Abstract
Maladaptive behaviors are challenging and a source of stress for caregivers of individuals with Angelman Syndrome (AS). There is limited information on how these maladaptive behaviors vary over time among individuals with AS due to different genetic etiologies. In this study, caregivers of 301 individuals with AS were asked questions about their child’s behavior and completed the Aberrant Behavior Checklist-Community version (ABC-C). Developmental functioning was evaluated with either the Bayley Scales of Infant Development, Third Edition (Bayley-III) or the Mullen Scales of Early Learning (MSEL). Family functioning was assessed using the parent-completed Parenting Stress Index (PSI) and the Family Quality of Life questionnaire (FQoL). Approximately 70% of participants had AS due to a deletion on the maternally-inherited copy of chromosome 15q11q13. Results revealed that at baseline, individuals with AS had low scores in the domains of lethargy (mean: 2.6–4.2 depending on genotype) and stereotypy (mean: 2.3–4.2 depending on genotype). Higher cognitive functioning was associated with increased irritability (r = 0.32, p < .01). Hyperactivity (p < .05) and irritability (p < .05) increased with age across all genotypes and should be ongoing targets for both behavioral and pharmacological treatment. Concerns for short attention span were endorsed by more than 70% of caregivers at baseline. Maladaptive behaviors, particularly hyperactivity, irritability and aggression, adversely affected parental stress, and family quality of life.
Keywords: behavior rating scale, behavioral symptoms, developmental disabilities, problem behavior
1 |. INTRODUCTION
Angelman Syndrome (AS) is a rare neurogenetic disorder that results in severe intellectual disability, minimal or absent speech, seizures, and ataxia (Dagli, Mueller, & Williams, 2017). Characteristic behaviors include frequent laughter that is easily provoked, mouthing of objects, sleep disturbances, and a fascination with water and plastic or paper items that make crinkly noises when manipulated (Dagli, Buiting, & Williams, 2011; Williams, 2010). AS is caused by lack of expression of the maternally inherited UBE3A on chromosome 15q11q13 due to one of four etiologies: deletion of the critical region of the maternally inherited chromosome 15 that encompasses UBE3A, paternal uniparental disomy (UPD) for chromosome 15q11q13, imprinting defects that alter expression of the maternally inherited copy of UBE3A, or a pathogenic variant in the maternally inherited UBE3A (Clayton-Smith & Laan, 2003). Approximately 70–75% of individuals with AS have a deletion, 8–9% have UPD, 7–8% have imprinting defects, and 11% have UBE3A mutations. Among those with a deletion, about 40% have a 5.9 Mb deletion (i.e., Class I), and about 50–55% have a smaller 5.0 Mb deletion (i.e., Class II); the remaining 5–10% of individuals with a deletion have an unusually small or large deletion (i.e., the “atypical deletion”; Bird, 2014). Phenotypic differences among the genotypes have been identified, with deletion individuals typically having more severe developmental delay and more frequent seizures compared to those with AS due to other etiologies (Gentile et al., 2010; Tan et al., 2011).
Despite their apparently happy demeanor, individuals with AS exhibit maladaptive or challenging behaviors, which are often dysfunctional and interfere with their ability to interact well in social environments. These behaviors include, but are not limited to, restlessness, hyperactivity, noncompliance, irritability, temper tantrums, and stereotypy (Clarke & Marston, 2000; Summers, Allison, Lynch, & Sandier, 1995). Hyperactivity is often the most frequent concern reported by caregivers of individuals with AS irrespective of age (Berry, Leitner, Clarke, & Einfeld, 2005; Clarke & Marston, 2000; Wheeler, Sacco, & Cabo, 2017). Aggression has been reported in approximately 70% of individuals with AS (Arron, Oliver, Moss, Berg, & Burbidge, 2011; Larson, Shinnick, Shaaya, Thiele, & Thibert, 2015) and is often thought to result from frustration due to challenges with communication (Didden et al., 2009). Approximately 50% of individuals with AS exhibit self-injurious behavior (Arron et al., 2011; Larson et al., 2015). In terms of stereotypies, while hand flapping and licking inedible objects occur in approximately 55% of individuals with AS (Walz, 2007), other stereotypies that are commonly witnessed in individuals diagnosed with autism such as spinning, lining up of objects, and restricted preferences, are less commonly observed within the AS population (Moss, Oliver, Arron, Burbidge, & Berg, 2009). Anxiety symptoms become more prominent with age, with 57% of adults demonstrating anxiety that is sufficiently severe to interfere with daily functioning and leading to challenging behaviors (Prasad, Grocott, Parkin, Larson, & Thibert, 2018).
These maladaptive behaviors are often a cause of significant concern and contribute to increased parental stress and diminished quality of life in parents and caregivers (Miodrag & Peters, 2015). Identification of the types of problem behaviors prevalent in AS will help guide the development of behavioral and pharmacological treatments targeting these specific behaviors. Theoretically, once maladaptive behaviors are better controlled, self-help skills and attention span can improve (Summers et al., 1995), which can have a positive impact on the family system, leading to decreased parental stress as well as improved child and parental quality of life.
Research on the changes in problem behaviors with age in individuals with developmental disabilities has been inconsistent, with some studies noting stability of these behaviors in childhood (Matson, Mahan, Hess, Fodstad, & Neal, 2010), while others noting a decrease in irritability and hyperactivity over time (Brown, Aman, & Havercamp, 2002). A study in the AS population noted a decrease in hyperactivity in adolescence and adulthood (Clayton-Smith, 2001), while another study examing AS adolescents and adults noted an increase in anxiety in adults and a decrease in defiant behaviors (Prasad, Grocott, Parkin, Larson &Thibert, 2018). However, to date, no published studies have examined changes in a variety of maladaptive behaviors in AS over time.
In this study, we assessed whether maladaptive behaviors within the AS population vary in severity based on genotype, developmental level, chronological age, or sex. Our study provides a comprehensive understanding of maladaptive behaviors that are prevalent among individuals with different genotypes of AS. Assessment of maladaptive behaviors is often an important outcome measure in clinical trials examining the use of novel therapies for individuals with AS. This study will help establish baseline behavioral norms for maladaptive behaviors using standardized validated instruments against which future clinical treatments can be evaluated, identify behavioral targets for the development of new therapies, and formulate specialized interventions to help reduce these maladaptive behaviors in individuals with AS based on their molecular etiology, thereby potentially improving the overall family quality of life.
2 |. MATERIALS AND METHODS
2.1 |. Participants and study sites
Participants were recruited as part of the AS Natural History study (ClinicalTrials.gov identifier: NCT00296764), which was conducted under the auspices of the National Institutes of Health Rare Diseases Clinical Research Network from January 2006 to July 2014 at one of six study sites: Rady Children’s Hospital San Diego, Texas Children’s Hospital, Greenwood Genetic Center, Boston Children’s Hospital, Vanderbilt University Medical Center, and Cincinnati Children’s Hospital Medical Center. Eligibility criteria for this study included a clinical or molecular diagnosis of AS, absence of other comorbid disorders that might obscure the AS phenotype (such as severe prematurity or an additional genetic diagnosis), and age between 1 day and 60 years; only patients with a molecular diagnosis of AS were included in the analyses reported herein. Each participant was seen at one of the study sites for a baseline visit, then approximately annually thereafter for developmental, behavioral, and medical assessments.
2.2 |. Developmental, behavioral and family functioning outcome measures
Maladaptive behaviors were assessed using two sources of information: (a) a structured parental interview designed by the study investigators, and (b) a standardized questionnaire, the Aberrant Behavior Checklist-Community version (ABC-C; Aman, Singh, Stewart, & Field, 1985). During the structured parental interview, parents were provided with a list of 10 behaviors and were asked whether or not their child exhibited the specific behavior; additionally, parents were invited to report other behaviors that were not listed. The ABC-C is a 58-item questionnaire that measures maladaptive behaviors in individuals with intellectual disability. The ABC-C includes five scales (irritability: 15 items, hyperactivity: 16 items, lethargy: 16 items, stereotypy: seven items, and inappropriate speech: four items); “inappropriate speech” was irrelevant to the AS population given that the vast majority of individuals are nonverbal, hence we omitted the inappropriate speech scale in this study. Each item on the ABC-C is scored from 0 to 3, with higher scores indicating greater magnitude of problem behaviors. The range of possible scores in the different subscales is as follows: hyperactivity: 0–48; lethargy: 0–48, irritability 0–45, and stereotypy: 0–21. The ABC-C has been an effective measure for children and young adults with fragile X syndrome (Sansone et al., 2012) as well as those with cognitive disability (Freund & Reiss, 1991). It has been used in prior studies in the AS population (Clarke & Marston, 2000; Summers & Feldman, 1999). The ABC-C is often used as an outcome measure in intervention studies and clinical trials targeting challenging behaviors in individuals with developmental disabilities (Sansone et al., 2012; Freund & Reiss, 1991).
Developmental functioning was assessed at each visit by a psychologist using either the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III; Bayley, 2005) or for participants who exceeded performance thresholds on the Bayley-III, the Mullen Scales of Early Learning (MSEL; Mullen, 1995). The Bayley-III provides a quantitative assessment of cognitive, language, and motor functioning that has been standardized for children up to 42 months of age. The MSEL provides an objective assessment of the gross motor, fine motor, expressive language, receptive language, and visual reception and has been standardized for children up to 68 months (Mullen, 1995). Both the Bayley-III and MSEL have been used beyond the normative age range in several studies of individuals with AS (Gentile et al., 2010) as individuals with intellectual disability should be evaluated with measures that are better suited for their developmental rather than chronological age (Lichtenberger, 2005). Age equivalents in the domains of cognitive functioning, receptive language, and gross motor skills are reported.
Parenting stress was assessed using the standardized Parenting Stress Index (PSI; Abidin, 1995), a 120-item inventory that focuses on perceived stress in the parent–child relationship. The PSI includes two domains, a child domain which assesses six child characteristics (distractibility/hyperactivity, adaptability, reinforces parents, demandingness, mood, and acceptability) that may be contributing to overall stress, and a parent domain which assesses seven stressors related to parenting and family (competence, isolation, attachment, health, role restriction, depression, and spouse) that may contribute to overall stress, the sum of which forms the total stress score. Each item is scored on a 5-point scale ranging from strongly agree to strongly disagree, and the higher the score, the greater the stress (Abidin, 1995).
Quality of life was assessed using a validated Family Qualify of Life (FQoL) questionnaire (Beach Center on Disability, 2006). This is a 25-item questionnaire specifically designed for use in families of children with disabilities. It consists of five subscales: family interaction, parenting, emotional well-being, physical/material well-being, and disability-related support. Each item is answered on a 5-point scale, and the higher the score, the higher the satisfaction and presumably quality of life.
2.3 |. Statistical methods
Descriptive statistics, including means, standard deviations, and ranges for continuous variables, and counts and percentages for categorical variables, were reported. Data were compared across three genotypes: (a) deletion, (b) UBE3A, and (c) UPD and imprinting defects (ImpD) as a single group, as in previous studies in this population (Tan et al., 2011).
Baseline ABC-C scores and baseline rates of maladaptive behavior were summarized by genotype and sex. Analysis of variance (ANOVA) was used to test for significant differences in mean baseline ABC-C scores between genotypes. Baseline ABC-C scores were also examined between Class I and II deletion using a two-sample t-test. To determine whether there were significant differences in maladaptive behaviors by genotype or sex, logistic regression models were fitted with effects for genotype, sex and the genotype-by-sex interaction. Pearson product moment correlation coefficients were used to evaluate baseline associations between ABC-C scores and age, Bayley-III or MSEL age equivalents, parenting PSI subscales, and FQoL satisfaction subscales.
To determine whether there were statistically significant changes in ABC-C scores or rates of maladaptive behavior with increasing age, generalized mixed effects models were fitted with random slope and intercept. Growth models were used to fit mean ABC-C scores over time while mixed effects models for binary responses were used to fit the log-odds of the maladaptive behavior over time. Each outcome variable was modeled as a function of genotype (deletion, UBE3A mutation, and UPD/imprinting defects), age, sex (male and female), the genotype-by-age-interaction, and the sex-by-age interaction.
Statistical tests were performed at the 0.05 level of significance, except for tests of interaction which were performed at the 0.10 level of significance. Nonsignificant interaction terms were dropped from statistical models before testing main effects. Statistical analyses were performed using SAS (version 9.4, SAS Institute, Cary, NC).
3 |. RESULTS
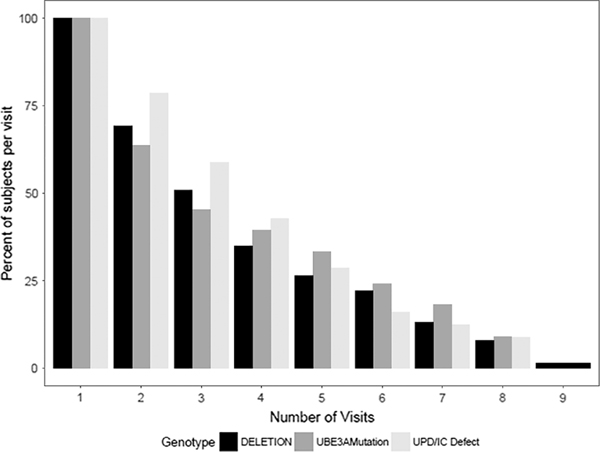
Caregivers of 301 participants with AS completed the ABC-C questionnaire. Characteristics of the AS participants are listed in Table 1. Approximately 70% of the participants had a deletion on the maternally-inherited copy of chromosome 15q11q13, 34% of whom have a Class I deletion, 47% have a Class II deletion, 17% have atypical deletions and in 2%, the deletion subclass was unknown. Mean age at the first visit was 6.0 years. Approximately 95% of the participants were less than 20 years of age at the first visit. The distribution of the number of visits across genotype is depicted in Figure 1. Out of the entire cohort, 24% had only one visit. All other participants had at least two visits.
TABLE 1.
Demographics of participants in the study
| Demographics | Deletion (N = 212) 70% | UPD/ImpD (N = 56) 19% | UBE3A (N = 33) 11% | Total N = 301 | p-value |
|---|---|---|---|---|---|
| Sex (male: Female) | 96:116 (45%:55%) | 29:27 (52%:48%) | 19:14 (58%:42%) | 144:157 (48%:52%) | 0.342 |
| Age at baseline (years) | |||||
| Mean (SD) | 6.0 (6.6) | 6.3 (4.2) | 5.7 (3.9) | 6.0 (5.9) | 0.867 |
| Range | 0.9–40.6 | 2.1–21.0 | 0.4–14.6 | 0.4–40.6 | |
| Median (Q1, Q3) | 3.5 (2.1, 7.2) | 4.9 (3.4, 8.1) | 4.4 (2.7, 8.2) | 4.1 (2.3, 8.0) | |
| Age at final visit (years) | |||||
| Mean (SD) | 8.6 (6.9) | 9.1 (4.5) | 8.2 (4.1) | 8.7 (6.3) | 0.819 |
| Range | 1.1–40.6 | 2.2–26.7 | 1.4–18.6 | 1.1–40.6 | |
| Median (Q1, Q3) | 6.9 (3.9,11.0) | 7.9 (6.3,11.3) | 8.2 (4.9,10.8) | 7.5 (4.3,11.1) | |
| No. of visits | 0.819 | ||||
| Mean (SD) | 3.3 (2.1) | 3.5 (2.0) | 3.3 (2.4) | 3.3 (2.1) | |
| Median | 3.0 | 3.0 | 3.0 | 3.0 | |
| Range | 1–9 | 1–8 | 1–8 | 1–9 | |
Abbreviations: UPD, paternal uniparental disomy for chromosome 15q11q13; ImpD, imprinting defects that alter expression of the maternally inherited copy of UBE3A.
FIGURE 1.
Percentage of participants at each visit, by genotype Abbreviations: UPD, Paternal uniparental disomy for chromosome 15q11q13; IC, Imprinting defects that alter expression of the maternally inherited copy of UBE3A
Caregivers were asked about the presence of 10 behaviors that have been observed in the participants (Table 2). The percentage of caregivers who endorsed each of the behaviors was calculated. The common behavioral features of AS such as easy excitability, mouthing behaviors, and fascination with water, were endorsed frequently (70–92%) by caregivers of individuals across the different genotypes. Short attention span was endorsed by 87% of the caregivers.
TABLE 2.
Percentage of individuals with the specific maladaptive behaviors stratified by genotype at baseline, as reported by parents
| Behavior | Deletion % (N = 211) | UPD/ImpD % (N = 56) | UBE3A % (N = 33) | Total % (N = 300) | Difference in genotypes (p valuea) |
|---|---|---|---|---|---|
| Mouthing behaviors | 92 | 89 | 70 | 89 | .002 |
| Easy excitability | 90 | 91 | 70 | 88 | .005 |
| Short attention span | 89 | 88 | 73 | 87 | .069 |
| Fascination with water | 79 | 75 | 70 | 77 | .486 |
| Hand flapping | 68 | 80 | 61 | 70 | .111 |
| Hyperactive | 69 | 59 | 55 | 65 | .155 |
| Frequent laughter | 69 | 54 | 42 | 63 | .005 |
| Aggressive behavior | |||||
| Overall | 51 | 84 | 70 | 59 | <.001 |
| Bitingb | 27 | 45 | 49 | 33 | .012 |
| Hair pullingb | 44 | 55 | 36 | 45 | .161 |
| Pinchingc | 23 | 43 | 27 | 27 | .025 |
| Anxiety | 19 | 45 | 36 | 26 | <.001 |
| Temper tantrums | 17 | 25 | 52 | 22 | <.001 |
Abbreviations: UPD, paternal uniparental disomy for chromosome 15q11q13; ImpD, imprinting defects that alter expression of the maternally inherited copy of UBE3A.
The p-value presented is for genotype, based on models that included both sex and genotype.
Sex differences found to be statistically significant.
The interaction term between sex and genotype was found to be significant. The interaction was tested for all models but removed from all models except for the outcome of pinching.
Differences in the prevalence of maladaptive behaviors across genotypes were noted in the domains of overall aggressive behavior (including biting and pinching), anxiety, easy excitability, frequent laughter, mouthing behaviors, and temper tantrums. Differences between the sexes were noted only in the domains of hair pulling (of another individual) (male = 54%, female = 37%; p = .003) and biting (male = 43%, female = 24%; p = .001). While there were no statistical differences in the overall prevalence of pinching between males and females in the entire cohort (male = 27%, female = 28%; p = 0.80), there was a significant interaction of sex and genotype for pinching behaviors (p = .046) with a higher rate of pinching for females (59%) than for males (28%) in the UPD/ImpD genotype, but a lower rate of pinching for females than for males in the other genotypes (Deletion: male = 25%, female = 22%; UBE3A mutation: male = 32%, female = 21%).
In examining ABC-C scores, we found that individuals with AS had low scores in the domains of irritability, lethargy, and stereotypy (Table 3). Pairwise comparisons indicated that individuals with deletion have lower irritability scores compared to those with UPD/ImpD (p = .012) and those with UBE3A mutations (p < .001), but higher lethargy and stereotypy scores compared to those with UPD/ImpD (p = .008 and p = .002, respectively) and not significantly different from those with UBE3A mutations (p = .116 and p = .092, respectively). No differences between deletion Class I and deletion Class II were found across the different ABC domains and hence deletion Classes I and II were combined for all further analyses. Participant’s sex impacted their lethargy scores with females being more lethargic than males (Male = 3.2; Female = 4.3; p = .014).
TABLE 3.
Relationship between genotype and ABC-C scores at baseline
| ABC-C Subscales: Mean (SD) Range |
||||
|---|---|---|---|---|
| Genotype | Irritability [0–45]a | Lethargy [0–48]a | Stereotypy [0–21]a | Hyperactivity [0–48]a |
| Deletion (N = 197) | 4.5 (5.5) 0–30 | 4.2 (4.0) 0–25 | 4.2 (3.9) 0–18 | 13.9 (9.9) 0–39 |
| UPD/ImpD (N = 53) | 7.0 (6.5)* 0–29 | 2.6 (3.4)** 0–13 | 2.3 (3.2)** 0–16 | 17.0 (11.2) 1–44 |
| UBE3A mutation (N = 31) | 8.6 (9.6)** 0–33 | 3.0 (3.8) 0–13 | 3.0 (3.9) 0–14 | 17.1 (14.1) 0–48 |
Abbreviations: ABC-C, Aberrant Behavior Checklist-Community; UPD, paternal uniparental disomy for chromosome 15q11q13; ImpD, imprinting defects that alter expression of the maternally inherited copy of UBE3A.
Theoretical range of subscale scores.
Significant difference from Deletion genotype at
p < .05 and
p < .01.
When correlating age and level of developmental functioning with ABC-C scores, we found that higher cognitive functioning was associated with increased irritability (r = .32, p < .01) and hyperactivity (r = .20, p < .01). Chronological age correlated positively with irritability (r = .35, p < .01; Table 4).
TABLE 4.
Correlation between ABC-C scores at baseline and developmental functioning and chronological age, PSI, and FQoL
| ABC-C subscales |
||||
|---|---|---|---|---|
| Developmental functioning (age equivalent) | Irritability | Lethargy | Stereotypy | Hyperactivity |
| Cognitive functioning (N = 276) | 0.32** | −0.19** | −0.16** | 0.27** |
| Receptive language functioning (N = 276) | 0.23** | −0.23** | −0.19** | 0.15* |
| Gross motor functioning (N = 279) | 0.40** | −0.08 | −0.12* | 0.37** |
| Chronological age (N = 281)a | 0.35** | 0.13* | 0.08 | 0.20** |
| PSI scores at baseline | ||||
| PSI child domain (N = 255) | 0.64** | 0.29** | 0.25** | 0.67** |
| PSI parent domain (N = 255) | 0.28** | 0.15* | 0.14* | 0.27** |
| PSI Total stress score (N = 254) | 0.51** | 0.25** | 0.22** | 0.52** |
| FQoL at baseline | ||||
| FQoL family interaction (N = 238) | −0.10 | −0.09 | 0.002 | −0.10 |
| FQoL parenting (N = 239) | −0.15* | −0.05 | 0.01 | −0.14* |
| FQoL emotional well-being (N = 242) | −0.14* | 0.02 | −0.03 | −0.17** |
| FQoL physical material well-being (N = 246) | −0.13* | −0.05 | −0.13* | −0.11 |
| FQoL disability related support (N = 243) | −0.21** | 0.02 | 0.03 | −0.16* |
| FQoL Avg satisfaction (N = 225) | −0.18** | −0.07 | −0.03 | −0.18** |
ABC-C: Aberrant Behavior Checklist-Community; PSI: Parenting Stress Index; FQoL: Family Quality of Life questionnaire.
Missing data for 20 individuals (281/301).
p < .05;
p < .01.
Strong positive correlations (r = .51 to .67) were noted between the irritability and hyperactivity domains of the ABC and the Child Domain and Total Stress domain of the PSI, indicating that increased irritability and hyperactivity are associated with the child characteristics of the PSI that impact parental stress as well as increased overall stress in the parent–child relationships. The ABC domains of irritability and hyperactivity had low negative correlations (r = −.17 to −.10) with most scales of the FQoL (Table 4).
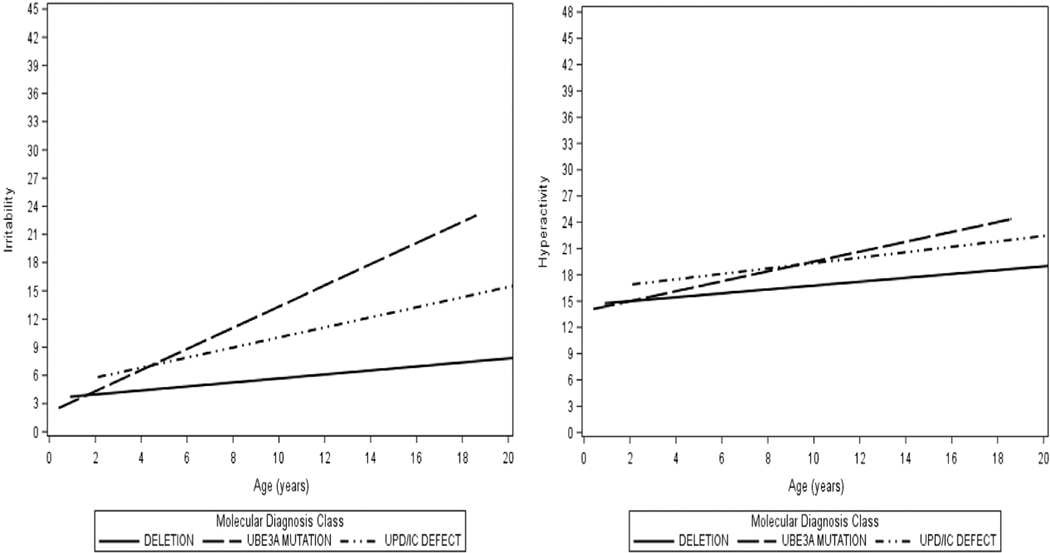
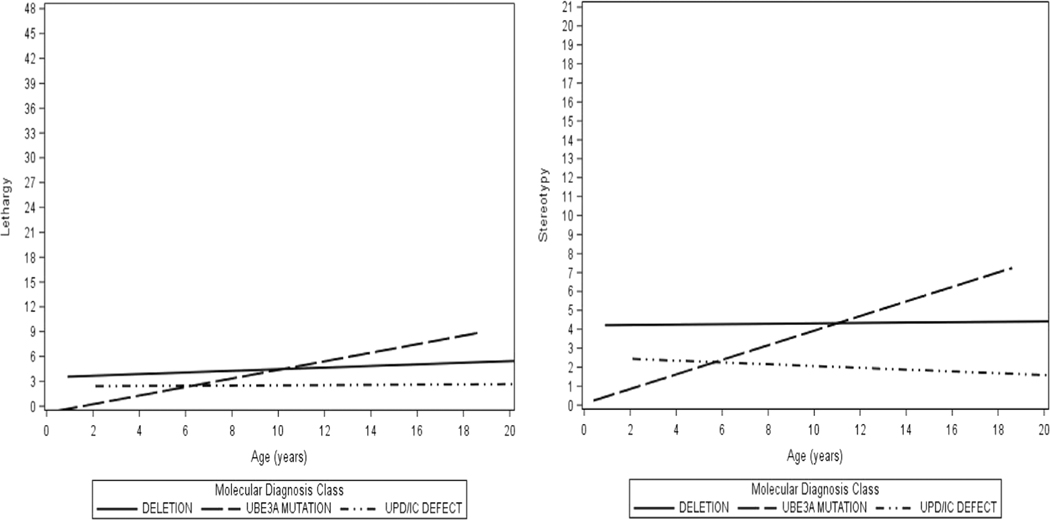
Results of growth models, which showed how ABC-C subscale scores changed with time indicated that on average across genotypes, as subjects increased in age, there was a greater tendency to exhibit irritability, hyperactivity, lethargy, and stereotypy (all p < .05; Table 5). Significant genotype-by-age interaction effects were found for the subscales of irritability. All participants experienced an increase in irritability scores with advancing age, with the greatest increase seen among those with a UBE3A mutation and the smallest increase for individuals with deletion (Figure 2). For lethargy, scores remained low over time, that is, they started off low and remained stable for individuals with UPD/ImpD, and increased only slightly with age in individuals with deletion and UBE3A mutation (Figure 3). Scores on stereotypy increased with age for the UBE3A mutation genotype and remained relatively stable for the deletion and the UPD/ImpD genotypes (Figure 3). Across all genotypes, hyperactivity scores started off as moderately elevated at baseline and increased with age (Figure 2).
TABLE 5.
Changes in ABC-C scores over time based on genotype
| Genotype slope estimateb (95% CI) | Irritabilityd | Lethargyc | Stereotypyc | Hyperactivitye |
|---|---|---|---|---|
| Deletion | 0.41 (0.27, 0.56)a | 0.16 (0.07, 0.26)a | 0.05 (−0.02, 0.11) | 0.67 (0.43, 0.90)a |
| UBE3A mutation | 1.45 (1.06, 1.83)a | 0.40 (0.14, 0.66)a | 0.31 (0.10, 0.52)a | 1.18 (0.54, 1.81)a |
| UPD /ImpD | 0.76 (0.45, 1.07)a | −0.07 (−0.28, 0.15) | −0.03 (−0.19, 0.13) | 0.59 (0.07, 1.10)a |
Abbreviations: ABC-C, Aberrant Behavior Checklist-Community; UPD, paternal uniparental disomy for chromosome 15q11q13; ImpD, imprinting defects that alter expression of the maternally inherited copy of UBE3A.
Change in ABC-C subscale score found to be significant over time.
Slope estimate indicates average change in score per year.
Fixed effect p-value <.05 for age, genotype, and age*genotype interaction.
Fixed effect p-value <.05 for age and age*genotype interaction only.
Fixed effect p-value <.05 for age only.
FIGURE 2.
Expected ABC-C irritability and hyperactivity scores over time, by genotype
Abbreviations: ABC-C, Aberrant behavior checklist-community; UPD, Paternal uniparental disomy for chromosome 15q11q13; IC, Imprinting defects that alter expression of the maternally inherited copy of UBE3A
FIGURE 3.
Expected ABC-C lethargy and stereotypy scores over time, by genotype
Abbreviations: ABC-C, Aberrant behavior checklist-community; UPD, Paternal uniparental disomy for chromosome 15q11q13; IC, Imprinting defects that alter expression of the maternally inherited copy of UBE3A
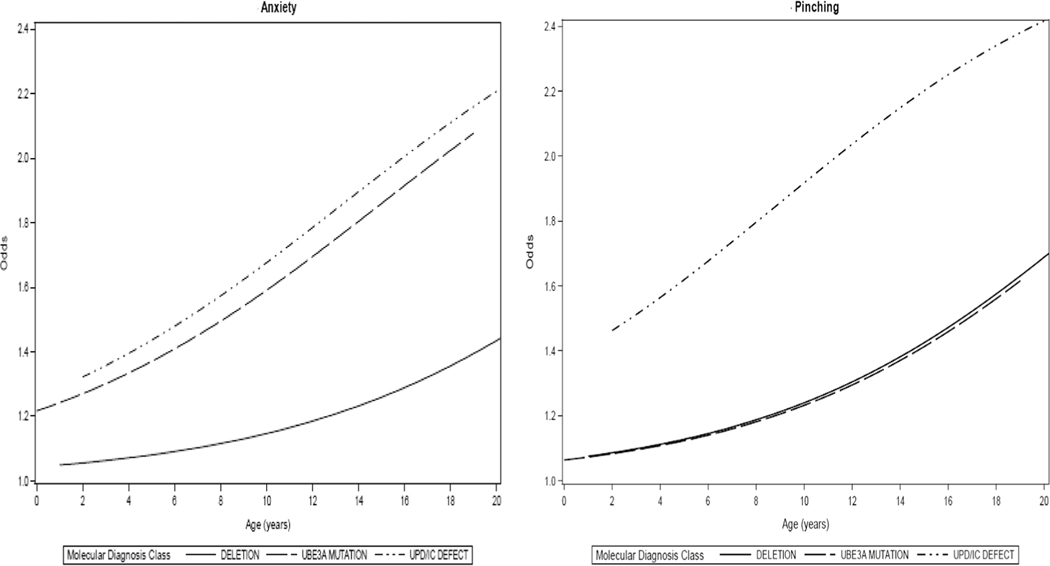
Changes in maladaptive behaviors with age within each of the genotypes as reported by parent interview were also examined. Although there were differences in prevalence among participants with different genotypes at baseline, the rate of change for mouthing behaviors, easy excitability, short attention span, hand flapping, hyperactivity, aggressive behavior, biting, pinching, anxiety, and temper tantrums across genotypes was similar over time (Table 6). Across all genotypes, aggressive behavior (including pinching), hand flapping, and anxiety increased significantly with age (Figure 4). Changes in the rates of short attention span and easy excitability over time differed significantly for males and females, with the likelihood increasing with age for males, but remaining more stable for females. Results showed significant differences among the genotypes with respect to changes in the likelihood of fascination with water, frequent laughter, and hair pulling with increasing age. Within the deletion genotype, fascination with water and frequent laughter increased significantly with age, while hair pulling showed no significant change over time. Within the UPD/ImpD genotype, fascination with water increased significantly with age but hair pulling decreased significantly with age (Table 6).
TABLE 6.
Rate of change in odds of parental report of maladaptive behavior over time by genotype
| Behavior | Deletion (N = 211) | UPD/ImpD (N = 56) | UBE3A (N = 33) |
|---|---|---|---|
| Factor by which odds of behavior changes with 1 year increase in age (95% CI)a,b | |||
| Mouthing behaviors | 0.85 (0.76, 0.96)c | ||
| Easy excitability | |||
| Males | 1.30 (1.00, 1.69) | ||
| Females | 1.07 (0.81, 1.41) | ||
| Short attention span | |||
| Males | 1.17 (0.94, 1.46) | ||
| Females | 0.96 (0.81, 1.13) | ||
| Fascination with water | 2.51 (1.60, 3.94)c | 1.65 (1.05, 2.61)c | 1.18 (0.76, 1.84) |
| Hand flapping | 1.25 (1.11, 1.41)c | ||
| Hyperactive | 0.94 (0.89, 1.00) | ||
| Frequent laughter | 1.30 (1.10, 1.53)c | 0.86 (0.68, 1.10) | 0.84 (0.63, 1.12) |
| Aggressive behavior (overall) | 1.30 (1.13, 1.48)c | ||
| Biting | 0.93 (0.86, 0.99)c | ||
| Pinching | 1.15 (1.04, 1.27)c | ||
| Hair pulling | 1.01 (0.95, 1.07) | 0.84 (0.73, 0.97)c | 1.10 (0.91, 1.33) |
| Anxiety | 1.14 (1.04, 1.24)c | ||
| Temper tantrums | 1.08 (0.98, 1.18) | ||
Abbreviations: UPD, paternal uniparental disomy for chromosome 15q11q13; ImpD, imprinting defects that alter expression of the maternally inherited copy of UBE3A.
A value greater than 1 indicates an increase in odds of exhibiting behavior over time, while a value less than 1 indicates a decrease in odds over time. A confidence interval that includes 1.0 indicates that the change in odds over time is not significant.
For behaviors where there is only one value across the different genotypes, the rate of change does not differ based on genotype, hence results are for all genotypes combined.
Log odds of displaying behavior found to change significantly over time.
FIGURE 4.
Odds of displaying anxiety and pinching over time, by genotype
Abbreviations: UPD, Paternal uniparental disomy for chromosome 15q11q13; IC, Imprinting defects that alter expression of the maternally inherited copy of UBE3A
4 |. DISCUSSION
This is the first published study to examine the prevalence and severity of a wide range of maladaptive behaviors and how these behaviors vary over time by molecular etiology in a large cohort of individuals with AS. Caregiver ratings on the ABC-C from our study cohort provide normative baseline behavioral ratings for the different ABC scales and identify specific maladaptive behaviors in AS individuals that can be targeted in clinical trials. Consistent with findings from other studies examining ABC-C scores in AS individuals (Clarke & Marston, 2000; Summers & Feldman, 1999), we found that participants with AS had low lethargy and stereotypy scores. In addition, we found genotypic differences in the severity of these symptoms across the different ABC domains, which prior studies had not examined. In examining these changes in ABC-C scores with age, while rates of lethargy and stereotypy remained low over time, all participants experienced an increase in irritability and hyperactivity with advancing age. This increase in hyperactivity with age in our study is not consistent with other studies, which had found that adults had lower hyperactivity scores than adolescents (Clarke & Marston, 2000; Clayton-Smith, 2001). However, given the relatively young ages of the participants in our cohort, our findings should be interpreted with caution for adults. We speculate that the increase in irritability in cognitively higher functioning individuals is related to their increased self-awareness and subsequently increased frustration in expressing themselves and interacting with their social environment.
Compared to our patients with AS in whom irritability and hyperactivity increased with age, cross-sectional studies in individuals with Fragile X syndrome suggest that irritability may increase with age in both males and females, while stereotypy and hyperactivity may decrease with age, but only in males (Wheeler et al., 2014). Among individuals with Down syndrome and Prader-Willi syndrome, cross-sectional studies suggest that lethargy may increase with age (Salehi et al., 2018). Thus, changes in the ABC profile over time are dependent on the specific genetic syndrome.
In examining the specific behaviors commonly reported in AS, short attention span emerged as an area of significant concern across the different genotypes, which over time increased for males and decreased for females. Other studies on AS have also commented on the limited attention span in individuals with AS and noted that it was comparable to other disorders with moderate to profound disability (Berry et al., 2005). Consistent with other studies, we saw moderately elevated rates of aggression in our cohort (Arron et al., 2011), which increased over time across all genotypes. Interestingly, not only does the frequency of aggressive behaviors increase with age, the manifestations of aggression also changed over time in our cohort. With advancing age, there was an increase in the frequency of pinching behaviors and a reduction in the frequency of biting behaviors.
Overall, across both parental measures, hyperactivity, short attention span, and irritability/aggression emerged as areas of major concern for caregivers of individuals with AS, and they were correlated with increased parental stress and decreased family quality of life, consistent with other studies examining parental stress in individuals with developmental disabilities (Tomanik, Harris, & Hawkins, 2004). Treatments that alleviate some of these challenging behaviors would be expected to reduce parental stress and improve quality of life.
Although not examined as part of this study, it is critical to discuss some of the possible biomedical and psychosocial triggers of these maladaptive behaviors in individuals with AS to identify effective behavioral and psychopharmacological treatments. It is possible that attentional difficulties may be affected by the prevalence of seizures in this population (Pelc, Cheron, & Dan, 2008). Studies in children with autism spectrum disorder (ASD) have found that children with seizures have higher scores on the hyperactivity and irritability domains of the ABC compared to children without seizures (Hartley-McAndrew & Weinstock, 2010), although no studies have examined similar associations in the AS population. Increased irritability and poor impulse control may be a result of sleep disturbances. Several studies have documented sleep dysfunction in individuals with AS (Spruyt, Braam, & Curfs, 2017), so an association between increased irritability and poorer sleep should be examined in future studies. Some studies have noted that frustration over inability to communicate, escape, and socially engage often appear to be the reasons for aggressive behaviors (Radstaake et al., 2013; Strachan et al., 2009). Functional communication training (Radstaake et al., 2013) has been shown to help alleviate some of these behavioral challenges in individuals with AS. In addition, providing individuals with AS access to appropriate alternative and augmentative communication (AAC) to communicate their needs may also help reduce some of their frustration.
There are several limitations to the current study. Maladaptive behaviors were assessed using only parental report. Limited information on school placement and services received by the AS individual was collected in our study. Detailed information on the school environment such as teacher: student ratio and class sizes was not collected. It is important to corroborate these findings with multiple independent respondents, including reports from other caregivers such as teachers, as well as to investigate other methodologies besides questionnaires such as direct home or school-based observation to obtain a better understanding of the frequency, severity, and the duration of these challenging behaviors. Comprehensive information on the school environment would also be helpful to assess the prevalence of maladaptive behaviors from a multisystemic perspective. Moreover, given the young ages of our participants at the time of the first visit, these results should be generalized to the adult AS population with caution. The psychometric properties of the ABC should be further examined specifically for individuals with AS. It is possible that an AS-specific factor structure is warranted as is the case with some other developmental disabilities, including fragile X syndrome and autism (Wheeler et al., 2014) and would be a more sensitive measure for identifying behavior problems in AS individuals.
In summary, maladaptive behaviors in individuals with AS are characterized by hyperactivity, short attention span, irritability and aggressive outbursts. These findings have important implications for upcoming clinical trials by providing normative baseline information for maladaptive behaviors and identifying behavioral targets for treatment.
ACKNOWLEDGMENTS
This study was supported by NIH U54 RR019478 (awarded to Arthur L. Beaudet) from the National Center for Research Resources (NCRR) and NIH U54 HD061222 (awarded to Alan Percy) from the National Institute of Child Health and Human Development (NICHD), both components of the National Institutes of Health (NIH). We would like to thank our study participants and their families for their continued commitment to this longitudinal study. A special thanks to Ovid Therapeutics for assistance with statistical analyses and to Erin Sheldon and Terry Jo Bichell for their detailed review of our manuscript. The data that support the findings of this study are available from the corresponding author upon reasonable request. We would also like to thank all the site principal investigators and coordinators who facilitated the collection of these data.
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: U54 HD061222; National Center for Research Resources, Grant/Award Number: U54 RR019478; National Institutes of Health; National Institute of Child Health and Human Development; National Center for Research Resources, Grant/Award Number: NIH U54 HD061222; National Institutes of Health, Grant/Award Numbers: RR019478, U54
REFERENCES
- Abidin RR (1995). Parenting stress index manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Aman M, Singh N, Stewart A, & Field C (1985). The aberrant behavior checklist: A behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency, 89(5), 485–491. [PubMed] [Google Scholar]
- Arron K, Oliver C, Moss J, Berg K, & Burbidge C (2011). The prevalence and phenomenology of self-injurious and aggressive behaviour in genetic syndromes. Journal of Intellectual Disability Research, 55(2), 109–120. [DOI] [PubMed] [Google Scholar]
- Bird LM (2014). Angelman syndrome: Review of clinical and molecular aspects. The Application of Clinical Genetics, 7, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach Center on Disability. (2006). Family quality of life scale. Lawrence, KS: Beach Center on Disabilities. [Google Scholar]
- Bayley N (2005). Bayley scales of infant and toddler development, third edition (Bayley-III). San Antonio, TX: Harcourt Assessment, Inc. [Google Scholar]
- Berry RJ, Leitner RP, Clarke AR, & Einfeld SL (2005). Behavioral aspects of Angelman syndrome: A case control study. American Journal of Medical Genetics Part a, 132(1), 8–12. [DOI] [PubMed] [Google Scholar]
- Brown EC, Aman MG, & Havercamp SM (2002). Factor analysis and norms for parent ratings on the aberrant behavior checklist-community for young people in special education. Research in Developmental Disabilities, 23(1), 45–60. [DOI] [PubMed] [Google Scholar]
- Clarke DJ, & Marston G (2000). Problem behaviors associated with 15q-Angelman syndrome. American Journal on Mental Retardation, 105 (1), 25–31. [DOI] [PubMed] [Google Scholar]
- Clayton-Smith J (2001). Angelman syndrome: Evolution of the phenotype in adolescents and adults. Developmental Medicine and Child Neurology, 43(7), 476–480. [DOI] [PubMed] [Google Scholar]
- Clayton-Smith J, & Laan L (2003). Angelman syndrome: A review of the clinical and genetic aspects. Journal of Medical Genetics, 40(2), 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagli A, Buiting K, & Williams C (2011). Molecular and clinical aspects of Angelman syndrome. Molecular Syndromology, 2(3–5), 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagli AI, Mueller J, & Williams CA (2017). Angelman syndrome GeneReviews®[Internet]: University of Washington, Seattle. [PubMed] [Google Scholar]
- Didden R, Sigafoos J, Korzilius H, Baas A, Lancioni GE, O’Reilly MF, & Curfs LM (2009). Form and function of communicative behaviours in individuals with Angelman syndrome. Journal of Applied Research in Intellectual Disabilities, 22(6), 526–537. [Google Scholar]
- Freund LS, & Reiss AL (1991). Rating problem behaviors in outpatients with mental retardation: Use of the aberrant behavior checklist. Research in Developmental Disabilities, 12(4), 435–451. [DOI] [PubMed] [Google Scholar]
- Gentile JK, Tan W-H, Horowitz LT, Bacino CA, Skinner SA, Barbieri-Welge R, … Lee H-S (2010). A neurodevelopmental survey of Angelman syndrome with genotype-phenotype correlations. Journal of Developmental and Behavioral Pediatrics, 31(7), 592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley-McAndrew M, & Weinstock A (2010). Autism spectrum disorder: Correlation between aberrant behaviors, EEG abnormalities and seizures. Neurology International, 2(1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberger EO (2005). General measures of cognition for the preschool child. Mental Retardation and Developmental Disabilities Research Reviews, 11(3), 197–208. [DOI] [PubMed] [Google Scholar]
- Larson AM, Shinnick JE, Shaaya EA, Thiele EA, & Thibert RL (2015). Angelman syndrome in adulthood. American Journal of Medical Genetics Part A, 167(2), 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson JL, Mahan S, Hess JA, Fodstad JC, & Neal D (2010). Progression of challenging behaviors in children and adolescents with autism spectrum disorders as measured by the autism spectrum disorders-problem behaviors for children (ASD-PBC). Research in Autism Spectrum Disorders, 4(3), 400–404. [Google Scholar]
- Miodrag N, & Peters S (2015). Parent stress across molecular subtypes of children with Angelman syndrome. Journal of Intellectual Disability Research, 59(9), 816–826. [DOI] [PubMed] [Google Scholar]
- Moss J, Oliver C, Arron K, Burbidge C, & Berg K (2009). The prevalence and phenomenology of repetitive behavior in genetic syndromes. Journal of Autism and Developmental Disorders, 39(4), 572–588. [DOI] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning. Circle Pines, MN: AGS Circle Pines. [Google Scholar]
- Pelc K, Cheron G, & Dan B (2008). Behavior and neuropsychiatric manifestations in Angelman syndrome. Neuropsychiatric Disease and Treatment, 4(3), 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Grocott O, Parkin K, Larson A, & Thibert RL (2018). Angelman syndrome in adolescence and adulthood: A retrospective chart review of 53 cases. American Journal of Medical Genetics. Part a, 176 (6), 1327–1334. [DOI] [PubMed] [Google Scholar]
- Radstaake M, Didden R, Lang R, O’Reilly M, Sigafoos J, Lancioni GE, … Curfs LM (2013). Functional analysis and functional communication training in the classroom for three children with Angelman syndrome. Journal of Developmental and Physical Disabilities, 25(1), 49–63. [Google Scholar]
- Sansone SM, Widaman KF, Hall SS, Reiss AL, Lightbody A, Kaufmann WE, … Hessl D (2012). Psychometric study of the aberrant behavior checklist in fragile X syndrome and implications for targeted treatment. Journal of Autism and Developmental Disorders, 42 (7), 1377–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi P, Herzig L, Capone G, Lu A, Oron AP, & Kim SJ (2018). Comparison of aberrant behavior checklist profiles across Prader-Willi syndrome, down syndrome, and autism spectrum disorder. American Journal of Medical Genetics. Part A, 176(12), 2751–2759. [DOI] [PubMed] [Google Scholar]
- Spruyt K, Braam W, & Curfs LM (2017). Sleep in Angelman syndrome: A review of evidence. Sleep Medicine Reviews, 37, 69–84. [DOI] [PubMed] [Google Scholar]
- Strachan R, Shaw R, Burrow C, Horsler K, Allen D, & Oliver C (2009). Experimental functional analysis of aggression in children with Angelman syndrome. Research in Developmental Disabilities, 30(5), 1095–1106. [DOI] [PubMed] [Google Scholar]
- Summers JA, Allison D, Lynch P, & Sandier L (1995). Behaviour problems in Angelman syndrome. Journal of Intellectual Disability Research, 39(2), 97–106. [DOI] [PubMed] [Google Scholar]
- Summers JA, & Feldman MA (1999). Distinctive pattern of behavioral functioning in Angelman syndrome. American Journal on Mental Retardation, 104(4), 376–384. [DOI] [PubMed] [Google Scholar]
- Tan WH, Bacino CA, Skinner SA, Anselm I, Barbieri Welge R, Bauer Carlin A, … Glaze DG (2011). Angelman syndrome: Mutations influence features in early childhood. American Journal of Medical Genetics Part a, 155(1), 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanik S, Harris GE, & Hawkins J (2004). The relationship between behaviours exhibited by children with autism and maternal stress. Journal of Intellectual and Developmental Disability, 29(1), 16–26. [Google Scholar]
- Walz NC (2007). Parent report of stereotyped behaviors, social interaction, and developmental disturbances in individuals with Angelman syndrome. Journal of Autism and Developmental Disorders, 37(5), 940–947. [DOI] [PubMed] [Google Scholar]
- Wheeler A, Raspa M, Bann C, Bishop E, Hessl D, Sacco P, & Bailey DB (2014). Anxiety, attention problems, hyperactivity, and the aberrant behavior checklist in fragile X syndrome. American Journal of Medical Genetics Part A, 164(1), 141–155. [DOI] [PubMed] [Google Scholar]
- Wheeler AC, Sacco P, & Cabo R (2017). Unmet clinical needs and burden in Angelman syndrome: A review of the literature. Orphanet Journal of Rare Diseases, 12(1), 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CA (2010). The behavioral phenotype of the Angelman syndrome. Paper presented at the. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 154C, 432–437. [DOI] [PubMed] [Google Scholar]