Abstract
Background
The COVID-19 pandemic has necessitated the social isolation of the population and the rapid implementation of remote care for patients with neurodegenerative diseases. The objective of this study was to explore the perceived impact of confinement in patients with Parkinson's disease and document the effects of gender and living environment.
Methods
We recruited two cohorts from the Canadian provinces of Québec and Alberta, which differed in the dynamics of COVID-19 spreading at the time of the study, and administered a questionnaire on the perceived effects of confinement on daily living and disease management.
Results
The data reveals that approximately half of the patients experienced a change in one or more clinical symptoms, with differences observed between gender (e.g. day-to-day changes in slowness in men, aggravated headaches in women) and geographic location (e.g. increased depression in Alberta but reduced sleep quality in Québec). Furthermore, participants identifying as women or living in Alberta implemented more frequently home or online exercise. Lastly, high levels of satisfaction with phone or video consultations did not translate into a sustained interest to pursue this mode of healthcare.
Conclusions
This study suggests that COVID-19-related confinement affected Parkinson's disease manifestation and management. Patients also reported varying levels of interest to continue remote care. A number of differences reported in our study were seemingly related to gender and living environment.
Keywords: Pandemic, Isolation, Telemedicine, Gender effect, Neurodegenerative diseases
1. Introduction
The unforeseen COVID-19 pandemic has imposed the confinement of the global population. This sudden disruption of lifestyle and daily activities has raised concerns regarding the wellbeing of patients affected by chronic diseases such as neurodegenerative disorders [[1], [2], [3], [4]]. However, these unprecedented circumstances offered an opportunity to evaluate several important factors which contribute to the welfare and comfort of patients with Parkinson's disease (PD). These include social interactions and daily routines, ability to manage life-disrupting motor and non-motor symptoms and continuity of healthcare. Recent reports from a number of countries have shown that confinement due to COVID-19 has aggravated motor and non-motor symptoms in patients diagnosed with PD [[5], [6], [7], [8], [9]]. Accumulating evidence from past outbreaks and the current pandemic also revealed gender disparities [[10], [11], [12]] such as differences in disease burden and response to health risk [13,14]. These observations suggest that women's overall health status and quality of life during the COVID-19 pandemic may deteriorate more significantly compared to men and need to be monitored closely to appropriately address health disparities in the population. In addition, clinical studies have previously reported that PD-related symptoms can manifest differently in men and women patients [[15], [16], [17], [18]]. As such, the existence of a gender effect in response to confinement in patients with PD has yet to be characterized and understanding gender-based differences is essential to implement adequate PD-related public health responses. Furthermore, regional differences in the perception of COVID-19-related risk and impact on chronic disease management have emerged in the United States and appear to reflect COVID-19 burden in these communities [19]. In Canada, the number of diagnosed COVID-19 cases reported during the first wave of contaminations (March–July 2020) also differed dramatically between provinces, which provided a unique opportunity to compare how PD patients living in regions with fast or slow COVID-19 spread perceived the confinement. The objective of this study was to expand on previous findings and explore different variables that may impact self-reported changes in motor and non-motor symptoms, such as gender (i.e. self-identification as man, woman or other gender identity) or living in geographic areas with different rates of COVID-19 community transmission. In parallel, we assessed the level of satisfaction of PD patients with remote consultations and their interest in pursuing this alternative mode of care in the future.
2. Methods
The Canadian Open Parkinson Network (C-OPN) [20], the Quebec Parkinson Network (QPN) [21] and the Calgary Parkinson Research Initiative (CaPRI) [22] collaborated to collect the responses of 417 PD patients to an online questionnaire. These networks or initiatives were created with the goal to accelerate PD research and each have a registry component whereby potential patients are recruited. Patients may be referred by a neurologist or an investigator conducting other research studies at local Movement Disorders Clinics, or recruited via advertisements displayed in these clinics or on their Facebook page. Patients can also join the various initiatives via self-referral through the websites.
Participants were invited to fill out the online survey if they were actively enrolled in the networks and had consented to be contacted for future research. To ensure a research outcome that is inclusive and representative of all participants, patients were asked to select their gender from a list of options (man, woman, transgender, other). All participants identified as either “man” or “woman”, and none of the patients selected the “transgender” or “other” option. The questionnaire, which was available between May 20th and September 16th 2020, assessed the impact of the COVID-19 confinement on perceived wellbeing, management of symptoms and continuity of health care. Sarah Bogard (QPN) and Catherine Normandeau (C-OPN) designed and submitted the questions to a panel of movement disorder clinicians affiliated with C-OPN in order to evaluate adequacy and relevance. The survey was subsequently modified by changing or adding items until a consensus was reached by the panel of experts. The final survey consisted of 36 questions divided in three main sections related to physical and mental wellbeing, daily activities and PD symptoms during the COVID-19 confinement (full questionnaire available as a Supplementary Material). The beginning of confinement was defined as the date when provincial governments enforced social isolation measures and travel restrictions, which corresponded to March 13th 2020 for participants living in Québec and March 17th 2020 for those in Alberta. For the purpose of this questionnaire, the terms “virtual care” and “virtual visits” refer to both phone and videoconferences. To capture the responses of patients with PD during the early stages of confinement, we did not test the survey in a pilot group.
The online questionnaire was administered through REDCap electronic data capture tool software [23] or the Longitudinal Online Research and Imaging System (LORIS) [24] to all enrolled parkinsonian patients via C-OPN, CaPRI and QPN that consented to be contacted for future research. Responses from patients diagnosed with atypical parkinsonism syndromes (e.g. progressive supranuclear palsy or multiple system atrophy) were excluded from the analysis presented here. Ethical approval (REB19-1688, REB16-0545, Pro00091716, MUHC-15-944) and informed consent (either electronically, verbally, or implicitly) were obtained in accordance with appropriate institutional policies. Implied consent consists in providing a written explanation, at the beginning of the questionnaire, stating that participants accepting to complete the survey also give their consent to take part in the study. It is an abbreviated version of all relevant material normally included in a consent form, including that participation is voluntary, but does not require a signature and informs that participation is voluntary (see Questionnaire, Supplementary Material). This procedure is approved by local Research Ethics Boards and is commonly used for de-identified questionnaires that present minimal risk or harm to participants. Eligible patients (patients with PD older than 18 years of age) were invited to complete the online questionnaire either via email or over the phone with trained research personnel (coordinator or research assistant) based on their preference. Overall, 89% (383/430) of questionnaires were self-administered by patients.
Categorical data between groups were analyzed using a Pearson exact Chi-square test with Monte Carlo estimation when appropriate and continuous data were analyzed using a Student's t-test. The interest in future virtual care was modeled using univariate and multivariate log-binomial regression models with and without interaction to analyze the relationships between the variables province and education or living environment. Statistical analysis was performed using the SPSS statistics software (version 27.0 of the IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, USA) and the SAS® software (version 9.4 of the SAS system for Windows, SAS Institute Inc., Cary, NC, USA) with significance level set at p ≤ 0.05.
3. Results
3.1. Population characteristics
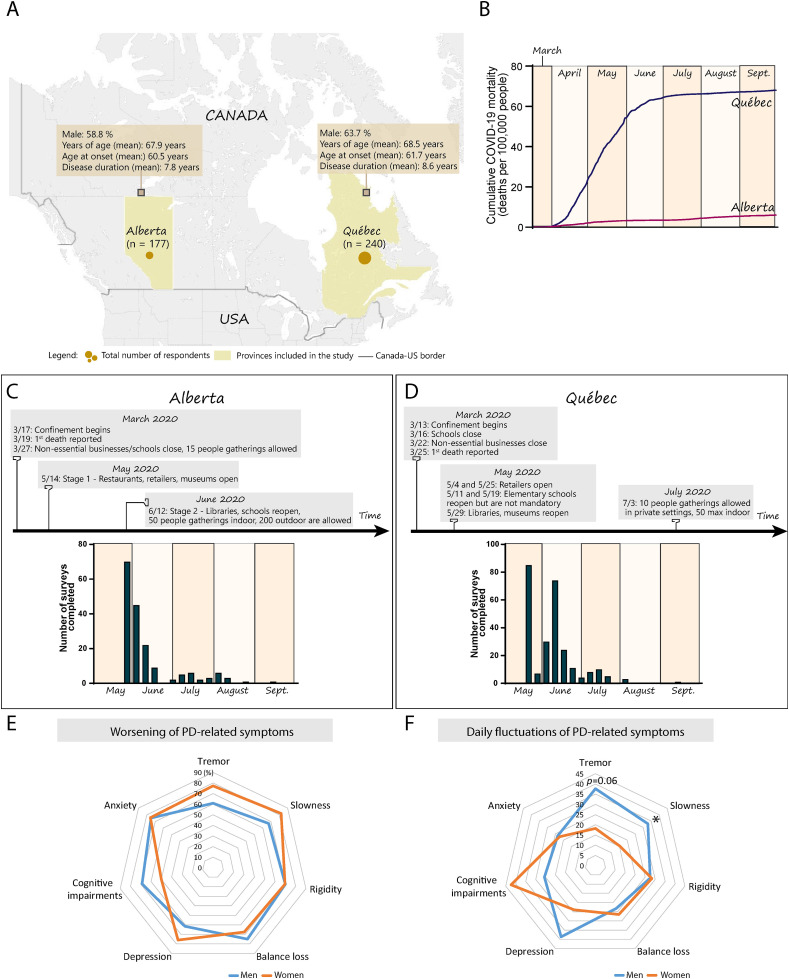
A total of 417 patients with PD were recruited, with an average age of 68.2 ± 9.7 years, disease duration of 8 ± 5.4 years, disease onset at 60.9 ± 11.4 years and 61.3% were men. The cohorts recruited from Québec (n = 240) and Alberta (n = 177) had a similar demographic profile (Québec: men, 63.7%; age, 68.5 ± 9.5; PD duration, 8.6 ± 6.4 years. Alberta: men, 58.8%; age, 67.9 ± 9.5; PD duration, 7.8 ± 4.9 years) with comparable distribution of gender (p = 0.31), age (p = 0.495), duration of PD (p = 0.256), presence of comorbidities (p = 0.650), and only a small proportion of patients was diagnosed with dementia (Québec, 0%; Alberta, 2.9%; men, 1.5%; women, 1.6%) (Supplementary Table 1, Fig. 1 A). Men and women participants also had a similar distribution of age (p = 0.885), duration of PD (p = 0.941) and presence of comorbidities (p = 0.664). The median Hoehn and Yahr (H&Y) stage was 2 (scale of 1–5) for the Alberta and Québec cohorts and remained unchanged when the participants were stratified between men and women (Supplementary Table 1). H&Y scores were available for 91% of patients in Alberta and for 56% in Québec. The number of patients with a University degree was equivalent in both provinces (∼45%), but people without such degree more frequently held a high school diploma in Québec or a non-university diploma (e.g. trade certificate) in Alberta (p = 0.005). In addition, more participants in Alberta reported living in urban areas, i.e. where the total population count exceeded 10,000 people (78.1%, Québec; 86.9%, Alberta; p = 0.026) (Table 1 ).
Fig. 1.
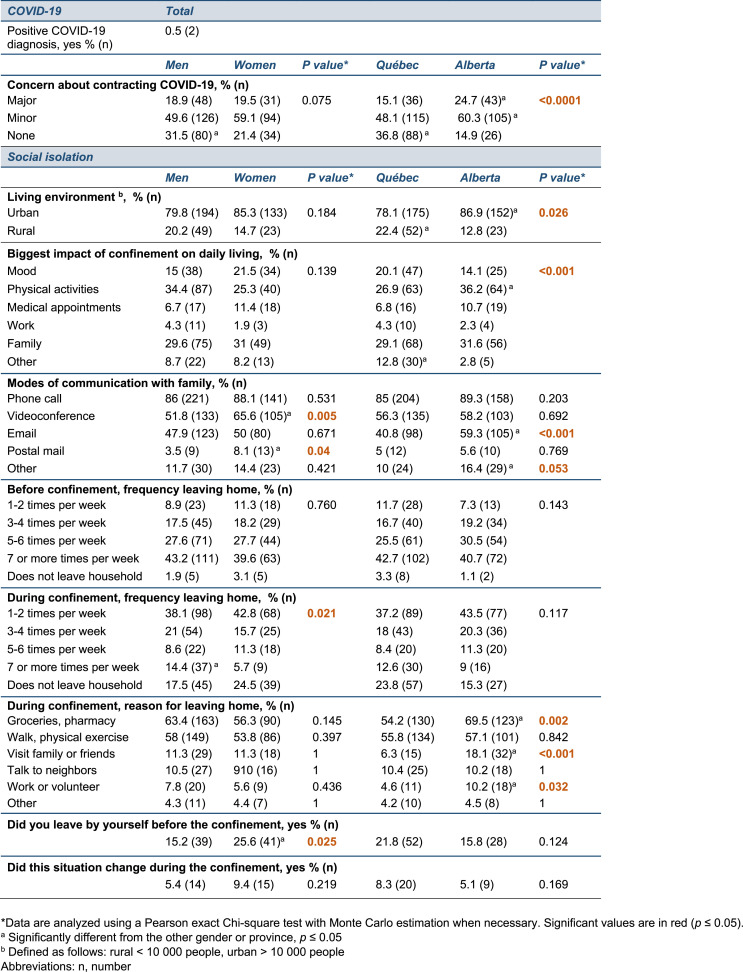
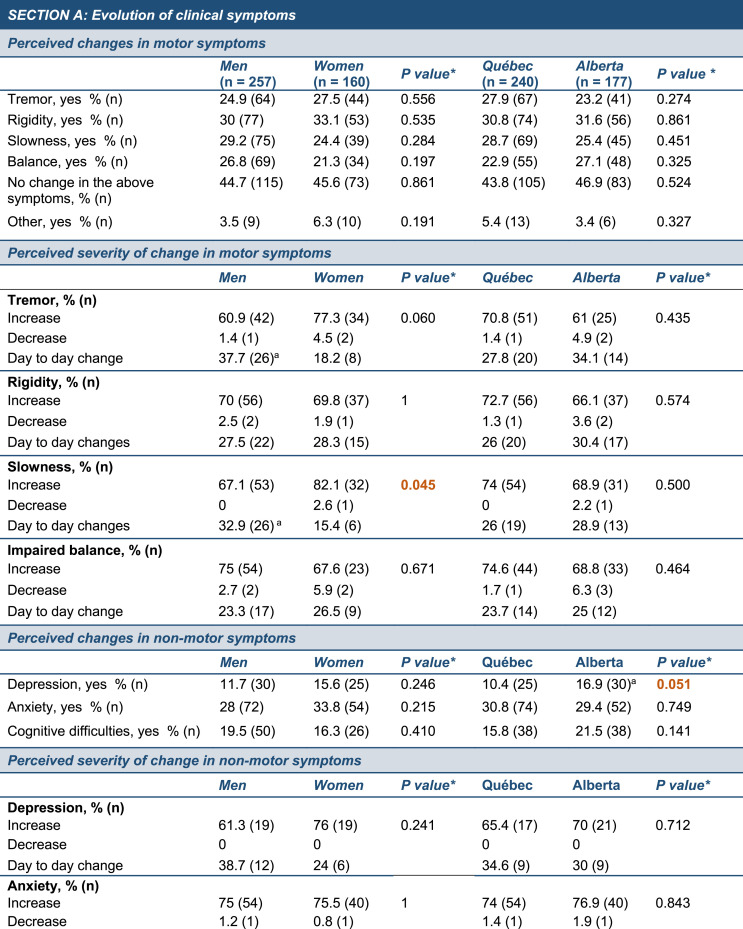
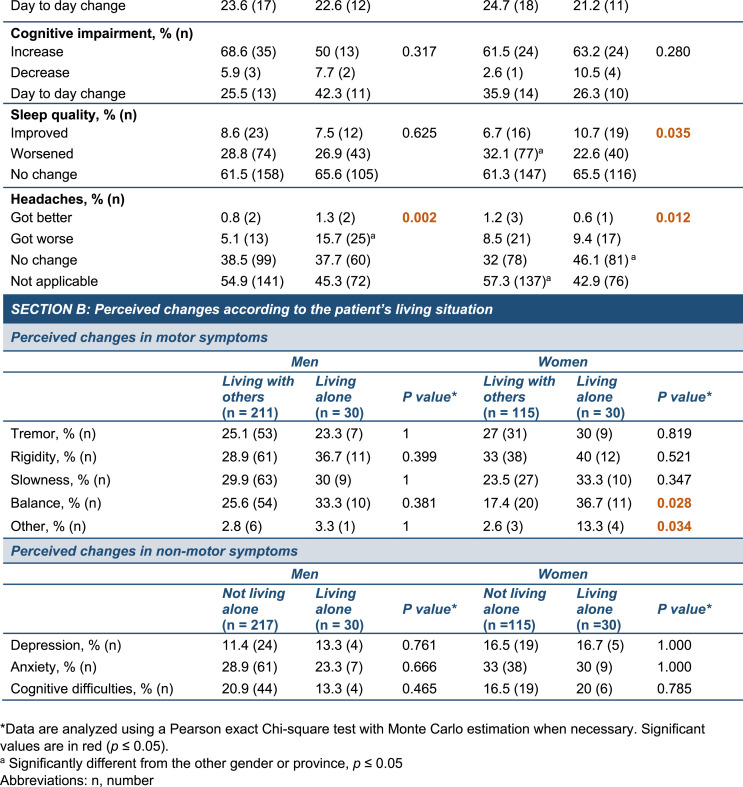
Changes to daily routines and PD-related symptoms during COVID-19 confinement. (A) Map depicting the geographic location of the two provinces included in the study (yellow). The size of the circles is proportional to the number of participants in each province. Information in the yellow squares summarizes the characteristics of the cohorts recruited in Alberta and Québec. (B) Graph showing the cumulative mortality associated with COVID-19 infection in the global population in Alberta (red line) and Québec (blue line) during the survey period (March to September 2020). Census data obtained from provincial sources [46], COVID-19 mortality data obtained from the COVID-19 Canada Open Data Working Group [47]. (C,D) The timeline provides key dates related to COVID-19 confinement in Alberta (C) and Québec (D) and the histograms depict the number of responses to the surveys during the duration of the study with each bar of the histogram corresponding to a specific week. (E,F) Radar chart showing the percentage of self-reported worsening (E) or daily fluctuations (F) in motor and non-motor symptoms in men (blue line) and women (orange line) since COVID-19-related confinement. Each grey line of the axis corresponds to a percentage value. Data analyzed using a Pearson exact Chi-square test with Monte Carlo estimation when necessary, *p ≤ 0.05. Abbreviations: n, number; PD, Parkinson's disease; Sept., September.
Table 1.
Impact of COVID-19 confinement on daily living.
3.2. Impact of COVID-19 on daily living
The data were collected in an epidemiological context where COVID-19 cumulative mortality in Québec totaled 5,834 (68 per 100,000) people by the end of September 2020, compared to 267 (6 per 100,000) in Alberta (illustrated in Fig. 1B) and at a time where provinces had implemented different policies and timeline/schedule of restrictions (illustrated in Fig. 1C and D). A majority of responses were collected in May and June for both Alberta and Québec, suggesting that the responses to the survey are concentrated at the beginning of the study period (Fig. 1C and D). Our survey only reported 2 cases officially diagnosed with COVID-19 and no participants were hospitalized during the study for PD or COVID-19-related health reasons (Supplementary Table 1). Physical activities, family and changes in mood were the three categories most impacted by the confinement, regardless of the province or gender.
Environment. Participants in both provinces expressed concerns about contracting COVID-19, but those living in Alberta reported significantly greater concerns (p < 0.001). Differences in the preferred mode of communication were observed, with participants in Alberta communicating more frequently by email compared to those living in Québec (p < 0.001) (Table 1).
Gender. Phone calls were the most utilized mode of communication with family members but women engaged more frequently in videoconferences (p = 0.005) and postal mail (p = 0.04) compared to men. It should also be noted that a greater number of women reported living alone compared to men (p = 0.025) and this living situation changed for 7.4% of all participants during confinement. Before the implementation of social isolation measures, there were no differences in the frequency at which participants left their home, but during the confinement, 14.4% of men reported going out at least seven times a week compared to only 5.7% of women (p = 0.021) (Table 1).
3.3. Self-reported aggravation of motor and non-motor symptoms in PD patients
We then investigated the impact of the confinement on PD-related symptoms and questioned whether changes in motor and non-motor symptoms would vary as a result of geographic location or gender (Table 2 ).
Table 2.
Self-reported evolution of motor and non-motor symptoms in PD patients during COVID-19 confinement.
Environment. Across all provinces, participants reported experiencing changes in motor symptoms during the confinement, including tremor (group average: 26.6%), rigidity (group average: 31.6%), slowness (group average: 26.8%) and balance (group average: 24.1%). A general worsening of symptoms was most frequent regardless of the province, but patients also reported day-to-day changes (Table 2, Fig. 1E and F). Furthermore, changes in depression were more common in Alberta (p = 0.051) while participants in Québec observed a diminution in their quality of sleep (p = 0.035).
Gender. We found gender-based differences in the evolution of slowness (p = 0.045), with men experiencing daily fluctuations (15.4% women, 32.9% men), while women more often experienced a persistent aggravation of this symptom (82.1% women, 67.1% men). There was a trend towards differences in the evolution of tremor between men and women (p = 0.06), with men frequently reporting day-to-day changes (18.2% women, 37.7% men) while women noted a worsening (77.3% women, 60.9% men). In addition, women described the worsening of headaches compared to men (p = 0.002) (Table 2). We found that there were more women participants living alone than men and this situation could have exacerbated the social isolation already imposed on them during confinement. An analysis of the perceived changes in symptoms according to gender and living arrangements revealed that women living alone before and during the confinement experienced significantly greater changes in balance (p = 0.028) and “other” symptoms (p = 0.034) compared to women not living alone (Table 2, Section B). The “other” category allowed participant to detail unlisted symptoms which included fatigue, lower endurance and insomnia. However, analysis did not unveil an effect of the living situation in the perception of symptoms by men participants.
3.4. Disease management and telemedicine
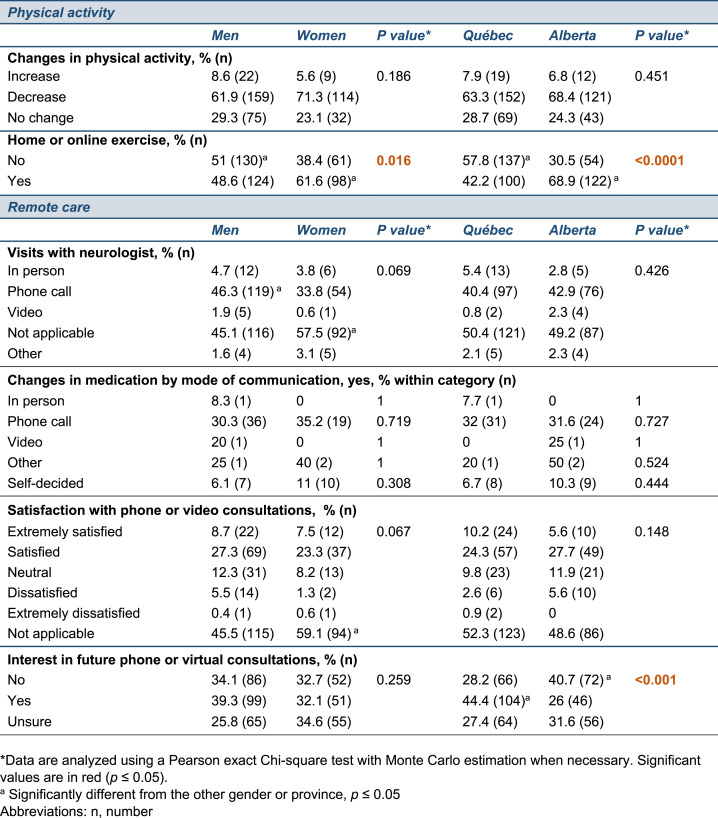
As part of the questionnaire, we inquired about changes in physical activity, as regular exercise is essential to manage PD symptoms, but a majority of participants reported a decrease in their level of physical activity as a result of confinement. However, most participants implemented home or online exercise during this period (men, 48.6%; women, 61.6%) and we found a greater interest in home-based exercise in Alberta compared to Québec (p < 0.0001) and in women compared to men (p = 0.016) (Table 3 ). During the study period, scheduled appointments were largely carried out by phone and approximately 30% of patients who communicated with their neurologist adjusted their PD medication, without significant differences by province or gender. When asked about their remote care experience, patients admitted to high levels of satisfaction regardless of province or gender and ∼45% of the participants in Québec showed an interest in pursuing remote consultations (i.e. phone or videoconference), compared to 26% in Alberta (p < 0.001) (Table 3). These varying levels of interest could depend on a person's education, as shown in other studies [25], or on the living environment, with rural patients facing transportation challenges or having to travel longer distances to visit their providers. Given the findings that the Alberta and Québec cohorts significantly differed in living environments and educational profiles, we assessed the influence of these variables on the participant's interest in future remote care. Log-binomial regression models revealed the absence of statistically significant interactions between provinces and education, or provinces and living environment, suggesting that these variables do not account for the different interest in future phone- or videoconference-based care between provinces. However, some trends were observed and participants with a University degree or living in an urban area were more likely to express an interest in future remote care if they were located in Québec compared to Alberta (positive responses increased by 65% [95% CL: 17%; 134%] with a University degree, or 56% [95% CL:18%; 106%] with urban living).
Table 3.
Changes in physical activity and medical care during COVID-19 confinement.
4. Discussion
4.1. Effects of confinement on PD-related symptoms
Two distinct cohorts of patients were recruited in Québec and Alberta, provinces with different dynamics of COVID-19 transmission at the time of the study. Access to this large dataset enabled a stratification based on gender and/or geographic location, complementing existing studies and offering a unique insight into the susceptibility or resilience of different sub-populations of PD patients to the COVID-19 confinement. Approximately half of the participants noted changes in motor and non-motor symptoms, in accordance with previous studies reporting an aggravation of behavioral and psychological symptoms, a worsening of sleep disorders, depression and anxiety as well as reduced physical activity of patients with PD as a result of confinement or loneliness [[5], [6], [7],[26], [27], [28], [29], [30], [31], [32]]. Patients also reported increased anxiety and worsened cognitive abilities (30.9% and 17.9% positive responses, respectively) during confinement, without significant differences between gender and living environment. Changes in clinical presentation and decreased low intensity exercise have also been reported for patients with neuromuscular diseases [33]. This suggests that patients affected by chronic disorders experience similar detrimental effects of COVID-19 confinement on wellbeing and disease management strategies. However, the influence of inherent and extrinsic factors on these observations remained unclear and our study found that at least five PD-related symptoms were differentially perceived as a result of province or gender. For example, our data highlight a strong association between living alone and perceived changes in motor symptoms in women, but not men participants. Living alone had previously been identified as a factor aggravating the effects of the COVID-19 pandemic in patients with PD [27], supporting our observation that lifestyle and social interactions could explain the gender effect observed. Our results relative to the fluctuation or continuous aggravation of motor symptoms observed in men vs. women is not readily accounted for by treatment changes, as men and women equally modified their medication after a visit with their care provider. In further support of our observations, previous studies suggested that women were more likely to perceive COVID-19 as a significant health risk [14], which could in turn influence their physical and psychological responses to confinement. In contrast to other studies [[15], [16], [17], [18]], pre-confinement motor symptom assessment of the patients included in our cohorts did not identify statistically significant gender differences. This may explain the relatively small number of symptoms perceived differently between men and women during confinement. However, the reported aggravation of slowness, headaches and balance (Table 2) in women vs. men warrants further investigation of post-COVID-19 changes to PD-related symptoms in a larger cohort.
The observed differences between provinces suggest an association between the sociocultural environment, local COVID-19 transmission dynamics and the response to confinement, corroborating previous findings on the role of social contexts to risk perception [[34], [35], [36]]. For example, three independent studies carried out in the spring of 2020 found varying levels of COVID-19-related risk perception in distinct populations of PD patients. In these reports, 42% of surveyed Spanish patients reported high concerns about contracting COVID-19 [8], compared to 8% in India [4] and 59% of patients reporting fear/worries in Germany [37]. These observations indicate that geographical location, such as Alberta vs. Québec in our study, along with different sociocultural backgrounds could affect a patient's perceived risk to contract the virus. Lastly, a recent study assessed the association between worsening of motor or non-motor features during the COVID-19 pandemic and four variables related to lifestyle or medical support namely (1) “available home support during lockdown”, (2) “duration of home confinement”, (3) “duration of Parkinson's disease” and (4) “difficulty seeking formal neurologist's opinion and/or medicines for Parkinson's disease” [28]. It was found that some – but not all – clinical features are affected by these variables, emphasizing that clinical manifestations of PD are complex and can be influenced by more than one external factor. Overall, our data indicate that patients may have different susceptibilities to COVID-19 confinement, and could inform on measures relative to disease management in these different populations.
4.2. Post-COVID-19 care and telemedicine
This survey reports that scheduled consultations were carried out most frequently via phone calls, suggesting that videoconference-related technologies may have been inaccessible to most patients and/or care providers at the time of the study. However, caution is warranted as this proportion may only reflect clinical practices at early stages of the pandemic and could be specific to the selected subset of clinics. The cohort living in Québec appeared more inclined to continue remote neurological care (i.e. phone or videoconference) compared to Alberta. A recent study found that 46% of PD patients in the United States expressed the desire to pursue telehealth even after the end of the pandemic [38], which is similar to the level of interest observed in the Québec cohort. Furthermore, a study carried out shortly before the COVID-19 pandemic questioned 18 patients with PD located in rural British Columbia (Canada) and found that living in a large urban center was largely perceived as an advantage to receive the best PD care, and more than 70% of the participants would consider using videoconferencing for follow-up appointments with their neurologist [39]. The different interest in future remote care observed between patients living in Québec or Alberta is not readily explained by the rural or urban living environment, but future studies could (i) recruit a larger cohort and (ii) collect data on travel burden, as published before [39]. We can, however, propose that the basis for inter-regional differences could involve a combination of culture and comfort with digital tools, but also reflect the structure of care in different clinics across provinces. For example, patients in some centers can conveniently access multiple services (e.g. dieticians, physical therapists) on the same day for in-person visits, while these may be more difficult to schedule during a single telemedicine appointment. The varying levels of expressed interest in telemedicine suggest a potential for a more personalized approach to healthcare delivery, by offering patients the choice between remote or in-person consultations. North American clinicians reported patient resistance to videoconferencing in the early COVID-19 crisis as the second barrier to its implementation, after the lack of access to technology [40]. It will therefore be critical to ensure that deploying video-based consultations does not create disparities between patients from different socioeconomic backgrounds, not only for their medical care but also for their inclusion into research studies [41]. Lastly, clinical studies have suggested that telemedicine for PD patients is as effective as in-person care [[42], [43], [44]]. These promising findings would benefit from additional studies designed to evaluate the differences between in-person vs. virtual visits in a neurologist's ability to adjust medication [45].
5. Conclusions
Our findings have been collected from a brief observational survey, which presents with strengths and limitations. For instance, the documentation of perceived consequences of the confinement is not accompanied by clinical assessments by movement disorder specialists and the data is not controlled for socioeconomic status. Strengths include the fact that this is an inclusive study that compares two cohorts recruited from provinces with very different dynamics of COVID-19 progression. We also surveyed a large number of participants, which further enabled a gender-based analysis. Our study showed that slowness, depression, sleep quality, headache and balance were differently perceived as a result of gender and/or localization, suggesting that patients with PD can be segregated into sub-populations with different sensitivities to confinement and related changes to their routine and living conditions. The impact of the environment on the observed differences between provinces is complex and likely to be attributed to more than one factor among which are the rate of COVID-19 community transmission in each province and the sociocultural environment. To draw firm conclusions on how gender and/or geographical location affect clinical symptoms during COVID-19-related social isolation, the findings presented in this article will need to be validated by a follow-up study that includes a larger sample size, or re-analyzed as part of a global meta-analysis of published literature. Additional survey will also be critical to understand the long-term impact of the COVID-19-related restrictions on mental and physical resilience of both patients and their caregivers, as well as the consequences of this rapid shift from in-person to virtual care from the perspective of neurologists and caretakers.
Acknowledgements and financial disclosures of all authors
The authors would like to warmly thank participants in this study for their time and invaluable insight into the impact of the confinement on disease management. We also thank the Clinical Biological Imaging and Genetic Repository (C-BIG, McGill University, Montréal, QC, Canada), the teams/investigators associated with the Canadian Open Parkinson Network, Québec Parkinson Network and Calgary Parkinson Research Initiative who were vital collaborators on this project. We would also like to thank Dr. Fadi Abu Ahmad, MD (McGill University, Montréal, Canada) for helpful discussions and David Simonyan for assistance with statistical analysis (Clinical and Evaluative Research Platform, CHU de Québec-Université Laval Research Centre, Québec, Canada). Data collected via the QPN initiative were stored in the C-BIG Repository (Open Science Clinical Biological Imaging and Genetic Repository, Montréal Neurological Institute, Canada). The Canadian Open Parkinson Network project has been made possible by Brain Canada through the Canada Brain Research Fund, with financial support of Health Canada and Parkinson Canada.
A.d.R.J.: Postdoctoral fellowship from the Parkinson's Foundation. N.D.: Research funding through Ataxie Canada – Fondation Claude St-Jean; ALS Society of Canada, Brain Canada Foundation; Fonds de recherche du Québec - Santé (FRQS); Fondation de l'ataxie Charlevoix-Saguenay; Canadian Institutes of Health Research (CIHR) Project grant; Association de la neurofibromatose du Québec (ANFQ); Brain Canada, Technology and Platform Application; ALS Canada & Brain Canada, Arthur J. Hudson Translational Team Grant; Fondation du CHU de Québec; Michael J. Fox Foundation for Parkinson's Disease; CQDM/Brain Canada. R.B.P.: Grants and personal fees from Fonds de Recherche du Québec – Santé, the Canadian Institute of Health Research, Parkinson Canada, the W. Garfield Weston Foundation, the Michael J. Fox Foundation, the Webster Foundation, and Roche, and personal fees from Takeda, Roche/Prothena, Teva Neurosciences, Novartis Canada, Biogen, Boehringer Ingelheim, Theranexus, GE HealthCare, Jazz Pharmaceuticals, Abbvie, Jannsen, and Otsuko, outside the submitted work. E.A.F: CIHR foundation grants; Healthy Brains for Healthy Lives International Partnership; Canadian Consortium on Neurodegeneration in Aging; Michael J Fox Foundation for Parkinson's Research; ALS Canada/Brain Canada Hudson Team grant; Centre Québecois du Médicament (CQDM)/Takeda/SGC; Parkinson Canada/Brain Canada. F.C.: Operating grants from CIHR; Fondation du CHU de Québec; Huntington Society of Canada; Michael J Fox Foundation for Parkinson's Research; Research Chair from the FRQS.
Ethical compliance statement
Ethical approval and informed consent (either electronically, verbally or implicitly) were obtained in accordance with appropriate institutional policies: (i) University of Calgary Conjoint Health Research Ethics Board (CHREB), REB19-1688, REB16-0545; (ii) University of Alberta Health Research Ethics Board, Pro00091716; (iii) McGill University Health Centre Research Ethics Board, MUHC-15-944. Implied consent consists in providing a written explanation, at the beginning of the questionnaire, stating that participants accepting to complete the survey also give their consent to take part in the study (see Questionnaire, Supplementary Material).
Author contributions
Manuscript: A. Study design and data collection, B. Data and statistical analysis, C. Writing of the First Draft, D. Review and Critique, E. Editing, F. Final Edits.
A.d.R.J.: B, C, F.
S.B.: A, D, E.
C.N.: A, B, D, E.
C.D.: A.
R.B.P.: D, E.
N.D.: D, E.
O.M.: A, D, E.
J.M.: A, D, E.
D.M.: A, D, E.
E.F.: A.
F.C.: D, E, F.
Funding related to this study/conflict of interest
The study was funded by the “Canadian-Open Parkinson Network” platform grant from Brain Canada and Parkinson Canada (PI: OM) and the Fonds de recherche du Québec – Santé (FRQS) “Quebec Parkinson Network” (QPN) (PI: E.A.F., N.D.). A.d.R.J., S.B., C.P.N., C.D., R.B.P., D.M.: No funding related to this study or conflict of interest to disclose. J.M.: Associate Editor, Parkinsonism and Related Disorders, Canadian Consortium on Neurodegeneration in Aging 2018-present, Canadian Open Parkinson Network, Brain Canada 2018–2022, Parkinson Foundation research grant funding 2019–2020. O.M.: Tourmaline Oil Chair in Parkinson's disease; Canada Research Chair in non-motor symptoms of Parkinson's disease; project grant from the Canadian Institutes of Health Research (CIHR) (PJT-166123); Natural Sciences and Engineering Council Discovery Grant. E.A.F.: Canadian Institutes of Health Research (CIHR) Foundation grant (FDN-154301), the Canadian Consortium on Neurodegeneration in Aging and Canada Research Chair (Tier 1) in Parkinson's disease. F.C.: Research Chair from the FRQS.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.parkreldis.2021.08.018.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Helmich R.C., Bloem B.R. The impact of the COVID-19 pandemic on Parkinson's disease: hidden sorrows and emerging opportunities. J. Parkinsons Dis. 2020;10(2):351–354. doi: 10.3233/JPD-202038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoessl A.J., Bhatia K.P., Merello M. Movement disorders in the world of COVID-19. Mov. Disord. 2020;35(5):709–710. doi: 10.1002/mds.28069. [DOI] [PubMed] [Google Scholar]
- 3.Papa S.M., Brundin P., Fung V.S.C., Kang U.J., Burn D.J., Colosimo C., Chiang H.L., Alcalay R.N., Trenkwalder C., Committee M.D.-S.I. Impact of the COVID-19 pandemic on Parkinson's disease and movement disorders. Mov. Disord. 2020;35(5):711–715. doi: 10.1002/mds.28067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prasad S., Holla V.V., Neeraja K., Surisetti B.K., Kamble N., Yadav R., Pal P.K. Parkinson's disease and COVID-19: perceptions and implications in patients and caregivers. Mov. Disord. 2020;35(6):912–914. doi: 10.1002/mds.28088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salari M., Zali A., Ashrafi F., Etemadifar M., Sharma S., Hajizadeh N., Ashourizadeh H. Incidence of anxiety in Parkinson's disease during the coronavirus disease (COVID-19) pandemic. Mov. Disord. 2020;35(7):1095–1096. doi: 10.1002/mds.28116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shalash A., Roushdy T., Essam M., Fathy M., Dawood N.L., Abushady E.M., Elrassas H., Helmi A., Hamid E., Mental Health Physical activity, and quality of life in Parkinson's disease during COVID-19 pandemic. Mov. Disord. 2020;35(7):1097–1099. doi: 10.1002/mds.28134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar N., Gupta R., Kumar H., Mehta S., Rajan R., Kumar D., Kandadai R.M., Desai S., Wadia P., Basu P., Mondal B., Juneja S., Rawat A., Meka S.S., Mishal B., Prashanth L.K., Srivastava A.K., Goyal V. Impact of home confinement during COVID-19 pandemic on sleep parameters in Parkinson's disease. Sleep Med. 2021;77:15–22. doi: 10.1016/j.sleep.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos-Garcia D., Oreiro M., Perez P., Fanjul G., Paz Gonzalez J.M., Feal Painceiras M.J., Cores Bartolome C., Valdes Aymerich L., Garcia Sancho C., Castellanos Rodrigo M.D.M. Impact of coronavirus disease 2019 pandemic on Parkinson's disease: a cross-sectional survey of 568 Spanish patients. Mov. Disord. 2020;35(10):1712–1716. doi: 10.1002/mds.28261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo D., Han B., Lu Y., Lv C., Fang X., Zhang Z., Liu Z., Wang X. Influence of the COVID-19 pandemic on quality of life of patients with Parkinson’s disease. Parkinsons Dis. 2020;2020:1216568. doi: 10.1155/2020/1216568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simba H., Ngcobo S. Are pandemics gender neutral? Women's health and COVID-19. Front. Glob. Womens Health. 2020;1:570666. doi: 10.3389/fgwh.2020.570666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenham C., Smith J., Morgan R., Gender, Group C.-W. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395(10227):846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor J., Madhavan S., Mokashi M., Amanuel H., Johnson N.R., Pace L.E., Bartz D. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc. Sci. Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Sanguino C., Ausin B., Castellanos M.A., Saiz J., Lopez-Gomez A., Ugidos C., Munoz M. Mental health consequences during the initial stage of the 2020 Coronavirus pandemic (COVID-19) in Spain. Brain Behav. Immun. 2020;87:172–176. doi: 10.1016/j.bbi.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galasso V., Pons V., Profeta P., Becher M., Brouard S., Foucault M. Gender differences in COVID-19 attitudes and behavior: panel evidence from eight countries. PNAS. 2020;117(44):27285–27291. doi: 10.1073/pnas.2012520117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefanova E., Ziropadja L., Petrovic M., Stojkovic T., Kostic V. Screening for anxiety symptoms in Parkinson disease: a cross-sectional study. J. Geriatr. Psychiatr. Neurol. 2013;26(1):34–40. doi: 10.1177/0891988713476368. [DOI] [PubMed] [Google Scholar]
- 16.Yoon J.E., Kim J.S., Jang W., Park J., Oh E., Youn J., Park S., Cho J.W. Gender differences of nonmotor symptoms affecting quality of life in Parkinson disease. Neurodegener. Dis. 2017;17(6):276–280. doi: 10.1159/000479111. [DOI] [PubMed] [Google Scholar]
- 17.Szewczyk-Krolikowski K., Tomlinson P., Nithi K., Wade-Martins R., Talbot K., Ben-Shlomo Y., Hu M.T. The influence of age and gender on motor and non-motor features of early Parkinson’s disease: initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Park. Relat. Disord. 2014;20(1):99–105. doi: 10.1016/j.parkreldis.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Scott B., Borgman A., Engler H., Johnels B., Aquilonius S.M. Gender differences in Parkinson's disease symptom profile. Acta Neurol. Scand. 2000;102(1):37–43. doi: 10.1034/j.1600-0404.2000.102001037.x. [DOI] [PubMed] [Google Scholar]
- 19.Mehta B., Jannat-Khah D., Mancuso C.A., Bass A.R., Moezinia C.J., Gibofsky A., Goodman S.M., Ibrahim S. Geographical variations in COVID-19 perceptions and patient management: a national survey of rheumatologists. Semin. Arthritis Rheum. 2020;50(5):1049–1054. doi: 10.1016/j.semarthrit.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canadian Open Parkinson Network https://copn-rpco.ca/ accessed online December 21 2020.
- 21.Gan-Or Z., Rao T., Leveille E., Degroot C., Chouinard S., Cicchetti F., Dagher A., Das S., Desautels A., Drouin-Ouellet J., Durcan T., Gagnon J.F., Genge A., Karamchandani J., Lafontaine A.L., Sun S.L.W., Langlois M., Levesque M., Melmed C., Panisset M., Parent M., Poline J.B., Postuma R.B., Pourcher E., Rouleau G.A., Sharp M., Monchi O., Dupre N., Fon E.A. The Quebec Parkinson network: a researcher-patient matching platform and multimodal biorepository. J. Parkinsons Dis. 2020;10(1):301–313. doi: 10.3233/JPD-191775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calgary Parkinson research initiative. https://capriresearch.org/ accessed online December 20 2020.
- 23.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L., McLeod L., Delacqua G., Delacqua F., Kirby J., Duda S.N., Consortium R.E. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inf. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das S., Zijdenbos A.P., Harlap J., Vins D., Evans A.C. LORIS: a web-based data management system for multi-center studies. Front. Neuroinf. 2012;5(37) doi: 10.3389/fninf.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viers B.R., Pruthi S., Rivera M.E., O'Neil D.A., Gardner M.R., Jenkins S.M., Lightner D.J., Gettman M.T. Are patients willing to engage in telemedicine for their care: a survey of preuse perceptions and acceptance of remote video visits in a urological patient population. Urology. 2015;85(6):1233–1239. doi: 10.1016/j.urology.2014.12.064. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian I., Farahnik J., Mischley L.K. Synergy of pandemics-social isolation is associated with worsened Parkinson severity and quality of life. NPJ Parkinsons Dis. 2020;6:28. doi: 10.1038/s41531-020-00128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown E.G., Chahine L.M., Goldman S.M., Korell M., Mann E., Kinel D.R., Arnedo V., Marek K.L., Tanner C.M. The effect of the COVID-19 pandemic on people with Parkinson's disease. J. Parkinsons Dis. 2020;10(4):1365–1377. doi: 10.3233/JPD-202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar N., Gupta R., Kumar H., Mehta S., Rajan R., Kumar D., Kandadai R.M., Desai S., Wadia P., Basu P., Mondal B., Sanchita, Rawat A., Meka S.S., Mishal B., Prashanth L.K., Srivastava A.K., Goyal V. Impact of home confinement during COVID-19 pandemic on Parkinson’s disease. Park. Relat. Disord. 2020;80:32–34. doi: 10.1016/j.parkreldis.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Heide A., Meinders M.J., Bloem B.R., Helmich R.C. The impact of the COVID-19 pandemic on psychological distress, physical activity, and symptom severity in Parkinson's disease. J. Parkinsons Dis. 2020;10(4):1355–1364. doi: 10.3233/JPD-202251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oppo V., Serra G., Fenu G., Murgia D., Ricciardi L., Melis M., Morgante F., Cossu G. Parkinson's disease symptoms have a distinct impact on caregivers' and patients' stress: a study assessing the consequences of the COVID-19 lockdown. Mov. Disord. Clin. Pract. 2020;7(7):865–867. doi: 10.1002/mdc3.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rábano-Suárez P., Martínez-Fernández R., Natera-Villalba E., Pareés I., Martínez-Castrillo J.C., Alonso-Canovas A. Impulse control disorders in Parkinson’s disease: has COVID-19 related lockdown been a trigger? Mov. Disord. Clin. Pract. 2021;8:940–943. doi: 10.1002/mdc3.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Prete E., Francesconi A., Palermo G., Mazzucchi S., Frosini D., Morganti R., Coleschi P., Raglione L.M., Vanni P., Ramat S., Novelli A., Napolitano A., Battisti C., Giuntini M., Rossi C., Menichetti C., Ulivelli M., De Franco V., Rossi S., Bonuccelli U., Ceravolo R., Tuscany Parkinson C.-P. Prevalence and impact of COVID-19 in Parkinson's disease: evidence from a multi-center survey in Tuscany region. J. Neurol. 2021;268(4):1179–1187. doi: 10.1007/s00415-020-10002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Stefano V., Battaglia G., Giustino V., Gagliardo A., D'Aleo M., Giannini O., Palma A., Brighina F. Significant reduction of physical activity in patients with neuromuscular disease during COVID-19 pandemic: the long-term consequences of quarantine. J. Neurol. 2021;268(1):20–26. doi: 10.1007/s00415-020-10064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bish A., Michie S. Demographic and attitudinal determinants of protective behaviours during a pandemic: a review. Br. J. Health Psychol. 2010;15(4):797–824. doi: 10.1348/135910710X485826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bavel J.J.V., Baicker K., Boggio P.S., Capraro V., Cichocka A., Cikara M., Crockett M.J., Crum A.J., Douglas K.M., Druckman J.N., Drury J., Dube O., Ellemers N., Finkel E.J., Fowler J.H., Gelfand M., Han S., Haslam S.A., Jetten J., Kitayama S., Mobbs D., Napper L.E., Packer D.J., Pennycook G., Peters E., Petty R.E., Rand D.G., Reicher S.D., Schnall S., Shariff A., Skitka L.J., Smith S.S., Sunstein C.R., Tabri N., Tucker J.A., Linden S.V., Lange P.V., Weeden K.A., Wohl M.J.A., Zaki J., Zion S.R., Willer R. Using social and behavioural science to support COVID-19 pandemic response. Nat. Hum Behav. 2020;4(5):460–471. doi: 10.1038/s41562-020-0884-z. [DOI] [PubMed] [Google Scholar]
- 36.Dryhurst S., Schneider C.R., Kerr J., Freeman A.L.J., Recchia G., van der Bles A.M., Spiegelhalter D., van der Linden S. Risk perceptions of COVID-19 around the world. J. Risk Res. 2020;23(7–8) [Google Scholar]
- 37.Zipprich H.M., Teschner U., Witte O.W., Schonenberg A., Prell T. Knowledge, attitudes, practices, and burden during the COVID-19 pandemic in people with Parkinson’s disease in Germany. J. Clin. Med. 2020;9(6) doi: 10.3390/jcm9061643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feeney M.P., Xu Y., Surface M., Shah H., Vanegas-Arroyave N., Chan A.K., Delaney E., Przedborski S., Beck J.C., Alcalay R.N. The impact of COVID-19 and social distancing on people with Parkinson’s disease: a survey study. NPJ Parkinsons Dis. 2021;7 doi: 10.1038/s41531-020-00153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peacock D., Baumeister P., Monaghan A., Siever J., Yoneda J., Wile D. Perception of healthcare access and utility of telehealth among Parkinson's disease patients. Can. J. Neurol. Sci. 2020;47(5):700–704. doi: 10.1017/cjn.2020.99. [DOI] [PubMed] [Google Scholar]
- 40.Shivkumar V., Subramanian T., Agarwal P., Mari Z., Mestre T.A., Parkinson Study G. Uptake of telehealth in Parkinson’s disease clinical care and research during the COVID-19 pandemic. Park. Relat. Disord. 2021;86:97–100. doi: 10.1016/j.parkreldis.2021.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naito A., Wills A.M., Tropea T.F., Ramirez-Zamora A., Hauser R.A., Martino D., Turner T.H., Rafferty M.R., Afshari M., Williams K.L., Vaou O., McKeown M.J., Ginsburg L., Ezra A., Iansek R., Wallock K., Evers C., Schroeder K., DeLeon R., Yarab N., Alcalay R.N., Beck J.C. Expediting telehealth use in clinical research studies: recommendations for overcoming barriers in North America. NPJ Parkinsons Dis. 2021;7 doi: 10.1038/s41531-021-00177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beck C.A., Beran D.B., Biglan K.M., Boyd C.M., Dorsey E.R., Schmidt P.N., Simone R., Willis A.W., Galifianakis N.B., Katz M., Tanner C.M., Dodenhoff K., Aldred J., Carter J., Fraser A., Jimenez-Shahed J., Hunter C., Spindler M., Reichwein S., Mari Z., Dunlop B., Morgan J.C., McLane D., Hickey P., Gauger L., Richard I.H., Mejia N.I., Bwala G., Nance M., Shih L.C., Singer C., Vargas-Parra S., Zadikoff C., Okon N., Feigin A., Ayan J., Vaughan C., Pahwa R., Dhall R., Hassan A., DeMello S., Riggare S.S., Wicks P., Achey M.A., Elson M.J., Goldenthal S., Keenan H.T., Korn R., Schwarz H., Sharma S., Stevenson E.A., Zhu W., Parkinson I. Connect. National randomized controlled trial of virtual house calls for Parkinson disease. Neurology. 2017;89(11):1152–1161. doi: 10.1212/WNL.0000000000004357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cubo E., Gabriel-Galan J.M., Martinez J.S., Alcubilla C.R., Yang C., Arconada O.F., Perez N.M. Comparison of office-based versus home Web-based clinical assessments for Parkinson's disease. Mov. Disord. 2012;27(2):308–311. doi: 10.1002/mds.24028. [DOI] [PubMed] [Google Scholar]
- 44.Korn R.E., Wagle Shukla A., Katz M., Keenan H.T., Goldenthal S., Auinger P., Zhu W., Dodge M., Rizer K., Achey M.A., Byrd E., Barbano R., Richard I., Andrzejewski K.L., Schwarz H.B., Dorsey E.R., Biglan K.M., Kang G., Kanchana S., Rodriguez R., Tanner C.M., Galifianakis N.B. Virtual visits for Parkinson disease: a multicenter noncontrolled cohort. Neurol. Clin. Pract. 2017;7(4):283–295. doi: 10.1212/CPJ.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulroy E., Menozzi E., Lees A.J., Lynch T., Lang A.E., Bhatia K.P. Telemedicine in Movement Disorders: lecons du COVID-19. Mov. Disord. 2020;35(11):1893–1896. doi: 10.1002/mds.28297. [DOI] [PubMed] [Google Scholar]
- 46.Government of Alberta Population statistics. https://www.alberta.ca/population-statistics.aspx Accessed online December 22 2020.
- 47.Berry I., Soucy J.P.R., Tuite A., Fisman D. Open access epidemiologic data and an interactive dashboard to monitor the COVID-19 outbreak in Canada. CMAJ. 2020;192(15) doi: 10.1503/cmaj.75262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.