Abstract
Background and Objectives:
Group B streptococcus (GBS) can cause severe and invasive infections in pregnant women, infants, and adults. This study aimed to investigate the risk factors of GBS colonization in pregnant women and determine the macrolide resistance and capsular type of isolates.
Materials and Methods:
In a cross-sectional study, a total of 200 pregnant women were screened for GBS colonization by phenotypic methods. Antibiotic susceptibility pattern of colonizing isolates and ermB, ermTR, mefA/E genes were detected. Also, molecular capsular types of isolates were distinguished.
Results:
The overall prevalence of colonization of participates with GBS was 13.5%. Statistical analysis showed that there was no association between risk factors and colonization with GBS. The highest resistance was observed to erythromycin (44.4%) followed by clindamycin (29.6%), penicillin, ampicillin, and ceftriaxone (18.5%), levofloxacin (11.1%), and 29.6% isolates were multidrug-resistant. ermTR and mefA/E genes were detected in 37% and 11.1% isolates; respectively and the ermB gene was not detected. The most common capsular type was type Ib (44.4%) followed by type III (40.7%), type II (11.1), and type Ia (3.7%).
Conclusion:
In the present study, the prevalence of GBS was in the medium range. Resistance to key antibiotic agents was relatively high. Also, capsular serotype Ib was the predominant serotype, which emphasizes the importance of monitoring the molecular typing of the GBS isolates regularly.
Keywords: Streptococcus agalactiae, Drug resistance, Pregnant women, Bacterial capsules, Iran
INTRODUCTION
Streptococcus agalactiae is known as group B streptococcus (GBS) and is usually found subclinically in the gastrointestinal tract and genital system. However, this pathogen can cause severe and invasive infections such as bacteremia, urinary tract infections (UTIs), chorioamnionitis, endometritis, puerperal sepsis, meningitis, and septic thrombophlebitis in pregnant women, infants, and adults (1–3). Indeed, 30% of pregnant women are colonized by GBS, and mother-to-child transmission can occur 29 times higher in colonized mothers than non-colonized (4, 5). According to the centers for disease control and prevention (CDC) guidelines, all pregnant women should be screened for GBS recto-vaginal infections at 35–37 weeks of gestation to receive prophylactic antibiotic therapies. While successful intrapartum antibiotic prophylaxis is performed, the incidence of GBS infection in infants will be reduced by 86 to 89% (6, 7). Beta-lactam antibiotics such as penicillin and ampicillin are considered as the first choice for prophylaxis and treatment of GBS infections. However, observation of resistance to beta-lactam antibiotics and penicillin-allergy caused to approach other antibiotics such as macrolides and lincosamides. During the last decade, resistance to these agents also has frequently reported (8). Resistance to macrolide-lincosamide-streptogramin B (MLSB) in GBS strains is commonly caused by ermA (subclass TR) and ermB genes. The mechanism which gives resistance to these antibiotics is the modification of the target site (methylation of the 23SrRNA binding site) (9). Also, the production of macrolide efflux pumps mediated by the mef (A and E) gene leads to the macrolide-only (M) resistance phenotype (9). Polysaccharide capsule as a virulence factor is an anti-phagocytic and immune evasion element in GBS pathogenicity and can be an important target for the development of the vaccine. Until now, GBS are clustered into ten serotypes (Ia, Ib, and II–IX, dominated Ia, Ib, II, III, and V) according to the capsular antigens (10). Regarding the importance of the issue, in this survey, we aimed to determine the macrolide resistance, the capsular genotyping, and the risk factors of GBS colonization in pregnant women.
MATERIALS AND METHODS
Study design.
This cross-sectional study was conducted in Isfahan city, Iran, from April to November 2017 and was approved by the Ethics Committee of Isfahan University of Medical Sciences (project number: 293322). All participants filled and signed an informed consent form.
The targeted population was pregnant women with gestational age 35 to 37 weeks that attending Al-Zahra and Shahid Beheshti Hospital clinics for a routine clinical checkup. In our country, routine GBS screening for pregnant women is not established yet. During the study, a total of 200 pregnant women were enrolled. Persons with a history of antibiotic use during the last 4 weeks, acute and severe diseases such as diabetes mellitus, women who had used traditional disinfection techniques like vinegar bath in the ten days before sampling, and patients who have been banned from vaginal tests were excluded from this survey. A checklist including age, number of previous pregnancies (parity), history of previous abortion, history of preterm delivery, history of urinary tract infection (UTI) during the current pregnancy, and the educational level (primary, secondary and higher education) of the pregnant women were filled by researchers to investigate the associated GBS maternal colonization risk factors.
Sample collection and bacterial identification.
Two sterile cotton swabs, one from the vaginal (the lower third vagina) and the other from the rectum, were collected from each participant according to CDC (Centers for Disease Control and Prevention) guideline (7) and placed separately in the Amies transport medium (HiMedia, India) without charcoal and transmitted within 4 hours to the bacteriological laboratory of Isfahan Infectious Diseases and Tropical Medicine Research Center. If any of the vaginal and/or rectal swab cultures were positive, that participate would be considered colonized.
All swabs were transferred to Trans-Vag media as a selective and enrichment medium containing: Todd Hewitt Broth (India, HiMedia) supplemented with 8 μg/ml gentamicin and 15 μg/ml nalidixic acid. After 24 h incubation at 37°C, the enriched colonies were streaked on the defibrinated sheep blood agar (DSBA). After 24 hours, all colonies were investigated by Gram staining, catalase test, CAMP reaction, sodium hippurate hydrolysis test, and cfb gene amplification by PCR (GenBank: X72754.1) with 154 bp product size (11). Gram-positive colonies with negative catalase, positive CAMP reaction, positive sodium hippurate hydrolysis test, and positive PCR result for the cfb gene were considered as GBS.
Antimicrobial susceptibility test.
Antimicrobial susceptibility testing for confirmed GBS isolates was carried out using the Kirby-Bauer method. The following antibiotics were tested: penicillin (10Units), ampicillin (10 μg), ceftriaxone (30 μg), vancomycin (30 μg), erythromycin (15 μg), clindamycin (2 μg), and levofloxacin (5 μg) (UK, Mast). Performance and interpretation were done according to the Clinical and Laboratory Standards Institute (CLSI) guideline 2017 (12). We used the double-disc diffusion testing (Inducible Clindamycin Resistance; D-zone) to identify the cMLS, iMLS, M phenotype, and L phenotype (lincosamides-resistance) phenotypes. The interpretation was performed according to CLSI 2017 guideline (12).
Molecular assays.
All GBS isolates were screened by multiplex PCR for detection of the known GBS capsular serotypes (Ia, Ib, and II–IX) as described by Imperi et al. (13) and ermB, ermTR, mefA/E antibiotic resistance genes (14–16).
DNA extraction was done using the YTA Genomic DNA Extraction Mini Kit (Yekta Tajhiz Azma, Iran), according to the manufacturer’s instructions.
Statistical analysis.
SPSS software version 20 (SPSS Inc., Chicago, IL, USA) was used to analyze the results. Descriptive statistics were used to summarize the pregnant women’s characteristics. Bi-variable and multi-variable logistic regression analysis was applied to investigate the association between the risk factors and colonization. A P-Value of <0.05 was considered statistically significant.
RESULTS
The mean age of participated pregnant women was 31.1 ± 5.7 years (ranging from 18 to 42), and 16.5% (33/200) of them had academic education. Seventy-nine (39.5%) participates were experiencing their first pregnancy, 18% (36/200) had a history of previous abortion, 13.5% (27/200) had a preterm delivery, and 13.5% (27/200) had a history of UTI during the pregnancy.
The overall prevalence of colonization of participates with GBS was 27/200 (13.5% (95% CI 9.1–19)). Among the 27 pregnant women who were colonized with GBS, 10 (37%) women were colonized in the vaginal area, 7 (26%) women in the rectal area, and 10 (37%) women in both areas. In Fig. 1. cfb gene PCR for 4 isolates was presented. Binary and multivariable logistic regression analysis showed that there was no association between risk factors and colonization with GBS (Table 1).
Fig. 1.
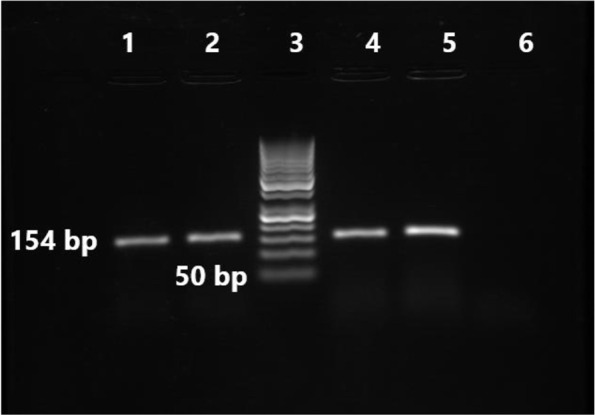
Gel electrophoresis results of cfb gene PCR for three GBS isolates.
Lanes 1, 2, and 4: isolates with cfb gene in 154 bp. Lane 3: 50 bp DNA ladder (Iran, Sinaclon), Lane 5: positive control (S. agalactiae, ATCC 49619). Lane 6: negative control (distilled water).
Table 1.
Comparison of associated factors among colonized and non-colonized pregnant women with GBS
| Associated factors | Colonized cases | Non-colonized cases | P-value | OR | Confidence interval 95% | |
|---|---|---|---|---|---|---|
| Mean age | 30.04 ± 6.334 | 31.36 ± 5.652 | 0.428 | 1.031 | 0.956–1.112 | |
| Number of previous pregnancies | one | 14 | 65 | 0.243 | 1.702 | 0.697–4.156 |
| Two or more | 13 | 108 | ||||
| History of Abortion | Yes | 7 | 29 | 0.103 | 2.319 | 0844–6.370 |
| No | 20 | 144 | ||||
| History of UTI during pregnancy | Yes | 11 | 62 | 0.824 | 1.104 | 0.460–2.650 |
| No | 16 | 111 | ||||
| History of Preterm delivery | Yes | 1 | 16 | 0.362 | 0.375 | 0.046–3.082 |
| No | 26 | 157 | ||||
| Educational level | Elementary | 3 | 42 | 0.664 | 0.859 | 0.431–1.710 |
| High school | 21 | 101 | ||||
| Academic | 3 | 30 |
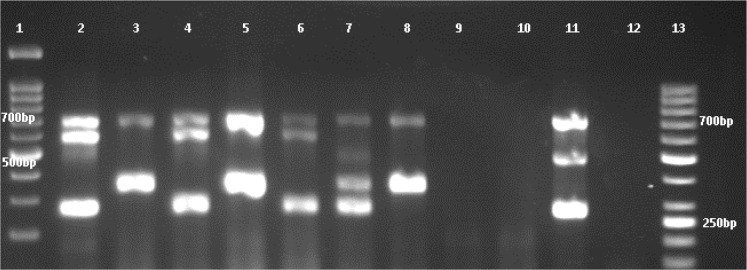
Among the 27 GBS isolates identified in the present study, none of the isolates was resistant to vancomycin. The highest resistance was observed to erythromycin (12/27; 44.4%) followed by clindamycin (8/27; 29.6%), penicillin, ampicillin, and ceftriaxone (5/27; 18.5%), levofloxacin (3/27; 11.1%), and 29.6% (8/27) isolates were multidrug-resistant (MDR) (resistance to ≥2 agents). From 12 erythromycin-resistant isolates, eight isolates were cMLSB, one isolate was iMLSB and presented D-zone in double disk diffusion test, and three isolates were M-phenotype. L phenotype was not found. ermTR and mefA/E genes were detected in 10 (37%) and three (11.1%) isolates; respectively, and the ermB gene was not detected. The most common capsular type was type Ib (12/27; 44.4%) followed by type III (11/27; 40.7%), type II (3/27; 11.1), and type Ia (1/27; 3.7%) (Fig. 2). The serotypes IV, V, VI, VII, VIII, and IX were not found. The phenotypic and genotypic characteristics of GBS isolates are presented in Table 2.
Fig. 2.
Gel electrophoresis of the multiplex PCR amplification products of numbers of GBS isolates.
lane 1: 100 bp DNA Ladder (Iran, Sinaclon), lane 2: Ib); lane 2, 4, and 6 capsular type Ib, lane 3, 5 and 8 capsular types III, lane 7 non-typeable, lane 11 capsular type II, lane 9 and 10 non- S. agalactiae strain, lane 12 negative control (distilled water), lane 13: 50 bp DNA ladder (Iran, Sinaclon).
Table 2.
Phenotypic and genotypic characteristics of GBS isolates
| No. | Sample ID | CT | Antibiotic agents | Phenotype | Resistance genes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||
| PEN | AMP | CRO | LVX | VAN | ERY | CLI | cMLSB | iMLSB | M | ermB | ermTR | mefA/E | |||
| 1 | SA10 | III | S | S | S | S | S | S | S | - | - | - | - | - | - |
| 2 | SA17 | Ib | R | R | R | S | S | R | R | + | - | - | - | + | - |
| 3 | SA26 | III | S | S | S | S | S | S | S | - | - | - | - | - | - |
| 4 | SA30 | Ib | S | S | S | S | S | S | S | - | - | - | - | - | - |
| 5 | SA37 | Ib | S | S | S | S | S | R | R | + | - | - | - | + | - |
| 6 | SA48 | Ia | S | S | S | S | S | S | S | - | - | - | - | - | - |
| 7 | SA51 | III | S | S | S | S | S | S | S | - | - | - | - | - | - |
| 8 | SA61 | III | S | S | S | S | S | R | S | - | + | - | - | + | - |
| 9 | SA72 | Ib | S | S | S | S | S | R | R | + | - | - | - | + | - |
| 10 | SA76 | III | R | R | R | R | S | R | R | + | - | - | - | + | - |
| 11 | SA79 | Ib | S | S | S | S | S | S | S | - | - | - | - | - | - |
| 12 | SA87 | III | R | R | R | R | S | R | R | + | - | - | - | + | - |
| 13 | SA93 | II | R | R | R | R | S | R | R | + | - | - | - | + | - |
| 14 | SA101 | Ib | S | S | S | S | S | R | S | - | - | + | - | - | + |
| 15 | SA122 | Ib | S | S | S | S | S | I | S | - | - | - | - | - | - |
| 16 | SA137 | III | S | S | S | S | S | S | S | - | - | - | - | - | - |
| 17 | SA144 | Ib | S | S | S | S | S | I | S | - | - | - | - | + | - |
| 18 | SA151 | III | S | S | S | S | S | S | S | - | - | - | - | - | - |
| 19 | SA158 | II | S | S | S | S | S | S | S | - | - | - | - | - | - |
| 20 | SA166 | III | S | S | S | S | S | R | S | - | - | + | - | - | + |
| 21 | SA170 | Ib | S | S | S | S | S | R | S | - | - | + | - | - | + |
| 22 | SA175 | III | S | S | S | S | S | S | S | - | - | - | - | - | - |
| 23 | SA177 | Ib | R | R | R | S | S | R | R | + | - | - | - | + | - |
| 24 | SA179 | II | S | S | S | S | S | S | S | - | - | - | - | - | - |
| 25 | SA186 | III | S | S | S | S | S | S | S | - | - | - | - | - | - |
| 26 | SA190 | Ib | S | S | S | S | S | S | S | - | - | - | - | - | - |
| 27 | SA194 | Ib | S | S | S | S | S | R | R | + | - | - | - | + | - |
Number of isolates; No, Capsular type; CT, Penicillin; PEN, Ampicillin; AMP, Ceftriaxone; CRO, Levofloxacin; LVX, Erythromycin; ERY, Clindamycin; CLI.
DISCUSSION
In this study, 13.8% pregnant women were colonized by GBS. In the last ten years in our country, prevalence of GBS colonization among pregnant women was reported differently and in the latest meta-analysis from Iran, prevalence of GBS colonization in Iranian pregnant women was calculated as 13.65% (17). In another meta-analysis, maternal GBS colonization worldwide was estimated at 18% (18). According to these, our finding is in the Iranian pregnant women colonization range but lower than the worldwide estimation. It should be noted that differences in the sampling sites, diagnostic methods, statistical communities, and geographical variations can result in differences in the prevalence of colonization with GBS.
In the present study, there was no association between risk factors and colonization with GBS. Similar to our survey, in a study by Darabi et al. there were no significant differences between colonized and non-colonized women concerning age, educational level, and parity (19). Also, in a study by Ahmadi et al. no risk factor correlation was seen in colonized pregnant women and those with spontaneous abortion (20). Despite our study, Yasini et al. found a significant correlation between GBS colonization in pregnant women and parity (21). Since, in our country, there was not GBS maternal screening during pregnancy guidelines, knowing GBS colonization risk factors are necessary, and clinicians should pay special attention to risk factors.
β-lactam antibiotics, including penicillin G, are the first-choice agents for the treatment of GBS infections and prophylaxis. GBS is considered susceptible to penicillin in vitro. Although, there have been reports of reduced penicillin sensitivity (22–24). Also, other β-lactam antibiotics such as ampicillin, cephalosporins, and carbapenems are used for GBS prophylaxis and treatment but resistance to these agents was more reported (25). In the present study, 18.5% of isolated GBS were resistant to penicillin, ampicillin, and ceftriaxone. Different β-lactam resistance percentage in commensal GBS has been reported from Iran. In one study, high-level resistance to penicillin (88.6%) was reported (26), while in other studies no resistant isolates were detected (21, 27). It is noteworthy that in most of the studies minimum inhibitory concentration (MIC) of β-lactam agents was not investigated, and in the only one study from Iran, 27% of GBS isolates were resistant to penicillin at MIC of 1.5 μg/ml (28). It seems that the mechanism of penicillin resistance should be investigated in GBS isolates in Iran.
In patients with a severe allergy to β-lactam antibiotics, alternative therapies or prophylaxis include clindamycin, erythromycin, fluoroquinolones, and vancomycin. Resistance to vancomycin was not seen in this study, and resistance to levofloxacin was low. However, our findings showed that 44.4% and 29.6% isolates were resistant to erythromycin and clindamycin; respectively, and among these, one isolate (3.7%) had an iMLSB phenotype. In Iran, the erythromycin resistance rate in invasive and commensal GBS isolates was reported from 19.5 to 100%, and clindamycin resistance rate from 16.6 to 100% (21, 26–33). Unfortunately, the mechanism of resistance to MLSB was less investigated in our region. In our study, 37% and 11.1% of isolates harbored ermTR and mefA/E genes; respectively, and none of the GBS isolates had the ermB gene. All M phenotype isolates carried mefA/E. In a few studies from Iran, the erm- TR and ermB genes were detected, and the mefA/E gene was only reported in one study (27, 30, 34).
In the present study, the most common capsular type detected was type Ib (44.4%), but three capsular types III, II, and Ia accounted for more than half of the collected GBS isolates. Over half of maternal diseases were caused by capsular type Ia and III followed by V, Ib, and II (25). Dominant detected capsular types in our study and other studies from Iran were Ib, Ia, III, and II (27, 29, 34). Since the vaccine development is according to accurate population serotype distribution data, so screening the molecular type of the colonizing and invasive GBS isolates are crucial.
This study has one limitation that was the small sample size of GBS strains. For accurate detection of antibiotic susceptibility patterns and capsular types, more GBS strains should be considered.
In conclusion, the prevalence of GBS in participant pregnant women was in the medium range. However, resistance to key antibiotic agents was relatively high. Besides, capsular serotype Ib was the predominant serotype, which emphasizes the importance of monitoring the molecular typing of the GBS isolates regularly.
ACKNOWLEDGEMENTS
This work was supported by the deputy vice-chancellor for research affairs of Isfahan University of Medical Sciences (grant number 293322). We would like to thank the Nosocomial infection Research Center laboratory staff for supporting the practical work.
REFERENCES
- 1.Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA 2008;299:2056–2065. [DOI] [PubMed] [Google Scholar]
- 2.McCord N, Owen P, Powls A, Lunan B. A complete audit cycle of intrapartum group B streptococcus prophylaxis. Health Bull (Edinb) 2001; 59:263–267. [PubMed] [Google Scholar]
- 3.Verani JR, Schrag SJ. Group B streptococcal disease in infants: progress in prevention and continued challenges. Clin Perinatol 2010; 37:375–392. [DOI] [PubMed] [Google Scholar]
- 4.Heath PT, Feldman RG. Vaccination against group B streptococcus. Expert Rev Vaccines 2005; 4:207–218. [DOI] [PubMed] [Google Scholar]
- 5.Picard FJ, Bergeron MG. Laboratory detection of group B Streptococcus for prevention of perinatal disease. Eur J Clin Microbiol Infect Dis 2004; 23:665–671. [DOI] [PubMed] [Google Scholar]
- 6.Brozanski BS, Jones JG, Krohn MA, Sweet RL. Effect of a screening-based prevention policy on prevalence of early-onset group B streptococcal sepsis. Obstet Gynecol 2000; 95:496–501. [DOI] [PubMed] [Google Scholar]
- 7.Verani JR, McGee L, Schrag SJ, Division of Bacterial Diseases. National Center for Immunization and Respiratory Diseases. Centers for Disease Control and Prevention (CDC) . Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep 2010; 59:1–36. [PubMed] [Google Scholar]
- 8.Capanna F, Emonet SP, Cherkaoui A, Irion O, Schrenzel J, Martinez de Tejada B. Antibiotic resistance patterns among group B Streptococcus isolates: implications for antibiotic prophylaxis for early-onset neonatal sepsis. Swiss Med Wkly 2013; 143:w13778. [DOI] [PubMed] [Google Scholar]
- 9.De Francesco MA, Caracciolo S, Gargiulo F, Manca N. Phenotypes, genotypes, serotypes and molecular epidemiology of erythromycin-resistant Streptococcus agalactiae in Italy. Eur J Clin Microbiol Infect Dis 2012; 31:1741–1747. [DOI] [PubMed] [Google Scholar]
- 10.Gajic I, Plainvert C, Kekic D, Dmytruk N, Mijac V, Tazi A, et al. Molecular epidemiology of invasive and non-invasive group B Streptococcus circulating in Serbia. Int J Med Microbiol 2019; 309:19–25. [DOI] [PubMed] [Google Scholar]
- 11.Ke D, Menard C, Picard FJ, Boissinot M, Ouellette M, Roy PH, et al. Development of conventional and real-time PCR assays for the rapid detection of group B streptococci. Clin Chem 2000; 46:324–331. [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute (CLSI) . Performance Standards for Antimicrobial Susceptibility Testing. 27th ed. CLSI supplement M100. Wayne, Pennsylvania: 2017. [Google Scholar]
- 13.Imperi M, Pataracchia M, Alfarone G, Baldassarri L, Orefici G, Creti R. A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae. J Microbiol Methods 2010; 80:212–214. [DOI] [PubMed] [Google Scholar]
- 14.de la Pedrosa EG, Morosini MI, van der Linden M, Ruiz-Garbajosa P, Galan JC, Baquero F, et al. Polyclonal population structure of Streptococcus pneumoniae isolates in Spain carrying mef and mef plus erm(B). Antimicrob Agents Chemother 2008; 52:1964–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florindo C, Viegas S, Paulino A, Rodrigues E, Gomes JP, Borrego MJ. Molecular characterization and antimicrobial susceptibility profiles in Streptococcus agalactiae colonizing strains: association of erythromycin resistance with subtype III-1 genetic clone family. Clin Microbiol Infect 2010; 16:1458–1463. [DOI] [PubMed] [Google Scholar]
- 16.Reinert RR, Franken C, van der Linden M, Lutticken R, Cil M, Al-Lahham A. Molecular characterisation of macrolide resistance mechanisms of Streptococcus pneumoniae and Streptococcus pyogenes isolated in Germany, 2002–2003. Int J Antimicrob Agents 2004; 24:43–47. [DOI] [PubMed] [Google Scholar]
- 17.Hossein YektaKooshali M, Hamidi M, Mohammad Taghi Razavi Tousi S, Nikokar I. Prevalence of group B streptococcus colonization in Iranian pregnant women: A systematic review and meta-analysis. Int J Reprod Biomed 2019; 16(12): ijrm.v16i12.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell NJ, Seale AC, O’Driscoll M, O’Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J, et al. Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis 2017; 65(suppl_2):S100–S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darabi R, Tadi S, Mohit M, Sadeghi E, Hatamizadeh G, Kardeh B, et al. The prevalence and risk factors of group B streptococcus colonization in Iranian pregnant women. Electron Physician 2017; 9:4399–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmadi A, Farhadifar F, Rezaii M, Zandvakili F, Seyedoshohadaei F, Zarei M, et al. Group B Streptococci and Trichomonas vaginalis infections in pregnant women and those with spontaneous abortion at Sanandaj, Iran. Iran J Microbiol 2018; 10:166–170. [PMC free article] [PubMed] [Google Scholar]
- 21.Yasini M, Moniri R, Ghorbaali Z, Ansaripour L, Movahedinejad M, Yadegarsalehi M. Prevalence rate, antibiotic susceptibility and colonization risk factors of group B Streptococcus in genital tract of pregnant women. MJMS 2014; 57:676–683. [Google Scholar]
- 22.Assefa S, Desta K, Lema T. Group B streptococci vaginal colonization and drug susceptibility pattern among pregnant women attending in selected public antenatal care centers in Addis Ababa, Ethiopia. BMC Pregnancy Childbirth 2018; 18:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moroi H, Kimura K, Kotani T, Tsuda H, Banno H, Jin W, et al. Isolation of group B Streptococcus with reduced beta-lactam susceptibility from pregnant women. Emerg Microbes Infect 2019; 8:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campo CH, Martinez MF, Otero JC, Rincon G. Vagino-rectal colonization prevalence by Streptococcus agalactiae and its susceptibility profile in pregnant women attending a third-level hospital. Biomedica 2019; 39:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raabe VN, Shane AL. Group B Streptococcus (Streptococcus agalactiae). Microbiol Spectr 2019; 7: 10.1128/microbiolspec.GPP3-0007-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahraee S, Milani F, Atrkar Roushan Z, Hedayati Ch M, Rostami S, Shoja S, et al. The prevalence of rectovaginal colonization and antibiotic susceptibility pattern of Streptococcus agalactiae in pregnant women in Al-Zahra hospital, Rasht, Iran. Infect Dis Clin Pract 2019; 27:143–147. [Google Scholar]
- 27.Emaneini M, Jabalameli F, Mirsalehian A, Ghasemi A, Beigverdi R. Characterization of virulence factors, antimicrobial resistance pattern and clonal complexes of group B streptococci isolated from neonates. Microb Pathog 2016; 99:119–122. [DOI] [PubMed] [Google Scholar]
- 28.Malek-Jafarian M, Hosseini FS, Ahmadi AR. Pattern of infection and antibiotic activity among Streptococcus agalactiae isolates from adults in Mashhad, Iran. Rep Biochem Mol Biol 2015; 3:89–93. [PMC free article] [PubMed] [Google Scholar]
- 29.Khodaei F, Najafi M, Hasani A, Kalantar E, Sharifi E, Amini A, et al. Pilus-encoding islets in S. agalactiae and its association with antibacterial resistance and serotype distribution. Microb Pathog 2018; 116:189–194. [DOI] [PubMed] [Google Scholar]
- 30.Mousavi SM, Nasaj M, Hosseini SM, Arabestani MR. Survey of strain distribution and antibiotic resistance pattern of group B streptococci (Streptococcus agalactiae) isolated from clinical specimens. GMS Hyg Infect Control 2016; 11: Doc18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goudarzi G, Ghafarzadeh M, Shakib P, Anbari K. Culture and real-time PCR based maternal screening and antibiotic susceptibility for group B Streptococcus: an Iranian experience. Glob J Health Sci 2015; 7:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jalalifar S, Havaei SA, Motallebirad T, Moghim S, Fazeli H, Esfahani BN. Determination of surface proteins profile, capsular genotyping, and antibiotic susceptibility patterns of Group B Streptococcus isolated from urinary tract infection of Iranian patients. BMC Res Notes 2019; 12:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saffar H, Rajabiani A, Abdollahi A, Habibi S, Baseri Z. Frequency of inducible clindamycin resistance among Gram-positive cocci in a tertiary hospital, Tehran, Iran. Iran J Microbiol 2016; 8:243–248. [PMC free article] [PubMed] [Google Scholar]
- 34.Emaneini M, Mirsalehian A, Beigvierdi R, Fooladi AA, Asadi F, Jabalameli F, et al. High incidence of macrolide and tetracycline resistance among Streptococcus agalactiae strains isolated from clinical samples in Tehran, Iran. Maedica (Bucur) 2014; 9:157–161. [PMC free article] [PubMed] [Google Scholar]