Abstract
Introduction:
Zygoma defects are a challenging clinical problem and are frequently connected with the alteration of facial harmony, horizontal asymmetry of the face, and significant functional deficit. The application of patient-specific implants (PSIs) has the potential to improve the effectiveness of zygoma defect management. The aim of this study was to evaluate the anatomic, esthetic, and functional outcomes of PSI application for zygoma reconstruction.
Materials and Methods:
A retrospective study was conducted on data from 11 patients with zygoma defects who underwent a reconstruction procedure in which a PSI was applied and was followed for >1 year after surgery with the evaluation of esthetic and functional outcomes. Precision of PSI position and anatomy reconstruction was estimated by superimposition of the models with automatic point-to-point measurement and determination of the existing deviations between models.
Results:
The mean follow-up period in our study was 21.6 ± 6.2 months (range 14–39 months). No major complications occurred in the postoperative period: There were no clinical or computed tomography symptoms of maxillary sinusitis, implant-related infection, or implant exposure. The mean deviation between the planned and real positions of PSIs in our series was 0.72 ± 0.41 mm. The mean deviation between the reconstructed zygomatic complex and the mirrored intact side in our series was 1.45 ± 0.7 mm. The mean volume difference between the intact and damaged orbits was 1.7 ± 0.8 mm3.
Discussion:
The results of the present study support the wider clinical application of PSIs in orbital and zygoma reconstructions, as it is an effective option to achieve precise reconstruction of the complex zygoma anatomy.
Keywords: Computer-aided design/computer-aided manufacturing technologies, orbital reconstruction, patient-specific implants, zygoma reconstruction
INTRODUCTION
The reconstruction of zygoma defects is a challenging clinical problem due to the unique geometric shape and structural complexity of zygomas. Zygoma defects occur after tumour ablation surgery, trauma management, or congenital anomalies, and frequently lead to alteration of facial harmony, horizontal asymmetry of the face, and significant functional deficit.[1,2,3,4] Different approaches to zygoma reconstruction have been described in the literature.[5,6] They include nonvascularized autologous bone grafting, free tissue transfer on microvascular anastomosis, preformed titanium plates and meshes, custom-made patient-specific implants (PSIs), or a combination of the above methods.[1,7,8,9] In recent decades, the autologous bone grafting procedures have been considered the gold standard for reconstruction of midface bone defects.[4,10,11] Several donor sites are available for bone graft harvesting for zygoma reconstructions, such as iliac crest, fibula, and scapula.[3,9,11] However, this approach has some significant disadvantages, including the inability to restore the true-to-original anatomy of the zygomatic complex, unpredictable volume loss due to graft resorption during the postoperative period, possible delayed bone healing with an incomplete integration of the bone graft, and morbidity of the donor site.[12,13,14,15,16] Thus, the assessment and justification of the alternative approaches to zygoma defect management is a pertinent subject of scientific research.
In recent years, the development of computer-aided design/computer-aided manufacturing (CAD/CAM) technology has raised new possibilities in the reconstruction of maxillofacial defects.[2,17,18,19] The application of custom-made PSIs made from titanium, polyethylene, polyether ether ketone, and other bioinert materials became an alternative tool for facial reconstruction.[20,21] The effectiveness of PSIs in the reconstruction of mandible, skull, and orbital defects is well documented in the literature. However, there are only a few publications concerning zygoma reconstructions with PSIs, most of which present single clinical cases without long-term follow-up.[12,13,20]
The aim of this retrospective study was to present our experience in the application of PSIs for primary and secondary zygoma reconstruction; to describe its advantages and the indications; and to estimate the anatomic, esthetic, and functional outcomes during the early postoperative period and long term.
MATERIALS AND METHODS
Between 2016 and 2019, 13 patients with zygoma defects underwent primary or secondary reconstruction with a PSI. Patients’ medical records and computed tomography (CT) data were collected for further analysis. Written informed consent for treatment and participation in the study was obtained from all of the patients. All procedures and analyses performed were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards. The research was approved by the Bioethics Committee of Bogomolets National Medical University, Kyiv, Ukraine (Protocol № 126).
The inclusion criteria for the study were as follows: Presence of total or subtotal zygoma defect with an area of >6 cm2 involving the zygomatic body, arch, or bony orbit; complete clinical and imaging data; a follow-up period of >12 months; reconstruction of the affected bony structures with a PSI; and written informed consent of the patient.
The exclusion criteria were: Age under 16 years, active malignancy after the reconstructive surgery, defects of the cranial bones associated with zygoma defects, active radiation therapy or chemotherapy, mental illness, noncompliance with medical recommendations and lack of interaction with a doctor during the postoperative period, incomplete medical or imaging records, and refusal to participate in the study. Of the 13 cases analyzed, 11 patients (8 males and 3 females, aged from 17 to 54 years with a mean age of 37.6 ± 10.5 years) met the inclusion criteria and lacked exclusion criteria and were thus included in the study.
Patients were examined preoperatively; during the 1st week and 1 month, 6 months, and 1 year after the surgery; and then once per year using the standard protocol, which includes local status examination and evaluation of vision (visual acuity, diplopia, and ocular motility). CT was performed 1 week and 1 year after surgery. The occurrence of any early and long-term postoperative complications was thoroughly documented. Esthetic results were estimated by two independent observers (one male and one female, neither of whom had any information about the details of the surgery) according to the following scale: (1) unsatisfactory results: The residual cosmetic defect was significant, obvious to both observers, and required a secondary surgical correction; (2) satisfactory: Surgical elimination of the residual cosmetic defect was not necessary or only a small correction in the periorbital soft tissues was required; and (3) good: Both of the observers and the patient were satisfied with the result obtained. Ocular motility and diplopia were assessed using the “follow my finger” test [Table 1].
Table 1.
Patient demographic and clinico-pathological features
| Patient | Age (years) | Sex | Diagnosis | Type reconstruction | Approach | Cutting guide | Type of defect | Symmetry of Eyeball | Aesthetic outcomes | Complication |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | Male | Angiofibroma | Secondary | Direct | Computer generated physical cutting guide | Total | Eyeball position did not changed | Good | No |
| 2 | 53 | Male | Posttraumatic (gun-shot) deformity | Secondary | Direct | No | Subtotal | Did not changed | Satisfactory | No |
| 3 | 48 | Male | Posttraumatic (nongun-shot) deformity | Secondary | Coronal subciliar intra oral | No | Subtotal | Symmetrical | Satisfactory | Aperanse of fistula: second procedure - secvestrectomy |
| 4 | 38 | Male | Fibrous dysplasia | Primery | Subciliar intraoral | No | Partial | Symmetrical | Good | No |
| 5 | 41 | Female | Fibrous dysplasia | Primery | Subciliar | Computer generated physical cutting guide | Partial | Symmetrical | Good | No |
| 6 | 33 | Male | Traumatic (gun-shot) deformity | Secondary | Coronal subciliar intraoral | No | Subtotal | Symmetrical | Good | No |
| 7 | 32 | Male | Fibrous dysplasia | Primery | Coronal | Computer generated physical cutting guide | Partial | Did not changed | Good | No |
| 8 | 24 | Male | Fibrous dysplasia | Primery | Subciliar | No | Partial | Symmetrical | Good | No |
| 9 | 25 | Female | Congenital anomaly | Secondary | Coronal | No | Subtotal | Did not changed | Good | No |
| 10 | 27 | Male | Traumatic (nongun-shot) deformity | Secondary | Coronal, subciliar intraoral | No | Partial | Symmetrical | Good | No |
| 11 | 40 | Female | Traumatic (nongun-shot) deformity | Secondary | Coronal | No | Total | Symmetrical | Good | No |
Design and manufacturing of the implants
Virtual simulation of the surgical procedures and design of the PSIs were carried out in a close collaboration between surgeons and biomedical engineers. Preoperative CT data (DICOM files without compression) were provided to the manufacturer of the implants. Segmentation of the CT data and creation of the virtual models of the facial bones were performed in the SimPlant13.02 software environment (Materialise Dental, Leuven, Belgium, and D2P 1.0.2.53 Simbionix Ltd/3D Systems Inc., Beit Golan, Israel). In cases of primary zygoma reconstruction after tumour ablation surgery, virtual tumour resection was performed after the precise determination of the bone resection margins based on CT data. The mirroring of the intact side of the midfacial area was then carried out. STL models (file format describes the surface geometry of a three-dimensional object) (real and mirrored midfacial area) were exported to CAD software (Geomagic Freeform, Rock Hill, South Carolina, USA).
The design of the implants was aimed at the elimination of the zygoma defects, thus restoring a true-to-original shape of the injured structures. An appropriate determination of the desirable anatomical shape for the PSI was performed using several different methods. Minor zygoma defects, associated with orbital wall injuries, were replaced during the segmentation and mask editing procedures in accordance with the contour of the mirrored intact side. Major or complex defects (including total and subtotal defects) were eliminated using “virtual donors” (a part of virtual model of the mirrored intact opposite side zygoma, which can be incorporated to the virtual model of the damaged zygoma with minor modification).
In all cases, retention points for the precise intraoperative positioning of the implants and their stable retention after the installation, as well as additional elements with holes for screw fixation, were modeled and created. After careful validation and correction performed by clinicians, virtual 3D models of the PSI (STL files) were sent for manufacturing.
In cases of primary reconstruction, tumour resection with cutting guides was performed, followed by a reduction of the herniated orbital soft tissues. The bone areas around the defect were exposed for correct implant placement. Implant insertion and fixation to the orbital rim were accomplished by placing the eyeball in the proper position. In all cases, a careful soft-tissue dissection was performed to avoid the perforation of the maxillary sinus mucoperiosteum and to preserve it intact. In cases where the small (<5 mm) perforations appeared no specific procedures were applied to close them.
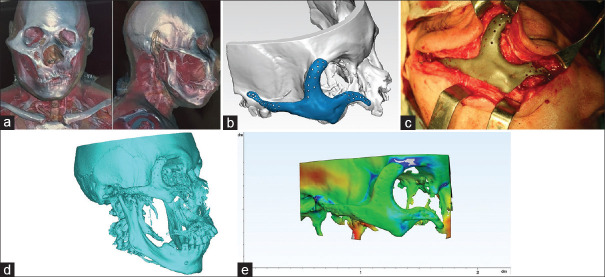
To evaluate the PSI position, the postsurgical CT data were segmented and the virtual models of the midfacial area with PSI were acquired, imported to Geomagic Freeform Plus software, and superimposed onto the models used for surgical planning along with the mirrored intact side models. The outer surfaces of both models were selected for comparison. The program automatically identified the corresponding points from both models, determined the distance between them, and generated the colour-graded error map of the superimposed images, which demonstrated the existing deviations between the models. The mean deviation in mm between the referent points was also measured and analyzed. This parameter was used to evaluate the accuracy of the PSI positioning according to the presurgical plan and to compare the outcome with the mirrored intact side [Figure 1].[22,23]
Figure 1.
Patient with total zygoma defect (a) computed tomography before zygoma reconstruction. (b) Virtual model with designed patient-specific implant. (c) Intraoperative photo of patient-specific implant. (d) Computed tomography after zygoma reconstruction. (e) Colour map comparison analysis between planned and real position of patient-specific implant
Statistical analysis of the data included the calculation of mean values and standard deviation for each parameter evaluated. The Mann–Whitney U-test and Pearson's Chi-squared test were used to compare differences in the parameters measured. The level of significance was set at P < 0.05. The calculations were performed using SPSS Statistics software (IBM, Armonk, New York, USA).
RESULTS
The demographic data and pathological features of the 11 patients included in the study are presented in Table 1. The mean follow-up period was 21.6 ± 6.2 months (range 16–39 months). Secondary reconstruction was performed on seven patients and primary reconstruction after tumour resection was performed in four cases. The design of the PSI determined its proper location, as its complex shape resembled the anatomy of the reconstructed areas and the presence of retention elements. The mean deviation between the planned and real positions of PSIs in our series was 0.72 ± 0.41 mm. The mean deviation between the reconstructed zygomatic complex and the mirrored intact side in our series was 1.45 ± 0.7 mm. Esthetic outcomes were considered to be good by two independent observers in 81.8% of the cases, while the other 18.2% were deemed satisfactory.
No major complications occurred during the postoperative period. In one case (patient № 7), a fistula was noticed during the 3-month follow-up, with signs of chronic infection of the injured bone. A secondary procedure (surgical revision of the infected area with sequestrectomy and preservation of the implant) was performed. Further follow-up did not reveal any other complications.
Six cases (patients № 1, 2, 3, 6, 10, 11) required lateral orbital wall reconstruction. The position of the lateral canthal ligament and its point of fixation were restored in all cases. An additional orbital floor reconstruction was performed in eight cases (patients № 1, 3, 4, 5, 6, 8, 10, 11). The mean volume difference between the intact and damaged orbits in these cases was 1.7 ± 0.8 mm3. The mean difference in mean orbital volume between the intact and damaged orbits after reconstruction was found to be statistically significant (P < 0.05); however, this did not affect the occurrence of enophthalmos in our group of patients (P > 0.05). Temporary diplopia during the 1st month after surgery was observed in two cases (patients № 4, 10). In one patient, who had diplopia before the surgery (case № 3), moderate improvement was observed, but not complete in the long-term follow-up.
In 7 cases (patients № 1, 2, 3, 6, 10), the PSIs were in direct contact with the maxillary sinus. However, there were no clinical or CT symptoms of maxillary sinusitis or implant-related infection during the entire follow-up period. In addition, no signs of implant exposure were detected.
CONCLUSION
High-energy trauma, extended ablative surgery, or congenital malformations of the midface can lead to the occurrence of zygoma defects, which result in severe facial disfigurement and functional disability.[1,2,5,11] A 3D reconstruction of the zygoma is one of the most challenging procedures in craniofacial surgery due to its complex anatomy with a unique combination of concave and convex surfaces; low osteogenic capacity; and proximity to the maxillary sinus, eye globe, orbit, and cranial base.[3,4,13,18,21]
There are many surgical techniques applied for restoring the midfacial bones. Since the 1980s, bone grafts combined with free vascularized tissue transplantation were considered to be the standard option for zygoma reconstruction.[9,11,24] This approach was based on the principals of recreating the vertical and horizontal buttresses using rigid fixation techniques.[1,5,11] Since then, research on fibula, scapula, and iliac crest osteomyocutaneous free flaps or free calvarial bone grafts in combination with free vascularized soft tissue to cover the bone scaffold has been conducted.[3,24,25] In this approach, major attention was focused on reconstruction of the buttress with bar-shaped grafts; however, the restoration of the original 3D shape of the zygomatic complex and orbital walls remained a highly problematic issue.[5,10] Further modifications of this strategy aimed at replacing the convexity of the zygomatic body with the convex surface of the iliac crest or calvarial bone grafts were not precise and were time-consuming due to the necessity of adapting the graft shape to a complex defect configuration.[3,4,25] In addition, insufficient blood supply of the donor site or compromised recipient vessels were the main contraindications for free tissue transferring.[5,26] Nevertheless, many authors advocated the use of bone grafting procedures for zygoma reconstruction due to the possible disadvantages of implant application, including foreign body reaction, infection risk, possibility of exposure, high cost, and complexity of the prebending procedure. These drawbacks were documented for the conventional titanium reconstructive plates, standard meshes, and polymeric implants.[3,4,11,24,25]
In recent years, the philosophy of facial bone reconstruction has been significantly changed by the development and wide application of CAD/CAM technology, which provides opportunities for computer simulation of the surgical procedures, virtual reconstruction, and precise bone osteotomies with surgical guides, as well as the use of the PSI for bone defect repair.[18,27] Scolozzi P[17] reported that using CAD/CAM technology resulted in significantly shorter operating times, a decrease in the risks that may occur during operation. Furthermore, this method is associated with precise 3D reconstruction in orthognathic surgery and in correcting orbital and mandibular defects.[2,14,18,19,21] Many authors focused on this approach as an alternative to traditional reconstruction with free vascularized flaps, especially for the treatment of compromised patients. Mommaerts et al.[28] recommended PSIs as the treatment modality of first choice to avoid donor site morbidity and long reconstructive surgery, considering the free tissue transferring to be the second choice in cases of implant failure.
The present study reports the results of zygoma reconstruction with the application of a PSI in a series of 11 cases. The main goal was to estimate conformity of the preoperative virtual plan and postoperative PSI position depending on the existing clinical conditions, to evaluate the symmetry of the reconstructed midfacial zone, and to present long-term clinical outcomes of this treatment strategy.
The patients involved in the study had different etiologies of the defects; however, all had similar indications for the PSI as a treatment option: Significant length of the defect (zygomatic arch and body) or combined involvement of the zygomatic body, orbital rim, and orbital walls. In such cases, PSI reconstruction is more technically simple and predictable than bone transplantation techniques.[8,13,29]
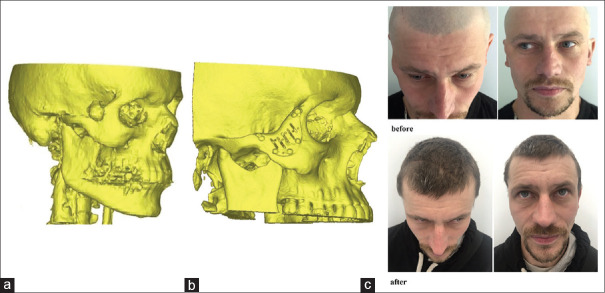
The algorithm applied for PSI modeling resulted in highly precise reconstructions. The mean deviation between the referent points in our series correlated with the results of Modabber et al.[3] and He et al.[27] However, in the cases of primary reconstruction after tumour resection, the application of the surgical guides was mandatory for more precise determination of the defect margins and prevention of PSI misplacement. [Figure 2]
Figure 2.
Patient with partial zygoma defect (a) Computed tomography before tumour ablative surgery. (b) Computed tomography after zygoma reconstruction with patient-specific implant. (c) Photo, of the patient before and after surgery
The PSI design in the present study was significantly influenced by the clinical possibilities of implant insertion. The direction of implant placement, on the other hand, determined which specific surgical approaches were used. Despite the complex shape of the implants (some of them had two-sectional [puzzled] structure), we did not have difficulties with implant placement or positioning in any of the cases.
Our data demonstrated the high performance of the PSI from the esthetic point of view. The mean deviation between the reconstructed zygoma and the mirrored intact side in our series was 1.45 ± 0.7 mm. This result corresponds to the data presented by Klug et al.[23] and He et al.[27] in their clinical studies, which reported mean deviations of 1.1 mm and 1.2 mm, respectively. The mean deviation observed in our study was lower than the 3.5 ± 3.14 mm mean difference obtained by Modabber et al.[3] with vascularized iliac crest. According to Moubayed et al.[22] and Zingg et al.,[15] a 2 mm difference in zygoma positioning can be detected by an experienced observer in only 50% of cases.[22,27] Thus, the deviations obtained in our study are clinically insignificant.
Functional results from this study obtained during a long-term follow-up revealed no cases of limited mouth opening, exposure of the implant, maxillary sinusitis, or other inflammatory complications related to the PSI. Three patients had temporary diplopia 3 months after surgery, with complete reduction in one case. The PSI application in zygoma and orbital reconstruction provided the opportunity for restoration of the exact orbital shape and volume. In our series, the mean difference between the intact and damaged orbits was 1.7 ± 0.8 mm3. Such a result is even more precise than those obtained by Zimmerer et al.[29] and Zhang et al.[30] in orbital trauma surgery.[21,31] Relatively high survival rate of PSI in our study with a long-term follow-up compared to the results reported for the mandible with PSI failure from 12.5% to 27% could be explained by the influence of predominantly static bio-mechanical loading of the implants with low stress and strains values.[32,33] However, excessive tension of skin above the implant can be considered as risk factor of its exposure and should be prevented during at CAD stage of the treatment planning.[1,7,25]
Thus, the main advantage of using PSIs in zygoma reconstruction is the precise restoration of its 3D anatomy without requiring any bone grafting procedures, which are associated with a complicated and time-consuming tissue transfer and donor-site morbidity. However, this study had several limitations: The most important are the relatively small number of patients and absence of the control group for comparison of different treatment approaches. This allows to consider only about potential capability of this methods. At the same time, the potential disadvantages, such as high cost and possible exposure of the PSI, limit their application and require further clinical research to determine the exact indications and contraindications for their usage. The results of the present study support the wider clinical application of PSIs in orbital and zygoma reconstructions. It effectively achieves precise reconstruction of the complex zygoma anatomy and is an alternative option to bone grafting procedures. Another possibility is the combined application of PSI for the 3D restoration of the complex anatomy and bone grafts or survived bone fragments to provide the nutrition and blood supply for the overlying soft tissues. Further biomechanical evaluation of PSI application for zygoma reconstruction is required for the deeper understanding of the causes of possible complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Markiewicz MR, Gelesko S, Bell RB. Zygoma reconstruction. Oral Maxillofac Surg Clin North Am. 2013;25:167–201. doi: 10.1016/j.coms.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Schramm A, Suarez-Cunqueiro MM, Rücker M, Kokemueller H, Bormann KH, Metzger MC, et al. Computer-assisted therapy in orbital and mid-facial reconstructions. Int J Med Robot. 2009;5:111–24. doi: 10.1002/rcs.245. [DOI] [PubMed] [Google Scholar]
- 3.Modabber A, Gerressen M, Ayoub N, Elvers D, Stromps JP, Riediger D, et al. Computer-assisted zygoma reconstruction with vascularized iliac crest bone graft. Int J Med Robot. 2013;9:497–502. doi: 10.1002/rcs.1557. [DOI] [PubMed] [Google Scholar]
- 4.Nicot R, Schlund M, Sentucq C, Raoul G. A new orbito-zygomatic complex reconstruction technique using computer-aided design and manufacturing-assisted harvest of autologous calvarial bone in cases of orbito-zygomatic benign tumor. J Oral Maxillofac Surg. 2019;77:1082–91. doi: 10.1016/j.joms.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Trosman SJ, Haffey TM, Couto RA, Fritz MA. Large orbital defect reconstruction in the setting of globe-sparing maxillectomy: The titanium hammock and layered fibula technique. Microsurgery. 2018;38:354–61. doi: 10.1002/micr.30199. [DOI] [PubMed] [Google Scholar]
- 6.Massa AF, Otero-Rivas M, Rodríguez-Prieto MÁ. Titanium mesh in the reconstruction of a malar defect: A case report. Int J Dermatol. 2014;53:1278–80. doi: 10.1111/ijd.12551. [DOI] [PubMed] [Google Scholar]
- 7.Parthasarathy J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann Maxillofac Surg. 2014;4:9–18. doi: 10.4103/2231-0746.133065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Visscher DO, Farré-Guasch E, Helder MN, Gibbs S, Forouzanfar T, van Zuijlen PP, et al. Advances in bioprinting technologies for craniofacial reconstruction. Trends Biotechnol. 2016;34:700–10. doi: 10.1016/j.tibtech.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Rogers GF, Greene AK. Autogenous bone graft: Basic science and clinical implications. J Craniofac Surg. 2012;23:323–7. doi: 10.1097/SCS.0b013e318241dcba. [DOI] [PubMed] [Google Scholar]
- 10.Tessier P, Kawamoto H, Matthews D, Posnick J, Raulo Y, Tulasne JF, et al. Autogenous bone grafts and bone substitutes – Tools and techniques: I.A 20,000-case experience in maxillofacial and craniofacial surgery. Plast Reconstr Surg. 2005;116:6S–24. doi: 10.1097/01.prs.0000173862.20563.12. [DOI] [PubMed] [Google Scholar]
- 11.Rohner D, Tan BK, Song C, Yeow V, Hammer B. Repair of composite zygomatico-maxillary defects with free bone grafts and free vascularized tissue transfer. J Craniomaxillofac Surg. 2001;29:337–43. doi: 10.1054/jcms.2001.0253. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Li P, Lu H, Shen L, Tian W, Long J, et al. Digital design and individually fabricated titanium implants for the reconstruction of traumatic zygomatico-orbital defects. J Craniofac Surg. 2013;24:363–8. doi: 10.1097/SCS.0b013e3182701243. [DOI] [PubMed] [Google Scholar]
- 13.Moiduddin K, Al-Ahmari A, Kindi MA, Nasr ES, Mohammad A, Ramalingam S. Customized porous implants by additive manufacturing for zygomatic reconstruction. Biocybern Biomed Eng. 2016;36:719–30. [Google Scholar]
- 14.Hernando J, Geijo D, Leizaola-Cardesa IO, Aguilar-Salvatierra A, Gómez MC, Erce C, et al. Reconstruction of liposarcoma resection defect with a made-to-measure polyethylene prosthesis using three-dimensional digital technology. J Craniofac Surg. 2018;29:e16–7. doi: 10.1097/SCS.0000000000004013. [DOI] [PubMed] [Google Scholar]
- 15.Zingg M, Laedrach K, Chen J, Chowdhury K, Vuillemin T, Sutter F, et al. Classification and treatment of zygomatic fractures: A review of 1,025 cases. J Oral Maxillofac Surg. 1992;50:778–90. doi: 10.1016/0278-2391(92)90266-3. [DOI] [PubMed] [Google Scholar]
- 16.Kasper FK, Melville J, Shum J, Wong M, Young S. Tissue engineered prevascularized bone and soft tissue flaps. Oral Maxillofac Surg Clin North Am. 2017;29:63–73. doi: 10.1016/j.coms.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Scolozzi P. Maxillofacial reconstruction using polyetheretherketone patient-specific implants by “mirroring” computational planning. Aesthetic Plast Surg. 2012;36:660–5. doi: 10.1007/s00266-011-9853-2. [DOI] [PubMed] [Google Scholar]
- 18.Chepurnyi Y, Chernohorskyi D, Prykhodko D, Poutala A, Kopchak A. Reliability of orbital volume measurements based on computed tomography segmentation: Validation of different algorithms in orbital trauma patients. J Craniomaxillofac Surg. 2020;48:574–81. doi: 10.1016/j.jcms.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Gibelli D, Cellina M, Gibelli S, Oliva AG, Termine G, Pucciarelli V, et al. Assessing symmetry of zygomatic bone through three-dimensional segmentation on computed tomography scan and “mirroring” procedure: A contribution for reconstructive maxillofacial surgery. J Craniomaxillofac Surg. 2018;46:600–4. doi: 10.1016/j.jcms.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Rotaru H, Schumacher R, Kim SG, Dinu C. Selective laser melted titanium implants: A new technique for the reconstruction of extensive zygomatic complex defects. Version 2. Maxillofac Plast Reconstr Surg. 2015;37:1. doi: 10.1186/s40902-015-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chepurnyi Y, Chernohorskyi D, Zhukovtceva O, Poutala A, Kopchak A. Automatic evaluation of the orbital shape after application of conventional and patient-specific implants: Correlation of initial trauma patterns and outcome. J Oral Biol Craniofac Res. 2020;10:733–7. doi: 10.1016/j.jobcr.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moubayed SP, Duong F, Ahmarani C, Rahal A. A novel technique for malar eminence evaluation using 3-dimensional computed tomography. Arch Facial Plast Surg. 2012;14:403–7. doi: 10.1001/archfacial.2012.510. [DOI] [PubMed] [Google Scholar]
- 23.Klug C, Schicho K, Ploder O, Yerit K, Watzinger F, Ewers R, et al. Point-to-point computer-assisted navigation for precise transfer of planned zygoma osteotomies from the stereolithographic model into reality. J Oral Maxillofac Surg. 2006;64:550–9. doi: 10.1016/j.joms.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 24.Ahn SJ, Hong JW, Kim YO, Lew DH, Lee WJ. Treatment of fibrous dysplasia of the zygomaticomaxillary complex with radical resection and three-dimensional reconstruction with autologous calvarial bone graft. Arch Craniofac Surg. 2018;19:200–4. doi: 10.7181/acfs.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day KM, Phillips PM, Sargent LA. Correction of a posttraumatic orbital deformity using three-dimensional modeling, virtual surgical planning with computer-assisted design, and three-dimensional printing of custom implants. Craniomaxillofac Trauma Reconstr. 2018;11:78–82. doi: 10.1055/s-0037-1601432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolff KD, Hölzle F, Kolk A, Hohlweg-Majert B, Steiner T, Kesting MR. Raising the osteocutaneous fibular flap for oral reconstruction with reduced tissue alteration. J Oral Maxillofac Surg. 2011;69:E260–7. doi: 10.1016/j.joms.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 27.He Y, Zhang Y, An JG, Gong X, Feng ZQ, Guo CB. Zygomatic surface marker-assisted surgical navigation: A new computer-assisted navigation method for accurate treatment of delayed zygomatic fractures. J Oral Maxillofac Surg. 2013;71:2101–14. doi: 10.1016/j.joms.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Mommaerts MY, Nicolescu I, Dorobantu M, De Meurechy N. Extended total temporomandibular joint replacement with occlusal adjustments: Pitfalls, patient-reported outcomes, subclassification, and a New Paradigm. Ann Maxillofac Surg. 2020;10:73–9. doi: 10.4103/ams.ams_245_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmerer RM, Ellis E, Aniceto GS, Schramm A, Wagner ME, Grant MP, et al. A prospective multicenter study to compare the precision of posttraumatic internal orbital reconstruction with standard preformed and individualized orbital implants. J Craniomaxillofac Surg. 2016;44:1485–97. doi: 10.1016/j.jcms.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, He Y, Zhang ZY, An JG. Evaluation of the application of computer-aided shape-adapted fabricated titanium mesh for mirroring-reconstructing orbital walls in cases of late post-traumatic enophthalmos. J Oral Maxillofac Surg. 2010;68:2070–5. doi: 10.1016/j.joms.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 31.Rahimov CR, Ahmadov SG, Rahimli MC, Farzaliyev IM. Three-dimensional diagnosis in orbital reconstructive surgery. Ann Maxillofac Surg. 2020;10:3–9. doi: 10.4103/ams.ams_183_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavlychuk T, Chernogorskyi D, Chepurnyi Y, Neff A, Kopchak A. Biomechanical evaluation of type P condylar head osteosynthesis using conventional small-fragment screws reinforced by a patient specific two-component plate. Head Face Med. 2020;16:25. doi: 10.1186/s13005-020-00236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilde F, Hanken H, Probst F, Schramm A, Heiland M, Cornelius CP. Multicenter study on the use of patient-specific CAD/CAM reconstruction plates for mandibular reconstruction. Int J Comput Assist Radiol Surg. 2015;10:2035–51. doi: 10.1007/s11548-015-1193-2. [DOI] [PubMed] [Google Scholar]