Abstract
PURPOSE
In pancreatic cancer (PC), the RAF family alterations define a rare subset of patients that may predict response to inhibition of the BRAF/MEK/ERK signaling pathway. A comprehensive understanding of the molecular and clinical characteristics of RAF-mutated PC may support future development of RAF-directed strategies.
METHODS
Clinical outcomes were assessed across a multi-institutional case series of 81 patients with RAF family-mutated PC. Mutational subgroups were defined on the basis of RAF alteration hotspots and therapeutic implications.
RESULTS
The frequency of RAF alterations in PC was 2.2% (84 of 3,781) within a prevalence cohort derived from large molecular databases where BRAF V600E (Exon 15), BRAF ΔNVTAP (Exon 11), and SND1-BRAF fusions were the most common variants. In our retrospective case series, we identified 17 of 81 (21.0%) molecular profiles with a BRAF V600/Exon 15 mutation without any confounding drivers, 25 of 81 (30.9%) with BRAF or RAF1 fusions, and 18 of 81 (22.2%) with Exon 11 mutations. The remaining 21 of 81 (25.9%) profiles had atypical RAF variants and/or multiple oncogenic drivers. Clinical benefit from BRAF/MEK/ERK inhibitors was observed in 3 of 3 subjects within the V600 subgroup (two partial responses), 4 of 6 with fusions (two partial responses), 2 of 6 with Exon 11 mutations (one partial response), and 0 of 3 with confounding drivers. Outcomes analyses also suggested a trend favoring fluorouracil-based regimens over gemcitabine/nab-paclitaxel within the fusion subgroup (P = .027).
CONCLUSION
Prospective evaluation of RAF-directed therapies is warranted in RAF-mutated PC; however, differential responses to targeted agents or standard regimens for each mutational subgroup should be a consideration when designing clinical trials.
INTRODUCTION
Pancreatic cancer (PC) has a 5-year survival of 9% and is projected to be the second leading cause of cancer-related mortality in the United States before 2030.1,2 Despite the widespread availability of genomic profiling, US Food and Drug Administration (FDA)–approved therapies specifically for PC are mostly limited to combinatorial cytotoxic regimens including FOLFIRINOX,3 gemcitabine with nab-paclitaxel (Gem/nab-P),4 and nal-irinotecan with fluorouracil (5-FU).5 Beyond tumor-agnostic markers for PD-1 inhibitors and Trk inhibitors, each of which is rare in PC (< 1%),6-11 olaparib remains the only targeted therapy for a molecularly defined subset of PC.12 The molecular landscape of PC is dominated by a preponderance of KRAS mutations (92%-93%),6,13-22 limiting the scope of molecularly targeted strategies in PC. In KRAS wild-type PC, activating alterations in oncogenic drivers such as BRAF have been reported as potentially actionable6,13,16,23-26; however, clinical outcomes on standard therapies and targeted therapies are difficult to capture for these rare molecularly defined PC subgroups.
CONTEXT
Key Objective
To quantify the prevalence of RAF alterations in epithelial pancreatic cancer and report real-world outcomes to standard and targeted therapies from a multi-institution case series.
Knowledge Generated
The frequency of RAF alterations in pancreatic cancer was 2.2% (84 of 3,781) within a prevalence cohort derived from large molecular databases where BRAF V600E (Exon 15), BRAF ΔNVTAP (Exon 11), and SND1-BRAF fusions were the most common variants. Clinical benefit from targeted therapies occurred in patients with BRAF V600E mutations, RAF fusion abnormalities, and Exon 11 mutations.
Relevance
In KRAS wild-type pancreatic cancer, certain RAF alterations predict benefit from map-kinase targeted therapy. Further prospective trials are warranted.
Beyond PC, recurrent RAF family alterations are enriched in solid tumors including lung, colon, thyroid, and melanoma.27,28 Multiple BRAF inhibitors (eg, dabrafenib/encorafenib/vemurafenib) have been approved for use as a single agent or in combination with a MEK inhibitor (eg, trametinib/binimetinib/cobimetinib) across multiple disease types including metastatic melanoma, non–small-cell lung cancer and anaplastic thyroid cancer.29-32 Recently, a BRAF inhibitor combined with an anti-EGFR antibody was also approved by the FDA for use in BRAF V600E–mutated colorectal cancer.33
Although BRAF inhibitor combinations have shown promising activity across a broad range of BRAF V600E–mutated tumor types, the feasibility of targeting RAF in PC has not yet been established given the relative rarity of KRAS wild-type tumors, the diversity of RAF alterations seen across PC subtypes, and limited outcomes available from those who have received BRAF/MEK/ERK inhibitors.17,18,34,35
Here, we provide an overview of RAF family alterations in epithelial pancreatic malignancies. By aggregating real-world molecular, clinical, treatment data from multiple institutions and a national registry, we describe the largest case series of RAF-altered PC. By establishing PC-specific RAF mutational subgroups (BRAF Exon 15, BRAF Exon 11, Fusions, and Other) on the basis of potential therapeutic implications, we summarize preliminary outcomes and responses to RAF-directed and standard therapies.
METHODS
RAF Family Alteration Frequency in a Real-World Cohort With Genomic Testing Results
To assess the frequency of RAF alterations in PC, real-world data were obtained via the Perthera Platform, which includes 1,802 patients who underwent molecular profiling as part of the Know Your Tumor Program15 and other hospital initiatives.36 Additional public data were obtained from 1,979 patients with genomic testing results available via the AACR GENIE project37 (release 6.1.0). Genomic profiles from this aggregated cohort of 3,781 patients with PC were analyzed to assess the prevalence of BRAF alterations (see Prevalence Cohort described in Table 1). Molecular profiles with fewer than three genomic variants detected were removed from the aggregated prevalence cohort to exclude low-quality profiles. Tumors with predominantly neuroendocrine features were excluded, whereas any epithelial histologies were allowed including ductal adenocarcinoma, acinar cell carcinoma, solid pseudopapillary neoplasm, and pancreatoblastoma.
TABLE 1.
Overview of Four RAF Subgroups With Distinct Implications for Therapy That Are Defined on the Basis of Certain BRAF (or RAF1) Alterations Identified in Pancreatic Tumors via Genomic Profiling as well as the Presence or Absence of Confounding Drivers (eg, KRAS mutations) Which Might Otherwise Affect the Actionability of Therapies Targeting the MAPK Pathway
Case Series of RAF-Driven PC From Know Your Tumor Program and Academic Collaborators
Deidentified patient and genomic information was collected by collaborators from Dana-Farber, MD Anderson Cancer Center, Memorial Sloan Kettering (MSK), PanCAN, Inova Schar Cancer Institute, and Cedars-Sinai Medical Center. Individual patient charts were retrospectively reviewed, and clinical information was extracted.
PanCAN and Perthera initiated an institutional review board–approved observational registry trial to capture real-world outcomes across all lines of therapies and NGS testing results from Clinical Laboratory Improvement Amendments–certified commercial laboratories in addition to proteomics and/or phosphoproteomics data, as previously described.38 Additional subjects with MSK-IMPACT Assay (MSK-Integrated Mutation Profiling of Actionable Cancer Targets) results were identified at MSK.27 MD Anderson Cancer Center subjects were identified using the Molecular and Clinical Data Integration Platform of the Khalifa Institute for Personalized Cancer Therapy. At Dana-Farber, an institutionally supported clinical assay (OncoPanel) was used.29 Additional genomic findings were abstracted from commercial laboratory reports.
Patient Outcomes Data
A total of 81 patients with RAF-mutated PC were identified. Demographic data, diagnosis date, staging information, treatment history, and response to therapy were collected under institution-specific institutional review board–approved protocols for each individual site. All data from sites were deidentified before analysis. Overall survival (OS) represents the time of the patient's diagnosis of advanced PC until death (survival event) or last follow-up (censored event) for those received at least one therapy in the advanced setting. Progression-free survival (PFS) was calculated from treatment initiation until discontinuation because of disease progression (survival event), cessation because of tolerability issues (censored event), or the last follow-up (censored event).
All analyses were implemented in an R/Bioconductor programming environment. Survival was assessed using Cox proportional hazards regression models with survival and survminer packages. Multivariate Cox regression models were used to account for line of therapy and histology as potentially confounding factors. Differences in frequencies were assessed using Fisher's exact test.
RESULTS
RAF Alterations Are Recurrent Events in PC
In a real-world cohort of 3,781 patients with PC having genomic testing results available,37 we identified 84 patients (2.2% of 3,781 with PC) whose tumors harbored RAF family alterations within this Prevalence Cohort (Table 1). We categorized each patient's molecular profile with an RAF alteration into one of four subgroups intended to distinguish the actionability for therapies targeting the MAPK pathway: BRAF Exon 15 mutations, BRAF Exon 11 mutations, BRAF/RAF1 fusions/rearrangements, or Other (Table 1). This Other subgroup includes nonactionable molecular profiles where the actionability of the RAF alteration is confounded by the presence of another oncogenic driver or the RAF alteration did not align to any of the other three subgroups.
For those without any confounding drivers, the most common BRAF alterations identified within the Prevalence Cohort included the canonical BRAF V600E mutation in Exon 15 (17 of 3,781, 0.45%), a recurring five-amino-acid in-frame deletion in the BRAF β3-αC loop within Exon 11 commonly referred to as ΔNVTAP or N486_P490del (16 of 3,781, 0.42%), and SND1-BRAF fusions (9 of 3,781, 0.24%). These three specific BRAF alterations have distinct implications for targeted therapy and form the basis of the Exon 15, Exon 11, and Fusion subgroups considered throughout this study.
Within the Prevalence Cohort, the proportion of RAF alterations was higher (P = .000000691, Fisher's exact test) in pancreatic acinar cell carcinoma (9 of 49, 18.4%) relative to pancreatic adenocarcinoma (64 of 3,298, 1.9%), and they frequently harbored RAF fusion events (6 of 49, 12.2%). BRAF Exon 15 mutations were also observed in rare PC histologies, with one in a pancreatoblastoma and in a solid pseudopapillary neoplasm (Data Supplement).
Patient Outcomes in RAF-Altered PC
In this retrospective case series of patients with PC with clinically annotated outcomes data, we identified 81 patients with genomic alterations in RAF family genes (Fig 1). In this Clinical Cohort, the median age at diagnosis was 64 (42-86) years and 40 of 81 were women (Table 3). The majority of patients presented with advanced disease: 61 of 81 at initial diagnosis. The histologies were 62 of 81 adenocarcinoma, 14 of 81 acinar cell carcinoma, 4 of 81 IPMN (excluded from the analysis cohort), and one pancreatoblastoma (Table 3). The distributions of BRAF alterations were concentrated within Exons 11 and 15 (Fig 1A), similar to the Prevalence Cohort (Table 1). Notably, 69 of the 81 tumor genomic profiles were KRAS wild-type. Additional genomic testing results were available for other commonly mutated genes in PC (Fig 1B). The median overall survival of the analysis cohort (excluding IPMNs and cases with missing information) who presented with advanced disease (n = 54) was 1.51 years [95% CI = 1.11 to 2.01] with a median follow-up of 1.22 years and 38 total events. The median number of lines of therapy was 2.
FIG 1.

Genomic profiling results from pancreatic tumors harboring RAF pathway alterations. (A) Lollipop plot highlighting amino acid positions along the BRAF gene where alterations were most commonly found in this case series (n = 81). Each stemmed circle represents the numbers of patients with a BRAF alteration at each position (or type for structural variants), counted separately on the basis of either the presence (downward lollipop) or absence (upward lollipop) of a confounding alteration in another oncogenic driver (eg, KRAS mutation). RAF-altered molecular profiles were categorized into four subgroups that have been associated with distinct implications for therapy: Exon 15 (blue; V600 mutations that have been associated with responsiveness to canonical BRAF inhibitors), Exon 11 (red; non-V600 mutations that confer RAS-independent activity but are likely vemurafenib-insensitive), Fusions (teal; intergenic structural variants), and Other (orange; structural and/or short variants, either uncharacterized, characterized as RAS-dependent mutations, or found alongside confounding driver mutations). The three most common variants (BRAF V600E, BRAF N486_P490del also known as ΔNVTAP, and SND1-BRAF fusions) are highlighted at three hotspots that form the basis for the subgroups. (B) Molecular matrix organized by RAF subgroup shows genomic testing results for each patient including specific BRAF variants, RAF fusions, confounding drivers, and p53/CDKN2A/SMAD4 mutations.
TABLE 3.
Summary of Patients With RAF-Mutated Pancreatic Cancer in the Clinical Case Series Cohort and Overall Survival Analysis Cohorts
Actionability of RAF Variants and Confounding Drivers
To assess response to BRAF-directed therapy, genomic profiles from the Clinical Cohort were classified into one of four subgroupings (Table 2), as described above for the Prevalence Cohort: Exon 15 (17 of 81, 21.0%), Fusions (25 of 81, 30.9%), Exon 11 (18 of 81, 22.2%), or Other (21 of 81, 25.9%). The resulting distributions of potentially actionable RAF variants in PC were enriched in BRAF Exons 11 and 15 (Fig 1A).
TABLE 2.
Overview of Recurring RAF Variants and Histological Subtypes Within Each RAF Subgroup Within the Clinical Cohorta
Within each actionable subgroup, the most common variants were BRAF V600E (Exon 15), BRAF N486_P490del (Exon 11), and SND1-BRAF fusion (Fusions; Fig 1A). Before subgroup assignments, we identified confounding drivers in three of 17 tumor profiles with BRAF V600E mutations (KRAS G12V, NTRK fusion, and SND1-BRAF fusion; Fig 1B). Activating KRAS mutations were notably mutually exclusive with BRAF N486_P490del (0 of 17) and BRAF fusion events (0 of 25).
Despite the many nuances to the biology of RAF variants (see the Data Supplement for variant-specific details related to each case), the presence of a confounding driver was the key defining feature of the RAF Other subgroup (17 of 21). One notable exception to these subgroup definitions is for the class 3 kinase-dead BRAF D594G variant, which does not confer similar actionability as the class 1 BRAF V600E despite its position nearby within Exon 15.39,40 This distinction is important because these BRAF variants are considered RAS-dependent and enriched for co-occurrence with KRAS mutations. In the Clinical Cohort, all but two of the BRAF short variants identified in protein-coding regions outside of Exons 11 and 15 were found alongside a confounding driver (Fig 1A). In contrast to RAS-dependent BRAF variants, RAS-independent variants (ie, class 1 or 2) are expected to be mutually exclusive of KRAS mutations.
Survival Analysis by RAF Subgroup
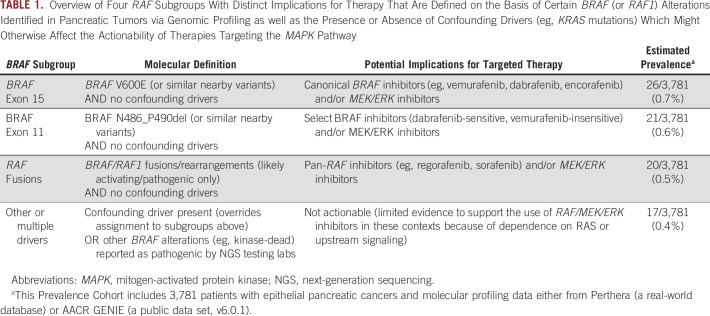
We performed exploratory analyses to assess the prognostic impact of each RAF subgrouping (Fig 2) across the 54 patients who presented with metastatic disease (see OS Cohort in Table 3). No significant differences in median overall survival was observed (Fig 2).
FIG 2.
OS of advanced PC subjects by RAF subgroup. No significant differences in OS (from initial diagnosis) were observed across these four categories (P > .05, pairwise comparisons evaluated by Cox regression), suggesting that these functional classifications of RAF alterations are not likely prognostic. For this survival analysis, patients diagnosed with IPMN's and resected disease were excluded (see OS Cohort in Table 3 for additional baseline characteristics). IPMN, intraductal papillary mucinous neoplasm; NR, not reached; OS, overall survival.
MEK and RAF Inhibitors Have Activity in Patients With KRAS Wild-Type and RAF Family-Mutated PC
Response to molecularly targeted agents against the MAPK pathway was evaluated in 18 subjects who received targeted therapies (Fig 3). The most commonly implemented agents included: canonical BRAF inhibitors (n = 7), pan-RAF inhibitors (n = 2), MEK inhibitors (n = 13), and/or ERK inhibitors (n = 2). The most common combination and single agent regimens were dabrafenib plus trametinib (n = 5) and trametinib (n = 7), respectively.
FIG 3.

PFS data while on BRAF/MEK/ERK inhibitors for patients with RAF-mutated pancreatic cancer. Responses to 17 patients treated with RAF-directed therapy categorized by RAF subgroup, including six patients treated with combination BRAF and MEK and eight patients with MEK inhibitor therapy alone. Each horizontal bar represents the time on therapy without disease progression (ie, PFS). Teal represents treatment lines with partial response as best response, blue are patients who achieved stable disease, light red bars are patients with rapidly progressive disease (no response), and gray bars indicate unevaluable subjects who had discontinued due to tolerability issues (censored event). Bars capped with arrows indicate lines of therapy that were continuing as of the last available progress note (censored event). It is important to note that vemurafenib plus chemotherapy given to PBRAF-211 is considered an unmatched therapy (see Table 1 for actionability definitions), as is considered for all targeted therapies given to patients in the Other subgroup. PFS, progression-free survival.
The clinical benefit rate was highest at 100% in the BRAF Exon 15 subgroup in which three patients received dual BRAF/MEK-targeted therapy (Fig 3) including 1 with noncanonical BRAF T599_V600insT. In the BRAF/RAF1 fusions subgroup, 80% (4 of 5) of evaluable patients had clinical benefit including 2 with partial responses with single-agent MEK inhibitors. Within the BRAF Exon 11 subgroup, 40% (2 of 5) had clinical benefit on single-agent MEK inhibitors with one partial response. Patients with confounding drivers or other RAF alterations did not appear to derive any significant clinical benefit from targeted therapy consisting of MEK inhibitors given in combination with either a BRAF inhibitor (2/3) or immunotherapy (1/3) in this cohort (Fig 3).
RAF Alterations May Predict Response to Standard Chemotherapy
As an exploratory analysis, we evaluated median PFS across the clinical cohort and within each RAF subgroup on standard chemotherapy consisting of either 5FU-based regimens (n = 46) or Gem/nab-P (n = 40; Fig 4). In the first-line setting, patients with RAF-mutated PC receiving FOLFIRINOX (Fig 4A) or Gem/nab-P (Fig 4C) had a median PFS of 6.5 months (95% CI, 4.4 to not reached [NR]; n = 28) or 4.7 months (95% CI, 2.3 to NR; n = 19), respectively. In subsequent lines of therapy (limit 1 per patient), 5FU-based therapies or Gem/nab-P had a median PFS of 4.7 months (95% CI, 3.5 to NR; Fig 4B) or 4.0 months (95% CI, 2.8 to 6.5; Fig 4D). In patients receiving gemcitabine plus nab-paclitaxel, PFS did not significantly differ across subgroups. We observed a modest trend in favor of 5FU-based therapies that appeared to be specific to the Fusion subgroup (Appendix Fig A1).
FIG 4.

PFS analysis across RAF classes for two types of standard therapies commonly implemented in pancreatic cancer. PFS while receiving either (A) first-line FOLFIRINOX, (B) 5FU-based chemotherapy (10 on FOLFIRINOX; two on FOLFOX; two on FOLFIRI; four on 5FU/nal-irinotecan) in second-line or later, or gemcitabine/nab-paclitaxel given in (C) first line or (D) later lines were analyzed for each of the four categories of BRAF alterations with median PFS values (95% CIs) shown. FOLFIRI, fluorouracil, leucovorin, and irinotecan; FOLFIRINOX, infusional fluorouracil, leucovorin, irinotecan, and oxaliplatin; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; FU, fluorouracil; NA, not applicable; NR, not reached; PFS, progression-free survival.
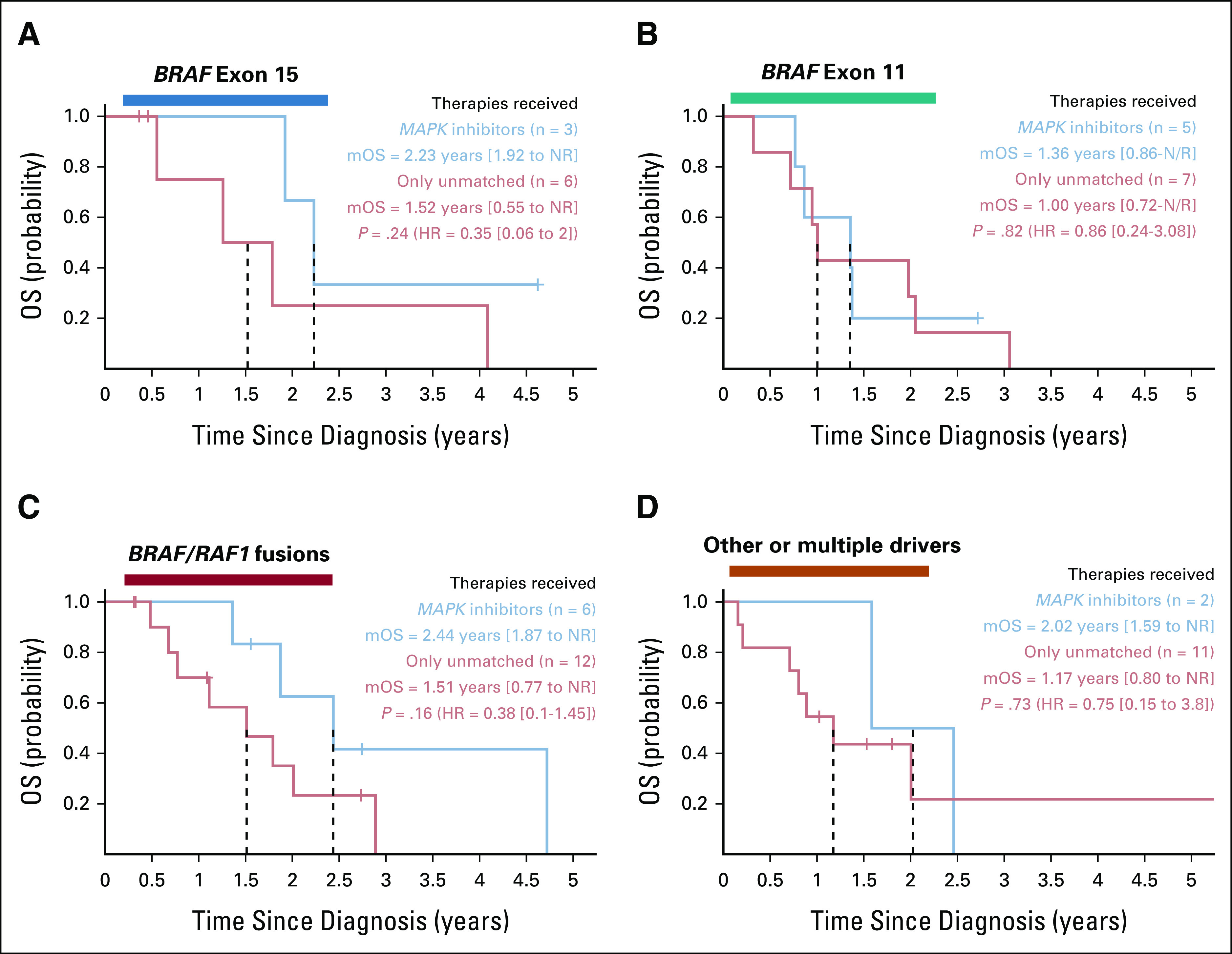
In a follow-up analysis focusing on the Fusion subgroup, we identified a significant difference in PFS (P = .0051; hazard ratio [HR] = 0.1 [0.02 to 0.50]) between FOLFIRINOX (mPFS = 8.9 months [7.5 to NR], n = 14, first line or later) and Gem/nab-P (mPFS = 2.8 months [1.9 to NR], n = 12, first line or later) via univariate Cox regression (Appendix Fig A2A). This subgroup analysis included only the patients who received the entire FOLFIRINOX regimen, and these differences remained significant when applying a multivariate Cox model (P = .027; HR = 0.08 [0.01 to 0.75]) factoring in line of therapy (first line v later lines: P = .75; HR = 1.34 [0.22 to 8.26]; Appendix Fig A2B) with or without a third term accounting for differences in adenocarcinoma versus acinar cell carcinoma histology (see Appendix Fig A2).
DISCUSSION
With this multi-institutional retrospective case series, we report the first comprehensive evaluation of patients with RAF-altered PC including clinical outcomes on MAPK pathway inhibitors as well as standard of care. Although rare, KRAS wild-type PC tumors are enriched for potentially actionable RAF alterations34,37,38,41-43 centered around three hotspot variants: BRAF V600E in Exon 15, BRAF N486_P490del in Exon 11,44 and SND1-BRAF fusions. We categorize RAF subgroups around these three hotspot mutations (plus a fourth subgroup for any nonactionable or RAS-dependent profiles), each of which is rare in PC (0.4%-0.7%) and has distinct implications for therapy.40
In this cohort, we confirm previous reports of clinical responses to MEK and BRAF inhibition in subjects with biologically significant RAF alterations.43,45-49 Benefit was well aligned with the classification system described by Yaeger et al,50 as many subjects within the BRAF Exon 15 and RAF Fusion subgroups responded to targeted therapies. All three subjects in the nonactionable BRAF Other subgroup had rapidly progressive disease on targeted combinations which is likely attributable to the presence of confounding drivers.
The proportion of BRAF ΔNVTAP deletions was unexpectedly high in this case series given limited reports on Exon 11 mutations in cancers enriched for BRAF V600E mutations (eg, melanoma, thyroid, lung, and colon). In this study, single-agent MEK inhibitors demonstrated limited activity within the Exon 11 subgroup; however, next-generation agents with selectivity against BRAF N486_P490del warrant further investigation. Importantly, BRAF ΔNVTAP does not confer sensitivity to the BRAF inhibitor vemurafenib despite an increase in RAS-independent dimerization-dependent kinase activity for cells with this in-frame deletion.13,51 However, there are clinical case reports of significant activity with dabrafenib in patients with BRAF ΔNVTAP deletions,52 which aligns with the observation that dabrafenib fits better than vemurafenib inside the BRAF pocket at the conformational binding-level. Interestingly, there is an important structural paralogy between BRAF and EGFR where BRAF V600E mutations in Exon 15 and BRAF ΔNVTAP deletions in Exon 11 conceptually mirror EGFR L858R mutations in Exon 21 and various EGFR deletions in Exon 19, which have represented the core actionable subset of activating EGFR variants in non–small-cell lung cancer.13
In our cohort, only individuals receiving approved MEK inhibitors or approved combinations of MEK and BRAF inhibitors had clinical benefit. In a recently published study, three patients with PC were enrolled in a 172-patient BRAF V600 basket trial and the results were consistent with our findings.24 In the NCI-MATCH subprotocol H arm (N = 31), two patients with PC were enrolled (n = 1 unevaluable with progressive disease, n = 1 stable disease).53 Notably, these RAF alterations occur across a spectrum of epithelial pancreatic tumors underscoring the importance of routine molecular profiling, irrespective of histology across PCs, particularly acinar cell carcinomas (which commonly harbor BRAF fusions) and other pancreaticobiliary tumors (eg, cholangiocarcinoma, ampullary, and duodenal carcinomas).38,54,55
We examined RAF categorization as a prognostic or predictive factor. In our cohort, RAF categorization was not associated with differences in overall survival. Unlike previous reports in colon cancer and lung cancer,47,56 BRAF V600E alterations were not predictive of poor response to chemotherapy. However, we found that RAF fusion abnormalities may speculatively represent a predictive marker of improved response to FOLFIRINOX and poor response to gemcitabine and nab-paclitaxel. These findings are limited by sample sizes not sufficiently large to account for potentially confounding factors. We were unable to find any evaluation of chemotherapeutic response to tumors harboring fusion abnormalities outside of pemetrexed therapy in lung cancers with ROS1 fusion abnormalities.57
Our data set is limited by its retrospective design and modest sized cohort as the abnormality of interest is rare. There are selection biases for those who receive RAF-directed therapy that cannot be accounted for in this design. Observational bias can occur when recording responses to therapy in select groups of patients. These case reports were collected from academic medical centers. Therefore, this case series may not adequately represent important population-level factors (eg, differences in insurance coverage, socioeconomic status, urban v rural cohorts, and academic v community settings) that can influence patient outcomes as well as access to targeted therapies either off label or on a clinical trial.
Nonetheless, because of the high unmet need in the PC patient population and the infrequency of BRAF alterations, a single-arm prospective trial confirming substantial response rates and durability of responses would likely be sufficient to pursue an application to expand FDA-approved labels for BRAF inhibitor combinations with MEK inhibitors to include patients with BRAF-mutated PC within the Exon 15 subgroup. Following the recent approval of a BRAF inhibitor plus an EGFR antibody (but not for the triple targeted approach that included a MEK inhibitor) in BRAF V600E–mutated colon cancer,58 multipronged strategies targeting BRAF alongside other signaling components beyond the RAF/MEK/ERK signaling cascade may warrant further investigation. Profiling the activation of upstream receptors following MAPK pathway inhibition may provide clues into adaptive resistance mechanisms that could be exploited in a disease-specific manner.59 As future generations of BRAF-directed therapies enter clinical trials, it will be imperative to understand the binding affinity of these novel agents for different RAF variant subgroups and to screen for potential mechanisms of acquired (MEK mutation) or intrinsic (KRAS mutation) resistance.31
Herein, we have described a cohort of RAF-mutated PC that comprises 2% of PC cases. We report promising treatment responses and encouraging outcomes in patients within BRAF Exon 15 and BRAF/RAF1 fusions receiving MAPK pathway-directed therapies. Prospective studies are warranted to confirm these hypothesis-generating results and establish the optimal treatment approaches for BRAF-mutated PC taking into account current standards of care.
APPENDIX
FIG A1.
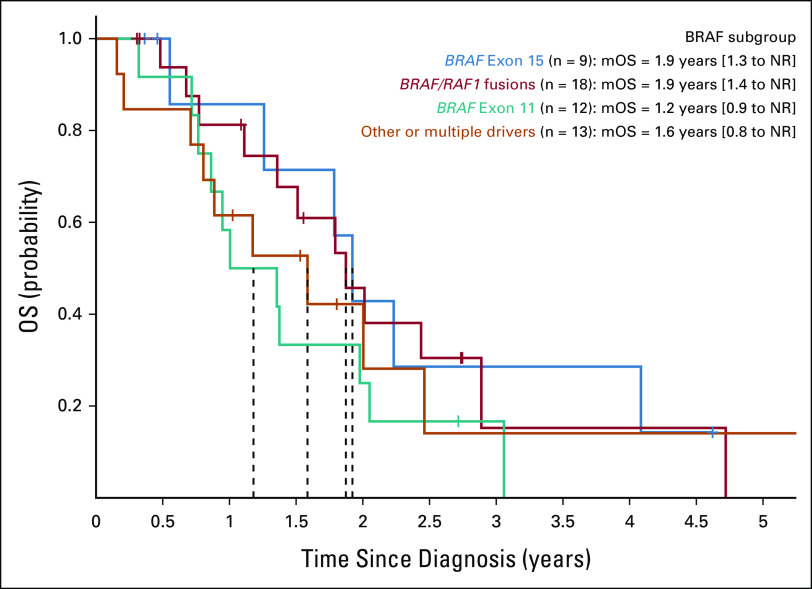
OS analysis comparing patients who received a molecularly matched therapy targeting the MAPK signaling pathway (eg, BRAF/MEK/ERK inhibitors) versus those who only received unmatched therapies in the advanced treatment setting. OS differences between matched and unmatched subgroups were not considered statistically significant for either RAF subgroups (A) Exon 15, (B) Exon 11, (C) Fusions, or (D) Other alterations when analyzed individually (P > .05). For the broader subset of patients, mOS differences were trending toward benefit but not considered significant (P = .07252; HR = 0.48 [0.21 to 1.07]) when comparing matched (mOS = 1.92 years [1.37 to NA], n = 14) and unmatched (mOS = 1.51 years [0.95 to 2.89], n = 25) subgroups. Only patients who were initially diagnosed with metastatic disease were included in these analyses (see OS Matched and OS Unmatched subgroups in Table 3 for additional baseline characteristics across the combined cohort). HR, hazard ratio; MAPK, mitogen-activated protein kinase; mOS, median overall survival; NA, not applicable; OS, overall survival.
FIG A2.
PFS analyses highlighting favorable trends for 5FU-based therapies versus gemcitabine/nab-paclitaxel in patients with RAF fusions or (B) separated for first line of therapy versus later lines. A significant difference in mPFS was observed for FOLFIRINOX versus gemcitabine/nab-paclitaxel within the BRAF fusion subgroup (A) using a univariate Cox regression model across all lines of therapy (P = .0051; HR = 0.1 [0.02 to 0.50]) or (B) using a multivariate model (P = .027) that factored in therapies given in first line of therapy versus later lines. Although these trends were considered significant, prospective evaluation is warranted when considering the imbalance between treatment choices for first line, an unexpected trend of longer PFS for later lines versus first line (note that this term was not significant in the multivariate model), the relatively small sample sizes, among other potentially confounding factors. Within this subset of the BRAF fusion analysis cohort, acinar cell carcinoma histology was seen in five (36% of 14) and six (50% of 12) for 5FU-based versus gemcitabine-based therapies, respectively (the rest were adenocarcinoma). This variable was not significantly enriched by Fisher's exact test (P = .69), and its addition to the multivariate Cox regression model yielded similar results for the contrast between regimens (P = .0405, HR = 0.1 [0.01 to 0.9]). FOLFIRINOX, infusional fluorouracil, leucovorin, irinotecan, and oxaliplatin; FU, fluorouracil; HR, hazard ratio; PFS, progression-free survival.
Andrew Hendifar
Consulting or Advisory Role: Novartis, Ipsen, Perthera, Celgene, AbbVie, Eisai
Research Funding: Ipsen, NGM Biopharmaceuticals
Travel, Accommodations, Expenses: Halozyme
Edik M. Blais
Employment: AstraZeneca, Perthera, Emerald Cloud Labs
Stock and Other Ownership Interests: Perthera
Patents, Royalties, Other Intellectual Property: Named as an inventor on patents filed by Perthera Inc (as an employee)
Brian Wolpin
Honoraria: G1 Therapeutics, Celgene
Consulting or Advisory Role: Genentech, G1 Therapeutics, BioLineRx, GRAIL, Celgene
Research Funding: Celgene, Lilly
Vivek Subbiah
Consulting or Advisory Role: MedImmune, Helsinn Therapeutics, Loxo, R-Pharm, QED Therapeutics
Research Funding: Novartis, GlaxoSmithKline, NanoCarrier, Northwest Biotherapeutics, Genentech/Roche, Berg Pharma, Bayer, Incyte, Fujifilm, PharmaMar, D3 Oncology Solutions, Pfizer, Amgen, AbbVie, Multivir, Blueprint Medicines, LOXO, Vegenics, Takeda, Alfasigma, Agensys, Idera, Boston Biomedical, Inhibrx, Exelixis, Turning Point Therapeutics
Travel, Accommodations, Expenses: PharmaMar, Bayer, Novartis, Helsinn Therapeutics
Other Relationship: Medscape
Eric Collisson
Stock and Other Ownership Interests: Guardant Health, BloodQ, Tatara Therapeutics, Clara Health, Hint Health
Consulting or Advisory Role: Merck
Research Funding: Merck KGaA, Loxo/Bayer, Ferro Oncology/Bridge Bio, AstraZeneca
Expert Testimony: NantBioScience Inc
Timothy Cannon
Honoraria: Deciphera, AstraZeneca
Consulting or Advisory Role: Loxo, Navican
Other Relationship: Navican/Intermountain Healthcare
Kenna Shaw
Consulting or Advisory Role: Guidepoint Global
Research Funding: Guardant Health, Tempus, Philips Healthcare
Emanuel F. Petricoin
Leadership: Perthera, Ceres Nanosciences
Stock and Other Ownership Interests: Perthera, Ceres Nanosciences, Theralink Technologies, Inc
Consulting or Advisory Role: Perthera, Ceres Nanosciences, TheraLink Technologies, Inc
Research Funding: Ceres Nanosciences, AbbVie, Mirati Therapeutics, Genentech, G1 Therapeutics
Patents, Royalties, Other Intellectual Property: NIH Patents Licensing Fee Distribution/Royalty, University assigned patent licensing fee/royalty
Travel, Accommodations, Expenses: Perthera, Ceres Nanosciences
Samuel Klempner
Stock and Other Ownership Interests: TP Therapeutics
Honoraria: Natera
Consulting or Advisory Role: Lilly, Boston Biomedical, Astellas Pharma, Foundation Medicine, Bristol Myers Squibb, Pieris Pharmaceuticals, Merck, Daiichi Sankyo/UCB Japan
Speakers' Bureau: Foundation Medicine
Research Funding: Leap Therapeutics, BeiGene
Other Relationship: NCCN
Emily Lyons
Research Funding: Midwinter Solutions, Repare Therapeutics, InVitae, Novocure, AstraZeneca, NCgenetics, Indegene, 2nd.MD, Novartis, Ipsen, Engitix, Abbott, BiolineRx, Speratum, Phase V Consulting, Amarex Clinical Research, RenovoRx, Merck, FibroGen
Andrea Wang-Gillam
Employment: Jacobio
Stock and Other Ownership Interests: Jacobio
Consulting or Advisory Role: Jacobio, AstraZeneca, Repugene, Merck, Eisai, Bayer, Inxmed
Research Funding: AstraZeneca, Pfizer, Lilly, Verastem, Merck, BioMed Valley Discoveries, Roche, Bristol Myers Squibb, Xcovery, Boston Biomedical, Hutchison MediPharma, Rafael Pharmaceuticals, Gossamer Bio
Michael J. Pishvaian
Stock and Other Ownership Interests: Perthera
Honoraria: Halozyme
Consulting or Advisory Role: Perthera, AstraZeneca/MedImmune, Merck
Research Funding: Tesaro, Seattle Genetics, Pfizer
Patents, Royalties, Other Intellectual Property: Perthera patient matching algorithm, Use patent for veliparib and FOLFOX
Eileen M. O'Reilly
Consulting or Advisory Role: Adicet Bio, Agios, AstraZeneca, Alnylam, Autem Medical, Bayer, BeiGene, Berry Genomics, Center for Emerging & Neglected Diseases (CEND), Celgene, CytomX Therapeutics, Eisai, Exelixis, Flatiron Health, Genentech/Roche, Genoscience Pharma, Helio, Incyte, Ipsen, Legend Biotech, Loxo, Merck, MiNA Therapeutics, Nerviano Medical Sciences, QED Therapeutics, RedHill Biopharma, Refael, Silenseed, Sillajen, SOBI, Surface Oncology, TheraBionic, twoXAR, Vector Health, Yiviva
Research Funding: AstraZeneca/MedImmune, Celgene, Genentech, Roche, Silenseed, Arcus Ventures, BioNTech AG
No other potential conflicts of interest were reported.
Footnotes
A.H. and E.M.B. are co-lead authors.
M.J.P. and E.M.O. are co-senior authors.
AUTHOR CONTRIBUTIONS
Conception and design: Andrew Hendifar, Edik M. Blais, Vivek Subbiah, Eric Collisson, Kenna Shaw, Emanuel F. Petricoin III, Andrea Wang-Gilliam, Michael J. Pishvaian, Eileen M. O'Reilly
Administrative support: Vivek Subbiah, Kenna Shaw, Emanuel F. Petricoin III, Emily Lyons
Provision of study materials or patients: Brian Wolpin, Vivek Subbiah, Isha Singh, Timothy Cannon, Kenna Shaw, Emanuel F. Petricoin III, Samuel Klempner, Eileen M. O'Reilly
Collection and assembly of data: Andrew Hendifar, Edik M. Blais, Brian Wolpin, Vivek Subbiah, Isha Singh, Timothy Cannon, Kenna Shaw, Emanuel F. Petricoin III, Samuel Klempner, Emily Lyons, Andrea Wang-Gillam, Michael J. Pishvaian, Eileen M. O'Reilly
Data analysis and interpretation: Andrew Hendifar, Edik M. Blais, Vivek Subbiah, Eric Collisson, Emanuel F. Petricoin III, Samuel Klempner, Andrea Wang-Gillam, Michael J. Pishvaian, Eileen M. O'Reilly
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Andrew Hendifar
Consulting or Advisory Role: Novartis, Ipsen, Perthera, Celgene, AbbVie, Eisai
Research Funding: Ipsen, NGM Biopharmaceuticals
Travel, Accommodations, Expenses: Halozyme
Edik M. Blais
Employment: AstraZeneca, Perthera, Emerald Cloud Labs
Stock and Other Ownership Interests: Perthera
Patents, Royalties, Other Intellectual Property: Named as an inventor on patents filed by Perthera Inc (as an employee)
Brian Wolpin
Honoraria: G1 Therapeutics, Celgene
Consulting or Advisory Role: Genentech, G1 Therapeutics, BioLineRx, GRAIL, Celgene
Research Funding: Celgene, Lilly
Vivek Subbiah
Consulting or Advisory Role: MedImmune, Helsinn Therapeutics, Loxo, R-Pharm, QED Therapeutics
Research Funding: Novartis, GlaxoSmithKline, NanoCarrier, Northwest Biotherapeutics, Genentech/Roche, Berg Pharma, Bayer, Incyte, Fujifilm, PharmaMar, D3 Oncology Solutions, Pfizer, Amgen, AbbVie, Multivir, Blueprint Medicines, LOXO, Vegenics, Takeda, Alfasigma, Agensys, Idera, Boston Biomedical, Inhibrx, Exelixis, Turning Point Therapeutics
Travel, Accommodations, Expenses: PharmaMar, Bayer, Novartis, Helsinn Therapeutics
Other Relationship: Medscape
Eric Collisson
Stock and Other Ownership Interests: Guardant Health, BloodQ, Tatara Therapeutics, Clara Health, Hint Health
Consulting or Advisory Role: Merck
Research Funding: Merck KGaA, Loxo/Bayer, Ferro Oncology/Bridge Bio, AstraZeneca
Expert Testimony: NantBioScience Inc
Timothy Cannon
Honoraria: Deciphera, AstraZeneca
Consulting or Advisory Role: Loxo, Navican
Other Relationship: Navican/Intermountain Healthcare
Kenna Shaw
Consulting or Advisory Role: Guidepoint Global
Research Funding: Guardant Health, Tempus, Philips Healthcare
Emanuel F. Petricoin
Leadership: Perthera, Ceres Nanosciences
Stock and Other Ownership Interests: Perthera, Ceres Nanosciences, Theralink Technologies, Inc
Consulting or Advisory Role: Perthera, Ceres Nanosciences, TheraLink Technologies, Inc
Research Funding: Ceres Nanosciences, AbbVie, Mirati Therapeutics, Genentech, G1 Therapeutics
Patents, Royalties, Other Intellectual Property: NIH Patents Licensing Fee Distribution/Royalty, University assigned patent licensing fee/royalty
Travel, Accommodations, Expenses: Perthera, Ceres Nanosciences
Samuel Klempner
Stock and Other Ownership Interests: TP Therapeutics
Honoraria: Natera
Consulting or Advisory Role: Lilly, Boston Biomedical, Astellas Pharma, Foundation Medicine, Bristol Myers Squibb, Pieris Pharmaceuticals, Merck, Daiichi Sankyo/UCB Japan
Speakers' Bureau: Foundation Medicine
Research Funding: Leap Therapeutics, BeiGene
Other Relationship: NCCN
Emily Lyons
Research Funding: Midwinter Solutions, Repare Therapeutics, InVitae, Novocure, AstraZeneca, NCgenetics, Indegene, 2nd.MD, Novartis, Ipsen, Engitix, Abbott, BiolineRx, Speratum, Phase V Consulting, Amarex Clinical Research, RenovoRx, Merck, FibroGen
Andrea Wang-Gillam
Employment: Jacobio
Stock and Other Ownership Interests: Jacobio
Consulting or Advisory Role: Jacobio, AstraZeneca, Repugene, Merck, Eisai, Bayer, Inxmed
Research Funding: AstraZeneca, Pfizer, Lilly, Verastem, Merck, BioMed Valley Discoveries, Roche, Bristol Myers Squibb, Xcovery, Boston Biomedical, Hutchison MediPharma, Rafael Pharmaceuticals, Gossamer Bio
Michael J. Pishvaian
Stock and Other Ownership Interests: Perthera
Honoraria: Halozyme
Consulting or Advisory Role: Perthera, AstraZeneca/MedImmune, Merck
Research Funding: Tesaro, Seattle Genetics, Pfizer
Patents, Royalties, Other Intellectual Property: Perthera patient matching algorithm, Use patent for veliparib and FOLFOX
Eileen M. O'Reilly
Consulting or Advisory Role: Adicet Bio, Agios, AstraZeneca, Alnylam, Autem Medical, Bayer, BeiGene, Berry Genomics, Center for Emerging & Neglected Diseases (CEND), Celgene, CytomX Therapeutics, Eisai, Exelixis, Flatiron Health, Genentech/Roche, Genoscience Pharma, Helio, Incyte, Ipsen, Legend Biotech, Loxo, Merck, MiNA Therapeutics, Nerviano Medical Sciences, QED Therapeutics, RedHill Biopharma, Refael, Silenseed, Sillajen, SOBI, Surface Oncology, TheraBionic, twoXAR, Vector Health, Yiviva
Research Funding: AstraZeneca/MedImmune, Celgene, Genentech, Roche, Silenseed, Arcus Ventures, BioNTech AG
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States Cancer Res 742913–29212014 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A.Cancer statistics, 2018 CA Cancer J Clin 687–302018 [DOI] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer N Engl J Med 3641817–18252011 [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine N Engl J Med 3691691–17032013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial Lancet 387545–5572016 [DOI] [PubMed] [Google Scholar]
- 6.Singhi AD, George B, Greenbowe JR, et al. Real-time targeted genome profile analysis of pancreatic ductal adenocarcinomas identifies genetic alterations that might be targeted with existing drugs or used as biomarkers Gastroenterology 1562242–2253.e42019 [DOI] [PubMed] [Google Scholar]
- 7.Latham A, Srinivasan P, Kemel Y, et al. Microsatellite instability is associated with the presence of Lynch syndrome pan-cancer J Clin Oncol 37286–2952019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu ZI, Shia J, Stadler ZK, et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: Challenges and recommendations Clin Cancer Res 241326–13362018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demetri GD, Paz-Ares L, Farago AF, et al. Efficacy and safety of entrectinib in patients with NTRK fusion-positive (NTRK-fp) tumors: Pooled analysis of STARTRK-2, STARTRK-1 and ALKA-372-001. https://doi.org/10.1093/annonc/mdy483.003 ESMO, 2018 Congress 2018.
- 10.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children N Engl J Med 378731–7392018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta M, Sherrow C, Krone ME, et al. Targeting the NTRK fusion gene in pancreatic acinar cell carcinoma: A case report and review of the Literature J Natl Compr Canc Netw 1910–152021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer N Engl J Med 381317–3272019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster SA, Whalen DM, Özen A, et al. Activation mechanism of oncogenic deletion mutations in BRAF, EGFR, and HER2 Cancer Cell 29477–4932016 [DOI] [PubMed] [Google Scholar]
- 14.Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer Nature 518495–5012015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pishvaian MJ, Bender RJ, Halverson D, et al. Molecular profiling of patients with pancreatic cancer: Initial results from the know your tumor initiative Clin Cancer Res 245018–50272018 [DOI] [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas Research Network Integrated genomic characterization of pancreatic ductal adenocarcinoma Cancer Cell 32185–203.e132017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowery MA, Jordan EJ, Basturk O, et al. Real-time genomic profiling of pancreatic ductal adenocarcinoma: Potential actionability and correlation with clinical phenotype Clin Cancer Res 236094–61002017 [DOI] [PubMed] [Google Scholar]
- 18.Aguirre AJ, Nowak JA, Camarda ND, et al. Real-time genomic characterization of advanced pancreatic cancer to enable precision medicine Cancer Discov 81096–11112018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pishvaian MJ, Petricoin E., III Molecular profiling of pancreatic cancer patients-response. Clin Cancer Res. 2018;24:6612. doi: 10.1158/1078-0432.CCR-18-2645. [DOI] [PubMed] [Google Scholar]
- 20.Heeke AL, Pishvaian MJ, Lynce F, et al. Prevalence of homologous recombination-related gene mutations across multiple cancer types JCO Precis Oncol 20181–132018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes Nature 491399–4052012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy Nat Med 17500–5032011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heining C, Horak P, Uhrig S, et al. NRG1 fusions in KRAS wild-type pancreatic cancer Cancer Discov 81087–10952018 [DOI] [PubMed] [Google Scholar]
- 24.Subbiah V, Puzanov I, Blay JY, et al. Pan-cancer efficacy of vemurafenib in BRAF V600-mutant non-melanoma cancers Cancer Discov 10657–6632020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: A retrospective analysis of the Know Your tumor registry trial Lancet Oncol 21508–5182020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishimura N, Yamasawa K, Karim Rumi MA, et al. BRAF and K-ras gene mutations in human pancreatic cancers Cancer Lett 199169–1732003 [DOI] [PubMed] [Google Scholar]
- 27.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer Nature 417949–9542002 [DOI] [PubMed] [Google Scholar]
- 28. El-Osta H, Falchook G, Tsimberidou A, et al. BRAF mutations in advanced cancers: Clinical characteristics and outcomes. PLoS One. 2011;6:e25806. doi: 10.1371/journal.pone.0025806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations N Engl J Med 373726–7362015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF V600E-mutant metastatic non-small cell lung cancer: An open-label, multicentre phase 2 trial Lancet Oncol 17984–9932016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Cutsem E, Huijberts S, Grothey A, et al. Binimetinib, encorafenib, and cetuximab triplet therapy for patients with BRAF V600E-mutant metastatic colorectal cancer: Safety lead-in results from the phase III BEACON colorectal cancer study J Clin Oncol 371460–14692019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kopetz S, Grothey A, Van Cutsem E, et al. BEACON CRC: A randomized, 3-arm, phase 3 study of encorafenib and cetuximab with or without binimetinib vs. choice of either irinotecan or FOLFIRI plus cetuximab in BRAF V600E-mutant metastatic colorectal cancer. Ann Oncol. 2019;30(suppl 4):iv154. [Google Scholar]
- 33.Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600e-mutated colorectal cancer N Engl J Med 3811632–16432019 [DOI] [PubMed] [Google Scholar]
- 34. Witkiewicz AK, McMillan EA, Balaji U, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744. doi: 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pishvaian MJ, Bender RJ, Halverson D, et al. Molecular profiling of pancreatic cancer patients: Initial results from the know your tumor initiative Clin Cancer Res 215018–50272018 [DOI] [PubMed] [Google Scholar]
- 36.Lowder CY, Dhir T, Goetz AB, et al. A step towards personalizing next line therapy for resected pancreatic and related cancer patients: A single institution's experience Surg Oncol 33118–1252020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.AACR Project GENIE Consortium AACR project GENIE: Powering precision medicine through an international consortium Cancer Discov 7818–8312017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: A retrospective analysis of the Know Your tumor registry trial Lancet Oncol 21508–5182020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cope NJ, Novak B, Liu Z, et al. Analyses of the oncogenic BRAFD594G variant reveal a kinase-independent function of BRAF in activating MAPK signaling J Biol Chem 2952407–24202020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS Nature 548234–2382017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karnoub AE, Weinberg RA.Ras oncogenes: Split personalities Nat Rev Mol Cell Biol 9517–5312008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen SH, Zhang Y, Van Horn RD, et al. Oncogenic BRAF deletions that function as homodimers and are sensitive to inhibition by RAF dimer inhibitor LY3009120 Cancer Discov 6300–3152016 [DOI] [PubMed] [Google Scholar]
- 43.Ross JS, Wang K, Chmielecki J, et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy Int J Cancer 138881–8902016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gysin S, Salt M, Young A, et al. Therapeutic strategies for targeting ras proteins Genes Cancer 2359–3722011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim KB, Semrad T, Schrock AB, et al. Significant clinical response to a MEK inhibitor therapy in a patient with metastatic melanoma harboring an RAF1 fusion JCO Precis Oncol 21–62018 [DOI] [PubMed] [Google Scholar]
- 46.Busser B, Leccia MT, Gras-Combe G, et al. Identification of a novel complex BRAF mutation associated with major clinical response to vemurafenib in a patient with metastatic melanoma JAMA Dermatol 1491403–14062013 [DOI] [PubMed] [Google Scholar]
- 47.Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer Clin Cancer Res 194532–45402013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Trudel S, Odolczyk N, Dremaux J, et al. The clinical response to vemurafenib in a patient with a rare BRAFV600DK601del mutation-positive melanoma. BMC Cancer. 2014;14:727. doi: 10.1186/1471-2407-14-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marchand A, Tallet A, Collin C, et al. A rare BRAF T599dup mutation conferring sensitivity to BRAF inhibitor in a patient with metastatic melanoma Br J Dermatol 179528–5292018 [DOI] [PubMed] [Google Scholar]
- 50.Yaeger R, Corcoran RB.Targeting alterations in the RAF-MEK pathway Cancer Discov 9329–3412019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yaeger R, Kotani D, Mondaca S, et al. Response to anti-EGFR therapy in patients with BRAF non-V600-mutant metastatic colorectal cancer Clin Cancer Res 257089–70972019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wrzeszczynski KO, Rahman S, Frank MO, et al. Identification of targetable BRAF DeltaN486_P490 variant by whole-genome sequencing leading to dabrafenib-induced remission of a BRAF-mutant pancreatic adenocarcinoma. Cold Spring Harb Mol Case Stud. 2019;5:a004424. doi: 10.1101/mcs.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salama AKS, Li S, Macrae ER, et al. Dabrafenib and trametinib in patients with tumors with BRAFV600E mutations: Results of the NCI-MATCH trial subprotocol H J Clin Oncol 383895–39042020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lowery MA, Ptashkin R, Jordan E, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: Potential targets for intervention Clin Cancer Res 244154–41612018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Watari J, Mitani S, Ito C, et al. Molecular alterations and PD-L1 expression in non-ampullary duodenal adenocarcinoma: Associations among clinicopathological, immunophenotypic and molecular features. Sci Rep. 2019;9:10526. doi: 10.1038/s41598-019-46167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Innocenti F, Ou FS, Qu X, et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome J Clin Oncol 371217–12272019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen YF, Hsieh MS, Wu SG, et al. Efficacy of pemetrexed-based chemotherapy in patients with ROS1 fusion-positive lung adenocarcinoma compared with in patients harboring other driver mutations in east asian populations J Thorac Oncol 111140–11522016 [DOI] [PubMed] [Google Scholar]
- 58.Tabernero J, Grothey A, Van Cutsem E, et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600e-mutant metastatic colorectal cancer: Updated survival results and subgroup analyses from the BEACON study J Clin Oncol 39273–2842021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindberg JM, Newhook TE, Adair SJ, et al. Co-treatment with panitumumab and trastuzumab augments response to the MEK inhibitor trametinib in a patient-derived xenograft model of pancreatic cancer Neoplasia 16562–5712014 [DOI] [PMC free article] [PubMed] [Google Scholar]