Abstract
3,3′-Diindolylmethane (DIM), a major phytochemical derived from ingestion of cruciferous vegetables, is also a dietary supplement. In preclinical models, DIM is an effective cancer chemopreventive agent and has been studied in a number of clinical trials. Previous pharmacokinetic studies in preclinical and clinical models have not reported DIM metabolites in plasma or urine after oral dosing, and the pharmacological actions of DIM on target tissues is assumed to be solely via the parent compound. Seven subjects (6 males and 1 female) ranging from 26–65 years of age, on a cruciferous vegetable-restricted diet prior to and during the study, took 2 BioResponse DIM 150-mg capsules (45.3 mg DIM/capsule) every evening for one week with a final dose the morning of the first blood draw. A complete time course was performed with plasma and urine collected over 48 hours and analyzed by UPLC-MS/MS. In addition to parent DIM, two monohydroxylated metabolites and 1 dihydroxylated metabolite, along with their sulfate and glucuronide conjugates, were present in both plasma and urine. Results reported here are indicative of significant phase 1 and phase 2 metabolism and differ from previous pharmacokinetic studies in rodents and humans, which reported only parent DIM present after oral administration. 3-((1H-indole-3-yl)methyl)indolin-2-one, identified as one of the monohydroxylated products, exhibited greater potency and efficacy as an aryl hydrocarbon receptor agonist when tested in a xenobiotic response element-luciferase reporter assay using Hepa1 cells. In addition to competitive phytochemical-drug adverse reactions, additional metabolites may exhibit pharmacological activity highlighting the importance of further characterization of DIM metabolism in humans.
SIGNIFICANCE STATEMENT
3,3′-Diindolylmethane (DIM), derived from indole-3-carbinol in cruciferous vegetables, is an effective cancer chemopreventive agent in preclinical models and a popular dietary supplement currently in clinical trials. Pharmacokinetic studies to date have found little or no metabolites of DIM in plasma or urine. In marked contrast, we demonstrate rapid appearance of mono- and dihydroxylated metabolites in human plasma and urine as well as their sulfate and glucuronide conjugates. The 3-((1H-indole-3-yl)methyl)indolin-2-one metabolite exhibited significant aryl hydrocarbon receptor agonist activity, emphasizing the need for further characterization of the pharmacological properties of DIM metabolites.
Introduction
Cruciferous vegetables are a rich source of phytochemicals, such as sulforaphane and indole-3-carbinol (I3C), which are effective cancer chemoprevention agents in preclinical models (Wattenberg and Loub, 1978; Bradfield and Bjeldanes, 1987a; Higdon et al., 2007; Hayes et al., 2008). Currently, there are 65 ongoing or completed clinical trials involving cruciferous vegetables or I3C/3,3′-diindolylmethane (DIM) (www.ClinicalTrials.gov, accessed 06/26/20). Results in clinical trials and epidemiology studies with cruciferous vegetables have a mixed record of success, typically exhibiting a moderate degree of protection from cancer (van Poppel et al., 1999; Minich and Bland, 2007; Ambrosone and Tang, 2009; Kim and Park, 2009; Maruthanila et al., 2014). The variation in previous study results may in part depend upon experimental design but are also likely a function of dose. For example, to achieve a DIM dose equivalent to two 150-mg capsules of BR-DIM, each containing 45.3 mg of DIM, it would require consumption of 1 kg of freeze-dried Brussels sprout powder (approximately 10.7 kg of fresh Brussels sprouts) (Shorey et al., 2013).
I3C taken orally undergoes acid condensation reactions forming the dimer DIM as well as linear and cyclic trimers (Bradfield and Bjeldanes, 1987a; Bjeldanes et al., 1991). Little or no I3C is found in systemic circulation of rats or humans after oral dosing, and thus, the pharmacological effects of I3C are assumed to be due to one or more acid condensation products (Stresser et al., 1995b; Reed et al., 2006). Other than DIM, the pharmacology and toxicology of few acid condensation products have been described. DIM, the initial and major product (∼30% of I3C dose) formed by acid condensation of I3C, is not subject to further condensation reactions and is often reported as the only I3C-derived indole in plasma after oral dosing (Reed et al., 2006).
DIM has been employed in numerous animal models of cancer prevention as well as in human clinical trials with a major focus on breast and prostate cancer (Dalessandri et al., 2004; Higdon et al., 2007; Bradlow, 2008; Heath et al., 2010; Hwang et al., 2016; Li and Sarkar, 2016; Paltsev et al., 2016; Thomson et al., 2017; Donovan et al., 2018). Among many pathways impacted by DIM are those mediated by the aryl hydrocarbon receptor (AHR) and estrogen receptor (ER) (Higdon et al., 2007; Thomson et al., 2017). The Kd (90 nM) of DIM for AHR is modest compared with other I3C condensation products such as 6-formylindolo[3,2-b]carbazole (FICZ) or [3,2-b]indolocarbazole (0.07 and 0.19 nM, respectively), but maximum concentrations achievable in vivo are typically in the low µM range (Rannug et al., 1987; Bjeldanes et al., 1991; Faust et al., 2017) Protection against estrogen-driven breast cancer is thought to be due in part to AHR induction of the P450 1 family with resultant enhancement of the ratio of 2-hydroxy-β-estradiol (E2) to 16α-hydroxy-E2 (Jellinck et al., 1993; Lord et al., 2002; Auborn et al., 2003).
Cruciferous vegetable-derived indoles including I3C and DIM are described as “blocking agents” in preclinical models, as greatest efficacy is seen when given prior to and/or during carcinogen exposure (Stresser et al., 1994a,b; Takahashi et al., 1995; Fujioka et al., 2016). The proposed mechanism is thought to be AHR-dependent induction of both phase 1 (P450s) and 2 (glutathione-S-transferases and UDP-glucuronosyltransferases) enzymes (Bradfield and Bjeldanes, 1987b; Lampe et al., 2000; Walters et al., 2004; Navarro et al., 2009; Peterson et al., 2009).
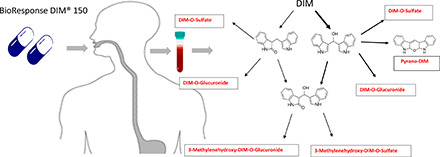
This research is part of a larger study using UPLC-accelerator mass spectrometry to examine [14C]-benzo[a]pyrene (BaP) toxicokinetics after oral microdosing, and the possible effects of dietary intake on kinetics (Madeen et al., 2019). One component of this study involved administration of DIM (BioResponse, BR-DIM 150) daily for 1 week prior to [14C]BaP dosing to assess the importance of the blocking mechanism in humans toward an important dietary carcinogen. We performed UPLC-MS/MS analysis of plasma and urine prior to supplementation (T: −7 days), the morning (overnight fast) of [14C]BaP microdosing (T: 0), and at subsequent timed intervals (plasma, 0.25, 0.50, 1.0, 1.5, 2.0, 3.0, 4.0, 8.0, 24, and 48 hours; urine 0–6, 6–12, 12–24, and 24–48 hours). In contrast to previously published studies (Sepkovic et al., 2001; Anderton et al., 2004; Reed et al., 2006, 2008; Paltsev et al., 2013), we found rapid and significant sulfate and glucuronide conjugates of DIM in plasma and urine characterized by a marked interindividual variability. These conjugates were derived from 3-methylenehydroxy-DIM (tentatively identified as the breakdown product pyrano-DIM) with lesser amounts of 2-ox-DIM and 3-methylenehydroxy-2-ox-DIM. These findings support a rapid and significant metabolism after oral administration of BR-DIM 150.
Materials and Methods
All chemicals and solvents were HPLC, LC-MS, or Optima grade. DIM, as well as deconjugating enzymes (all from Helix pomatia) sulfatase, β-glucuronidase, and β-glucuronidase/arylsulfatase were purchased from Sigma Aldrich (St. Louis, MO). Surine was purchased from Cerillium (Round Rock, TX). [2H2]-DIM was a gift from Stephen S. Hecht (University of Minnesota, Minneapolis, MN). BR-DIM (BioResponse DIM 150), a commercially available formulation with clinically demonstrated enhanced bioavailability, was generously provided by Dr. Michael Zeligs of BioResponse, L.L.C. under a material transfer agreement with Oregon State University. 1H (400 MHz, Varian) spectrum was recorded in DMSO-d6. 1H NMR chemical shifts are reported in ppm (δ) relative to tetramethylsilane, with the solvent resonance employed as the internal standard [DMSO-d6, (δ = 2.50 ppm)]. Data are reported as follows: chemical shift, multiplicity (s = singlet, bs = broad singlet, d = doublet, t = triplet, m = multiplet), coupling constants (Hz), and integration.
Synthesis of (3-((1H-indol-3-yl)methyl)indolin-2-one).
The 2-ox-DIM was synthesized according to a modification of Pillaiyar et al. (2017). Indole-3-carbaldehyde (4 mmol) and 2-oxindole (4 mmol) were dissolved in ethanol (20 ml). Benzylamine (44 µL, 0.4 mmol) and acetic acid (23 µL, 0.4 mmol) were added and refluxed for 5 hours. The reaction was monitored by thin-layer chromatography (hexanes/ethyl acetate = 1:1). The resulting mixture was cooled to 0°C, sodium borohydride (0.76 g, 20 mmol) added in small portions over 5 minutes at 0°C, and the mixture stirred at room temperature overnight. The reaction was quenched with a saturated aqueous solution of ammonium chloride, extracted with ethyl acetate three times, washed with brine, and the ethyl acetate layer dried over anhydrous sodium sulfate. The ethyl acetate was then evaporated under reduced pressure, and the residue purified by flash column chromatography (silica gel, hexanes/ethyl acetate = 5:1 to 3:1) to give product (442 mg, 42%, 2-ox-DIM, Rf = 0.39, hexanes/ethyl acetate = 1:1). 1H NMR (400 MHz, DMSO-d6) δ 10.75 (bs, -N1-H, 1H), 10.26 (bs, -N2-H, 1H), 7.53 (d, J = 7.95 Hz, C3-H, 1H), 7.29 (d, J = 8.02 Hz, C4-H, 1H), 7.11–7.00 (m, C5-H, 2H), 6.98 (m,C6-H, 2H), 6.92 (m, C7-H, 1H), 6.82 (td, J = 7.52, 0.96 Hz, C8-H, 1H), 6.72 (d, J = 7.74 Hz, C9-H, 1H), 3.78 (dd, J = 7.52, 4.73 Hz, C10-H, 1H), 3.41 (dd, J = 14.7, 7.52 Hz, C11-H, 1H), 3.12 (dd, J = 14.7, 7.52 Hz, C12-H, 1H) (Supplemental Fig. 1). High resolution mass spectrometry (time-of-fligth-mass spectrometry/electro-spray ionization (HRMS (TOF-MS/ESI)): m/z calculated for C17H15N2O+ [M+H]+, 263.1179; found, 263.1179.
Recruitment and Enrollment of Volunteers, Dietary Restrictions, and Food Diary.
All protocols and procedures, including plans for recruitment and volunteer informed consent documents, followed the Declaration of Helsinki and were approved by the Oregon State University Institutional Review Board [protocol number 8789 under Food and Drug Administration Investigational New Drug #117175 and registered at ClinicalTrials.gov (identifier NCT03802721)]. Healthy men and women ages 21–65 were recruited from the local community. Exclusion criteria included smoking of tobacco or other substances or use of smokeless tobacco in the past 3 months; a history of kidney, liver, or gastrointestinal diseases such as Crohn’s disease, ulcerative colitis, Celiac disease, or gastrointestinal surgery; the use of medications that affect gut motility or nutrient absorption; and allergy to cruciferous vegetables. Additionally, female volunteers were required to be postmenopausal or surgically sterile. Seven individuals were screened for eligibility, and all were enrolled after a health history review and physical exam by the study physician. Volunteers included six males and one female, ranged from ages 26–65 years, and BMI 22.4–37.4. The demographics of volunteers are given in Table 1.
TABLE 1.
Volunteer demographics
| Volunteer | Age | Sex | BMI at Screening Visit | Race | Hispanic (Y/N) |
|---|---|---|---|---|---|
| BaP021 | 27 | M | 24.3 | White | N |
| BaP022 | 65 | M | 32.5 | White | N |
| BaP025 | 44 | M | 27.5 | White | N |
| BaP028 | 49 | M | 28.1 | White | N |
| BaP031 | 59 | F | 33.7 | White | N |
| BaP041 | 26 | M | 22.4 | Black/AA | N |
| BaP042 | 43 | M | 37.4 | White | N |
Volunteers were asked to avoid cruciferous vegetables and condiments (Supplemental Table 1) such as mustard, horseradish, and wasabi or any supplements containing I3C or DIM for two weeks prior to time 0 hour and through the 48-hour study cycle. All foods and nonwater beverages were recorded in a food diary that spanned 3 days prior to and throughout the 48-hour study cycle. Volunteers also completed the Arizona Cruciferous Vegetable Food Frequency Questionnaire to assess their usual intake of cruciferous vegetables and condiments in the previous three months (Thomson et al., 2016).
Dosing of Volunteers with BioResponse DIM 150 and Collection of Blood and Urine.
Volunteers were instructed to consume two capsules (300 mg total) of BioResponse DIM 150 (45.3 mg DIM/capsule) each evening with dinner for 7 days prior to the 48-hour study cycle. Based on self-report capsule intake diaries, compliance was 100%. Volunteers also consumed 2 BR-DIM capsules at the start of the 48-hour pharmacokinetic study (T: 0).
Prior to consumption of BR-DIM capsules (T: −7 days), spot urine and 25 ml of blood were collected for baseline analysis. Subjects were instructed to fast overnight (10–12 hours) prior to the 48-hour study cycle. In the morning, prior to final DIM dosing, an indwelling catheter was placed in an antecubital vein, and a spot urine and 25 ml of blood collected. Blood was sampled at 0, 0.25 0.5, 1.0, 1.5, 2, 3, 4, 8, 24, and 48 hours. Samples at 0–4 hours were collected from the catheter and 8–48-hour samples by straight stick phlebotomy. Breakfast was provided after 2 hours. Volunteers were instructed to collect all urine from 0–48 hours in a separate amber glass container for each voiding. Urine was pooled for the 0–6, 6–12, 12–24, and 24–48 hour collections. To protect confidentiality, all specimens were deidentified at time of collection.
Extraction of Plasma and Urine for DIM and Metabolite Quantitation.
Urine or plasma (1 ml) was adjusted to pH 5.0 with 0.06 M sulfuric acid. A mixture of β-glucuronidase (100,00 Fishman units/ml) and arylsulfatase (800,000 Roy units/ml) was added and incubated (37°C) overnight (18–20 hours). An internal standard, [2H2]-DIM, was added and samples extracted with 4 ml methyl tert-butyl ether (plasma) or ethyl acetate (urine) (Staub et al., 2006). For extraction, samples were mixed by hand inversion for 1 minute, centrifuged for 5 minutes at 2,000 g, and aliquots evaporated to dryness under a gentle stream of nitrogen. Samples were reconstituted in 200 µL water:acetonitrile (90:10, v:v), centrifuged at 2,000 g for 10 minutes, and transferred to low-volume injection vials. Spiked synthetic urine (Surine) was used to prepare quality control samples. Samples were stored at −20°C until UPLC-MS/MS analysis.
UPLC-MS/MS Quantitation of DIM and Metabolites in Plasma and Urine.
Quantitative analysis was performed on a Shimadzu (Shimadzu Scientific, Inc. Columbia, MD) Nexera LC-30AD UPLC system interfaced to a Shimadzu LCMS-8060 triple quadrupole mass spectrometer (MS/MS). The quantitation method was adapted from previously published methods (Staub et al., 2006; Fujioka et al., 2014; Baenas et al., 2017). Briefly, chromatographic separation was performed using a Waters (Milford, MA) ACQUITY UPLC BEH (2.1 × 50 mm, 1.7 µm) C18 column with a gradient of water (13 mM ammonium acetate, pH 4.0 with acetic acid; mobile phase A) and acetonitrile (0.1% acetic acid; MPB). The gradient conditions were as follows: 10% MPB 0–0.2 minutes, linear increase to 60% MPB 0.2–5.0 minutes, 60% MPB 5.0–9.0 minutes, return to initial 9.0–9.1 minutes, and hold 9.0–10.0 minutes at a flow rate of 0.18 ml/min. The column oven temperature was 40°C. Quantitation of DIM and 2-ox-DIM was determined daily by running a standard curve of instrument response versus injection (0–10 pmol) for the two standards.
Positive ion electrospray mass spectrometry and multiple reaction monitoring was used for quantitative analysis. Compound-dependent parameters such as precursor/product ion information, voltage potentials (Q1 and Q3), and collision energy are shown in Supplemental Table 2. The dwell time was set at 20 milliseconds. The optimal ESI conditions for detection of the analytes were: nebulizer gas, 2.9 L/min; heating gas, 10 L/min; drying gas, 10 L/min; interface temperature, 300°C; desolvation line temperature, 250°C; heat block temperature, 400°C. LabSolutions LCMS Ver.5.80 (Shimadzu Scientific, Inc. Columbia, MD) was used for data collection and quantitation.
Extraction of Non-deconjugated Plasma and Urine for Structural Analysis.
Plasma or urine (200 µL) was combined with cold 100% methanol (400 µL), mixed on a vortex for 30 seconds, and incubated at −20°C for 1 hour to precipitate proteins. Samples were centrifuged 10,000 g 15 minutes at 4°C. Aliquots (525 µL) of supernatant were transferred to clean vials, evaporated to dryness with a nitrogen stream, and solubilized in 200 µL of 100% methanol. Samples were centrifuged at 2,000 g for 5 minutes and transferred to low-volume injection vials.
UPLC-MS/MS Analysis of DIM, Oxygenated DIM, and Conjugates in Plasma and Urine.
UPLC was performed with a Shimadzu Nexera system (Shimadzu; Columbia, MD) coupled to a high-resolution hybrid quadrupole time of flight (TOF) mass spectrometer (TripleTOF 5600; SCIEX; Framingham, MA). UPLC separations were as described above with a sample injection volume of 5 µL and flow rate of 0.18 ml/min. TOF-MS was operated with an acquisition time of 0.15 second and a scan range of 60–1000 Da. MS/MS acquisition was performed with collision energy set at 40 V and collision energy spread of 15 V. Each MS/MS scan had an accumulation time of 0.1 second and a range of 40–1000 Da using information-dependent acquisition. The source temperature was 500°C with IonSpray voltage at 5.5 kV in positive ion mode and −4.5 kV in negative ion mode, respectively. Data were processed using Peak View software Ver. 2.1 (SCIEX). Chromatographic peaks of metabolites were annotated using the extract ion chromatogram lists based on high accuracy MS, MS/MS fragmentation, and isotopic distribution.
Pharmacokinetic Analysis.
Pharmacokinetics of DIM and metabolites were evaluated using noncompartmental and compartmental analyses. Area under the curve (AUC) of DIM and metabolite concentrations in plasma from time zero to the last measured time point and extrapolated to infinity (using last 3 time points) were calculated using the trapezoidal rule (Gibaldi and Perrier, 1982). Mean residence times were calculated as a ratio of AUC under the 1st moment curve extrapolated to infinity to AUC extrapolated to infinity. Noncompartmental half-lives were calculated as the product of mean resident times with the natural log of two. A one-compartment pharmacokinetic model was used to evaluate the time course of DIM concentrations in plasma (eqs. 1–3), where “A0,” “A1,” and “A2” represent the amount DIM in the absorption/formation and central compartments, respectively; “ka,” and “ke” are first-order rate constants. The concentration of the central compartment (C1) was calculated by normalizing the amount with the apparent volume of distribution (V1, L, eq. 3).
 |
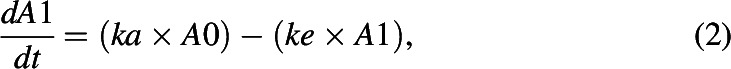 |
 |
Optimizations of model parameters were obtained using a maximum log likelihood objective and the Nelder-Mead algorithm. Initial values were set by adjusting parameters visually. Software used to statistically analyze data were “R: A language and environment for statistical computing” version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
AHR Reporter Assay.
Hepa1 mouse hepatoma cells, transfected with a xenobiotic response element-luciferase reporter, were plated in 96-well plates at 1 × 104 cells per well. After growth overnight, each well was treated with 0.1, 1.0, or 50 µM DIM or 2-ox-DIM (each concentration in triplicate in 0.1% DMSO) and incubated for 18–24 hours. Cells were lysed in 100 µL passive cell lysis reagent (Promega Corp., Madison, WI) for 15 minutes, and luciferase activity measured using a luminometer (Tropix TR717 microplate luminometer, Applied Biosystem, Bedford, MA). The potent AHR indole ligand FICZ was used as a positive control at 0.1 µM.
Results
Metabolites in Plasma.
No DIM or DIM metabolites were detected prior to initiation of the one week of supplementation (T: −7 hour). After an overnight fast (10–12 hours after last daily DIM dose), urine and plasma were collected (T: 0 hour), and subjects given a final dose of DIM along with 50 ng of [14C]BaP. When plasma samples were treated with β-glucuronidase/sulfatase followed by extraction and UPLC-MS/MS, both 2H2-DIM (249.10 > 132.05) and DIM (247.10 > 130.05) eluted at 7.00 minutes, whereas the major polar metabolite (5.85 minutes), initially thought to be 2-ox-DIM (263.12 > 130.15), on closer examination eluted slightly later than the 2-ox-DIM standard (5.65 minutes) (Fig. 1). Standard curves of instrument response versus pmol of DIM and 2-ox-DIM standards established that the latter generated a 70-fold greater signal (Fig. 1). Utilizing the Sciex 5600 quadropole time of flight (QTOF), 2-ox-DIM in the samples was confirmed by mass (<5 ppm error), retention time, MS/MS fragmentation, and isotope distribution. The major peak was tentatively identified as pyrano-DIM, formed spontaneously from 3-methylenehydroxy-DIM based on fragmentation pattern (Fig. 2). Estimation of pyrano-DIM (3-methylenehydroxy-DIM) was done with the assumption that the instrument response was approximately equivalent to 2-ox-DIM. In addition to the two monohydroxylated metabolites, a significant amount of the dihydroxylated metabolite, 3-methylenehydroxy-2-ox-DIM (279.09 > 146.06), was detected after deconjugation (Supplemental Fig. 2). The identity of this metabolite was confirmed by mass accuracy [m/z 279.1128, MS/MS fragmentation (m/z 146.0592)] and isotope distribution. This metabolite was not quantified owing to the lack of a di-hydroxylated standard. Our TOF-MS/MS results are not consistent with hydroxylation of the phenyl ring in agreement with metabolism of DIM in vitro by MCF-7 cells.
Fig. 1.
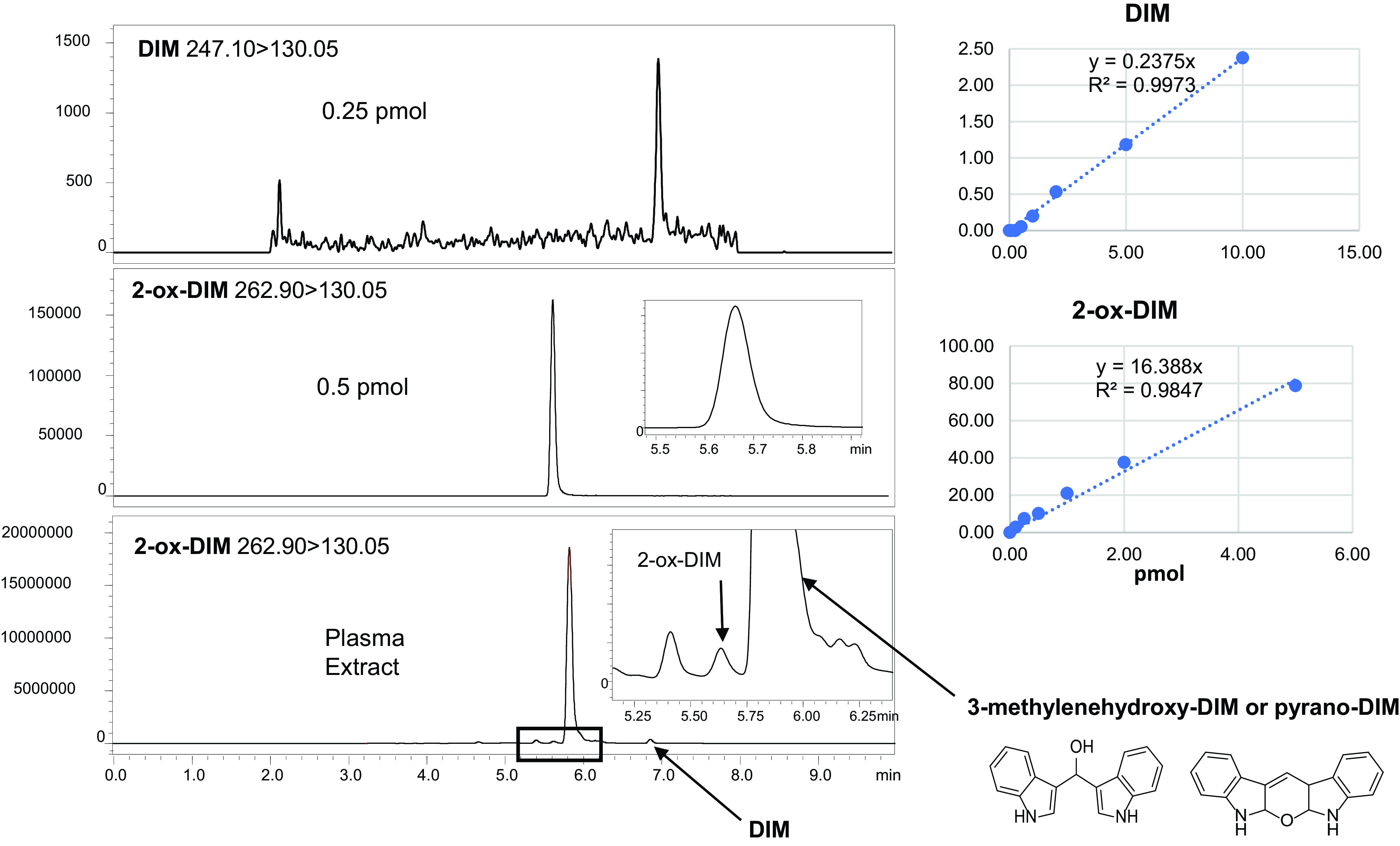
Quantitation of DIM and hydroxylated metabolites in plasma by UPLC-MS/MS after deconjugation. Top panels: DIM and 2-ox-DIM standards; DIM (m/z 246.95 > 130.15), 0.25 pmol with instrument response with standards on right; 2-ox-DIM (m/z 263.12→130.15), 0.5 pmol with instrument response with standards on right; Bottom panel, UPLC of plasma extract from a single individual at T= 0 hour. In total, 1 ml of plasma was adjusted to pH 5.0 and treated with β-glucuronidase and sulfatase. After an overnight incubation, the extract was analyzed by UPLC-MS/MS as described in Materials and Methods.
Fig. 2.
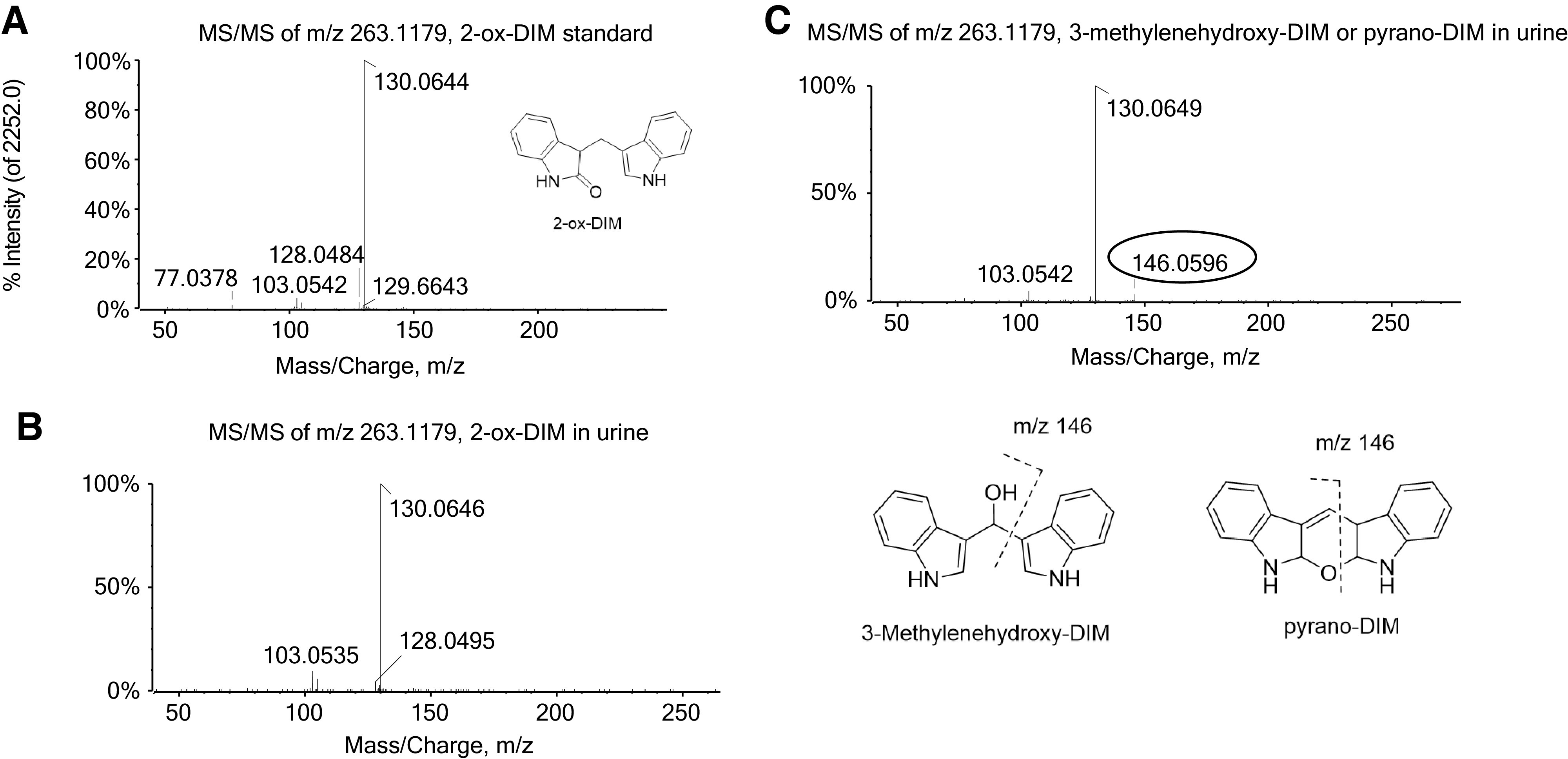
MS/MS spectrum of mono-oxygenated DIM at m/z 263.1179. The fragmentation pattern for the oxygenated product of DIM was obtained from (A) the 2-ox-DIM standard; (B) the peak corresponding to the retention time of the 2-ox-DIM standard from an extract from urine; (C) proposed oxygenated DIM distinct from 2-ox-DIM from urine (3-methylenehydroxy-DIM or pyrano-DIM) with specific fragment ion m/z 146.0596.
The time course for appearance of DIM, 2-ox-DIM, and pyrano-DIM in plasma treated with deconjugation enzymes is shown in Fig. 3 for each of the seven subjects. A large interindividual variability is apparent with two individuals exhibiting little or no parent plasma DIM with robust yield of the pyrano-DIM. Pharmacokinetic analysis of the data were performed with both one- and noncompartmental models (Table 2). The mean Tmax values for DIM and pyrano-DIM and conjugates are 2.67 ± 0.98 and 2.96 ± 2.44 hours, respectively (Table 2). The mean Cmax for DIM and the monohydroxylated metabolites and their respective conjugates are 423 ± 610 and 1910 ± 3190 pmol/ml plasma, respectively (Table 3). The Tmax and Cmax (average ∼0.4 µM) for DIM is consistent with previous studies (Reed et al., 2008; Paltsev et al., 2013). Mean noncompartmental half-life, apparent clearance, and apparent volume of distribution for DIM were 4.29 ± 2.48 hours, 161 ± 132 L x hr−1, and 1010 ± 693 L, respectively. The relatively large apparent volume of distribution would indicate that little of the administered dose was in the central plasma compartment, suggesting DIM was poorly absorbed or extensively distributed to other compartments. For comparison, noncompartmental half-lives for monohydroxylated metabolites and their conjugates was 9.34 ± 3.13.
Fig. 3.
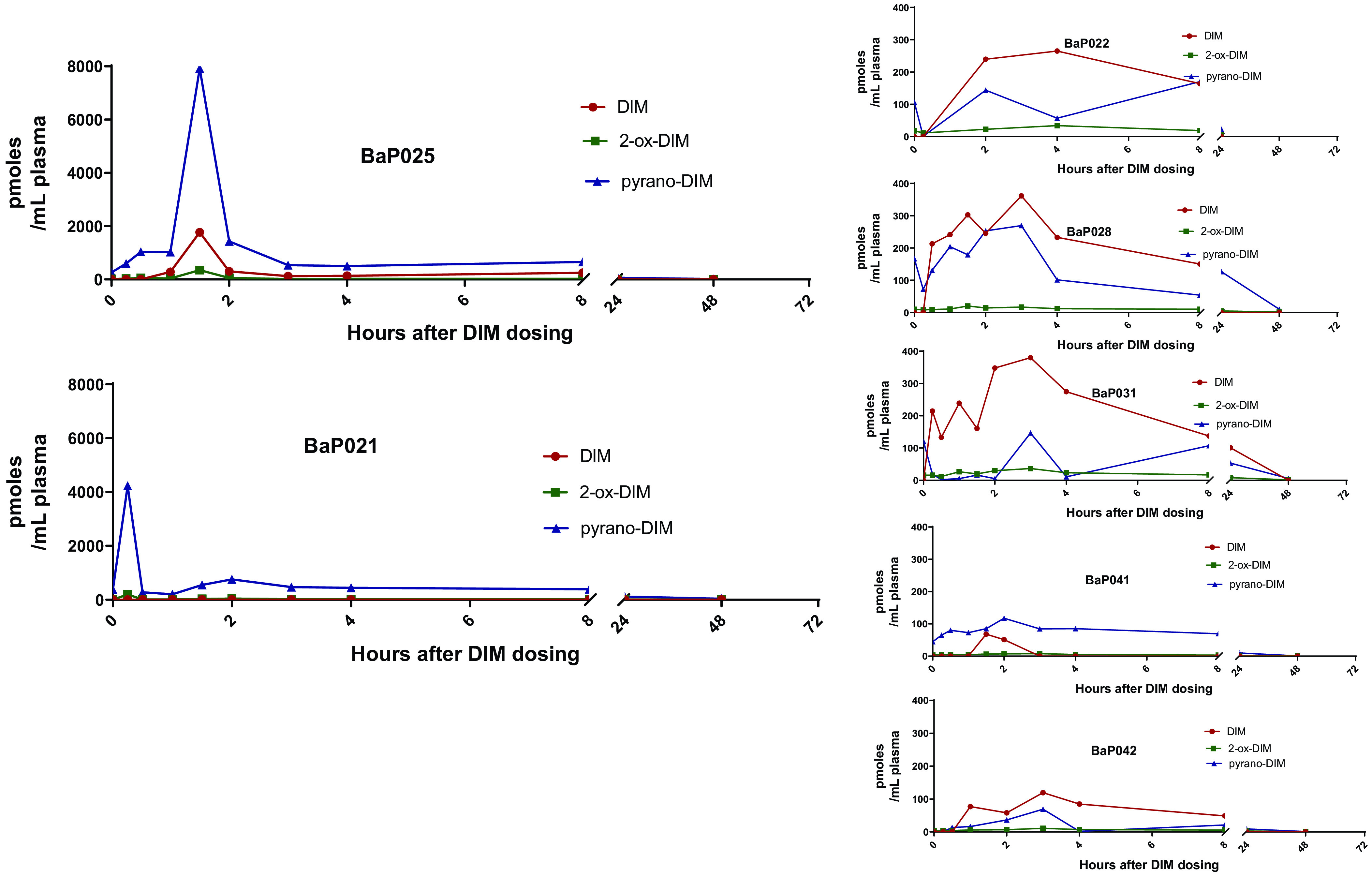
Plasma levels of DIM, 2-ox-DIM, and pyrano-DIM over time. Blood was collected from each of the 7 individuals at time points indicated. Plasma was isolated, treated with sulfatase/β-glucuronidase, extracted, and analyzed by UPLC-MS/MS as described in Material and Methods. DIM and 2-ox-DIM were quantified utilizing standard curves; pyrano-DIM was estimated based on instrument response with 2-ox-DIM.
TABLE 2.
Individual pharmacokinetics of DIM, 2-ox-DIM, plus pyrano-DIM (3-methylenehydroxy-DIM): Noncompartmental model
| Volunteer | Tmax DIM | Tmax OH-DIMa | Cmax DIM | Cmax OH-DIMb | AUC0-48hr DIM | AUC0-48hr OH-DIM | t1/2 DIM | t1/2 OH-DIMa | Cl DIMa | Vdis DIMa |
|---|---|---|---|---|---|---|---|---|---|---|
| h | h | pmol/ml | pmol/ml | pmol x h x ml−1 | pmol x h x ml−1b | h | h | L/h | L | |
| BaP021 | — | 0.25 | — | 4230 | — | 11,100 | — | 9.84 | — | — |
| BaP022 | 4.0 | 8 | 265 | 205 | 2890 | 2740 | 4.13 | 8.36 | 127 | 759 |
| BaP025 | 1.5 | 1.5 | 1770 | 8280 | 4190 | 16,600 | 3.87 | 5.12 | 87.7 | 490 |
| BaP028 | 3.0 | 3.0 | 362 | 290 | 2990 | 4510 | 3.76 | 14.1 | 123 | 669 |
| BaP031 | 3.0 | 3.0 | 380 | 184 | 5020 | 2910 | 8.85 | 11.9 | 73.3 | 937 |
| BaP041 | 1.5 | 2.0 | 68 | 125 | 72 | 1500 | 1.22 | 6.14 | — | — |
| BaP042 | 3.0 | 3.0 | 120 | 80 | 935 | 699 | 3.92 | 9.93 | 393 | 2220 |
| 2.67 ± 0.98 | 2.96 ± 2.44 | 423 ± 610 | 1910 ± 3190 | 2300 ± 2000 | 5730 ± 5900 | 4.29 ± 2.48 | 9.34 ± 3.13 | 161 ± 132 | 1010 ± 693 |
a The reported Tmax is for pyrano-DIM.
b The reported Cmax and AUC0-48hr are for the sum of 2-ox-DIM and pyrano-DIM.
TABLE 3.
Individual pharmacokinetics of DIM: One-compartmental model
| Volunteer | Ka DIM | K1e DIM | V1 DIM | t1/2 DIM |
|---|---|---|---|---|
| h−1 | h−1 | L | h | |
| BaP021 | — | — | — | — |
| BaP022 | 0.28 | 0.28 | 513 | 2.45 |
| BaP025 | 0.70 | 0.70 | 222 | 0.99 |
| BaP028 | 0.18 | 0.84 | 166 | 0.82 |
| BaP031 | 0.08 | 1.16 | 72.1 | 0.60 |
| BaP041 | — | — | — | — |
| BaP042 | 0.29 | 0.29 | 1490 | 2.37 |
| 0.30 ± 0.23 | 0.65 ± 0.37 | 492 ± 580 | 1.45 ± 0.89 |
a The reported Tmax is for pyrano-DIM.
A one-compartment model was used to estimate rate constants, apparent volume of distribution, and half-life for DIM (Table 3). The compartmental modeling approach estimated lower apparent volumes of distribution compared with the noncompartmental modeling approach (492 versus 1010 L, respectively); however, they were still relatively high compared with body weights of volunteers, suggesting low fraction of absorption and/or high distribution to tissues. Again, the large S.D. of the means for these pharmacokinetic constants reflect a large interindividual variability.
The metabolic profile was also analyzed for plasma not treated with deconjugation enzymes. The time course for appearance in plasma of the sulfate and glucuronide conjugates of monohydroxylated and dihydroxylated DIM is shown in Fig. 4. It is apparent that a significant amount of the DIM dose is present in plasma as sulfate and glucuronide conjugates of mono- (and di-) hydroxylated metabolites (Fig. 4) throughout the time course, and 24 hours is sufficient to mostly clear the DIM dose from blood. 3-((1H-indole-3-yl)methyl)indolin-2-one-O-sulfate and/or bis(1H-indol-3-yl)methanol-O-sulfate and 3-((1H-indole-3-yl)methyl)indolin-2-one-O-glucuronide and/or bis(1H-indol-3-yl)methanol-O-glucuronide represent conjugation at the 3-methylenehydroxy- and/or 2-ox-position. Although we cannot make a definitive assignment by TOF-MS/MS, based on the relative amounts of 2-ox-DIM and pyrano-DIM after deconjugation, we can assume the large majority of conjugation occurred at the 3-methylenehydroxy position (Fig. 3). The plasma levels at T: 0 for each metabolite includes the contribution from the previous night’s DIM dose (little or no parent DIM present at T: 0). The Tmax for the conjugated metabolites is 2 to 3 hours, similar to parent DIM. If one assumes a comparable instrument response, it appears that sulfate conjugates predominate.
Fig. 4.
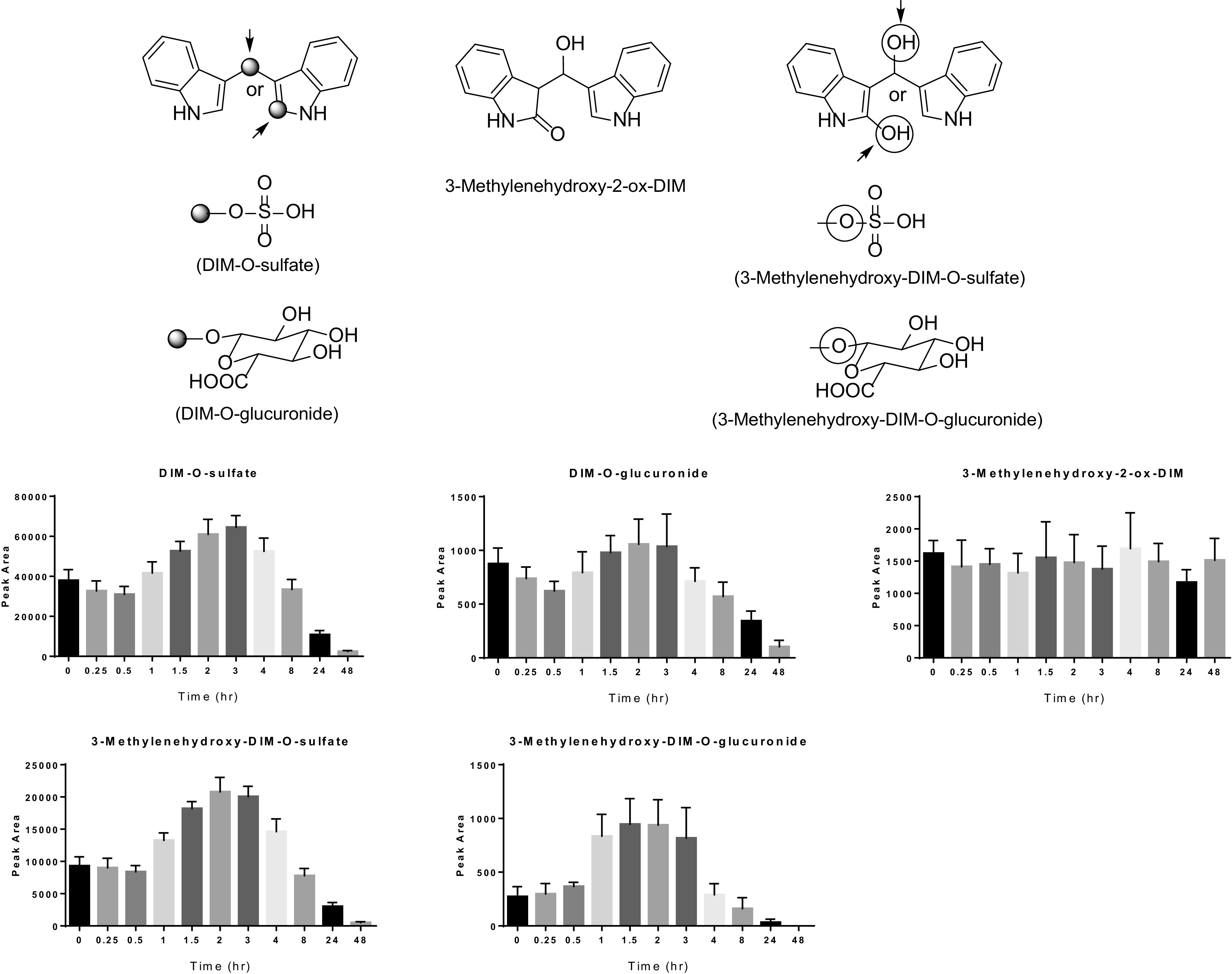
Plasma levels of sulfate and glucuronide conjugates of mono- and dihydroxylated DIM and free dihydroxylated DIM. Blood was collected from the 7 individuals at the time points indicated. Plasma was isolated, extracted, and analyzed by UPLC-MS/MS as described in Materials and Methods. Bars indicate the mean (n = 7) peak area of instrument response and the bars the S.D.
Urinary Metabolites.
Urine was collected 10–12 hours after the last of the 7 daily DIM doses (T: 0) and examined for DIM and DIM metabolites. As in plasma, upon deconjugation, pyrano-DIM was predominant (Fig. 5) with much smaller amounts of 2-ox-DIM. Of the identified components in urine, parent DIM (29%) and 3-methylenehydroxy-DIM conjugates (70%) made up almost the entire profile (Fig. 5). The relatively large apparent volume of distribution would indicate that little of the orally administered dose was in the plasma compartment, which could suggest low oral absorption of DIM. In urine, we accounted for 5% of the administered DIM dose in urinary elimination of DIM, pyrano-DIM, and 2-ox-DIM. With respect to the time course, DIM, 2-ox-DIM, and pyrano-DIM in deconjugated samples from urine were observed at T: 0 (10–12 hours after the last of the daily doses) and peaked in the 6–12 hour pool. As in plasma, levels of pyrano-DIM were 2- to 3-fold higher than parent DIM.
Fig. 5.
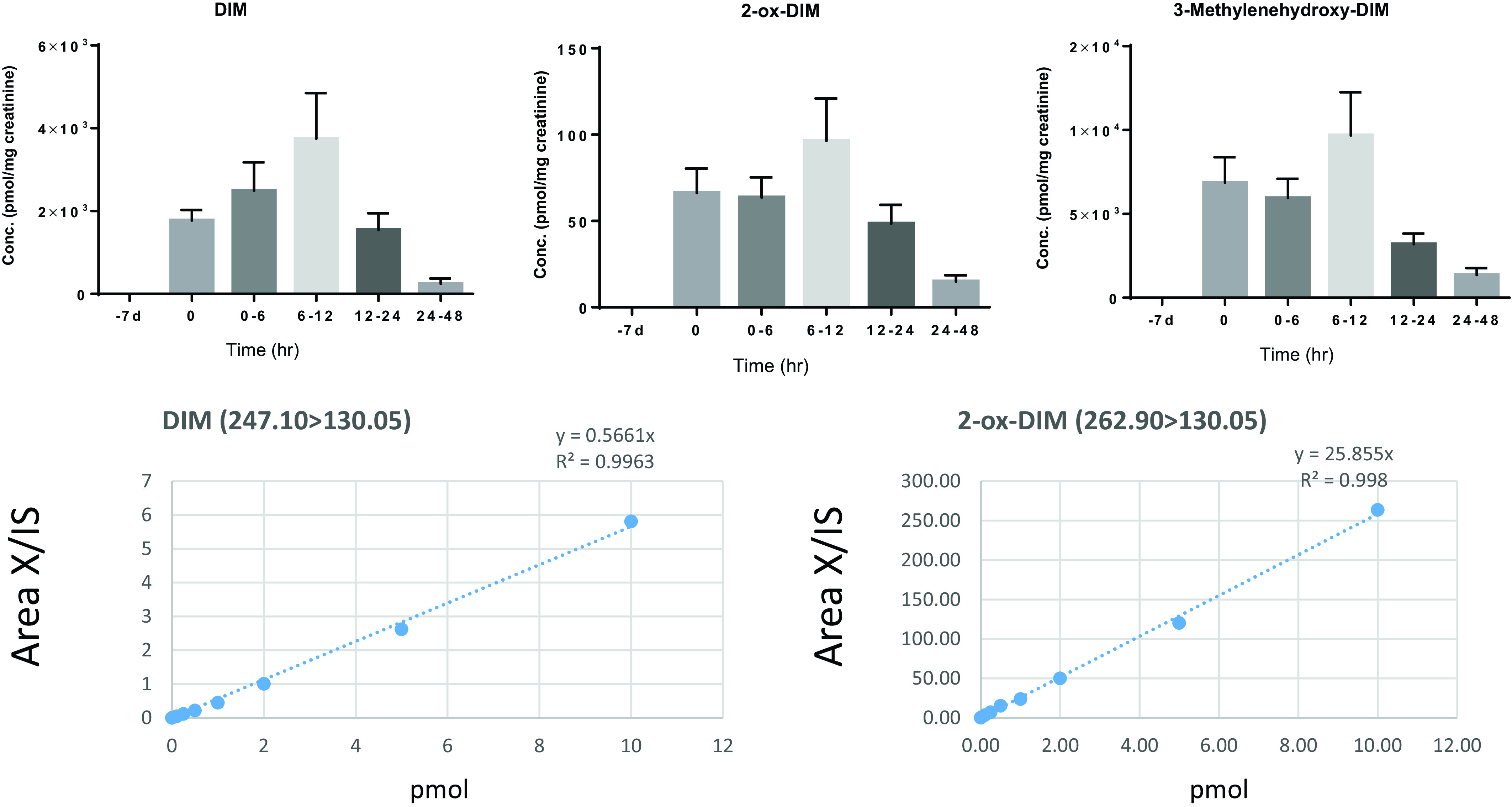
Urine levels of DIM, 2-ox-DIM, and 3-methylenehydroxy(pyrano)-DIM over time. All urine was collected over 48 hours postdosing and pooled in fractions of 0–6, 6–12, 12–24, and 24–48 hours. Aliquots of 1 ml were treated with sulfatase/β-glucuronidase, extracted, and analyzed by UPLC-MS/MS as described in Materials and Methods. No DIM or DIM metabolites were seen prior to initiation of 1 week of DIM supplementation (−7d). The 0-hour time point was urine collected just prior to the last dose (morning of day 8). Bars indicate the mean (n = 7) in pmol/mg creatinine and the bars the S.D. DIM and 2-ox-DIM were quantified utilizing standard cures; pyrano-DIM (from 3-methylenehydroxy-DIM) was estimated based on instrument response with 2-ox-DIM standards.
In urine samples not treated with deconjugation enzymes, there were abundant amounts of sulfate (Fig. 6) and glucuronide (Fig. 7) conjugation metabolites of 3-methylenehydroxy-DIM and 3-methylenehydroxy-2-ox-DIM. The morning after the last dose, urine was collected prior (T: 0 hour) to the final acute dose and pooled over the time periods 0–6, 6–12, 12–24, and 24–48 hours. With DIM supplementation, there were still significant amounts of parent DIM, conjugated monohydroxylated metabolites, and the dihydroxylated metabolite after the 10–12 hour fast just prior to the acute dose at T: 0 hours. The levels of metabolites decreased with time, although there were still small amounts present in the 24–48 hour fraction. Urine collected from the seven volunteers just prior to initiation of DIM supplementation (T: −7 days) did not indicate the presence of DIM or any DIM metabolites (Fig. 5) confirming the effectiveness of the implemented dietary restriction.
Fig. 6.
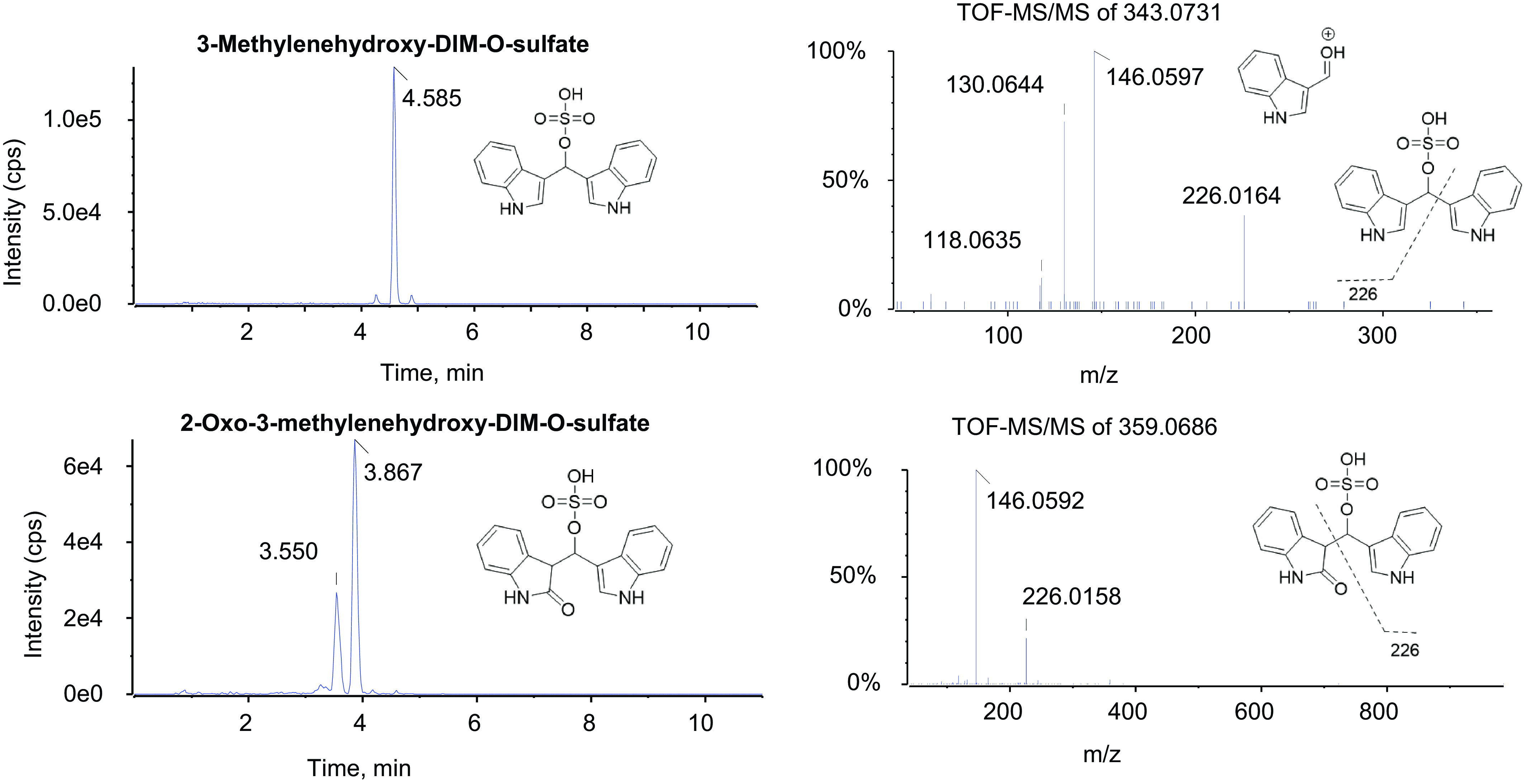
Sulfate conjugates of mono- and dihydroxylated DIM metabolites in urine. The 1-mL aliquots of urine collected from a single individual (BaP022), 6–12 hours postdosing, was treated with sulfatase, extracted, and analyzed by UPLC-MS/MS as described in Materials and Methods. The instrument response is given as intensity in units of counts per seconds (cps). The molecular ions exhibited m/z values of 343.0731 (peak eluting at 4.585 minutes) for the sulfate conjugate of monohydroxylated DIM and 359.0686 for the sulfate conjugates of dihydroxylated DIM (peaks eluting at 3.550 and 3.867 minutes). The fragment ion at 146.0596 is diagnostic for pyrano(3-methylenehydroxy)-DIM and is not seen with 2-ox-DIM.
Fig. 7.
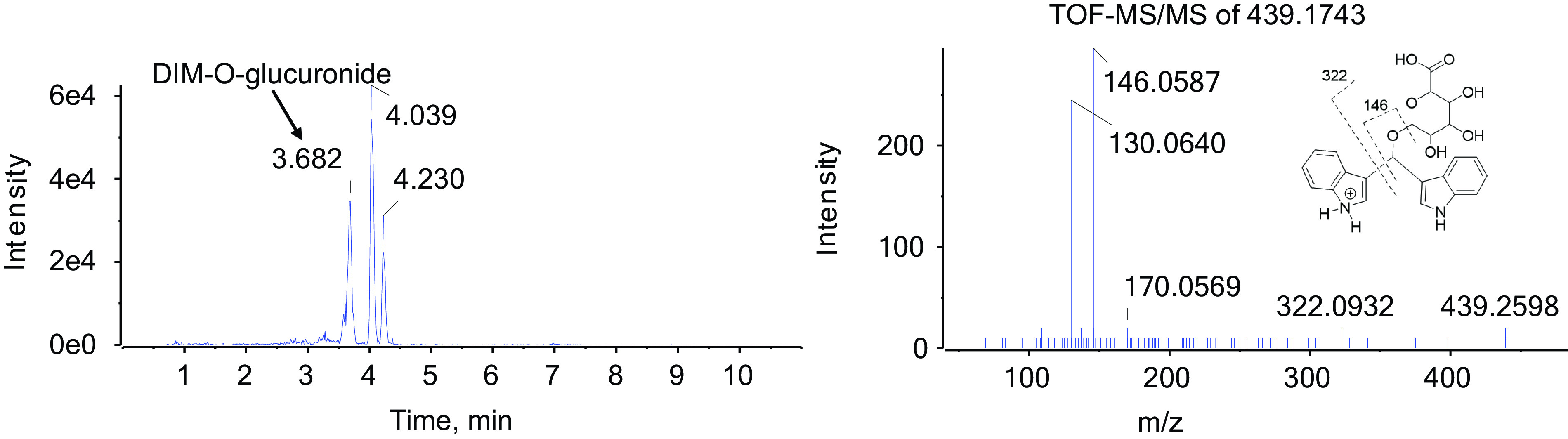
Glucuronide metabolites of monohydroxylated DIM. One 1 ml aliquots of urine collected from a single individual (BaP022) 6–12 hours postdosing was treated with β-glucuronidase, extracted, and analyzed by UPLC-MS/MS as described in Materials and Methods. The peak eluting at 3.682 minutes exhibited an m/z value of 439.1743. The fragment ion at 146.0596 is diagnostic for pyrano(3-methylenehydroxy)-DIM and is not seen with 2-ox-DIM.
We compared noncompartmental half-lives of DIM and DIM metabolites in plasma and urine using a compartmental model. DIM metabolite half-lives in plasma and urine demonstrated good agreement [12.4 and 11.2 hours, respectively for 2-ox-DIM and for 3-methylenehydroxy-(pyrano)-DIM], 9.3 and 9.8 hours, respectively, suggesting urine is the primary route of elimination for the monohydroxylated metabolites (data not shown). In contrast, plasma and urine half-lives for DIM differed by 2-fold (4.3 and 8.6 hours, respectively), suggesting other processes, like metabolism, were at least partially responsible for clearing DIM. In this study, we measured urinary elimination of DIM, 3-methylenehydroxy-(pyrano)-DIM, and 2-ox-DIM and their conjugates (Tables 2 and 3), but have not accounted for potential routes of elimination such as feces or additional metabolites such as 3-methylenehydroxy-2-ox-DIM and all conjugates.
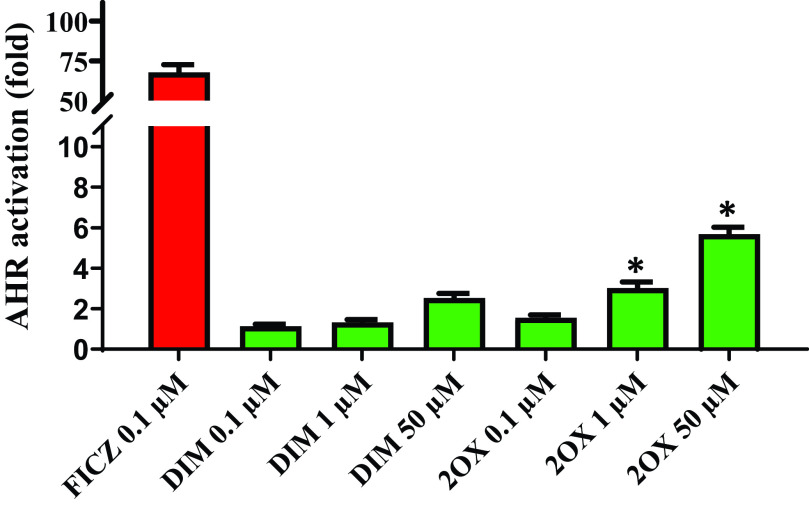
We tested the only metabolite standard available to us, 2-ox-DIM, in a mouse Hepa1 AHR reporter assay and found significantly greater efficacy with the metabolite, significant at 1 (P = 0.017) and 50 (P = 0.031) µM (Fig. 8).
Fig. 8.
AHR activation in a Hepa1 reporter system by DIM and 2-ox-DIM. DIM and 2-ox-DIM were incubated with a mouse hepatoma cell line (Hepa1) with a xenobiotic response element containing luciferase reporter in a 96-well plate. FICZ was included as a positive control. Test compounds (in 0.1% DMSO) were added to 200 µL of media in wells and incubated for 18–24 hours prior to analyzing luciferase activity as reported in Materials and Methods. The bars are the mean of triplicates (± S.D.). Significant differences between identical concentrations of DIM and 2-ox-DIM were determined with a two-tail t test and are indicated by *(P < 0.05).
Discussion
I3C is formed from hydrolysis of glucobrassicin by the plant and bacterial enzyme myrosinase. DIM is the major (∼30%) condensation product formed from I3C in the stomach after oral ingestion and is considered the major bioactive indole from cruciferous vegetables, the dietary source of glucobrassicin. In fact, urinary levels of DIM have been shown to be an accurate biomarker of human dietary crucifer ingestion (Fujioka et al., 2016). DIM and I3C are popular dietary supplements and effective cancer chemopreventive agents in preclinical models. Clinical trials show promise especially with respect to ER-responsive breast cancer and androgen receptor-dependent prostate cancer (Heath et al., 2010; Hwang et al., 2016; Li and Sarkar, 2016; Thomson et al., 2016). I3C and DIM are also effective in treatment of cervical intraepithelial neoplasia (Ashrafian et al., 2015).
There are numerous mechanisms proposed for DIM’s cancer chemoprevention including AHR-dependent induction of CYP1A2 with resultant increases in the ratio of 2-hydroxy- to 16α-hydroxy-E2 (Jellinck et al., 1993; Lord et al., 2002; Auborn et al., 2003). DIM can also modulate ER and androgen receptor signaling, important in prevention of breast and prostate cancers (Marconett et al., 2010; Hwang et al., 2016; Thomson et al., 2017). Other targets include components of cell cycle control and apoptosis (Bonnesen et al., 2001; Firestone and Bjeldanes, 2003; Aggarwal and Ichikawa, 2005; Kim and Milner, 2005; Hsu et al., 2008; Weng et al., 2008; Rajoria et al., 2011).
The AHR-dependent induction of P450s 1A1, 1A2, and 1B1 alter the toxicokinetics of BaP in rodent models, but at BaP doses orders of magnitude higher than daily human exposure. To examine the potential for DIM supplementation in alteration of the toxicokinetics of oral BaP (>95% exposure is dietary) in humans at relevant environmental exposures, we have used UPLC-accelerator mass spectrometry at Lawrence Livermore National Laboratory in a National Institutes of Health–funded study with a Food and Drug Administration Investigational New Drug approval (Madeen et al., 2019). The study reported here was not designed as a DIM pharmacokinetic study, although it resembles a previous protocol with I3C (Reed et al., 2006) in which women in a phase 1 trial were given 200 mg I3C for 4 weeks followed by 4 weeks of 400 mg daily I3C. The pharmacokinetic study was then performed after an acute 400-mg dose after an overnight fast. Subsequent analysis of plasma only quantified DIM (no acid condensation products of I3C or DIM metabolites were detected). In a subsequent study, Reed et al. (2008) found the Cmax for an acute dose of 200 mg DIM (104 ± 94 ng/ml) was similar (93 ± 30 ng/ml) to 600 mg I3C (Reed et al., 2006), although there was significant interindividual variation and a small (n = 3 plus 1 placebo for each of 6 different dose groups) sample size. Again, no DIM metabolites were reported. The interindividual variation across the six dosage groups was not related to sex, age, or BMI (Reed et al., 2008). Heath et al. (2010) also found high interindividual variation (n = 12 total men divided into 4 dose groups) in a phase 1 dose-escalation study of castrate-resistant, nonmetastatic prostate cancer. In the present study, we report a similar plasma Cmax (111 ± 160 ng/ml, n = 7) for parent DIM in individuals dosed with 2 × 150 mg BR-DIM 150 capsules each containing 45.3 mg DIM.
In this study, upon examination of plasma and urine levels after an acute dose of DIM, 10–12 hours subsequent to 1 week of supplementation (2 × 150 mg BioResponse DIM 150 in the evening with dinner), we were struck by the amount of sulfate and glucuronide conjugate metabolites present in plasma and urine. Again, previous pharmacokinetic studies of orally administered DIM in rodent models and human trials reported all DIM to be present as parent compound with no metabolites reported. Given that the [14C]BaP was a microdose (50 ng at T: 0), an impact on DIM metabolism or pharmacokinetics is extremely unlikely.
The DIM metabolites in plasma were sulfate and glucuronide conjugates of 3-methylenehydroxy-DIM, 2-ox-DIM, and 3-methylenehydroxy-2-ox-DIM. Lacking standards for conjugates, 3-methylenehydroxy-DIM (pyrano-DIM) or the dihydroxylated metabolite, 3-methylenehydroxy-(pyrano)-2-ox-DIM, we are not reporting quantities but can report amounts of 2-ox-DIM and estimate pyrano-DIM (assuming the latter monohydroxylated metabolites elicits a similar ionization response from the mass spectrometer) in samples treated with β-glucuronidase/sulfatase. Pyrano-DIM is present at much higher levels than 2-ox-DIM. Appreciable amounts of the conjugated (and small amounts of the free) 3-methylenehydroxy-(pyrano)-2-ox-DIM are also present in plasma.
In urine not treated with deconjugation enzymes, there were significant amounts of the same sulfate and glucuronides observed in plasma with sulfate conjugates appearing to be dominant. As with resveratrol, the difference observed between sulfate and glucuronide conjugates may be partly due to a greater instrument response [area of analyte (X) Internal standard (IS)] to the sulfate (Muzzio et al., 2012). The ratio of sulfate/glucuronide may also be lower than predicted by instrument response, as assessed by the yield of 2-ox-DIM and 3-methylenehydroxy-2-ox-DIM after incubation of urine samples with pure sulfatase or β-glucuronidase as well as β-glucuronidase containing sulfatase (Supplemental Fig. 2). As pyrano-DIM would be blocked from further oxygenation at the 2-position, and also could not be conjugated, the 3-methylenehydroxy-DIM must be stable long enough for the second oxygenation or conjugations by UGT and SULT to occur. We did not find any disulfate (exact mass 437.0119), diglucuronide (exact mass 628.1551), or mixed sulfate and glucuronide metabolites in plasma or urine.
Our results strongly suggest robust metabolism of DIM in these subjects after oral administration (Fig. 9), again, conflicting with previous studies in the literature. In an in vitro study with human MCF-7 breast cancer cells (Staub et al., 2006), incubation with DIM resulted in a metabolite pattern very similar to our present in vivo study, although the hydroxylation and sulfation of DIM was not as robust. It took 48 hours of incubation with 1 µM [3H]-DIM for levels of metabolites in media to exceed parent DIM. After 72 hours in media, 35% was present as DIM with the remainder sulfate conjugates, whereas in the cell 81–93% of [3H] was parent DIM, perhaps indicating active transport of sulfate conjugates from MCF-7 cells. Staub et al. (2006) demonstrated, via the use of specific CYP1A2 inducers and inhibitors, that CYP1A2 was primarily responsible for initial hydroxylation. CYP1A2, unlike CYP1A1 and CYP1B1, is constitutively expressed at significant levels in liver, as are numerous sulfotransferases and β-glucuronidases which may explain the robust first-pass metabolism (intestine may also play a role). I3C/DIM is known to induce hepatic CYP1A2 in rodents (Manson et al., 1997; Katchamart et al., 2000) and I3C supplementation (400–800 mg daily for 8 weeks) in women (measured as DIM in vivo), was effective in induction (4-fold) of CYP1A2 as determined by caffeine metabolism (Reed et al., 2005). Similarly, administration of resveratrol to humans at 1000 mg daily for 4 weeks resulted in induction of CYP1A2 activity (Chow et al., 2010). During human supplementation with DIM, coadministration of inhibitors or inducers of CYP1A2 such as quercetin, resveratrol, or caffeine could inhibit first-pass metabolism with possible resultant increased levels of parent DIM in plasma.
Fig. 9.
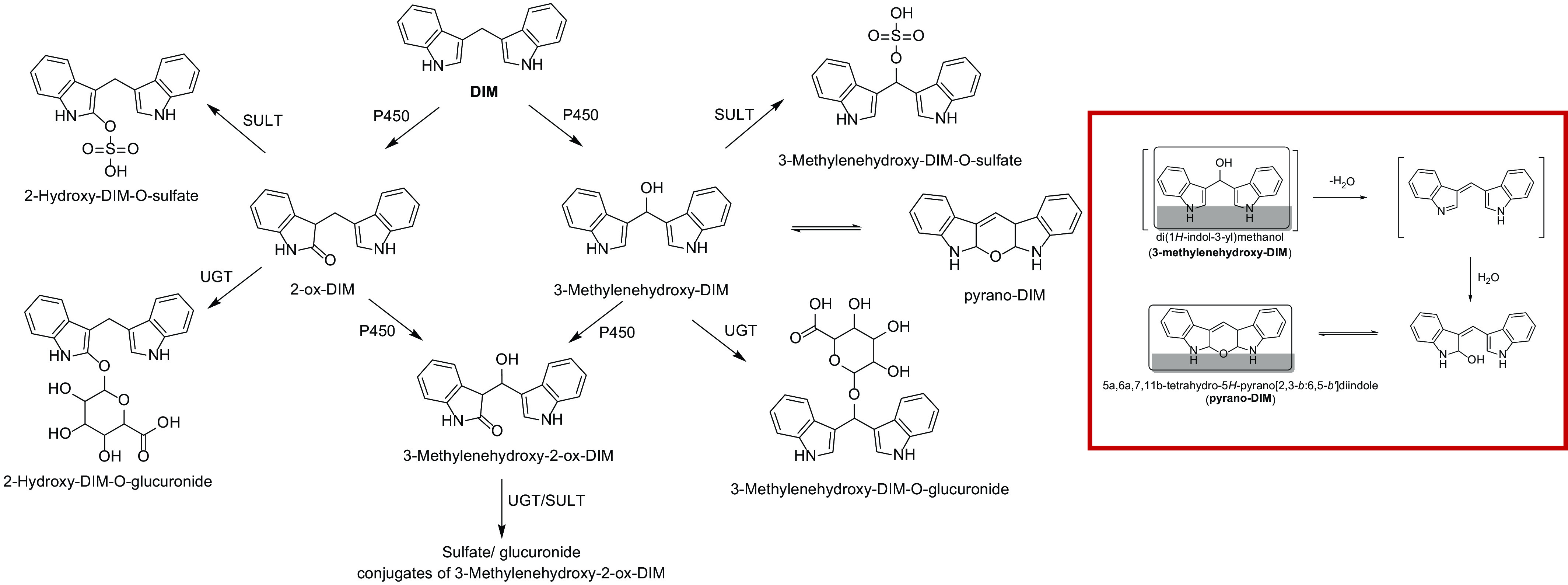
Phase 1 (P450) and phase 2 (UGT and SULT) of DIM in humans. The pathway for formation of two mono-oxygenated metabolites and one dioxygenated metabolite is shown with subsequent conjugation by UGT and SULT. The identity of the 2-ox-DIM is confirmed with a synthesized standard. The second hydroxylated metabolite is tentatively identified as 3-methylenehydroxy-DIM, which spontaneously is converted to a pyrano product. The dioxygenated product is tentatively identified as 3-methylenehydroxy-2-ox-DIM. Highlighted figure on right is the proposed pathway for spontaneous conversion of 3-methylenehydroxy-DIM to pyrano-DIM.
Given the robust metabolism, physiologically-based pharmacokinetic models developed in rodents (Anderton et al., 2004) may have to be modified for humans, and further work on potential pharmacological properties of metabolites deserve attention. Staub et al. (2006) tested 2-ox-DIM as a potential ER ligand and found no activity. In this study, 2-ox-DIM was effective in activating mouse AHR in a Hepa1 hepatoma cell line to a degree significantly greater than DIM and was comparable to the well known tryptophan metabolites kynurenine, indole-3-aldehyde, and indole-3-acetate (data not shown).
As the pharmacological activity of DIM metabolites has not previously been reported, a comparison with indole and 3-methylindole is useful. Indole and 2-ox-indole are the major indoles found in both mouse and human feces and production in mice is entirely microbial (Dong et al., 2020). Indole and 2-ox-indole are human selective AHR agonists but somewhat active in a Hepa1 reporter system (Dong et al., 2020). If 2-ox-DIM is also human AHR-selective, the fact that we see significant AHR agonist activity with mouse AHR in Hepa1 cells suggest 2-ox-DIM would be even more active with human AHR. The potent mutagen and pulmonary toxicant, 3-methylindole, also produces 2-ox-3-methylindole through P450 oxygenation, as well as hydroxylation at the 3-methyl position to generate I3C. 2-Ox-3-methylindole formation is efficiently catalyzed by the CYP2A family (D’Agostino et al., 2009; Hartog et al., 2019) whereas CYP1A1, 1A2, and 1B1 are active in 3-methyl hydroxylation (Lanza and Yost, 2001).
In vitro studies with trout liver slices demonstrated that the relatively potent (Kd, low µM) estrogenic activity of DIM could be significantly blocked by the P450 inhibitor SKF-525A (Shilling et al., 2001). This observation, along with those by our laboratory and others, demonstrating DIM is an inhibitor of multiple rat and humans P450s (Stresser et al., 1995a; Parkin et al., 2008), raises the possibility that DIM metabolites, in addition to activating AHR, may exhibit significant drug (phytochemical)-drug interactions as seen with reduced tamoxifen metabolites in serum of women taking DIM long-term (Thomson et al., 2017). With respect to conjugates, SULT conjugation of 3-hydroxyindole produces the potent uremic toxicant indoxyl sulfate (Banoglu and King, 2002). The potential for sulfate or glucuronide metabolites of DIM to exhibit any pharmacological activity is not known.
A recently completed unpublished study (Dr. Emily Ho, Oregon State University and Drs. Cynthia A. Thomson and H.H. Sherry Chow, University of Arizona, personal communication) saw results (urine only) similar to the present study. After 6 months of BR-DIM 150 (twice daily to women), an untargeted metabolomics study demonstrated urine contained substantial amounts of glucuronide and sulfate conjugates of hydroxylated DIM.
In the present study, we employed the commercially available BioResponse 150 DIM supplement, as it has been shown in clinical trials to have enhanced oral bioavailability in mice (Anderton et al., 2004). There is no rationale to expect that the BioResponse DIM 150 formulation (30% DIM in microencapsulation containing phosphatidylcholine and polyethylene glycol carriers to stimulate absorption) would be metabolized differently than pure DIM.
In addition to examination of a broader range of individuals in vivo, we plan to conduct in vitro studies with human intestinal and hepatic S9, as well as individually expressed P450s, SULTs, and UGTs followed by testing of metabolites for pharmacological activity to better understand the mechanism(s) of chemoprevention by DIM supplementation.
Acknowledgments
The authors would like to thank Dr. Michael Zeligs of BioResponse L.L.C. for providing the BioResponse DIM 150 capsules used in this study. Dr. Stephen S. Hecht, University of Minnesota kindly provided us with [2H2]-DIM. Drs. Cynthia Thomson and H.H. Sherry Chow, University of Arizona, provided us with data on DIM metabolites in urine. Dr. Paul R. Blakemore, Department of Chemistry at Oregon State University, provided the hypothesized pathway for formation of pyrano-DIM from 3-methylenehydroxy-DIM.
Abbreviations
- AHR
aryl hydrocarbon receptor
- AUC
area under the curve
- BaP
benzo[a]pyrene
- BMI
body mass index
- BR-DIM
BioResponse DIM 150
- bs
broad singlet
- d
doublet
- DIM
3,3′-diindolylmethane
- E2
β-estradiol
- ER
estrogen receptor
- FICZ
6-formylindolo[3,2-b]carbazole
- I3C
indole-3-carbinol
- m
multiplet
- 3-methylenehydroxy-DIM
bis(1H-indol-3-yl)methanol
- 3-methylenehydroxy-2-ox-DIM
3-[hydroxy-(1H-indol-3-yl)-methyl]-1,3-dihydro-2-oxindole
- MPB
mobile phase B
- MS
mass spectrometer
- 2-ox-DIM
3-((1H-indole-3-yl)methyl)indolin-2-one
- P450
cytochrome P450
- pyrano-DIM
5a,6a,11b-tetrahydro-5-H-pyrano[,2,3-b:6,5-b’]diindole
- s
singlet
- SULT
sulfotransferase
- t
triplet
- TOF
time of flight
- UGT
UDP-glucuronosyltransferase
Authorship Contributions
Participated in research design: Vermillion Maier, Siddens, Uesugi, Tilton, Ho, Williams.
Conducted experiments: Vermillion Maier, Siddens, Uesugi, Choi, Leonard, Chow, Pennington, Nguyen, Kolluri.
Performed data analysis: Vermillion Maier, Siddens, Choi, Leonard, Tilton, Smith, Nguyen, Kolluri, Williams.
Wrote or contributed to the writing of the manuscript: Vermillion Maier, Williams.
Footnotes
Funding support for this article was provided by the Public Health Service NIH National Institute of Food and Agriculture (NIFA) (P42ES016465, R01ES028600, P30ES030287, T32ES07060, Projects W4002 and W4122)
This study was funded by Public Health Service National Institute of Health National Institute of Environmental Health Sciences [Grant P42ES016465] and National Cancer Institute [Grant P30CA23074] and Multistate NIFA projects [W4002] and [W4122] from the Oregon Agricultural Experiment Station.
The authors have no conflict of interest to declare.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Aggarwal BB, Ichikawa H (2005) Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 4:1201–1215. [DOI] [PubMed] [Google Scholar]
- Ambrosone CB, Tang L (2009) Cruciferous vegetable intake and cancer prevention: role of nutrigenetics. Cancer Prev Res (Phila) 2:298–300. [DOI] [PubMed] [Google Scholar]
- Anderton MJ, Manson MM, Verschoyle R, Gescher A, Steward WP, Williams ML, Mager DE (2004) Physiological modeling of formulated and crystalline 3,3′-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab Dispos 32:632–638. [DOI] [PubMed] [Google Scholar]
- Ashrafian L, Sukhikh G, Kiselev V, Paltsev M, Drukh V, Kuznetsov I, Muyzhnek E, Apolikhina I, Andrianova E (2015) Double-blind randomized placebo-controlled multicenter clinical trial (phase IIa) on diindolylmethane’s efficacy and safety in the treatment of CIN: implications for cervical cancer prevention. EPMA J 6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auborn KJ, Fan S, Rosen EM, Goodwin L, Chandreskaren A, Williams DE, Chen D, Carter TH (2003) Indole-3-carbinol is a negative regulator of estrogen. Nutritional genomics and proteomics in cancer prevention. J Nutr 133S (Suppl):1S–6S. [DOI] [PubMed] [Google Scholar]
- Baenas N, Suárez-Martínez C, García-Viguera C, Moreno DA (2017) Bioavailability and new biomarkers of cruciferous sprouts consumption. Food Res Int 100:497–503. [DOI] [PubMed] [Google Scholar]
- Banoglu E, King RS (2002) Sulfation of indoxyl by human and rat aryl (phenol) sulfotransferases to form indoxyl sulfate. Eur J Drug Metab Pharmacokinet 27:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjeldanes LF, Kim J-Y, Grose KR, Bartholomew JC, Bradfield CA (1991) Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci USA 88:9543–9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnesen C, Eggleston IM, Hayes JD (2001) Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res 61:6120–6130. [PubMed] [Google Scholar]
- Bradfield CA, Bjeldanes LF (1987a) High-performance liquid chromatographic analysis of anticarcinogenic indoles in Brassica oleracea. J. Agric. Food Chem 35:46–49. [Google Scholar]
- Bradfield CA, Bjeldanes LF (1987b) Structure-activity relationships of dietary indoles: a proposed mechanism of action as modifiers of xenobiotic metabolism. J Toxicol Environ Health 21:311–323. [DOI] [PubMed] [Google Scholar]
- Bradlow HL (2008) Review. Indole-3-carbinol as a chemoprotective agent in breast and prostate cancer. In Vivo 22:441–445. [PubMed] [Google Scholar]
- Chow H-H, Garland LL, Hsu C-H, Vining DR, Chew WM, Miller JA, Perloff M, Crowell JA, Alberts DS (2010) Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila) 3:1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino J, Zhuo X, Shadid M, Morgan DG, Zhang X, Humphreys WG, Shu Y-Z, Yost GS, Ding X (2009) The pneumotoxin 3-methylindole is a substrate and a mechanism-based inactivator of CYP2A13, a human cytochrome P450 enzyme preferentially expressed in the respiratory tract. Drug Metab Dispos 37:2018–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF (2004) Pilot study: effect of 3,3′-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer 50:161–167. [DOI] [PubMed] [Google Scholar]
- Dong F, Hao F, Murray IA, Smith PB, Koo I, Tindall AM, Kris-Etherton PM, Gowda K, Amin SG, Patterson AD, Perdew GH (2020) Intestinal microbiota-derived tryptophan metabolites are predictive of Ah receptor activity. Gut Microbes 12: 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan MG, Selmin OI, Romagnolo DF (2018) Aryl hydrocarbon receptor diet and breast cancer risk. Yale J Biol Med 91:105–127. [PMC free article] [PubMed] [Google Scholar]
- Faust D, Nikolova T, Wätjen W, Kaina B, Dietrich C (2017) The Brassica-derived phytochemical indolo[3,2-b]carbazole protects against oxidative DNA damage by aryl hydrocarbon receptor activation. Arch Toxicol 91:967–982. [DOI] [PubMed] [Google Scholar]
- Firestone GL, Bjeldanes LF (2003) Indole-3-carbinol and 3-3′-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions. J Nutr 133(7, Suppl)2448S–2455S. [DOI] [PubMed] [Google Scholar]
- Fujioka NAinslie-Waldman CEUpadhyaya PCarmella SGFritz VARohwer CFan YRauch DLe CHatsukami DK, et al. (2014) Urinary 3,3′-diindolylmethane: a biomarker of glucobrassicin exposure and indole-3-carbinol uptake in humans. Cancer Epidemiol Biomarkers Prev 23:282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka N, Fritz V, Upadhyaya P, Kassie F, Hecht SS (2016) Research on cruciferous vegetables, indole-3-carbinol, and cancer prevention: A tribute to Lee W. Wattenberg. Mol Nutr Food Res 60:1228–1238. [DOI] [PubMed] [Google Scholar]
- Gibaldi M, Perrier D (1982) Pharmacokinetics, M, Dekker, New York. [Google Scholar]
- Hartog M, Zhang Q-Y, Ding X (2019) Role of mouse cytochrome P450 enzymes of the CYP2ABFGS subfamilies in the induction of lung inflammation by cigarette smoke exposure. Toxicol Sci 172:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Kelleher MO, Eggleston IM (2008) The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Nutr 47 (Suppl 2):73–88. [DOI] [PubMed] [Google Scholar]
- Heath EI, Heilbrun LK, Li J, Vaishampayan U, Harper F, Pemberton P, Sarkar FH (2010) A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3′- Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. Am J Transl Res 2:402–411. [PMC free article] [PubMed] [Google Scholar]
- Higdon JV, Delage B, Williams DE, Dashwood RH (2007) Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res 55:224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu EL, Chen N, Westbrook A, Wang F, Zhang R, Taylor RT, Hankinson O (2008) CXCR4 and CXCL12 down-regulation: a novel mechanism for the chemoprotection of 3,3′-diindolylmethane for breast and ovarian cancers. Cancer Lett 265:113–123. [DOI] [PubMed] [Google Scholar]
- Hwang CSethi SHeilbrun LKGupta NSChitale DASakr WAMenon MPeabody JOSmith DWSarkar FH, et al. (2016) Anti-androgenic activity of absorption-enhanced 3, 3′-diindolylmethane in prostatectomy patients. Am J Transl Res 8:166–176. [PMC free article] [PubMed] [Google Scholar]
- Jellinck PH, Forkert PG, Riddick DS, Okey AB, Michnovicz JJ, Bradlow HL (1993) Ah receptor binding properties of indole carbinols and induction of hepatic estradiol hydroxylation. Biochem Pharmacol 45:1129–1136. [DOI] [PubMed] [Google Scholar]
- Katchamart S, Stresser DM, Dehal SS, Kupfer D, Williams DE (2000) Concurrent flavin-containing monooxygenase down-regulation and cytochrome P-450 induction by dietary indoles in rat: implications for drug-drug interaction. Drug Metab Dispos 28:930–936. [PubMed] [Google Scholar]
- Kim YS, Milner JA (2005) Targets for indole-3-carbinol in cancer prevention. J Nutr Biochem 16:65–73. [DOI] [PubMed] [Google Scholar]
- Kim MK, Park JHY (2009) Cruciferous vegetable intake and the risk of human cancer: Epidemiology evidence. Proc Nutr Soc 68:103–110. [DOI] [PubMed] [Google Scholar]
- Lampe JW, Chen C, Li S, Prunty J, Grate MT, Meehan DE, Barale KV, Dightman DA, Feng Z, Potter JD (2000) Modulation of human glutathione S-transferases by botanically defined vegetable diets. Cancer Epidemiol Biomarkers Prev 9:787–793. [PubMed] [Google Scholar]
- Lanza DL, Yost GS (2001) Selective dehydrogenation/oxygenation of 3-methylindole by cytochrome p450 enzymes. Drug Metab Dispos 29:950–953. [PubMed] [Google Scholar]
- Li Y, Sarkar FH (2016) Role of BioResponse 3,3′-diindolylmethane in the treatment of human prostate cancer: Clinical experience. Med Princ Pract 25 (Suppl 2):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord RS, Bongiovanni B, Bralley JA (2002) Estrogen metabolism and the diet-cancer connection: rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern Med Rev 7:112–129. [PubMed] [Google Scholar]
- Madeen ESiddens LKUesugi SMcQuistan TCorley RASmith JWaters KMTilton SCAnderson KAOgnibene T, et al. (2019) Toxicokinetics of benzo[a]pyrene in humans: Extensive metabolism as determined by UPLC-accelerator mass spectrometry following oral micro-dosing. Toxicol Appl Pharmacol 364:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson MM, Ball HWL, Barrett MC, Clark HL, Judah DJ, Williamson G, Neal GE (1997) Mechanism of action of dietary chemoprotective agents in rat liver: induction of phase I and II drug metabolizing enzymes and aflatoxin B1 metabolism. Carcinogenesis 18:1729–1738. [DOI] [PubMed] [Google Scholar]
- Marconett CN, Sundar SN, Poindexter KM, Stueve TR, Bjeldanes LF, Firestone GL (2010) Indole-3-carbinol triggers aryl hydrocarbon receptor-dependent estrogen receptor (ER)alpha protein degradation in breast cancer cells disrupting an ERalpha-GATA3 transcriptional cross-regulatory loop. Mol Biol Cell 21:1166–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruthanila VL, Poornima J, Mirunalini S (2014) Attenuation of carcinogenesis and the mechanism underlying by the influence of indole-3-carbinol and its metabolite 3,3′-diindolylmethane: A therapeutic marvel. Adv Pharmacol Sci 2014:832161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minich DM, Bland JS (2007) A review of the clinical efficacy and safety of cruciferous vegetable phytochemicals. Nutr Rev 65:259–267. [DOI] [PubMed] [Google Scholar]
- Muzzio M, Huang Z, Hu S-C, Johnson WD, McCormick DL, Kapetanovic IM (2012) Determination of resveratrol and its sulfate and glucuronide metabolites in plasma by LC-MS/MS and their pharmacokinetics in dogs. J Pharm Biomed Anal 59:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro SL, Peterson S, Chen C, Makar KW, Schwarz Y, King IB, Li SS, Li L, Kestin M, Lampe JW (2009) Cruciferous vegetable feeding alters UGT1A1 activity: diet- and genotype-dependent changes in serum bilirubin in a controlled feeding trial. Cancer Prev Res (Phila) 2:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltsev M, Kiselev V, Muyzhnek E, Drukh V, Kuznetsov I, Pchelintseva O (2013) Comparative preclinical pharmacokinetics study of 3,3′-diindolylmethane formulations: is personalized treatment and targeted chemoprevention in the horizon? EPMA J 4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltsev M, Kiselev V, Drukh V, Muyzhnek E, Kuznetsov I, Andrianova E, Baranovskiy P (2016) First results of the double-blind randomized placebo-controlled multicenter clinical trial of DIM-based therapy designed as personalized approach to reverse prostatic intraepithelial neoplasia (PIN). EPMA J 7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DR, Lu Y, Bliss RL, Malejka-Giganti D (2008) Inhibitory effects of a dietary phytochemical 3,3′-diindolylmethane on the phenobarbital-induced hepatic CYP mRNA expression and CYP-catalyzed reactions in female rats. Food Chem Toxicol 46:2451–2458. [DOI] [PubMed] [Google Scholar]
- Peterson S, Schwarz Y, Li SS, Li L, King IB, Chen C, Eaton DL, Potter JD, Lampe JW (2009) CYP1A2, GSTM1, and GSTT1 polymorphisms and diet effects on CYP1A2 activity in a crossover feeding trial. Cancer Epidemiol Biomarkers Prev 18:3118–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillaiyar T, Köse M, Sylvester K, Weighardt H, Thimm D, Borges G, Förster I, von Kügelgen I, Müller CE (2017) Diindolylmethane derivatives: Potent agonists of the immunostimulatory orphan G Protein-Coupled Receptor GPR84. J Med Chem 60:3636–3655. [DOI] [PubMed] [Google Scholar]
- Rajoria S, Suriano R, Wilson YL, Schantz SP, Moscatello A, Geliebter J, Tiwari RK (2011) 3,3′-diindolylmethane inhibits migration and invasion of human cancer cells through combined suppression of ERK and AKT pathways. Oncol Rep 25:491–497. [DOI] [PubMed] [Google Scholar]
- Rannug A, Rannug U, Rosenkranz HS, Winqvist L, Westerholm R, Agurell E, Grafström AK (1987) Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J Biol Chem 262:15422–15427. [PubMed] [Google Scholar]
- Reed GA, Arneson DW, Putnam WC, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A (2006) Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiol Biomarkers Prev 15:2477–2481. [DOI] [PubMed] [Google Scholar]
- Reed GA, Peterson KS, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A (2005) A phase I study of indole-3-carbinol in women: tolerability and effects. Cancer Epidemiol Biomarkers Prev 14:1953–1960. [DOI] [PubMed] [Google Scholar]
- Reed GA, Sunega JM, Sullivan DK, Gray JC, Mayo MS, Crowell JA, Hurwitz A (2008) Single-dose pharmacokinetics and tolerability of absorption-enhanced 3,3′-diindolylmethane in healthy subjects. Cancer Epidemiol Biomarkers Prev 17:2619–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepkovic DW, Bradlow HL, Bell M (2001) Quantitative determination of 3,3′-diindolylmethane in urine of individuals receiving indole-3-carbinol. Nutr Cancer 41:57–63. [DOI] [PubMed] [Google Scholar]
- Shilling AD, Carlson DB, Katchamart S, Williams DE (2001) 3,3′-diindolylmethane, a major condensation product of indole-3-carbinol, is a potent estrogen in the rainbow trout. Toxicol Appl Pharmacol 170:191–200. [DOI] [PubMed] [Google Scholar]
- Shorey LE, Madeen EP, Atwell LL, Ho E, Löhr CV, Pereira CB, Dashwood RH, Williams DE (2013) Differential modulation of dibenzo[def,p]chrysene transplacental carcinogenesis: maternal diets rich in indole-3-carbinol versus sulforaphane. Toxicol Appl Pharmacol 270:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub RE, Onisko B, Bjeldanes LF (2006) Fate of 3,3′-diindolylmethane in cultured MCF-7 human breast cancer cells. Chem Res Toxicol 19:436–442. [DOI] [PubMed] [Google Scholar]
- Stresser DM, Bailey GS, Williams DE (1994a) Indole-3-carbinol and β-naphthoflavone induction of cytochromes P450 associated with bioactivation and detoxication of aflatoxin B1 in the rat. Drug Metab Dispos 22:383–391. [PubMed] [Google Scholar]
- Stresser DM, Bjeldanes LF, Bailey GS, Williams DE (1995a) The anticarcinogen 3,3′-diindolylmethane is an inhibitor of cytochrome P-450. J Biochem Toxicol 10:191–201. [DOI] [PubMed] [Google Scholar]
- Stresser DM, Williams DE, McLellan LI, Harris TM, Bailey GS (1994b) Indole-3-carbinol induces a rat liver glutathione transferase subunit (Yc2) with high activity toward aflatoxin B1 exo-epoxide. Association with reduced levels of hepatic aflatoxin-DNA adducts in vivo. Drug Metab Dispos 22:392–399. [PubMed] [Google Scholar]
- Stresser DM, Williams DE, Griffin DA, Bailey GS (1995b) Mechanisms of tumor modulation by indole-3-carbinol. Disposition and excretion of [3H]-indole-3-carbinol in male Fischer 344 rats. Drug Metab Dispos 23:965–975. [PubMed] [Google Scholar]
- Takahashi N, Stresser DM, Williams DE, Bailey GS (1995) Induction of hepatic CYP1A by indole-3-carbinol in protection against aflatoxin B1 hepatocarcinogenesis in rainbow trout. Food Chem Toxicol 33:841–850. [DOI] [PubMed] [Google Scholar]
- Thomson CAChow HHSWertheim BCRoe DJStopeck AMaskarinec GAltbach MChalasani PHuang CStrom MB, et al. (2017) A randomized, placebo-controlled trial of diindolylmethane for breast cancer biomarker modulation in patients taking tamoxifen. Breast Cancer Res Treat 165:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson CA, Ho E, Strom MB (2016) Chemopreventive properties of 3,3′-diindolylmethane in breast cancer: evidence from experimental and human studies. Nutr Rev 74:432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Poppel G, Verhoeven DT, Verhagen H, Goldbohm RA (1999) Brassica vegetables and cancer prevention. Epidemiology and mechanisms. Adv Exp Med Biol 472:159–168. [DOI] [PubMed] [Google Scholar]
- Walters DG, Young PJ, Agus C, Knize MG, Boobis AR, Gooderham NJ, Lake BG (2004) Cruciferous vegetable consumption alters the metabolism of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in humans. Carcinogenesis 25:1659–1669. [DOI] [PubMed] [Google Scholar]
- Wattenberg LW, Loub WD (1978) Inhibition of polycyclic aromatic hydrocarbon-induced neoplasia by naturally occurring indoles. Cancer Res 38:1410–1413. [PubMed] [Google Scholar]
- Weng J-R, Tsai C-H, Kulp SK, Chen C-S (2008) Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett 262:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]