Abstract
Background
Pseudomonas aeruginosa (P. aeruginosa) is prevalent in hospital-acquired surgical wound infections. It exhibits both innate and acquired resistance to a broad range of antimicrobials and remains a principal problem in clinical practice.
Methods
In total, 284 sterile surgical wound swabs (142 each) were collected from two government hospitals: Central Hospital Benin (CHB) and University of Benin Teaching Hospital (UBTH) in Benin City, Nigeria. Pseudomonas spp. isolated from both hospitals were screened with eight different antibiotics by way of disk diffusion method. Polymerase chain reaction (PCR) amplification of 34 multiple drug-resistant isolates was carried out using genus-specific primer set on extracted genomic DNA for the identification of Pseudomonas spp. and substituent 16S rRNA sequencing to determine the prevailing strains in the two locations.
Results
Sixty-two Pseudomonas spp. were isolated from the two locations (27 isolates from CHB and 35 isolates from the UBTH). Surgical wound infections screened with regularly used antibiotics revealed that 17 (62.9%) isolates from CHB and 20 (57.1%) isolates from UBTH were multiple drug resistant Pseudomonas spp. PCR identification using Pseudomonas spp. specific primer showed that 16 (94.1%) isolates from CHB and 18 (90%) isolates from UBTH were confirmed. The 16S DNA sequencing revealed that P. aeruginosa strain H25883 was dominant in both locations.
Conclusion
High antibiotic resistance among P. aeruginosa isolates was established in our study. PCR technique revealed a more reliable method of bacterial identification. H25883 strain of P. aeruginosa is the prevalent strain in both locations and it should be given attention in nosocomial surgical wound infections.
Keywords: 16S rRNA sequencing, molecular characterisation, Pseudomonas aeruginosa, wound infection, nosocomial infections
Introduction
Post-surgical wound infection is the major source of nosocomial infection in surgical patients, accounting for 39.9% of all infections. It mainly causes post-operative morbidity, resulting in longer hospital stay, increased hospital bill and incidences of postoperative death. Generally, wound infections are a result of wound contamination caused by endogenous bacteria from the patient’s skin, mucous membrane or hollow viscera (1). The development of an infection in any wound is subjective largely to the virulent nature of the microorganism and immunity of the patient. Nevertheless, when pus oozes from a closed surgical opening along with signs of inflammation in the adjoining tissues, it is referred to as wound infection (2, 3).
Pseudomonas aeruginosa (P. aeruginosa) is a gram-negative bacterium. It is non-sporous, motile and a facultative anaerobe. It is accountable for a wide range of diseases in both humans and animals (4). Generally, P. aeruginosa is an opportunistic and nosocomial infectious organism that could develop infections in burns, injury, surgical wounds and in immunocompromised subjects (5, 6). Incidences of P. aeruginosa infections are on the rise worldwide due to their mechanisms of survival, adaptation and resistance to different types of antibiotics (7). Wound infection caused by P. aeruginosa is considered a major cause of morbidity and mortality (8). The immense use of routine broad-spectrum antibiotics has increased the resistance of P. aeruginosa to clinical drugs, which has led to serious therapeutic problems (9). Thus, timely and precise diagnosis is essential for proper treatment and also to control future disease outbreaks. A wide range of diagnostic methods have been established for P. aeruginosa identification. They include phenotypic methods (10), electrochemical techniques (11) such as enzyme-linked immunosorbent assay (12), and molecular methods such as polimerase chain reaction (PCR) (13), real-time PCR (14, 15), and particularly 16S DNA sequencing (16). Despite the existing and extensive reports on the prevalence of P. aeruginosa in hospital environments, there is still a paucity of research finding on molecular identification of multidrug P. aeruginosa strains from surgical wounds particularly in Benin City, Nigeria. Therefore, the present study sought to identify prevailing multidrug P. aeruginosa strains from the surgical wound using the 16S rRNA sequencing technique.
Methods
Sample Collection
A total of 284 random swab samples of post-operative surgical wound patients (142 from each) were collected from Central Hospital Benin (CHB) and University of Benin Teaching Hospital (UBTH), Benin City.
Bacteriological Procedures/Identification of Isolates
All samples were aseptically inoculated onto blood, MacConkey, nutrient agar and incubated aerobically at 37 °C for 24 h and checked for colonial growth. Different P. aeruginosa strains were isolated from surgical wound samples. They were further identified by using morphological and physiological test (gram staining; oxidase; indole, methyl red, Voges-Proskauer and citrate [IMViC] test; nitrate reduction test and catalase; carbohydrate fermentation test for glucose, maltose, lactose, galactose and sucrose). All specimens were processed at the Lahor Research Laboratories, Benin City, Nigeria using standard microbiological methods. All isolates were identified using conventional techniques as described by Cheesbrough (17).
Screening Method for Multidrug Resistant P. aeruginosa
Antibiotic screening of Pseudomonas spp. isolated from surgical wound swab were carried out with commonly used antibiotic by the Kirby-Bauer disk diffusion method to identify multidrug resistant (MDR) Pseudomonas spp. The following antibiotic disks were used: augmentin (AUG 30 μg), ofloxacin (OFL 5 μg), cefixime (CXM 5 μg), gentamycin (GEN 30 μg), cefuroxime (CRX 30 μg), ceftazidime (CAZ 30 μg), ciprofloxacin (CPR 5 μg), nitrofurantion (NIT 300 μg) and interpretation of zones of inhibition according to the Clinical and Laboratory Standards Institute guidelines (18).
Bacteria Genomic DNA Extraction
All multidrug resistant P. aeruginosa isolates were subcultured overnight in Luria-Bertani broth (Merck, Germany) and DNA was extracted from typical colonies of P. aeruginosa strains using Zymo research DNA extraction kits (Irvine, CA, USA), according to manufacturer’s instructions.
Polymerase Chain Reaction Technique
PCR was employed for the amplification of Pseudomonas spp. and 16S rRNA primers separately (Table 1) in ABI9700 thermal cycler PCR machine at Lahor Research Laboratories, Benin City, Nigeria. All primers and PCR master mix 2× (New England Biolab, USA) was purchased from lnqaba Biotech, Hartfield, South Africa and was used according to the manufacturer’s instruction. The PCR run was performed in 25 μL reaction mixture containing one part of Quick load (2×) master mix, 1.25 μL of each forward and reverse primer (20 μM), 5.0 μL of nuclease free water and 5 μL of DNA template was added last. The PCR was started immediately as follows: Initial denaturation at 94 °C for 3 min; denaturation at 94 °C for 30 sec; annealing at 50 °C and 54 °C for 30 sec, respectively; extension at 72 °C for 1 min, for 35 cycles; final extension at 72 °C for 10 min; and final holding at 4 °C forever. The amplified PCR products (10 μL) were separated on a 1.0% agarose gel containing ethidium bromide in Tris/Borate/EDTA (TBE) buffer. Electrophoresis was performed at 90 volts for 60 min. Products were visualised in a UV transilluminator and photographed. Amplicon weights were calculated using size maker.
Table 1.
Sequence information of primer used
PCR Product Purification and Sequencing
Amplification and sequencing were done as described by Agbonlahor et al. (21), with the following modifications: Purification was done with the Applied Biosystems Incorporation (ABI) V3.1 Big dye kit according to manufacturer’s instructions. The labeled products were then cleaned with the Zymo Seq clean-up kit (USA) in accordance with manufacturer’s instructions. The ultra-pure DNA was sequenced with ABI3500XL analyser at Functional Bioscience, Madison, USA. Sequences data generated were analysed with Geneious version 9.0.5 and phylogenetic tree were constructed using neighbour-joining method as described by Agbonlahor et al. (21).
Statistical Analysis
Percentage multiple drug resistance isolates was calculated using the following equation:
Results
Biochemical Characterisation of Bacterial Isolates and Distribution of Etiologic Agents of Surgical Wound Infection
Two hundred and eighty-four postoperative wound swabs specimens were collected from patients in CHB and UBTH both in Benin City and analysed. A total of 99 (35%) of patients studied had wound infections. Phenotypic identification of these bacterial isolates using morphological and biochemical tests revealed rod shaped, Gram negative, motile, catalase, oxidase, glucose and citrate positive isolates as well lactose, urease, mannitol, coagulase negative which was suggestive of Pseudomonas spp. as shown in Table 2. From both locations, 62 (21.8%) patients (27 from CHB and 35 from UBTH) had Pseudomonas spp., 18 (6.3%) patients (8 from CHB and 10 from UBTH) had Escherichia coli, 12 (4.2%) patients (5 from CHB and 7 from UBTH) had Staphylococcus aureus. Pseudomonas spp. is the most isolated pathogen.
Table 2.
Morphological and biochemical characterisation of bacterial isolates
| Characterisation | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| Shape | Rods | Rods | Rods | Cocci |
| Gram’s staining | −ve | −ve | −ve | +ve |
| Motility | Motile | Motile | Motile | Non- motile |
| Catalase | +ve | +ve | +ve | +ve |
| Oxidase | −ve | +ve | −ve | −ve |
| Glucose | +ve | +ve | +ve | +ve |
| Sucrose | −ve | −ve | −ve | +ve |
| Maltose | +ve | −ve | −ve | +ve |
| Lactose | +v | −ve | −ve | +ve |
| Oxidation fermentation | Fermenter | Oxidiser | Facultative anaerobes | Fermenter |
| Mannitol | +ve | −ve | −ve | +ve |
| Urease | −ve | −ve | +ve | +ve |
| Citrate | −ve | +ve | −ve | +ve |
| Nirate | +ve | +ve | +ve | +ve |
| Indole | +ve | −ve | −ve | −ve |
| Methyl red | +ve | −ve | +ve | −ve |
| Coagulase | −ve | −ve | −ve | +ve |
| Bacteria suspected | Escherichia coli | Pseudomonas spp. | Proteus mirabilis | Staphylococcus aureus |
Notes: +ve = positive; −ve = negative; Group A, B, C, D = different isolates
Table 3.
Distribution of etiologic agents of surgical wound infection
| S/N | Bacterial isolates | UBTH | CHB |
|---|---|---|---|
| 1 | Pseudomonas spp. | 35 | 27 |
| 2 | Escherichia coli | 8 | 10 |
| 3 | Proteus mirabilis | 4 | 3 |
| 4 | Staphylococcus aureus | 7 | 5 |
|
| |||
| Total | 54 | 45 | |
Antibiotic Susceptibility and Resistance
Antibiotic susceptibility profile of all Pseudomonas spp. from both locations showed that 17 (63.0%) from CHB and 20 (57.1%) from UBTH had MDR ability against the tested antibiotics. Ceftazidime recorded highest resistance (85.2%) in isolates from CHB while isolates from UBTH showed highest resistance against nitrofuration (77.1%) followed by (68.6%) observed for gentamycin (Table 4).
Table 4.
Susceptibility profile of suspected Pseudomonas spp. isolates to tested antibiotics
| Class of antibiotics | Type of antibiotics | CHB n = 27 | UBTH n = 35 | ||
|---|---|---|---|---|---|
|
|
|
||||
| R | S | R | S | ||
| Penicillin | Augmentin (30 μg) | 17 | 10 | 22 | 13 |
| Aminoglycoside | Gentamycin (30 μg) | 19 | 8 | 24 | 11 |
| Cephalosporin | Ceftazidime (30 μg) | 23 | 4 | 21 | 14 |
| Cefuroxime (30 μg) | 19 | 8 | 20 | 15 | |
| Cefixime (5 μg) | 22 | 5 | 20 | 15 | |
| Nitrofuran | Nitrofuration (300 μg) | 18 | 9 | 27 | 8 |
| Quinolones | Ofloxacin (5 μg) | 16 | 11 | 18 | 17 |
| Ciprofloxacin (5 μg) | 13 | 14 | 13 | 22 | |
Notes: n = number of bacteria tested; R = number of bacteria resistant; S = number of bacteria sensitive
Amplification of Pseudomonas spp
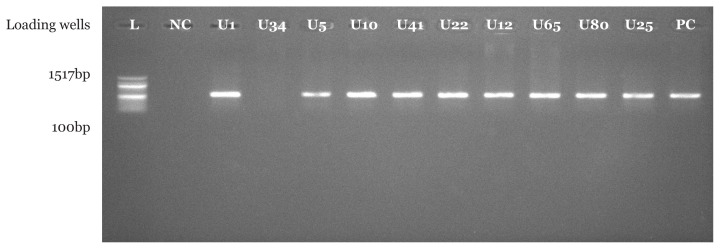
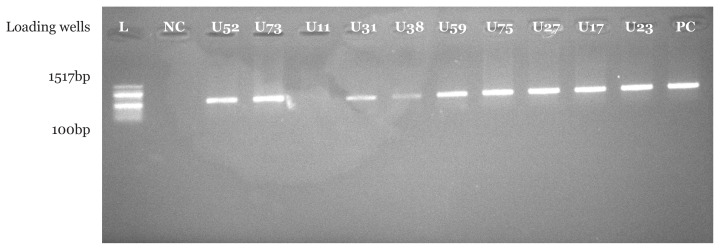
PCR amplification using Pseudomonas spp. specific primer set indicated that 16 (94.1%) suspected Pseudomonas spp. isolates from CHB and 18 (90.0%) suspected Pseudomonas spp. isolates from UBTH were confirmed to be Pseudomonas spp. with bands at 618 base pair which were clearly visible under UV transilluminator. In addition, a similar band was also seen for positive control strain with American type culture collection number 27852. As expected, no band was seen in the negative control where nuclease free water was used instead of bacterial DNA as shown in Figures 1–4.
Figure 1.
Molecular confirmation of Pseudomonas spp. using PCR technique. Isolates C20, C44, C31, C45, C52, C28, C84, C10 and C92 are positive control with bands at 618bp, isolate C2 is not Pseudomonas spp.
Notes: NC = negative control; PC is a positive control strain with American type culture collection number 27852
Figure 2.
Molecular confirmation of Pseudomonas spp. using PCR technique. Isolates C14, C77, C33, C60, C18, C15 and C78 are positive control with bands at 618bp
Notes: NC = negative control; PC = positive control strain with American type culture collection number 27852
Figure 3.
Molecular confirmation of Pseudomonas spp. using PCR technique. Isolates U1, U5, U10, U41, U22, U12, U65, U80 and U25 are positive with bands at 618bp. Isolates U34 is negative control for Pseudomonas sp. and PC is a positive control strain with American type culture collection number 27852
Figure 4.
Molecular confirmation of Pseudomonas spp. using PCR technique. Isolates U52, U73, U31, U38, U59, U75, U27, U17 and U23 are positive with bands at 618 bp. NC is a negative control; isolates U11 is negative control for Pseudomonas spp. and PC is a positive control strain with American type culture collection number 27852
16S rRNA Sanger Sequencing of Pseudomonas spp
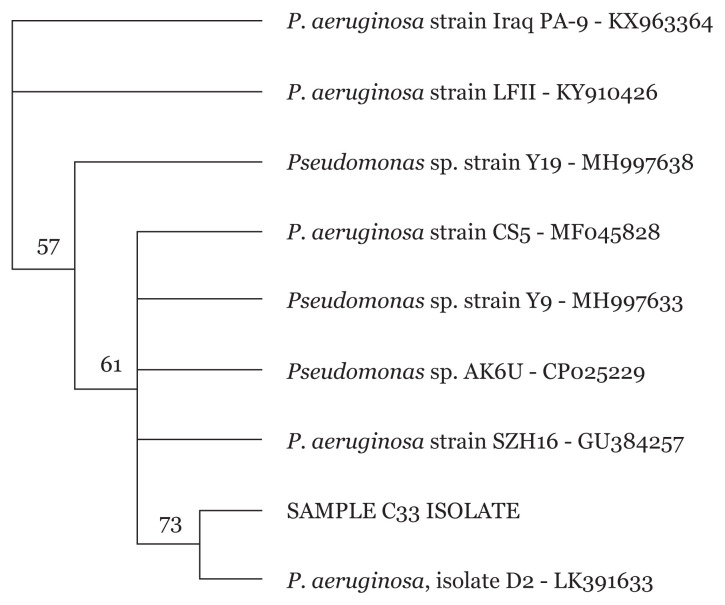
Sequencing of Pseudomonas spp. isolates were carried out to further identify the MDR Pseudomonas spp. isolates to the strain level. Phylogenetic tree of isolates revealed different P. aeruginosa strains for all 34 Pseudomonas spp. as exemplified in Figures 5–8.
Figure 5.
Phylogenetic analysis of clinical isolate based on the nucleotide sequence of part of the 16S rRNA. The phylogenetic tree was constructed by the neighbour-joining method programme in the Geneious package (version 9.0.5). The numbers at the forks show the numbers of occurrences of the repetitive groups to the right out of 100 bootstrap samples. Sample C33 isolate show close relation to NCBI-Blast P. aeruginosa strain SZH16 with accession number GU384267
Figure 6.
Phylogenetic analysis of clinical isolate based on the nucleotide sequence of part of the 16S rRNA. The phylogenetic tree was constructed by the neighbour-joining method programme in the Geneious package (version 9.0.5). The numbers at the forks show the numbers of occurrences of the repetitive groups to the right out of 100 bootstrap samples. Sample C78 isolate show close relation to NCBI-Blast P. aeruginosa isolate D2 with accession number LK391633
Figure 7.
Phylogenetic analysis of clinical isolate based on the nucleotide sequence of part of the 16S rRNA. The phylogenetic tree was constructed by the neighbour-joining method programme in the Geneious package (version 9.0.5). The numbers at the forks show the numbers of occurrences of the repetitive groups to the right out of 100 bootstrap samples. Sample U1 isolate show close relation to NCBI-Blast P. aeruginosa strain SWD with accession number DQ859983
Figure 8.
Phylogenetic analysis of clinical isolate based on the nucleotide sequence of part of the 16S rRNA. The phylogenetic tree was constructed by the neighbour-joining method programme in the Geneious package (version 9.0.5). The numbers at the forks show the numbers of occurrences of the repetitive groups to the right out of 100 bootstrap samples. Sample U80 isolate show close relation to NCBI-Blast P. aeruginosa strain DKH-3 with accession number JQ773433.1
Prevalence of MDR P. aeruginosa Strains from CHB and UBTH
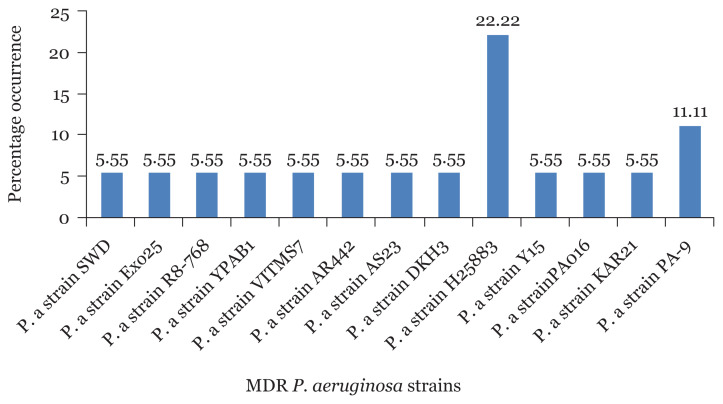
The percentage occurrence of MDR P. aeruginosa strains among sequenced isolates from CHB revealed that P. aeruginosa strains H25883 had the highest percentage occurrence of 18.75% followed by P. aeruginosa strains AR7-520 and PA006 with 12.5%, respectively (Figure 9). In the same vein, P. aeruginosa strains H25883 also recorded the highest percentage occurrence of (22.22%) in UBTH followed by P. aeruginosa strains KAR21 with 11% as shown in Figure 10.
Figure 9.
Percentage occurrence of MDR P. aeruginosa strains isolated from surgical wound in CHB
Figure 10.
Percentage occurrence of MDR P. aeruginosa strains isolated from surgical wound in UBTH
Discussion
P. aeruginosa, a non-fermentative gram-negative bacterium, is currently the second most widespread nosocomial bacterium, after Acinetobacter species (22). P. aeruginosa broadly exists in hospital environments (23) and medical equipment (22). Infections caused by P. aeruginosa are particularly tough to treat as the microbe has intrinsic resistance to a large number of antimicrobial agents. Furthermore, with the acquisition of antibiotic-resistant genes, it is becoming more difficult to cure infections caused by this organism (24).
Despite the existing and extensive reports on the prevalence of P. aeruginosa in hospital environments, there is still a paucity of research finding on molecular identification of multidrug P. aeruginosa strains from surgical wounds, particularly in Benin City, Nigeria. Hence, data from the present study revealed that the P. aeruginosa strain showed the highest antibiotic resistance to ceftazidime in isolates from CHB and nitrofurantoin in isolates from the UBTH. Lowest resistance was observed for ciprofloxacin for both locations. In comparison to previously reported data, the result of the present study corroborates the finding of Carroll et al. (25) and Leone et al. (26) that also reported a high antibiotic resistance rate towards ceftazidime and gentamycin antibiotics in both clinical and environmental isolates. Additionally, the study of Ruiz et al. (27) reported that clinical bacterial isolates are less susceptible to antimicrobial agents than environmental bacterial isolates due to their selective action (27).
Molecular characterisation of Pseudomonas spp. isolated from surgical wound infections specimens from both locations showed that 16 (94.1%) out of 17 isolates were confirmed to be Pseudomonas spp. in CHB, while 18 (90%) out of 20 were confirmed to be Pseudomonas spp. with bands at 618 base pair for test isolates and positive control strains. The above-mentioned genus-specific PCR assays indicated that three clinical isolates had been misidentified using phenotypic laboratory methods. This signifies the efficiency of the molecular characterisation method over phenotypic characterisation. With regard to a study by Spilker et al. (19), the genus-specific PCR assays indicated that several of the 66 clinical isolates were misidentified by the referring laboratories (19).
Sequence analysis of 16S rRNA is now being used as a taxonomic ‘gold standard’ in determining the phylogenies of bacterial species (28). The 16S rRNA gene sequences comprise hypervariable regions with high conservation that can differentiate species-specific signature sequences helpful in the classification of bacteria (29, 30). Going forward, 34 (91.9%) Pseudomonas spp. were further examined by 16S rRNA sequence analysis, and in each case, the PCR assay results were consistent. Thus, when this set of isolates was assessed against the 16S rRNA sequence, the sensitivity and specificity of both PCR assays were again 100%. It has also been reported that selective amplification of Pseudomonas 16S rRNA analysis is used to detect and differentiate Pseudomonas species from clinical and environmental samples (30). The present results agree with the finding of Didelot et al. (32) who reported that 16S rRNA gene sequencing is now common in medical microbiology as a quick and inexpensive alternative to phenotypic approaches of bacterial identification.
The result from the phylogenetic trees showed that MDR P. aeruginosa strain H25883 was the predominant strain in both locations (CHB and UBTH) with 18.75% and 22.22%, respectively. P. aeruginosa strains AR7-520 and PA006 with 12.5% were observed in CHB, making it the second predominant strains. However, many studies reported that P. aeruginosa has been mostly isolated from post-operative surgical wounds regardless of the site of infection and location of samples as a result of its high survival uniqueness in the hospital setting (33, 34). It has been ranked second among nosocomial disease-causing microbes. They are isolated from hospitals frequently, contaminating hospital equipment such as sinks used for wound dressing and other surgical tools. Furthermore, many antimicrobial-resistant strains continue to exist in apparently sterile hospital equipment, therefore, making it a precarious nosocomial pathogen broadly dispersed in the hospital environments where they are most difficult to eliminate (35).
The 16S rRNA sequence analysis on all identified MDR isolates was carried out and the sequence data confirmed the PCR results. Though our PCR and DNA sequence analyses showed bacterial isolates that were misidentified by phenotypic testing, we must point out that this study was not intended to determine the incidence of misidentification of post-surgical wound bacterial isolates neither to evaluate the relative precision of different phenotypic identification systems. Both assays have 100% sensitivity and specificity for their intended targets. We have also established the utility of these PCR assays in precisely identifying P. aeruginosa strains among isolates not correctly identified by phenotypic analyses. These assays should serve as a valuable accessory in the evaluation of gram-negative non-fermenting bacteria recovered from surgical wound isolates.
Conclusion
The results obtained from our study revealed that the 16S rRNA-based PCR and sequencing are highly sensitive, precise and consistent for the identification of P. aeruginosa strains isolated from post-operative surgical wound infections than conventional bacterial phenotypic methods. Our finding further highlights the use of DNA sequencing of the 16S rRNA gene as an effective tool to study bacterial phylogeny and taxonomy associations between bacteria and bacterial detection as well. Thus, early identification and control of this pathogen have become increasingly important.
Acknowledgements
Our sincere gratitude to Professor Dennis E. Agbonlahor, MD/CEO of Lahor Research Laboratories and Medical Centre, Head of Laboratory Department, Dr Joy Ehiaghe for her encouragement and the entire staff of Lahor Research Laboratories and Medical Centre for their contributions.
Footnotes
Conflict of Interest
None.
Funds
None.
Authors’ Contributions
Conception and design: EOA, SOH
Analysis and interpretation of the data: EOA, NSU
Drafting of the article: EOA
Critical revision of the article for important intellectual content: SOH, NSU
Final approval of the article: EOA, SOH, NSU
Provision of study materials or patients: EOA
Statistical expertise: EOA
Ethics of Study
Approval was obtained from the UBTH and CHB ethical committee, and all the patients consented to collection of samples after being educated on the objectives of the study.
References
- 1.Mohammed A, Adeshina GO, Ibrahim YKE. Retrospective incidence of wound infections and antibiotic sensitivity pattern: a study conducted at the Aminu Kano Teaching Hospital, Kano, Nigeria. Int J Med Sci. 2013;55:60–66. doi: 10.5897/IJMMS12.114. [DOI] [Google Scholar]
- 2.Heggars JP. Assessing and controlling wound infections. Clin Plas Surg. 2003;30(1):25–35. doi: 10.1016/s0094-1298(02)00072-x. [DOI] [PubMed] [Google Scholar]
- 3.Anaya DA, Dellinger EP. Challenges in the prevention of surgical site infections. Infections in Medicine. 2006;23:12–26. [Google Scholar]
- 4.Pallavali RR, Degati VL, Dakshayani L, Reddy MC, Durbaka VRP. Isolation and in vitro evaluation of bacteriophages against MDR-bacterial isolates from septic wound infections. PLoS One. 2017;12(7):e0179245. doi: 10.1371/journal.pone.0179245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garbe D, Thiel IV, Mootz HD. Protein transsplicing on an M13 bacteriophage: towards directed evolution of a semisynthetic split intein by phage display. J Pept Sci. 2010;16(10):575–581. doi: 10.1002/psc.1243. [DOI] [PubMed] [Google Scholar]
- 6.Kwiatek M, Mizak L, Parasion S, Gryko R, Olender A, Niemcewicz M. Characterization of five newly isolated bacteriophages active against Pseudomonas aeruginosa clinical strains. Folia Microbiol (Praha) 2015;60(1):7–14. doi: 10.1007/s12223-014-0333-3. [DOI] [PubMed] [Google Scholar]
- 7.Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7(39):1–29. doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehiaghe JI, Nwobu RAU, Ehiaghe FA, Agbakoba NR, Agbonlahor DE, Uwabor CI. Plasmid profiling of multidrug resistant bacteria isolates from surgical site infection in Nigeria. Am J Biotechnol Mol Sci. 2016;5(1):23–32. [Google Scholar]
- 9.Peng Y, Bi J, Shi J, Li Y, Ye X, Chen X. Multidrug-resistant Pseudomonas aeruginosa infections pose growing threat to health care-associated infection control in the hospitals of Southern China: a case-control surveillance study. Am J Infect Control. 2014;42:1308–1311. doi: 10.1016/j.ajic.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Jin WY, Jang SJ, Lee MJ, Park G, Kim MJ, Kook JK. Evaluation of VITEK2, MicroScan, and Phoenix for identification of clinical isolates and reference strains. Diagn Microbiol Infect Dis. 2011;70:442–447. doi: 10.1016/j.diagmicrobio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Webster TA, Sismaet HJ, Conte JL, Chan IP, Goluch ED. Electrochemical detection of Pseudomonas aeruginosa in human fluid samples via pyocyanin. Biosens Bioelectron. 2014;60:265–270. doi: 10.1016/j.bios.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Mauch RM, Rossi CL, Ribeiro JD, Ribeiro AF, NolascodaSilva MT, Levy CE. Assessment of IgG antibodies to Pseudomonas aeruginosa in patients with cystic fibrosis by an enzyme-linked immunosorbent assay (ELISA) Diagn Pathol. 2014;9:158. doi: 10.1186/s13000-014-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aghamollaei H, Moghaddam MM, Kooshki H, Heiat M, Mirnejad R, Barzi NS. Detection of Pseudomonas aeruginosa by a triplex polymerase chain reaction assay based on lasI/RandgyrB genes. J Infect Public Health. 2015;8:314–322. doi: 10.1016/j.jiph.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Qin X, Emerson J, Stapp J, Stapp L, Abe P, Burns JL. Use of real-time PCR with multiple targets to identify Pseudomonas aeruginosa and other non-fermenting gram-negative bacilli from patients with cystic fibrosis. J Clin Microbiol. 2003;41:4312–4317. doi: 10.1128/JCM.41.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deschaght P, VanDaele S, DeBaets F, Vaneechoutte M. PCR and the detection of Pseudomonas aeruginosa in respiratory samples of CF patients: a literature review. J Cyst Fibros. 2011;10:293–297. doi: 10.1016/j.jcf.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Cai Y, Zhou G, Shi X, Su J, Guanwu C, et al. Rapid Sanger sequencing of the 16S rRNA gene for identification of some common pathogens. PLoS One. 2014;9(2):e88886. doi: 10.1371/journal.pone.0088886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheesbrough M. District laboratory practice manual in tropical countries. Part 2. New York: Cambridge University Press; 2000. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Twenty first informational supplement. Wayne PA: Clinical and Laboratory Standards Institute; 2011. (CLSI document M100-S21). [Google Scholar]
- 19.Spilker T, Coenye T, Vandamme P, LiPuma JJ. PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J Clin Microbiol. 2004;42:2074–2079. doi: 10.1128/jcm.42.5.2074-2079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank AJ, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol. 2008;74(8):2461–2470. doi: 10.1128/AEM.02272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agbonlahor DE, Ehiaghe JI, Eremwanarue OA, Ehiaghe AF, Oviasogie FE, Iyen R, et al. A primal incrimination of Cedecea davisae with post-prostatectomy urinary tract infection in Nigeria. International Journal of Biological and Chemical Science. 2018;12(2):676–688. [Google Scholar]
- 22.Dong D, Zou D, Liu H, Yang Z, Huang S, Liu N, et al. Rapid detection of Pseudomonas aeruginosa targeting the toxA gene in intensive care unit patients from Beijing, China. Front Microbiol. 2015;6(6):1100. doi: 10.3389/fmicb.2015.01100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loveday HP, Wilson JA, Kerr K, Pitchers R, Walker JT, Browne J, et al. Association between healthcare water systems and Pseudomonas aeruginosa infections: a rapid systematic review. J Hosp Infect. 2014;86:7–15. doi: 10.1016/j.jhin.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Potron A, Poirel L, Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int J Antimicrob Agents. 2015;45:568–585. doi: 10.1016/j.ijantimicag.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Carroll MR, Syrmis MW, Wainwright CE, Greer RM, Mitchell P, Coulter C. Clonal strains of Pseudomonas aeruginusa in paediatric and adult cystic fibrosis units. Eur Respir J. 2004;24:101–106. doi: 10.1183/09031936.04.00122903. [DOI] [PubMed] [Google Scholar]
- 26.Leone I, Chirillo MG, Raso T, Zucca M, Savoia D. Phenotypic and genotypic characterization of Pseudomonas aeruginosa from cystic fibrosis patients. Eur J Clin Microbiol Infect Dis. 2008;27(11):1093–1099. doi: 10.1007/s10096-008-0551-1. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz L, Domínguez MA, Ruiz N, Viñas M. Relationship between clinical and environmental isolates of Pseudomonas aeruginosa in a hospital setting. Arch Med Res. 35(3):251–257. doi: 10.1016/j.arcmed.2004.02.005. 200. [DOI] [PubMed] [Google Scholar]
- 28.Trivedi MK, Branton A, Trivedi D, Nayak G, Gangwar M, Snehasis J. Bacterial identification using 16S rDNA gene sequencing and antibiogram analysis on biofield treatedPseudomonas fluorescens. Clin Med Biochem Open Access. 2015;1:101. doi: 10.4172/2471-2663.1000101. [DOI] [Google Scholar]
- 29.Guasp C, Moore ER, Lalucat J, Bennasar A. Utility of internally transcribed 16S-23S rDNA spacer regions for the definition of Pseudomonas stutzeri genomovars and other Pseudomonas species. Int J Syst Evol Microbiol. 2000;50(4):1629–1639. doi: 10.1099/00207713-50-4-1629. [DOI] [PubMed] [Google Scholar]
- 30.Adékambi T, Colson P, Drancourt M. rpoB-based identification of non-pigmented and late-pigmenting rapidly growing mycobacteria. J Clin Microbiol. 2003;41(12):5699–708. doi: 10.1128/jcm.41.12.5699-5708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duineveld BM, Kowalchuk GA, Keijzer A, van Elsas JD, van Veen JA. Analysis of bacterial communities in the rhizosphere of chrysanthemum via denaturing gradient gel electrophoresis of PCR-amplified 16S rRNA as well as DNA fragments coding for 16S rRNA. Appl Environ Microbiol. 2001;67(1):172–178. doi: 10.1128/AEM.67.1.172-178.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Didelot X, Bowden R, Wilson DJ, Peto TEA, Crook DW. Transforming clinical microbiology with bacterial genome sequencing. Nat Rev Genet. 2012;13(9):601–612. doi: 10.1038/nrg3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christopher AE, Omoregie R, Igbarumah IO, Onemu S. Microbiology of wound infections among patients of a tertiary hospital in Benin City, Nigeria. J Res Health Sci. 2011;11(2):109–113. [PubMed] [Google Scholar]
- 34.Dalhatu A, Yunusa U, Ahmad S, Timothy G, Jari S, Musa M. Bacterial agents of abdominal surgical site infections in General Hospital Funtua, Katsina State, North-Western Nigeria. J Dent Med Sci. 2014;13(6):48–52. [Google Scholar]
- 35.Masaadeh HA, Jaran AS. Incident of Pseudomonas aeruginosa in postoperative wound infection. Am J Infect Dis. 2009;5(1):1–6. [Google Scholar]