Abstract
Objectives
Relapses affect 30–50% of patients with ANCA-associated vasculitis (AAV) over 5 years, necessitating long-term treatment. Although there have been studies looking at predictors of relapse in AAV, this research has yet to translate clinically into guidance on tailored therapy. The aim of this systematic review was to identify and meta-analyse existing risk factors from the literature and produce a model to calculate individualised patient risk of relapse.
Method
A search strategy was developed to include all studies identifying predictors of AAV relapse using multivariate analysis. Individual risk factors were extracted and pooled hazard ratios (HRs) calculated. A model to predict the time to first relapse based on identified risk factors was tested retrospectively using a cohort of patients with AAV.
Results
The review of 2674 abstracts identified 117 papers for full text review, with 16 eligible for inclusion. Pooled HRs were calculated from significant risk factors, including anti-PR3 ANCA positivity [HR 1.69 (95% CI 1.46, 1.94)], cardiovascular involvement [HR 1.78 (95% CI 1.26, 2.53)], creatinine >200 µmol/l (relative to creatinine ≤100) [HR 0.39 (95% CI 0.22, 0.69)] and creatinine 101–200 µmol/l [HR 0.81 (95% CI 0.77, 0.85)]. Using data from 182 AAV patients to validate the model gave a C-statistic of 0.61.
Conclusion
Anti-PR3 ANCA positivity, lower serum creatinine and cardiovascular system involvement are all associated with an increased risk of relapse, and a combination of these risk factors can be used to predict the individualised risk of relapse. In order to produce a clinically useful model to stratify risk, we need to identify more risk factors, with a focus on robust biomarkers.
Keywords: ANCA, vasculitis, relapse, immunosuppression, maintenance
Key messages
Anti-PR3 ANCA positivity, lower serum creatinine and cardiovascular involvement are associated with increased risk of relapse.
A combination of these risk factors can be used to predict individual relapse.
There is a need to focus on identifying more robust biomarkers to produce a model to predict relapse.
Introduction
Survival from ANCA-associated vasculitis (AAV) has improved significantly with the introduction of immunosuppressive therapies, changing our perception of vasculitides from acutely fatal diseases to chronic relapsing–remitting conditions [1, 2]. Although survival rates now approach 80% at 5 years, there is still considerable morbidity and mortality associated with AAV; the mortality ratio remains 2.6 times worse than the age- and sex-matched general population [3].
Despite initial disease control, relapse occurs in 30–50% of patients over 5 years, necessitating repeated courses of immunotherapy and, in many, long-term treatment [4]. Relapses are associated with accumulation of disease- and treatment-related damage and morbidity [4]. However, prolonged maintenance therapy to reduce the risk of relapse is also coupled with toxicity [5]. Current recommendations suggest ≥24 months of remission-maintenance therapy once remission has been achieved, although the optimal duration remains unknown [6, 7].
Although there have been randomized control trials (RCTs) and a number of observational studies looking at clinical, histological and biochemical predictors of relapse in AAV, there is variability in the risk factors identified, and thus far, no meta-analysis of these has been performed to quantify their risk [8–10]. Furthermore, this research has yet to translate clinically into guidance on tailored therapy dependent on individualised risk of relapse.
The primary aim of our study was to identify risk factors for relapse in AAV through a systematic review and meta-analysis. The secondary aim was to develop a model to predict the risk of relapse.
Methods
This systematic review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on 28 June 2018 (registration number: CRD42018102716).
Search strategies
A literature search strategy for MEDLINE and EMBASE was developed, with the help of a librarian, to include all studies identifying predictors of AAV relapse from database inception to December 2020. Database-specific indexing was used for MEDLINE and EMBASE. The main search concepts were AAV and all its derivatives for disease terminology combined with relapse/recurrence. We included only those studies written in English and involving adult humans. The search strategies can be found in the supplementary material (for MEDLINE, Supplementary Table S1, and for EMBASE, Supplementary Table S2, available at Rheumatology Advances in Practice online).
Abstracts and titles for all studies identified from the literature search were reviewed by two reviewers (C.K. and E.K.) for relevance. They had to involve patients with AAV with reference to risk factors for relapse to be endorsed for full-paper review. Full papers of selected abstracts were then assessed independently for eligibility based on stipulated inclusion and exclusion criteria (C.K. and K.L.D.), and any disagreements were resolved by a referee (E.K.). The main inclusion criteria were adult patients with a new diagnosis of AAV with ≥12 months of follow-up. Patients had to have achieved remission with remission-induction treatment and subsequently received maintenance therapy. Studies had to have identified and quantified independent predictors of AAV relapse using multivariable analysis or an RCT with relapse or sustained remission as an endpoint. Studies were excluded if there was no definition of relapse, if their immunosuppression was not clearly defined with the induction and maintenance agent and duration, or if they were a case report, review or conference abstract.
We defined a risk factor as any measurable variable that was associated with a relapse event during the follow-up period of the study.
Further searches were also performed of the references in relevant papers and review articles to identify any additional studies that might also address the research question. Where the same study published results for a risk factor in more than one manuscript, the results from the most recent publication were used.
Data extraction
For the eligible studies, data were extracted by two authors (C.K. and K.L.D.) using a standardized form. Data extracted from the studies included sample size, sampling frame, data collection, follow-up duration, study design and aims. Details of induction and maintenance treatment were extracted, along with patient characteristics including gender, age, anti-PR3 positivity, BVAS and creatinine at study entry. Outcome data were extracted on risk factors with their hazard ratio (HR) and P-values. Where more than one model was presented in the same study, the model with the greatest number of significant risk factors was used.
On reviewing the sampling frame from the data-extraction form, it became apparent that there were 11 studies deemed eligible that had included patients from the same RCTs in their pooled study cohorts. The RCT cohorts included in more than one eligible study were CYCAZEREM, CYCLOPS, NORAM, MEPEX, WEGENT and IMPROVE; many of the original RCTs did not meet the initial inclusion criteria [11–16]. In order to ensure that there was no data duplication in the meta-analysis, further studies were excluded from the meta-analysis after a sensitivity analysis, assessment of quality using the Quality In Prognosis Studies (QUIPS) tool and discussion among all authors. The sensitivity analysis was performed by taking a single risk factor and calculating the pooled HR based on the inclusion of each duplicated study.
Statistical analysis
Only those risk factors that were identified in more than one eligible study were included in the meta-analysis. Studies presenting risk factors in the form of a HR and 95% CI, or those in which such estimates could be derived from the presented statistics, were included in the meta-analysis. Categorical risk factors were standardized to the same reference category and continuous variables to the same units. Pooled HRs for individual risk factors were calculated using REVMAN software. Heterogeneity was assessed using I2 statistics.
The quality of the studies was assessed using the QUIPS tool, which is designed for use in systematic reviews of prognostic factor studies [17]. QUIPS is composed of six categories to assess the risk of bias: study participation; study attrition; prognostic factor measurement; outcome measurement; study confounding; and statistical analysis and reporting. For each study, all six categories were scored separately as being of high, moderate or low quality. Assessment of bias was completed independently by two authors (C.K. and K.L.D.) for each study included in the meta-analysis, with disagreements being resolved by discussion. All studies were included in the analysis, but where data duplication occurred, quality was used to differentiate studies for inclusion.
We constructed a funnel plot, in which a measure of the study size was plotted against the HR. We used the logarithm of the HRs from individual studies and the logarithm of precision (1/variance). The distribution on the funnel plot was used to assess for publication bias and small-study effect in the meta-analysis.
Concordance statistics were used to validate a model based on the identified risk factors at diagnosis, using the data collected retrospectively from the electronic patient records of 182 patients with AAV under the care of the vasculitis clinic at University Hospitals Birmingham National Health Service Foundation Trust in 2018. Patients were included if they had achieved remission after induction treatment and if data were available for the risk factors identified after the meta-analysis. We defined relapse in our cohort as new or worsening disease activity that required a change in treatment. BVAS was used to identify organ involvement, but this remained a clinical decision. Cox regression analysis was applied to test the possible score combinations of combined risk factors, with analysis truncated at 5 years of follow-up.
Results
Systematic review
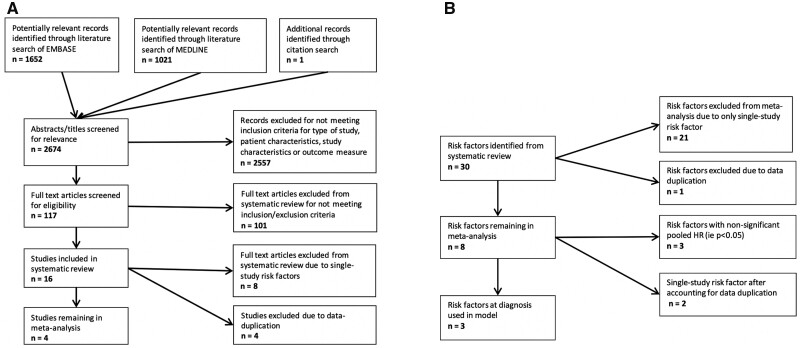
We reviewed 2674 abstracts (1652 EMBASE, 1021 MEDLINE and 1 reference list), of which 2557 were excluded (Fig. 1A). The complete list of abstracts reviewed and their outcomes are available on request from the corresponding author.
Fig. 1.
Flowcharts demonstrating the number of studies (A) and risk factors (B) identified, screened and included in the meta-analysis
One hundred and seventeen full-text studies were then screened for eligibility. Of these, 101 were excluded, primarily owing to a lack of multivariate analysis or poor identification of specific treatment regimens, as outlined in Fig. 1A.
The 16 studies eligible for the systematic review included a total of 2785 patients [8–13, 18–27]. The studies were published between 2005 and 2018, and the designs included nine RCTs, five post-hoc analyses and two cohort studies.
Meta-analysis
Risk factors identified
Thirty risk factors were identified in total from the studies in the systematic review, of which 21 were identified in only one study and were not included in the meta-analysis (Fig. 1B). Thirteen of these single risk factors were not significant (P > 0.05). As demonstrated in Fig. 1A, eight full-text articles were excluded because they identified these single-study risk factors. The single-study risk factors are included in Supplementary Table S3, available at Rheumatology Advances in Practice online.
Quality assessment
The quality of the remaining nine studies was assessed using the QUIPS tool. This can be found in Supplementary Table S4, available at Rheumatology Advances in Practice online.
None of the studies scored a low risk of bias in all six quality categories assessed using QUIPS. Study confounding was the area with the highest degree of bias across the studies owing to the variation in confounding factors assessed in the multivariate analysis of the studies. Only one RCT remained in the analysis, which reflects the overall poorer quality of the studies [12].
Studies excluded owing to data duplication
Seven of nine of the remaining studies included patient cohorts from the same trials, and therefore studies had to be excluded from the analysis to avoid duplication of patient data. Supplementary Table S5, available at Rheumatology Advances in Practice online, summarizes this duplication of patient cohorts.
The CYCLOPS trial was the most frequently used cohort, with four of the eligible studies including patients from this trial; only one of these could be included in the meta-analysis [8, 9, 19, 24]. The three largest studies, with the longest durations of follow-up, were those by De Joode et al. [19], Walsh et al. [8] and Morgan et al. [9]; they were all post-hoc analyses including pooled patient cohorts from multiple trials including CYCLOPS, each identifying multiple risk factors.
Anti-PR3 ANCA positivity at diagnosis was the most frequently identified risk factor. Using this risk factor, we performed a sensitivity analysis (Supplementary Table S6, available at Rheumatology Advances in Practice online). This demonstrated a difference of only 0.62 between the largest (2.03) and smallest (1.41) HR, with the inclusion of the study by Walsh et al. [8] and exclusion of the other three duplicate studies producing a HR 1.69 (95% CI 1.46, 1.94) with low heterogeneity (I2 7%). The study by Walsh et al. [8] had the largest sample size in the systematic review, with 535 patients and a median follow-up of 40 months. The studies by Walsh et al. [8] and Morgan et al. [9] were both assessed to have a moderate risk of bias using the QUIPs tool, compared with a high risk in the study by De Joode et al. [19] (Supplementary Table S4, available at Rheumatology Advances in Practice online). Overall, our consensus opinion was that selecting the study by Walsh et al. [8] produced a more reliable and clinically relevant meta-analysis.
The same process was followed for the WEGENT trial, excluding the study by Puéchal et al. [20] from further analysis.
Risk factors included in the meta-analysis
Pulsed vs oral CYC was excluded as a risk factor after all three of the studies that identified this risk factor were excluded owing to data duplication [9, 19, 24]. Six risk factors remained in the meta-analysis identified from four studies, as listed in Table 1 [8, 18, 22, 25]. Three of these risk factors produced non-significant (P < 0.05) pooled HRs (Table 1). These risk factors were composed of non-significant HRs from individual studies and despite pooling, remained non-significant.
Table 1.
All risk factors remaining in the meta-analysis
| Risk factor | Number of studies | Effect size [HR (fixed, 95% CI)] | P-value | I² (%) |
|---|---|---|---|---|
| Anti-PR3 ANCA positive at diagnosis | 4 | 1.69 (1.46, 1.94) | <0.00001 | 7 |
| Lung involvement at diagnosis | 3 | 1.18 (0.90, 1.86) | 0.24 | 0 |
| Age at diagnosis (per year) | 3 | 1.00 (0.99, 1.01) | 0.79 | 84 |
| Cardiovascular system involvement at diagnosis | 2 | 1.78 (1.26, 2.53) | 0.001 | 43 |
| Upper respiratory tract involvement at diagnosis | 2 | 1.39 (0.91, 2.13) | 0.13 | 0 |
|
Creatinine at diagnosis >200 µmol/l (relative to creatinine ≤100 µmol/l) Creatinine at diagnosis 100–200 µmol/l (relative to creatinine ≤100 µmol/l) |
2* |
0.39 (0.22, 0.69) 0.81 (0.77, 0.85) |
0.001 <0.001 |
n/a n/a |
Creatinine was identified as a risk factor in two studies, but owing to the different units of measurement the data could not be pooled and were selected from one study.
HR: hazard ratio; n/a: not assessed.
The measurement unit of creatinine varied between studies, making it difficult to pool this relapse risk. Two studies remaining in the meta-analysis demonstrated the negative trend for increasing relapse risk with a lower serum creatinine at diagnosis, although their units could not be pooled [8, 18]. Likewise, an additional study in the meta-analysis, by Pierrot-Deseilligny Despujol et al. [22], identified a reduced risk of relapse associated with an estimated glomerular filtration rate <30 ml/min. We included the data from the study by Walsh et al. [8] because this included the larger number of patients and it graded the risk factor, producing two HRs.
Thus, the meta-analysis identified three significant risk factors for relapse in patients with AAV, namely anti-PR3 ANCA positivity (Fig. 2A), cardiovascular system (CVS) involvement (Fig. 2B) and a lower serum creatinine, all at diagnosis.
Fig. 2.
Forest plots for risk factors included in the meta-analysis
(A) Relapse risk of pooled hazard ratio for anti-PR3 ANCA positive. (B) Relapse risk of pooled hazard ratio for cardiovascular system involvement.
Publication bias
A funnel plot was constructed for the risk factor shown in the most studies, anti-PR3 ANCA positivity, in which a measure of the study size was plotted against the HR. This was done to detect publication bias or the small-study effect. We had only four studies plotted on the chart; therefore, it was difficult to assess for asymmetry and this has not been included. During the screening for this review and when assessing the quality of the included studies, it became apparent that the HR for non-significant risk factors was not always published, which might have introduced an element of reporting bias to this review.
How useful are risk factors for predicting relapse in a real-world setting?
Creating the model
The secondary aim of this study was to develop a model to predict relapse. The three significant risk factors included in this model were anti-PR3 ANCA positivity [HR 1.69 (95% CI 1.46, 1.94)], CVS involvement [HR 1.78 (95% CI 1.26,2.53)], creatinine >200 µmol/l (relative to creatinine ≤100 µmol/l) [HR 0.39 (95% CI 0.22, 0.69)] and creatinine 101–200 µmol/l (relative to creatinine ≤100 µmol/l) [HR 0.81 (95% CI 0.77, 0.85)]. These HRs were combined to estimate HRs of every combination, as shown in Supplementary Table S7, available at Rheumatology Advances in Practice online.
Testing the model
Patient cohort
Data were used from 182 patients with AAV from a single tertiary centre to validate the model based on the three risk factors; the patient demographics of this cohort are shown in Table 2 and compared with the pooled patient cohorts included in the meta-analysis. One hundred and four (57%) of the patients relapsed with a median follow-up time of 133 months; 75 (41%) relapsed within 5 years (Table 2). One hundred and eleven (61%) of the patients were anti-PR3 positive at diagnosis with a median creatinine of 146 µmol/l; 33% had a creatinine at diagnosis ≤100 µmol, 32% 101–200 µmol/l and 35% >200 µmol/l. Only 11 of 182 patients (6%) had CVS organ involvement at diagnosis.
Table 2.
Comparison of patient demographics of the cohort used to test the model with pooled cohorts from the meta-analysis
| Demographic | Patient cohort used to test model (n = 182) | Pooled median values from patient cohorts included in meta-analysis (n = 1041) |
|---|---|---|
| Age at diagnosis, median (range), years | 57 (17–85) | 59 |
| Male gender, % | 58 | 55 |
| White British ethnicity, % | 89 | 87 |
| Anti-PR3 positive at diagnosis, % | 61 | 64 |
| Creatinine at diagnosis, median (range), µmol/l | 146 (44–1137) | 190 |
| Cardiovascular involvement at diagnosis, % | 6 | 7 |
| Relapse in first 5 years, % | 41 | 41 |
| Duration of follow-up, median (range), months | 133 (7–329) | 46 |
Concordance statistics
Testing our model using these data produced a concordance (C-statistic) of 0.61, with a standard error of 0.029. The model was truncated at 5 years of follow-up; there was a very small improvement in the C-statistic to 0.62, with a standard error of 0.032.
Cox regression
We used Cox regression to assess the risk of relapse in our cohort of patients relative to the risk factors present in the patient at diagnosis, as shown in Table 3. We were unable to test CVS involvement reliably owing to the low incidence rate of 6% in our patients. We performed Cox regression analysis, truncated at 5 years, for the other six combinations of risk factors. Comparing the risk of relapse in our patient cohort for each possible outcome with the highest risk of relapse outcome (anti-PR3 ANCA positive and low creatinine), all but one was statistically significant (P < 0.05; Table 3). The HRs estimated from the data were consistent with those derived from the results of the meta-analysis, as demonstrated in Table 3.
Table 3.
Results of Cox regression analysis of relapse-free survival in the 5 years following diagnosis for our patient cohort
| Risk combination | P-value | HR (fixed, 95% CI) | Theoretical HR calculated from meta-analysis |
|---|---|---|---|
| High creatinine, anti-PR3 negative, CVS negative | 0.002 | 0.23 (0.09, 0.60) | 0.23 |
| High creatinine, anti-PR3 positive, CVS negative | 0.002 | 0.35 (0.19, 0.67) | 0.39 |
| Medium creatinine, anti-PR3 negative, CVS negative | 0.001 | 0.22 (0.09, 0.54) | 0.48 |
| Medium creatinine, anti-PR3 positive, CVS negative | 0.014 | 0.40 (0.20, 0.83) | 0.81 |
| Low creatinine, anti-PR3 negative, CVS negative | 0.059 | 0.45 (0.20, 1.03) | 0.59 |
| Low creatinine, anti-PR3 postive, CVS negative | Ref. | Ref. | Ref. |
Comparison of relapse risk for the patient cohort calculated through Cox regression analysis, with the theoretical HR for the same combinations of risk factors identified from the meta-analysis. The theoretical HRs for the risk factor combinations were estimated by multiplying the HRs for the constituent risk factors identified from the meta-analysis.
High creatinine, >200 µmol/l; medium creatinine, 100–200 µmol/l; low creatinine, <100 µmol/l.
CVS: cardiovascular system; HR: hazard ratio; Ref.: reference category.
Kaplan–Meier curve for relapse-free survival
The Kaplan–Meier curve (Fig. 3) demonstrates relapse-free survival in the 5 years following diagnosis for our patients for the different combinations of risk factors. Although there is some overlap on the curve between outcomes, those patients with the highest risk (i.e. anti-PR3 positive and low creatinine) are clearly defined. We did not censor for death for the cumulative relapse-free survival because none of the patients in our cohort died without relapsing.
Fig. 3.

Kaplan–Meier curve for relapse-free survival dependent on risk factors
Discussion
This is the first meta-analysis to study risk factors for relapse in AAV. We identified three significant risk factors from the meta-analysis: anti-PR3 ANCA positivity, CVS involvement and a lower serum creatinine at diagnosis. A pooled HR was calculated for each risk factor, which enabled us to attribute risk to specific combinations of risk factors in AAV and create a model.
The model we created appeared to be a modest approximation of relapse risk, but we could not test CVS involvement reliably within the model because its incidence was rare. We have demonstrated clearly through the Kaplan–Meier curve that the combination of anti-PR3 ANCA positivity and preserved renal function in patients with AAV carries the greatest risk of relapse. Clinicians when presented with patients with both risk factors must consider a prolonged duration of maintenance therapy, but a model based around only two risk factors is unlikely to be clinically useful.
A previous systematic review by Mukhtyar et al. [28] looked at outcomes in AAV and identified similar risk factors for relapse, but a meta-analysis was not performed. Additionally, Tomasson et al. [29] produced a meta-analysis looking specifically at ANCA measurements during remission to predict relapse. However, ours is the first meta-analysis identifying multiple risk factors for relapse in AAV. Multiple studies have shown that anti-PR3 ANCA-positive patients are at an increased risk of relapse compared with anti-MPO ANCA-positive patients. Much less explored, and arguably contrary to what we might expect, is the reduced relapse risk associated with poorer renal function. It has been suggested that this might be attributable to the immune dysfunction caused by renal failure [30–32].
A strength of this review is testing the model using our patient cohort. This cohort appears to be comparable to the patient cohorts identified in the meta-analysis, providing assurance the results are valid (Table 2) [33]. The definition of relapse and cardiovascular organ involvement in our cohort matched each of the studies included in the meta-analysis.
Our meta-analysis has several limitations. A substantial proportion of the studies included in the systematic review had to be excluded from the meta-analysis, predominantly owing to data duplication. This highlights the limited data sets we have looking at relapse risk in this cohort of patients. The evidence for pulsed CYC as a risk factor for relapse compared with oral CYC is strongly established; however, this was not included after all of the studies identifying it were removed owing to data duplication. Although this is an important risk factor that is not included in the meta-analysis, we could not have validated this in the model using our cohort, because all of our patients are now managed with pulsed CYC owing to the increased side-effect profile associated with oral use. In addition, many of the newer trials, such as RAVE, MAINRITSAN and PEXIVAS, recruited patients with new and relapsing disease [34–36]. Relapsing disease was one of the exclusion criteria for this meta-analysis, because previous relapse can be a confounding factor to further relapse. There should be scope for post-hoc analysis of more recent trials, such as RITUXVAS, to recognize new factors and endorse already identified factors affecting relapse outcome in AAV. A greater focus on biomarkers might provide more robust risk factors.
Excluding studies to account for data duplication might have introduced bias, but the sensitivity analysis performed for the risk factors was reassuring. The largest study included in this review contained data from a pooled post-hoc analysis from multiple RCTs. Including the data from the original RCTs would have been preferable, but many of these RCTs did not meet the inclusion criteria for the systematic review. Additionally, pooled analyses looked at multiple risk factors with a much larger number of patients over a longer period of follow-up compared with the original RCTs.
We did not include risk factors in the meta-analysis that were identified in only a single study in order to improve the reliability of the model; many of these were not significant and, by definition, not therefore a risk factor. Although excluding significant factors might have introduced selection bias, we do not feel that the addition of these factors would have changed the clinical usefulness of the model.
Initially, patients with Eosinophilic granulomatosis with polyangiitis (EGPA) were included in our search strategy. Although EGPA is classified as an AAV, it behaves differently from Granulomatosis with Polyangiitis and Microscopic Polysangiitis and it is rarer; therefore, there has been less research into this subgroup. It became apparent that the predictors of relapse for this vasculitis, such as eosinophil count and anti-MPO positivity, were different, and we made a decision to exclude patients with EGPA from the meta-analysis [37]. Not having stipulated this from the outset might have introduced selection bias.
A common limitation with meta-analyses is the comparability of the cohorts and the appropriateness of the comparison. The included cohorts differed in terms of the induction and maintenance treatment and durations, AAV subgroups and disease severity. None of the four studies included in the meta-analysis was a RCT. For these studies, the quality of the multivariate analysis to identify risk factors was reliant upon adjusting for confounding factors, and there was some variation between studies. Although this is commented on in the quality assessment of each study, studies were not excluded entirely based on this quality assessment. Additionally, relapse risk appears to change over time; there was a range of follow-up for the studies between 35 and 50 months. When testing the model, we truncated the follow-up for our cohort of patients to 5 years given that Cox regression relies on the assumption that the HR stays the same over time. Despite this variation within the meta-analysis, the heterogeneity was generally low.
Conclusion
Anti-PR3 positivity, a lower serum creatinine and CVS involvement at diagnosis are all associated with an increased risk of relapse, and a combination of these risk factors can be used to predict an individualised relapse risk. To produce a clinically useful model to stratify risk and guide the duration of maintenance treatment, we need to identify a greater number of risk factors, with a focus towards more robust biomarkers.
Supplementary Material
Acknowledgements
We thank Mary Ingram (librarian at the Centre for Epidemiology Versus Arthritis, University of Manchester) for her help with formulating the search strategy.
Catherine King is funded by the National Institute for Health Research (NIHR) Academic Clinical Fellow programme for this research project.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: Neil Basu has grants from Vifor, personal fees from Roche; outside the submitted work. The other authors have declared no conflicts of interest.
Data availability statement
Additional data underlying this article which are not already available in the article or in its online supplementary material, will be shared on reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology Advances in Practice online.
References
- 1.Fauci AS, Haynes BF, Katz P, Wolff SM.. Wegener's granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med 1983;98:76–85. [DOI] [PubMed] [Google Scholar]
- 2.Walton EW.Giant-cell granuloma of the respiratory tract (Wegener's granulomatosis). Br Med J 1958;2:265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flossmann O, Berden A, de Groot K. et al. ; for the European Vasculitis Study Group. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011;70:488–94. [DOI] [PubMed] [Google Scholar]
- 4.Robson J, Doll H, Suppiah R. et al. Damage in the anca-associated vasculitides: long-term data from the European vasculitis study group (EUVAS) therapeutic trials. Ann Rheum Dis 2015;74:177–84. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman GS, Kerr GS, Leavitt RY. et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992;116:488–98. [DOI] [PubMed] [Google Scholar]
- 6.Yates M, Watts RA, Bajema IM. et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis 2016;75:1583–94. [DOI] [PubMed] [Google Scholar]
- 7.Ntatsaki E, Carruthers D, Chakravarty K. et al. ; BSR and BHPR Standards, Guidelines and Audit Working Group. BSR and BHPR guideline for the management of adults with ANCA-associated vasculitis. Rheumatology 2014;53:2306–9. [DOI] [PubMed] [Google Scholar]
- 8.Walsh M, Flossmann O, Berden A. et al. ; European Vasculitis Study Group. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2012;64:542–8. [DOI] [PubMed] [Google Scholar]
- 9.Morgan MD, Szeto M, Walsh M. et al. ; European Vasculitis Society. Negative anti-neutrophil cytoplasm antibody at switch to maintenance therapy is associated with a reduced risk of relapse. Arthritis Res Ther 2017;19:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karras A, Pagnoux C, Haubitz M. et al. Randomised controlled trial of prolonged treatment in the remission phase of ANCA-associated vasculitis. Ann Rheum Dis 2017;76:1662–8. [DOI] [PubMed] [Google Scholar]
- 11.De Groot K, Rasmussen N, Bacon PA. et al. ; for the European Vasculitis Study Group. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005;52:2461–9. [DOI] [PubMed] [Google Scholar]
- 12.Hiemstra TF, Walsh M, Mahr A. et al. ; European Vasculitis Study Group (EUVAS). Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA 2010;304:2381–8. [DOI] [PubMed] [Google Scholar]
- 13.Pagnoux C, Mahr A, Hamidou MA. et al. ; French Vasculitis Study Group. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med 2008;359:2790–803. [DOI] [PubMed] [Google Scholar]
- 14.Jayne DR, Gaskin G, Rasmussen N. et al. ; European Vasculitis Study Group. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol 2007;18:2180–8. [DOI] [PubMed] [Google Scholar]
- 15.Jayne D, Rasmussen N, Andrassy K. et al. ; European Vasculitis Study Group. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003;349:36–44. [DOI] [PubMed] [Google Scholar]
- 16.de Groot K, Harper L, Jayne DRW. et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2009;150:670–80. [DOI] [PubMed] [Google Scholar]
- 17.Hayden JA, Cote P, Bombardier C.. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427–37. [DOI] [PubMed] [Google Scholar]
- 18.Hessels AC, Rutgers A, Sanders JSF, Stegeman CA.. Thiopurine methyltransferase genotype and activity cannot predict outcomes of azathioprine maintenance therapy for antineutrophil cytoplasmic antibody associated vasculitis: a retrospective cohort study. PLoS One 2018;13:e0195524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Joode AAE, Sanders JSF, Puéchal X. et al. Long term azathioprine maintenance therapy in ANCA-associated vasculitis: combined results of long-term follow-up data. Rheumatology 2017;56:1894–901. [DOI] [PubMed] [Google Scholar]
- 20.Puéchal X, Pagnoux C, Perrodeau E. et al. ; French Vasculitis Study Group. Long-term outcomes among participants in the Wegent trial of remission-maintenance therapy for granulomatosis with polyangiitis (Wegener's) or microscopic polyangiitis. Arthritis Rheumatol 2016;68:690–701. [DOI] [PubMed] [Google Scholar]
- 21.Walsh M, Faurschou M, Berden A. et al. ; European Vasculitis Study Group. Long-term follow-up of cyclophosphamide compared with azathioprine for initial maintenance therapy in ANCA-associated vasculitis. Clin J Am Soc Nephrol 2014;9:1571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierrot-Deseilligny Despujol C, Pouchot J, Pagnoux C, Coste J, Guillevin L.. Predictors at diagnosis of a first Wegener's granulomatosis relapse after obtaining complete remission. Rheumatology 2010;49:2181–90. [DOI] [PubMed] [Google Scholar]
- 23.Metzler C, Miehle N, Manger K. et al. ; German Network of Rheumatic Diseases. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener's granulomatosis. Rheumatology 2007;46:1087–91. [DOI] [PubMed] [Google Scholar]
- 24.Harper L, Morgan MD, Walsh M. et al. ; EUVAS investigators. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann Rheum Dis 2012;71:955–60. [DOI] [PubMed] [Google Scholar]
- 25.McGregor JG, Hogan SL, Hu Y. et al. Glucocorticoids and relapse and infection rates in anti-neutrophil cytoplasmic antibody disease. Clin J Am Soc Nephrol 2012;7:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders JS, de Joode AA, DeSevaux RG. et al. Extended versus standard azathioprine maintenance therapy in newly diagnosed proteinase-3 anti-neutrophil cytoplasmic antibody-associated vasculitis patients who remain cytoplasmic anti-neutrophil cytoplasmic antibody-positive after induction of remission: a randomized clinical trial. Nephrol Dial Transplant 2016;31:1453–9. [DOI] [PubMed] [Google Scholar]
- 27.Jones RB, Furuta S, Tervaert JW. et al. ; European Vasculitis Society (EUVAS). Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis 2015;74:1178–82. [DOI] [PubMed] [Google Scholar]
- 28.Mukhtyar C, Flossmann O, Hellmich B. et al. ; European Vasculitis Study Group (EUVAS). Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism systemic vasculitis task force. Ann Rheum Dis 2008;67:1004–10. [DOI] [PubMed] [Google Scholar]
- 29.Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA.. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis—a meta-analysis. Rheumatology 2012;51:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girndt M, Sester M, Sester U, Kaul H, Köhler H.. Molecular aspects of T- and B-cell function in uremia. Kidney Int Suppl 2001;59:S206–11. [DOI] [PubMed] [Google Scholar]
- 31.Cohen G, Rudnicki M, Hörl WH.. Uremic toxins modulate the spontaneous apoptotic cell death and essential functions of neutrophils. Kidney Int Suppl 2001;78:S48–52. [DOI] [PubMed] [Google Scholar]
- 32.Cohen G, Hörl WH.. Immune dysfunction in uremia—an update. Toxins (Basel) 2012;4:962–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pagnoux C, Carette S, Khalidi NA. et al. Comparability of patients with ANCA-associated vasculitis enrolled in clinical trials or in observational cohorts. Clin Exp Rheumatol 2015;33(2 Suppl 89):S-77-83. [PMC free article] [PubMed] [Google Scholar]
- 34.Stone JH, Merkel PA, Spiera R. et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh M, Merkel PA, Jayne DRW.. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. Reply. N Engl J Med 2020;382:2169. [DOI] [PubMed] [Google Scholar]
- 36.Guillevin L, Pagnoux C, Karras A. et al. ; French Vasculitis Study Group. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 2014;371:1771–80. [DOI] [PubMed] [Google Scholar]
- 37.Samson M, Puéchal X, Devilliers H. et al. Long-term outcomes of 118 patients with eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome) enrolled in two prospective trials. J Autoimmun 2013;43:60–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data underlying this article which are not already available in the article or in its online supplementary material, will be shared on reasonable request to the corresponding author.