Abstract
Dryland degradation is a persistent and accelerating global problem. Although the mechanisms initiating and maintaining dryland degradation are largely understood, returning productivity and function through ecological restoration remains difficult. Water limitation commonly drives slow recovery rates within drylands; however, the altered biogeochemical cycles that accompany degradation also play key roles in limiting restoration outcomes. Addressing biogeochemical changes and resource limitations may help improve restoration efforts within this difficult-to-restore biome. In the present article, we present a synthesis of restoration literature that identifies multiple ways biogeochemical understandings might augment dryland restoration outcomes, including timing restoration around resource cycling and uptake, connecting heterogeneous landscapes, manipulating resource pools, and using organismal functional traits to a restoration advantage. We conclude by suggesting ways to incorporate biogeochemistry into existing restoration frameworks and discuss research directions that may help improve restoration outcomes in the world's highly altered dryland landscapes.
Restoring degraded drylands is a critical challenge for the twenty-first century. Drylands, defined by an aridity index below 0.65 and including hyper arid, arid, semiarid, and subhumid regions (Middleton and Thomas 1992), cover 45% of the Earth's land surface (Pravalie 2016) and are home to more than 2 billion people (Safriel et al. 2005). Precise estimates are difficult, but at least 20% of drylands are considered degraded (Reynolds et al. 2007a, Safriel 2009, Bestelmeyer et al. 2015), which we define as a persistent reduction in ecological productivity, biodiversity, and ecosystem services, such as soil conservation, water regulation, and forage (Safriel et al. 2005) due to land-use practices and climate change. Although the problem of dryland degradation is widely recognized (United Nations 2011), the ability to restore productivity and ecosystem services to degraded drylands has been poor (James et al. 2013); for example, seed germination and seedling survival can be as low as 5%–10% after seeding in some dryland types (Kildisheva et al. 2019).
Dryland restoration is challenging because aboveground and belowground biomass are constrained by low overall precipitation, high climate variability, and low soil fertility (Safriel et al. 2005). When degradation causes changes in the biomass or distribution of ecological communities, such as in some cases of woody-plant encroachment (Puttock et al. 2014) or annual plant invasion (Miller et al. 2012), an ecosystem's ability to retain resources (e.g., soil nutrients, moisture, native plant seeds) can be reduced (but see Archer et al. 2001, Maestre et al. 2009). This reduced capacity to retain resources can result in a feedback of resource loss that is difficult to reverse (Bestelmeyer et al. 2015). When this feedback is occuring, it is often very challenging to meet restoration goals (Monaco et al. 2012, Svejcar and Kildisheva 2017), such as returning plant and soil crust cover and soil stability (Antoninka et al. 2016, Fick et al. 2020, Havrilla et al. 2020).
In the present article, we explore the possibility that the discipline of biogeochemistry may help advance restoration goals and outcomes within dryland restoration. Biogeochemistry is defined as the biologic, geologic, and chemical processes that dictate the composition of an environment (Schlesinger and Bernhardt 2013). The simplifying principle that underlies biogeochemistry is that, within a given ecological state, essential requirements for chemical elements such as carbon (C), nitrogen (N), and phosphorus (P) are unchanging; therefore, by tracking their quantities, fluxes, chemical conversions, and ratios, constraints can be identified that lead to system-level understanding. For example, ecologists have long understood that multiple resources can limit rates of ecosystem processes such as plant growth (Rietkerk and van de Koppel 1997), whereas basic stoichiometric requirements can limit the distribution and abundance of producers, consumers, and decomposers (Güsewell 2004, Schmidt et al. 2016, Leroux et al. 2017). Although some of these concepts have been applied to restoration ecology (Suding et al. 2004), there is an opportunity to further incorporate biogeochemical understandings into dryland restoration frameworks and actions.
Biogeochemistry has the potential to help improve dryland restoration outcomes for several reasons. First, recent biogeochemical insights in drylands have illuminated important biogeochemical principles relevant to restoration (figure 1). For example, although water limitations have received the most attention in explaining productivity in drylands, other limiting resources, such as nutrients, are increasingly being recognized as important drivers in dryland productivity, species composition, and ecological processes (Austin 2011, Eskelinen and Harrison 2015). Second, biogeochemical approaches may offer insight into the difficult issue of restoration timing. Correctly timing dryland restoration efforts so they coincide with both periods of prolonged soil moisture (Hardegree et al. 2012) and nutrient availability for target organisms may aid restoration outcomes such as plant or biocrust germination and establishment (figure 2). Third, biogeochemical insights provide the opportunity to examine organismal traits that can be used and manipulated to affect biogeochemical cycling in a restoration setting. For example, the use of N-fixing biocrusts or plants can be used to increase soil N availability when increasing primary production is the restoration goal (Evans and Ehleringer 1993).
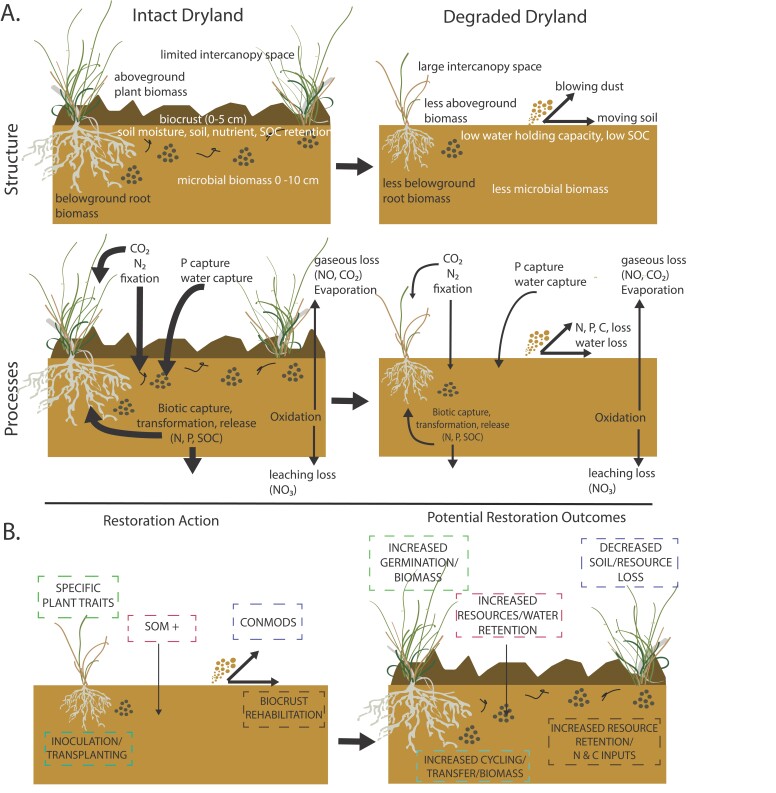
Figure 1.
Diagram depicting general biogeochemical properties within some dryland types. (a) The left panel is an example of a semiarid grassland in the absence of recent severe disturbance, which we are calling “intact.” On the right is an example of the same dryland after degradation, which we define as a reduction in productivity and ecosystem services due to land-use practices or climate change. The biogeochemical components change noticeably from the intact to degraded state and involve changes in the structure (biomass pools and organismal types) and processes (degree of nutrient, carbon, and water retention, loss, and capture). The arrow widths indicate hypothesized differences in the amount of nutrient and carbon cycling occurring and the amount of water capture or loss. Generally, degradation decreases biomass, amounts of cycling, and soil moisture retention. (b) The left panel is an example of restoration actions that can be applied to degraded drylands to jumpstart biogeochemical processes when water is available, such as adding soil organic matter (SOM +), using plant traits that allow for higher rates of germination and establishment, (specific plant traits), transplanting vascular plants and inoculating with soil microorganisms (inoculation or transplanting), adding biocrust propagules back onto the soil surface (biocrust rehabilitation), and changing resource connectivity (conmods). On the right are the potential biogeochemical outcomes of those restoration actions. Throughout this review, we highlight the importance of spatial and temporal components, multiple limitations, and organismal traits related to each restoration action and how they may increase the likelihood of the biogeochemical outcomes shown in the figure.
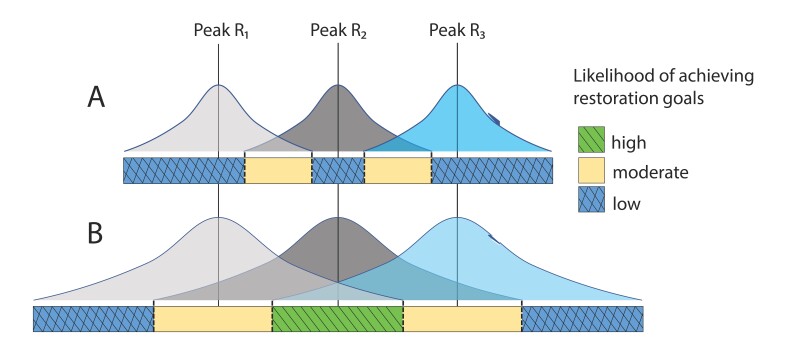
Figure 2.
Drylands can be limited by multiple resources that are heterogeneous across space and over time. (a) In areas in which the availability of limiting resources, such as water, nutrients, or organic carbon (shown as R1, R2, and R3), have limited overlap, achieving restoration goals such as increased productivity and ecosystem functioning may be more difficult (indicated as low). (b) When restoration actions increase the duration or magnitude of multiple limiting resources, the likelihood of achieving restoration goals is greater (indicated as high). For example, restoration actions that add resources such as nutrients and mulch can increase resource retention and create more overlap in resource availability when soil moisture becomes available. In addition, restoration actions that collect resources, such as ConMods, can maintain resources, such as soil nutrients, creating more overlap during periods of prolonged soil moisture.
For these reasons, incorporating a biogeochemical perspective into restoration frameworks and actions has the potential to improve restoration outcomes (but see Maestre et al. 2006); however, an examination of studies covering these topics is lacking. In the present article, we present a summary of the restoration literature that addresses biogeochemistry in degraded drylands and examine dryland biogeochemical research in a restoration context. From the literature, we identified four primary ways that restoration action addresses biogeochemistry in degraded regions: timing restoration around resource cycling and uptake, connecting heterogeneous landscapes, manipulating resource pools, and using organismal functional traits to a restoration advantage. Within each of these categories, we provide examples of how these tactics have been used in restoration and how new insights in biogeochemistry may relate to restoration action.
Timing around resource cycling and uptake
Timing restoration actions around precipitation events or periods of prolonged soil moisture is an effective way to increase plant regeneration or survival in drylands (Abbott et al. 2003), such as during monsoonal El Niño events in the US Southwest or projected times of increased or reliable soil moisture in other regions (Holmgren and Scheffer 2001, Hardegree et al. 2018). Restoration actions planned in accordance with water availability can increase the establishment of native seeds (Shriver et al. 2018), influenced vegetation recovery trajectories (Copeland et al. 2018), and may aid in the establishment of inoculated biological soil crust (biocrust; Young et al. 2019, Fick et al. 2020)—surface-dwelling photosynthetic communities that support primary production and soil stabilization in drylands (Chaudhary et al. 2009, Darrouzet-Nardi et al. 2015).
However, timing restoration action so that it occurs during periods when water and other resources are available is a strategy that has not received much attention, despite its potential impact on restoration outcomes (Seastedt and Knapp 1993, Blair 1997). Precipitation events can be decoupled from photosynthesis, N transformations, and organic matter inputs that stimulate biological responses in drylands. This decoupling is likely because of a temporal lag in nutrient cycling following precipitation pulses or a differential response to precipitation from plant or microbial functional types (Schwinning and Sala 2009, Winkler et al. 2020). During precipitation events or periods when water is less limiting, a rapid drawdown in soil nutrients can occur as plants capitalize on moisture to acquire important resources. This drawdown in nutrients may change the limiting resource from water to nutrients over relatively short time periods (Seastedt and Knapp 1993). These patterns, combined with data suggesting strong nutrient controls over dryland systems (Hooper and Johnson 1999, James et al. 2005), point to the need to plan around the availability of other limiting resources in addition to water availability when attempting to establish and maintain vegetation and biocrust communities.
Alternatively, planning restoration actions around times when resources are naturally limiting may be an effective strategy for reducing biomass of annual invasive species. For example, in the southwestern United States, nutrient limitation for exotic cheatgrass (Bromus tectorum L.) occurs during the late winter and early spring, although water limitation occurs during late spring and fall (Miller et al. 2006). In a restoration context, lowering the availability of nutrients (e.g., by adding C to stimulate microbial immobilization of N) at times when invasive species are already nutrient limited or adding soil amendments that alter soil chemistry, such as calcium dichloride and zeolite, may reduce the likelihood of increased invasion, although the effectiveness may vary with factors such as soil type and precipitation (Newingham and Belnap 2006). Planting native annual plants that reduce soil resource availability at the same time exotic annual plants are seeking the resources represents a potential restoration practice that uses a biogeochemical mechanism. Furthermore, data suggests that some bunchgrass species, which are common restoration target species, and have a stronger growth response to nitrate (NO3-) over ammonium (NH4+; Monaco et al. 2003), providing an opportunity to target N additions or sequestration strategies toward specific plant types, however, these patterns may not hold across systems (James et al. 2008). Although we know N can increase plant growth in drylands (Yahdjian et al. 2011), further research determining whether, how, and when different forms of N or other limiting resources affect restoration outcomes could provide more biogeochemically informed management options.
The timing and asynchrony of resource limitations is likely going to be more pronounced under climate change, with the potential to make dryland restoration more difficult in the future. For example, concentrations of soil organic C and total N are expected to decrease with aridity, whereas the concentrations of inorganic P are expected to rise (Delgado-Baquerizo et al. 2013). This decoupling in biogeochemical cycles is attributed to the predominant role of water and biological processes on concentrations of soil organic C and N and the predominant role of rock weathering on P concentrations. Nonlinear changes in resource availability in drylands may shift the balance of limiting resources and require additional interventions through time in the form of resource additions, such as organic matter, or resource retention methods, such as small barrier structures that serve as connectivity modifiers (ConMods; Okin et al. 2015). Predictive models that forecast multiple resource fluctuations (soil moisture, N, C, and P) under a more arid climate could aid in restoration planning and could allow practitioners to plan ahead to restore dryland ecosystems so that they are more resilient to future fluctuations in resources availability (Bradford et al. 2018).
Connecting heterogeneous landscapes
Heterogeneity, at both large and small scales, is a defining characteristic of drylands (Bestelmeyer et al. 2006). Within drylands, plant canopies are often discontinuous and soil properties vary widely from microsites to landscapes (Buxbaum and Vanderbilt 2007). With this heterogeneity come differences in soil nutrient and water content, retention, and cycling rates at multiple scales that may help or hinder restoration efforts (Prober et al. 2002, Valladares and Gianoli 2007). In some cases, restoration action seeks to turn heterogeneous landscapes into more homogeneous landscapes in which resources are spread evenly over an area. This type of intervention can combat resource accumulation in specific areas, as in the case of shrub islands that concentrate nutrients and organic C under shrubs and leave interspaces bare (Schlesinger et al. 1996). Inserting physical barriers that collect wind- and water-borne organic matter is a longstanding restoration tactic to reduce resource loss and maintain an even spread of resources (Ludwig and Tongway 1996). Implementing physical barriers, such as ConMods or straw checkerboards can interrupt connected pathways that remove litter and topsoil (Li et al. 2006, Rachal et al. 2015) and change nutrient content at the retention point (Jacobs 2015). These types of interventions have been effective at increasing plant and biocrust establishment and germination across a variety of settings (Fick et al. 2016, Peters et al. 2020).
Increasingly, restoration tactics are using background heterogeneity at a variety of scales to augment restoration action and outcomes. One such tactic is the use of restoration islands. Restoration islands are nucleated sites that require high management inputs but serve as areas of high biodiversity and functioning that can radiate out into larger areas or be connected over time (Hulvey et al. 2017). Targeting soil types, textures, and microsites that have desirable soil nutrient and water cycling characteristics in addition to other important abiotic variables such as soil depth, slope, aspect, and solar radiation levels (Breshears et al. 1997) may help practitioners plan where and when to create restoration islands. Soil characteristics were shown to strongly affect revegetation outcomes in a restored sagebrush ecosystem in Wyoming, in the United States, where soil-related variables correctly predicted revegetation performance on 82.4% of plots (Boyd and Davies 2012). Similarly, initiating restoration in depressions or vegetated areas that create physical breaks across a landscape and accumulate resources, such as plant litter or soil moisture through run-on, may increase plant regeneration (Field et al. 2012, Havrilla et al. 2020). These types of approaches may allow practitioners to take advantage of the landscape-scale resource redistribution and accumulation patterns that occur during dryland degradation (Schlesinger et al. 1990).
Heterogeneity associated with soil characteristics can also influence biocrust community presence and function (Belnap et al. 2003, Pietrasiak et al. 2011). Using background soil heterogeneity to determine where to introduce biocrust propagules may be a tool for successfully reintroducing these important ecosystem engineers (Bowker et al. 2006). Evidence suggests that soil textures, nutrient availabilities, and water-holding capacities interact to influence biocrust species presence and, ultimately, function (Williams et al. 2013, Bowker et al. 2016). For example, some biocrust types can develop more rapidly on fine fraction soils (smaller than 125 micrometers) than on coarse fraction soils (Rozenstein et al. 2014), and some biocrust species may favor specific soil micronutrients (Bowker et al. 2005). Considering these variables during restoration planning may help with the establishment of biocrust communities.
An important consideration when using background heterogeneity to plan restoration action is the influence of increasing aridity on the relationships between soil characteristics and nutrient cycling. The relationships between soil properties, N mineralization, and net primary production can change over aridity gradients. For example, soil texture's influence on N mineralization can diminish under very arid conditions because of small soil C and N pools across soil textures but increase in semiarid conditions and subhumid conditions where soil C and N pools are larger and the differences between N turnover are greater between soil textures (Austin et al. 2004). The ways in which these background biogeochemical processes may change under a future climate is an important consideration when attempting to restore plant and biocrust communities in specific locations.
Manipulating resource pools
Most forms of dryland degradation redistribute resource pools (Yates et al. 2000, Michaelides et al. 2012). In a restoration setting, whether to add nutrients or bind nutrients will depend on the ecological transition that has occurred and the ultimate restoration goal. Efforts to reintroduce nutrients to increase plant productivity in drylands have been met with mixed outcomes. Although adding N in the form of fertilizer can increase primary production (Yahdjian et al. 2011), N-specific additions can increase the dominance of undesired annual species that quickly capitalize on higher nutrient levels (Chen et al. 2017) or decrease plant species diversity (Suding et al. 2005).
The addition of organic C can have multiple applications in dryland restoration. When the restoration goal is to reduce invasive species cover, restoration projects have used C-rich soil amendments (e.g., sawdust, sugar) to reduce nutrients—specifically, N—by immobilizing nutrients within soil microbes, making them less available to exotic plants (Bleier and Jackson 2007, Perry et al. 2010, Morris and Barse, De 2013). However, this approach varies in effectiveness depending on the form of C, as well as the characteristics of the site and plant traits (Vasquez et al. 2008). In addition, sucrose addition can reduce biocrust lichen and moss cover and biocrust species richness (Chiquoine et al. 2020). When the restoration goal is soil moisture retention, adding organic C amendments, such as mulch, can increase the percentage volumetric water content and soil roughness by increasing microtopography, resulting in higher infiltration and lower rates of soil erosion (Eldridge et al. 2012, Hueso-Gonzalez et al. 2018). However, the degree to which mulch influences soil moisture can depend on the amount of mulch added, with lower mulching rates having the smallest effect (Jordán et al. 2010).
The application of organic amendments to soils, such as sewage sludge or manure, can increase plant productivity, retain soil moisture, increase soil microbial community biomass, and increase soil stability (for a meta-analysis, see Gravuer et al. 2019). However, the effect of the amendment on soil properties can vary widely depending on the amendment origin (for a review, see Hueso-Gonzalez et al. 2018). Like fertilizer additions, organic amendments run the risk of increasing undesirable or invasive plant species through increased nutrient availability (Martínez et al. 1997, Hanke et al. 2015) and may also reduce biocrust survival due to burial (Chiquoine et al. 2016). The possibility for undesirable outcomes with amendment additions, such as an increase in invasive annual plant species, highlights the need for site-specific amendment application strategies. Tailored strategies can take into account amendment type, minimum effective doses, and the possibility of using low-N amendments to reduce invasive species presence but maintain increases in plant productivity (Hueso-Gonzalez et al. 2018, Gravuer et al. 2019). Restoration outcomes may be improved through a greater understanding of the interactions between the timing, form, and amount of amendments to add to a site and their interactions with climate. Site-specific amendment recommendations attained through modeling may represent an effective way to achieve restoration goals, such as increased primary productivity and biodiversity.
Examining less frequently used amendments may be useful for manipulating specific soil properties. For example, gypsum and urea showed promise in increasing plant biomass and restoring desired soil properties such as N availability and pH in a postmining site (Bateman et al. 2019). The latter property can influence plant composition during restoration because of the limited pH tolerance of some plants and can contribute to the binding or release of essential nutrients (Costantini et al. 2016). However, benefits of these amendments decreased with water scarcity. More research is needed to determine the efficacy of these amendments as drylands continue to become more arid under climate change.
There is a clear need to better predict ecological responses to nutrient inputs in drylands on the basis of the mixed outcomes of resource additions in restoration. Beneficial future research directions include testing for thresholds of N addition or comparisons of N forms (organic N versus NH4+ versus NO3-) that could improve restoration objectives, such as biodiversity, without increasing undesired species (Bai et al. 2010). Furthermore, addressing relationships between precipitation, temperature, and nutrients in a restoration context will become more important as precipitation regimes change and aridity increases (Grossiord et al. 2018). A meta-analysis of N fertilization studies found that both water and N limit primary production in drylands, but at different times of the year, with the effect of N becoming smaller as annual precipitation decreases (Yahdjian et al. 2011). Building from these types of insights will be useful in dryland areas in which N deposition is increasing, allowing managers to begin to predict how N deposition may change plant composition and soil communities under future climates (Fenn et al. 2003, McHugh et al. 2017) and how this could affect restoration options and outcomes.
Using organismal functional traits
Plant functional traits (e.g., height, specific leaf area, seed mass) can be used to predict plant performance and measure outcomes of restoration actions (Clark et al. 2012). Across ecosystems, incorporating plant functional traits into restoration planning and predictions represents an important and growing component of ecological restoration. For example, in some mesic grasslands, traits such as competitive ability, vegetative growth, and seed bank persistence can be determinants of restoration success (Pywell et al. 2003). Efforts to match plant functional traits to environmental and biogeochemical variables in dryland settings is a potential way to maximize revegetation success (Balazs et al. 2020).
Identifying and understanding how plant functional traits affect biogeochemical cycling is an underexplored area of trait-based research (Bardgett 2017). In a degraded Mediterranean site, species with deep roots, low leaf to total photosynthetic area ratios, and N-fixing bacteria associations had the highest survival rates in nutrient poor soils, which was attributed to the species’ abilities to maximize resource uptake (Padilla et al. 2009). In southeastern Australia, the plant species Themeda australis suppressed soil NO3- concentrations and the presence of exotic annual species by producing low N litter and having high N capture through extensive root systems with year-round activity (Prober and Lunt 2009). Across geographic locations, leaf traits such as growth rate, specific leaf area, and tissue strength can affect decomposition and subsequent soil C and nutrient cycling, whereas root traits such as root length density, root depth, and specific root length can influence C inputs into soils, microbial biomass, and resource retention through reductions in erosion (see review in Bardgett 2017). Furthermore, efforts relating specific plant traits to nutrient cycling in dryland systems can provide practitioners with a greater understanding of how plant traits are going to affect restoration outcomes. Terrestrial biogeochemical and dynamic vegetation models could help these efforts by providing more links between plant traits and soil processes. However, these biogeochemical responses to plant traits are predicated on seedling survival, which currently represents a bottleneck in dryland restoration (James et al. 2011) and has been attributed to a lack of understand of plant seed traits such as dormancy and germination and their relationship with climate (Kildisheva et al. 2019).
Considering biological traits outside of plant species can also be beneficial. Specific traits within biocrust species can be used to achieve desired biogeochemical outcomes in dryland restoration (Mallen-Cooper and Eldridge 2016, Mallen‐Cooper et al. 2020). For example, many species within biocrust communities can fix N (Torres-Cruz et al. 2018) and capture airborne macro- and micronutrients through dust more readily than others (Belnap 2003, Lan et al. 2012), potentially increasing nutrient or C availability within soils (Evans and Ehleringer 1993, Barger et al. 2016). Restored cyanobacterial biocrusts can sequester C in mine waste soils (Muñoz-Rojas et al. 2018) and the composition and stage of development within some biocrust community types can alter albedo and, ultimately, the energy balance for a given area, a trait that influences soil temperature and feedbacks to local climate (Couradeau et al. 2016, Rutherford et al. 2017). Similar to selecting plant species traits, biocrust traits may be a valuable tool to enhance C sequestration, increase nutrient capture and cycling, and create microclimates to promote resource retention during restoration.
The manipulation of subsurface soil organisms and their functional traits to achieve specific restoration outcomes remains complex. Inoculating with arbuscular mycorrhizal fungi or other growth-promoting microorganisms to increase nutrient acquisition is an established practice in dryland restoration (Bashan and de-Bashan 2010, De-Bashan et al. 2012). However, outcomes can vary with the origin of the soil organisms (native or commercial varieties) and the response variables measured (Caravaca et al. 2003, Chaudhary et al. 2019). For example, mycorrhizal addition can improve plant growth but does not always improve soil quality (Alguacil et al. 2003). Important to note is that the fungal or microbial consortia associated with dryland plants are not well categorized, and many root-associated fungi show strong plant preferences, implying that adding arbuscular mycorrhizal fungi generalists to soils may not be a one-size-fits-all approach for plant success (Klironomos 2003). Most mutually beneficial mycorrhizal associations are locally adapted, and inoculation with nonlocalized fungi may affect soil microbial community composition and may hinder restoration goals (Schwartz et al. 2006).
When transplanting vascular plants or growing plants from seed, inoculating with native soil microorganisms may increase plant establishment and growth when compared with controls (Jeffries and Barea 2001, Requena et al. 2001), particularly in a warming and drying climate (Remke et al. 2020). This can be achieved through transplanting native soil into pots or transplant areas, providing a potentially cost-effective and low-consequence solution for practitioners. There are, however, many outstanding biogeochemical questions related to the relationship between vascular plants, associated soil microbes, and their functions, including questions about when microbes immobilize nutrients (Gallardo and Schlesinger 1995), when microbes move from mutualists to parasites within plants (Johnson et al. 1997), and which conditions best prime microbial activity (Blagodatskaya and Kuzyakov 2008). Answering these questions in a restoration context could bolster our ability to restore with advantageous soil organisms, in correct proportions, and at opportune times.
Relating functional traits across organisms may be important to achieving desired restoration outcomes. For example, plant traits that result in low-quality litter, such as low specific leaf area, can increase the growth of fungi relative to bacteria, slowing rates of nutrient cycling and increasing nutrient retention (Bardgett 2017), which is a common goal in dryland restoration. Restoration actions that seek to restore both biocrust and plants may want to account for the complex interactions between plant traits and biocrust traits. Biocrusts can be either a facilitator or competitor of plant species, depending on plant traits and biocrust community types (Zhang et al. 2016). For example, plants without N-fixing symbionts exhibited a more positive response to biocrusts presence than plants with N-fixing symbionts (Havrilla et al. 2019). Considering how traits within plants, biocrusts, and soil microorganisms interact to influence biogeochemical cycling and specific restoration goals is an underexplored and potentially important area of research.
Incorporating biogeochemistry into dryland restoration
In the present synthesis, we examined restoration action that addresses biogeochemistry in four primary ways: timing restoration around resource cycling and uptake, connecting heterogeneous landscapes, manipulating resource pools, and using organismal functional traits to a restoration advantage. Our overall conclusion is that specific restoration actions within each category show strong potential for achieving restoration goals, including planning restoration around periods of resource availability and cycling, using restoration islands and connectivity modifiers, adding fertilizer or organic amendments, and using trait-based restoration approaches. Another key insight is that each of these actions should be implemented in the context of resource availability at specific locations and should take into consideration resource changes through time. Because resource availability is often asynchronous in drylands, synchronizing resource availability to benefit plant and biocrust communities may be an important restoration action. Although it is complex, this type of multiresource planning may help increase resource overlap, reduce the likelihood of limitations, and aid in the establishment of plant and biocrust species.
Currently, multiple frameworks exist to help plan restoration actions and predict restoration outcomes. These include state and transition models, as well as quantitative models based on processes and mechanisms driving restoration outcomes (Reynolds et al. 2007b, James et al. 2013, Okin et al. 2015, James and Carrick 2016, Svejcar and Kildisheva 2017). Biogeochemical insights, specifically ones that aid in synchronizing multiple resources through time and across locations, may help to augment these frameworks in useful ways. For example, insights into temporal considerations and colimitation may be effectively incorporated into frameworks that address propagule dispersal and generation, plant establishment, and biocrust restoration. The temporal and spatial components of nutrient and C availability that influence plant demographic transitions can be included alongside more traditionally considered drivers, such as water and propagule availability, in restoration action. As another example, state and transition models can further incorporate soil nutrient dynamics into ecological site descriptions (Duniway et al. 2016), given the increasing evidence that drylands are often limited by nutrients. Practices that time restoration action around precipitation events, such as El Niño events in the Southwest United States (Holmgren and Scheffer 2001) can also consider manipulating additional limiting resources while planning around soil moisture availability. Adaptive and anticipatory management and concepts within “prestoration,” or planning restoration with future climate in mind, could incorporate biogeochemical concepts, such as colimitations or specific plant traits, when planning for increase aridity and precipitation uncertainty (Butterfield et al. 2017, Bradford et al. 2018, Shriver et al. 2018).
Research directions for incorporating biogeochemistry into dryland restoration
Despite the benefits of incorporating biogeochemistry into restoration frameworks and actions, there are clear gaps in our understanding of dryland biogeochemistry that need to be addressed. These important research gaps include gaining more comprehensive understandings of colimitations over space and across time, understanding interactions between limiting resources and increasing aridity, predicting plant and soil community outcomes of resource additions, and determining organismal functional traits that affect nutrient availability. Although most empirical research in this synthesis focused on manipulating resource pools and organismal traits, fewer experiments examined preexisting and manipulable temporal and spatial components into research questions, such as the seasonality of restoration or using naturally occurring pockets of high resources to begin restoration action. There is a need for more experiments addressing these questions, because the limited data suggests planning around areas and times of increased resource and soil moisture availability may be a determining factor in dryland restoration outcomes. Hypotheses such as the transient maximum hypothesis support this assertion by demonstrating that biotic responses—or a lack thereof—can often be explained by shifts in multiple limiting resources over time and across space (Seastedt and Knapp 1993, Blair 1997). Future research questions that integrate these concepts with iterative hypothesis testing and report “negative” restoration outcomes could go far in advancing our understanding of biogeochemistry in a restoration setting. In addition, concerted efforts by ecosystem ecologists to incorporate restoration components into experiments would advance understandings in both disciplines.
Many outstanding questions surround how dryland ecosystems will respond to climate change. Climate driven changes in ecosystem structure such as vegetation composition (Allen et al. 2010), biocrust cover (Ferrenberg et al. 2015), and insect and mammal distributions (Ye et al. 2018, Eldridge et al. 2020), will result in functional changes to ecosystem, such as changes in resource cycling and distribution (de Graaff et al. 2014). The novel functional ecosystems that emerge will become the baseline for predicting and gauging restoration outcomes, because concepts such as ecological reference states loose meaning under new climate regimes (Harris et al. 2006). To inform restoration action and predict restoration success, it will be essential to understand how ecosystem processes and functions affected by climate change will alter biogeochemical cycling. Specifically, long-term manipulative experiments that examine cover change and functional responses are necessary to understanding, managing, and restoring this rapidly changing biome (de Graaff et al. 2014).
Conclusions
There is an increasing need to restore productivity and ecosystem functions in global drylands. Integrating biogeochemistry into dryland restoration could be a key to achieving a variety of targeted restoration outcomes. The need to restore drylands will only grow as global change accelerates (Ye et al. 2019). Dryland regions are expected to expand over the next century (Huang et al. 2015) and face the continued pressures of climate change, accelerated land use, and species invasions (Hoover et al. 2019). The limited capacity to effectively restore dryland regions implies the need to explore new approaches to returning desired function and productivity to these socially, economically, and ecologically important regions (Reynolds et al. 2007b). Finally, the difficulties of restoring drylands and the widespread changes in basic biogeochemical structure that accompanies degradation highlights the need for sustainable use and conservation within dryland regions. These types of efforts, in addition to an increased understanding of the processes and functions occurring within intact and degraded drylands, are necessary to reduce degradation and negate the need for perpetual restoration of these valuable ecosystems.
Acknowledgments
Thanks to Eva Stricker, Brandon Bestelmeyer, and Jayne Belnap for conducting early reviews of the manuscript and to the anonymous reviewers who greatly improved the manuscript. SCR and DEW were supported by the US Geological Survey Ecosystems Mission area. DEW was also supported by the Bureau of Land Management. KEY, CC, and AD were supported by National Science Foundation grants no. 1557162 and no. 1557135. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US government. SF acknowledges support from the U.S. Department of Agriculture, National Institute of Food and Agriculture, Agriculture and Food Research Initiative under Award Number 2019-67020-29320. For additional funding, we acknowledge BUILDing SCHOLARS program: NIH RL5GM118969, TL4GM118971, and UL1GM118970.
Author Biographical
Kristina Young (keyoung1@miners.utep.edu), Catherine Cort, and Anthony Darrouzet-Nardi are affiliated with the Department of Biological Sciences at the University of Texas at El Paso, in El Paso, Texas, in the United States. Sasha Reed and Daniel Winkler are affiliated with the US Geological Survey, Southwest Biological Science Center, in Moab, Utah, in the United States. Scott Ferrenberg is affiliated with the Department of Biology at New Mexico State University, in Las Cruces, New Mexico, in the United States. Akasha Faist is affiliated with the Department of Animal and Range Sciences at New Mexico State University, in Las Cruces, New Mexico, in the United States.
Contributor Information
Kristina E Young, Department of Biological Sciences, University of Texas, El Paso, El Paso, Texas, United States.
Sasha C Reed, US Geological Survey, Southwest Biological Science Center, Moab, Utah, United States.
Scott Ferrenberg, Department of Biology, New Mexico State University, Las Cruces, New Mexico, United States.
Akasha Faist, Department of Animal and Range Sciences, New Mexico State University, Las Cruces, New Mexico, United States.
Daniel E Winkler, US Geological Survey, Southwest Biological Science Center, Moab, Utah, United States.
Catherine Cort, Department of Biological Sciences, University of Texas, El Paso, El Paso, Texas, United States.
Anthony Darrouzet-Nardi, Department of Biological Sciences, University of Texas, El Paso, El Paso, Texas, United States.
References cited
- Abbott LB, Roundy BA, Abbott LB, Roundy BA.. 2003. Available water influences field germination and recruitment of seeded grasses. Society of Range Management 56: 56–64. [Google Scholar]
- Alguacil MM, Caravaca F, Azcon R, Pera J, Diaz G, Roldan A.. 2003. Improvements in soil quality and performance of mycorrhizal Cistus albidus L. seedlings resulting from addition of microbially treated sugar beet residue to a degraded semiarid Mediterranean soil. Soil Use and Management 19: 277–283. [Google Scholar]
- Allen CD, et al. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259: 660–684. [Google Scholar]
- Antoninka A, Bowker MA, SC Reed, Doherty K.. 2016. Production of greenhouse-grown biocrust mosses and associated cyanobacteria to rehabilitate dryland soil function. Restoration Ecology 24: 324–335. [Google Scholar]
- Archer S, Boutton TW, Hibbard KA. 2001. Trees in grasslands: Biogeochemical consequences of woody plant expansion. Pages 115–1337 in Schulze E-D, Harrison S P, Heimann M, Holland EA, Lloyd J, Prentice IC, Schimel D, eds. Global Biogeochemical Cycles in the Climate System, Academic Press. [Google Scholar]
- Austin AT. 2011. Has water limited our imagination for aridland biogeochemistry? Trends in Ecology and Evolution 26: 229–235. [DOI] [PubMed] [Google Scholar]
- Austin AT, Yahdjian L, Stark JM, Belnap J, Porporato A, Norton U, Ravetta DA, Schaeffer SM.. 2004. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141: 221–235. [DOI] [PubMed] [Google Scholar]
- Bai Y, Ji Wu, Clark C, Naeem S, Pan Q, Huang J, Zhang L, Han X. 2010. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: Evidence from inner Mongolia Grasslands. Global Change Biology 16: 358–372. [Google Scholar]
- Balazs KR, Kramer AT, Munson SM, Talkington N, Still S, Butterfield BJ.. 2020. The right trait in the right place at the right time: Matching traits to environment improves restoration outcomes. Ecological Applications 30: 1–7. [DOI] [PubMed] [Google Scholar]
- Bardgett RD. 2017. Plant trait-based approaches for interrogating belowground function. Biology and Environment 117B: 1–13. [Google Scholar]
- Barger NN, Weber B, Garcia-Pichel F, Zaady E, Belnap J. 2016. Patterns and controls on nitrogen cycling of biological soil crusts. Pages 257–285 in Weber, B, Büdel, B, Belnap, J, eds. Biological Soil Crusts: An Organizing Principle in Drylands. Springer. [Google Scholar]
- Bashan Y, de-Bashan LE.. 2010. Microbial populations of arid lands and their potential for restoration of deserts. Pages 109–137 in Dion P, ed. Soil Biology and Agriculture in the Tropics. Soil Biology Series. Springer. [Google Scholar]
- Bateman AM, Erickson TE, Merritt DJ, Veneklaas EJ, Muñoz-Rojas M.. 2019. Water availability drives the effectiveness of inorganic amendments to increase plant growth and substrate quality. Catena 182: 104116. [Google Scholar]
- Belnap J. 2003. The world at your feet: Desert biological soil crusts. Frontiers in Ecology and the Environment 1: 181–189. [Google Scholar]
- Belnap J, Büdel B, Lange OL.. 2003. Biological soil crusts: Characteristics and distribution. Pages 3–30 in Belnap J, Lange O, eds. Biological Soil Crusts: Structure, Function, and Management. Springer. [Google Scholar]
- Bestelmeyer BT, Okin GS, Duniway MC, Archer SR, Sayre NF, Williamson JC, Herrick JE.. 2015. Desertification, land use, and the transformation of global drylands. Frontiers in Ecology and the Environment 13: 28–36. [Google Scholar]
- Bestelmeyer BT, Ward JP, Havstad KM.. 2006. Soil-geomorphic heterogeneity governs patchy vegetation dynamics at an arid ecotone. Ecology 87: 963–973. [DOI] [PubMed] [Google Scholar]
- Blagodatskaya E, Kuzyakov Y.. 2008. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biology and Fertility of Soils 45: 115–131. [Google Scholar]
- Blair JM. 1997. Fire, N availability, and plant response in grasslands: A test of the transient maxima hypothesis. Ecology 78: 2359–2368. [Google Scholar]
- Bleier JS, Jackson RD.. 2007. Manipulating the quantity, quality, and manner of C addition to reduce soil inorganic N and increase C4:C3 grass biomass. Restoration Ecology 15: 688–695. [Google Scholar]
- Bowker MA, Belnap J, Davidson DW, Phillips SL.. 2005. Evidence for micronutrient limitation of biological soil crusts: Importance to arid-lands restoration. Ecological Applications 15: 1941–1951. [Google Scholar]
- Bowker MA, Belnap J, Miller ME.. 2006. Spatial modeling of biological soil crusts to support rangeland assessment and monitoring. Rangeland Ecology and Management 59: 519–529. [Google Scholar]
- Bowker MA, Belnap J, Budel B, Sannier C, Pietrasiak N, Eldridge DJ, Rivera-Aguilar V.. 2016. Controls on distribution patterns of biological soil crusts at micro- to global scales. Page183 in Weber B, Büdel B, Belnap J, eds. Biological Soil Crusts: An Organizing Principle in Drylands. [Google Scholar]
- Boyd CS, Davies KW.. 2012. Spatial variability in cost and success of revegetation in a Wyoming big sagebrush community. Environmental Management 50: 441–450. [DOI] [PubMed] [Google Scholar]
- Bradford JB, Betancourt JL, Butterfield BJ, Munson SM, Wood TE.. 2018. Anticipatory natural resource science and management for a changing future. Frontiers in Ecology and the Environment 16: 295–303. [Google Scholar]
- Breshears DD, Rich Paul M, Barnes FJ, Campbell K. 1997. Overstory-imposed heterogeneity in solar radiation and soil moisture in a semiarid woodland. Ecological Applications 7: 1201–1215. [Google Scholar]
- Butterfield BJ, Copeland SM, Munson SM, Roybal CM, Wood TE.. 2017. Prestoration: Using species in restoration that will persist now and into the future. Restoration Ecology 25: S155–S163. [Google Scholar]
- Buxbaum CAZ, Vanderbilt K.. 2007. Soil heterogeneity and the distribution of desert and steppe plant species across a desert-grassland ecotone. Journal of Arid Environments 69: 617–632. [Google Scholar]
- Caravaca F, Barea JM, Palenzuela J, Figueroa D, Alguacil MM, Roldán A.. 2003. Establishment of shrub species in a degraded semiarid site after inoculation with native or allochthonous arbuscular mycorrhizal fungi. Applied Soil Ecology 22: 103–111. [Google Scholar]
- Chaudhary VB, Akland K, Johnson NC, Bowker MA.. 2019. Do soil inoculants accelerate dryland restoration? A simultaneous assessment of biocrusts and mycorrhizal fungi. Restoration Ecology 28: S115–S126. [Google Scholar]
- Chaudhary VB, Bowker MA, Dell TEO, Grace JB, Redman E, Rillig MC, Johnson NC.. 2009. Untangling the biological contributions to soil stability in semiarid shrublands. Ecological Applications 19: 110–122. [DOI] [PubMed] [Google Scholar]
- Chen Q, Hooper DU, Li H, Ying X, Fei G. 2017. Effects of resource addition on recovery of production and plant functional composition in degraded semiarid grasslands. Oecologia 184: 13–24. [DOI] [PubMed] [Google Scholar]
- Chiquoine LP, Abella SR, Bowker MA.. 2016. Rapidly restoring biological soil crusts and ecosystem functions in a severely disturbed desert ecosystem. Ecological Applications 26: 1260–1272. [DOI] [PubMed] [Google Scholar]
- Chiquoine LP, Abella SR, Greenwood JL, DeCorte A.. 2020. Unexpected side effects in biocrust after treating non-native plants using carbon addition. Restoration Ecology 28: S32–S44. [Google Scholar]
- Clark DL, Wilson M, Roberts R, Dunwiddie PW, Stanley A, Kaye TN.. 2012. Plant traits: A tool for restoration? Applied Vegetation Science 15: 449–458. [Google Scholar]
- Copeland SM, Munson SM, Butterfield BJ, Bradford JB.. 2018. Influence of climate, post-treatment weather extremes, and soil factors on vegetation recovery after restoration treatments in the southwestern US. Applied Vegetation Science 85–95. [Google Scholar]
- Costantini EAC, Branquinho C, Nunes A, Schwilch G, Stavi I, Valdecantos A, Zucca C.. 2016. Soil indicators to assess the effectiveness of restoration strategies in dryland ecosystems. Solid Earth 7: 397–414. [Google Scholar]
- Couradeau E, Karaoz U, Lim HC, Nunes da Rocha U, Northen T, Brodie E, Garcia-Pichel F.. 2016. Bacteria increase arid-land soil surface temperature through the production of sunscreens. Nature Communications 7: 10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrouzet-Nardi A, Reed SC, Grote EE, Belnap J.. 2015. Observations of net soil exchange of CO2 in a dryland show experimental warming increases carbon losses in biocrust soils. Biogeochemistry 126: 363–378. [Google Scholar]
- De-Bashan LE, Hernandez J-P, Bashan Y.. 2012. The potential contribution of plant growth-promoting bacteria to reduce environmental degradation: A comprehensive evaluation. Applied Soil Ecology 61: 171–189. [Google Scholar]
- Delgado-Baquerizo M, et al. 2013. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 502: 672–676. [DOI] [PubMed] [Google Scholar]
- Duniway MC, Nauman TW, Johanson JK, Green S, Miller ME, Williamson JC, Bestelmeyer BT.. 2016. Generalizing ecological site concepts of the Colorado plateau for landscape-level applications. Rangelands 38: 342–349. [Google Scholar]
- Eldridge DJ, Oliver I, Val J, Travers SK, Delgado-Baquerizo M.. 2020. Grazing and aridity have contrasting effects on the functional and taxonomic diversity of ants .Basic and Applied Ecology 48: 73–82. [Google Scholar]
- Eldridge JD, Redente EF, Paschke M.. 2012. The use of seedbed modifications and wood chips to accelerate restoration of well pad sites in Western Colorado, U.S.A. Restoration Ecology 20: 524–531. [Google Scholar]
- Eskelinen A, Harrison SP.. 2015. Resource colimitation governs plant community responses to altered precipitation. Proceedings of the National Academy of Sciences 112: 13009–13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans ARD, Ehleringer JR.. 1993. A break in the nitrogen cycle in aridlands? Evidence from δ 15N of soils. Oecologia 94: 314–317. [DOI] [PubMed] [Google Scholar]
- Fenn ME, et al. 2003. Ecological effects of nitrogen deposition in the western United States. BioScience 53: 404–420. [Google Scholar]
- Ferrenberg S, Reed SC, Belnap J.. 2015. Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proceedings of the National Academy of Sciences 112: 12116–12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick SE, Day N, Duniway MC, Hoy-Skubik S, Barger NN.. 2020. Microsite enhancements for soil stabilization and rapid biocrust colonization in degraded drylands. Restoration Ecology 28: S139–S149. [Google Scholar]
- Fick SE, Decker C, Duniway MC, Miller ME.. 2016. Small-scale barriers mitigate desertification processes and enhance plant recruitment in a degraded semiarid grassland. Ecosphere 7: 1–16. [Google Scholar]
- Field JP, Breshears DD, Whicker JJ, Zou CB.. 2012. Sediment capture by vegetation patches: Implications for desertification and increased resource redistribution. Biogeosciences 117: 1–9. [Google Scholar]
- Gallardo A, Schlesinger WH.. 1995. Factors Determining Soil Microbial Biomass and Nutrient Immobilization in Desert Soils. Biogeochemistry 28: 55–68. [Google Scholar]
- de Graaff MA, Throop HL, Verburg PSJ, Arnone JA, Campos X.. 2014. A synthesis of climate and vegetation cover effects on biogeochemical cycling in shrub-dominated drylands. Ecosystems 17: 931–945. [Google Scholar]
- Gravuer K, Gennet S, Throop HL.. 2019. Organic amendment additions to rangelands: A meta-analysis of multiple ecosystem outcomes. Global Change Biology 25: 1152–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossiord C, et al. 2018. Reductions in tree performance during hotter droughts are mitigated by shifts in nitrogen cycling. Plant Cell and Environment 41: 2627–2637. [DOI] [PubMed] [Google Scholar]
- Güsewell S. 2004. N:P ratios in terrestrial plants: Variation and functional significance. New Phytologist 164: 243–266. [DOI] [PubMed] [Google Scholar]
- Hanke W, Wesuls D, Schmiedel U. 2015. Tradeoffs in the rehabilitation of a Succulent Karoo. Land Degradation and Development 842: 833–842. [Google Scholar]
- Hardegree SP, Abatzoglou JT, Brunson MW, Germino MJ, Hegewisch KC, Moffet CA, Pilliod DS, Roundy BA, Boehm AR, Meredith GR.. 2018. Weather-centric rangeland revegetation planning. Rangeland Ecology and Management 71: 1–11. [Google Scholar]
- Hardegree SP, Schneider JM, Moffet CA.. 2012. Weather variability and adaptive management for rangeland restoration. Rangelands 34: 53–56. [Google Scholar]
- Harris JA, Hobbs RJ, Higgs E, Aronson J.. 2006. Ecological restoration and global climate change. Restoration Ecology 14: 170–176. [Google Scholar]
- Havrilla CA, et al. 2019. Towards a predictive framework for biocrust mediation of plant performance: A meta-analysis. Journal of Ecology 107: 2789–2807. [Google Scholar]
- Havrilla CA, Munson SM, McCormick ML, Laushman KM, Balazs KR, Butterfield BJ.. 2020. RestoreNet: An emerging restoration network reveals controls on seeding success across dryland ecosystems. Journal of Applied Ecology 57: 11 2191–2202. [Google Scholar]
- Holmgren M, Scheffer M.. 2001. El Niño as a window of opportunity for the restoration of degraded arid ecosystems. Ecosystems 4: 151–159. [Google Scholar]
- Hooper DU, Johnson L. 1999. Nitrogen limitation in dryland ecosystems: Responses to geographical and temporal variation in precipitation. Biogeochemistry 46: 247–293. [Google Scholar]
- Hoover D, Bestelmeyer B, Grimm N, Huxman T, Reed S, Sala O, Seastedt T, Wilmer H, Ferrenberg S. 2019. Traversing the wasteland: A framework for assessing ecological threats to drylands. BioScience 70: 35–47. [Google Scholar]
- Huang J, Yu H, Guan X, Wang G, Guo R.. 2015. Accelerated dryland expansion under climate change. Nature Climate Change 6: 166–171. [Google Scholar]
- Hueso-Gonzalez P, Munoz-Rojas M, Martinez-Murillo JF.. 2018. The role of organic amendments in drylands restoration. Current Opinion in Environmental Science and Health 5: 1–6. [Google Scholar]
- Hulvey KB, Leger EA, Porensky LM, Roche LM, Veblen KE, Fund A, Shaw J, Gornish ES.. 2017. Restoration islands: A tool for efficiently restoring dryland ecosystems? Restoration Ecology 25: S124–S134. [Google Scholar]
- Jacobs BF. 2015. Restoration of degraded transitional (piñon–juniper) woodland sites improves ecohydrologic condition and primes understory resilience to subsequent disturbance. Ecohydrology 8: 1417–1428. [Google Scholar]
- James JJ, Carrick PJ.. 2016. Toward quantitative dryland restoration models. Restoration Ecology 24: S85–S90. [Google Scholar]
- James JJ, Davies KW, Sheley RL, Aanderud ZT.. 2008. Linking nitrogen partitioning and species abundance to invasion resistance in the Great Basin. Oecologia 156: 637–648. [DOI] [PubMed] [Google Scholar]
- James JJ, Sheley RL, Erickson T, Rollins KS, Taylor H, Dixon KW.. 2013. A systems approach to restoring degraded drylands. Journal of Applied Ecology 50: 730–739. [Google Scholar]
- James JJ, Svejcar TJ, Rinella MJ.. 2011. Demographic processes limiting seedling recruitment in arid grassland restoration. Journal of Applied Ecology 48: 961–969. [Google Scholar]
- James JJ, Tiller RL, Richards JH.. 2005. Multiple resources limit plant growth and function in a saline-alkaline desert community. Journal of Ecology 93: 113–126. [Google Scholar]
- Jeffries P, Barea JM.. 2001. Arbuscular Mycorrhiza: A key component of sustainable plant–soil ecosystems. Pages 95–113 in Hock B, ed. Fungal Associations. Springer. [Google Scholar]
- Johnson BYNC, Graham JH, Smith FA.. 1997. Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytologist 135: 575–585. [Google Scholar]
- Jordán A, Zavala LM, Gil J. 2010. Effects of mulching on soil physical properties and runoff under semi-arid conditions in southern Spain. Catena 81: 77–85. [Google Scholar]
- Kildisheva OA, Erickson TE, Madsen MD, Dixon KW, Merritt DJ.. 2019. Seed germination and dormancy traits of forbs and shrubs important for restoration of North American dryland ecosystems. Plant Biology 21: 458–469. [DOI] [PubMed] [Google Scholar]
- Klironomos JN. 2003. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84: 2292–2301. [Google Scholar]
- Lan S, Wu L, Zhang D, Hu C.. 2012. Successional stages of biological soil crusts and their microstructure variability in Shapotou region (China). Environmental Earth Sciences 65: 77–88. [Google Scholar]
- Leroux SJ, et al. 2017. Stoichiometric distribution models: Ecological stoichiometry at the landscape extent. Ecology Letters 20: 1495–1506. [DOI] [PubMed] [Google Scholar]
- Li XR, Xiao HL, He MZ, Zhang JG.. 2006. Sand barriers of straw checkerboards for habitat restoration in extremely arid desert regions. Ecological Engineering 28: 149–157. [Google Scholar]
- Ludwig JA, Tongway DJ.. 1996. Rehabilitation of semiarid landscapes in Australia. II. Restoring vegetation patches. Restoration Ecology 4: 398–406. [Google Scholar]
- Maestre FT, et al. 2009. Shrub encroachment can reverse desertification in semi-arid Mediterranean grasslands. Ecology Letters 12: 930–941. [DOI] [PubMed] [Google Scholar]
- Maestre FT, Cortina J, Vallejo R.. 2006. Are ecosystem composition, structure, and functional status related to restoration success? A test from semiarid Mediterranean steppes. Restoration Ecology 14: 258–266. [Google Scholar]
- Mallen-Cooper M, Eldridge DJ.. 2016. Laboratory-based techniques for assessing the functional traits of biocrusts. Plant and Soil 406: 131–143 [Google Scholar]
- Mallen-Cooper M, Bowker MA, Antoninka AJ, Eldridge DJ.. 2020. A practical guide to measuring functional indicators and traits in biocrusts. Restoration Ecology 28: S56–S66. [Google Scholar]
- Martínez F, Cuevas G, Calvo R, Walter I.. 1997. Biowaste effects on soil and native plants in a semiarid ecosystem. Journal of environmental quality 32: 472–479. [DOI] [PubMed] [Google Scholar]
- McHugh TA, Morrissey EM, Mueller RC, Gallegos-Graves LV, Kuske CR, Reed SC.. 2017. Bacterial, fungal, and plant communities exhibit no biomass or compositional response to two years of simulated nitrogen deposition in a semiarid grassland. Environmental Microbiology 19: 1600–1611. [DOI] [PubMed] [Google Scholar]
- Michaelides K, Lister D, Wainwright J, Parsons AJ. 2012. Linking runoff and erosion dynamics to nutrient fluxes in a degrading dryland landscape. Biogeosciences 117: 2012JG002071. [Google Scholar]
- Middleton NJ, Thomas DSG, eds. 1992. World Atlas of Desertification. Edward Arnold. [Google Scholar]
- Miller ME, Belnap J, Beatty SW, Reynolds RL.. 2006. Performance of Bromus tectorum L. in relation to soil properties, water additions, and chemical amendments in calcareous soils of southeastern Utah, USA. Plant and Soil 288: 1–18. [Google Scholar]
- Miller ME, Bowker MA, Reynolds RL, Goldstein HL. 2012. Post-fire land treatments and wind erosion: Lessons from the Milford Flat Fire, UT, USA. Aeolian Research 7: 29–44. [Google Scholar]
- Monaco TA, Johnson DA, Norton JM, Jones TA, Connors KJ, Norton JB, Redinbaugh MB.. 2003. Contrasting responses of Intermountain West grasses to soil nitrogen. Journal of Range Management 56: 282–290. [Google Scholar]
- Monaco TA, Jones TA, Thurow TL.. 2012. Identifying rangeland restoration targets: An appraisal of challenges and opportunities. Rangeland Ecology and Management 65: 599–605. [Google Scholar]
- Morris EC, Barse De M. 2013. Carbon, fire and seed addition favour native over exotic species in a grassy woodland. Austral Ecology 38: 413–426. [Google Scholar]
- Muñoz-Rojas M, Román JR, Roncero-ramos B, Erickson TE, Merritt DJ.. 2018. Cyanobacteria inoculation enhances carbon sequestration in soil substrates used in dryland restoration. Science of the Total Environment 636: 1149–1154. [DOI] [PubMed] [Google Scholar]
- Newingham BA, Belnap J.. 2006. Direct effects of soil amendments on field emergence and growth of the invasive annual grass Bromus tectorum L. and the native perennial grass Hilaria jamesii (Torr.) Benth. Plant and Soil 280: 29–40. [Google Scholar]
- Okin GS, De Las Heras MM, Saco PM, Throop HL, Vivoni ER, Parsons AJ, Wainwright J, Peters DPC.. 2015. Connectivity in dryland landscapes: Shifting concepts of spatial interactions. Frontiers in Ecology and the Environment 13: 20–27. [Google Scholar]
- Padilla FM, Ortega R, Sánchez J, Pugnaire FI.. 2009. Rethinking species selection for restoration of arid shrublands. Basic and Applied Ecology 10: 640–647. [Google Scholar]
- Perry LG, Blumenthal DM, Monaco TA, Paschke MW, Redente EF.. 2010. Immobilizing nitrogen to control plant invasion. Oecologia 163: 13–24. [DOI] [PubMed] [Google Scholar]
- Peters DPC, Okin GS, Herrick JE, Savoy HM, Anderson JP, Scroggs SLP, Zhang J.. 2020. Modifying connectivity to promote state change reversal: The importance of geomorphic context and plant–soil feedbacks. Ecology 101: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrasiak N, Johansen JR, Drenovsky RE.. 2011. Geologic composition influences distribution of microbiotic crusts in the Mojave and Colorado Deserts at the regional scale. Soil Biology and Biochemistry 43: 967–974. [Google Scholar]
- Pravalie R. 2016. Drylands extent and environmental issues: A global approach. Earth-Science Reviews 161: 259–278. [Google Scholar]
- Prober SM, Lunt ID.. 2009. Restoration of Themeda australis swards suppresses soil nitrate and enhances ecological resistance to invasion by exotic annuals. Biological Invasion 11: 171–181. [Google Scholar]
- Prober SM, Lunt ID, Thiele KR.. 2002. Determining reference conditions for management and restoration of temperate grassy woodlands: Relationships among trees, topsoils and understorey flora in little-grazed remnants. Australian Journal of Botany 50: 687–697. [Google Scholar]
- Puttock A, Dungait JAJ, Macleod CJA, Bol R, Brazier RE.. 2014. Woody plant encroachment into grasslands leads to accelerated erosion of previously stable organic carbon from dryland soils. Biogeosciences 119: 2345–2357. [Google Scholar]
- Pywell RF, Bullock JM, Roy DB, Man LIZWAR, Walker KJ, Rothery P.. 2003. Plant traits as predictors of performance in ecological restoration. Journal of Applied Ecology 40: 65–77. [Google Scholar]
- Rachal DM, Okin GS, Alexander C, Herrick JE, Peters DPC.. 2015. Modifying landscape connectivity by reducing wind driven sediment redistribution, Northern Chihuahuan Desert, USA. Aeolian Research 17: 129–137. [Google Scholar]
- Remke MJ, Hoang T, Kolb T, Gehring C, Johnson NC, Bowker MA.. 2020. Familiar soil conditions help Pinus ponderosa seedlings cope with warming and drying climate. Restoration Ecology 28: S344–S354. [Google Scholar]
- Requena N, Perez-Solis E, Azcón-Aguilar C, Jeffries P, Barea JM.. 2001. Management of indigenous plant–microbe symbioses aids restoration of desertified ecosystems. Applied and Environmental Microbiology 67: 495–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JF, Maestre FT, Kemp PR, Stafford-Smith DM, Lambin E.. 2007a. Natural and human dimensions of land degradation in drylands: Causes and consequences. Pages 247–257 in Canadell JG, Pataki DE, Pitelka LF, eds. Terrestrial Ecosystems in a Changing World. Global Change: The IGBP Series. Springer. [Google Scholar]
- Reynolds JF, et al. 2007b. Global desertification: Building a science for dryland development. Science 316: 847–851. [DOI] [PubMed] [Google Scholar]
- Rietkerk M, van de Koppel J.. 1997. Alternate stable states and threshold effects in semi-arid grazing systems. Oikos 79: 69–76. [Google Scholar]
- Rozenstein O, Zaady E, Katra I, Karnieli A, Adamowski J, Yizhaq H.. 2014. The effect of sand grain size on the development of cyanobacterial biocrusts. Aeolian Research 15: 217–226. [Google Scholar]
- Rutherford WA, Painter TH, Ferrenberg S, Belnap J, Okin GS, Flagg C, Reed SC.. 2017. Albedo feedbacks to future climate via climate change impacts on dryland biocrusts. Scientific Reports 7: 44188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safriel UN. 2009. Status of Desertification in the Mediterranean Region. Pages 33–73 in Rubio JL, Safriel U, Daussa R, Blum W, Pedrazzini F, eds. Water Scarcity, Land Degradation and Desertification in the Mediterranean Region. NATO Science for Peace and Security Series C: Environmental Security. Springer. [Google Scholar]
- Safriel U, et al. 2005. Dryland systems. Pages 623–662 in Watson RT, et al., eds. Ecosystems and Human Well-Being: Current State and Trends. Island Press. [Google Scholar]
- Schlesinger WH, Bernhardt ES.. 2013. Biogeochemistry: An Analysis of Global Change, 3rd ed.Academic Press. [Google Scholar]
- Schlesinger WH, Raikes JA, Hartley AE, Cross AF.. 1996. On the spatial pattern of soil nutrients in desert ecosystems. Ecology 77: 364–374. [Google Scholar]
- Schlesinger WH, Reynolds JF, Cunningham GL, Huenneke LF, Jarrell WM, Virginia RA, Whitford WG.. 1990. Biological feedbacks in global desertification. Science 247: 1043–1048. [DOI] [PubMed] [Google Scholar]
- Schmidt SK, Porazinska D, Concienne BL, Darcy JL, King AJ, Nemergut DR.. 2016. Biogeochemical stoichiometry reveals P and N limitation across the post-glacial landscape of Denali National Park, Alaska. Ecosystems 19: 1164–1177. [Google Scholar]
- Schwartz MW, Hoeksema JD, Gehring CA, Johnson NC, Klironomos JN, Abbott LK, Pringle A.. 2006. The promise and the potential consequences of the global transport of mycorrhizal fungal inoculum. Ecology Letters 9: 501–515. [DOI] [PubMed] [Google Scholar]
- Schwinning S, Sala OE.. 2009. Hierarchy of responses to resource pulses in arid and semi-arid ecosystems. Oecologia 139: 459–466. [DOI] [PubMed] [Google Scholar]
- Seastedt TR, Knapp AK. 1993. Consequences of nonequilibrium resource availability across multiple time scales: The transient maxima hypothesis. American Society of Naturalists 141: 621–633. [DOI] [PubMed] [Google Scholar]
- Shriver RK, Andrews CM, Pilliod DS, Arkle RS, Welty JL, Germino MJ, Duniway MC, DA Pyke, Bradford JB.. 2018. Adapting management to a changing world: Warm temperatures, dry soil, and interannual variability limit restoration success of a dominant woody shrub in temperate drylands. Global Change Biology 24: 4972–4982. [DOI] [PubMed] [Google Scholar]
- Suding KN, Collins SL, Gough L, Clark C, Cleland EE, Gross KL, Milchunas DG, Pennings S. 2005. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proceedings of the National Academy of Sciences 102: 4387–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suding KN, Gross KL, Houseman GR.. 2004. Alternative states and positive feedbacks in restoration ecology. Trends in Ecology and Evolution 19: 46–53. [DOI] [PubMed] [Google Scholar]
- Svejcar LN, Kildisheva OA.. 2017. The age of restoration: Challenges presented by dryland systems. Plant Ecology 218: 1–6. [Google Scholar]
- Torres-Cruz TJ, Howell AJ, Reibold RH, McHugh TA, Eickhoff MA, Reed SC.. 2018. Species-specific nitrogenase activity in lichen-dominated biological soil crusts from the Colorado Plateau, USA. Plant and Soil 429: 113–125. [Google Scholar]
- United Nations . 2011. Global Drylands: A UN System-Wide Response. United Nations. [Google Scholar]
- Valladares F, Gianoli E.. 2007. How much ecology do we need to know to restore Mediterranean ecosystems? Restoration Ecology 15: 363–368. [Google Scholar]
- Vasquez AE, Sheley R, Svejcar T, Vasquez E, Sheley R, Svejcar T.. 2008. Creating invasion resistant soils via nitrogen management. Invasive Plant Science and Management 1: 304–314. [Google Scholar]
- Williams AJ, Buck BJ, Soukup DA, Merkler DJ.. 2013. Geomorphic controls on biological soil crust distribution: A conceptual model from the Mojave Desert (USA). Geomorphology 195: 99–109. [Google Scholar]
- Winkler DE, Belnap J, Duniway MC, Hoover D, Reed SC, Yokum H, Gill R.. 2020. Seasonal and individual event-responsiveness are key determinants of carbon exchange across plant functional types. Oecologia 193: 811–825. [DOI] [PubMed] [Google Scholar]
- Yahdjian L, Gherardi L, Sala OE.. 2011. Nitrogen limitation in arid-subhumid ecosystems: A meta-analysis of fertilization studies. Journal of Arid Environments 75: 675–680. [Google Scholar]
- Yates CJ, Norton DA, Hobbs RJ.. 2000. Grazing effects on plant cover, soil and microclimate in fragmented woodlands in south-western Australia: Implications for restoration. Austral Ecology 25: 36–47. [Google Scholar]
- Ye JS, Delgado-Baquerizo M, Soliveres S, Maestre FT.. 2019. Multifunctionality debt in global drylands linked to past biome and climate. Global Change Biology 25: 2152–2161. [DOI] [PubMed] [Google Scholar]
- Ye X, Yu X, Yu C, Tayibazhaer A, Xu F, Skidmore A, Wang T.. 2018. Impacts of future climate and land cover changes on threatened mammals in the semi-arid Chinese Altai Mountains. Science of the Total Environment 612: 775–787. [DOI] [PubMed] [Google Scholar]
- Young KE, Bowker MA, Reed SC, Duniway MC, Belnap J.. 2019. Temporal and abiotic fluctuations may be preventing successful rehabilitation of soil-stabilizing biocrust communities. Ecological Applications 29: 5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Aradottir AL, Serpe M, Boeken B.. 2016. Interactions of biological soil crusts with vascular plants. Pages 385–406 in Weber B, Büdel B, Belnap J, eds. Biological Soil Crusts: An Organizing Principle in Drylands. Springer. [Google Scholar]