Abstract
Objectives:
To examine a set of proposed eligibility factors for hemi-ablative focal therapy in prostate cancer and to determine the likelihood of residual extensive disease.
Methods:
We retrospectively analyzed data from 98 patients with unilateral prostate cancer on biopsy with detailed tumor maps from whole-mount slides and preoperative magnetic resonance imaging (MRI) data. These patients met the focal therapy consensus meeting inclusion criteria (prostate-specific antigen <15 ng/mL, clinical stage T1c-T2a, and Gleason score 3+3 or 3+4 on needle biopsy) and underwent radical prostatectomy between 2000 and 2014. Extensive disease was defined as having Gleason pattern 4/5 in bilateral lobes, any extraprostatic extension, seminal vesicle or lymph node invasion. Both lobes of the prostate were scored on MRI. Preoperative characteristics including biopsy and MRI data were used to predict extensive disease.
Results:
Among our cohort of 98 patients, 40% (95% CI 30%, 50%) had extensive disease. Thirty-three percent (95% CI 24%, 43%) had Gleason pattern 4/5 in both lobes with a median Gleason pattern 4/5 tumor volume in the biopsy negative lobe of 0.06 cm3, 17 patients had pathologic tumor stage ≥3, and one had lymph node invasion.
Conclusions:
An important number of patients meeting the focal therapy consensus meeting inclusion criteria can present extensive disease. Further studies using targeted biopsies may provide more accurate information about the selection of focal therapy candidates.
Keywords: ablation techniques, biopsy, magnetic resonance imaging, pathology, prostatic neoplasms
INTRODUCTION
Current options for patients with localized prostate cancer are active surveillance (AS) and whole-gland therapy using surgery or radiation. The primary aim of AS is to prevent overtreatment. However, the cumulative incidence of treatment 10 year after the start of AS has been reported to be 37−50% 1, 2, thus many AS patients manage to delay but not prevent whole-gland therapy. Meanwhile, whole-gland therapy is associated with significant and lasting morbidity: at 15-year follow-up, approximately 20% and 40% of patients reported being bothered by urinary incontinence and sexual dysfunction, respectively 3.
Focal therapy is a treatment modality that aims to reduce side effects while maintaining oncologic efficacy. The ablative therapies include high-intensity focused ultrasound (HIFU), cryotherapy, laser ablation, radiofrequency ablation, irreversible electroporation, and photodynamic therapy 4. To minimize collateral damage to the urinary sphincter, urethra, bladder, rectum, and neurovascular bundles, focal treatment strategies consist of targeted ablation, hemi-ablation, and zonal ablation 4. Several investigators have reported that focal cryotherapy, HIFU, and brachytherapy are feasible and safe, although they only examined small numbers of patients and short-term outcomes 5–7.
The most frequently performed focal therapy is hemi-ablative, which involves ablation of an entire half of the prostate. The ideal candidates are patients with organ-confined unilateral cancer, but hemi-ablative focal therapy is also offered to patients who have insignificant lesions in the lobe to be spared. Detailed tumor maps outlining tumor location are useful in identifying all areas of cancer and were deemed necessary for inclusion into this study. Among patients undergoing radical prostatectomy (RP), we reviewed tumor maps and pre-RP magnetic resonance imaging (MRI) scans for patients diagnosed with unilateral prostate cancer. From this pool of potential candidates for hemi-ablative focal therapy, we sought to determine preoperative predictors of extensive (bilateral or non-organ-confined) disease to identify the inappropriate candidates.
METHODS
After receiving institutional review board approval, we identified 9519 patients who underwent RP at Memorial Sloan Kettering Cancer Center between 2000–2014. Of these patients, 770 patients (a) were diagnosed with unilateral prostate cancer with a minimum 10-core biopsy, (b) met the focal therapy consensus meeting inclusion criteria (prostate-specific antigen [PSA] <15 ng/mL, clinical stage T1c-T2a, and Gleason score (GS) 3+3 or 3+4 on needle biopsy) 4, and (c) had no history of other treatments before RP. Patients with GS 4+3 or more on needle biopsy were not included. Among these patients, 109 had detailed tumor maps available. Tumor maps were prepared by our pathology department in 3-mm sections from apex to base from whole-mount slides8 wherein all tumor borders were outlined using two different colors for Gleason pattern 3 versus Gleason 4/5, and tumor volume of Gleason pattern 3 and Gleason pattern 4/5 was calculated. There are no selection criteria at our institution for which patients have a tumor map created and as such they are not created as part of routine pathology report, but instead specially created for research purposes. We therefore assessed differences between patient who did and did not have tumor maps created and observed that patients with tumor maps had lower pathologic tumor stage (83% vs. 73% with pT2; p=0.040) and a smaller proportion had extraprostatic extension (EPE) (17% vs. 27%; p=0.018) and positive surgical margins (4.6% vs. 12%; p=0.020) using Fisher’s exact and Wilcoxon rank-sum test for categorical and continuous variables, respectively. As a result, our cohort consisted of patients with lower-risk disease.
All MRI scans (GE Medical Systems, Waukesha, USA) were retrospectively reviewed by a radiologist blinded to patients’ identity and RP pathology. The radiologist scored both lobes of the prostate to determine the likelihood of the patient having tumors using the standardized 5-point Prostate Imaging Reporting and Data System version 2 (PI-RADS v2) (1 − definitely no tumor, 5 − definitely tumor) 9. Consequently, we excluded 11 patients whose MRI did not include diffusion-weighted imaging, which is necessary for PI-RADS v2. Our final cohort consisted of 98 patients with 3-Tesla (3T) multiparametric MRI (mpMRI) data, who underwent the MRI less than 6 months prior to surgery. Most patients had their MRI performed after biopsy. In particular, 10% of patients had their MRI performed before biopsy. The likelihood of EPE and seminal vesicle involvement (SVI) were also scored using a 5-point Likert scale (1 − Unlikely, 2 − Less likely, 3 − Possible, 4 − Suspicious for/probable, 5 − Consistent with). Our postoperative determination of extensive disease was based on RP results showing Gleason pattern 4/5 in bilateral lobes, EPE in any lobe, SVI, or lymph node invasion (LNI) on the tumor map and RP pathology (illustration shown in Figure 1). Any of these features would indicate that the patient’s disease was too extensive for hemi-ablative therapy. As a sensitivity analysis, we considered a more restrictive definition of extensive disease: having ≥0.5mL Gleason pattern 4/5 in bilateral lobes, EPE, SVI, or LNI. This threshold is based on incidentally detected prostate cancer in a radical cystoprostatectomy series by Stamey et al based on an 8% lifetime risk of being diagnosed with clinically significant prostate cancer 10. Tumor volume was calculated by multiplying the tumor area by the slice thickness with computerized planimetry using image analysis and measurement software Photoshop CS6 (Adobe Systems, San Jose, CA, USA) 11.
Figure 1.
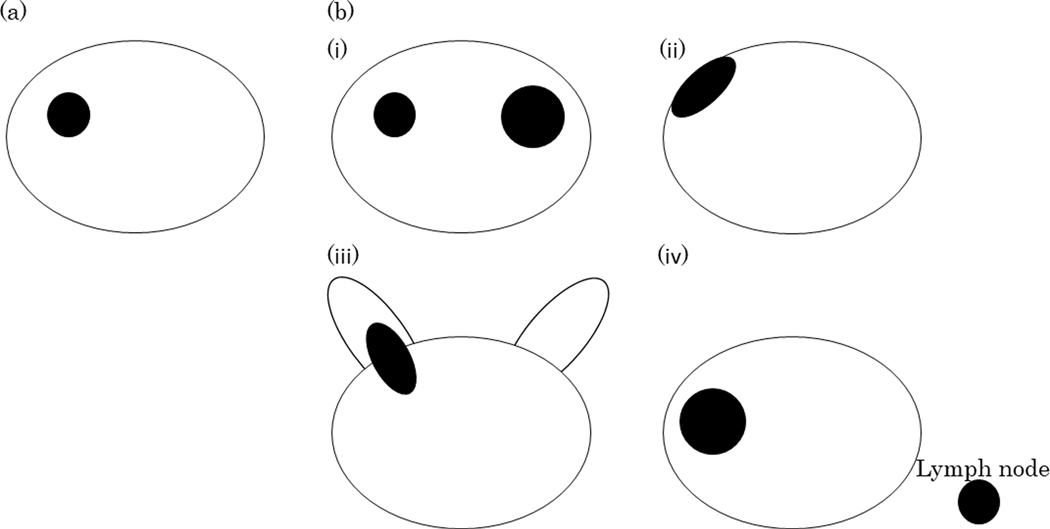
The illustration of tumor location of (a) no extensive disease and (b) extensive disease. No extensive disease was defined as no Gleason pattern 4/5 in bilateral lobes, no extraprostatic extension, no seminal vesicle involvement, and no lymph node invasion. Extensive disease was defined as (i) Gleason pattern 4/5 in bilateral lobes, or (ii) extraprostatic extension, or (iii) seminal vesicle involvement, or (iv) lymph node invasion.
To avoid an overfitted predictive model due to a low number of men with extensive disease (N=39), we used a forward selection stepwise logistic regression with a significance level of <0.15 indicating addition into the model. With this method, odds ratios from the logistic regression would be inflated and would need to be interpreted accordingly. Our list of potential predictors included age at diagnosis, percent positive biopsy cores, prostate volume on MRI, bilateral MRI tumor score ≥3, bilateral MRI tumor score ≥4, MRI EPE score ≥3, MRI EPE score ≥4, MRI SVI score ≥3, MRI SVI score ≥4, and Kattan nomogram score for risk of recurrence after RP. The Kattan nomogram is a prediction tool assessing the risk of disease recurrence based on PSA, clinical stage, and biopsy GS 12. We tested the nomogram for nonlinearity using restricted cubic splines with knots at the tertiles, but since we found no evidence of a non-linear relationship, it was included as a linear variable.
To evaluate the discriminative accuracy of our predictive model, we calculated the area under the receiver-operating characteristics curve (AUC). We used decision curve analysis to determine the clinical value of our predictive model, focusing on a particular range of threshold probability. As clinicians may have varying opinions of when they may offer focal therapy as a treatment option, and patients would weigh potential side effects from other treatments compared to potential undertreatment of focal therapy differently, this threshold would vary among clinicians and between clinicians and patients. The threshold probability would indicate at what probability of having extensive disease a patient would nonetheless choose to undergo focal therapy. For example, a patient with a threshold probability of 15% would choose focal therapy if the probability of having extensive disease were ≤15% and forego focal treatment, likely choosing something more aggressive, if the probability were >15%. We investigated the range of threshold probabilities from 1% to 20%. Both the discrimination and decision curve were corrected for overfit using 10-fold cross validation, which included the forward stepwise logistic regression. All statistical analyses were conducted using STATA 13.0 (StataCorp, College Station, TX).
RESULTS
Patient and tumor characteristics stratified by the primary definition of extensive disease are shown in Table 1. Among our cohort of 98 patients who met the focal therapy consensus meeting inclusion criteria 4, 39 (40%; 95% CI 30%, 50%) had extensive disease based on their tumor maps and RP specimen, 32 (33%; 95% CI 24%, 43%) had Gleason pattern 4/5 in both lobes, 17 had pathologic tumor stage ≥3, and one had LNI. In these 32 patients with Gleason pattern 4/5 in both lobes, dominant tumor volume in biopsy negative lobe was 0.2 cm3 (26 patients with GS 3+4) and 0.1 cm3 (6 patients with GS 4+3), and median tumor volume of Gleason pattern 4/5 in biopsy negative lobe was 0.06 cm3. Table 2 depicts the MRI scores for the biopsy-positive and negative lobe, 42% (95% CI 32%, 52%) of patients had biopsy-negative lobe assigned an MRI tumor score of 4 or 5. Our model consisted of the Kattan nomogram score (OR 1.10; 95% CI 1.02, 1.19; p=0.020) and MRI EPE score ≥3 (OR 5.08; 95% CI 1.84, 14.00; p=0.002) and exhibited a discrimination of 0.698 after 10-fold cross-validation. Although the variables in our predictive model are significantly associated with extensive disease, it does not add value within our range of threshold probabilities between 1–20% on decision curve analysis (Figure 2).
Table 1 –
Characteristics of 98 prostate cancer patients treated with radical prostatectomy but retrospectively evaluated as candidates for focal therapy with primary definition of extensive disease. All values are median (interquartile range) or frequency (proportion)
| No Extensive Disease at RP (N=59; 60%) | Extensive Disease at RP (N=39; 40%) | |
|---|---|---|
| Age at diagnostic biopsy (yr) | 57 (52, 63) | 58 (53, 65) |
| Gleason score at diagnostic biopsy | ||
| 6 | 42 (71%) | 14 (36%) |
| 3+4 | 17 (29%) | 25 (64%) |
| Percentage of positive cores at diagnostic biopsy | 13.3 (7.7, 25.0) | 16.7 (8.3, 30.8) |
| Clinical stage | ||
| T1c | 52 (88%) | 32 (82%) |
| T2a | 7 (12%) | 7 (18%) |
| Kattan nomogram score* | 8.7 (8.0, 15.1) | 14.9 (12.1, 19.6) |
| Prostate volume on MRI (cm3) | 35.2 (26.8, 48.0) | 33.0 (25.6, 38.2) |
| Pre-RP PSA level (ng/ml) | 4.5 (3.3, 6.2) | 5.6 (4.1, 7.6) |
| Gleason score at RP | ||
| 6 | 26 (44%) | 0 |
| 3+4 | 33 (56%) | 39 (100%) |
| Pathologic stage at RP | ||
| T2 | 59 (100%) | 22 (56%) |
| T3a | 0 | 14 (36%) |
| T3b | 0 | 2 (5%) |
| T4 | 0 | 1 (2.6%) |
| Gleason pattern 4/5 in bilateral lobes | 0 | 32 (82%) |
| Tumor volume of Gleason pattern 4/5 in biopsy negative lobe (cm3) (N=32) | - | 0.06 (0.02,0.14) |
| Dominant tumor volume with Gleason pattern 4 or 5 in biopsy negative lobe (cm3) (N=32) | - | 0.2 (0.1, 0.5) |
| Gleason score 3+4 (cm3) (N=26) | - | 0.2 (0.2, 0.5) |
| Gleason score 4+3 (cm3) (N=6) | - | 0.1 (0.1, 0.4) |
| Extraprostatic extension | 0 | 17 (44%) |
| Seminal vesicle involvement | 0 | 2 (5.1%) |
| Lymph node invasion | 0 | 1 (2.6%) |
MRI = magnetic resonance imaging; PSA = prostate-specific antigen; RP = radical prostatectomy.
The Kattan nomogram score incorporates PSA, clinical stage, and biopsy Gleason score.
Table 2 –
Tumor characteristics on MRI for 98 patients diagnosed with unilateral prostate cancer. All values are frequency (proportion)
| Biopsy-positive lobe | Biopsy-negative lobe | |
|---|---|---|
| Tumor score on MRI | ||
| 1 (no tumor) | 0 | 1 (1.0%) |
| 2 | 10 (10%) | 24 (24%) |
| 3 | 21 (21%) | 32 (33%) |
| 4 | 43 (44%) | 37 (38%) |
| 5 (definitely tumor) | 24 (24%) | 4 (4.1%) |
| EPE score on MRI | ||
| 1 (no EPE) | 42 (43%) | 67 (68%) |
| 2 | 10 (10%) | 15 (15%) |
| 3 | 21 (21%) | 9 (9.2%) |
| 4 | 19 (19%) | 7 (7.1%) |
| 5 (definitely EPE) | 6 (6.1%) | 0 |
| SVI score on MRI | ||
| Absence of SV | 1 (1.0%) | 0 |
| 1 (no SVI) | 92 (94%) | 94 (96%) |
| 2 | 2 (2.0%) | 3 (3.1%) |
| 3 | 1 (1.0%) | 1 (1.0%) |
| 4 | 1 (1.0%) | 0 |
| 5 (definitely SVI) | 1 (1.0%) | 0 |
EPE = extraprostatic extension; MRI = magnetic resonance imaging; SVI = seminal vesicle involvement.
Figure 2.
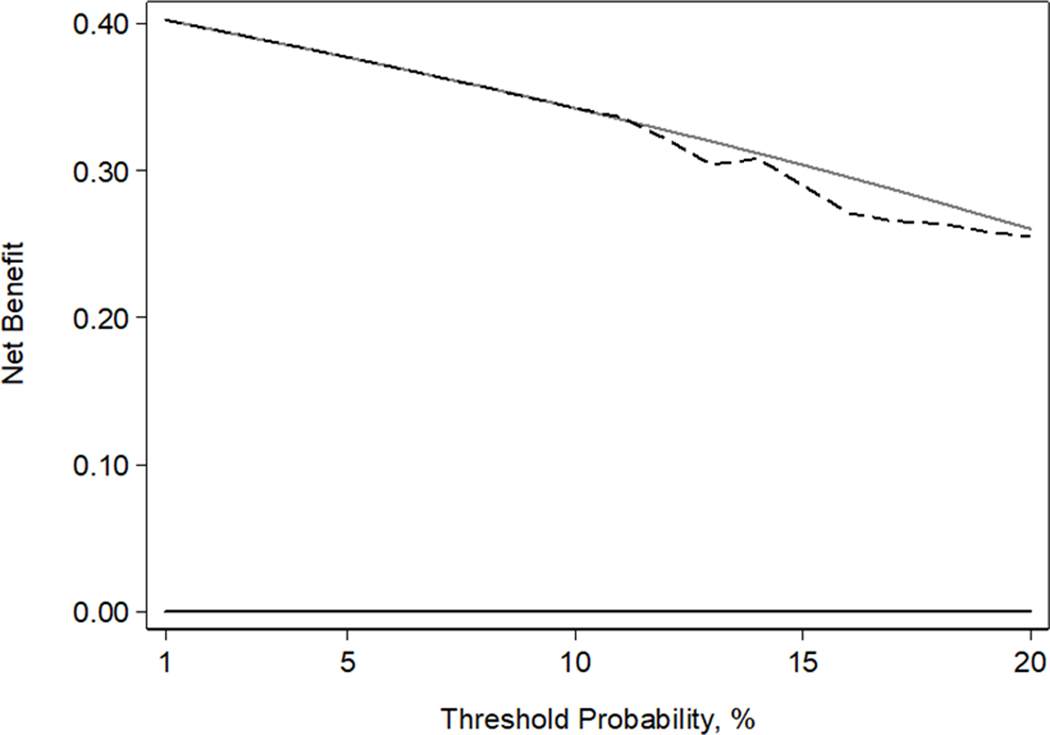
Decision curve analysis plot. The black dashed line represents net benefit of our predictive model on primary analysis (extensive disease defined as Gleason pattern 4/5 in bilateral lobes, extraprostatic extension, seminal vesicle involvement, or lymph node invasion), the grey solid line represents the treat-all strategy, and the black solid line represents the treat-none strategy.
On sensitivity analysis, 17 (17%; 95% CI 10%, 26%) patients had extensive disease. There were only two patients with ≥0.5 mL Gleason pattern 4/5 in bilateral lobes, both had EPE, and only one had LNI and SVI (Table 3). This model incorporated the Kattan nomogram score (OR 1.16; 95% CI 1.03, 1.32; p=0.017) and MRI EPE score >3 (OR 6.79; 95% CI 1.46, 31.66; p=0.015) and exhibited a discrimination of 0.809 after 10-fold cross-validation. These predictors are significantly associated with this more restrictive definition of extensive disease and adds value on decision curve analysis (Figure 3).
Table 3 –
Characteristics of 98 prostate cancer patients treated with radical prostatectomy but retrospectively evaluated as candidates for focal therapy with the more restrictive definition of extensive disease. All values are median (interquartile range) or frequency (proportion)
| No Extensive Disease at RP (N=81; 83%) | Extensive Disease at RP (N=17; 17%) | |
|---|---|---|
| Age at diagnostic biopsy (yr) | 58 (53, 63) | 58 (53, 65) |
| Gleason score at diagnostic biopsy | ||
| 6 | 54 (67%) | 2 (12%) |
| 3+4 | 27 (33%) | 15 (88%) |
| Percentage of positive cores at diagnostic biopsy | 15.4 (8.3, 25.0) | 16.7 (8.3, 33.3) |
| Clinical stage | ||
| T1c | 72 (89%) | 12 (71%) |
| T2a | 9 (11%) | 5 (29%) |
| Kattan nomogram score* | 9.8 (8.2, 15.4) | 18.0 (14.5, 20.9) |
| Prostate volume on MRI (cm3) | 35.0 (27.0, 44.9) | 29.0 (24.9, 36.0) |
| Pre-RP PSA level (ng/ml) | 4.7 (3.6, 6.5) | 5.0 (4.0, 7.6) |
| Gleason score at RP | ||
| 6 | 26 (32%) | 0 (0%) |
| 3+4 | 55 (68%) | 17 (100%) |
| Gleason pattern 4/5 in bilateral lobes | 18 (22%) | 9 (53%) |
| ≥0.5 mL Gleason pattern 4/5 in bilateral lobes | 0 | 2 (12%) |
| Pathologic stage at RP | ||
| T2 | 81 (100%) | 0 (0%) |
| T3a | 0 (0%) | 14 (82%) |
| T3b | 0 (0%) | 2 (12%) |
| T4 | 0 (0%) | 1 (5.9%) |
| Extraprostatic extension | 0 (0%) | 17 (100%) |
| Seminal vesicle involvement | 0 (0%) | 2 (12%) |
| Lymph node invasion | 0 (0%) | 1 (5.9%) |
MRI = magnetic resonance imaging; PSA = prostate-specific antigen; RP = radical prostatectomy.
The Kattan nomogram score incorporates PSA, clinical stage, and biopsy Gleason score.
Figure 3.
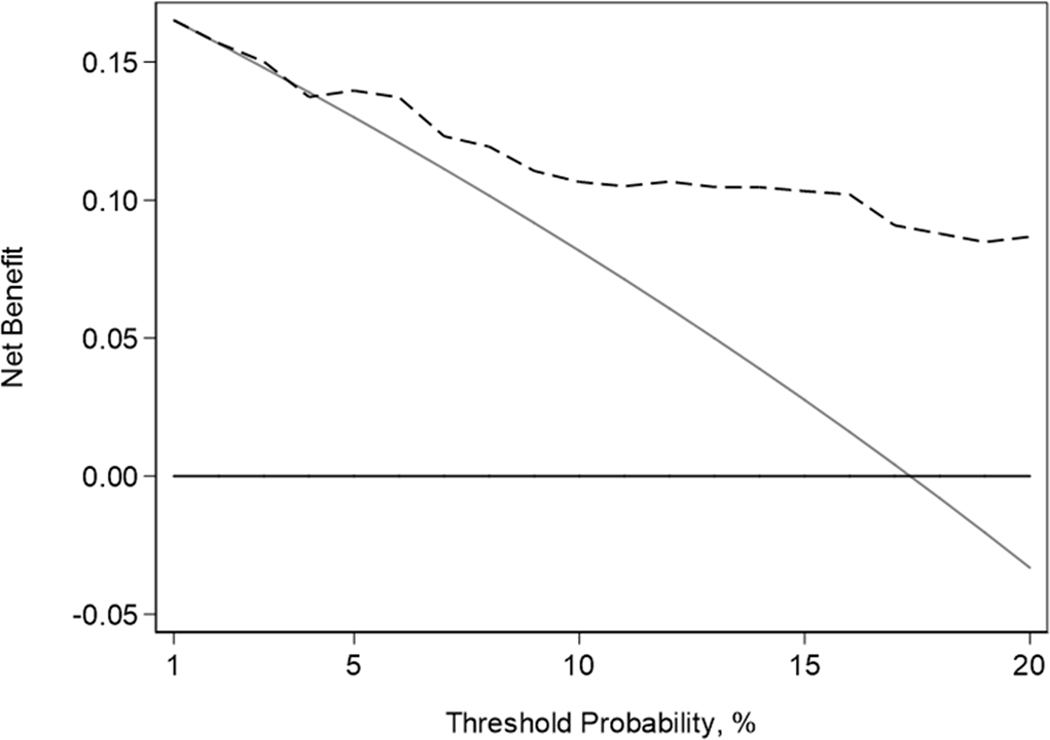
Decision curve analysis plot. The black dashed line represents net benefit of our predictive model on sensitivity analysis (extensive disease defined as having ≥0.5mL Gleason pattern 4/5 in bilateral lobes, extraprostatic extension, seminal vesicle involvement, or lymph node invasion), the grey solid line represents the treat-all strategy, and the black solid line represents the treat-none strategy.
DISCUSSION
We constructed a model to identify patients with extensive disease among men with biopsy-proven unilateral tumor who met criteria for focal therapy. Our primary model had only moderate discrimination and was demonstrated to have no clinical value, however on sensitivity analysis, utilizing a more restrictive definition of extensive disease where we required a minimum of 0.5mL Gleason pattern 4/5 in bilateral lobes, our model added clinical value on decision analysis.
The ideal candidates for hemi-ablative focal therapy are patients with organ-confined unilateral disease. Nevertheless, hemi-ablative focal therapy can also be offered to patients who have insignificant lesions in the biopsy-negative lobe. Several papers have evaluated the accuracy of the sextant and/or extended biopsy in predicting unilateral tumors. Unfortunately, the rate of agreement on unilaterality of prostate cancer between biopsy and RP specimen was less than 30% 13, 14. Other investigators have evaluated independent predictors of unilateral prostate cancer by multivariable analysis and found that PSA, SVI, biopsy unilaterality, negative family history, and percent of positive cores were independent predictors 15–17. Although these papers had the same aim, which was to attempt to predict true unilateral tumors, and none of them included MRI data in their analysis. In our study, we evaluated 3T mpMRI data as well as biopsy data. Rather than predicting only unilaterality of tumors, we reviewed tumor maps and defined organ-confined GS 6 cancer with any volume as insignificant in primary analysis. Therefore, patients with insignificant cancer on the biopsy-negative lobe were deemed to also be candidates for hemi-ablative focal therapy. Finally, we took a more comprehensive look at our predictive factors by evaluating whether our overall model was useful in identifying candidates for hemi-ablative focal therapy.
The classical Epstein definition that considers <0.5 cm3 of organ-confined GS 6 cancer as insignificant has been accepted for more than 20 years 18, but several recent studies have suggested changing the definition of insignificant cancer. Wolters et al. and Ting et al. suggested increasing the threshold of GS 6 total tumor volume to 2.5 cm3 19. Van der Kwast and Roobol stated that essentially all GS 6 prostate cancers could be included in insignificant prostate cancers, irrespective of their volume 20. On the other hand, Kryvenko and Epstein, and Schiffmann et al. did not recommend increasing the threshold of GS 6 tumor volume because of the higher rates of biochemical recurrence, EPE, and positive surgical margins 21, 22. The American Society of Clinical Oncology proposed offering AS to select patients with low-volume GS 3+4 prostate cancer on needle biopsy 23; however, the threshold of volume for considering Gleason pattern 3+4 as insignificant is still controversial. In our study, both definitions of extensive disease considered GS 6 cancer with any volume as insignificant, and our secondary definition considered <0.5mL Gleason pattern 4/5 as insignificant. Although our model with latter definition added clinical value on decision analysis, further evaluation is warranted regarding GS and tumor volume in the spared lobe which is treated with AS.
Currently, mpMRI, including T2-weighted imaging, diffusion-weighted imaging, and dynamic contrast-enhanced MRI, is considered the best imaging setting for prostate cancer detection. A recent meta-analysis showed that mpMRI has sensitivity of 74% and specificity of 88% for prostate cancer detection 24. In terms of staging, mpMRI has a sensitivity and specificity of 57% and 91%, respectively, for EPE detection, and 58% and 96%, respectively, for SVI detection 25.
Nevertheless, mpMRI has better sensitivity in tumor detection than staging. Two papers have evaluated the use of mpMRI and biopsy for hemi-ablative focal therapy: Matsuoka et al. and Tran et al. reported negative predictive values of 96% and 91% for predicting lobes with significant prostate cancer 26, 27. Both studies included tumors with Gleason pattern 4/5 as insignificant cancer: Matsuoka et al. defined <0.5 cm3 ≤ GS3+4 without Gleason pattern 5 as insignificant, and Tran et al. defined GS7 with ≤5% grade 4 or GS8–10 with <0.7 cm3 as insignificant. In the present study, the MRI EPE score ≥3 was an independent predictor of extensive disease. However, bilateral MRI tumor scores of ≥3 or ≥4 were not selected as independent predictors in the model for either definition of extensive disease.
We had 32 patients whose RP results showed Gleason pattern 4/5 in both lobes. Dominant tumor volume with Gleason pattern 4/5 in biopsy negative lobe of these 32 patients was 0.2 cm3 (26 patients with GS 3+4) and 0.1 cm3 (6 patients with GS 4+3), but MRI yielded a tumor score of 4/5 in the biopsy-negative lobe in only 17 of these patients (53%). It may be that MRI does not reliably detect small volumes of cancer in the biopsy-negative lobe, even if it has a Gleason pattern 4/5. Vargas et al. reported that 3T mpMRI could not identify over half of the GS ≥4+3 tumors with a volume <0.5 cm3 28. Furthermore, another paper has reported that tumor detectability on MRI is volume-dependent 29. Improving the diagnostic accuracy of mpMRI for small volumes of high GS cancer would greatly increase its usefulness for staging purposes.
Our study has the following limitations. It was retrospective, based on data from a single institution, and included only patients who underwent RP. This inclusion criterion was necessary to ensure the more accurate results from RP and detailed tumor maps, allowing a more direct comparison to the MRI scan data. The criteria would also exclude patients who underwent previous treatment and radiologic imaging (EPE or SVI, Lymph node or bone metastasis). However, we did not implement the exclusion criteria so that we could evaluate MRI factors in the present study. If we used this exclusion criteria, 30 patients with EPE score ≥4, 2 of whom had an SVI score ≥4 would have been excluded. If we were to exclude these 30 patients, 3 in the remaining 68 patients have extensive disease (4.4%).
Additionally, with no standard recommendation for which patients have tumor maps created, when considering possible selection bias, we found that patients with tumor maps had less aggressive pathologic characteristics than the patients without. Moreover, although we included patients with minimum 10-core biopsy, current consensus guidelines recommend MRI/ultrasound fusion-guided targeted biopsies in addition to systematic biopsies 4. Targeted biopsy diagnosed 30% more GS ≥4+3 cancers vs. standard biopsy 30; combining the 3T mpMRI with targeted biopsy might improve overall accuracy for predicting which patients are eligible for focal therapy. Lastly, in utilizing stepwise selection to choose the variables to include in our model, the coefficients are biased upwards.
In conclusion, based on the tumor maps created from radical prostatectomy data, we tested the proposed set of criteria designed to select patients for hemi-ablative therapy and found that 40% (95% CI 30%, 50%) had extensive disease, 82% of whom would have Gleason pattern 4/5 in the untreated lobe. While focal therapy is an important addition to the treatment of prostate cancer, further studies using targeted biopsies may provide more accurate selection criteria for candidates of this treatment.
Acknowledgment
This study was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers and the NIH/NCI Cancer Center Support Grant P30 CA008748
Abbreviations:
- AS
active surveillance
- AUC
area under the receiver-operating characteristics curve
- EPE
extraprostatic extension
- HIFU
high-intensity focused ultrasound
- mpMRI
multiparametric magnetic resonance imaging
- MRI
magnetic resonance imaging
- PI-RADS v2
Prostate Imaging Reporting and Data System version 2
- PSA
prostate-specific antigen
- RP
radical prostatectomy
- SVI
seminal vesicle involvement
- 3T
3-Tesla
Footnotes
Conflict of Interest
The authors declare no conflict of interests.
REFERENCES
- 1.Klotz L, Vesprini D, Sethukavalan P et al. : Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015; 33: 272–7 [DOI] [PubMed] [Google Scholar]
- 2.Tosoian JJ, Mamawala M, Epstein JI et al. : Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol 2015; 33: 3379–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Resnick MJ, Koyama T, Fan KH et al. : Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med 2013; 368: 436–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Bos W, Muller BG, Ahmed H et al. : Focal therapy in prostate cancer: international multidisciplinary consensus on trial design. Eur Urol 2014; 65: 1078–83 [DOI] [PubMed] [Google Scholar]
- 5.van Velthoven R, Aoun F, Marcelis Q et al. : A prospective clinical trial of HIFU hemiablation for clinically localized prostate cancer. Prostate Cancer Prostatic Dis 2016; 19: 79–83 [DOI] [PubMed] [Google Scholar]
- 6.Ward JF, Jones JS: Focal cryotherapy for localized prostate cancer: a report from the national Cryo On-Line Database (COLD) Registry. BJU Int 2012; 109: 1648–54 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen PL, Chen MH, Zhang Y et al. : Updated results of magnetic resonance imaging guided partial prostate brachytherapy for favorable risk prostate cancer: implications for focal therapy. J Urol 2012; 188: 1151–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fine SW, Al-Ahmadie HA, Gopalan A et al. : Anatomy of the anterior prostate and extraprostatic space: a contemporary surgical pathology analysis. Adv Anat Pathol 2007; 14: 401–7 [DOI] [PubMed] [Google Scholar]
- 9.American College of Radiology. PIRADS v2. Reston, Va: American College of Radiology, 2014. [Google Scholar]
- 10.Stamey TA, Freiha FS, McNeal JE et al. : Localized prostate cancer. Relationship of tumor volume to clinical significant for treatment of prostate cancer. Cancer 1993; 71: 933–8 [DOI] [PubMed] [Google Scholar]
- 11.Wibmer A, Hricak H, Gondo T, et al. : Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur Radiol 2015; 25: 2840–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kattan MW, Eastham JA, Stapleton AM et al. : A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst 1998; 90: 766–71 [DOI] [PubMed] [Google Scholar]
- 13.Tareen B, Godoy G, Sankin A et al. : Can contemporary transrectal prostate biopsy accurately select candidates for hemi-ablative focal therapy of prostate cancer? BJU Int 2009; 104: 195–9 [DOI] [PubMed] [Google Scholar]
- 14.Gallina A, Maccagnano C, Suardi N et al. : Unilateral positive biopsies in low risk prostate cancer patients diagnosed with extended transrectal ultrasound-guided biopsy schemes do not predict unilateral prostate cancer at radical prostatectomy. BJU Int 2012; 110: E64–8 [DOI] [PubMed] [Google Scholar]
- 15.Tareen B, Sankin A, Godoy G et al. : Appropriate candidates for hemiablative focal therapy are infrequently encountered among men selected for radical prostatectomy in contemporary cohort. Urology 2009; 73: 351–4 [DOI] [PubMed] [Google Scholar]
- 16.Briganti A, Tutolo M, Suardi N et al. : There is no way to identify patients who will harbor small volume, unilateral prostate cancer at final pathology. implications for focal therapies. Prostate 2012; 72: 925–30 [DOI] [PubMed] [Google Scholar]
- 17.Polascik TJ, Mayes JM, Schroeck FR et al. : Patient selection for hemiablative focal therapy of prostate cancer: variables predictive of tumor unilaterality based upon radical prostatectomy. Cancer 2009; 115: 2104–10 [DOI] [PubMed] [Google Scholar]
- 18.Epstein JI, Walsh PC, Carmichael M et al. : Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA 1994; 271: 368–74 [PubMed] [Google Scholar]
- 19.Wolters T, Roobol MJ, van Leeuwen PJ et al. : A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol 2011; 185: 121–5 [DOI] [PubMed] [Google Scholar]
- 20.Van der Kwast TH, Roobol MJ: Defining the threshold for significant versus insignificant prostate cancer. Nat Rev Urol 2013; 10: 473–82 [DOI] [PubMed] [Google Scholar]
- 21.Kryvenko ON, Epstein JI: Definition of insignificant tumor volume of Gleason Score 3+3=6 (Grade Group 1) prostate cancer at radical prostatectomy - is it time to increase the threshold? J Urol 2016; 196: 1664–9 [DOI] [PubMed] [Google Scholar]
- 22.Schiffmann J, Connan J, Salomon G et al. : Tumor volume in insignificant prostate cancer: increasing threshold gains increasing risk. Prostate 2015; 75: 45–9 [DOI] [PubMed] [Google Scholar]
- 23.Chen RC, Rumble RB, Loblaw DA et al. : Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol 2016; 34: 2182–90 [DOI] [PubMed] [Google Scholar]
- 24.de Rooij M, Hamoen EH, Futterer JJ et al. : Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol 2014; 202: 343–51 [DOI] [PubMed] [Google Scholar]
- 25.de Rooij M, Hamoen EH, Witjes JA. et al. : Accuracy of magnetic resonance imaging for local staging of prostate cancer: a diagnostic meta-analysis. Eur Urol 2016; 70: 233–45 [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka Y, Numao N, Saito K et al. : Combination of diffusion-weighted magnetic resonance imaging and extended prostate biopsy predicts lobes without significant cancer: application in patient selection for hemiablative focal therapy. Eur Urol 2014; 65: 186–92 [DOI] [PubMed] [Google Scholar]
- 27.Tran M, Thompson J, Bohm M et al. : Combination of multiparametric MRI and transperineal template-guided mapping biopsy of the prostate to identify candidates for hemi-ablative focal therapy. BJU Int 2016; 117: 48–54 [DOI] [PubMed] [Google Scholar]
- 28.Vargas HA, Hotker AM, Goldman DA et al. : Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol 2016; 26: 1606–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vargas HA, Akin O, Shukla-Dave A et al. : Performance characteristics of MR imaging in the evaluation of clinically low-risk prostate cancer: a prospective study. Radiology 2012; 265: 478–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siddiqui MM, Rais-Bahrami S, Turkbey B et al. : Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313: 390–7 [DOI] [PMC free article] [PubMed] [Google Scholar]


