. Journal of Urmia University of Medical Sciences 2013;24(1):58-64. [ISSN: 1027-3727] [umj.umsu.ac.ir/article-1-1628-en.html] [Google Scholar]
Jain 2016 {published data only}
- Jain S, Mukhopadhyay K, Jain V, Kumar P. Slow versus rapid enteral feed in preterm neonates with antenatal absent end diastolic flow. Journal of Maternal-Fetal & Neonatal Medicine 2016;29(17):2828-33. [DOI: 10.3109/14767058.2015.1105954] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay K, Jain S. Feed intolerance and necrotising enterocolitis in rapid vs slow feeding in preterm neonates with absent end diastolic flow: a randomised controlled trial. In: Pediatric Academic Societies and Asian Society for Pediatric Research; 2014 May 3-6; Vancouver. 2014. [CENTRAL: CN-01344272]
Karagol 2013 {published data only}
- Karagol BS, Zenciroglu A, Okumus N, Polin RA. Randomized controlled trial of slow versus rapid enteral feeding advancements on the clinical outcomes of preterm infants with 750-1250g. Journal of Parenteral and Enteral Nutrition 2013;37(2):223-8. [DOI: 10.1177/0148607112449482] [PMID: ] [DOI] [PubMed] [Google Scholar]
Krishnamurthy 2010 {published data only}
- Krishnamurthy S, Gupta P, Debnath S, Gomber S. Slow versus rapid enteral feeding advancement in preterm newborn infants 1000-1499 g: a randomized controlled trial. Acta Paediatrica 2010;99(1):42-6. [DOI: 10.1111/j.1651-2227.2009.01519.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Modi 2015 {published data only}
- Modi M, Ramji S, Jain A, Kumar P, Gupta N. Early aggressive enteral feeding in neonates weighing 750–1250 grams: a randomized controlled trial. Indian Pediatrics 2019;56(4):294-8. [PMID: ] [PubMed] [Google Scholar]
- Modi M, Ranji S, Jain A, Sharma P, Gupta N. A randomised trial of aggressive feeding regimen in infants with birth weight ≤ 1250 grams. In: Pediatric Academic Societies Annual Conference; 2015 Apr 25-28; San Diego, CA. 2015. [CENTRAL: CN-01344271]
Montealegre‐Pomar 2021 {published data only}
- Montealegre‐Pomar AP, Bertolotto-Cepeda AM, Romero-Marquez Y, Munoz-Ramirez KJ. Effectiveness and safety of fast enteral advancement in preterm infants between 1000 and 2000 g of birth weight. Journal of Parenteral and Enteral Nutrition 2021;45(3):578-86. [DOI: 10.1002/jpen.1925] [PMID: ] [DOI] [PubMed] [Google Scholar]
Raban 2014a {published data only}
- Raban MS, Santhakumaran S, Keraan Q, Joolay Y, Uthaya S, Horn AR, et al. A randomised controlled trial of high or low volume initiation and rapid or slow advancement of milk feeds for infants ≤ 1000 g. In: South African Paediatric Association Congress; 2014 Sept 10-14; Cape Town. 2014.
- Raban S, Santhakumaran S, Keraan Q, Joolay Y, Uthaya S, Horn A, et al. A randomised controlled trial of high vs low volume initiation and rapid vs slow advancement of milk feeds in infants with birthweights ≤ 1000 g in a resource-limited setting. Paediatrics and International Child Health 2016;36(4):288-95. [DOI: 10.1179/2046905515Y.0000000056] [PMID: ] [DOI] [PubMed] [Google Scholar]
Raban 2014b {published data only}
- Raban MS, Santhakumaran S, Keraan Q, Joolay Y, Uthaya S, Horn AR, et al. A randomised controlled trial of high or low volume initiation and rapid or slow advancement of milk feeds for infants ≤ 1000 g. In: South African Paediatric Association Congress; 2014 Sept 10-14; Cape Town. 2014.
- Raban S, Santhakumaran S, Keraan Q, Joolay Y, Uthaya S, Horn A, et al. A randomised controlled trial of high vs low volume initiation and rapid vs slow advancement of milk feeds in infants with birthweights ≤ 1000 g in a resource-limited setting. Paediatrics and International Child Health 2016;36(4):288-95. [DOI: 10.1179/2046905515Y.0000000056] [PMID: 26369284] [DOI] [PubMed] [Google Scholar]
Rayyis 1999 {published data only}
- Rayyis SF, Ambalavanan N, Wright L, Carlo WA. Randomized trial of "slow" versus "fast" feed advancements on the incidence of necrotizing enterocolitis in very low birth weight infants. Journal of Pediatrics 1999;134(3):293-7. [DOI: 10.1016/s0022-3476(99)70452-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Saha 2019 {published data only}
- Saha LC, Yaesmin R, Hoque M, Chowdhury MA. Slow versus rapid advancement of enteral feeding in preterm infants less than 34 weeks: a randomized controlled trial. Journal of Neonatology and Clinical Pediatrics 2019;6:029. [DOI: 10.24966/NCP-878X/100029] [DOI] [Google Scholar]
Salhotra 2004 {published data only}
- Salhotra A, Ramji S. Slow versus fast enteral feed advancement in very low birth weight infants: a randomized control trial. Indian Pediatrics 2004;41(5):435-41. [PMID: ] [PubMed] [Google Scholar]
SIFT 2016 {published data only}
- Abbott J, Berrington J, Bowler U, Boyle E, Dorling J, Embleton N, et al, SIFT Investigators Group. The Speed of Increasing milk Feeds: a randomised controlled trial. BMC Pediatrics 2017;17(1):39. [DOI: 10.1186/s12887-017-0794-z] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIFT Investigators Group. Early enteral feeding strategies for very preterm infants: current evidence from Cochrane reviews. Archives of Disease in Childhood - Fetal and Neonatal Edition 2013;98(6):F470-2. [DOI: 10.1136/archdischild-2012-303260] [PMID: ] [DOI] [PubMed] [Google Scholar]
- SIFT Investigators Group. SIFT: Outcomes at discharge. In: BAPM Annual General & Scientific Meeting; 2016 Sep 15-16; Watershed, Bristol. 2016.
- SIFT Investigators Group. SIFT: Speed of increasing feeds trial. In: Hot Topics in Neonatology Annual Meeting; 2016 Dec 5-7; Washington, DC. 2016.
- SIFT Investigators Group. The Speed of Increasing milk Feeds Trial (SIFT); results at hospital discharge. In: Pediatric Academic Societies Annual Meeting; 2017 May 6-9; San Francisco, CA. 2017. [registration.pas-meeting.org/2017/reports/rptPAS17_Abstracts.asp]
References to studies excluded from this review
Ahmed 2020 {published data only}
- Ahmed F, Dey SK, Shahidullah M, Mannan MA, Raj AY, Sharmin S. Early versus delayed enteral feeding for achieving full feeding in preterm growth-restricted infants: a randomized clinical trial. Mymensingh Medical Journal 2020;29(3):638-45. [PubMed] [Google Scholar]
Berseth 2003 {published data only}
- Berseth CL, Bisquera JA, Paje VU. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2003;111(3):529-34. [DOI: 10.1542/peds.111.3.529] [PMID: ] [DOI] [PubMed] [Google Scholar]
Book 1976 {published data only}
- Book LS, Herbst JJ, Jung A. Comparison of fast- and slow-feeding rate schedules to the development of necrotizing enterocolitis. Journal of Pediatrics 1976;89(3):463-6. [DOI: 10.1016/s0022-3476(76)80552-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gray 2017 {published data only}
- Gray MM, Medoff-Cooper B, Enlow EM, Mukhopadhyay S, DeMauro SB. Every three-hour versus every six-hour oral feeding in preterm infants: a randomised clinical trial. Acta Paediatrica 2017;106(2):236-41. [DOI: 10.1111/apa.13658] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ibrahim 2017 {published data only}
- Ibrahim NR, Kheng TH, Nasir A, Ramli N, Foo JLK, Syed Alwi SH, et al. Two-hourly versus 3-hourly feeding for very low birthweight infants: a randomised controlled trial. Archives of Disease in Childhood - Fetal and Neonatal Edition 2017;102(3):F225-9. [DOI: 10.1136/archdischild-2015-310246] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jayaraman 2017 {published data only}
- Jayaraman D, Mukhopadhyay K, Bhalla AK, Dhaliwal LK. Randomized controlled trial on effect of intermittent early versus late kangaroo mother care on human milk feeding in low-birth-weight neonates. Journal of Human Lactation 2017;33(3):533-9. [DOI: 10.1177/0890334416685072] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kadam 2016 {published data only}
- Kadam RM, Prasad VS, Santosh M. Rapid versus slow advancement of feeds in preterm babies less than 34 weeks in incidence of NEC and feed intolerance. Journal of Neonatal Biology 2016;5(1):1000214. [DOI: 10.4172/2167-0897.1000214] [DOI] [Google Scholar]
Nangia 2019 {published data only}
- Nangia S, Vadivel V, Thukral A, Saili A. Early total enteral feeding versus conventional enteral feeding in stable very-low-birth-weight infants: a randomised controlled trial. Neonatology 2019;115(3):256-62. [DOI: 10.1159/000496015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tewari 2018 {published data only}
- Tewari VV, Dubey SK, Kumar R, Vardhan S, Sreedhar CM, Gupta G. Early versus late enteral feeding in preterm intrauterine growth restricted neonates with antenatal doppler abnormalities: an open-label randomized trial. Journal of Tropical Pediatrics 2018;64(1):4-14. [DOI: 10.1093/tropej/fmx018] [PMID: ] [DOI] [PubMed] [Google Scholar]
Viswanathan 2017 {published data only}
- Viswanathan S, Merheb R, Wen X, Collin M, Groh-Wargo S. Standardized slow enteral feeding protocol reduces necrotizing enterocolitis in micropremies. Journal of Neonatal-Perinatal Medicine 2017;10(2):171-80. [DOI: 10.3233/NPM-171680] [PMID: ] [DOI] [PubMed] [Google Scholar]
Additional references
Battersby 2018
- Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Archives of Disease in Childhood - Fetal and Neonatal Edition 2018;103(2):F182-9. [DOI: 10.1136/archdischild-2017-313880] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bernstein 2000
- Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 1):198-206. [DOI: 10.1016/s0002-9378(00)70513-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Berrington 2012
- Berrington JE, Hearn RI, Bythell M, Wright C, Embleton ND. Deaths in preterm infants: changing pathology over 2 decades. Journal of Pedodontics 2012;160(1):49-53.e1. [DOI: 10.1016/j.jpeds.2011.06.046] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bertino 2009
- Bertino E, Giuliani F, Prandi G, Coscia A, Martano C, Fabris C. Necrotizing enterocolitis: risk factor analysis and role of gastric residuals in very low birth weight infants. Journal of Pediatric Gastroenterology and Nutrition 2009;48(4):437-42. [DOI: 10.1097/mpg.0b013e31817b6dbe] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cobb 2004
- Cobb BA, Carlo WA, Ambalavanan N. Gastric residuals and their relationship to necrotizing enterocolitis in very low birth weight infants. Pediatrics 2004;113(1 Pt 1):50-3. [DOI: 10.1542/peds.113.1.50] [PMID: ] [DOI] [PubMed] [Google Scholar]
Conde‐Agudelo 2016
- Conde-Agudelo A, Diaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD002771. [DOI: 10.1002/14651858.CD002771.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Waard 2018
- Waard M, Li Y, Zhu Y, Ayede AI, Berrington J, Bloomfield FH, et al. Time to full enteral feeding for very low-birth-weight infants varies markedly among hospitals worldwide but may not be associated with incidence of necrotizing enterocolitis: the NEOMUNE-NeoNutriNet cohort study. Journal of Parenteral and Enteral Nutrition 2018;43:658-67. [DOI: 10.1002/jpen.1466] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorling 2005
- Dorling J, Kempley S, Leaf A. Feeding growth restricted preterm infants with abnormal antenatal Doppler results. Archives of Disease in Childhood - Fetal and Neonatal Edition 2005;90(5):F359-63. [DOI: 10.1136/adc.2004.060350] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Embleton 2013
- Embleton ND. Early nutrition and later outcomes in preterm infants. World Review of Nutrition and Dietetics 2013;106:26-32. [DOI: 10.1159/000342553] [PMID: ] [DOI] [PubMed] [Google Scholar]
Embleton 2017
- Embleton ND, Berrington JE, Dorling J, Ewer AK, Juszczak E, Kirby JA, et al. Mechanisms affecting the gut of preterm infants in enteral feeding trials. Frontiers in Nutrition 2017;4:14. [DOI: 10.3389/fnut.2017.00014] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flidel‐Rimon 2004
- Flidel-Rimon O, Friedman S, Lev E, Juster-Reicher A, Amitay M, Shinwell ES. Early enteral feeding and nosocomial sepsis in very low birthweight infants. Archives of Disease in Childhood - Fetal and Neonatal Edition 2004;89(4):F289-92. [DOI: 10.1136/adc.2002.021923] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flidel‐Rimon 2006
- Flidel-Rimon O, Branski D, Shinwell ES. The fear of necrotizing enterocolitis versus achieving optimal growth in preterm infants - an opinion. Acta Paediatrica 2006;95(11):1341-4. [DOI: 10.1080/08035250600719713] [PMID: ] [DOI] [PubMed] [Google Scholar]
Garite 2004
- Garite TJ, Clark R, Thorp JA. Intrauterine growth restriction increases morbidity and mortality among premature neonates. American Journal of Obstetrics and Gynecology 2004;191(2):481-7. [DOI: 10.1016/j.ajog.2004.01.036] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 12 May 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Härtel 2009
- Härtel C, Haase B, Browning-Carmo K, Gebauer C, Kattner E, Kribs A, et al. Does the enteral feeding advancement affect short-term outcomes in very low birth weight infants? Journal of Pediatric Gastroenterology and Nutrition 2009;48(4):464-70. [DOI: 10.1097/MPG.0b013e31818c5fc3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hay 2018
- Hay WW Jr. Nutritional support strategies for the preterm infant in the neonatal intensive care unit. Pediatric Gastroenterology, Hepatology & Nutrition 2018;21(4):234-47. [DOI: 10.5223/pghn.2018.21.4.234] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henderson 2009
- Henderson G, Craig S, Brocklehurst P, McGuire W. Enteral feeding regimens and necrotising enterocolitis in preterm infants: a multicentre case-control study. Archives of Disease in Childhood - Fetal and Neonatal Edition 2009;94(2):F120-3. [DOI: 10.1136/adc.2007.119560] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hickey 2018
- Hickey M, Georgieff M, Ramel S. Neurodevelopmental outcomes following necrotizing enterocolitis. Seminars in Fetal and Neonatal Medicine 2018;23(6):426-32. [DOI: 10.1016/j.siny.2018.08.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Horbar 2012
- Horbar JH, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 2012;129(6):1019-26. [DOI: 10.1542/peds.2011-3028] [PMID: ] [DOI] [PubMed] [Google Scholar]
Klingenberg 2012
- Klingenberg C, Embleton ND, Jacobs SE, O'Connell LA, Kuschel CA. Enteral feeding practices in very preterm infants: an international survey. Archives of Disease in Childhood - Fetal and Neonatal Edition 2012;97(1):F56-61. [DOI: 10.1136/adc.2010.204123] [PMID: ] [DOI] [PubMed] [Google Scholar]
Leaf 2013
- Leaf A. Introducing enteral feeds in the high-risk preterm infant. Seminars in Fetal and Neonatal Medicine 2013;18(3):150-4. [DOI: 10.1016/j.siny.2013.03.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Luig 2005
- Luig M, Lui K, NSW & ACT NICUS Group. Epidemiology of necrotizing enterocolitis - part II: risks and susceptibility of premature infants during the surfactant era: a regional study. Journal of Paediatrics and Child Health 2005;41(4):174-9. [DOI: 10.1111/j.1440-1754.2005.00583.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Maas 2018
- Maas C, Franz AR, Krogh S, Arand J, Poets CF. Growth and morbidity of extremely preterm infants after early full enteral nutrition. Archives of Disease in Childhood - Fetal and Neonatal Edition 2018;103(1):F79-81. [DOI: 10.1136/archdischild-2017-312917] [PMID: ] [DOI] [PubMed] [Google Scholar]
Martin 2010
- Martin CR, Dammann O, Allred EN, Patel S, O'Shea TM, Kuban KC, et al. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. Journal of Pediatrics 2010;157(5):751-6. [DOI: 10.1016/j.jpeds.2010.05.042] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mihatsch 2002
- Mihatsch WA, Schoenaich P, Fahnenstich H, Dehne N, Ebbecke H, Plath C, et al. The significance of gastric residuals in the early enteral feeding advancement of extremely low birth weight infants. Pediatrics 2002;109(3):457-9. [DOI: 10.1542/peds.109.3.457] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morgan 2013a
- Morgan J, Bombell S, McGuire W. Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD000504. [DOI: 10.1002/14651858.CD000504.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2014a
- Morgan J, Young L, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD001970. [DOI: 10.1002/14651858.CD001970.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pammi 2017
- Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 2017;5(1):31. [DOI: 10.1186/s40168-017-0248-8] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patole 2005
- Patole SK, Klerk N. Impact of standardised feeding regimens on incidence of neonatal necrotising enterocolitis: a systematic review and meta-analysis of observational studies. Archives of Disease in Childhood - Fetal and Neonatal Edition 2005;90(2):F147-51. [DOI: 10.1136/adc.2004.059741] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Premji 2011
- Premji SS, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: CD001819. [DOI: 10.1002/14651858.CD001819.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quigley 2019
- Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD002971. [DOI: 10.1002/14651858.CD002971.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samuels 2017
- Samuels N, de Graaf RA, Jonge RC, Reiss IKM, Vermeulen MJ. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatrics 2017;17(1):105. [DOI: 10.1186/s12887-017-0847-3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sankar 2008
- Sankar MJ, Agarwal R, Mishra S, Deorari AK, Paul VK. Feeding of low birth weight infants. Indian Journal of Pediatrics 2008;75(5):459-69. [DOI: 10.1007/s12098-008-0073-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shah 2012
- Shah TA, Meinzen-Derr J, Gratton T, Steichen J, Donovan EF, Yolton K, et al. Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. Journal of Perinatology 2012;32(7):552-8. [DOI: 10.1038/jp.2011.176] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soll 2019
- Soll RF, McGuire W. Evidence-based practice: improving the quality of perinatal care. Neonatology 2019;116(3):193-8. [DOI] [PubMed] [Google Scholar]
Tyson 2007
- Tyson JE, Kennedy KA, Lucke JF, Pedroza C. Dilemmas initiating enteral feedings in high risk infants: how can they be resolved? Seminars in Perinatology 2007;31(2):61-73. [DOI: 10.1053/j.semperi.2007.02.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 1986
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric Clinics of North America 1986;33(1):179-201. [DOI: 10.1016/s0031-3955(16)34975-6] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2019
- Walsh V, McGuire W. Immunonutrition for preterm infants. Neonatology 2019;115(4):398-405. [DOI: 10.1159/000497332] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 2020
- Walsh V, Brown JVE, Copperthwaite BR, Oddie SJ, McGuire W. Early full enteral feeding for preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD013542. [DOI: 10.1002/14651858.CD013542.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2021
- Walsh V, McGuire W, Halliday HL. Evaluation of the quality of perinatal trials: making the GRADE. Neonatology 2021 ;118:378-83. [DOI: 10.1159/000516239] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
McGuire 2008
- McGuire W, Bombell S. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD001241. [DOI: 10.1002/14651858.CD001241.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morgan 2011
- Morgan J, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD001241. [DOI: 10.1002/14651858.CD001241.pub3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morgan 2013b
- Morgan J, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD001241. [DOI: 10.1002/14651858.CD001241.pub4] [DOI] [PubMed] [Google Scholar]
Morgan 2014b
- Morgan J, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD001241. [DOI: 10.1002/14651858.CD001241.pub4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morgan 2015
- Morgan J, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD001241. [DOI: 10.1002/14651858.CD001241.pub6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Oddie 2017
- Oddie SJ, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD001241. [DOI: 10.1002/14651858.CD001241.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]


