Abstract
Objective: Stress is a significant risk factor for various diseases such as hypertension, heart attack, stroke, and even sudden death. Stress can also lead to psychological and behavioral disorders. Heart rate variability (HRV) can reflect changes in stress levels while other physiological factors, like blood pressure, are within acceptable ranges. Electroencephalogram (EEG) is a vital technique for studying brain activities and provides useful data regarding changes in mental status. This study incorporates EEG and a detailed HRV analysis to have a better understanding and analysis of stress. Investigating the correlation between EEG and HRV under stress conditions is valuable since they provide complementary information regarding stress. Methods: Simultaneous electrocardiogram (ECG) and EEG recordings were obtained from fifteen subjects. HRV /EEG features were analyzed and compared in rest, stress, and meditation conditions. A one-way ANOVA and correlation coefficient were used for statistical analysis to explore the correlation between HRV features and features extracted from EEG. Results: The HRV features LF (low frequency), HF (high frequency), LF/HF, and rMSSD (root mean square of the successive differences) correlated with EEG features, including alpha power band in the left hemisphere and alpha band power asymmetry. Conclusion: This study demonstrated five significant relationships between EEG and HRV features associated with stress. The ability to use stress-related EEG features in combination with correlated HRV features could help improve detecting stress and monitoring the progress of stress treatments/therapies. The outcomes of this study could enhance the efficiency of stress management technologies such as meditation studies and bio-feedback training.
Keywords: Stress, EEG, ECG, HRV, meditation
I. Introduction
Stress is a process that burdens a person’s adaptive capacity and leads to both psychological and biological changes, which are potential risks for diseases [1]. These diseases might include hypertension, coronary artery disease, cardiac arrest, stroke, and mental disorders such as depression and anxiety [2]. The stress condition could be classified into short-term acute stress and long-term chronic stress types. Finally, the parasympathetic nervous system (PSNS) returns the body to normalcy [3].
The stress reaction can be measured and evaluated in terms of perceptual, behavioral, and physical responses. For measuring an individual’s perceptual level of stress, self-report questionnaires are commonly used [4]. Several physical and physiological features sensitive to stress have been studied in the past. For example, the salivary cortisol test is routinely used as a biomarker test [5]. Also, the HR, BP, Galvanic skin response (GSR), and respiratory sinus arrhythmia (RSA) are expanded during stress while skin temperature (ST) is decreased during stress [6], [7]. The ECG records the electrical changes on the skin that appear because of the depolarization of the heart muscle [8]. Heart Rate Variability (HRV) is computed from RR time intervals (the time difference between two ECG R peaks) [9].
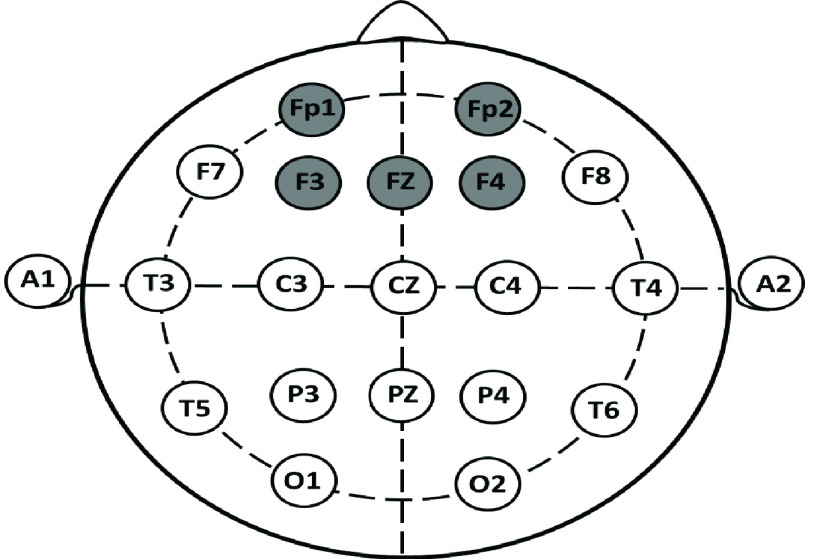
The sympathetic nervous system (SNS) is responsible for increases, whereas the PSNS is responsible for decreases in the heart rate (HR). The HR is continuously modified due to the interactions between the PSNS and the SNS. Therefore, the HR is a measure of the autonomic nervous system (ANS) and a marker for assessment of the balance between the SNS and the PSNS. For example, when the SNS dominates, the HR is increased, and when the PSNS dominates, the HR is reduced [9]. It has been shown that a low HRV suggests that the body is under stress, and high HRV means the body has a strong ability to tolerate stress [10]. The HRV measures investigated in this study are summarized in Table 1. The neuroimaging modalities such as EEG [11], functional magnetic resonance imaging (fMRI) [12], and positron emission tomography (PET) [13] examine the functional changes in brain activity. However, EEG is the most convenient modality to analyze the cortical response to stress due to its low-cost and practical use. Additionally, since EEG has a high temporal resolution, it provides useful information to analyze variability in a mental state [14]. EEG also serves as a useful tool for neurofeedback-based rehabilitation. The 10–20 system shown in Figure 1 is the standard electrode location method used to collect EEG data and is the standard for most current databases [15]. Based on that system, each electrode placement site is represented by a letter to classify the lobe. Also, even numbers indicate the right side and odd numbers indicate the left side of the brain.
TABLE 1. HRV Feature Calculations in Time and Frequency Domain.
| Feature | Equation | Unit | Description |
|---|---|---|---|
| SDNN | 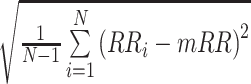 |
ms | Standard deviation of all normal RR (NN) intervals [29] |
| rMSSD | 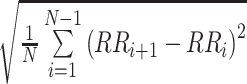 |
ms | Square root of the mean squared differences of successive RR intervals [29] |
| pNN50 | 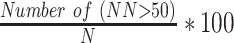 |
% | Percentage of adjacent NN intervals that differ from each other by more than 50 ms in a 5-min epoch [29] |
| LF | Summation of power from 0.04 to 0.15 Hz | ms2 | Low frequency power of HRV [30] |
| HF | Summation of power from 0.15 to 0.40 Hz | ms2 | High frequency power of HRV [30] |
| LF/HF | LF/HF Ratio | - | Ratio of low to high frequency power of HRV [30] |
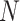 : Number of RR intervals,
: Number of RR intervals, 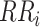 : Interval between two heartbeats,
: Interval between two heartbeats, 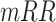 : Mean of RR
: Mean of RR
FIGURE 1.
The 10–20 electrode placements.
The EEG signal amplitude is in the microvolt range. The raw EEG time series data are transformed into the frequency data and classified into five frequency bands: delta band (0.2–4 Hz), theta band (4–8 Hz), alpha band (8–13 Hz), beta band (13–30 Hz), and gamma band (>30 Hz). There have been several EEG based studies that analyze stress. For example, one study has shown that the right hemisphere of the brain becomes dominant in comparison to the left hemisphere of the brain with the onset of stress, and the right hemisphere is more associated with the processing of negative emotions [16]. These differences are illustrated by an emotional processing model in which the frontal cortex performs a key role [17]. Alpha power asymmetry is defined as the functional difference between the left and the right hemispheres; it measures the difference in EEG band power between the measurements from the homologous electrodes located on these hemispheres [18]. It has been shown that alpha power asymmetry and inter-hemispheric asymmetry indicate mental stress [19]. Previous research has focused on different EEG features, including frequency band power, the ratio of power spectral densities of alpha and beta bands, and cross-correlation between band powers [20]. It has been reported that EEG activity is well correlated with mental stress in terms of reduction in alpha power [21] and an increase in beta power [22]. In this study, EEG recording from the four electrodes F3, F4, Fp1, Fp2 were investigated with Fz as the reference electrode as shown in Figure 1. The EEG measures evaluated in this study are listed in Table 2. A previous study has reported that meditation leads to increased power of alpha waves [23]. Therefore, we also included a meditation session after the stress-inducing period. The goal was to include a sequence of rest, stress, and meditation sessions and to compare the resultant HRV and EEG measurements. There are many studies that use either EEG or ECG for stress assessment [24]. However, there are only three previous studies that combined ECG and EEG for stress assessment [25]–[27]. In the first study, they used only one HRV feature and found that there was a significant negative correlation between SDNN (the standard deviation of NN intervals) and relatively high beta power [25]. The second study focused on building a brain device with HRV and EEG recording from three electrodes. Only one electrode was used on the forehead for stress assessment [26]. This study concluded that stress level detection accuracy was significantly higher using support vector machine algorithms when EEG features were used in combination with ECG features [26]. However, we recorded data from frontal lobe and five frontal pole electrodes as these areas are known to be the major sites in terms of response to stressors, as mentioned before. The third study [27] does not involve HRV analysis other than the mean and standard deviation of heart rate data, and it used only one EEG feature (theta Fz/alpha Pz) for stress assessment. In this study, six HRV features and ten EEG features were extracted and evaluated (Table 1 and Table 2). We increased the number of features for EEG to discover unforeseen relationships between stress and the frontal brain area. In addition, we used short time meditation after a stress-inducing condition to evaluate its effect on the four mentioned electrodes. The alpha band power and beta band power were calculated from each electrode and for both hemispheres. The alpha asymmetry was also tested from left to right hemisphere for (F3, F4) and (Fp1, Fp2) electrodes.
TABLE 2. EEG Features.
| Feature | Equation | Unit | Description |
|---|---|---|---|
| LAPF3 | 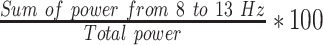 |
% | Normalized left hemisphere alpha band power (F3) |
| RAPF4 | 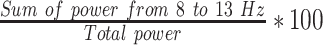 |
% | Normalized right hemisphere alpha band power (F4) |
| LAPFP1 | 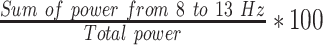 |
% | Normalized left hemisphere alpha band power (Fp1) |
| RAPFP2 | 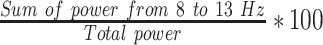 |
% | Normalized right hemisphere alpha band power (Fp2) |
| LBPF3 | 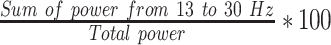 |
% | Normalized left hemisphere beta band power (F3) |
| RBPF4 | 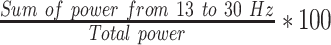 |
% | Normalized right hemisphere beta band power (F4) |
| LBPFp1 | 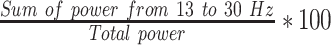 |
% | Normalized left hemisphere beta band power (Fp1) |
| RBPFp2 | 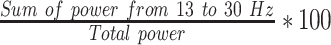 |
% | Normalized right hemisphere beta band power (Fp2) |
| APA1 | 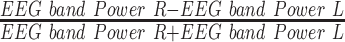 |
- | Alpha band power asymmetry (F3, F4) |
| APA2 | 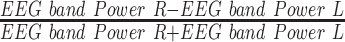 |
- | Alpha band power asymmetry (Fp1, Fp2) |
II. Methods and Procedures
This study proposes to examine EEG, and HRV features simultaneously and their roles in the determination of stress levels. Fifteen healthy volunteers participated in the study. The Stroop Color-Word Test (SCWT) technique was employed to induce a level of stress. The Stroop Color and Word Test have long been an approved test in neuropsychological assessment. It measures cognitive processing and provides quantifiable diagnostic knowledge on brain dysfunction. The SCWT is developed on the concept that individuals can read words much faster than they can recognize and name colors. The test is quick, and its easy administration, validity, and reliability make it a highly useful tool [28]. EEG and HRV were measured simultaneously during rest, stress, and meditation sessions.
EEG measurements were performed using a DSI-24 dry electrode EEG headset (DSI-24, Wearable Sensing, San Diego, CA, USA). The DSI-24 is a wireless EEG headset that contains 21 sensors at positions corresponding to the 10–20 international system, and the EEG signals were acquired using DSI-streamer (Wearable Sensing, San Diego, CA, USA) at a sampling rate of 300 Hz [31].
The ECG was acquired using a wireless data acquisition system named BioRadio 150 (BioRadio Great Lakes Neuro Technologies, OH, USA) during the three conditions at 960 samples/s [32]. The BioRadio is a 12-channel system that displays and records physiological signals, including the ECG. Three single-use dynamic ECG electrodes, (PB-50-060, Myovision, Seattle, USA) were placed as follows: One was placed about 2 inches below the left underarm; the second electrode was placed approximately 2 inches below the right underarm. These two locations were selected instead of the arms to reduce motion artifacts. The ground electrode was placed on the right side of the abdominal cavity, above the iliac crest to minimize power line noise from interfering with the ECG signal. Lead I ECG was used for computing the R-R intervals.
A. Subjects
A total number of 15 EEG and ECG recordings were obtained from 15 right-handed participants. There were seven female and eight male subjects with mean ages of 19.7 ± 1.5 years and 21.3 ± 3.4 years, respectively. A signed consent form was obtained from all the subjects. A digital questionnaire (PSS) that provided general medical and anthropometric information was completed by each person. All the testing procedures with human subjects and the recording sites were approved on August 26, 2018 by the Office of Compliance and Risk Management Institutional Review Board (IRB Approval Number: 18–125) at Florida Institute of Technology, Melbourne, FL, USA.
B. Study Design and Human Subject Protocol
Physiological and anthropometrical features such as weight, height, age, and sex were gathered from the questionnaire that the subjects filled out. The participants had their EEG signals and HRV recorded for a total duration of 15 minutes. The test began with 5 minutes of ‘rest session’, and then 5 minutes of ‘stress session’ (which is induced by the elaborate Stroop Color-Word Test). Finally, 5 minutes of ‘meditation session’ was performed (where they were watching and listening to soothing ocean waves video and breathing slowly). We used this video because recent studies show that the beach can be a healing treatment for a patient who is suffering from stress.
C. Data Analysis
For the EEG signal preprocessing stage, the raw EEG data were separated into three sections: (1) rest (2) stress, and (3) meditation. Band-pass filtering (1–35 Hz) was applied to remove physiological and non-physiological artifacts. The EEG analysis was performed using MATLAB (ver. R2017a, MathWorks, MA, USA). Table 2 lists the name, description, and equation of each feature extracted from the EEG. The EEG data computation starts by selecting the data from the four electrodes: two electrodes in the left-hemisphere (F3, Fp1), and two electrodes in the right-hemisphere (F4, Fp2) in reference to electrode Fz. For power spectral density (PSD) estimation, the Welch’s method, a nonparametric method which is a modified approach of Fast Fourier Transform (FFT) algorithm [33] was used to classify the signals based on frequency into five frequency bands: Delta (0.2 – 3 Hz), Theta (3 – 8 Hz), Alpha (8 – 13 Hz), Beta (13 – 30 Hz) and Gamma from 30 Hz and up. The alpha band power asymmetry between the left and right hemisphere was calculated using the equation in Table 2. The HRV frequency and time-domain analyses were performed using Kubios HRV software (ver. 2.2, Kubios, Eastern Finland).
For ECG signal preprocessing stage, the Pan-Tompkins algorithm was used for QRS detection, R-peak identification, and the HRV analysis [30]. The HRV was interpolated at 4 Hz, and a cubic spline interpolation was used to improve the time resolution of the detection [31].
The ECG signals come with noise, including baseline wanders, powerline interference, electromyographic (EMG) noise, and electrode motion artifact noise. To reduce noise, the cutoff frequencies of the high-pass filter and low-pass filter were set to 0.5 Hz and 70 Hz, respectively.
The HRV signals were classified based on their frequency ranges and separated into low frequency (LF), high frequency (HF), and LF/HF ratios were calculated based on those.
D. Statistical Analysis
One-way ANOVA and Pearson’s correlation coefficient were used as two statistical approaches in this study. All groups of datasets were checked for homoscedasticity/heteroscedasticity condition using Levene’s Absolute Deviation test. ANOVA was used for all significant difference tests since three groups (rest, stress, and meditation) were compared with each other. The significance level was set at 5% Type I Error level for all the statistical tests. Post-hoc analysis for pairwise comparisons was performed using the Tukey-Kramer test which is the default test for ANOVA in MATLAB. Pearson’s correlation was used to determine the significance of the correlation between EEG and ECG-derived features. All statistical analyses were performed using MATLAB R2017a software.
III. Results
Levene’s Absolute Deviation test results showed that only one feature (LBPFp1) was statistically significant for heteroscedasticity (p-value: 0.045) indicating unequal variance and all the rest of the datasets demonstrated homoscedasticity condition. Based on the Levene’s Absolute Deviation test results, Welch correction was applied for one feature (LBFp1). Using Welch’s correction, the p-value for LBFp1 was calculated. Welch correction indicated that the differences in LBPFp1 values among the three groups were statistically insignificant (p = 0.6317). The stress-related features obtained from HRV, and their trends over different conditions are shown in Table 3. The results shown in Table 3 and Table 4 are expressed as the mean ± the standard error of the mean (SEM). ANOVA analysis showed that there were no significant differences (p > 0.05) among any of the conditions in any of the time domain features (SDNN, pNN50, and rMSSD) even though the trends were as expected. However, there were significant differences among all the frequency domain features considered in this study.
TABLE 3. Effects of Stress and Meditation on HRV Features (Sample Size = 15).
| Feature | Rest Mean± SEM | Stress Mean ± SEM | Trend | Meditation Mean ± SEM | Trend |
|---|---|---|---|---|---|
| SDNN | 68.96 (±8.32) | 66.86 (±5.70) |  |
80.46 (±6.10) |  |
| rMSSD | 64.12 (±10.5) | 61.59 (±9.23) |  |
67.51 (±9.08) |  |
| pNN50 | 17.89 (±4.25) | 13.85 (±3.05) |  |
15.52 (±3.17) |  |
| LF | 50.79 (±3.18) | 64.5 (±2.93)Δ |  |
62.57 (±2.51)* |  |
| HF | 48.86 (±3.16) | 34.29 (±2.92)Δ |  |
39.71 (±2.80)* |  |
| LF/HF | 1.18 (±0.18) | 2.18 (±0.26) |  |
1.68 (±0.15)* |  |
Significant difference between the rest and stress
Significant difference between the rest and meditation
TABLE 4. Effects of Stress and Meditation on EEG Features (Sample Size = 15).
| Feature | Rest Mean± SEM | Stress Mean ± SEM | Trend | Meditation Mean ± SEM | Trend |
|---|---|---|---|---|---|
| LAPF3 | 0.26 (±0.09) | 0.37 (±0.09) |  |
0.20 (±0.12) |  |
| RAPF4 | 0.23 (±0.08) | 0.27 (±0.09) |  |
0.26 (±0.09) |  |
| LAPFP1 | 0.34 (±0.08) | 0.60 (±0.23) |  |
0.48 (±0.17) |  |
| RAPFP2 | 0.40 (±0.10) | 0.48 (±0.14) |  |
0.43 (±0.23) |  |
| LBPF3 | 1.11 (±0.38) | 2.15 (±0.54) |  |
1.52 (±0.35) |  |
| RBPF4 | 1.30 (±0.47) | 1.50 (±0.46) |  |
1.45 (±0.45) |  |
| LBPFp1 | 1.60 (±0.38) | 2.64 (±0.99) |  |
1.93 (0.57) |  |
| RBPFp2 | 2.00 (±0.49) | 2.56 (±0.81) |  |
2.22 (±1.07) |  |
| APA1 | −0.02 (±0.09) | −0.13 (±0.10) |  |
−0.12 (±0.10) |  |
| APA2 | −0.01 (±0.11) | −0.06 (±0.08) |  |
−0.01 (±0.10) |  |
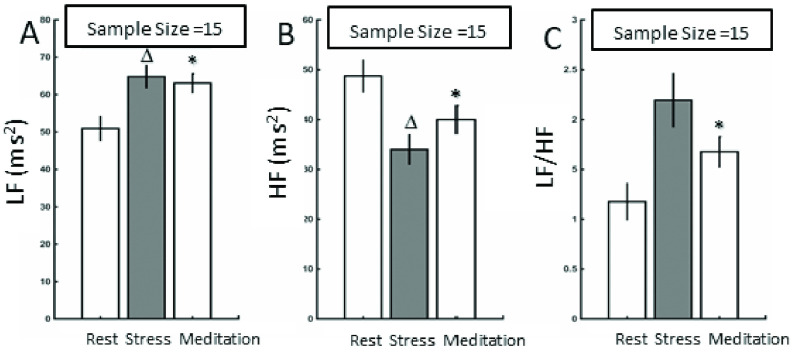
LF under stress (p = 0.007) and meditation (p < 0.001) were significantly different from the ‘rest’ session. HF under stress (p = 0.006) and meditation (p < 0.001) were significantly different from the ‘rest’ session. LF/HF under no-stress was significantly different from the meditation session (p < 0.003) as shown in Figure 2. The error bars in Figure 2 show the SEMs for LF, HF, and LF/HF during rest, stress, and meditation.
FIGURE 2.
A) Bar graph that compares the LF values for rest, stress and meditation sessions B) Bar graph that compares the HF values for rest, stress and meditation sessions C) Bar graph that compares the LF/HF values for rest, stress and meditation sessions. The error bars refer to the SEMs.
The stress-related features computed from the EEG and their trends over different sessions are shown in Table 4. The EEG results from F3, F4, Fp1 and Fp2 show an increase in normalized left hemisphere alpha band power (F3, Fp1), normalized right hemisphere alpha band power (F4, Fp2), normalized left hemisphere beta band power (F3, Fp1), and normalized right hemisphere beta band power (F4, Fp2) with the onset of stress.
In addition, alpha band power asymmetry (F3, F4), and alpha band power asymmetry (Fp1, Fp2) amplified during stress. The short time meditation results show various trends among the frontal brain lobes. The alpha band power asymmetry (F3, F4), and alpha band power asymmetry (Fp1, Fp2) were less negative in meditation situation. The other EEG features showed decreasing trend in meditation sessions.
Using Pearson’s correlation coefficients, five correlations were computed between the HRV and EEG features in the stress condition, as shown in Table 5.
TABLE 5. HRV and EEG Correlation Results During Stress (Sample Size = 15).
| HRV Feature | EEG Feature | r | p |
|---|---|---|---|
| LF/HF | LAPF3 | −0.30 | 0.00001 |
| HF | LAPFp1 | 0.53 | 0.04 |
| HF | LAPF3 | 0.25 | 0.008 |
| LF | LAPFp1 | −0.54 | 0.04 |
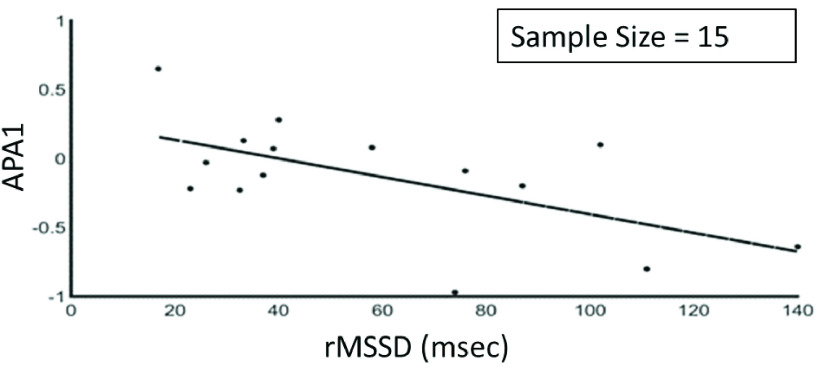
| rMSSD | APA1 | −0.60 | 0.006 |
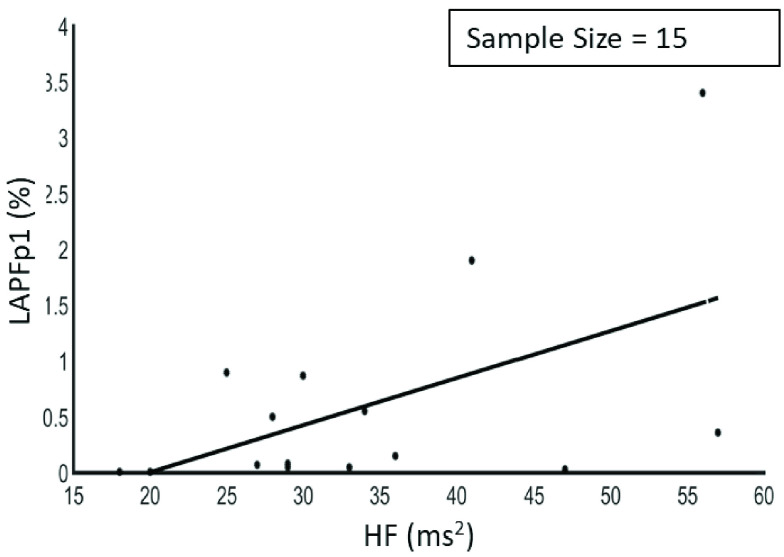
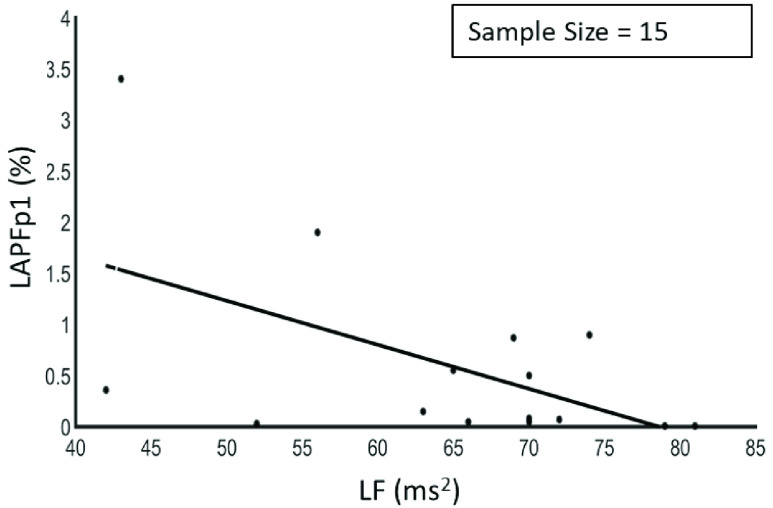
The results showed that there was a negative correlation between LF/HF and LAPF3, a positive correlation between HF and LAPFp1 as shown in Figure 3, a positive correlation between HF and LAPF3, a negative correlation between LF and LAPFp1 as shown in Figure 4 and between rMSSD and APA1 as shown in Figure 5.
FIGURE 3.
Relationship between high frequency and left hemisphere alpha band power (Fp1) (r = 0.53, p < 0.05).
FIGURE 4.
Relationship between low frequency and left hemisphere alpha band power (Fp1) (r = −0.54, p < 0.05).
FIGURE 5.
Relationship between the rMSSD and APA1 (based on F3 and F4 electrodes) (r = −0.60, p < 0.05).
IV. Discussion
In this study, a comprehensive set of comparisons was performed between the extracted parameters from the ECG and the EEG signals. The goal of these comparisons was to examine the correlation between the response of the autonomic nervous system (HRV) and the response of the brain (EEG) to stress and meditation.
As discussed in the introduction section, there are only three previous studies that combined ECG and EEG for short-term stress assessment. The first study used only one HRV feature and found a significant negative correlation between SDNN and relatively high beta power [25]. The second study used only three electrodes with one electrode on the forehead for stress assessment and concluded that stress level detection accuracy using a machine learning algorithm (support vector machine) was significantly higher when EEG features were used in combination with HRV features [26]. We recorded data from frontal lobe and five frontal pole electrodes as these areas are known to be the major sites in terms of response to stressors, as mentioned before. The third study [27] included HRV analysis with only the mean and standard deviation of heart rate data, and it used only one EEG feature (theta Fz/alpha Pz) for stress assessment. These studies did not include meditation sessions either.
This present study demonstrated that there are five considerable relationships among EEG and HRV features associated with stress. The four HRV frequency domain features (LF, HF, LF/HF and rMSSD) correlated with left alpha and beta bands during stress sessions and rMSSD correlated with alpha power asymmetry. Those results confirmed that cardiac stimulation during stress was followed with cortical activation. In addition to confirming the results of previous studies, our results suggest that stress may be reliably assessed by frequency-domain features and relative left alpha EEG power at anterior frontal sites. Indeed, these correlations could be used as markers for diseases associated with stress.
The results showed that the HRV time-domain features (SDNN, rMSSD, and pNN50) decreased under the ‘stress’ condition compared to the ‘rest’ condition indicating sympathetic activation. Conversely, the short time meditation session showed the opposite direction in those HRV time features as expected.
The frequency domain features with stress showed an increase in the LF and LF/HF which represented sympathetic activation and a decrease in HF which indicated lowered parasympathetic activation. The results were similar to the findings in a previous review of HRV analysis and mental stress [24]. The short time meditation showed an increase in HF, and a decrease in LF and LF/HF in comparison to the stress condition suggesting a parasympathetic activation and a sympathetic reduction.
The frontal activity is heavily involved in the emotional stress regulation [16], [32]. During rest, the frontal alpha amplitude symmetry is associated with lower stress. With the onset of stress, alpha power asymmetry becomes more evident. As indicated, both APA1 and APA2 were more negative during stress than at rest and meditation which demonstrate that the right alpha power was reduced considerably more than the left alpha power during stress situation. These results are in line with the physiological expectations that increased cortical activity in the right hemisphere is associated with processing of negative emotions such as stress [16], [32], [33].
One of the limitations of this study might be the number of subjects; however, this study provides a profound preliminary data analysis. Data from a larger sample size might lead to a more robust statistical analysis and it might reveal or de-emphasize other correlations. In addition, mental stress might increase with duration, but in terms of HRV analysis, all the previous studies were performed for either 5-minutes (short-term) or 24-hour (long-term) [10]. Long-term stress analysis might reveal different dynamics; for example, long-term stress is a better predictor of depressive symptoms as compared to short-term stress [39]. A general problem with EEG can be the low spatial resolution on the scalp and a reduced signal-to-noise ratio. Thus, relatively large amounts of subjects will improve our findings. For our future study, we plan to build a wearable device with ECG and EEG measurements to monitor and manage people suffering from stress.
V. Conclusion
Investigating the correlation between EEG and HRV under stress conditions is significant since they provide complementary information regarding stress. The ability and outcomes of EEG and ECG might allow for improved diagnosis and monitoring of the progress of treatment/ therapy, performance, learning, and decision making in people that suffer from stress. Another contribution of this study can be stress management with the HRV and EEG data as inputs for treatment applications, including meditation studies, bio-feedback training, attention disorder, attention deficit hyperactivity disorder, depression, and anxiety disorders.
Funding Statement
The work of Eyad Talal Attar was supported by King Abdul-Aziz University (KAU) Doctoral Scholarship Academic for the Ph.D. Program at Florida Institute of Technology.
References
- [1].Cohen S., Kessler R. C., and Gordon L. U., “Strategies for measuring stress in studies of psychiatric and physical disorders,” in Measuring Stress: A Guide for Health and Social Scientists. New York, NY, USA: Oxford Univ. Press, 1995, pp. 3–26. [Google Scholar]
- [2].Duman R. S., “Neurobiology of stress, depression, and rapid acting antidepressants: Remodeling synaptic connections,” Depression Anxiety, vol. 31, no. 4, pp. 291–296, Apr. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Staal M. A., “Stress, cognition, and human performance: A literature review and conceptual framework,” NASA Tech. Memorandum, Washington, DC, USA, Tech. Rep. NASA/TM–2004–212824, Aug. 2004, p. 168. [Google Scholar]
- [4].Short M. E.et al. , “How accurate are self-reports? Analysis of self-reported health care utilization and absence when compared with administrative data,” J. Occupational Environ. Med., vol. 51, no. 7, pp. 786–796, Jul. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sinha R., “Chronic stress, drug use, and vulnerability to addiction,” Ann. New York Acad. Sci., vol. 1141, no. 1, pp. 105–130, Oct. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sinha A., Das P., Gavas R., Chatterjee D., and Saha S. K., “Physiological sensing based stress analysis during assessment,” in Proc. IEEE Frontiers Educ. Conf. (FIE), Oct. 2016, pp. 1–8. [Google Scholar]
- [7].Sioni R. and Chittaro L., “Stress detection using physiological sensors,” Computer, vol. 48, no. 10, pp. 26–33, Oct. 2015. [Google Scholar]
- [8].Becker D. E., “Fundamentals of electrocardiography interpretation,” Anesthesia Prog., vol. 53, no. 2, pp. 53–64, Jun. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Task Force of the European Society of Cardiology the North American Society of Pacing Electroaphysiology, “Heart rate variability,” Circulation, vol. 93, no. 5, pp. 1043–1065, Mar. 1996. [PubMed] [Google Scholar]
- [10].Kim H.-G., Cheon E.-J., Bai D.-S., Lee Y. H., and Koo B.-H., “Stress and heart rate variability: A meta-analysis and review of the literature,” Psychiatry Invest., vol. 15, no. 3, pp. 235–245, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].St Louis E.et al. , “Electroencephalography (EEG): An introductory text and atlas of normal and abnormal findings in adults, children, and infants,” Amer. Epilepsy Soc., Chicago, IL, USA, Tech. Rep., 2016. [PubMed] [Google Scholar]
- [12].Dubois J. and Adolphs R., “Building a science of individual differences from fMRI,” Trends Cognit. Sci., vol. 20, no. 6, pp. 425–443, Jun. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vanitha N. V., “Positron emission tomography in neuroscience research,” Ann. Neurosci., vol. 18, no. 2, p. 36, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].He B., Yang L., Wilke C., and Yuan H., “Electrophysiological imaging of brain activity and connectivity—Challenges and opportunities,” IEEE Trans. Biomed. Eng., vol. 58, no. 7, pp. 1918–1931, Jul. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Silverman D., “The rationale and history of the 10–20 system of the international federation,” Amer. J. EEG Technol., vol. 3, no. 1, pp. 17–22, Mar. 1963. [Google Scholar]
- [16].Wheeler R. E., Davidson R. J., and Tomarken A. J., “Frontal brain asymmetry and emotional reactivity: A biological substrate of affective style,” Psychophysiology, vol. 30, no. 1, pp. 82–89, Jan. 2007. [DOI] [PubMed] [Google Scholar]
- [17].Horlings R., Datcu D., and Rothkrantz L. J. M., “Emotion recognition using brain activity,” in Proc. 9th Int. Conf. Comput. Syst. Technol. Workshop PhD Students Comput. (CompSysTech), 2008, p. 1. [Google Scholar]
- [18].Smith E. E., Reznik S. J., Stewart J. L., and Allen J. J. B., “Assessing and conceptualizing frontal EEG asymmetry: An updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry,” Int. J. Psychophysiol., vol. 111, pp. 98–114, Jan. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].P C.et al., “EEG alpha asymmetry in virtual environments for the assessment of stress-related disorders,” Stud. Health Technol. Inform., vol. 173, pp. 102–104, 2012. [PubMed] [Google Scholar]
- [20].Hjorth B., “EEG analysis based on time domain properties,” Electroencephalogr. Clin. Neurophysiol., vol. 29, no. 3, pp. 306–310, 1970. [DOI] [PubMed] [Google Scholar]
- [21].Ryu K. and Myung R., “Evaluation of mental workload with a combined measure based on physiological indices during a dual task of tracking and mental arithmetic,” Int. J. Ind. Ergonom., vol. 35, no. 11, pp. 991–1009, Nov. 2005. [Google Scholar]
- [22].Bhoria R., Singal P., and Verma D., “Analysis of effect of sound levels on EEG,” Int. J. Adv. Technol. Eng. Res., vol. 2, no. 2, pp. 121–124, 2012. [Google Scholar]
- [23].Cahn B. R. and Polich J., “Meditation states and traits: EEG, ERP, and neuroimaging studies,” Psychol. Consciousness, Theory, Res., Pract., vol. 1, no. S, pp. 48–96, Aug. 2013. [DOI] [PubMed] [Google Scholar]
- [24].Castaldo R., Melillo P., Bracale U., Caserta M., Triassi M., and Pecchia L., “Acute mental stress assessment via short term HRV analysis in healthy adults: A systematic review with meta-analysis,” Biomed. Signal Process. Control, vol. 18, pp. 370–377, Apr. 2015. [Google Scholar]
- [25].Seo S.-H. and Lee J.-T., “Stress and EEG,” in Convergence and Hybrid Information Technologies. Rijeka, Croatia: InTech, 2010. [Google Scholar]
- [26].Ahn J. W., Ku Y., and Kim H. C., “A novel wearable EEG and ECG recording system for stress assessment,” Sensors, vol. 19, no. 9, p. 1991, Apr. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Asif A., Majid M., and Anwar S. M., “Human stress classification using EEG signals in response to music tracks,” Comput. Biol. Med., vol. 107, pp. 182–196, Apr. 2019. [DOI] [PubMed] [Google Scholar]
- [28].Scarpina F. and Tagini S., “The stroop color and word test,” Frontiers Psychol., vol. 8, p. 557, Apr. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xiao M., Yan H., Song J., Yang Y., and Yang X., “Sleep stages classification based on heart rate variability and random forest,” Biomed. Signal Process. Control, vol. 8, no. 6, pp. 624–633, 2013, doi: 10.1016/j.bspc.2013.06.001. [DOI] [Google Scholar]
- [30].Shaffer F. and Ginsberg J. P., “An overview of heart rate variability metrics and norms,” Frontiers Public Health, vol. 5, p. 258, Sep. 2017, doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].(2018). DSI-24 Dry Electrode EEG Headset. Wearable Sensors. [Online]. Available: http:www.wearablesensing.com/DSI24.php
- [32].(2020). BioRadio 150. Gt. Lakes NeuroTechnologies. [Online]. Available: http://glneurotech.com/docrepo/quicknotes/Note_BioCaptureECG_final.pdf
- [33].Alam R. U., Zhao H., Goodwin A., Kavehei O., and McEwan A., “Differences in power spectral densities and phase quantities due to processing of eeg signals,” Sensors, vol. 20, no. 21, pp. 1–20, 2020, doi: 10.3390/s20216285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Arzeno N. M., Deng Z.-D., and Poon C.-S., “Analysis of first-derivative based QRS detection algorithms,” IEEE Trans. Biomed. Eng., vol. 55, no. 2, pp. 478–484, Feb. 2008, doi: 10.1109/TBME.2007.912658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Echeverría J. C., Crowe J. A., Woolfson M. S., and Hayes-Gill B. R., “Application of empirical mode decomposition to heart rate variability analysis,” Med. Biol. Eng. Comput., vol. 39, no. 4, pp. 471–479, Jul. 2001, doi: 10.1007/BF02345370. [DOI] [PubMed] [Google Scholar]
- [36].Zhang X.et al. , “Emotional stress regulation: The role of relative frontal alpha asymmetry in shaping the stress response,” Biol. Psychol., vol. 138, pp. 231–239, Oct. 2018, doi: 10.1016/j.biopsycho.2018.08.007. [DOI] [PubMed] [Google Scholar]
- [37].Arnsten A. F. T., “Stress signalling pathways that impair prefrontal cortex structure and function,” Nature Rev. Neurosci., vol. 10, no. 6, pp. 410–422, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brouwer A. M., Neerincx M. A., Kallen V., van der Leer L., and Ten Brinke M., “EEG alpha asymmetry, heart rate variability and cortisol in response to virtual reality induced stress,” J. Cyber Ther. Rehabil., vol. 4, no. 1, pp. 27–40, 2011. [Google Scholar]
- [39].Saeed S. M. U., Anwar S. M., Khalid H., Majid M., and Bagci U., “EEG based classification of long-term stress using psychological labeling,” Sensors, vol. 20, no. 7, p. 1886, Mar. 2020, doi: 10.3390/s20071886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Attar E. and Kaya M., “Quantitative assessment of stress levels with biomedical sensors,” in Proc. 45th Northeast Bioeng. Conf. (NEBEC), 2020, p. 270. [Google Scholar]