Abstract
Introduction:
India is a high-risk region for oropharyngeal cancer (OC) due to high prevalence of tobacco, betel nut, and alcohol and accounts for 30% of all new cases of oral cancer annually.
Materials and Methods:
Records of all 73 diagnosed cases of different types of OC and oropharynx patients were analyzed who reported in “Tobacco cessation center”’ of the Institute between January 2017 and December 2019. The patients’ demographic details, blood groups, oral habits, and clinicohistological records were obtained from the medical records available in the hospital.
Results:
OC incidence was 3.75 cases/year with male-to-female ratio 3.29:1. Mean age was 51.25 ± 13.6 years. The most common site of tumour presentation was mandibular alveolar ridge. Combined use of tobacco/betal nut/alcohol constituted the major cause for the development of oral squamous cell carcinoma (OSCC). Majority patients were presented in Stage II (43.8%). Histopathological reports were suggestive of maximally well-differentiated (52.1%) OSCC. People with blood group A+ve had 3.22 times higher risk of developing OSCC compared to people of other blood groups.
Discussion:
Male: female ratio was reported higher than in most of other studies. Mandibular alveolus was the most frequent site because most of the patients tend to keep the tobacco quid in the buccal vestibule with close proximity to alveolus. The relative downregulation of glycosyl transferase that is involved in the biosynthesis of A and B antigens as seen in association with tumour development could be the reason for increased OC reported in blood group A subjects.
Keywords: ABO blood groups, oral cancer, staging, tobacco
INTRODUCTION
According to Wills,[1] neoplasm is an abnormal mass of tissue, the growth of which exceeds and is uncoordinated with that of normal tissues and persists in the same excessive manner after cessation of the stimuli which evoked the change. Squamous cell carcinomas of head–neck cancer come under the category of biologically heterogeneous group of cancers. Oral squamous cell carcinoma (OC) is the most common form of cancer in oral cavity because oral cavity has been frequently exposed to many carcinogenic agents such as tobacco, alcohol, betel nut, and virus (human papilloma virus) which serve as major etiological factors. About 75% of all OCs are attributable to tobacco use and alcohol consumption.[2] Regardless of the different modalities of tobacco consumption (chewing or smoking), its use is more strongly associated with oral cancer than alcohol alone. The high levels of carcinogenic tobacco-specific nitrosamines, e.g., nitro-nor-nicotine and 4-methyl-nitrosamino-1-(3 pyridyl)-1-butanone (NNK) were reported in the saliva of oral snuff users.[3] The chewing of the areca nut (betel quid) releases large amounts of reactive oxygen species, especially while the quid is present in the oral cavity. Both tobacco specific nitrosamines and reactive oxygen species are major genotoxic agents involved in oral cancer associated with the use of chewing tobacco/betel nut.
The dietary deficiencies, poor oral hygiene, genetics, and lower socioeconomic strata of society are minor etiological factors of oral carcinoma. The ABO blood group is one of such genetic factors that have been related in the etiology of various chronic diseases. The association between ABO blood groups and malignancy was first explored in 1921 by Alexander.[4] In India, studies done by Gilmiyarova et al.,[5] Mittal and Gupta,[6] Ewald and Sumner,[7] and Baruah and Gogoi[8] have shown that individuals with blood Group A have predisposition for oral cancer. Hypermethylation of ABO gene promoter and modification of ABO blood group antigen expression on cancer cells is related to the invasiveness or metastatic potential of tumour. Moreover, OC is one of the leading causes of death all over the world with its incidence rate varying by geographic location. According to the reports of the World Health Organization,[9] oral cancer ranks sixth among all malignancies in the world. Incidence rate is 12.8% men and 7% women. It commonly occurs in elderly from fifth to sixth decades of life but rarely seen in younger age group. In oral cavity, tongue and buccal mucosa have been noted to be quiet common sites in India.
The rich lymphatic supply of the oral cavity results in many cases being detected first in the advanced stages. Furthermore, the late presentation of the carcinoma patients may be attributed due to ignorance of symptoms and lack of concern for the disease.[10] Thus, the knowledge of the varied presentations of OC and an experienced eye can go a long way in decreasing the high morbidity and mortality associated with oral cancers. Various classifications have been proposed to categorize OC and treatment planning are given based upon them. Usually, chemotherapy, surgery, and radiotherapy are the most common form of treatment given, and prognosis is dependent on age, gender, site, and histopathological grading.[11] Younger individuals, histopathogically well-differentiated lesions involve good prognosis while elder individuals, histopathologically poorly differentiated lesions have poor prognosis.
The spectrum of oral cancer varies from region to region. With this background, the study was planned with the rationale to assess the relationship between ABO blood groups and different types of oral cancer, which might contribute to determine the susceptibility of an individual to oral cancer and to assess the utility of ABO blood group as a preclinical marker.
MATERIALS AND METHODS
Study design
This was a retrospective cross-sectional clinicopathological study.
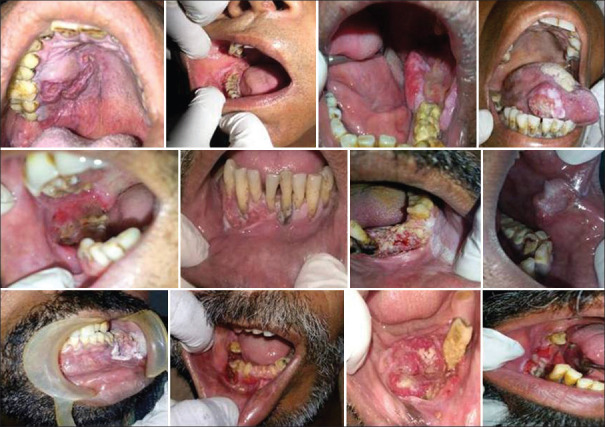
The records of 73 patients with a histologically confirmed diagnosis of OC and oropharynx (including both primary and secondary malignancies) were reviewed thoroughly, who reported to “Tobacco Cessation Centre” of the Institute, located in Sri Ganganagar (Rajasthan) from January 2017 to December 2019 by an experienced Head and Neck Oncologist [Figure 1]. The informed and written consent in both local and English language was taken from each subject and was ensured about the confidentiality of the data. The study was conducted in accordance with the declaration of Helsinki and was approved by the local Ethical Committee of the Institute (SDCRI/IEC/2016/157 dated December 23, 2016). The inclusion criteria of the study consisted of (1) patient records with complete clinicopathological data of OC, including age, sex, stage, tobacco habit, alcohol consumption, histologic differentiation, radiographs, the ABO blood group, and treatment status. (2) Malignant lesions were restaged using records according to the 2002 Union for International Cancer Control cancer staging system. Exclusion criteria included: (1) Patient records showing initial treatment with curative intent by one or a combination of surgery, radiation therapy, and chemotherapy; (2) patients with salivary gland tumours; (3) patient records without biopsy reports.
Figure 1.
Various Oral Cancer sites noted in the study.
The data collected from these patients were entered in a standardized format. This included age, gender, ABO blood group, habits of tobacco/betel nut/alcohol consumption, site of the tumour (9 different sites, i.e., buccal mucosa, tongue, floor of mouth, alveolar ridge, Gingivo-buccal sulcus, pharynx/peritonsillar region, palate, lip, and retromolar trigone), TNM staging (Tumour-Node-Metastasis classification of Union of International Cancer Control staging of carcinoma of oral cavity and Border's histopathological descriptive grading based on differentiation of malignant cells (Grade I: well differentiated, Grade II: moderately differentiated, Grade III: poorly differentiated, and Grade IV: anaplastic/pleomorphic). In case, the patient was not aware of his/her ABO blood group, the same was obtained during routine hematological investigations using mouse-derived monoclonal antibodies (Ortho Bioclones Anti-A, B, and O; Ortho Diagnostic Systems Inc., Raritan, NJ, USA). After cessation of treatment, each patient was followed up every 3 months at the hospital or by telephone contact for an interview. The last follow-up was December 31, 2019. The data thus obtained were tabulated and subjected for further statistical analysis using the Statistical Package for the Social Sciences software (SPSS version 20.0; SPSS Inc., Chicago, IL, USA) and the results were formulated.
RESULTS
The study comprised 73 oral squamous cell carcinoma (OSCC) patients, out of which 56 (76.7%) were males and 17 (23.3%) were females. The mean age of male patients was 52.1 ± 14.2 years and female 48.4 ± 11.7 years [Table 1]. The OSCC incidence was found to be 3.75 cases/year with male to female ratio of 3.29:1 noted in the considered 2 years study time. The highest peak of OSCC (28.8%) in the study was observed in the age group of 41–50 years [Table 2]. The maximum cases of primary OSCC patients (83.6%) clinically diagnosable were found out of five categories of oral malignancies recorded in the study. Moreover, majority of the patients (43.8%) were in Stage II and very less patients (13.7%) reported with stage IV OSCC malignancy [Table 3]. On comparing the biopsy histopathological reports based on differentiation of cells, it was found that maximum OCs were well-differentiated (46.6%) oral cancers [Table 4].
Table 1.
Age and gender distribution of patients
| Gender | Number of patients (n) | Age (years), mean±SD |
|---|---|---|
| Female | 17 | 48.41±11.710 |
| Male | 56 | 52.11±14.183 |
| Total | 73 | 51.25±13.660 |
SD: Standard deviation
Table 2.
Age groups of the study population
| Age group (years) | Number of patients, n (%) |
|---|---|
| 20-30 | 6 (8.22) |
| 31-40 | 13 (17.81) |
| 41-50 | 21 (28.77) |
| 51-60 | 14 (19.18) |
| 61-70 | 14 (19.18) |
| >70 | 5 (6.85) |
Table 3.
Oral cancer diagnosis according to tumour-node-metastasis staging
| Diagnosis | Stage of OSCC | Total, n (%) | |||
|---|---|---|---|---|---|
|
| |||||
| I, n (%) | II, n (%) | III, n (%) | IV, n (%) | ||
| OSMF with SCC | 0 | 4 (5.5) | 1 (1.4) | 0 | 5 (6.9) |
| PIOC | 1 (1.4) | 1 (1.4) | 2 (2.7) | 0 | 4 (5.5) |
| PVL | 2 (2.7) | 0 | 0 | 0 | 2 (2.7) |
| SCC | 12 (16.4) | 26 (35.6) | 13 (17.8) | 10 (13.7) | 61 (83.6) |
| Verrucous carcinoma | 0 | 1 (1.4) | 0 | 0 | 1 (1.4) |
| Total | 15 (20.5) | 32 (43.8) | 16 (21.9) | 10 (13.7) | 73 (100.0) |
OSMF: Oral submucous fibrosis, SCC: Squamous cell carcinoma, PVL: Proliferative verrucous leukoplakia, OSCC: Oral SCC, PIOC: Primary intraosseous carcinoma
Table 4.
Border’s classification according to tumour-node-metastasis staging
| Border’s classification | Stage | Total, n (%) | |||
|---|---|---|---|---|---|
|
| |||||
| I, n (%) | II, n (%) | III, n (%) | IV, n (%) | ||
| Anaplastic | 0 | 0 | 2 (2.7) | 2 (2.7) | 4 (5.5) |
| Moderately differentiated | 5 (6.9) | 12 (16.4) | 2 (2.7) | 0 | 19 (26.1) |
| Poorly differentiated | 1 (1.4) | 4 (5.5) | 6 (8.2) | 5 (6.8) | 16 (21.9) |
| Well differentiated | 9 (12.3) | 16 (21.9) | 6 (8.2) | 3 (4.1) | 34 (46.6) |
| Total | 15 (20.5) | 32 (43.8) | 16 (21.9) | 10 (13.7) | 73 (100.0) |
Out of considered nine sites, the most common presentation of OC was found on alveolar ridge (45.2%) with 37% (27 cases) on mandibular alveolar ridge, out of which 24 cases reported in males and only three cases in female patients. The second most common site observed in our study was the buccal mucosa (19.2%) with equal number of cases recorded on the right and left side (7 each). The deleterious habit of combined use of tobacco/betel nut/alcohol constituted (39.7%) most common in OC patients, followed by tobacco chewing alone habit (27.4%). Only one case of OC was found secondary to metastasis from carcinoma liver. Furthermore, one case each of OC was recorded because of chronic denture irritation and alcohol use alone [Table 5].
Table 5.
Distribution of oral cancer sites according to deleterious habits
| Habits | Site | Total, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Alveolar ridge, n (%) | Buccal mucosa, n (%) | Floor of mouth, n (%) | Gingivo-buccal sulcus, n (%) | Lip, n (%) | Palate, n (%) | Pharynx, n (%) | Retromolar trigone, n (%) | Tongue, n (%) | ||
| Alcoholic | 1 (1.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.4) |
| Betalnut chewer | 1 (1.4) | 4 (5.5) | 0 | 0 | 0 | 1 (1.4) | 1 (1.4) | 0 | 1 (1.4) | 8 (11.0) |
| Combined | 14 (19.2) | 6 (8.2) | 2 (2.7) | 3 (4.1) | 0 | 0 | 1 (1.4) | 2 (2.7) | 1 (1.4) | 29 (39.7) |
| Denture irritation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.4) | 1 (1.4) |
| Metastatic | 1 (1.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.4) |
| No habit | 3 (4.1) | 0 | 0 | 0 | 0 | 1 (1.4) | 0 | 0 | 1 (1.4) | 5 (6.8) |
| Tobacco chewer | 11 (15.1) | 4 (5.5) | 0 | 1 (1.4) | 0 | 1 (1.4) | 0 | 0 | 3 (4.1) | 20 (27.4) |
| Tobacco smoker | 2 (2.7) | 0 | 0 | 0 | 2 (2.7) | 1 (1.4) | 0 | 3 (4.1) | 0 | 8 (11.0) |
| Total | 33 (45.2) | 14 (19.2) | 2 (2.7) | 4 (5.5) | 2 (2.7) | 4 (5.5) | 2 (2.7) | 5 (6.8) | 7 (9.6) | 73 (100.0) |
| P | 0.02* | 0.28 | 0.41 | 0.31 | 0.58 | 0.78 | 0.46 | 0.61 | 0.44 | |
*Statistically significant
Table 6 shows the distribution of ABO blood groups among OC patients. Out of 73 Oral cancer cases, 38 (52.1%) had blood group A+ve, 14 (19.2%) had B+ve and O+ve each, 3 (1.4%) had AB+ve, 2 (2.7%) had O−ve, and 01 (1.4%) had B−ve and A−ve each. ABO blood groups and OSCC were assessed by odds ratio, it was found that people with blood group A had 3.22 times higher risk of developing oral cancer compared to people of other blood groups, while the relative risk for people with blood group O was 0.58 times. A statistically significant correlation (P = 0.02) was found only between blood group A+ve and diagnosis of OSCCs [Table 6].
Table 6.
Correlation of ABO blood groups and diagnosis of oral cancer
| Blood group | Diagnosis of OSCC | Total, n (%) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| OSMF with SCC, n (%) | PIOC, n (%) | PVL, n (%) | SCC, n (%) | Verrucous carcinoma, n (%) | ||
| A −ve | 0 | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) |
| A +ve | 3 (4.1) | 1 (1.4) | 1 (1.4) | 33 (45.2) | 0 | 38 (52.1) |
| AB +ve | 0 | 0 | 0 | 3 (4.1) | 0 | 3 (4.1) |
| B −ve | 0 | 0 | 0 | 1 (1.4) | 0 | 1 (1.4) |
| B +ve | 0 | 2 (2.7) | 1 (1.4) | 11 (14.1) | 0 | 14 (19.2) |
| O −ve | 0 | 0 | 0 | 2 (2.7) | 0 | 2 (2.7) |
| O +ve | 2 (2.8) | 1 (1.4) | 0 | 10 (13.7) | 1 (1.4) | 14 (19.2) |
| Total | 5 (6.9) | 4 (5.5) | 2 (2.7) | 61 (83.6) | 1 (1.4) | 73 (100.0) |
|
| ||||||
| Blood group | OR | P | ||||
|
| ||||||
| A | 3.22 | |||||
| All other blood groups | 0.02* | |||||
*Statistically significant. OSMF: Oral submucous fibrosis, SCC: Squamous cell carcinoma, PVL: Proliferative verrucous leukoplakia, OSCC: Oral squamous cell carcinoma, PIOC: Primary intraosseous carcinoma, OR: Odds Ratio
DISCUSSION
Oral cancer is considered to be the sixth most common cancer worldwide, with India having highest prevalence.[12] The good understanding of the risk factors and epidemiology of OC can be helpful in early identification and prompt treatment of these patients. The OC early detection is important as it leads to early institution of therapy that translates into a better prognosis. Delayed detection and diagnosis of OC are directly proportional to increased morbidity and mortality.[13] The male: female ratio (3.29:1) was reported higher than in most of other studies, except for a Greek populated-based study, where they found a ratio of 9.2:1.[14] The higher incidence of OC among males can be attributed to the easy acceptance of habits by males. The deleterious habits of tobacco and betel nut consumption as a means of stimulants renders males more susceptible to the development of OCs. Although the consumption of tobacco and betel nut in India is considered a taboo among females, the custom is gradually fading nowadays as females cutting across socioeconomic lines and age are turning to these habits.
The mean age recorded for OC patients is 65 years according to US National cancer Institute.[15] In the present study, the most predominantly affected age group was 41–50 years, youngest of all OC patients reported was 23 years old, and the oldest was 80 years. In a study conducted by Chattopadhyay[16] in Eastern India, the mean age for OC patients was 52.07 years which was almost same 51.25 ± 13.66 years as in the present study. According to Philips et al.,[17] it was believed that 25-year latency exists between the initial exposure to a carcinogen and the development of a clinically recognizable cancer. Sankaranarayan found that the peak age frequency of occurrence of oral cancer in India was fifth decade of life, which is at least a decade earlier than that reported in the Western literature.[18] Gupta et al. also observed that there is an increase in the incidence of OC in the younger age group in developing countries, especially India.[19] This could be explained by the fact that, ease with which tobacco, betel nut, and related products are available at very affordable prices in the market, leading to youth adopting this pernicious habit in the country.
The occurrence of OC differs widely according to various epidemiological studies. In this study, alveolar ridges (45.2%) especially mandibular alveolus were the most frequent site because most of the patients tend to keep the tobacco quid in the buccal vestibule with close proximity to alveolus. This leads to chronic irritation by chemical and physical insult of gingiva. A study in western Uttar Pradesh reported that the buccal mucosa was the most common OC site, followed by retromolar area, floor of mouth, lateral border of tongue, and least being the palate.[20] In the present study, buccal mucosa (19.2%) was reported as the second most common site of OC and palate and pharynx OC being reported least, this could be due to regional difference in consumption of various deleterious habits. The rampant use of chewable tobacco can be linked to the relatively high incidence of involvement of buccal tissues, as associated potentially malignant mucosal lesions located in the cheeks are also more common in South Central Asian countries, possibly related to the tobacco chewing habits.[21] In our study, the third most commonly involved anatomical site observed was the tongue (9.6%), while in western countries, it was observed as the most common subsite of OC in every race/ethnicity. In India, besides betel nut quid chewing, the trends of alcohol consumption show an increasing intake in recent decade, a sign of the westernization of cultural habits that influence OC incidence. The retromolar trigone area, which is an ill-defined triangular area in the oral cavity posterior to the upper and lower third molar teeth with maxillary tuberosity at its apex was the next most common site for OC (6.8%) reported in present study. This could be explained by the fact that posterior parts of both jaws are most common sites for chewing tobacco or betelnut, and the associated mucosal lesions occurring in these regions sometimes extend through the alveolar crest and lingual cortex and spread through the inferior alveolar canal to involve retromolar trigone.[22] According to our data, 2.7% of cases were reported as carcinoma of lower lip. No case of upper lip carcinoma was reported. A risk factor associated with carcinoma of the lower lip, apart from tobacco chewing and smoking is prolonged exposure to ultraviolet (UV) rays,[23] which is common in the Northwestern parts of India. Moreover, in our study, the majority of patients we encountered were rural people doing farming, who work under direct sunlight throughout the day and thus are the ones most exposed to UV rays.
In our study, maximum number (39.7%) of OC patients reported with combined habituation with tobacco chewing, smoking betel nut, and alcohol, the results were in accordance with previous studies. The harmful carcinogenic effect of consuming tobacco and dehydrating effect of alcohol on oral tissues leads to a dangerous synergy of expression of the oral cancer. Sanghvi et al.[24] also observed that the risk of oral cancer was three fold in tobacco chewers, two fold in smokers, and four-fold in chewers and smokers both. The habit of tobacco chewing alone (27.4%) was noted as the second most common cause of OC in the present study. Studies[24,25] have shown that tobacco chewers also include slaked lime in tobacco chewing, so combined use has carcinogenic (due to N-nitroso compounds of tobacco) and genotoxic effects on human oral epithelium cells. Furthermore, the hydrogen peroxide and oxygen production (source of ROS) occurs due to alkaline pH that arises from the addition of slaked lime when chewing these products. The betel nut chewing habit alone was the third most common deleterious habit noted among the OC patients (11%), as long-term areca nut chewing can cause oral submucous fibrosis which itself is a premalignant condition.
Most of the OC patients reported in Stage II and with well differentiated dysplastic changes of tissues. The delay in diagnosis of OC could be correlated to patient delay (in looking for professional care), professional delay (in reaching a diagnosis), or both. This can also be attributed to the fact that because of poverty, illiteracy, and possibly resorting to home remedies, all leading to delay by the patients. Hence, these patients refer late as compared to Western data.[26]
Many researchers have studied the relationship between ABO blood groups and oral cancer.[27,28] More than twenty genetically determined blood group systems are known today, but ABO blood groups are sensitive than other blood grouping systems in detecting antigen responsible for cancers as ABO blood group genes are mapped at 9q34.2 region, in which genetic alteration is common in many cancers.[28] The present study clearly demonstrates that people having blood group A+ve were found to have a greater tendency to develop oral cancer while blood group O has the least. This would be explained by the fact that blood group antigens, in addition to being present on red blood cell membranes, are also found on epithelial cells of various other tissues including the oral mucosa. The relative downregulation of glycosyl transferase that is involved in the biosynthesis of A and B antigens as seen in association with tumour development.[29] Moreover, H antigen is a blood group antigen present in all the individuals irrespective of blood group types. It is the precursor for the formation of A and B antigens. In people belonging to A and B blood groups, the precursor H antigen is converted into A and B antigens, respectively, whereas O+ve blood group individuals, it remains in its original form.[30] Hence, people with O blood group have the highest amount of H antigen which affords protection against OC.
Our study had many limitations. First, it was a retrospective study based on single hospital records; it may not be truly representative of all oral cancer cases in the community. Second, to detect an association between the ABO blood group and oral cancer, salivary gland, and other soft-tissue malignancies of maxillofacial region were not included. Third, healthy subjects (controls) were not considered for comparison. Future studies with larger sample size in different geographical areas are required to better elucidate this topic. Similar studies regarding the relationship of ABO blood group and other types of cancers are also recommended.
CONCLUSION
Blood group A+ve patients were found to have a greater tendency to develop oral cancer while blood group O has the least The most common age group of oral cancer was 41–50 years with male predominance. Most of the patients were indulging in combined habituation with tobacco chewing, smoking, betel nut and alcohol. Majority of OC patients diagnosed with Stage II with well-differentiated dysplastic cell morphology. Hence, by employing simple blood grouping test during community field programs, public health program must be undertaken for the prevention of oral cancer and a multidisciplinary approach should be followed in early diagnosis of OC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Willis RA. Pathology of Tumors. Butterworth and Co.,Ltd. St Louis: The CV Mosby Company; 1948. p. 881. [Google Scholar]
- 2.Tandon P, Dadhich A, Saluja H, Bawane S, Sachdeva S. The prevalence of squamous cell carcinoma in different sites of oral cavity at our Rural Health Care Centre in Loni, Maharashtra – A retrospective 10-year study. Contemp Oncol (Pozn) 2017;21:178–83. doi: 10.5114/wo.2017.68628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yalcin E, de la Monte S. Tobacco nitrosamines as culprits in disease: Mechanisms reviewed. J Physiol Biochem. 2016;72:107–20. doi: 10.1007/s13105-016-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jalili L, Zarabadipour M, Azmoudeh F, Esfehani M, Tamiz P. ABO Blood group distribution In patients with Oral squamous cell carcinoma. Ann Dent Special. 2018;6:149–52. [Google Scholar]
- 5.Gilmiyarova FN, Kolotyeva NA, Kuzmicheva VI, Gusyakova OA, Borodina IA, Baisheva GM, et al. Blood group and human diseases (review of literature) Klin Lab Diagn. 2020;65:216–21. doi: 10.18821/0869-2084-2020-65-4-216-221. [DOI] [PubMed] [Google Scholar]
- 6.Mittal VP, Gupta S. The study of ABO blood groups in Oral cancer. J Cancer. 1969;6:34–7. [PubMed] [Google Scholar]
- 7.Ewald DR, Sumner SC. Blood type biochemistry and human disease. Wiley Interdiscip Rev Syst Biol Med. 2016;8:517–35. doi: 10.1002/wsbm.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baruah BD, Gogoi BC. Blood groups in cancer in Assam. Indian J Cancer. 1977;14:6–9. [PubMed] [Google Scholar]
- 9.World Health Organization. Control of Oral cancer in developing countries. A WHO meeting. Bull World Health Organ. 1984;62:817–30. [PMC free article] [PubMed] [Google Scholar]
- 10.Basharat S, Shaikh BT, Rashid HU, Rashid M. Health seeking behaviour, delayed presentation and its impact among oral cancer patients in Pakistan: A retrospective qualitative study. BMC Health Serv Res. 2019;19:2–9. doi: 10.1186/s12913-019-4521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin NC, Hsu JT, Tsai KY. Survival and clinicopathological characteristics of different histological grades of oral cavity squamous cell carcinoma: A single-center retrospective study. PLoS One. 2020;15:e0238103. doi: 10.1371/journal.pone.0238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena S, Gupta KK, Meena P. Association of ABO blood groups in relation to oral cavity cancers in Western Rajasthan. Int J Contemp Med Res. 2016;3:2661–4. [Google Scholar]
- 13.Singh V, Tudu R, Kumar A. Demographic, histopathological patterns and clinical profile of oral squamous cell carcinoma at a tertiary level referral hospital in Jharkhand. J Dent Med Sci. 2018;5:68–72. [Google Scholar]
- 14.Antoniades DZ, Styanidis K, Papanayotou P, Trigonidis G. Squamous cell carcinoma of the lips in a northern Greek population. Evaluation of prognostic factors on 5-year survival rate – I. Eur J Cancer B Oral Oncol. 1995;31B:333–9. doi: 10.1016/0964-1955(95)00030-5. [DOI] [PubMed] [Google Scholar]
- 15.Morais-Faria K, Palmier NR, de Lima Correia J, de Castro Júnior G, Dias RB, da Graça Pinto H, et al. Young head and neck cancer patients are at increased risk of developing oral mucositis and trismus. Support Care Cancer. 2020;28:4345–52. doi: 10.1007/s00520-019-05241-x. [DOI] [PubMed] [Google Scholar]
- 16.Chattopadhyay A. Epidemiologic study of oral cancer in eastern India. Indian J Dermatol. 1989;34:59–65. [PubMed] [Google Scholar]
- 17.Philips PA. The Basic Science of Oncology. New York: Pergamon Press; 1987. p. 25. [Google Scholar]
- 18.Sankaranarayan R. Oral cancer in India, an epidemiologic and clinic review. Oral Surg Oral Med Oral Pathol. 1990;69:325–30. doi: 10.1016/0030-4220(90)90294-3. [DOI] [PubMed] [Google Scholar]
- 19.Gupta PC, Murti PR, Bhonsle RB, Mehta FS, Pindborg JJ. Effect of cessation of tobacco use on the incidence of oral mucosal lesion in a 10 yr follow-up study of 12,212 users. Oral Dis. 1995;1:54–8. doi: 10.1111/j.1601-0825.1995.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 20.Sharma P, Saxena S, Aggarwal P. Trends in the epidemiology of oral squamous cell carcinoma in Western UP: An institutional study. Indian J Dent Res. 2010;21:316–9. doi: 10.4103/0970-9290.70782. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Kumar SK, Sedghizadeh PP, Jayakar AN, Shuler CF. Oral squamous cell carcinoma incidence by subsite among diverse racial and ethenic populations in California. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:470–80. doi: 10.1016/j.tripleo.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Binahmed A, Nason RW, Abdoh AA, Sándor GK. Population-based study of treatment outcomes in squamous cell carcinoma of the retromolar trigone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:662–5. doi: 10.1016/j.tripleo.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Kumar M, Nanavati R, Modi TG, Dobariya C. Oral cancer: Etiology and risk factors: A review. J Cancer Res Ther. 2016;12:458–63. doi: 10.4103/0973-1482.186696. [DOI] [PubMed] [Google Scholar]
- 24.Sanghvi LD, Rao KC, Khanolkar VR. Smoking and chewing of tobacco in relation to cancer of the upper alimentary tract. Br Med J. 1955;1:1111–4. doi: 10.1136/bmj.1.4922.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar GK, Abidullah M, Elbadawi L, Dakhil S, Mawardi H. Epidemiological profile and clinical characteristics of oral potentially malignant disorders and oral squamous cell carcinoma: A pilot study in Bidar and Gulbarga Districts, Karnataka, India. J Oral Maxillofac Pathol. 2019;23:90–6. doi: 10.4103/jomfp.JOMFP_116_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozlü T, Bülbül Y, Oztuna F, Can G. Time course from first symptom to the treatment of lung cancer in the Eastern Black Sea Region of Turkey. Med Princ Pract. 2004;13:211–4. doi: 10.1159/000078318. [DOI] [PubMed] [Google Scholar]
- 27.Franchini M, Liumbruno GM, Lippi G. The prognostic value of ABO blood group in cancer patients. Blood Transfus. 2016;14:434–40. doi: 10.2450/2015.0164-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramesh G, Katiyar A, Raj A, Kumar A, Nagarajappa R, Pandey A. Assessment of relationship of ABO blood groups among tobacco induced oral cancer patients of Kanpur Population, Uttar Pradesh. J Exp Ther Oncol. 2017;12:129–35. [PubMed] [Google Scholar]
- 29.Kellokumpu S, Hassinen A, Glumoff T. Glycosyltransferase complexes in eukaryotes: Long-known, prevalent but still unrecognized. Cell Mol Life Sci. 2016;73:305–25. doi: 10.1007/s00018-015-2066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin T, Li PJ, Chen XZ, Hu WH. ABO blood group is a predictor of survival in patients with laryngeal cancer. Chin J Cancer. 2016;35:2–7. doi: 10.1186/s40880-016-0152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]