Abstract
Objective:
This study evaluates differences in child healthcare utilization by maternal fertility status in the first four years of life.
Study Design:
The retrospective cohort evaluated Massachusetts (MA) live born infants using data linked from clinical assisted reproductive technology (ART) data, birth certificates, and hospital discharge records. Hospital records of infants born 2004-2017 to mothers of fertile (no infertility treatments or indicators of infertility), unassisted subfertile (UF, indicators of infertility but no fertility treatment), medically assisted reproduction (MAR, non-ART assistance with reproduction) and ART treatment were studied. Adjusted relative risk (aRR) was calculated using multivariable log binomial regression models.
Results:
We included 339,426 singleton live-born infants discharged from birth hospitalization. Compared to children born to fertile mothers, those born to UF, MAR and ART-treated mothers were more likely to have hospital-based care (aRR 1.06-1.21) in their first 4 years.
Conclusions:
Maternal subfertility with and without treatment was associated with small increases in child healthcare utilization.
Introduction
Use of assisted reproductive technology (ART)—those treatments that involve removal of eggs from a woman’s body and their manipulation in vitro—has continued to increase over many years1. Despite the increase in usage, numerous studies have demonstrated health risks for infants born of ART including increased rates of prematurity, low birthweight, and congenital anomalies2–6. These risks persist even in singletons7–11. Preterm birth is known to carry a lifelong influence on survivors12, nevertheless, the effect of ART on health outcomes of offspring after birth hospitalization, has been studied less extensively. Furthermore, subfertility itself, in the absence of ART treatment, has been shown to increase risks for prematurity, low birthweight, and congenital anomalies and could also be associated with additional morbidity in offspring13,14.
There are mixed results for studies of risks for children through early childhood. A review article by Chen and Heilbronn in 201715 summarized studies a number of which showed no increase in adverse health risk for children conceived through ART while others showed those children to have increased risk of respiratory disease, incidence of cerebral palsy as well as height, weight and growth differences with most comparing ART to unassisted conception in fertile women. Some studies in this review also suggested increased risk of metabolic and cardiac abnormalities. Similarly, Bergh and Wennerholm16 reviewed child health studies and found that even in singletons, many studies show no difference but a few demonstrate increased risk of some health conditions, in particular cardiovascular abnormalities and diabetes. Other studies have reviewed whether ART treatment parameters such as culture media17–19, and Prenatal Genetic Diagnosis (PGD)20 effect child health; again, results are mixed.
Clearly there is a need for additional studies to conclusively determine whether ART affects child health; and if so, how. One way of evaluating health is to examine hospitalizations experienced by children. In a recent study, we found that the incidence of respiratory and gastrointestinal conditions during the birth hospitalization were increased following deliveries to ART-treated and subfertile women21. In addition, this study demonstrated that hospitalizations for conditions of infectious disease and cardiovascular conditions were increased in those born in some gestational age groups (primarily >37 weeks) but not in other gestational age groups. Whether these risks continue into early childhood has yet to be fully evaluated. Thus, the goal of this study was to understand whether a mother’s subfertility and/or treatment with ART or other medically assisted reproduction (MAR) treatments resulted in increased health care utilization among singleton, liveborn children to the age of four.
Methods:
Data Source:
The Massachusetts Outcome Study of Assisted Reproductive Technology (MOSART) is a longitudinal population-based cohort of all births in Massachusetts. The details of the MOSART cohort have been previously described [6, 13, 14]. Briefly, MOSART is a database that combines the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS) (15), a clinical database of treatment information on ART cycles and the Pregnancy to Early Life Longitudinal (PELL) data system (16–18), which links birth certificates to hospital discharge records for mothers and infants in Massachusetts. PELL is a unique, longitudinal, population-based data system that links multiple sources of data, capturing diagnostic codes for hospital stays and health care utilization using hospital related discharge data.
Linkage
The MOSART database links the SART CORS and PELL data systems for all children born in Massachusetts hospitals to Massachusetts resident women between July 1, 2004 and December 31, 2013. We chose the starting date based on the availability of SART CORS data (January 1, 2004) to allow us to capture any births associated with ART and the end date reflected the latest available data from both SART and PELL when this analysis was initiated and allowed for appropriate offspring follow up (i.e. up to four years). A deterministic five phase linkage algorithm was used with matching based on the baby’s date of birth, mother’s date of birth, mother’s first name and last name, and father/partner’s last name22. The linkage rate was 90.2% overall and 94.6% for deliveries in which both mother’s zip code and clinic were in Massachusetts.
Study Sample
The sample for these analyses included Massachusetts children born as singleton births to mothers ≥18 years of age between July 1, 2004 and December 31, 2012. The sample was limited to children born at ≥23 weeks gestational age who survived their birth hospitalization and whose mothers were on private insurance at the time of delivery. The sample was limited to private insurance in order to minimize the effect of utilization of emergency room visits in the population that is publicly insured, where primary care/medical home may not be as well established. In addition, since this is a state with mandated infertility insurance, the vast majority of ART-treated or subfertile women have insurance. Children were followed until 12/31/15. We excluded patients with a missing covariate and/or outcome (Figure 1).
Figure:
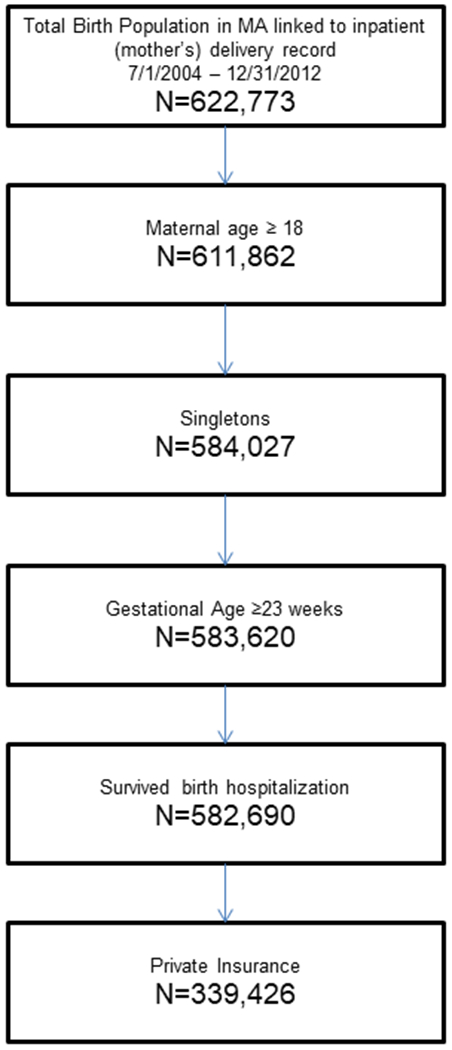
Population Cohort
Independent variable:
The primary independent variable of interest was the maternal fertility group. Women were categorized in one of four mutually exclusive groups: (1) fertile - those without ART or other non-ART MAR techniques or other indicators of infertility; (2) unassisted subfertile - those without ART, but who had other indicators of infertility, including a diagnosis of infertility (i.e. ICD9 code 628.9 or V23 code) in maternal hospitalization records during the 5 years prior to the birth, a prior ART cycle in SART CORS, or a prior birth or fetal death certificate indicating use of non-ART or non-ART MAR in an earlier delivery but who did not have ART or MAR on the delivery for this child); (3) MAR—those with indication of MAR on the birth certificate but whose delivery did not link to SART CORS and (4) ART-treated - those deliveries linked to ART cycles within the SART CORS online database. The definition of subfertility has been published previously23 and is closely aligned with the recent publication by the International committee for Monitoring Assisted Reproductive Technologies24. The term ‘subfertility’ was used instead of ‘infertility’ because a strict definition of infertility (one year of unprotected intercourse without conception) was not confirmed for many of the women in this group. The period of five years to help define the unassisted subfertile group was chosen based on earliest availability of PELL data.
Primary Outcomes and Co-variates:
The outcome was receipt of hospital-based care, measured by inpatient hospitalizations, observational stays, and emergency room (ER) visits for children through age four. Of note, 9.9% of patients were born in year 2012 and only have follow up to 4 years since the PELL database was only available through December 31, 2015. Birth hospitalization was not included, as we have previously reported these results21, 25. Due to potential differences in healthcare utilization over the first four years of life, we evaluated not only overall hospitalization but also each segment of life span by dividing into the first year, years one to three, and 4th year of life. The age of follow up was selected for two primary reasons: (1) the pre-school age is a critical stage of development and would allow to pick up on more subtle increase in inpatient healthcare utilization that may not be apparent at the birth hospitalization, particularly if the child was born at near term gestation; and (2) the availability of long enough follow up between the aforementioned linked databases to allow for sufficient follow up.
Covariates included maternal demographics (age, race, education, parity), maternal health conditions (chronic hypertension and pre-pregnancy diabetes), and year of birth. The reason for adjustment for year of birth was to account for the changes in ART technology over time and account for potential impact on utilization, as well as to account for any financial or clinical differences that may impact utilization year to year. Results were stratified by birth characteristics (gestational age, birthweight). As previously described in our prior studies, covariates for maternal health conditions were obtained from a combination of the birth certificate and hospital ICD 9 codes, all other covariates were obtained from the birth certificate21, 26.
Statistical Analyses:
Chi-square tests and ANOVA were applied for categorical and continuous variables respectively to assess the unadjusted relationships between the covariates and across maternal fertility groups. Mean length of stay was reported as least square means and standard error (SE) and was calculated using gamma log link regression. We report overall results and results stratified by GA (<37 weeks or >/=37 weeks) and birthweight (BW) (<2,500g or >/=2,500g). Multivariable log binomial regression models were adjusted for maternal age, race and ethnicity, education, chronic diabetes mellitus, chronic hypertension, parity, and birth year. We report percent and adjusted relative risk (aRR) and 95% Confidence Intervals (CI). SAS software version 9.4 (SAS Institute, Inc, Cary, NC) was used to perform all statistical analyses. The study was approved by the Institution Review Board of the Massachusetts Department of Public Health and the Dartmouth-Hitchcock Health IRB. Code availability for statistical methods can be requested from the corresponding author.
Results
There were 339,426 children in our cohort. Of these 316,187 were born to fertile, 6,308 to unassisted subfertile, 3,802 to non-ART MAR treated, and 13,129 to ART treated women. Maternal demographics for the four fertility groups are shown in Table 1. The ART-treated and subfertile mothers were older, more often non-Hispanic white, and had completed a higher level of education than the fertile mothers. In addition, ART-treated and subfertile mothers had more chronic diabetes and hypertension and lower parity. In addition, infants born to ART-treated and subfertile mothers were more likely to be preterm and low birthweight than babies born to fertile mothers (Table 1).
Table 1:
Maternal Demographics, Health, Delivery and Infant Outcome
| Demographic Characteristic | Category | Total | Fertile | Unassisted Subfertile | MAR | ART |
|---|---|---|---|---|---|---|
| Total N | 339426 | 316187 | 6308 | 3802 | 13129 | |
| Percent | % | % | % | % | % | |
| Age | 18-29 | 29 | 30.6 | 6.6 | 14.1 | 7.2 |
| 30-34 | 40.7 | 41.3 | 32.1 | 38.5 | 31.7 | |
| 35-37 | 17.9 | 17.3 | 28.6 | 22.1 | 24.8 | |
| 38-40 | 9 | 8.2 | 20.6 | 16.6 | 20.4 | |
| 41-42 | 2.4 | 2 | 8.2 | 5.4 | 8.6 | |
| 43+ | 1.1 | 0.8 | 3.9 | 3.2 | 7.2 | |
| Race/Ethnicity | Hispanic | 5.1 | 5.2 | 3.5 | 3.5 | 3.5 |
| Non-Hispanic White | 79.7 | 79.4 | 85.8 | 85 | 84.8 | |
| Non-Hispanic Black | 4.6 | 4.7 | 2.6 | 2.4 | 2.8 | |
| Non-Hispanic Asian | 10.6 | 10.7 | 8.2 | 9.1 | 8.9 | |
| Education | < HS/HS graduate | 21.9 | 22.6 | 14.8 | 11.4 | 12.6 |
|
|
Some college | 11.8 | 11.9 | 11.6 | 10.1 | 9.9 |
| College graduate | 66.3 | 65.5 | 73.6 | 78.4 | 77.5 | |
| Non-gestational diabetes | No | 98.9 | 98.9 | 98.4 | 98.4 | 98.2 |
| Yes | 1.1 | 1.1 | 1.6 | 1.6 | 1.8 | |
| Chronic hypertension | No | 98.2 | 98.2 | 97.7 | 97.3 | 97 |
| Yes | 1.8 | 1.8 | 2.3 | 2.7 | 3 | |
| Parity | 1 | 47.5 | 47.1 | 21.8 | 65.3 | 63 |
| 2 | 36.3 | 36.4 | 47.6 | 27.3 | 30.1 | |
| 3+ | 16.2 | 16.4 | 30.6 | 7.4 | 6.9 | |
| Method of Delivery | Vaginal | 64.5 | 65.3 | 50.3 | 58.6 | 52.2 |
| VBAC | 1.9 | 1.9 | 4 | 1.4 | 1.2 | |
| Primary CS | 19.2 | 18.6 | 14.2 | 28.9 | 32.4 | |
| Repeated CS | 14.3 | 14 | 31.2 | 11.1 | 14.1 | |
| Missing | 0.1 | 0.1 | 0.2 | 0 | 0.1 | |
| Year of birth | 2004 | 6.7 | 6.9 | 4.7 | 10 | 3.3 |
| 2005 | 12.9 | 13.1 | 9.3 | 12.3 | 11.1 | |
| 2006 | 12.6 | 12.7 | 11.7 | 11.1 | 11.4 | |
| 2007 | 12.4 | 12.4 | 13.1 | 9.2 | 11.8 | |
| 2008 | 12.3 | 12.3 | 14 | 7.3 | 11.4 | |
| 2009 | 11.5 | 11.5 | 12.3 | 8.9 | 12.2 | |
| 2010 | 11.2 | 11.1 | 12.3 | 8.3 | 13.8 | |
| 2011 | 10.5 | 10.3 | 11.7 | 14.7 | 12.2 | |
| 2012 | 9.9 | 9.7 | 11 | 18.1 | 12.8 | |
| Infant Characteristics | ||||||
| Infant Sex | Male | 51.3 | 51.3 | 51 | 51.2 | 51.6 |
| Female | 48.7 | 48.7 | 49 | 48.8 | 48.4 | |
| Gestational Age | Overall | 39 (2) | 39 (2) | 39 (2) | 39 (2) | 39 (2) |
| ≥37 weeks | 94.4 | 94.6 | 93.4 | 91.9 | 90.1 | |
| <37 week | 5.6 | 5.4 | 6.6 | 8.1 | 9.9 | |
| Birthweight | Overall | 3415 (530) | 3420 (526) | 3431 (542) | 3360 (571) | 3325 (596) |
| >2,500g | 95.8 | 95.9 | 95.7 | 93.6 | 92.5 | |
| ≤2,500g | 4.2 | 4.1 | 4.3 | 6.4 | 7.5 |
Demographics for the patient population. All p-values for comparison across the 4 groups are <0.0001 except for infant sex.
In the overall cohort, 50.9% of children had at least one hospital encounter during the first four years of life, however there was no difference among the fertility groups (Table 2). Nevertheless, when evaluated by type of hospitalization, although there was no difference among groups in ER visits, children of ART-treated and subfertile mothers had more observational stays and inpatient hospitalizations. This pattern was fairly consistent in all age categories (first year of life, 1–3 years, and 4th year) with ER visits being no different but either observational stays, hospitalization or both being more common in the ART-treated and subfertile groups.
Table 2:
Unadjusted healthcare utilization overall and by age of children
| Total | Fertile | Unassisted Subfertile | MAR | ART | p-value | |
|---|---|---|---|---|---|---|
| N | 339,426 | 316,187 | 6,308 | 3,802 | 13,129 | |
| Overall | % | % | % | % | % | |
| ER Visit | 47.6 | 47.6 | 47.1 | 47.3 | 48.0 | 0.6162 |
| Observation | 4.6 | 4.6 | 5.2 | 5.5 | 5.2 | <.0001 |
| Hospitalization | 9.5 | 9.4 | 10.1 | 10.1 | 10.2 | 0.0086 |
| Length of stay (days (SE))* | -- | 4.72 (0.08) | 5.19 (0.89) |
5.38 (0.89) | 4.60 (0.33) | 0.3218 |
| Any hospital encounter | 50.9 | 50.9 | 51.2 | 51.1 | 51.7 | 0.3155 |
| First year (Excluding birth hospitalization) | ||||||
| ER Visit | 21.9 | 21.9 | 20.6 | 21.6 | 21.9 | 0.0836 |
| Observation | 6.0 | 5.9 | 6.6 | 6.3 | 6.2 | 0.0586 |
| Hospitalization | 2.1 | 2.1 | 2.4 | 2.2 | 2.3 | 0.0685 |
| Length of stay (days (SE))* | -- | 4.63 (0.03) | 5.37 (0.27) | 4.46 (0.30) | 4.95 (0.18) | 0.0069 |
| Any hospital encounter | 26.0 | 26.0 | 25.5 | 26.0 | 26.4 | 0.5198 |
| Utilization in the years 1-3 | ||||||
| ER Visit | 32.7 | 32.7 | 31.2 | 32.7 | 32.6 | 0.0784 |
| Observation | 3.5 | 3.5 | 3.8 | 3.6 | 3.8 | 0.0771 |
| Hospitalization | 2.0 | 2.0 | 2.3 | 2.4 | 2.2 | 0.024 |
| Length of stay (days (SE))* | -- | 4.19 (0.04) | 3.72 (0.26) | 6.64 (0.61) | 3.61 (0.17) | <.0001 |
| Any hospital encounter | 34.3 | 34.3 | 33.3 | 34.7 | 34.4 | 0.3357 |
| Utilization in the 4th year | ||||||
| ER Visit | 15.0 | 14.9 | 15.6 | 14.8 | 15.6 | 0.0763 |
| Observation | 1.1 | 1.1 | 1.2 | 1.2 | 1.4 | 0.0355 |
| Hospitalization | 0.8 | 0.8 | 0.9 | 1.2 | 1.0 | 0.0071 |
| Length of stay (days (SE))* | -- | 3.91 (0.07) | 3.60 (0.41) | 2.93 (0.43) | 3.17 (0.24) | 0.0138 |
| Any hospital encounter | 15.9 | 15.9 | 16.7 | 16.1 | 16.8 | 0.0096 |
Unadjusted rates of healthcare utilization.
Hospital encounter=ER visit, observation, or hospitalization;
Length of stay is calculate only for the patients who had a hospitalization encounter; significance across fertility groups for length of stay was calculated using gamma log link regression; least square means and standard error are reported
Hospitalization data stratified by gestational age and birthweight are shown in Table 3. Overall, observational stays and in-patient hospitalizations were more common in children of ART-treated and subfertile women (both unassisted subfertile and MAR), however, when stratified, these differences only persisted for observational stays in children born at term or with birthweight >=2500 grams.
Table 3:
Unadjusted healthcare utilization stratified by gestational age and birthweight
| Total | Fertile | Unassisted Subfertile | MAR | ART | p-value | |
|---|---|---|---|---|---|---|
| GA | ||||||
| <37 weeks | ||||||
| N | 18686 | 16706 | 407 | 301 | 1272 | |
| ER | 51.7 | 51.7 | 47.2 | 55.1 | 52.2 | 0.1794 |
| Observation | 16.2 | 16.3 | 16 | 14.6 | 15.7 | 0.824 |
| Hospitalization | 7.6 | 7.6 | 5.7 | 11 | 8 | 0.0625 |
| No hospital utilization | 43.1 | 43.1 | 48.2 | 40.2 | 42.4 | 0.1359 |
| ≥37 weeks | ||||||
| N | 315794 | 295112 | 5763 | 3407 | 11512 | |
| ER | 47.4 | 47.4 | 47.1 | 46.8 | 47.6 | 0.845 |
| Observation | 9.1 | 9 | 9.7 | 9.7 | 9.5 | 0.0765 |
| Hospitalization | 4.4 | 4.4 | 5.1 | 5 | 4.9 | 0.0011* |
| No hospital utilization | 49.4 | 49.4 | 48.8 | 49.5 | 49 | 0.6022 |
| BW | ||||||
| ≤2500 grams | ||||||
| N | 14360 | 12861 | 271 | 243 | 985 | |
| ER | 50.8 | 50.8 | 48 | 56 | 50.6 | 0.3226 |
| Observation | 15.9 | 15.9 | 18.1 | 14.4 | 16.3 | 0.6846 |
| Hospitalization | 7.9 | 7.9 | 5.5 | 9.9 | 8.3 | 0.3005 |
| No hospital utilization | 44.3 | 44.3 | 46.5 | 40.7 | 44.2 | 0.6181 |
| ≤2500 grams | ||||||
| N | 323861 | 302224 | 5996 | 3542 | 12099 | |
| ER | 47.4 | 47.4 | 47 | 46.7 | 47.8 | 0.621 |
| Observation | 9.2 | 9.1 | 9.7 | 9.8 | 9.6 | 0.0763 |
| Hospitalization | 4.5 | 4.4 | 5.2 | 5.2 | 5 | <0.0001* |
| No hospital utilization | 49.3 | 49.3 | 48.9 | 49.4 | 48.6 | 0.4377 |
Unadjusted healthcare utilization stratified for prematurity and birthweight
denotes statistical significance with p<0.05
Table 4 presents the aRRs and 95% CIs for comparisons of each of the fertility groups with the fertile group as reference. The most notable differences were found when the ART-treated group was compared with the fertile group however differences were also seen between the MAR and fertile and the unassisted subfertile and fertile groups. Again, as in Table 2 differences were most common for children born of term deliveries and of normal birthweight. Comparisons within fertility groups are shown in Supplemental Table 1. No significant differences were seen between ART and the subfertile groups.
Table 4:
Adjusted Relative Risk (aRR) and 95% Confidence Intervals (95%CI) for Stratified Utilization1
| Unassisted subfertile vs Fertile | MAR vs Fertile | ART vs Fertile | |
|---|---|---|---|
| aRR (95%CI) | aRR (95%CI) | aRR (95%CI) | |
| Overall | |||
| ER | 1.03 (1.00,1.06) | 1.04 (1.00,1.07) | 1.06 (1.04,1.08)* |
| Hospitalization | 1.07 (0.99,1.15) | 1.17 (1.07,1.29)* | 1.17 (1.11,1.24)* |
| Observation | 1.15 (1.03,1.28*) | 1.26 (1.10,1.44)* | 1.21 (1.12,1.31)* |
| No Hospitalization | 0.96 (0.93,0.98)* | 0.95 (0.92,0.98)* | 0.94 (0.92,0.96)* |
| GA <37 weeks | |||
| ER | 0.95 (0.86,1.06) | 1.14 (1.03,1.26)* | 1.09 (1.03,1.15)* |
| Hospitalization | 0.99 (0.79,1.24) | 0.96 (0.73,1.27) | 1.04 (0.91,1.19) |
| Observation | 0.77 (0.51,1.15) | 1.45 (1.04,2.02)* | 1.10 (0.90,1.35) |
| No Hospitalization | 1.07 (0.96,1.18) | 0.88 (0.77,1.01) | 0.91 (0.85,0.98)* |
| ≥37 weeks | |||
| ER | 1.04 (1.01,1.07)* | 1.03 (0.99,1.07) | 1.06 (1.04,1.08)* |
| Hospitalization | 1.07 (0.99,1.16) | 1.18 (1.06,1.31)* | 1.15 (1.09,1.22)* |
| Observation | 1.17 (1.04,1.31)* | 1.19 (1.03,1.38)* | 1.19 (1.10,1.30)* |
| No Hospitalization | 0.95 (0.93,0.98)* | 0.95 (0.92,0.98)* | 0.94 (0.93,0.96)* |
| BW ≤2500 grams | |||
| ER | 0.95 (0.84,1.08) | 1.18 (1.05,1.31)* | 1.07 (1.00,1.14) |
| Hospitalization | 1.09 (0.84,1.42) | 0.99 (0.73,1.35) | 1.09 (0.94,1.27) |
| Observation | 0.67 (0.41,1.10) | 1.30 (0.88,1.92) | 1.1 (0.88,1.38) |
| No Hospitalization | 1.03 (0.91,1.17) | 0.86 (0.73,1.00) | 0.93 (0.86,1.00) |
| >2500 grams | |||
| ER | 1.04 (1.01,1.06)* | 1.03 (0.99,1.07) | 1.06 (1.04,1.08)* |
| Hospitalization | 1.06 (0.98,1.15) | 1.18 (1.07,1.31)* | 1.16 (1.09,1.23)* |
| Observation | 1.18 (1.06,1.32)* | 1.24 (1.08,1.43)* | 1.21 (1.11,1.31)* |
| No Hospitalization | 0.95 (0.93,0.98)* | 0.95 (0.92,0.98)* | 0.94 (0.92,0.96)* |
Fertile is the reference for all comparisons. Models adjusted for mother’s age, race, education, parity. birth year, pre-pregnancy diabetes and chronic hypertension.
Significantly different (95% Confidence Intervals do not cross 1).
Discussion
In this paper we demonstrated that maternal subfertility and fertility treatment are associated with a small increase in healthcare utilization in the first four years of life of their offspring. Hospitalizations and observational stays, but not ER visits, were increased in offspring of mothers in all 3 non-fertile groups. This pattern was particularly notable in the term population and in children born at birth weight >=2500 grams.
The ART-treated group showed the most notable differences from fertile overall and in the various gestational age and birthweight groups. We hypothesize that this association may be due to the likelihood that women undergoing ART have more underlying medical pathology than those in the other groups, although it does not eliminate the possibility that the ART treatment itself contributed to the increased risk. Nevertheless, the children of the non-ART subfertile groups also had an increased usage of hospital services suggesting that a woman’s underlying pathology associated with infertility was also a key factor. These observations are consistent with previous observations of neonatal outcomes as well as early childhood health that show both children conceived through ART and those of subfertile women at increased risk of adverse outcomes13, 14.
Previous studies have demonstrated varied results on whether child health is influenced by a mother’s subfertility or ART-treatment. While some studies show no difference in child health others have demonstrated differences between ART and fertile groups as to cardiovascular health, diabetes, and cerebral palsy15,16. There are also studies demonstrating that some risks may persist to the age of 8 or more years19, 27,28. It should be recognized that the magnitude of differences demonstrated in these studies has been small.
Hospital based utilization such as inpatient hospitalization, observational stays and ER visits, was used in this study as a marker of child health. Nevertheless, use of the ER as the location for primary care in those who have no insurance could increase use of these services without resulting from increases in underlying disease29,30. Given that Massachusetts is an infertility insurance mandated state, a disproportionate number of the uninsured women would have been in the fertile group23. We sought to limit this confounding effect by confining our cohort to the privately insured population. In this study, children born of ART-treatment, non-ART MAR and to untreated subfertile women, had a small (between 6% and 21%) increase in overall hospitalization (all categories) suggesting a small increase in underlying pathology in these children. Nevertheless, it is also possible that some hospitalization in these children resulted from an overzealousness on the part of parents of these “precious” children, in many cases conceived with difficulty, to deal with any conditions that might threaten the health of their children31.
Our study showed the greatest increases in hospital usage for children of subfertile women to be found among the term deliveries and children born of birthweight >=2500 grams. The Barker hypothesis12 suggests that being born small or premature can have ongoing consequences for long term health. Thus, some of the differences in the in the low birthweight and premature populations based on the maternal fertility group may be obscured by the expected increase in healthcare utilization in those two strata.
This study has several limitations. First, MOSART is composed of hospital data from administrative databases which can contain inaccuracies and incomplete information, although the number of hospitalizations is likely highly accurate. In addition, the definitions of our subfertile groups are dependent on birth certificate information, known to be imperfect as described previously32. Furthermore, 9.4% of the ART group was conceived via donor ovum, with an apparent reduction in any utilization within the ART group between donor (48.2%) and non-donor (51.6%) ovum strata (unadjusted p=0.02). A more comprehensive analysis of hospitalizations by ART parameters requires further exploration in future publications. We are missing some information that could be helpful such as BMI of the mothers and details of fetal growth. The last year birth year of the study group (2012) did not have follow up through four years of age given that the PELL data was not available beyond 2015 at the time of the analysis. Given that only 9.9% of the patients were born in 2012 and the hospital encounters in the 4th year of life is less than 20%, the lack of complete forth year affects a relatively small percentage of our study group and unlikely to impact our results. Lastly, the study was specific to Massachusetts and it is also the possible that some families could have moved out of state during the study period making hospitalizations of those children impossible to capture. In addition, a small portion of patients who reside close to the border of the neighboring states in New England may seek some care in those states out of convenience and thus their utilization is not captured. Nevertheless, it is unlikely the percentage that left the state differed in the different fertility groups. Finally, the study population was a Massachusetts cohort which may not be generalizable to other states or countries.
Despite these limitations, this study includes one of the largest cohorts of children born to subfertile, MAR, and ART-treated mothers. Moreover, children born from ART treatment were identified through linkage to the SART CORS database, the gold standard for ART treatment. Limiting our population to those with private insurance only, also allowed us to overcome the issues associated with overuse of the hospital system by uninsured individuals, who would have been more prevalent in the fertile group.
Conclusions
In summary, our study showed a small but significant increase in utilization of hospital services among children of both ART-treated mothers and mothers who were subfertile but did not receive ART treatment. Further study will be needed to determine the significance of these differences and the long-term influence on child health and development. We are currently extending our studies of child health to use of the Massachusetts All Payors Claims Database to determine whether disease diagnosed during outpatient visits is equally increased in these groups. We are also currently investigating the relative costs for these groups which in order to provide additional information on underlying disease and resource usage.
Supplementary Material
Acknowledgment:
Society for Assisted Reproductive Technology (SART) wishes to thank all of its members for providing clinical information to the SART CORS database for use by patients and researchers. Without the efforts of SART members, this research would not have been possible.
This study was supported by a grant from the National Institutes of Health RO1HD67270.
Funding Source: National Institutes of Health R01HD067270
Abbreviations:
- ART
assisted reproductive technology
- LOS
Length of Stay
- MOSART
Massachusetts Outcome Study of Assisted Reproductive Technology
- PELL
Pregnancy to Early Life Longitudinal
- SART CORS
Society for Assisted Reproductive Technology Clinic Outcome Reporting System
Footnotes
Financial Disclosures: The authors have indicated they have no relevant financial relationships to disclose.
Conflict of Interests: The authors have indicated they have no conflict of interests to this article.
REFERENCES:
- 1.Adamson GD, de Mouzon J, Chambers GM, Zegers-Hochschild F, Mansour R, Ishihara O, et al. International Committee for Monitoring Assisted Reproductive Technology: world report on assisted reproductive technology, 2011. Fertil Steril 2018;110:1067–1079 10.1016/j.fertnstert.2018.06.039 [DOI] [PubMed] [Google Scholar]
- 2.Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med 2002;346:725–30. [DOI] [PubMed] [Google Scholar]
- 3.Helmerhorst FM, Perquin DAM, Donker D, Keirse JNC. Perinatal outcome of singletons and twins after assisted conception: A systematic review of controlled studies. BMJ 2004;328:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen M, Bower C, Milne E, de Klerk N, Kurinczuk JJ. Assisted reproductive technologies and the risk of birth defects—a systematic review. Hum Reprod 2005;20:328–38. [DOI] [PubMed] [Google Scholar]
- 5.Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA, and the National Birth Defects Prevention Study. Assisted reproductive technology and major structural birth defects in the United States. Hum Reprod 2009;24:360–6. [DOI] [PubMed] [Google Scholar]
- 6.El-Chaar D, Yang Q, Gao J, Bottomley J, Leader A, Wen SW, Walker M. Risk of birth defects increased in pregnancies conceived by assisted human reproduction. Fertility and Sterility 2009;92:1557–61. [DOI] [PubMed] [Google Scholar]
- 7.Schieve LA, Ferre C, Peterson HB, Macaluso M, Reynolds MA, Wright VC. Perinatal Outcome Among Singleton Infants Conceived Through Assisted Reproductive Technology in the United States. Obstetrics & Gynecology. 2004;103(6):1144–11538. [DOI] [PubMed] [Google Scholar]
- 8.Sutcliffe AG, Ludwig M. Outcome of assisted reproduction. Lancet. 2007;370:351–359. [DOI] [PubMed] [Google Scholar]
- 9.Henningsen AK, Pinborg A, Lidegaard O, Vestergaard C, Forman JL, Andersen AN. Perinatal outcome of singleton siblings born after assisted reproductive technology and spontaneous conception: Danish national sibling cohort study. Fertil Steril. 2011;95:959–963 [DOI] [PubMed] [Google Scholar]
- 10.Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Soderstrom-Antilla V, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2013;19:87–104. [DOI] [PubMed] [Google Scholar]
- 11.Dunetz GL, Holzman C, McKane P, Li C, Boulet SL, Todem D, et al. Assisted reproductive technology and the risk of preterm birth among primiparas. Fertil Steril. 2015;103:974–9 doi: 10.1016/j.fertnstert.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raju TNK, Buist AS, Blaisdell CJ, Moxey-Mims M, Saigal S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatr. 2017. 106(9):1409–1437. doi: 10.1111/apa.13880. [DOI] [PubMed] [Google Scholar]
- 13.Declercq E, Luke B, Belanoff C, Cabral H, Diop H, Gopal D, et al. Perinatal Outcomes Associated with Assisted Reproductive Technology: the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART). Fertility and Sterility 2015;103:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luke B, Gopal D, Cabral H, Stern JE, Diop H. Pregnancy, Birth, and Infant Outcomes by Maternal Fertility Status: The Massachusetts Outcomes Study of Assisted Reproductive Technology. American Journal Obstetrics and Gynecology. 2017;217:327.e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M, Heilbronn LK. The health outcomes of human offspring conceived by assisted reproductive technologies (ART). Journal of Developmental Origins of Health and Disease. 2017;8(4):388–402. [DOI] [PubMed] [Google Scholar]
- 16.Bergh C, Wennerholm UB. Long-term health of children conceived after assisted reproductive technology. Journal of Medical Sciences: 2020:125 DOI: 10.1080/03009734.2020.1729904: 10.1080/03009734.2020.1729904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinborg A, Loft A, Schmidt L, Andersen AN. Morbidity in a Danish National cohort of 472 IVF/ICSI twins, 1132 non-IVF/ICSI twins and 634 IVF/ICSI singletons: health-related and social implications for the children and their families. Human Reproduction. 2003:18(6):1234–1243. [DOI] [PubMed] [Google Scholar]
- 18.Catford SR, McLachlan RI, O’Bryan MK, Halliday JL. Long-term follow-up of intra-cytoplasmic sperm injection conceived offspring compared with in vitro fertilization-conceived offspring: a systematic review of health outcomes beyond the neonatal period. Andrology:2017;5:610–621. [DOI] [PubMed] [Google Scholar]
- 19.Zandstra H; Brentjens LBPM; Spauwen B; Touwslager RNH; Bons JAP; Mulder AL; et al. Association of culture medium with growth, weight and cardiovascular development of IVF children at the age of 9 years. Human Reproduction.2018;33(9):1645–1656. [DOI] [PubMed] [Google Scholar]
- 20.Heijligers M, Peeters A, van Montfoort A, Nijsten J, Janssen E, Gunnewiek FK, et al. Growth, health, and motor development of 5-year-old children born after preimplantation genetic diagnosis. Fertil Steril:2019;111:1151–8. [DOI] [PubMed] [Google Scholar]
- 21.Hwang SS. Dukhovny D, Gopal D, Cabral H, Missmer SA, Diop H, et al. Health of infants following ART-treated, subfertile and fertile deliveries in Massachusetts. Pediatrics 2018:142(2);e20174069; DOI: 10.1542/peds.2017-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotelchuck M, Hoang L, Stern JE, Diop H, Belanoff C, Declercq E. The MOSART database: linking the SART CORS clinical database to the population-based Massachusetts PELL reproductive public health data system. Matern Child Health J. 2014;18(9):2167–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Declercq ER, Belanoff C, Diop H, Gopal D, Hornstein MD, Kotelchuk M, et al. Identifying women with indicators of subfertility in a statewide population database: operationalizing the missing link in assisted reproductive technology research. Fertil Steril. 2014;101(2):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393–406. [DOI] [PubMed] [Google Scholar]
- 25.Dukhovny D, Hwang SS, Gopal D, Cabral H, Missmer S, Diop H, et al. .Length of stay and cost of birth hospitalization: effects of subfertility and ART. JPerinatol:2018; August: 10.1038/s41372-018-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern JE, Liu CL, Cabral H, Gopal D, Harvey E, Missmer SA, et al. Hospitalization before and after delivery in fertile, subfertile, and ART-treated women. JARG. 2019;36(10):1989–1997. DOI: 10.1007/s10815-019-01562-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceelen M, van Weissenbruch MM, Prein J, Smit JJ, Vermeiden JPW, Spreeuwenberg M, et al. Growth during infancy and early childhood in relation to blood pressure and body fat measures at age 8–18 years of IVF children and spontaneously conceived controls born to subfertile parents, Human Reproduction 2009;24(11):2788–2795, https://academic.oup.com/humrep/article/24/11/2788/627120 [DOI] [PubMed] [Google Scholar]
- 28.Hart R, Norman RJ. The longer-term health outcomes for children born as a result of IVF treatment: Part I–General health outcomes, Human Reproduction Update 2013;19(3):232–243. https://academic.oup.com/humupd/article/19/3/232/727781 [DOI] [PubMed] [Google Scholar]
- 29.Dovey S, Weitzman M, Fryer G, Green, Lawn B, Lanier D, et al. The ecology of medical care for children in the United States. Pediatrics May 2003, 111 (5) 1024–1029; DOI: https://pediatrics.aappublications.org/content/111/5/1024 [DOI] [PubMed] [Google Scholar]
- 30.Simpson L, Owens PL, Zodet MW, Chevarley FM, Dougherty D, Elixhauser A, et al. Health care for children and youth in the United States: annual report on patterns of coverage, utilization, quality, and expenditures by income. Ambul Pediatr. 2005;5(1):6–44. doi: 10.1367/A04-119R.1 [DOI] [PubMed] [Google Scholar]
- 31.Srebnik N, Miron-Shatz T, Rolison JJ, Hanoch Y, Tsafrir A. Physician recommendation for invasive prenatal testing: the case of the ‘precious baby Human Reprod 2013;28:3007–11. [DOI] [PubMed] [Google Scholar]
- 32.Bacal V, Russo M, Fell DB, Shapiro H, Walker M, Gaudet LM. A systematic review of database validation studies among fertility populations. 2019: Human Reproduction Open, pp. 1–14. doi: 10.1093/hropen/hoz010 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


