PURPOSE
Constitutional mismatch repair deficiency syndrome (CMMRD) is a lethal cancer predisposition syndrome characterized by early-onset synchronous and metachronous multiorgan tumors. We designed a surveillance protocol for early tumor detection in these individuals.
PATIENTS AND METHODS
Data were collected from patients with confirmed CMMRD who were registered in the International Replication Repair Deficiency Consortium. Tumor spectrum, efficacy of the surveillance protocol, and malignant transformation of low-grade lesions were examined for the entire cohort. Survival outcomes were analyzed for patients followed prospectively from the time of surveillance implementation.
RESULTS
A total of 193 malignant tumors in 110 patients were identified. Median age of first cancer diagnosis was 9.2 years (range: 1.7-39.5 years). For patients undergoing surveillance, all GI and other solid tumors, and 75% of brain cancers were detected asymptomatically. By contrast, only 16% of hematologic malignancies were detected asymptomatically (P < .001). Eighty-nine patients were followed prospectively and used for survival analysis. Five-year overall survival (OS) was 90% (95% CI, 78.6 to 100) and 50% (95% CI, 39.2 to 63.7) when cancer was detected asymptomatically and symptomatically, respectively (P = .001). Patient outcome measured by adherence to the surveillance protocol revealed 4-year OS of 79% (95% CI, 54.8 to 90.9) for patients undergoing full surveillance, 55% (95% CI, 28.5 to 74.5) for partial surveillance, and 15% (95% CI, 5.2 to 28.8) for those not under surveillance (P < .0001). Of the 64 low-grade tumors detected, the cumulative likelihood of transformation from low-to high-grade was 81% for GI cancers within 8 years and 100% for gliomas in 6 years.
CONCLUSION
Surveillance and early cancer detection are associated with improved OS for individuals with CMMRD.
INTRODUCTION
Constitutional mismatch repair deficiency (CMMRD) syndrome (OMIM #276300)1 is a cancer predisposition syndrome associated with inheritance of biallelic pathogenic variants in mismatch repair (MMR) genes (MLH1, PMS2, MSH2, and MSH6), leading to deficient MMR during DNA replication.2 Loss of functional MMR results in a rapid accumulation of mutations and the development of cancers that are highly aggressive and display a hypermutant phenotype.2-4 Because of the nature of these tumors, commonly used chemotherapies and radiation are largely ineffective.5 Consequently, many patients with CMMRD develop symptomatic cancers and die regardless of treatment approach.6-12
CONTEXT
Key Objective
Do the current surveillance recommendations for constitutional mismatch repair deficiency (CMMRD) syndrome improve early tumor detection and overall survival in individuals with CMMRD? This has never been tested prospectively.
Knowledge Generated
Early presymptomatic detection of CMMRD cancers using the current recommended surveillance modalities was associated with improved survival. Overall survival was significantly better in individuals with CMMRD who fully adhered to surveillance guidelines compared with individuals who did not undergo surveillance (P < .0001). All low-grade brain and most GI lesions transformed to malignant cancers during the study period indicating a potential benefit of early detection and intervention.
Relevance
Current surveillance recommendations appear effective for early detection and intervention that can improve outcome for patients with CMMRD. These guidelines can be implemented in most centers around the world.
The most common tumors observed in these patients are early-onset CNS, GI, and hematologic malignancies with higher penetrance than other cancer predisposition syndromes.13 Although other cancer types have been reported in children and adults with CMMRD,13-16 the frequency and extent of these malignancies are not as well characterized.
Surveillance protocols have previously been developed for several cancer predisposition syndromes such as hereditary breast-ovarian cancer and Li-Fraumeni syndromes and have significantly improved survival for screened patients.17-20 Effective surveillance relies on three central pillars.21 First, a surveillance protocol should include modalities that identify most cancer types presenting in at-risk individuals. Second, the recommended modalities should prove effective at early detection of asymptomatic tumors that can be resected or managed effectively with reduced treatment related morbidity. Third, a surveillance protocol must demonstrate that early asymptomatic tumor detection ultimately improves overall patient survival.
Initial efforts to establish surveillance for individuals with CMMRD began with anecdotal cases incorporating GI surveillance22,23 and serial brain magnetic resonance imaging (MRI), although surveillance approaches varied widely.22,24,25 Proposed surveillance guidelines for CMMRD were recently revised26,27 to include more comprehensive recommendations. However, current guidelines are based on expert opinion and no study has systematically evaluated the efficacy of surveillance and whether adherence affects overall survival (OS).
The International Replication Repair Deficiency Consortium (IRRDC) was formed in 2007 and currently includes pediatric and adult patients from more than 45 countries. Since 2008, surveillance recommendations were implemented as part of the management of these patients. Data from all registered IRRDC patients are collected prospectively from the time of enrollment.
The goal of this study was to systematically evaluate the proposed surveillance guidelines for CMMRD27 to determine whether current modalities are appropriate to detect the full spectrum of tumors seen and whether detecting asymptomatic tumors results in improved outcome.
PATIENTS AND METHODS
Inclusion Criteria
All patients were enrolled in the IRRDC with patient consent and institutional research ethics board approval (SickKids, Research Ethics Board no. 1000048813). Patients were diagnosed as having CMMRD through established genetic, clinical, and other molecular diagnostic criteria by the consortium genetic counselor (M.A.) based on criteria established by an international consensus.13,28 This group was termed full study cohort (Fig 1). Patients who did not completely fulfill these established criteria including suspected yet unconfirmed CMMRD were excluded. Since current evidence on CMMRD does not suggest an association between the cancer susceptibility of a patient and which of the four MMR genes is affected, we included all patients with CMMRD in this study and added the gene affected to our multivariable analysis.
FIG 1.
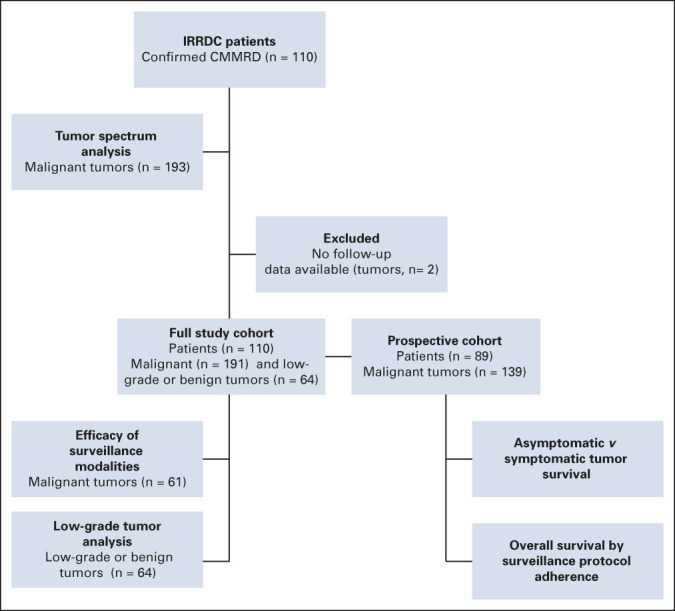
Study overview. CMMRD, constitutional mismatch repair deficiency; IRRDC, International Replication Repair Deficiency Consortium.
Tumor Spectrum
Cancer diagnoses were collected for the full study cohort (n = 110). These were compared with patients with CMMRD previously published in the literature through a computer-aided search of MEDLINE (2008 to December 2019). The search used CMMRD syndrome and biallelic mismatch repair deficiency syndrome as keywords and was restricted to English language publications. Malignant lesions and age of diagnosis were recorded. Authors were contacted to clarify patient identification to minimize duplication of cases.
Surveillance Data
The surveillance protocol used by the consortium was published in 2017 and includes the following: Brain MRI is to be implemented at diagnosis and repeated every 6 months. Whole-body MRI (WBMRI) should begin at age 6 years and is to be performed annually. Abdominal ultrasound and CBC should be performed at diagnosis and repeated every 6 months. Upper and lower endoscopy are recommended annually starting at 4-6 years of age. Further details of the protocol are outlined in the Data Supplement (online only).27 Data were obtained through ongoing follow-up for the full study cohort. In addition, a surveillance questionnaire was sent to each patient's physician (Data Supplement). Medical documents, including pathology, surgical, endoscopy, and autopsy reports, were also reviewed. The end of patient follow-up date for the study was November 1, 2019.
To evaluate the efficacy of early cancer detection by the recommended guidelines, tumors from patients undergoing surveillance from the full study cohort were identified either as asymptomatic or symptomatic at diagnosis and the modalities that detected each tumor were reported. Data were also collected on all benign and low-grade lesions found in the full study cohort.
Prospective Cohort and Outcome Measures
To assess whether surveillance guidelines and early detection affect survival, we evaluated patients who were followed prospectively from 2008 (n = 89), the year surveillance was initiated (Fig 1). Patients in this prospective cohort were categorized as either full, partial, or no surveillance based on the level of surveillance they received. The full surveillance cohort (n = 33) comprised individuals who underwent routine screening using all the modalities recommended. The partial surveillance cohort (n = 20) included individuals who did not consistently undergo all screening modalities or from whom modalities were not performed at recommended intervals. Examples include individuals in whom only colonoscopy and CBC were performed, or for whom brain MRIs were performed at intervals > 6 months. The no surveillance cohort (n = 36) included individuals who did not receive any routine screening. Overall patient survival was determined for these three groups. Patients who were enrolled in the consortium before 2008 (n = 21) and whose data were retrospectively collected were excluded from survival analysis.
To test the role of early asymptomatic tumor detection and considering that patients with CMMRD develop synchronous and metachronous cancers, a secondary separate analysis of patient outcome was measured per tumor. Tumors were divided into two groups, symptomatic or asymptomatic, based on presentation at diagnosis. Tumor-specific survival was subsequently measured by comparing these two groups. To avoid further bias introduced by low-grade tumors, patient- and tumor-specific survival analyses were determined solely using malignant tumors from the prospective cohort. Patients who were enrolled in the consortium before 2008 (n = 21) and whose data were retrospectively collected were excluded from survival analysis. Patient country of origin was classified as low resource or high resource based on the World Bank Global Index LMIC List 2020.
Statistical Analysis
For analysis of asymptomatic versus symptomatic tumor-specific survival, each tumor was treated as an independent entity and the unit of analysis was the tumor rather than the individual. Tumor-specific survival was defined as time to death or last follow-up from the diagnosis of brain, GI, or other tumors. For participants with multiple tumors, cause of death was attributed to one of the tumors and we censored tumors that were not the cause of death at the time of death. For patients, time to OS was defined in years from date of CMMRD diagnosis to the date of death or last follow-up.
Categorical variables such as sex, gene affected, and tumor types were summarized with counts and percentages. Continuous variables such as age at first cancer diagnosis and follow-up duration were summarized with median and range. One-way analysis of variance, Fisher's exact test, and N–1 chi-squared test (for comparison of proportions) were used where appropriate for analysis of the prospective cohort demographics tables.
OS rates were calculated using the Kaplan-Meier product-limit method. Logrank test was used as a univariate analysis to assess potential prognostic factors. Cox proportional hazards model was also used to assess the joint effect of prognostic factors. All tests were two-tailed with a probability of < .05 considered statistically significance. Statistical analyses were performed using version 9.4 of the SAS System for Windows (Copyright 2002-2012 SAS Institute Inc, Cary, NC), and SPSS, Statistics for Windows, Version 22.0., IBM Corp (Armonk, NY).
RESULTS
Overall, 110 patients with confirmed CMMRD and 193 malignant tumors were identified (Fig 1). Two tumors were excluded from the remaining analysis as full information was not available. The full study cohort comprised 110 patients and 191 tumors that were used to determine the efficacy of the proposed surveillance modalities in malignant tumors and for analysis of low-grade lesions. Ongoing prospective data were collected for 89 patients. This established the prospective cohort for the survival analysis. Five patients did not develop cancer during the study with a median follow-up of 1.6 years (range, 0.68-4.98 years) and 84 developed 139 malignant tumors during the study time.
Tumor Spectrum for Patients With CMMRD
The spectrum of malignant tumors identified for IRRDC patients (full study cohort) is presented in Figure 2A. Additional demographics are detailed in the Data Supplement. Overall, the median age of first cancer diagnosis was 9.2 years (range, 1.7-39.5 years). CNS tumors were the most common cancers observed (44%, n = 85; median age at diagnosis 9.9 years, range 2.3-38.5 years) followed by GI (27%, n = 52; median age at diagnosis 15.9 years, range 8.5-49.9 years) and hematologic malignancies (19%, n = 37; median age at diagnosis 10.5 years, range 2.2-29.9 years). We compared the prevalence of cancers in individuals with CMMRD from the literature (Data Supplement) to our cohort and revealed similar ratios. Ten percent of tumors (n = 19; median age at diagnosis 16.1 years, range 1.7-52.3 years) occurred in other organs, such as bone, soft tissue, genitourinary, and embryonal cancers.
FIG 2.
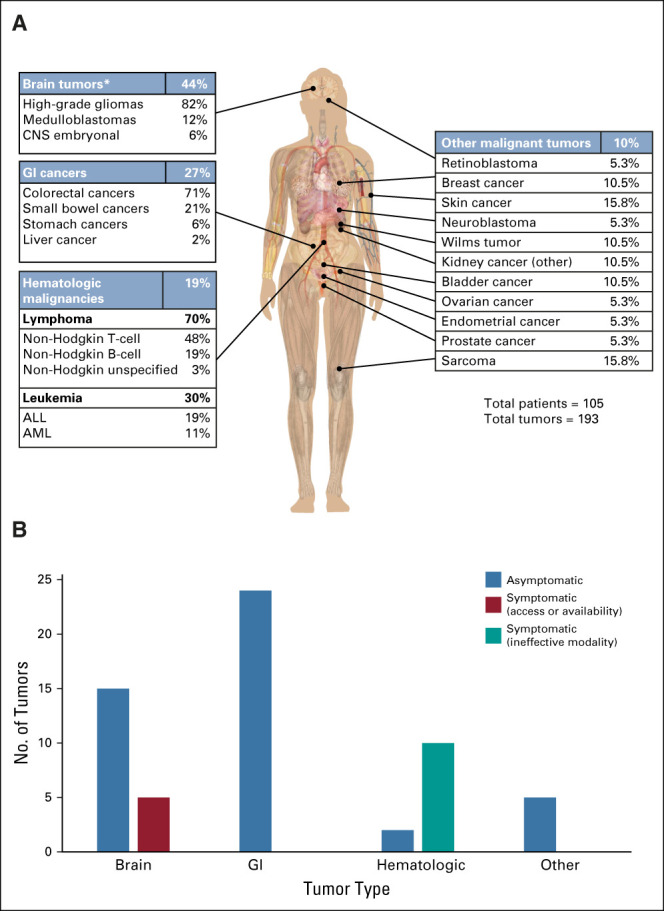
Tumor spectrum and efficacy of the surveillance modalities to detect asymptomatic malignant tumors in the full study cohort. (A) Distribution of malignant tumors from individuals with CMMRD. (B) Tumors detected during surveillance per tumor type. *P = .03 (brain) compared with literature cohort (brain) determined using “N–1” chi-squared test. ALL, acute lymphoblastic leukemia; AML, acute myelomonocytic leukemia; CMMRD, constitutional mismatch repair deficiency; IRRDC, International Replication Repair Deficiency Consortium.
In addition, adult type tumors, such as colorectal, breast, and genitourinary tumors, were observed at young ages (31%, median age 17.9 years, range 8.5-52.3 years). Of the 52 patients who survived their initial cancers, 36 (69%) developed metachronous cancers (> 2 years between cancers). A detailed list of the malignant tumors identified for each IRRDC patient along with basic demographics is listed in the Data Supplement. Together, the high frequency of numerous cancers in multiple organ systems at a young age highlights the importance of implementing a multimodal surveillance protocol early in life.
Efficacy of Surveillance for Asymptomatic Tumor Detection
To test the efficacy of recommended modalities in detecting asymptomatic tumors, we looked specifically at individuals undergoing surveillance within the full study cohort (n = 56). We then analyzed the cancers developed during this period to determine which tumors were detected asymptomatically or symptomatically (Fig 2B). Twenty CNS tumors were identified, including both malignant gliomas and embryonal tumors such as medulloblastoma. Both brain-specific and WBMRI were able to detect 15 (75%) asymptomatic CNS cancers. The five brain tumors that developed symptomatically while on surveillance were in patients who had intervals longer than six months between scans (n = 1, 1.5 years), or interruptions because of access or availability to surveillance modalities (n = 4). All 24 GI tumors were identified asymptomatically by surveillance. Similarly, all five of the other solid tumors detected by surveillance were asymptomatic. By contrast, only two of 12 (16%) hematopoietic malignancies were detected by current surveillance methods, both of which were identified incidentally.
Survival Benefit for Asymptomatic Detection of Malignant Cancers
Having established the tumor spectrum and potential benefit of the surveillance recommendations for patients with CMMRD, we examined the impact of early detection of asymptomatic malignant cancers on survival. Within the prospective cohort, 139 tumors were detected. Of those, 39 tumors (28%) were detected by surveillance tools before symptoms arose. All other tumors (72%, n = 100) were diagnosed after initial symptoms or signs. Survival analysis was performed per tumor. Five-year OS was 90% (95% CI, 78.6 to 100) and 50% (95% CI, 39.2 to 63.7) for asymptomatic versus symptomatic detection, respectively (P = .001, Fig 3A). Having a large cohort in this study allowed for evaluation of the two most common cancer types. For CNS tumors (92% high-grade glioma, 6% medulloblastoma, and 2% CNS embryonal tumors), 5-year OS was 72% (95% CI, 49.5 to 100) for asymptomatic tumors (n = 15) and 33% (95% CI, 20.5 to 55.6) for symptomatic tumors (n = 53; P = .04, Fig 3B). For GI cancers (n = 33, Fig 3C), OS was 100% (95% CI, 100 to 100) for tumors detected asymptomatically (n = 18) during surveillance compared with 81% (95% CI, 71.1 to 100) survival for symptomatic tumors (n = 15; P = .18). Multivariable analysis for all tumors (Data Supplement) and for brain (Data Supplement) and colon (Data Supplement) separately confirmed the independent value of asymptomatic tumor detection in all cancers and brain tumors specifically.
FIG 3.
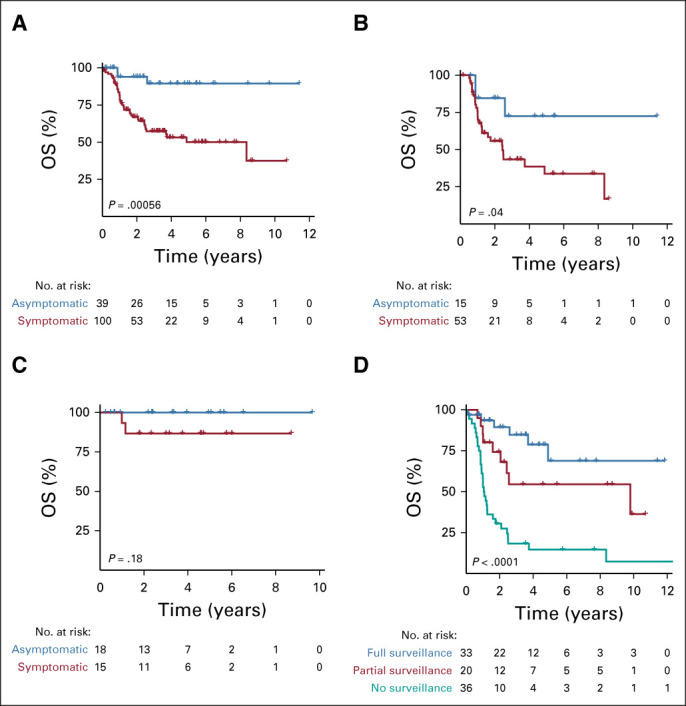
Survival for individuals with constitutional mismatch repair deficiency according to surveillance status. (A) Survival for all tumors stratified by symptomatic and asymptomatic detection. (B) Survival for brain tumors stratified by symptomatic and asymptomatic detection. (C) Survival for GI tumors stratified by symptomatic and asymptomatic detection. (D) Survival for patients stratified by surveillance adherence. P values were calculated by log-rank test. OS, overall survival.
Survival Benefit for Patients With CMMRD Undergoing Surveillance
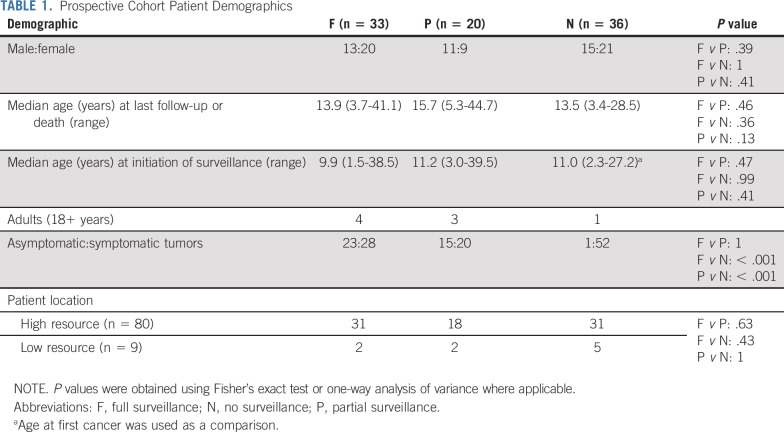
We then tested the ability of the surveillance protocol to improve survival for patients with CMMRD. Patients in the prospective cohort (n = 89) were further divided into three groups: individuals undergoing full surveillance (n = 33), partial surveillance (n = 20), and no surveillance (n = 36; see Methods, Data Supplement). Patient demographics were similar between groups (Table 1). Only eight patients were older than 18 years when the surveillance was initiated.
TABLE 1.
Prospective Cohort Patient Demographics
Four-year OS was 79% (95% CI, 54.8 to 90.9) and 15% (95% CI, 5.2 to 28.8) for patients with CMMRD undergoing full surveillance versus those not undergoing surveillance (P < .0001, Fig 3D). Patients undergoing partial surveillance had favorable outcomes with 4-year OS of 54% (95% CI, 28.5 to 74.5; P = .001).
To examine whether the observed differences in OS was a result of resource differences that affect availability or adherence to the surveillance protocol, we analyzed the distribution of patients from the full, partial, and no surveillance groups by country of origin (Table 1). The distribution of patients who did or did not undergo surveillance did not differ between high- and low-resource settings.
To address the lead time bias, the mortality ratio was calculated for the median follow-up of 25.4 months (Data Supplement). For the full surveillance cohort, mortality was 30% (10 of 33), which was significantly less (P = .001) than the nonsurveillance cohort at 72% (26 of 36). The partial surveillance cohort had a mortality of 40% (8 of 20) compared with the no surveillance cohort (P = .02). There was no significant difference in mortality between the full surveillance and partial surveillance cohorts. Eighty-nine patients from the prospective cohort were from 72 families. The penetrance of this syndrome and familial clustering did not affect presentation, tumor type, and outcome of patients in this study.
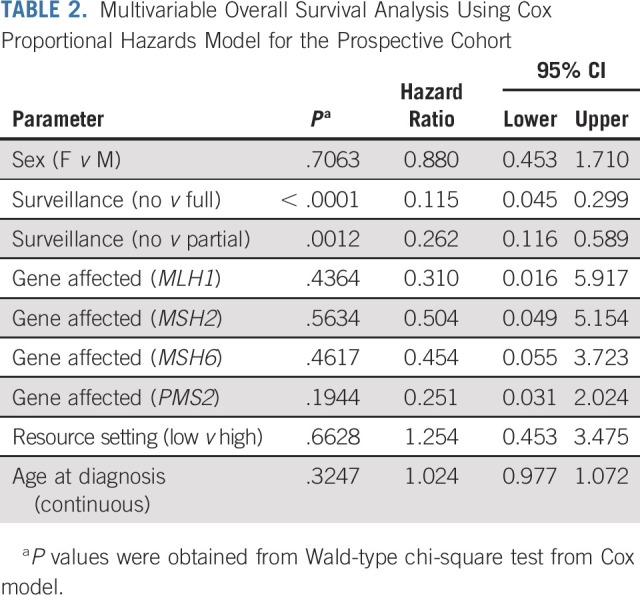
In multivariable analysis, including age, sex, gene affected, and resources available, surveillance was the single variable associated with improved OS in patients with CMMRD (P < .0001; Table 2).
TABLE 2.
Multivariable Overall Survival Analysis Using Cox Proportional Hazards Model for the Prospective Cohort
Low-Grade CMMRD Tumors Transform to High-Grade Cancers
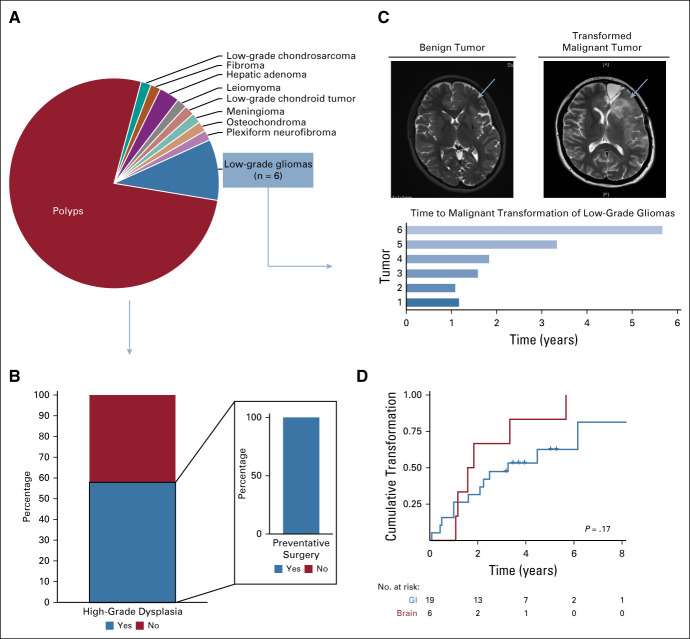
A total of 64 benign and low-grade lesions were detected in 54 patients (Fig 4A, Data Supplement). Polyposis and low-grade gliomas (LGG) were the most common tumors. To assess the risk of malignant transformation, we analyzed data for patients undergoing GI surveillance for at least 3 years (n = 19). Of these, 58% (n = 11) developed dysplastic polyps, all of which resulted in prophylactic colectomy (Fig 4B) by age 13.2 years (range, 9.2-25 years). Of the six nonresected LGG identified by surveillance, all revealed universal transformation to high-grade glioma at a median of 1.7 years (range, 1-5.7 years; Figs 4C and 4D). Cumulative likelihood of transformation over time revealed an 81% probability of colorectal polyps undergoing high-grade dysplastic changes within 8 years (Fig 4D). Similarly, for LGG, the likelihood of transformation into high-grade glioma was 100% in 6 years (Fig 4D).
FIG 4.
Low-grade tumors detected in patients with constitutional mismatch repair deficiency undergoing surveillance. (A) Distribution of low-grade tumors in the full study cohort (n = 64). (B) Percentage of polyps with high-grade dysplasia and the frequency of preventative surgery. (C) Magnetic resonance imaging scans showing tumor transformation and time to transformation of six nonresected low-grade gliomas. (D) Cumulative likelihood of high-grade transformation over time for brain (red) and GI (blue) tumors.
DISCUSSION
This is the first prospective report on the impact of surveillance for individuals with CMMRD and demonstrates that a multimodal approach is associated with a survival benefit when these individuals adhere to the recommended surveillance guidelines.27 The large number of tumors that can develop in affected individuals during a short period enabled us to examine several important concepts associated with current surveillance recommendations.
With increased awareness and diagnosis of CMMRD, the tumor-risk spectrum of this syndrome is expanding (Fig 2A). This study confirms that CNS, GI, and hematologic cancers are the most common tumor types, but also highlights that a significant number of tumors in other tissues, including embryonal tumors such as Wilms tumor and neuroblastoma, are not uncommon during early childhood. In addition, malignancies such as breast cancer, sarcomas, and genitourinary tumors are observed particularly in patients beyond the pediatric years. Overall, the tumor spectrum for CMMRD, including 576 CMMRD-associated cancers (literature and consortium cohorts), supports the recommended screening modalities.
The benefit from early detection of asymptomatic cancers is thought to be related to complete surgical resection and possibly less aggressive adjuvant therapy. Although early detection of tumors has not shown to improve survival in other cancer syndromes and sporadic cancer types,29-31 our study reveals that early asymptomatic tumor detection is associated with a significant OS benefit, including in malignant gliomas where the role of gross total resection is still debated (Figs 3A-3C).
Another important observation in this study is that some patients with CMMRD have developed multiple metachronous cancers and as a result of prior therapies, exceeded the maximum tolerated lifetime dose limits for radiation and certain chemotherapy agents. Surveillance enables these patients to be managed with surgery and follow-up alone.
Lead time bias must be considered, as it may skew the observed survival benefit for the surveillance cohorts. To address this, we reported mortality ratios for the prospective cohort at the median follow-up (Data Supplement) and further demonstrated a significant increase in survival for the full surveillance cohort compared with the nonsurveillance cohort (P = .001). In addition, lead time bias is typically related to cancers where there is a long time between diagnosis and death. As the majority of CMMRD-associated cancers are rapidly lethal, this bias is less likely in this study.
The proposed modalities and screening frequency identified a large majority of asymptomatic brain tumors (75%), and all of the GI and other solid tumors in patients with CMMRD undergoing surveillance (Fig 2B). Perhaps, the most disappointing part of this study is the inability of current methods to reliably detect asymptomatic hematologic malignancies. Similar observations have been described in other leukemia-related cancer syndromes.32 Several options may be more successful including metabolic tests such as lactate dehydrogenase or the use of molecular assays such T-cell rearrangement in circulating tumor DNA. Strategies are needed as 43% of the deaths in patients undergoing surveillance were because of hematologic malignancies, whereas these cancers accounted for only 16% of the deaths in the nonsurveillance cohort (while brain tumors predominate 75%).
Low-grade malignancies are invariably detected in all surveillance protocols.17 Controversy exists regarding whether the transformation risk of these lesions is high enough to offer survival benefit.17,18,29 We observed a strikingly high rate of transformation into malignant and premalignant lesions for both LGG and colorectal polyps (Fig 4). This may be because of the extremely high rate of mutation accumulation especially with secondary mutations in POLE and TP53.3,33,34 Complete surgical resection of low-grade tumors, and total colectomy for patients with multiple high-grade dysplastic polyps may be lifesaving by preventing the development to higher-grade tumors.
Although aggressive, the benefit of the currently recommended surveillance protocol on OS of patients with CMMRD is striking (Fig 3D). This is justified as many patients develop multiple cancers in a synchronous or metachronous fashion resulting in extremely low long-term survival. The increased survival benefit associated with partial surveillance for patients with CMMRD is an intriguing finding. This may have management implications for patients with CMMRD worldwide where not all of the recommended modalities are readily available. Relatively simple but consistent follow-up with fewer modalities may still improve outcome for this devastating syndrome.35
Although surveillance recommendations were available to all patients with CMMRD since 2008, many centers decided not to burden their patients with such a cumbersome protocol because of the lack of robust evidence supporting the benefit of CMMRD surveillance recommendations. Other reasons for nonadherence to full and consistent surveillance may include poor access to care, lack of education, awareness, social and cultural norms, and physician opinions regarding the role of these protocols in cancer predisposition. However, a lack of awareness and resources on a systemic level does not appear to have influenced the results of this study (Table 1). As observed in other cancer syndromes,17,18 we hope this study provides strong evidence leading to a wider use and adherence to the current protocol.
There are several limitations to this study that should be discussed. First, as most patients with CMMRD do not reach adulthood, the spectrum of cancers that may present in adults is limited. As the cancer risks become more defined, modification of the protocol should be considered. Second, in our cohort, only one malignant tumor was identified with WBMRI. Nevertheless, the tumor spectrum observed in patients with CMMRD (Fig 2A) clearly demonstrates a need for extended modalities outside of CNS, GI, and hematologic screening. In Li-Fraumeni17,18 and hereditary pheochromocytoma or paraganglioma sydndromes,36 WBMRI has been studied prospectively for efficacy and has been shown to play an important role in the detection of both benign and malignant lesions.37 Ultimately, a longer follow-up time and a larger cohort of long-term survivors would be required to determine efficacy of WBMRI for CMMRD. In addition, it is important to recognize that the level of surveillance adherence was the decision of the patient and clinician and may be based on various factors. Although we considered systemic differences (ie, resource setting) and found no correlation, future studies should investigate other potential factors that may be involved. Finally, it will be important to assess the psychosocial impact of living with this condition and following this aggressive surveillance protocol.
In summary, this study provides justification for the current protocol and demonstrates a significant survival benefit associated with undergoing surveillance in patients with CMMRD. Further work is required to enable better detection of some malignancies.
Hagit N. Baris
Consulting or Advisory Role: Sanofi, Igentify Ltd
Speakers' Bureau: Sanofi, Takeda, Pfizer
Donald Basel
Honoraria: BioMarin
Consulting or Advisory Role: iQvia
Deborah T. Blumenthal
Consulting or Advisory Role: AstraZeneca, Novocure, Takeda
Miriam Bornhorst
Consulting or Advisory Role: AstraZeneca/MedImmune
Sara Rhode
Honoraria: Myriad Genetics
Speakers' Bureau: Myriad Genetics
Travel, Accommodations, Expenses: Myriad Genetics
Roula Farah
Honoraria: Novo Nordisk
Consulting or Advisory Role: Novo Nordisk
William D. Foulkes
Research Funding: AstraZeneca
David Gass
Employment: X4 Pharmaceuticals
Honoraria: X4 Pharmaceuticals
Speakers' Bureau: Precisionscientia
Heather Hampel
Stock and Other Ownership Interests: Genome Medical
Consulting or Advisory Role: InVitae, Genome Medical, Promega, 23andMe
Jordan R. Hansford
Consulting or Advisory Role: Bayer
Craig Harlos
Travel, Accommodations, Expenses: GlaxoSmithKline
Nobuko Hijiya
Honoraria: Novartis
Consulting or Advisory Role: Novartis, Incyte
Research Funding: Pfizer
Junne Kamihara
Stock and Other Ownership Interests: PanTher Therapeutics, ROME Therapeutics, TellBio
Honoraria: Pfizer, NanoString Technologies, Third Rock Ventures, Foundation Medicine
Consulting or Advisory Role: ROME Therapeutics, Third Rock Ventures
Research Funding: PureTech, Ribon Therapeutics, ACD Biotechne
Patents, Royalties, Other Intellectual Property: Patent on drug delivery device licensed to PanTher Therapeutics, Patents on Repeat RNA biomarkers and therapeutics licensed to Rome Therapeutics, Patents on Circulating Tumor Cell Biomarkers Licensed to TellBio Inc
Jeffrey Knipstein
Employment: PRA Health Sciences
Consulting or Advisory Role: Atheneum
Alvaro Lassaletta
Stock and Other Ownership Interests: Gilead Sciences
Consulting or Advisory Role: Shire, Jazz Pharmaceuticals, Roche, Servier
Travel, Accommodations, Expenses: Shire, Gilead Sciences
Simon C. Ling
Honoraria: Abbvie
Research Funding: Abbvie, Gilead Sciences
Michael P. Link
Consulting or Advisory Role: Incyte, ADC Therapeutics, Lilly, Steba Biotech, Mesoblast, GlaxoSmithKline, Syndax
Research Funding: Seattle Genetics, Janssen Oncology
Rebecca Loret de Mola
Employment: Huron Consulting
Maura Massimino
Consulting or Advisory Role: Oncoscience, Novartis
Gary Mason
Employment: Janssen Research & Development, Merck
Stock and Other Ownership Interests: Johnson & Johnson, Merck
Travel, Accommodations, Expenses: Janssen Research & Development
Kim E. Nichols
Stock and Other Ownership Interests: Incyte
Research Funding: Incyte/Novartis, Alpine Immune Sciences
Enrico Opocher
Consulting or Advisory Role: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Michael Osborn
Travel, Accommodations, Expenses: Amgen, Pfizer
Benjamin Oshrine
Honoraria: Mesoblast
Alyssa Reddy
Consulting or Advisory Role: Novartis, AstraZeneca
Lara Reichman
Research Funding: Illumina
Marc Remke
Stock and Other Ownership Interests: Bayer, BB Biotech Ventures, BioNTech AG, InVitae, IDEXX Laboratories
Kami Wolfe Schneider
Other Relationship: Journal of Genetic Counseling
Duncan Stearns
Consulting or Advisory Role: AstraZeneca
Open Payments Link: https://openpaymentsdata.cms.gov/physician/792397
Patrick Tomboc
Honoraria: Unicare Health Plan
Consulting or Advisory Role: UniCare Health Plan
An Van Damme
Consulting or Advisory Role: Octapharm, Pfizer, Bayer
Research Funding: Johnson & Johnson
Travel, Accommodations, Expenses: Pfizer, Sobi, Shire, Roche
Ira Winer
Research Funding: Oncoceutics
David S. Ziegler
Consulting or Advisory Role: Bayer, Amgen
Travel, Accommodations, Expenses: Bayer
Cynthia Hawkins
Consulting or Advisory Role: Bayer
Patents, Royalties, Other Intellectual Property: IP for low-grade glioma and sarcoma fusion panels as well as medulloblastoma subgrouping panel
David Malkin
Consulting or Advisory Role: Bayer
Eric Bouffet
Consulting or Advisory Role: Novartis
Research Funding: Roche, Bristol Myers Squibb
No other potential conflicts of interest were reported.
SUPPORT
Supported by a Stand Up to Cancer—Bristol Meyers Squibb Catalyst Research Grant (Grant Number: SU2C-AACR-CT07-17), which is administered by the American Association for Cancer Research, the scientific partner of SU2C. This work was also supported by the Canada-Israel Health Research initiative, jointly funded by the Canadian Institutes of Health Research, the Israel Science Foundation, the International Development Research Centre, Canada, and the Azrieli Foundation. Additional financial support was provided by the Canadian Institutes of Health Research (CIHR), Meagan's Walk (MW-2014-10), LivWise, The Zane Cohen Center, and BRAINchild.
C.D., A.B.E., and V.B. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Carol Durno, Ayse Bahar Ercan, Vanessa Bianchi, Melissa Edwards, Melyssa Aronson, Shlomi Constantini, Heather Hampel, Scott Lindhorst, Magnus Sabel, Kami Wolfe Schneider, David Malkin, Eric Bouffet, Anita Villani, Uri Tabori
Administrative support: Jan Rapp
Provision of study materials or patients: Gadi Abebe-Campino, Musa Alharbi, Vahid Fallah Azad, Hagit N. Baris, Donald Basel, Raymond Bedgood, Anne Bendel, Deborah T. Blumenthal, Maude Blundell, Elizabeth Cairney, Shlomi Constantini, Anirban Das, Roula Farah, William D. Foulkes, Zehavit Frenkel, David Gass, Mithra Ghalibafian, Yael Goldberg, Syed Ahmer Hamid, Jordan R. Hansford, Nobuko Hijiya, Junne Kamihara, Rejin Kebudi, Jeffrey Knipstein, Alvaro Lassaletta, Michael P. Link, Rebecca Loret De Mola, Rebecca Luiten, Michal Lurye, Vanan MagimairajanIssai, Ossama M. Maher, Maura Massimino, Rose B. McGee, Naureen Mushtaq, Garth Nicholas, Michael Osborn, Rachel Pearlman, Marc Remke, Gabriel Robbins, Magnus Sabel, David Samuel, Santanu Sen, Duncan Stearns, Carol Swallow, Helen Toledano, Michal Yalon, Lee Yi Yen, Michal Zapotocky, Shayna Zelcer, David S. Ziegler, Stefanie Zimmermann
Collection and assembly of data: Ayse Bahar Ercan, Vanessa Bianchi, Melissa Edwards, Melyssa Aronson, Melissa Galati, Gadi Abebe-Campino, Abeer Al-Battashi, Musa Alharbi, Vahid Fallah Azad, Hagit N. Baris, Donald Basel, Raymond Bedgood, Anne Bendel, Shay Ben-Shachar, Deborah T. Blumenthal, Maude Blundell, Miriam Bornhorst, Annika Bronsema, Elizabeth Cairney, Sara Carroll, Aghiad Chamdin, Stefano Chiaravalli, Shlomi Constantini, Anirban Das, Rina Dvir, Roula Farah, William D. Foulkes, Zehavit Frenkel, Sharon Gardner, David Gass, Mithra Ghalibafian, Catherine Gilpin, Yael Goldberg, Syed Ahmer Hamid, Heather Hampel, Jordan R. Hansford, Craig Harlos, Nobuko Hijiya, Saunders Hsu, Junne Kamihara, Jeffrey Knipstein, Carl Koschmann, Christian Kratz, Valerie Larouche, Alvaro Lassaletta, Simon C. Ling, Michael P. Link, Rebecca Loret De Mola, Rebecca Luiten, Michal Lurye, Jamie L. Maciaszek, Vanan MagimairajanIssai, Ossama M. Maher, Maura Massimino, Rose B. McGee, Naureen Mushtaq, Gary Mason, Monica Newmark, Garth Nicholas, Kim E. Nichols, Theodore Nicolaides, Enrico Opocher, Michael Osborn, Benjamin Oshrine, Rachel Pearlman, Daniel Pettee, Jan Rapp, Mohsin Rashid, Alyssa Reddy, Lara Reichman, Marc Remke, Gabriel Robbins, Sumita Roy, Magnus Sabel, David Samuel, Isabelle Scheers, Santanu Sen, Duncan Stearns, David Sumerauer, Carol Swallow, Leslie Taylor, Gregory Thomas, Helen Toledano, Patrick Tomboc, An Van Damme, Ira Winer, Michal Yalon, Lee Yi Yen, Michal Zapotocky, Shayna Zelcer, David S. Ziegler, Stefanie Zimmermann, Cynthia Hawkins, Eric Bouffet, Uri Tabori
Data analysis and interpretation: Ayse Bahar Ercan, Vanessa Bianchi, Melissa Edwards, Melissa Galati, Eshetu G. Atenafu, Anne Bendel, Aghiad Chamdin, Bruce Crooks, Roula Farah, Bailey Gallinger, Catherine Goudie, Jordan R. Hansford, Rejin Kebudi, Vanan MagimairajanIssai, Gary Mason, Marc Remke, Magnus Sabel, Ira Winer, Cynthia Hawkins, David Malkin, Eric Bouffet, Anita Villani, Uri Tabori
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Survival Benefit for Individuals With CMMRD Undergoing Surveillance
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Hagit N. Baris
Consulting or Advisory Role: Sanofi, Igentify Ltd
Speakers' Bureau: Sanofi, Takeda, Pfizer
Donald Basel
Honoraria: BioMarin
Consulting or Advisory Role: iQvia
Deborah T. Blumenthal
Consulting or Advisory Role: AstraZeneca, Novocure, Takeda
Miriam Bornhorst
Consulting or Advisory Role: AstraZeneca/MedImmune
Sara Rhode
Honoraria: Myriad Genetics
Speakers' Bureau: Myriad Genetics
Travel, Accommodations, Expenses: Myriad Genetics
Roula Farah
Honoraria: Novo Nordisk
Consulting or Advisory Role: Novo Nordisk
William D. Foulkes
Research Funding: AstraZeneca
David Gass
Employment: X4 Pharmaceuticals
Honoraria: X4 Pharmaceuticals
Speakers' Bureau: Precisionscientia
Heather Hampel
Stock and Other Ownership Interests: Genome Medical
Consulting or Advisory Role: InVitae, Genome Medical, Promega, 23andMe
Jordan R. Hansford
Consulting or Advisory Role: Bayer
Craig Harlos
Travel, Accommodations, Expenses: GlaxoSmithKline
Nobuko Hijiya
Honoraria: Novartis
Consulting or Advisory Role: Novartis, Incyte
Research Funding: Pfizer
Junne Kamihara
Stock and Other Ownership Interests: PanTher Therapeutics, ROME Therapeutics, TellBio
Honoraria: Pfizer, NanoString Technologies, Third Rock Ventures, Foundation Medicine
Consulting or Advisory Role: ROME Therapeutics, Third Rock Ventures
Research Funding: PureTech, Ribon Therapeutics, ACD Biotechne
Patents, Royalties, Other Intellectual Property: Patent on drug delivery device licensed to PanTher Therapeutics, Patents on Repeat RNA biomarkers and therapeutics licensed to Rome Therapeutics, Patents on Circulating Tumor Cell Biomarkers Licensed to TellBio Inc
Jeffrey Knipstein
Employment: PRA Health Sciences
Consulting or Advisory Role: Atheneum
Alvaro Lassaletta
Stock and Other Ownership Interests: Gilead Sciences
Consulting or Advisory Role: Shire, Jazz Pharmaceuticals, Roche, Servier
Travel, Accommodations, Expenses: Shire, Gilead Sciences
Simon C. Ling
Honoraria: Abbvie
Research Funding: Abbvie, Gilead Sciences
Michael P. Link
Consulting or Advisory Role: Incyte, ADC Therapeutics, Lilly, Steba Biotech, Mesoblast, GlaxoSmithKline, Syndax
Research Funding: Seattle Genetics, Janssen Oncology
Rebecca Loret de Mola
Employment: Huron Consulting
Maura Massimino
Consulting or Advisory Role: Oncoscience, Novartis
Gary Mason
Employment: Janssen Research & Development, Merck
Stock and Other Ownership Interests: Johnson & Johnson, Merck
Travel, Accommodations, Expenses: Janssen Research & Development
Kim E. Nichols
Stock and Other Ownership Interests: Incyte
Research Funding: Incyte/Novartis, Alpine Immune Sciences
Enrico Opocher
Consulting or Advisory Role: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Michael Osborn
Travel, Accommodations, Expenses: Amgen, Pfizer
Benjamin Oshrine
Honoraria: Mesoblast
Alyssa Reddy
Consulting or Advisory Role: Novartis, AstraZeneca
Lara Reichman
Research Funding: Illumina
Marc Remke
Stock and Other Ownership Interests: Bayer, BB Biotech Ventures, BioNTech AG, InVitae, IDEXX Laboratories
Kami Wolfe Schneider
Other Relationship: Journal of Genetic Counseling
Duncan Stearns
Consulting or Advisory Role: AstraZeneca
Open Payments Link: https://openpaymentsdata.cms.gov/physician/792397
Patrick Tomboc
Honoraria: Unicare Health Plan
Consulting or Advisory Role: UniCare Health Plan
An Van Damme
Consulting or Advisory Role: Octapharm, Pfizer, Bayer
Research Funding: Johnson & Johnson
Travel, Accommodations, Expenses: Pfizer, Sobi, Shire, Roche
Ira Winer
Research Funding: Oncoceutics
David S. Ziegler
Consulting or Advisory Role: Bayer, Amgen
Travel, Accommodations, Expenses: Bayer
Cynthia Hawkins
Consulting or Advisory Role: Bayer
Patents, Royalties, Other Intellectual Property: IP for low-grade glioma and sarcoma fusion panels as well as medulloblastoma subgrouping panel
David Malkin
Consulting or Advisory Role: Bayer
Eric Bouffet
Consulting or Advisory Role: Novartis
Research Funding: Roche, Bristol Myers Squibb
No other potential conflicts of interest were reported.
REFERENCES
- 1.McKusick VA, Knifflin CL: Mismatch Repair Cancer Syndrome. Baltimore, MD, John Hopkins University Press, 2015 [Google Scholar]
- 2.Preston BD, Albertson TM, Herr AJ: DNA replication fidelity and cancer. Semin Cancer Biol 20:281-293, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shlien A Campbell BB de Borja R, et al. : Combined hereditary and somatic mutations of replication error repair genes result in rapid onset of ultra-hypermutated cancers. Nat Genet 47:257-262, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Loeb LA: Human cancers express mutator phenotypes: Origin, consequences and targeting. Nat Rev Cancer 11:450-457, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Touat M Li YY Boynton AN, et al. : Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 580:517-523, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiricny J: The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 7:335-346, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Karran P, Attard N: Thiopurines in current medical practice: Molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer 8:24-36, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Attarbaschi A Carraro E Abla O, et al. : Non-Hodgkin lymphoma and pre-existing conditions: Spectrum, clinical characteristics and outcome in 213 children and adolescents. Haematologica 101:1581, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fedier A, Fink D: Mutations in DNA mismatch repair genes: Implications for DNA damage signaling and drug sensitivity (review). Int J Oncol 24:1039-1047, 2004 [PubMed] [Google Scholar]
- 10.Cahill DP Levine KK Betensky RA, et al. : Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res 13:2038, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFaline-Figueroa JL Braun CJ Stanciu M, et al. : Minor changes in expression of the mismatch repair protein MSH2 exert a major impact on glioblastoma response to temozolomide. Cancer Res 75:3127, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter C Smith R Cahill DP, et al. : A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res 66:3987, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakry D Aronson M Durno C, et al. : Genetic and clinical determinants of constitutional mismatch repair deficiency syndrome: Report from the Constitutional Mismatch Repair Deficiency Consortium. Eur J Cancer 50:987-996, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Wimmer K, Kratz CP: Constitutional mismatch repair-deficiency syndrome. Haematologica 95:699, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavoine N Colas C Muleris M, et al. : Constitutional mismatch repair deficiency syndrome: Clinical description in a French cohort. J Med Genet 52:770-778, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Durno CA Sherman PM Aronson M, et al. : Phenotypic and genotypic characterisation of biallelic mismatch repair deficiency (BMMR-D) syndrome. Eur J Cancer 51:977-983, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Villani A Shore A Wasserman JD, et al. : Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol 17:1295-1305, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Villani A Tabori U Schiffman J, et al. : Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: A prospective observational study. Lancet Oncol 12:559-567, 2011 [DOI] [PubMed] [Google Scholar]
- 19.de Jong AE Hendriks YMC Kleibeuker JH, et al. : Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 130:665-671, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Saslow D Boetes C Burke W, et al. : American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 57:75-89, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Dobrow MJ Hagens V Chafe R, et al. : Consolidated principles for screening based on a systematic review and consensus process. CMAJ 190:E422-E429, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durno CA Aronson M Tabori U, et al. : Oncologic surveillance for subjects with biallelic mismatch repair gene mutations: 10 year follow-up of a kindred. Pediatr Blood Cancer 59:652-656, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Sjursen W Bjornevoll I Engebretsen LF, et al. : A homozygote splice site PMS2 mutation as cause of Turcot syndrome gives rise to two different abnormal transcripts. Fam Cancer 8:179-186, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Aronson M Gallinger S Cohen Z, et al. : Gastrointestinal findings in the largest series of patients with hereditary biallelic mismatch repair deficiency syndrome: Report from the International Consortium. Am J Gastroenterol 111:275-284, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Vasen HFA Ghorbanoghli Z Bourdeaut F, et al. : Guidelines for surveillance of individuals with constitutional mismatch repair-deficiency proposed by the European Consortium “Care for CMMR-D” (C4CMMR-D). J Med Genet 51:283, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Durno C Boland CR Cohen S, et al. : Recommendations on surveillance and management of biallelic mismatch repair deficiency (BMMRD) syndrome: A consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 152:1605-1614, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Tabori U Hansford JR Achatz MI, et al. : Clinical management and tumor surveillance recommendations of inherited mismatch repair deficiency in childhood. Clin Cancer Res 23:e32, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Wimmer K Kratz CP Vasen HF, et al. : Diagnostic criteria for constitutional mismatch repair deficiency syndrome: Suggestions of the European consortium “care for CMMRD” (C4CMMRD). J Med Genet 51:355-365, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Evans DGR Salvador H Chang VY, et al. : Cancer and central nervous system tumor surveillance in pediatric neurofibromatosis 1. Clin Cancer Res 23:e46, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Martin RM Donovan JL Turner EL, et al. : Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: The CAP Randomized Clinical Trial. JAMA 319:883-895, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schilling FH Spix C Berthold F, et al. : Neuroblastoma screening at one year of age. N Engl J Med 346:1047-1053, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Porter CC Druley TE Erez A, et al. : Recommendations for surveillance for children with leukemia-predisposing conditions. Clin Cancer Res 23:e14, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Campbell BB Light N Fabrizio D, et al. : Comprehensive analysis of hypermutation in human cancer. Cell 171:1042-1056.e10, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouffet E Larouche V Campbell BB, et al. : Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol 34:2206-2211, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Kebudi R Amayiri N Abedalthagafi M, et al. : Position paper: Challenges and specific strategies for constitutional mismatch repair deficiency syndrome in low-resource settings. Pediatr Blood Cancer 67:e28309, 2020 [DOI] [PubMed] [Google Scholar]
- 36.Jasperson KW Kohlmann W Gammon A, et al. : Role of rapid sequence whole-body MRI screening in SDH-associated hereditary paraganglioma families. Fam Cancer 13:257-265, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Anupindi SA Bedoya MA Lindell RB, et al. : Diagnostic performance of whole-body MRI as a tool for cancer screening in children with genetic cancer-predisposing conditions. Am J Roentgenol 205:400-408, 2015 [DOI] [PubMed] [Google Scholar]