Abstract
PURPOSE
To evaluate the use of early assessment of chemotherapy responsiveness by positron emission tomography (PET) imaging to tailor therapy in patients with esophageal and esophagogastric junction adenocarcinoma.
METHODS
After baseline PET, patients were randomly assigned to an induction chemotherapy regimen: modified oxaliplatin, leucovorin, and fluorouracil (FOLFOX) or carboplatin-paclitaxel (CP). Repeat PET was performed after induction; change in maximum standardized uptake value (SUV) from baseline was assessed. PET nonresponders (< 35% decrease in SUV) crossed over to the alternative chemotherapy during chemoradiation (50.4 Gy/28 fractions). PET responders (≥ 35% decrease in SUV) continued on the same chemotherapy during chemoradiation. Patients underwent surgery at 6 weeks postchemoradiation. Primary end point was pathologic complete response (pCR) rate in nonresponders after switching chemotherapy.
RESULTS
Two hundred forty-one eligible patients received Protocol treatment, of whom 225 had an evaluable repeat PET. The pCR rates for PET nonresponders after induction FOLFOX who crossed over to CP (n = 39) or after induction CP who changed to FOLFOX (n = 50) was 18.0% (95% CI, 7.5 to 33.5) and 20% (95% CI, 10 to 33.7), respectively. The pCR rate in responders who received induction FOLFOX was 40.3% (95% CI, 28.9 to 52.5) and 14.1% (95% CI, 6.6 to 25.0) in responders to CP. With a median follow-up of 5.2 years, median overall survival was 48.8 months (95% CI, 33.2 months to not estimable) for PET responders and 27.4 months (95% CI, 19.4 months to not estimable) for nonresponders. For induction FOLFOX patients who were PET responders, median survival was not reached.
CONCLUSION
Early response assessment using PET imaging as a biomarker to individualize therapy for patients with esophageal and esophagogastric junction adenocarcinoma was effective, improving pCR rates in PET nonresponders. PET responders to induction FOLFOX who continued on FOLFOX during chemoradiation achieved a promising 5-year overall survival of 53%.
BACKGROUND
With the rising incidence of esophageal and esophagogastric junction (EGJ) adenocarcinoma, the global burden from this aggressive malignancy is expected to grow dramatically over the next decade, underscoring the critical need for more effective therapies.1 Neoadjuvant chemoradiotherapy followed by surgery has been shown to confer superior survival and enhanced local tumor control compared with surgery alone and is an accepted standard of care for operable esophageal adenocarcinoma.2,3 However, distant failure is common and adjuvant therapy trials have demonstrated poor tolerance and low rates of delivery for additional systemic therapy following preoperative therapy and surgery and decreased efficacy as compared with preoperative chemotherapy.4,5 Moreover, although a variety of chemotherapeutic agents have been included in neoadjuvant chemoradiotherapy trials, the optimal regimen remains to be defined.6-14
CONTEXT
Key Objective
The optimal chemotherapy regimen in the neoadjuvant setting for resectable esophageal and esophagogastric junction (EGJ) cancers is not known, and distant failure rates remain high. Moreover, the efficacy of the concurrent chemotherapy with neoadjuvant chemoradiation cannot be discerned by evaluating the pathologic response because of the use of concurrent radiotherapy. The Alliance/CALGB 80803 trial evaluated whether metabolic response assessment by positron emission tomography (PET) imaging after induction chemotherapy could be used to direct the decision to change chemotherapy during preoperative chemoradiotherapy with the goal of improving pathologic complete response and survival outcomes among PET nonresponders.
Knowledge Generated
With mature follow-up, CALGB 80803 demonstrated that early metabolic response assessment after induction chemotherapy can help tailor neoadjuvant therapy to a patient's individual tumor biology. The primary end point of pathologic complete response improvement in the PET nonresponders was achieved, and this translated into promising 5-year overall survival rates for these patients.
Relevance
This trial demonstrates a viable and promising strategy for patients with resectable esophageal and EGJ cancers that highlights several key principles, including incorporation of induction chemotherapy prior to chemoradiation and the utility of early response assessment by PET that can inform subsequent selection of therapy. Moreover, the particularly robust results observed in modified oxaliplatin, leucovorin, and fluorouracil responders suggest that this chemotherapy backbone may have potential advantages in this clinical context over the more commonly used CROSS regimen (carboplatin-paclitaxel), although such comparisons require further study.
Induction chemotherapy followed by chemoradiotherapy has been evaluated in some studies15,16 and allows for early exposure to systemic therapy prior to undergoing surgery, thereby addressing subclinical systemic disease and increasing the likelihood that patients will receive the chemotherapy. In addition, up-front chemotherapy prior to chemoradiotherapy allows for the assessment of tumor response to the chemotherapeutic regimen rather than to combined chemoradiotherapy, thus providing a window of opportunity to evaluate response and adapt therapy in the setting of suboptimal tumor response.17
One option for evaluating tumor response is the use of metabolic imaging such as 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging. FDG-PET scans are routine for staging esophageal and EGJ cancers and PET scan parameters, such as the maximum standardized uptake value (SUVmax), have been shown to be predictive and prognostic markers.17 The MUNICON phase II trial used metabolic response by PET, defined as a 35% or greater reduction in SUVmax from baseline to 14 days after initiation of chemotherapy, to direct either continuation of current chemotherapy or moving directly to surgery in patients with esophageal and EGJ cancers who were metabolic nonresponders. Adapting therapy and going directly to surgery was associated with an improved median overall survival (OS).17-19 Thus, PET response–adapted treatment may help to tailor multimodality therapy on the basis of early identification of patients who can benefit from changing the treatment regimen in the absence of response. Additionally, this concept of treatment adaption leads to a personalized or tailored treatment approach.
It has been reliably demonstrated that pathologic complete response (pCR) to preoperative treatment for esophageal cancer is prognostic for improved local control and survival.3,10 Although the eradication of disease in the esophagus ensures a complete resection, a pCR also predicts for better systemic response of micrometastatic disease to an active preoperative chemotherapy regimen.3 Therefore, efforts to enhance pCR rates are presumed to translate into more complete resections, fewer local failures, and improved survival. This study evaluated the use of PET response–adapted therapy in patients with esophageal and EGJ adenocarcinoma with the goal of improving oncologic outcomes for these high-risk patients.
METHODS
Study Design and Participants
The Cancer and Leukemia Group B (CALGB) 80803 trial was a randomized, open-label, phase II study designed to evaluate the use of early assessment of chemotherapy responsiveness by metabolic imaging to direct further therapy in patients with esophageal and EGJ adenocarcinoma to improve their response as demonstrated by pCR rates. The study was conducted by the CALGB, which is now a part of Alliance for Clinical Trials in Oncology (Alliance), and enrolled patients at 69 outpatient cancer centers in the United States (Appendix Table A1, online only). The study Protocol (online only) was approved by the institutional review board at each participating center, and all patients provided written, informed consent.
Patients were eligible if they were at least 18 years of age and had surgically resectable, histologically confirmed esophageal adenocarcinoma, including Siewert EGJ adenocarcinomas types 1 and 2, with stage cT1N1-3M0 or T2-4NanyM0 according to the 2010 (7th edition) staging criteria of the American Joint Commission on Cancer. Patients were also required to have Eastern Cooperative Oncology Group performance status 0-1 and adequate renal, hepatic, and cardiac functions. Staging included computed tomography (CT) scan of the chest and abdomen, and locoregional staging was determined by endoscopic ultrasound if technically feasible. All disease (tumor and nodes) was required to be both surgically resectable and capable of inclusion in a radiotherapy field; thus, patients with involved cervical or supraclavicular lymph nodes were not eligible and any T4 tumors with clear evidence of invasion of the vertebral column, heart, great vessels, or tracheobronchial tree were excluded. Patients were required to have an FDG-avid tumor with a maximum standardized uptake value (SUVmax) of ≥ 5.0 in the primary tumor on baseline combined PET-CT scan that conformed to Protocol guidelines.
Eligible patients were enrolled by treating physicians at the participating hospitals and then randomly assigned with equal probability (1:1) to induction treatment with modified FOLFOX6 (oxaliplatin, leucovorin, and fluorouracil [5-FU; FOLFOX]) or CP. Treatment was assigned by computerized central random allocation using a permuted block method with block size of six, stratified by T-stage (T1-2 and T3-4) and nodal status (N0 and N+).
Procedures
Prior to enrollment of patients, institutions were required to be credentialed to participate by the Imaging Core Laboratory (ICL) at The Ohio State University Medical Center if they had not been previously credentialed for PET imaging for any other Alliance study (PET credentialing details are in Appendix 1, online only). All patients underwent a baseline PET scan to evaluate the metabolic activity of the primary tumor as measured by the SUVmax. Prior to establishing eligibility, there was a mandatory central review of the baseline PET scan by imaging experts at the ICL. If a PET scan was not performed per Protocol requirements, repeat PET imaging was required. Once the PET was deemed to have met Protocol requirements and any additional evaluation for study inclusion was complete, patients were randomly allocated to one of two induction chemotherapy arms. Patients were randomly assigned to receive either FOLFOX or CP; details of the chemotherapy administration are in Appendix 1. After completion of induction chemotherapy, a repeat PET was performed during days 36-42 and the change in SUVmax from baseline was assessed. The day 36-42 PET scan was centrally reviewed and patients were categorized as a PET responder or PET nonresponder by the ICL prior to reregistration to proceed with concurrent chemoradiotherapy. PET responders (≥ 35% decrease in SUV) continued on with the same chemotherapy regimen during chemoradiotherapy, whereas PET nonresponders (< 35% decrease in SUV) crossed over to the alternative chemotherapy regimen during chemoradiotherapy.
Site credentialing for radiotherapy was required through Imaging and Radiation Oncology Core Rhode Island, and treatment plans were reviewed and approved centrally prior to the start of radiotherapy. Radiation treatment planning details are in Appendix 1. The prescription dose of radiotherapy was 5,040 cGy in 180-cGy fractions. Radiotherapy was delivered 5 days per week, once per day, for 5 weeks. Digital submission of radiotherapy treatment plans for central review was required. Radiotherapy was started on the first day of the concurrent chemotherapy.
Toxicity was assessed as per National Cancer Institute's Common Terminology Criteria for Adverse Events (version 4.0). Restaging CT scans were undertaken 4-6 weeks after chemoradiotherapy, and surgery was performed at 6-8 weeks after completion of chemoradiotherapy. The type of surgery was not mandated; however, surgical quality assurance data were collected and reviewed by the Surgical Quality Assurance Committee of Alliance. The resection specimens were evaluated by the local pathologists as per detailed trial-specific guidelines. In those cases with a complete response on the resection specimen, the preoperative biopsy was submitted to confirm the initial cancer diagnosis.
After completion of Protocol treatment, follow-up physical examinations and routine laboratory tests were performed every 3 months for 2 years, then every 6 months for additional 3 years. Patients underwent an upper endoscopy annually for 5 years. CT scans were obtained every 6 months for 2 years, then annually until 5 years posttreatment. Patients were followed until their time of death for a maximum of 5 years after Protocol treatment.
Statistical Analysis
The primary end point of this study was the pCR rate of PET nonresponders within each induction treatment group. pCR was defined as complete absence of tumor in the entire resected specimen (ypT0N0). Secondary end points included a comparison of PET response between induction treatment arms, comparison of pCR rates between induction treatment arms among PET responders and nonresponders, and OS for all patients and by PET response and induction therapy group. OS was defined as time from registration to death because of any cause. When examining OS by PET response group, a landmark approach was chosen for OS analysis since the response status was an intermediate outcome and not known at the time of registration. In the landmark OS analysis, OS was landmarked at restaging PET scan date. Dose intensity was defined as the cumulative (total) dose of each agent divided by the expected total dose. Other secondary end points, including anastomotic leak rates, quality of life, and correlative studies will be reported at a later time point.
The primary objective was to induce a pCR rate of 20% in PET nonresponders treated with either induction FOLFOX or CP, who had crossed over to the alternative regimen during radiotherapy. PET nonresponders were defined as patients whose SUV decreased < 35% from baseline after induction chemotherapy. A two-stage Simon's20 design (minimax) was used to test the null hypothesis that the pCR rate among PET nonresponders within each induction regimen is 5% versus 20%. The null hypothesis of 5% was based on the MUNICON 2 trial and a phase II study from Memorial Sloan Kettering Cancer Center that demonstrated a pCR rate of 4% for patients who were PET nonresponders and continued on the same chemotherapy with preoperative radiotherapy.21,22
RESULTS
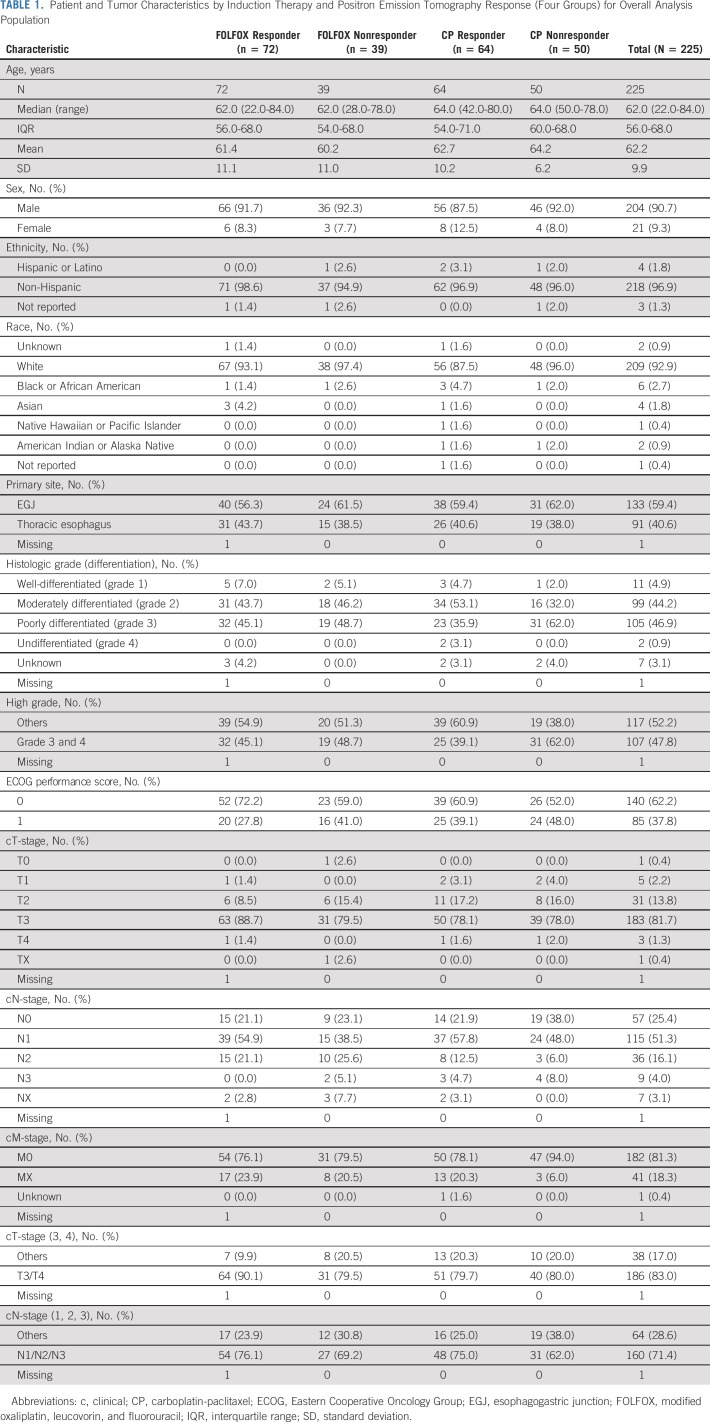
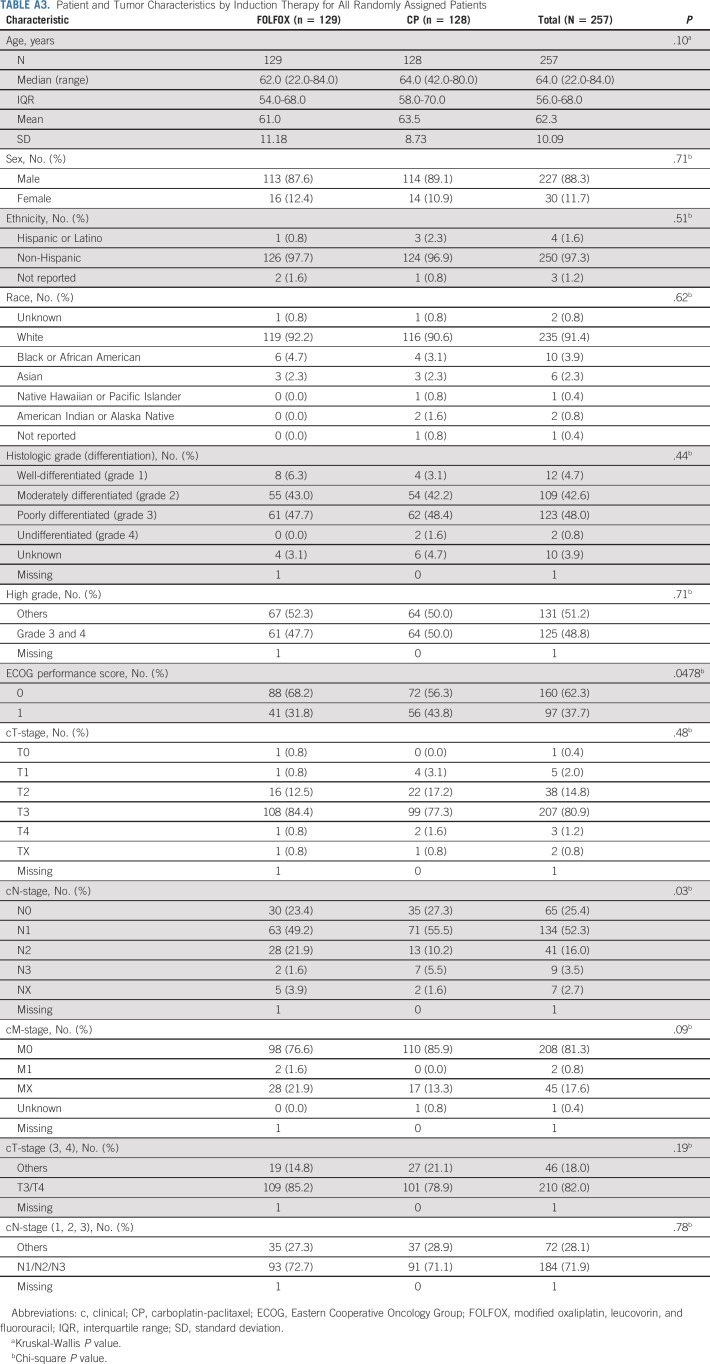
Between November 9, 2011 and May 7, 2015, a total of 257 patients were enrolled and randomly assigned to induction FOLFOX or CP chemotherapy (Fig 1). The final data cutoff analysis date was March 3, 2020. Seven patients on each arm were found to be ineligible, and one eligible patient on each arm declined treatment. A total of 241 eligible patients received induction chemotherapy, and 225 patients had interpretable PET scan after completing induction chemotherapy. Patient and tumor baseline characteristics were balanced between the induction chemotherapy and PET response groups (Table 1). The median age was 62.0 years (interquartile range [IQR]: 56.0-68.0), 204 (90.7%) were male, 209 (92.9%) were White, and 62.2% had Eastern Cooperative Oncology Group performance score 0. A significant majority of patients had T3 (81.7%) and/or node-positive (71.4%) tumors located at the EGJ (59.4%).
FIG 1.
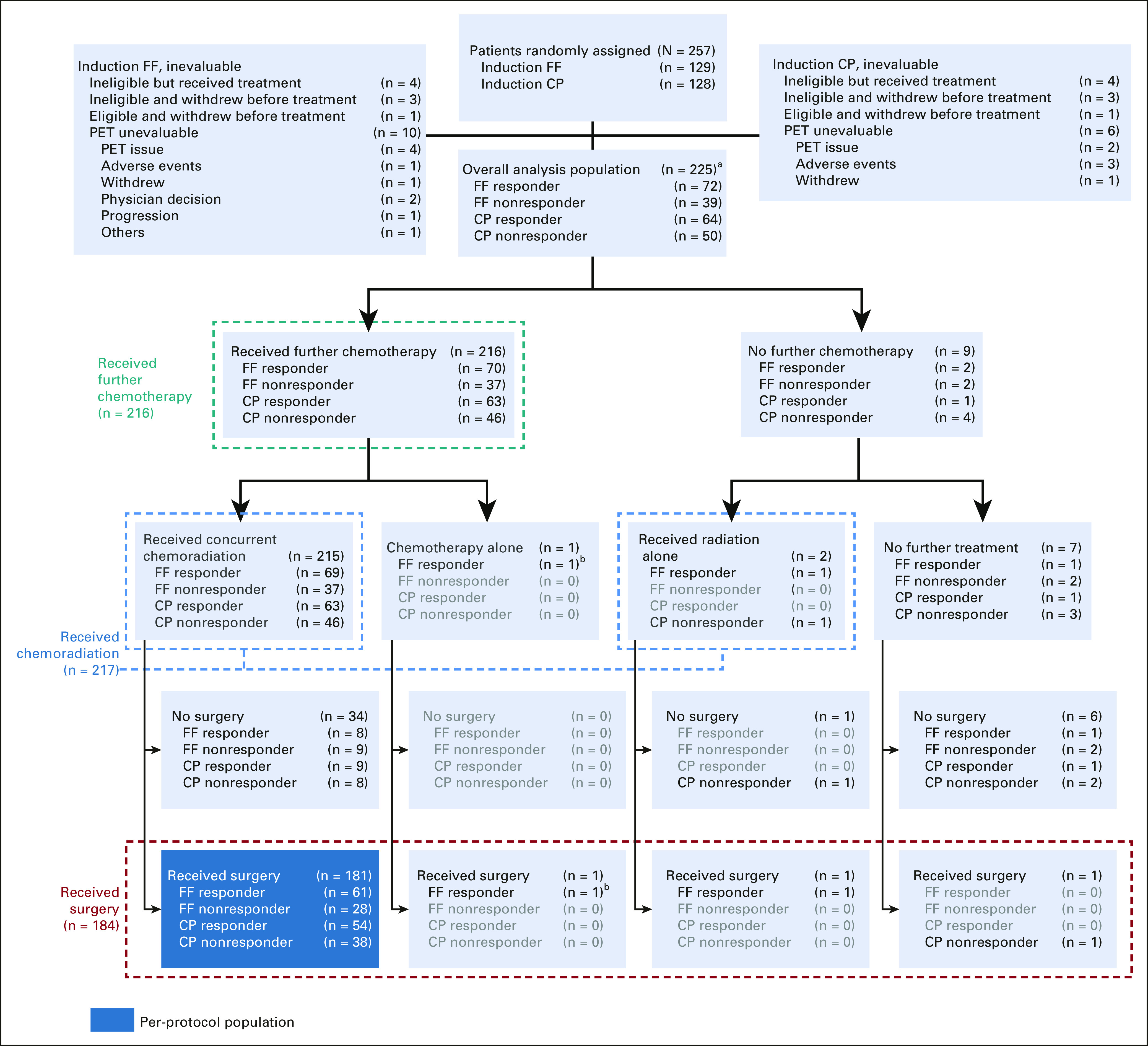
CONSORT diagram. aAll 225 patients received induction chemotherapy; bReceived FOLFOX as the chemotherapy. The induction chemotherapy and PET response groups with zero counts have been grayed out. CP, carboplatin-paclitaxel; FF, FOLFOX (modified oxaliplatin, leucovorin, and fluorouracil); PET, positron emission tomography.
TABLE 1.
Patient and Tumor Characteristics by Induction Therapy and Positron Emission Tomography Response (Four Groups) for Overall Analysis Population
Central review of PET scans (458 scans reviewed) demonstrated that the most common causes for noncompliance with Protocol specifications were as follows: fasting period of < 4 hours (n = 35), blood glucose levels ≥ 200 mg/dL (n = 35), timing between FDG administration and scan time (uptake time outside the window of 60 minutes ± 10 minutes postinjection, n = 14), out-of-window scheduling of the baseline or postinduction PET scan (n = 5), and inconsistency in uptake time between baseline and postinduction scan (n = 2). Overall, adherence to the Protocol requirements for PET procedures and performance metrics was quite good. A weighted compliance score with a maximum score of 100 on the basis of all these criteria was applied to the 458 PET scans performed. Ninety-nine percent of the scans scored in the range of 80-100, which was deemed acceptable. Thus, only 1% scored between 70 and 79, because of more substantial Protocol deviations.
During induction FOLFOX chemotherapy, the median percentage of Protocol dose of 5-FU was 99.3% (IQR: 96.5-100.1) and 99.2% (IQR: 97.3-100.3) for the oxaliplatin. For induction CP, the median Protocol dose of carboplatin and paclitaxel was 100.0% (IQR: 89.08-107.4) and 98.6% (IQR: 94.6-100.2), respectively. There was no statistically significant difference in the rate of PET responders after induction FOLFOX as compared with induction CP (64.9% v 56.1%; P = .22). Of the 225 patients who received induction chemotherapy and had a PET response assessed, 217 proceeded to radiotherapy either with the same chemotherapy for the PET responders (n = 132) or crossing over to the alternative chemotherapy for the PET nonresponders (n = 83) and two patients received radiation alone. One patient who responded to FOLFOX continued on FOLFOX without concurrent radiotherapy. During chemoradiation, the median percentage of Protocol dose was 82.0% (IQR: 60.7-95.6) for 5-FU and 97.6% (IQR: 90.3-99.8) for oxaliplatin. The median percentage of Protocol dose of carboplatin and paclitaxel was 101.9% (IQR: 86.8-114.9) and 98.1% (IQR: 94.4-100.6), respectively. Radiotherapy treatment plans for all patients were centrally reviewed and approved by Imaging and Radiation Oncology Core Rhode Island prior to the start of radiotherapy. The median radiotherapy dose was 5,040 cGy (IQR: 5,040-5,040 cGy and range: 3,600-5,040 cGy) for all patients and 94% were able to receive the planned total of 28 fractions and 98% were able to receive at least 4,140 cGy, which was not significantly different across the four subgroups.
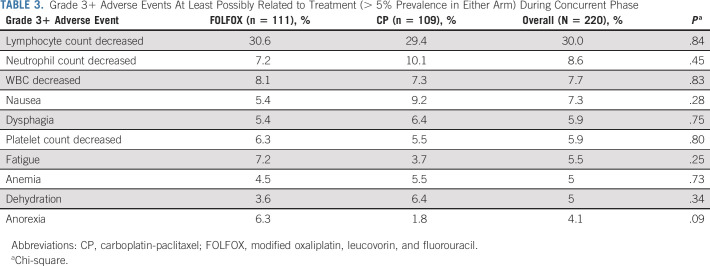
Treatment-related grade 3 or higher adverse events during induction chemotherapy and chemoradiation are shown in Tables 2 and 3. Grade 3 or higher neutropenia occurred in 4.8% of patients on induction FOLFOX and 5.7% during induction CP. Treatment-related adverse events during concurrent chemoradiation were primarily related to lymphopenia (30% grade 3+ in combined concurrent chemoradiation groups). Overall, grade 3+ hematologic toxicity occurred in 37% of patients in both the concurrent FOLFOX and the concurrent CP arm. Nonhematologic adverse events were also similar for both groups and included nausea (7.3%), dysphagia (5.9%), fatigue (5.5%), dehydration (5%), and anorexia (4.1%). Other grade 3 or higher GI toxicities were rare and reported in Appendix 1. There were 5 of 218 (2.2%) patients who died during or after concurrent chemoradiation, prior to surgery, among which only one death is treatment-related (esophageal perforation, probably related to treatment).
TABLE 2.
Grade 3+ Adverse Events At Least Possibly Related to Treatment (> 5% Prevalence in Either Arm) During Induction Phase
TABLE 3.
Grade 3+ Adverse Events At Least Possibly Related to Treatment (> 5% Prevalence in Either Arm) During Concurrent Phase
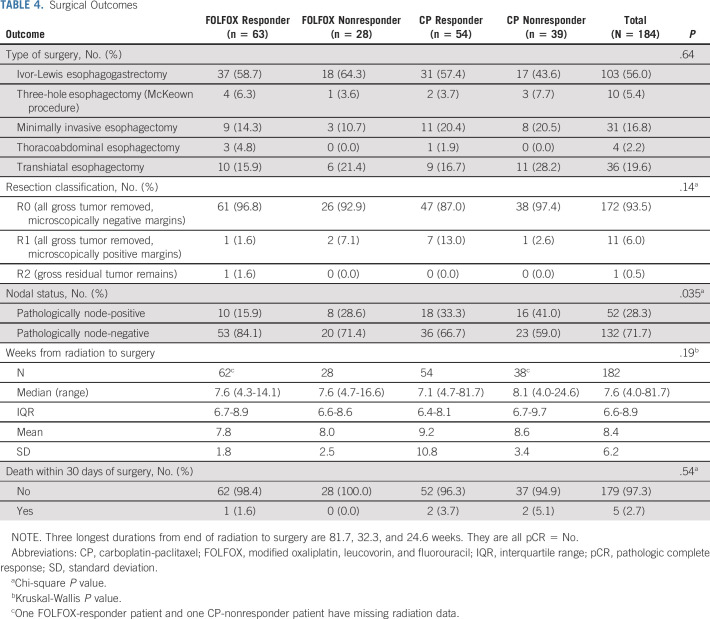
One hundred eighty-four patients underwent surgical resection at a median of 53 days (7.5 weeks) following completion of chemoradiation. Among 111 patients who received induction FOLFOX, 63 of 72 PET responders (87.5%) and 28 of 39 PET nonresponders (71.8%) went to surgery. Among the 114 in the induction CP group, 54 of 64 PET responders (84.4%) and 39 of 50 PET nonresponders (78%) went to surgery. Reasons for not undergoing surgical resection (19 PET responders and 22 PET nonresponders) were disease progression (48.8%), medical concerns and/or poor performance status (24.4%), patient refusal or alternative therapy (14.6%), early death unrelated to their cancer (7.3%), or adverse event (4.9%). Of the 184 patients who proceeded to surgery, 172 patients (93.5%) had R0 resection, 11 patients (6.0%) had microscopically involved margins (R1), and one patient (0.5%) had gross residual tumor at the margin (R2). The R0 resection rates ranged from 87% in the CP responder group and 93% in the FOLFOX nonresponder group to 97% in both the FOLFOX responder and CP nonresponder group (Table 4). The majority (56%) underwent an Ivor-Lewis esophagogastrectomy; the remainder had a transhiatal esophagectomy (19.6%), a minimally invasive esophagectomy (16.8%), 3-hole McKeown procedure (5.4%), or thoracoabdominal esophagectomy (2.2%). There were 6 (3.3%) perioperative deaths (90-day mortality), three in the CP responder group, two in the CP nonresponder group, one in the FOLFOX responder group, and none in the FOLFOX nonresponder group.
TABLE 4.
Surgical Outcomes
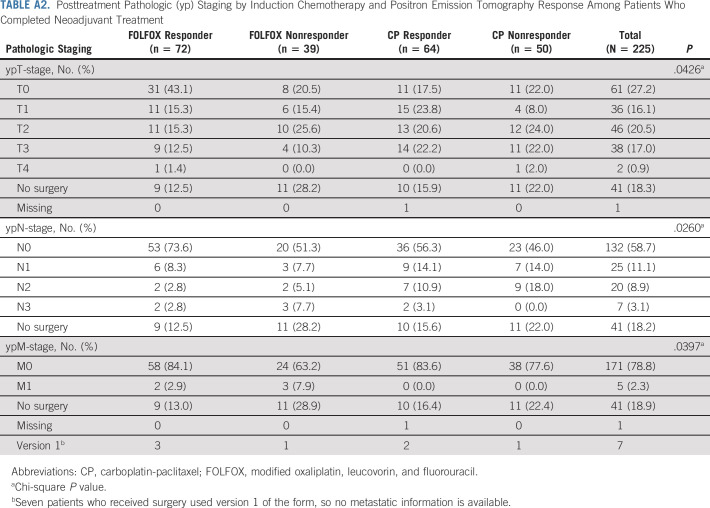
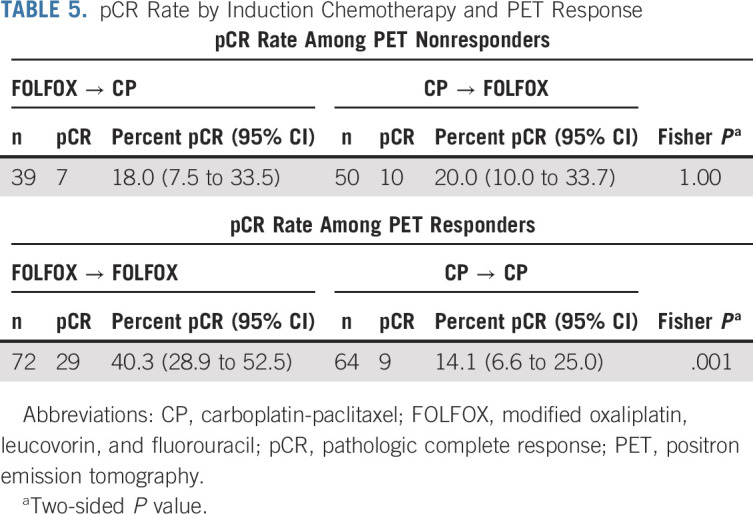
The trial passed the preplanned interim analysis and was fully accrued. Efficacy criteria were met for the primary end point of pCR in the PET nonresponders in both induction groups (prespecified efficacy cutoff pCR ≥ 5). Among the first 38 evaluable FOLFOX nonresponders, six patients had pCR. Among the first 38 evaluable CP nonresponders, six patients had pCR. For all evaluable PET nonresponders after induction FOLFOX who crossed over to CP (n = 39), the pCR was 18.0% (95% CI, 7.5 to 33.5), and for all evaluable PET nonresponders after CP who received FOLFOX (n = 50), pCR was 20% (95% CI, 10 to 33.7) (Table 5). For PET responders in the FOLFOX induction group who continued FOLFOX with chemoradiation (n = 72), the pCR was 40.3% (95% CI, 28.9 to 52.5), whereas in PET responders who received induction CP (n = 9), only 14.1% (95% CI, 6.6 to 25.0) achieved a pCR; this difference was statistically significant (P = .001). The pathologic staging (ypT and ypN) for patients undergoing surgery is shown in Appendix Table A2 (online only). Pathologic node-negative (ypN0) rates were higher for the patients receiving induction FOLFOX, 84.1% for PET responders and 71.4% for PET nonresponders versus those receiving induction CP, 66.7% for responders and 59.0% for nonresponders.
TABLE 5.
pCR Rate by Induction Chemotherapy and PET Response
At the time of data cutoff, with a median follow-up of 5.17 years (IQR: 4.71-5.42), the median OS for evaluable patients was 41.2 months (95% CI, 30.9 to not estimable [NE]) and the 2- and 5-year OS was 63.5% (95% CI, 57.4 to 70.1) and 44.9% (95% CI, 38.8 to 52.0), respectively.
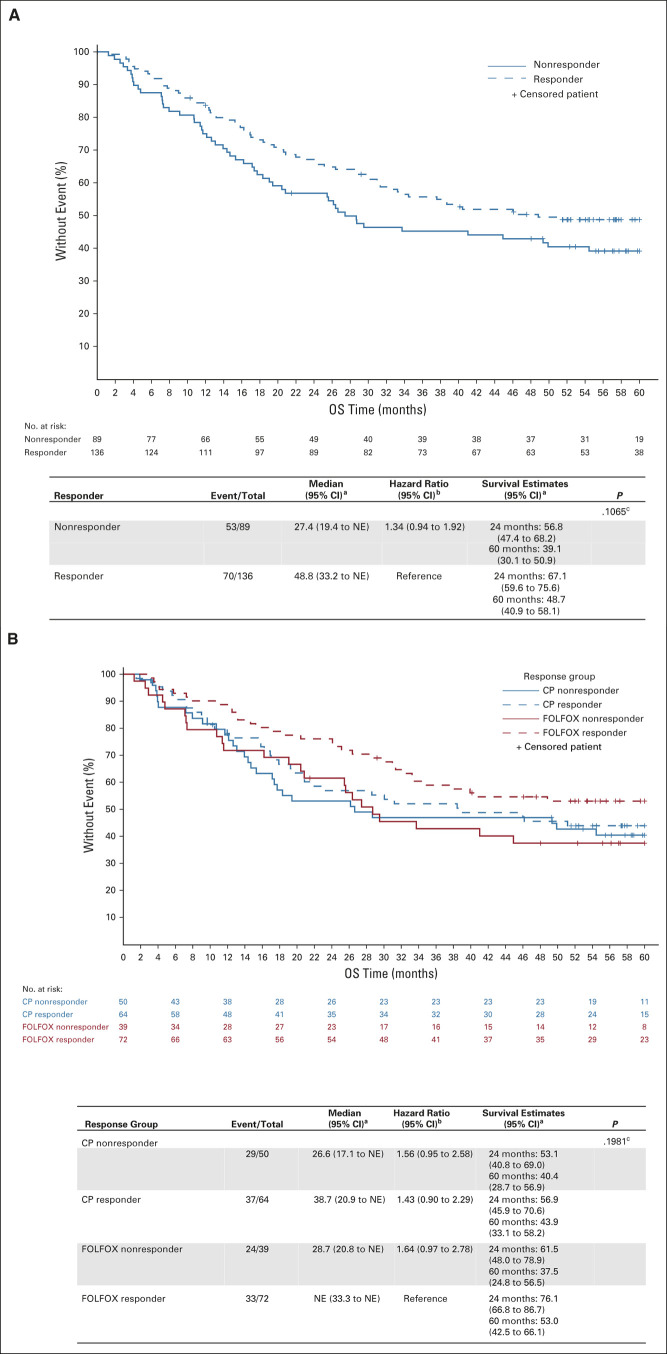
For the PET responders versus nonresponders, median OS was 48.8 months (95% CI, 33.2 to NE) and 27.4 months (95% CI, 19.4 to NE), respectively (Fig 2B). The 2- and 5-year OS rates were 67.1% (95% CI, 59.6 to 75.6) and 48.7% (95% CI, 40.9 to 58.1) for the PET responders and 56.8% (95% CI, 47.4 to 68.2) and 39.1% (95% CI, 30.1 to 50.9) for the PET nonresponders. The hazard ratio for OS between the responders and nonresponders was 1.34 (0.94 to 1.92), which was not statistically significant. For the induction FOLFOX patients who had a PET response and continued on FOLFOX during chemoradiation, the median survival was not reached and the 5-year OS was 53.0% (95% CI, 42.5 to 66.1). In the CP responder group, the median OS was 38.7 months and the 5-year OS was 43.9% (95% CI, 33.1 to 58.2).
FIG 2.
(A) OS by PET response after induction chemotherapy. (B) OS by induction chemotherapy and PET response groups (four groups). aKaplan-Meier method; bCox model; cLog-rank test. CP, carboplatin-paclitaxel; FOLFOX, modified oxaliplatin, leucovorin, and fluorouracil; NE, not estimable; OS, overall survival; PET, positron emission tomography.
DISCUSSION
This phase II randomized trial demonstrated that early response assessment using PET imaging following a short course of induction chemotherapy to direct the change to alternative chemotherapy during preoperative chemoradiation for PET nonresponders was effective and met the primary end point of improving pCR rates in PET nonresponders. Metabolic nonresponders represent a poor-risk group with an expected pCR rate of only 5%; however, by intervening early and tailoring therapy during chemoradiation, the PET nonresponders were able to achieve a pCR rate of 18% among those who switched from FOLFOX to CP and 20% for those who switched from CP to FOLFOX. The mature survival data reflect this improvement in pCR with a median OS of 29 months for PET nonresponders. The median OS in the PET nonresponder group is not significantly worse than the median OS for PET responders (27.4 months v 48.8 months; P = .107), thus suggesting that changing therapy in this poor-risk group brings their outcomes closer to the PET responder group.
The highest pCR rate of 40.3% occurred in PET responders who received both induction and concurrent FOLFOX, and they also achieved a 53% 5-year OS with a median survival that has not been reached, thus demonstrating that enriching the population on the basis of an imaging biomarker to identify responders resulted in an excellent outcome for this group. These results compare very favorably with the 5-year OS rate of approximately 43% for patients with esophageal adenocarcinoma treated with neoadjuvant chemoradiation on the CROSS trial.3 The 30-day postoperative mortality rate in this study was 2.7%, similar to that of other contemporary studies, 5.9% in the CROSS trial and 2.6% in the NEOSCOPE trial, which included induction oxaliplatin-capecitabine followed by random assignment to oxaliplatin-capecitabine or CP with preoperative radiotherapy.3,12
Despite the lower pCR rate in the induction CP responder group, their 5-year OS was 44% and median OS was 39 months, suggesting that the surrogate end point of pCR may not be as reliable for studies evaluating systemic therapy options because of the impact on micrometastatic disease rather than on the response in the primary tumor.23 Although this study was not powered for a head-to-head comparison of the induction chemotherapy groups, the results suggest that the FOLFOX responders may have better long-term outcomes and this induction regimen should be the focus of further studies.
The NRG/RTOG 1010 trial, which enrolled patients during the same time period and evaluated the addition of trastuzumab to preoperative carboplatin and paclitaxel for esophageal adenocarcinoma with human epidermal growth factor receptor 2 overexpression, was recently reported in abstract form. This study had a similar resection rate of 82% and the pCR rate for all patients was 28%, similar to prior studies where patients received up-front chemoradiation.24
The use of induction chemotherapy is a rational approach for patients with esophageal and EGJ adenocarcinomas given the high rate of systemic spread that argues for early intervention for subclinical micrometastases. Moreover, preoperative chemotherapy has additional advantages including downsizing the tumor to allow for better tolerance of chemoradiation by improving dysphagia leading to better oral intake before starting radiotherapy, improved dose delivery versus adjuvant chemotherapy, and increased rates of R0 resection.4-6,25 Although the initial results of the randomized phase II trial of trimodality therapy with or without induction chemotherapy for esophageal cancer at MD Anderson Cancer Center did not initially show a benefit to induction chemotherapy, a secondary analysis in patients with long-term follow-up demonstrated that induction chemotherapy significantly prolonged OS in patients with well-differentiated or moderately differentiated esophageal cancers.14
Biomarker-driven therapy to individualize therapeutic options for patients has been a major focus in oncology; however, the minority of patients with cancer have identifiable and actionable molecular or genetic targets to direct therapy, particularly in esophageal and gastric cancers. Molecular imaging, such as FDG-PET imaging, has been demonstrated to be a promising imaging biomarker in many types of cancers and since PET is considered a standard part of the staging evaluation for esophageal and EGJ cancers, it has been relatively seamlessly integrated into the evaluation of treatment response at various time points. Change in metabolic activity after treatment as measured by a reduction in the SUVmax of ≥ 35% from baseline has been found to be both predictive and prognostic of outcomes.17,18 Ilson et al confirmed that a ≥ 35% decline in SUV on PET scan was still valid in predicting outcomes for patients receiving induction chemotherapy followed by the same chemotherapy with radiation even at 6 weeks.20 The median OS of PET nonresponders from the Alliance/CALGB 80803 trial improves upon the outcomes from previous studies where patients either continued the same regimen for a total of 12 weeks before surgery (median OS = 18 months) or discontinued chemotherapy and went directly to surgery on the MUNICON trial (median OS = 25.8 months).17,19
A limitation of this study is that there was no random assignment between using the PET findings to change therapy versus not changing therapy on the basis of PET. Although the pCR rate improvement of the PET nonresponders as compared with historical controls is suggestive of a benefit, causation cannot be definitively determined. In addition, the small numbers of patients in each subgroup makes the interpretation of the pCR rate difficult, particularly in the CP responder group. Of note, the low pCR rate of 14% in the CP responder group was not consistent with the pCR rates in other studies using concurrent CP, including the CROSS trial2,3 and the NEOSCOPE trial.12
Furthermore, the baseline and postinduction chemotherapy PET scans were performed according to stringent Protocol guidelines and reviewed centrally by expert nuclear medicine physicians at the Alliance ICL. Although molecular imaging with PET has emerged as a powerful imaging tool for assessing therapy response, standardization of imaging and reconstruction protocols, image processing, and analysis to ensure quality control was necessary for a multicenter trial. Central review of PET scans was performed and the rigorous PET scan protocol standards helped to improve the quality of the scans on this study. Nonetheless, as PET imaging is integrated into routine clinical practice for response assessment, the quality issues will need to be addressed to allow for PET to be an accurate biomarker.
The Alliance/CALGB 80803 trial validates the use of early response assessment using PET imaging as a biomarker of response to induction chemotherapy to individualize therapy for patients receiving combined modality therapy for esophageal and EGJ adenocarcinoma and demonstrates that PET response–adapted therapy can improve outcomes in esophageal and EGJ cancers. The FOLFOX regimen followed by 5-FU and oxaliplatin during radiotherapy may have advantages over CP and should be considered for further studies of chemoradiation for esophageal and EGJ adenocarcinomas. Moreover, since PET response–adapted treatment can help to tailor multimodality therapy on the basis of early identification of patients who might benefit from changing the treatment regimen in the absence of response, this approach could be used to introduce newer regimens and targeted therapies into the armamentarium of systemic therapies for this disease.
APPENDIX 1
Supplemental Methods
Additional information on positron emission tomography credentialing
Prior to enrollment of patients, institutions were required to be credentialed to participate by the Imaging Core Laboratory (ICL) at The Ohio State University Medical Center if they had not been previously credentialed for positron emission tomography (PET) imaging for any other Alliance study. This entailed a virtual site visit to review the local PET performance characteristics and infrastructure requirements so that the Protocol specifications for PET scans could be met for the trial patients. If previously credentialed, the ICL required a brief WebEx refresher prior to enrolling patients. Given the potential impact of heterogeneity in the PET performance characteristics in a multicenter study, stringent guidelines for the PET imaging metrics were described in the Protocol to maintain the integrity of the use of PET as a biomarker (see sections 6.0 and 11.0 of Protocol). Oversight and central review by the ICL provided central quality assurance and the ability to document the compliance with the imaging guidelines.
Additional information on radiation treatment planning
Radiation treatment plans were reviewed and approved centrally prior to the start of radiotherapy through Imaging and Radiation Oncology Core Rhode Island. CT-based treatment planning was mandated, and either 3-dimensional conformal or intensity-modulated radiotherapy plans were acceptable. The gross tumor volume encompassed the primary esophageal tumor and enlarged nodes and was based on the prechemotherapy extent of disease using the initial PET scan, endoscopy and/or endoscopic ultrasound report, and CT scan. The clinical target volume (CTV) included the primary esophageal tumor with a proximal and distal margin of 3-4 cm and a 1-cm radial margin, as well as the regional lymphatics. For distal esophageal tumors and GE junction tumors, the CTV included the celiac lymph nodes. The planning target volume margins were 0.5 cm beyond the CTV but could extend to 0.7-1.0 cm superiorly and inferiorly for esophagogastric junction tumors to account for respiratory motion.
Additional information on chemotherapy dosing
Modified oxaliplatin, leucovorin, and fluorouracil (5-FU) (FOLFOX) was administered intravenously (IV) every 2 weeks for three cycles with oxaliplatin (85 mg/m2), leucovorin (400 mg/m2) or levoleucovorin (200 mg/m2), and 5-FU (400 mg/m2 bolus), followed by 5-FU infusion over 48 hours (2,400 mg/m2). For patients 60 years of age or older, the oxaliplatin was administered IV at 70 mg/m2, leucovorin at 300 mg/m2 (or levoleucovorin 150 mg/m2), and the 5-FU bolus at 300 mg/m2, followed by 5-FU 48-hour infusion of 2,000 mg/m2. Patients randomly assigned to carboplatin-paclitaxel (CP) received two 3-week cycles of CP with carboplatin area under the receiver operator characteristic curve (AUC) 2* IV on day 1 and day 8 and paclitaxel 90 mg/m2 IV on day 1 and day 8. Dose modification for patients 60 years of age or older was carboplatin AUC 2* IV on day 1 and day 8 and paclitaxel 70 mg/m2 IV on day 1 and day 8.
The modifications for concurrent treatment with FOLFOX was 5-FU 300 mg/m2/d, over 96 hours via continuous IV infusion every week during radiotherapy. Oxaliplatin 85 mg/m2 IV was given on day 1 every 2 weeks for three cycles. The concurrent CP was administered as carboplatin AUC 2* IV and paclitaxel 50 mg/m2 IV, weekly × 5 weeks. If chemotherapy doses were reduced during induction therapy, those reduced doses were used as the starting doses for combined chemoradiation.
Additional information on statistical design
Assuming a PET nonresponder rate of 50% and that 25% of patients would be nonevaluable, because of uninterpretable PET imaging or failure to go to surgery for reasons unrelated to treatment, approximately 51 nonresponders were initially projected to be required in each induction chemotherapy group to obtain 38 evaluable PET nonresponders. Data review for the interim analysis on this trial revealed a 10% lower than expected proportion of patients who were PET nonresponders. Thus, with only approximately 40% of treated patients who were PET nonresponders, the statistical plan was updated to require 127 patients on each induction therapy to yield 38 evaluable PET nonresponders. Therefore, the sample size of the study was revised to be 254 patients.
On the basis of the Simon's two-stage design, stage 1 ended after 29 evaluable PET nonresponders were randomly assigned to each induction regimen. If one or fewer of those first 29 PET nonresponders in a particular induction regimen experienced a pathologic complete response (pCR), then that treatment regimen would be closed to further accrual because of lack of efficacy. If two or more patients experienced a pCR among the first 29 PET nonresponders, nine additional evaluable patients were to be enrolled to the induction regimen.
Under this design, 38 evaluable PET nonresponders in each induction regimen provided 90% power to detect the specified difference in pCR (approximate one-sided α = .05). Total sample size was determined to be 254. Patients who were evaluable for primary end point were those who were eligible, consented, received induction chemotherapy with Protocol-specified PET scans at both baseline and restaging (ie, prior to reregistration) and were determined to be nonresponder by PET. Patients who had inoperable disease because of disease progression, death, or treatment-related toxicity were considered treatment failures (ie, not a pCR) and were evaluable for the primary end point. Patients who did not go to surgery for reasons unrelated to treatment were considered nonevaluable for the primary end point. The interim analysis was carried out in each induction regimen separately, and it was determined that the study should be fully accrued. For the second stage, with 38 evaluable PET nonresponders, an induction regimen was considered efficacious if five or more patients (13%) achieved a pCR.
Patient and treatment characteristics were examined by induction therapy for all randomly assigned patients (Appendix Table A3, online only) and were reported by induction therapy and PET response resulting in four subgroups (Table 1) among patients with interpretable PET scans. Descriptive statistics are reported. Comparisons of continuous variables were performed using Wilcoxon rank-sum test and Kruskal-Wallis test. Comparisons of categorical variables were performed using chi-square test and Fisher's exact test. Overall survival was estimated with Kaplan-Meier survival curves and differences were tested using log-rank tests. The hazard ratio from Cox proportional hazard model and its 95% CI are also reported. The landmark approach was used for overall survival comparison involving PET responder versus nonresponder. All analyses were conducted using SAS (version 9.4) at a significance level of .05. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
Supplemental Results
GI toxicities during induction chemotherapy were less than 5%, with only 1.6% in FOLFOX and 0.8% in CP experiencing vomiting and no grade 3+ weight loss reported. Grade 3+ GI toxicities that occurred in < 5% of patients during concurrent therapy included the following: esophagitis (4.5% of patients receiving FOLFOX and 0.9% of patients receiving CP), vomiting (0% in FOLFOX and 3.7% in CP), and weight loss (0.9% in FOLFOX and 0.9% in CP).
TABLE A1.
Protocol Sites and Patient Enrollment
TABLE A2.
Posttreatment Pathologic (yp) Staging by Induction Chemotherapy and Positron Emission Tomography Response Among Patients Who Completed Neoadjuvant Treatment
TABLE A3.
Patient and Tumor Characteristics by Induction Therapy for All Randomly Assigned Patients
Karyn A. Goodman
Consulting or Advisory Role: RenovoRx
Tanios Bekaii-Saab
Consulting or Advisory Role: Amgen, Ipsen, Lilly, Bayer, Roche/Genentech, AbbVie, Incyte, Immuneering, Seattle Genetics, Pfizer, Boehringer Ingelheim, Janssen, Eisai, Daiichi Sankyo/UCB Japan, AstraZeneca, Exact Sciences, Natera, Treos Bio, Celularity, SOBI, BeiGene, Foundation Medicine
Patents, Royalties, Other Intellectual Property: Patent WO/2018/183488 and Patent WO/2019/055687
Other Relationship: Exelixis, Merck, AstraZeneca, Lilly, Pancreatic Cancer Action Network
Daniel J. Boffa
Research Funding: Epic Sciences
Wendy L. Frankel
Patents, Royalties, Other Intellectual Property: Patent title: Automated Identification of Tumor Buds. Application No.: 16/230,118. Filing date: December 21, 2018. Publication date: July 4, 2019. Applicant(s): Ohio State Innovation Foundation, Columbus, OH. Inventor(s): Metin Gurcan, Winston-Salem, NC; Wendy Frankel, Columbus, OH; Wei Chen, Columbus, OH; Ahmad Fauzi and Mohammad Faizal, Selangor, MY
Anne Noonan
Consulting or Advisory Role: Helsinn Healthcare, QED Therapeutics, Exelixis, Eisai
Yelena Y. Janjigian
Stock and Other Ownership Interests: Rgenix
Consulting or Advisory Role: Pfizer, Merck, Bristol Myers Squibb, Merck Serono, Daiichi Sankyo, Rgenix, Bayer, Imugene, AstraZeneca, Lilly, Zymeworks, Seattle Genetics, Merck Sharpe and Dohme Corp, Michael J. Hennessy Associates, Jounce Therapeutics, Ono Pharmaceutical
Research Funding: Boehringer Ingelheim, Bayer, Roche, Genentech, Rgenix, Bristol Myers Squibb, Merck, Lilly
Other Relationship: Paradigm, Clinical Care Options, Axis Medical Education, Research to Practice
Paul J. Thurmes
Consulting or Advisory Role: US Oncology
Travel, Accommodations, Expenses: US Oncology
Alan P. Venook
Consulting or Advisory Role: Merck Sharp & Dohme, Array BioPharma
Research Funding: Genentech/Roche, Bristol Myers Squibb, Amgen
Patents, Royalties, Other Intellectual Property: Royalties from Now-UptoDate for authoring and maintaining two chapters
Travel, Accommodations, Expenses: Genentech, Roche
Jeffrey A. Meyerhardt
Honoraria: Cota Healthcare, Taiho Pharmaceutical
Research Funding: Boston Biomedical
Eileen M. O'Reilly
Consulting or Advisory Role: Merck, Agios, AstraZeneca, Bayer, BeiGene, Berry Genomics, Celgene, CytomX Therapeutics, Debiopharm Group, Eisai, Exelixis/Ipsen, Flatiron Health, Incyte, Janssen, LAM Therapeutics, Lilly, Loxo, Genentech/Roche, Minapharma, QED Therapeutics, RedHill Biopharma, Sillajen, SOBI, Yiviva, Autem Medical, Gilead Sciences, Ipsen, Silenseed, TheraBionic, twoXAR, Vector Health
Research Funding: AstraZeneca/MedImmune, Acta Biologica, Bristol Myers Squibb, Celgene, Genentech, Halozyme, MabVax, Roche, Silenseed
David H. Ilson
Consulting or Advisory Role: Lilly/ImClone, Roche/Genentech, Bristol Myers Squibb, Pieris Pharmaceuticals, Merck, Bayer, AstraZeneca, Taiho Pharmaceutical, Astellas Pharma, IQvia, Macrogenics
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented as an oral presentation at the 2017 Gastrointestinal Cancers Symposium San Francisco, CA, January 19, 2017, and in a poster discussion session at the 2018 ASCO Annual Meeting, Chicago, IL, June 1-5, 2018.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology; https://acknowledgments.alliancefound.org), UG1CA233180, UG1CA233253, UG1CA233290, UG1CA233331, UG1CA233337, UG1CA233373, and UG1CA232760. Funding for the PET scans and central pathology review was provided through the Biomarker, Imaging and Quality of Life Studies Funding Program (BIQSFP) from the NCI to support randomized treatment trials with an integral biomarker (PET) or imaging study.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
All data used in the manuscript can be requested. Data can be requested via Alliance data share: https://www.allianceforclinicaltrialsinoncology.org/main/public/standard.xhtml?path=%2FPublic%2FDatasharing.
AUTHOR CONTRIBUTIONS
Conception and design: Karyn A. Goodman, Nathan C. Hall, Tanios Bekaii-Saab, Michael O. Meyers, Donna Niedzwiecki, Alan P. Venook, David H. Ilson
Provision of study materials or patients: Karyn A. Goodman, Tanios Bekaii-Saab, Briant Fruth, Daniel J. Boffa, Anne Noonan, Paul J. Thurmes
Collection and assembly of data: Karyn A. Goodman, Nathan C. Hall, Tanios Bekaii-Saab, Erin Twohy, Michael O. Meyers, Kisha Mitchell, Donna Niedzwiecki, Anne Noonan, Paul J. Thurmes, David H. Ilson
Data analysis and interpretation: Karyn A. Goodman, Fang-Shu Ou, Nathan C. Hall, Tanios Bekaii-Saab, Briant Fruth, Erin Twohy, Michael O. Meyers, Daniel J. Boffa, Wendy L. Frankel, Donna Niedzwiecki, Yelena Y. Janjigian, Alan P. Venook, Jeffrey A. Meyerhardt, Eileen M. O'Reilly
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase II Study of PET Response–Adapted Combined Modality Therapy for Esophageal Cancer: Mature Results of the CALGB 80803 (Alliance) Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Karyn A. Goodman
Consulting or Advisory Role: RenovoRx
Tanios Bekaii-Saab
Consulting or Advisory Role: Amgen, Ipsen, Lilly, Bayer, Roche/Genentech, AbbVie, Incyte, Immuneering, Seattle Genetics, Pfizer, Boehringer Ingelheim, Janssen, Eisai, Daiichi Sankyo/UCB Japan, AstraZeneca, Exact Sciences, Natera, Treos Bio, Celularity, SOBI, BeiGene, Foundation Medicine
Patents, Royalties, Other Intellectual Property: Patent WO/2018/183488 and Patent WO/2019/055687
Other Relationship: Exelixis, Merck, AstraZeneca, Lilly, Pancreatic Cancer Action Network
Daniel J. Boffa
Research Funding: Epic Sciences
Wendy L. Frankel
Patents, Royalties, Other Intellectual Property: Patent title: Automated Identification of Tumor Buds. Application No.: 16/230,118. Filing date: December 21, 2018. Publication date: July 4, 2019. Applicant(s): Ohio State Innovation Foundation, Columbus, OH. Inventor(s): Metin Gurcan, Winston-Salem, NC; Wendy Frankel, Columbus, OH; Wei Chen, Columbus, OH; Ahmad Fauzi and Mohammad Faizal, Selangor, MY
Anne Noonan
Consulting or Advisory Role: Helsinn Healthcare, QED Therapeutics, Exelixis, Eisai
Yelena Y. Janjigian
Stock and Other Ownership Interests: Rgenix
Consulting or Advisory Role: Pfizer, Merck, Bristol Myers Squibb, Merck Serono, Daiichi Sankyo, Rgenix, Bayer, Imugene, AstraZeneca, Lilly, Zymeworks, Seattle Genetics, Merck Sharpe and Dohme Corp, Michael J. Hennessy Associates, Jounce Therapeutics, Ono Pharmaceutical
Research Funding: Boehringer Ingelheim, Bayer, Roche, Genentech, Rgenix, Bristol Myers Squibb, Merck, Lilly
Other Relationship: Paradigm, Clinical Care Options, Axis Medical Education, Research to Practice
Paul J. Thurmes
Consulting or Advisory Role: US Oncology
Travel, Accommodations, Expenses: US Oncology
Alan P. Venook
Consulting or Advisory Role: Merck Sharp & Dohme, Array BioPharma
Research Funding: Genentech/Roche, Bristol Myers Squibb, Amgen
Patents, Royalties, Other Intellectual Property: Royalties from Now-UptoDate for authoring and maintaining two chapters
Travel, Accommodations, Expenses: Genentech, Roche
Jeffrey A. Meyerhardt
Honoraria: Cota Healthcare, Taiho Pharmaceutical
Research Funding: Boston Biomedical
Eileen M. O'Reilly
Consulting or Advisory Role: Merck, Agios, AstraZeneca, Bayer, BeiGene, Berry Genomics, Celgene, CytomX Therapeutics, Debiopharm Group, Eisai, Exelixis/Ipsen, Flatiron Health, Incyte, Janssen, LAM Therapeutics, Lilly, Loxo, Genentech/Roche, Minapharma, QED Therapeutics, RedHill Biopharma, Sillajen, SOBI, Yiviva, Autem Medical, Gilead Sciences, Ipsen, Silenseed, TheraBionic, twoXAR, Vector Health
Research Funding: AstraZeneca/MedImmune, Acta Biologica, Bristol Myers Squibb, Celgene, Genentech, Halozyme, MabVax, Roche, Silenseed
David H. Ilson
Consulting or Advisory Role: Lilly/ImClone, Roche/Genentech, Bristol Myers Squibb, Pieris Pharmaceuticals, Merck, Bayer, AstraZeneca, Taiho Pharmaceutical, Astellas Pharma, IQvia, Macrogenics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Arnold M, Laversanne M, Brown LM.Predicting the future burden of esophageal cancer by histological subtype: International trends in incidence up to 2030 Am J Gastroenterol 1121247–12552017 [DOI] [PubMed] [Google Scholar]
- 2.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer N Engl J Med 3662074–20842012 [DOI] [PubMed] [Google Scholar]
- 3.Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial Lancet Oncol 161090–10982015 [DOI] [PubMed] [Google Scholar]
- 4.Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: A Japan Clinical Oncology Group Study—JCOG9204 J Clin Oncol 214592–45962003 [DOI] [PubMed] [Google Scholar]
- 5.Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907) Ann Surg Oncol 1968–742012 [DOI] [PubMed] [Google Scholar]
- 6.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781 J Clin Oncol 261086–10922008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leichman LP, Goldman BH, Bohanes PO, et al. S0356: A phase II clinical and prospective molecular trial with oxaliplatin, fluorouracil, and external-beam radiation therapy before surgery for patients with esophageal adenocarcinoma J Clin Oncol 294555–45602011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus N Engl J Med 337161–1671997 [DOI] [PubMed] [Google Scholar]
- 9.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: A randomised controlled phase III trial Lancet Oncol 6659–6682005 [DOI] [PubMed] [Google Scholar]
- 10.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma J Clin Oncol 19305–3132001 [DOI] [PubMed] [Google Scholar]
- 11.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma N Engl J Med 335462–4671996 [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee S, Hurt CN, Gwynne S, et al. NEOSCOPE: A randomised phase II study of induction chemotherapy followed by oxaliplatin/capecitabine or CP based pre-operative chemoradiation for resectable oesophageal adenocarcinoma Eur J Cancer 7438–462017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang TC, Hsu CH, Lin CC, et al. Systematic review and network meta-analysis: Neoadjuvant chemoradiotherapy for locoregional esophageal cancer Jpn J Clin Oncol 451023–10282015 [DOI] [PubMed] [Google Scholar]
- 14.Wang T, Yu J, Liu M, et al. The benefit of taxane-based therapies over fluoropyrimidine plus platinum (FP) in the treatment of esophageal cancer: A meta-analysis of clinical studies Drug Des Devel Ther 13539–5532019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ajani JA, Xiao L, Roth JA, et al. A phase II randomized trial of induction chemotherapy versus no induction chemotherapy followed by preoperative chemoradiation in patients with esophageal cancer Ann Oncol 242844–28492013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimodaira Y, Slack RS, Harada K, et al. Influence of induction chemotherapy in trimodality therapy-eligible oesophageal cancer patients: Secondary analysis of a randomised trial Br J Cancer 118331–3372018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ott K, Weber WA, Lordick F, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction J Clin Oncol 244692–46982006 [DOI] [PubMed] [Google Scholar]
- 18.Weber WA, Ott K, Becker K, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging J Clin Oncol 193058–30652001 [DOI] [PubMed] [Google Scholar]
- 19.Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: The MUNICON phase II trial Lancet Oncol 8797–8052007 [DOI] [PubMed] [Google Scholar]
- 20.Simon R.Optimal two-stage designs for phase II clinical trials Control Clin Trials 101–101989 [DOI] [PubMed] [Google Scholar]
- 21.zum Büschenfelde CM, Herrmann K, Schuster T, et al. (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: The MUNICON II trial J Nucl Med 521189–11962011 [DOI] [PubMed] [Google Scholar]
- 22.Ilson DH, Minsky BD, Ku GY, et al. Phase 2 trial of induction and concurrent chemoradiotherapy with weekly irinotecan and cisplatin followed by surgery for esophageal cancer Cancer 1182820–28272012 [DOI] [PubMed] [Google Scholar]
- 23.Petrelli F, Tomasello G, Barni S.Surrogate end-points for overall survival in 22 neoadjuvant trials of gastro-oesophageal cancers Eur J Cancer 768–162017 [DOI] [PubMed] [Google Scholar]
- 24.Safran H, Winter KA, Wigle DA, et al. Trastuzumab with trimodality treatment for esophageal adenocarcinoma with HER2 overexpression: NRG oncology/RTOG 1010. J Clin Oncol. 2020;38 doi: 10.1016/S1470-2045(21)00718-X. suppl; abstr 4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sunde B, Johnsen G, Jacobsen AB, et al. Effects of neoadjuvant chemoradiotherapy vs chemotherapy alone on the relief of dysphagia in esophageal cancer patients: Secondary endpoint analysis in a randomized trial Dis Esophagus 321–92019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in the manuscript can be requested. Data can be requested via Alliance data share: https://www.allianceforclinicaltrialsinoncology.org/main/public/standard.xhtml?path=%2FPublic%2FDatasharing.