Abstract
PURPOSE
Sixty percent of newly diagnosed patients with acute myeloid leukemia (ND-AML) receiving frontline therapy attain a complete response (CR), yet 30%-40% of patients relapse. Relapsed or refractory AML (R/R-AML) remains a particularly adverse population necessitating improved therapeutic options. This phase Ib/II study evaluated the safety and efficacy of fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin combined with the B-cell lymphoma-2 inhibitor venetoclax in ND-AML and R/R-AML.
MATERIALS AND METHODS
The phase IB portion (PIB) enrolled patients with R/R-AML using a 3 + 3 dose escalation and de-escalation algorithm for identification of maximum tolerated dose and dose-limiting toxicities. The phase II portion enrolled patients into two arms to evaluate response and time-to-event end points: phase IIA (PIIA): ND-AML and phase IIB (PIIB): R/R-AML.
RESULTS
Sixty-eight patients have enrolled to date (PIB, 16; PIIA, 29; PIIB, 23). Median age was 46 years (range, 20-73). Grade 3 and 4 adverse events occurring in ≥ 10% of patients included febrile neutropenia (50%), bacteremia (35%), pneumonia (28%), and sepsis (12%). The overall response rate for PIB, PIIA, and PIIB was 75%, 97%, and 70% with 75%, 90%, and 61%, respectively, achieving a composite CR. Measurable residual disease–negative composite CR was attained in 96% of ND-AML and 69% of R/R-AML patients. After a median follow-up of 12 months, median overall survival (OS) for both PII cohorts was not reached. Fifty-six percent of patients proceeded to allogeneic hematopoietic stem-cell transplantation (ND-AML, 69%; R/R-AML, 46%). In R/R-AML, allogeneic hematopoietic stem-cell transplantation resulted in a significant improvement in OS (median OS, NR; 1-year OS, 87%). One-year survival post-HSCT was 94% in ND-AML and 78% in R/R-AML.
CONCLUSION
Fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin + venetoclax represents an effective intensive treatment regimen in ND-AML and R/R-AML patients, associated with deep remissions and a high rate of transition to successful transplantation.
INTRODUCTION
Induction chemotherapy (IC) in acute myeloid leukemia (AML) typically combines an anthracycline (ie, daunorubicin or idarubicin) with the antimetabolite cytarabine (the often termed 7 + 3 regimen), resulting in complete remission (CR) rates of approximately 60%.1 Anthracycline dose augmentation and development of synergistic multidrug regimens improves CR rates to 70%-80%2-6; however, 30%-40% of patients still ultimately relapse.7,8 Treatment regimens capable of producing long-term remissions are needed.
CONTEXT
Key Objective
Is the addition of the B-cell lymphoma-2 inhibitor venetoclax (VEN) to standard fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin (FLAG-IDA) induction and consolidation (FLAG-IDA + VEN) therapy safe and effective for patients with newly diagnosed acute myeloid leukemia (ND-AML) and/or relapsed or refractory AML (R/R-AML)?
Knowledge Generated
FLAG-IDA + VEN resulted in high rates of measurable residual disease–negative composite complete remission in patients with both ND-AML and R/R-AML, with a majority of patients able to effectively transition to allogeneic stem-cell transplantation. The combination was associated with an expected and manageable myelosuppression-related toxicity profile.
Relevance
VEN in combination with intensive induction and consolidation therapy in AML demonstrates the regimen is effective in both the ND-AML and R/R-AML setting. The high rates of observed measurable residual disease–negative responses suggest this regimen induces deep remissions and is effective for successfully bridging patients with ND-AML and R/R-AML to allogeneic transplantation.
The multiagent regimen of fludarabine, cytarabine, granulocyte colony-stimulating factor (G-CSF), and idarubicin (FLAG-IDA) is an effective frontline treatment in fit patients with AML. Compared with alternate IC regimens, frontline FLAG-IDA induction results in composite CR (CRc) rates of 85%, a reduced cumulative incidence of relapse (38% v 55%), improved relapse-free survival, and median overall survival (OS) of approximately 5 years, albeit with increased myelosuppression.3 Patients with high-risk myelodysplastic syndrome or secondary AML (sAML) receiving FLAG-IDA achieved CR rates of 78%; 63% achieved a morphologic and cytogenetic CR after one or two treatment cycles.9
Patients with relapsed and/or refractory (R/R) AML experience inferior CR rates (20%-60%) with IC reinduction, and few patients obtain durable remissions with salvage therapy.10,11 Although no proven optimal salvage regimen exists, FLAG-IDA is commonly used, resulting in a sobering CR or CR with incomplete hematologic recovery (CRi) rate of 21%, 3.5-month median OS, and 30-day mortality exceeding 10%.10-12
The B-cell lymphoma-2 (BCL-2) inhibitor venetoclax (VEN) combined with lower-intensity treatment regimens (azacitidine, decitabine, or low-dose cytarabine) is approved for unfit, older patients with AML13,14 and has rapidly emerged as a standard-of-care treatment option for this challenging patient population.13-16
Congruent with the synergy observed with VEN combinations incorporating lower-intensity treatments, VEN demonstrated preclinical synergy with standard chemotherapeutic agents, suggesting benefit beyond the older unfit AML population.17
Attenuated cytarabine and idarubicin (the 5 + 2 regimen) combined with VEN in older, adverse-risk patients with newly diagnosed acute myeloid leukemia (ND-AML) resulted in CRc (CR + CRi) rates of 72% and 30-day mortality of 6%.18 In R/R-AML, 69% of patients treated with FLA-IDA with VEN (days 1-7) achieved a CRc without significantly increased hematologic toxicity compared with a matched FLA-IDA cohort,19 suggesting optimization of VEN with IC may increase efficacy without untoward toxicity. Herein, we provide results of a phase Ib/II study of FLAG-IDA + VEN as frontline or salvage AML therapy.
MATERIALS AND METHODS
Inclusion Criteria
Patients age > 18 years with ND-AML (de novo AML, sAML, treated secondary AML [ts-AML], and therapy-related AML [t-AML]), high-risk myelodysplastic syndrome (defined by the presence of > OR = 10% blasts), or R/R-AML (defined as persistent leukemia without achievement of an International Working Group–defined response following at least one cycle of induction chemotherapy or patients in first relapse or beyond) were eligible. Only R/R-AML patients were eligible for the phase Ib (PIB) portion. Patients with acute promyelocytic leukemia, significant cardiovascular comorbidities, known malabsorption syndromes, or who had received prior BCL-2 inhibitor therapy were excluded (full eligibility available within the Appendix, online only). All investigations were conducted under the approval of the institutional review committee and in accordance with the Declaration of Helsinki.
Cytogenetic and Molecular Analysis
Cytogenetic evaluation using standard metaphase karyotype analysis and molecular analysis via an 81-gene institutional next-generation sequencing platform was performed at study enrollment. Measurable residual disease (MRD) was assessed by 8-color multiparameter flow cytometry (FC) using leukemia-associated immunophenotype or different from normal assessment20 with a minimum sensitivity of 10−3 to 10−4 (0.1%-0.01%).
Safety and Efficacy
The PIB (dose-escalation) portion applied a 3 + 3 dose escalation and de-escalation algorithm to determine the maximal tolerated dose (MTD) (details of PIB cohorts are provided in the Appendix) starting at the −1 dose level (VEN 200 mg) and escalating to dose level 0 (VEN 400 mg). Patients receiving at least one dose of VEN were included in the intention-to-treat safety and efficacy analysis. PIB patients receiving a minimum of 80% of planned VEN doses and at least 3 days of FLAG-IDA were evaluable for dose-limiting toxicity (DLT). The phase II (dose-expansion) portion enrolled separate cohorts of ND-AML (PIIA) and R/R-AML (PIIB) patients at the recommended PII dose.
Treatment Administration
FLAG-IDA induction consisted of 28-day cycles of intravenous (IV) fludarabine (30 mg/m2) and cytarabine (1.5-2 g/m2 IV) on days (D) 2-6, idarubicin (IV; ND-AML, 8 mg/m2 D4-6; R/R-AML, 6 mg/m2 D4-5), and filgrastim (5 mcg/kg D1-7). Consolidation used reduced durations of fludarabine and cytarabine (D2-4) and filgrastim (D1-5); idarubicin was permitted (D3-4) in up to two consolidation cycles at the discretion of the treating physician. At the recommended phase II dosing, VEN was administered on D1-14 during induction and D1-7 in consolidation. PEGylated filgrastim was permitted after D5 (induction) or D3 (consolidation) to replace remaining G-CSF doses. VEN dose adjustments for patients receiving CYP3A inhibitors such as azole antifungals followed US prescribing information recommendations (Appendix). Antimicrobial prophylaxis was recommended during periods of neutropenia.
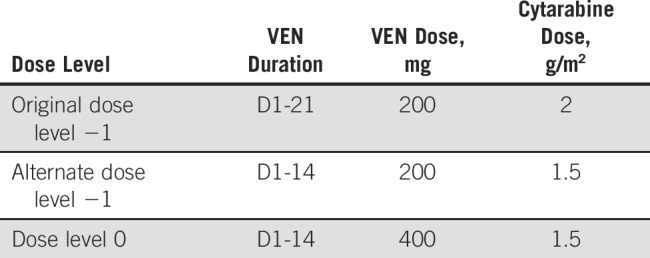
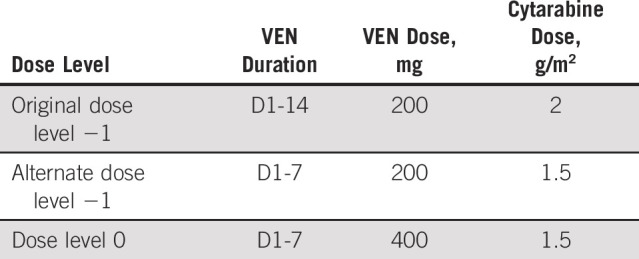
Because of pronounced grade 3 and 4 neutropenia-related infectious complications and one DLT (typhlitis) in the original PIB dose −1 level (n = 8), the Protocol (online only) was amended to evaluate an alternate dose level −1, reducing the VEN induction duration to 14 days (from 21) and with attenuated cytarabine (1.5 g/m2 from 2 g/m2). Venetoclax (D1-14) was then administered at 200 mg (alternate dose level −1, n = 5) and 400 mg (dose level 0, n = 3), and dose level 0 was confirmed as the recommended phase II dose for expansion. Additional details are provided in the Appendix. Following completion of induction or consolidation, continuous daily VEN maintenance was permitted on D1-28 of each 28-day cycle for up to 1 year in patients not proceeding to stem-cell transplantation.
Statistical Considerations
The dual primary objectives included safety and tolerability of FLAG-IDA + VEN, with identification of DLTs and determination of MTD (PIB), and assessment of overall activity (overall response rate [ORR]) per modified International Working Group criteria (PII).21 Secondary objectives included additional assessments of efficacy: CRc (CR + CRi + CR with partial hematologic recovery [CRh]), ORR (CR + CRh + CRi + morphologic leukemia-free state + partial response), OS (time from treatment initiation to death), event-free survival (EFS; time from treatment initiation until death or relapse, whichever occurred first; nonresponding patients were considered as progressing on cycle 1 day 1 for EFS), and duration of response (DOR; time from best response to relapse or death in responding patients only). Exploratory objectives included identification of biomarkers (ie cytogenetic and molecular mutations) predictive of VEN activity.
Futility and toxicity monitoring used a Bayesian method,22 applying monitoring rules to each arm separately (Appendix); 95% credible intervals were calculated for the primary objective for each PII arm and 95% exact CIs were computed for other response outcomes. Descriptive statistics were assessed using the Fisher's exact test. Time-to-event analyses were estimated using the Kaplan-Meier method and were compared using the log-rank test.
RESULTS
Demographics
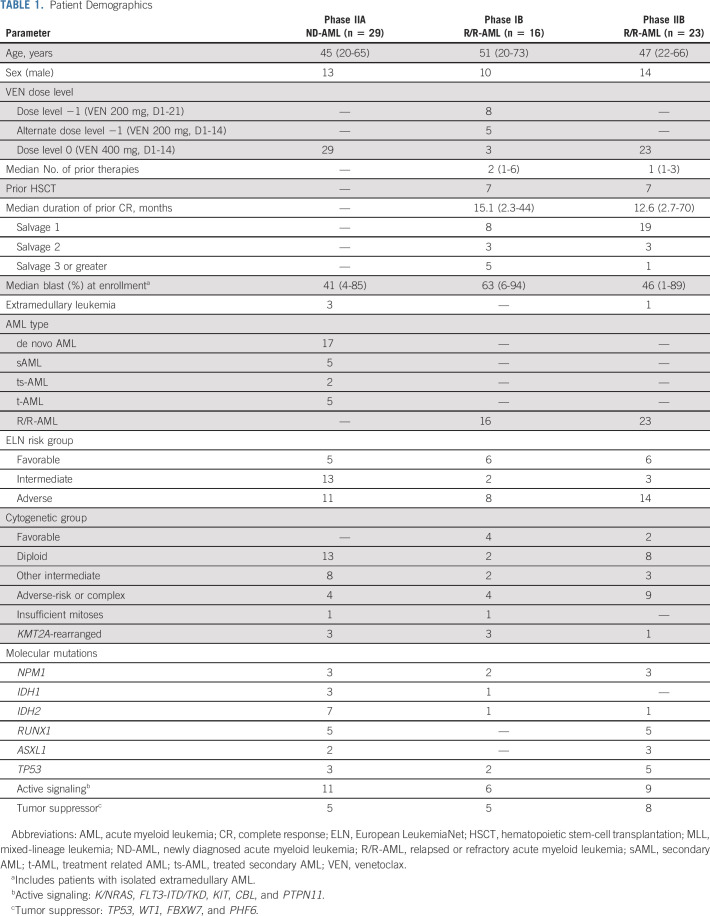
Sixty-eight patients (median age 46 years [range, 20-73]) have been enrolled (Table 1). Forty-one percent of ND-AML had sAML, ts-AML, or t-AML. European LeukemiaNet (ELN) risk across PIB, PIIA, and PIIB cohorts was favorable in 37.5%, 17%, and 26% of patients; intermediate in 12.5%, 45%, and 13%; and adverse in 50%, 38%, and 61%. Most R/R-AML patients (69%) were in salvage 1; 15% were in salvage 2 and 15% were in salvage 3 or greater. Forty-four percent PIB and 30% PIIB patients had received prior allogeneic hematopoietic stem-cell transplantation (alloHSCT).
TABLE 1.
Patient Demographics
Cytogenetic and Molecular Mutations at Study Enrollment
Diploid or other intermediate-risk cytogenetics (76%) were frequent in ND-AML, whereas adverse-risk or complex cytogenetics were common in R/R-AML (41%). Ten percent of patients harbored KMT2A rearrangements (KMT2A+). Epigenetic mutations (DNMT3A, TET2, IDH1, and IDH2) were more common in ND-AML compared with R/R-AML (41% v 15%; P value, .025), with enrichment of IDH2 in ND-AML (24% v 5%; P value, .03). Conversely, mutations in TP53 (18%) and WT1 (13%) were frequent in R/R-AML (Appendix Fig A1, online only).
Treatment Characteristics
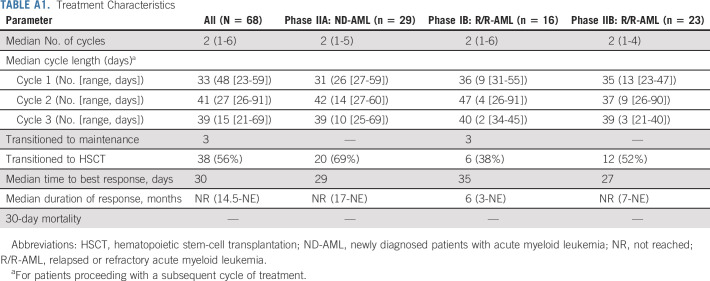
Patients received a median of 2 treatment cycles (range, 1-6; Appendix Table A1, online only). Sixty-nine percent (n = 47) received 1-2 cycles; 31% (n = 21) received ≥ 3 cycles. Four patients required reinduction (R/R-AML, 3; ND-AML, 1) with only the ND-AML patient responding (CRi). Median time to count recovery following induction (absolute neutrophil count ≥ 500 and platelet count ≥ 50,000) for PIB, PIIA, and PIIB patients was 37, 31, and 37 days (Appendix Figs A2A-F, online only) and was prolonged across all cohorts following cycle 2. Median time to count recovery for R/R-AML patients who underwent prior alloHSCT for cycles 1, 2, and 3 was 36, 41, and 37 days. Fifty-six percent of patients (n = 38; PIB: 38%, PIIA: 69%, and PIIB: 52%) transitioned to alloHSCT in remission after a median of 2 (range, 1-4) cycles. Sixty-seven percent (n = 20) of patients not undergoing alloHSCT received 1-2 cycles of therapy; 33% (n = 10) received ≥ 3 cycles.
Cycle lengths extending ≥ 40 days occurred in 19%, 59%, and 47% of patients completing cycles 1, 2, and 3. Myelosuppression was the leading cause of cycle delays, particularly following C2. Delayed count recovery requiring dose reductions in consolidation occurred in 24% (n = 11) of patients, and most frequently occurred in patients with s-AML, t-AML, ts-AML, or R/R-AML following C1 (89%) and C2 (63%), possibly reflecting poor marrow reserve in these populations. Sixty-one percent (n = 23) of patients transitioned to alloHSCT without full hematologic recovery (ie, absolute neutrophil count < 500 and/or platelet count < 50,000).
Adverse Events
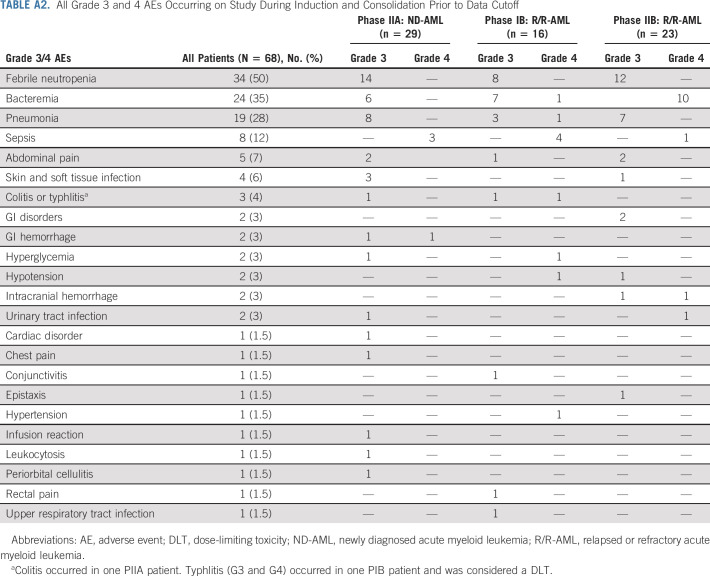
Grade 3 and 4 adverse events (AEs) occurring in ≥ 2 patients are displayed in Figure 1; all grade 3 and 4 AEs are provided in Appendix Table A2 (online only). Grade 3 and 4 AEs occurring in ≥ 10% of patients included febrile neutropenia (50%), bacteremia (35%), pneumonia (28%), and sepsis (12%). Febrile neutropenia and pneumonia occurred at similar frequencies in R/R-AML and ND-AML. Bacteremia was more common in R/R-AML (46% v 21%; P = .04), particularly PIB patients (50%; Fig 1B). Typhlitis was only observed in the original PIB cohort. Grade 3 and 4 AEs in R/R-AML patients who received prior alloHSCT were predominantly infectious (90%). Across all cohorts, 30- and 60-day mortality was 0% and 4.4%. Deaths on study or within one week of discontinuation all occurred in R/R patients, including four nonresponding (sepsis, n = 2; pneumonia, n = 1; pulmonary hemorrhage, n= 1) and two responding patients (sepsis and hemophagocytic syndrome).
FIG 1.
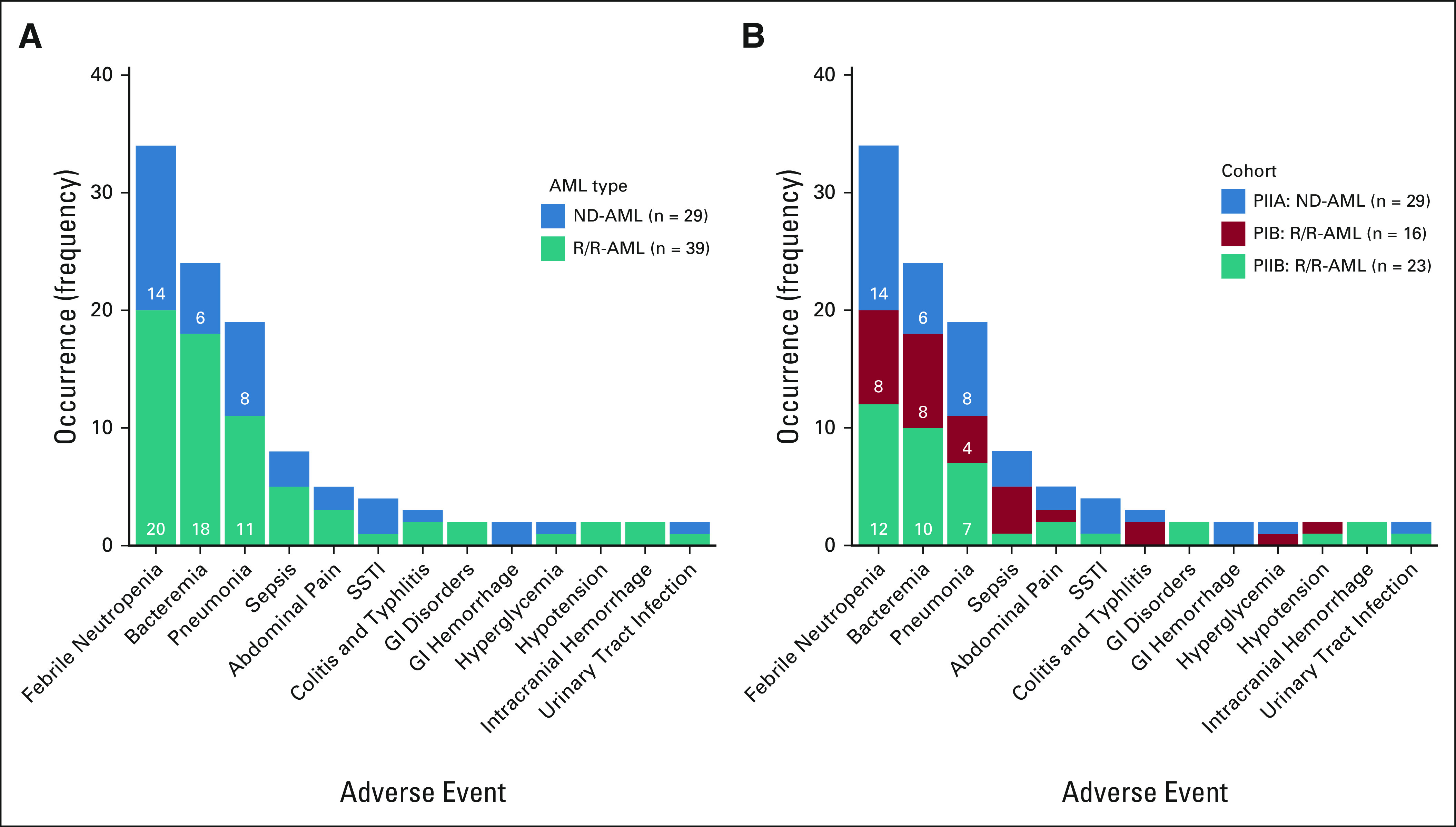
Adverse events by (A) AML type and (B) cohort. AML, acute myeloid leukemia; ND-AML, newly diagnosed acute myeloid leukemia; R/R-AML, relapsed or refractory acute myeloid leukemia; SSTI, skin and soft tissue infection.
Efficacy
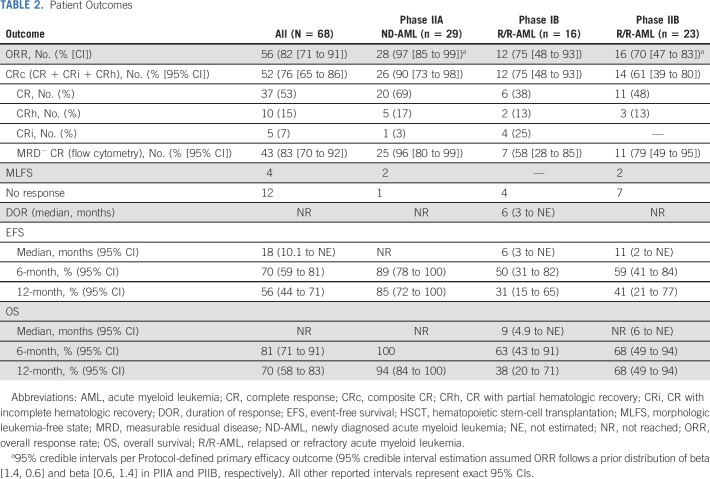
The ORR for PIB, PIIA, and PIIB cohorts was 75% (95% CI, 48 to 93), 97% (95% credible interval, 85 to 99), and 70% (95% credible interval, 47 to 83]) (Table 2) with CRc attained in 75% (48 to 93), 90% (73 to 98), and 61% (39 to 80; Fig 2); 67% of patients with R/R-AML (including 57% [n = 8] patients receiving prior alloHSCT) and 83% with sAML, t-AML, or ts-AML attained a CRc. No significant response difference was observed between patients with refractory versus relapsed AML (56% v 70%; P, .53). Median time to best response was 30 days, with ongoing responses in 70% of patients. Twelve patients (R/R-AML, 11; ND-AML, 1) were refractory to FLAG-IDA-VEN induction. Eighty-three percent (95% CI, 70 to 92) of patients in CRc attained MRD negativity (MRD−) including 96% (95% CI, 80 to 99) and 69% of patients with ND-AML (de novo AML: 94%; sAML, t-AML, or ts-AML: 100%) and R/R-AML, respectively (PIB: 58% [95% CI, 28 to 85]; PIIB: 79% [95% CI, 49 to 95]).
TABLE 2.
Patient Outcomes
FIG 2.
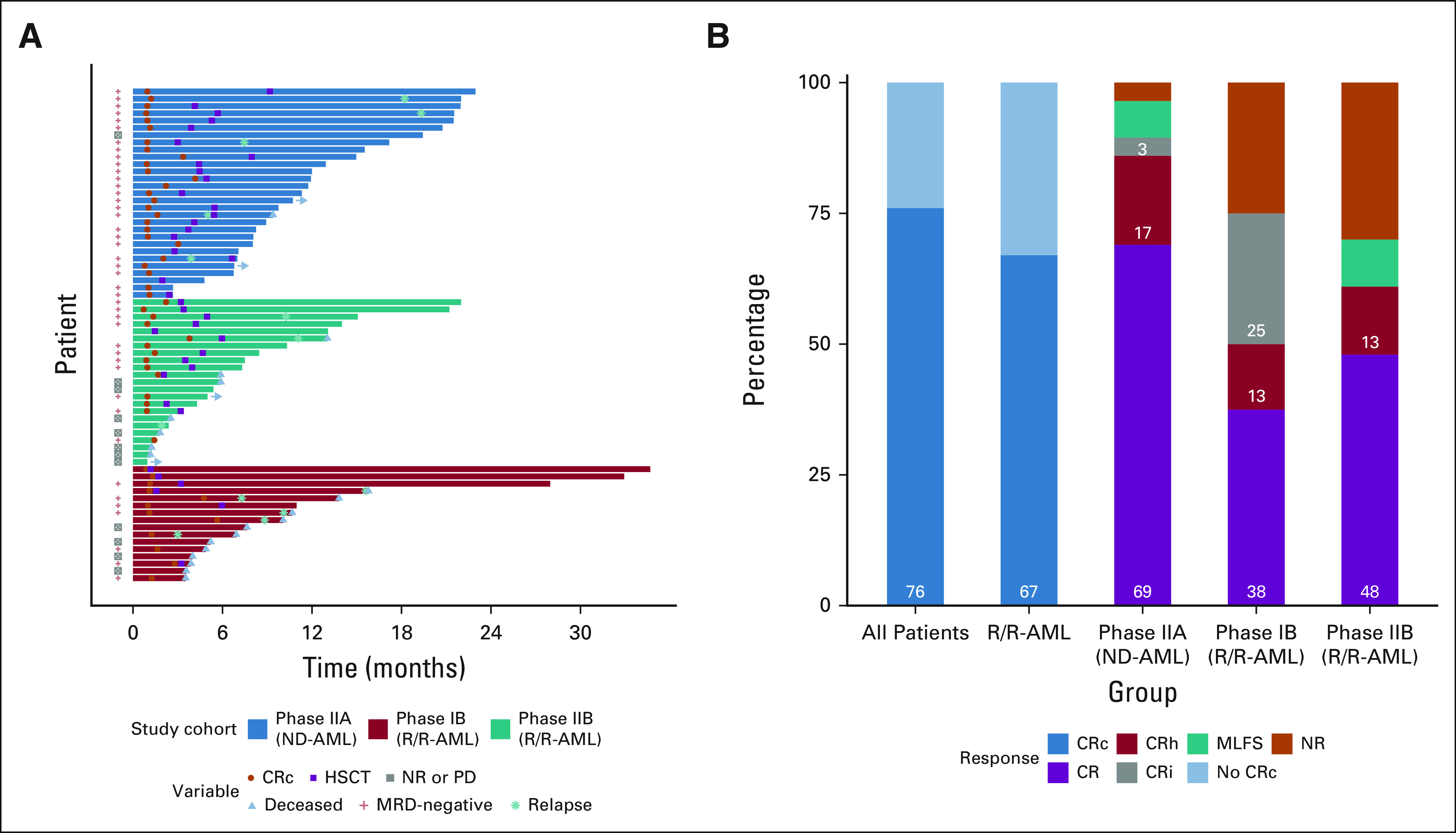
(A) Swimmers plot of all study participants and (B) response across study cohorts. Horizontal arrows indicate patients remaining on study. CR, complete response; CRc, composite CR; CRh, CR with partial hematologic recovery; CRi, CR with incomplete count recovery; HSCT, hematopoietic stem-cell transplantation; MLFS, morphologic leukemia-free state; MRD, measurable residual disease; ND-AML, newly diagnosed acute myeloid leukemia; NR, not reached; PD, progressive disease; R/R-AML, relapsed or refractory acute myeloid leukemia.
After a median follow-up of 12 months, 12-month OS for the overall population, PIIA patients, and PIIB patients was 70% (95% CI, 58 to 83), 94% (95% CI, 84 to 100), and 68% (95% CI, 49 to 94). Median EFS and OS were 6 (95% CI, 3 to not estimated [NE]) and 9 (95% CI, 4.9 to NE) months in PIB versus 11 (95% CI, 2 to NE) months and not reached (NR; 95% CI, 6 to NE) in PIIB patients (Figs 3A and 3B). Median DOR was 6 months (95% CI, 3 to NE) in PIB and NR in PII patients. The study population was predominantly composed of younger patients (median age, 46 years); however; 73% (n = 8) of patients age ≥ 60 years (including 100% age ≥ 65 [n = 4]) attained a CRc. No survival difference was observed between patients age ≤ 60 (n = 57) or age > 60 years (n = 11).
FIG 3.
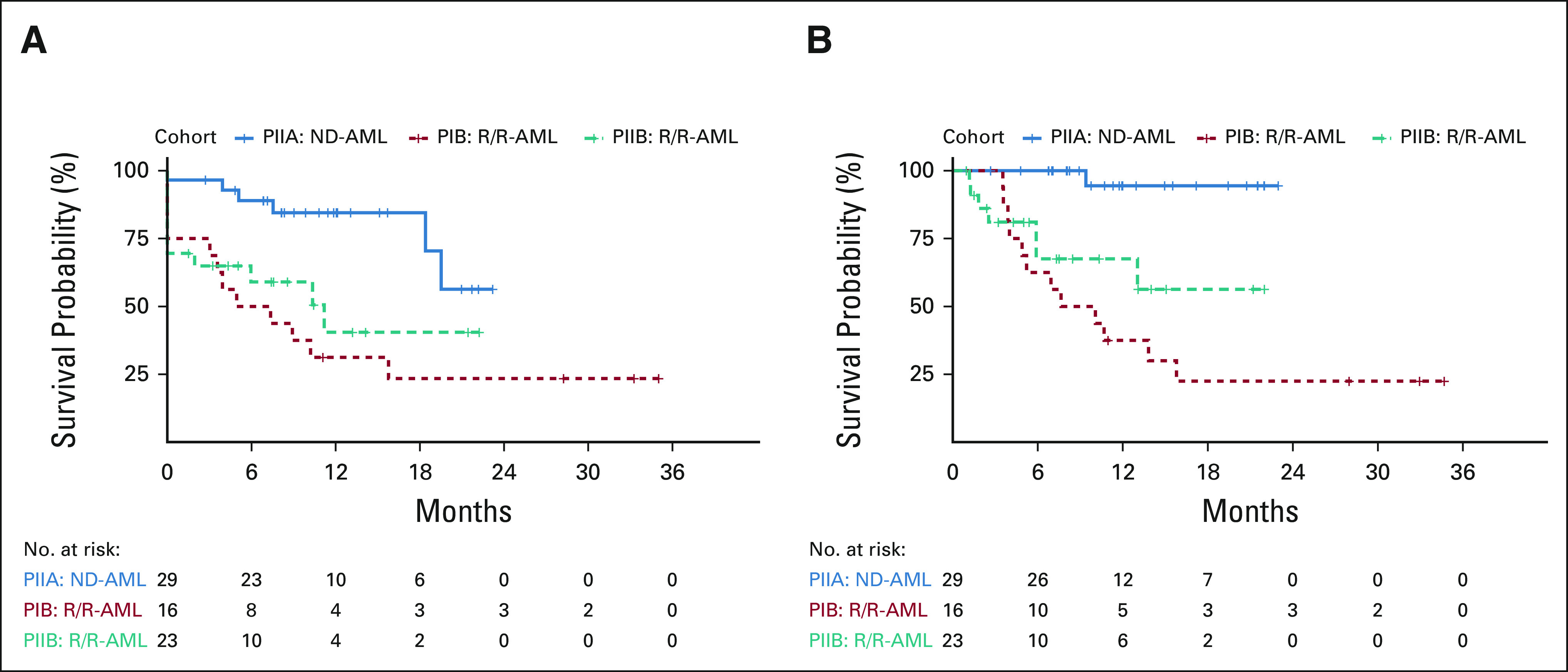
(A) EFS and (B) OS by cohort. EFS, event-free survival; ND-AML, newly diagnosed acute myeloid leukemia; OS, overall survival; R/R-AML, relapsed or refractory acute myeloid leukemia.
Median OS in R/R-AML was 13 months (95% CI, 7 to NR). Survival was not influenced on the basis of receipt of prior alloHSCT (median OS 13 months [95% CI, 7.64 to NE]) or prior CR duration, although survival in patients with prior CR durations < 12 months was similar to patients refractory at study enrollment (n = 9; 7 [6 to NE] v 8 [5 to NE] months; P = .94). Seventy-six percent of patients in salvage 1 or 2 and 17% in salvage 3 or greater achieved a CRc. Median OS in R/R-AML in salvage 1 (n = 27) or salvage 2 (n = 6) was significantly longer than that in patients in salvage 3 or greater (n = 6; median OS: 14 [10 to NE] v 4 [4 to NE] months; P = .003; Fig 4A).
FIG 4.
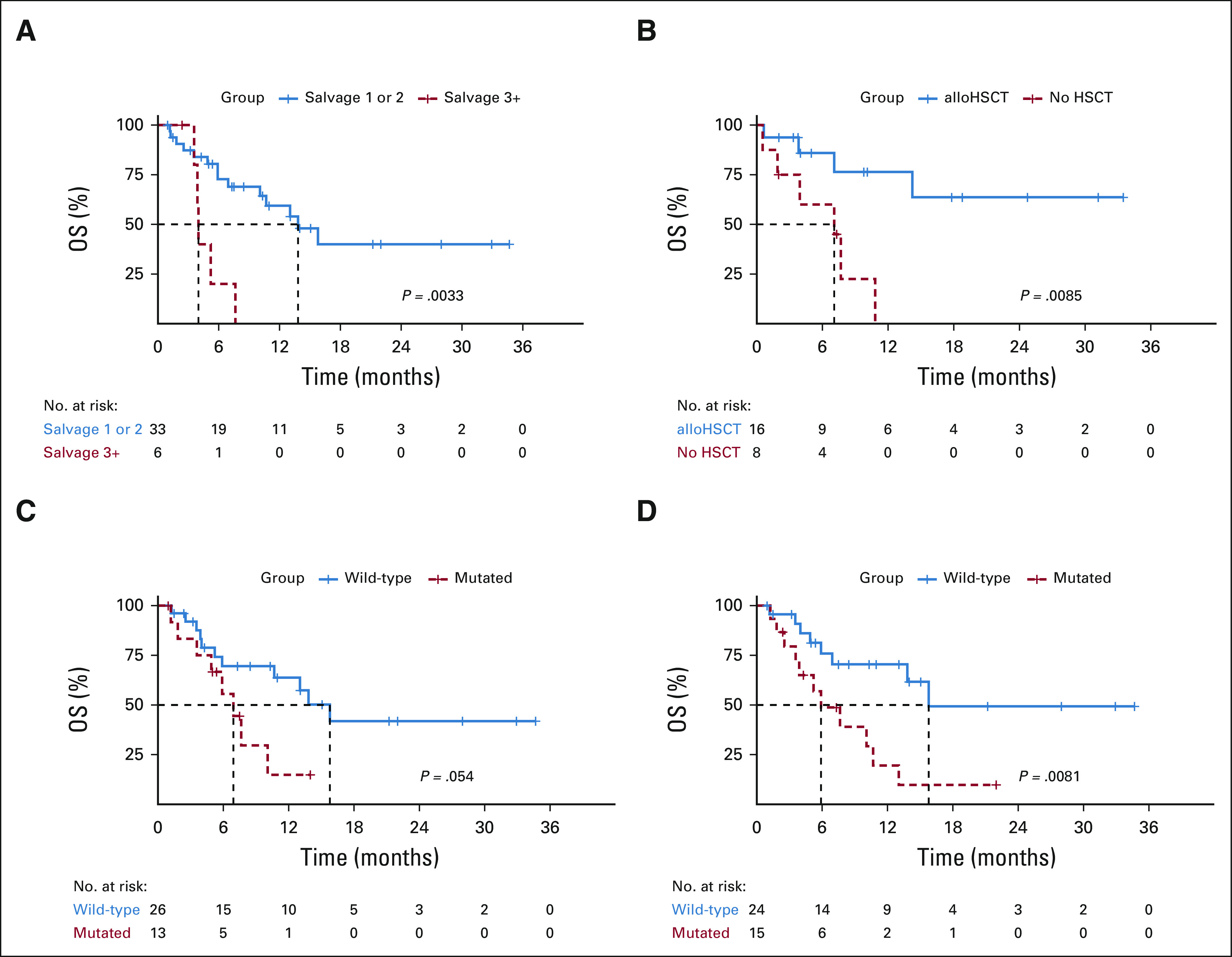
Outcomes in R/R-AML on the basis of (A) salvage number and (B) 3-month landmark analysis of HSCT in patients attaining CRc. Outcomes in R/R-AML with versus without mutations in (C) tumor suppressor and (D) active signaling genes. alloHSCT, allogeneic hematopoietic stem-cell transplantation; HSCT, hematopoietic stem-cell transplantation; ND-AML, newly diagnosed acute myeloid leukemia; OS, overall survival; R/R-AML, relapsed or refractory acute myeloid leukemia.
Cytogenetic and Molecular Subgroups
Overall, 100%, 85%, and 91% of ND-AML and 83%, 60%, and 59% of R/R-AML patients with ELN favorable-, intermediate-, and adverse-risk disease achieved a CRc. Four patients with extramedullary AML (ND-AML, 3; R/R-AML, 1) had durable responses, with three transitioning to alloHSCT. KMT2A-rearranged patients (n = 7; ND-AML, 3; R/R-AML, 4) attained a 100% CRc rate (80% MRD− by reverse transcriptase polymerase chain reaction for KMT2A) with a resultant 12-month OS of 80% (95% CI, 52 to 100).
Biomarkers predictive of response were only discriminatory within R/R-AML, given the high response rate (97%) in ND-AML. R/R-AML with favorable-risk cytogenetics (n = 6) correlated with an unpredictably poor median EFS and OS of 4 (95% CI, 4 to NE) and 7.6 (95% CI, 4 to NE) months; median EFS and OS were 7 (95% CI, 3 to NE) and 11 (95% CI, 5 to NE) months in R/R-AML patients with complex or adverse-risk cytogenetics (n = 16).
Molecular subgroups (NPM1, IDH1, or IDH2; n = 7) conferring sensitivity to VEN-based therapy13,23,24 demonstrated a 100% CRc rate and a 12-month OS of 83% in R/R-AML. Conversely, tumor suppressor mutations (TP53, WT1, FBXW7, and PHF6; n = 13) associated with treatment resistance (CRc with mutation: 38% v without: 77%; P = .021) were enriched in nonresponders (73%) versus responders (18%; P = .002) and trended toward inferior OS (7 [5 to NE] v 16 [11 to NE] months; P = .054; Fig 4C). Signaling mutations (RAS, FLT3, PTPN11, CBL, and KIT; n = 15) similarly correlated with inferior survival in R/R-AML (median OS, 6 [4 to NE] v 16 [14 to NE] months; P = .0081; Fig 4D).
Ten patients (ND-AML, 3; R/R-AML, 7) had detectable TP53 mutations (TP53+) at baseline. Sixty percent attained a CRc (ND-AML, 3/3; R/R-AML, 3/7) including 4 with MRD− CRc by FC. Median DOR and OS in ND-AML were 3.4 (95% CI, 2 to NE) and 9 (95% CI, 9 to NE) months. In R/R-AML, median DOR and OS were 3.2 (95% CI, 2 to NE) and 7 (95% CI, 5 to NE) months. Of interest, TP53+ persisted in all four patients with MRD− CRc and was identified in 64% (n = 7) of relapsed patients with available NGS, including three initially TP53 wild-type patients who developed TP53+ clones despite achieving a prior MRD− CRc by FC.
Role of HSCT
Thirty-eight patients (ND-AML, 20 [69%]; R/R-AML, 18 [46%]) transitioned to alloHSCT, including 75% (n = 6) of responding R/R patients who had received a prior alloHSCT. A 3-month landmark analysis in R/R-AML demonstrated improved OS with consolidative alloHSCT in CRc versus without (median OS: NR [14 to NE] v 7 [4 to NE] months; P value, .009; Fig 4B). Twelve-month OS was 87% in patients undergoing HSCT. After a median post-HSCT follow-up of 9 months, 12-month post-HSCT survival was 94% in ND-AML and 78% in R/R-AML. Thirty and 60-day post-HSCT mortality was 3%.
Three PIB patients transitioned to VEN maintenance, two patients who experienced significant infectious complications during induction precluding additional intensive chemotherapy and one following four cycles of FLAG-IDA + VEN induction or consolidation without plans for alloHSCT. Median EFS and OS with maintenance VEN were 8.8 (95% CI, 7 to NE) and 10.7 (95% CI, 10 to NE) months.
DISCUSSION
Despite AML induction regimens resulting in CR rates of 75%-85%,4,7,9 relapse remains the primary cause of mortality.25 Overall, 90% of ND-AML patients receiving FLAG-IDA + VEN achieved a CRc, including 81% with ongoing responses, with 12-month EFS of 85%. FLAG-IDA + VEN demonstrated a robust CRc rate of 83% in patients with sAML, ts-AML, or t-AML, an improvement compared with standard IC regimens in this higher-risk population.9
MRD-negative remissions translate into improved outcomes in ND-AML and R/R-AML and are increasingly considered an optimal IC end point, stratifying pre- and posttransplantation relapse risk.26-29 Development of potent frontline and salvage regimens capable of achieving this end point remain of particular interest. Ninety-six percent of ND-AML and 69% of R/R-AML receiving FLAG-IDA + VEN attained an MRD− CRc, highlighting the regimen's capability of producing MRD-negative remissions. Additional follow-up is warranted to confirm the survival impact of MRD− CRc within the ND-AML and R/R-AML cohorts.
Responses in R/R-AML vary greatly (CR rate, 20%-60%) by treatment selection and line of salvage therapy and often lack durablility.10,12,30 FLAG-IDA salvage in older (median age, 62 years) patients results in CR rates of approximately 20% and OS of 3.5 months10 Here, FLAG-IDA + VEN in a largely younger R/R-AML population improves upon historical outcomes with a CRc rate of 67% and median OS of 13 months. Patients receiving salvage 1 or 2 attained a 76% CRc rate, with a median OS of 14 months. Patients receiving FLAG + IDA + VEN as salvage 3 or greater experienced reduced CRc rates (17%) and median OS (4 months), reflecting the unmet therapeutic need in this population.12
Across ELN risk groups, FLAG-IDA + VEN resulted in high CRc rates. Diploid and intermediate-risk cytogenetics predicted favorable outcomes. Inferior survival was observed in patients with adverse-risk or complex cytogenetics, although FLAG-IDA + VEN improved outcomes compared with contemporary analyses of IC in this cytogenetic subgroup.31,32 Favorable-risk cytogenetics, implicated in upregulation of alternative BCL-2 proteins and a monocytic phenotype, may contribute to VEN resistance, partially accounting for the poor outcomes observed within this favorable but multiply relapsed subgroup.33,34
NPM1-, IDH1-, or IDH2-mutated AML had favorable responses to FLAG-IDA + VEN, whereas tumor suppressor mutations, in particular TP53, resulted in primary and secondary resistance and similar to signaling mutations predicted inferior survival in R/R-AML. Acknowledging the small sample sizes and exploratory nature of included molecular subgroup analyses, cautious interpretation and confirmation within larger study populations are warranted.
FLAG-IDA + VEN permitted transition to alloHSCT in 69% of ND-AML and 46% of R/R-AML patients, improving OS in R/R-AML compared with those not undergoing alloHSCT in CRc. Comparisons are limited by confounding reasons for patients not receiving alloHSCT; however, the high pretransplant MRD− CRc rate and favorable posttransplant survival (1-year post-HSCT OS, 78%) suggest FLAG-IDA + VEN is effective to bridge R/R-AML patients to alloHSCT.
At the phase II dosing regimen, FLAG + IDA + VEN was tolerable, with no early mortality observed. Grade 3 and 4 AEs occurring with FLAG-IDA + VEN were primarily infectious. Febrile neutropenia, bacteremia, and pneumonia accounted for the majority of AEs with observed rates similar to induction regimens across varying AML types, particularly sAML or R/R-AML.3,4,10,35 Despite G-CSF utilization, delayed count recovery following C2 was common, with cycle lengths exceeding 40 days in 59% of patients, similar to prior investigations of FLAG-IDA.3 Approximately one quarter of patients required dose reductions in consolidation, and 61% transitioned to HSCT without complete count recovery. Myelosuppression was most pronounced in patients with sAML or t-AML, or R/R-AML. G-CSF administration, frequent monitoring of peripheral blood counts, antimicrobial prophylaxis, and surveillance for infectious complications are essential for optimal patient support.
FLAG-IDA + VEN represents an effective intensive induction regimen for ND-AML and R/R-AML, with particular utility as a bridge to alloHSCT in the R/R-AML population. Confirmation of these results in ongoing dose-expansion cohorts and through randomized comparison with standard-of-care induction regimens is necessary to confirm the safety and effectiveness of FLAG-IDA + VEN both as a frontline induction regimen in newly diagnosed AML and an optimal salvage regimen in fit patients with relapsed or refractory AML.
APPENDIX. SUPPLEMENTARY METHODS
PIB Dosing Cohorts
The PIB (dose-escalation) arm applied a 3 + 3 dose escalation and de-escalation algorithm to determine the MTD starting at the −1 dose level (venetoclax [VEN] 200 mg) and escalating to dose level 0 (VEN 400 mg). Fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin (FLAG-IDA) induction consisted of 28-day cycles of intravenous (IV) fludarabine (30 mg/m2) and cytarabine (1.5-2 g/m2 IV) on days (D) 2-6, idarubicin (IV; newly diagnosed acute myeloid leukemia [ND-AML]: 8 mg/m2 D4-6; relapsed or refractory acute myeloid leukemia [R/R-AML]: 6 mg/m2 D4-5), and filgrastim (5 mcg/kg D1-7). Consolidation used reduced durations of fludarabine and cytarabine (D2-4) and filgrastim (D1-5); idarubicin was permitted (D3-4) in up to two consolidation cycles at the discretion of the treating physician. PEGylated filgrastim was permitted after D5 (induction) or D3 (consolidation) to replace remaining granulocyte colony-stimulating factor doses. VEN dose adjustments for patients receiving azole antifungals followed FDA recommendations.
Because of increased rates of grade 3 and 4 infectious complications in the original PIB patients treated at dose level −1 (n = 8) including one dose-limiting toxicity (DLT) of typhlitis, the Protocol was amended to use a reduced duration of venetoclax during induction and attenuated cytarabine (1.5 g/m2 in induction and consolidation). At this alternate dose level −1, no DLTs occurred and the dose was escalated to dose level 0, evaluating 400 mg of venetoclax. Dose level 0 was also determined to be safe and was selected for the phase II dose-expansion arms.
Note that in the original study design, the definition of DLT evaluable required the receipt of two cycles of therapy. Because of efficacy observed at even the dose −1 level, many patients in remission transitioned to allogeneic hematopoietic stem-cell transplantation after one induction cycle, rendering them unevaluable for DLT and requiring replacement. A Protocol amendment, which updated the DLT evaluation period to one cycle of therapy, was approved by all applicable regulatory bodies in August 2018 and dose level 0 opened for patient enrollment in September 2018.
PIB Treatment Administration
Induction dosing schema after Protocol amendment
Consolidation dosing schema
Additional Statistical Considerations
Futility and Toxicity Monitoring for Dose-Expansion Cohorts (PIIA and PIIB)
Futility and toxicity monitoring used a Bayesian method,22 applying monitoring rules to each arm separately. The ND-AML cohort will be stopped early if a > 98% probability exists that the overall response rate (ORR) with FLAG-IDA + VEN was less than the overall response rate under standard-of-care treatment (SOC) plus 15% (ie, it is less likely that the study treatment will improve ORR by 15% over SOC) or if a > 88% probability existed that the DLT rate was > 30%. Similarly, the R/R-AML cohort will be stopped early if there is a > 99% probability that the ORR with FLAG-IDA + VEN was less than the ORR under SOC plus 10% or if > 90% probability existed that the DLT rate was > 30%.
FIG A1.
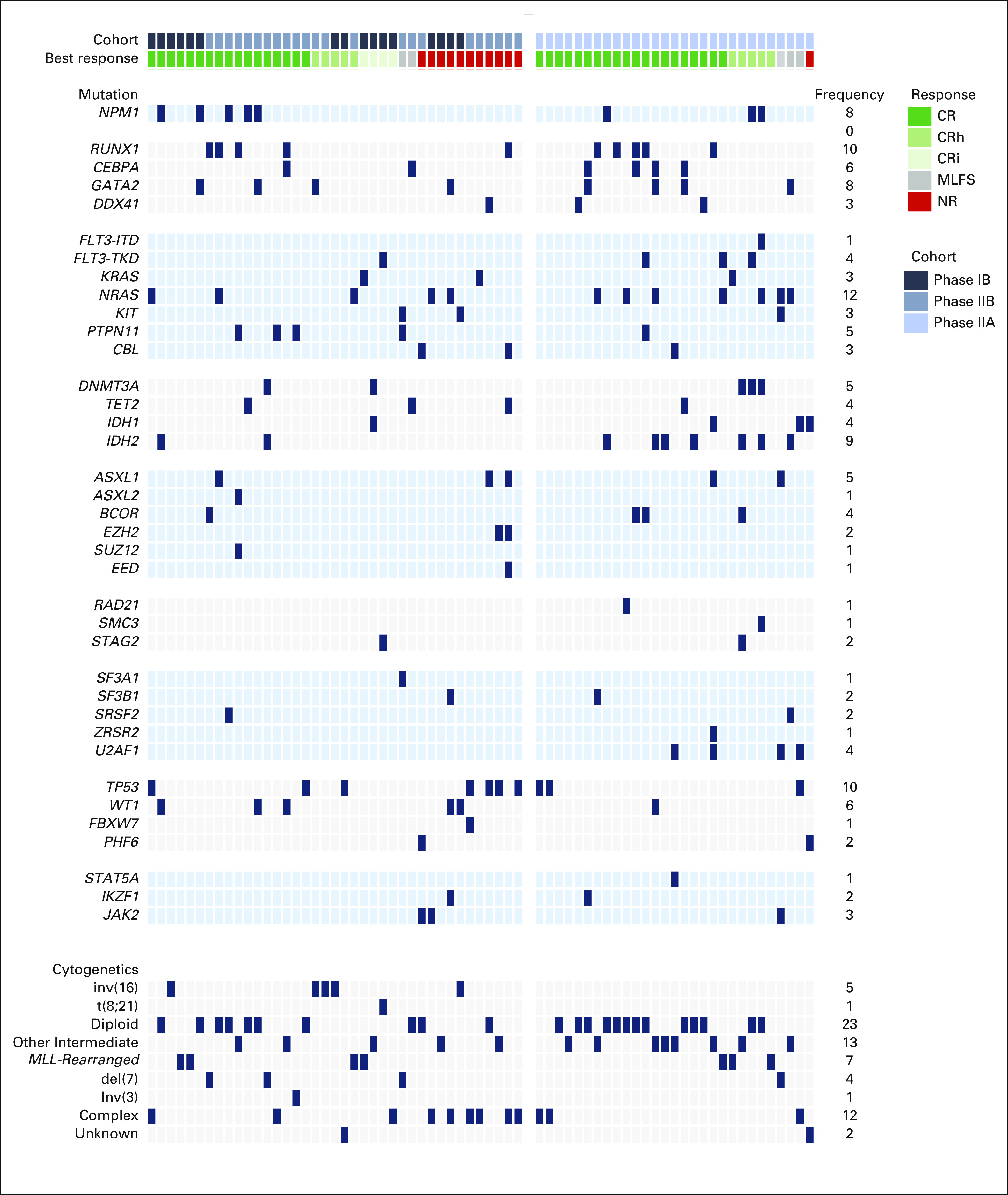
Genomic landscape of AML cohorts. AML, acute myeloid leukemia; CR, complete response; CRh, CR with partial hematologic recovery; CRi, CR with incomplete count recovery; MLFS, morphologic leukemia-free state; NR, not reached.
FIG A2.
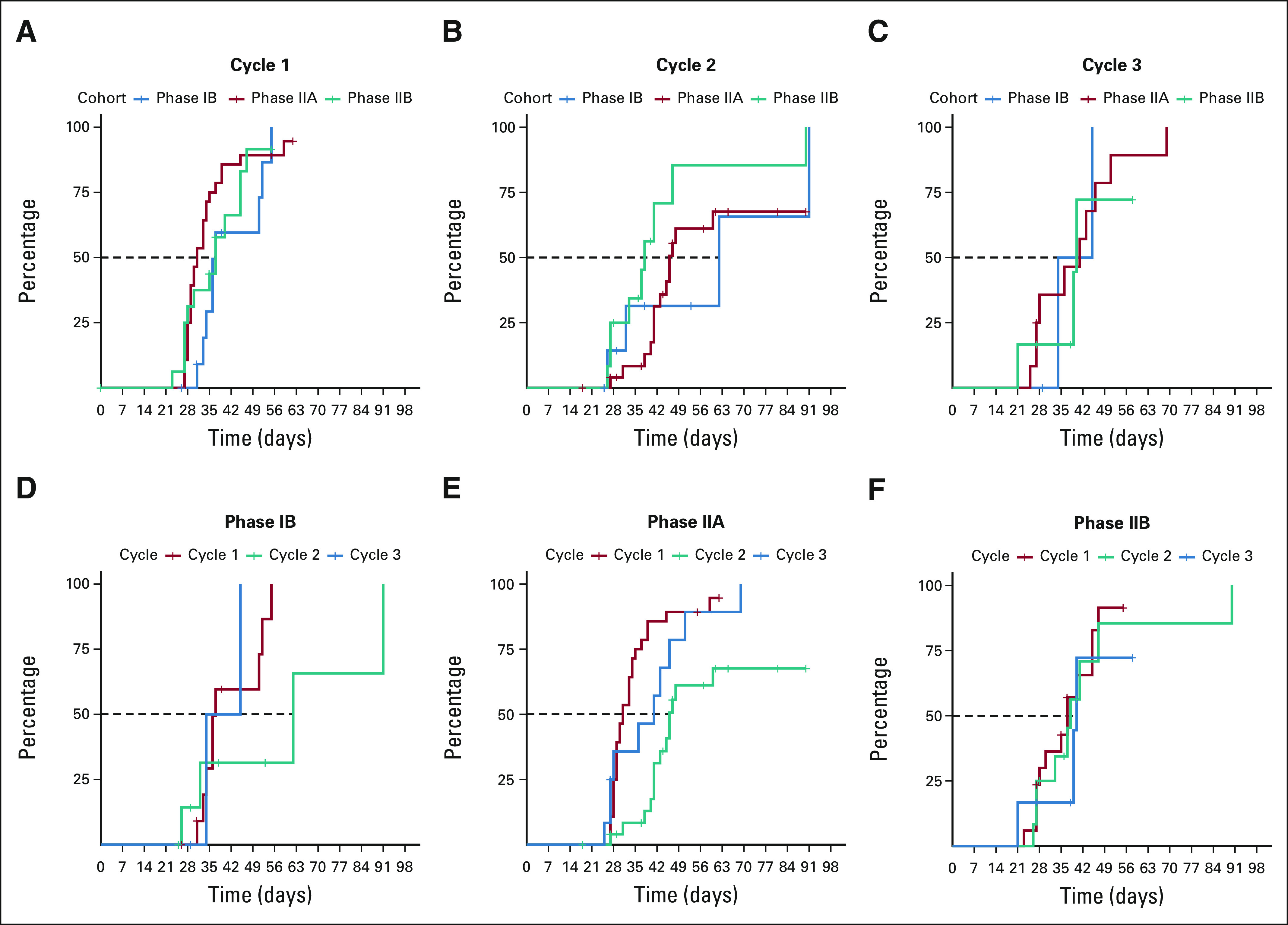
Median time to count recovery (ANC ≥ 500; platelet count ≥ 50,000 cells/µL) by (A-C) cycle and (D-F) study cohort. ANC, absolute neutrophil count.
TABLE A1.
Treatment Characteristics
TABLE A2.
All Grade 3 and 4 AEs Occurring on Study During Induction and Consolidation Prior to Data Cutoff
Courtney D. DiNardo
Leadership: Notable Labs
Stock and Other Ownership Interests: Notable Labs
Honoraria: Agios, Celgene, AbbVie, Jazz Pharmaceuticals, Daiichi Sankyo, Novartis, Takeda
Consulting or Advisory Role: Celgene, Agios, AbbVie
Research Funding: AbbVie, Agios, Celgene, Daiichi Sankyo
Koichi Takahashi
Honoraria: Celgene, Novartis, GlaxoSmithKline, SymBio Pharmaceuticals, DAVA Pharmaceuticals
Consulting or Advisory Role: SymBio Pharmaceuticals, Novartis, GlaxoSmithKline, Celgene
Research Funding: Onconova Therapeutics
Travel, Accommodations, Expenses: SymBio Pharmaceuticals, Celgene, GlaxoSmithKline, DAVA Pharmaceuticals
Sanam Loghavi
Stock and Other Ownership Interests: AbbVie, PureTech
Honoraria: Curio Science
Consulting or Advisory Role: Seattle Genetics, Gerson Lehrman Group, Guidepoint Global
Travel, Accommodations, Expenses: Curio Science
Tapan Kadia
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Pfizer, AbbVie/Genentech, Agios
Research Funding: Bristol Myers Squibb, Celgene, Sanofi, Amgen, BiolineRx, Incyte, Genentech/AbbVie, Pfizer, Jazz Pharmaceuticals, AstraZeneca, Astellas Pharma, Ascentage Pharma, Genfleet, Cyclacel
Naval Daver
Honoraria: BMS, Jazz Pharmaceuticals, Novartis, Incyte, Otsuka, Immunogen, Pfizer, Astellas Pharma, AbbVie
Consulting or Advisory Role: Celgene, Agios, Jazz Pharmaceuticals, Pfizer, AbbVie, Astellas Pharma, Daiichi Sankyo, Novartis, Bristol Myers Squibb, Otsuka, Incyte, Karyopharm Therapeutics, Sunesis Pharmaceuticals, Amgen, Immunogen, Genentech, Servier, Syndax, Trillium Therapeutics
Research Funding: Bristol Myers Squibb, Pfizer, Immunogen, Genentech, Nohla Therapeutics, AbbVie, Astellas Pharma, Servier, Daiichi Sankyo, Novartis, Karyopharm Therapeutics, Incyte, Sunesis Pharmaceuticals, Sobi
Nicholas J. Short
Honoraria: Amgen
Consulting or Advisory Role: AstraZeneca
Research Funding: Takeda, Astellas Pharma
Koji Sasaki
Honoraria: Otsuka
Consulting or Advisory Role: Novartis, Pfizer, Takeda
Research Funding: Novartis
Travel, Accommodations, Expenses: Otsuka
Sa Wang
Consulting or Advisory Role: Boston Biomedical, GlaxoSmithKline, Blueprint Medicines, Caprico
Research Funding: GlaxoSmithKline, Autolus Ltd, Genmab
Gautam Borthakur
Consulting or Advisory Role: Argenx, PTC Therapeutics, BiolineRx, BioTheryX, Nkarta, Treadwell Therapeutics, Novartis, Catamaran Bio, Takeda
Research Funding: Incyte, GlaxoSmithKline, Cyclacel, BiolineRx, MedImmune, Lilly, Oncoceutics, Ryvu Therapeutics, Janssen Scientific Affairs, Bristol Myers Squibb, AbbVie, Novartis, AstraZeneca, Mundipharma Research, PTC Therapeutics, BioTheryX, XBiotech, Arvinas, Astex Pharmaceuticals, TCR2 Therapeutics, Nkarta, Treadwell Therapeutics, Cellestia Biotech
Ghayas Issa
Consulting or Advisory Role: Novartis, Kura Oncology
Research Funding: Novartis, Syndax, Kura Oncology
Abhishek Maiti
Research Funding: Celgene
Yesid Alvarado
Research Funding: Jazz Pharmaceuticals, FibroGen, Sun Pharma, BerGenBio, Daiichi Sankyo/Lilly, Astex Pharmaceuticals
Naveen Pemmaraju
Employment: MD Anderson Cancer Center
Leadership: ASH, ASCO
Honoraria: Incyte, Novartis, LFB Biotechnologies, Stemline Therapeutics, Celgene, AbbVie, MustangBio, Roche Molecular Diagnostics, Blueprint Medicines, DAVA Pharmaceuticals, Springer
Consulting or Advisory Role: Blueprint Medicines, Pacylex, Immunogen, Bristol Myers Squibb
Research Funding: Novartis, Stemline Therapeutics, Samus Therapeutics, AbbVie, Cellectis, Affymetrix/Thermo Fisher Scientific, Daiichi Sankyo, Plexxikon
Travel, Accommodations, Expenses: Stemline Therapeutics, Celgene, AbbVie, DAVA Oncology, MustangBio
Uncompensated Relationships: Dan's House of Hope, Oncology Times
Guillermo Bravo
Research Funding: IFM Therapeutics
Musa Yilmaz
Research Funding: Daiichi Sankyo, Pfizer
Nitin Jain
Honoraria: Pharmacyclics, ADC Therapeutics, Adaptive Biotechnologies, AbbVie/Genentech, Janssen, AstraZeneca/MedImmune, Servier, Precision Biosciences, BeiGene, TG Therapeutics, Cellectis, Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Pharmacyclics, ADC Therapeutics, Adaptive Biotechnologies, AbbVie/Genentech, Janssen, AstraZeneca/MedImmune, Servier, Precision Biosciences, BeiGene, TG Therapeutics, Cellectis, Bristol Myers Squibb/Celgene
Research Funding: Pfizer, Pharmacyclics, AbbVie, Genentech/Roche, Incyte, Infinity Pharmaceuticals, Bristol Myers Squibb, Seattle Genetics, Celgene, ADC Therapeutics, Servier, AstraZeneca/MedImmune, Cellectis, Adaptive Biotechnologies, Precision Biosciences, Aprea Therapeutics, Fate Therapeutics, Kite, a Gilead company
Michael Andreef
Stock and Other Ownership Interests: Reata Pharmaceuticals, Aptose Biosciences, Eutropics, Senti Biosciences, Oncoceutics, Oncolyze
Consulting or Advisory Role: Daiichi Sankyo, Jazz Pharmaceuticals, Celgene, Amgen, AstraZeneca
Research Funding: Daiichi Sankyo, Breast Cancer Research Foundation, United Therapeutics, Ono Pharmaceutical, Karyopharm Therapeutics, CPRIT, NIH/NCI, Amgen, AstraZeneca
Patents, Royalties, Other Intellectual Property: Patent with Reata
Travel, Accommodations, Expenses: Aptose Biosciences, Oncoceutics, Amgen, AstraZeneca
Elias Jabbour
Consulting or Advisory Role: Pfizer, Takeda, Amgen, AbbVie, Bristol Myers Squibb, Incyte, Adaptive Biotechnologies, Astellas Pharma, Genentech
Research Funding: Pfizer, AbbVie, Amgen, Takeda, Adaptive Biotechnologies, Ascentage Pharma Group
Guillermo Garcia-Manero
Honoraria: Celgene, Astex Pharmaceuticals, Acceleron Pharma, Helssin, AbbVie
Consulting or Advisory Role: Celgene, Astex Pharmaceuticals, Acceleron Pharma, Jazz Pharmaceuticals, Bristol Myers Squibb, Helsinn Therapeutics
Research Funding: Celgene, Astex Pharmaceuticals, Amphivena, Helsinn Therapeutics, Novartis, AbbVie, Bristol Myers Squibb, Onconova Therapeutics, H3 Biomedicine, Merck
Farhad Ravandi
Honoraria: Amgen, Pfizer, Astellas Pharma, Orsenix, Celgene, Agios, AbbVie/Genentech, AstraZeneca, Bristol Myers Squibb, Takeda, Jazz Pharmaceuticals, Novartis
Consulting or Advisory Role: Amgen, Astellas Pharma, Orsenix, Celgene, Jazz Pharmaceuticals, Agios, AbbVie/Genentech, Bristol Myers Squibb, AstraZeneca, Taiho Oncology, Syros Pharmaceuticals, Certara Inc
Research Funding: Bristol Myers Squibb, Amgen, Macrogenics, Xencor, Selvita, Cellerant
Marina Y. Konopleva
Honoraria: AbbVie, Genentech, Roche, Amgen, Stemline Therapeutics, KisoJi Biotechnology
Consulting or Advisory Role: AbbVie, Genentech/Roche, Stemline Therapeutics, Amgen, Forty Seven, KisoJi Biotechnology, Roche, Janssen
Research Funding: AbbVie, Genentech, Roche, Lilly, Cellectis, Calithera Biosciences, Ablynx, Agios, Ascentage Pharma, AstraZeneca, Sanofi
Patents, Royalties, Other Intellectual Property: Novartis—Pending, Eli Lilly—Research Funding; Issued/Licensed, Reata Pharmaceutical—Research Funding (Issued, Licensed, Royalities)
Hagop M. Kantarjian
Honoraria: AbbVie, Amgen, ARIAD, Bristol Myers Squibb, Immunogen, Orsenix, Pfizer, Agios, Takeda, Actinium Pharmaceuticals
Research Funding: Pfizer, Amgen, Bristol Myers Squibb, Novartis, ARIAD, Astex Pharmaceuticals, AbbVie, Agios, Cyclacel, Immunogen, Jazz Pharmaceuticals
No other potential conflicts of interest were reported.
Listen to the podcast by Dr Dillon at jcopodcast.libsynpro.com
PRIOR PRESENTATION
Presented at the American Society of Hematology Annual Meeting, Orlando, FL, December 7-10, 2019, and December 5-8, 2020.
SUPPORT
C.D.D. is a recipient of the Lloyd Family/V Foundation Clinical Scholar Award MD Anderson Cancer Center Leukemia SPORE CA10063.
C.D.D. and C.A.L. are first authors; M.Y.K. and H.M.K are last authors.
AUTHOR CONTRIBUTIONS
Conception and design: Courtney D. DiNardo, Sanam Loghavi, Lianchun Xiao, Musa Yilmaz, Elias Jabbour, Guillermo Garcia-Manero, Farhad Ravandi, Marina Y. Konopleva
Financial support: Courtney D. DiNardo
Administrative support: Courtney D. DiNardo
Provision of study materials or patients: Courtney D. DiNardo, Koichi Takahashi, Tapan Kadia, Naval Daver, Maria Adeoti, Gautam Borthakur, Ghayas Issa, Yesid Alvarado, Lucia Masarova, Michael Andreef, Guillermo Garcia-Manero, Farhad Ravandi, Marina Y. Konopleva, Hagop M. Kantarjian
Collection and assembly of data: Courtney D. DiNardo, Curtis A. Lachowiez, Koichi Takahashi, Sanam Loghavi, Tapan Kadia, Naval Daver, Maria Adeoti, Nicholas J. Short, Sa Wang, Gautam Borthakur, Yesid Alvarado, Naveen Pemmaraju, Guillermo Montalban Bravo, Nitin Jain, Guillermo Garcia-Manero, Steven Kornblau, Marina Y. Konopleva
Data analysis and interpretation: Courtney D. DiNardo, Curtis A. Lachowiez, Sanam Loghavi, Lianchun Xiao, Tapan Kadia, Naval Daver, Koji Sasaki, Sa Wang, Ghayas Issa, Abhishek Maiti, Naveen Pemmaraju, Guillermo Montalban Bravo, Lucia Masarova, Michael Andreef, Elias Jabbour, Guillermo Garcia-Manero, Marina Y. Konopleva, Hagop M. Kantarjian
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Venetoclax Combined With FLAG-IDA Induction and Consolidation in Newly Diagnosed and Relapsed or Refractory Acute Myeloid Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Courtney D. DiNardo
Leadership: Notable Labs
Stock and Other Ownership Interests: Notable Labs
Honoraria: Agios, Celgene, AbbVie, Jazz Pharmaceuticals, Daiichi Sankyo, Novartis, Takeda
Consulting or Advisory Role: Celgene, Agios, AbbVie
Research Funding: AbbVie, Agios, Celgene, Daiichi Sankyo
Koichi Takahashi
Honoraria: Celgene, Novartis, GlaxoSmithKline, SymBio Pharmaceuticals, DAVA Pharmaceuticals
Consulting or Advisory Role: SymBio Pharmaceuticals, Novartis, GlaxoSmithKline, Celgene
Research Funding: Onconova Therapeutics
Travel, Accommodations, Expenses: SymBio Pharmaceuticals, Celgene, GlaxoSmithKline, DAVA Pharmaceuticals
Sanam Loghavi
Stock and Other Ownership Interests: AbbVie, PureTech
Honoraria: Curio Science
Consulting or Advisory Role: Seattle Genetics, Gerson Lehrman Group, Guidepoint Global
Travel, Accommodations, Expenses: Curio Science
Tapan Kadia
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Pfizer, AbbVie/Genentech, Agios
Research Funding: Bristol Myers Squibb, Celgene, Sanofi, Amgen, BiolineRx, Incyte, Genentech/AbbVie, Pfizer, Jazz Pharmaceuticals, AstraZeneca, Astellas Pharma, Ascentage Pharma, Genfleet, Cyclacel
Naval Daver
Honoraria: BMS, Jazz Pharmaceuticals, Novartis, Incyte, Otsuka, Immunogen, Pfizer, Astellas Pharma, AbbVie
Consulting or Advisory Role: Celgene, Agios, Jazz Pharmaceuticals, Pfizer, AbbVie, Astellas Pharma, Daiichi Sankyo, Novartis, Bristol Myers Squibb, Otsuka, Incyte, Karyopharm Therapeutics, Sunesis Pharmaceuticals, Amgen, Immunogen, Genentech, Servier, Syndax, Trillium Therapeutics
Research Funding: Bristol Myers Squibb, Pfizer, Immunogen, Genentech, Nohla Therapeutics, AbbVie, Astellas Pharma, Servier, Daiichi Sankyo, Novartis, Karyopharm Therapeutics, Incyte, Sunesis Pharmaceuticals, Sobi
Nicholas J. Short
Honoraria: Amgen
Consulting or Advisory Role: AstraZeneca
Research Funding: Takeda, Astellas Pharma
Koji Sasaki
Honoraria: Otsuka
Consulting or Advisory Role: Novartis, Pfizer, Takeda
Research Funding: Novartis
Travel, Accommodations, Expenses: Otsuka
Sa Wang
Consulting or Advisory Role: Boston Biomedical, GlaxoSmithKline, Blueprint Medicines, Caprico
Research Funding: GlaxoSmithKline, Autolus Ltd, Genmab
Gautam Borthakur
Consulting or Advisory Role: Argenx, PTC Therapeutics, BiolineRx, BioTheryX, Nkarta, Treadwell Therapeutics, Novartis, Catamaran Bio, Takeda
Research Funding: Incyte, GlaxoSmithKline, Cyclacel, BiolineRx, MedImmune, Lilly, Oncoceutics, Ryvu Therapeutics, Janssen Scientific Affairs, Bristol Myers Squibb, AbbVie, Novartis, AstraZeneca, Mundipharma Research, PTC Therapeutics, BioTheryX, XBiotech, Arvinas, Astex Pharmaceuticals, TCR2 Therapeutics, Nkarta, Treadwell Therapeutics, Cellestia Biotech
Ghayas Issa
Consulting or Advisory Role: Novartis, Kura Oncology
Research Funding: Novartis, Syndax, Kura Oncology
Abhishek Maiti
Research Funding: Celgene
Yesid Alvarado
Research Funding: Jazz Pharmaceuticals, FibroGen, Sun Pharma, BerGenBio, Daiichi Sankyo/Lilly, Astex Pharmaceuticals
Naveen Pemmaraju
Employment: MD Anderson Cancer Center
Leadership: ASH, ASCO
Honoraria: Incyte, Novartis, LFB Biotechnologies, Stemline Therapeutics, Celgene, AbbVie, MustangBio, Roche Molecular Diagnostics, Blueprint Medicines, DAVA Pharmaceuticals, Springer
Consulting or Advisory Role: Blueprint Medicines, Pacylex, Immunogen, Bristol Myers Squibb
Research Funding: Novartis, Stemline Therapeutics, Samus Therapeutics, AbbVie, Cellectis, Affymetrix/Thermo Fisher Scientific, Daiichi Sankyo, Plexxikon
Travel, Accommodations, Expenses: Stemline Therapeutics, Celgene, AbbVie, DAVA Oncology, MustangBio
Uncompensated Relationships: Dan's House of Hope, Oncology Times
Guillermo Bravo
Research Funding: IFM Therapeutics
Musa Yilmaz
Research Funding: Daiichi Sankyo, Pfizer
Nitin Jain
Honoraria: Pharmacyclics, ADC Therapeutics, Adaptive Biotechnologies, AbbVie/Genentech, Janssen, AstraZeneca/MedImmune, Servier, Precision Biosciences, BeiGene, TG Therapeutics, Cellectis, Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Pharmacyclics, ADC Therapeutics, Adaptive Biotechnologies, AbbVie/Genentech, Janssen, AstraZeneca/MedImmune, Servier, Precision Biosciences, BeiGene, TG Therapeutics, Cellectis, Bristol Myers Squibb/Celgene
Research Funding: Pfizer, Pharmacyclics, AbbVie, Genentech/Roche, Incyte, Infinity Pharmaceuticals, Bristol Myers Squibb, Seattle Genetics, Celgene, ADC Therapeutics, Servier, AstraZeneca/MedImmune, Cellectis, Adaptive Biotechnologies, Precision Biosciences, Aprea Therapeutics, Fate Therapeutics, Kite, a Gilead company
Michael Andreef
Stock and Other Ownership Interests: Reata Pharmaceuticals, Aptose Biosciences, Eutropics, Senti Biosciences, Oncoceutics, Oncolyze
Consulting or Advisory Role: Daiichi Sankyo, Jazz Pharmaceuticals, Celgene, Amgen, AstraZeneca
Research Funding: Daiichi Sankyo, Breast Cancer Research Foundation, United Therapeutics, Ono Pharmaceutical, Karyopharm Therapeutics, CPRIT, NIH/NCI, Amgen, AstraZeneca
Patents, Royalties, Other Intellectual Property: Patent with Reata
Travel, Accommodations, Expenses: Aptose Biosciences, Oncoceutics, Amgen, AstraZeneca
Elias Jabbour
Consulting or Advisory Role: Pfizer, Takeda, Amgen, AbbVie, Bristol Myers Squibb, Incyte, Adaptive Biotechnologies, Astellas Pharma, Genentech
Research Funding: Pfizer, AbbVie, Amgen, Takeda, Adaptive Biotechnologies, Ascentage Pharma Group
Guillermo Garcia-Manero
Honoraria: Celgene, Astex Pharmaceuticals, Acceleron Pharma, Helssin, AbbVie
Consulting or Advisory Role: Celgene, Astex Pharmaceuticals, Acceleron Pharma, Jazz Pharmaceuticals, Bristol Myers Squibb, Helsinn Therapeutics
Research Funding: Celgene, Astex Pharmaceuticals, Amphivena, Helsinn Therapeutics, Novartis, AbbVie, Bristol Myers Squibb, Onconova Therapeutics, H3 Biomedicine, Merck
Farhad Ravandi
Honoraria: Amgen, Pfizer, Astellas Pharma, Orsenix, Celgene, Agios, AbbVie/Genentech, AstraZeneca, Bristol Myers Squibb, Takeda, Jazz Pharmaceuticals, Novartis
Consulting or Advisory Role: Amgen, Astellas Pharma, Orsenix, Celgene, Jazz Pharmaceuticals, Agios, AbbVie/Genentech, Bristol Myers Squibb, AstraZeneca, Taiho Oncology, Syros Pharmaceuticals, Certara Inc
Research Funding: Bristol Myers Squibb, Amgen, Macrogenics, Xencor, Selvita, Cellerant
Marina Y. Konopleva
Honoraria: AbbVie, Genentech, Roche, Amgen, Stemline Therapeutics, KisoJi Biotechnology
Consulting or Advisory Role: AbbVie, Genentech/Roche, Stemline Therapeutics, Amgen, Forty Seven, KisoJi Biotechnology, Roche, Janssen
Research Funding: AbbVie, Genentech, Roche, Lilly, Cellectis, Calithera Biosciences, Ablynx, Agios, Ascentage Pharma, AstraZeneca, Sanofi
Patents, Royalties, Other Intellectual Property: Novartis—Pending, Eli Lilly—Research Funding; Issued/Licensed, Reata Pharmaceutical—Research Funding (Issued, Licensed, Royalities)
Hagop M. Kantarjian
Honoraria: AbbVie, Amgen, ARIAD, Bristol Myers Squibb, Immunogen, Orsenix, Pfizer, Agios, Takeda, Actinium Pharmaceuticals
Research Funding: Pfizer, Amgen, Bristol Myers Squibb, Novartis, ARIAD, Astex Pharmaceuticals, AbbVie, Agios, Cyclacel, Immunogen, Jazz Pharmaceuticals
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rai KR, Holland JF, Glidewell OJ, et al. Treatment of acute myelocytic leukemia: A study by cancer and leukemia group B Blood 581203–12121981 [PubMed] [Google Scholar]
- 2.Estey EH.Acute myeloid leukemia: 2019 update on risk‐stratification and management Am J Hematol 931267–12912018 [DOI] [PubMed] [Google Scholar]
- 3.Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: Results of the medical research council AML15 trial J Clin Oncol 313360–33682013 [DOI] [PubMed] [Google Scholar]
- 4.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia N Engl J Med 3611249–12592009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holowiecki J, Grosicki S, Giebel S, et al. Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: A multicenter, randomized phase III study J Clin Oncol 302441–24482012 [DOI] [PubMed] [Google Scholar]
- 6.Jabbour E, Short NJ, Ravandi F, et al. A randomized phase 2 study of idarubicin and cytarabine with clofarabine or fludarabine in patients with newly diagnosed acute myeloid leukemia Cancer 1234430–44392017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnett AK, Russell NH, Hills RK, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: Results from the UK NCRI AML17 trial in 1206 patients Blood 1253878–38852015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rollig C, Bornhauser M, Thiede C, et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: Evaluation of the proposed reporting system J Clin Oncol 292758–27652011 [DOI] [PubMed] [Google Scholar]
- 9.Parker JE, Pagliuca A, Mijovic A, et al. Fludarabine, cytarabine, G‐CSF and idarubicin (FLAG‐IDA) for the treatment of poor‐risk myelodysplastic syndromes and acute myeloid leukaemia Br J Haematol 99939–9441997 [DOI] [PubMed] [Google Scholar]
- 10.Roboz GJ, Rosenblat T, Arellano M, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia J Clin Oncol 321919–19262014 [DOI] [PubMed] [Google Scholar]
- 11.Cortes JE, Goldberg SL, Feldman EJ, et al. Phase II, multicenter, randomized trial of CPX‐351 (cytarabine:daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML Cancer 121234–2422015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantarjian HM, DiNardo CD, Nogueras‐Gonzalez GM, et al. Results of second salvage therapy in 673 adults with acute myelogenous leukemia treated at a single institution since 2000 Cancer 1242534–25402018 [DOI] [PubMed] [Google Scholar]
- 13.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia Blood 1337–172019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei AH, Strickland SA, Jr, Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: Results from a phase Ib/II study J Clin Oncol 371277–12842019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia J Clin Oncol 302670–26722012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dombret H, Seymour JF, Butrym A, et al. International phase III study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with> 30% blasts Blood 126291–2992015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teh TC, Nguyen NY, Moujalled DM, et al. Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1 Leukemia 32303–3122018 [DOI] [PubMed] [Google Scholar]
- 18.Chua CC, Roberts AW, Reynolds J, et al. Chemotherapy and venetoclax in elderly acute myeloid leukemia trial (CAVEAT): A phase Ib dose-escalation study of venetoclax combined with modified intensive chemotherapy J Clin Oncol 383506–35172020 [DOI] [PubMed] [Google Scholar]
- 19.Shahswar R, Beutel G, Klement P, et al. FLA-IDA salvage chemotherapy combined with a seven-day course of venetoclax (FLAVIDA) in patients with relapsed/refractory acute leukaemia Br J Haematol 188e11–e152019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Jorgensen JL, Wang SA.How do we use multicolor flow cytometry to detect minimal residual disease in acute myeloid leukemia? Clin Lab Med 37787–8022017 [DOI] [PubMed] [Google Scholar]
- 21.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia J Clin Oncol 214642–46492003 [DOI] [PubMed] [Google Scholar]
- 22.Thall PF, Simon RM, Estey EH.Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes Stat Med 14357–3791995 [DOI] [PubMed] [Google Scholar]
- 23.Lachowiez CA, Loghavi S, Kadia TM, et al. Outcomes of older patients with NPM1-mutated AML: Current treatments and the promise of venetoclax-based regimens Blood Adv 41311–13202020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falini B, Mecucci C, Tiacci E, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype N Engl J Med 352254–2662005 [DOI] [PubMed] [Google Scholar]
- 25.Röllig C, Bornhäuser M, Thiede C, et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: Evaluation of the proposed reporting system J Clin Oncol 292758–27652011 [DOI] [PubMed] [Google Scholar]
- 26.Short NJ, Zhou S, Fu C, et al. Association of measurable residual disease with survival outcomes in patients with acute myeloid leukemia: A systematic review and meta-analysis JAMA Oncol 61890–18992020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Short NJ, Rafei H, Daver N, et al. Prognostic impact of complete remission with MRD negativity in patients with relapsed or refractory AML Blood Adv 46117–61262020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter RB, Buckley SA, Pagel JM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission Blood 1221813–18212013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hourigan CS, Dillon LW, Gui G, et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease J Clin Oncol 381273–12832020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel Blood 129424–4472017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Döhner H, Dolnik A, Tang L, et al. Cytogenetics and gene mutations influence survival in older patients with acute myeloid leukemia treated with azacitidine or conventional care Leukemia 322546–25572018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solh MM, Solomon SR, Morris LE, et al. Improved Post remission survival of non-favorable risk acute myelogenous leukemia (AML) patients following initial remission induction therapy with FLAG+/−idarubicin versus 3+7 (anthracycline + cytarabine) Leuk Res. 2020;93:106318. doi: 10.1016/j.leukres.2020.106318. [DOI] [PubMed] [Google Scholar]
- 33.Wunderlich M, Krejci O, Wei J, et al. Human CD34+ cells expressing the inv (16) fusion protein exhibit a myelomonocytic phenotype with greatly enhanced proliferative ability Blood 1081690–16972006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chou FS, Griesinger A, Wunderlich M, et al. The thrombopoietin/MPL/Bcl-xL pathway is essential for survival and self-renewal in human preleukemia induced by AML1-ETO Blood 120709–7192012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia J Clin Oncol 362684–26922018 [DOI] [PMC free article] [PubMed] [Google Scholar]