Abstract
The whitefly, Bemisia tabaci (Gennadius), is responsible for significant yield losses in many crops, including potato, by sucking the phloem sap and transmitting a number of plant viruses. B. tabaci is a complex of cryptic species which is commonly designated as genetic groups. The B. tabaci genetic groups differ biologically with respect to host plant preference, insecticidal resistance, reproduction capacity, and ability to transmit begomoviruses. Therefore, understanding genetic variation among populations is important for establishing crop-specific distribution profile and management. We sequenced the mitochondrial cytochrome oxidase I (mtCOI) gene of B. tabaci collected from major potato growing areas of India. BLAST analysis of the 24 mtCOI sequences with reference Gene Bank sequences revealed four B. tabaci genetic groups prevailing in this region. mtCOI analysis exhibited the presence of Asia II 1, Asia II 5, Asia 1, and MEAM1 B. tabaci genetic groups. Our study highlighted that a new genetic group Asia II 5 has been detected in Indo-Gangetic Plains. Further virus–vector relationship study of ToLCNDV with Asia II 5 B. tabaci revealed that females are efficient vector of this virus as compared to males. This behavior of females might be due to their ability to acquire more virus titer than males. This study will help in better understanding of whitefly genetic group mediated virus diseases.
Keywords: Bemisia tabaci, mtCOI, Genetic groups, ToLCNDV, Begomoviruses, Indo-Gangetic plains
Introduction
The worldwide distribution of insect vector is the major driving force for the dissemination of more than 70% of plant viruses in major horticultural and field crops (Hogenhout et al. 2008). The whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae), is one such highly notorious insect vector and a polyphagous pest which is responsible for significant yield losses in many crops, including potato (Naranjo and Ellsworth 2001). Whitefly may cause enormous direct losses by sucking plant sap and reducing plant vigor or indirectly by transmitting several plant viruses (Bedford et al. 1994; Briddon et al. 2003; Segev et al. 2004). B. tabaci has ability to ingest viruses belonging to at least five genera––Begomovirus, Ipomovirus, Crinivirus, Carlavirus, and Torradovirus (Navas-Castillo et al. 2011)––and can transmit more than hundred plant viruses (Czosnek et al. 2017). The transmission of several plant viruses along with its ability to develop resistance to pesticides makes it a most devastating pest in agriculture. Begomoviruses and B. tabaci have been considered as one of the most economically important virus–vector complexes during the last two decades. This virus–vector complex poses a serious threat to major vegetable and field crops across the developing world where availability of food remains scare (Paredes-Montero et al. 2019). Several diseases caused by these whitefly-transmitted begomoviruses have reached to an epidemic status in various countries and became a consistent threat to global food security (Saeed et al. 2017). In Latin America, more than 5 million hectare area is directly affected by about 30 different begomoviruses causing huge crop loss. Economic losses due to whitefly-transmitted geminivirus infections are estimated to be US $300 million in India, US $5 billion in Pakistan, US $1300–2300 million in Africa, and US $140 million in Florida (Saeed et al. 2017).
It is difficult to morphologically distinguish the cryptic B. tabaci species; hence, molecular markers are the most common tools being used to distinguish the B. tabaci populations. Molecular markers based on mitochondrial cytochrome oxidase I (mtCOI) and internal transcribed spacer (ITS1) gene give insight into genetic variability within B. tabaci. Initially Boykin et al. (2007) distinguished 12 major genetic groups based on mtCOI and ITS1 gene sequences. Thereafter Dinsdale et al. (2010) fixed a quantifiable limits for grouping of B. tabaci based on mtCOI gene sequences and this resolved the global B. tabaci populations into 24 putative species based on 3.5% divergence and so far 42 distinct species have been reported on the basis of divergence in mtCOI sequences within B. tabaci species (Kanakala and Ghanim 2019). The whitefly genetic groups reflect phenotypic variability in terms of several biological and molecular features, like host plant preference (Bedford et al. 1994; Brown et al. 1995) insecticidal resistance (Naveen et al. 2017), reproduction capacity (Drost et al. 1998), ability to transmit begomoviruses (Bedford et al. 1994; Brown et al. 1995; Shi et al. 2018), and mitochondrial cytochrome oxidase I (mtCOI) DNA sequences (De Barro 2005; De Barro et al. 2011; Dinsdale et al. 2010; Kanakala and Ghanim 2019). Nowadays, potato apical leaf curl disease is becoming one of the major challenges in potato production in Indo-Gangetic plains (Jeevalatha et al. 2014). This disease was reported for the first time in northern India by Garg et al. (2001). Further, Usharani et al. (2004) confirmed that the associated virus with this disease is a strain of tomato leaf curl New Delhi virus (ToLCNDV) (Begomovirus:Geminiviridae).The causal virus of potato apical leaf curl disease (PALCD) has 93–95% sequence identity with ToLCNDV isolates and less than 75% sequence similarity with other viruses, like tomato leaf curl virus isolates and potato yellow mosaic virus (Usharani et al. 2004). The incidence of ToLCNDV has immensely increased year after year and it has occupied the first position among Indian potato viruses during the last two decades. The higher record of disease incidence in Indo-Gangetic plains (40–100% infection) along with reports of heavy yield losses in susceptible varieties raised alarms for this emerging disease (Lakra 2003; Venkatasalam et al. 2011). The reduction in tuber size and number of tubers per plant in infected cultivars may lead to a yield loss up to 60.8% (Chandel et al. 2010; Lakra 2003). Besides, up to 40% incidence of PALCD was reported from West Bengal (Saha and Saha 2014). There are recent reports of increased severity of potato apical leaf curl disease in areas of higher whitefly, B. tabaci population (Jeevalatha et al. 2013; Shah et al. 2019). Information regarding ability of vectors to acquire, retain, and transmit a virus is crucial for understanding and managing outbreak of viral diseases (Polston 1990). Hence, it is important to monitor the prevalent B. tabaci genetic groups in the major potato growing areas of India and establishing relationship with ToLCNDV virus which will help in understanding the epidemiology of potato apical leaf curl disease. The present study was carried out to determine the prevalence of whitefly genetic groups in the major potato growing regions of India and establish relationship of ToLCNDV with newly reported whitefly genetic group in this region. The virus acquisition ability was also estimated to know the causes of the variation in the transmission abilities of adult male and female whiteflies.
Materials and methods
Sample collection
Whitefly samples were collected during 2018, from 12 locations representing nine potato growing states of India (Table 1). Whitefly samples were collected from six host plants, viz., potato, moong bean, brinjal, okra, Parthenium, and bottle gourd, for genetic group identification.
Table 1.
Details of field survey for whitefly, B. tabaci collection
| SN | Location | State | Host | Field | Genetic group | Accession No. |
|---|---|---|---|---|---|---|
| 1 | Modipuram | Uttar Pradesh | Potato | I | Asia II 1 | MW513950 |
| 2 | Moongbean | I, II | Asia II 1 | MW513946, MW513941 | ||
| 3 | Brinjal | I, II | Asia II 1 | MW513948, MW513952 | ||
| 4 | Okra | I, II, III | Asia II 5 | MW513960, MW513947, MW513942 | ||
| Parthenium | I, II | Asia II 1, Asia II 5 | MW513949, MW513954 | |||
| 5 | ||||||
| 6 | Pantnagar | Uttarakhand | Potato | I | Asia II 1 | MW513953 |
| 7 | Hisar | Haryana | Potato | I | Asia II 1 | MW517838 |
| 8 | Kurukshetra | Haryana | Potato | I | Asia II 1 | MW513951 |
| 9 | Ludhiana | Punjab | Parthenium | I | Asia II 1 | MW513944 |
| 10 | Jalandhar | Punjab | Potato | I | Asia II 5 | MW513959 |
| 11 | Bottle gourd | I, II | Asia II 1, Asia II 5 | MW517845, MW517840 | ||
| 12 | Brinjal | I | Asia I | MW517844 | ||
| 13 | Parthenium | I | Asia II 1 | MW517842 | ||
| 14 | Pune | Maharashtra | Potato | I | Asia II 5 | MW513955 |
| 15 | Jalgaon | Maharashtra | Potato | I | Asia II 5 | MW513945 |
| 16 | Gwalior | Madhya Pradesh | Potato | I | Asia I | MW513943 |
| 17 | Kalyani | West Bengal | Potato | I | Asia I | MW513956 |
| 18 | Banaskantha | Gujarat | Potato | I | Asia I | MW517839 |
| 19 | Jaipur | Rajasthan | Brinjal | I | MEAM I | MW542521 |
Across each location, three samples were taken from two fields separated with at least one kilometer distance. B. tabaci populations were collected as adults using a hand-held aspirator. Samples for genetic group identification were collected in 70% ethanol and stored at − 80 °C until samples were processed for DNA isolation.
Whitefly samples for virus transmission experiment were collected from Jalandhar (Punjab) from potato host. The adults were aspirated in a 50-ml Falcon tubes containing potato leaves with moist cotton wrapped around the petiole. Whitefly populations were maintained in insect proof cages in a glasshouse at 26 ± 2 °C temperature conditions at ICAR-CPRI, Shimla. Single pair of male and female whiteflies was caged to develop uniform population and further raised on virus-free potato plants. About ten random individuals from the progeny of this population was tested for the presence of virus using the method described in detail in further section (Jeevalatha et al. 2013) and same population was also included for B. tabaci genetic group identification.
B. tabaci genetic groups in Indo-Gangetic plains
Whitefly samples were washed with distilled water; then DNA isolation was carried out from three individual whiteflies from each location using DNeasy Blood & Tissue (Qiagen) by following the manufacturer guidelines. The DNA was quantified using NanoDrop 2000/2000c Spectrophotometer (Thermo Fisher Scientific, USA). Polymerase Chain Reaction (PCR) mix comprised 2 × Emerald Amp® GT PCR Master Mix 12.5 µl, 1 µl of forward and reverse primer (10 pmol) each, and 2 µl DNA template (25 ng/ µl), and nuclease-free water was added to make the total reaction volume of 25 µl. PCR was performed using forward primer C1-J-2195 (-TTGATTTTTTGGTCATCCAGAAGT) and reverse primer L2-N-3014 TCCAATGCAC- TAATCTGCCATATTA (Simon et al. 1994).
PCR was carried out with an initial denaturation at 94 °C for 4 min followed by 35 cycles of 94 °C for 30 s, 50 °C for 45 s, 72 °C for 1 min, and a final extension at 72 °C for 7 min. The PCR products were visualized in 1% agarose gel stained with ethidium bromide (EtBR). The mtCOI gene fragments were excised and purified using Qiaquick gel extraction kit (Qiagen). The purified gene fragment was cloned into pTZ57R/T vector (Thermo scientific) and plasmid was isolated from three positive transformants which were further sequenced using genetic analyzer 3500 (Applied Biosystems). The cycle sequencing reactions were performed using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Luthiana).
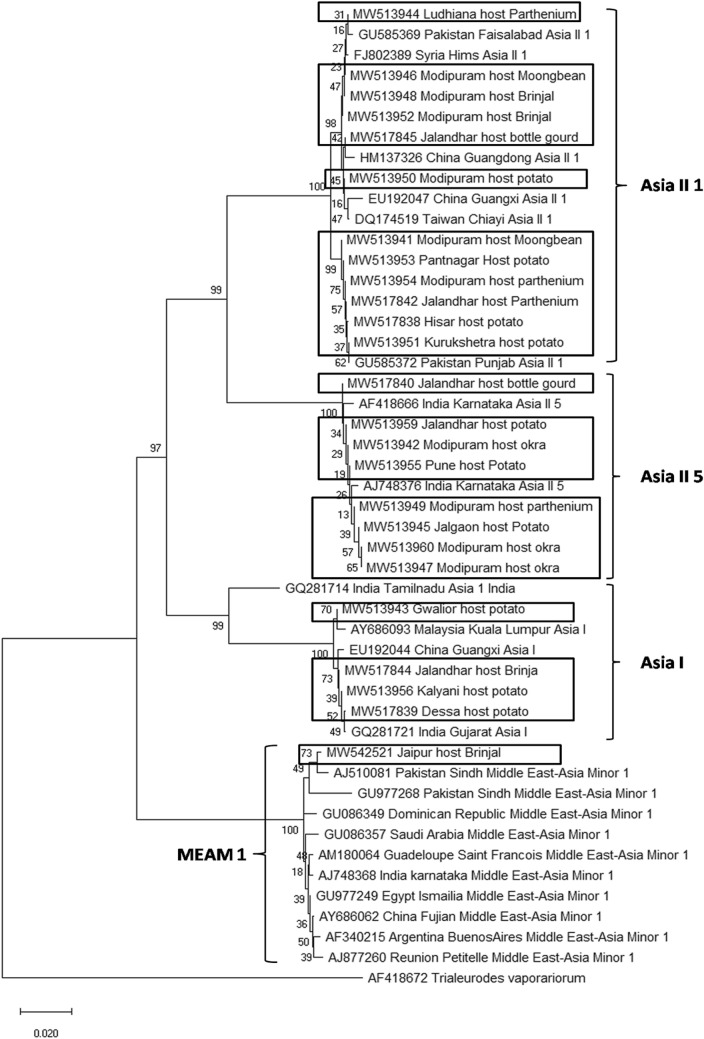
Sequence analysis was performed on 657 bp of mtCOI gene sequences from 115 taxa (data not shown) (Boykin and De Barro 2014; Lee et al. 2013) and was added with 700 bp sequence of the mtCOI gene of our samples collected during the present study to identify the B. tabaci populations in major potato growing areas of India. The mtCOI gene sequences generated in the present study were submitted to the GeneBank (NCBI) database (Table 1). Before carrying out the sequence analysis all mtCOI gene sequences were checked for stop codons by translating into putative amino acid sequence. The mtCOI gene sequences were aligned in MEGA X using ClustalW and polygenetic tree was constructed (Hussain et al. 2019; Thompson et al. 1994) (Fig. 1). The genetic distance (p-distance) within and between species groups was calculated estimating variance with 1000 bootstraps.
Fig. 1.
Molecular phylogenetic placements of mtCOI sequences of whitefly samples collected and consensus sequences for each of these previously determined genetic groups. Multiple sequence alignment by clustalW followed by phylogenic tree construction using the minimum evolution method and keeping 1000 bootstrap value. The B. tabaci mtCOI sequences of the present study have been highlighted in boxes
Transmission of ToLCNDV by B. tabaci
Transmission method of ToLCNDV by B. tabaci was followed as suggested by Ning et al. (2015) with slight modifications. Transmission of ToLCNDV was carried out using Asia II 5 B. tabaci genetic group. The adult whiteflies were removed from the culture plants leaving only immature stages. Such plants carrying only immature stages were further shifted to new cages and newly emerged adults (2–3 days old) were used for this study. Newly emerged whiteflies were allowed to mass feed on ToLCNDV infected source plants obtained from virus pure culture facility (ICAR-CPRI, Shimla) for 48-h acquisition access periods (AAPs). Following virus acquisition, male and female whiteflies were separated as suggested by Shi et al. (2018). A 50-ml Falcon tube (Tarsons) excised at terminal end and wrapped with muslin cloth was used to hold male and female whiteflies in this experiment. These falcon tubes containing single, three, six, and 12 male and female whiteflies separately were then kept inverted (open end) on virus-free potato plants (5–6 days old) raised from microtubers. Whiteflies were allowed to feed on the healthy potato plants for 48-h inoculation access periods (IAPs). Subsequently, the transmission was terminated by application of Imidacloprid 17.8 SL @ 0.03%. The application of same insecticide was repeated after 7 days to kill the immature stages emerged out of eggs laid by whiteflies during IAPs. The virus-transmitted plants were grown under insect proof cages at 27 ± 3 °C for one month. After one month the plants were tested for the presence of ToLCNDV using polymerase chain reaction (PCR) as previously described by Jeevalatha et al. (2013) for detection of ToLCNDV virus. DNA isolation was performed using GeneJET Plant Genomic DNA Purification Kit (Thermo Fisher) and quantified using NanoDrop 2000/2000c Spectrophotometer® (Thermo Fisher Scientific, India). The PCR reaction mixture consisted of 10 µl of 2xEmeraldAmp pcr master mix (Takara), 1 µl of each forward (LCVCPF 5′-AAAGTCATGTGTGTTAGTGATGTTACC-3′) and reverse primer (LCVCPR 5′-TAGAAATAGATCCGGATTTTCAAAGTA-3′) (10 pmol/ µl), and 1 µl of DNA template (100 ng), and final reaction volume was adjusted to 20 µl using nuclease-free water. The PCR conditions were 94 °C for four min as initial denaturation, followed by 35 cycles of 94 °C for 30 s, 62 °C for 1 min, and 72 °C for 1 min followed by a final extension of 72 °C for 10 min. The PCR products were visualized in 1% agarose gel stained with EtBR.
Virus acquisition by male and female whiteflies
Whiteflies (Asia II 5 genetic group) were allowed to feed in clip cages on ToLCNDV-positive plant for three, six, 12, 24, 48, and 72 h. Subsequently after above mentioned acquisition period whiteflies were removed along with clip cages and killed by keeping at − 20 °C for 10 min. The male and female whiteflies were separated by observing the tail tip and genitalia structure under stereo zoom microscope and transferred in separate 1.5-ml eppendorf tubes with proper label and further kept at -80 °C until processed for virus load estimation using qPCR.
qPCR mediated virus load estimation in male and female whiteflies
Male and female whiteflies collected after each acquisition periods (3, 6, 12, 24, 48, and 72 h) were used for determination of virus acquisition ability. Ten whiteflies of each set of experiment were clubbed and used for DNA isolation using blood and tissue kit (Qaigen) following the manufacturer guidelines. The estimation of virus titer was performed using qPCR (Step One Plus Applied Biosystems real-time PCR, USA) by following the method previously established for ToLCNDV (Naga et al. 2020). The qPCR reactions mix included 1 µl DNA template (9 ng/µl), 10 μl of Power SYBR Green Master Mix (Applied biosystems, UK), 1 μM of each forward and reverse primer (5’ TAAGGTGCAGTCCTTTGAATCT3’—Sense and 5’CTCCTCGGGTAACATCACTAAC3’-AntiSense), and nuclease-free H2O to attain final reaction volume of 20 μl. The qPCR cycling condition included 10 min activation at 94 °C followed by 40 cycles of 15 s at 94 °C and 30 s at 60 °C for both annealing and extension.
Standard curve was generated by following the method previously mentioned by Segev et al. (2004). To generate standard curve 770 bp sequence of coat protein gene of ToLCNDV was cloned in pTZ57R/T vector (Thermo Scientific®) following the manufacturer’s protocol. The plasmid was isolated from positive colonies using Qiagen Plasmid Miniprep kit and linearized by digesting EcoR1 (New England Biolabs). Linearized plasmid was further purified using Qiagen PCR purification kit. The concentration of plasmid was converted to copy number using a dsDNA copy number calculator (https://cels.uri.edu/gsc/cndna.html) and total six serial dilutions (tenfold) of plasmid were prepared with a starting concentration of 1.1 × 10–6. This serially diluted plasmid samples were loaded in the same reaction plate along DNA samples of male and female whiteflies of different acquisition periods. The quantification reaction was performed in 96-well optical plates in the Thermocycler (Step One Plus Applied Biosystems real-time PCR, USA). Each qPCR reaction replicated thrice along with DNA from healthy whitefly used as control while reactions mix without templates as negative control.
Results
B. tabaci genetic groups in Indo-Gangetic plains
Whitefly samples were collected from 11 locations and six host plants representing major potato growing regions except one location Jaipur (Rajasthan) (Table 1). During this study the majority of samples had been taken from the potato host, i.e., 10 locations (Table 1). However, to decipher the off season survival of identified whitefly genetic groups, the samples were also collected from other host plants in summer season. The PCR reaction amplified ~ 850 bp mtCOI gene fragment and after sequence trimming approximately 700 bp sequence was used for alignment with reference sequences retrieved from GenBank. Total 25 representative sequences were submitted to GenBank and accession numbers were obtained which are mentioned in Table 1.
Originally 115 consensus sequences of already determined B. tabaci genetic groups were used for correct assignment of sequences obtained in the present study. However, we have presented only 48 mtCOI sequences in the phylogenetic tree (Fig. 1). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches (Felsenstein 1985). The tree has been drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree (Fig. 1).
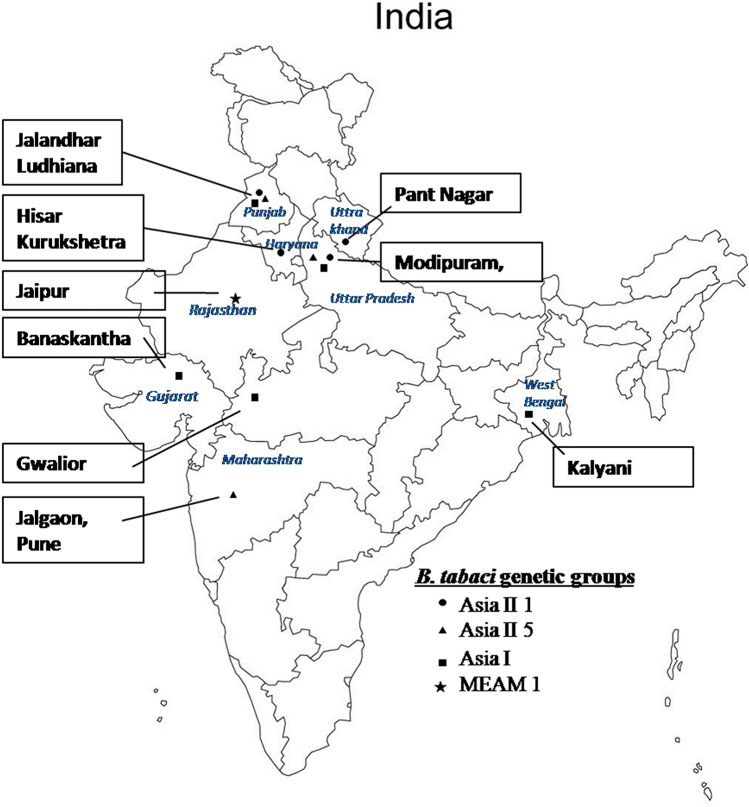
Analysis of the mtCOI sequences of our samples with sequences from GenBank revealed four whitefly genetic groups (Asia II 1, Asia II 5, Asia I, and Middle East Asia Minor 1) in the present study. Asia II 1 was observed at six locations (Modipuram, Pantnagar, Hisar, Kurukshetra, Ludhiana, and Jalandhar) over 5 host plants (potato, moong bean, brinjal, Parthenium, and bottle gourd), whereas Asia II 5 and Asia I both were observed at four locations (Table 1). Whitefly samples collected from Jaipur (Rajasthan) were recorded as an invasive species MEAM 1, though the potato is not grown in this region. Overall Asia II 1 was found most dominating genetic groups followed by Asia II 5 and Asia I in the major potato seed and crop production (Table 1, Fig. 2).
Fig. 2.
Distribution of whitefly genetic groups at major potato growing areas
Transmission of ToLCNDV by B. tabaci
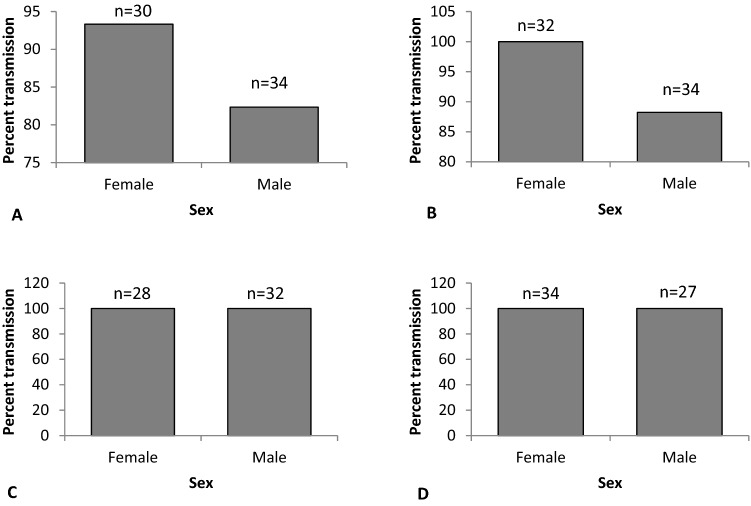
Transmission of ToLCNDV was carried out separately with male and female whiteflies of Asia II 5 genetic group. In our experiment we have observed a clear cut difference in the virus transmission abilities of male and female whiteflies. The female transmitted the virus with higher efficiencies than male (Fig. 3A, B). Single female and male individuals transmitted the ToLCNDV with 93.33% and 82.35% efficiencies, respectively. The virus transmission increased with increasing number of the male and female whitefly individuals. The virus was transmitted with 100% efficiency by three female whiteflies, whereas three males could transmit with 88.24% efficiencies. Six and above male whiteflies can transmit the virus with cent percent efficiencies (Fig. 3; C and D).
Fig. 3.
Transmission efficiency of ToLCNDV-potato by male and female (Asia II 5 genetic group) with different numbers. A transmission with single male and female; B transmission with three individuals; C transmission with 6 individuals; D transmission with 12 individuals. The n above the bars are the numbers of plants tested for the presence of ToLCNDV-potato at the end of the experiment
qPCR mediated virus load estimation in male and female whiteflies
One of the important factors to test the virus transmission potential is to understand the ability to acquire the virus. Our results revealed that the females can acquire significantly higher virus (P < 0.05) at different acquisitions periods (3, 12, 24, 48, and 72 h) except at 6 h of acquisition. In both male and female the virus accumulation increased with increase in the acquisition periods till 48 h and decreased afterward. The virus titer in terms of copy number increased from 2554.48 ± 397.31 to 293,498.24 ± 6777.27 (Virus copies/23.0 ng of DNA) during 48 h of virus acquisition by female whitefly (Fig. 4). Likewise, copy number varied from 458.71 ± 189.24 to 161,347.76 ± 4645.80 (Virus copies/ 23.0 ng of DNA) during same periods of virus acquisition by male whitefly (Fig. 4). The virus titer estimated after 72 h of acquisition revealed reduction in copy numbers, i.e., 107,169.73 ± 3095.33 and 141,978.97 ± 4268.69 (Virus copies/23.0 ng of DNA) in both male and female whiteflies, respectively (Fig. 4).
Fig. 4.
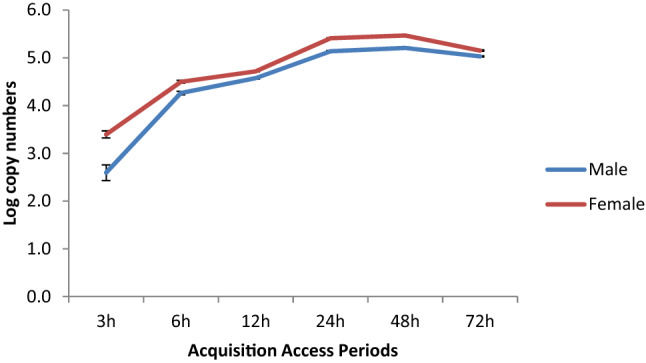
Accumulation of ToLCNDV-potato by male and female Asia II 5 genetic group at different acquisition periods. The values are Log virus DNA copies (Mean ± SE)/23.0 ng in the male and female B. tabaci DNA. Virus accumulation at different acquisition periods were measured by qPCR as described in Materials and Methods based on standard curves. Quantification of ToLCNDV-potato is given by Slope: − 3.33, Y-intercept: 3.252, R2: 0.98, Efficiency%: 99.64. The log transformed virus copies at each acquisition period in both male and female were compared using Student t-test
Discussion
Our study revealed the prevalence of four whitefly genetic groups, viz., Asia II 1, Asia II 5, Asia 1, and MEAM 1 in the major potato growing areas of parts of North and Central India. Asia II1 and Asia 1 have been reported as one of the major whitefly genetic groups in the Indo-Gangetic plains which is consistent with the previous reports (Ellango et al. 2015). The present study reveals that Asia II 5 was found at four locations Modipuram (Uttar Pradesh), Jalandhar (Punjab), Pune (Maharashtra), and Jalgaon (Maharashtra) of major potato growing regions. Out of these four locations, Asia II 5 reported for the first time at Modipuram (Uttar Pradesh) and Jalandhar (Punjab); however, there are reports of its prevalence in Pune (Maharashtra) (Ellango et al. 2015). The Asia II 5 genetic group has also been previously reported from southern India and West Bengal (Ellango et al. 2015). The detection of the Asia II 5 B. tabaci genetic group in the North India may change the prevalence of the whitefly-transmitted ToLCNDV virus which is responsible for causing potato apical leaf curl disease in potato (Kreuze et al. 2020). The invasive B. tabaci genetic group MEAM I which was earlier known as biotype B has only observed at Jaipur (Rajasthan). This genetic group was initially reported for the first time in India at Bangalore (Banks et al. 2001; Rekha et al. 2005) then in some parts of Gujarat (Chowda-Reddy et al. 2012). In our study we reported the occurrence MEAM 1 genetic group from western part of India (Jaipur, Rajasthan); however, the absence of this genetic group in major potato growing Indo-Gangetic region minimizes the risk of losses due to associated viruses. So far nine genetic groups (Asia I, Asia II 1, Asia II 5, Asia II 7, Asia II 8, Asia II 11, China 3, and one invasive MEAM 1) have been identified in different crops across India (Ellango et al. 2015). However, the whitefly samples for genetic group identification have not been drawn from potato crop. Our study involving the potato as host crop for whitefly genetic group identification revealed that there is no crop-specific genetic group in the major potato growing Indo-Gangetic plains.
The detection of Asia II 5 genetic group in the Indo-Gangetic plains warns for more severe outbreak of potato apical leaf curl disease. The virus acquisition ability of B. tabaci varies in sex-dependent manner. We observed that the ability to acquire ToLCNDV was higher in females compared to males in Asia II 5 genetic group. Our results corroborate with previous reports where female whiteflies can acquire more virus than males (Byrne and Bellows 1991; Ning et al. 2015; Peng et al. 2020). The higher virus acquisition of females may positively affect the ToLCNDV virus transmission efficiencies as compared to males. To validate this assumption we have carried out transmission of ToLCNDV separately with male and female of newly detected B. tabaci Asia II 5 genetic group. Our finding reveals that Asia II5 genetic group acts as an efficient vector of ToLCNDV and female individuals are superior in transmitting this virus than males. Limited literature is available where association of ToLCNDV established with B. tabaci in genetic group-dependent manner. Our findings were concordant with previous reports where single whiteflies were able to acquire and transmit ToLCV-Ban4 virus to tomato test plants. Moreover, females were observed far more efficient (93–100%) in infecting tomato plants than males (23–29%) in a one insect/one plant inoculation regime (Muniyappa et al. 2000). Our results are also concurrent with those obtained for other insects and viruses where females are more efficient vectors than males (Xie et al. 2012). Shi et al. (2018) also observed superior virus acquisition and transmission efficiency of tomato chlorosis virus with invasive MEAM1 and MED B. tabaci where females of both the genetic groups were more efficient than male populations. Ning et al. (2015) also observed variation in transmission of tomato yellow leaf curl virus by whitefly, B. tabaci, based on differences of sex and biotypes (B and Q). Similarly, females transmitted the ramie mosaic virus with greater efficiency than by males of MED cryptic species of B. tabaci (Peng et al. 2020). Besides, in other virus vector systems similar observations have been made, for example, females of plant hopper, Peregrinus maidis, are superior vectors of rice stripe virus than males (Narayana et al. 1996). However, there are many reports where male transmitted the virus with greater efficiencies than females (Rotenberg et al. 2009; Van De Wetering et al. 1999). Various workers have studied the probing behavior of the male and female whiteflies, B. tabaci, and observed that female salivated more and for longer time than males regardless of the biotype; this could affect the virus ingestion in the males and females (Jiang et al. 2000; Ning et al. 2015). Still there are many factors that affect the virus transmission abilities, like level of virus penetration across the midgut (Guo et al. 2018), presence of specific endosymbionts (Gottlieb et al. 2010; Kliot and Ghanim 2013; Rana et al. 2016; Van den Heuvel et al. 1994), and titer of the virus in the host plant (Wintermantel et al. 2016) that need to be taken into consideration. Newly added vectors in the Indo-Gangetic plains can change the epidemiology of the ToLCNDV-borne diseases in this region, which contribute for major part of potato production. There are numerous examples where invasion of whitefly strains had caused severe impact on the agriculture. During 1991 vast numbers of whiteflies were recorded on the winter vegetable crops caused estimated loss of $500 million in California (USA) alone. Later studies also revealed that invasion of biotype B in this region and associated Gemini viruses (Brown 1991). Introduction of MEAM1 whitefly genetic group (India) results in endemic occurrence of tomato leaf curl virus disease in Bangalore (Banks et al. 2001). The rapid spread of tomato chlorosis virus was associated with introduction of MED populations of B. tabaci in China (Shi et al. 2018). Recent reports suggested the association of only Asia II 5 B. tabaci genetic group with chrysanthemum in the Karnataka (Ashwathappa et al. 2020). This host might be responsible for potential geographical expansion of the Asia II 5 genetic group in the Indo-Gangetic region or North India. Among flowering plants, chrysanthemum is commercially cultivated through terminal cuttings, root suckers. Transport of planting material (terminal cuttings) harboring developmental stages of Asia II 5 genetic group might be responsible for spread of this genetic group in Indo-Gangetic plains. Besides, this genetic group (Asia II 5) has also been recorded on other host plants, like brinjal, beans, sunflower, cassava, Ageratum spp., and tobacco, from south India (Chowda-Reddy et al. 2012; Ellango et al. 2015). However, chances of migration mediated through these host plants are limited due to seed propagated nature of brinjal, beans, sunflower, Ageratum spp, and tobacco. Moreover, other host, like cassava, is not being cultivated in the major potato growing area of North India. However, there is a possibility of Asia II 5 B. tabaci getting migrated with other hosts which have not been recorded yet. This study highlights the changing distribution of the B. tabaci genetic group in India which may change the dynamics of vector-borne diseases in potato production system.
Conclusion
700 bp mtCOI gene sequence analysis of the B. tabaci samples collected from major potato growing regions of India revealed the prevalence of three B. tabaci genetic groups in the region. The study also highlights that Asia II 5 genetic group has expanded its geographical distribution from south India to Indo-Gangetic plains. This geographical expansion of new B. tabaci genetic group warns for outbreak and spread of B. tabaci-transmitted viruses in potato as well as other host plants. Furthermore, females of Asia II genetic group are efficient vectors as compared to males. Still there is a need for extensive and continuous survey for understanding the distribution and associated host plants of B. tabaci genetic groups in India.
Acknowledgements
This study was supported by grants from Indian Council of Agricultural Research, New Delhi, India to ICAR-CPRI, Shimla.
Author contributions
KCN and SSH conceptualization. KCN, SK, RMS, GMB, AS and GKP methodology. KCN, SSI, RK, TB experimentation. GV software analysis. KCN, RKT, SS, AB writing-original draft.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Contributor Information
Kailash C. Naga, Email: Kailashnaga3j@gmail.com
Sundaresha Siddappa, Email: sundareshas8@gmail.com.
Ravinder Kumar, Email: chauhanravinder97@gmail.com.
Rahul K. Tiwari, Email: rahultiwari226@gmail.com
S. Subhash, Email: subhashsram@gmail.com
Gaurav Verma, Email: gaurav14000@gmail.com.
Tanuja Buckseth, Email: tanujagbpuat@gmail.com.
Aarti Bairwa, Email: aarticpri13@gmail.com.
Sanjeev Sharma, Email: sanjeevsharma.cpri@gmail.com.
Subhash Katare, Email: Subash.Katare@icar.gov.in.
R. M. Srivastava, Email: ravimohanento@gmail.com
G. M. Bansode, Email: ganeshban@rediffmail.com
Anirban Sarkar, Email: asarkar1920@gmail.com.
J. K. Patel, Email: jkpatel2489@gmail.com
References
- Ashwathappa KV, Venkataravanappa V, Reddy CNL, Reddy MK. Association of tomato leaf curl New Delhi virus with mosaic and leaf curl disease of Chrysanthemum and its whitefly cryptic species. Indian Phytopathol. 2020;73:533–542. doi: 10.1007/s42360-020-00214-1. [DOI] [Google Scholar]
- Banks GK, Colvin J, Reddy RVC, et al. First report of the Bemisia tabaci B biotype in India and an associated tomato leaf curl virus disease Epidemic. Plant Dis. 2001;85:231–231. doi: 10.1094/pdis.2001.85.2.231c. [DOI] [PubMed] [Google Scholar]
- Bedford ID, Briddon RW, Brown JK, et al. Geminivirus transmission and biological characterisation of Bemisia tabaci (Gennadius) biotypes from different geographic regions. Ann Appl Biol. 1994;125:311–325. doi: 10.1111/j.1744-7348.1994.tb04972.x. [DOI] [Google Scholar]
- Boykin LM, De Barro PJ. A practical guide to identifying members of the Bemisia tabaci species complex: and other morphologically identical species. Front Ecol Evol. 2014;2:45. doi: 10.3389/FEVO.2014.00045. [DOI] [Google Scholar]
- Boykin LM, Shatters RG, Rosell RC, et al. Global relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) revealed using Bayesian analysis of mitochondrial COI DNA sequences. Mol Phylogenet Evol. 2007;44:1306–1319. doi: 10.1016/J.YMPEV.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Briddon RW, Bull SE, Amin I, et al. Diversity of DNA β, a satellite molecule associated with some monopartite begomoviruses. Virology. 2003;312:106–121. doi: 10.1016/S0042-6822(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Brown JK. An update on the whitefly-transmitted geminiviruses in the Americas and the Caribbean basin. FAO Plant Prot Bull. 1991;39:5–23. [Google Scholar]
- Brown JK, Coats SA, Bedford ID, et al. Characterization and distribution of esterase electromorphs in the whitefly, Bemisia tabaci (Genn.) (Homoptera: Aleyrodidae) Biochem Genet. 1995;33:205–214. doi: 10.1007/BF02401851. [DOI] [PubMed] [Google Scholar]
- Byrne DN, Bellows TS. Whitefly biology. Annu Rev Entomol. 1991;36(1):431–457. doi: 10.1146/annurev.ento.36.1.431. [DOI] [Google Scholar]
- Chandel RS, Banyal DK, Singh BP, et al. Integrated management of whitefly, Bemisia tabaci (Gennadius) and potato apical leaf curl virus in India. Potato Res. 2010;53:129–139. doi: 10.1007/s11540-010-9152-3. [DOI] [Google Scholar]
- Chowda-Reddy RV, Kirankumar M, Seal SE, et al. Bemisia tabaci phylogenetic groups in India and the relative transmission efficacy of tomato leaf curl Bangalore virus by an indigenous and an exotic population. J Integr Agric. 2012;11:235–248. doi: 10.1016/S2095-3119(12)60008-2. [DOI] [Google Scholar]
- Czosnek H, Hariton-Shalev A, Sobol I, et al. The incredible journey of begomoviruses in their whitefly vector. Viruses. 2017;9(10):273. doi: 10.3390/v9100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Barro PJ. Genetic structure of the whitefly Bemisia tabaci in the Asia-Pacific region revealed using microsatellite markers. Mol Ecol. 2005;14:3695–3718. doi: 10.1111/j.1365-294X.2005.02700.x. [DOI] [PubMed] [Google Scholar]
- De Barro PJ, Liu S-S, Boykin LM, Dinsdale AB. Bemisia tabaci : A statement of species status. Annu Rev Entomol. 2011;56:1–19. doi: 10.1146/annurev-ento-112408-085504. [DOI] [PubMed] [Google Scholar]
- den Heuvel V, Johannes FJM, Verbeek M, Van der Wilk F. Endosymbiotic bacteria associated with circulative transmission of potato leafroll virus by Myzus persicae. J Gen Virol. 1994;75:2559–2565. doi: 10.1099/0022-1317-75-10-2559. [DOI] [PubMed] [Google Scholar]
- Dinsdale A, Cook L, Riginos C, et al. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann Entomol Soc Am. 2010;103:196–208. doi: 10.1603/an09061. [DOI] [Google Scholar]
- Drost YC, van Lenteren JC, van Roermund HJW. Life-history parameters of different biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae) in relation to temperature and host plant: a selective review. Bull Entomol Res. 1998;88(3):219–230. doi: 10.1017/s0007485300025840. [DOI] [Google Scholar]
- Ellango R, Singh ST, Rana VS, et al. Distribution of Bemisia tabaci genetic groups in India. Environ Entomol. 2015;44:1258–1264. doi: 10.1093/ee/nvv062. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution (n y) 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Garg ID, Khurana SP, Shiv K, Lakra BS. Association of a geminivirus with potato apical leaf curl in India and its immuno-electron microscopic detection. J Indian Potato Assoc. 2001;28:227–232. [Google Scholar]
- Gottlieb Y, Zchori-Fein E, Mozes-Daube N, et al. The transmission efficiency of tomato yellow leaf curl virus by the whitefly, Bemisia tabaci is correlated with the presence of a specific symbiotic bacterium species. J Virol. 2010;84:9310–9317. doi: 10.1128/jvi.00423-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Zhao J, Pan LL, et al. The level of midgut penetration of two begomoviruses affects their acquisition and transmission by two species of Bemisia tabaci. Virology. 2018;515:66–73. doi: 10.1016/j.virol.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Hogenhout SA, Ammar ED, Whitfield AE, Redinbaugh MG. Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol. 2008;46:327–359. doi: 10.1146/annurev.phyto.022508.092135. [DOI] [PubMed] [Google Scholar]
- Hussain S, Farooq M, Malik HJ, et al. Whole genome sequencing of Asia II 1 species of whitefly reveals that genes involved in virus transmission and insecticide resistance have genetic variances between Asia II 1 and MEAM1 species. BMC Genom. 2019;20(1):1–13. doi: 10.1186/s12864-019-5877-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevalatha A, Kaundal P, Venkatasalam EP, et al. Uniplex and duplex pcr detection of geminivirus associated with potato apical leaf curl disease in India. J Virol Methods. 2013;193:62–67. doi: 10.1016/j.jviromet.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Jeevalatha A, Singh BP, Kaundal P, et al. RCA-PCR: A robust technique for the detection of tomato leaf curl New Delhi virus-potato at ultra low virus titre. Potato J. 2014;41:76–80. [Google Scholar]
- Jiang YX, De Blas C, Barrios L, Fereres A. Correlation between whitefly (Homoptera: Aleyrodidae) feeding behavior and transmission of tomato yellow leaf curl virus. Ann Entomol Soc Am. 2000;93:573–579. doi: 10.1603/0013-8746(2000)093[0573:CBWHAF]2.0.CO;2. [DOI] [Google Scholar]
- Kanakala S, Ghanim M. Global genetic diversity and geographical distribution of Bemisia tabaci and its bacterial endosymbionts. PLoS ONE. 2019;14(3):e0213946. doi: 10.1371/journal.pone.0213946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliot A, Ghanim M. The role of bacterial chaperones in the circulative transmission of plant viruses by insect vectors. Viruses. 2013;5:1516–1535. doi: 10.3390/v5061516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuze JF, Souza-Dias JAC, Jeevalatha A, Figueira AR, Valkonen JP, Jones RA. Viral diseases in potato. In: Campos H, Ortiz O, editors. The potato crop. Cham: Springer; 2020. pp. 389–430. [Google Scholar]
- Lakra BS. Potato apical leaf curl begomovirus-symptoms, appraisal of a scale and losses in potato crop. Potato J. 2003;30:119–120. [Google Scholar]
- Lee W, Park J, Lee GS, et al. Taxonomic status of the Bemisia tabaci complex (Hemiptera: Aleyrodidae) and reassessment of the number of its constituent species. PLoS One. 2013;8(5):e63817. doi: 10.1371/journal.pone.0063817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyappa V, Venkatesh HM, Ramappa HK, et al. Tomato leaf curl virus from Bangalore (ToLCV-Ban4): sequence comparison with Indian ToLCV isolates, detection in plants and insects, and vector relationships. Arch Virol. 2000;145:1583–1598. doi: 10.1007/s007050070078. [DOI] [PubMed] [Google Scholar]
- Naga KC, Subhash S, Bhatnagar A, Tiwari RK, et al. Influence of host plants on virus acquisition and endosymbionts of whitefly, Bemisia tabaci (Gennadius) Indian J Entomol. 2020;83:1–5. doi: 10.5958/0974-8172.2020.00206.0. [DOI] [Google Scholar]
- Naranjo SE, Ellsworth PC. Challenges and opportunities for pest management of Bemisia tabaci in the new century. Crop Prot. 2001;9(20):707. doi: 10.1016/S0261-2194(01)00107-7. [DOI] [Google Scholar]
- Narayana YD, Muniyappa V, Narayana YD. Virus vector relationships of a planthopper (Peregrinus maidis) borne sorghum stripe tenuivirus. Int J Pest Manag. 1996;42:165–170. doi: 10.1080/09670879609371990. [DOI] [Google Scholar]
- Navas-Castillo J, Fiallo-Olivé E, Sánchez-Campos S. Emerging virus diseases transmitted by whiteflies. Annu Rev Phytopathol. 2011;49:219–248. doi: 10.1146/annurev-phyto-072910-095235. [DOI] [PubMed] [Google Scholar]
- Naveen NC, Chaubey R, Kumar D, et al. Insecticide resistance status in the whitefly, Bemisia tabaci genetic groups Asia-I, Asia-II-1 and Asia-II-7 on the Indian subcontinent. Sci Rep. 2017;7:1–15. doi: 10.1038/srep40634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning W, Shi X, Liu B, et al. Transmission of tomato yellow leaf curl virus by Bemisia tabaci as affected by whitefly sex and biotype. Sci Rep. 2015;5:1–8. doi: 10.1038/srep10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes-Montero JR, Hameed U, Zia-Ur-Rehman M, et al. Demographic expansion of the predominant Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) mitotypes associated with the cotton leaf curl virus epidemic in Pakistan. Ann Entomol Soc Am. 2019;112(3):265–280. doi: 10.1093/aesa/saz002. [DOI] [Google Scholar]
- Peng J, Xie G, Zhang S, et al. Higher ramie mosaic virus transmission efficiency by females than by males of Bemisia tabaci MED. Sci Rep. 2020;10:5–10. doi: 10.1038/s41598-019-57343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polston JE. Association of the nucleic acid of squash leaf curl Geminivirus with the whitefly, Bemisia Tabaci. Phytopathology. 1990;80(9):850–856. doi: 10.1094/Phyto-80-850. [DOI] [Google Scholar]
- Rana VS, Popli S, Saurav GK, et al. A Bemisia tabaci midgut protein interacts with begomoviruses and plays a role in virus transmission. Cell Microbiol. 2016;18(5):663–678. doi: 10.1111/cmi.12538. [DOI] [PubMed] [Google Scholar]
- Rekha AR, Maruthi MN, Muniyappa V, Colvin J. Occurrence of three genotypic clusters of Bemisia tabaci and the rapid spread of the B biotype in south India. Entomol Exp Appl. 2005;117:221–233. doi: 10.1111/J.1570-7458.2005.00352.X. [DOI] [Google Scholar]
- Rotenberg D, Kumar NKK, Ullman DE, et al. Variation in tomato spotted wilt virus titer in Frankliniella occidentalis and its association with frequency of transmission. Phytopathology. 2009;99:404–410. doi: 10.1094/PHYTO-99-4-0404. [DOI] [PubMed] [Google Scholar]
- Saeed ST, Kumar B, Shasany AK, Samad A. Molecular identification of chilli leaf curl india virus along with betasatellite molecule causing leaf curl disease of menthol mint (Mentha arvensis var. Kosi) in India. J Gen Plant Pathol. 2017;83:333–336. doi: 10.1007/s10327-017-0730-y. [DOI] [Google Scholar]
- Saha A, Saha D. Molecular detection and partial characterization of a begomovirus causing leaf curl disease of potato in sub-Himalayan West Bengal, India. Artic J Environ Biol. 2014;35:601–606. [PubMed] [Google Scholar]
- Segev L, Wintermantel WM, Polston JE, Lapidot M. First report of tomato chlorosis virus in Israel. Plant Dis. 2004;88:1160–1160. doi: 10.1094/pdis.2004.88.10.1160a. [DOI] [PubMed] [Google Scholar]
- Shah MA, Malik K, Bhatnagar A, et al. Effect of temperature and cropping sequence on the infestation pattern of Bemisia tabaci in potato. Indian J Agric Sci. 2019;89:1802–1807. [Google Scholar]
- Shi X, Tang X, Zhang X, et al. Transmission efficiency, preference and behavior of Bemisia tabaci MEAM1 and MED under the influence of tomato chlorosis virus. Front Plant Sci. 2018;8:1–9. doi: 10.3389/fpls.2017.02271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Frati F, Beckenbach A, et al. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am. 1994;87:651–701. doi: 10.1093/aesa/87.6.651. [DOI] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usharani KS, Surendranath B, Paul-Khurana SM, et al. Potato leaf curl—a new disease of potato in northern India caused by a strain of tomato leaf curl New Delhi virus. Plant Pathol. 2004;53(2):235–235. doi: 10.1111/j.0032-0862.2004.00959.x. [DOI] [Google Scholar]
- Van De Wetering F, Van Der Hoek M, Goldbach R, Peters D. Differences in tomato spotted wilt virus vector competency between males and females of Frankliniella occidentalis. Entomol Exp Appl. 1999;93:105–112. doi: 10.1046/j.1570-7458.1999.00567.x. [DOI] [Google Scholar]
- Venkatasalam EP, Singh S, Sivalingam PN, et al. Polymerase chain reaction and nucleic acid spot hybridisation detection of begomovirus(es) associated with apical leaf curl disease of potato. Arch Phytopathol Plant Prot. 2011;44:987–992. doi: 10.1080/03235401003633741. [DOI] [Google Scholar]
- Wintermantel WM, Gilbertson RL, McCreight JD, Natwick ET. Host-specific relationship between virus titer and whitefly transmission of cucurbit yellow stunting disorder virus. Plant Dis. 2016;100:92–98. doi: 10.1094/PDIS-11-14-1119-RE. [DOI] [PubMed] [Google Scholar]
- Xie W, Xu YX, Jiao XG, Zhang YJ. High efficient of females of B-type Bemisia tabaci as males in transmitting the whitefly-borne tomato yellow leaf curl virus to tomato plant with Q-PCR method confirmation. Commun Integr Biol. 2012;5:543–545. doi: 10.4161/cib.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]