Abstract
Background
Clinical significance of red blood cell distribution (RDW) as a predictive marker for the incidence of postoperative morbidity after esophagectomy for esophageal cancer has not been established.
Methods
This study included 634 consecutive patients who underwent three-incisional esophagectomy with lymphadenectomy for esophageal cancer between April 2005 and November 2020. Correlation between pretreatment RDW and patient background, cancer background, and short-term outcome after esophagectomy were retrospectively investigated.
Results
Eighty patients (12.6%) had a high pretreatment RDW (> 14.2), which correlated with malnutrition estimated by body mass index, hemoglobin, total lymphocyte count, albumin, and total cholesterol. High pretreatment RDW was an independent risk factor for postoperative severe morbidity of grade IIIb or higher based on the Clavien–Dindo classification (hazard ratio [HR] 3.90, 95% confidence interval [CI] 1.707–8.887; p = 0.0012) and reoperation (HR 4.39, 95% CI 1.552–12.390; p = 0.0053) after open esophagectomy (OE). However, RDW was not associated with postoperative morbidity incidence after minimally invasive esophagectomy (MIE).
Conclusions
Pretreatment RDW may be a surrogate marker for nutritional status and could be a predictive marker for postoperative severe morbidity, reoperation, and possibly pneumonia after OE. On the contrary, the lower invasiveness of MIE may have reduced the effect of pretreatment malnutrition on morbidity incidence, which could explain the insignificant relationship between RDW and poor short-term outcomes in MIE.
Supplementary Information
The online version contains supplementary material available at 10.1245/s10434-021-10719-2.
Esophagectomy for esophageal cancer is among the most invasive surgeries associated with frequent postoperative morbidity compared with other gastrointestinal cancer surgeries. The preoperative prediction of the incidence of postoperative morbidity is considered important because of its prophylactic value and the possible reduction of surgery-related mortality. Several predictive markers for postoperative morbidity have been previously reported.1–3 Notably, measurable markers based on common blood tests are useful because these could be performed at any institute and be objectively evaluated. To date, the mean corpuscular volume (MCV) and several nutritional markers are suggested as useful predictors of postoperative morbidity after esophagectomy.4–6
Red blood cell distribution width (RDW) is a parameter in red blood cell size variability and is used for estimating the pathogenesis of anemia.7 High RDW may also be associated with several cardiovascular diseases8–10 and poor prognosis of inflammatory diseases.11–15 Recently, the correlation between elevated RDW and high mortality in patients with coronavirus disease 2019 (COVID-19) has attracted increasing attention.16 However, the mechanism underlying the association of RDW with the incidence and prognoses of these diseases remains unclear.
RDW has been considered an indicator of inflammation, malnutrition, microvascular disorder, oxidative stress, and prothrombotic effect.17 Although the progression of these conditions could be a risk for postoperative morbidities after highly invasive surgery, there have been no studies regarding the effect of RDW on short-term outcomes after gastroenterological surgeries. Thus, this study aimed to clarify the correlation between pretreatment RDW and the short-term outcomes after esophagectomy for esophageal cancer. Moreover, the correlation between pretreatment RDW and patient and cancer backgrounds was also investigated to elucidate how high RDW reflects the frequent incidence of postoperative morbidities.
Material and Methods
Patients
A total of 803 consecutive three-incisional esophagectomies with lymphadenectomy were performed for esophageal cancer at the Kumamoto University Hospital between April 2005 and November 2020. Of these, 24 cases of two-stage esophagectomies, 43 cases of salvage esophagectomies after definitive chemoradiotherapy (CRT), and 102 cases with insufficient clinical data (70 data points for alcohol status, 1 for smoking status, 12 for pretreatment data of total cholesterol, 9 for RDW, 6 for C-reactive protein [CRP], and 4 for total lymphocyte count) were excluded. Eventually, 634 patients were enrolled in this study (Fig. 1). RDW examined in this study was measured before the administration of any treatments. Patients were divided into two groups according to the institutional standard value of pretreatment RDW—high (14.2 <) and normal (≤ 14.2). A retrospective investigation for the association between RDW and clinicopathological features, blood tests, and short-term outcomes after surgery was performed using a prospectively entered institutional clinical database. This study was conducted in accordance with the ethical standards of the Helsinki Declaration of 1975. The institutional Ethics Committee approved the study procedures (registry number 2193). Written informed consent was waived for the patients because of the retrospective nature of this study.
Fig. 1.
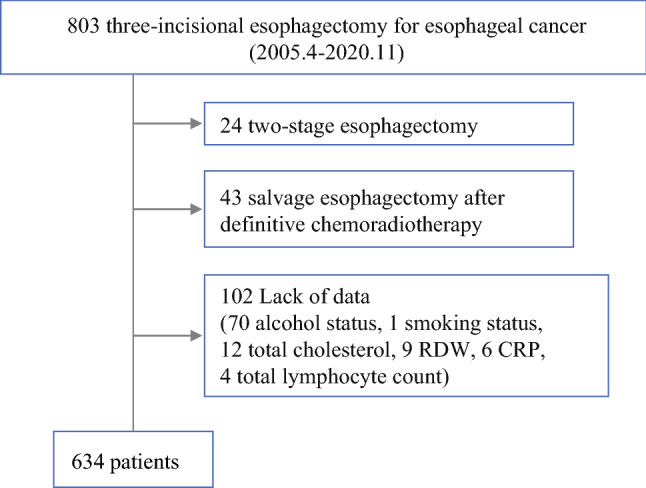
Flow chart of analyzed patients. RDW red blood cell distribution width, CRP C-reactive protein
Treatment Strategy
Treatment strategy details have been previously described.4 For non-T4 node-positive tumors, neoadjuvant chemotherapy has been administered since August 2008, whereas for T4 tumors, neoadjuvant CRT has been generally performed. The Union for International Cancer Control TNM staging (version 7) was used to classify the pretreatment clinical stage.18
Surgery
Esophagectomy was defined as three-incisional (in the neck, chest, and abdomen) esophagectomy with lymphadenectomy. The extent of lymphadenectomy was determined in accordance with the 2012 guidelines formulated by the Japan Esophageal Society.19 Regarding manipulation in the thorax, open esophagectomy (OE) was performed before April 2011. Minimally invasive esophagectomy (MIE) for clinical stage T1 and T2 cancers was initiated in May 2011. With progress in the operator’s and team’s skills, we have routinely performed MIE for cancers of all stages since September 2011. During MIE, chest manipulation was performed from the right thorax in the left semi-prone position.
Perioperative Management
Details regarding perioperative management have been previously described.20 Bolus administration of methylprednisolone and continuous intravenous administration of neutrophil elastase inhibitors for 24 h were routinely conducted at the start of surgery. Moreover, precautionary antibiotics were administered every 4 h during surgery. Extubation was performed in the operating room shortly after surgery, and enteral nutrition was generally started on the first day after surgery.
Definitions of Morbidities
The morbidity details have been previously described.5 Severe morbidity was defined as a complication of grades IIIb or higher according to the Clavien–Dindo classification (CDc) system.21 Initial ventilatory support for > 48 h or reintubation for respiratory failure, need for tracheostomy, and pneumonia were defined as respiratory morbidities. Moreover, any respiratory morbidity requiring intervention or surgical treatment was included under this definition.
Statistical Methods
Statistical analyses were performed using the StatView™ version 5.0 software package (Abacus Concepts, Inc., Berkeley, CA, USA). The Chi-square test and Mann–Whitney U test were used to compare groups and unpaired samples, respectively. Fisher's exact test was used for the comparisons of groups containing a matrix with fewer than five patients. Data on pretreatment blood tests were divided into two groups according to the institutional standard value. Logistic regression analysis was performed to estimate the hazard ratio (HR) with a 95% confidence interval (CI) for each morbidity. The following data were adopted to analyze independent risk factors for the incidence of severe morbidity, reoperation, and pneumonia among patients who underwent OE: age (per 10 years), sex (male vs. female), body mass index (BMI; < 18.5 vs. ≥ 18.5 kg/m2), performance status (PS; 0 vs. 1 and 2), American Society of Anesthesiologists physical status (ASAPS; 1 and 2 vs. 3), Brinkman index (tobacco number/day × year, for a 100-point increase), chronic obstructive pulmonary disease (COPD; yes vs. no), neoadjuvant treatment (chemotherapy and CRT vs. none), dissection field (≤ 2 vs. 3), conduit (stomach vs. others), operative time (per 60 min increase), blood loss (per 100 g increase), clinical stage (II, III, and IV vs. I), and RDW (> 14.2 vs. ≤ 14.2). Factors with a probability level of ≤ 0.1 were adopted for subsequent multivariate analyses. Variables with a p value < 0.05 were presumed to be independent risk factors.
Results
Association Between Pretreatment Red Blood Cell Distribution Width and Clinicopathological Features
Among all patients, 80 (12.6%) had a high pretreatment RDW (> 14.2). High RDW significantly correlated with lower BMI (p = 0.045); however, high RDW was not related to any other clinicopathological factors, such as age, sex, past smoking and drinking, PS, ASAPS, comorbidity, clinical stage, and histological type of cancer (Table 1). The blood test revealed that high RDW was significantly associated with lower hemoglobin (p < 0.0001) and total lymphocyte count (p = 0.012). Moreover, high RDW exhibited a trend toward lower serum albumin and total cholesterol. Nevertheless, high RDW was also irrelevant to inflammation, as suggested by white blood cell count and CRP (Table 2).
Table 1.
Association between pretreatment red blood cell distribution width and patient characteristics
| Clinical, epidemiological, and pathological feature | Total N | Pretreatment red blood cell distribution | p value | |
|---|---|---|---|---|
| ≤ 14.2 | 14.2 < | |||
| All cases | 634 | 554 | 80 | |
| Age, years [mean ± SD] | 66.5 ± 8.3 | 66.7 ± 8.2 | 65.2 ± 8.7 | 0.14 |
| Male patients | 556 (88) | 483 (87) | 73 (91) | 0.30 |
| Body mass index [mean ± SD] | 21.9 ± 3.1 | 22.0 ± 3.1 | 21.3 ± 3.1 | 0.045 |
| Estimated ethanol consumptiona [mean ± SD] | 2450 ± 2040 | 2450 ± 2060 | 2450 ± 1900 | 0.98 |
| Brinkman indexb [mean ± SD] | 770 ± 570 | 770 ± 580 | 800 ± 460 | 0.64 |
| Performance status | 0.82 | |||
| 0 | 565 (89) | 495 (89) | 70 (88) | |
| 1 | 64 (10) | 55 (10) | 9 (11) | |
| 2 | 5 (1) | 4 (1) | 1 (1) | |
| ASA physical status | 0.24 | |||
| 1 | 125 (20) | 111 (20) | 14 (18) | |
| 2 | 483 (76) | 423 (76) | 60 (75) | |
| 3 | 26 (4) | 20 (4) | 6 (8) | |
| Comorbidity | ||||
| Diabetes mellitus | 133 (21) | 117 (21) | 16 (20) | 0.82 |
| Chronic obstructive pulmonary disease | 196 (31) | 167 (30) | 29 (36) | 0.27 |
| Cardiovascular | 334 (53) | 295 (53) | 39 (49) | 0.45 |
| Clinical stage | 0.25 | |||
| 0, I | 276 (44) | 248 (45) | 28 (35) | |
| II | 113 (18) | 94 (17) | 19 (24) | |
| III | 204 (32) | 178 (32) | 26 (33) | |
| IV | 41 (6) | 34 (6) | 7 (9) | |
| Pathology | 0.70 | |||
| Squamous cell carcinoma | 577 (91) | 505 (91) | 72 (90) | |
| Adenocarcinoma | 36 (6) | 30 (5) | 6 (8) | |
| Others | 21 (3) | 19 (3) | 2 (3) | |
| Neoadjuvant treatment | 0.83 | |||
| None | 359 (57) | 315 (57) | 44 (55) | |
| Chemotherapy | 208 (33) | 182 (33) | 26 (33) | |
| Chemoradiotherapy | 67 (11) | 57 (10) | 10 (13) | |
| Surgical procedure, MIE | 355 (56) | 318 (57) | 37 (46) | 0.060 |
Data are expressed as n(%) unless otherwise specified
MIE minimally invasive esophagectomy, SD standard deviation, ASA American Society of Anesthesiologists
aEthanol consumption was calculated as follows: estimated daily ethanol consumption [(0.4 × whisky + 0.2 × distilled spirit + 0.15 × wine and sake + 0.04 × beer) × year]
bBrinkman index was calculated as follows: number of cigarettes/day × smoking duration (year)
Table 2.
Association between pretreatment red blood cell distribution width and blood parameter test results
| Blood test | Total N | Pretreatment red blood cell distribution | p value | |
|---|---|---|---|---|
| ≤ 14.2 | 14.2 < | |||
| All cases | 634 | 554 | 80 | |
| Hemoglobin, g/dL [mean ± SD] | 13.6 ± 1.5 | 13.8 ± 1.4 | 12.7 ± 1.7 | < 0.0001 |
| Hemoglobin, <11.6 g/dL | 50 (8%) | 33 (6%) | 17 (21%) | < 0.0001 |
| White blood cell count, /μL [mean ± SD] | 6490 ± 2740 | 6540 ± 2830 | 6110 ± 1940 | 0.19 |
| White blood cell count, < 3300 /μL | 11 (2%) | 10 (2%) | 1 (1%) | > 0.99 |
| Total lymphocyte count, /μL [mean ± SD] | 1740 ± 630 | 1770 ± 640 | 1580 ± 550 | 0.012 |
| Total lymphocyte count, <1600 /μL | 282 (44%) | 229 (43%) | 43 (54%) | 0.074 |
| C-reactive protein, mg/dL [mean ± SD] | 0.45 ± 0.97 | 0.47 ± 1.02 | 0.32 ± 0.44 | 0.21 |
| C-reactive protein, ≥ 1.00 mg/dL | 72 (11%) | 67 (12%) | 5 (6%) | 0.12 |
| Serum albumin, g/dL [mean ± SD] | 4.04 ± 0.39 | 4.05 ± 0.39 | 3.97 ± 0.39 | 0.074 |
| Serum albumin, < 3.5 g/dL | 45 (7%) | 38 (7%) | 7 (9%) | 0.54 |
| Total cholesterol, mg/dL [mean ± SD] | 197 ± 35 | 197 ± 35 | 193 ± 39 | 0.33 |
| Total cholesterol, < 180 g/dL | 212 (33%) | 178 (32%) | 34 (43%) | 0.071 |
SD standard deviation
Short-Term Outcomes After Surgery
Because the distribution of OE and MIE was considerably different between the high and normal RDW groups, the short-term outcomes for OE and MIE were separately investigated. Table 3 shows the short-term outcomes in patients who underwent OE, according to pretreatment RDW. Operative time and blood loss were significantly higher in the high RDW group, possibly due to frequent use of the colon conduit (high RDW, 19%; normal RDW, 6%; p = 0.0046). Moreover, postoperative severe morbidity, pneumonia, and reoperation were significantly frequent in the high RDW group. The association of high RDW with frequent severe morbidity, reoperation, and pneumonia was also seen in patients who underwent reconstruction using a gastric conduit (electronic supplementary Table 1). By contrast, short-term outcomes in patients who underwent MIE were statistically equivalent between the high and normal RDW groups (Table 4).
Table 3.
Pretreatment red blood cell distribution width, surgical feature, and short-term surgical outcomes of patients who underwent open esophagectomy
| Surgical data and morbidities | Total N | Pretreatment red blood cell distribution | p value | |
|---|---|---|---|---|
| ≤ 14.2 | 14.2 < | |||
| All cases | 279 | 236 | 43 | |
| Operation time, min [mean ± SD] | 540 ± 110 | 540 ± 110 | 570 ± 100 | 0.034 |
| Blood loss, g [mean ± SD] | 580 ± 440 | 560 ± 380 | 740 ± 670 | 0.012 |
| Postoperative morbidity | ||||
| Any morbidity, CDc grade II or higher | 111 (40) | 89 (38) | 22 (51) | 0.097 |
| Severe morbidity, CDc grade IIIb or higher | 33 (12) | 20 (8) | 13 (30) | <0.0001 |
| Respiratory morbidity | 48 (17) | 37 (16) | 11 (26) | 0.11 |
| Pneumonia | 21 (8) | 14 (6) | 7 (16) | 0.018 |
| Cardiovascular morbidity | 14 (5) | 13 (6) | 1 (2) | 0.70 |
| Leak | 32 (11) | 25 (11) | 7 (16) | 0.28 |
| Reoperation | 19 (7) | 10 (4) | 9 (21) | <0.0001 |
Data are expressed as n (%) unless otherwise specified
SD standard deviation, CDc Clavien–Dindo classification
Table 4.
Pretreatment red blood cell distribution width, surgical features, and short-term surgical outcomes of patients who underwent minimally invasive esophagectomy
| Surgical data and morbidities | Total N | Pretreatment red blood cell distribution | p value | |
|---|---|---|---|---|
| ≤ 14.2 | 14.2 < | |||
| All cases | 355 | 318 | 37 | |
| Operation time, min [mean ± SD] | 580 ± 100 | 580 ± 100 | 570 ± 100 | 0.54 |
| Blood loss, g [mean ± SD] | 230 ± 290 | 240 ± 300 | 190 ± 230 | 0.36 |
| Postoperative morbidity | ||||
| Any morbidity, CDc grade II or higher | 121 (34) | 108 (34) | 13 (35) | 0.89 |
| Severe morbidity, CDc grade IIIb or higher | 46 (13) | 42 (13) | 4 (11) | 0.80 |
| Pulmonary morbidity | 49 (14) | 44 (14) | 5 (14) | 0.96 |
| Pneumonia | 31 (9) | 29 (9) | 2 (5) | 0.76 |
| Cardiovascular morbidity | 26 (7) | 26 (8) | 0 | 0.092 |
| Leak | 46 (13) | 40 (13) | 6 (16) | 0.53 |
| Reoperation | 24 (7) | 22 (7) | 2 (5) | >0.99 |
Data are expressed as n (%) unless otherwise specified
SD standard deviation, CDc Clavien–Dindo classification
Risk Factors for Postoperative Morbidities of Grades IIIb or Higher According to the Clavien–Dindo Classification, Reoperation, and Pneumonia in Patients who Underwent Open Esophagectomy
Table 5 shows the results of multivariate analyses for postoperative morbidities in patients who underwent OE. High pretreatment RDW was an independent risk factor for postoperative severe morbidity of CDc grade IIIb or higher (HR 3.90, 95% CI 1.707–8.887; p = 0.0012) and reoperation (HR 4.39, 95% CI 1.552–12.390; p = 0.0053). It also exhibited a trend toward frequent incidence of pneumonia, although it was not statistically significant (HR 2.70, 95% CI 0.995–7.351; p = 0.051).
Table 5.
Logistic regression analysis (backward stepwise elimination) for morbidities of grades IIIb or higher according to the Clavien–Dindo classification, reoperation, and pneumonia in patients who underwent open esophagectomy
| Morbidity | Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| CDc grade IIIb or higher | Dissection field ≤ 2 (vs. 3) | 0.42 (0.157–1.139) | 0.089 | 0.45 (0.159–1.262) | 0.13 |
| Conduit stomach (vs. others) | 0.24 (0.090–0.646) | 0.0046 | 0.33 (0.103–1.061) | 0.063 | |
| Operation time (for 60 min increase) | 1.17 (0.979–1.401) | 0.085 | 1.03 (0.823–1.277) | 0.82 | |
| Red blood cell distribution 14.2< (vs. ≤14.2) | 4.68 (2.111–10.373) | 0.0001 | 3.90 (1.707–8.887) | 0.0012 | |
| Reoperation | Conduit stomach (vs. others) | 0.11 (0.036–0.305) | <0.0001 | 0.17 (0.047–0.590) | 0.0055 |
| Operation time (for 60 min increase) | 1.28 (1.031–1.589) | 0.026 | 1.06 (0.803–1.387) | 0.70 | |
| Blood loss (for 100 g increase) | 1.08 (0.997–1.179) | 0.059 | 1.03 (0.934–1.138) | 0.54 | |
| Red blood cell distribution 14.2 < (vs. ≤ 14.2) | 5.98 (2.268–15.78) | 0.0003 | 4.39 (1.552–12.390) | 0.0053 | |
| Pneumonia | Operation time (for 60 min increase) | 1.17 (0.979–1.401) | 0.085 | 1.03 (0.823–1.277) | 0.82 |
| Red blood cell distribution 14.2 < (vs. ≤ 14.2) | 3.08 (1.165–8.161) | 0.023 | 2.70 (0.995–7.351) | 0.051 | |
HR hazard ratio, CI confidence interval, CDc Clavien–Dindo classification
Discussion
In this study, several interesting results were obtained for the clinical value of pretreatment RDW in esophagectomy for esophageal cancer. First, high RDW may be a surrogate marker for malnutrition estimated by BMI, hemoglobin, total lymphocyte count, albumin, and total cholesterol. Second, high RDW was not relevant to factors other than nutrition, such as age, sex, past smoking and drinking, comorbidities, cancer stage, and histological type. Third, high RDW was an independent risk factor for severe morbidity, reoperation, and possibly pneumonia after OE, but not after MIE.
RDW is an indicator of variation in red blood cell size. In patients with anemia, RDW increases because of the mixture of different erythrocyte sizes via erythrocyte turnover. In patients with chronic anemia, the size of erythrocytes is uniform, irrespective of macrocytic or microcytic anemia; hence, RDW becomes normalized. RDW also reflects bone marrow function and is used in estimating the pathogenesis of anemia.7 RDW could also reflect inflammation and may be a predictive marker for activity level and survival in several inflammatory diseases, such as inflammatory bowel disease,11 chronic hepatitis,12 COPD,13 acute pancreatitis,14 acute respiratory distress syndrome,15 and several cardiovascular diseases.8–10 Recently, several studies on the significance of RDW as a predictive marker for COVID-19 mortality have been attracting increasing attention.16 Moreover, RDW could be a prognostic marker for several malignancies, including esophageal cancer.22,23 However, the mechanism behind the significant association between high RDW and the prognosis of the aforementioned diseases has not been established.
To date, no previous studies regarding the association of pretreatment RDW and short-term outcomes after gastroenterological surgery are available. To the best of our knowledge, this is the first study to disclose that high RDW may correlate with the incidence of postoperative morbidity after esophagectomy. In this study, RDW seemed a mere surrogate marker of nutritional status. Although a huge cohort study suggested that smoking habit may affect RDW level,24 no such associations were observed in this study. Other factors that could affect the incidence of postoperative morbidities were equivalent between the high and normal RDW groups. The same result was obtained in several studies reporting that pretreatment malnutrition increased postoperative morbidities in highly invasive gastroenterological procedures, such as esophagectomy,25–27 pancreatoduodenectomy,28 and rectal surgery.29
The Controlling Nutritional Status (CONUT) score is one indicator for the nutritional status estimation and could be a predictive marker for postoperative morbidity after several gastroenterological surgeries.5,30,31 In this study, the CONUT score in the high RDW group was significantly higher than that in the normal RDW group (high RDW, 1.6 ± 1.6; normal RDW, 1.2 ± 1.3; p = 0.0096). Moreover, the percentage of patients with malnutrition estimated by the CONUT score was significantly higher in the high RDW group (high RDW, 41%; normal RDW, 32%; p = 0.025) [data not shown]. The Prognostic Nutritional Index (PNI) is the first identified nutrition-related indicator and could predict complications after esophagectomy.6 In this study, the PNI was also significantly worse in the high RDW group than in the normal RDW group (high RDW, 47.5 ± 5.1; normal RDW, 49.3 ± 5.3; p = 0.0050) [data not shown]. These results support that RDW reflected nutritional status and affected short-term outcomes after esophagectomy.
RDW reportedly increases in several diseases, such as heart failure, atrial fibrillation, peripheral vascular disease, coronary artery disease, stroke, and pulmonary hypertension.8–10 Li et al. have reviewed the effects of high RDW on cardiovascular disease.17 This study discussed how high RDW reflected microvascular disorder, high inflammatory cytokine, oxidative stress, and prothrombotic effects. Thus, patients with high RDW may have several latent disadvantages other than malnutrition, which could have influenced the frequent incidence of postoperative morbidities.
OE is considered more invasive and associated with more frequent postoperative morbidities than MIE.29,32,33 No studies have clarified the correlation between preoperative malnutrition and poor short-term outcomes in MIE to date. In general, poor preoperative conditions could severely affect the incidence of postoperative morbidity as the surgery becomes more invasive. The lower invasiveness of MIE may have reduced the effect of pretreatment malnutrition on the incidence of morbidities, which could explain the irrelevance between high RDW and poor short-term outcomes in MIE.
We previously reported that high MCV was associated with low BMI and higher frequency of habitual alcohol and tobacco use, which significantly increased pulmonary morbidities after esophagectomy.4 We investigated the association of MCV with patient’s backgrounds and short-term outcomes using the current cohort. Consequently, high MCV was correlated with lower BMI (p = 0.040) and more frequent habitual alcohol (p < 0.0001) and tobacco use (p = 0.0002); however, MCV was not related to total lymphocyte count (p = 0.74), serum albumin (p = 0.13) and cholesterol (p = 0.13) levels, and malnutrition in CONUT (p = 0.47). Although RDW could reflect malnutrition based on the blood test, CONUT, and PNI, it was not related to habitual alcohol and tobacco use. Thus, we consider that both MCV and RDW reflect different backgrounds. Finally, we conducted multivariate analysis to calculate the incidence of postoperative pneumonia, including both MCV and RDW, as an element. Only RDW was identified as an independent risk factor for pneumonia after OE (HR 2.94, 95% CI 1.079–7.988; p = 0.035). Based on the result, RDW may be superior to MCV in the prediction of postoperative pneumonia in patients with OE.
Nevertheless, this study has several limitations. First, this was a retrospective study at a single institute and with a comparatively long study period; hence, there could have been historical bias regarding treatment strategy and perioperative management. Second, many patients had to be excluded due to insufficient data, which could be a selection bias.
Conclusion
Pretreatment RDW may be a surrogate marker for nutritional status and could be a predictive marker for postoperative severe morbidity, reoperation, and possibly pneumonia after OE; however, further multi-institutional investigation with a large cohort is necessary to establish the importance of pretreatment RDW for predicting short-term outcomes after esophagectomy.
Supplementary Information
Below is the link to the electronic supplementary material.
Disclosures
Naoya Yoshida and Takatsugu Ishimoto are affiliated to a department supported by Chugai Pharmaceutical Co., Ltd and Yakuruto Honsya Co., Ltd, but declare no conflicts of interest in relation to this current research. Tomo Horinouchi, Tasuku Toihata, Kazuto Harada, Kojiro Eto, Hiroshi Sawayama, Masaaki Iwatsuki, Yohei Nagai, Yoshifumi Baba, Yuji Miyamoto, and Hideo Baba have no conflicts of interest or financial ties to disclose regarding this current research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yoshida N, Baba Y, Hiyoshi Y, et al. Duration of smoking cessation and postoperative morbidity after esophagectomy for esophageal cancer: how long should patients stop smoking before surgery? World J Surg. 2016;40:142–147. doi: 10.1007/s00268-015-3236-9. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida N, Morito A, Nagai Y, et al. Clinical importance of sputum in the respiratory tract as a predictive marker of postoperative morbidity after esophagectomy for esophageal cancer. Ann Surg Oncol. 2019;26:2580–2586. doi: 10.1245/s10434-019-07477-7. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida N, Baba Y, Kuroda D, et al. Clinical utility of exhaled carbon monoxide in assessing preoperative smoking status and risks of postoperative morbidity after esophagectomy. Dis Esophagus. 2018 doi: 10.1093/dote/doy024. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida N, Kosumi K, Tokunaga R, et al. Clinical importance of mean corpuscular volume as a prognostic marker after esophagectomy for esophageal cancer: a retrospective study. Ann Surg. 2020;271:494–501. doi: 10.1097/SLA.0000000000002971. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida N, Baba Y, Shigaki H, et al. Preoperative nutritional assessment by controlling nutritional status (CONUT) is useful to estimate postoperative morbidity after esophagectomy for esophageal cancer. World J Surg. 2018;40:1910–1917. doi: 10.1007/s00268-016-3549-3. [DOI] [PubMed] [Google Scholar]
- 6.Filip B, Scarpa M, Cavallin F, et al. Postoperative outcome after oesophagectomy for cancer: Nutritional status is the missing ring in the current prognostic scores. Eur J Surg Oncol. 2015;41:787–794. doi: 10.1016/j.ejso.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Buttarello M. Laboratory diagnosis of anemia: are the old and new red cell parameters useful in classification and treatment, how? Int J Lab Hematol. 2016;38(Suppl 1):123–132. doi: 10.1111/ijlh.12500. [DOI] [PubMed] [Google Scholar]
- 8.Parizadeh SM, Jafarzadeh-Esfehani R, Bahreyni A, et al. The diagnostic and prognostic value of red cell distribution width in cardiovascular disease; current status and prospective. Biofactors. 2019;45:507–516. doi: 10.1002/biof.1518. [DOI] [PubMed] [Google Scholar]
- 9.Pilling LC, Atkins JL, Kuchel GA, Ferrucci L, Melzer D. Red cell distribution width and common disease onsets in 240,477 healthy volunteers followed for up to 9 years. PLoS One. 2018;13(9):e0203504. doi: 10.1371/journal.pone.0203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrauskas LA, Saketkoo LA, Kazecki T, et al. Use of red cell distribution width in a population at high risk for pulmonary hypertension. Respir Med. 2019;150:131–135. doi: 10.1016/j.rmed.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsaros M, Paschos P, Giouleme O. Red cell distribution width as a marker of activity in inflammatory bowel disease: a narrative review. Ann Gastroenterol. 2020;33:348–354. doi: 10.20524/aog.2020.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Huang R, Yan X, et al. Red blood cell distribution width: A promising index for evaluating the severity and long-term prognosis of hepatitis B virus-related diseases. Dig Liver Dis. 2020;52:440–446. doi: 10.1016/j.dld.2019.12.144. [DOI] [PubMed] [Google Scholar]
- 13.Kalemci S, Akin F, Sarihan A, Sahin C, Zeybek A, Yilmaz N. The relationship between hematological parameters and the severity level of chronic obstructive lung disease. Pol Arch Intern Med. 2018;128:171–177. doi: 10.20452/pamw.4198. [DOI] [PubMed] [Google Scholar]
- 14.Goyal H, Awad H, Hu ZD. Prognostic value of admission red blood cell distribution width in acute pancreatitis: a systematic review. Ann Transl Med. 2017;5:342. doi: 10.21037/atm.2017.06.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu XS, Chen ZQ, Hu YF, et al. Red blood cell distribution width is associated with mortality risk in patients with acute respiratory distress syndrome based on the Berlin definition: a propensity score matched cohort study. Heart Lung. 2020;49:641–645. doi: 10.1016/j.hrtlng.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Foy BH, Carlson JCT, Reinertsen E, et al. Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS-CoV-2 infection. JAMA Netw Open. 2020;3:e2022058. doi: 10.1001/jamanetworkopen.2020.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li N, Zhou H, Tang Q. Red blood cell distribution width: a novel predictive indicator for cardiovascular and cerebrovascular diseases. Dis Markers. 2017;2017:7089493. doi: 10.1155/2017/7089493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobin LH, Gospodarowicz MK, Wittekind C. International Union against Cancer. In: TNM classification of malignant tumours, 7th ed. Chichester, West Sussex; Hoboken, NJ: Wiley-Blackwell; 2010.
- 19.Kuwano H, Nishimura Y, Oyama T, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1–30. doi: 10.1007/s10388-014-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida N, Nakamura K, Kuroda D, et al. Preoperative smoking cessation is integral to the prevention of postoperative morbidities in minimally invasive esophagectomy. World J Surg. 2018;42:2902–2909. doi: 10.1007/s00268-018-4572-3. [DOI] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirahara N, Tajima Y, Fujii Y, et al. Prognostic significance of red cell distribution width in esophageal squamous cell carcinoma. J Surg Res. 2018;230:53–60. doi: 10.1016/j.jss.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 23.Wang PF, Song SY, Guo H, Wang TJ, Liu N, Yan CX. Prognostic role of pretreatment red blood cell distribution width in patients with cancer: a meta-analysis of 49 studies. J Cancer. 2019;10:4305–4317. doi: 10.7150/jca.31598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayasuriya NA, Kjaergaard AD, Pedersen KM, et al. Smoking, blood cells and myeloproliferative neoplasms: meta-analysis and Mendelian randomization of 2·3 million people. Br J Haematol. 2020;189:323–334. doi: 10.1111/bjh.16321. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida N, Harada K, Iwatsuki M, Baba Y, Baba H. Precautions for avoiding pulmonary morbidity after esophagectomy. Ann Gastroenterol Surg. 2020;4:480–484. doi: 10.1002/ags3.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang P, Li Y, Sun H, et al. Predictive Value of Body Mass Index for Short-Term Outcomes of Patients with Esophageal Cancer After Esophagectomy: a Meta-analysis. Ann Surg Oncol. 2019;26:2090–2103. doi: 10.1245/s10434-019-07331-w. [DOI] [PubMed] [Google Scholar]
- 27.Papaconstantinou D, Vretakakou K, Paspala A, et al. The impact of preoperative sarcopenia on postoperative complications following esophagectomy for esophageal neoplasia: a systematic review and meta-analysis. Dis Esophagus. 2020 doi: 10.1093/dote/doaa002. [DOI] [PubMed] [Google Scholar]
- 28.Kim E, Lee DH, Jang JY. Effects of preoperative malnutrition on postoperative surgical outcomes and quality of life of elderly patients with periampullary neoplasms: a single-center prospective cohort study. Gut Liver. 2019;13:690–697. doi: 10.5009/gnl18469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf JH, Ahuja V, D'Adamo CR, Coleman J, Katlic M, Blumberg D. Preoperative nutritional status predicts major morbidity after primary rectal cancer resection. J Surg Res. 2020;255:325–331. doi: 10.1016/j.jss.2020.05.081. [DOI] [PubMed] [Google Scholar]
- 30.Ahiko Y, Shida D, Horie T, et al. Controlling nutritional status (CONUT) score as a preoperative risk assessment index for older patients with colorectal cancer. BMC Cancer. 2019;19:946. doi: 10.1186/s12885-019-6218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takagi K, Domagala P, Polak WG, Buettner S, Ijzermans JNM. The controlling nutritional status score and postoperative complication risk in gastrointestinal and hepatopancreatobiliary surgical oncology: a systematic review and meta-analysis. Ann Nutr Metab. 2019;74:303–312. doi: 10.1159/000500233. [DOI] [PubMed] [Google Scholar]
- 32.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida N, Yamamoto H, Baba H, et al. Can minimally invasive esophagectomy replace open esophagectomy for esophageal cancer?: latest analysis of 24,233 esophagectomies from the Japanese national clinical database. Ann Surg. 2020;272:118–124. doi: 10.1097/SLA.0000000000003222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


