Abstract
Tat stimulates human immunodeficiency virus type 1 (HIV-1) transcriptional elongation by recruitment of the human transcription elongation factor P-TEFb, consisting of Cdk9 and cyclin T1, to the HIV-1 promoter via cooperative binding to the nascent HIV-1 transactivation response RNA element. The Cdk9 kinase activity has been shown to be essential for P-TEFb to hyperphosphorylate the carboxy-terminal domain (CTD) of RNA polymerase II and mediate Tat transactivation. Recent reports have shown that Tat can also interact with the multisubunit transcription factor TFIIH complex and increase the phosphorylation of CTD by the Cdk-activating kinase (CAK) complex associated with the core TFIIH. These observations have led to the proposal that TFIIH and P-TEFb may act sequentially and in a concerted manner to promote phosphorylation of CTD and increase polymerase processivity. Here, we show that under conditions in which a specific and efficient interaction between Tat and P-TEFb is observed, only a weak interaction between Tat and TFIIH that is independent of critical amino acid residues in the Tat transactivation domain can be detected. Furthermore, immunodepletion of CAK under high-salt conditions, which allow CAK to be dissociated from core-TFIIH, has no effect on either basal HIV-1 transcription or Tat activation of polymerase elongation in vitro. Therefore, unlike the P-TEFb kinase activity that is essential for Tat activation of HIV-1 transcriptional elongation, the CAK kinase associated with TFIIH appears to be dispensable for Tat function.
Human immunodeficiency virus type 1 (HIV-1) encodes a small regulatory protein, Tat, which strongly stimulates HIV-1 transcriptional elongation by interacting with the transactivation response (TAR) RNA stem-loop structure located at the 5′ end of the nascent viral transcripts (12, 13). A protein phosphorylation event which can be inhibited by specific kinase inhibitors has been recognized as a key step in Tat transactivation (21, 22). It has been shown that hyperphosphorylation of the carboxy-terminal domain (CTD) of the largest subunit of RNA polymerase II correlates closely with the production of highly processive polymerase elongation complexes (7) and that Tat activation of HIV-1 elongation requires CTD (5, 26, 28, 39). Based on these observations, it has been proposed that Tat activation is mediated by a cellular kinase, whose phosphorylation of CTD and perhaps other components of the polymerase elongation complex is essential for the generation of highly processive polymerase elongation complexes (12, 40).
Among many cellular kinases that are capable of phosphorylating pol II CTD in vitro, two Cdk-cyclin pairs present in two transcription factor complexes have been implicated as Tat coactivators to facilitate Tat stimulation of polymerase elongation. The first one, a Cdk9-cyclin T1 pair, was recently found to constitute the human positive-acting transcription elongation factor P-TEFb, and it can support both basal transcriptional elongation (32) as well as Tat activation (4, 27). P-TEFb was first identified and purified from Drosophila extracts (24), and it functions by hyperphosphorylating pol II CTD and preventing polymerase arrest (23). Immunodepletion of Cdk9 from HeLa nuclear extract eliminated basal HIV-1 transcription elongation and Tat transactivation (21, 42, 45), and the addition of affinity-purified human P-TEFb complex completely restored these two processes (42). Human P-TEFb was shown to interact with the activation domain of Tat (11, 45), suggesting that it may be a direct target of Tat. In fact, Tat was recently found to stimulate polymerase elongation by recruitment of the P-TEFb complex to the HIV-1 promoter through a Tat-TAR interaction (4, 42). In addition to forming a complex with Tat, P-TEFb was also found to interact with and phosphorylate Tat-SF1, a transcription elongation factor required for Tat transactivation (16, 42). Recently, a novel cyclin C-related protein called cyclin T1 has been shown to be a major partner of Cdk9 in human cells (32, 38). Importantly, Wei et al. (38) have demonstrated that recombinant cyclin T1 interacted specifically with the transactivation domain of Tat and that this association mediated the high-affinity binding of the Tat-cyclin T1 complex to TAR RNA dependent on sequences in the TAR apical loop.
In addition to the Cdk9-cyclin T1 dimer that constitutes the P-TEFb complex, recent studies have also implicated TFIIH as a Tat-specific coactivator. TFIIH is comprised of nine polypeptides (ERCC3, ERCC2, p62, p54, p44, Cdk7, cyclin H, Mat1, and p34) and has dual roles in transcriptional regulation and DNA repair (for a review see reference 20). In transcription reactions, TFIIH is part of the preinitiation complex and functions at the stages of initiation and promoter clearance when the RNA transcript is less than 30 to 50 bases long (41). This is in contrast to P-TEFb, which does not associate with the preinitiation complex and works at the stage of RNA chain elongation after the polymerase clears the promoter (14).
TFIIH has a kinase activity, and this activity resides in the Cdk7 subunit, which interacts with cyclin H and Mat1 to form a stable trimeric Cdk-activating kinase (CAK) complex. CAK has been shown to exist in three distinct complexes (20, 37). While the majority is present as free CAK, it was also found to exist as a CAK-ERCC2 complex as well as in association with the core TFIIH (ERCC3, ERCC2, p62, p54, p44, and p34) to form the holo-TFIIH complex. In addition to having a role in cell cycle control, CAK has been widely postulated to function as a major CTD kinase in transcription reactions (2, 37), leading several groups to examine whether Tat may target TFIIH-CAK directly. In fact, Tat has been shown to interact with TFIIH and to stimulate phosphorylation of pol II CTD by the TFIIH kinase, although different groups have different opinions on which subunit of TFIIH mediates Tat binding (3, 6, 9, 28). Recently, Cujec et al. (6) showed that Tat binds directly to Cdk7 and that Tat activation can be blocked by a Cdk7 pseudosubstrate peptide inhibitor. Based on these results, a two-stage model for CTD phosphorylation, suggesting that both TFIIH and P-TEFb act sequentially and in a concerted manner to promote hyperphosphorylation of CTD and increase polymerase processivity, has been proposed (12, 40).
Because two distinct Cdk-cyclin pairs have been demonstrated to be coactivators of Tat by different groups under different experimental conditions, we decided to investigate their roles in Tat transactivation under the same condition. Our data indicate that while the human P-TEFb complex is essential for Tat transactivation, neither free CAK nor CAK associated with core-TFIIH seems to be required for Tat function in vitro. Correlating with their roles in transcription reactions, we show here that under the same condition where a specific and efficient interaction between P-TEFb and Tat is observed, only weak interactions between Tat and CAK and between Tat and core-TFIIH that are independent of several critical amino acid residues in the Tat transactivation domain can be detected. These results further underscore the importance of P-TEFb in Tat transactivation and also reveal that the CAK kinase complex associated with TFIIH is dispensable for Tat function in vitro.
MATERIALS AND METHODS
Antibodies.
Commercial preparations of rabbit polyclonal antibodies directed against Cdk7, Mat1, and ERCC3 were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). The anti-cyclin H antiserum was raised in rabbits. Anti-Cdk7 and anti-Mat1 polyclonal antibodies or monoclonal antibody (MAb) 12CA5 recognizing the hemagglutinin (HA) epitope tag was directly coupled to protein A-Sepharose with dimethylpimelimidate as described previously (10).
Immunoaffinity-purification of Cdk7-HA and associated proteins.
Thirty micrograms of HA-tagged Cdk7 construct (6) was transfected into human 293T cells by the calcium phosphate precipitation method. Forty-eight hours posttransfection, cells were washed in phosphate-buffered saline and lysed with either high-salt lysis buffer (500 mM NaCl, 1% Nonidet P-40 [NP-40], 50 mM HEPES-KOH [pH 7.9], 0.5 mM EDTA, 2 mM dithiothreitol [DTT], and 0.5 mM phenylmethylsulfonyl fluoride [PMSF]) or low-salt lysis buffer (150 mM NaCl, 1% NP-40, 50 mM HEPES-KOH [pH 7.9], 0.5 mM EDTA, 2 mM DTT, and 0.5 mM PMSF), as specified in the text and figure legend. Precleared cell lysates were subjected to immunoprecipitation with immobilized MAb 12CA5 under the same buffer conditions as for cell lysis. After extensive washes, Cdk7-HA-containing complexes were eluted from the antibody column with the elution solution containing 1 mg of HA epitope peptide/ml as described previously (43).
Immunodepletion of Cdk7 or Mat1 from HeLa nuclear extract.
Immunodepletion was carried out by incubating HeLa nuclear extract containing 0.15% NP-40 and either 0.1 M or 0.8 M KCl with anti-Cdk7 or anti-Mat1 antibodies immobilized on protein A-Sepharose. Incubation was carried out at 4°C for 1 h, and the supernatant was incubated with fresh antibody beads two more times. The depleted extracts were subjected to spin column (Sephadex G-25) desalting prior to analysis in transcription reactions.
Tat binding assays.
Cdk9-HA and its associated proteins (labeled P-TEFb fraction) were affinity-purified from a stable human 293-derived cell line (B4) expressing Cdk9-HA as previously described (42). Cdk7-HA and associated proteins (labeled TFIIH fraction) were affinity-purified as described above. These two fractions were incubated with wild-type GST-Tat(1-48), mutant GST-Tat(1-48, C22G), GST-Tat(1-48, K41A), GST-Tat(1-48, H33A), or glutathione S-transferase (GST) bound to glutathione-Sepharose at 23°C for 20 min. The binding buffer contains 20 mM Tris-HCl (pH 8.0), 20% glycerol, 500 mM KCl, 0.5% NP-40, 0.05% sodium dodecyl sulfate (SDS), 0.2 mM EDTA, 0.2 mg of bovine serum albumin/ml, 0.2 mM ZnCl2, 1 mM DTT, and 0.5 mM PMSF. After extensive washes in the same buffer, the bound proteins were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting.
Transcription assay.
In vitro transcription reactions containing HeLa nuclear extract and HIV-1 promoter templates were carried out as previously described (28, 44). G-less RNA fragments derived from HIV transcripts were isolated after RNase T1 treatment and analyzed on 6% sequencing gels.
RESULTS
P-TEFb, but not TFIIH, interacts with Tat specifically and efficiently.
HIV-1 Tat has been shown to interact with TFIIH (3, 6, 9, 28) and P-TEFb (11, 45) in different reports following different protocols. We decided to compare the efficiency and specificity of these interactions under exactly the same conditions. CAK and its associated core-TFIIH in the holo-TFIIH complex were purified from human 293T cells expressing an HA-tagged Cdk7 (Cdk7-HA) by immunoprecipitation with MAb 12CA5 followed by elution with HA epitope peptide (42, 43). The affinity-purified complex was shown by Western blotting to contain Cdk7, Mat1 (Fig. 1C), and cyclin H (see Fig. 4), the three subunits of CAK. It also contained ERCC3 (Fig. 1C), one of the core-TFIIH subunits. A similar procedure was used to obtain affinity-purified P-TEFb complex from a stable human 293 cell line (B4) expressing HA-tagged Cdk9 (Cdk9-HA) (4, 27). In addition to the Cdk9-HA-cyclin T1 heterodimer that constitutes the mature and active form of P-TEFb and supports Tat transactivation, the affinity-purified Cdk9-HA fraction also contained two other complexes that consist of Cdk9-HA/Hsp90/Cdc37 and Cdk9-HA/Hsp70, which function as precursors of the active P-TEFb complex (27). Because both Cdk7-HA of TFIIH and Cdk9-HA of P-TEFb contained an HA tag, Western blotting with MAb 12CA5 was used to normalize their levels in Tat-binding reactions.
FIG. 1.
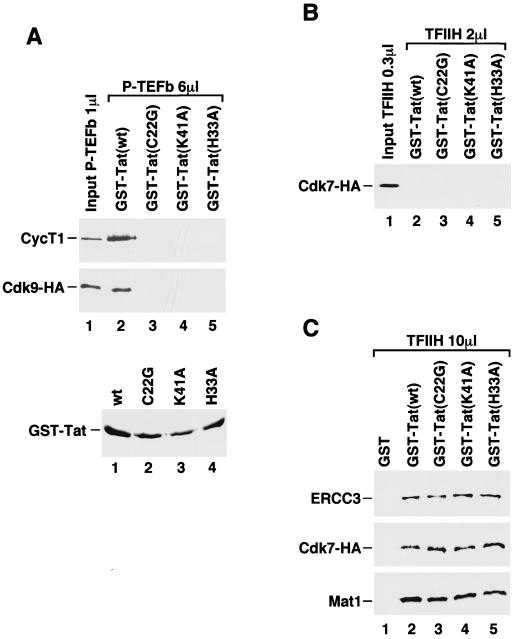
Strong and specific binding of Tat to P-TEFb but not to TFIIH. (A) Binding of P-TEFb to Tat. Affinity-purified P-TEFb complex fraction (6 μl) was incubated with wild-type GST-Tat(1-48) and three GST-Tat(1-48) mutants (C22G, K41A, and H33A) bound to glutathione-Sepharose beads. After extensive washes, the bound proteins were analyzed by SDS-PAGE and Western blotting for the presence of cyclin T1 and Cdk9-HA with antibodies specific for cyclin T1 and HA (MAb 12CA5), respectively. One microliter of the input P-TEFb fraction was used in lane 1 as a reference. The lower panel is a Coomassie-stained SDS gel showing the relative amounts of wild-type and mutant GST-Tat(1-48) proteins bound to glutathione-Sepharose beads. (B) Binding of TFIIH to Tat. TFIIH fraction (2 μl) with associated Cdk7-HA at a level similar to that of Cdk9-HA in P-TEFb (6 μl) was tested for binding to Tat under the same conditions as for P-TEFb. After washes, Western blotting with 12CA5 was used to detect Cdk7-HA bound to the GST-Tat beads. (C) Five times more TFIIH fraction (10 μl) was tested for binding to GST, wild-type, or mutant GST-Tat(1-48) beads. Bound proteins were examined by Western blotting with antibodies specific for ERCC3, the HA tag of Cdk7-HA, and Mat1.
FIG. 4.
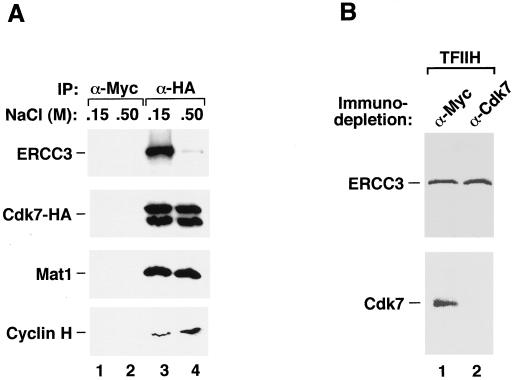
Efficient removal of CAK from holo-TFIIH by anti-Cdk7 depletion in the presence of high salt concentrations. (A) CAK can be dissociated from holo-TFIIH by high salt concentrations. Human 293T cells transiently transfected with an HA-tagged Cdk7 (Cdk7-HA) construct were lysed with buffers containing either 500 mM or 150 mM NaCl. Immunoprecipitation with anti-HA tag MAb 12CA5 was carried out at the same salt concentrations as in the lysis buffers. The immunoprecipitated proteins were analyzed by Western blotting with antibodies directed against Cdk7, Mat1, cyclin H, and ERCC3 as indicated. Occasionally, Cdk7-HA can be seen as a doublet probably because it can be modified differently under different conditions. (B) Cdk7-CAK in highly purified TFIIH complex can be immunodepleted in the presence of high salt concentrations. Highly purified TFIIH preparation was incubated with immobilized Cdk7 antibodies in the presence of 0.8 M KCl. Western blotting with antibodies directed against Cdk7 and ERCC3 was carried out to examine the presence of these two proteins in the highly purified TFIIH preparation after immunodepletion.
When the Cdk9-HA fraction (labeled P-TEFb) was incubated with immobilized wild-type GST-Tat(1-48) that lacks the RNA-binding C terminus but contains an intact transactivation domain (11, 33), approximately 17% of Cdk9-HA and 60% of cyclin T1 present in the input fraction were found to bind to the Tat column (Fig. 1A). The apparent difference between Cdk9-HA and cyclin T1 in Tat-binding efficiency can be explained by our recent observation that among the three Cdk9-HA complexes in the affinity-purified Cdk9-HA fraction, only the Cdk9-HA-cyclin T1 dimer (P-TEFb) demonstrated high-affinity interaction with Tat (27). Importantly, interaction of P-TEFb with Tat was absolutely dependent on an intact Tat activation domain (Fig. 1A), as three point mutations (C22G, H33A, and K41A) that are located in the Tat activation domain and were shown previously to destroy Tat transactivation (33) also inhibited the Tat–P-TEFb interaction.
When affinity-purified TFIIH fraction, which contained a similar amount of Cdk7-HA as Cdk9-HA in the P-TEFb fraction, was tested for binding to Tat, virtually no Cdk7-HA was found to associate with the GST-Tat column (Fig. 1B, upper panel). When five times more TFIIH fraction was used in the binding reactions, about 2% of Cdk7-HA, Mat1, and ERCC3 from the input fraction were found to bind to the wild-type GST-Tat(1-48) column but not to a column with GST only (Fig. 1C). However, unlike the interaction between Tat and P-TEFb, the weak binding of TFIIH to Tat was not disrupted by any of the three point mutations in the Tat activation domain (Fig. 1C). Thus, under the same condition, where a specific and efficient interaction between Tat and P-TEFb was observed, a much weaker interaction between Tat and TFIIH that is insensitive to mutations of several critical amino acid residues in the Tat activation domain was detected.
The binding condition used above contained a high salt concentration (500 mM KCl) and detergents (0.5% NP-40 and 0.05% SDS), which may prevent a specific interaction of CAK-TFIIH with Tat. To test this possibility, we also examined the binding of TFIIH to Tat under less stringent conditions in which an interaction between Tat and TFIIH was previously observed (28). Moreover, we also varied the concentration of GST-Tat on the beads in reactions containing 100 mM KCl and no detergents. Under these mild conditions, CAK and core-TFIIH were found to bind to GST-Tat readily, but these interactions were only slightly affected by the C22G point mutation in the Tat activation domain (data not shown). Therefore, neither the stringent nor the mild salt conditions revealed a specific Tat-TFIIH interaction that is dependent on the wild-type Tat activation domain.
Immunodepletion of Cdk7 at different salt concentrations has different effects on Tat transactivation.
Human P-TEFb not only bound tightly and specifically to the Tat activation domain, it was also required for Tat activation of HIV-1 transcription in vitro (Fig. 2A). Compared with mock-depleted HeLa nuclear extract, which supported a Tat-specific and TAR-dependent activation of HIV-1 transcriptional elongation (44) (Fig. 2A, left panel, lanes 1 and 2), immunodepletion of Cdk9–P-TEFb from HeLa nuclear extract under high-salt conditions (0.8 M KCl) eliminated HIV-1 transcription (Fig. 2A, lanes 3 and 4). Importantly, the addition of affinity-purified human P-TEFb complex into the depleted extract resulted in a complete recovery of both basal and Tat-activated HIV-1 transcriptional elongation (lanes 5 and 6). To determine whether P-TEFb is really a Tat-specific coactivator or simply a general elongation factor that plays a basic role during polymerase elongation, we recently generated and examined the activities of human-rodent “hybrid” P-TEFb complexes (4). Our results indicated that human P-TEFb is both a basal elongation factor as well as a Tat-specific coactivator and that these two activities can be separated. Moreover, the specific interaction of human P-TEFb with Tat at the HIV-1 TAR RNA element is crucial for P-TEFb to mediate a Tat-specific and species-restricted activation of HIV-1 transcription (4).
FIG. 2.
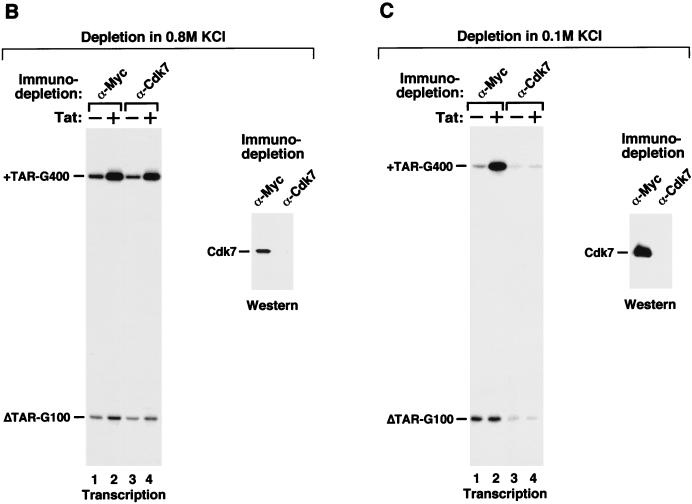
Tat transactivation in HeLa nuclear extract depleted of Cdk7 or Cdk9. (A [left panel]) Transcription reactions containing both templates pHIV+TAR-G400 and pHIVΔTAR-G100 were performed in the absence (−) or presence (+) of Tat and mock-depleted HeLa nuclear extract (lanes 1 and 2) or HeLa nuclear extract immunodepleted of the Cdk9 subunit of P-TEFb (lanes 3 to 6). Affinity-purified P-TEFb complex was added to Cdk9-depleted reactions as indicated. Immunodepletion was performed in the presence of 0.8 M KCl. (Right panel) The depleted extracts were examined by Western blotting with Cdk9 antibodies. (B and C) Cdk7, a subunit of the CAK ternary complex, was removed from HeLa nuclear extract by immunodepletion with anti-Cdk7 antibodies under high-salt (0.8 M KCl [B]) or low-salt (0.1 M KCl [C]) conditions. Immobilized anti-Myc antibody was used in control depletion reactions. (Left halves of panels B and C) Transcription reactions containing depleted extracts and transcription templates were carried out in the absence (−) or presence (+) of Tat. (Right halves of panels B and C) The depleted extracts were analyzed by Western blotting with anti-Cdk7 antibodies.
With the demonstration that Tat activation depends on P-TEFb and Tat interacts specifically with P-TEFb but not with CAK or core-TFIIH, we decided to investigate whether CAK of holo-TFIIH is actually required for Tat activation of HIV-1 transcription. HeLa nuclear extract was subjected to immunodepletion with anti-Cdk7 antibodies immobilized on protein A-Sepharose beads at two different salt concentrations (0.8 M and 0.1 M KCl). A column containing immobilized anti-Myc antibody was used as a control. As indicated by Western analyses (right panels of Fig. 2B and C), no detectable Cdk7 remained in HeLa nuclear extract after anti-Cdk7 immunodepletion at both salt concentrations. Moreover, Mat1, a subunit of the CAK ternary complex, was also removed from the Cdk7-depleted extracts (data not shown), indicating that the anti-Cdk7 immunodepletion was able to remove both Cdk7 and its associated proteins in the CAK ternary complex from HeLa nuclear extracts.
Next, the Cdk7-depleted extracts were analyzed in transcription reactions for their abilities to mediate Tat activation (left panels of Fig. 2B and C). Depending on the conditions of depletion, significant differences between the two Cdk7-depleted extracts in their abilities to mediate basal HIV-1 transcription and Tat activation were observed (Fig. 2B and C). In reactions containing HeLa nuclear extract mock-depleted with the control anti-Myc column at either of the two salt conditions, Tat specifically activated transcriptional elongation from HIV-1 template containing wild-type TAR element (pHIV+TAR-G400 [44]) but not from an internal control template (pHIVΔTAR-G100) with a mutant TAR (Fig. 2B and C, lanes 1 and 2). Importantly, unlike anti-Cdk9 immunodepletion (Fig. 2A), removal of Cdk7-CAK from HeLa nuclear extract under high-salt conditions (0.8 M KCl) did not have a significant effect on either basal or Tat-activated HIV-1 transcription compared with the mock-depleted reactions (Fig. 2B, left panel, lanes 1 and 2 showed 5.5-fold and lanes 3 and 4 showed 6.3-fold Tat activation when normalized to internal controls). In contrast, immunodepletion of Cdk7 in the presence of 0.1 M KCl significantly reduced basal transcription and virtually eliminated Tat activation (Fig. 2C, left panel). As discussed below, the low-level basal transcription produced by the depleted extract may derive from a minor form of transcription complex whose assembly on the HIV-1 long terminal repeat does not require a TATA box, TFIIH, or a few other basal transcription factors (18, 29, 31). Together, these results suggest that although Cdk7 can be immunodepleted efficiently from HeLa nuclear extract in the presence of either 0.1 or 0.8 M KCl, different forms of Cdk7 complexes were probably depleted under these two conditions. Proteins complexed with Cdk7 under mild salt concentrations are most likely required for HIV-1 transcription.
Different sizes of Cdk7-containing complexes exist at different salt concentrations.
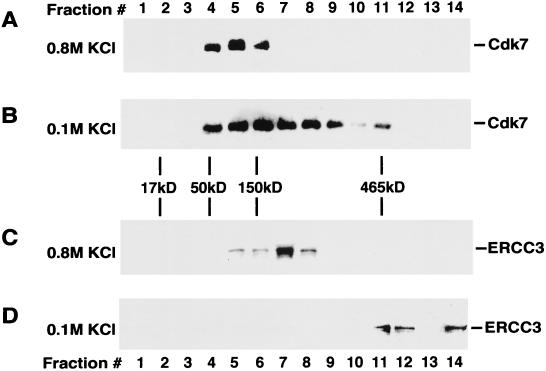
To examine how different salt concentrations used in the immunodepletion reactions affected the association of Cdk7 with other nuclear proteins, HeLa nuclear extract was sedimented through a glycerol gradient containing either 0.1 M or 0.8 M KCl. The resultant gradient fractions were analyzed by immunoblotting with antibodies specific for Cdk7 of the CAK complex and ERCC3 of core-TFIIH (Fig. 3). In the presence of 0.8 M KCl, Cdk7 was detected in fractions 4 to 6 with an estimated molecular mass of ∼100 kDa, which is similar to the calculated molecular mass (115 kDa) of the CAK ternary complex (Fig. 3A). Both cyclin H and Mat1 were also detected in these fractions (data not shown), suggesting that CAK is likely to be the predominant Cdk7-containing complex in nuclear extract at this salt concentration. In contrast, when nuclear extract was analyzed in a glycerol gradient containing 0.1 M KCl, Cdk7 was detected in many fractions (Fig. 3B), suggesting that it may be present in several different complexes, including the CAK complex. Approximately 10% of Cdk7 was found in fraction 11, corresponding to a molecular mass of ∼465 kDa, which is similar to the calculated molecular mass (∼510 kDa) of the holo-TFIIH complex. These experiments demonstrate that while the association of Cdk7 with cyclin H and Mat1 in the CAK complex was stable in the presence of 0.8 M KCl, the interaction of Cdk7-CAK with other nuclear proteins was disrupted by a high salt concentration.
FIG. 3.
Cdk7-containing complexes have different sizes at different salt concentrations. HeLa nuclear extracts were subjected to ultracentrifugation sedimentation through a 13 to 30% glycerol gradient containing either 0.1 M (B and D) or 0.8 M KCl (A and C). Gradient fractions were analyzed by Western blotting using antibodies specific for Cdk7 (A and B) or ERCC3 (C and D). A mixture of molecular mass marker proteins were sedimented in a parallel gradient. Their positions in the gradient were determined by SDS-PAGE and silver staining and are indicated in between panels B and C.
We also examined the effect of different salt concentrations on the distribution of ERCC3, a core-TFIIH subunit, in glycerol gradient fractions. In the presence of 0.8 M KCl, most of ERCC3 was found in a complex of ∼250 kDa (Fig. 3C). In the presence of 0.1 M KCl, however, ∼50% of ERCC3 was found in fractions 11 and 12 with an apparent molecular mass similar to that of holo-TFIIH (Fig. 3D). About half of ERCC3 was also found at the bottom of the centrifuge tube in protein complexes or aggregates with very high molecular masses (Fig. 3D). Therefore, like Cdk7-containing complexes, an increase in salt concentration also decreased the size of ERCC3-containing complexes in HeLa nuclear extract.
CAK can be efficiently removed from holo-TFIIH by anti-Cdk7 immunodepletion in the presence of high salt concentrations.
The above experiment revealed salt-sensitive interactions of Cdk7-CAK and ERCC3 with HeLa nuclear proteins. When a partially purified TFIIH fraction was loaded onto a glycerol gradient containing 1 M KCl, the peak fractions of CAK and core-TFIIH were found to be partially separated from each other (1). Although this may suggest a high-salt-concentration-mediated disruption of the interaction of CAK with core-TFIIH, it is also possible that the partially purified TFIIH fraction was contaminated with some free CAK or that the process of column fractionations, including a hydrophobic column, caused a partial dissociation of CAK from core-TFIIH. To confirm the salt-sensitive nature of the interaction between CAK and core-TFIIH, we transfected human 293T cells with a construct expressing Cdk7-HA and immunoprecipitated Cdk7-HA and its associated proteins from cell lysates with MAb 12CA5 under two different salt concentrations (0.15 M or 0.5 M NaCl). The immunoprecipitates were analyzed by Western blotting with antibodies specific for ERCC3 and the three subunits of CAK (Fig. 4A). At both salt concentrations, MAb 12CA5 efficiently precipitated Cdk7-HA and associated cyclin H and Mat1 in the CAK complex (Fig. 4A, lanes 3 and 4), whereas a control anti-Myc column did not precipitate these proteins (lanes 1 and 2). Importantly, while 0.5 M NaCl had no effect on the composition of the CAK ternary complex, it significantly reduced the association of ERCC3–core-TFIIH with CAK (Fig. 4A, lane 4).
While the majority of CAK was separated from core-TFIIH by 0.5 M NaCl, a small fraction of CAK appeared to interact with core-TFIIH in a salt-resistant manner (Fig. 4A, lane 4). Furthermore, a TFIIH fraction (30) purified extensively from HeLa nuclear extract by multiple column chromatography steps, a few of which involved relatively high-salt conditions, also contained some cdk7-CAK (Fig. 4B, lane 1). We were concerned with the possibility that perhaps a small fraction of holo-TFIIH with tightly bound CAK was somehow resistant to anti-Cdk7 immunodepletion and was fully responsible for the transcriptional activity observed in Fig. 2B. To examine this possibility, we subjected a highly purified TFIIH preparation (kindly provided by Jae-sang Kim and Phillip Sharp) (30) to anti-Cdk7 immunodepletion under the same high-salt condition (0.8 M KCl) used for the depletion of HeLa nuclear extract (Fig. 2B). Western blotting indicated that while Cdk7 was efficiently removed from this fraction, the core-TFIIH subunit ERCC3 was left behind (Fig. 4B). This, together with the previous results, indicated that CAK can be dissociated from core-TFIIH and quantitatively removed from HeLa nuclear extract by a combination of anti-Cdk7 immunodepletion and high-salt-concentration treatment. Importantly, the CAK-free core-TFIIH appeared to be fully active in mediating both basal and Tat-activated HIV-1 transcription (Fig. 2B).
In contrast to depletion in the presence of high concentrations of salt, depletion of Cdk7-CAK in 0.1 M KCl probably also removed associated core-TFIIH, which resulted in a major loss of basal transcription and an elimination of Tat transactivation (Fig. 2C). The low-level, apparently TFIIH-independent transcription in the depleted reaction may derive from a previously observed minor form of transcription complex specified by the HIV-1 long terminal repeat that is not regulated by Tat and does not need a TATA box for the assembly (18). This form of complex may even lack a few other “basal” factors, as Parvin and Sharp (29) have noticed that basal transcription can proceed from some promoters and under certain conditions in the absence of several “basal” factors such as TFIIE, TFIIH, and TFIIA. Nevertheless, it is important to stress that although the data in Fig. 2C revealed the importance of core-TFIIH as a general transcription factor in basal and Tat-activated transcription, it provided no evidence in support of a Tat-specific role of core-TFIIH.
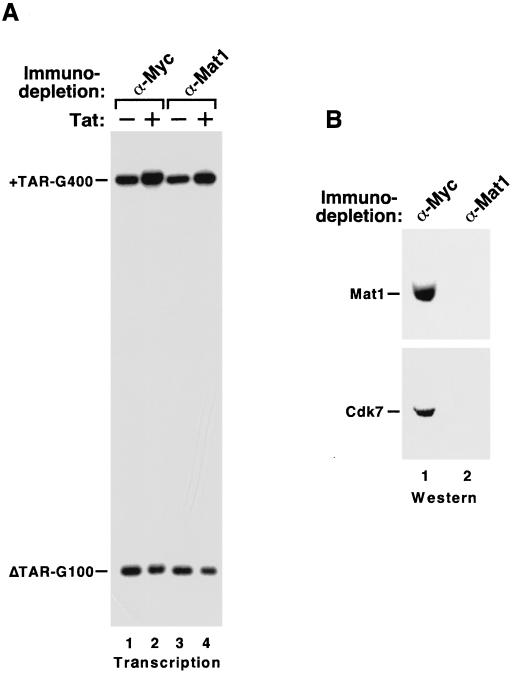
Removal of CAK from holo-TFIIH with anti-Mat1 antibodies does not affect Tat transactivation.
Mat1 has been shown to be an integral part of the CAK ternary complex and it can stabilize CAK and enhance Cdk7 kinase activity (1, 8). Recently, it was reported that anti-Mat1 antibodies inhibited Tat function in transcription reactions in vitro (9). To further investigate the role of Mat1 in Tat activation of HIV transcription, we subjected HeLa nuclear extract to anti-Mat1 immunodepletion in the presence of 0.8 M KCl and examined the ability of the Mat1-depleted extract to mediate Tat activation. Western blotting results shown in Fig. 5B indicate a quantitative removal of both Mat1 and Cdk7 from HeLa nuclear extract after depletion. Importantly, just like the Cdk7-depleted extract, Mat1-depleted nuclear extract was also capable of supporting both basal HIV-1 transcription and Tat transactivation (Fig. 5A; lanes 1 and 2 showed 3.6-fold and lanes 3 and 4 showed 4.1-fold Tat activation when normalized to internal controls). Therefore, using antibodies directed against two different subunits of CAK, our data strongly argue that CAK, either free or associated with core-TFIIH, was dispensable for basal HIV-1 transcription elongation as well as Tat transactivation in vitro.
FIG. 5.
Immunodepletion of CAK from holo-TFIIH with anti-Mat1 antibodies does not affect Tat activation. Immobilized anti-Mat1 antibodies were used to immunodeplete Mat1 from HeLa nuclear extract containing 0.8 M KCl. Immobilized anti-Myc MAb was used in a control depletion reaction. (A) The depleted extracts were analyzed for their abilities to mediate Tat activation in transcription reactions as described in the legend for Fig. 1. (B) The removal of Mat1 and its associated Cdk7 in the CAK complex was confirmed by Western blotting with anti-Mat1 and anti-Cdk7 antibodies.
DISCUSSION
Contrary to several recent reports suggesting an important role for CAK in Tat activation of HIV-1 transcription, we show here that while core-TFIIH subunits are essential for transcription, neither free CAK ternary complex nor CAK associated with core-TFIIH appeared to be required for basal and Tat-activated HIV-1 transcription. In contrast, the human P-TEFb kinase complex was shown to be essential for both processes. Complementing the functional studies of CAK, our data indicated that under the conditions in which a specific and efficient interaction is observed between Tat and P-TEFb, only weak interactions between Tat and CAK and between Tat and core-TFIIH that are independent of several critical amino acid residues in the Tat transactivation domain can be detected.
A requirement for CAK in Tat transactivation was demonstrated mainly by inhibiting CAK activity using kinase inhibitors such as DRB and H-8 (9, 28), a Cdk7 pseudosubstrate peptide derived from a mutant Cdk2 (6), or anti-Mat1 antibodies (9). However, both kinase inhibitors and the Cdk7 pseudosubstrate peptide could potentially block Tat transactivation through inhibiting the activity of other related kinases important for Tat function. For example, the kinase activity of Cdk9, which interacts with cyclin T1 to form the P-TEFb complex, has been shown to be essential for Tat activation of HIV-1 transcriptional elongation (42). In kinase reactions, phosphorylation of pol II CTD by P-TEFb was found to be more sensitive to DRB inhibition than phosphorylation by Cdk7-CAK of holo-TFIIH (21). In fact, the inhibition profile of a group of kinase inhibitors on the activity of Cdk9-P-TEFb, but not on Cdk7-TFIIH, correlated very well with their abilities to block Tat function (21). Given the fact that both Cdk7 and Cdk9 belong to the same Cdk kinase superfamily, it is possible that the kinase activity of Cdk9 was also inhibited when excess amounts of a pseudosubstrate inhibitor of Cdk7 (a Cdk2 substrate peptide containing a point mutation at Thr-160) was used to block Tat activation (6). In addition to the kinase inhibitors and pseudosubstrate peptide, HIV-1 transcription and Tat transactivation were also found to be inhibited by Mat1 antibodies (9). Similarly, antibodies against the other subunits of CAK (Cdk7 and cyclin H) were shown to inhibit basal transcription from certain promoters in vivo and in vitro (2, 34, 36). This inhibition is difficult to interpret, as it could be due to either sequestration of CAK kinase activity, disruption of the holo-TFIIH complex, or sterically blocking the critical enzymatic activities of the core-TFIIH subunits by the antibodies.
The role of CAK in TFIIH-mediated transcription remains controversial. In yeast, Cdk7 kinase activity is encoded by an essential gene called KIN28. Recently, a set of genes have been shown to require neither KIN28 kinase nor normally critical components of the RNA polymerase II holoenzyme for their activated transcription (15, 25). In reconstituted transcription reactions in vitro, Cdk7 kinase activity is required for transcription from the murine dihydrofolate reductase promoter (2), but is completely dispensable for both basal and activated transcription from the adenovirus major late promoter (2, 19). Based on these studies, it has been proposed that transcription from various pol II promoters is accomplished by different mechanisms and the Cdk7-CAK kinase associated with core-TFIIH may contribute to transcription in a promoter- or transactivator-specific manner (15, 25). Unlike many DNA sequence-specific transcription factors which activate transcription primarily by increasing the rate of initiation, Tat interacts with the nascent TAR RNA stem-loop structure and enhances HIV-1 transcription by stimulating the efficiency of elongation by RNA polymerase II (12, 13). It has been shown that activation of transcription initiation by Gal4-VP16 from the adenovirus major late promoter can be mediated by a mutant TFIIH with a kinase-inactive Cdk7 subunit (19). As an important extension of this earlier observation, we show here that stimulation of polymerase elongation by Tat in vitro can also be mediated by core-TFIIH free of Cdk7-CAK. However, our data cannot rule out an indirect role of CAK in phosphorylating and activating certain critical transcription factors (e.g., P-TEFb), whose modification may be essential for Tat function in vivo. The requirement for CAK in transcription reactions in vitro is eliminated probably because these critical transcription factors are already in an activated state when isolated from the cell.
Hyperphosphorylation of polymerase CTD has been implicated in the generation of highly processive polymerase elongation complexes (7). Consistent with this notion, Tat activation of HIV-1 elongation has been shown to require CTD (5, 26, 28, 39), although there has yet to be a direct demonstration of a Tat-dependent increase of CTD phosphorylation during transcription. In in vitro kinase reactions, CTD has been shown to be an excellent substrate for many cellular kinases, including several Cdk kinases, such as Cdk7 of CAK-TFIIH, Cdk8 of the SRB-mediator complex, and Cdk9 of P-TEFb (17, 23, 35, 36). Whether these different kinases also phosphorylate CTD during transcription and how they may affect transcription at different stages of the transcription cycle remain largely unknown. Our results suggest that phosphorylation of CTD and perhaps other components of the transcriptional machinery by Cdk7-TFIIH is not essential for Tat activation of HIV-1 transcription in vitro. It remains to be tested whether other related kinases in Cdk7-depleted nuclear extract can functionally replace the Cdk7-TFIIH kinase activity and mediate Tat transactivation. Future studies may reveal other functional substrates of the Cdk7-TFIIH kinase and help us understand the mechanism by which this kinase affects transcription in a promoter- or transactivator-specific fashion.
ACKNOWLEDGMENTS
We thank M. Peterlin and T. Cujec for the Cdk7-HA constructs, J. Kim and P. A. Sharp for a highly purified TFIIH fraction, and A. Rice for Tat mutant constructs. We thank B. O’Keeffe, K. Luo, and R. Tjian for valuable comments on the manuscript.
This work was supported by grants from the National Institutes of Health (AI-41757) and the University of California Universitywide AIDS Research Program (R97-B-113) to Q.Z.
REFERENCES
- 1.Adamczewski J P, Rossignol M, Tassan J P, Nigg E A, Moncollin V, Egly J M. MAT1, cdk7 and cyclin H form a kinase complex which is UV light-sensitive upon association with TFIIH. EMBO J. 1996;15:1877–1884. [PMC free article] [PubMed] [Google Scholar]
- 2.Akoulitchev S, Makela T P, Weinberg R A, Reinberg D. Requirement for TFIIH kinase activity in transcription by RNA polymerase II. Nature. 1995;377:557–560. doi: 10.1038/377557a0. [DOI] [PubMed] [Google Scholar]
- 3.Blau J, Xiao H, McCracken S, O’Hare P, Greenblatt J, Bentley D. Three functional classes of transcriptional activation domain. Mol Cell Biol. 1996;16:2044–2055. doi: 10.1128/mcb.16.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, D., Y. Fong, and Q. Zhou. Specific interaction of Tat with the human but not rodent P-TEFb complex mediates the species-specific Tat activation of HIV-1 transcription. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 5.Chun R F, Jeang K T. Requirements for RNA polymerase II carboxyl-terminal domain for activated transcription of human retroviruses human T-cell lymphotropic virus I and HIV-1. J Biol Chem. 1996;271:27888–27894. doi: 10.1074/jbc.271.44.27888. [DOI] [PubMed] [Google Scholar]
- 6.Cujec T P, Okamoto H, Fujinaga K, Meyer J, Chamberlin H, Morgan D O, Peterlin B M. The HIV transactivator TAT binds to the CDK-activating kinase and activates the phosphorylation of the carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997;11:2645–2657. doi: 10.1101/gad.11.20.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahmus M E. Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J Biol Chem. 1996;271:19009–19012. doi: 10.1074/jbc.271.32.19009. [DOI] [PubMed] [Google Scholar]
- 8.Fisher R P, Jin P, Chamberlin H M, Morgan D O. Alternative mechanisms of CAK assembly require an assembly factor or an activating kinase. Cell. 1995;83:47–57. doi: 10.1016/0092-8674(95)90233-3. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Martinez L F, Mavankal G, Neveu J M, Lane W S, Ivanov D, Gaynor R B. Purification of a Tat-associated kinase reveals a TFIIH complex that modulates HIV-1 transcription. EMBO J. 1997;16:2836–2850. doi: 10.1093/emboj/16.10.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 11.Herrmann C H, Rice A P. Lentivirus Tat proteins specifically associate with a cellular protein kinase, TAK, that hyperphosphorylates the carboxyl-terminal domain of the large subunit of RNA polymerase II: candidate for a Tat cofactor. J Virol. 1995;69:1612–1620. doi: 10.1128/jvi.69.3.1612-1620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones K A. Taking a new TAK on Tat transactivation. Genes Dev. 1997;11:2593–2599. doi: 10.1101/gad.11.20.2593. [DOI] [PubMed] [Google Scholar]
- 13.Jones K A, Peterlin B M. Control of RNA initiation and elongation at the HIV-1 promoter. Annu Rev Biochem. 1994;63:717–743. doi: 10.1146/annurev.bi.63.070194.003441. [DOI] [PubMed] [Google Scholar]
- 14.Kephart D D, Marshall N F, Price D H. Stability of Drosophila RNA polymerase II elongation complexes in vitro. Mol Cell Biol. 1992;12:2067–2077. doi: 10.1128/mcb.12.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee D, Lis J T. Transcriptional activation independent of TFIIH kinase and the RNA polymerase II mediator in vivo. Nature. 1998;393:389–392. doi: 10.1038/30770. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Green M R. The HIV-1 Tat cellular coactivator Tat-SF1 is a general transcription elongation factor. Genes Dev. 1998;12:2992–2996. doi: 10.1101/gad.12.19.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao S M, Zhang J, Jeffery D A, Koleske A J, Thompson C M, Chao D M, Viljoen M, van Vuuren H J, Young R A. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature. 1995;374:193–196. doi: 10.1038/374193a0. [DOI] [PubMed] [Google Scholar]
- 18.Lu X, Welsh T M, Peterlin B M. The human immunodeficiency virus type 1 long terminal repeat specifies two different transcription complexes, only one of which is regulated by Tat. J Virol. 1993;67:1752–1760. doi: 10.1128/jvi.67.4.1752-1760.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makela T P, Parvin J D, Kim J, Huber L J, Sharp P A, Weinberg R A. A kinase-deficient transcription factor TFIIH is functional in basal and activated transcription. Proc Natl Acad Sci USA. 1995;92:5174–5178. doi: 10.1073/pnas.92.11.5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maldonado E, Reinberg D. News on initiation and elongation of transcription by RNA polymerase II. Curr Opin Cell Biol. 1995;7:352–361. doi: 10.1016/0955-0674(95)80090-5. [DOI] [PubMed] [Google Scholar]
- 21.Mancebo H S, Lee G, Flygare J, Tomassini J, Luu P, Zhu Y, Peng J, Blau C, Hazuda D, Price D, Flores O. P-TEFb kinase is required for HIV Tat transcriptional activation in vivo and in vitro. Genes Dev. 1997;11:2633–2644. doi: 10.1101/gad.11.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marciniak R A, Sharp P A. HIV-1 Tat protein promotes formation of more-processive elongation complexes. EMBO J. 1991;10:4189–4196. doi: 10.1002/j.1460-2075.1991.tb04997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall N F, Peng J, Xie Z, Price D H. Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J Biol Chem. 1996;271:27176–27183. doi: 10.1074/jbc.271.43.27176. [DOI] [PubMed] [Google Scholar]
- 24.Marshall N F, Price D H. Purification of P-TEFb, a transcription factor required for the transition into productive elongation. J Biol Chem. 1995;270:12335–12338. doi: 10.1074/jbc.270.21.12335. [DOI] [PubMed] [Google Scholar]
- 25.McNeil J, Agah H, Bentley D. Activated transcription independent of the RNA polymerase II holoenzyme in budding yeast. Genes Dev. 1998;12:2510–2521. doi: 10.1101/gad.12.16.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto H, Sheline C T, Corden J L, Jones K A, Peterlin B M. Trans-activation by human immunodeficiency virus Tat protein requires the C-terminal domain of RNA polymerase II. Proc Natl Acad Sci USA. 1996;93:11575–11579. doi: 10.1073/pnas.93.21.11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Keeffe, B., Y. Fong, D. Chen, and Q. Zhou. Requirement for a kinase-specific chaperone pathway in the production of a Cdk9/cyclin T1 heterodimer responsible for P-TEFb-mediated Tat stimulation of HIV-1 transcription. Submitted for publication. [DOI] [PubMed]
- 28.Parada C A, Roeder R G. Enhanced processivity of RNA polymerase II triggered by Tat-induced phosphorylation of its carboxy-terminal domain. Nature. 1996;384:375–378. doi: 10.1038/384375a0. [DOI] [PubMed] [Google Scholar]
- 29.Parvin J D, Sharp P A. DNA topology and a minimal set of basal factors for transcription by RNA polymerase II. Cell. 1993;73:533–540. doi: 10.1016/0092-8674(93)90140-l. [DOI] [PubMed] [Google Scholar]
- 30.Parvin J D, Shykind B M, Meyers R E, Kim J, Sharp P A. Multiple sets of basal factors initiate transcription by RNA polymerase II. J Biol Chem. 1994;269:18414–18421. [PubMed] [Google Scholar]
- 31.Parvin J D, Timmers H T, Sharp P A. Promoter specificity of basal transcription factors. Cell. 1992;68:1135–1144. doi: 10.1016/0092-8674(92)90084-p. [DOI] [PubMed] [Google Scholar]
- 32.Peng J, Zhu Y, Milton J T, Price D H. Identification of multiple cyclin subunits of human P-TEFb. Genes Dev. 1998;12:755–762. doi: 10.1101/gad.12.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice A P, Carlotti F. Structural analysis of wild-type and mutant human immunodeficiency virus type 1 Tat proteins. J Virol. 1990;64:6018–6026. doi: 10.1128/jvi.64.12.6018-6026.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy R, Adamczewski J P, Seroz T, Vermeulen W, Tassan J P, Schaeffer L, Nigg E A, Hoeijmakers J H, Egly J M. The MO15 cell cycle kinase is associated with the TFIIH transcription-DNA repair factor. Cell. 1994;79:1093–1101. doi: 10.1016/0092-8674(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 35.Serizawa H, Makela T P, Conaway J W, Conaway R C, Weinberg R A, Young R A. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature. 1995;374:280–282. doi: 10.1038/374280a0. [DOI] [PubMed] [Google Scholar]
- 36.Shiekhattar R, Mermelstein F, Fisher R P, Drapkin R, Dynlacht B, Wessling H C, Morgan D O, Reinberg D. Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature. 1995;374:283–287. doi: 10.1038/374283a0. [DOI] [PubMed] [Google Scholar]
- 37.Svejstrup J Q, Vichi P, Egly J M. The multiple roles of transcription/repair factor TFIIH. Trends Biochem Sci. 1996;21:346–350. [PubMed] [Google Scholar]
- 38.Wei P, Garber M E, Fang S M, Fischer W H, Jones K A. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 39.Yang X, Herrmann C H, Rice A P. The human immunodeficiency virus Tat proteins specifically associate with TAK in vivo and require the carboxyl-terminal domain of RNA polymerase II for function. J Virol. 1996;70:4576–4584. doi: 10.1128/jvi.70.7.4576-4584.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yankulov K, Bentley D. Transcriptional control: Tat cofactors and transcriptional elongation. Curr Biol. 1998;8:447–449. doi: 10.1016/s0960-9822(98)70289-1. [DOI] [PubMed] [Google Scholar]
- 41.Zawel L, Kumar K P, Reinberg D. Recycling of the general transcription factors during RNA polymerase II transcription. Genes Dev. 1995;9:1479–1490. doi: 10.1101/gad.9.12.1479. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Q, Chen D, Pierstorff E, Luo K. Transcription elongation factor P-TEFb mediates Tat activation of HIV-1 transcription at multiple stages. EMBO J. 1998;17:3681–3691. doi: 10.1093/emboj/17.13.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Q, Lieberman P M, Boyer T G, Berk A J. Holo-TFIID supports transcriptional stimulation by diverse activators and from a TATA-less promoter. Genes Dev. 1992;6:1964–1974. doi: 10.1101/gad.6.10.1964. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Q, Sharp P A. Novel mechanism and factor for regulation by HIV-1 Tat. EMBO J. 1995;14:321–328. doi: 10.1002/j.1460-2075.1995.tb07006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Y, Pe’ery T, Peng J, Ramanathan Y, Marshall N, Marshall T, Amendt B, Mathews M B, Price D H. Transcription elongation factor P-TEFb is required for HIV-1 Tat transactivation in vitro. Genes Dev. 1997;11:2622–2632. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]