Incidence dynamics of emerging infectious diseases are essentially non-linear: in a population with no pre-existing natural immunity, a horizontally transmitted pathogen spreads more than linearly (and exponentially in the purely theoretical situation where each case infects the same number of individuals). Conversely, healthcare capacities can only grow linearly, which means that after some time they are bound not to cope with disease incidence. Quantitative epidemiology and modelling can shed light on different scenarios on near and medium future trends and help us better understand the past to describe the present [1], [2].
The COVID-19 pandemic caught the vast majority of countries unawares in early 2020, thereby challenging public health actors. In France, the first lockdown was implemented on the 17th of March 2020. At that time, population RT-qPCR testing was not sufficient to accurately estimate the size of the epidemic but it was sufficient to detect a near-exponential growth that had been going on for weeks. The main questions rose by hospital staff, public health authorities, and the general public (or the media) revolved around the date and height of the peak in hospital capacity strain, the actual cumulative incidence (following the hypothesis of hidden herd immunity), and the lockdown efficiency. These created an urgent need for mathematical epidemiology insights.
In a popularisation of science's perspective, our team released the first public estimate of the COVID-19 basic reproduction of COVID-19 in France [3]. We also published an online simulator (on the 6th of April 2020) to allow users to explore a variety of control scenarios that could differ from the full lockdown implemented at the time [4]. This counterfactual exploration made it possible to apprehend the impact of anticipating or delaying the implementation of non-pharmaceutical measures. It could also be used to explore the consequence of an earlier lockdown release, e.g., in the context of an implementation of a short lockdowns series. One of the important goals was to help defining, once the urgency of the first wave had passed, an optimal strategy (in terms of timing and intensity, or even age stratification, see e.g., [5], [6]) for the use of non-pharmaceutical interventions (NPIs) that would be less restrictive than the long and strict national lockdown. Therefore, contrary to what the media exposure suggested, detailed mathematical models were intended as a tool for anticipating and exploring less drastic solutions, at least in terms of their spatiotemporal application.
Our simulator involves a discrete-time model and is designed to be as parsimonious as possible while capturing the memory effect of infectious history [7]. Its inferential statistical component, which relies on hospital time series, has been refined over time and adapted to subnational levels as well [8]. Hospital time series are less subject to fluctuations in the testing effort than screening time series and their lag behind the events of associated infections is on average two weeks, which is less than mortality time series. The projections provided by our framework were used as support to political decision-makers and hospital service planning, especially the Montpellier University Hospital.
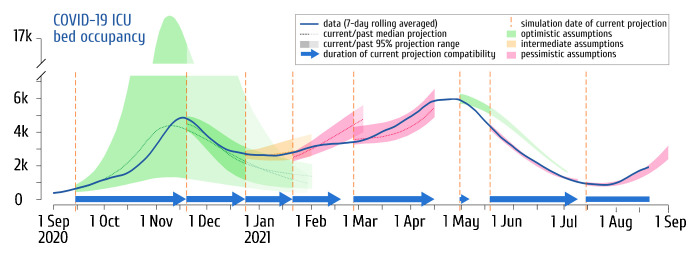
In Fig. 1 , we show a retrospective view of the ICU occupancy projections we made since early September 2020. Whether it was based on optimistic or pessimistic assumptions, the closest simulation of each period anticipated hospitals dynamics 38 days ahead on average. In two of the seven phases considered, however, none of the simulated scenarios was compatible with the observations after one month. In both cases, these were situations where the signal characterising the effect of newly introduced measures was still insufficient: the introduction of the national curfew on the 16th of January 2021, and the third lockdown on the 5th of April 2021. Based on then-current estimates of the number of reproductions, the most optimistic assumptions overestimated the future dynamics. Note that in both cases, analyses performed with the updated data less than two weeks after the dropout accurately anticipated the following trends.
Fig. 1.
COVIDSIM projections of the French COVID-19 ICU bed occupancy confronted to data (September 2020–June 2021).
The solid blue line shows the 7-day rolling averaged nationwide COVID-19 bed occupancy from the Santé Publique France (SI-VIC) database. The shaded areas and the dotted lines (when provided) correspond respectively to the range spanned by 95% of the COVIDSIM simulations and the median projection of future ICU bed occupancy. Among the pool of (2.5 on average) scenarios investigated for each period, only the closest to reality is shown and the color of the projection indicates whether the scenario was the most optimistic (green), the most pessimistic (pink) or based on intermediate assumptions (orange) within the pool of projections performed the day corresponding to the vertical dashed orange line on the left. When projections overlap, the former projections are depicted in lighter color. Only the main projections publicly released (in [9], [16], the French media, or on social networks) are here shown and no simulation was performed more than a day after that of the last available data point. The blue arrows shows the duration the current projection accurately anticipates the ICU dynamics within the range of the simulations. Note that the model was improved over time, which explains the strong confidence interval reduction from the second projection and that each run of the model simulates the whole epidemic curve from January 2020, explaining why the inferred values are not always centred on the data corresponding to the initial time point of the projection.
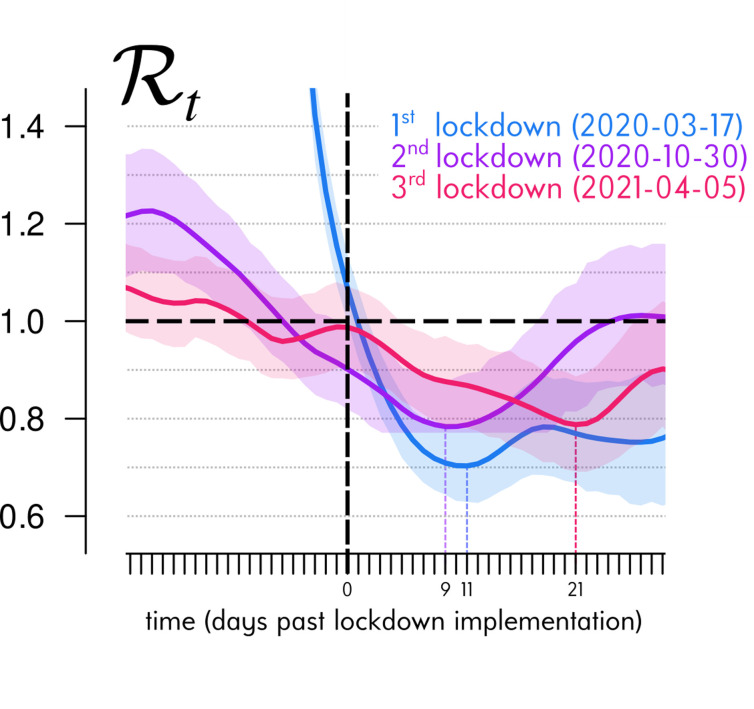
Let us focus on the projections published in the previous issue of Anaesthesia Critical Care and Pain Medicine [9]. These investigated the end of the third lockdown, which was initiated on the 3rd of April 2021 (with a tolerance until the 5th due to the Easter weekend) and released in several stages three weeks later. On the day the projections were made, on the 29th of April, hospital data could only reflect the first ten days of the lockdown (there is an average 14-day delay between infections and critical care admissions [7]). For the first two lockdowns, this period was sufficient to reach the full effect of the lockdown and, therefore, formulate an appropriate working hypothesis to simulate the further dynamics. As shown in Fig. 2 , the kinetics of the impact of the third lockdown measures on the estimated temporal reproduction number – calculated on daily critical care admissions nationally – was much slower than the first two, and the virus circulation minimum was reached only 21 days later (compared to 11 and 9 for the first two).
Fig. 2.
ICU-based COVID-19 reproduction number dynamics around lockdown implementations.
The solid curves represent the mean temporal reproduction number Rt estimated by the Wallinga & Tenuis approach [13], in R [14] with the R0 package [15]) from the daily COVID-19 ICU admissions in France provided by Santé Publique France (7-day rolling averaged to smooth weekly artefacts and 14-day earlier shifted to recover the mean infection time [7]). The shaded areas correspond to the daily 95% confidence interval of the mean. To compare the dynamics of stimated Rt around each of the three metropolitan France lockdowns, the times series are here overlapped and synchronised on their associated implementation day (vertical dashed black line), i.e., the 17th of March 2020, the 30th of October 2020 and the 5th of April 2021, respectively (though the third lockdown officially began on Apr 3th 2021, interregional mobility was tolerated during the Easter weekend). The horizontal dashed black line represents the Rt = 1 threshold under which the epidemic is under control. We see that the lag between lockdown implementation and its maximum effect (i.e., the minimum value reached by the estimated Rt shown with a vertical dotted line) doubled for a third lockdown.
Retrospective analyses stratified by age and space, including various contextual variables, will be necessary to shed light on the interrelated phenomena and their relative contributions to this particular kinetics. However, some hypotheses can be put forward such as a delayed effect of school closures in the transmission chains or weather conditions less favourable to virus transmission. The fact that the successive lifting of the measures did not give rise to a rebound (even if a signal attributable to the reopening of schools could be detected) and gave rise to the most optimistic scenario envisaged testifies to the effectiveness of the vaccination campaign and the low risk of transmission of SARS-CoV-2 in the open air (possibly amplified by the gradual realisation of the contribution of the airborne route in its transmission [10]).
Deviations between observations and simulations are always informative and provide opportunities to improve the model. In this particular case, the readjustment of the dynamics once the viral circulation during the 3rd lockdown was well-estimated suggests that the problem came from the choice of parametric hypotheses and not from the structure of the model itself. From a formal point of view, models are analogous to a logical implication: if condition A is fulfilled, then situation B can be expected to occur. If the working hypotheses are not satisfied, the scenarios produced become invalid and the simulations must be updated. This issue mainly arises when the signal of public health policy change is still incomplete in the hospital data.
Although at the time the extrapolation of the dynamics using the latest data was a reasonable choice under the parsimony principle and leaning on the experience from the two previous lockdowns, it is important to ask whether models could have done better. One possibility could have been to access robust early signals of epidemic trends such as weather variations, population Ct values [11], [12], or random screening in the population. In the United Kingdom, for example, epidemiological surveillance in schools, monitoring of contact chains, random population screening, and sequencing provide valuable sources of signal enrichment and model parameterisation. However, such complementary, dense, and stratified datasets are not available for France. Another option could have been to add even more mechanistic details in the model, e.g., explicitly capturing school attendance dynamics. This sounds appealing on paper but is extremely hard to achieve with a parsimonious model. Indeed, it could be that adding a specific component to the model perfectly captures the effect of the third lockdown but very poorly that of the previous ones or even the rest of the epidemic. More generally, and somehow paradoxically, moving away from the parsimony principle informs us on specific trends but also estranges us from the possibility to use data useful to set up relevant scenarios.
Even if they are not the most accurate in the short term, parsimonious models can easily explore all the possibilities in the medium term, a time frame that is of particular interest for decision-making. In this respect, they are suitable for informing anticipation strategies, particularly in the context of an epidemic outbreak, where the health impact of a delay can grow near-exponentially, even if it means re-evaluating the timetable every fortnight as the estimates are consolidated. This consolidation can be accelerated if the spatial heterogeneity of the epidemic allows delaying the implementation or the lifting of measures depending on the territory, a source of valuable data to improve the models and inform decision-making.
Even when they are mechanistic, i.e., here based on the explicit dynamics of transmission, all models are wrong because they greatly simplify the studied phenomena. Their ambition is, therefore, not to predict precisely how many hospitalisations there will be within a given number of days in a given place, but rather to know, for example, how much slack can be allowed without fearing ICU overload or the potential morbimortality impact of a next wave.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
We thank the ETE modelling team for discussion, as well as the University of Montpellier, the CNRS, the IRD, the South Green computational platform, France Bioinformatique, for logistical support.
References
- 1.Panovska-Griffiths J. Can mathematical modelling solve the current Covid-19 crisis? BMC Public Health. 2020;20:551. doi: 10.1186/s12889-020-08671-z. [s12889-020-08671-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Djidjou-Demasse R., Selinger C., Sofonea M.T. Épidémiologie mathématique et modélisation de la pandémie de Covid-19 : enjeux et diversité. Rev Francoph Lab. 2020;2020:63–69. doi: 10.1016/S1773-035X(20)30315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danesh G., Alizon S. 2020. Estimation du nombre de reproduction de l’épidémie de COVID-19 en France.https://hal.archives-ouvertes.fr/hal-02882680 [Google Scholar]
- 4.Sofonea M.T., Reyné B., Alizon S. 2020. COVIDSIM–Combining statistical analysis of hospital data and parsimonious non Markovian modelling for infering epidemiological parameters and simulating NPI of the COVID-19 epidemic in France. [Google Scholar]
- 5.Djidjou-Demasse R., Michalakis Y., Choisy M., Sofonea M.T., Alizon S. Optimal COVID-19 epidemic control until vaccine deployment. medRxiv. 2020 doi: 10.1101/2020.04.02.20049189. [2020.04.02.20049189] [DOI] [Google Scholar]
- 6.Richard Q., Alizon S., Choisy M., Sofonea M.T., Djidjou-Demasse R. Age-structured non-pharmaceutical interventions for optimal control of COVID-19 epidemic. PLOS Comput Biol. 2021;17:e1008776. doi: 10.1371/journal.pcbi.1008776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sofonea M.T. Memory is key in capturing COVID-19 epidemiological dynamics. Epidemics. 2021;35:100459. doi: 10.1016/j.epidem.2021.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boennec C., Alizon S., Sofonea M.T. 2021. COVIDici–Visualization & projection tool of the COVID-19 populational data in France at national, regional and departmental levels.https://cloudapps.france-bioinformatique.fr/covidici/ [Google Scholar]
- 9.Sofonea M.T., Boennec C., Michalakis Y., Alizon S. Two waves and a high tide: the COVID-19 epidemic in France. Anaesth Crit Care Pain Med. 2021;40:100881. doi: 10.1016/j.accpm.2021.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenhalgh T. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 2021;397:1603–1605. doi: 10.1016/S0140-6736(21)00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hay J.A. Estimating epidemiologic dynamics from cross-sectional viral load distributions. Science. 2021 doi: 10.1126/science.abh0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alizon S. Epidemiological and clinical insights from SARS-CoV-2 RT-PCR cycle amplification values. medRxiv. 2021 doi: 10.1101/2021.03.15.21253653. [2021.03.15.21253653] [DOI] [Google Scholar]
- 13.Wallinga J., Teunis P. Different epidemic curves for severe acute respiratory syndrome reveal similar impacts of control measures. Am J Epidemiol. 2004;160:509–516. doi: 10.1093/aje/kwh255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2020).
- 15.Obadia T., Haneef R., Boëlle P.-Y. The R0 package: a toolbox to estimate reproduction numbers for epidemic outbreaks. BMC Med Inform Decis Mak. 2012;12:147. doi: 10.1186/1472-6947-12-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alizon S, Haim-Boukobza S, Foulongne V, Verdurme L, Trombert-Paolantoni S, , Lecorche E, Roquebert B, Sofonea MT. (2021) Rapid spread of the SARS-CoV-2 delta variant in French regions in June 2021. Eurosurveillance (https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.28.2100573). [DOI] [PMC free article] [PubMed]