Abstract
Purpose
Despite management advances, accelerated atherosclerotic cardiovascular disease (ACVD) remains a major cause of morbimortality in systemic lupus erythematosus (SLE) patients; that is not fully explained by traditional risk factors. Fibroblast growth factor-23 (FGF23) is a bone-derived phosphaturic hormone with multiple klotho-dependent and independent effects, including promotion of atherosclerosis and vascular calcification, particularly in the context of chronic kidney disease. Increased circulating FGF23 was reported in SLE patients, particularly with lupus nephritis (LN); but its atherogenic role in these disorders was not explored.
Subjects and Methods
Three study groups of predominantly middle-aged females were categorized by the 2012 SLE International Collaborating Clinics (SLICC) criteria as SLE (without LN), LN, or controls matching for traditional CVD risk profile. Measures of SLE activity, damage, steroid therapy, and glomerular filtration rate were calculated. Fasting blood samples were checked for serum lipid profile, anti-DNA, urea, creatinine, uric acid, proteins, albumin, calcium, phosphorus, C3, C4, CRP, vitamin-D3, intact parathyroid hormone and FGF23 (iFGF23). By carotid ultrasonography, mean common carotid artery intima-media thickness (CC-IMT), plaque score (PS) and internal carotid resistive index (ICRI) were recorded.
Results
CC-IMT, ICRI and serum iFGF23 differed along the study groups (LN>SLE>controls). In both SLE and LN patients, serum iFGF23 had a significant positive correlation with serum phosphorus, CC-IMT and PS. On multivariate analysis, the strongest predictor of increased CC-IMT was cumulative steroid dose in SLE and serum iFGF23 in LN patients. Most significant independent predictors of increased serum iFGF23 were hyperphosphatemia in SLE and proteinuria in LN patients.
Conclusion
FGF23-phosphate axis has a key role in accelerated ACVD in SLE patients. Serum phosphorus and iFGF23 should be included in ACVD risk profile assessment of these patients. Prospective studies shall define the role of dietary and/or pharmacologic control of hyperphosphatemia and proteinuria in reducing circulating iFGF23 and ACVD in them.
Keywords: atherosclerosis, FGF23, hyperphosphataemia, lupus, nephritis, ultrasonography
Introduction
Systemic lupus erythematosus (SLE) is a prototype autoimmune disease predominantly affecting middle-aged women, typically with multiple organ-system involvement, and a protracted course with remissions, exacerbation and cumulative tissue damage. Despite marked geographical disparities, a review of recent epidemiologic studies denoted a trend for increasing global prevalence.1 Although patients` life expectancy has improved in line with advances in diagnosis and treatment, it remains considerably lower compared with the general population.2 Accelerated atherosclerotic cardiovascular disease (ACVD) constitutes the major comorbidity and the leading cause of death, with a frightening risk of myocardial infarction and stroke disproportionately striking young female patients, who are otherwise known to have low ACVD risk.3 It has been long- recognized that this amplified risk cannot be fully accounted for by traditional (Framingham) risk factors, like hypertension, obesity and dyslipidaemia.4 The immunologically geared chronic inflammatory status that typify the disease is a major inducer of ACVD in itself.3 Corticosteroids, that have long constituted the backbone of effective anti-inflammatory therapy, are also atherogenic in the long-term.5 The inclusion of SLE diagnosis and corticosteroid use in the newly developed QRISK3 score has significantly improved its CVD risk prediction in SLE patients.6 Looking for novel, non-traditional, ACVD risk factors, with a focus on recently identified molecular biomarkers, is an evolving area of SLE research.7,8
There is now ample evidence for a link between bone disease and ACVD, both in the general population,9 and in patients with SLE and other autoimmune diseases.10,11 This disturbed bone- vascular axis may result from perturbations of vitamin D,12 parathyroid hormone (PTH),13 or fibroblast growth factor-23 (FGF23).14 Principally secreted from osteocytes and osteoblasts in response to phosphorus loading, FGF23 has been identified as the major phosphaturic hormone (phosphatonin), that decreases proximal renal tubular phosphate reabsorption, intestinal phosphorus absorption, and vitamin D activation.15 It was soon realized that FGF23 is a pleiotropic molecule, with a host of klotho-dependent and independent autocrine, paracrine and endocrine effects on almost all body tissues.16 Increased circulating FGF23 is now recognized as the earliest biochemical abnormality in chronic kidney disease – mineral bone disorder (CKD-MBD).17 Progressive renal impairment is paralleled with an exponential increase in circulating FGF23, approaching several thousands of normal in patients with end-stage renal disease.18
Epidemiologic studies have inked increased circulating FGF23 in subjects with normal renal function with both early functional alterations (impaired forearm flow-mediated dilatation)14 and established carotid ultrasonographic (US) changes (increased intima-media thickness [IMT] and plaque presence)19,20 of atherosclerosis in the community. Similar associations were confirmed in CKD patients.21,22 Therefore, serum FGF23 could improve the power of Framingham risk score to predict increased carotid IMT.23 FGF23 may induce/augment some traditional atherosclerosis risk factors, such as hypertension,24 dyslipidaemia,25 insulin resistance and obesity.26 Of more importance, however, is the association of FGF23 with a host of non-traditional ACVD risk factors such as chronic inflammation,27 hypovitaminosis D,28 and vascular calcification.29 These latter factors become particularly prominent in presence of CKD. Increased FGF23 is also a significant predictor of CKD occurrence30 and progression.31
Two small studies reported significantly higher serum FGF23 in SLE patients compared with controls, with a still higher level in patients with lupus nephritis (LN).32,33 The inflammatory reaction of active lupus upregulates FGF23 production by osteocytes.34 The development of LN is conductive for a further rise of circulating FGF23, possibly induced by hyperparathyroidism,35 hyperphosphataemia,36 impaired renal clearance,37 and klotho deficiency.38 Therefore, LN patients typically have in parallel significantly higher level of FGF23 and higher burden of ACVD, compared with SLE patients without nephritis.33 FGF23 may thus prove to be an important mediator of accelerated ACVD in SLE patients, with a particularly more prominent role in patients with LN. Controlling the circulating level of this key molecule may then become a novel therapeutic approach to reduce cardiovascular morbimortality in SLE patients. Of note, however, studies exploring the cardiovascular risk of circulating FGF23 in SLE patients are lacking.39
Materials and Methods
Study Design and Participants
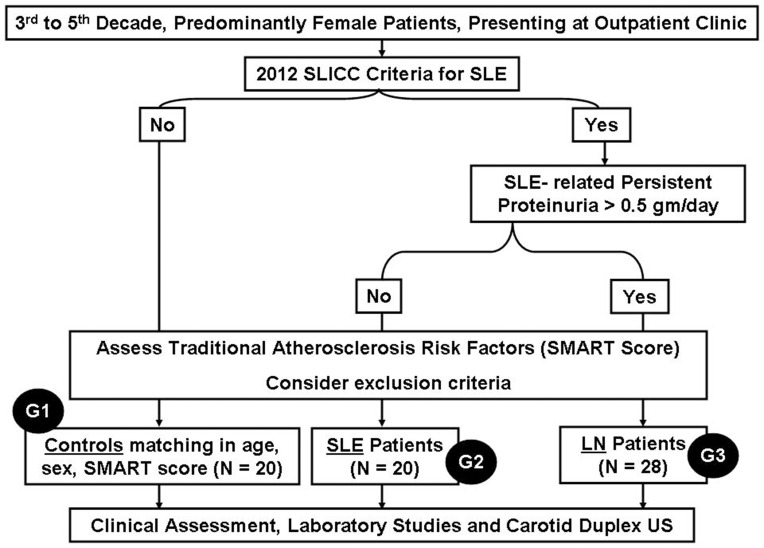
This was a cross-sectional, case control study, conducted in accordance with the Declaration of Helsinki and approved by the institutional medical ethics committee. Between March and November 2018, eligible participants providing written informed consent were recruited from the outpatient clinics of the Medical Research Institute, Alexandria University, Egypt, and subjected to full clinical evaluation and medical records review, followed by laboratory and imaging studies (Figure 1).
Figure 1.
Study flowchart.
Abbreviation: SLICC, SLE International Collaborating Clinics.
Diagnosis of SLE was based on the 2012 SLE International Collaborating Clinics (SLICC) criteria.40 LN was defined by persistent proteinuria > 0.5gm/day in the context of SLE.41 SLE disease activity was assessed by SLE Disease Activity Index (SLEDAI),42 whereas the extent of established damage was assessed by SLICC Damage Index.43 Average steroid dose (Av_S) was calculated as the average equivalent prednisone dose in mg/day. Cumulative steroid dose (Cum_S) was obtained by multiplying Av_S by disease duration in years. Steroid pulses were defined as short-term (3–5 days) intensification of basic steroid dose, mostly given parenterally during in-hospital admission. Traditional atherosclerosis risk factors (age decade, male sex, hypertension, dyslipidaemia, obesity, treatment for these conditions, and presence of ACVD in different vascular beds) were defined by standard criteria and given one point each. The total score (termed Second Manifestations of ARTerial disease, or SMART score) provided a validated semiquantitative estimate of the burden of traditional ACVD risk factors.44,45
We excluded patients with diabetes mellitus, morbid obesity, smoking (current or past), pregnancy, estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73m2, age > 50 years or insufficient data. Finally, 68 subjects (age 20–50 years, 9 males) were triaged into 3 study groups:
G1 (Controls) (N = 20): selected on individual basis to be matching with the other groups in age, sex and SMART score.
G2 (SLE) (N = 20): having SLE without LN.
G3 (LN) (N = 28): having LN.
Laboratory Studies46
After 12 hours fast, blood samples were drawn into EDTA tubes (for complete blood count) and serum separator tubes that were immediately transported and centrifuged, keeping serum at −80 OC until batch analysis was made for levels of serum low-density lipoproteins (LDL), high-density lipoproteins (HDL), triglycerides (TGs), anti-double-stranded DNA (anti-DNA), complement components C3 and C4, C-reactive protein (CRP), urea, creatinine, uric acid, total proteins, albumin, calcium, phosphorus, vitamin D3, intact parathyroid hormone (iPTH) (3rd generation assay), and intact FGF23 (iFGF23) using commercially available kits according to manufacturers` instructions. Morning void urine was used for complete urinalysis. Twenty-four hours urine collections were used for quantitation of proteinuria. eGFR was calculated from stable serum creatinine using CKD-epidemiology collaboration (CKD-EPI) equation.47
Carotid Duplex Ultrasonography48
It was done by an experienced sonographer blinded to the patients` data, using Acuson X300 US System, Siemens Healthineers (formerly Siemens Healthcare), USA. The patient was placed in a supine position with a slight head tilt away from the examined side. A linear-array transducer (VF10-5) with a frequency of 8 MHz was used to scan the carotid system on both sides and calculate the following:
Plaque Score (PS):49 A carotid plaque was defined as either a focal protrusion of the intima-media layer encroaching into the arterial lumen (protuberant plaque), or a diffuse thickening of the intima-media layer measuring > 1.5 mm (diffuse plaque). These were looked for thoroughly in three distinct segments (distal common carotid, bulb, and proximal internal carotid arteries). The total number of plaque-bearing segments on both sides represented the PS, which ranged 0 to 6.
Mean Common Carotid Artery Intima-Media Thickness (CC-IMT):50 The distal segment of the common carotid artery on each side was scanned with the probe in 3 directions (anterior, lateral, and posterior). Each time, a short clip was saved in which the intima-lumen and the media-adventitia interfaces on the arterial far wall were clearly delineated. Some clearly captured image frames were then analysed by the Syngo Arterial Health Package (AHP), an automatic edge detection software, to calculate the mean intima-media thickness along a one-centimetre plaque-free arterial segment. The composite mean CC-IMT was calculated by averaging the 6 readings (3 from each side).
Internal Carotid Artery Resistive Index (ICRI):51 The insonation angle (between transducer beam and axis of blood flow) was kept <60O and the pulse repetition frequency was adjusted to prevent aliasing while maximizing sensitivity and waveform size. Aided by colour flow mapping, the sampling gate was placed completely within the internal carotid flow. The spectral velocity-time curve was recorded while the patient holds breath. The ICRI was calculated as:[(peak-systolic flow velocity – end-diastolic flow velocity) divided by (peak-systolic flow velocity)] (Pourcelot’s equation, measured from the same waveform). Two readings were obtained on each side and the average of the four readings was recorded.
Test-retest variabilities of the ultrasonographic measurements were generally < 5%.
Statistical Methods
Data were analysed using SPSS software package version 20 (SPSS Inc., Chicago, Illinois, USA). Categorical data were expressed as absolute numbers (percentages) and compared by Chi-square or Fisher exact test. Continuous data were tested for normality using Shapiro Wilk test. Parametric data were presented as mean ± SD and compared by analysis of variance (ANOVA). Non-parametric data were presented as median (interquartile range) and compared by Mann–Whitney U or Kruskal–Wallis H-test. Post-hoc analysis was performed for pairwise comparisons. Correlations were tested by the Spearman’s rank correlation coefficient. Models for multivariate linear regression analysis were constructed separately in G2 and G3, introducing clinically relevant predictors with unadjusted P < 0.08. Significance was judged at the 5% level.
Results
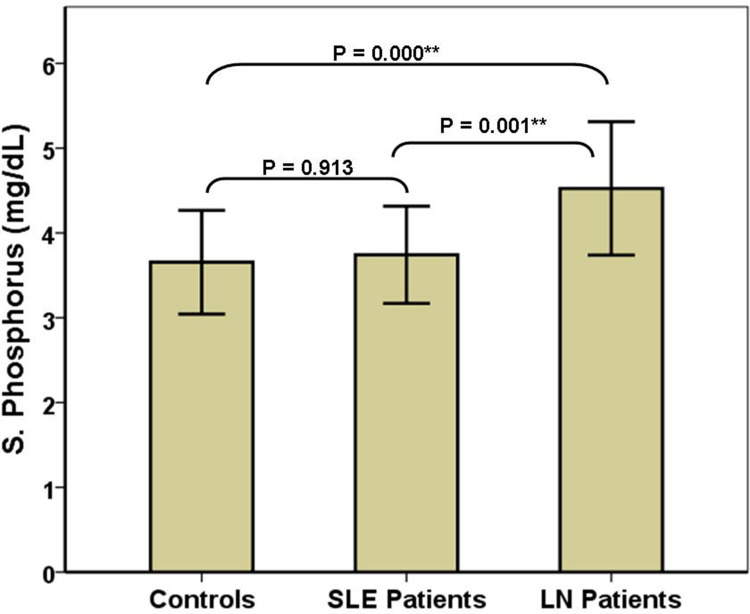
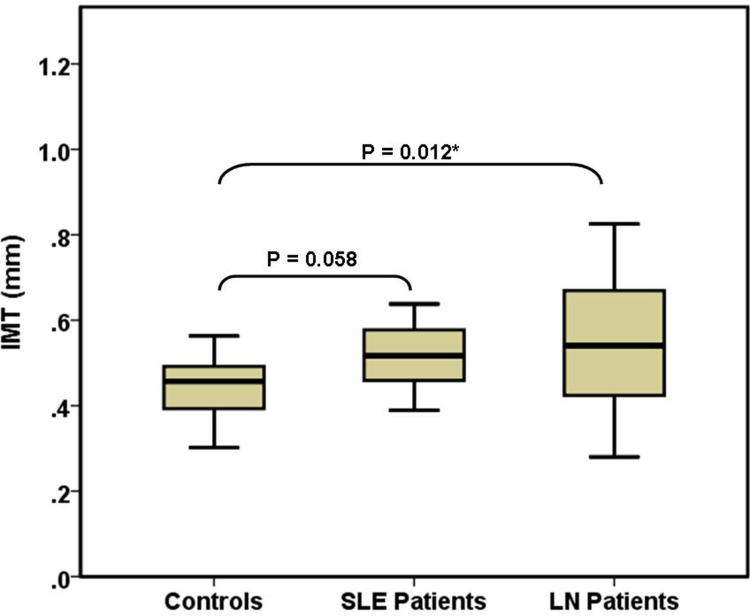
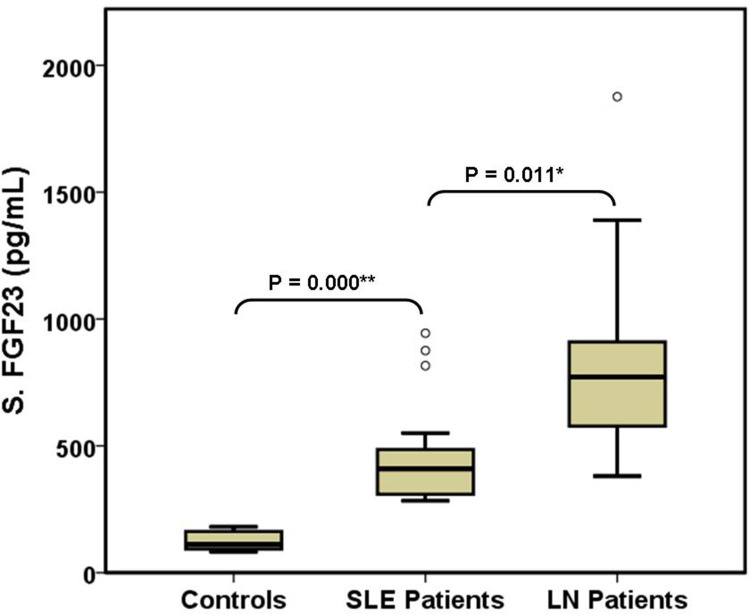
The three study groups were comparable regarding sex, age, frequency of hypertension and dyslipidaemia, SMART score, white blood cells, serum TGs, HDL, uric acid, and PS (Table 1). Compared with the other two groups, controls had significantly higher haemoglobin, serum C4 and vitamin D3, and significantly lower serum anti-DNA, CRP, and iPTH. SLE (G2) patients had significantly higher serum proteins and significantly lower platelets than the other two groups. LN patients had significantly higher proteinuria, serum phosphorus and ICRI and significantly lower serum calcium than the other two groups (Figure 2). Compared with controls, LN patients had significantly higher serum urea and CC-IMT (Figure 3). Compared with SLE (G2), LN patients had significantly higher serum LDL and creatinine and significantly lower eGFR and serum albumin. Serum C3 and iFGF23 showed significant changes between the 3 groups (Figure 4). Compared with SLE (G2), LN patients had significantly higher Cum_S and SLEDAI (Table 2).
Table 1.
Comparison Between the 3 Study Groups
| Parameter | Controls (Cont) (N = 20) | Lupus Only (SLE) (N = 20) | Lupus Nephritis (LN) (N = 28) | Statistical Test | P value (Post- hoc Differences) |
|---|---|---|---|---|---|
| Male | 3 (15%) | 2 (10%) | 4 (14.3%) | Fisher exact test | 0.57 |
| Female | 17 (85%) | 18 (90%) | 24 (85.7%) | ||
| Age (Years) | 35.56 ± 7.5 | 31.15 ± 7.8 | 36.11 ± 9.3 | ANOVA | 0.127 |
| HTN | 6 (30%) | 3 (15%) | 11 (39.3%) | Chi square | 0.19 |
| No HTN | 14 (70%) | 17 (85%) | 17 (60.7%) | ||
| DL | 8 (40%) | 6 (30%) | 15 (53.6%) | Chi square | 0.524 |
| No DL | 12 (60%) | 14 (70%) | 13 (46.4%) | ||
| SMART Score | 5 (4–6) | 4.5 (4–5) | 4 (4–6) | KWH | 0.743 |
| S. LDL (mg/dL) | 121.89 ± 21 | 108.6 ± 15.2 | 130.96 ± 33.1 | ANOVA | 0.016* (LN > SLE) |
| S. TGs (mg/dL) | 170.5 (151–188.3) | 160.5 (136–171.3) | 155 (120–198.3) | KWH | 0.311 |
| S. HDL (mg/dL) | 44 (41.3–49.8) | 44.5 (42.8–47) | 45 (41.8–49.3) | KWH | 0.828 |
| Haemoglobin (gm/dL) | 13.54 ± 0.9 | 9.87 ± 1.9 | 10.18 ± 1.4 | ANOVA | < 0.001** (Cont > Others) |
| WBCs (k/uL) | 7 (4.5–7.5) | 7.8 (3.7–9.5) | 6.9 (5.5–9.2) | KWH | 0.627 |
| Platelets (k/uL) | 288.5 (263.5–309) | 217 (192.3–273.5) | 297 (245–320.5) | KWH | 0.018* (SLE < Others) |
| S. Anti-DNA (IU/mL) | 37 (28.3–43.5) | 71 (48.8–102.8) | 135 (69.5–210.3) | KWH | < 0.001** (Cont < Others) |
| S. C3 (mg/dL) | 132 ± 29.7 | 93.85 ± 24.4 | 56.83 ± 26.4 | ANOVA | 0.001** (LN < SLE < Cont) |
| S. C4 (mg/dL) | 48.67 ± 9.3 | 21.33 ± 9.1 | 18.89 ± 9.9 | ANOVA | < 0.001** (Cont > Others) |
| S. CRP (mg/L) | 3.2 (2.4–4) | 5.5 (4–7) | 7 (5.8–8.3) | KWH | < 0.001** (Cont < Others) |
| S. Urea (mg/dL) | 23.5 (22–30) | 31 (27.8–44.5) | 48 (29.8–80.3) | KWH | < 0.001** (LN > Cont) |
| S. Creatinine (mg/dL) | 0.9 (0.8–1.1) | 0.8 (0.7–1) | 1.1 (0.9–1.6) | KWH | 0.011* (LN > SLE) |
| eGFR (mL/min/1.73) | 89.2 (66.9–103.3) | 99.6 (75.2–120.7) | 70.2 (44.7–95.8) | KWH | 0.006** (LN < SLE) |
| S. Uric Acid (mg/dL) | 5.2 (4.2–6.3) | 4.7 (4.3–5.4) | 5.7 (4.8–7.2) | KWH | 0.237 |
| Proteinuria (gm/day) | 0.24 (0.2–0.3) | 0.15 (0.1–0.3) | 2.5 (0.86–4.78) | KWH | < 0.001** (LN > Others) |
| S. Proteins (gm/dL) | 6.4 (5.8–7.2) | 7.3 (6.6–7.6) | 6.6 (6.2–7) | KWH | 0.007** (SLE > Others) |
| S. Albumin (gm/dL) | 3.4 (3.2–3.7) | 4 (3.4–4.2) | 3.2 (2.7–3.7) | KWH | < 0.001** (LN < SLE) |
| S. Calcium (mg/dL) | 10 (9.5–10.5) | 10.1 (9–10.6) | 8.8 (8.3–8.9) | KWH | < 0.001** (LN < Others) |
| S. Phosphorus (mg/dL) | 3.73 ± 0.6 | 3.74 ± 0.6 | 4.53 ± 0.8 | ANOVA | 0.001** (LN > Others) |
| S. Vitamin D3 (ng/mL) | 34.5 (29.6–40.8) | 12.5 (11–15.8) | 12 (10.9–13.1) | KWH | < 0.001** (Cont > Others) |
| S. iPTH (pg/mL) | 46.5 (32.5–51.8) | 107.5 (84–121.3) | 105 (92.3–120) | KWH | < 0.001** (Cont < Others) |
| S. iFGF23 (pg/mL) | 108.5 (92.4–152.2) | 409.5 (313–483.2) | 771.7 (579.2–900) | KWH | < 0.001** (LN > SLE > Cont) |
| PS | 0 (0–0) | 0 (0–1) | 0 (0–1) | KWH | 0.427 |
| CC-IMT (mm) | 0.47 (0.4–0.5) | 0.517 (0.464–0.577) | 0.541 (0.425–0.661) | KWH | 0.01* (LN > Cont) |
| ICRI | 0.61 (0.6–0.6) | 0.62 (0.58–0.63) | 0.66 (0.61–0.71) | KWH | 0.001** (LN > Others) |
Notes: Data are expressed as mean + SD or median (interquartile range), *Significant (P < 0.05), **Highly significant (P < 0.01).
Abbreviations: HTN, hypertension; DL, dyslipidemia; S, serum; LDL, low-density lipoproteins; TGs, triglycerides; HDL, high-density lipoproteins; WBCs, white blood cells; k, X1000; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; iPTH, intact parathyroid hormone; iFGF23, intact fibroblast growth factor-23; PS, Plaque score; CC-IMT, common carotid intima-media thickness; ICRI, internal carotid resistive index; ANOVA, analysis of variance; KWH, Kruskal–Wallis H-Test.
Figure 2.
Comparison of serum phosphorus in the 3 study groups. **Highly significant (P < 0.01).
Figure 3.
Comparison of common carotid intima-media thickness in the 3 study groups. *Significant (P < 0.05).
Figure 4.
Comparison of serum iFGF23 in the 3 study groups. *Significant (P < 0.05), **Highly significant (P < 0.01).
Table 2.
Comparison of Lupus-Related Parameters in SLE Patients with and without Lupus Nephritis (LN)
| Parameter | SLE (without LN) (N = 20) | LN (N = 28) | P (Mann Whitney U-Test) |
|---|---|---|---|
| Lupus Duration (Y) | 3 (2–4.3) | 3.5 (2.4–6) | 0.194 |
| Average Steroid Dose | 22.5 (18.8–30) | 30 (20–30) | 0.272 |
| Cumulative Steroid Dose | 60 (40–105) | 90 (60–165) | 0.049* |
| Steroid Pulses | 1 (0–1.3) | 1 (0–2) | 0.217 |
| SLEDAI Activity Index | 6.5 (4–9.3) | 10 (6–12) | 0.046* |
| SLICC Damage Index | 0 (0–1.3) | 1 (0–2) | 0.628 |
Notes: Average Steroid Dose: Expressed as average equivalent prednisone dose in mg/day. Cumulative Steroid Dose: Average steroid dose multiplied by disease duration in years. Steroid Pulses: Total number of pulses given throughout the disease history; a pulse is defined as an exceptionally high steroid dose given for 3–5 days. *Significant (P < 0.05).
Abbreviations: SLEDAI, systemic lupus erythematosus disease activity index; SLICC, SLE International Collaborating Clinics damage index.
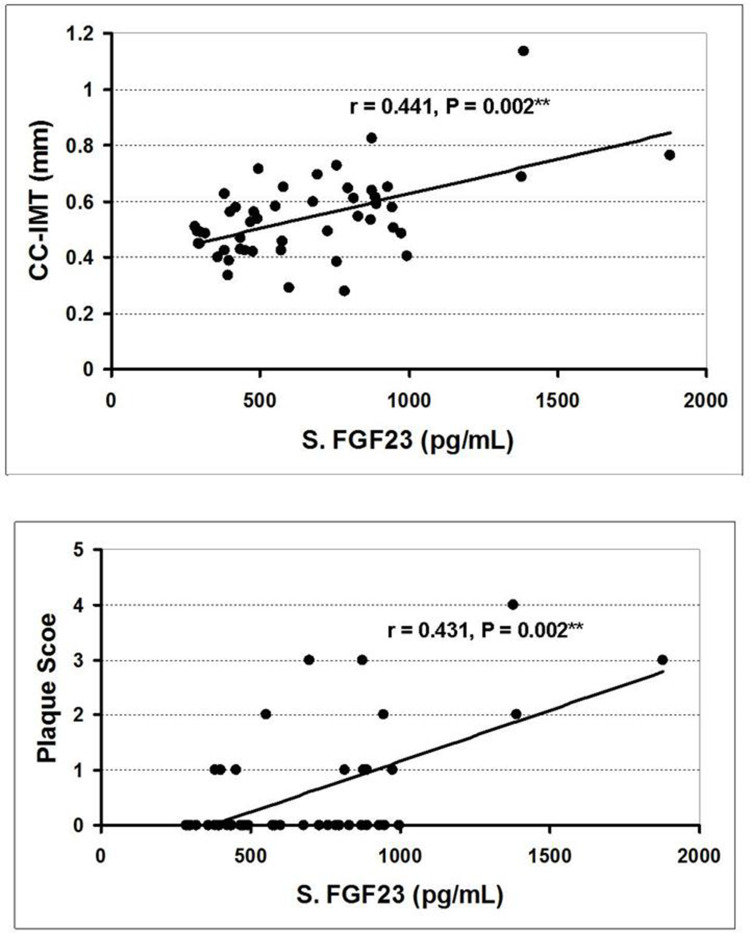
In both G2 and G3, serum iFGF23 had a statistically significant positive correlation with serum phosphorus, CC-IMT and PS; and these two US parameters significantly correlated with each other (Table 3, Figure 5). Serum iFGF23 had a statistically significant negative correlation with eGFR (in G2), and a statistically significant positive correlation with proteinuria (in G3). Serum phosphorus had a statistically significant positive correlation with CC-IMT and PS (in G2), and a statistically significant negative correlation with eGFR (in G3). Cum_S had a statistically significant positive correlation with CC-IMT in G2; this correlation was reversed and of weaker significance in G3.
Table 3.
Statistical Correlations Between Some Parameters in SLE Patients with and without Lupus Nephritis (LN)
| eGFR | Proteinuria | S. Phosphorus | S. iFGF23 | Cum_S | PS | CC-IMT | ||
|---|---|---|---|---|---|---|---|---|
| eGFR | r |
SLE LN |
− 0.334 | − 0.153 | − 0.446 | − 0.56 | − 0.245 | − 0.297 |
| P | 0.15 | 0.519 | 0.049* | 0.01* | 0.299 | 0.203 | ||
| Proteinuria | r | − 0.139 |
SLE LN |
− 0.184 | 0.244 | 0.479 | 0.245 | 0.152 |
| P | 0.48 | 0.437 | 0.301 | 0.033* | 0.297 | 0.523 | ||
| S. Phosphorus | r | −0.458 | 0.34 |
SLE LN |
0.513 | 0.295 | 0.552 | 0.539 |
| P | 0.014* | 0.076 | 0.021* | 0.207 | 0.012* | 0.014* | ||
| S. iFGF23 | r | − 0.31 | 0.479 | 0.383 |
SLE LN |
0.414 | 0.675 | 0.568 |
| P | 0.108 | 0.01* | 0.044* | 0.069 | 0.001** | 0.009** | ||
| Cum_S | r | − 0.234 | 0.087 | 0.115 | − 0.12 |
SLE LN |
0.263 | 0.689 |
| P | 0.23 | 0.659 | 0.56 | 0.543 | 0.263 | 0.001** | ||
| PS | r | 0.315 | 0.295 | − 0.054 | 0.431 | − 0.281 |
SLE LN |
0.485 |
| P | 0.102 | 0.128 | 0.784 | 0.022* | 0.147 | 0.03* | ||
| CC-IMT | r | 0.057 | 0.338 | − 0.06 | 0.422 | − 0.396 | 0.478 |
SLE LN |
| P | 0.774 | 0.078 | 0.76 | 0.025* | 0.037* | 0.01* |
Notes: *Significant (P < 0.05), **Highly significant (P < 0.01). Correlations in G2 (SLE without LN, N = 20) and G3 (LN, N = 28) are shown in right upper and left lower halves of the table, respectively.
Abbreviations: S, serum; eGFR, estimated glomerular filtration rate; iFGF23, intact fibroblast growth factor 23; Cum_S, cumulative steroid dose; PS, plaque score; CC-IMT, common carotid intima-media thickness.
Figure 5.
Correlation of serum iFGF23 with common carotid intima-media thickness (upper) and plaque score (lower) in SLE patients (groups 2 and 3 together). **Highly significant (P < 0.01).
In the regression analysis, the strongest predictor of increased CC-IMT was Cum_S in G2 and serum iFGF23 in G3. The strongest predictor of increased serum iFGF23 was hyperphosphatemia in G2 and proteinuria in G3 (Table 4).
Table 4.
Multivariate Linear Regression Analysis for Predictors of Increased CC-IMT and S. iFGF23 in SLE Patients with and without Lupus Nephritis (LN)
| A: Outcome: Increased CC-IMT | |||||
| G2: SLE Patients (N = 20) | G3: LN Patients (N = 28) | ||||
| Model R2 | 0.614 | Model R2 | 0.339 | ||
| Model Significance | 0.029* | Model Significance | 0.042* | ||
| Predictors | β Coefficient | Adjusted P | Predictors | β Coefficient | Adjusted P |
| Cum_S | 0.833 | 0.05* | S. iFGF23 | 0.442 | 0.09 |
| S. iFGF23 | 0.195 | 0.475 | Cum_S | − 0.053 | 0.792 |
| S. Phosphorus | 0.043 | 0.86 | Dyslipidaemia | 0.163 | 0.43 |
| Haemoglobin | − 0.420 | 0.089 | Proteinuria | 0.099 | 0.64 |
| Dyslipidaemia | − 0.364 | 0.214 | |||
| Age | − 0.308 | 0.292 | |||
| *Significant (P < 0.05). | |||||
| B: Outcome: Increased S. iFGF23 | |||||
| G2: SLE Patients (N = 20) | G3: LN Patients (N = 28) | ||||
| Model R2 | 0.611 | Model R2 | 0.463 | ||
| Model Significance | 0.005** | Model Significance | 0.028* | ||
| Predictors | β Coefficient | Adjusted P | Predictors | β Coefficient | Adjusted P |
| S. LDL | 0.072 | 0.73 | SMART Score | 0.233 | 0.277 |
| S. Phosphorus | 0.397 | 0.049* | Dyslipidaemia | 0.231 | 0.209 |
| S. Creatinine | 0.276 | 0.197 | Proteinuria | 0.466 | 0.026* |
| Cum_S | 0.314 | 0.121 | S. Creatinine | − 0.046 | 0.828 |
| Av_S | 0.059 | 0.762 | |||
| S. Phosphorus | 0.011 | 0.954 | |||
| *Significant (P < 0.05), **Highly significant (P < 0.01). | |||||
Abbreviations: CC-IMT, common carotid intima-media thickness; Cum_S, cumulative steroid dose; S, serum; iFGF23, intact fibroblast growth factor 23; LDL, low-density lipoproteins; Av_S, average steroid dose.
Discussion
This study confirms previous reports that serum FGF23 is elevated in SLE patients (more so in LN),32,33 and generates a conceptual framework for its atherogenic role in these patients. We excluded subjects with exaggerated ACVD risk profile because of diabetes mellitus, obesity, advanced renal disease or age. Controls (G1) were individually selected to match with lupus patients (G2,3) in the traditional ACVD risk profile, so that inter-group differences are largely determined by the lupus disease and related factors. We used CKD-EPI equation for eGFR calculation as it performed best in SLE patients, compared with other creatinine-based formulas.52 Subclinical atherosclerosis was evaluated in the carotid arteries by 3 integrative US measures, of proven benefit for quantitation of atherosclerosis extent,53 and progression,54,55 as well as prediction of future CVD events in SLE patients.56 IMT is a sensitive early marker of generalised atherosclerosis.57 Its measurement at the distal common carotid artery offers easier accessibility and higher accuracy and reproducibility, over other carotid segments.58 However, IMT may increase because of media thickening as a function of age and increased blood pressure, rather than atherosclerosis.59 Therefore, the measurement of established focal atherosclerotic protuberances by PS was intended to further improve the quantitation of subclinical atherosclerosis and CVD risk.60 The simplified PS score we adopted may be less prone to assessment errors, and has repeatedly proved useful for quantification of carotid atherosclerosis in SLE patients and assessment of its progression.54,61 Carotid US included a measurement of ICRI, a potentially more sensitive measure for subtle early functional vascular alterations,62 with comparable cardiovascular morbimortality predictor power to the standard IMT.51
Among these predominantly middle-aged females with relatively low traditional ACVD risk profile, both CC-IMT and ICRI showed a graded rise along the 3 study groups (LN > SLE > Controls). LN patients had significantly higher CC-IMT compared with controls, and significantly higher ICRI compared with the other 2 groups. These results conform with the previous reports that SLE patients suffer an increased burden of subclinical atherosclerosis3,5,7,13 and that this burden is much significantly amplified and to a large extent determined, by the presence of LN.63–66 Contrary to some previous studies,67,68 we did not find significant differences between the study groups regarding PS, which may be explained by the relatively young age, low overall ACVD risk and low plaque occurrence, in the study subjects. Serum iFGF23 varied the same way as CC-IMT and ICRI (LN > SLE > Controls), with significant differences between the 3 study groups, also in line with the few previous reports.32,33
Our study revealed multiple possible correlates for the increased ACVD risk in SLE patients. Some correlates encompassed all SLE patients (G 2,3); and some others were particularly distinguished among LN patients. Correlates common for all SLE patients were mainly indicative of the overwhelming immunologically driven, chronic inflammatory nature of the disease; and have been reported by previous studies, like increased serum anti-DNA,69 and CRP,70 and depressed serum C3 and C4.63 Clearly, the lupus-related parameters included in Table 2 also tackle SLE patients as whole; and may constitute an indirect measure of the immune-inflammatory disease pathogenesis, which is thought to progress in parallel with atherogenesis.71 Hypovitaminosis D was also common to all SLE (G2,3) patients, a nearly universal finding in previous studies, that is mainly ascribed to insufficient sun exposure and drug-induced accelerated vitamin D catabolism.12,72–74 Somewhat unexpectedly, PTH was significantly increased in all SLE patients (including those without LN). Increased circulating PTH and its association with ACVD in SLE patients was only recently reported,13 and may be the result of concomitant hypovitaminosis D.75,76
As shown in Table 1, LN patients have, in comparison with other SLE patients, the added risks of proteinuria, renal impairment, hyperphosphatemia and a higher FGF23 elevation. In community studies, both proteinuria,77–80 and GFR decline,81–84 have significant, additive, dose-dependent associations with subclinical atherosclerosis burden and CVD events. These associations were firmly reproduced in studies of LN patients.63–66 Clearly, the development of LN engenders a more aggressive lupus phenotype, evidenced in the present cohort by having significantly lower serum C3, and significantly higher LDL, SLEDAI, and Cum_S, compared with SLE (G2) patients.
An intriguing finding is that Cum_S had a statistically significant positive correlation with CC-IMT in G2 (SLE only) that remained marginally significant (P = 0.05) in the adjusted model, whereas this correlation was reversed (still significant but somewhat weaker) in G3 (LN patients). Corticosteroids in SLE represent a double-edged sword.85 It is possible that their pro- atherogenic effects (insulin resistance, dyslipidaemia, obesity, and hypertension) dominated in the absence of renal disease.5 LN, as a hallmark of more aggressive disease, determined a greater need for the immunosuppressive and anti-inflammatory steroid actions, imparting a better benefit/risk ratio and a net anti-atherogenic action.86 Possibly also the steroid dose was not precisely tailored for the different patient needs, being relatively overdosed in absence, and underdosed in presence, of LN; or there may be endogenous individual variations that determine the patient`s response to these medications.85
The FGF23/klotho axis is the principal regulator of phosphorus metabolism.87 Tight regulation of serum phosphorus is essential since hyperphosphatemia, and even minor elevations of serum phosphorus within the normal range, have been increasingly linked in community studies with impaired vasoreactivity,88 subclinical atherosclerosis,89,90 vascular calcification,91 cardiovascular events,92,93 and mortality.94 Similar strong associations were also found in CKD patients.95–97 Growing evidence for cardiovascular phosphorus toxicity led some authors suggest it the “new cholesterol”.98 Surprisingly, serum phosphorus was rarely reported in SLE patients. Similar to the present study, one study reported serum phosphorus to be significantly higher in LN patients compared with lupus patients without nephritis and controls.99 There is evidence that increased serum phosphorus in LN patients would augment further systemic inflammation, vascular calcification and faster ACVD.100–103 Another study found serum phosphorus to be marginally elevated in SLE patients with atheromatous plaques or increased arterial IMT compared with patients lacking these signs, but the differences were insignificant.13 In the present study, serum phosphorus in G2 (SLE only) had a significant correlation with CC-IMT and PS, although it was within normal range (< 4.5 mg/dL) in all but one patient. A Finnish population study found a significant direct correlation between dietary phosphorus intake and CC-IMT.90 Unawareness about excessive dietary phosphorus consumption is a recently recognised global health problem,104 particularly afflicting less-privileged societies habitually ingesting highly absorbable phosphorus-rich food additives.105
Normally, increased secretion of the counter-regulatory phosphaturic hormone, FGF23, occurs in a robust response to phosphorus loading.15,106 This adaptive FGF23 rise (typically not exceeding 200 pg/mL), utilizing klotho-dependent pathways, generates a controlled phosphaturic response destined to prevent hyperphosphatemia and subsequent perturbations of the bone-vascular axis (bone disease, atherosclerosis, and vascular calcification). A maladaptive FGF23 surge, largely signalling through klotho-independent pathways, approaches much higher values but fails to prevent hyperphosphatemia and rather produces off target effects like hypovitaminosis D, bone rarefaction, ACVD, and myocardial hypertrophy.107,108 A full-blown picture of this maladaptive FGF23 increase occurs in CKD, when klotho deficiency, low GFR, and hypovitaminosis D generate a state of progressive FGF23 resistance;109 leading in advanced CKD to a marked rise of both serum phosphorus and FGF23 in a characteristic strong direct correlation.110,111 A hallmark for the disturbed FGF23-phosphorus axis in SLE patients (G2 and 3) in the present study was the rise of both biomarkers and the presence of a significant direct correlation between them. The maladaptive FGF23 rise was not restricted to LN patients; it involved SLE patients without evidence of renal disease as well. Indeed, G2 patients (SLE only) had a significantly increased serum iFGF23 over controls (exceeding the typical level of adaptive increase and matching with a previous study),32 and an even stronger direct correlation between serum iFGF23 and phosphorus compared to this correlation in LN patients. In multivariate analysis, serum phosphorus persisted in G2 as the most (and only) significant independent predictor of increased serum iFGF23. We thus infer that the maladaptive FGF23 hypersecretion can occur in SLE patients as an effect of the lupus phenotype itself, independently of renal disease. At least two salient, renal function independent, features of SLE can synergistically reduce klotho gene expression and induce significant FGF23 resistance and hypersecretion, namely, chronic inflammation,27,34,112,113 and hypovitaminosis D.114,115 The latter might also result in increased serum PTH which provides another stimulus for FGF23 hypersecretion,116 a possibility supported by the finding of increased serum PTH in SLE patients in the present study and a previous one.13 The issue of FGF23 resistance in SLE requires a separate study.
Previous studies built a consensus that ACVD in SLE mainly (but by no means exclusively) develops in conjunction with LN, with or without renal impairment.63–66 Consistent with this, we found the median CC-IMT in SLE patients, with or without LN, increased over controls; but the difference was statistically significant only with LN patients. Furthermore, we found a significantly increased ICRI in LN patients over the other two groups, denoting a more generalized vascular pathology conductive for atherogenesis in LN patients.62 Serum iFGF23 had a statistically significant positive correlation with CC-IMT and PS, both in G2 and G3 (Table 3), and a similar correlation with ICRI in G2 (not shown). Regression analysis in LN patients revealed that the strongest independent predictor of increased CC-IMT was the increased serum iFGF23; and that proteinuria was the strongest (and the only significant) independent predictor of increased serum iFGF23 in these patients. Therefore, increased FGF23 may, at least in part, explain the strong association between proteinuria and ACVD observed in LN patients;63–66 an association that has been reported as well in type 2 diabetic patients,117 and even in the healthy population.118 The explanation for FGF23 rise in proteinuric nephropathies was based on results of the KNOW-CKD119 and subsequent studies.120,121 Proteinuria decreases the biologic activity of FGF23 on renal tubules, independent of renal function.120 Albuminuria induces a state endoplasmic reticulum stress in renal tubular cells, leading to downregulation of klotho production.122 This leads to FGF23 hypersecretion that fails to correct hyperphosphatemia (more so if renal function is impaired as well). In these cases, an enormous ACVD risk burden would result from the combined effects of increased FGF23, hyperphosphatemia, hypovitaminosis D, and renal impairment.123 Identification of this multifactorial model, with the focal role of the proteinuria-hyperphosphatemia axis, is an important addition to our understanding of the ACVD in SLE patients.
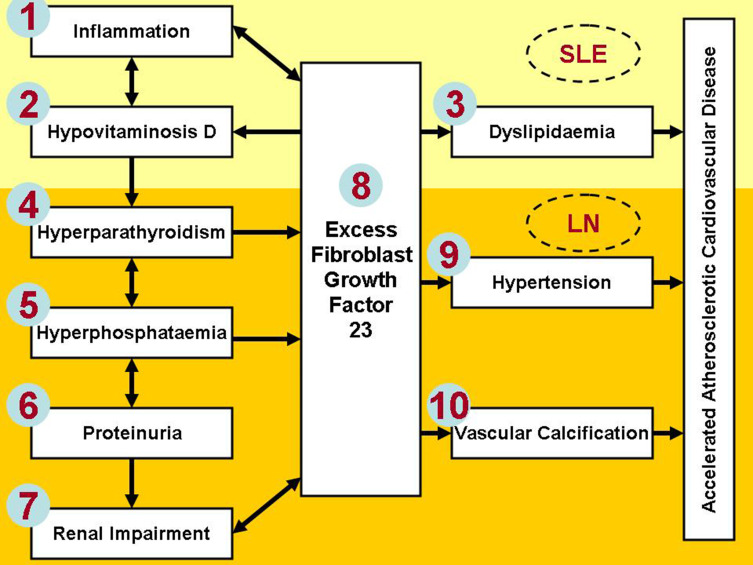
Based on the present work and current knowledge, we constructed a simplified conceptual framework (Figure 6) for the role of FGF23 in the pathogenesis of ACVD in SLE patients. Although not all inclusive, the factors numbered one through ten may be regarded as the key players in this paradigm. Factors in the upper (yellow) pane are nearly always present in all SLE patients since the disease beginning. Inflammation is meant to denote the SLE disease process itself, as well as markers of disease activity and treatment; which continue to have a significant impact on ACVD risk and long-term outcome.3,5,6 Factors in the lower (gold) pane occur mainly or exclusively in patients with LN, when all risk factors become significantly amplified. FGF23 lies at the focal point of intersection of several self-perpetuating (vicious) cycles. For example, increased circulating FGF23 further exaggerates hypovitaminosis D, which is present in most SLE patients.28,72–74 Hypovitaminosis D exaggerates inflammation12,124 and hyperparathyroidism75,76 which independently complete two vicious circles for FGF23 hypersecretion. We can likewise describe another vicious circle in which proteinuria causes hyperphosphatemia and FGF23 hypersecretion;120,121 then FGF23 fails to correct hyperphosphatemia which even continues to progress causing further renal damage,14,30,31 and resistance to the renoprotective effects of angiotensin converting enzyme inhibitors,125 and low protein diet.126 Unless one or more of these vicious circles is interrupted, FGF23 levels would continue to rise inexorably with increased burden of ACVD and further CKD progression. We propose that breaking these cycles to control circulating FGF23 level (for example by controlling hyperphosphatemia and proteinuria) might provide novel approaches for reduction of ACVD risk in SLE patients. It should be noticed, however, that directly targeting the FGF23 itself by neutralizing antibodies in a rat model of CKD-MBD led to a dose-dependent increase in serum phosphorus, aortic calcification, and mortality.127 Therefore, well-designed prospective studies shall define the optimal circulating FGF23 range offering the best compromise between adaptive and maladaptive effects and resulting in the best measures of long-term outcome.
Figure 6.
Simplified schema for the role of FGF23 in atherogenesis in SLE patients. Factors in the upper (yellow) part are typical early findings in all (inflammation) or a large proportion of (hypovitaminosis D and dyslipidaemia) SLE patients, whereas the other factors (lower gold part) are either dependent on (proteinuria and renal impairment), or largely determined by, the presence and severity of LN.
To the best of our knowledge, this is the first study to explore the role of FGF23 and related parameters in subclinical atherosclerosis in SLE patients with or without LN. We acknowledge the limitations of the relatively small sample size and the cross-sectional design of the study; so that a cause-effect relationship between the study parameters cannot be readily inferred. The simplified PS we adopted does not account for the multiplicity of plaques in one arterial segment or variations in their area and size, as offered by more elaborate scores.49 Subclinical atherosclerosis assessed in only one vascular bed might not sufficiently reflect its generalized burden. Better exploration of FGF23 actions would have required assessment of dietary phosphorus intake, urinary phosphorus excretion and circulating klotho level.
Conclusion
The FGF23-phosphate axis has a key role in accelerated ACVD in SLE patients. A maladaptive FGF23 hypersecretion coupled with renal tubular resistance to its phosphaturic action operates several self-perpetuating cycles leading to progressive hyperphosphatemia, hyperparathyroidism, hypovitaminosis D and ACVD. Most significant independent predictors of FGF23 hypersecretion were hyperphosphatemia and proteinuria, in SLE patients without and with LN, respectively. Serum phosphorus and iFGF23 should be included in the ACVD risk profile assessment of SLE patients. Dietary and/or pharmacologic control of hyperphosphatemia and proteinuria can be feasible therapeutic targets to reduce circulating FGF23 and ACVD burden in SLE patients. Large-scale prospective studies are needed to define the circulating FGF23 target range in SLE/LN patients providing the best compromise between its adaptive and maladaptive effects, and to assess the effects of reduction of serum phosphorus, iFGF23 and proteinuria on ACVD progression and CVD events in SLE/LN patients. Dietary vigilance for phosphorus-rich food additives may be a readily feasible intervention that can be broadly promulgated in less-privileged communities.
Acknowledgments
This study has been presented in an abstract form in the 56th ERA-EDTA Congress (June 13–16, 2019, Budapest, Hungary) and published in Nephrology Dialysis Transplantation, Volume 34, Issue Supplement_1, June 2019, gfz106.FP220, https://doi.org/10.1093/ndt/gfz106.FP220.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rees F, Doherty M, Grainge MJ, Lanyon P, Zhang W. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology. 2017;56(11):1945–1961. doi: 10.1093/rheumatology/kex260 [DOI] [PubMed] [Google Scholar]
- 2.Urowitz MB, Gladman DD, Tom BD, Ibanez D, Farewell VT. Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol. 2008;35(11):2152–2158. doi: 10.3899/jrheum.080214 [DOI] [PubMed] [Google Scholar]
- 3.Appleton BD, Major AS. The latest in systemic lupus erythematosus-accelerated atherosclerosis: related mechanisms inform assessment and therapy. Curr Opin Rheumatol. 2021;33(2):211–218. doi: 10.1097/BOR.0000000000000773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2331–2337. doi: [DOI] [PubMed] [Google Scholar]
- 5.Doria A, Shoenfeld Y, Wu R, et al. Risk factors for subclinical atherosclerosis in a prospective cohort of patients with systemic lupus erythematosus. Ann Rheum Dis. 2003;62(11):1071–1077. doi: 10.1136/ard.62.11.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards N, Langford-Smith AW, Parker BJ, et al. QRISK3 improves detection of cardiovascular disease risk in patients with systemic lupus erythematosus. Lupus Sci Med. 2018;5(1):e000272. doi: 10.1136/lupus-2018-000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teixeira V, Tam L-S. Novel insights in systemic lupus erythematosus and atherosclerosis. Front Med. 2018;4:262. doi: 10.3389/fmed.2017.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rho YH, Chung CP, Oeser A, et al. Novel cardiovascular risk factors in premature coronary atherosclerosis associated with systemic lupus erythematosus. J Rheumato. 2008;35(9):1789–1794. [PMC free article] [PubMed] [Google Scholar]
- 9.den Uyl D, Nurmohamed MT, van Tuyl LH, Raterman HG, Lems WF. (Sub) clinical cardiovascular disease is associated with increased bone loss and fracture risk; a systematic review of the association between cardiovascular disease and osteoporosis. Arthritis Res Ther. 2011;13(1):1–19. doi: 10.1186/ar3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ajeganova S, Gustafsson T, Jogestrand T, Frostegård J, Hafström I. Bone mineral density and carotid atherosclerosis in systemic lupus erythematosus: a controlled cross-sectional study. Arthritis Res Ther. 2015;17(1):1–13. doi: 10.1186/s13075-015-0595-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bultink IE. Bone disease in connective tissue disease/systemic lupus erythematosus. Calcif Tissue Int. 2018;102(5):575–591. doi: 10.1007/s00223-017-0322-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiemstra TF, Lim K, Thadhani R, Manson JE. Vitamin D and atherosclerotic cardiovascular disease. J Clin Endocrinol Metab. 2019;104(9):4033–4050. doi: 10.1210/jc.2019-00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giannelou M, Skarlis C, Stamouli A, Antypa E, Moutsopoulos HM, Mavragani CP. Atherosclerosis in SLE: a potential role for serum parathormone levels. Lupus Sci Med. 2020;7(1):e000393. doi: 10.1136/lupus-2020-000393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205(2):385–390. doi: 10.1016/j.atherosclerosis.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 15.Kocełak P, Olszanecka-Glinianowicz M, Chudek J. Fibroblast growth factor 23–structure, function and role in kidney diseases. Adv Clin Exp Med. 2012;21(3):391–401. [PubMed] [Google Scholar]
- 16.Erben RG. Update on FGF23 and Klotho signaling. Mol Cell Endocrinol. 2016;432:56–65. doi: 10.1016/j.mce.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 17.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–1378. doi: 10.1038/ki.2011.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chathoth S, Al-Mueilo S, Cyrus C, et al. Elevated fibroblast growth factor 23 concentration: prediction of mortality among chronic kidney disease patients. Cardiorenal Med. 2016;6(1):73–82. doi: 10.1159/000440984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodríguez-Ortiz ME, Alcalá-Díaz JF, Canalejo A, et al. Fibroblast growth factor 23 predicts carotid atherosclerosis in individuals without kidney disease. The CORDIOPREV study. Europ J Int Med. 2020;74:79–85. doi: 10.1016/j.ejim.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 20.Shah NH, Dong C, Elkind MS, et al. Fibroblast growth factor 23 is associated with carotid plaque presence and area: the Northern Manhattan Study. Arterioscler Thromb Vasc Biol. 2015;35(9):2048–2053. doi: 10.1161/ATVBAHA.115.305945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yilmaz MI, Sonmez A, Saglam M, et al. FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int. 2010;78(7):679–685. doi: 10.1038/ki.2010.194 [DOI] [PubMed] [Google Scholar]
- 22.Yilmaz G, Ustundag S, Temizoz O, et al. Fibroblast growth factor-23 and carotid artery intima media thickness in chronic kidney disease. Clin Lab. 2015;61(8):1061–1070. [DOI] [PubMed] [Google Scholar]
- 23.Hu X, Ma X, Luo Y, et al. Contribution of fibroblast growth factor 23 to Framingham risk score for identifying subclinical atherosclerosis in Chinese men. Nutr Metab Cardiovasc Dis. 2017;27(2):147–153. doi: 10.1016/j.numecd.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 24.Fyfe-johsnon AL, Alonso A, Selvin E, et al. Serum fibroblast growth factor-23 and incident hypertension: the atherosclerosis risk in communities study. J Hypertens. 2016;34(7):1266–1272. doi: 10.1097/HJH.0000000000000936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirza MA, Alsiö J, Hammarstedt A, et al. Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler Thromb Vasc Biol. 2011;31(1):219–227. doi: 10.1161/ATVBAHA.110.214619 [DOI] [PubMed] [Google Scholar]
- 26.Hanks LJ, Casazza K, Judd SE, Jenny NS, Gutiérrez OM. Associations of fibroblast growth factor-23 with markers of inflammation, insulin resistance and obesity in adults. PLoS One. 2015;10(3):e0122885. doi: 10.1371/journal.pone.0122885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharaf El Din U, Salem MM, Abdulazim DO. FGF23 and inflammation. World J Nephrol. 2017;6(1):57–58. doi: 10.5527/wjn.v6.i1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prié D, Friedlander G. Reciprocal control of 1, 25-dihydroxyvitamin D and FGF23 formation involving the FGF23/Klotho system. Clin J Am Soc Nephrol. 2010;5(9):1717–1722. doi: 10.2215/CJN.02680310 [DOI] [PubMed] [Google Scholar]
- 29.Donate-Correa J, Martín-Núñez E, Hernández-Carballo C, et al. Fibroblast growth factor 23 expression in human calcified vascular tissues. Aging. 2019;11(18):7899–7913. doi: 10.18632/aging.102297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Jong MA, Eisenga MF, van Ballegooijen AJ, et al. Fibroblast growth factor 23 and new-onset chronic kidney disease in the general population: the Prevention of Renal and Vascular Endstage Disease (PREVEND) study. Nephrol Dial Transplant. 2021;36(1):121–128. doi: 10.1093/ndt/gfz266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18(9):2600–2608. doi: 10.1681/ASN.2006080936 [DOI] [PubMed] [Google Scholar]
- 32.Falcini F, Masi L, Capannini S, et al. Fibroblast growth factor (FGF23) serum levels in juvenile onset systemic lupus erythematosus (JSLE): a possible link with renal involvement. Arthritis Rheum. 2010;2:62. [Google Scholar]
- 33.Resende AL, Elias RM, Wolf M, et al. Serum levels of fibroblast growth factor 23 are elevated in patients with active lupus nephritis. Cytokine. 2017;91:124–127. doi: 10.1016/j.cyto.2016.12.022 [DOI] [PubMed] [Google Scholar]
- 34.Zhou M, Li S, Pathak JL. Pro-inflammatory cytokines and osteocytes. Curr Osteoporos Rep. 2019;17(3):97–104. doi: 10.1007/s11914-019-00507-z [DOI] [PubMed] [Google Scholar]
- 35.Rhee Y, Bivi N, Farrow E, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49(4):636–643. doi: 10.1016/j.bone.2011.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burnett SAM, Gunawardene SC, Bringhurst FR, Jüppner H, Lee H, Finkelstein JS. Regulation of C‐terminal and intact FGF‐23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21(8):1187–1196. doi: 10.1359/jbmr.060507 [DOI] [PubMed] [Google Scholar]
- 37.van Ballegooijen AJ, Rhee EP, Elmariah S, de Boer IH, Kestenbaum B. Renal clearance of mineral metabolism biomarkers. J Am Soc Nephrol. 2016;27(2):392–397. doi: 10.1681/ASN.2014121253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu X, Hu MC. Klotho/FGF23 axis in chronic kidney disease and cardiovascular disease. Kidney Dis. 2017;3(1):15–23. doi: 10.1159/000452880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirchler C, Husar-Memmer E, Rappersberger K, Thaler K, Fritsch-Stork R. Type I Interferon as cardiovascular risk factor in systemic and cutaneous lupus erythematosus: a systematic review. Autoimmun Rev. 2021;102794. doi: 10.1016/j.autrev.2021.102794 [DOI] [PubMed] [Google Scholar]
- 40.Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hahn BH, Mcmahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. 2012;64(6):797–808. doi: 10.1002/acr.21664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum. 1992;35(6):630–640. doi: 10.1002/art.1780350606 [DOI] [PubMed] [Google Scholar]
- 43.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–369. doi: 10.1002/art.1780390303 [DOI] [PubMed] [Google Scholar]
- 44.Simons PC, Algra A, Bots ML, Grobbee DE, van der Graaf Y. Common carotid intima-media thickness and arterial stiffness: indicators of cardiovascular risk in high-risk patients the SMART study (Second Manifestations of ARTerial Disease). Circulation. 1999;100(9):951–957. doi: 10.1161/01.CIR.100.9.951 [DOI] [PubMed] [Google Scholar]
- 45.Uthoff H, Staub D, Socrates T, et al. PROCAM-, FRAMINGHAM-, SCORE-and SMART-risk score for predicting cardiovascular morbidity and mortality in patients with overt atherosclerosis. Vasa. 2010;39(4):325–333. doi: 10.1024/0301-1526/a000057 [DOI] [PubMed] [Google Scholar]
- 46.Burtis CA, Ashwood ER, Bruns DE. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics-e-Book. Elsevier Health Sciences; 2012. [Google Scholar]
- 47.Levey AS, Stevens LA. Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622. doi: 10.1053/j.ajkd.2010.02.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21(2):93–111. [DOI] [PubMed] [Google Scholar]
- 49.Johri AM, Nambi V, Naqvi TZ, et al. Recommendations for the assessment of carotid arterial plaque by ultrasound for the characterization of atherosclerosis and evaluation of cardiovascular risk: from the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33(8):917–933. doi: 10.1016/j.echo.2020.04.021 [DOI] [PubMed] [Google Scholar]
- 50.Rivera LMS, Ríos LV, Díaz CH, Villaseñor CP. Usefulness of measuring common carotid intima-media thickness: ultrasound diagnosis of sub-clinical atherosclerosis in rheumatic diseases. A literature review. Rev Colomb Reumatol. 2016;23(2):92–101. [Google Scholar]
- 51.Staub D, Meyerhans A, Bundi B, Schmid HP, Frauchiger B. Prediction of cardiovascular morbidity and mortality: comparison of the internal carotid artery resistive index with the common carotid artery intima-media thickness. Stroke. 2006;37(3):800–805. doi: 10.1161/01.STR.0000202589.47401.c6 [DOI] [PubMed] [Google Scholar]
- 52.Martínez-Martínez MU, Martínez-Martínez MU, Mandeville P, Llamazares-Azuara L, Abud-Mendoza C. CKD-EPI is the most reliable equation to estimate renal function in patients with systemic lupus erythematosus. Nefrología. 2013;33(1):99–106. [DOI] [PubMed] [Google Scholar]
- 53.Belibou C, Ancuţa C, Ancuţa E, Filoş C, Chirieac R. Carotid intima-media thickness and plaque as surrogate biomarkers of atherosclerosis among consecutive women with systemic lupus erythematosus. Rom J Morphol Embryol. 2012;53(1):29–34. [PubMed] [Google Scholar]
- 54.Telles RW, Lanna CC, Sousa AJ, et al. Progression of carotid atherosclerosis in patients with systemic lupus erythematosus. Clin Rheumatol. 2013;32(9):1293–1300. doi: 10.1007/s10067-013-2264-9 [DOI] [PubMed] [Google Scholar]
- 55.Ajeganova S, Gustafsson T, Lindberg L, Hafström I, Frostegård J. Similar progression of carotid intima–media thickness in 7-year surveillance of patients with mild SLE and controls, but this progression is still promoted by dyslipidaemia, lower HDL levels, hypertension, history of lupus nephritis and a higher prednisolone usage in patients. Lupus Sci Med. 2020;7(1):e000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kao AH, Lertratanakul A, Elliott JR, et al. Relation of carotid intima-media thickness and plaque with incident cardiovascular events in women with systemic lupus erythematosus. Am J Cardiol. 2013;112(7):1025–1032. doi: 10.1016/j.amjcard.2013.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salonen JT, Salonen R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation. 1993;87(3 Suppl):II56–65. [PubMed] [Google Scholar]
- 58.Touboul P, Hennerici M, Meairs S, Adams H, Amarenco P, Bornstein N. Mannheim carotid intima-media thickness consensus (2004–2006). Cerebrovasc Dis. 2007;23(1):75–80. doi: 10.1159/000097034 [DOI] [PubMed] [Google Scholar]
- 59.Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol. 2010;30(2):177–181. doi: 10.1161/ATVBAHA.108.173609 [DOI] [PubMed] [Google Scholar]
- 60.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220(1):128–133. doi: 10.1016/j.atherosclerosis.2011.06.044 [DOI] [PubMed] [Google Scholar]
- 61.Roman MJ, Crow MK, Lockshin MD, et al. Rate and determinants of progression of atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2007;56(10):3412–3419. doi: 10.1002/art.22924 [DOI] [PubMed] [Google Scholar]
- 62.Frauchiger B, Schmid HP, Roedel C, Moosmann P, Staub D. Comparison of carotid arterial resistive indices with intima-media thickness as sonographic markers of atherosclerosis. Stroke. 2001;32(4):836–841. doi: 10.1161/01.STR.32.4.836 [DOI] [PubMed] [Google Scholar]
- 63.Gustafsson JT, Herlitz Lindberg M, Gunnarsson I, et al. Excess atherosclerosis in systemic lupus erythematosus,—a matter of renal involvement: case control study of 281 SLE patients and 281 individually matched population controls. PLoS One. 2017;12(4):e0174572. doi: 10.1371/journal.pone.0174572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hermansen M-L, Lindhardsen J, Torp-Pedersen C, Faurschou M, Jacobsen S. The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatology. 2017;56(5):709–715. [DOI] [PubMed] [Google Scholar]
- 65.Hermansen M-L, Sandholt B, Fuchs A, et al. Atherosclerosis and renal disease involvement in patients with systemic lupus erythematosus: a cross-sectional cohort study. Rheumatology. 2018;57(11):1964–1971. doi: 10.1093/rheumatology/key201 [DOI] [PubMed] [Google Scholar]
- 66.Falaschi F, Ravelli A, Martignoni A, et al. Nephrotic‐range proteinuria, the major risk factor for early atherosclerosis in juvenile‐onset systemic lupus erythematosus. Arthritis Rheum. 2000;43(6):1405–1409. doi: [DOI] [PubMed] [Google Scholar]
- 67.Souza A, Hatta FS, Miranda JF, Sato EI. Atherosclerotic plaque in carotid arteries in systemic lupus erythematosus: frequency and associated risk factors. Sao Paulo Med J. 2005;123(3):137–142. doi: 10.1590/S1516-31802005000300010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmad Y, Shelmerdine J, Bodill H, et al. Subclinical atherosclerosis in systemic lupus erythematosus (SLE): the relative contribution of classic risk factors and the lupus phenotype. Rheumatology. 2007;46(6):983–988. doi: 10.1093/rheumatology/kem002 [DOI] [PubMed] [Google Scholar]
- 69.Skaggs BJ, Hahn BH, McMahon M. Accelerated atherosclerosis in patients with SLE—mechanisms and management. Nat Rev Rheumatol. 2012;8(4):214–223. doi: 10.1038/nrrheum.2012.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Neill SG, Isenberg DA, Rahman A. Could antibodies to C-reactive protein link inflammation and cardiovascular disease in patients with systemic lupus erythematosus? Ann Rheum Dis. 2007;66(8):989–991. doi: 10.1136/ard.2007.073312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manzi S. Systemic lupus erythematosus: a model for atherogenesis? Rheumatology. 2000;39(4):353–359. doi: 10.1093/rheumatology/39.4.353 [DOI] [PubMed] [Google Scholar]
- 72.Kamen DL, Cooper GS, Bouali H, Shaftman SR, Hollis BW, Gilkeson GS. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev. 2006;5(2):114–117. doi: 10.1016/j.autrev.2005.05.009 [DOI] [PubMed] [Google Scholar]
- 73.Kamen DL. Vitamin D in lupus: new kid on the block? Bull NYU Hosp Jt Dis. 2010;68(3):218–222. [PMC free article] [PubMed] [Google Scholar]
- 74.Ruiz-Irastorza G, Egurbide M, Olivares N, Martinez-Berriotxoa A, Aguirre C. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology. 2008;47(6):920–923. doi: 10.1093/rheumatology/ken121 [DOI] [PubMed] [Google Scholar]
- 75.Oh R. Vitamin D insufficiency as a cause of hyperparathyroidism. Am Fam Physician. 2005;71(1):46–49. [PubMed] [Google Scholar]
- 76.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocrine Rev. 2001;22(4):477–501. doi: 10.1210/edrv.22.4.0437 [DOI] [PubMed] [Google Scholar]
- 77.Currie G, Delles C. Proteinuria and its relation to cardiovascular disease. Int J Nephrol Renovasc Dis. 2013;7:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perkovic V, Verdon C, Ninomiya T, et al. The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. PLoS Med. 2008;5(10):e207. doi: 10.1371/journal.pmed.0050207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sung JK, Kim J-Y, Youn YJ, et al. Urine albumin creatinine ratio is associated with carotid atherosclerosis in a community based cohort: atherosclerosis risk of rural area in Korean general population study. J Cardiovasc Ultrasound. 2010;18(4):134–138. doi: 10.4250/jcu.2010.18.4.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jorgensen L, Jenssen T, Johnsen SH, et al. Albuminuria as risk factor for initiation and progression of carotid atherosclerosis in non-diabetic persons: the Tromso Study. Eur Heart J. 2007;28(3):363. doi: 10.1093/eurheartj/ehl394 [DOI] [PubMed] [Google Scholar]
- 81.Xu R, Cai H, Fan Z, Wan Y, Gao X. The change in kidney function was associated with carotid artery plaque in a community-based population: a cohort study. Nutr Metab Cardiovasc Dis. 2021;31(1):119–126. doi: 10.1016/j.numecd.2020.08.016 [DOI] [PubMed] [Google Scholar]
- 82.Kajitani N, Uchida HA, Suminoe I, et al. Chronic kidney disease is associated with carotid atherosclerosis and symptomatic ischaemic stroke. J Int Med Res. 2018;46(9):3873–3883. doi: 10.1177/0300060518781619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ohara T, Kokubo Y, Toyoda K, et al. Impact of chronic kidney disease on carotid atherosclerosis according to blood pressure category: the Suita study. Stroke. 2013;44(12):3537–3539. doi: 10.1161/STROKEAHA.113.002957 [DOI] [PubMed] [Google Scholar]
- 84.Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bruce I. ‘Not only … but also’: factors that contribute to accelerated atherosclerosis and premature coronary heart disease in systemic lupus erythematosus. Rheumatology. 2005;44(12):1492–1502. doi: 10.1093/rheumatology/kei142 [DOI] [PubMed] [Google Scholar]
- 86.Ng M, Celermajer D. Glucocorticoid Treatment and Cardiovascular Disease. BMJ Publishing Group Ltd; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Donate-Correa J, Muros-de-fuentes M, Mora-Fernández C, Navarro-González JF. FGF23/Klotho axis: phosphorus, mineral metabolism and beyond. Cytokine Growth Factor Rev. 2012;23(1–2):37–46. doi: 10.1016/j.cytogfr.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 88.Shuto E, Taketani Y, Tanaka R, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20(7):1504–1512. doi: 10.1681/ASN.2008101106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Onufrak SJ, Bellasi A, Shaw LJ, et al. Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis. 2008;199(2):424–431. doi: 10.1016/j.atherosclerosis.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 90.Itkonen ST, Karp HJ, Kemi VE, et al. Associations among total and food additive phosphorus intake and carotid intima-media thickness–a cross-sectional study in a middle-aged population in Southern Finland. Nutr J. 2013;12(1):1–10. doi: 10.1186/1475-2891-12-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sheridan K, Logomarsino JV. Effects of serum phosphorus on vascular calcification in a healthy, adult population: a systematic review. J Vasc Nurs. 2017;35(3):157–169. doi: 10.1016/j.jvn.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 92.Foley RN. Phosphate levels and cardiovascular disease in the general population. Clin J Am Soc Nephrol. 2009;4(6):1136–1139. doi: 10.2215/CJN.01660309 [DOI] [PubMed] [Google Scholar]
- 93.Osuka S, Razzaque MS. Can features of phosphate toxicity appear in normophosphatemia? J Bone Miner Metab. 2012;30(1):10–18. doi: 10.1007/s00774-011-0343-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bai W, Li J, Liu J. Serum phosphorus, cardiovascular and all-cause mortality in the general population: a meta-analysis. Clinica Chimica Acta. 2016;461:76–82. doi: 10.1016/j.cca.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 95.Disthabanchong S. Phosphate and cardiovascular disease beyond chronic kidney disease and vascular calcification. Int J Nephrol. 2018;1:3162806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou C, Shi Z, Ouyang N, Ruan X. Hyperphosphatemia and cardiovascular disease. Front Cell Dev Biol. 2021;9:644363. doi: 10.3389/fcell.2021.644363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ritter CS, Slatopolsky E. Phosphate toxicity in CKD: the killer among us. Clin J Am Soc Nephrol. 2016;11(6):1088–1100. doi: 10.2215/CJN.11901115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ellam TJ, Chico TJ. Phosphate: the new cholesterol? The role of the phosphate axis in non-uremic vascular disease. Atherosclerosis. 2012;220(2):310–318. doi: 10.1016/j.atherosclerosis.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 99.Keshk W, Soliman N, Esheba N. Study of some biomarkers changes in patients with lupus nephritis and their correlation with disease activity and progression. Bull Egypt Soc Physiol Sci. 2013;33(1):17–34. [Google Scholar]
- 100.Cunningham SE, Czaya BE, Faul C. Elevated phosphate levels induce markers of systemic inflammation and anemia in murine hepatocytes. THE FASEB J. 2020;34(S1):1. [Google Scholar]
- 101.Yamada S, Tokumoto M, Tatsumoto N, et al. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. Am J Physiol Renal Physiol. 2014;306(12):F1418–F1428. doi: 10.1152/ajprenal.00633.2013 [DOI] [PubMed] [Google Scholar]
- 102.Czaya B, Richter B, Yanucil C, Campos I, Heitman K, Faul C. Hyperphosphatemia contributes to inflammation and iron dysregulation in models of normal and impaired renal function. Blood. 2019;134(Suppl 1):2238. doi: 10.1182/blood-2019-122144 [DOI] [Google Scholar]
- 103.Voelkl J, Egli-Spichtig D, Alesutan I, Wagner CA. Inflammation: a putative link between phosphate metabolism and cardiovascular disease. Clin Sci. 2021;135(1):201–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Erem S, Razzaque MS. Dietary phosphate toxicity: an emerging global health concern. Histochem Cell Biol. 2018;150(6):711–719. doi: 10.1007/s00418-018-1711-8 [DOI] [PubMed] [Google Scholar]
- 105.Gutiérrez OM, Anderson C, Isakova T, et al. Low socioeconomic status associates with higher serum phosphate irrespective of race. J Am Soc Nephrol. 2010;21(11):1953–1960. doi: 10.1681/ASN.2010020221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wöhrle S, Bonny O, Beluch N, et al. FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF‐23 signaling and regulating FGF‐23 expression in bone. J Bone Miner Res. 2011;26(10):2486–2497. doi: 10.1002/jbmr.478 [DOI] [PubMed] [Google Scholar]
- 107.Quarles LD. FGF-23 and α-klotho co-dependent and independent functions. Curr Opin Nephrol Hypertens. 2019;28(1):16–25. doi: 10.1097/MNH.0000000000000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–2215. doi: 10.1681/ASN.2005010052 [DOI] [PubMed] [Google Scholar]
- 109.Koh N, Fujimori T, Nishiguchi S, et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280(4):1015–1020. doi: 10.1006/bbrc.2000.4226 [DOI] [PubMed] [Google Scholar]
- 110.Kritmetapak K, Losbanos L, Berent TE, et al. Hyperphosphatemia with elevated serum PTH and FGF23, reduced 1, 25 (OH) 2 D and normal FGF7 concentrations characterize patients with CKD. BMC Nephrol. 2021;22(1):1–8. doi: 10.1186/s12882-021-02311-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vogt I, Haffner D, Leifheit-Nestler M. FGF23 and phosphate–cardiovascular toxins in CKD. Toxins. 2019;11(11):647. doi: 10.3390/toxins11110647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martín-Núñez E, Donate-Correa J, López-Castillo A, et al. Soluble levels and endogenous vascular gene expression of KLOTHO are related to inflammation in human atherosclerotic disease. Clin Sci. 2017;131(21):2601–2609. doi: 10.1042/CS20171242 [DOI] [PubMed] [Google Scholar]
- 113.Rodríguez-Ortiz ME, Díaz-Tocados JM, Muñoz-Castañeda JR, et al. Inflammation both increases and causes resistance to FGF23 in normal and uremic rats. Clin Sci. 2020;134(1):15–32. doi: 10.1042/CS20190779 [DOI] [PubMed] [Google Scholar]
- 114.Forster RE, Jurutka PW, Hsieh J-C, et al. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun. 2011;414(3):557–562. doi: 10.1016/j.bbrc.2011.09.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Haussler MR, Whitfield GK, Haussler CA, et al. 1, 25-Dihydroxyvitamin D and Klotho: a tale of two renal hormones coming of age. Vitam Horm. 2016;100:165–230. [DOI] [PubMed] [Google Scholar]
- 116.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299(4):F882–F889. doi: 10.1152/ajprenal.00360.2010 [DOI] [PubMed] [Google Scholar]
- 117.Bae J, Lee Y-H, Kang ES, Cha B-S, Lee B-W. Proteinuria is associated with carotid artery atherosclerosis in non-albuminuric type 2 diabetes: a cross-sectional study. J Clin Med. 2020;9(1):136. doi: 10.3390/jcm9010136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Furtner M, Kiechl S, Mair A, et al. Urinary albumin excretion is independently associated with carotid and femoral artery atherosclerosis in the general population. Europ Heart J. 2005;26(3):279–287. doi: 10.1093/eurheartj/ehi014 [DOI] [PubMed] [Google Scholar]
- 119.Oh K-H, Park SK, Park HC, et al. KNOW-CKD (KoreaN cohort study for Outcome in patients With Chronic Kidney Disease): design and methods. BMC Nephrol. 2014;15(1):1–9. doi: 10.1186/1471-2369-15-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Seigneux S, Courbebaisse M, Rutkowski JM, et al. Proteinuria increases plasma phosphate by altering its tubular handling. J Am Soc Nephrol. 2015;26(7):1608–1618. doi: 10.1681/ASN.2014010104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jung JY, Ro H, Chang JH, et al. Mediation of the relationship between proteinuria and serum phosphate: insight from the KNOW-CKD study. PLoS One. 2020;15(6):e0235077. doi: 10.1371/journal.pone.0235077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Delitsikou V, Jarad G, Rajaram RD, et al. Klotho regulation by albuminuria is dependent on ATF3 and endoplasmic reticulum stress. FASEB J. 2020;34(2):2087–2104. doi: 10.1096/fj.201900893R [DOI] [PubMed] [Google Scholar]
- 123.de Seigneux S, Delitsikou V, Martin P-Y. The KNOW-CKD study: evidence for a link between proteinuria and alterations of mineral metabolism. Nephrol Dial Transplant. 2020;35(3):382–385. doi: 10.1093/ndt/gfz083 [DOI] [PubMed] [Google Scholar]
- 124.Leal LKAM, Lima LA, de Aquino PEA, et al. Vitamin D (VD3) antioxidative and anti-inflammatory activities: peripheral and central effects. Europ J Pharmacol. 2020;879:173099. doi: 10.1016/j.ejphar.2020.173099 [DOI] [PubMed] [Google Scholar]
- 125.Zoccali C, Ruggenenti P, Perna A, et al. Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J Am Soc Nephrol. 2011;22(10):1923–1930. doi: 10.1681/ASN.2011020175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Di Iorio BR, Bellizzi V, Bellasi A, et al. Phosphate attenuates the anti-proteinuric effect of very low-protein diet in CKD patients. Nephrol Dial Transplant. 2013;28(3):632–640. doi: 10.1093/ndt/gfs477 [DOI] [PubMed] [Google Scholar]
- 127.Shalhoub V, Shatzen EM, Ward SC, et al. FGF23 neutralization improves chronic kidney disease–associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012;122(7):2543–2553. doi: 10.1172/JCI61405 [DOI] [PMC free article] [PubMed] [Google Scholar]