Here, we report the complete genome sequence of fowl adenovirus D (FAdV-D) isolated from a preserved 24-year-old pancreas sample of a broiler chicken embryo. The results of the sequence showed that the viral genome is 44,079 bp long.
ABSTRACT
Here, we report the complete genome sequence of fowl aviadenovirus D (FAdV-D) isolated from a preserved 24-year-old pancreas sample of a broiler chicken embryo. The results of the sequence showed that the viral genome is 44,079 bp long.
ANNOUNCEMENT
Fowl aviadenovirus (FAdV) is a member of the genus Aviadenovirus within the family Adenoviridae (1). Adenoviruses can infect a wide range of hosts; however, avian adenoviruses are reported to infect only avian species (2). FAdVs have been categorized into 5 species (FAdV-A to FAdV-E) on the basis of their genome structure and are further divided into 12 serotypes, based on a cross-neutralization test (3, 4). FAdVs are widely distributed and cause various degrees of associated clinical disease (5, 6). Some species of FAdVs cause inclusion body hepatitis (1, 7–14), hepatitis-hydropericardium syndrome (15–17), adenoviral gizzard erosions (18, 19), and possibly hypoglycemia and spiking mortality syndrome (H-SMS) (20, 21) in chickens. To date, only a few complete genomes of FAdV-D from the United States are available in public databases (22, 23). In this study, we report the complete genome sequence of an FAdV-D isolate from the United States.
A fecal sample was collected from a broiler chicken with hypoglycemia and spiking mortality syndrome (H-SMS) at a commercial farm in Georgia in 1995 (24). At that time, in order to experimentally reproduce severe H-SMS, a series of embryo-passaged preparations were performed. Briefly, H-SMS was experimentally reproduced by inoculating crude feces to 1-day-old chicks. Virus particles from their intestines, which were collected at 12 to 14 days postinoculation, were banded in a discontinuous Renografin gradient and inoculated into 7-day-old specific-pathogen-free embryonating chicken eggs (SPF ECE). Four days postinoculation, the embryos were harvested, homogenized in sterile phosphate-buffered saline, filtered, and then inoculated into 7-day-old SPF ECE. The embryos from this passage died between 48 and 96 hours postinoculation and then were harvested and processed as described before to create a third passage. The pancreases of the embryos from the third passage were homogenized, filtered, and stored at −70°C for 24 years. In 2019, viral RNA and total nucleic acids were isolated from a preserved pancreas sample using the QIAamp viral RNA minikit and the DNeasy blood and tissue kit (Qiagen, Germany), respectively, after first undergoing DNase treatment with the Turbo DNA-free kit (Ambion, USA) to remove host DNA according to the manufacturer’s recommendations. Sequence-independent single-primer amplification (25–27) was used to produce random amplicons that were processed using the Nextera XT DNA library preparation kit (Illumina, USA). The distribution size and concentration of the prepared library were checked on a 2100 bioanalyzer, using the high-sensitivity (HS) DNA kit (Agilent Technologies, Germany), and on a Qubit fluorometer, using the double-stranded DNA (dsDNA) HS assay kit (Life Technologies, USA), respectively. Two next-generation paired-end sequencing (2 × 150 and 2 ×250 bp) runs were performed on an Illumina MiSeq instrument using the 300- and 500-cycle MiSeq reagent kit v.2 (Illumina), respectively. Sequence data from the two runs were combined, and de novo assembly was performed utilizing MIRA v.3.4.1 (28) within a customized workflow on the Galaxy platform (29); all tools were run with default parameters, as described previously (30, 31). A total of 1,113,717 raw paired-end reads (904,985 and 208,732 reads of 150- and 250-bp reads, respectively) were generated. The de novo-generated contigs of interest were subjected to BLASTn search and aligned with the full-length reference genome MX95-S11 (GenBank accession number KU746335.1) to obtain a draft genome scaffold. The genome consensus was then recalled from 200,733 FAdV reads using BWA-MEM (32) mapping of trimmed but unnormalized reads to the genome scaffold. The median read depth of the assembly was 220, and the maximum depth was 5,226. The final genome consensus of the isolate designated GA/1358/1995 was 44,079 nucleotides long (100% genome coverage) with a GC content of 53.6% and coded 37 putative open reading frames (ORFs) (Table 1). The ORFs were identified using the Geneious 11.1.5 and confirmed by alignment with published FAdV genomes. BLAST comparison to the currently available full-length FAdV genome sequences showed the highest (99.23%) nucleotide identity to the FAdV-D serotype 2 prototype ATCC reference strain P7-A (GenBank accession number MK572866.1) (23, 33) (Fig. 1).
TABLE 1.
Characteristics of the full-length genome of FAdV-D isolate GA/1358/1995
| Gene name | Strand directiona | Start codon position | End codon position | No. of codons | Closest viral homology (GenBank accession no.) | Amino acid identity (%) |
|---|---|---|---|---|---|---|
| ORF0 | R | 524 | 808 | 94 | FAdV-2 (ANJ02325.1) | 100 |
| ORF1 | R | 848 | 1,339 | 163 | FAdV-2 (ANJ02326.1) | 100 |
| ORF1B | R | 1,501 | 1,731 | 76 | FAdV-3 (ANJ02402.1) | 100 |
| ORF1C | R | 1,679 | 1,879 | 66 | FAdV-3 (ANJ02403.1) | 100 |
| ORF2 | R | 1,953 | 2,756 | 267 | FAdV-9 (NP597818.1) | 100 |
| ORF7 | L | 2,348 | 2,668 | 106 | FAdV-11 (QFR45452.1) | 100 |
| ORF24 | L | 2,839 | 3,519 | 228 | FAdV-11 (QIM09468.1) | 99.56 |
| 16,294 | 16,299 | |||||
| ORF14 | L | 3,538 | 4,224 | 230 | FAdV-9 (AP000373.1) | 100 |
| 16,294 | 16,299 | |||||
| ORF13 | L | 4,263 | 5,249 | 330 | FAdV-2 (ANJ02370.1) | 100 |
| 16,294 | 16,299 | |||||
| ORF12 | L | 5,245 | 6,162 | 307 | FAdV-9 (AP000375.1) | 100 |
| 16,294 | 16,299 | |||||
| IVa2 | L | 6,131 | 7,351 | 406 | FAdV-9 (NP050280.1) | 99.01 |
| DNA polymerase | L | 7,348 | 11,262 | 1,304 | FAdV-2 (QGQ62975.1) | 99.39 |
| pTP | L | 11,259 | 13,220 | 655 | FAdV-11 (ANJ02596.1) | 99.23 |
| 16,294 | 16,299 | |||||
| 52k | R | 13,259 | 14,467 | 402 | FAdV-11 (AIS19821.1) | 100 |
| pIIIa | R | 14,454 | 16,229 | 591 | FAdV-11 (AKR76192.1) | 100 |
| Penton base | R | 16,310 | 17,947 | 545 | FAdV-11 (AIS19822.1) | 100 |
| pVII | R | 17,987 | 18,223 | 78 | FAdV-11 (AKR76194.1) | 100 |
| pX | R | 18,458 | 19,057 | 199 | FAdV-9 (NP050285.1) | 100 |
| pVI | R | 19,187 | 19,873 | 228 | FAdV-9 (NP050286.1) | 100 |
| Hexon | R | 19,985 | 22,834 | 949 | FAdV-2 (QGQ62983.1) | 100 |
| Protease | R | 22,848 | 23,465 | 205 | FAdV-11 (AIS19826.1) | 100 |
| DNA-binding protein | L | 23,580 | 25,009 | 557 | FAdV-11 (AKR76199.1) | 98.56 |
| 25,107 | 25,350 | |||||
| 100k | R | 25,414 | 28,398 | 994 | FAdV-11 (AKR76200.1) | 93.55 |
| 33k | R | 28,079 | 28,409 | 221 | FAdV-11 (AKR76201.1) | 99.55 |
| 28,655 | 28,989 | |||||
| 22k | R | 28,079 | 28,624 | 181 | FAdV-11 (ALS87111.1) | 99.45 |
| pVIII | R | 29,029 | 29,754 | 241 | FAdV-11 (AIS19829.1) | 100 |
| U exon | L | 29,638 | 30,006 | 122 | FAdV-11 (QIM09482.1) | 98.36 |
| Fiber | R | 30,005 | 31,717 | 570 | FAdV-11 (ANQ43489.1) | 98.60 |
| ORF22 | L | 31,777 | 32,349 | 190 | FAdV-11 (AIS19831.1) | 100 |
| ORF20A | L | 32,353 | 32,832 | 166 | FAdV-11 (AKR76206.1) | 100 |
| 33,846 | 33,866 | |||||
| ORF20 | L | 32,833 | 33,750 | 312 | FAdV-2 (ANJ02355.1) | 100 |
| 33,846 | 33,866 | |||||
| ORF19 | L | 34,067 | 36,193 | 714 | FAdV-11 (QGQ63065.1) | 99.58 |
| 36,272 | 36,289 | |||||
| GAM-1 | R | 37,747 | 38,580 | 277 | FAdV-2 (QGQ63210.1) | 100 |
| ORF17 | L | 39,674 | 40,144 | 156 | FAdV-11 (QFR45478.1) | 99.36 |
| ORF11 | R | 40,519 | 40,879 | 261 | FAdV-2 (QGQ63176.1) | 100 |
| 40,957 | 41,201 | |||||
| 41,261 | 41,442 | |||||
| ORF23 | L | 41,675 | 42,610 | 311 | FAdV-11 (ANJ02619.1) | 100 |
| ORF25 | R | 43,067 | 43,075 | 169 | FAdV-11 (QIM09485.1) | 99.41 |
| 43,154 | 43,654 |
R, rightward-transcribed strand; L, leftward-transcribed strand.
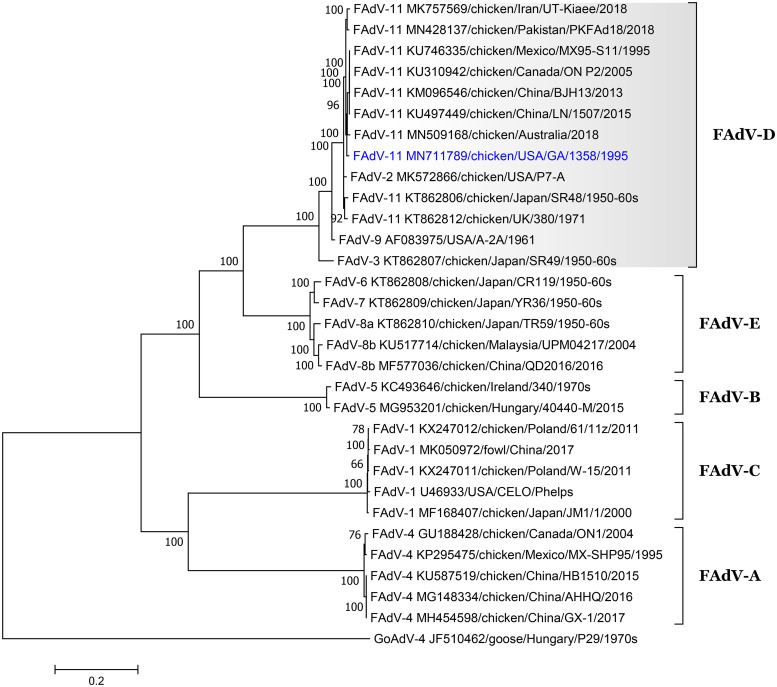
FIG 1.
Phylogenetic analysis of fowl aviadenovirus isolates based on the complete genome sequences constructed with the maximum likelihood method based on the general time-reversible model in MEGA v.7.0. The tree with the highest log likelihood (−217,618.18) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. A discrete gamma distribution was used to model evolutionary rate differences among sites (5 categories [+G, parameter = 1.4043]). The rate variation model allowed for some sites to be evolutionarily invariable ([+I], 17.27% sites). The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 31 nucleotide sequences (sequences of goose adenovirus [GoAdV] is included as an outgroup). All positions containing gaps and missing data were eliminated. There were a total of 29,569 positions in the final data set. The isolate used in this study is shown in blue.
Fowl aviadenoviruses appear to be widely endemic in poultry and have been associated with clinical disease, but full-genome sequences of FAdV-D circulating in the United States are scarce. More complete genome sequence information is necessary to understand how fowl aviadenoviruses contribute to disease in poultry and how to control it.
Data availability.
The complete genome sequence of isolate GA/1358/1995 of FAdV-D has been deposited in GenBank under the accession number MN711789. Raw data were deposited in the SRA under accession number SRR10500667, BioSample number SAMN13338320, and BioProject number PRJNA590745.
ACKNOWLEDGMENTS
The mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
This study was supported by USDA CRIS project 6040-32000-072 and USDA APHIS interagency agreement 60-6040-5-009.
REFERENCES
- 1.Şahindokuyucu İ, Çöven F, Kılıç H, Yılmaz Ö, Kars M, Yazıcıoğlu Ö, Ertunç E, Yazıcı Z. 2020. First report of fowl aviadenovirus serotypes FAdV-8b and FAdV-11 associated with inclusion body hepatitis in commercial broiler and broiler-breeder flocks in Turkey. Arch Virol 165:43–51. doi: 10.1007/s00705-019-04449-w. [DOI] [PubMed] [Google Scholar]
- 2.Slaine PD, Ackford JG, Kropinski AM, Kozak RA, Krell PJ, Nagy É. 2016. Molecular characterization of pathogenic and nonpathogenic fowl aviadenovirus serotype 11 isolates. Can J Microbiol 62:993–1002. doi: 10.1139/cjm-2016-0297. [DOI] [PubMed] [Google Scholar]
- 3.Hess M. 2000. Detection and differentiation of avian adenoviruses: a review. Avian Pathol 29:195–206. doi: 10.1080/03079450050045440. [DOI] [PubMed] [Google Scholar]
- 4.Hess M, Prusas C, Bergmann V, Mazaheri A, Raue R. 2000. [Epizootiology of fowls adenoviruses]. Berl Munch Tierarztl Wochenschr 113:202–208. [PubMed] [Google Scholar]
- 5.Absalón AE, Morales-Garzón A, Vera-Hernández PF, Cortés-Espinosa DV, Uribe-Ochoa SM, García LJ, Lucio-Decanini E. 2017. Complete genome sequence of a non-pathogenic strain of Fowl Adenovirus serotype 11: minimal genomic differences between pathogenic and non-pathogenic viruses. Virology 501:63–69. doi: 10.1016/j.virol.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Toro H, Gonzalez C, Cerda L, Hess M, Reyes E, Geissea C. 2000. Chicken anemia virus and fowl adenoviruses: association to induce the inclusion body hepatitis/hydropericardium syndrome. Avian Dis 44:51–58. doi: 10.2307/1592507. [DOI] [PubMed] [Google Scholar]
- 7.Mase M, Nakamura K, Minami F. 2012. Fowl adenoviruses isolated from chickens with inclusion body hepatitis in Japan, 2009–2010. J Vet Med Sci 74:1087–1089. doi: 10.1292/jvms.11-0443. [DOI] [PubMed] [Google Scholar]
- 8.Alvarado IR, Villegas P, El-Attrache J, Jensen E, Rosales G, Perozo F, Purvis LB. 2007. Genetic characterization, pathogenicity, and protection studies with an avian adenovirus isolate associated with inclusion body hepatitis. Avian Dis 51:27–32. doi: 10.1637/0005-2086(2007)051[0027:GCPAPS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Zhong Q, Zhao Y, Hu YX, Zhang GZ. 2015. Pathogenicity and complete genome characterization of fowl adenoviruses isolated from chickens associated with inclusion body hepatitis and hydropericardium syndrome in China. PLoS One 10:e0133073. doi: 10.1371/journal.pone.0133073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shivaprasad HL, Woolcock PR, McFarland MD. 2001. Group I avian adenovirus and avian adeno-associated virus in turkey poults with inclusion body hepatitis. Avian Pathol 30:661–666. doi: 10.1080/03079450120092152. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Zaheer I, Saleemi MK, Qi X, Gao Y, Cui H, Li K, Gao L, Fayyaz A, Hussain A, Liu C, Zhang Y, Wang X, Pan Q. 2020. The first complete genome sequence and pathogenicity characterization of fowl adenovirus 11 from chickens with inclusion body hepatitis in Pakistan. Vet Microbiol 244:108670. doi: 10.1016/j.vetmic.2020.108670. [DOI] [PubMed] [Google Scholar]
- 12.Hosseini H, Langeroudi AG, FallahMehrabadi MH, Ziafati Kafi Z, Dizaji RE, Ghafouri SA, Hamadan AM, Aghaiyan L, Hajizamani N. 2020. The fowl adenovirus (Fadv-11) outbreak in Iranian broiler chicken farms: the first full genome characterization and phylogenetic analysis. Comp Immunol Microbiol Infect Dis 70:101365. doi: 10.1016/j.cimid.2019.101365. [DOI] [PubMed] [Google Scholar]
- 13.Abghour S, Zro K, Mouahid M, Tahiri F, Tarta M, Berrada J, Kichou F. 2019. Isolation and characterization of fowl aviadenovirus serotype 11 from chickens with inclusion body hepatitis in Morocco. PLoS One 14:e0227004. doi: 10.1371/journal.pone.0227004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wibowo MH, Sahesty A, Mahardika BK, Purwanto B, Lestariningsih CL, Kade Suardana IB, Oka Winaya IB, Irine I, Suryanggono J, Jonas M, Murwijati T, Mahardika GN. 2019. Epizootiology, clinical signs, and phylogenetic analysis of fowl adenovirus in chicken farms in Indonesia from 2018 to 2019. Avian Dis 63:619–624. doi: 10.1637/aviandiseases-D-19-00127. [DOI] [PubMed] [Google Scholar]
- 15.Mazaheri A, Prusas C, Voss M, Hess M. 1998. Some strains of serotype 4 fowl adenoviruses cause inclusion body hepatitis and hydropericardium syndrome in chickens. Avian Pathol 27:269–276. doi: 10.1080/03079459808419335. [DOI] [PubMed] [Google Scholar]
- 16.Vera-Hernández PF, Morales-Garzón A, Cortés-Espinosa DV, Galiote-Flores A, García-Barrera LJ, Rodríguez-Galindo ET, Toscano-Contreras A, Lucio-Decanini E, Absalón AE. 2016. Clinicopathological characterization and genomic sequence differences observed in a highly virulent fowl Aviadenovirus serotype 4. Avian Pathol 45:73–81. doi: 10.1080/03079457.2015.1125443. [DOI] [PubMed] [Google Scholar]
- 17.Asthana M, Chandra R, Kumar R. 2013. Hydropericardium syndrome: current state and future developments. Arch Virol 158:921–931. doi: 10.1007/s00705-012-1570-x. [DOI] [PubMed] [Google Scholar]
- 18.Marek A, Schulz E, Hess C, Hess M. 2010. Comparison of the fibers of Fowl adenovirus A serotype 1 isolates from chickens with gizzard erosions in Europe and apathogenic reference strains. J Vet Diagn Invest 22:937–941. doi: 10.1177/104063871002200613. [DOI] [PubMed] [Google Scholar]
- 19.Domanska-Blicharz K, Tomczyk G, Smietanka K, Kozaczynski W, Minta Z. 2011. Molecular characterization of fowl adenoviruses isolated from chickens with gizzard erosions. Poult Sci 90:983–989. doi: 10.3382/ps.2010-01214. [DOI] [PubMed] [Google Scholar]
- 20.Mendelson C, Nothelfer HB, Monreal G. 1995. Identification and characterization of an avian adenovirus isolated from a “spiking mortality syndrome” field outbreak in broilers on the Delmarva Peninsula, USA. Avian Pathol 24:693–706. doi: 10.1080/03079459508419108. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin MA, Hill DL, Dekich MA, Putnam MR. 1993. Multisystemic adenovirus infection in broiler chicks with hypoglycemia and spiking mortality. Avian Dis 37:625–627. doi: 10.2307/1591701. [DOI] [PubMed] [Google Scholar]
- 22.Schrenzel M, Oaks JL, Rotstein D, Maalouf G, Snook E, Sandfort C, Rideout B. 2005. Characterization of a new species of adenovirus in falcons. J Clin Microbiol 43:3402–3413. doi: 10.1128/JCM.43.7.3402-3413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schachner A, Gonzalez G, Endler L, Ito K, Hess M. 2019. Fowl adenovirus (FAdV) recombination with intertypic crossovers in genomes of FAdV-D and FAdV-E, displaying hybrid serological phenotypes. Viruses 11:1094. doi: 10.3390/v11121094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis JF, Castro AE, de la Torre JC, Barnes HJ, Doman JT, Metz M, Lu H, Yuen S, Dunn PA, Teng MN. 1996. Experimental reproduction of severe hypoglycemia and spiking mortality syndrome using field-derived and embryo-passaged preparations. Avian Dis 40:158–172. doi: 10.2307/1592385. [DOI] [PubMed] [Google Scholar]
- 25.Chrzastek K, Lee DH, Smith D, Sharma P, Suarez DL, Pantin-Jackwood M, Kapczynski DR. 2017. Use of sequence-independent, single-primer-amplification (SISPA) for rapid detection, identification, and characterization of avian RNA viruses. Virology 509:159–166. doi: 10.1016/j.virol.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng WX, Li JS, Huang CP, Yao DP, Liu N, Cui SX, Jin Y, Duan ZJ. 2010. Identification and nearly full-length genome characterization of novel porcine bocaviruses. PLoS One 5:e13583. doi: 10.1371/journal.pone.0013583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Victoria JG, Kapoor A, Li L, Blinkova O, Slikas B, Wang C, Naeem A, Zaidi S, Delwart E. 2009. Metagenomic analyses of viruses in stool samples from children with acute flaccid paralysis. J Virol 83:4642–4651. doi: 10.1128/JVI.02301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chevreux B, Wetter T, Suhai S. 1999. Genome sequence assembly using trace signals and additional sequence information, p 45–56. InComputer science and biology. The German Conference on Bioinformatics (GCB '99), Hanover, Germany. [Google Scholar]
- 29.Afgan E, Baker D, van den Beek M, Blankenberg D, Bouvier D, Čech M, Chilton J, Clements D, Coraor N, Eberhard C, Grüning B, Guerler A, Hillman-Jackson J, Von Kuster G, Rasche E, Soranzo N, Turaga N, Taylor J, Nekrutenko A, Goecks J. 2016. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res 44:W3–W10. doi: 10.1093/nar/gkw343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimitrov KM, Sharma P, Volkening JD, Goraichuk IV, Wajid A, Rehmani SF, Basharat A, Shittu I, Joannis TM, Miller PJ, Afonso CL. 2017. A robust and cost-effective approach to sequence and analyze complete genomes of small RNA viruses. Virol J 14:72. doi: 10.1186/s12985-017-0741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goraichuk IV, Msoffe PLM, Chiwanga GH, Dimitrov KM, Afonso CL, Suarez DL. 2019. First complete genome sequence of a subgenotype Vd Newcastle disease virus isolate. Microbiol Resour Announc 8:e00436-19. doi: 10.1128/MRA.00436-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khanna PN. 1966. Occurrence of avian adenoviruses in Hungary. Acta Vet Acad Sci Hung 16:351–356. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The complete genome sequence of isolate GA/1358/1995 of FAdV-D has been deposited in GenBank under the accession number MN711789. Raw data were deposited in the SRA under accession number SRR10500667, BioSample number SAMN13338320, and BioProject number PRJNA590745.