Abstract
Background
Exposure to respirable crystalline silica is suggested to increase the risk of autoimmune rheumatic diseases. We examined the association between respirable crystalline silica exposure and systemic sclerosis, rheumatoid arthritis, systemic lupus erythematosus and small vessel vasculitis.
Methods
In a cohort study of the total Danish working population, we included 1 541 505 male and 1 470 769 female workers followed since entering the labour market 1979–2015. Each worker was annually assigned a level of respirable crystalline silica exposure estimated with a quantitative job exposure matrix. We identified cases of autoimmune rheumatic diseases in a national patient register and examined sex-specific exposure-response relations by cumulative exposure and other exposure metrics.
Results
We identified 4673 male and 12 268 female cases. Adjusted for age and calendar year, men exposed to high levels of respirable crystalline silica compared with non-exposed showed increased incidence rate ratio (IRR) for the four diseases combined of 1.53 [95% confidence interval (CI): 1.39–1.69], for systemic sclerosis of 1.62 (1.08–2.44) and rheumatoid arthritis of 1.57 (1.41–1.75). The overall risk increased with increasing cumulative exposure attained since entering the workforce [IRR: 1.07 (1.05–1.09) per 50 µg/m3-years]. Female workers were less exposed to respirable crystalline silica, but showed comparable risk patterns with overall increased risk with increasing cumulative exposure [IRR: 1.04 (0.99–1.10) per 50 µg/m3-years].
Conclusions
This study shows an exposure-dependent association between occupational exposure to respirable crystalline silica and autoimmune rheumatic diseases and thus suggests causal effects, most evident for systemic sclerosis and rheumatoid arthritis.
Keywords: Respirable crystalline silica, autoimmune, systemic sclerosis, rheumatoid arthritis, cohort
Key Messages
Inhalation of respirable crystalline silica has since the 1930s repeatedly been suggested in the aetiology of rheumatoid arthritis and other autoimmune rheumatic diseases.
In a cohort of 3 million workers, we show an exposure-dependent association between respirable crystalline silica and systemic sclerosis, rheumatoid arthritis and possibly also systemic lupus erythematosus and small vessel vasculitis, supporting a causal role of this widespread occupational exposure.
Introduction
Crystalline silica (SiO2) is a major element of earth's crust and found in soil, sand and rocks, and in concrete, ceramics, glass and other industrial materials. Worldwide, a considerable number of especially male workers employed in construction, the metal industry, farming and other industries are exposed at high levels, whenever these materials are used, moved, crushed, drilled in or processed in the production of new materials.1,2 Since 1997, silica has been classified as a group 1 human lung carcinogen by the International Agency for Research on Cancer (IARC)3 and inhalation of fine particles of silica is furthermore a well-recognized risk factor for silicosis.4
A causal link of rheumatic diseases with occupational exposure to crystalline silica was already suggested from the 1930s.5 More recently, respirable crystalline silica has repeatedly been reported to increase the risk of several autoimmune rheumatic diseases: systemic sclerosis in men and women6–9 and rheumatoid arthritis in men;9–15 however, findings for women are unclear and based on few studies.12,15 Exposure to respirable crystalline silica may also increase the risk of systemic lupus erythematosus16–18 and small vessel vasculitis in men and women.19–24 These diseases affect people of working age, women more often than men.25–29 Low concordances between monozygotic twins indicate environmental factors as of aetiological importance.30,31 Thus we have much to learn about the complex pathogenesis, which potentially includes interaction between genetic, environmental and epigenetic factors.30,32
Limited quantitative information on silica exposure levels characterizes most studies, and only few have examined exposure-response relations,13,17,18,20 which are important before any conclusions on causation can be drawn. We combined a large and detailed nationwide occupational cohort with workplace surveillance exposure measurements, and examined the risk of systemic sclerosis, rheumatoid arthritis, systemic lupus erythematosus and small vessel vasculitis, following occupational exposure to respirable crystalline silica in men and women.
Methods
Register studies in Denmark without biological materials do not need approval from the National Committee of Health Research Ethics. This study is approved by the Danish Data Protection Agency (j.no: 1–16-02–196-17)
Study population
The study population comprised all Danish residents, born 1956 or later, with a minimum of 1 year of gainful employment 1977–2015 and a valid job code according to the Danish version of the International Standard Classification of Occupations from 1988 (ISCO 88) as registered in the Danish Occupational Cohort (DOC*X).33 DOC*X includes annual, harmonized information on employment and job code for all Danish citizens. The information is based on several data sources, such as union membership, self-report to the civil registration authorities, tax records and employers' mandatory reporting of occupation to Statistics Denmark of all employees.33 If the ISCO code was missing in a year with active employment, we assigned the latest valid ISCO code up to 5 years back. All Danish citizens hold a unique social security number which is used by all official authorities and allows linkage with national registers. Through linkage with the national civil registration system,4 we excluded those who died, disappeared or emigrated before the start of follow-up in 1979.
Autoimmune rheumatic diseases
Incident cases of autoimmune rheumatic diseases were identified in the National Patient Registry. Since 1977 the register holds information on all inpatient contacts and, since 1995, outpatient contacts with any Danish hospitals,35 all coded according to the 8th (1977–93) or 10th (1994–2015) version of the International Classification of diseases. Cases were defined according to Table 1.
Table 1.
Summary of the International Classification of Diseases (ICD) codes, 8th and 10th versions for the studied autoimmune rheumatic diseases
| Disease | ICD 8 (1977–93) | ICD 10 (1994–2015) |
|---|---|---|
| Systemic sclerosis | 73400, 73401, 73402, 73408, 73409, 73491 | M34, M340, M341, M342, M342A, M342B, M348, M348B, M349 |
| Rheumatoid arthritis | 71219, 71229, 71238, 71239 | M05, M050, M051, M051A-F, M052, M053, M058, M059, M06, M060, M068, M069 |
| Seropositive rheumatoid arthritisa | M05, M050, M051, M051A-F, M052, M053, M058, M059 | |
| Seronegative rheumatoid arthritisa | M06, M060, M068, M069 | |
| Systemic lupus erythematosus | 73419 | M32, M320, M321, M328, M329 |
| Small vessel vasculitis | 22709, 44619, 44629, 44649, 44799, 44808, 44809 | M301, M310, M310A-B, M311, M311A, M313, M317, M318, M318A, M319 |
Rheumatoid arthritis is split into seropositive and seronegative rheumatoid arthritis in ICD 10.
Exposure assessment
Each worker was assigned a quantitative estimate of respirable crystalline silica exposure for each year of employment, based on the SYNJEM job exposure matrix (JEM, developed for the SYNERGI study).36,37 The SYNJEM originally provided time- and region-specific respirable crystalline silica exposure estimates for all job codes included in the 1968 version of ISCO, based on the modelling of 23 640 personal measurements of respirable crystalline silica from several European countries and Canada, together with expert assessments. For the current study, the SYNJEM was modified to provide exposure estimates for ISCO 88 job codes and was restricted to estimates for the Nordic countries. For each year of follow-up, we constructed the following exposure metrics based on each worker’s exposure history since entry: (i) cumulative exposure (µg/m3-year) as the sum of exposure levels for all exposed years; (ii) mean exposure intensity (µg/m3) as cumulative exposure divided by the number of exposed years; (iii) highest attained exposure intensity (µg/m3); and (iv) duration of exposure (years).
Statistical methods
Follow-up started the year following the first year of employment, because of no available information on month or day of employment. For the same reason, all independent variables were lagged by 1 year. We furthermore started follow-up at the earliest in 1979, 2 years after information on autoimmune rheumatic diseases was available from the National Patient Registry. We included this 2-year washout period (1977–78) to reduce number of prevalent cases. Study participants were followed until the year of the first diagnosis of systemic sclerosis, small vessel vasculitis, systemic lupus erythematosus or rheumatoid arthritis, death, emigration or end of follow-up on 31 December 2015, whichever came first.
Associations between respirable crystalline silica exposure and each of the autoimmune rheumatic diseases, as well as the studied diseases combined, were analysed in separate discrete time hazard models in a logistic regression procedure, with person-years as unit of analysis yielding incidence rate ratios that were presented with 95% confidence intervals (CI).38 All exposures and covariates were treated as time-varying variables.
Table 2 presents the distribution of all male and female person-years cumulated during follow-up and classified by time worker characteristics and cumulative respirable crystalline silica exposure level. Separately for each exposure metric, study participants were grouped as exposed or non-exposed. The exposed were further grouped into tertiles based on the combined female and male distribution of exposed person-years. We also analysed respirable crystalline silica exposure accrued during three confined time windows (the previous 1–10, 11–20 and >20 years). In these analyses any silica exposure accrued outside each time window was classified as zero, and only exposure received in the years within the time windows were divided by the median into two exposure groups.39
Table 2.
Distribution of person-years at risk (%) by time-varying worker characteristics and cumulative respirable crystalline silica exposure level among 1 541 505 men and 1 470 769 women, Denmark, 1979–2015
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cumulative respirable crystalline silica (µg/m3-years) |
Cumulative respirable crystalline silica (µg/m3-years) |
|||||||
| 0 | 2.0–29.2 | 29.3–93.9 | 94.0–1622 | 0 | 2.0–29.2 | 29.3–93.9 | 94.0–1622 | |
| 28 596 448 | 1 581 413 | 1 644 508 | 1 790 255 | 30 957 666 | 342 405 | 280 298 | 134 819 | |
| Worker characteristics | Person-years | Person-years | Person-years | Person-years | Person-years | Person-years | Person-years | Person-years |
| Occupationa | ||||||||
| Armed forces | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| White-collar workers | 40 | 17 | 13 | 12 | 63 | 36 | 32 | 29 |
| Skilled blue-collar workers | 17 | 26 | 28 | 41 | 1 | 12 | 14 | 21 |
| Unskilled blue-collar workers | 16 | 42 | 45 | 36 | 12 | 32 | 35 | 34 |
| Others | 12 | 13 | 10 | 7 | 14 | 18 | 16 | 12 |
| Missing | 12 | 1 | 3 | 4 | 10 | 2 | 3 | 4 |
| Age | ||||||||
| <25 | 38 | 26 | 21 | 8 | 35 | 20 | 13 | 5 |
| 26–35 | 32 | 36 | 35 | 31 | 33 | 34 | 35 | 29 |
| >36 | 29 | 38 | 44 | 61 | 32 | 46 | 52 | 66 |
| Calendar year | ||||||||
| 1979–84 | 7 | 2 | 6 | 2 | 6 | 2 | 3 | 1 |
| 1985–94 | 22 | 12 | 19 | 21 | 21 | 12 | 16 | 18 |
| 1995–2004 | 30 | 29 | 30 | 32 | 30 | 28 | 33 | 33 |
| 2005–15 | 41 | 57 | 45 | 45 | 43 | 58 | 48 | 48 |
| Probability of smoking | ||||||||
| 5–25% | 24 | 23 | 18 | 21 | 35 | 37 | 29 | 28 |
| 26–35% | 28 | 39 | 34 | 34 | 29 | 38 | 40 | 40 |
| 36–74% | 32 | 38 | 48 | 45 | 24 | 25 | 31 | 32 |
| Missing | 16 | – | – | – | 12 | – | – | – |
| Educationb | ||||||||
| Lower secondary | 27 | 43 | 44 | 30 | 26 | 38 | 40 | 41 |
| Vocational or high secondary | 46 | 44 | 45 | 61 | 44 | 43 | 45 | 46 |
| Short cycle higher | 5 | 3 | 3 | 3 | 3 | 4 | 4 | 4 |
| Medium cycle higher | 9 | 5 | 4 | 4 | 17 | 10 | 7 | 6 |
| Long cycle higher | 7 | 2 | 1 | 0 | 6 | 3 | 2 | 1 |
| Unknown | 6 | 3 | 3 | 2 | 4 | 2 | 2 | 2 |
| Duration (year) | ||||||||
| 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| 1 | 0 | 58 | 4 | 0 | 0 | 60 | 3 | 0 |
| 2–5 | 0 | 41 | 68 | 13 | 0 | 40 | 72 | 20 |
| 6–39 | 0 | 1 | 28 | 87 | 0 | 0 | 25 | 80 |
Grouped according to ISCO 88 = International Standard Classification of Occupations, 1988 revision: Armed forces (ISCO 88 codes 0110), White-collar workers (ISCO 88 codes 1000–5999), Skilled blue-collar workers (ISCO 88 codes 6000–7999), Unskilled blue-collar workers (ISCO 88 codes 8000–9999), Others (unemployed or retired).
Highest attained educational level.
All analyses were stratified by sex and adjusted for age (≤25, 26–35, ≥36 years), and calendar year of follow-up (1979-84, 1985–94, 1995–2004, 2005–15). We did not have information on smoking at an individual level, but in supplementary analyses we used a smoking JEM developed for the DOC*X cohort used in this study.40 This JEM provided sex- and calendar year-specific estimates of smoking prevalence for all ISCO 88 job codes, based on self-reported smoking habits reported in four large Danish population-based surveys. Years without employment were assigned the same smoking habit as in the latest job period. We furthermore conducted analyses adjusted for educational level (lower secondary, vocational or higher secondary, short-, medium- or long-cycle higher education, unknown) and analyses restricted to blue-collar workers (ISCO major categories 6–9) as defined at baseline, to obtain a more homogeneous population with respect to smoking and socioeconomic factors.
We analysed log-linear relations between respirable crystalline silica exposure and the autoimmune rheumatic diseases with continuous exposure variables. These analyses included the total study populations as well as the exposed populations only, with the low exposed as the reference. We fitted restricted cubic splines to the models, placing the knots at the 40, 60 and 80 percentiles. All analyses were carried out using Stata v.15 and v.16.
Results
The study population included 1 541 505 male workers cumulating 4673 cases of autoimmune rheumatic diseases during follow-up: systemic sclerosis (n = 252), rheumatoid arthritis (n = 3490), systemic lupus erythematosus (n = 255) and small vessel vasculitis (n = 749). The corresponding figures for 1 470 769 female workers were 12 268 cases of autoimmune rheumatic diseases: systemic sclerosis (n = 746), rheumatoid arthritis (n = 9190), systemic lupus erythematosus (n = 1821) and small vessel vasculitis (n = 869). Some participants were diagnosed with more than one autoimmune rheumatic disease and hence the number of specific diseases summed up to more than all autoimmune rheumatic diseases. Analyses for each disease were conducted separately and the respective study populations differed slightly. Only person-years at risk for the analyses of the studied autoimmune diseases combined are shown in the tables. The distribution of persons included in each exposure stratum is shown in Supplementary Table S3, available as Supplementary data at IJE online.
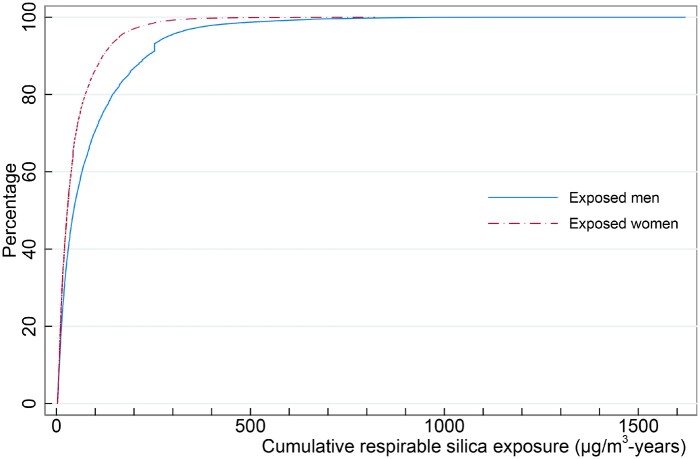
Among men, 17% ever held a job with exposure to respirable crystalline silica, and this was the case for 3% of the women. Furthermore, women were less exposed than men, with median cumulative exposure of 33 µg/m3-years (25-75% centiles: 16-72 µg/m3-years) versus 60 µg/m3-years (23–135 µg/m3-years) for men (Figure 1).
Figure 1.
Cumulative plot of the distribution of cumulative exposure level (μg/m3-years) at end of follow-up among 266 325 men and 42 914 women ever exposed to respirable crystalline silica
High exposure levels were associated with greater age, as expected, and with a higher probability of smoking (Table 2). There is an increasing time trend for being diagnosed with one of the studied autoimmune rheumatic diseases. In the time period 2005–15 compared with 1979–84, men had an increased risk (1.58, 95% CI: 1.30-1.92) of being diagnosed with one the studied diseases.
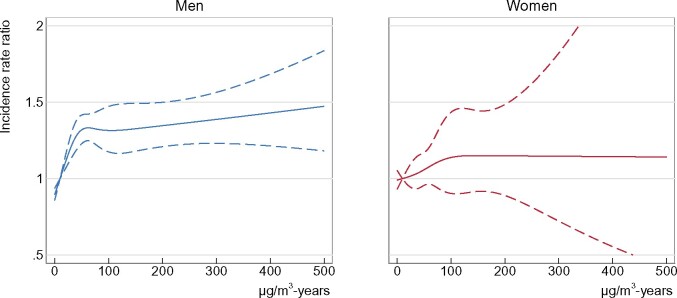
Among men, we observed an increased overall incidence rate ratio of the studied autoimmune rheumatic diseases combined of 1.53 (95% CI: 1.39-1.69) in analyses comparing the highest cumulative exposure stratum with non-exposure (Figure 2 and Table 3). Similar results were seen for mean exposure intensity, highest attained exposure intensity and duration of exposure. Furthermore, in the analysis of cumulative exposure, we observed an increasing trend of 1.07 (95% CI: 1.05-1.09) per 50 µg/m3-years. The corresponding trend computed among the exposed only was 1.03 (95% CI: 1.00-1.05) per 50 µg/m3-years. Similar risk patterns were seen for the respective diseases and most clearly for systemic sclerosis and rheumatoid arthritis. Cumulative exposure received more than 20 years earlier appears to be more influential for the exposure-response relation than cumulative exposure received more recently (Table 4).
Figure 2.
Restricted cubic spline fits of the age- and calendar year-adjusted overall incidence rate ratios of autoimmune rheumatic diseases by cumulated respirable crystalline silica among 1 541 505 men and 1 470 769 women, 1979–2015
Table 3.
Incidence rate ratios (IRR) of the studied autoimmune rheumatic diseases combined, systemic sclerosis, rheumatoid arthritis, systemic lupus erythematosus and small vessel vasculitis following exposure to respirable crystalline silica among 1 541 505 men and 1 470 769 women, Denmark, 1979–2015
|
The studied diseases combined
a
|
Systemic sclerosis |
Rheumatoid arthritis |
Systemic lupus erythematosus |
Small vessel vasculitis |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | Person-years b | Cases | IRR c (95% CI) | Cases | IRR c (95% CI) | Cases | IRR c (95% CI) | Cases | IRR c (95% CI) | Cases | IRR c (95% CI) |
|
Men | |||||||||||
| Cumulative exposure (µg/m3-years) | |||||||||||
| 0 | 28 527 938 | 3563 | 1 | 203 | 1 | 2630 | 1 | 198 | 1 | 587 | 1 |
| 2.0–29.2 | 1 576 698 | 283 | 1.23 (1.09–1.39) | 8 | 0.69 (0.34–1.40) | 218 | 1.24 (1.08–1.43) | 18 | 1.42 (0.88–2.31) | 46 | 1.34 (0.99–1.80) |
| 29.3–93.9 | 1 639 692 | 351 | 1.42 (1.27–1.58) | 14 | 1.04 (0.60–1.79) | 267 | 1.42 (1.25–1.61) | 16 | 1.22 (0.73–2.04) | 57 | 1.54 (1.17–2.02) |
| 94.0–1622 | 1 784 974 | 476 | 1.53 (1.39–1.69) | 27 | 1.62 (1.08–2.44) | 375 | 1.57 (1.41–1.75) | 23 | 1.46 (0.94–2.27) | 59 | 1.34 (1.02–1.76) |
| Per 50 µg/m3-years | 1.07 (1.05–1.09) | 1.10 (1.03–1.18) | 1.07 (1.05–1.10) | 1.09 (1.01–1.17) | 1.06 (1.01–1.11) | ||||||
| Per 50 µg/m3-years (exposed only) | 1.03 (1.00–1.05) | 1.11 (1.02–1.21) | 1.02 (0.99–1.05) | 1.06 (0.96–1.18) | 0.99 (0.93–1.07) | ||||||
| Mean exposure (µg/m3) | |||||||||||
| 0 | 28 527 938 | 3563 | 1 | 203 | 1 | 2630 | 1 | 198 | 1 | 587 | 1 |
| 2.0–10.7 | 1 612 428 | 397 | 1.42 (1.28–1.57) | 11 | 0.85 (0.46–1.57) | 317 | 1.45 (1.29–1.63) | 24 | 1.64 (1.06–2.52) | 53 | 1.37 (1.03–1.83) |
| 10.8–18.0 | 1 654 722 | 366 | 1.41 (1.26–1.57) | 16 | 1.15 (0.69–1.92) | 277 | 1.39 (1.23–1.58) | 22 | 1.60 (1.03–2.50 | 58 | 1.55 (1.18–2.03) |
| 18.1–122.0 | 1 734 214 | 347 | 1.39 (1.25–1.56) | 22 | 1.46 (0.94–2.27) | 266 | 1.43 (1.26–1.62) | 11 | 0.84 (0.45–1.55) | 51 | 1.30 (0.98–1.74) |
| Per 50 µg/m3 | 2.27 (1.88–2.74) | 1.90 (0.86–4.19) | 2.34 (1.88–2.91) | 1.57 (0.65–3.79) | 2.27 (1.42–3.61) | ||||||
| Per 50 µg/m3 (exposed only) | 1.13 (0.75–1.70) | 2.37 (0.44–12.72) | 1.03 (0.65–1.65) | 0.38 (0.48–2.93) | 1.42 (0.50–4.04) | ||||||
| Highest attained exposure (µg/m3) | |||||||||||
| 0 | 28 527 938 | 3563 | 1 | 203 | 1 | 2630 | 1 | 198 | 1 | 587 | 1 |
| 2.0–12.0 | 1 581 211 | 356 | 1.37 (1.23–1.53) | 12 | 0.98 (0.55–1.77) | 279 | 1.39 (1.22–1.57) | 20 | 1.44 (0.90–2.28) | 52 | 1.43 (1.07–1.91) |
| 12.1–21.9 | 1 645 575 | 357 | 1.38 (1.24–1.55) | 10 | 0.73 (0.39–1.38) | 283 | 1.44 (1.27–1.62) | 20 | 1.47 (0.93–2.33) | 52 | 1.39 (1.04–1.84) |
| 22.0–122 | 1 774 578 | 397 | 1.46 (1.31–1.62) | 27 | 1.69 (1.12–2.54) | 298 | 1.45 (1.29–1.64) | 17 | 1.22 (0.74–2.01) | 58 | 1.40 (1.06–1.84) |
| Per 50 µg/m3 | 1.95 (1.69–2.25) | 1.85 (1.02–3.39) | 1.97 (1.68–2.32) | 1.78 (0.93–3.40) | 1.87 (1.29–2.70) | ||||||
| Per 50 µg/m3 (exposed only) | 1.29 (0.98–1.70) | 2.62 (0.87–7.90) | 1.20 (0.87–1.65) | 1.41 (0.39–5.06) | 1.20 (0.57–2.54) | ||||||
| Duration (years) | |||||||||||
| 0 | 28 527 938 | 3563 | 1 | 203 | 1 | 2630 | 1 | 198 | 1 | 587 | 1 |
| 1 | 974 370 | 145 | 1.09 (0.92–1.29) | 6 | 0.84 (0.37–1.89) | 108 | 1.08 (0.89–1.31) | 9 | 1.24 (0.63–2.41) | 23 | 1.11 (0.73–1.69) |
| 2–5 | 1 993 555 | 395 | 1.38 (1.24–1.53) | 14 | 0.90 (0.52–1.55) | 304 | 1.41 (1.25–1.59) | 21 | 1.36 (0.86–2.13) | 65 | 1.48 (1.15–1.92) |
| 6–39 | 2 003 439 | 570 | 1.54 (1.41–1.69) | 29 | 1.54 (1.03–2.29) | 448 | 1.56 (1.41–1.73) | 27 | 1.44 (0.96–2.17) | 74 | 1.46 (1.14–1.87) |
| Per 5 year | 1.16 (1.13–1.20) | 1.17 (1.02–1.35) | 1.17 (1.13–1.21) | 1.20 (1.04–1.37) | 1.11 (1.02–1.22) | ||||||
| Per 5 year (exposed only) | 1.07 (1.02–1.12) | 1.21 (0.98–1.49) | 1.07 (1.02–1.13) | 1.15 (0.94–1.41) | 0.97 (0.84–1.11) | ||||||
|
Women | |||||||||||
| Cumulative exposure (µg/m3-years) | |||||||||||
| 0 | 30 800 795 | 11 888 | 1 | 716 | 1 | 8906 | 1 | 1767 | 1 | 846 | 1 |
| 2.0–29.2 | 340 301 | 156 | 0.99 (0.84–1.16) | 12 | 1.36 (0.77– 2.40) | 114 | 0.93 (0.78– 1.12) | 25 | 1.18 (0.79–1.75) | 9 | 0.87 (0.45–1.69) |
| 29.3–93.9 | 278 490 | 148 | 1.12 (0.95–1.31) | 12 | 1.56 (0.88–2.76) | 110 | 1.07 (0.88–1.29) | 22 | 1.26 (0.83–1.93) | 8 | 0.94 (0.47–1.88) |
| 94.0–1622 | 133 920 | 76 | 1.09 (0.87–1.37) | 6 | 1.46 (0.65–3.27) | 60 | 1.10 (0.85–1.42) | 7 | 0.82 (0.39–1.73) | 6 | 1.38 (0.62–3.08) |
| Per 50 µg/m3-years | 1.04 (0.99–1.10) | 1.14 (0.95–1.36) | 1.05 (0.98–1.11) | 1.04 (0.89–1.22) | 1.03 (0.82–1.29) | ||||||
| Per 50 µg/m3-years (exposed only) | 1.03 (0.96–1.12) | 1.04 (0.78–1.38) | 1.05 (0.97–1.15) | 0.98 (0.78–1.24) | 1.10 (0.82–1.47) | ||||||
| Mean exposure (µg/m3) | |||||||||||
| 0 | 30 800 795 | 11888 | 1 | 716 | 1 | 8906 | 1 | 1767 | 1 | n.r. | 1 |
| 2.0–10.7 | 300 872 | 149 | 0.96 (0.82–1.13) | 7 | 0.86 (0.41–1.81) | 113 | 0.92 (0.77–1.11) | 20 | 1.01 (0.65–1.57) | n.r. | 1.15 (0.63–2.08) |
| 10.8–18.0 | 266 425 | 145 | 1.16 (0.99–1.37) | 13 | 1.77 (1.02–3.07) | 106 | 1.10 (0.91–1.33) | 23 | 1.39 (0.92–2.10) | n.r. | 0.99 (0.49–1.99) |
| 18.1–122.0 | 185 414 | 86 | 1.07 (0.87–1.33) | 10 | 1.92 (1.03–3.61) | 65 | 1.07 (0.84–1.36) | 11 | 1.01 (0.56–1.84) | n.r. | 0.72 (0.27–1.93) |
| Per 50 µg/m3 | 1.27 (0.91–1.77) | 3.53 (1.28–9.74) | 1.20 (0.82–1.75) | 1.55 (0.66–3.65) | 0.67 (0.16–2.87) | ||||||
| Per 50 µg/m3 (exposed only) | 1.42 (0.67–2.99) | 5.05 (0.62–41.25) | 1.60 (0.70–3.67) | 1.42 (0.18–11.25) | 0.37 (0.01–13.49) | ||||||
| Highest attained exposure (µg/m3) | |||||||||||
| 0 | 30 800 795 | 11 888 | 1 | 716 | 1 | 8906 | 1 | 1767 | 1 | 846 | 1 |
| 2.0–12.0 | 333 072 | 167 | 0.99 (0.85–1.16) | 8 | 0.90 (0.45–1.81) | 127 | 0.97 (0.81–1.15) | 22 | 1.01 (0.67–1.55) | 12 | 1.15 (0.65–2.03) |
| 12.1–21.9 | 257 420 | 129 | 1.08 (0.90–1.28) | 12 | 1.69 (0.95–2.99) | 97 | 1.05 (0.86–1.28) | 19 | 1.19 (0.76–1.88) | 6 | 0.77 (0.34–1.71) |
| 22.0–122 | 162 219 | 84 | 1.16 (0.93–1.44) | 10 | 2.15 (1.15–4.01) | 60 | 1.08 (0.84–1.39) | 13 | 1.36 (0.79–2.35) | 5 | 1.01 (0.42–2.44) |
| Per 50 µg/m3 | 1.23 (0.92–1.64) | 2.90 (1.16–7.26) | 1.16 (0.83–1.63) | 1.46 (0.68–3.14) | 0.84 (0.24–2.89) | ||||||
| Per 50 µg/m3 (exposed only) | 1.29 (0.68–2.45) | 3.39 (0.46–24.96) | 1.40 (0.68–2.89) | 1.32 (0.22–7.93) | 1.10 (0.07–17.82) | ||||||
| Duration (years) | |||||||||||
| 0 | 30 800 795 | 11 911 | 1 | 716 | 1 | 8906 | 1 | 1767 | 1 | n.r. | 1 |
| 1 | 210 515 | 93 | 1.00 (0.81–1.22) | 10 | 1.86 (1.00–3.48) | 70 | 0.98 (0.77–1.24) | 11 | 0.86 (0.47–1.55) | n.r. | 0.64 (0.24–1.72) |
| 2–5 | 363 012 | 181 | 1.07 (0.93–1.24) | 11 | 1.12 (0.62–-2.04) | 130 | 1.00 (0.84–1.18) | 32 | 1.42 (1.00–2.01) | n.r. | 1.18 (0.68–2.04) |
| 6–39 | 179 184 | 106 | 1.08 (0.89–1.31) | 9 | 1.65 (0.85–3.18) | 84 | 1.08 (0.87–1.34) | 11 | 0.93 (0.51–1.69) | n.r. | 1.01 (0.45–2.25) |
| Per 5 year | 1.05 (0.97–1.14) | 1.19 (0.89–1.59) | 1.05 (0.95–1.15) | 0.99 (0.77–1.28) | 1.11 (0.81–1.51) | ||||||
| Per 5 year (exposed only) | 1.03 (0.92–1.16) | 0.99 (0.61–1.59) | 1.05 (0.92–1.20) | 0.82 (0.54–1.23) | 1.24 (0.81–1.90) | ||||||
n.r. not reported, cells with less than five cases.
The studied diseases combined: systemic sclerosis, rheumatoid arthritis, systemic lupus erythematosus, and small vessel vasculitis.
Number of person-years used for each analysis of the different outcomes differed slightly. Only total person-years from the analysis of all autoimmune rheumatic disease combined are shown in the tables.
Adjusted for age (≤25, 26–35,≥36) and calendar year (1979–84, 1985–94, 1995–2004, 2005–15).
Table 4.
Incidence rate ratios (IRR) of the studied autoimmune rheumatic diseases combined, systemic sclerosis, rheumatoid arthritis, systemic lupus erythematosus and small vessel vasculitis following respirable crystalline silica exposure accrued during the previous 1–10, 11–20 and >20 years time windows among 1 541 505 men and 1 470 769 women, Denmark, 1979–2015
|
The studied diseases combined
a
|
Systemic sclerosis |
Rheumatoid arthritis |
Systemic lupus erythematosus |
Small vessel vasculitis |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | Person-years b | Cases | IRR c (95% CI) | Cases | IRR c (95% CI) | Cases | IRR c (95% CI) | Cases | IRR c (95% CI) | Cases | IRR c (95% CI) |
|
Men | |||||||||||
| Cumulative exposure (µg/m3-years) | |||||||||||
| 1–10 years | |||||||||||
| 0 | 29 829 503 | 3975 | 1 | 217 | 1 | 2953 | 1 | 217 | 1 | 650 | 1 |
| 2.0–37.1 | 1 779 056 | 355 | 1.36 (1.22–1.51) | 19 | 1.45 (0.90–2.31) | 271 | 1.36 (1.20–1.54) | 18 | 1.26 (0.78–2.04) | 55 | 1.38 (1.05–1.82) |
| 37.2–875.2 | 1 920 743 | 343 | 1.30 (1.16–1.45) | 16 | 1.02 (0.61–1.70) | 266 | 1.36 (1.20–1.55) | 20 | 1.37 (0.86–2.17) | 44 | 1.03 (0.76–1.41) |
| Per 50 µg/m3-years | 1.10 (1.04–1.16) | 1.07 (0.87–1.31) | 1.12 (1.06–1.19) | 1.14(0.93–1.39) | 1.00 (0.87–1.16) | ||||||
| 11–20 years | 31 276 025 | 4038 | 1 | 222 | 1 | 2986 | 1 | 223 | 1 | 668 | 1 |
| 03.5–47.6 | 1 081 784 | 302 | 1.42 (1.27–1.60) | 16 | 1.64 (0.98–2.75) | 227 | 1.36 (1.19–1.56) | 15 | 1.40 (0.82–2.37) | 51 | 1.80 (1.35–2.41) |
| 47.7–875.2 | 1 171 493 | 333 | 1.46 (1.30–1.63) | 14 | 1.27 (0.73–2.20) | 277 | 1.54 (1.36–1.75) | 17 | 1.54 (0.93–2.55) | 30 | 1.00 (0.69–1.45) |
| Per 50 µg/m3-years | 1.13 (1.08–1.18) | 1.16 (0.97–1.38) | 1.14 (1.09–1.20) | 1.14 (0.94–1.37) | 1.01 (0.88–1.16) | ||||||
| >20 years | |||||||||||
| 0 | 32 434 659 | 4242 | 1 | 230 | 1 | 3153 | 1 | 236 | 1 | 689 | 1 |
| 6.1–66.6 | 521 145 | 184 | 1.42 (1.23–1.66) | 7 | 1.28 (0.59–2.75) | 145 | 1.40 (1.18–1.66) | 10 | 1.72 (0.90–3.29) | 25 | 1.52 (1.01–2.29) |
| 66.7–1338.5 | 573 498 | 247 | 1.70 (1.49–1.94) | 15 | 2.48(1.44–4.27) | 192 | 1.65 (1.42–1.92) | 9 | 1.37 (0.69–2.71) | 35 | 1.87 (1.32–2.66) |
| Per 50 µg/m3-years | 1.13 (1.10–1.17) | 1.22 (1.09–1.36) | 1.12 (1.08–1.16) | 1.15 (1.00–1.32) | 1.17 (1.08–1.26) | ||||||
| Mean exposure (µg/m3) | |||||||||||
| 1–10 years | |||||||||||
| 0 | 29 829 503 | 3975 | 1 | 217 | 1 | 2953 | 1 | 217 | 1 | 650 | 1 |
| 0.1–9.2 | 1 836 924 | 490 | 1.42 (1.29–1.56) | 22 | 1.43 (0.91–2.23) | 392 | 1.45 (1.30–1.61) | 217 | 1.77 (1.13–2.49) | 56 | 1.19 (0.90–1.57) |
| 9.3–122.0 | 1 862 875 | 208 | 1.15 (1.00–1.33) | 13 | 0.97 (0.55–1.72) | 145 | 1.17 (0.99–1.39) | 29 | 0.77 (0.39–1.52) | 43 | 1.22 (0.89–1.67) |
| Per 50 µg/m3 | 1.77 (1.24–2.53) | 1.09 (0.28–4.17) | 1.96 (1.26–3.04) | 9 | 1.20 (0.25–5.76) | 1.57 (0.73–3.38) | |||||
| 11–20 years | |||||||||||
| 0 | 31 276 025 | 4038 | 1 | 222 | 1 | 2986 | 1 | 223 | 1 | 668 | 1 |
| 0.1–8.1 | 1 148 078 | 373 | 1.56 (1.40–1.74)) | 20 | 2.45 (1.55–3.87) | 292 | 1.55 (1.37–1.75) | 23 | 1.95 (1.26–3.03) | 45 | 1.40 (1.03–1.91) |
| 8.2–110 | 1 105 199 | 262 | 1.30 (1.15–1.48)) | 10 | 1.27 (0.68–2.40) | 212 | 1.34 (1.16–1.54) | 9 | 0.90 (0.46–1.76) | 36 | 1.38 (0.98–1.95) |
| Per 50 µg/m3 | 2.72 (1.90–3.88) | 1.76 (0.32–9.54) | 2.90 (1.95–4.32) | 2.02 (0.38–10.63) | 2.49 (0.92–6.76) | ||||||
| >20 years | |||||||||||
| 0 | 32 434 659 | 4242 | 1 | 230 | 1 | 3153 | 1 | 236 | 1 | 689 | 1 |
| 0.2–11.7 | 561 913 | 184 | 1.56 (1.36–1.80) | 14 | 2.37 (1.36–4.15) | 170 | 1.54 (1.31–1.80) | 10 | 1.61 (0.84–3.08) | 26 | 1.48 (0.99–2.21) |
| 11.8–110 | 532 730 | 247 | 1.58 (1.37–1.81) | 8 | 1.41 (0.69–2.91) | 167 | 1.53 (1.31–1.80) | 9 | 1.46 (0.74–2.88) | 34 | 1.93 (1.35–2.76) |
| Per 50 µg/m3 | 2.95 (2.19–3.98) | 4.86 (1.37–17.24) | 2.74 (1.95–3.85) | 1.94 (0.41–9.18) | 4.06 (1.88–8.74) | ||||||
| Highest attained exposure (µg/m3) | |||||||||||
| 1–10 years | |||||||||||
| 0 | 29 829 503 | 3975 | 1 | 217 | 1 | 2953 | 1 | 217 | 1 | 650 | 1 |
| 2.0–12.5 | 1 776 923 | 441 | 1.41 (1.28–1.56) | 15 | 1.05 (0.62–1.78) | 352 | 1.45 (1.30–1.62) | 23 | 1.41 (0.92–2.18) | 60 | 1.38 (1.05–1.80) |
| 12.6–121.9 | 1 922 876 | 257 | 1.21 (1.06–1.37) | 20 | 1.39 (0.87–2.21) | 185 | 1.23 (1.05–1.42) | 15 | 1.19 (0.70–2.03) | 39 | 1.01 (0.72–1.40) |
| Per 50 µg/m3 | 1.91 (1.48–2.46) | 1.69 (0.66–4.31) | 2.08 (1.54–2.82) | 1.78 (0.62–5.15) | 1.40 (0.76–2.59) | ||||||
| 11–20 years | |||||||||||
| 0 | 31 276 025 | 4038 | 1 | 222 | 1 | 2986 | 1 | 223 | 1 | 668 | 1 |
| 3.5–15.8 | 1 047 317 | 352 | 1.56 (1.39–1.74) | 13 | 1.30 (0.74–2.31) | 279 | 1.55 (1.37–1.76) | 21 | 1.92 (1.21–3.04) | 50 | 1.68 (1.25–2.27) |
| 15.9–121.9 | 1 205 960 | 282 | 1.32 (1.17–1.49) | 17 | 1.58 (0.95–2.61) | 225 | 1.35 (1.18–1.55) | 11 | 1.02 (0.55–1.88) | 31 | 1.09 (0.76–1.57) |
| Per 50 µg/m3 | 2.10 (1.72–2.57) | 2.17 (0.91–5.00) | 2.18 (1.74–2.74) | 2.13 (0.89–5.11) | 1.62 (0.91–2.89) | ||||||
| >20 years | |||||||||||
| 0 | 32 434 659 | 4242 | 1 | 230 | 1 | 3153 | 1 | 236 | 1 | 689 | 1 |
| 6.1–23.4 | 504 415 | 207 | 1.60 (1.39–1.84) | 8 | 1.49 (0.72–3.08) | 164 | 1.59 (1.35–1.86) | 10 | 1.71 (0.89–3.27) | 30 | 1.80 (1.23–2.62) |
| 23.5–121.9 | 590 228 | 224 | 1.54 (1.34–1.77) | 14 | 2.26 (1.29–3.95) | 173 | 1.49 (1.27–1.74) | 9 | 1.38 (0.70–2.73) | 30 | 1.63 (1.12–2.37) |
| Per 50 µg/m3 | 2.04 (1.71–2.44) | 2.97 (1.41–6.25) | 1.95 (1.60–2.39) | 1.85 (0.77–4.42) | 2.26 (1.40–3.66) | ||||||
|
Women | |||||||||||
| Cumulative exposure (µg/m3-years) | |||||||||||
| 1–10 years | |||||||||||
| 0 | 31 051 236 | 12 066 | 1 | 731 | 1 | 9045 | 1 | 1790 | 1 | 854 | 1 |
| 2.0–37.1 | 319 807 | 134 | 0.98 (0.82–1.16) | 10 | 1.26 (0.68–2.36) | 93 | 0.89 (0.72–1.09) | 24 | 1.23 (0.82–1.83) | 10 | 1.08 (0.58–2.02) |
| 37.2–875.2 | 182 463 | 68 | 0.97 (0.76–1.23) | 5 | 1.08 (0.45–2.61) | 52 | 1.00 (0.76–1.31) | 7 | 0.65 (0.31–1.36) | 5 | 1.00 (0.41–2.40) |
| Per 50 µg/m3-years | 1.00 (0.87–1.15) | 0.92 (0.52–1.63) | 1.00 (0.85–1.19 | 0.96 (0.67–1.38) | 0.99 (0.60–1.65) | ||||||
| 11–20 years | |||||||||||
| 0 | 31 252 372 | 12 085 | 1 | 732 | 1 | 9050 | 1 | 1798 | 1 | n.r. | 1 |
| 3.5–47.6 | 194 665 | 118 | 1.09 (0.91–1.31) | 9 | 1.54 (0.79–2.97) | 88 | 1.02 (0.83–1.26) | 15 | 1.14 (0.69–1.90) | n.r. | 1.40 (0.73–2.71) |
| 47.7–875.2 | 106 469 | 65 | 1.08 (0.84–1.38) | 5 | 1.51 (0.62–3.64) | 52 | 1.08 (0.82–1.42) | 8 | 1.14 (0.57–2.29) | n.r. | 0.58 (0.14–2.31) |
| Per 50 µg/m3-years | 1.03 (0.92–1.16) | 1.16 (0.75–1.77) | 1.02 (0.89–1.17) | 1.06 (0.76–1.48) | 0.96 (0.56–1.65) | ||||||
| >20 year | |||||||||||
| 0 | 31 417 074 | 12 150 | 1 | 736 | 1 | 9096 | 1 | n.r | 1 | n.r. | 1 |
| 6.1–66.6 | 92 154 | 79 | 1.27 (1.01–1.58) | 5 | 1.48 (0.61–3.57) | 62 | 1.22 (0.95–1.57) | n.r | 1.91 (1.08–3.38) | n.r. | 1.09 (0.41–2.93) |
| 66.7–1338.5 | 44 278 | 39 | 1.30 (0.95–1.78) | 5 | 3.06 (1.27–7.40) | 32 | 1.31 (0.92–1.85) | n.r | 0.66 (0.17–2.65) | n.r. | 1.69 (0.54–5.27) |
| Per 50 µg/m3-years | 1.12 (1.02–1.24) | 1.36 (1.06–1.74) | 1.14 (1.02–1.26) | 1.15 (0.86–1.53) | 1.13 (0.77–1.66) | ||||||
| Mean exposure (µg/m3) | |||||||||||
| 1–10 years | |||||||||||
| 0 | 31 051 236 | 12 066 | 1 | 731 | 1 | 9045 | 1 | 1790 | 1 | n.r. | 1 |
| 0.1–9.2 | 261 915 | 129 | 0.94 (0.82–1.16) | 8 | 1.11 (0.55–2.23) | 97 | 0.90 (0.74–1.10) | 14 | 0.81 (0.478–1.37) | n.r. | 1.57 (0.91–2.72) |
| 9.3–122.0 | 240 355 | 73 | 1.03 (0.76–1.23) | 7 | 1.31 (0.62–2.77) | 48 | 0.98 (0.73–1.30) | 17 | 1.30 (0.81–2.11) | n.r. | 0.34 (0.08–1.35) |
| Per 50 µg/m3 | 0.78 (0.39–1.55) | 2.18 (0.31–15.40) | 0.65 (0.28–1.54) | 0.99 (0.21–4.57) | 0.19 (0.1–3.64) | ||||||
| 11–20 years | |||||||||||
| 0 | 31 252 372 | 12 085 | 1 | n.r. | 1 | 9050 | 1 | 1798 | 1 | n.r. | 1 |
| 0.1–8.1 | 128 933 | 83 | 1.11 (0.89–1.37) | 5 | 1.23 (0.51–2.96) | 65 | 1.09 (0.85–1.39) | 10 | 1.14 (0.61–2.12) | 858 | 0.33 (0.60–2.98) |
| 8.2–110 | 172 201 | 100 | 1.07 (0.88–1.30) | 9 | 1.77 (0.91–3.42) | 75 | 1.01 (0.80–1.27) | 13 | 1.14 (0.66–1.98) | 6 | 0.93 (0.38–2.24) |
| Per 50 µg/m3 | 1.24 (0.68–2.26) | 5.37 (0.93–31.02) | 1.03 (0.51–2.07) | 1.30 (0.24–6.87) | 5 | 0.28 (0.11–15.32) | |||||
| >20 years | |||||||||||
| 0 | 31 417 074 | 12 150 | 1 | n.r. | 1 | 9096 | 1 | 1807 | 1 | n.r. | 1 |
| 0.2–11.7 | 54 240 | 50 | 1.37 (1.04–1.81) | n.r. | 2.03 (0.76–5.43) | 39 | 1.31 (0.96–1.80) | 5 | 1.36 (0.56–3.28) | n.r. | 1.89 (0.70–5.05) |
| 11.8–110 | 82 192 | 68 | 1.21 (0.95–1.54) | n.r. | 1.97 (0.88–4.42) | 55 | 1.20 (0.92–1.57) | 9 | 1.60 (0.83–3.09) | n.r. | 0.91 (0.29–2.82) |
| Per 50 µg/m3 | 1.91 (1.14–3.20) | 4.79 (0.94–24.47) | 1.95 (1.11–3.44) | 3.30 (0.84–12.98) | 1.11 (0.10–12.74) | ||||||
| Highest attained exposure (µg/m3) | |||||||||||
| 1–10 years | |||||||||||
| 0 | 31 051 236 | 12 066 | 1 | 731 | 1 | 9045 | 1 | 1790 | 1 | n.r. | 1 |
| 2.0–12.5 | 311 925 | 148 | 0.97 (0.82–1.14) | 9 | 1.10 (0.57–2.13) | 109 | 0.91 (0.76–1.10) | 20 | 0.99 (0.64–1.54) | n.r. | 1.38 (0.79–2.38) |
| 12.6–121.9 | 190 345 | 54 | 0.98 (0.75–1.29) | 6 | 1.37 (0.61–3.08) | 36 | 0.96 (0.69–1.34) | 11 | 1.08 (0.59–1.95) | n.r. | 0.42 (0.10–1.68) |
| Per 50 µg/m3 | 0.83 (0.47–1.46) | 1.63 (0.28–9.42) | 0.73 (0.37–1.46) | 0.93 (0.25–3.49) | 0.40 (0.04–3.54) | ||||||
| 11–20 years | |||||||||||
| 0 | 31 252 372 | 12 085 | 1 | 732 | 1 | 9050 | 1 | 1798 | 1 | n.r. | 1 |
| 3.5–15.8 | 183 189 | 114 | 1.04 (0.87–1.25) | 8 | 1.37 (0.68–2.76) | 87 | 0.99 (0.80–1.23) | 15 | 1.19 (0.72–1.99) | n.r. | 1.08 (0.51–2.28) |
| 15.9–121.9 | 117 945 | 69 | 1.17 (0.92–1.48) | 6 | 1.80 (0.80–4.02) | 53 | 1.14 (0.87–1.49) | 8 | 1.05 (0.53–2.11) | n.r. | 1.17 (0.44–3.13) |
| Per 50 µg/m3 | 1.29 (0.84–1.97) | 2.90 (0.69–12.27) | 1.18 (0.72–1.93) | 1.62 (0.52–5.01) | 1.26 (0.22–7.36) | ||||||
| >20 years | |||||||||||
| 0 | 31 417 074 | 12 150 | 1 | n.r. | 1 | 9096 | 1 | 1807 | 1 | n.r. | 1 |
| 6.1–23.4 | 84 633 | 73 | 1.26 (1.00–1.58) | n.r. | 1.27 (0.47–3.40) | 60 | 1.27 (0.98–1.64) | 9 | 1.55 (0.80–2.99) | n.r. | 1.16 (0.43–3.12) |
| 23.5–121.9 | 51 799 | 45 | 1.31 (0.97–1.75) | n.r. | 3.22 (1.44–7.21) | 34 | 1.21 (0.86–1.70) | 5 | 1.43 (0.59–3.45) | n.r. | 1.50 (0.48–4.69) |
| Per 50 µg/m3 | 1.66 (1.12–2.46) | 4.13 (1.19–14.32) | 1.62 (1.04–2.51) | 2.52 (0.85–7.45) | 1.75 (0.35–8.74) | ||||||
n.r. not reported, cells with less than five cases.
The studied diseases combined: systemic sclerosis, rheumatoid arthritis, systemic lupus erythematosus, small vessel vasculitis .
Number of person-years used for each analysis of the different outcomes differed slightly. Only total person-years from the analysis of all autoimmune rheumatic disease combined are shown in the tables.
Adjusted for age (≤25, 26–35, ≥36) and calendar year (1979–84, 1985–94, 1995–2004, 2005–15).
Among women, we observed a slightly increased incidence rate ratio of 1.09 (95% CI: 0.87-1.37) for all the studied autoimmune rheumatic diseases combined, for the highest cumulative exposure stratum compared with no exposure, and a trend estimate of 1.04 (95% CI: 0.99-1.10) per 50 µg/m3-years (Figure 2 and Table 3). Among women, there were also indications of a latency effect of more than 20 years; however, this was less evident than among men (Table 4).
In subanalyses of seropositive and seronegative rheumatoid arthritis (only possible for cases classified according to ICD 10), we observed an equally elevated incidence rate ratio for both serotypes in both sexes (Supplementary Table S1, available as Supplementary data at IJE online).
In additional analysis of men only, we added job-, sex-, and calendar year-specific estimates of smoking prevalence to the models, and observed an increased incidence rate ratio of 1.44 (95% CI: 1.31-1.59) for all autoimmune rheumatic disease when comparing high cumulative exposure with no exposure (Supplementary Table S2, available as Supplementary data at IJE online). In age-, calendar year- and education-adjusted analysis, comparing the highest cumulative exposed men with the unexposed, we observed a similar increased risk ratio of 1.37 (95% CI: 1.24-1.51). A sensitivity analysis restricted to male blue-collar workers showed an incidence rate ratio of 1.44 (95% CI: 1.31-1.59) for high versus no cumulative silica exposure (Supplementary Table S2).
Discussion
Principal findings
Among men, we observed increasing risk of autoimmune rheumatic diseases following increasing occupational exposure to respirable crystalline silica. Findings were strongest for systemic sclerosis and rheumatoid arthritis. Similar, but less evident, results were seen for women. However, few women were exposed at high levels.
Strengths and weaknesses of the study
The quantitative estimates of silica exposure based on job-exposure matrix derived from an extensive number of measurements allowed exposure response analyses, a prerequisite for causal inference. The long follow-up of a national working population combined with national health registers allowed us to study these rare diseases. However, the study still included a relatively limited number of exposed cases, especially few exposed female cases due to the rarity of silica exposure among women, and therefore the outcome still comes with considerable statistical uncertainty. The almost complete high coverage of the health registers precluded major selection bias. Information on occupation obtained from national labour marked registers, combined with exposure assessment based on a job exposure matrix, largely limited recall bias.
We identified cases in a national hospital register with positive predictive values of 79% for rheumatoid arthritis,41 94% for systemic sclerosis42 and 73% for systemic lupus erythematosus, when compared with medical records as the gold standard.43 Thus false-positive cases, except perhaps for systemic sclerosis, may have biased measures of association most likely towards the null.
Smoking is a well-documented risk factor for rheumatoid arthritis and probably also for systemic lupus erythematosus44,45 and could have confounded our risk estimates, as could other factors related to social class. However, we still observed increased risks of the studied diseases when adjusting by: estimates of smoking prevalence via a smoking JEM; highest attained educational level; and in analyses restricted to blue-collar workers expected to have fairly comparable life style patterns across different occupations and silica exposure levels.
Comparison with other studies
Our results are in line with extensive evidence linking occupational exposure to respirable crystalline silica and autoimmune rheumatic diseases.44–46 To our knowledge, only few studies have examined the association with quantitative exposure levels.12,13 Vihlborg et al.13 observed a doubled risk of seropositive rheumatoid arthritis of [standardized incidence ratio of 2.59 (95% CI: 1.24-4.76)] at exposure levels of respirable crystalline silica above 50 µg/m3 and exposure-response relation in a cohort of male foundry workers. Others have observed increasing risk with increasing duration of exposure and semi-quantified exposure levels (never, low, high).6,8,17,18,20 Turner et al.12 did not, however, observe an association between quantitative levels of silica exposure and rheumatoid arthritis in a cohort of pottery, sandstone and refractory material workers.
Whereas the prevalence of autoimmune rheumatic diseases is higher among women, the association with respirable crystalline silica exposure is most evident among men in our study, most likely because fewer women were exposed and when exposed their cumulative exposure was lower. Exposure-response patterns were similar for men and women though.
In a meta-analysis by Rubio-Rivas et al. of respirable crystalline silica exposure and systemic sclerosis, they found a slightly higher risk among men than women.47 Similarly, the risk of rheumatoid arthritis among men was slightly higher than the risk for men and women combined in a meta-analysis by Khuder et al.48 A single study on systemic lupus erythematosus found a higher risk among men than among women.18 However, an animal model with male and female lupus-prone mice did not demonstrate sex-related differences in outcomes after exposure to crystalline silica.49
We observed increased risks of several of the studied autoimmune rheumatic diseases at mean exposure intensity levels well below the current European occupational exposure limit of 100 µg/m3,50 indicating that this limit provides insufficient protection of workers exposed to crystalline silica.
Possible mechanisms
Following inhalation, respirable crystalline silica particles are deposited in the alveoli.1 Animal models have shown that macrophages phagocyte the particles, activating the immune system by secretion of cytokines, chemokines and lysosomal enzymes, which activate antigen-presenting and in turn antibody-producing cells.46,51 In susceptible individuals, a disturbed control mechanism and breaking of tolerance result in continuous production of auto-antibodies.32,51 Apoptosis of macrophages results in release of silica particles and new uptake by antigen-presenting cells, contributing to chronic inflammation.46 For silicosis it has been shown that most of the disease progression takes place after termination of exposure to crystalline silica.52 Retained silica in lung tissue, and other similar or partly overlapping mechanisms as for silicosis, may explain the increased risks observed in this study more than 20 years after exposure. Furthermore, auto-antibodies are present years before clinical symptoms of systemic lupus erythematosus develop,53,54 and it has been suggested that triggering exposures in susceptible individuals first lead to serological autoimmunity and later to overt clinical disease.32 This could also explain the highest risks we observed following exposure accrued more than 20 years earlier.
Conclusions
This study shows an exposure-dependent association between respirable crystalline silica, systemic sclerosis and rheumatoid arthritis, and possibly also systemic lupus erythematosus and small vessel vasculitis. Findings were most evident in men, but few women were exposed at high levels.
Supplementary data
Supplementary data are available at IJE online.
Funding
This work was supported by a grant from the Danish Working Environment Research Fund (grant no. 34–2016-09). SP and HK received a grant from the Deutsche Gesetzliche Unfallversicherung to elaborate SYNJEM.
Supplementary Material
Acknowledgements
The authors would like to thank Lützen Portengen for help with understanding and interpretation of the statistical methods used.
Conflicts of interest
None declared.
References
- 1.Roney N, Faroon O, Williams M. et al. Toxicological Profile for Silica. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service: Agency for Toxic Substances and Disease Registry (ATSDR), 2019. [Google Scholar]
- 2.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, Metals, Fibres, and Dusts. Lyon, France: IARC, 2012. [PMC free article] [PubMed] [Google Scholar]
- 3.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Silica, Some Silicates, Coal Dust and Para-Aramid Fibrils. Lyon, France: IARC, 1997. [PMC free article] [PubMed] [Google Scholar]
- 4.T Mannetje A, Steenland K, Attfield M. et al. Exposure-response analysis and risk assessment for silica and silicosis mortality in a pooled analysis of six cohorts. Occup Environ Med 2002;59:723–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collis EL, Gu Y.. The mortality experience of an occupational group exposed to silica dust, compared with that of the general population and an occupational group exposed to dust not containing silica. J Indust Hyg 1933;15:395–417. [Google Scholar]
- 6.Diot E, Lesire V, Guilmot JL. et al. Systemic sclerosis and occupational risk factors: a case-control study. Occup Environ Med 2002;59:545–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Englert H, Small-McMahon J, Davis K, O'Connor H, Chambers P, Brooks P.. Male systemic sclerosis and occupational silica exposure - a population-based study. Aust N Z J Med 2000;30:215–20. [DOI] [PubMed] [Google Scholar]
- 8.Marie I, Gehanno JF, Bubenheim M. et al. Prospective study to evaluate the association between systemic sclerosis and occupational exposure and review of the literature. Autoimmun Rev 2014;13:151–56. [DOI] [PubMed] [Google Scholar]
- 9.Blanc PD, Jarvholm B, Toren K.. Prospective risk of rheumatologic disease associated with occupational exposure in a cohort of male construction workers. Am J Med 2015;128:1094–101. [DOI] [PubMed] [Google Scholar]
- 10.Klockars M, Koskela RS, Jarvinen E, Kolari PJ, Rossi A.. Silica exposure and rheumatoid arthritis: a follow up study of granite workers 1940-81. Br Med J (Clin Res Ed) 1987;294:997–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stolt P, Yahya A, Bengtsson C. et al. ; the EIRA Study Group. Silica exposure among male current smokers is associated with a high risk of developing ACPA-positive rheumatoid arthritis. Ann Rheum Dis 2010;69:1072–76. [DOI] [PubMed] [Google Scholar]
- 12.Turner S, Cherry N.. Rheumatoid arthritis in workers exposed to silica in the pottery industry. Occup Environ Med 2000;57:443–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vihlborg P, Bryngelsson IL, Andersson L, Graff P.. Risk of sarcoidosis and seropositive rheumatoid arthritis from occupational silica exposure in Swedish iron foundries: a retrospective cohort study. BMJ Open 2017;7:e016839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yahya A, Bengtsson C, Larsson P. et al. Silica exposure is associated with an increased risk of developing ACPA-positive rheumatoid arthritis in an Asian population: evidence from the Malaysian MyEIRA case-control study. Mod Rheumatol 2014;24(2):271–74 [DOI] [PubMed] [Google Scholar]
- 15.Ilar A, Alfredsson L, Wiebert P, Klareskog L, Bengtsson C.. Occupation and risk of developing rheumatoid arthritis: results from a population-based case-control study. Arthritis Care Res 2018;70:499–509. [DOI] [PubMed] [Google Scholar]
- 16.Cooper GS, Wither J, Bernatsky S; CaNIOS GenES Investigators et al. Occupational and environmental exposures and risk of systemic lupus erythematosus: silica, sunlight, solvents. Rheumatology (Oxf) 2010;49:2172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finckh A, Cooper GS, Chibnik LB. et al. Occupational silica and solvent exposures and risk of systemic lupus erythematosus in urban women. Arthritis Rheum 2006;54:3648–54. [DOI] [PubMed] [Google Scholar]
- 18.Parks CG, Cooper GS, Nylander-French LA. et al. Occupational exposure to crystalline silica and risk of systemic lupus erythematosus: a population-based, case-control study in the southeastern United States. Arthritis Rheum 2002;46:1840–50. [DOI] [PubMed] [Google Scholar]
- 19.Gregorini G, Ferioli A, Donato F. et al. Association between silica exposure and necrotizing crescentic glomerulonephritis with P-Anca and Anti-Mpo antibodies - a hospital-based case-control study. Anca-Associated Vasculitides 1993;336:435–40. [DOI] [PubMed] [Google Scholar]
- 20.Hogan SL, Cooper GS, Savitz DA. et al. Association of silica exposure with anti-neutrophil cytoplasmic autoantibody small-vessel vasculitis: a population-based, case-control study. CJASN 2007;2:290–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogan SL, Satterly KK, Dooley MA. et al. Silica exposure in anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and lupus nephritis. J Am Soc Nephrol 2001;12:134–42. [DOI] [PubMed] [Google Scholar]
- 22.Lane SE, Watts RA, Bentham G, Innes NJ, Scott DG.. Are environmental factors important in primary systemic vasculitis? A case-control study. Arthritis Rheum 2003;48:814–23. [DOI] [PubMed] [Google Scholar]
- 23.Nuyts GD, Van Vlem E, De Vos A. et al. Wegener granulomatosis is associated to exposure to silicon compounds: a case-control study. Nephrol Dial Transplant 1995;10:1162–65. [PubMed] [Google Scholar]
- 24.Stratta P, Messuerotti A, Canavese C. et al. The role of metals in autoimmune vasculitis: epidemiological and pathogenic study. Sci Total Environ 2001;270:179–90. [DOI] [PubMed] [Google Scholar]
- 25.Denton CP, Khanna D.. Systemic sclerosis. Lancet 2017;390:1685–99. [DOI] [PubMed] [Google Scholar]
- 26.Scott DL, Wolfe F, Huizinga TW.. Rheumatoid arthritis. Lancet 2010;376:1094–108. [DOI] [PubMed] [Google Scholar]
- 27.Lisnevskaia L, Murphy G, Isenberg D.. Systemic lupus erythematosus. Lancet 2014;384:1878–88. [DOI] [PubMed] [Google Scholar]
- 28.Jennette JC.Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol 2013;17:603–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watts RA, Lane S, Scott DG.. What is known about the epidemiology of the vasculitides? Best Pract Res Clin Rheumatol 2005;19:191–207. [DOI] [PubMed] [Google Scholar]
- 30.Gourley M, Miller FW.. Mechanisms of disease: Environmental factors in the pathogenesis of rheumatic disease. Nat Rev Rheumatol 2007;3:172–80. [DOI] [PubMed] [Google Scholar]
- 31.Selmi C, Leung PS, Sherr DH. et al. Mechanisms of environmental influence on human autoimmunity: a National Institute of Environmental Health Sciences expert panel workshop. J Autoimmun 2012;39:272–84. [DOI] [PubMed] [Google Scholar]
- 32.Wahren-Herlenius M, Dorner T.. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 2013;382:819–31. [DOI] [PubMed] [Google Scholar]
- 33.Flachs EM, Petersen SEB, Kolstad HA. et al. Cohort Profile: DOCX: a nationwide Danish occupational cohort with eXposure data—an open research resource. Int J Epidemiol 2019;48:1413–k. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen CB.The Danish Civil Registration System. Scand J Public Health 2011;39:22–25. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT.. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters S, Kromhout H, Portengen L. et al. Sensitivity Analyses of Exposure Estimates from a Quantitative Job-exposure Matrix (SYN-JEM) for use in community-based studies. Ann Occup Hyg 2013;57:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peters S, Vermeulen R, Portengen L. et al. Modelling of occupational respirable crystalline silica exposure for quantitative exposure assessment in community-based case-control studies. J Environ Monit 2011;13:3262–68. [DOI] [PubMed] [Google Scholar]
- 38.Richardson DB.Discrete time hazards models for occupational and environmental cohort analyses. Occup Environ Med 2010;67:67–71. [DOI] [PubMed] [Google Scholar]
- 39.Checkoway H, Pearce N, Hickey JL, Dement JM.. Latency analysis in occupational epidemiology. Arch Environ Health 1990;45:95–100. [DOI] [PubMed] [Google Scholar]
- 40.Bondo Petersen S, Flachs EM, Prescott EIB. et al. Job-exposure matrices addressing lifestyle to be applied in register-based occupational health studies. Occup Environ Med 2018;75:890–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibfelt EH, Sorensen J, Jensen DV. et al. Validity and completeness of rheumatoid arthritis diagnoses in the nationwide DANBIO clinical register and the Danish National Patient Registry. Clin Epidemiol 2017;9:627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butt SA, Jeppesen JL, Fuchs C. et al. Trends in incidence, mortality, and causes of death associated with systemic sclerosis in Denmark between 1995 and 2015: a nationwide cohort study. BMC Rheumatol 2018;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hermansen ML, Lindhardsen J, Torp-Pedersen C, Faurschou M, Jacobsen S.. Incidence of systemic lupus erythematosus and lupus nephritis in Denmark: a nationwide cohort study. J Rheumatol 2016;43:1335–39. [DOI] [PubMed] [Google Scholar]
- 44.Miller FW, Alfredsson L, Costenbader KH. et al. Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun 2012;39:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parks CG, Miller FW, Pollard KM. et al. Expert panel workshop consensus statement on the role of the environment in the development of autoimmune disease. Int J Mol Sci 2014;15:14269–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper GS, Miller FW, Germolec DR.. Occupational exposures and autoimmune diseases. Int Immunopharmacol 2002;2:303–13. [DOI] [PubMed] [Google Scholar]
- 47.Rubio-Rivas M, Moreno R, Corbella X.. Occupational and environmental scleroderma. Systematic review and meta-analysis. Clin Rheumatol 2017;36:569–82. [DOI] [PubMed] [Google Scholar]
- 48.Khuder SA, Peshimam AZ, Agraharam S.. Environmental risk factors for rheumatoid arthritis. Rev Environ Health 2002;17:307–15. [DOI] [PubMed] [Google Scholar]
- 49.Brown JM, Archer AJ, Pfau JC, Holian A.. Silica accelerated systemic autoimmune disease in lupus-prone New Zealand mixed mice. Clin Exp Immunol 2003;131:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.European Parliament and the Council of the European Union, Official Journal of the European Union (L 345/87). DIRECTIVE (EU) 2017/2398 amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. Brussels: European Parliament and the Council of the European Union, 2017.
- 51.Pollard KM.Silica, silicosis, and autoimmunity. Front Immunol 2016;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller BG, Hagen S, Love RG. et al. Risks of silicosis in coalworkers exposed to unusual concentrations of respirable quartz. Occup Environ Med 1998;55:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eriksson C, Kokkonen H, Johansson M, Hallmans G, Wadell G, Rantapää-Dahlqvist S.. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther 2011;13:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rantapää-Dahlqvist S, de Jong BAW, Berglin E. et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.