Abstract
Pyrazinamide (PZA) is an important component of a standard combination therapy against tuberculosis. However, PZA is hepatotoxic, and the underlying mechanisms are poorly understood. Biotransformation of PZA in the liver was primarily suggested behind its hepatoxicity. This review summarizes the knowledge of the key enzymes involved in PZA metabolism and discusses their contributions to PZA hepatotoxicity.
SIGNIFICANCE STATEMENT
This review outlines the current understanding of PZA metabolism and hepatotoxicity. This work also highlights the gaps in this field, which can be used to guide the future studies on PZA-induced liver injury.
Introduction
Tuberculosis (TB), mostly caused by Mycobacterium tuberculosis, is a leading infectious disease killing around 4000 people worldwide daily (https://www.who.int/teams/global-tuberculosis-programme/tb-reports). Pyrazinamide (PZA), first synthesized in 1936 as a structural analog of nicotinamide, made its way into clinical use against TB since 1952. PZA has still been in use till today as an essential element of a standard anti-TB combination therapy together with isoniazid (INH), rifampicin (RIF), ethambutol, or streptomycin (Stout, 2004; https://www.who.int/teams/global-tuberculosis-programme/tb-reports). In the recommended 6-month anti-TB regimen by World Health Organization, PZA is used in the intensive phase of 2 months (Table 1). Same as INH and RIF, PZA is on the World Health Organization’s list of essential medicines for TB treatment.
TABLE 1.
The preferred regimen for TB treatment in adults and the risk of drug-induced liver injury (DILI) from each anti-TB drug
| mg/kg/day | Treatment Period | DILI Score* | |
|---|---|---|---|
| RIF | 8–12 | 6 months | A |
| INH | 4–6 | 6 months | A |
| PZA | 20–30 | first 2 months | A |
| Ethambutol (EMB) | 15–25 | first 2 months | C |
*DILI scores were adapted from LiverTox [LiverTox - NCBI Bookshelf (nih.gov)]. A, well established cause of clinically apparent liver injury; C, probable cause of clinically apparent liver injury.
Despite the important contribution of PZA to anti-TB success for the past seven decades, it can cause liver injury and even liver failure (https://www.ncbi.nlm.nih.gov/books/NBK547856/). In comparison with other potential hepatotoxic anti-TB drugs such as INH and RIF, PZA was initially reported safe (Girling, 1982). However, according to recent studies, PZA turns out to be more hepatotoxic than previously considered and could be more hepatotoxic than either INH or RIF (Schaberg et al., 1996; Yee et al., 2003; Chang et al., 2007; Tostmann et al., 2008). Unfortunately, very little is known about the mechanisms of PZA hepatotoxicity. One reason behind this is that PZA is often used together with other anti-TB drugs which are also hepatotoxic making it difficult to distinguish its individual contribution to liver damage (Table 1). Evidence from animal studies and clinical trials suggests that metabolites derived from PZA biotransformation are related to its hepatoxicity (Kudo et al., 2008; Shih et al., 2013; Rawat et al., 2018). In this review, we summarize the major enzymes involved in PZA metabolism and discuss the current understanding of PZA hepatotoxicity.
Metabolism and Disposition of PZA
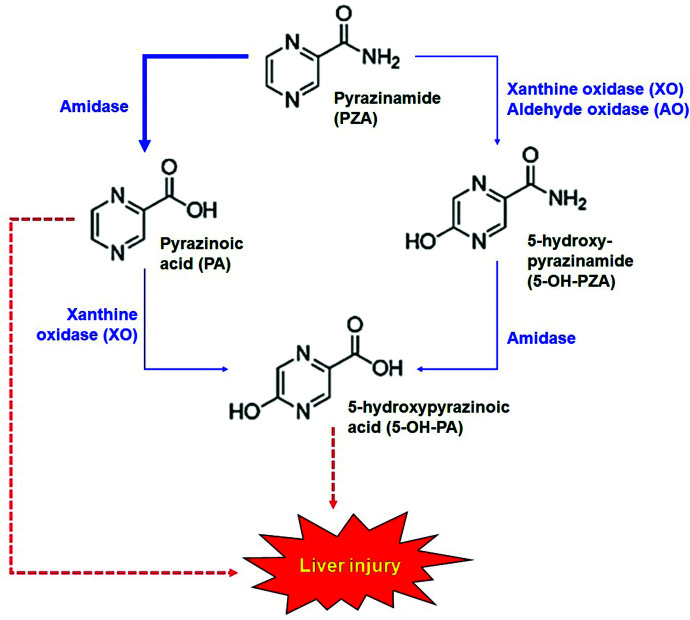
The oral dosage of PZA for adults is 20–30 mg/kg/day (https://www.ncbi.nlm.nih.gov/books/NBK247416/). It is well absorbed from the gastrointestinal tract and widely distributed in the body, including the liver. PZA is mainly metabolized in the liver by amidase, which converts PZA to pyrazinoic acid (PA) (Aoki et al., 1957). PA can be further oxidized by xanthine oxidase (XO) to form 5-hydroxy-pyrazinoic acid (5-OH-PA). Alternatively, PZA can first be oxidized to 5-hydroxy-pyrazinamide (5-OH-PZA) by XO followed by amidase-mediated hydrolysis to form 5-OH-PA (Fig. 1). Furthermore, PA can be conjugated with glycine to form trace amounts of pyrazinuric acid (Lacroix et al., 1989). PZA and its metabolites are predominantly excreted by the kidney. Within 36 hours, ∼70% of an oral dose of PZA is excreted in urine, and the relative abundances are as follows: PA (36%), 5-OH-PZA (15.4%), 5-OH-PA (l3.8%), and PZA (3.8%) (Lacroix et al., 1988).
Fig. 1.
Metabolic map of PZA and proposed mechanisms for hepatotoxicity. Amidase is the primary enzyme in PZA metabolism that produces PA. PA can be further metabolized by XO to produce 5-OH-PA. 5-OH-PA can also be produced from PZA through XO and AO-mediated oxidation followed by amidase-mediated hydrolysis. PA and 5-OH-PA are proposed as the causes of PZA hepatotoxicity.
In subjects with normal renal and hepatic functions, the plasma half-life of PZA is 9.6 hours (Lacroix et al., 1989). The half-life of PZA is significantly prolonged in patients with pre-existing liver or kidney diseases. In subjects with insufficient hepatic functions, a marked reduction of PZA clearance was observed, and the half-life of PZA was increased to 15.07 hours. In addition, the clearance rate of PA, a major and active metabolite of PZA, was also significantly decreased in patients with hepatic insufficiency (Lacroix et al., 1990). In chronic uremic patients, bioavailability of PZA was slightly increased, but bioavailability of PA was markedly increased (Stamatakis et al., 1988). These data suggest that a reduction of PZA dosage is needed in patients with hepatic or renal insufficiency.
Amidase and Its Role in PZA Metabolism.
Amidase, classified as EC 3.5.1.4, has several aliases such as acylamide amidohydrolase (systematic name), acylamidase, fatty acylamidase, acylase (although misleading), as well as some ambiguous names including amidohydrolase, deaminase, and N-acetylaminohydrolase (International Union of Biochemistry and Molecular Biology Enzyme Nomenclature). Enzymes of this group not only hydrolyze amide compounds but can also hydrolyze carboxyl esters in several cases and share a similar catalytic mechanism with esterases (Wang, 1994; Wang et al., 2016b). Amidases exhibit broad substrate specificity and have diverse biologic roles in mammals, including control of pain and neuromodulation by fatty acid amide hydrolase as well as regulation of inflammation by N-acylethanolamine–hydrolyzing acid amidase (Cravatt et al., 1996; Tsuboi et al., 2005).
A hepatic amidase has been proposed to metabolize PZA to produce PA and ammonia (Aoki et al., 1957). Later, the activity of PZA amidase was found in various tissues of mice, rats, guinea pigs, and rabbits (Toida, 1973). The activity of PZA amidase was the highest in the liver of all tested tissues, and the rabbit liver showed an outstandingly high activity of PZA amidase among these tested species. In addition, the PZA amidase was mostly localized in the microsomal fraction of the liver (Toida, 1973). Despite significant importance of hepatic microsomal amidase in PZA metabolism, the genetic identity of this enzyme is still unknown. Pyrazinamidase/nicotinamidase (EC 3.5.1.19, PNC1, I6XD65) is responsible for bacterial PZA metabolism (French et al., 2010). NCBI (National Center for Biotechnology Information) blast search with the amino acid sequence of mycobacterial pyrazinamidase showed 40% homology to yeast nicotinamidase and 27% homology to A. thaliana nicotinamidase, whereas no homology was found to any major mammalian proteins. Thus, discovering the mammalian amidase responsible for PZA metabolism remains a great challenge for the researchers in this field.
XO and Its Role in PZA Metabolism.
XO (EC 1.17.3.2) is expressed in almost all tissues with high levels in the intestine and the liver (Harrison, 2004). XO oxidizes hypoxanthine/xanthine to uric acid (Wang et al., 2016a). In addition, XO hydroxylates heterocyclic compounds including PZA and PA to produce 5-OH-PZA and 5-OH-PA, respectively (Fig. 1). In mammals, XO predominantly exists as xanthine dehydrogenase (EC 1.17.1.4), which essentially acts on the same substrates of XO but can use either NAD+ or O2 as an electron acceptor, whereas XO can only use O2 as an electron acceptor resulting in reactive oxygen species (ROS) formation. The conversion from xanthine dehydrogenase to XO proceeds either reversibly by the oxidation of its certain cysteine thiols to form cystine disulfide bonds or irreversibly by specific proteolysis. Despite these differences between the two forms of this enzyme, the term XO was often used as a general name for both throughout the literatures (Harrison, 2004).
Differences in XO activity among individuals and various ethnicities as evaluated by caffeine metabolic rate suggest interesting polymorphic behavior of this enzyme. A low activity of liver XO was observed in 20% Caucasian as well as in 11% Japanese (Kudo et al., 2008). In addition, males showed higher XO activities than females. Classic xanthinuria type 1 is a rare autosomal recessive disorder, caused by arginine149cysteine substitution resulting in loss of activity of XO leading to chronic renal failure (Kudo et al., 2008). On the contrary, two different point mutations, isoleucine703valine or histidine1221arginine, were reported to increase XO activity by around 2-fold in a study conducted in Japanese population (Kudo et al., 2008). However, limited information is available for the associations of XO polymorphisms with PZA metabolism. Furthermore, enhanced XO expression and activity were found in mouse liver and other tissues treated with interferon (IFN) and IFN-inducers (Ghezzi et al., 1984). Human XO gene was also reported to contain IFN-γ response elements (Xu et al., 1996). Therefore, further studies are needed to determine the potential impact of XO polymorphisms and/or induction on PZA metabolism.
It is here worthwhile to mention that rabbits do not express XO and thereby do not respond to allopurinol, an XO inhibitor, which explained the unique pharmacokinetics and high exposure of PA in rabbits when compared with mice and other mammals (Via et al., 2015). This fact is consistent with previous studies in human volunteers who showed increased levels of PA and decreased levels of 5-OH-PZA as well as 5-OH-PA when cotreated with PZA and allopurinol (Lacroix et al., 1988; Naftalin et al., 2017). Allopurinol is commonly used to treat hyperuricemia or gout but is not suggested to treat PZA-induced hyperuricemia, a common side effect of PZA, which occurs because of inhibition of renal excretion of uric acid by PA and thus counterbalancing the effect of allopurinol (Lacroix et al., 1988).
The ability of patients with xanthinuria caused by XO deficiency to oxidize PZA raised the possibility of the existence of an alternative enzyme other than classic XO in PZA metabolism. Moriwaki et al. (1993) showed that aldehyde oxidase (AO; EC 1.2.3.1) could potentially convert PZA to 5-OH-PZA but not PA to 5-OH-PA (Fig. 1). In addition, the Km value of XO for PZA was about 10 times higher than that of AO. These results indicate that AO is catalytically distinct from XO. However, the relative contribution of AO to PZA metabolism in mammals was not investigated so far.
Mechanisms of PZA-Induced Hepatotoxicity
The contribution of PZA to liver damage during anti-TB therapy is not fully clear, because PZA is used only in combination with other anti-TB drugs that are hepatotoxic, such as INH and RIF. However, mounting evidence supports that PZA is hepatotoxic. For example, the use of combination therapy with RIF and PZA for latent TB was abandoned because of the frequency of severe liver injury (https://www.ncbi.nlm.nih.gov/books/NBK547856/). The pattern of PZA hepatotoxicity is typically acute hepatitis with hepatocellular necrosis, inflammation, and variable degrees of cholestasis, which negatively impacts the outcomes of anti-TB therapy (https://www.ncbi.nlm.nih.gov/books/NBK547856/). Unfortunately, no approach is currently available to predict and prevent PZA hepatotoxicity because its mechanisms are poorly understood, especially when compared with that of INH and RIF.
PZA hepatotoxicity is dose-dependent, especially at daily doses above 40 mg/kg, and the extent of PZA hepatotoxicity is correlated with its hepatic metabolism, suggesting a direct toxic effect, but not a hypersensitive or immune-mediated effect (Tostmann et al., 2008; Shih et al., 2013). Recent in vitro and in vivo reports suggest that amidase-mediated production of PA from PZA was responsible for PZA hepatotoxicity (Fig. 1). Experiments with Wistar rats treated with PZA or PA showed hepatotoxicity as observed in elevated serum levels of alanine aminotransferase, aspartate transaminase, and galactose single point (Shih et al., 2013). Amidase inhibitor bis-p-nitrophenyl phosphate decreased PZA-induced, but not PA-induced, hepatotoxicity, suggesting amidase as the initiator of PZA hepatotoxicity (Shih et al., 2013). Consistently, PA levels in the urine were highly correlated with PZA hepatotoxicity in TB patients (Shih et al., 2013). However, because the genetic identity of PZA amidase remains unknown, it is difficult to investigate the role of PZA amidase in PZA hepatotoxicity using genetic approaches.
PA can be further metabolized by XO to produce 5-OH-PA, that was suggested to be more hepatotoxic than PA (Fig. 1) (Shih et al., 2013; Rawat et al., 2018). Shih et al. (2013) treated human hepatoma cell line HepG2 cells with PZA and its metabolites and found 5-OH-PA being the most toxic metabolite of PZA. A recent study in rats showed that 5-OH-PA caused liver damage accompanied with aberrant metabolic shifts (Rawat et al., 2018). In addition, the patients with severe hepatotoxicity showed much higher ratio of 5-OH-PA to PZA in the urine than other patients with mild or no hepatotoxicity (Shih et al., 2013; Rawat et al., 2018). These data indicate that XO may play an important role in PZA hepatotoxicity by producing 5-OH-PA. However, inhibition of XO by allopurinol increased PZA toxicity in HepG2 cells, suggesting that the hydroxy metabolites of PZA and/or PA, products of XO, were not responsible for PZA hepatotoxicity (Tostmann et al., 2010). With these controversial data, further studies are needed to determine the role of XO in PZA hepatotoxicity.
Physiologic significances of XO-catalyzed reactions are to increase hydrophilicity of purine catabolic end products by forming uric acid to be excreted through urine. XO-null mice developed renal interstitial fibrosis through aberrant lipid and purine accumulation in renal tubules resulting in premature death (Ohtsubo et al., 2009). In a recent study, hepatocyte-specific ablation of XO in mice was found to correct obesity-induced systemic hyperuricemia despite other metabolic abnormalities being unchanged (Harmon et al., 2019). In addition, ROS produced as a by-product of XO, especially in immune cells, are used to kill microbes as a part of innate immunity of the host. However, due to being responsible for ROS formation, XO is also implicated to several pathophysiological processes such as oxidative stress, hypertension, and ischemia reperfusion injury (Wang et al., 2016a). Therefore, it is worthwhile to investigate the contributions of XO-mediated production of ROS as well as 5-OH-PA to PZA hepatotoxicity. In addition, further studies are also suggested to explore whether PZA and/or PA disrupt XO-dependent metabolism of endobiotics in the liver and result in liver dysfunction.
Genetic studies have been conducted to explore the impact of PZA on the liver. In rats treated with PZA for 28 days, it was found that PZA upregulates CYP2b1, epoxide hydrolase 1, and heme oxygenase, and downregulates two peroxisome proliferator activated receptor (PPAR)-dependent genes including carnitine palmitoyltransferase 1b and fatty acid binding protein 7 (Zhang et al., 2013). In a follow-up study using the same model, PPARα expression was shown to be inversely correlated with PZA-induced liver injury (Zhang et al., 2016). However, in a very recent study conducted in TB patients with or without liver injury taking standard anti-TB drug regimen, no association was found between various single nucleotide polymorphisms in PPARα gene and liver injury (Zhang et al., 2020). Interestingly, two polymorphic variants in pregnane X receptor, a nuclear transcription factor known to regulate the expression of various drug-metabolizing enzymes, were associated with the decreased risk of anti-TB drug-induced hepatotoxicity, suggesting that drug-metabolizing enzymes regulated by pregnane X receptor are involved in the hepatotoxicity of the standard anti-TB drug regimen (Wang et al., 2019).
Since some case reports of PZA-induced hepatotoxicity showed evidence of cholestasis, a recent study investigated its mechanisms in rats (Guo et al., 2016). When rats were orally treated with PZA (2 g/kg/day) for 1 week, total bile acids increased 10-fold in the serum, whereas alanine aminotransferase and aspartate aminotransferase increased 2-fold. The farnesoid X receptor (FXR), a bile acid-responsive transcription factor, which plays a key role in the regulation of bile acid synthesis, excretion, and transport, was found to be downregulated. Interestingly, treatment with the FXR agonist obeticholic acid attenuated PZA hepatotoxicity, suggesting that PZA-induced cholestatic liver injury was related to FXR suppression (Guo et al., 2016).
Conclusion
Metabolism of PZA, a first-line anti-TB drug, is worth interesting in clinical context, because increased PZA metabolites such as PA and 5-OH-PA were found to be highly correlated to the extent of hepatotoxicity. Out of two major enzymes metabolizing PZA, amidase catalyzed the production of PA, but the molecular identity of amidase is unknown. In addition, XO is suggested to play an important role in PZA hepatotoxicity by producing 5-OH-PA. However, the preclinical data of 5-OH-PA- and/or PA-mediated hepatotoxicity are not conclusive. Thus, more research is needed to elucidate the metabolic pathways of PZA and determine their contributions to PZA hepatotoxicity.
Abbreviations
- AO
aldehyde oxidase
- EC
Enzyme Commission
- FXR
farnesoid X receptor
- IFN
interferon
- INH
isoniazid
- 5-OH-PA
5-hydroxy-pyrazinoic acid
- 5-OH-PZA
5-hydroxy-pyrazinamide
- PA
pyrazinoic acid
- PPAR
peroxisome proliferator activated receptor
- PZA
pyrazinamide
- RIF
rifampicin
- ROS
reactive oxygen species
- TB
tuberculosis
- XO
xanthine oxidase
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Hussain, Zhu, Ma.
Footnotes
This work was supported in part by the National Institutes of Health National Institute of Allergy and Infectious Diseases [Grant R01AI131983] and the National Center for Complementary and Integrative Health [Grant R21AT011088].
The authors have declared that no conflict of interest exists.
References
- Aoki T, Nishio K, Ito F (1957) [Research on the metabolic fate of pyrazinamide; pyrazinoic acid formation by liver homogenates in vitro]. Kekkaku 32:469–471. [PubMed] [Google Scholar]
- Chang KC, Leung CC, Yew WW, Tam CM (2007) Standard anti-tuberculosis treatment and hepatotoxicity: do dosing schedules matter? Eur Respir J 29:347–351. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87. [DOI] [PubMed] [Google Scholar]
- French JB, Cen Y, Vrablik TL, Xu P, Allen E, Hanna-Rose W, Sauve AA (2010) Characterization of nicotinamidases: steady state kinetic parameters, classwide inhibition by nicotinaldehydes, and catalytic mechanism. Biochemistry 49:10421–10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi P, Bianchi M, Mantovani A, Spreafico F, Salmona M (1984) Enhanced xanthine oxidase activity in mice treated with interferon and interferon inducers. Biochem Biophys Res Commun 119:144–149. [DOI] [PubMed] [Google Scholar]
- Girling DJ (1982) Adverse effects of antituberculosis drugs. Drugs 23:56–74. [DOI] [PubMed] [Google Scholar]
- Guo HL, Hassan HM, Zhang Y, Dong SZ, Ding PP, Wang T, Sun LX, Zhang LY, Jiang ZZ (2016) Pyrazinamide induced rat cholestatic liver injury through inhibition of FXR regulatory effect on bile acid synthesis and transport. Toxicol Sci 152:417–428. [DOI] [PubMed] [Google Scholar]
- Harmon DBMandler WKSipula IJDedousis NLewis SEEckels JTDu JWang YHuckestein BRPagano PJ, et al. (2019) Hepatocyte-specific ablation or whole-body inhibition of xanthine oxidoreductase in mice corrects obesity-induced systemic hyperuricemia without improving metabolic abnormalities. Diabetes 68:1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R (2004) Physiological roles of xanthine oxidoreductase. Drug Metab Rev 36:363–375. [DOI] [PubMed] [Google Scholar]
- Kudo M, Moteki T, Sasaki T, Konno Y, Ujiie S, Onose A, Mizugaki M, Ishikawa M, Hiratsuka M (2008) Functional characterization of human xanthine oxidase allelic variants. Pharmacogenet Genomics 18:243–251. [DOI] [PubMed] [Google Scholar]
- Lacroix C, Guyonnaud C, Chaou M, Duwoos H, Lafont O (1988) Interaction between allopurinol and pyrazinamide. Eur Respir J 1:807–811. [PubMed] [Google Scholar]
- Lacroix C, Hoang TP, Nouveau J, Guyonnaud C, Laine G, Duwoos H, Lafont O (1989) Pharmacokinetics of pyrazinamide and its metabolites in healthy subjects. Eur J Clin Pharmacol 36:395–400. [DOI] [PubMed] [Google Scholar]
- Lacroix C, Tranvouez JL, Phan Hoang T, Duwoos H, Lafont O (1990) Pharmacokinetics of pyrazinamide and its metabolites in patients with hepatic cirrhotic insufficiency. Arzneimittelforschung 40:76–79. [PubMed] [Google Scholar]
- Moriwaki Y, Yamamoto T, Nasako Y, Takahashi S, Suda M, Hiroishi K, Hada T, Higashino K (1993) In vitro oxidation of pyrazinamide and allopurinol by rat liver aldehyde oxidase. Biochem Pharmacol 46:975–981. [DOI] [PubMed] [Google Scholar]
- Naftalin CMVerma RGurumurthy MLu QZimmerman MYeo BCMTan KHLin WYu BDartois V, et al. (2017) Coadministration of allopurinol to increase antimycobacterial efficacy of pyrazinamide as evaluated in a whole-blood bactericidal activity model. Antimicrob Agents Chemother 61:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo T, Matsumura K, Sakagami K, Fujii K, Tsuruya K, Noguchi H, Rovira II, Finkel T, Iida M (2009) Xanthine oxidoreductase depletion induces renal interstitial fibrosis through aberrant lipid and purine accumulation in renal tubules. Hypertension 54:868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat AChaturvedi SSingh AKGuleria ADubey DKeshari AKRaj VRai APrakash AKumar U, et al. (2018) Metabolomics approach discriminates toxicity index of pyrazinamide and its metabolic products, pyrazinoic acid and 5-hydroxy pyrazinoic acid. Hum Exp Toxicol 37:373–389. [DOI] [PubMed] [Google Scholar]
- Schaberg T, Rebhan K, Lode H (1996) Risk factors for side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur Respir J 9:2026–2030. [DOI] [PubMed] [Google Scholar]
- Shih TY, Pai CY, Yang P, Chang WL, Wang NC, Hu OY (2013) A novel mechanism underlies the hepatotoxicity of pyrazinamide. Antimicrob Agents Chemother 57:1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis G, Montes C, Trouvin JH, Farinotti R, Fessi H, Kenouch S, Méry JP (1988) Pyrazinamide and pyrazinoic acid pharmacokinetics in patients with chronic renal failure. Clin Nephrol 30:230–234. [PubMed] [Google Scholar]
- Stout JE (2004) Safety of rifampin and pyrazinamide for the treatment of latent tuberculosis infection. Expert Opin Drug Saf 3:187–198. [DOI] [PubMed] [Google Scholar]
- Toida I (1973) Metabolism of pyrazinamide. Pyrazinamide deamidase of animal tissues. Am Rev Respir Dis 107:630–638. [DOI] [PubMed] [Google Scholar]
- Tostmann A, Aarnoutse RE, Peters WH, Richard PN, Boeree MJ (2010) Xanthine oxidase inhibition by allopurinol increases in vitro pyrazinamide-induced hepatotoxicity in HepG2 cells. Drug Chem Toxicol 33:325–328. [DOI] [PubMed] [Google Scholar]
- Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R (2008) Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol 23:192–202. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Sun YX, Okamoto Y, Araki N, Tonai T, Ueda N (2005) Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J Biol Chem 280:11082–11092. [DOI] [PubMed] [Google Scholar]
- Via LESavic RWeiner DMZimmerman MDPrideaux BIrwin SMLyon EO’Brien PGopal PEum S, et al. (2015) Host-mediated bioactivation of pyrazinamide: implications for efficacy, resistance, and therapeutic alternatives. ACS Infect Dis 1:203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Zhang C, Xing XH (2016a) Xanthine dehydrogenase: An old enzyme with new knowledge and prospects. Bioengineered 7:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY (1994) Microsomal amidases and carboxylesterases, in Conjugation—deconjugation reactions in drug metabolism and toxicity (Kauffman FC, ed) pp 161–187, Springer, Berlin, Heidelberg. [PubMed] [Google Scholar]
- Wang P, Pradhan K, Zhong XB, Ma X (2016b) Isoniazid metabolism and hepatotoxicity. Acta Pharm Sin B 6:384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xiang X, Huang WW, Sandford AJ, Wu SQ, Zhang MM, Wang MG, Chen G, He JQ (2019) Association of PXR and CAR polymorphisms and antituberculosis drug-induced hepatotoxicity. Sci Rep 9:2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Huecksteadt TP, Hoidal JR (1996) Molecular cloning and characterization of the human xanthine dehydrogenase gene (XDH). Genomics 34:173–180. [DOI] [PubMed] [Google Scholar]
- Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D (2003) Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med 167:1472–1477. [DOI] [PubMed] [Google Scholar]
- Zhang CJiao LBai HZhao ZHu XWang MWu TPeng WLiu TSong J, et al. (2020) Association of POR and PPARα polymorphisms with risk of anti-tuberculosis drug-induced liver injury in Western Chinese Han population. Infect Genet Evol 79:104147. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guo H, Hassan HM, Ding PP, Su Y, Song Y, Wang T, Sun L, Zhang L, Jiang Z (2016) Pyrazinamide induced hepatic injury in rats through inhibiting the PPARα pathway. J Appl Toxicol 36:1579–1590. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jiang Z, Su Y, Chen M, Li F, Liu L, Sun L, Wang Y, Zhang S, Zhang L (2013) Gene expression profiling reveals potential key pathways involved in pyrazinamide-mediated hepatotoxicity in Wistar rats. J Appl Toxicol 33:807–819. [DOI] [PubMed] [Google Scholar]