Abstract
Background:
Pelvic lymph node dissection (PLND) is the most reliable procedure for lymph node staging. However, the therapeutic benefit remains unproven; although most radical prostatectomies at academic centers are accompanied by PLND, there is no consensus regarding the optimal anatomical extent of PLND.
Objective:
To evaluate whether extended PLND results in a lower biochemical recurrence rate.
Design, setting, and participants:
We conducted a single-center randomized trial. Patients, enrolled between October 2011 and March 2017, were scheduled to undergo radical prostatectomy and PLND. Patients were assigned to limited or extended PLND by cluster randomization. Specifically, surgeons were randomized to perform limited or extended PLND for 3-mo periods.
Intervention:
Randomization to limited (external iliac nodes) or extended (external iliac, obturator fossa and hypogastric nodes) PLND.
Outcome measurements and statistical analysis:
The primary endpoint was the rate of biochemical recurrence.
Results and limitations:
Of 1440 patients included in the final analysis, 700 were randomized to limited PLND and 740 to extended PLND. The median number of nodes retrieved was 12 (interquartile range [IQR] 8–17) for limited PLND and 14 (IQR 10–20) extended PLND; the corresponding rate of positive nodes was 12% and 14% (difference −1.9%, 95% confidence interval [CI] −5.4% to 1.5%; p = 0.3). With median follow-up of 3.1 yr, there was no significant difference in the rate of biochemical recurrence between the groups (hazard ratio 1.04, 95% CI 0.93–1.15; p = 0.5). Rates for grade 2 and 3 complications were similar at 7.3% for limited versus 6.4% for extended PLND; there were no grade 4 or 5 complications.
Conclusions:
Extended PLND did not improve freedom from biochemical recurrence over limited PLND for men with clinically localized prostate cancer. However, there were smaller than expected differences in nodal count and the rate of positive nodes between the two templates. A randomized trial comparing PLND to no node dissection is warranted.
Patient summary:
In this clinical trial we did not find a difference in the rate of biochemical recurrence of prostate cancer between limited and extended dissection of lymph nodes in the pelvis.
This study is registered on ClinicalTrials.gov as NCT01407263.
Keywords: Prostatic neoplasms, Lymphatic metastasis, Pelvic lymph node dissection, Prognosis
1. Introduction
The role of pelvic lymph node dissection (PLND) in the surgical treatment of clinically localized prostate cancer has remained a controversial topic over the last three decades. Although PLND provides valuable staging information and prognostic insights that help guide further treatment decisions, the hypothesis that removing regional metastatic lymph nodes provides a therapeutic benefit remains unproven.
The widespread use of prostate-specific antigen (PSA)-based prostate cancer screening led to a downward stage migration, with a higher proportion of men diagnosed with less aggressive cancers and lower prevalence of lymph node metastasis [1]. Therefore, a number of urologists have advocated for a limited anatomical template for PLND in a select group of patients at risk of unfavorable pathology [1,2] and cite a higher risk of complications with extended PLND. Others, however, argue that a more extended PLND identifies twice as many men with nodal metastases as a limited PLND and may help to cure some with nodal micrometastases [3–5]. The body of evidence supporting both sides of this argument is based on retrospective data and personal experience.
The guidelines of both the European Association of Urology [6] and the National Comprehensive Cancer Network [7] recommend an extended PLND. However, the American Urological Association (AUA) guidelines do not recommend one PLND template over the other and balance the risks and benefits of each approach. The AUA recognizes PLND as the most effective way to detect nodal metastasis, but stresses the lack of evidence supporting its therapeutic benefit [8].
To the best of our knowledge, there are no sufficiently powered randomized trials comparing the risks and benefits of the two anatomical PLND templates for prostate cancer. We undertook this prospective randomized trial to assess whether extended PLND improves biochemical recurrence (BCR)-free survival compared to limited PLND among men undergoing radical prostatectomy for clinically localized prostate cancer.
2. Patients and methods
2.1. Trial oversight
The study was designed by members of the Prostate Cancer Working Group in the Urology Service at Memorial Sloan Kettering Cancer Center (MSK) and was approved by the MSK institutional review board. All participants gave written informed consent. The authors attest that the study was conducted and monitored as specified by the protocol and vouch for the accuracy and completeness of the data and analyses.
2.2. Study design
The study was designed as a clinically integrated randomized trial: both patients and clinicians had experiences very similar to what would have occurred without the trial. Specifically, there were no additional tests, questionnaires, or procedures for patients participating in the trial; all data used for trial analysis were captured as part of routine care [9]. We used the MSK institutional Caisis data management system, an electronic platform for routine clinical documentation. Initial consultation, follow-up visits, patient-reported quality-of-life information, operative reports, perioperative complications, pathology results, and laboratory values designed in discrete data elements or synoptic format are directly integrated into Caisis to allow direct download into research databases. Patient-reported outcomes were captured using an electronic interface as part of routine care, as previously reported [10].
2.3. Patients
Patients were eligible for participation in the trial if they had clinically localized prostate cancer with no evidence of systemic metastases and were scheduled for radical prostatectomy and bilateral PLND with one of the consenting surgeons.
2.4. Intervention: pelvic lymph node dissection
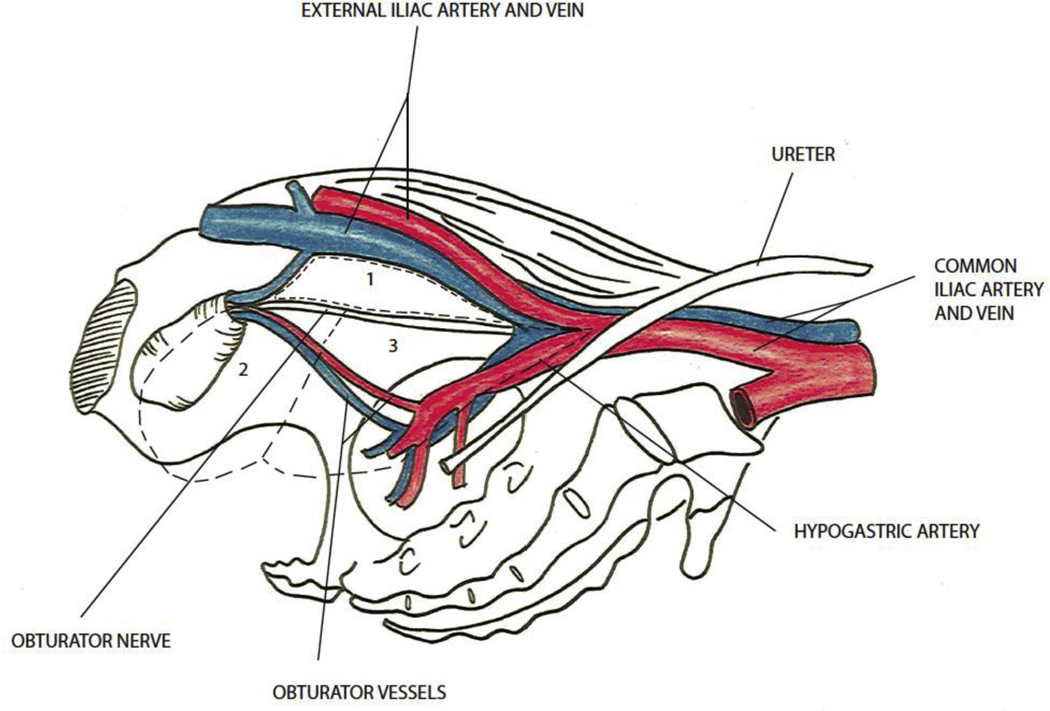
The trial compared two anatomical templates for PLND performed at the time of radical prostatectomy. The limited template included surgical removal of the nodal packet under the external iliac vein and above the obturator nerve. The extended template included removal of the external iliac, hypogastric, and obturator fossa nodal groups (Fig. 1).
Fig. 1 –
Pelvic lymph node template. Limited node dissection includes region 1 (external iliac nodes), and the extended dissection includes regions 1 (external iliac nodes), 2 (obturator fossa nodes), and 3 (hypogastric nodes).
Pretrial sessions were held with all participating surgeons to define the anatomical templates to be studied in the trial and to standardize the anatomical terminology and surgical performance of PLND. Anatomical schemas (Fig. 1) and surgical videos from participating surgeons were used during this preparation process.
All surgical approaches (open and laparoscopic with or without robotic assistance) were included in the trial. This is a clinically integrated protocol. Our care pathway was not modified for the protocol. Our perioperative routine care for prostate surgery is standardized. This includes antibiotic prophylaxis on the day of surgery (cephalosporin or an alternative in cases of allergy) a short course of antibiotics on the day of catheter removal, sequential compressive devices for venous thromboembolism prophylaxis, and an overnight stay for laparoscopic cases. All surgeons in the trial had considerable experience in performing radical prostatectomy and PLND. Therefore, no patient received suboptimal care because he was randomized to a procedure for which a surgeon had insufficient experience. A total of nine surgeons participated in this trial. All lymph node specimens removed during surgery were fixed in neutral buffered 4% formaldehyde for 24 h, and then searched and counted manually. Each node retrieved was cut into 3-mm slices that were then separately embedded in paraffin, stained with hematoxylin and eosin, and examined microscopically. The results were stated as the number of lymph nodes involved and the total number of lymph nodes retrieved per side (right and left).
2.5. Randomization and masking
Beginning in October 2011, for the first 8 mo of the study, enrolled patients were initially randomized 1:1 to receive either a limited or an extended PLND. In June 2012, the randomization schema was modified from individual patient randomization to surgeon-level cluster crossover randomization. The change to cluster randomization was introduced to ease the consent discussion and the logistics of trial execution. In both cases, randomization was conducted by the Biostatistics Service at MSK.
For the remainder of the trial, surgeons were cluster-randomized every 3 mo using randomly permuted blocks of four. The surgeons were contacted at the start of each 3-mo period and informed of the allocation, and were asked to confirm their understanding of the randomization allocation in writing. Patients were informed of their allocated template for PLND if they requested it. Surgeons were to use their clinical judgment as to the best interest of the patient: if there were clear reasons to use or avoid a given PLND template, surgeons were to act accordingly; otherwise, the randomization assignment was to be followed. Both the surgeons and the patients were unblinded as to allocation.
2.6. Trial endpoints
In this superiority trial, the primary endpoint was the BCR rate, defined as any postoperative PSA of ≥0.2 ng/ml with a confirmatory PSA rise, or initiation of treatment with hormones, radiotherapy, or chemotherapy for prostate cancer. Secondary endpoints were the rate of postoperative complications and erectile function recovery.
2.7. Sample size considerations
The power calculation was based on an estimated 5-yr recurrence rate of 23%. This estimation was based on an analysis of the MSK database, taking into account changes in risk in the presenting cohort. A two-group trial with 1700 patients undergoing lymph node dissection would have close to 90% power for detecting a hazard ratio (HR) of 0.67. However, during the trial we found that event rates were higher than expected because of changes in practice patterns that led to fewer patients with lower risk undergoing surgery. A decision was made to close the trial at the end of the first quarter of 2017, when close to 1500 patients had been accrued, as more events had been observed than were required in the sample size calculation.
2.8. Statistical analysis
Cox proportional-hazards regression was used to assess the impact of the limited and extended PLND templates on oncologic outcomes after radical prostatectomy. Our primary endpoint was the BCR rate. Cox regression was used to compare groups, using the sandwich estimator to account for the cluster design. Kaplan-Meier methods were used to estimate BCR-free survival probability stratified by PLND template.
Two surgeons were removed from the study in June 2015 for having low compliance to randomized assignments. For sensitivity analysis, the Cox model was rebuilt to exclude patients enrolled before this date. In addition, analyses were repeated separately for the 73 patients enrolled before the implementation of cluster randomization and the remaining patients. These results were combined using meta-analytic approaches in which heterogeneity between the study periods were assessed. A model was also built that included adjustment for biopsy grade group, PSA, and clinical stage to estimate the effect of PLND template. As cluster randomization means that allocation cannot be concealed, we sought to assess whether there was any bias as to which patients were included in the trial according to surgeon, current randomization (ie, limited or extended PLND template), and patient risk. All analyses were on an intent-to-treat basis, with patients analyzed according to randomization, irrespective of the PLND template used. Analyses were performed in R v3.6 (R Foundation for Statistical Computing, Vienna, Austria) and Stata 15 (StataCorp, College Station, TX, USA).
3. Results
3.1. Patients and procedures
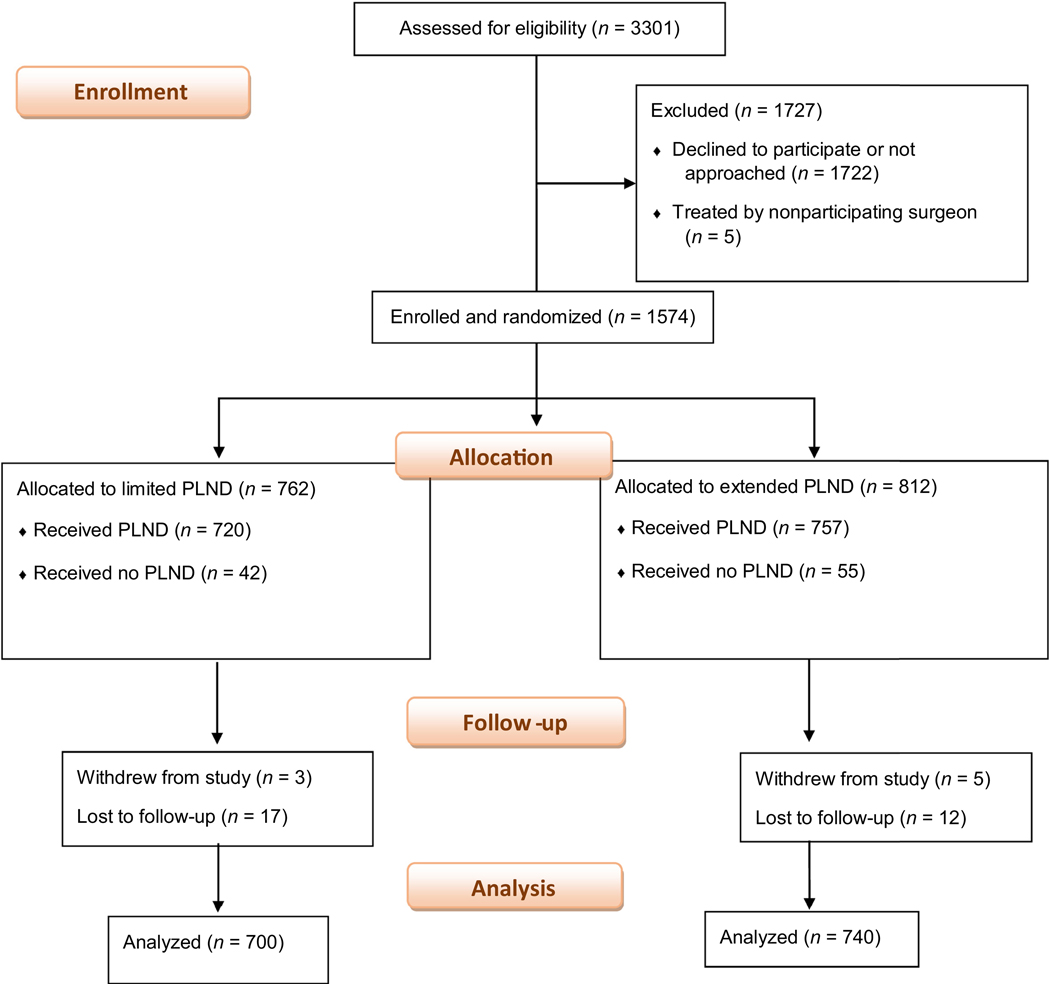
From October 2011 to March 2017, 1574 men who underwent radical prostatectomy gave written informed consent to participate in the trial. Of these men, 1477 underwent bilateral PLND: 720 were randomized to the limited PLND template and 757 to the extended PLND template (Fig. 2). A total of 37 patients were excluded: eight withdrew consent (three in the limited PLND group and five in the extended PLND group) and 29 patients were lost to follow-up (17 in the limited PLND group and 12 in the extended PLND group).
Fig. 2 –
Recruitment, randomization, and patient flow. MSK = Memorial Sloan Kettering Cancer Center; PLND = pelvic lymph node dissection; RP = radical prostatectomy.
The characteristics of the patients were well balanced between the two groups (Table 1). The rate of pathologic Gleason score ≥8 (International Society of Urological Pathology grade groups 4 and 5) at biopsy was 12% and 16% for the limited and extended PLND groups, respectively.
Table 1 –
Baseline characteristics of the participantsa
| Limited PLND (n = 700) | Extended PLND (n = 740) | |
|---|---|---|
| Median age, yr (IQR) | 62 (57–67) | 63 (57–67) |
| Median preoperative prostate-specific antigen, ng/ml (IQR) b | 5.9 (4.3–8.6) | 5.7 (4.2–8.2) |
| Biopsy ISUP grade group, n (%) | ||
| 1 | 72 (10) | 69 (9.4) |
| 2 | 362 (52) | 383 (52) |
| 3 | 135 (19) | 125 (17) |
| ≥4 | 129 (18) | 160 (22) |
| Unknown | 2 | 3 |
| Clinical tumor stage, n (%) | ||
| T1c | 400 (61) | 418 (61) |
| T2a | 88 (13) | 108 (16) |
| T2b | 94 (14) | 98 (14) |
| T2c | 24 (3.7) | 27 (3.9) |
| T3a | 34 (5.2) | 24 (3.5) |
| T3b | 15 (2.3) | 15 (2.2) |
| Unknown | 45 | 50 |
| Prostatectomy ISUP grade group, n (%) | ||
| 1 | 40 (5.9) | 53 (7.3) |
| 2 | 399 (58) | 385 (53) |
| 3 | 160 (23) | 173 (24) |
| ≥4 | 84 (12) | 118 (16) |
| Unknown | 17 | 11 |
| N stage, n (%) | ||
| N0 | 619 (88) | 640 (86) |
| N1 | 81 (12) | 100 (14) |
| Extracapsular extension, n (%) | 375 (54) | 364 (49) |
| Unknown | 1 | 4 |
| Seminal vesicle invasion, n (%) | 85 (12) | 89 (12) |
| Unknown | 4 | 0 |
| Median number of nodes removed, n (IQR) | 12 (8–17) | 14 (10–20) |
| Number of positive nodes removed, n (%) | ||
| 0 | 619 (88) | 640 (86) |
| 1 | 41 (5.9) | 51 (6.9) |
| 2 | 18 (2.6) | 14 (1.9) |
| ≥3 | 22 (3.1) | 35 (4.7) |
IQR = interquartile range; ISUP, International Society of Urological Pathology; PLND, pelvic lymph node dissection.
Data are for participants who were randomized, received PLND, and were included in the final analysis (n = 1440).
Data for preoperative prostate-specific antigen missing for three patients (2 in the limited PLND group, 1 in the extended PLND group).
We first looked for any evidence that the cluster design had led to selection bias or differential treatment on the basis of patient risk factors. Enrollment was slightly higher for patients with lower-risk disease compared to those with higher-risk disease. Enrollment was also slightly higher when the randomization allocation was to the extended PLND template compared to the limited template. For example, the probability of accrual to the limited versus extended PLND arms was 46% versus 55% for the patients with lower risk (10% baseline risk) and 42% versus 46% for patients with high risk (80% baseline risk; Supplementary Fig. 1). However, the modest change by risk level was similar for both arms, which thus provides evidence against selection bias based on risk. Similarly, we saw an increase in the number of nodes retrieved in higher-risk disease. This increase was observed in both the limited and extended PLND groups. The mean number of nodes in the limited versus extended PLND arms was 12.6 versus 14.1 for patients with lower risk, and 14.3 versus 17.5 for patients with higher risk (Supplementary Fig. 2).
A median of 12 nodes were removed in the limited PLND arm versus 14 in the extended arm (Supplementary Fig. 3). The rate of lymph node metastasis was 12% in the limited and 14% in the extended PLND arm (difference −1.9%, 95% confidence interval [CI] −5.4% to 1.5%; p = 0.3). Some 3.1% of patients in the limited PLND group had three or more positive nodes, compared to 4.7% of patients in the extended PLND group.
3.2. Biochemical recurrence
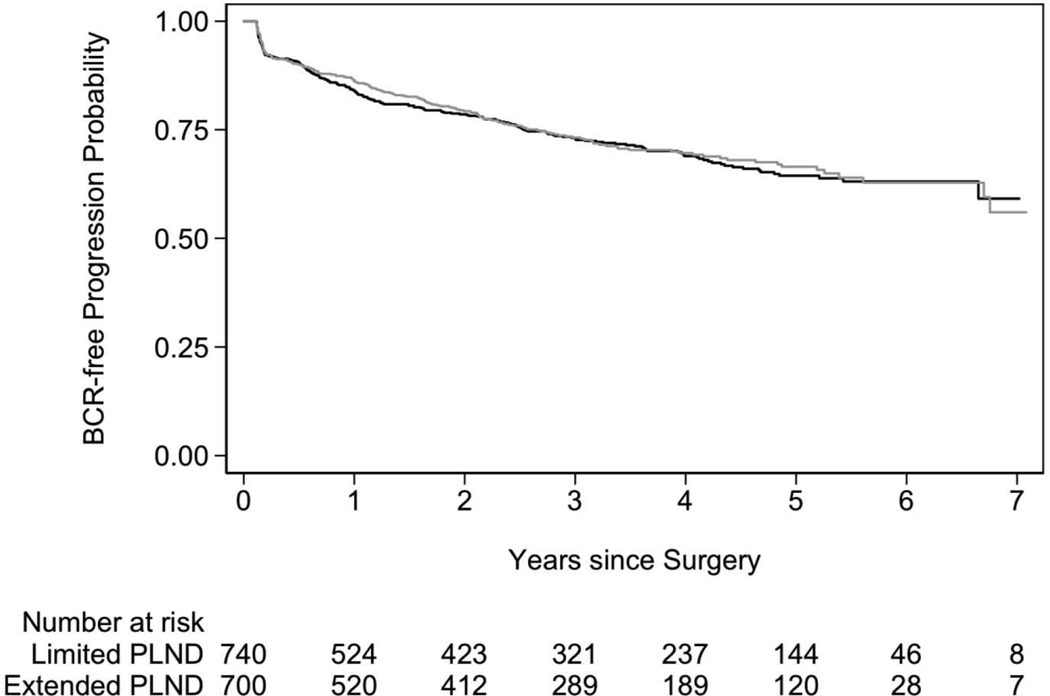
With median follow-up of 3.1 yr (interquartile range [IQR] 1.5–5.0) for patients without BCR and 388 BCR events observed, we did not see a difference in the BCR rate between the randomization groups (HR 1.04, 95% CI 0.93–1.15; p = 0.5 for limited PLND as the reference group; Fig. 3). Results were similar in our sensitivity analyses (Table 2). Table 2 presents four different analyses. First is the primary analysis for this trial, including all men; the second excluded men enrolled before two low-compliance surgeons were removed from the trial in 2015; the third presents the meta-analytic approach; and the fourth adjusts for PSA, biopsy grade group, and clinical stage as potential confounding factors.
Fig. 3 –
Biochemical recurrence (BCR)-free survival after radical prostatectomy for limited (gray line) versus extended (black line) pelvic lymph node dissection (PLND).
Table 2.
Biochemical recurrence–free survival for extended versus limited PLND
| Model | Hazard ratio (95% CI) | p value |
|---|---|---|
| All evaluable patients (n = 1440) | 0.5 | |
| Patients enrolled after June 2015 (n = 431) | 1.18 (0.76–1.86) | 0.5 |
| Meta-analysis combining results from before and after cluster randomization began in June 2012 (n = 1440) | 1.03 (0.94–1.13) | 0.8 |
| Model adjusted for PSA, biopsy grade group, and clinical stage (n = 1339) | 0.95 (0.84–1.07) | 0.4 |
CI = confidence interval; PLND = pelvic lymph node dissection; PSA = prostate-specific antigen.
3.3. Complications and erectile function
Fuller data on complications and erectile function are presented in the Supplementary material. In brief, there were no grade 4 or 5 complications. Overall, the rates of grade 2 and grade 3 complications were comparable between the limited (7.3%) and extended PLND groups (6.4%; Supplementary Tables 1 and 2). There were no differences in erectile function between the groups. In all analyses, the 95% CI excluded a >1 point reduction in International Index of Erectile Function scores for the extended template group (Supplementary Table 3).
4. Discussion
To the best of our knowledge, this is the first adequately powered randomized clinical trial to address the question of therapeutic benefit of extended versus limited PLND in prostate cancer. We found that men with clinically localized prostate cancer who received extended PLND did not have lower recurrence rates than those who received limited PLND. We determined the anatomical templates to be compared in this trial on the basis of well-established prostate cancer lymph-node metastasis mapping studies showing that external iliac, hypogastric, and obturator fossa nodal packets harbor 36%, 58%, and 60% of the positive nodes, respectively [11,12]. Moreover, data from retrospective studies have indicated that extended PLND improves staging over limited PLND, with a twofold to threefold increase in detection of lymph node metastasis [5,13].
While this trial did not detect a significant difference in BCR rate between the limited and extended PLND groups, the small difference in the median number of nodes retrieved (12 vs 14) and the rate of positive nodes detected (12% vs 14%) suggest that the limited PLND performed in this trial may have been more extended than anticipated. The number of lymph nodes removed and the rate of lymph node metastasis in the limited PLND group are clearly higher than results reported in the literature (12 vs 6–9 nodes retrieved, and 12% vs 1–6.1% for the incidence of positive nodes) [14,15]. Surgery is a complex, operator-dependent intervention subject to substantial heterogeneity. Ensuring that the intervention is delivered as intended is a well-recognized challenge in surgical randomized trials [16]. Personal audit of the surgeon in the operating room was suggested as an effective method for addressing this factor, but it remains costly and difficult to implement in large trials [17].
That said, there are reasons to believe that the smaller than anticipated difference in node templates did not have an important impact on our findings. We can view our results using an instrumental variables perspective [18]. Informally, if compliance with treatment is c and the effect size is d, the anticipated effect size for perfect compliance would be . However, if d is close to zero, as seen in this trial, adjustment for compliance would make little difference, as when d is small and c is in any plausible range. For instance, if compliance is only 50%, the treatment effect under perfect compliance would be twice as great as that observed. However, in this trial, the treatment effect is close to zero, and so would remain close to zero after any reasonably adjustment for compliance.
There is a possibility of selection bias and contamination in an unblinded cluster randomized trial. However, the two groups were well balanced for important prognostic variables at baseline and there was no evidence of differential accrual or node removal depending on risk. In an era in which it is common for even relatively small trials to fail to accrue [19], it is of note that we accrued a very large number of patients (>1500) at a single institution in a relatively short period of time with a minimal budget. We attribute our success in completing our trial to the clinically integrated trial design [9,20]. The principle of a clinically integrated trial is that the experience of patients and clinicians is almost identical irrespective of whether a patient is on or off study. This necessitates a highly streamlined trial design, with minimal eligibility criteria and endpoints derived from routine clinical documentation. The use of cluster randomization dramatically simplifies clinical management of the trial. We also found during the consent process that this design had high patient acceptability.
A recently reported trial of limited (external iliac nodes) versus superextended PLND (external iliac, hypogastric, obturator fossa, lateral external iliac, common iliac, and presacral nodes) enrolled 300 patients with prostate cancer and showed no significant difference in biochemical recurrence rates between the two groups. In this trial the median number of nodes removed and the rate of positive nodes in the limited versus extended PLND were 3 (IQR 2–5) versus 17 (IQR 13–24) and 3.4% versus 17%, respectively [21]. Data from randomized trials in other cancers also cast doubt on the value of extended regional node dissections compared to limited or no node dissection [22–29]. In the Dutch Gastric Cancer Group Trial, patients were treated with curative intent; 380 were randomized to receive a limited D1 dissection and 331 an extended D2 dissection. After 11 yr, there was no significant difference in overall survival (30% vs 35%; p = 0.5). D2 dissection was associated with higher morbidity [30]. A trial by the Association of Urologic Oncology of the German Cancer Society randomized 401 patients undergoing radical cystectomy for bladder cancer to receive either limited or extended lymph node dissection. There were no significant differences in recurrence-free survival (HR 0.84, 95% CI 0.58–1.22; p = 0.4), cancer-specific survival (HR 0.70, 95% CI 0.46–1.07; p = 0.10), or overall survival (HR 0.78, 95% CI 0.57–1.07; p = 0.12), although CIs are wide and include a clinically relevant benefit for extended dissection [22]. In non–small-cell lung cancer, a randomized trial comparing mediastinal sampling to complete mediastinal lymph node dissection in 169 patients did not demonstrate a statistically significant improvement in overall survival (HR 0.76; 95% CI 0.47–1.24; p = 0.3), although the CIs are again wide [28].
The failure to demonstrate a therapeutic benefit of extended PLND may be because an individual’s prognosis is mainly driven by the biological properties of his cancer cells and less likely to be influenced by the surgical intervention [31]. Alternatively, perhaps the benefit of extended PLND is smaller than anticipated—our findings are consistent with an approximate 10% reduction in risk—and therefore detection of the benefit requires even larger trials. Given the challenges in surgical trials and the small size of some of the trials already published, the Halstedian concept that comprehensive surgical removal of nodal micrometastasis halts the disease progression process cannot be considered to have been refuted. The comparison of extended lymphadenectomy to no lymphadenectomy, as recently conducted in ovarian cancer by Harter et al [27], may be the best methodology for addressing the question of the therapeutic value of lymphadenectomy.
5. Conclusions
We did not find a difference in recurrence rates between extended and limited node dissection during radical prostatectomy. We recommend that subsequent trials compare extended to no node dissection in prostate cancer.
Supplementary Material
Acknowledgments:
The completed article was edited by Joyce Tsoi and Amy Plofker, employees of Memorial Sloan Kettering Cancer Center.
Funding/Support and role of the sponsor: This study was supported by The Sidney Kimmel Center for Prostate and Urologic Cancers at MSK, and by NIH/NCI grants P30 CA008748, R21 CA133869, and P50 CA092629. None of the sponsors played a direct role in the study.
Financial disclosures: Karim A. Touijer certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Karim A. Touijer is a co-inventor on a patent for a cancer imaging probe licensed to Elucida Oncology, and has received a speaker honorarium from Janssen. Peter T. Scardino and Andrew Vickers are named on a patent for a statistical method to detect prostate cancer (the 4KScore test) that has been commercialized by OPKO Health, and they receive royalties from sales of the test. Peter T. Scardino has stock in OPKO Health and Andrew Vickers has stock options in OPKO Health.
Footnotes
The remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bishoff JT, Reyes A, Thompson IM, et al. Pelvic lymphadenectomy can be omitted in selected patients with carcinoma of the prostate: development of a system of patient selection. Urology 1995;45:270–4. [DOI] [PubMed] [Google Scholar]
- 2.Bluestein DL, Bostwick DG, Bergstralh EJ, Oesterling JE. Eliminating the need for bilateral pelvic lymphadenectomy in select patients with prostate cancer. J Urol 1994;151:1315–20. [DOI] [PubMed] [Google Scholar]
- 3.Bader P, Burkhard FC, Markwalder R, Studer UE. Disease progression and survival of patients with positive lymph nodes after radical prostatectomy. Is there a chance of cure? J Urol 2003;169:849–54. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich A, Varga Z, Von Knobloch R. Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: high incidence of lymph node metastasis. J Urol 2002;167:1681–6. [PubMed] [Google Scholar]
- 5.Touijer K, Rabbani F, Otero JR, et al. Standard versus limited pelvic lymph node dissection for prostate cancer in patients with a predicted probability of nodal metastasis greater than 1%. J Urol 2007;178:120–4. [DOI] [PubMed] [Google Scholar]
- 6.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2017;71:618–29. [DOI] [PubMed] [Google Scholar]
- 7.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016. J Natl Compr Cancer Netw 2016;14:19–30. [DOI] [PubMed] [Google Scholar]
- 8.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part II: recommended approaches and details of specific care options. J Urol 2018;199:990–7. [DOI] [PubMed] [Google Scholar]
- 9.Vickers AJ, Scardino PT. The clinically-integrated randomized trial: proposed novel method for conducting large trials at low cost. Trials 2009;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vickers AJ, Savage CJ, Shouery M, Eastham JA, Scardino PT, Basch EM. Validation study of a web-based assessment of functional recovery after radical prostatectomy. Health Qual Life Outcomes 2010;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bader P, Burkhard FC, Markwalder R, Studer UE. Is a limited lymph node dissection an adequate staging procedure for prostate cancer? J Urol 2002;168:514–8. [DOI] [PubMed] [Google Scholar]
- 12.Godoy G, von Bodman C, Chade DC, et al. Pelvic lymph node dissection for prostate cancer: frequency and distribution of nodal metastases in a contemporary radical prostatectomy series. J Urol 2012;187:2082–6. [DOI] [PubMed] [Google Scholar]
- 13.Touijer K, Fuenzalida RP, Rabbani F, et al. Extending the indications and anatomical limits of pelvic lymph node dissection for prostate cancer: improved staging or increased morbidity? BJU Int 2011;108:372–7. [DOI] [PubMed] [Google Scholar]
- 14.Allaf ME, Palapattu GS, Trock BJ, Carter HB, Walsh PC. Anatomical extent of lymph node dissection: impact on men with clinically localized prostate cancer. J Urol 2004;172:1840–4. [DOI] [PubMed] [Google Scholar]
- 15.Choo MS, Kim M, Ku JH, Kwak C, Kim HH, Jeong CW. Extended versus standard pelvic lymph node dissection in radical prostatectomy on oncological and functional outcomes: a systematic review and meta-analysis. Ann Surg Oncol 2017;24:2047–54. [DOI] [PubMed] [Google Scholar]
- 16.Blencowe NS, Mills N, Cook JA, et al. Standardizing and monitoring the delivery of surgical interventions in randomized clinical trials. Br J Surg 2016;103:1377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landheer ML, Therasse P, van de Velde CJ. Quality assurance in surgical oncology(QASO) within the European Organization for Research and Treatment of Cancer (EORTC): current status and future prospects. Eur J Cancer 2001;37:1450–62. [DOI] [PubMed] [Google Scholar]
- 18.Baker SG, Kramer BS, Lindeman KS. Latent class instrumental variables: a clinical and biostatistical perspective. Stat Med 2016;35:147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennette CS, Ramsey SD, McDermott CL, Carlson JJ, Basu A, Veenstra DL. Predicting low accrual in the National Cancer Institute’s Cooperative Group clinical trials. J Natl Cancer Inst 2016;108:djv324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vickers AJ, Bennette C, Touijer K, et al. Feasibility study of a clinically-integrated randomized trial of modifications to radical prostatectomy. Trials 2012;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lestingi JFP GG, Trinh QD, Coelho RF, et al. Extended versus limited pelvic lymph node dissection during radical prostatectomy for intermediate- and high-risk prostate cancer: early oncological outcomes from a randomized phase 3 trial. Eur Urol. In press. 10.1016/j.eururo.2020.11.040 [DOI] [PubMed]
- 22.Gschwend JE, Heck MM, Lehmann J, et al. Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: survival results from a prospective, randomized trial. Eur Urol 2019;75:604–11. [DOI] [PubMed] [Google Scholar]
- 23.Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999;340:908–14. [DOI] [PubMed] [Google Scholar]
- 24.de Boer AG, van Lanschot JJ, van Sandick JW, et al. Quality of life after transhiatal compared with extended transthoracic resection for adenocarcinoma of the esophagus. J Clin Oncol 2004;22:4202–8. [DOI] [PubMed] [Google Scholar]
- 25.Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23–31. [DOI] [PubMed] [Google Scholar]
- 26.Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002;347:567–75. [DOI] [PubMed] [Google Scholar]
- 27.Harter P, Sehouli J, Lorusso D, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N Engl J Med 2019;380:822–32. [DOI] [PubMed] [Google Scholar]
- 28.Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998;227:138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riall TS, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma—part 3: update on 5-year survival. J Gastrointest Surg 2005;9:1191–204. [DOI] [PubMed] [Google Scholar]
- 30.Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004;22:2069–77. [DOI] [PubMed] [Google Scholar]
- 31.Cady B. Regional lymph node metastases; a singular manifestation of the process of clinical metastases in cancer: contemporary animal research and clinical reports suggest unifying concepts. Ann Surg Oncol 2007;14:1790–800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.