Abstract
Background:
IL-33 is an emerging key factor in development of allergic diseases. The IL-33 receptor (ST2) is a differentially expressed gene in pathogenic TH2 cells but its role in T cell effector function has not been elucidated.
Objective:
We investigated the role of IL-33 in modulating circulating allergen-specific T cell responses. We hypothesized selective ST2 expression on allergen-specific CD4+ T cells would confer susceptibility to the effects of IL-33.
Methods:
PBMCs from food allergic, inhalant allergic, and non-allergic subjects were obtained based on clinical history and serum IgE. A T cell receptor (TCR) dependent CD154 up-regulation assay and direct peptide-major histocompatibility complex class II (pMHC-II) tetramer staining were used to profile allergen specific CD4+ T cells by flow cytometry. Allergen specific CD4+ T cell cytokine production was evaluated during IL-33 exposure. ST2 expression was also tracked using a two-color flow-based assay.
Results:
ST2 expression on peripheral allergen-specific CD4+ T cells was confined to allergic subjects and restricted to TH2A cells. Comparison between direct pMHCII tetramer staining and the CD154 functional assay identified ST2 as a TH2A-activation marker. IL-33 exposure enhanced IL-4 and IL-5 secretion in allergen-reactive TH2A cells. Allergen-induced ST2 expression on peripheral CD4+ T cells can be used to track allergen-reactive TH2A cells from allergic donors.
Conclusion:
ST2 expression on circulating CD4+ T cells represents a transient phenotype associated with TH2A cell activation allowing these cells to sense locally-elicited tissue cytokines. IL-33 selectively amplifies pathogenic TH2 cell effector functions suggesting a tissue checkpoint that may regulate adaptive allergic immunity.
Keywords: T cell, ST2, TH2A, IL-33, CD154, allergy, epithelial cytokine, pMHCII tetramer
Graphical Abstract
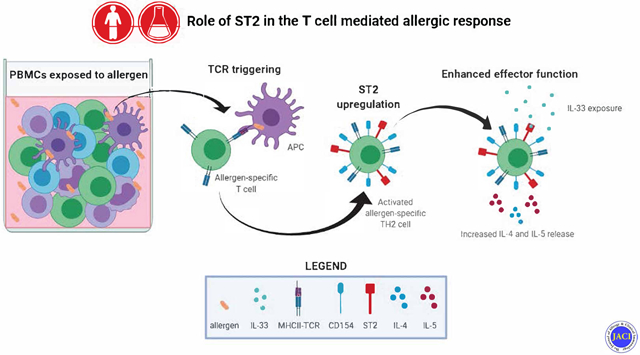
Capsule summary:
TH2A cell activation is associated with surface expression of ST2 providing a tissue checkpoint that regulates adaptive allergic immunity.
INTRODUCTION
CD4+ helper T cells comprise a variety of subsets with distinct effector function and homing properties to aid in host defense against pathogens. While T helper type 1 (TH1) and TH17 responses are involved in the adaptive immune response against bacteria, fungi and viruses, the TH2 cell subset evolved to defend against extracellular parasites such as helminths. Recently, our group identified a pathogenic TH2 cell subpopulation confined to individuals with allergic inflammation that are phenotypically and functionally distinct from conventional TH2 cells. These cells, termed TH2A cells, are defined by a unique surface marker expression profile characterized by expression of CRTH2 and CD161 and a lack of CD27 expression (1). Transcriptional profiling also revealed high gene expression of the IL-33 receptor (IL1RL1, also known as ST2) and the IL-25 receptor (IL-17RB) (1-3) suggesting that TH2A cells may sense epithelial cytokines. IL-33 is a nuclear cytokine expressed by epithelial and endothelial tissues as well as lymphoid organs (4). Under homeostatic conditions, IL-33 exhibits high intracellular expression in cells. However, in the presence of noxious stimuli such as allergen exposure in atopic individuals, tissue damage causes extracellular IL-33 release suggesting that it can function as an alarmin that alerts the immune system. IL-33 plays important roles in type-2 innate immunity via direct activation of eosinophils, basophils, mast cells, macrophages, and group 2 innate lymphoid cells (ILC2s) through its receptor ST2 (5, 6). These cell types sense damage through this pathway and respond by increasing transcription of pro-inflammatory genes, leading to the production of cytokines associated with innate type 2 immune responses (4, 6, 7).
IL-33 driven TH2 cytokine production via binding to ST2 in vitro and in vivo was first demonstrated in mice (8). While there is convincing evidence ST2 is expressed by murine TH2 cells (5, 9-12), data showing ST2 protein expression on the surface of human CD4+ T cells is still lacking and its role on human T cells in allergy has not yet been elucidated. Thus far, it is assumed ST2 is expressed by human TH2 cells due to the effects of IL-33 in the allergic process (12-15). The implications of IL-33 in the allergic process are significant and it has long been regarded as a novel therapeutic target. Considering that IL-33 release is an upstream event in the allergic process, there has been a growing interest in the development of antibodies that target alarmin cytokines or their corresponding receptors, thereby blocking the remainder of the allergic process.
In this study, we asked whether human allergen-specific CD4+ T cells in peripheral blood express ST2, and whether epithelial cytokines modulate effector function. We focused our study on IL-33 due to emerging trials investigating IL-33 blockade (16, 17). IL-33 acts on several cell types as a central mediator of atopic diseases including food allergies, asthma and atopic dermatitis. Here, we chose peanut allergy as a model for food allergy and included seasonal (timothy grass, alder pollen) and perennial (house dust mite) allergies as models for inhalant allergies. We demonstrate that allergen-specific TH2A cell activation is associated with the surface expression of ST2 and IL-17RB allowing these cells to sense epithelial cytokines. We also show IL-33 enhances TH2A effector function, suggesting a checkpoint that may modulate allergic responses at sites of tissue damage. As an enhancer of the allergic immune response, IL-33 represents an attractive therapeutic target in the treatment of atopic diseases.
METHODS
Study subjects
Fresh or frozen peripheral blood mononuclear cells (PBMCs) were obtained from subjects allergic to peanut, alder pollen, grass pollen, and/or house dust mite. Subjects were recruited based on clinical history, serum specific IgE > 0.35 kU/L, and positive skin prick test. We based our recruitment criteria on previous work demonstrating a positive serum specific IgE and clinical history of allergy being associated with the presence of TH2A cells or pathogenic antigen specific T cells identified by pMHCII tetramers in food allergy, inhalant allergy, or eosinophilic esophagitis (EoE) (1-3, 18-21). The study was approved by the Institutional Review Board at the Benaroya Research Institute and all subjects provided written informed consent.
CD154 functional assay
PBMCs were resuspended in 1ml of T cell medium (TCM; RPMI 1640 containing HEPES (ThermoFisher Scientific) 1% L-glutamine, 1%, sodium pyruvate, 1% penicillin + streptomycin, 10% human serum) and antigen presenting cells (APCs) were blocked with functional grade αCD40 (clone HB14, Milltenyi Biotec) for 10 minutes at RT at a concentration of 1μml/106 cells. Cells were then resuspended in TCM at a concentration of 10*106/ml. Approximately 1% of the PBMC count was taken for a pre-enrichment estimation of T cell counts. Remaining cells were stimulated with allergen in the form of crude extract obtained from Virginia Mason Medical Center (alder pollen, timothy grass pollen, house dust mite, peanut) or as peptide pools (peanut; Mimotopes) alongside allergen +/− 10ng/ml rIL-33 (R&D Systems). After 18 hours stimulation at 37°C, cells were harvested and labeled with PECF594-conjugated αCD154 mAb for 15 minutes at 4°C. Cells were then washed, labeled with anti-PE magnetic beads for 10 minutes and enriched using a magnetic column according to the manufacturer’s instructions (Miltenyi Biotec). Magnetically enriched cells were stained with antibodies against markers of interest (Table E1) before analysis. CD154+ CD4+ T cells were considered as allergen-reactive CD4+ T cells. The fixable viability dye eFluor520 (eBioscience), CD19, and CD14 antibodies were used to exclude B cells, and monocytes and dead cells from the analysis, respectively. For polyclonal stimulations, fresh or frozen PBMCs from allergic donors were treated the same way (αCD40 blocking, 18hr incubation) except for each experiment, one well was left blank (media only) and the other well was stimulated with ImmunoCult™ Human CD3/CD28 T Cell Activator (Stemcell Technologies) according to the manufacturer’s instructions. No enrichments were performed for polyclonal stimulation experiments.
MHC-II tetramer assay
PE-CF594 labeled HLA-DRB1*07:01 and HLA-DRB1*15:01 MHC class II tetramers loaded with a 13-mer Aln g 1 peptide (sequence = VGLLKAVESYLLA) were generated as described (22). Up to 20*106 fresh or frozen unstimulated PBMCs from HLA-DRB1*07:01 and/or HLA-DRB1*15:01 restricted alder allergic subjects were stained with the peptide-loaded HLA-matched tetramers at a 1:100 dilution in a volume of 100μl TCM for 2 hours at RT in the dark. Cells were then washed, labeled with anti-PE magnetic beads and enriched by using a magnetic column, according to the manufacturer’s instructions (Miltenyi Biotec). Alongside the tetramer assay, a functional CD154 upregulation assay utilizing the same 13-mer Aln g 1 peptide was performed with PBMCs from the same donors.
Intracellular cytokine analysis
For cytokine analysis, the protein transport inhibitor monensin (BD Biosciences) was added for the remaining 3 hours of incubation time for the CD154 assay. Recombinant human IL-33 (R&D Systems) was added to a final concentration of 10ng/ml at the same time as allergen; at the start of the 18hr incubation. Following incubation cells were stained for CD154 and enriched as described in “CD154 functional assay”. Surface staining was performed first on enriched cells followed by fixation/permeabilization using the Foxp3 Transcription Factor Staining Buffer Set Kit (ThermoFisher Scientific) according to the manufacturer’s instructions. Cells were then stained with various combinations of antibodies for IL-13, IL-4, IL-9, IL-5 or corresponding isotype-matched mAbs. After 30 min at 4°C, cells were washed and immediately analyzed by flow cytometry.
TH2A activation test
Up to 20*106 fresh or frozen PBMCs were stimulated with allergen extract as indicated in “CD154 functional assay” or with infectious disease peptide pool (CEF Pool, JPT Peptide Technologies), as a negative control. After 18 hours stimulation at 37°C, cells were harvested and labeled with PE-conjugated αST2 for 30 minutes in a 37°C water bath. Cells were then washed, labeled with anti-PE magnetic beads and enriched by using a magnetic column, according to the manufacturer’s instructions (Miltenyi Biotec). Magnetically enriched cells were stained with antibodies against markers of interest (Table E1) before analysis. ST2+ CD4+ T cells were considered as allergen-reactive TH2A cells.
Flow cytometry
Antibodies used for flow cytometry analysis are shown in Table E1. Data were acquired on a BD LSR Fortessa or BD FACSAria Fusion (BD Biosciences). Data were analyzed with FlowJo software (Tree Star, Inc).
Statistics
Statistical analyses were performed using GraphPad Prism 8 software. The following tests were performed where indicated: Wilcoxon matched-pairs signed rank test, Mann-Whitney U-test, Kruskal-Wallis test with Dunn’s multiple comparisons test, Friedman test with Dunn’s multiple comparisons test, and two-way ANOVA with Sidak’s multiple comparisons test. P values < 0.05 were considered significant.
RESULTS
ST2-expressing allergen-reactive memory T cells are restricted to allergic individuals
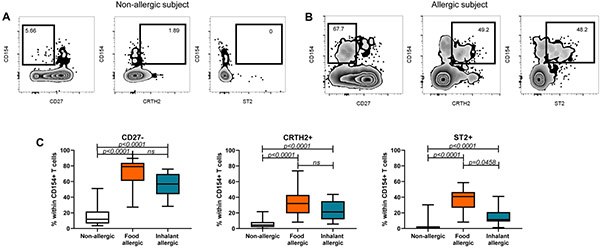
We have previously demonstrated the presence of a pathogenic memory TH2 subset confined to individuals with allergic disease, with a unique gene expression pattern including increased expression of ST2 (1). We asked whether circulating memory ST2+ allergen-specific CD4+ T cells would also be confined to allergic subjects. To this end, allergen-reactive memory (defined as CD45RA−) CD4+ T cells were assessed ex vivo using the CD154 up-regulation assay in food allergic, inhalant allergic, and in non-allergic subjects. Consistent with previous studies, CD27− allergen-reactive T cells were predominantly observed in allergic individuals (Figures 1A-C), whereas CD27+ allergen-reactive T cells were detected both in allergic and non-allergic subjects. Interestingly, we observed that expression of ST2 and the TH2-associated marker CRTH2 on allergen-reactive T cells (CD154+) were restricted to allergic individuals, regardless of the nature of the allergy (Figure 1C, p<0.0001 for CRTH2 and ST2). Altogether, these results confirm that the profile of circulating allergen-specific memory T cells can identify individuals with allergic disease.
Figure 1: Profile of allergen-reactive CD4+ memory T cells in non-allergic and allergic subjects.
FACS plots showing percentages of allergen reactive CD27− , CRTH2+, and ST2+ memory T cells in (A) a non-allergic subject, and (B) in an allergic subject. Memory T cells are defined as CD4+ CD45RA− and each plot represents a different visualization of the total memory T cell population. Numbers indicated on the plots represent percentages of CD27−, CRTH2+, or ST2+ allergen reactive cells within the total memory CD154+ subset. C: Graphical depictions of allergen reactive CD27−, CRTH2+, and ST2+ cells in non-allergic, food allergic, and inhalant allergic subjects. Expressed as % within CD154+ T cells. Statistics computed using the Kruskal-Wallis test.
ST2-expressing allergen-reactive T cells fall into the TH2A cell subset
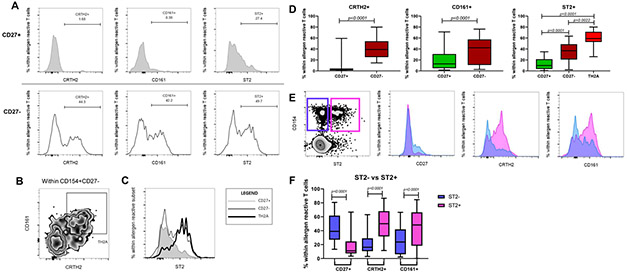
Our group previously demonstrated that CD27 expression defines functionally distinct allergen-specific T cell subsets in allergic individuals. Building on the finding that ST2+ allergen-reactive T cells are confined to allergic subjects, we next asked whether those cells fall into the TH2A cell subset or represent a distinct subset. As shown in Figures 2A-D, surface expression of CRTH2, CD161, and ST2 was significantly increased in CD27− allergen-reactive T cells relative to their CD27+ counterpart (p<0.0001 for each marker). Specifically, the proportion of ST2+ cells was the highest in the allergen-reactive TH2A cell subset (CD154+CD27−CRTH2+CD161+) (Figure 2D, p=0.0022 TH2A vs CD27−, p<0.0001 TH2A vs CD27+). Similarly, ST2+ allergen-reactive CD4+ T cells lack CD27 expression and exhibit increased expression of CRTH2 and CD161 (Figures 2E & 2F, p<0.0001 for each marker). Of note, we also found that the proportion of the IL-25 receptor, IL-17RB, was highest in allergen-reactive TH2A cells (Figures S1A & S1B, p<0.0001 TH2A vs CD27+). However, we did not detect expression of TSLP-R, the receptor for thymic stromal lymphopoietin (Figure S1C, p=ns) in allergen-reactive T cells. Altogether, our findings demonstrate that ST2 expression on peripheral allergen-reactive CD4+ T cells is restricted to the TH2A cell subset.
Figure 2: Characterization of ST2+ T cells.
A: FACS plots showing percentages of CRTH2+, CD161+, and ST2+ cells within allergen reactive CD27+ (top row) and CD27− (bottom row) memory T cells in an allergic subject. B: Representative gating strategy for TH2A cells (CD4+CD45RA−CD154+CD27−CRTH2+CD161+). The plot shows cells gated from CD154+CD27− memory T cells. C: FACS plots showing ST2 expression within TH2A cells (thick black line) compared CD154+CD27+ (gray shaded) and CD154+CD27− (thin black line). Expressed as % within allergen reactive subset. D: Graphical depictions of percentages of CRTH2+, CD161+, and ST2+ cells within allergen reactive CD27+ and CD27− memory T cells. Expressed as % within allergen reactive T cells. Statistics computed using the Wilcoxon test or Kruskal-Wallis test followed by Dunn’s multiple comparisons test. E: FACS plots showing allergen reactive ST2+ (pink) and ST2-(purple) memory T cells in a comparison of CD27, CRTH2, and CD161 expression. F:Graphical depiction of percentages of CD27+, CRTH2+, and CD161+ expressing cells within allergen reactive ST2+ and ST2−T cells. Expressed as % within allergen reactive T cells. Statistics computed using the Wilcoxon test.
TH2A activation is associated with the surface expression of ST2
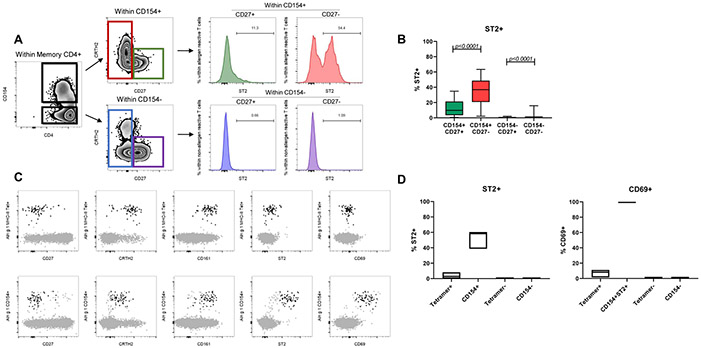
The CD154 assay requires short antigen stimulation to track reactive CD4+ T cells. During our T cell profiling we observed a lack of ST2 expression in the non-reactive (CD154−) compartment (Figures 3A & 3B, p<0.0001). We surmised TH2A cells require prior activation with the sensitized allergen to express ST2. To address this question, we performed a direct ex vivo tracking of unstimulated allergen-specific CD4+ T cells using pMHCII tetramer staining. Alder allergy was used as a model. We selected DRB1*07:01 and DRB1*15:01 as these alleles were prevalent in our cohort of alder pollen-allergic subjects and Aln g 1142–154 was previously described as minimal epitope for these alleles. In parallel, we performed the CD154 functional assay following stimulation with the same epitope. As expected, both the direct pMHCII tetramer staining and CD154 up-regulation assay tracked phenotypically similar allergen-specific CD4+ T cells (Figure 3C). However, only allergen-reactive T cells tracked by the CD154 assay expressed ST2 along with the activation marker CD69 (Figures 3C & 3D). Noteworthy, we also observed CRTH2 downregulation following activation in Aln g 1-reactive CD154+ T cells alongside ST2 upregulation (Figure 3C), suggesting a different role of these two molecules in disease pathogenesis. We also asked whether polyclonal activation of T cells without allergen would induce ST2. To this end, PBMCs from allergic donors were stimulated with αCD3 and αCD28 antibodies for 18 hours. While unstimulated total CD4+ T cells and TH2A cells did not express ST2, surprisingly this polyclonal stimulation did not lead to ST2 expression of a biologically meaningful magnitude on total CD4+ T cells or TH2A cells (Figure S2). Altogether, these results identified ST2 as an allergen-driven TH2A-activation marker and suggest these pathogenic TH2 cells are capable of sensing locally-elicited tissue cytokines.
Figure 3: ST2+ T cells arise through TCR triggering.
A: FACS plots showing percentages of ST2+ cells within allergen reactive CD154+CD27+ (green, top) and CD154+CD27− (red, top), and non-allergen reactive CD154−CD27+ (blue, bottom) and CD154−CD27− (purple, bottom) in an allergic subject. CD154+ and CD154− T cells are gated out of total memory CD4+ T cells. B: Graphical depictions of percentages of ST2+ cells within allergen reactive CD27+ (green), CD27− (red) and non-allergen reactive CD27+ (blue) and CD27− (purple). Expressed as % ST2+. Statistics computed with the Wilcoxon test. C: T cells tracked by direct pMHC-tetramer staining or CD154 staining. FACS plots showing unstimulated Aln g 1 MHC-II tetramer+ cells (top row, black) and Aln g 1 reactive CD154+ST2+ cells (bottom row, black) overlaid onto total memory CD4+T cells (gray). Total memory T cells are defined as CD4+CD45RA−. D: Graphical depictions of percentages of ST2+T cells within tetramer+, CD154+, tetramer−, and CD154−T cells (left) and percentages of CD69+T cells within tetramer+, CD154+ST2+, tetramer−, and CD154+T cells (right). Expressed as % ST2+ and % CD69+. Data are representative of 3 donors.
IL-33 enhances TH2A effector function
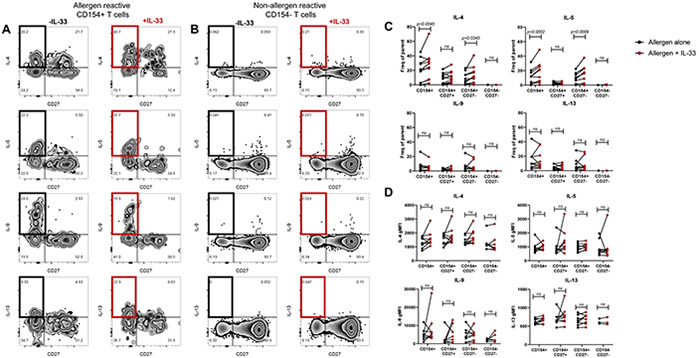
We next investigated the role of selective ST2 upregulation on activated TH2A cells. To this aim, we performed our CD154 assay with intracellular cytokine staining (ICS) on PBMCs from allergic subjects with or without the addition of IL-33 in culture and compared T cell effector function between conditions. Addition of IL-33 in culture significantly enhanced IL-4 and IL-5 production from both total and CD27− allergen-reactive T cells (Figures 4A & 4C, p=0.0045 and p=0.0340 for IL-4 from total and CD27−, p=0.0002 and p=0.0009 for IL-5 from total and CD27−). Consistent with selective ST2 expression on CD27− allergen-reactive T cells, this effect was not observed in the CD27+ counterpart (Figures 4A & 4C, p=ns). However, there were no significant differences in IL-9 or IL-13 production (Figures 4A & 4C, p=ns). Importantly, IL-33 had no effect on non-reactive T cells (CD154−) (Figures 4B & 4C, p=ns). While we observed an increase in the proportion of IL-4 and IL-5 secreting cells, we did not observe changes in MFI for any of the cytokines (Figure 4D), suggesting IL-33 increases the proportion of enhanced TH2A cells rather than modulating the amount of cytokine produced per cell. The addition of IL-33 alone did not activate T cells (Figure S3, p=0.0085 for IL-33 alone vs allergen, p=0.0002 for IL-33 alone vs allergen + IL-33 from CD154+CD27+, p=0.0085 for IL-33 alone vs allergen, p=0.0002 for IL-33 alone vs allergen + IL-33 from CD154+CD27−). These results indicate that IL-33 enhances TH2A effector function in a specific manner through allergen-induced ST2 expression.
Figure 4: Enhanced TH2 effector function of total allergen reactive and allergen reactive CD27− cells with IL-33.
A: FACS plots showing TH2 cytokine-expressing allergen reactive cells with allergen alone (left), and allergen + IL-33 (right). B: FACS plots showing TH2 cytokine-expressing non-allergen reactive cells with allergen alone (right) and allergen + IL-33 (right). C: Graphical depictions of TH2 cytokine secretion +/− IL-33 within the indicated subpopulations. D: Graphical depictions of TH2 cytokine MFI values +/− IL-33 within the indicated subpopulations. Statistics computed with the 2-way ANOVA followed by Sidak’s multiple comparisons test.
Allergen-induced ST2 expression on CD4+ T cells can be evaluated using a simple TH2A activation test
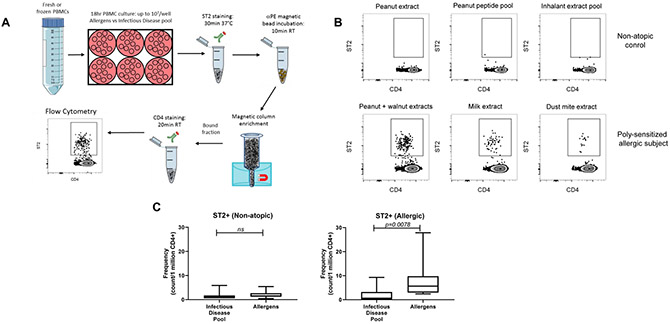
We next took advantage of selective surface expression on activated TH2A cells to develop a two-color flow cytometric-based assay for monitoring allergen-specific TH2 cells in allergic individuals. The method, referred to herein as the “TH2A activation test” was based on three steps: First, stimulation of the PBMCs from allergic individuals with the sensitized allergen. Second, staining and enrichment of the ST2-positive CD4+ T cells. Third, detection of the ST2+ CD4+ T cells by flow cytometry. The methodological workflow for the TH2A activation test is indicated in Figure 5A. As shown in Figures 5B & 5C, populations of ST2 positive and negative CD4+ T cells are easily separated by flow cytometry. Only allergic patients have a positive TH2A activation test and ST2+ T cells appeared to be detectable for allergens in which the subjects develop allergies (Figure 5B). Importantly, the proportion of ST2+ T cells was negligible in both non-atopic controls and allergic subjects following stimulation with a pool of infectious disease peptides as a control, confirming the allergen-specificity of the assay. (Figure 5C). These data demonstrate that surface expression of ST2 on activated TH2A cells can be easily detected and monitored on sensitized and allergic individuals using a simple flow-based method.
Figure 5: A simple flow-based method for detecting and tracking ST2+ CD4+ T cells.
A: Methodological workflow for the TH2A activation test. B: FACS plots showing the presence or absence of ST2+CD4+T cells after short term incubation with the indicated stimuli in a representative non-allergic control (top row) and allergic subject (bottom row). C: Graphical depictions of ST2+T cells in non-atopic subjects (left) and allergic subjects (right) after short term incubation with the indicated stimuli. Expressed as cell count/1 million CD4+. Statistics computed with the Wilcoxon test.
DISCUSSION
While the role of TSLP, IL-33, and IL-25 in type-2 innate immunity and in the initiation of allergic inflammation has been well described (4), their role and mode of action on adaptive immunity remains less defined. Effects of these epithelial cell-derived cytokines are mediated by their related receptor-mediated signaling pathways and were initially thought to act only early in allergic inflammation. Herein, we demonstrate that surface expression of epithelial cytokine receptors (i.e. ST2 and IL17-RB) represents a transient phenotype associated with TH2A cell activation. We focused our study on TH2A cells because they were the only CD4+ T cell subset observed to express ST2. We further show that IL-33/ST2 signaling modulates pro-inflammatory function of allergen-reactive TH2A cells by selectively increasing IL-4 and IL-5 cytokine production. While our data show that IL-33 enhances TH2A IL-4 and IL-5 production, IL-33 alone does not have this effect on T cells that have not undergone antigen-dependent activation, suggesting IL-33 has a role only on the functionality/quality of the T cells that have become receptive to IL-33 by upregulating ST2. Mechanistically, our data support a model in which epithelial cytokine signaling represents a tissue checkpoint that can modulate type-2 adaptive allergic immunity. In this model, TCR recognition of specific allergen by pathogenic TH2 cells is a prerequisite for allowing these cells to sense locally elicited tissue cytokines. These epithelial cytokine-responsive TH2A cells may then preferentially accumulate in tissue lesions where alarmins are released. For instance, in mice infected with N. brasiliensis worms, IL-25 was shown to act as a chemoattractant, recruiting TH2 cells to the lungs (23). It is commonly understood that the development of different TH subtypes relies on the appropriate external signals. Although TSLP, IL-33 and IL-25 appear to serve a similar function in type-2 immunity, experimental mouse models of asthma have elucidated some important differences and there are some subtle tissue-specific effects of these cytokines. For example, treatment of CD4+ T cells with IL-25 has been shown to regulate IL-9 expression in allergic airway inflammation (24). Previous studies have also determined that IL-33 influences the generation of IL-5 producing TH2 cells (12). Hence, it is interesting to speculate whether the downstream pathogenic TH2 effector profiles can be mediated in a tissue-dependent manner depending on qualitative differences in epithelial cell responses to allergen exposure.
TH2A cells share many common features with ILC2s; both cell types are receptive to epithelial cytokines, produce TH2 cytokines, share overlapping transcriptional and epigenetic profiles, and effector functions (23, 25, 26). However, TH2A cells and ILC2s are both ST2 expressing cells of different lineages and there are differences between the two cell types regarding signaling. In mice, IL-33 alone can induce TH2 cytokine secretion from ILC2s but not from ST2+ memory T cells. This is because the T cells express the MAP kinase phosphatase Dusp10, a negative regulator of IL-33-induced signaling which is absent in ILC2s (27). Whether an equivalent Dusp protein exists in TH2A cells and plays an important role in regulating ST2 expression in the allergic response remains to be determined. Whereas ILC2s mediate innate inflammation by secreting TH2 cytokines following direct activation by epithelial cytokines, it has been demonstrated in mice that primed TH2 cells can function in the absence of ILC2s to mediate type 2 inflammation (23). These data suggest that while ILC2s and TH2 cells respond to epithelial cytokines, ILC2s are not necessary to carry out the adaptive immune response in type 2 immune reactions. Instead, TH2 cells mediate the adaptive response, which is enhanced by epithelial cytokine presence. In our PBMC cultures, there are likely contributions from ILC2s and dendritic cells (DCs). Because ILC2s are activated through epithelial cytokines alone, it is reasonable to suspect initiation of an inflammatory feedforward polarizing loop with TH2 cytokines (28) in our IL-33 treatment cultures.
Interestingly, induction of ST2 expression was not observed in allergic donor TH2A cells or total CD4+ T cells upon polyclonal stimulation with a T cell activation cocktail comprised of αCD3 and αCD28 antibodies. These data suggest antigen-dependent T cell activation is necessary for ST2 expression to occur. Unexpectedly, antigen-dependent T cell activation with an infectious disease peptide cocktail did not result in ST2 upregulation, suggesting this signaling pathway is a TH2A-unique phenomenon confined to allergic individuals. The mechanisms leading to such a pathway to take hold in allergy and whether ST2 expression occurs from antigen-dependent stimulation in the context of other diseases are both open questions.
TH2A cells are generally defined as CD4+CD45RA−CRTH2+CD27−CD161+. Within the allergen reactive CD27− population, we observed some heterogeneity, particularly with CD161 and CRTH2. Notably, CRTH2 is downregulated following stimulation and activation. The reason why this occurs is not currently known but suggests a role of prostaglandin D2 (PGD2) in allergic immunity. Cells that constitutively express ST2 and are receptive to IL-33 such as basophils, mast cells, and eosinophils may be triggered by IL-33 to release PGD2. PGD2 may bind to CRTH2 on TH2A cells in the presence of allergen binding the TCR, aiding in recruitment to tissue resulting in CRTH2 downregulation and ST2 upregulation to become receptive to IL-33. Our results suggest that while PGD2 is a chemoattractant, IL-33 does not attract the cells to tissue but instead is more involved in modulation of the TH2 response. This potential mechanistic cascade warrants further investigation. Our results also encourage the search for more novel, stable biomarkers to identify TH2A cells. Such a biomarker that demonstrates less heterogeneity upon activation would have implications that are more meaningful for diagnostic purposes. What is clear is that TH2A cells fall within the CD27− population. Our study offers evidence that ST2 could be a key biomarker for primed TH2A cells. Observed heterogeneity with CD161 and CRTH2 could be attributed to factors such as activation kinetics, length of time the cells have been in circulation or even time since last natural exposure to antigen and warrants further investigation.
There are several limitations to the present study. First, IL-33 was shown to play both an inflammatory and a suppressive role. For instance, recent observations reveal that Foxp3+ regulatory T cells (Tregs) can also express the ST2 receptor either transiently or permanently to enhance suppressive function (29). Further studies will be helpful determining if there are physiological conditions triggering ST2 expression on Treg cells and to understanding the complex effect of this cytokine on T cell homeostasis. Second, this study focused on peripheral allergen-reactive T cells due to limited access to tissue biopsies in humans. However, a recent study by Wen et al (3) demonstrated that tissue-residing T cells in allergic patients with EoE are enriched in effector TH2 cells sharing feature of TH2A cell subset (i.e. GATA3+ IL-4+ IL-5+ HPGDS+ CRTH2+ CD161+ IL-17RB+ PPARγ+). Additionally, findings in TH2 cytokine reporter mice (23) support our model proposing a tissue checkpoint that occurs when epithelial cytokines are released at sites of tissue inflammation and primed ST2-expressing TH2A cells then recruit to the site of tissue damage. Finally, it will also be interesting to determine whether these epithelial cytokines influence the differentiation of a population of pathogenic human TH2 cells and whether the magnitude of epithelial cell responses correlates with disease severity.
In summary, our results highlight a crucial role of epithelial cytokines as a secreted immune checkpoint that can directly influence the effector function of pathogenic human TH2 cells. Selective upregulation of ST2 and IL-17RB on TH2A cells upon exposure to allergen may also indicate the importance of epithelial cytokines in T cell mobilization and retention during allergic inflammation. Altogether, our data provides a strong rationale for the clinical development of IL-33 blocking agents that can disrupt elevated TH2 responses thereby limiting disease exacerbation and its associated morbidities. Based on our findings, it will be important to clinically intervene with such a biologic at the stage that ST2 is expressed, which occurs after TCR stimulation. In the clinic, this would equate to intervention following an allergen challenge to inhibit full effector function of primed TH2A cells in tissues. Our data also highlight ST2 as an attractive marker to track peripheral allergen-reactive TH2A cells.
Supplementary Material
Clinical implication:
Allergen-primed TH2A cells upregulate ST2 to become a critical IL-33 target. Measurement of ST2+ peripheral CD4+ T cells may represent an interesting biomarker in allergy.
ACKNOWLEDGEMENTS
We thank the Flow Cytometry core at BRI for assistance with experiments. We thank Dr. Hannah DeBerg for statistical advice. We also thank Dr. Karen Cerosaletti and Dr. Steven F. Zeigler for stimulating discussion, critical review and feedback for this manuscript.
Funding: This work was supported by U19 AI125378-01; R01AI108839, Anaptys Bio Inc., and Food Allergy Research Education (FARE).
Abbreviations used:
- TH
T helper type
- ILC
innate lymphoid cell
- ST2
suppression of tumorigenicity / IL1RL1 / Interleukin 1 receptor-like 1
- TCR
T-cell receptor
- pMHCII
peptide-major histocompatibility complex class II
- AIT
allergen immunotherapy
- EoE
eosinophilic esophagitis
- PGD2
prostaglandin D2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure: EW received grant support from NIAID, Food Allergy Research and Education (FARE), Immune Tolerance Network (ITN), Regeneron Pharmaceuticals (research sponsorship), Astellas, AnaptysBio, and Aimmune Therapeutics. Marco Londei was an employee of Anaptys Bio during this study. JC, NG, VB, MF, DR, and DJ do not have any COIs to disclose.
REFERENCES
- 1.Wambre E, Bajzik V, DeLong JH, O’Brien K, Nguyen QA, Speake C, et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci Transl Med. 2017;9(401). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruiter B, Smith NP, Monian B, Tu AA, Fleming E, Virkud YV, et al. Expansion of the CD4(+) effector T-cell repertoire characterizes peanut-allergic patients with heightened clinical sensitivity. J Allergy Clin Immunol. 2020;145(1):270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen T, Aronow BJ, Rochman Y, Rochman M, Kc K, Dexheimer PJ, et al. Single-cell RNA sequencing identifies inflammatory tissue T cells in eosinophilic esophagitis. J Clin Invest. 2019;129(5):2014–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cayrol C, Girard J-P. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Current Opinion in Immunology. 2014;31:31–7. [DOI] [PubMed] [Google Scholar]
- 5.Griesenauer B, Paczesny S. The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases. Front Immunol. 2017;8:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lott JM, Sumpter TL, Turnquist HR. New dog and new tricks: evolving roles for IL-33 in type 2 immunity. J Leukoc Biol. 2015;97(6):1037–48. [DOI] [PubMed] [Google Scholar]
- 7.Pusceddu I, Dieplinger B, Mueller T. ST2 and the ST2/IL-33 signalling pathway-biochemistry and pathophysiology in animal models and humans. Clin Chim Acta. 2019;495:493–500. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–90. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Ramadan AM, Griesenauer B, Li W, Turner MJ, Liu C, et al. ST2 blockade reduces sST2-producing T cells while maintaining protective mST2-expressing T cells during graft-versus-host disease. Sci Transl Med. 2015;7(308):308ra160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10,and important for Th2 effector function. Proc Natl Acad Sci U S A. 1998;95(12):6930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187(5):787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endo Y, Hirahara K, Iinuma T, Shinoda K, Tumes DJ, Asou HK, et al. The interleukin-33-p38 kinase axis confers memory T helper 2 cell pathogenicity in the airway. Immunity. 2015;42(2):294–308. [DOI] [PubMed] [Google Scholar]
- 13.Murdaca G, Greco M, Tonacci A, Negrini S, Borro M, Puppo F, et al. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int J Mol Sci. 2019;20(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cayrol C, Girard JP. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol Rev. 2018;281(1):154–68. [DOI] [PubMed] [Google Scholar]
- 15.Cerosaletti K, Chen J, Johnson E, Garabatos N, Calise J, Robinson D, et al. Development of peanut allergy is associated with coding SNPs in the C-terminus of the IL-33 receptor that impact signaling and TH2 effector function. Journal of Allergy and Clinical Immunology. 2020;145(2):AB88. [Google Scholar]
- 16.Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest. 2019;129(4):1441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinthrajah S, Cao S, Liu C, Lyu SC, Sindher SB, Long A, et al. Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight. 2019;4(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwok WW, Roti M, Delong JH, Tan V, Wambre E, James EA, et al. Direct ex vivo analysis of allergen-specific CD4+ T cells. J Allergy Clin Immunol. 2010;125(6):1407–9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLong JH, Simpson KH, Wambre E, James EA, Robinson D, Kwok WW. Ara h 1-reactive T cells in individuals with peanut allergy. J Allergy Clin Immunol. 2011;127(5):1211–8 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wambre E, DeLong JH, James EA, LaFond RE, Robinson D, Kwok WW. Differentiation stage determines pathologic and protective allergen-specific CD4+ T-cell outcomes during specific immunotherapy. J Allergy Clin Immunol. 2012;129(2):544–51, 51 e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tu AA, Gierahn TM, Monian B, Morgan DM, Mehta NK, Ruiter B, et al. TCR sequencing paired with massively parallel 3’ RNA-seq reveals clonotypic T cell signatures. Nat Immunol. 2019;20(12):1692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J Clin Invest. 1999;104(12):R63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Dyken SJ, Nussbaum JC, Lee J, Molofsky AB, Liang HE, Pollack JL, et al. A tissue checkpoint regulates type 2 immunity. Nat Immunol. 2016;17(12):1381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan MH. Th9 cells: differentiation and disease. Immunol Rev. 2013;252(1):104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lombardi V, Beuraud C, Neukirch C, Moussu H, Morizur L, Horiot S, et al. Circulating innate lymphoid cells are differentially regulated in allergic and nonallergic subjects. J Allergy Clin Immunol. 2016;138(1):305–8. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd CM, Snelgrove RJ. Type 2 immunity: Expanding our view. Sci Immunol. 2018;3(25). [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, Endo Y, Onodera A, Hirahara K, Asou HK, Nakajima T, et al. DUSP10 constrains innate IL-33-mediated cytokine production in ST2(hi) memory-type pathogenic Th2 cells. Nat Commun. 2018;9(1):4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wambre E, James EA, Kwok WW. Characterization of CD4+ T cell subsets in allergy. Curr Opin Immunol. 2012;24(6):700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez F, Fritz JH, Piccirillo CA. Pleiotropic Effects of IL-33 on CD4(+) T Cell Differentiation and Effector Functions. Front Immunol. 2019;10:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.