Abstract
Korea’s overall food safety management policies reflect the Codex guidelines in terms of risk analysis and standards. Korea’s food safety management plan, which is established every 3 years, includes the pre-emptive management of risk factors and provides food safety information on the basis of scientific evidence. Since officially joining Codex as a member country in 1971, Korea has led the establishment of six Codex standards, including those for Kimchi and Gochujang. Moreover, Korea’s food standards are harmonized with Codex’s risk assessment system. This review aims to examine the current status and trends of Codex and to summarize and compare the food safety management systems and food standards between Korea and Codex.
Keywords: Food safety management, Codex, International standard
Introduction
Increasing levels of global food trade have led to fears regarding food safety that are felt in several countries. Incidents such as the introduction of melamine to milk in 2008, the occurrence of horse meat in hamburgers in 2013, and the presence of fipronil in eggs in 2017 affected global food industries (Winickoff and Bushey 2010). Thus, nontariff trade barriers have been raised as countries strengthen their food safety measures to protect consumers. The World Trade Organization (WTO), established in 1995, is the only intergovernmental organization that deals with international trade rules, and the body governs trade relations between member states. The WTO strives to reduce trade barriers between countries and plays a role in resolving trade disputes (WTO 2000b). Particular agreements have been developed to address hygiene and food safety [the Sanitary and Phytosanitary Measurement (SPS) Agreement] and Technical Barriers to Trade (TBT) (WTO 2000a). The SPS Agreement was established in 1995 to provide a set of standards for food safety and animal and plant health. Under the agreement, member states may individually establish national standards, but these must be based on scientific evidence and should comply with international standards, guidelines, and recommendations. The WTO SPS Agreement recommends the international food standard established by the Codex Alimentarius Commission (CAC) (Veggeland and Borgen, 2005).
Korea joined Codex in 1971 (Codex 1971), and to date, Korea has participated annually in commission summits and committee meetings to harmonize Korea’s food standards in line with those of Codex. The Korean delegation also recently attended the 32nd General Principles Committee meeting held as a virtual session due to COVID-19 in 2021, at which it was suggested that Codex member states discuss the meaning and development of the organization to mark its 60th anniversary in 2023 (Codex 2021e). Additionally, as the 2021 UN Food System Summit will be held this September, Codex recommended that member states focus on the themes of “Safe and nutritious food” and “Sustainable and healthy consumption” by looking over their food safety management systems (Codex 2021d). Codex strives toward several of the UN sustainable development goals adopted in 2015 including (1) No food poverty, (2) Zero hunger, (3) Good health and well-being, (4) Decent work and economic growth, (12) Responsible consumption and production, and (17) Partnerships for the goals (FAO and WHO 2020).
In this study, we review the current status of Korea’s participation in Codex and efforts toward the harmonization of food standards between Korea and the commission.
Status of the codex alimentarius commission
History of codex
The Food and Agriculture Organization (FAO)/World Health Organization (WHO) CAC held its first meeting in Rome between June 25 and July 3, 1963, with participants joining from 30 member countries and 16 international governmental and nongovernmental organizations (Codex 1963). Before the commission was established, a resolution was passed by the FAO in November 1961, and the commission was discussed at a joint FAO/WHO conference on food standards in Geneva in October 1962 (FAO and WHO 2018). At the 1962 conference, the operational guidelines for the CAC, its first meeting date, and financial statements were established (FAO and WHO 1962). After 6 months, articles from the CAC were adopted at the 16th World Health Assembly (FAO and WHO 2018).
The importance of the Codex standards has become increasingly highlighted since 1995. With the launch of the WTO in 1995, the SPS and TBT Agreements were brought into force. Codex standards are the only recognized international food safety guidelines, and they are used as an international reference in the event of trade disputes and friction between countries (Codex 2017f).
Purpose of codex
It is emphasized that Codex Alimentarius international food standards and guidelines are aimed at both protecting consumer safety and ensuring that fair trade occurs internationally. The report from the joint FAO/WHO conference in 1962 describes the purpose of the CAC and highlights that the dual aims of the Codex standards are to protect public health and safeguard fair food trade practices (FAO and WHO 1962). Because of these aims, there is difficulty in establishing a food standard if none of the criteria are met. For example, recently, there have been some in-depth discussions to establish a standard for grain size in quinoa. At the 42nd CAC, some opposed the removal of a regulation regarding the grain size of quinoa from the standards, because it is important to fair trade; however, the other side supported the removal of this regulation as the grain size of quinoa is more of a commercial issue than a matter of food safety (Codex 2019j). Consequently, the grain size of quinoa was removed from the standard (CXS 333–2019) (Codex 2020c).
Structure of codex
Executive committee
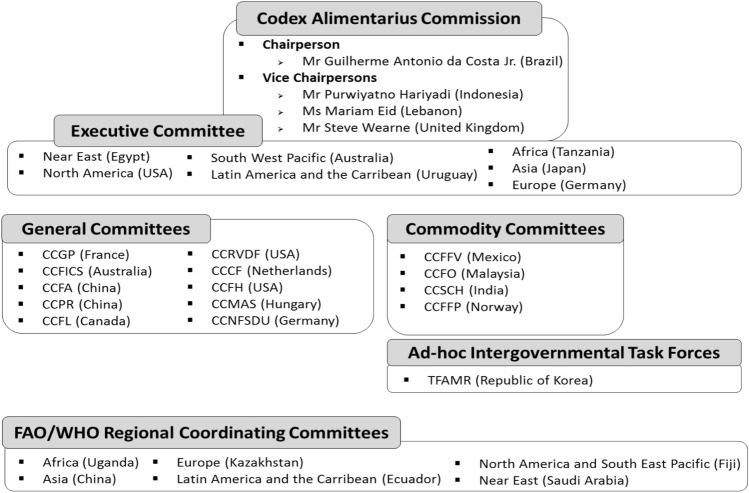
The Codex Executive Committee comprises the Chairperson, three vice chairmen, seven regional representatives, and six regional coordinators selected from the Regional Coordinating Committee and Codex subcommittees, and it serves to discuss international food standards by sector and support the overall activities of Codex (Fig. 1). The Executive Committee also responds to proposals from Codex member states and monitors operational regulations, the status of Codex strategic plans, and important events presented by the FAO and WHO (FAO and WHO 2019).
Fig. 1.
Current structure of the Codex Alimentarius Commission (2021)
The Codex Executive Committee currently meets twice a year. The meetings are attended by seven regional representatives and six regional coordinators, the chairperson of the CAC, three vice chairmen, the Codex secretariat, and staff from the FAO and WHO. The meeting is not open to member states as a real-time webinar, but its agenda and outcome reports are transparently posted on the Codex website. The discussions are reported to the CAC so that they may thoroughly consider the matters raised. Typically, an executive board meeting is held just before the Codex Conference to focus on critically reviewing the agenda for each Codex committee meeting and, particularly, matters that are to be adopted by the CAC (Codex 2020a).
Codex committees
There are currently 14 committees (Fig. 1) and one Ad hoc Codex Intergovernmental Task Force in active. Among these, 10 committees establish general regulations within the scope of each committee. Four committees serve as commodity committees and establish specifications such as definitions, quality standards, and manufacturing processing standards for food products within the scope of each subcommittee (FAO and WHO 2019). The Codex Committee on General Principles (CCGP) is a committee under French chairmanship that discusses amendments to the Codex Procedural Manual or general Codex operations (FAO and WHO 2019). The Codex Committee on Food Import and Export Inspection and Certification Systems (CCFICS) establishes import and export procedures, food product certification standards, and guidance for international food trade, with Australia as the host country (FAO and WHO 2019). The Codex Committee on Food Additives (CCFA) and Pesticide Residues (CCPR) respectively establish the maximum standards and residue limits for food additives and pesticide residues. They may also assess substances prioritized for evaluation by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) and Pesticide Residues (JMPR) for discussion of residue limits with member states on the basis of the results of risk assessments (Codex 2021a; FAO and WHO 2019). The CCPR works on the classification of animals and plants consumed as food and feed (Codex 2021b). The Codex Committee on Residues of Veterinary Drugs in Foods (CCRVDF) and Food Hygiene (CCFH), chaired by a member from the United States, respectively determine the standard allowance for the presence of residual veterinary drugs in food and establish guidelines on general food hygiene conditions and the management of microbial hazards (FAO and WHO 2019). The Codex Committee on Food Labelling (CCFL), which is chaired by Canada, discusses the labelling of all foods and, if necessary, approves the food labelling proposals requested by each Codex committee (FAO and WHO 2019). The CCFL works on guidelines for the e-commerce of foods that are actively traded internationally (Codex 2019j; Codex 2019a; Codex 2019k). The Codex Committee on Contaminants in Foods (CCCF) and Methods of Analysis and Sampling (CCMAS) are respectively chaired by the Netherlands and Hungary. The CCCF establishes maximum limits for contaminants in food and feed and develops practices for reducing contaminants in food, whereas the CCMAS examines whether the testing methods presented by Codex committees or member states concur with internationally recognized testing methods (FAO and WHO 2019). The Codex Committee on Nutrition and Foods for Special Dietary Uses (CCNFSDU), chaired by Germany, is responsible for discussing overall nutritional issues and establishing nutritional standards for food that is, particularly, given to infants or provided for people with special dietary needs (FAO and WHO 2019). The commodity committees, Codex Committee on Fresh Fruits and Vegetables (CCFFV), Fats and Oils (CCFO), and Spices and Culinary Herbs (CCSCH), respectively establish international food standards for fresh fruit and vegetables, edible oils, and spices and are chaired by Mexico, Malaysia, and India (Codex 2019h; FAO and WHO 2019). The Codex Committee on Fish and Fishery Products (CCFFP), the only committee that works on correspondence within active commodity committees, was previously adjourned but was approved by the 43rd Commission to add Sardinella lemuru to the Standard for Canned Sardines and Sardine-Type Products (CXS 94–1981) as proposed by the Philippines (Codex 2020c).
Regional coordinating committees
The Regional Coordinating Committee represents six regions, and their meeting is held every 2 years to investigate and discuss food-related issues of interest within each regional member state and to establish regional standards. In 2019, regional coordinators from five regions, excluding Europe (reappointed), were newly elected at the 43rd Commission. These coordinators were from Uganda (Africa), China (Asia), Kazakhstan (Europe), Ecuador (Latin America and the Caribbean), Fiji (North America and the South West Pacific), and Saudi Arabia (Near East) (Codex 2020c).
Procedures for the elaboration of codex standards
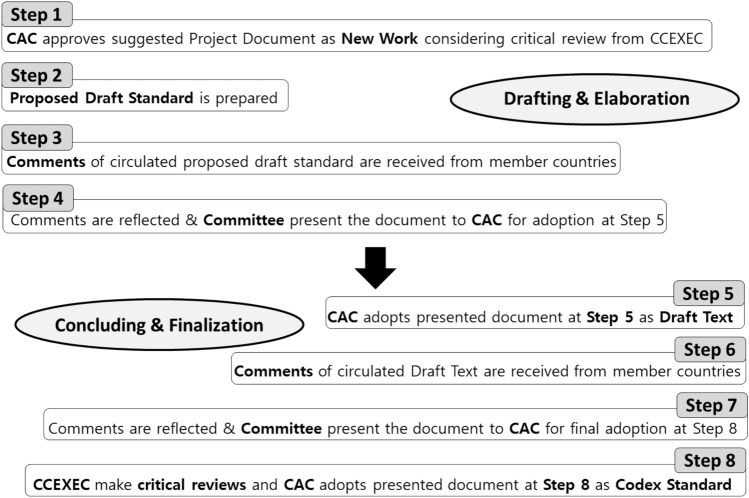
Codex establishes standards on the basis of the opinions of Codex member states following systematic procedures as shown in Fig. 2 to establish international food standards. Proposals for new work can be made by member states, agencies, and Codex committees. First of all, when proposing new work, a discussion paper is created and circulated to the relevant committees or the Executive Committee to identify and outline the key points. Based on this discussion paper, if details such as the definition, scope, redundancy, and quality standards of the specifications reach consensus, a project document is submitted to the related committee. Once this document has been submitted to the Executive Committee, the project has been approved through the related committee, and an agreement has been reached, the commission approves the new work. After approval, the committee in charge prepares draft standards and circulates documents to reflect the opinions of the member states. The revised draft standards, reflecting the opinions of the member states, are presented to the commission for adoption in step 5. If the final draft of the standard is prepared on the basis of a consensus within the related committee, it can be introduced for adoption by the commission at step 8 and then made into a final Codex document (FAO and WHO 2019). When there is no step-by-step agreement, it may take a long time for the draft standard to move forward, and sometimes, the issues may require further discussion back at the lower levels. Therefore, it can be difficult for an agreement between member states to be reached regarding a discussion paper or project document before it is approved as new work (Codex 2014a; Codex 2019i).
Fig. 2.
Uniform general procedure for the establishment of Codex Standard
Recent committee issues
Emerging issues in codex regions
The Codex regional committees conducted a survey in 2019 on emerging issues regarding food safety that may have huge impacts over the next 5–10 years. Interestingly, not only region-specific food safety issues but also common interests across the six regions were seen (Table 1). Four major concerns were antimicrobial resistance, weaknesses in national food management systems, climate change, and food fraud (Codex 2019c; Codex 2019b). In response to the worries of member countries regarding antimicrobial resistance, Codex organized the Ad hoc Codex Intergovernmental Task Force on Antimicrobial Resistance (TFAMR) and discussed a revision to the Code of Practice to Minimise and Contain Antimicrobial Resistance (CAC/RCP 61–2005) and development of the Guidance on Integrated Surveillance of Antimicrobial Resistance (Codex 2019e).
Table 1.
Emerging issues survey results from the FAO/WHO Regional Coordinating Committee 2019
| Regional committee | Emerging issues |
|---|---|
| CCAFRICA(a) | Antimicrobial resistance |
| Pesticides residues | |
| Weakness of food control systems/legal framework | |
| Food fraud | |
| Contamination of water used for food production and processing | |
| Consumer awareness | |
| Aflatoxins | |
| Climate change | |
| Others (risk analysis, biotechnology food/biofortification, heavy metal contamination of food, use of growth hormones, food waste, illegal animal slaughtering, food safety tool kits, budget allocation for food safety control, genetically modified organisms (GMO), marine biotoxins, street foods) | |
| CCASIA(b) | Pesticide residues |
| Veterinary drug residues and anti-microbial resistance (AMR) | |
| Risk-based control of imported food | |
| National food safety regulatory authorities | |
| Food standards bodies | |
| Climate change | |
| Food fraud and authenticity | |
| Food safety reporting in the media | |
| Online sale of food | |
| Novel foods | |
| CCEURO(c) | Food fraud |
| Antimicrobial resistance | |
| Contaminants and food additives | |
| New technologies/scientific progress | |
| Climate change and related issues | |
| Limited resources to manage food safety risks | |
| Noncommunicable diseases | |
| Legislation and implementation | |
| New distribution channels | |
| Problems of food safety management along the food chain | |
| CCLAC(d) | Antimicrobial resistance |
| Novel technologies | |
| Climate change | |
| Food control management | |
| Information education and training | |
| Inspection services | |
| Food law and regulations | |
| Laboratory services: food monitoring and epidemiological data | |
| CCNASWP(e) | Limited support to manage food regulatory systems |
| Climate change | |
| Innovative food technologies | |
| Noncommunicable diseases | |
| Limited national codex committee | |
| Risk communication | |
| Increased foodborne disease transmission | |
| Pesticide residues on food crops (MRLs) | |
| Antimicrobial resistance | |
| Veterinary drugs residues (MRLs) | |
| Human and environmental health risk from foreign food waste | |
| CCNE(f) | Chemical food contamination |
| Weakness of food control systems/legal framework | |
| Antimicrobial resistance | |
| Management of innovation/novel food | |
| Food fraud | |
| Others (general standards for food additives, climate change, regulation of food contact materials (FCM), rapid alert system for food safety, new distribution channels, e.g., internet food trade, spreading of food rumors, artificial intelligence, genetically modified organisms (GMO), food commodity standards) |
(a) Codex Alimentarius (Codex). Joint FAO/WHO Food Standards Program FAO/WHO Coordinating Committee for Africa, 23rd session, Agenda Item 3.1. Kenya (2019c)
(b) Codex Alimentarius (Codex). Joint FAO/WHO Food Standards Program FAO/WHO Coordinating Committee for Asia, 21st session, Agenda Item 3.1. India (2019b)
(c) Codex Alimentarius (Codex). Joint FAO/WHO Food Standards Program FAO/WHO Coordinating Committee for Europe, 31st session. Agenda Item 3.1. Kazakhstan (2019d)
(d) Codex Alimentarius (Codex). Joint FAO/WHO Food Standards Program FAO/WHO Coordinating Committee for Latin America and the Caribbean, 21st session. Agenda Item 3.1. Chile (2019e)
(e) Codex Alimentarius (Codex). Joint FAO/WHO Food Standards Program FAO/WHO Coordinating Committee for North America and the South West Pacific, 15th session. Agenda Item 3.1. Vanuatu (2019f)
(f) Codex Alimentarius (Codex). Joint FAO/WHO Food Standards Program FAO/WHO Coordinating Committee for the Near East, 10th session. Agenda Item 3.1. Italy (2019 g)
Additionally, the poor organization of food safety management systems was noted as a future food safety issue. Member countries in the Near East and Africa highlighted the lack of any obvious description of the roles played by the authorities regarding food safety management and collaboration among food safety-related agencies (Codex 2019c; Codex 2019g). Climate change was referred to as a direct threat to food safety and security (Codex 2019c; Codex 2019d). Rapid changes in temperature, abnormal rainfall, and sudden drought could cause imbalances in food production, which would have an economic impact and endanger public health. Regional member countries emphasized that climate change creates other biological issues associated with mycotoxins and microbes and mentioned the use of pesticides and veterinary drugs (Codex 2019c).
Lastly, the rapid flow of large amounts of information and the international food trade were indirectly increasing concerns regarding food fraud among member countries. In the European region, a survey found that food fraud represented one of the most pressing emerging issues in food safety (Codex 2019d). CCASIA also considered the seriousness of food fraud and noted that food and food chains that are vulnerable to food fraud must be identified and then managed (Codex 2019i).
Global pandemic and codex
Beginning in 2020, the unprecedented global pandemic made it difficult for Codex to implement its operational and strategic plans. Particularly, because of difficulties with international travel and various national responses to COVID-19, all the Codex committee meetings planned for 2020 were cancelled or postponed. In 2021, the Codex secretariat and chair countries are considering holding the meetings as video conferences to implement Codex policies (Codex 2021c).
Codex has discussed the need for further discussion and improvement due to the spread of COVID-19 in terms of the Codex Trust Fund project, implementation of strategic plans for 2020–2025, and budgeting plans post 2020 (Codex 2020b). In response to these concerns, the Codex Secretariat promised to provide information regarding the 2020–2021 working plan by closely examining the impact of the COVID-19 pandemic on the Codex budget (Codex 2020c).
Korea’s participation in codex activities
Korean membership of codex
Korea officially joined Codex as a member country in 1971 (Codex 1971) and attended its first meeting at the 10th commission, which convened in Rome, Italy (Codex 1974). Korea was already actively participating in the activities of FAO and WHO, organizations it joined in 1949 (FAO 2019; WHO 2016). The number of Codex members had increased from 75 to 89 countries compared with the 7th commission, including 28 countries from Europe, 2 from North America, 19 from Latin America, 19 from Africa, 2 from South West Pacific, and 19 from Asia including Korea (Codex 1971). Subsequently, Korea joined the first FAO/WHO Regional Coordinating Committee, which convened in India in 1978, along with India, Japan, Kuwait, Malaysia, the Philippines, and Thailand (Codex 1978).
Roles of competent agencies
Several Korean government and competent agencies cooperate and correspond with Codex meetings and activities. Korea’s government bodies that respond to Codex include the Ministry of Food and Drug Safety (MFDS), the Ministry of Agriculture, Food and Rural Affairs (MAFRA), the Ministry of Oceans and Fisheries (MOF), and the Korea Food Research Institute (KFRI). MFDS is a regulatory body that ensures the safety of food for the Korean people and manages the manufacturing, processing, distribution, and consumption of food through the implementation of risk assessment and risk management procedures and by communicating information regarding risks to food safety (MFDS 2021). The MFDS published The Food Sanitation Act, which includes basic legislation on food hygiene, and the Food Code, which establishes specific regulations and standards for individual food items. Additionally, the National Institute of Food and Drug Safety Evaluation (NIFDS) is an agency directly affiliated with the MFDS that specializes in food risk assessment (NIFDS 2021). Furthermore, MAFRA serves as Korea’s official Codex Contact Point (CCP), being involved in the quality control of agricultural products, promoting the food industry, distributing agricultural products, and stabilizing prices (MAFRA 2021). MOF manages water resources and fosters fishery industries in Korea (MOF 2021). Within MOF, the Fisheries Infrastructure and Aquaculture Policy Division is in charge of Codex affairs, and the International Commerce and Trade Division works to respond to Codex’s agenda on fisheries products. Finally, KFRI is a government-funded research institute that has conducted practical research to develop Codex standards for products ranging from Korean kimchi to dried persimmons. Particularly, KFRI undertakes research and development projects relating to the globalization of traditional Korean food by analyzing foodstuffs and conducting standardized research and development studies (KFRI 2021).
Hosting codex committees
Since joining Codex in 1971, Korea has held two Codex meetings, the FAO/WHO coordinating committee for Asia (CCASIA) and the Ad hoc Codex Intergovernmental TFAMR. Korea coordinated the 26th session of the Commission, the 14th session of CCASIA in September 2004, and the 15th session of CCASIA in 2006 (Codex 2004a; Codex 2006). The country chaired the Codex Ad hoc Intergovernmental TFAMR to develop guidelines for the “Development of science-based risk assessment guidance regarding foodborne antimicrobial resistant microorganisms” (Codex 2007). The 43rd session of the Commission adopted the revised code of practice in step 5 and recommended that the 8th session of TFAMR reach a consensus on the code of practice to be adopted at step 8 and diligently work on integrated monitoring guidance (Codex 2020c).
Contributions to codex
Since the 10th session of the Commission, Korea has regularly dispatched Korean officials to the Codex Secretariat, as well as attending general meetings, regional meetings, and committees. The country recently donated to the Codex Trust Fund, a fund that promotes Codex participation by developing countries. The MFDS has sent food safety experts to the Codex Secretariat every 3 years since October 1997 to continue its food safety-related partnership with the commission, and it signed a donor agreement with WHO in 2020 to deliver donations to the FAO/WHO Project and Fund for Enhanced Participation in Codex (Codex Trust Fund). The Codex Trust Fund project is a cooperative funding project instigated by the FAO and WHO that helps member countries, particularly developing countries, to actively participate in Codex meetings and activities (Codex 2019a; FAO and WHO, 2015).
Harmonization of standards between Korea and codex
Korean food code
The MFDS formulates and distributes the food code, which comprises the standards and specifications for food under the Food Sanitation Act article 14 in Korea. The standards refer to the regulations for the manufacturing, processing, use, cooking, and preservation of food, and the specifications refer to the regulations for components of food such as ingredients and additives. It includes not only beneficial or necessary components but also elements that are harmful to humans such as bacteria, heavy metals, pesticides, and antibiotics.
The Korean Food Code comprises eight chapters. Chapter 1 explains the general principles, the application of standards and specifications, and the terms associated with food ingredients and their classification. Chapter 2 sets out the standards for food ingredients and the manufacturing and specifications of hazard components. Chapters 3 and 4 contain the standards and specifications for food given to infants and young children and long-shelf-life foods respectively. All of these chapters comply with the general standards of the Codex. Chapter 5 describes the standards and specifications for food commodities according to Codex standards. Chapter 6 provides the standards and specifications for prepared foods in the food service business sector. This chapter does not correspond with Codex standards, since these foods are consumed in restaurants and cafes and are not traded internationally. Chapter 7 explains sampling and handling methods, and chapter 8 describes the official analytical methods used for the specifications stated in the food code (MFDS 2019).
Codex texts
Food standards
Codex food standards relate to food commodities, general standards, general methods, regional standards, group standards, and recommended methods. The food commodity text describes the standards for each type of food, including scope, product definition, classification, essential composition and quality factors, food additives, contaminants, hygiene, labelling, and methods for analysis and sampling (FAO and WHO 2005). The standards for 180 food commodities including agricultural, fishery, and livestock products are currently in place (Codex 2017b). The general standards describe the principles for food commodities such as production procedures, limits of contaminants, and usage of food additives. There are 11 general standards in the Codex regulations such as those for the labelling of packaged food, irradiated food, food additives, and contaminants and toxins in food and feed, etc. The general methods are the standards for analyzing contaminants, irradiated foods, and food additives. Regional committees can establish regional standards for food trade within the region. Regional standards for 19 food commodities have been established for items such as chanterelles, edible sago flour, and tempe. Regional standards are often extended to international standards. For example, the Asian regional standard for Gochujang was adopted to international standards in 2020. Group standards are in place for products such as cheeses in brine, unripened cheeses, and fresh cheeses. Finally, recommended methods have been established for analysis and sampling protocols (Codex 2017b).
Guidelines
The Codex guidelines assist in interpreting the principles or provision of general standards (FAO and WHO 2005). The guidelines are different from the standards in terms of the obligation associated with them; the guidelines act as suggestions. Codex has adopted 45 guidelines, four general guidelines, four regional guidelines, 12 principles, and one general principle in its guidelines (Codex 2017c). Both principles and guidelines are recommended by subsidiary committees and adopted by the CAC. The principles include the addition of essential nutrients to foods, food inspection and certification for the import and export of food, microbiological criteria for foods, microbiological risk assessment, and risk analysis of foods derived using modern biotechnology. The guidelines cover labelling, production, claims, sampling, and safety assessments (FAO and WHO 2005).
Code of practice
The codes of practice describe the guidelines and rules for Codex members including producers, manufacturers, deliverers, and consumers. The guidelines cover hygienic practice; the reduction of contaminants; and the packaging, production, and transport of individual food commodities. Codex has adopted 55 codes of practice developed by subsidiary committees, including three regional codes of practice for the preparation and sale of street foods in Latin America and the Caribbean; street-vended foods in the Near-East; and street-vended foods in Asia (Codex 2017a).
Differences between Korean standards and codex standards
Formation of food standards
Codex presents food commodity standards uniformly in 10 sections: the name of the standard, scope, description, essential composition and quality factors, food additives, contaminants, hygiene, weights and measures, labelling, and methods of analysis and sampling. The scope describes the food or foods to which the standard applies. This section makes it clear as to what products the general standards covering more than one food commodity apply. The description defines the foods of interest. This section describes the raw materials, manufacturing processes, and types and styles of food. The section on essential composition and quality factors describes the requirements for the composition of food or foods, including compulsory and optional ingredients. This section aims to qualify the raw materials and protect consumers. The section on food additives contains the names of the additives permitted in the food of interest, and the section on contaminants names specific contaminants and their permitted maximum levels in food. The hygiene section includes details of specific mandatory hygiene provisions. These latter two sections also present general standards, principles, guidelines, and codes of practice. The section of weights and measures details provisions regarding weights and measures except for labelling provisions. The section of labelling includes all provisions relating to labelling by referring to the general standard for the labelling of pre-packaged foods. The section on methods of analysis and sampling presents all recommended methods of analysis and sampling (FAO and WHO 2019).
The Korean food commodity standards are given in six sections: definition, requirements for ingredients, manufacturing/processing standards, types, specifications, and test methods. Most of the sections are similar to those offered by Codex. However, the Korean standard does not include the essential composition and quality factors and labelling sections. There is a separate labelling standard in Korea for all food. Legal analytical methods have been introduced to the Korean food code for all specifications, whereas Codex suggests that methods published in international journals such as AOAC are used.
Food classification
Korea considers the Codex standards to establish national guidelines, and several Codex standards have been adopted as national standards to assist in the global harmonization of standards. However, some differences between the Korean food code and the Codex standards remain because of variations in dietary habits between countries. Foods are classified into specific food categories so that certain standards and specifications such as maximum residue levels (MRLs) of pesticides can be efficiently applied to them. Food ingredient classifications in the Korean food code have been harmonized with those described by Codex since Korea amended it in 2010. However, there are still differences in the classifications of foods between Korea and Codex (Im 2013). Foods may be classified into certain food categories to enable the efficient application of food standards to them. Foods are categorized as commodities and ingredients, including those of plant and animal origin in Korea, whereas Codex categorizes foods according to whether they are primary food commodities of plant origin, primary food commodities of animal origin, processed foods of plant origin, or processed foods of animal origin. (Codex 1993; MFDS 2019). Tables 2, 3, and 4 show the differences in food and food ingredient classifications between Korea and Codex. Most food categories are similar between the Korean food code and Codex; however, the Korean food code describes more food ingredients than Codex because of the history of Korean traditional food. Codex adopts standards for food that is internationally, not domestically, traded. For example, the Korean code recognizes algae as a food ingredient of plant origin, whereas algae are not categorized as foods by Codex. Particularly, the Korean food code lists leafier vegetables, herbs, and marine fish categories than Codex. Korea has 23 types of classification for processed food, and each type of food is associated with some groups of food commodities. However, Codex has some classifications for processed food originating from plants and animals. One of the important differences between the Korean food classification and Codex is that simple processing is not considered as processing in Korea. Cereal grain milling fractions are categorized as processed foods in Codex but are categorized as cereal grains in Korea. Particularly, dried fruits, vegetables, and herbs are categorized as processed foods in Codex, whereas they are categorized as raw ingredients or processed foods according to the processing methods in Korea (Lee 2018; 2019a; 2019b).
Table 2.
Comparison of food classifications between Korean and Codex food standards (plant origin)
| Korean classification of foods | Codex classification of foods | |||
|---|---|---|---|---|
| Type | Group | Commodity a) | Type | Group |
| Cereal grains | Japanese barnyard millet | Grasses | Cereal grains | |
| Nuts and seeds | Peanut or nuts | Peanut b), acorn, ginkgo nut, | Nuts and seeds | Tree nuts |
| Oilseeds | Evening primrose seed, hempseed, perilla seed, pumpkin seed, | Oilseeds | ||
| Seed for beverage and sweets | Sicklepod seed, guarana | Seed for beverage and sweets | ||
| Fruits | Pome fruits | Pomegranate c) | Fruits | Pome fruits |
| Citrus fruits | Korean lemon (Citrus junos), Hardy orange | Citrus fruits | ||
| Stone fruits | Jujube d), San-su-yu, nanking cherry, schisandraberry | Stone fruits | ||
| Berries and other small fruits | Goji berry, arguta kiwifruit, fig e), aronia, akebia | Berries and other small fruits | ||
| Assorted tropical and subtropical fruits | Acai, dragon fruit | Assorted tropical and subtropical fruits—edible peel | ||
| Assorted tropical and subtropical fruits—inedible peel | ||||
| Potatoes | potato f), sweet potato f), yam f), chassava f), taro f), Konjac | |||
| Pulses | sword bean, red bean | Vegetables | Pulses | |
| Vegetables | Flowerhead brassicas | Korean cabbage g) | Brassica vegetables, Head cabbages, flowerhead cabbages | |
| Leafy vegetables g | Coastal hog fennel, mizuna, Sonchus leaf, Gondre, Korean wasabi, chili pepper leaves, narrow-head ragwort, shepherd’s purse, new green, tatsoi, dachungchae, danggwi, dolnamul, Polygonatum leaves, perilla leaves, butterbur, mulberry leaves, alpine leek leaves, Uleungdo aster, shinsuncho, Korean wormwood, Crown daisy, seumbagwi, Ssam cabbage, Ussuri thistle, Indian lettuce, Burdock leaves, Common day lily, Shiso leaves, Chamnamul, Chunchae, Chwinamul, East Asian wild parsley, Squash leaves | Leafy vegetables (including brassica leafy vegetables) | ||
| Stalk and stem vegetables | Salt sandspurry, Sweet potato vines, Royal fern, bracken, Dureup elater, Water dropwort, Hooker chives, Kohlrabi h), Taro stem, green garlic | Stalk and stem vegetables | ||
| Root and tuber vegetables | Korean wasabi,, Chinese bellflower, Polygonatum root, Mulbangkqi, ginseng, lotus root, tiger lily, wild parsnip | Root and tuber vegetables | ||
| Fruiting vegetables, cucurbits | Oriental melon | Fruiting vegetables, cucurbits | ||
| Fruiting vegetables, other than cucurbits | Fruiting vegetables, other than cucurbits | |||
| Bulb vegetables | ||||
| Legume vegetables | ||||
| Mushroom i) | ||||
| Herbs and spices | Herbs | Soursop, coriander leaves, five-leaf ginseng leaf, Ben moringa leaves, lemongrass, lemon myrtle, rooibos, Matari, field sow thistle, anise hyssop, Stevia, Ironwort, oregano, laurel leaves, Japanese pepper leaves, Culantro, honey bush | Herbs and Spices | Herbs |
| Spices, fruit or berry | Mastic-leaf prickly ash, Star anise | Spices | ||
| Spices, seeds | Mustard seed j), basil (seed), shiso (seed) | |||
| Spices, root or rhizome | ||||
| Other spices | Myrrha, Saffron, Cloves | |||
| Tea leaves | tea | Teas | ||
| Hops | Hop k) | |||
| Algae | Seaweed papulosa, sea lettuce, seaweed, laver, sea string, kelp, Chondracanthus tenellus, stone laver, Pelvetia siliquosa, seaweed fulvescens, Sargassum fulvellu, sea mustard, seaweed furcate, Campylaephora hypnaeoides, spirulina, Ceylon moss, Irish moss carrragheen, Sea staghorn, Chlorella, seaweed fusiforme, Ulva pertusa, etc | |||
| Other plants | Sweet sorghum L), sugar cane L) | |||
(a) Korean food commodities which are not classified in the Codex food classification
(b) Peanut is categorized as an oilseed in the Codex food classification
(c) Pomegranates are categorized as assorted tropical and subtropical fruits-inedible peel in the Codex food classification
(d) Jujubes are categorized as assorted tropical and subtropical fruits-edible peel in the Codex food classification
(e) Figs are categorized as assorted tropical and subtropical fruits-edible peel in the Codex food classification
(f) Potato, sweet potato, yam, cassava, and taro are categorized as root and tuber vegetables in the Codex food classification
(g) Korean cabbage is named kimchi cabbage in the Codex food classification
(h) Kohlrabi is categorized as brassica vegetables, head cabbages, and flowerhead brassicas in the Codex food classification
(i) Mushrooms are categorized as fruiting vegetables other than cucurbits in the Codex food classification
(j) Mustard seed is categorized as an oilseed in the Codex food classification
(k) Hops are categorized as herbs in the Codex food classification
(l) Sweet sorghum and sugar cane are categorized as grasses for sugar or syrup production in the Codex food classification
Table 3.
Comparison of food classifications between Korean and Codex food standards (animal origin)
| Korean Classification of foods | Codex classification of foods | |||
|---|---|---|---|---|
| Type | Group | Commodity a) | Type | Group |
| Livestock products | Eggs | Egg, Duck egg, Quail egg | Poultry products | Fats |
| Edible offal | ||||
| Meat | Beef, Pork, Lamb, Goat meat, Rabbit meat, Horse meat, Venison, Chicken, Pheasant meat, Duck meat, Goose meat, Turkey meat, Quail meat | Eggs | ||
| Meat | ||||
| Mammalian products | Meat | |||
| Milk | Cow’s milk, Goat milk | Milk | ||
| Mammalian fats | ||||
| Edible offal | ||||
| Fishery products | Freshwater fishes | Snakehead, Amur catfish, Chinese muddy lasch, Crusian carp, Crucian carp, Pondsmelt, Golden mandarin fish, Carp, Stone moroko, Arctic lamprey, Common carp | Aquatic animal products | Freshwater fish |
| Pelagic fishes | Cherry almond, Salmon, Ayu Sweetfish, Common eel | Diadromous fish | ||
| Marine fishes | Ray, Flounder, Pacific cutlass fish, Croaker, Chub mackerel, Pacific saury, Japanese Glyingfish, Bastard halibut, Spootybelly greenling, Seabass, Pacific cod, Japanese sandfish, Seabream, Goby, Anchovy, Alaska Pollack, Mi-iuy croaker, Tongue sole, Japanese amberjack, Japanese sardinella, glass fish, Silver pomfret, Puffer, Silverbelly sea perch, Korean rockfish, Dark-banded rockfish, Whitespotted conger, Japanese Spanish mackerel, Black cow-tongue, Common mullet, Crub fish, Korean sandlance, Okhotsk, atka mackerel, Japanese jack mackerel, Dotted gizzard shad, Sardine, Croaker, Slender shad, Threadsail Filefish, Pacific herring, Skate ray, Alfonsino, Broadnose sevengill shark, pelagic tresher, Salmon shark, Shortfin mako, Piked dogfish, smooth hammerhead, Silver chimaera, Blue shark, Blacktip reef shark, Sawedged perch, Inshore hagfish, silver warehou, Southern hake, black cod, tunas, Southern hake, black cod, tunas, Southern Bluefin tuna, Albacore, Bigeye tuna, yellowfin tuna, Longtail tuna, skipjack tuna, black skipjack, bullet tuna, Frigate tuna, Indo pacific sailfish, striped marlin, swordfish | Marine fish | ||
| Fish eggs | Pollack roe, salmon roe, caviar | Fish roe | ||
| Crustaceans | Shrimp, crab, lobster, crayfish, three-spined shore crab, krill | Crustaceans | ||
| Fish offal | ||||
| Marine mammals | ||||
| Echinoderms (sea urchins) | Sea urchin, Sea cucumber | |||
| Tunicates | Sea squirt, Warty sea squirt, Wrinkled sea squirt | |||
| Mollusks | Shellfishes: | Invertebrate animals | Molluscs (including Cephalopods) and other invertebrate animals | |
| Cephaolpods: | ||||
| Other mollusks: | ||||
| Other animals | Reptiles and amphibians | Edible snail | ||
| Edible soft-shelled turtle, Edible frog | Amphibians and reptiles | Frogs, lizards, snakes and turtles | ||
(a) Korean food commodities which are not classified in the Codex food classification
Table 4.
Comparison of food classifications between Korean and Codex food standards (Processed food)
| Korean Classification of foods | Codex classification of foods | ||
|---|---|---|---|
| Type | Group | Type | Group |
| Confectioneries, breads or rice cakes | Manufactured foods (multiingredient) of plant origin | Manufactured multiingredient cereal products | |
| Frozen confectioneries | Ice creams, Ice cream mixes, Frozen confectionery products, Ices | ||
| Cocoa products or chocolates | Processed cocoa products, Chocolates | Derived products of plant origin | Miscellaneous derived edible products of plant origin (Cocoa butter, Cocoa mass, Cocoa powder) |
| Saccharides | Sugars, Sugar syrups, Oligosaccharides, Glucose, Fructose, Taffies (Yeot), Processed saccharide products | ||
| Jams | |||
| Soybean curds or muk (starch jellies) | |||
| Edible fats and oils | Vegetables fats and oils, Animal fats and oils, Processed edible fat and oil products | Derived products of plant origin | Vegetable oils, (crude, refined) |
| Derived edible products of animal origin | Animal fats, processed | ||
| Noodles | |||
| Beverages | Teas, Coffee, Fruit/vegetable beverages, Carbonated beverages, Soy milks, Fermented beverages, Ginseng/red ginseng beverages, other beverages | Secondary food commodities of plant origin | Miscellaneous secondary food commodities of plant origin (coffee beans, roasted) |
| Derived products of plant origin | Teas, fruit juices, | ||
| Foods for special dietary uses | Milk formulas, Infant formulas, Follow-up formulas, Cereal formulas for infants/young children, Other foods for infants/young children, Foods for special medical purposes, weight control formulas, Foods for pregnant/lactating women | ||
| Soy sauces and pastes | |||
| Seasoning foods | Vinegars, Sauces, Curries, Hot pepper powder or shredded hot pepper, Spice products, Edible salts | Secondary food commodities of plant origin | Dried herbs |
| Pickled foods or boiled foods | Kimchi products, Picked food products, Boiled foods | ||
| Alcoholic beverages | Taku | ||
| Processed agricultural foods | Starch products, Wheat flour products, Processed peanut or nut products, Cereals, Parboiled rice, Enzyme food, Other processed agricultural products | Secondary food commodities of plant origin | Dried fruits, Dried vegetables |
| Derived products of plant origin | Cereal grain milling fractions, Miscellaneous derived edible products of plant origin | ||
| Processed meat products and packaged meats | Hams, Sausages, Bacons, Dry stored meats, seasoned meats, meat extract product, processed meat, packaged meat | ||
| Egg products | Egg product, processed egg containing product | ||
| Milk products | Milk, processed milk, goat milk, fermented milk, buttermilk, concentrated milk, Milk creams, butter, cheese, powdered milk, whey, lactose, hydrolyzed milk protein products | Secondary food commodities of animal origin | Secondary milk products |
| Derived edible products of animal origin | Milk fats, Derived milk products | ||
| Manufactured food (single-ingredients) of animal origin | Manufactured milk products (single-ingredient) | ||
| Manufactured food (multiingredient) of animal origin | Manufactured milk products (multiingredient) | ||
| Processed fishery foods | Processed fish meat products, salted and fermented seafood products, dried fish/shellfish fillet products, seasoned laver, agar, other processed fishery products | Secondary food commodities of animal origin | Dried meat and fish products |
| Derived edible products of animal origin | Crustaceans, processed | ||
| Processed animal food products | Other meat or other egg products, insect products, soft-shelled turtle products, processed extract product | ||
| Honey and pollen products | Honey, royal jellies, processed pollen foods | ||
| Prepared meals | Raw foods, ready-to-eat/convenience foods, Dumplings, | ||
| Other foods | Yeast foods, other processed products | ||
Food additives
Lee et al. compared the maximum use levels for food additives between Codex and Korea (Lee et al. 2006). There are some differences due to the different food category systems and the application of mixed food additives. In Korea, food additives are divided into four types: those allowed to be used in some foods with specifications, in all foods with specifications, in all foods at as low a rate as possible, and those not allowed to be used in some foods. However, food additives under the Codex standards may be applied to some foods with specifications (Lee et al. 2006). For instance, benzoic acid cannot be used in food but there is no maximum use level for it in Korea, whereas Codex indicates that it can be used at 200–3000 mg/kg. Moreover, food red No. 2 cannot be used in any foods in Korea but can be allowed in some foods at maximum levels under Codex guidelines (Codex 1995b; MFDS 2020).
Food contaminants
Codex defines contaminants as any substance unintentionally added to food from the environment and during production processes including manufacturing, processing, preparing, treating, packing, and transportation (Codex 1995a). Heavy metals, persistent organic pollutants, natural toxins, mycotoxin pesticides, veterinary medicines, and processing contaminants are all food contaminants (Li et al. 2020). Both Korea and Codex have established maximum legally permitted concentrations of certain contaminants to ensure food safety. However, processing contaminants are managed by reduction instead of setting maximum levels. Most of the maximum levels for food contaminants under Korean food standards are similar to those established by Codex; however, Korea has adopted maximum levels of contaminants in more food commodities than Codex. Particularly, Korea has agreed maximum levels for lead, cadmium, mercury, and arsenic in edible insects that are not in the Codex standards. Moreover, maximum levels of cadmium for livestock products and fish are set out in the Korean standards. Maximum levels of benzo(a)pyrene in various food commodities including fishery products in Korea have been established as a marker for polycyclic aromatic hydrocarbons, but these are not listed by Codex. In the case of mycotoxins, maximum levels of total aflatoxins in cereals, nuts, grains, beans, and processed foods; Ochratoxin A in cereals, beans, coffee, grape juice, and spices; fumonisins in corn, cereals, and snacks; and zearalenone in grains and snacks are set in the Korean standards but not in the Codex guidelines. Furthermore, Korea has set maximum levels for dioxins in meats of bovine, lamb, and pork (Codex 1995a; MFDS 2019).
Development of codex standards
South Korea led the development of a total of six Codex standards to February 2021, along with the establishment of the standard for Kimchi in 2001 (Codex 2001). The Korean-led Codex international standards include those for kimchi, ginseng products, gochujang, and dried persimmons and regional standards for laver products and fermented soybean paste.
Standard for kimchi
With the need for Korean Kimchi to have an international standard, in 1996 at the 10th session of the CCASIA meeting in Japan, the Korean delegation presented a draft of the Standard for Kimchi and the committee agreed to present it to the Executive Committee (CCEXEC) for approval as new work with the support of the member states (Codex 1996a). At the 43rd session of the CCEXEC, the new work was approved, and Korea began drafting the standard with Asian countries such as Japan (Codex 1996b). At the 11th session of CCASIA, it has been agreed that kimchi would not be included in the scope of the existing standard for pickles, and the draft Standard for Kimchi was adopted in step 5 at the 44th session of the CCEXEC (Codex 1997; Codex 1998). At the 20th session of the Codex Committee on Processed Fruits and Vegetables (CCPFV), a final agreement was reached on the kimchi specification, and it was finally adopted in step 8 at the 24th session of the CAC (Codex 2000; Codex 2001). It was meaningful for this traditional Korean food to be adopted as an international Codex standard with its Korean name. Approximately 20 years have passed since the establishment of the Standard for Kimchi; a revision will be carried out to harmonize the wording with current Codex texts and review food additives and quality standards.
Standard for ginseng products
Korea proposed a new Standard for Insam at the 20th session of the CCPFV, the name of which was revised to the Standard for Ginseng, but CCPFV decided to discuss this instead at an appropriate Codex committee because of conflict between member countries as to whether ginseng is used as food or for medicinal purposes (Codex 2002). At the 26th session of the CAC, taking into account the member states’ concerns and Korea’s proposals, it has been decided to allow Korea to prepare a comprehensive document on the Standard for Ginseng to be considered by the CCEXEC, noting that the working document states that ginseng is a food product that is not used for medicinal purposes (Codex 2003). Referring to the recommendations of the 54th session of the CCEXEC, at the 27th session of the CAC, it has been stated that CCASIA should begin work on ginseng product specifications within the scope of Asia and that determining the standard as regional or international would be decided after its adoption at step 5 (Codex 2004b). After a long discussion, at the 16th session of CCASIA, a final draft for adoption at step 8 was prepared, emphasizing that the scope and definition for a Regional standard for Ginseng Products were prepared as proposed by Korea but indicating that it should only refer to ginseng as a food product in consideration of the concerns of the various member countries (Codex 2008). Eventually, because of the concern regarding ginseng products being consumed for medicinal purposes, an agreement was reached to define ginseng products in compliance with Codex standards, and this was adopted at the 38th session of the CAC in 2015 (Codex 2014b). The international standard for ginseng products was established after a total of 15 years of long discussions.
Regional standard for laver products
A standard for laver products was first proposed at the 17th session of CCASIA in 2010, but the discussions were deferred to the CCFFP as laver products are traded internationally (Codex 2010). However, the CCFFP proposed that the standard be drafted by CCASIA, because laver is a seaweed product and does not fall within the scope of the CCFFP’s work. Moreover, China continued to oppose the standardization of laver products because they are considered to have no trade or food safety issues (Codex 2011a). Fortunately, CCASIA agreed to establish a Standard for Laver products, and the new work was approved at the 34th session of the CAC, and China and other interested Asian countries promised to participate in drafting the standard (Codex 2011b). There was difficulty in reaching a consensus on the subject title, labelling, and contaminant standards among Asian member states in drafting the standard, but a final agreement was reached at the 20th session of CCASIA in 2017 to be adopted at steps 5 and 8 (Codex 2016).
Regional standard for fermented soybean paste
Korea initially proposed a draft Standard for Fermented Soybean Paste to the CCPFV with the name Doenjang in the title (Codex 2002), but because of the heavy workload, the CCPFV requested that the standards be prepared by CCASIA (Codex 2004b). Accordingly, at the 14th session of CCASIA, the draft was started and the name Doenjang was removed so that the title covers all similar products within Asia (Codex 2004a). The draft standards were outlined in cooperation with countries such as Japan and Singapore and finally adopted at the 32nd session of the CAC in 2009 as a regional standard in Asia (Codex 2009).
Standard for gochujang
The Standard for Gochujang was also proposed at the 21st session of the CCPFV in 2002, together with that for Doenjang (Codex 2002). At the 27th session of the CAC, the commission approved the proposed standard as a working document for a regional standard (Codex 2004b), and the 14th session of CCASIA started the work by removing “Hot Pepper Fermented Soybean Paste” from its title, since soybean is optional in making Gochujang (Codex 2004a). Subsequently, after an agreement was reached by CCASIA on ingredients, food additives, and methods of analysis, the standard was finally adopted at step 8 during the 32nd CAC in 2009 (Codex 2009). In 2017, a proposal was submitted to the 73rd session of the CCEXEC to convert the Regional Standard for Gochujang to an international standard, and the new work was approved at the 40th session of CAC (Codex 2017e). With the CCPFV working remotely in 2020, Korea, as the chair of the electronic working group, attempted to reach an agreement with member states by drafting international standard for Gochujang, and the standard was finally adopted at steps 5 and 8 after revising the definition, quality standards, etc. from the regional standards (Codex 2020c). Consequently, the International Standard for Gochujang was established with its Korean name 20 years after the Standard for Kimchi was established in 2001.
Standard for dried fruits—annex E (dried persimmons)
The preparation of a standard for dried persimmons was proposed for critical review by the 73rd session of the CCEXEC, which suggested that dried persimmons should be worked within the standard for dried fruits (Codex 2017e). As proposed by the CCEXEC, at the 40th session of the CAC, it has been approved that the new work on dried persimmons should be considered within the standard for dried fruits (Codex 2017d). An electronic working group was held jointly by Thailand as chair and South Korea as co-chair to prepare standards for individual dried fruits presented by each member. At the 43rd session of the CAC, the standard for dried fruits including Korean dried persimmons was adopted at steps 5 and 8 (Codex 2020c).
Effects of codex on Korean food safety management systems
Food safety management plan of Korea
Korea has been establishing a food safety management plan every 3 years since 2009 with the cooperation of 10 government agencies including the Office for Government Policy Coordination, the Ministry of Education, the Ministry of Justice, the Ministry of Agriculture, Food and Rural Affairs, the Ministry of Health and Welfare, the Ministry of Environment, the Ministry of Oceans and Fisheries, the Ministry of Food and Drug Safety, Korea Customs Service, and the Rural Development Administration. The plan includes the pre-emptive safety management of risk factors, establishment of the basis for safe food production, food distribution management, providing information on the basis of science, and improvement to the promotion of healthy diets.
Food sanitation act
The food sanitation act is a key piece of legislation that ensures food safety in Korea. Several Codex concepts are present in the Korea Food Sanitation Act. The definitions of food in the Korea Food Sanitation Act are based on those found in the Codex guidelines. Codex defines “food” as any substance intended for human consumption. It excludes cosmetics, tobacco, or substances used only as drugs (FAO and WHO 2019). Food also incorporates all types of beverages. Those used as medicines are excluded from the Korea Food Sanitation Act (NLIC 2017). Some other countries including the EU, Australia, New Zealand, and the United States adopted the concept of food using different terms such as consumption and digestion (EC 2002; FRL 1991; OLRC 1938).
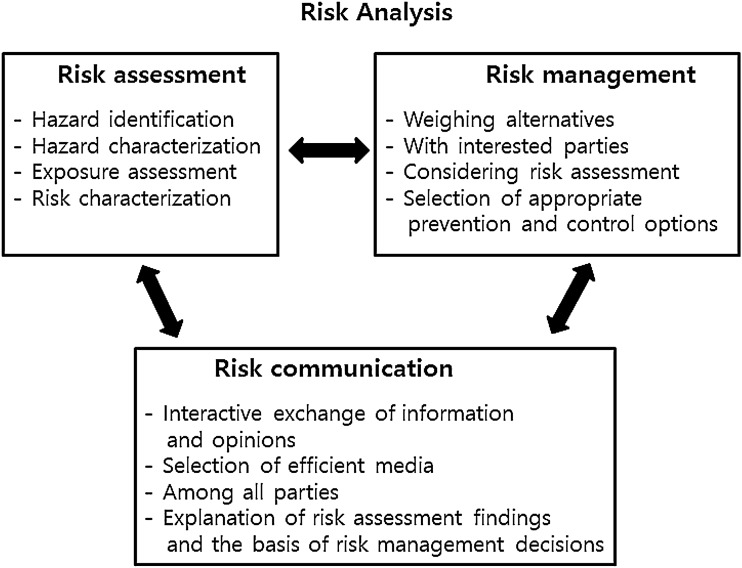
The Korea Food Sanitation Act adopts a risk analysis concept. Risk analysis has been recommended as one of the most effective measurements for managing food safety against globalized food scams and scandals. A risk analysis comprising three components, namely, risk assessment, risk management, and risk communication, is used to respond to food accidents and develop safety standards (Lee et al. 2015). Figure 3 shows the paradigm of risk analysis and its comprising factors (B.M.J. 2018). Codex recommends principles and procedures for risk analysis including methodologies for risk assessment (FAO and WHO 2019). The Korea Food Sanitation Act described the necessity for risk assessment to ensure food safety in 2005. The MFDS assesses the risks of food products suspected to contain harmful materials and can temporarily prohibit business operators from selling them. The act states that the risk assessment is conducted in four steps: hazard identification, hazard characterization, exposure assessment, and risk characterization, according to the Codex working principles for risk assessment (NLIC 2017). Particularly, risk assessment and risk management should be separated to reduce any conflict of interest, ensuring the scientific integrity of the risk assessment according to Codex (FAO and WHO 2019). The Korea National Institute of Food and Drug Safety Evaluation was established to assess risk in 2013 independently of the MFDS, which is in charge of risk management.
Fig. 3.
Paradigms of risk analysis and its components: risk assessment, risk management, and risk communication
The MFDS can set recommended specifications for food which standards and specifications have not been established for but might cause risks to the public. The recommended specification can adopt the specifications stipulated by the Codex and other countries’ legislation agencies. It is not a compulsory standard; however, MFDS can disclose the names of the business operators when they fail to comply with the recommended specifications (NLIC 2017).
The Hazard Analysis Critical Control Point (HACCP) system is a food safety assurance method, and it has played an essential role over the last 30 years. Codex adopted the guidelines for the application of the HACCP system in 1993 to harmonize the approach internationally (Motarjemi et al. 1996). In Korea, HACCP was implemented as the food safety management certification standard for food processing in 1996, meat processing in 1997, and milk processing in 2004 (Kim 2018; NLIC 2017). HACCP is a compulsory standard for managing harmful elements as a priority at all stages of food production in Korea.
The MFDS organizes and operates the food sanitation deliberation committee to investigate and deliberate on matters concerning the standards and specifications of foods, the prevention of food poisoning, and the maximum residue limits for toxic or harmful substances. Particularly, the food sanitation deliberation committee has research members studying the international standards and specifications for foods as prescribed by Codex (NLIC 2017).
Food code
The MFDS has been formulating master plans for the management of standards and specifications of food every 5 years. The second plan was announced in 2019 to cover the following 5 years, and it included the following strategies: to strengthen food safety management in preparation for population and environmental changes, to re-evaluate and advance standards and specifications, to manage standards and specifications according to structural changes in the food industry and the acceleration of technology, and to strengthen communication links with the public and the education system to ensure food safety (MWN 2020). Food standards and specifications are established to reduce exposure to hazardous elements when their risks, based on risk assessments, must be controlled (MFDS 2017). Risk assessments are conducted according to the Codex working principles for risk assessment.
Standards and specifications for food that have not yet been established in the Korean food code may be determined by considering those of Codex or major foreign standards and specifications. Analytical methods provided in the food code must be used for testing foods in Korea. However, the test methods approved by Codex can be used when the analytical methods are not provided in the Korean food code (MFDS, 2019).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Joon-Goo Lee and Yeonkyu Lee authors contributed equally to this work.
Contributor Information
Joon-Goo Lee, Email: jglee@dau.ac.kr.
Yeonkyu Lee, Email: yeonkyu@korea.kr.
Chun Soo Kim, Email: cskim94@korea.kr.
Sang Bae Han, Email: difco64@korea.kr.
References
- Codex Alimentarius (Codex). Report of the 1st Session of the Joint FAO/WHO Codex Alimentarius Commission. ALINORM 63/12. In: Joint FAO/WHO Codex Alimentarius Commission, 1st session. June 25–3, Rome, Italy. Codex Alimentarius, Rome, Italy (1963)
- Codex Alimentarius (Codex). Report of the 8th Session of the Joint FAO/WHO Codex Alimentarius Commission. ALINORM 71/31. In: Joint FAO/WHO Codex Alimentarius Commission, 8th session. June 30–July 9, Geneva, Switzerland. Codex Alimentarius, Rome, Italy (1971)
- Codex Alimentarius (Codex). Report of the 10th Session of the Joint FAO/WHO Codex Alimentarius Commission. ALINORM 74/44. In: Joint FAO/WHO Codex Alimentarius Commission, 10th session. July 1–11, Rome, Italy. Codex Alimentarius, Rome, Italy (1974)
- Codex Alimentarius (Codex). Report of the 1st Session of the Codex Coordinating Committee for Asia. ALINORM 78/15. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for Asia, 1st session. January 10–16, New Delhi, India. Codex Alimentarius (1978)
- Codex Alimentarius (Codex). Codex Classification of Foods and Animal Feeds. 2nd ed. Joint FAO/WHO Food Standards Programme Codex Committee on Pesticide Residues, Rome, Italy (1993)
- Codex Alimentarius (Codex). General Standard for Contaminants and Toxins in Food and Feed (Codex Stan 193–1995) (1995a). Available from: http://www.fao.org/fileadmin/user_upload/livestockgov/documents/1_CXS_193e.pdf. Accessed May. 28, 2021.
- Codex Alimentarius (Codex). General standards for food additives (Codex Stan 192–1995) (1995b). Available from: http://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/gsfa/en/. Accessed Dec. 23, 2020.
- Codex Alimentarius (Codex). Report of the 10th Session of the Codex Coordinating Committee For Asia. ALINORM 97/15. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for Asia, 10th session. March 5–8, Tokyo, Japan. Codex Alimentarius, Rome, Italy (1996a)
- Codex Alimentarius (Codex). Report of the 43rd Session of the Executive Committee of the Codex Alimentarius Commission. ALINORM 97/3. In: Joint FAO/WHO Executive Committee of the Codex Alimentarius Commission, 43rd session. June 4–7, Geneva, Switzerland. Codex Alimentarius, Rome, Italy (1996b)
- Codex Alimentarius (Codex). Report of the 11th Session of the Coordinating Committee For Asia. ALINORM 99/15. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for Asia, 11th session. December 16–19, Chiang Rai, Thailand. Codex Alimentarius, Rome, Italy (1997)
- Codex Alimentarius (Codex). Report of the 45th Session of the Executive Committee of the Codex Alimentarius Commission. ALINORM 99/3. In: Joint FAO/WHO Executive Committee of the Codex Alimentarius Commission, 45h session. June 3–5, Rome, Italy. Codex Alimentarius, Rome, Italy (1998)
- Codex Alimentarius (Codex). Report of the 20th Session of the Codex Committee on Processed Fruits and Vegetables. ALINORM 01/27. In: Joint FAO/WHO Food Standards Programme Codex Committee on Processed Fruits and Vegetables, 20th session. September 11–15, Washington, D.C. U.S.A. Codex Alimentarius, Rome, Italy (2000)
- Codex Alimentarius (Codex). Report of the 24th Session of the Joint FAO/WHO Codex Alimentarius Commission. ALINORM 01/41. In: Joint FAO/WHO Codex Alimentarius Commission, 24th session. July 2–7, Geneva, Switzerland. Codex Alimentarius, Rome, Italy (2001)
- Codex Alimentarius (Codex). Report of the 21st Session of the Codex Committee on Processed Fruits and Vegetables. ALINORM 03/27. In: Joint FAO/WHO Food Standards Programme Codex Committee on Processed Fruits and Vegetables, 21st session. September 23–27, San Antonio, Texas, U.S.A. Codex Alimentarius, Rome, Italy (2002)
- Codex Alimentarius (Codex). Report of the 26th Session of the Joint FAO/WHO Codex Alimentarius Commission. ALINORM 03/41. In: Joint FAO/WHO Codex Alimentarius Commission, 26th session. June 30-July 7, Rome, Italy. Codex Alimentarius, Rome, Italy (2003)
- Codex Alimentarius (Codex). Report of the 14th Session of the Coordinating Committee For Asia. ALINORM 05/28/15. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for Asia, 14th session. September 7–10, Jeju, Republic of Korea. Codex Alimentarius, Rome, Italy (2004a)
- Codex Alimentarius (Codex). Report of the 27, Rome, Italy Session of the Joint FAO/WHO Codex Alimentarius Commission. ALINORM 04/27/41. In: Joint FAO/WHO Codex Alimentarius Commission, 27th session. June 28–3, Geneva, Switzerland. Codex Alimentarius (2004b)
- Codex Alimentarius (Codex). Report of the 15th Session of the Coordinating Committee For Asia. ALINORM 07/30/15. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for Asia, 15th session. November 21–24, Seoul, Republic of Korea. Codex Alimentarius, Rome, Italy (2006)
- Codex Alimentarius (Codex). Report of the 1st Session of the Codex Ad hoc Intergovernmental Task Force on Antimicrobial Resistance. ALINORM 08/31/42. In: Joint FAO/WHO Food Standards Programme Codex Ad hoc Intergovernmental Task Force on Antimicrobial Resistance, 1st session. October 23–26, Seoul, Republic of Korea. Codex Alimentarius, Rome, Italy (2007)
- Codex Alimentarius (Codex). Report of the 16th Session of the Coordinating Committee For Asia. ALINORM 09/32/15. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for Asia, 16th session. November 17–21, Denpasar, Indonesia. Codex Alimentarius, Rome, Italy (2008)
- Codex Alimentarius (Codex). Report of the 32nd Session of the Joint FAO/WHO Codex Alimentarius Commission. ALINORM 09/32/REP. In: Joint FAO/WHO Codex Alimentarius Commission, 32nd session. June 29–July 4, Rome, Italy. Codex Alimentarius, Rome, Italy (2009)
- Codex Alimentarius (Codex). Report of the 17th Session of the Coordinating Committee For Asia. REP11/ASIA. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for Asia, 17th session. November 22–26, Bali, Indonesia. Codex Alimentarius, Rome, Italy (2010)
- Codex Alimentarius (Codex). Report of the 31st Session of the Codex Committee on Fish and Fishery Products. REP 11/FFP. In: Joint FAO/WHO Food Standards Programme Codex Committee on Fish and Fishery Products, 31t session. April 11–16, Tromsø, Norway. Codex Alimentarius, Rome, Italy (2011a)
- Codex Alimentarius (Codex). Report of the 34th Session of the Joint FAO/WHO Codex Alimentarius Commission. REP11/CAC. In: Joint FAO/WHO Codex Alimentarius Commission, 34th session. July 4–9, Geneva, Switzerland. Codex Alimentarius, Rome, Italy (2011b)
- Codex Alimentarius (Codex). Report of the 19th Session of the Coordinating Committee For Asia. REP15/ASIA. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for Asia, 19th session. November 3–7, Tokyo, Japan. Codex Alimentarius, Rome, Italy (2014a)
- Codex Alimentarius (Codex). Report of the 27th Session of the Codex Committee on Processed Fruits and Vegetables. REP15/PFV. In: Joint FAO/WHO Food Standards Programme Codex Committee on Processed Fruits and Vegetables, 27th session. September 8–12, Philadelphia U.S.A. Codex Alimentarius, Rome, Italy (2014b)
- Codex Alimentarius (Codex). Report of the 20th Session of the Coordinating Committee For Asia. REP17/ASIA. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for Asia, 20th session. September 26–30, New Delhi, India. Codex Alimentarius, Rome, Italy (2016)
- Codex Alimentarius (Codex). Codex Alimentarius codex of practice (2017a). Available from: http://www.fao.org/fao-who-codexalimentarius/codex-texts/guidelines/en/. Accessed May. 29, 2021.
- Codex Alimentarius (Codex). Codex Alimentarius international food standards (2017b). Available from: http://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en. Accessed Dec. 23, 2020.
- Codex Alimentarius (Codex). Codex Alimentarius international guidelines (2017c). Available from: http://www.fao.org/fao-who-codexalimentarius/codex-texts/guidelines/en/. Accessed May. 29, 2021.
- Codex Alimentarius (Codex). Report of the 40th Session of the Joint FAO/WHO Codex Alimentarius Commission. REP17/CAC. In: Joint FAO/WHO Codex Alimentarius Commission, 40th session. July 17–22, Geneva, Switzerland. Codex Alimentarius (2017d)
- Codex Alimentarius (Codex). Report of the 73rd Session of the Executive Committee of the Codex Alimentarius Commission. REP17/EXEC2. In: Joint FAO/WHO Executive Committee of the Codex Alimentarius Commission, 73rd session. July 10–13, Geneva, Switzerland. Codex Alimentarius, Rome, Italy (2017e)
- Codex Alimentarius (Codex). Timeline (2017f). Available from: http://www.fao.org/fao-who-codexalimentarius/about-codex/history/en. Accessed December 17, 2020.
- Codex Alimentarius (Codex). Discussion paper on Internet sale/e-commerce. Agenda Item 7. In: Joint FAO/WHO Food Standards Programme Codex Committee on Food Labelling, 45th session. May 13–17, Ottawa, Canada. Codex Alimentarius, Rome, Italy (2019a)
- Codex Alimentarius (Codex). Emerging Issues in Food Safety and Quality in Countries in the Region. Agenda Item 3.1. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for Asia, 21st session. September 23–27, Goa, India. Codex Alimentarius, Rome, Italy (2019b)
- Codex Alimentarius (Codex). Food Safety and Quality Situation in the Countries of the Region: Current and emerging issues in the region. Agenda Item 3.1. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for Africa, 23rd session. September 2–6, Nairobi, Kenya. Codex Alimentarius, Rome, Italy (2019c)
- Codex Alimentarius (Codex). Food Safety and Quality Situation in the Countries of the Region: Current and emerging issues in the region. Agenda Item 3.1. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for Europe, 31st session. September 30–October 4, Almaty, Kazakhstan. Codex Alimentarius, Rome, Italy (2019d)
- Codex Alimentarius (Codex). Food Safety and Quality Situation in the Countries of the Region: Current and emerging issues in the region. Agenda Item 3.1. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for Latin America and the Caribbean, 21st session. October 21–25, Santiago, Chile. Codex Alimentarius, Rome, Italy (2019e)
- Codex Alimentarius (Codex). Food Safety and Quality Situation in the Countries of the Region: Current and emerging issues in the region. Agenda Item 3.1. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for North America and the South West Pacific, 15th session. September 16–20, Port Vila, Vanuatu. Codex Alimentarius, Rome, Italy (2019f)
- Codex Alimentarius (Codex). Food Safety and Quality Situation in the Countries of the Region: Current and emerging issues in the region. Agenda Item 3.1. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for the Near East, 10th session. November 11–15, Rome, Italy. Codex Alimentarius, Rome, Italy (2019g)
- Codex Alimentarius (Codex). Report of the 21st Session of the Codex Committee on Fresh Fruits and Vegetables. REP20/FFV. In: Joint FAO/WHO Food Standards Programme Codex Committee on Fresh Fruits and Vegetables, 21st session. October 7–11, Monterrey, Mexico. Codex Alimentarius, Rome, Italy (2019h)
- Codex Alimentarius (Codex). Report of the 21st Session of the Coordinating Committee For Asia. REP20/ASIA. In: Joint FAO/WHO Food Standards Programme FAO/WHO Coordinating Committee for Asia, 21st session. September 23–27, Goa, India. Codex Alimentarius, Rome, Italy (2019i)
- Codex Alimentarius (Codex). Report of the 42nd Session of the Joint FAO/WHO Codex Alimentarius Commission. REP19/CAC. In: Joint FAO/WHO Codex Alimentarius Commission, 42nd Session. July 8–12, Geneva, Switzerland. Codex Alimentarius, Rome, Italy (2019j)
- Codex Alimentarius (Codex). Report of the 45th Session of the Codex Committee on Food Labelling. REP19/FL. In: Joint FAO/WHO Food Standards Programme Codex Committee on Food Labelling, 45th session. May 13–17. Ottawa, Canada. Codex Alimentarius, Rome, Italy (2019k)
- Codex Alimentarius (Codex). Critical Review Part I. Agenda Item 2. In: Joint FAO/WHO Food Standards Programme Executive Committee of the Codex Alimentarius Commission, 79th session. July 13–20, virtual. Codex Alimentarius, Rome, Italy (2020a)
- Codex Alimentarius (Codex). FAO/WHO Codex Trust Fund 2019 Annual Report. INF/1. In: Joint FAO/WHO Food Standards Programme Codex Alimentarius Commission, 43rd Session. September 24–26, October 12 and 19, November 5–6, virtual. Codex Alimentarius, Rome, Italy (2020b)
- Codex Alimentarius (Codex). Report of the 43rd Session of the Joint FAO/WHO Codex Alimentarius Commission. REP20/CAC. In: Joint FAO/WHO Codex Alimentarius Commission, 43rd Session. September 24–26, October 12 and 19, November 5–6, virtual. Codex Alimentarius, Rome, Italy (2020c)
- Codex Alimentarius (Codex). Matters of Interest arising from FAO/WHO and from the 87th Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Agenda Item 3.1. In: Joint FAO/WHO Food Standards Programme Codex Committee on Food Additives, 52nd session. September 1–10, virtual. Codex Alimentarius, Rome, Italy (2021a)
- Codex Alimentarius (Codex). Provisional Agenda. Agenda Item 1. In: Joint FAO/WHO Food Standards Programme Codex Committee on Pesticide Residues, 52nd session. September 1–10, virtual. Codex Alimentarius, Rome, Italy (2021b)
- Codex Alimentarius (Codex). Report of the 80th session of the Executive Committee of the Codex Alimentarius Commission. Rep21/EXEC1. In: 80th session of the Executive Committee of the Codex Alimentarius Commission. January 13–21, virtual. Codex Alimentarius, Rome, Italy (2021c)
- Codex Alimentarius (Codex). The role of Codex and food safety in the 2021 Food Systems Summit (2021d). Available from: http://www.fao.org/fao-who-codexalimentarius/news-and-events/news-details/en/c/1369557/. Jan 14 2021. Accessed Mar. 09, 2021.
- Codex Alimentarius (Codex). 60th Anniversary of the Codex Alimentarius Commission: 1963–2023. CRD24. In: Codex Committee on General Principles 32nd Session. February 8–17, virtual. Codex Alimentarius, Rome, Italy (2021e)
- European commission (EC). Commission regulation (EC) No. 178/2002 of the European parliament and of the council of 29 January 2002. (2002) Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:02002R0178-20210526&from=EN. Accessed May. 28. 2021
- Federal Register of Legislation (FRL). Meaning of food. No.118, Part 1- Section 5, pp. 7. In: Food standard Australia New Zealand Act 1991. Food Standards Australia New Zealand, Canberra, Australia (1991)
- Food and agricultural organization (FAO). Republic of Korea and FAO-partnering for food security and inclusive and sustainable rural development. ax287e. FAO, Rome, Italy (2019)
- Food and agricultural organization (FAO), World Health Organization (WHO). Report of the Joint FAO/WHO Conference on Food Standards. ALINORM 62/8. In: Joint FAO/WHO Conference on Food Standards. October 1-5, Geneva, Switzerland. FAO and WHO (1962)
- Food and agricultural organization (FAO), World Health Organization (WHO). Understanding the Codex Alimentarius. Rome. Italy (2005). Available from: http://www.fao.org/docrep/pdf/008/y7867e/y7867e00.pdf. Accessed Dec. 23, 2020.
- Food and agricultural organization (FAO), World Health Organization (WHO). Codex Trust Fund-2, Project Document. FAO and WHO, Geneva, Switzerland (2015)
- Food and agricultural organization (FAO), World Health Organization (WHO). Understanding Codex. 5th ed. FAO and WHO, Rome, Italy. pp. 9–29 (2018)
- Food and agricultural organization (FAO), World Health Organization (WHO). Elaboration of Codex Standards and Related Texts. 27th ed. pp. 27-95. In: Codex Alimentarius Commission-Procedural manual. FAO and WHO. FAO, Rome, Italy (2019)
- Food and agricultural organization (FAO), World Health Organization (WHO). Codex and the SDGs -How participation in Codex Alimentarius supports the 2030 Agenda for Sustainable Development. FAO and WHO, Rome, Italy. pp. 4-48 (2020)
- Im MH. Review of Codex Alimentarius and comparison between the US and Koren food classifications for pesticide residues of the US and Korea. J. Korean Soc. Appl. Biol. Chem. 2013;56:483–495. doi: 10.1007/s13765-013-3182-x. [DOI] [Google Scholar]
- Kim JH, Hur SH, Yim DG. Monitoring of microbial contaminants of beef, pork, and chicken in HACCP implemented meat processing plants of Korea. Korean J. Food Sci. Animal Resour. 2018;38:282–290. doi: 10.5851/kosfa.2018.38.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korea Food Research Institute (KFRI). About KFRI. Vision. Available from: https://www.kfri.re.kr/–r=en&c=129/135. Accessed Jan. 31, 2021
- Lee JG, Kim SH, Kim HJ, Yoon HJ. Total diet studies as a tool for ensuring food safety. Toxicol. Res. 2015;31:221–226. doi: 10.5487/TR.2015.31.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Lee SR, Lee TS, Jang YM, Hong KH, Park SK, Kwon YK, Han YJ. Comparison between use levels of food additives by Codex and Korea. J. Food Hyg. Saf. 2006;21:14–22. [Google Scholar]
- Lee MG. Classification and nomenclature of fruit commodities in South Korea and Codex alimentarius commission. J. Food Hyg. Saf. 2018;33:162–167. doi: 10.13103/JFHS.2018.33.3.162. [DOI] [Google Scholar]
- Lee MG. Classification of vegetable commodities by the Codex alimentarius commission. J. Food Hyg. Saf. 2019;34:87–93. doi: 10.13103/JFHS.2019.34.1.87. [DOI] [Google Scholar]
- Lee MG. Food classification by the Codex alimentarius commission: cereal grain, nuts and seeds, herbs and spices. J. Food Hyg. Saf. 2019;34:212–218. doi: 10.13103/JFHS.2019.34.2.212. [DOI] [Google Scholar]
- Medical World News (MWN). The 2nd master plan for management of food standards and specifications (2020). Available from: http://medicalworldnews.co.kr/news/view.php–idx=1510933947. Accessed Dec. 25. 23, 2020.
- Ministry of Agriculture, Food and Rural Affairs (MAFRA). About MAFRA. Organization. Available from: https://www.mafra.go.kr/english/754/subview.do. Accessed Jan. 30, 2021
- Ministry of Food and Drug Safety (MFDS). Principle for establishing food standards. 3rd ed. Cheongju, Korea (2017) Available from: https://www.mfds.go.kr/brd/m_218/view.do–seq=18437&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=22. Accessed Dec. 25. 23, 2020.
- Ministry of Food and Drug Safety (MFDS). Food Code (2019). Available: https://www.mfds.go.kr/eng/brd/m_15/down.do–brd_id=eng0001&seq=69982&data_tp=A&file_seq=1. Accessed Dec. 25. 23, 2020.
- Ministry of Food and Drug Safety (MFDS). Food additives code. Ministry of Food and Drug Safety Regulation 2020–59 (2020). Available from: https://www.mfds.go.kr/eng/brd/m_15/view.do–seq=72432. Accessed Dec. 23, 2020.
- Ministry of Food and Drug Safety (MFDS). About MFDS. Our Works. Available from: https://www.mfds.go.kr/eng/wpge/m_9/denofile.do. Accessed Jan. 30. 2021
- Ministry of Oceans and Fisheries (MOF). About MOF. Organization. Available from: https://www.mof.go.kr/en/page.do–menuIdx=1469. Accessed Jan. 30, 2021
- Motarjemi Y, Kaferstein F, Moy G, Miyagawa S, Miyagishima K. Importance of HACCP for public health and development the role of the World Health Organization. Food Control. 1996;7:77–85. doi: 10.1016/0956-7135(96)00003-5. [DOI] [Google Scholar]
- National Institute of Food and Drug Safety Evaluation (NIFDS). About NIFDS. Vision & Mission. Available from: https://www.nifds.go.kr/wpge/m_365/cont_04/cont_04_03.do. Accessed Jan. 30. 2021
- National Law Information Center (NLIC). Food sanitation act. Ministry of Food and Drug Safety, Cheongju, Korea (2017)
- Office of the Law Revision Counsel (OLRC). Federal food, drug, and cosmetic act. Food and Drug Administration, New Hampshire Ave Silver Spring, MD, U.S. (1938)
- van der Meulen. B.M.J. Codex Alimentarius: The impact of the joint FAO/WHO food standards programme on EU food law. European Institute for Food Law Working Paper Series 2018/04 (2018)
- Veggeland F, Borgen SO. Negotiating international food standards: the world trade organization’s impact on the Codex Alimentarius commission. Governance. 2005;18:675–708. doi: 10.1111/j.1468-0491.2005.00297.x. [DOI] [Google Scholar]
- Winickoff DE, Bushey BDM. Science and power in global food regulation: the rise of the Codex Alimentarius. Sci., Technol. Human Values. 2010;35:356–381. doi: 10.1177/0162243909334242. [DOI] [Google Scholar]
- World Health Organization (WHO). 70 Years Working Together for Health: The World Health Organization and the Republic of Korea. WHO Regional Office for the Western Pacific, Manila, Philippines (2016)
- World Trade Organization (WTO). Sanitary and Phytosanitary Measures (2000a). Available from: https://www.wto.org/english/tratop_e/sps_e/sps_e.htm. Accessed Feb. 22, 2021.
- World Trade Organization (WTO). What is the WTO (2000b). Available from: https://www.wto.org/english/thewto_e/whatis_e/whatis_e.htm. Accessed Feb. 22, 2021.