Abstract
Introduction:
The significance of membranes as wound dressing in oral surgeries has been reported by previous studies. The aim of the present split-mouth randomized clinical study was to assess and compare the wound dressing properties of acellular dermal matrix (ADM) and cryopreserved human amniotic membrane (AM) after reconstructive preprosthetic oral surgery.
Materials and Methods:
Twenty-eight patients with complete mandibular edentulism and resorbed alveolar bone were included. After taking mandibular impression, a clear acrylic splint with increased labial flange height was created. In each participant, labial vestibular depth was elevated using the Clark's technique. Subsequently, half of the exposed periosteum was covered with ADM while the other half was covered with cryopreserved human AM. Vestibule depth and relapse in the two sides were measured immediately after vestibuloplasty and at the end of the 1st week, 2nd week, 1st month, and 3rd months with graduations of 0.1 mm. Furthermore, after 3 and 7 days, samples were collected from graft material, and the macrophage population was analyzed by flow cytometry.
Results:
There was no significant difference in the relapse of vestibule depth between the two grafts at different time intervals. However, the frequency of wound-infiltrating macrophages (CD68+ cells) was significantly higher in areas covered by ADM after 3 and 7 days.
Discussion:
ADM is as effective as cryopreserved AM in terms of maintaining the postoperative vestibular depth. On the other hand, our results suggested that the onset of healing phase in ADM-covered areas occurs faster compared to the periosteum covered with cryopreserved human AM. This clinical trial showed significantly faster postoperative healing onset when ADM was used than when cryopreserved human AM was applied on the periosteum.
Keywords: Acellular dermal matrix, CD68+ cell, cryopreserved human amniotic membrane, vestibular depth, vestibuloplasty
INTRODUCTION
The height of lower anterior ridge plays a crucial role in stability, convenience, and function of the complete mandibular removable prostheses.[1] In order to obtain a satisfactory denture-bearing area, lower anterior ridges with insufficient quality and quantity can undergo different types of procedures such as implant placement and vestibuloplasty.[2,3] It has been shown that dental implants can not only provide appropriate stability and convenience but can relatively prevent alveolar bone resorption as well.[4,5,6,7] However, in addition to prohibitive cost and difficulties in providing the necessary hygiene, implant placement is contraindicated in patients with epilepsy, diabetes, endocarditis, osteoradionecrosis, cardiac transplant, myocardial infarction, immunosuppressive disorders, active treatment of malignancies, and drug abuse. Therefore, vestibuloplasty can be employed as a promising surrogate to overcome these contraindications.[4] Through this surgical procedure, the soft-tissue attachments are retracted to improve the residual bone height and to provide a nondisplaceable denture-bearing base.[8] Following this procedure, the exposure of periosteum can lead to common adverse consequences such as infection, relative relapse of initial attachments, patient discomfort, scar formation, and poor healing.[8,9,10] Previous studies suggested that covering the raw periosteal surfaces can prevent these complications. Among various covering materials, split-thickness skin grafts and human amniotic membrane (AM) are considered as the favorable covering materials in oral and maxillofacial surgical procedures.[11,12,13,14] However, there is no well-documented evidence that indicates which of one can serve as the candidate covering material in the oral cavity.
Applications of fresh and preserved forms of the human AM in clinical studies suggest that it is a descent membrane for wound covering, thanks to its low immunogenicity, low cost, availability, epithelialization-stimulating potential, and anti-inflammatory properties.[11,12,13,15,16,17,18,19,20] Although the acellular dermal matrix (ADM) was firstly developed for covering the full-thickness burn lesions, the histologic and clinical results from oral and craniofacial studies suggested that its unique characteristics including easy handling, keratinization inducing nature, appropriate root coverage, and scar-relieving potential make it a promising substitute for other covering materials in the dental and oral surgeries.[21,22,23,24,25,26]
It has been previously shown that both ADM and AM as wound-dressing membranes contribute to improve wound healing after surgical procedures in the oral cavity.[11,23,24,27,28,29] However, there was no study comparing the efficacy and wound-covering properties of these two membranes. The current split-mouth study aims to implement a reliable comparison between these two membranes on both macroscopic and molecular scales. For this purpose, the relapse of vestibular depth and the influx of macrophages to the periosteum were investigated and compared in the presence of cryopreserved AM and ADM.
MATERIALS AND METHODS
In this comparative study, twenty-eight patients consisting of 15 females and 13 males (mean age = 58 years), referred from the Department of Prosthodontics, were included between August 2017 and March 2018. The inclusion criteria were a minimum bone height of 2 cm and no previous alveolar bone augmentation procedure in the mandible. Patients with medical contraindications, those who smoke or consume alcohol, and those who are on medications were excluded to avoid their interaction with wound healing. Approval for the study was obtained from the Iranian Registry of Clinical Trials (IRCT 2017082835160N1).
Informed consent, approved by the research ethics board, was signed by each patient prior to the surgical procedure. The inclination of the anterior wall of the mandibular symphysis and the alveolar bone height were determined using a lateral cephalogram and a panoramic radiograph, respectively [Figure 1].
Figure 1.
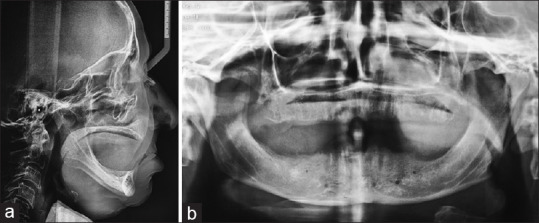
Preoperative radiographs: (a) Lateral cephalometric radiograph demonstrating the inclination of the anterior wall of the mandible symphysis. (b) Panoramic radiograph demonstrating adequate bone height for vestibuloplasty
In the first step, preoperative mandibular impression was taken, and a study cast was made for each patient for fabricating clear acrylic splints. The labial vestibular depth was increased 1 cm by scrapping the cast. Next, considering the inclination of the anterior wall of the body of the mandible, the splint was fabricated enough to cover the scrapped area, finished, and polished.
Two hours prior to the surgery, all patients received a preoperative dose of 2 g amoxicillin orally. After receiving local anaesthesia of lidocaine 2% with epinephrine 1/100,000, a labial-based mucosal supraperiosteal flap was released from the underlying periosteum through a horizontal incision of the mucogingival border, extending from the right to the left premolar area. The flap was then apically positioned and sutured with 4-0 Vicryl to the periosteum at the depth of newly created labial vestibule, which was 10 mm apical to the initial attachment. In each patient, the right and left exposed periostea were covered with cryopreserved human AM group (NEOX 100 Wound Allograft, AMNIOX) and ADM group (AlloDerm, BioHorizons), randomly. The splint, lined with the soft liner to establish minimal dead space, was fixed with two 7-mm bone screws [Figure 2]. Oral regimen of antibiotics of 0.5 g TID was continued for 7 days.
Figure 2.
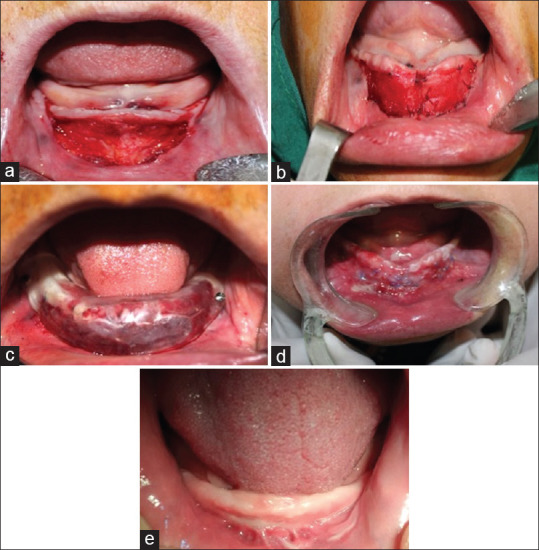
Vestibuloplasty: (a) Apically positioned supraperiosteal flap. (b) Suturing of amniotic membrane and acellular dermal matrix to denuded periosteum. (c) Fixing the lined splint by bone screws. (d) Surgical site 2 weeks postoperatively. (e) Surgical site 3 months postoperatively
Preoperative and postoperative assessments were performed by a blinded examiner. After removing the splint, vestibular depth and infection of the areas were examined on days 7, 14, 28, and 90. The labial vestibular depth measurement was performed during follow-up sessions, at three points, with graduations of 0.1 mm: at the proximal and distal end of the grafts as well as the center. The average height of these three points was reported as the final vestibular depth in each area.
To evaluate the infiltration of macrophages into the wound site, tissue samples, harvested from the graft margins at days 3 and 7, were analyzed by flow cytometry. The collected samples were washed in phosphate-buffered saline (PBS), containing antibiotics (penicillin and streptomycin). After measuring the sample's wet mass, they were cut into 1–3 mm pieces. The pieces were added to PBS on ice and washed 3 times. These were digested with 1.2 IU of Dispase (Roche, Indianapolis) for 1 h at 37°C. Minced tissues were then incubated with trypsin-ethylenediaminetetraacetic acid 0.05% solution for 10 min at room temperature. The cell suspension was sieved through a fine mesh to eliminate clumps and segments. This was followed by centrifugation of dissociated cells at 400 g for 10 min at 2°C and resuspension of the cell pellet in fluorescence activated cell sorting (FACS) buffer. Next, to count the living cells, 10 μl of the sample was mixed with trypan blue solution and counted using a hemocytometer. The results gave an estimate of 2.8–3 × 106 cells/gram of tissue. Then, 1 × 106 of the obtained cells were washed with staining buffer for 5 min. Fc receptors were blocked, and cells were incubated with phycoerythrin-conjugated anti-CD68 monoclonal antibody (Y1/82A; mouse IgG2b) or appropriate isotype control antibody for 20 min. Once washed with the staining buffer, flow cytometric assay was conducted and all data were analyzed using FlowJo software (Flowjo LLC, Ashland, OR, USA).
All results were expressed as mean ± standard deviation. Statistical analysis was performed by GraphPad Prism v6.07 software (GraphPad Software Inc., San Diego, CA, USA). First, the Shapiro–Wilk test was used to evaluate the normality of data sets acquired from each experiment. Comparisons between the two groups were made by Student's t-test, and P < 0.05 was considered to be statistically significant.
RESULTS
After 3 months, the tissues resulting from both membranes were clinically nonkeratinized and fixed to the underlying bone. There were no complications such as burning sensation, clinical evidence of infection, mental nerve paresthesia, or graft rejection on either side in all participants.
The Shapiro–Wilk test showed that the distribution of data collected from the vestibule depth and relapse in both types of the grafts was normal (P > 0.05). The results indicated that the reduction rate and vestibular depth loss were remarkably higher in the AM group comparing to that of the ADM group at different time intervals [Table 1]. Moreover, a similar trend of reduction in vestibular depth was observed on both sides [Figure 3]. These data suggested that during the follow-up period, ADM can maintain the vestibule depth more efficiently, resulting in less morbidity at the donor site.
Table 1.
Reduction rate in vestibule depth at different time intervals
| Time intervals | AM group | ADM group | P |
|---|---|---|---|
| 1 week | 0.173±0.021a | 0.085±0.013 | 0.011* |
| 2 weeks | 0.228±0.016 | 0.159±0.017 | 0.032* |
| 1 month | 0.264±0.014 | 0.168±0.020 | 0.008** |
| 3 months | 0.318±0.022 | 0.217±0.017 | 0.007** |
*P<0.05 and **P<0.01, respectively, aData are expressed as mean±SEM of the reduction rate (n=28 patients/group). ADM: Acellular dermal matrix, AM: Amniotic membrane, SEM: Standard error of mean
Figure 3.
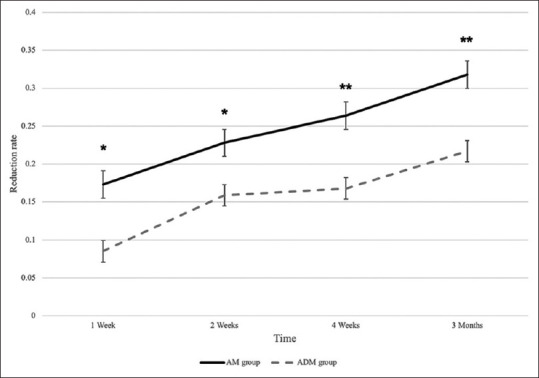
The trend of reduction rate in vestibule depth among AM and ADM groups. Assessing the relapse of vestibule depth showed that the reduction rate was significantly higher in amniotic membrane-covered regions than ADM group at four different points of follow-up within 3 months. The trend of reduction rate was similar in two groups. * and ** indicate P < 0.05 and P < 0.01, respectively.
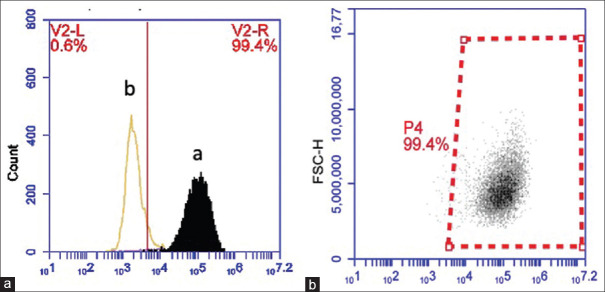
Flow cytometry analyses confirmed the presence of CD68+ in wound regions at different time intervals [Figure 4]. The Shapiro–Wilk test indicated that the distribution of all data obtained from flow cytometry analysis was normal (P > 0.05). The results were expressed as mean ± standard error of mean of absolute number of cells per gram of wet tissue. As it is shown in Figure 5, the frequency of wound-infiltrating macrophages (CD68+ cells) per gram of collected tissue was significantly higher in the ADM group after 3 and 7 days (P < 0.05), suggesting that the wound healing procedure was initiated earlier in the presence of ADM.
Figure 4.
Cell surface marker expression profile of extracted cells. Flow cytometric analysis demonstrating that obtained cells can be categorized into negative (a) and positive (b) for CD68. Only representative examples are shown here
Figure 5.
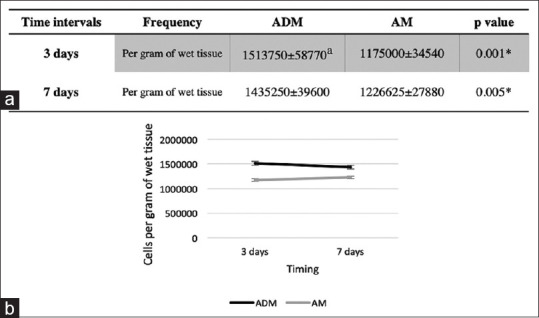
Macrophage infiltration assessment. (a) Number of macrophages at different sites and time intervals. (b) Representative data for CD68+ cells based on the CD68+ gate; the numbers indicate the absolute number of macrophages per gram of wet tissue. aData are expressed as mean ± standard error of the mean for 28 patients/group. *indicates P < 0.05
DISCUSSION
In the current study, the healing process in wounds covered by ADM and cryopreserved human AM as periosteum-covering membranes was investigated and compared. Since this study was performed in a split-mouth randomized clinical fashion, it allowed a good comparison between these membranes regardless of the status of the participants.
There have been reports on covering the periosteum postoperatively in the oral cavity. Samandari et al. used fresh AM in vestibuloplasty. In this study, the clinical and histological assays revealed that fresh AM is an appropriate membrane for covering the denuded periosteum after vestibuloplasty, accelerating the healing procedure, and preventing the depth reduction in the buccal vestibule.[13] Similarly, fresh AM was used after mandibular vestibuloplasty in the studies of Kothari et al. along with Sharma et al. They reported that the use of AM results in less postoperative morbidity.[19,30] In a study conducted by Güler et al., blood flow to the areas covered with lyophilized AM was measured after Clark's or Kazanjian's vestibuloplasty. Interestingly, it has been shown that it has promoted angiogenesis after 10–15 days.[31] Sikkerimath et al., along the same lines, reported that the application of amnion as a graft material after Clark's vestibuloplasty maintained the buccal vestibular depth more effectively in comparison with areas not covered with amnion.[32]
Successful uses of ADM in gingival recession have been widely reported in the literature. They suggested that the usage of this covering material could provide appropriate keratinized tissue and complete root coverage.[28,33,34,35] In addition, in a split-mouth design study, Hashemi et al. found that the width of fixed tissue after vestibuloplasty was significantly lower in the ADM-covered area than that in the mucosal graft-covered areas. Nevertheless, there was no significant difference in the relapse of the depth of labial vestibule between the two groups.[29]
In accordance with previous reports, the results of the current study demonstrated that unlike the similar clinical results of both covering materials, the healing response at cellular level was initiated faster in ADM-covered periosteum. The porosity of the covering materials is one of the most crucial parameters that play a key role in cell migration and directing the tissue formation.[36,37,38,39,40] Furthermore, a scaffold with appropriate level of porosity can result in more efficient angiogenesis at the wound site.[13,37,38,41] The hypothesis that the pore size and microarchitecture within the ADM are more favorable for covering the periosteum was supported by the flow cytometry data. These observations indicated a higher level of infiltrating macrophages per mass of tissue, owing to the specific structure of ADM, providing a favorable anchorage for cell migration.
Financial support and sponsorship
This work was supported by the Mashhad University of Medical Science (Grant number: ACE189021).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kawai Y, Ikeguchi N, Suzuki A, Kuwashima A, Sakamoto R, Matsumaru Y, et al. A double blind randomized clinical trial comparing lingualized and fully bilateral balanced posterior occlusion for conventional complete dentures. J Prosthodont Res. 2017;61:113–22. doi: 10.1016/j.jpor.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Singh O, Kaur R, Nanda S, Sethi E. Residual ridge resorption: A major oral disease entity in relation to bone density. Indian J Oral Sci. 2016;7:3–7. [Google Scholar]
- 3.Ku JK, Leem DH. Vestibuloplasty covering titanium mesh with grafted free gingiva on anterior mandible: Technical report and rationale. J Korean Assoc Oral Maxillofac Surg. 2019;45:369–73. doi: 10.5125/jkaoms.2019.45.6.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim HC, An SC, Lee DW. A retrospective comparison of three modalities for vestibuloplasty in the posterior mandible: Apically positioned flap only vs. free gingival graft vs. collagen matrix. Clin Oral Investig. 2018;22:2121–8. doi: 10.1007/s00784-017-2320-y. [DOI] [PubMed] [Google Scholar]
- 5.Schimmel M, Müller F, Suter V, Buser D. Implants for elderly patients. Periodontol 2000. 2017;73:228–40. doi: 10.1111/prd.12166. [DOI] [PubMed] [Google Scholar]
- 6.Aliabadi E, Tavanafar S, Khaghaninejad MS. Marginal bone resorption of posterior mandible dental implants with different insertion methods. BMC Oral Health. 2020;20:31–7. doi: 10.1186/s12903-020-1019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manor Y, Simon R, Haim D, Garfunkel A, Moses O. Dental implants in medically complex patients – A retrospective study. Clin Oral Investig. 2017;21:701–8. doi: 10.1007/s00784-016-1937-6. [DOI] [PubMed] [Google Scholar]
- 8.Fröschl T, Kerscher A. The optimal vestibuloplasty in preprosthetic surgery of the mandible. J Craniomaxillofac Surg. 1997;25:85–90. doi: 10.1016/s1010-5182(97)80050-9. [DOI] [PubMed] [Google Scholar]
- 9.Hillerup S. Preprosthetic mandibular vestibuloplasty with split-skin graft. A 2-year follow-up study. Int J Oral Maxillofac Surg. 1987;16:270–8. doi: 10.1016/s0901-5027(87)80147-9. [DOI] [PubMed] [Google Scholar]
- 10.Samit A, Popowich L. Mandibular vestibuloplasty: A clinical update. Oral Surg Oral Med Oral Pathol. 1982;54:141–7. doi: 10.1016/0030-4220(82)90208-0. [DOI] [PubMed] [Google Scholar]
- 11.Fénelon M, Catros S, Fricain JC. What is the benefit of using amniotic membrane in oral surgery? A comprehensive review of clinical studies. Clin Oral Investig. 2018;22:1881–91. doi: 10.1007/s00784-018-2457-3. [DOI] [PubMed] [Google Scholar]
- 12.Bansal N, Gupta ND. Human Amniotic Membrane Graft used in Vestibuloplasty: A Case Report. J Dent and Oral Disord. 2016;2:1038. [Google Scholar]
- 13.Samandari MH, Yaghmaei M, Ejlali M, Moshref M, Saffar AS. Use of amnion as a graft material in vestibuloplasty: A preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:574–8. doi: 10.1016/S107921040400006X. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt CM, Tudor C, Kiener K, Wehrhan F, Schmitt J, Eitner S, et al. Vestibuloplasty: Porcine collagen matrix versus free gingival graft: A clinical and histologic study. J Periodontol. 2013;84:914–23. doi: 10.1902/jop.2012.120084. [DOI] [PubMed] [Google Scholar]
- 15.Perepelkin J, Hayward K, Mokoena T, Bentley MJ, Ross-Rodriguez LU, Marquez-Curtis L, et al. Cryopreserved amniotic membrane as transplant allograft: Viability and post-transplant outcome. Cell Tissue Bank. 2016;17:39–50. doi: 10.1007/s10561-015-9530-9. [DOI] [PubMed] [Google Scholar]
- 16.Dehghani M, Azarpira N, Mohammad Karimi V, Mossayebi H, Esfandiari E. Grafting with cryopreserved amniotic membrane versus conservative wound care in treatment of pressure ulcers: A randomized clinical trial. Bull Emerg Trauma. 2017;5:249–58. doi: 10.18869/acadpub.beat.5.4.452.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizzo S, Caporossi T, Tartaro R, Finocchio L, Franco F, Barca F, et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina. 2019;39(Suppl 1):S95–03. doi: 10.1097/IAE.0000000000002320. [DOI] [PubMed] [Google Scholar]
- 18.Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting – A review. Cell Tissue Bank. 2017;18:193–204. doi: 10.1007/s10561-017-9618-5. [DOI] [PubMed] [Google Scholar]
- 19.Kothari CR, Goudar G, Hallur N, Sikkerimath B, Gudi S, Kothari MC. Use of amnion as a graft material in vestibuloplasty: A clinical study. Br J Oral Maxillofac Surg. 2012;50:545–9. doi: 10.1016/j.bjoms.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Waikakul S, Chumniprasas K, Setasubun S, Vajaradul Y. Application of freeze-dried amniotic membrane: A control trial at the donor site of split-thickness skin grafting. Bull Hosp Jt Dis Orthop Inst. 1990;50:27–34. [PubMed] [Google Scholar]
- 21.Lee KT, Mun GH. Updated evidence of acellular dermal matrix use for implant-based breast reconstruction: A meta-analysis. Ann Surg Oncol. 2016;23:600–10. doi: 10.1245/s10434-015-4873-9. [DOI] [PubMed] [Google Scholar]
- 22.Garvey PB, Giordano SA, Baumann DP, Liu J, Butler CE. Long-term outcomes after abdominal wall reconstruction with acellular dermal matrix. J Am Coll Surg. 2017;224:341–50. doi: 10.1016/j.jamcollsurg.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Guan W, Liao H, Guo L, Wang C, Cao Z. Root coverage using a coronally advanced flap with or without acellular dermal matrix: A meta-analysis. J Periodontal Implant Sci. 2016;46:22–34. doi: 10.5051/jpis.2016.46.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavelli L, Barootchi S, Di Gianfilippo R, Modarressi M, Cairo F, Rasperini G, et al. Acellular dermal matrix and coronally advanced flap or tunnel technique in the treatment of multiple adjacent gingival recessions. A 12-year follow-up from a randomized clinical trial. J Clin Periodontol. 2019;46:937–48. doi: 10.1111/jcpe.13163. [DOI] [PubMed] [Google Scholar]
- 25.de Resende DR, Greghi SLA, Siqueira AF, Benfatti CA, Damante CA, Ragghianti Zangrando MS. Acellular dermal matrix allograft versus free gingival graft: A histological evaluation and split-mouth randomized clinical trial. Clin Oral Investig. 2019;23:539–50. doi: 10.1007/s00784-018-2470-6. [DOI] [PubMed] [Google Scholar]
- 26.Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21:243–8. doi: 10.1016/0305-4179(95)93866-i. [DOI] [PubMed] [Google Scholar]
- 27.Rana D, Mehrotra N. Human amniotic membrane: Hope in periodontal regeneration. Int J Sci Res. 2016;5:564–9. [Google Scholar]
- 28.Cevallos CA, de Resende DR, Damante CA, Sant’Ana AC, de Rezende ML, Greghi SL, et al. Free gingival graft and acellular dermal matrix for gingival augmentation: A 15-year clinical study. Clin Oral Investig. 2020;24:1197–203. doi: 10.1007/s00784-019-02983-0. [DOI] [PubMed] [Google Scholar]
- 29.Hashemi HM, Parhiz A, Ghafari S. Vestibuloplasty: Allograft versus mucosal graft. Int J Oral Maxillofac Surg. 2012;41:527–30. doi: 10.1016/j.ijom.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Sharma Y, Maria A, Kaur P. Effectiveness of human amnion as a graft material in lower anterior ridge vestibuloplasty: A clinical study. J Maxillofac Oral Surg. 2011;10:283–7. doi: 10.1007/s12663-011-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Güler R, Ercan MT, Ulutunçel N, Devrim H, Uran N. Measurement of blood flow by the 133Xe clearance technique to grafts of amnion used in vestibuloplasty. Br J Oral Maxillofac Surg. 1997;35:280–3. doi: 10.1016/s0266-4356(97)90048-6. [DOI] [PubMed] [Google Scholar]
- 32.Jayapalan D, Sikkerimath B, Dandagi S, Gudi S. Comparison of vestibular sulcus depth in vestibuloplasty using standard Clark′s technique with and without amnion as graft material. Ann Maxillofac Surg. 2012;2:30–7. doi: 10.4103/2231-0746.95313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris RJ. A short-term and long-term comparison of root coverage with an acellular dermal matrix and a subepithelial graft. J Periodontol. 2004;75:734–43. doi: 10.1902/jop.2004.75.5.734. [DOI] [PubMed] [Google Scholar]
- 34.Woodyard JG, Greenwell H, Hill M, Drisko C, Iasella JM, Scheetz J. The clinical effect of acellular dermal matrix on gingival thickness and root coverage compared to coronally positioned flap alone. J Periodontol. 2004;75:44–56. doi: 10.1902/jop.2004.75.1.44. [DOI] [PubMed] [Google Scholar]
- 35.Chambrone L, Valenzuela FS, Oliveira L. Rationale for gingival tissue augmentation and vestibuloplasty around teeth and dental implants. Adv Periodontal Surg. 2020;7:157–76. [Google Scholar]
- 36.Murphy CM, Haugh MG, O’Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31:461–6. doi: 10.1016/j.biomaterials.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 37.Chan EC, Kuo SM, Kong AM, Morrison WA, Dusting GJ, Mitchell GM, et al. Three dimensional collagen scaffold promotes intrinsic vascularisation for tissue engineering applications. PLoS One. 2016;11:e0149799. doi: 10.1371/journal.pone.0149799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy CM, Duffy GP, Schindeler A, O’brien FJ. Effect of collagen-glycosaminoglycan scaffold pore size on matrix mineralization and cellular behavior in different cell types. J Biomed Mater Res A. 2016;104:291–304. doi: 10.1002/jbm.a.35567. [DOI] [PubMed] [Google Scholar]
- 39.Hollister SJ, Maddox RD, Taboas JM. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials. 2002;23:4095–103. doi: 10.1016/s0142-9612(02)00148-5. [DOI] [PubMed] [Google Scholar]
- 40.Sung HJ, Meredith C, Johnson C, Galis ZS. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials. 2004;25:5735–42. doi: 10.1016/j.biomaterials.2004.01.066. [DOI] [PubMed] [Google Scholar]
- 41.Metz C, Duda GN, Checa S. Towards multi-dynamic mechano-biological optimization of 3D-printed scaffolds to foster bone regeneration. Acta Biomater. 2020;101:117–27. doi: 10.1016/j.actbio.2019.10.029. [DOI] [PubMed] [Google Scholar]