Summary
Background
Robust age-specific estimates of anal human papillomavirus (HPV) and high-grade squamous intraepithelial lesions (HSIL) in men can inform anal cancer prevention efforts. We aimed to evaluate the age-specific prevalence of anal HPV, HSIL, and their combination, in men, stratified by HIV status and sexuality.
Methods
We did a systematic review for studies on anal HPV infection in men and a pooled analysis of individual-level data from eligible studies across four groups: HIV-positive men who have sex with men (MSM), HIV-negative MSM, HIV-positive men who have sex with women (MSW), and HIV-negative MSW. Studies were required to inform on type-specific HPV infection (at least HPV16), detected by use of a PCR-based test from anal swabs, HIV status, sexuality (MSM, including those who have sex with men only or also with women, or MSW), and age. Authors of eligible studies with a sample size of 200 participants or more were invited to share deidentified individual-level data on the above four variables. Authors of studies including 40 or more HIV-positive MSW or 40 or more men from Africa (irrespective of HIV status and sexuality) were also invited to share these data. Pooled estimates of anal high-risk HPV (HR-HPV, including HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), and HSIL or worse (HSIL+), were compared by use of adjusted prevalence ratios (aPRs) from generalised linear models.
Findings
The systematic review identified 93 eligible studies, of which 64 contributed data on 29 900 men to the pooled analysis. Among HIV-negative MSW anal HPV16 prevalence was 1·8% (91 of 5190) and HR-HPV prevalence was 6·9% (345 of 5003); among HIV-positive MSW the prevalences were 8·7% (59 of 682) and 26·9% (179 of 666); among HIV-negative MSM they were 13·7% (1455 of 10 617) and 41·2% (3798 of 9215), and among HIV-positive MSM 28·5% (3819 of 13 411) and 74·3% (8765 of 11 803). In HIV-positive MSM, HPV16 prevalence was 5·6% (two of 36) among those age 15–18 years and 28·8% (141 of 490) among those age 23–24 years (ptrend=0·0091); prevalence was 31·7% (1057 of 3337) among those age 25–34 years and 22·8% (451 of 1979) among those age 55 and older (ptrend<0·0001). HPV16 prevalence in HIV-negative MSM was 6·7% (15 of 223) among those age 15–18 and 13·9% (166 of 1192) among those age 23–24 years (ptrend=0·0076); the prevalence plateaued thereafter (ptrend=0·72). Similar age-specific patterns were observed for HR-HPV. No significant differences for HPV16 or HR-HPV were found by age for either HIV-positive or HIV-negative MSW. HSIL+ detection ranged from 7·5% (12 of 160) to 54·5% (61 of 112) in HIV-positive MSM; after adjustment for heterogeneity, HIV was a significant predictor of HSIL+ (aPR 1·54, 95% CI 1·36–1·73), HPV16-positive HSIL+ (1·66, 1·36–2·03), and HSIL+ in HPV16-positive MSM (1·19, 1·04–1·37). Among HPV16-positive MSM, HSIL+ prevalence increased with age.
Interpretation
High anal HPV prevalence among young HIV-positive and HIV-negative MSM highlights the benefits of gender-neutral HPV vaccination before sexual activity over catch-up vaccination. HIV-positive MSM are a priority for anal cancer screening research and initiatives targeting HPV16-positive HSIL+.
Funding
International Agency for Research on Cancer.
Research in context.
Evidence before this study
We searched MEDLINE, Embase, and the Cochrane Library on March 12, 2021, using the terms (“papillomaviridae” OR ”papillomavirus” OR “HPV”) AND (“anal canal” OR “anus” OR “anal”). We searched for primary research on anal human papillomavirus (HPV) infection in men published between Jan 1, 1986, and Feb 28, 2021, with no language restrictions. Persistent anal high-risk HPV (HR-HPV, including HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), particularly HPV16, is the established cause of anal cancer, for which anal high-grade squamous intraepithelial lesion (HSIL) is the assumed precursor. Several systematic reviews and relevant meta-analyses exist on the topic, one of which (published in 2012) focused on men who have sex with men (MSM). Other meta-analyses have since established HIV status and sexuality as important predictors of anal HPV prevalence and anal cancer incidence in men, as well as degree of immunosuppression and antiretroviral therapy in men living with HIV. However, these meta-analyses of summary-level data did not allow the analysis of patterns of anal HR-HPV and HSIL in risk groups by age and degree of HIV-related immunosuppression, nor combinations of anal HPV and HSIL, which require individual-level data and sample sizes larger than those available from any single study.
Added value of this study
To our knowledge, this is the first systematic reanalysis of the age-specific epidemiology of anal HPV in men, which offers a comprehensive picture of anal HPV infection in men before HPV vaccination. Our results show rapid increases in the prevalence of anal HPV16 in HIV-positive and HIV-negative MSM aged 15–24 years, and consistently high HPV16 prevalence in all MSM aged 25 years or older. We also provide evidence for the amplifying effect of HIV infection and HIV-related immunosuppression on anal HPV16 infection in men who have sex with women and MSM. The additional novelty of this collaborative reanalysis is the description of the epidemiology of HSIL in MSM by HIV status, including in combination with HPV16 data. This approach revealed important heterogeneity in HSIL detection, even across studies that used similar diagnostic strategies (eg, both cytology and high-resolution anoscopy). Despite this heterogeneity, HIV infection and degree of HIV immunosuppression were shown to be significant predictors of anal HSIL and HPV16-positive anal HSIL in MSM, as well as of anal HSIL among HPV16-positive MSM. Furthermore, the prevalence of anal HSIL slightly increased with age in HPV16-positive MSM.
Implications of all the available evidence
These data can inform the development of various anal cancer prevention efforts, both through primary prevention (ie, vaccination against HPV or prevention and control of HIV infection) and secondary prevention (eg, the potential screening of high-risk populations, such as people living with HIV), with the aim of detecting and managing HSIL, especially HPV16-positive HSIL. Of note, the rapid increase in anal HPV infection among young MSM highlights the benefits of gender-neutral HPV vaccination before sexual debut over catch-up HPV vaccination, regardless of HIV status. Our findings indicate that the prevalence of HPV16-positive anal HSIL in the HIV-positive MSM population is high, and that anal cancer screening research and initiatives should be prioritised in this group.
Introduction
Of 35 000 human papillomavirus (HPV)-related anal cancers diagnosed worldwide each year, 17 000 (48·6%) occur in men.1 Anal cancer is rare in the general male population (about one case per 100 000 person-years), but its incidence is elevated in HIV-negative men who have sex with men (MSM; about 20 cases per 100 000 person-years) and HIV-positive men who have sex with women (MSW; about 30 cases per 100 000 person-years), and can exceed 100 cases per 100 000 person-years in HIV-positive MSM due to increased anal HPV exposure and HIV-related immunosuppression.2 Indeed, the incidence of anal cancer is increasing in high-income countries,3 and a substantial proportion of this increase among men can be attributed to HIV.4
Persistent anal HPV infection is the major cause of anal cancer.5, 6 Although 13 high-risk HPV (HR-HPV; HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) types are classified as human carcinogens (on the basis of evidence from cervical cancer),6 HPV16 is the most carcinogenic type in the anus.5 HPV16 is more frequently identified in HPV-related anal cancer than in cervical cancer,1, 5 albeit in a larger majority of anal cancers in HIV-negative individuals than in HIV-positive individuals.5 HPV16 is also the dominant type in high-grade squamous intraepithelial lesions (HSIL), which is the precursor of anal cancer.5
Potential modalities for anal cancer prevention can be separated into those relevant to primary prevention (eg, vaccination against HPV7 and the prevention and control of HIV infection), and those relevant to secondary prevention (eg, screening of high-risk populations), which aim to detect and manage HSIL. Although there are parallels with modalities established for cervical cancer prevention, many differences exist, particularly for men.8 Thus, robust age-specific epidemiological data of anal HPV infection and HSIL in target populations can inform anal cancer prevention programmes for men and predict their potential effect.
To this end, we initiated a collaborative, individual-level, pooled analysis of anal HPV and HSIL in men according to HIV status, sexuality, and age. This analysis is complementary to a similar, previously published, collaborative, pooled analysis done in women according to HIV status.9 Our aim was to evaluate age-specific prevalence of HPV16, HSIL, and their combination, across the risk strata of HIV-positive MSM, HIV-negative MSM, HIV-positive MSW, and HIV-negative MSW.
Methods
Data collection
We did a systematic literature review for studies on anal HPV infection in men, by updating the strategy used in two previous meta-analyses.5, 10 We searched MEDLINE, Embase, and the Cochrane Library for primary research published between Jan 1, 1986, and Feb 28, 2021, using the search terms (“papillomaviridae” OR ”papillomavirus” OR “HPV”) AND (“anal canal” OR “anus” OR “anal”), with no language restrictions (appendix p 2).
To be eligible, studies were required to report type-specific HPV infection (for at least HPV16), detected by use of a PCR-based test of anal swabs; HIV status; sexuality (MSM, including those who have sex with men only or also with women, or MSW); and age. Authors of eligible studies with sample sizes of 200 individuals or more were invited to share deidentified individual-level data on the above four variables. Due to sparse data, authors of studies including 40 or more HIV-positive MSW, 40 or more men from Africa (irrespective of HIV status and sexuality), or both, were also invited to share deidentified individual-level data.
Although not strict inclusion requirements, other data were included when available on anal cytology or histology results collected at the same visit as (or within 6 months of) HPV sample collection and, for HIV positive men, on current and nadir CD4 cell counts and most recent HIV viral loads. The only exclusion criteria at the individual level were age younger than 15 years and having had one or more dose of HPV vaccine.
FW and CJA performed the literature search and possible conflicts were resolved by discussion with GMC.
Data analysis
Pooled anal HPV prevalence estimates were calculated for 13 individual HR-HPV types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), as well as for four groups of HPV types (HPV16 and 18; HPV6, 11, 16, and 18; HPV6, 11, 16, 18, 31, 33, 45, 52, and 58; and any 13 HR-HPV types). Not all studies tested for all HPV types, thus denominators vary by type or groups of types. Only studies testing for all 13 individual HR-HPV types contributed to analyses of prevalence for any or multiple HR-HPV types.
Studies with cytopathology were classified into four groups according to diagnostic strategy: high-resolution anoscopy for all participants; high-resolution anoscopy for participants with abnormal cytology only; anoscopy for all participants; and cytology only. Only men with a cytology result were included in analyses of HSIL or worse (HSIL+), defined as either (1) cytological diagnosis of atypical squamous cells, cannot exclude HSIL, HSIL, or anal cancer, or (2) histological diagnosis of grade 2 or 3 anal intraepithelial neoplasia or anal cancer. Prevalence estimates were also calculated for having HSIL+ and HPV16-positive anal swabs simultaneously (hereafter referred to as HPV16-positive anal HSIL+) in all MSM, and HSIL+ in HPV16-positive MSM. Sensitivity analyses of HSIL+ outcomes were done, in which studies were restricted to those that enrolled both HIV-positive and HIV-negative MSM and did high-resolution anoscopy for all participants. A random-effects meta-analysis and I2 were used to measure heterogeneity by study.
In HIV-positive men, estimates of HPV16 infection, HSIL+, and their combination, were stratified by current and nadir CD4 count (<200 cells per μL, 200–499 cells per μL, and ≥500 cells per μL), and HIV viral load (<200 copies per mL and ≥200 copies per mL).
The prevalence of HPV infection, HSIL+, and their combination, were stratified by risk group (HIV-positive MSM, HIV-negative MSM, HIV-positive MSW, and HIV-negative MSW) and age group. We estimated prevalence ratios (PRs) with corresponding 95% CIs by use of generalised linear models with a log-link function. To account for heterogeneity between studies, all PRs were adjusted by study (used as a fixed effect in the model), and by HIV and age group (used as covariables) in specific analyses, as appropriate. p values of age-specific trends in HPV prevalence were calculated with age group as a continuous variable. Stata (version 14) was used for statistical analyses, which were all two-sided.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
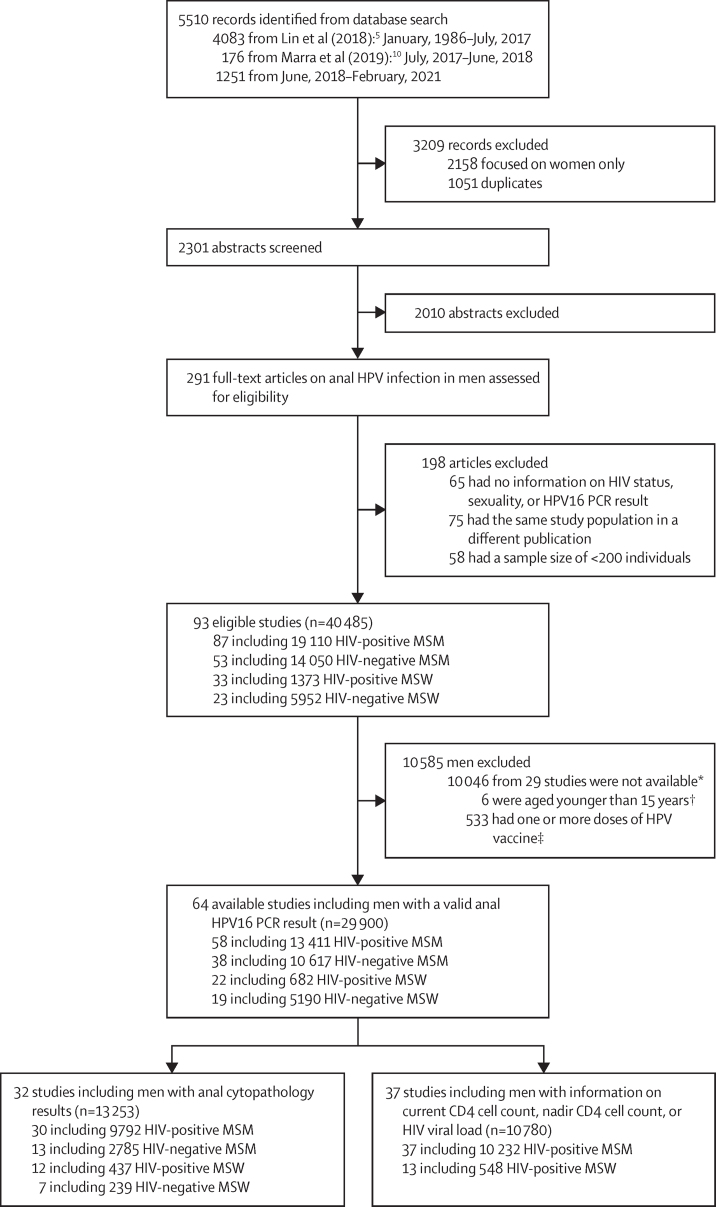
The systematic review identified 93 eligible studies (including 40 485 men), of which 64 (73·9%) contributed data on 29 900 men to the pooled analysis of HPV infection (figure 1). 44·9% (n=13 411) of all included men were HIV-positive MSM, 35·5% (n=10 617) HIV-negative MSM, 2·3% (n=682) HIV-positive MSW, and 17·4% (n=5190) HIV-negative MSW. 9877 men (33·0%) were from North America, 8028 (26·8%) from Asia, and 7524 (25·2%) from Europe (appendix pp 4–6); other regions were less well represented, but men from Africa contributed 46·2% of all HIV-positive MSW (315 of 682).
Figure 1.
Study selection
All studies included in this pooled analysis were mutually exclusive. HPV=human papillomavirus. MSM=men who have sex with men. MSW=men who have sex with women. *The authors of these studies did not share individual-level data. †Included five HIV-positive MSM and one HIV-positive MSW. ‡Included 247 HIV-positive MSM, 278 HIV-negative MSM, one HIV-positive MSW, and seven HIV-negative MSW.
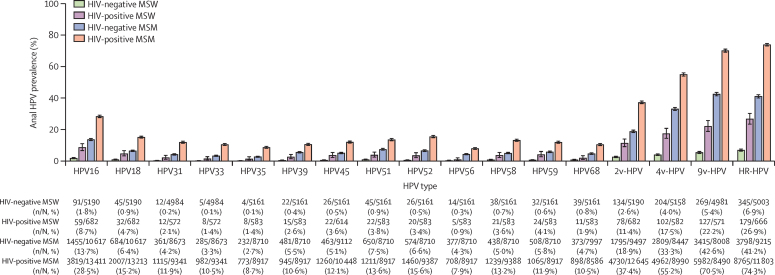
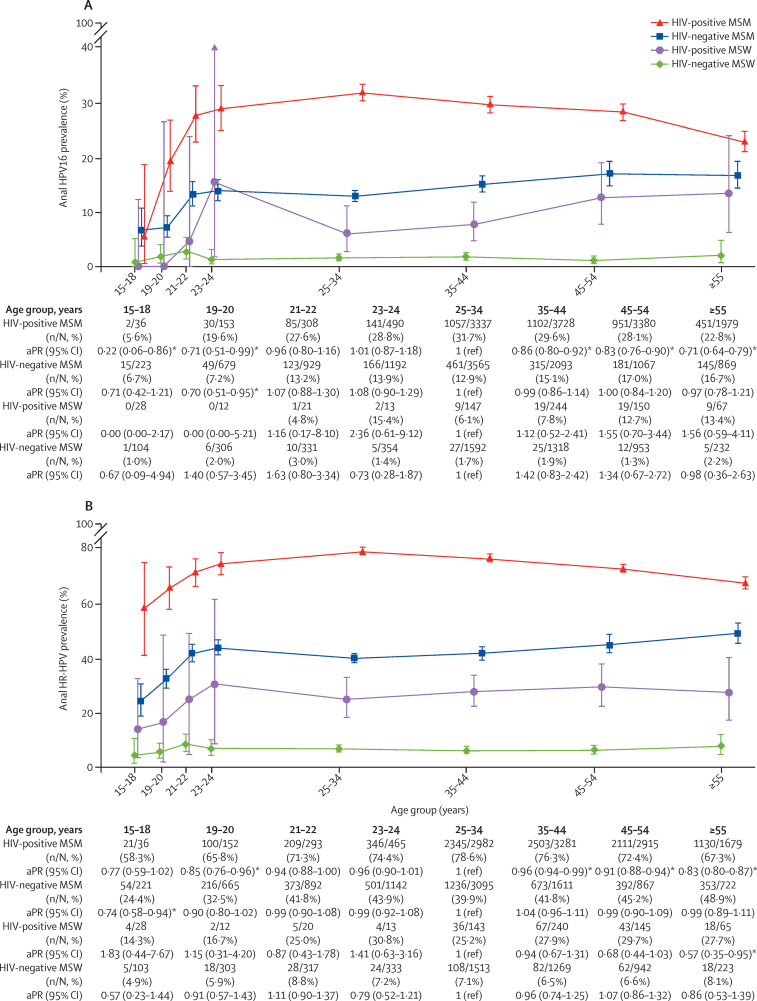
For all individual and groups of HPV types, anal HPV prevalence was lowest in HIV-negative MSW, followed by HIV-positive MSW and HIV-negative MSM, and was highest in HIV-positive MSM (figure 2). When comparing age-specific prevalence of HPV16 and HR-HPV, similar ranking among the four risk groups was consistently observed within each age group (figure 3). In HIV-positive MSM, the age-specific anal HPV16 prevalence increased from 5·6% at 15–18 years to 28·8% at 23–24 years (ptrend=0·0091), then decreased significantly with increasing age from 31·7% at 25–34 years to 22·8% at 55 years and older (adjusted PR [aPR] for ≥55 years vs 25–34 years 0·71, 95% CI 0·64–0·79; ptrend<0·0001; figure 3A). A similar age-specific pattern was observed for HR-HPV prevalence in HIV-positive MSM, increasing from 58·3% at 15–18 years to 74·4% at 23–24 years (ptrend=0·038), then decreasing significantly from 78·6% at 25–34 years to 67·3% at 55 years and older (aPR for ≥55 years vs 25–34 years 0·83, 95% CI 0·80–0·87, ptrend<0·0001; figure 3B). Similar increases in HPV16 (ptrend=0·0076) and HR-HPV (ptrend=0·011) prevalence from 15–18 years to 23–24 years were observed in HIV-negative MSM, but with no significant differences thereafter (from 25–34 years to ≥55 years HPV16 ptrend=0·72 and HR-HPV ptrend=0·73; figure 3). HIV-positive MSW were underrepresented in younger age groups, but prevalence of HPV16 increased from 6·1% at 25–34 years to 13·4% at 55 years and older (figure 3A), and prevalence of HR-HPV increased from 25·2% at 25–34 years to 27·7% at 55 years and older (figure 3B), albeit non-significantly. HPV16 and HR-HPV prevalence did not vary by age in HIV-negative MSW. Age-specific prevalence of individual HR-HPV excluding HPV16 and groups of HPV types by risk group are shown in the appendix (pp 9–14, 15–17).
Figure 2.
Prevalence of type-specific and grouped type HPV infection in four male risk groups
HR-HPV includes HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. Error bars show 95% CIs. HPV=human papillomavirus. 2v-HPV=HPV16 and 18. 4v-HPV=HPV6, 11, 16, and 18. 9v-HPV=HPV6, 11, 16, 18, 31, 33, 45, 52, and 58. HR-HPV=high-risk HPV. MSW=men who have sex with women. MSM=men who have sex with men.
Figure 3.
Age-specific prevalence of HPV16 (A) and HR-HPV (B) infection in four male risk groups
Error bars show 95% CIs. PRs were adjusted for study. HR-HPV includes HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. HPV=human papillomavirus. HR-HPV=high-risk HPV. MSM=men who have sex with men. MSW=men who have sex with women. aPR=adjusted prevalence ratio. *Significant aPRs relative to the reference group.
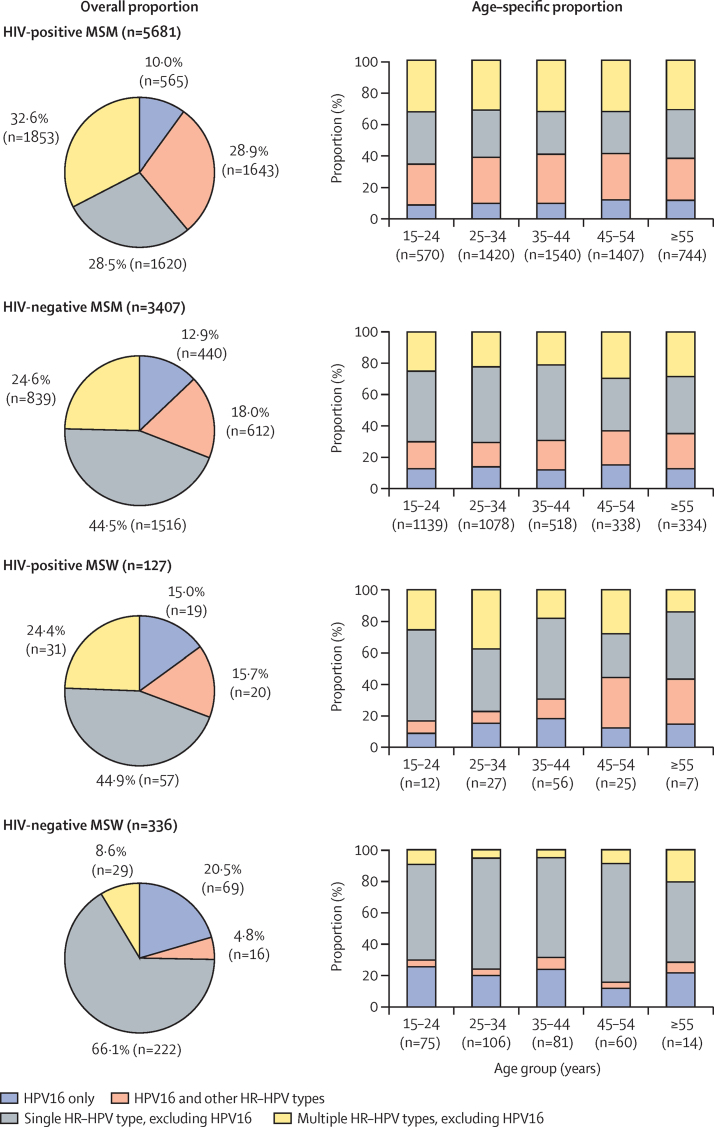
Among HR-HPV-positive men, infection with multiple HR-HPV types was observed in 61·5% HIV-positive MSM (28·9% including HPV16; 32·6% not including HPV16), 42·6% HIV-negative MSM (18·0%; 24·6%), 40·1% HIV-positive MSW (15·7%; 24·4%), and 13·4% HIV-negative MSW (4·8%; 8·6%), with no differences in the proportion of those with multiple HPV infections by age group (figure 4). Further details on the distribution of infection with different numbers of HR-HPV types in the four male risk groups, overall and by age group, are described in the appendix (p 18).
Figure 4.
Overall and age-specific proportion of men infected with HPV16 and other HR-HPV types
HR-HPV includes HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. HPV=human papillomavirus. HR-HPV=high-risk HPV. MSM=men who have sex with men. MSW=men who have sex with women.
In total, 32 studies (including 13 253 men) contributed data on cytopathology. 73·9% (n=9792) of men with cytopathology data were HIV-positive MSM, 21·0% (n=2785) were HIV-negative MSM, 3·3% (n=437) HIV-positive MSW, and 1·8% (n=239) HIV-negative MSW (figure 1; appendix pp 4–6). Prevalence of HPV16 and HR-HPV was lower in men without cytopathological data than in those with cytopathological data, in all risk groups (appendix p 19), especially for HIV-negative MSM aged 35 years and older (appendix p 20).
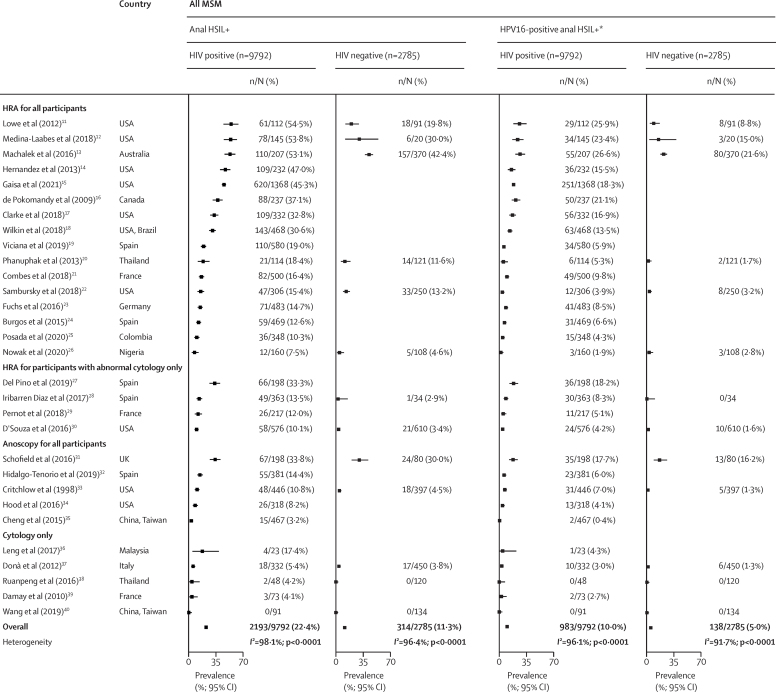
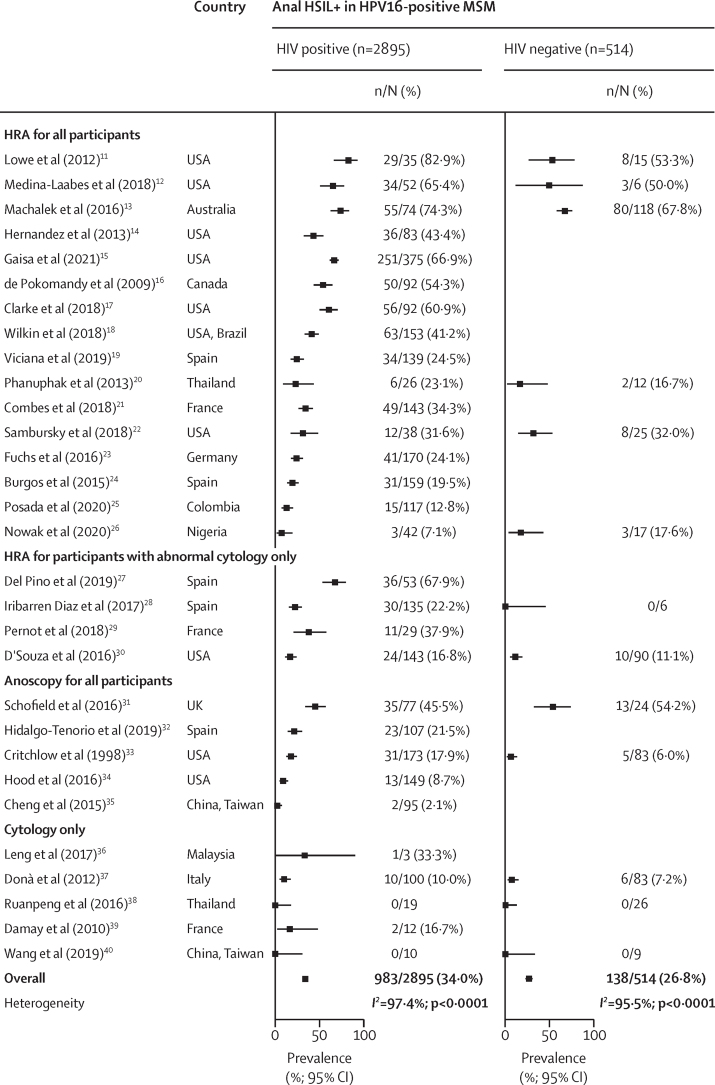
Because of small number of MSW, HSIL+ analyses were restricted to 30 studies including 12 577 MSM. With respect to diagnostic strategy, in addition to cytology, 16 studies, with 55·8% of all 12 577 MSM did high-resolution anoscopy in all participants; four, with 15·9%, did high-resolution anoscopy in participants with abnormal cytology only; five, with 18·2%, did anoscopy in all participants; and five, with 10·1%, did cytology only (figure 5). The pooled prevalence of anal HSIL+ was 22·4% in HIV-positive MSM, and 11·3% in HIV-negative MSM, with high study heterogeneity. The corresponding prevalence of HPV16-positive anal HSIL+ was 10·0% in HIV-positive MSM and 5·0% in HIV-negative MSM. When restricting the analysis to HPV16-positive MSM, HSIL+ prevalence was 34·0% in HIV-positive MSM and 26·8% in HIV-negative MSM. The high heterogeneity between studies for anal HSIL+ prevalence remained even when the analysis was restricted to studies in which HSIL+ was histologically confirmed by high-resolution anoscopy in all participants (appendix p 21).
Figure 5.
Prevalence of anal HSIL+
All men included in this analysis had anal cytology results. Studies are ranked in descending order of anal HSIL+ prevalence among HIV-positive MSM, stratified by diagnostic strategy. HPV=human papillomavirus. HRA=high-resolution anoscopy. HSIL+=high-grade squamous intraepithelial lesions or worse. MSM=men who have sex with men. *Only includes participants with HSIL+ plus HPV16-positive swabs.
HIV positivity was a significant risk factor for HSIL+ (aPR 1·54, 95% CI 1·36–1·73), HPV16-positive HSIL+ (1·66, 1·36–2·03), and HSIL+ in HPV16-positive MSM (1·19, 1·04–1·37; table). The prevalence of HSIL+ decreased significantly with age in all MSM (aPR 0·96 per 10 years increase in age, 95% CI 0·94–0·99), but increased significantly with age in HPV16-positive MSM (1·05, 1·01–1·09). The effects of HIV status and age on HSIL+ measures were materially unchanged in two sensitivity analyses (appendix pp 7–8).
Table.
Prevalence of anal HSIL+ in MSM
|
Anal HSIL+ in all MSM (n=12 577) |
HPV16-positive anal HSIL+*in all MSM (n=12 577) |
Anal HSIL+ in HPV16-positive MSM (n=3409) |
|||||
|---|---|---|---|---|---|---|---|
| n/N (%) | aPR (95% CI) | n/N (%) | aPR (95% CI) | n/N (%) | aPR (95% CI) | ||
| Age group, years | .. | .. | .. | .. | .. | .. | |
| 15–24 | 72/794 (9·1%) | 0·98 (0·78–1·22) | 23/794 (2·9%) | 0·76 (0·50–1·17) | 23/183 (12·6%) | 0·89 (0·62–1·29) | |
| 25–34 | 452/2847 (15·9%) | 1 (ref) | 200/2847 (7·0%) | 1 (ref) | 200/847 (23·6%) | 1 (ref) | |
| 35–44 | 725/3307 (21·9%) | 1·00 (0·91–1·10) | 334/3307 (10·1%) | 0·99 (0·84–1·17) | 334/965 (34·6%) | 1·13 (0·99–1·28) | |
| 45–54 | 778/3278 (23·7%) | 0·95 (0·86–1·05) | 343/3278 (10·5%) | 0·90 (0·76–1·07) | 343/903 (38·0%) | 1·09 (0·95–1·24) | |
| ≥55 | 480/2351 (20·4%) | 0·89 (0·79–0·99)† | 221/2351 (9·4%) | 0·91 (0·75–1·10) | 221/511 (43·2%) | 1·19 (1·03–1·36)† | |
| Age, per 10 years | .. | 0·96 (0·94–0·99)† | .. | 0·97 (0·92–1·02) | .. | 1·05 (1·01–1·09)† | |
| HIV status | .. | .. | .. | .. | .. | .. | |
| Negative | 314/2785 (11·3%) | 1 (ref) | 138/2785 (5·0%) | 1 (ref) | 138/514 (26·8%) | 1 (ref) | |
| Positive | 2193/9792 (22·4%) | 1·54 (1·36–1·73)† | 983/9792 (10·0%) | 1·66 (1·36–2·03)† | 983/2895 (34·0%) | 1·19 (1·04–1·37)† | |
PRs were adjusted for study, age group, and HIV status, as appropriate. aPR=adjusted prevalence ratio. HPV=human papillomavirus. HSIL+=high-grade squamous intraepithelial lesions or worse. MSM=men who have sex with men.
Only includes participants with HSIL+ plus HPV16-positive swabs.
Significant aPRs relative to the reference group.
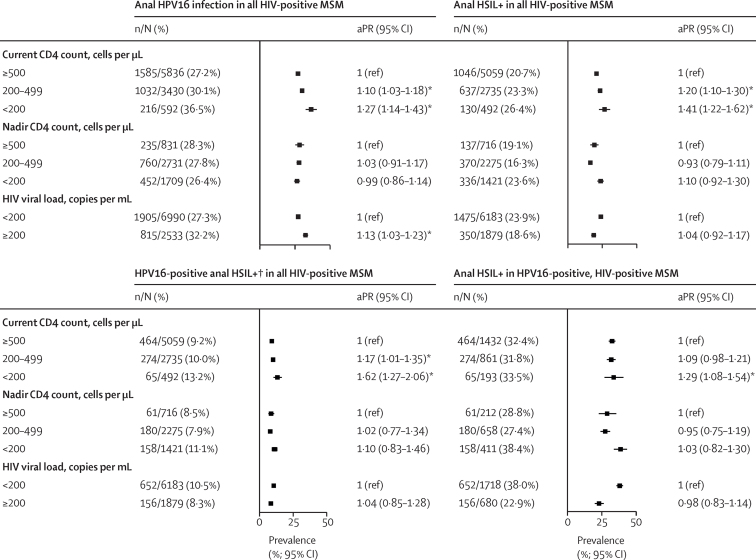
37 studies (including 10 780 HIV-positive men) provided data on current CD4 cell counts, nadir CD4 cell counts, or HIV viral load; most of which were from MSM (94·9%; figure 1; appendix pp 4–6). Low current CD4 count was significantly associated with HPV16 infection, HSIL+, and HPV16-positive HSIL+ in all HIV-positive MSM, and with HSIL+ in HPV16-positive, HIV-positive MSM (figure 6). After stratifying by age, the effects of low current CD4 count on HPV16 infection, anal HSIL+, and HPV16-positive anal HSIL+ were most clearly observed in the 25–54 years age group (appendix p 22). However, there were no associations between nadir CD4 cell count and HPV16 or HSIL+ outcomes (figure 6). HPV16 prevalence was higher in HIV-positive MSM with HIV viral loads of 200 copies per mL or higher than in those with lower viral loads, but HIV viral load was not significantly associated with HSIL+ outcomes. In HIV-positive MSW, prevalence of HPV16 was higher in men with CD4 counts of less than 200 cells per μL than in those with CD4 counts of 500 cells per μL or more (aPR 2·10, 0·98–4·49), although this difference was not significant (appendix p 23).
Figure 6.
Prevalence of anal HPV16 infection and anal HSIL+
PRs were adjusted for age group and study. Error bars show 95% CIs. aPR=adjusted prevalence ratio. HPV=human papillomavirus. HSIL+=high-grade squamous intraepithelial lesions or worse. MSM=men who have sex with men. *Significant aPRs relative to the reference group. †Only includes participants with HSIL+ plus HPV16-positive swabs.
Discussion
Pooling of individual-level data for 29 900 men provides a comprehensive picture of age-specific epidemiology of anal HPV infection in HIV-positive and HIV-negative men before HPV vaccination. Consistent with previous research,10 patterns of anal HR-HPV prevalence, most notably for HPV16, showed HIV status and sexuality to be important population-level determinants of anal HPV infection in men. Anal HPV data were particularly abundant for both HIV-negative and HIV-positive MSM, allowing robust estimates of age-specific changes in HPV infection, which notably indicated a rapid increase in prevalence from the age of 15 years to 24 years.
For HIV-negative MSW, HR-HPV prevalence was low relative to all other risk groups, but nonetheless present (including HPV16) across all age groups. To our knowledge, these data provide the most robust evidence to date on the prevalence of HPV infection in men without established risk factors, highlighting background transmission in the absence of anal sexual intercourse (as observed in women).41 This is an important concept, given that, because of population size, HIV-negative MSW still contribute a substantial proportion of anal cancer burden in men at a population level,42 despite having a low risk at the individual level. HPV vaccination is the only, albeit long-term, solution to anal cancer prevention in HIV-negative MSW. Although gender-neutral vaccination before sexual activity offers maximum protection, girls-only vaccination programmes provide substantial indirect protection (ie, herd immunity) in this low-risk group.43
HIV-positive MSW were the least represented risk group in our analysis, particularly those aged younger than 25 years. Nevertheless, for each age group, HPV prevalence was elevated multiple-fold in HIV-positive MSW versus that in HIV-negative MSW, consistent with a worsening effect of HIV on the natural history of HPV (as established in MSM),8 and resulting increased anal cancer risk.2 Indeed, HPV infection was associated with low current CD4 cell counts in this study. Of note, half of HIV-positive MSW in this analysis (but none of HIV-negative MSW) were from sub-Saharan Africa, the global epicentre of heterosexual HIV transmission. In HIV endemic settings, the long-term solution to anal cancer prevention in MSW remains direct and indirect protection from HPV vaccination. Nevertheless, our data also highlight how preventing HIV transmission could affect the future burden of male anal cancer in these settings.
Since establishment of the link between anal sexual intercourse and anal cancer risk,44 MSM have been the focus of anal HPV studies, and all age groups were well represented in this study. For every age category, pooled HPV prevalence was higher in HIV-negative MSM than in HIV-negative MSW, confirming the association with sexuality in immunocompetent men.45 High HPV exposure was already evident in young HIV-negative MSM, with approximately a quarter of those aged 15–18 years infected with HR-HPV. HPV prevalence increased rapidly with age, reaching a maximum by 23–24 years and remaining constant across older age groups. This pattern contrasts that observed for cervical HPV infection in immunocompetent women, which is characterised by a peak in young women (ie, those aged around 25 years) and a subsequent decline with increasing age.46 This difference could reflect biological factors (eg, impaired clearance of anal HPV compared with cervical HPV) or behavioural factors (eg, more new partners in older age groups in MSM than in women).47, 48
Anal HPV prevalence was consistently higher in HIV-positive MSM than in HIV-negative MSM, and this difference was already evident in the youngest men, with approximately half of HIV-positive MSM aged 15–18 years having been infected with HR-HPV. The prevalence of HPV increased rapidly with age, reaching a peak by 25 years. Although a significant decrease in prevalence was subsequently observed at older ages, it remained higher in HIV-positive MSM than in all other risk groups. Additional evidence for the role of HIV-related immunosuppression on HPV prevalence in MSM from this analysis, is the significantly higher prevalence of HPV16 in MSM with low versus high current CD4 cell counts and in those with high versus low HIV viral loads.49
In recognition of their elevated anal cancer risk and possible absence of herd immunity from girls-only vaccination programmes, some countries recommend targeted HPV vaccination of MSM. Nevertheless, given the absence of vaccine efficacy against previous HPV infection, the cost-benefit ratio of vaccination in this heavily exposed population decreases with age. For example, as HPV16 reaches peak prevalence by the age of 22–24 years, the incremental benefit of HPV vaccination might be expected to be minimal at older ages. Indeed, a clinical trial published in 2018, did not find effectiveness of HPV vaccine against anal HPV infection in HIV-infected adults aged 27 years or older.50 The potential limitation of a targeted approach for MSM is further highlighted by the high rate of current HPV detection, even in the youngest men who identify as homosexual, and in whom cumulative exposure (ie, including cleared and current infections) is expected to be even higher.51 Indeed, a large proportion of future anal cancers among young MSM are expected to occur among those with prevalent HPV16 infection. Hence, gender-neutral vaccination before sexual activity, rather than a targeted approach, is the long-term solution to anal cancer prevention for future generations of MSM, irrespective of HIV status.52
Current generations of MSM and HIV-positive men remain at high risk of anal cancer and could benefit from secondary prevention measures. In this report, we describe the epidemiology of anal HSIL in studies with concurrent assessment of cytology or histology, which, given the greater clinical imperative, tend to focus on HIV-positive MSM. Notably, however, even in this high-risk group with ubiquitously high HR-HPV infection, we observed wide variation in HSIL prevalence. Some of this heterogeneity could be explained by differences in the diagnostic strategy used between studies, with the lowest prevalence of anal HSIL+ observed between studies that used cytology only, consistent with reports of lower sensitivity for cytology versus high-resolution anoscopy-directed biopsy to diagnose HSIL.53 However, study-specific heterogeneity remained even in those that used high-resolution anoscopy in all participants, highlighting variability in the performance of this technique. Indeed, high-resolution anoscopy is a difficult technique characterised by a long learning period,54, 55 for which international standards were published only relatively recently, in 2016.56 Further support for differences in HSIL ascertainment, rather than underlying differences in risk, came from the observations that study-specific differences in HSIL were closely mirrored in HIV-negative MSM, and also when restricted to HPV16-positive MSM only (a homogeneously high-risk group of MSM).
We chose to study patient-level determinants (eg, HIV status and age) of HSIL+ outcomes in MSM, with a focus on relative, rather than absolute risk, and with adjustment by study. These analyses support HIV infection as a risk factor for HSIL in MSM.8 Further evidence for the worsening effect of HIV-related immunosuppression on HSIL came from the association between HSIL prevalence and low current CD4 cell counts. This finding highlights the potential effect of wider and earlier use of combined antiretroviral therapy (ART) to reduce risk of HSIL in HIV-positive populations.49
Recognising the unique anal carcinogenicity of HPV16, the novelty and power of our approach was to combine HSIL outcomes with concurrent HPV16 data. This combined analysis showed that HIV infection was associated with an increased risk of HPV16-positive HSIL, the most severe known anal cancer precursor that has a higher potential for persistence and for progression to cancer than HSIL with other HR-HPV types.5, 57 Even when restricted to HPV16-positive MSM, HIV infection (and low CD4 cell counts in HIV-positive MSM) conferred a significantly higher risk for HSIL compared with those who were HIV negative. Conversely, we found no evidence that nadir CD4 cell count affected the risk of HPV16-positive HSIL, and only a weak association between HPV16-positive HSIL risk with increasing age was observed. This result somewhat contrasts that for anal cancer risk, which is significantly associated with nadir CD4 cell count and increases significantly with age,2, 49 suggesting that not all prevalent HSIL (not even all HPV16-positive HSIL) are equally likely to progress to anal cancer, consistent with the report of increased spontaneous regression of HSIL observed in young MSM (those aged ≤35 years).58
Limitations of our analysis should be noted. We focused on characteristics associated with robust evidence on anal cancer risk that are pragmatic targets for public health programmes (ie, HIV status, sexuality, and age). Detailed sexual practices, for which data are less easily obtainable than for the included characteristics, were beyond the scope of our study. Nevertheless, studies of HIV-negative MSM could include important remaining heterogeneity, in terms of sexual behaviour, because of the over-represention of HIV-negative MSM recruited from sexual health clinics who are at higher risk of anal HPV infection than the whole HIV-negative MSM population.10 Indeed, the higher prevalence of HR-HPV in HIV-negative MSM from studies with cytopathology compared with those without cytopathology supports such selection bias, which could partly explain why age-specific anal HR-HPV prevalence was higher in HIV-negative MSM than in HIV-positive MSW, whereas relative anal cancer incidence appears to show an opposite pattern.2 External representativeness and internal consistency is expected to be better for HIV-positive MSM (who have a ubiquitously high risk and are recruited mainly from HIV treatment clinics) and HIV-negative MSW (who are predominantly recruited using community-based approaches). Time-dependent variables of duration of ART and immunosuppression, which are not typically available in studies, nor for pragmatic clinical decisions, were also beyond the scope of our study. For studies with HPV vaccination data,11, 15, 22, 30, 59 we excluded the few vaccinated men. Furthermore, combinations of age and country or calendar year of study recruitment preclude widespread HPV vaccination in other studies; therefore, we consider this analysis to represent HR-HPV prevalence in the absence of HPV vaccination. There were also insufficient data to allow robust comparison of age-specific prevalence within risk groups by region or other study-specific variables.
We addressed the major limitation of variability in HSIL ascertainment by adjusting relative risk estimates at an individual study level. We also did sensitivity analyses, which did not materially change the findings for MSM. By contrast, this problem did preclude the interpretation of HSIL outcomes by HIV status in MSW, for which data were too scarce to allow appropriate adjustment. Finally, we relied on a pragmatic definition of HPV16-positive HSIL, combining HPV16 obtained from anal swabs with the presence of composite HSIL, as detected from anal swabs or biopsies. Hence, we cannot establish true causality of HPV16 to HSIL, which, given the widespread multiplicity of HR-HPV highlighted by our analysis, would require laser capture microdissection of biopsies.60 For these reasons, we did not report on HSIL positive for non-HPV16 HR-HPV types, for which the causal links to anal cancer are less well established.5
Finally, findings from this pooled analysis can inform future anal screening efforts that involve HPV testing. Firstly, the results highlight the paucity of useful clinical stratification offered by testing 13 HR-HPV types defined as carcinogenic in the cervix; three quarters of HIV-positive MSM and half of HIV-negative MSM would test positive and require follow-up, despite the absence of strong evidence for anal carcinogenicity of non-HPV16 HR-HPV types. In a more specific approach of screening for HPV16 only, 20–30% of adult HIV-positive MSM and 10–15% of HIV-negative MSM would be positive at a single timepoint. If these men were referred for high-resolution anoscopy, our findings suggest that more than half could have HSIL, irrespective of HIV status, even if not all cases of HSIL would progress to cancer. Further improvements to risk stratification in high-risk men are therefore necessary, of which the most promising approaches include assessment of persistent, rather than prevalent, anal HPV16 infection57 or host methylation markers, which could have additional potential to prevent the few anal cancers caused by non-HPV16 HR-HPV types.61, 62
DData sharing
The data used for this analysis cannot be shared publicly because of legal and ethical requirements regarding the resharing of data from included studies. Researchers can request access to the summarised data from the corresponding author.
Declaration of interests
ARG received travel fees from Merck & Co to participate in scientific advisory board meetings, and her institution has received grants for research from Merck & Co. SEH is funded by the US National Institutes of Health and the Bill & Melinda Gates Foundation outside the submitted work; and is a consultant for In Bios and a grant reviewer for US National Institutes of Health Study Section outside the submitted work. SEG received an investigator-initiated grant from Merck & Co, Inovio, and Medtronic outside the submitted work; consulting fees from THD America outside the submitted work; and payment as a speaker and financial support for attending meetings from Merck & Co outside the submitted work. MFSvdL received funds awarded to his institution from the AidsFonds charity and from Merck Sharpe & Dohme for participation on a data safety monitoring board or advisory board. KN is the recipient of a Miguel Servet research grant (CPII18/00033) from the Instituto de Salud Carlos III (Madrid, Spain) outside the submitted work. AGN received an investigator-initiated grant from Merck & Co in 2010–11 awarded to his institution at the time (Moffitt Cancer Center, Tampa, FL, USA) to assess the prevalence and incidence of anal HPV among men, allowing genotyping of samples (these data are included in the current Article); received travel fees from EUROGIN to present at their conferences; and received donated swabs and vials from COPAN. UW received grant support from the German Federal Ministry of Health for a study included in this report. EPFC is supported by an Australian National Health and Medical Research Council Emerging Leadership Investigator Grant (GNT1172873) outside the submitted work. YC received financial support from the Canadian Institutes of Health Research scholarship/studentship (CGS-D) outside the submitted work. YH received funding from the Natural Science Foundation of China International/Regional Research Collaboration Project (72061137007) and the Natural Science Foundation of China (81673232 and 82073574) outside the submitted work. GM received funding from US National Institutes of Health/National Cancer Institute outside the submitted work. APO received funds awarded to her institution from Aids Malignancy Consortium (2UM1CA121947–14) outside the submitted work; funds awarded to her institution from California-Mexico-Puerto Rico Partnership Center for Prevention of HPV-related Cancer in HIV-positive Populations (5U54CA242646–02) and from UPR/MDACC: Partnership for Excellence in Cancer Research (5U54-CA096297–17 and 5R21DE027226–02) outside the submitted work; and consulting fees and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, and educational events from Merck & Co outside the submitted work. HZ received funding awarded to Sun Yat-sen University from the Natural Science Foundation of China Excellent Young Scientists Fund (82022064), the Natural Science Foundation of China International/Regional Research Collaboration Project (72061137001), and the Precision Targeted Intervention Studies among High Risk Groups for HIV Prevention in China, National Science and Technology Major Project of China (2018ZX10721102) outside the submitted work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by the International Agency for Research on Cancer. The authors alone are responsible for the views expressed in this paper and they do not necessarily represent the views, decisions, or policies of the institutions to which they are affiliated.
Contributors
GMC initiated and coordinated the study. FW and CJA collected and analysed the data. All other authors generated the data from the 62 original studies and contributed to the interpretation of the analysis. FW and GMC had full access to and verified all the data in the study. FW and GMC wrote the first draft of the manuscript. All authors provided input, approved the final manuscript, and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clifford GM, Georges D, Shiels MS. A meta-analysis of anal cancer incidence by risk group: towards a unified anal cancer risk scale. Int J Cancer. 2021;148:38–47. doi: 10.1002/ijc.33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Islami F, Ferlay J, Lortet-Tieulent J, Bray F, Jemal A. International trends in anal cancer incidence rates. Int J Epidemiol. 2017;46:924–938. doi: 10.1093/ije/dyw276. [DOI] [PubMed] [Google Scholar]
- 4.Shiels MS, Pfeiffer RM, Chaturvedi AK, Kreimer AR, Engels EA. Impact of the HIV epidemic on the incidence rates of anal cancer in the United States. J Natl Cancer Inst. 2012;104:1591–1598. doi: 10.1093/jnci/djs371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin C, Franceschi S, Clifford GM. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect Dis. 2018;18:198–206. doi: 10.1016/S1473-3099(17)30653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Biological agents. Volume 100B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100:1–475. [PMC free article] [PubMed] [Google Scholar]
- 7.Palefsky JM, Giuliano AR, Goldstone S. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 8.Machalek DA, Poynten M, Jin F. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13:487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 9.Lin C, Slama J, Gonzalez P. Cervical determinants of anal HPV infection and high-grade anal lesions in women: a collaborative pooled analysis. Lancet Infect Dis. 2019;19:880–891. doi: 10.1016/S1473-3099(19)30164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marra E, Lin C, Clifford GM. Type-specific anal human papillomavirus prevalence among men, according to sexual preference and HIV status: a systematic literature review and meta-analysis. J Infect Dis. 2019;219:590–598. doi: 10.1093/infdis/jiy556. [DOI] [PubMed] [Google Scholar]
- 11.Lowe B, Goldstone SE, Rus S. Detection of human papillomavirus in anal specimens using the hybrid capture 2 assay. Diagn Mol Pathol. 2012;21:150–156. doi: 10.1097/PDM.0b013e318249fd6b. [DOI] [PubMed] [Google Scholar]
- 15.Gaisa MM, Sigel KM, Deshmukh AA. Comparing anal cancer screening algorithms using cytology and HPV DNA testing in three high-risk populations. J Infect Dis. 2021 doi: 10.1093/infdis/jiaa801. https://doi.org//10.1093/infdis/jiaa801 published online Jan 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambursky JA, Terlizzi JP, Goldstone SE. Testing for human papillomavirus strains 16 and 18 helps predict the presence of anal high-grade squamous intraepithelial lesions. Dis Colon Rectum. 2018;61:1364–1371. doi: 10.1097/DCR.0000000000001143. [DOI] [PubMed] [Google Scholar]
- 30.D'Souza G, Wentz A, Wiley D. Anal cancer screening in men who have sex with men in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2016;71:570–576. doi: 10.1097/QAI.0000000000000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei F, Su Y, Cui X. Sequential acquisition of human papillomavirus infection at genital and anal sites, Liuzhou, China. Emerg Infect Dis. 2020;26:2387–2393. doi: 10.3201/eid2610.191646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daling JR, Madeleine MM, Johnson LG. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270–280. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 43.Drolet M, Benard E, Perez N, Brisson M, Group HPVVIS. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394:497–509. doi: 10.1016/S0140-6736(19)30298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daling JR, Weiss NS, Klopfenstein LL, Cochran LE, Chow WH, Daifuku R. Correlates of homosexual behavior and the incidence of anal cancer. JAMA. 1982;247:1988–1990. [PubMed] [Google Scholar]
- 45.Nyitray AG, Carvalho da Silva RJ, Baggio ML. Age-specific prevalence of and risk factors for anal human papillomavirus (HPV) among men who have sex with women and men who have sex with men: the HPV in Men (HIM) study. J Infect Dis. 2011;203:49–57. doi: 10.1093/infdis/jiq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiffman M, Doorbar J, Wentzensen N. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.86. [DOI] [PubMed] [Google Scholar]
- 47.Dona MG, Latini A, Benevolo M, Moretto D, Cristaudo A, Giuliani M. Anal human papillomavirus infection prevalence in men who have sex with men is age-independent: a role for recent sexual behavior? Future Microbiol. 2014;9:837–844. doi: 10.2217/fmb.14.44. [DOI] [PubMed] [Google Scholar]
- 48.Poynten IM, Machalek D, Templeton D. Comparison of age-specific patterns of sexual behaviour and anal HPV prevalence in homosexual men with patterns in women. Sex Transm Infect. 2016;92:228–231. doi: 10.1136/sextrans-2015-052032. [DOI] [PubMed] [Google Scholar]
- 49.Kelly H, Chikandiwa A, Alemany Vilches L, Palefsky JM, de Sanjose S, Mayaud P. Association of antiretroviral therapy with anal high-risk human papillomavirus, anal intraepithelial neoplasia, and anal cancer in people living with HIV: a systematic review and meta-analysis. Lancet HIV. 2020;7:e262–e278. doi: 10.1016/S2352-3018(19)30434-5. [DOI] [PubMed] [Google Scholar]
- 50.Wilkin TJ, Chen H, Cespedes MS. A randomized, placebo-controlled trial of the quadrivalent human papillomavirus vaccine in human immunodeficiency virus-infected adults aged 27 years or older: AIDS clinical trials group protocol A5298. Clin Infect Dis. 2018;67:1339–1346. doi: 10.1093/cid/ciy274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mooij SH, Landen O, van der Klis FR. HPV seroconversion following anal and penile HPV infection in HIV-negative and HIV-infected MSM. Cancer Epidemiol Biomarkers Prev. 2014;23:2455–2461. doi: 10.1158/1055-9965.EPI-14-0199. [DOI] [PubMed] [Google Scholar]
- 52.Chow EPF, Tabrizi SN, Fairley CK. Fall in human papillomavirus in young men who have sex with men after the implementation of gender-neutral HPV vaccination: a repeated cross-sectional study. Lancet Inf Dis. 2021 doi: 10.1016/S1473-3099(20)30687-3. published online May 24. [DOI] [PubMed] [Google Scholar]
- 53.Darragh TM, Winkler B. Anal cancer and cervical cancer screening: key differences. Cancer Cytopathol. 2011;119:5–19. doi: 10.1002/cncy.20126. [DOI] [PubMed] [Google Scholar]
- 54.Richel O, Prins JM, de Vries HJ. Screening for anal cancer precursors: what is the learning curve for high-resolution anoscopy? AIDS. 2014;28:1376–1377. doi: 10.1097/QAD.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 55.Siegenbeek van Heukelom ML, Marra E, Cairo I. Detection rate of high-grade squamous intraepithelial lesions as a quality assurance metric for high-resolution anoscopy in HIV-positive men. Dis Colon Rectum. 2018;61:780–786. doi: 10.1097/DCR.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 56.Hillman RJ, Cuming T, Darragh T. 2016 IANS international guidelines for practice standards in the detection of anal cancer precursors. J Low Genit Tract Dis. 2016;20:283–291. doi: 10.1097/LGT.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 57.Poynten IM, Jin F, Roberts JM. The natural history of anal high-grade squamous intraepithelial lesions in gay and bisexual men. Clin Infect Dis. 2021;72:853–861. doi: 10.1093/cid/ciaa166. [DOI] [PubMed] [Google Scholar]
- 58.Tong WW, Jin F, McHugh LC. Progression to and spontaneous regression of high-grade anal squamous intraepithelial lesions in HIV-infected and uninfected men. AIDS. 2013;27:2233–2243. doi: 10.1097/QAD.0b013e3283633111. [DOI] [PubMed] [Google Scholar]
- 59.Woestenberg PJ, van Benthem BHB, Bogaards JA. HPV infections among young MSM visiting sexual health centers in the Netherlands: opportunities for targeted HPV vaccination. Vaccine. 2020;38:3321–3329. doi: 10.1016/j.vaccine.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Cornall AM, Roberts JM, Molano M. Laser capture microdissection as a tool to evaluate human papillomavirus genotyping and methylation as biomarkers of persistence and progression of anal lesions. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-008439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Zee RP, Richel O, van Noesel CJM. Cancer risk stratification of anal intraepithelial neoplasia in HIV-positive men by validated methylation markers associated with progression to cancer. Clin Infect Dis. 2021;72:2154–2163. doi: 10.1093/cid/ciaa397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clarke MA, Wentzensen N. Strategies for screening and early detection of anal cancers: a narrative and systematic review and meta-analysis of cytology, HPV testing, and other biomarkers. Cancer Cytopathol. 2018;126:447–460. doi: 10.1002/cncy.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uncited References
- 12.Medina-Laabes DT, Suarez-Perez EL, Guiot HM. Human papillomavirus correlates with histologic anal high-grade squamous intraepithelial lesions in Hispanics with HIV. J Low Genit Tract Dis. 2018;22:320–325. doi: 10.1097/LGT.0000000000000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machalek DA, Poynten IM, Jin F. A composite cytology-histology endpoint allows a more accurate estimate of anal high-grade squamous intraepithelial lesion prevalence. Cancer Epidemiol Biomarkers Prev. 2016;25:1134–1143. doi: 10.1158/1055-9965.EPI-15-1106. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez AL, Efird JT, Holly EA, Berry JM, Jay N, Palefsky JM. Risk factors for anal human papillomavirus infection type 16 among HIV-positive men who have sex with men in San Francisco. J Acquir Immune Defic Syndr. 2013;63:532–539. doi: 10.1097/QAI.0b013e3182968f87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Pokomandy A, Rouleau D, Ghattas G. Prevalence, clearance, and incidence of anal human papillomavirus infection in HIV-infected men: the HIPVIRG cohort study. J Infect Dis. 2009;199:965–973. doi: 10.1086/597207. [DOI] [PubMed] [Google Scholar]
- 17.Clarke MA, Cheung LC, Lorey T. Five-year prospective evaluation of cytology, HPV testing, and biomarkers for detection of anal precancer in HIV+ MSM. Clin Infect Dis. 2018;69:631–638. doi: 10.1093/cid/ciy970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkin TJ, Chen H, Cespedes MS. A randomized, placebo-controlled trial of the quadrivalent human papillomavirus vaccine in human immunodeficiency virus-infected adults aged 27 years or older: AIDS clinical trials group protocol A5298. Clin Infect Dis. 2018;67:1339–1346. doi: 10.1093/cid/ciy274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viciana P, Milanes-Guisado Y, Fontillon M. High-risk human papilloma virus testing improves diagnostic performance to predict moderate- to high-grade anal intraepithelial neoplasia in human immunodeficiency virus-infected men who have sex with men in low-to-absent cytological abnormalities. Clin Infect Dis. 2019;69:2185–2192. doi: 10.1093/cid/ciz144. [DOI] [PubMed] [Google Scholar]
- 20.Phanuphak N, Teeratakulpisarn N, Keelawat S. Use of human papillomavirus DNA, E6/E7 mRNA, and p16 immunocytochemistry to detect and predict anal high-grade squamous intraepithelial lesions in HIV-positive and HIV-negative men who have sex with men. PLoS One. 2013;11 doi: 10.1371/journal.pone.0078291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Combes JD, Heard I, Poizot-Martin I. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus-positive men who have sex with men. J Infect Dis. 2018;217:1535–1543. doi: 10.1093/infdis/jiy059. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs W, Kreuter A, Hellmich M. Asymptomatic anal sexually transmitted infections in HIV-positive men attending anal cancer screening. Br J Dermatol. 2016;174:831–838. doi: 10.1111/bjd.14288. [DOI] [PubMed] [Google Scholar]
- 24.Burgos J, Curran A, Tallada N. Risk of progression to high-grade anal intraepithelial neoplasia in HIV-infected MSM. AIDS. 2015;29:695–702. doi: 10.1097/QAD.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 25.Posada DH, Acevedo LST, Arredondo MV, Vasquez GIS. High-risk human papillomavirus infection and associated factors in the anal canal of HIV-positive patients in Medellin, 2017–2018. Rev Saude Publica. 2020;54:93. doi: 10.11606/s1518-8787.2020054001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowak RG, Schumaker LM, Ambulos NP. Multiple HPV infections among men who have sex with men engaged in anal cancer screening in Abuja, Nigeria. Papillomavirus Res. 2020;10:0200. doi: 10.1016/j.pvr.2020.100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Pino M, Marti C, Gaber J. mRNA detection in anal cytology: a feasible approach for anal cancer screening in men who have sex with men living with HIV. Diagnostics. 2019;9:173. doi: 10.3390/diagnostics9040173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iribarren Diaz M, Ocampo Hermida A, Gonzalez-Carrero Fojon J. Preliminary results of a screening program for anal cancer and its precursors for HIV-infected men who have sex with men in Vigo-Spain. Rev Esp Enferm Dig. 2017;109:242–249. doi: 10.17235/reed.2017.4274/2016. [DOI] [PubMed] [Google Scholar]
- 29.Pernot S, Boucheron P, Pere H. Comparison of anal cancer screening strategies including standard anoscopy, anal cytology, and HPV genotyping in HIV-positive men who have sex with men. Br J Cancer. 2018;119:381–386. doi: 10.1038/s41416-018-0176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schofield AM, Sadler L, Nelson L. A prospective study of anal cancer screening in HIV-positive and negative MSM. AIDS. 2016;30:1375–1383. doi: 10.1097/QAD.0000000000001045. [DOI] [PubMed] [Google Scholar]
- 32.Hidalgo-Tenorio C, Gil-Anguita C, López Ruz MA, Omar M, López-Hidalgo J, Pasquau J. ART is key to clearing oncogenic HPV genotypes (HR-HPV) in anal mucosa of HIV-positive MSM. PLoS One. 2019;14 doi: 10.1371/journal.pone.0224183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Critchlow CW, Hawes SE, Kuypers JM. Effect of HIV infection on the natural history of anal human papillomavirus infection. AIDS. 1998;12:1177–1184. doi: 10.1097/00002030-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Hood JE, Gottlieb GS, Kiviat NB. The association between HPV, intraepithelial lesions and HIV-1 shedding in anogenital specimens in two contrasting populations: Senegalese women and American MSM. Int J STD AIDS. 2016;27:353–362. doi: 10.1177/0956462415580691. [DOI] [PubMed] [Google Scholar]
- 35.Cheng SH, Wang CC, Chang SL, Chu FY, Hsueh YM. Oncogenic human papillomavirus is not helpful for cytology screening of the precursor lesions of anal cancers in Taiwanese men who are infected with human immunodeficiency virus. Int J Clin Oncol. 2015;20:943–951. doi: 10.1007/s10147-015-0804-9. [DOI] [PubMed] [Google Scholar]
- 36.Leng CY, Low HC, Chua LL. Human papillomavirus 16 (HPV16) and HPV52 E6-specific immunity in HIV-infected adults on combination antiretroviral therapy. HIV Med. 2017;18:321–331. doi: 10.1111/hiv.12432. [DOI] [PubMed] [Google Scholar]
- 37.Donà MG, Benevolo M, Vocaturo A. Anal cytological abnormalities and epidemiological correlates among men who have sex with men at risk for HIV-1 infection. BMC Cancer. 2012;12:476. doi: 10.1186/1471-2407-12-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruanpeng D, Chariyalertsak S, Kaewpoowat Q. Cytological anal squamous intraepithelial lesions associated with anal high-risk human papillomavirus infections among men who have sex with men in northern Thailand. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damay A, Fabre J, Costes V. Human papillomavirus (HPV) prevalence and type distribution, and HPV-associated cytological abnormalities in anal specimens from men infected with HIV who have sex with men. J Med Virol. 2010;82:592–596. doi: 10.1002/jmv.21732. [DOI] [PubMed] [Google Scholar]
- 40.Wang CC, Chang SL, Chu FY, Cheng CY, Cheng SH. Human papillomavirus infection and anal cytology in Taiwanese homosexual men with and without HIV infection. J Infect Dev Ctries. 2019;13:318–325. doi: 10.3855/jidc.11162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used for this analysis cannot be shared publicly because of legal and ethical requirements regarding the resharing of data from included studies. Researchers can request access to the summarised data from the corresponding author.