Abstract
Objectives
Psychological symptoms of menopause may impose a negative effect on the quality of life of menopausal women. Thus, the management of these symptoms can improve the quality of life and psychological health of such woman. The present study aimed to determine the effect of evening primrose on psychological symptoms in menopausal women.
Methods
In this triple-blind randomized clinical trial, the effect of the evening primrose oil on postmenopausal psychological symptoms was investigated. The subjects were 100 menopausal women, referred to a healthcare center in Dastena city (Chaharmahal and Bakhtiari Province, Iran), who were randomly assigned to two groups. The intervention group used two 1 g pearls of evening primrose oil daily. The study instruments included a sub-scale of Menopause Rating Scale (MRS). Menopause data were analyzed using an independent t-test and Friedman test by the SPSS software. P values < 0.05 were considered statistically significant.
Results
The two groups were balanced in demographic characteristics and psychological disorder severity before the intervention. The median (interquartile range) MRS score in the intervention group before the intervention and 2 and 4 weeks after the intervention were 11 (10–12), 6 (5–7), and 3 (2–4), respectively, and in the placebo, they were 11 (9–11), 10 (9–11), and 11 (10–12). A significant reduction was observed in the intervention group compared with the placebo group 2 and 4 weeks post-intervention.
Conclusion
The use of evening primrose oil can decrease postmenopausal psychological symptoms.
Keywords: Evening primrose oil, Psychological, Post-menopausal
INTRODUCTION
In recent years, psychological health has been on the agenda all over the world because psychological disorders, besides involving all age groups, can impose a great burden of costs on the society [1,2]. World Health Organization (WHO) considers psychological health as one of the health dimensions, and psychological disorders as the second reason for disabilities by 2020 [3,4]. Psychological disorders have a high prevalence so that up to 25% of all people may experience them during their lives [2]. Menopause transition is one stage in women's lives that can affect the psychological condition and increase depression prevalence in them [5,6]. Currently, around 74% to 80% of women suffer from menopausal symptoms, and menopause is regarded as a universal phenomenon [7,8,9]. As the life expectancy increases in women, approximately one third of their life span is spent in the menopause transition [10,11]. There is no exact statistics on the prevalence of postmenopuusul psychological disorders. Cray et al. [12] reported the prevalence of psychological disorders in menopause women to be more than 30%. Beside depression, a wide spectrum of psychological disorders occurs during the menopause transition including decreased energy, decreased concentration power and academic performance, fatigue, aggressiveness, mental fatigue, irritability, a sense of vanity and inefficiency, loneliness, intolerance, anti-social behaviors, sleep disorder, and decreased sexual desire [12,13,14]. Since a great part of symptoms can be due to the complications of lack of estrogen production [14], making up for this deficiency can certainly prevent from the creation and worsening of these complications. Schmidt et al. [15] showed that use of estradiol improved the mood and decreased depression in pre-menopausal women. Although women are encouraged to use alternative hormone methods to alleviate the menopausal symptoms including depression [15,16], most women who choose not to use hormone therapy do so for perceived risks of hormone therapy use, and some women are reluctant to use hormones [17,18]. Research has shown that use of alternative hormone methods may increases breast and endometrial cancers, coronary heart diseases, stroke, thrombo embolism, etc. [17,19]. Given the above concerns, women are more willing to use alternative therapies including herbal drugs. In addition, Kwee et al. [20] has clearly shown that Chinese traditional medicine can be helpful in comparison of hormone therapy and placebo in removing menopause-related problems. In parallel, few studies have investigated the influence of herbal drugs on the improvement of post-menopausal psychological symptoms. For example, Taavoni et al. [21] found that psychological symptoms of menopause improved upon the three-month consumption of red clover. Furthermore, investigation of the influence of red ginseng on post-menopausal psychological complications of menopause revealed the effect of this herbal supplement [22]. Oenothera biennis L. is a plant of the Onagraceae family, usually known as the evening primrose. The oil extracted from this plant seeds is a full source of unsaturated fatty acids, especially linoleic acid and gamma linoleic acid, which are used widely in the pharmacy industry. It has been shown that this oil is widely used in the improvement of rheumatic diseases, dermatitis, psoriasis, premenstrual syndrome and the treatment of menopausal symptoms [23,24]. Psychological symptoms in menopause women can negatively influence their quality of life, and management of these symptoms can improve their life quality and psychological health [21,25]. In addition, according to different studies, the compounds containing unsaturated fatty acids have anti-depression effects [26,27]. So, in the present research, we decided to investigate the effect of evening primrose oil pearl on psychological symptoms in menopause women.
MATERIALS AND METHODS
The present triple blind randomized clinical trial was carried out on 100 menopause women referring to the health care center of Dastena city (Charmahal o Bakhtiyari Province) from August 2015 to December 2015. All the healthy menopause women with no background disease and no positive pap-smear test within the last one year, consuming no hormone drugs, no background of psychological diseases resulted from a non-menopausal reason, not complaining about menopausal symptoms, willing to participate in the research, and having a minimum literacy education were included in the study. The exclusion criteria included severe digestive disorders, weight increase probability, and sensitivity to pearl.
The study proposal was approved by the Ethics Committee of Shahrekord University of Medical Sciences (No. 6-11-89). It was also registered at the Iranian Center for Clinical Trials (No. IRCT2017012432161N1).
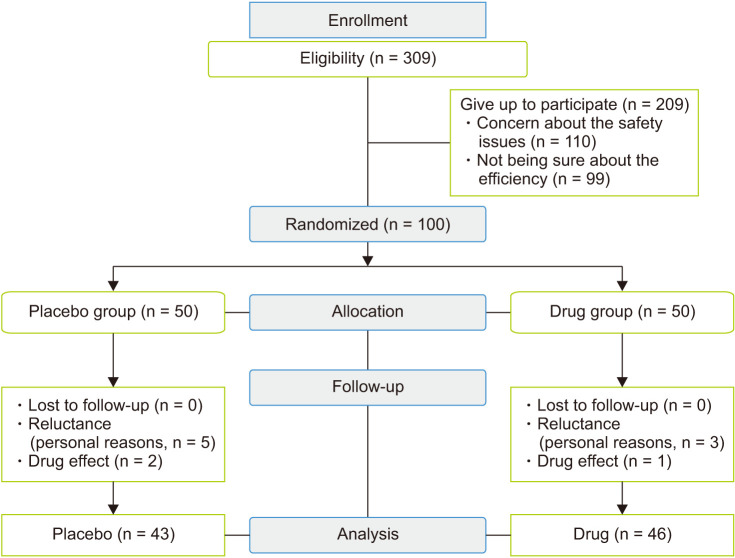
Among the 309 women possessing the competency to participate in this study, 209 individuals gave up because of concern about the safety issues and not being sure about the efficiency of the research. The present research was a triple blind study, i.e., the participants, the researcher and the data analyzer were unaware of the performed interventions, and both group used drugs in similar boxes with coded stickers. Since the manufacturing company (Barij Esans, Kashan, Iran) had placed both the evening primrose pearl and the placebo in similar boxes in the same shapes and number, while coding the boxes from 1–100 (50 number assigned to drugs and 50 number to placebo), it was impossible for the researcher to distinguish between the drug (Each capsule contained 1,000 mg of evening primrose oil. Evening primrose oil has two types of omega-6-fatty acid including linoleic acid [60%–80%] and γ-linoleic acid [8%–14%]. Each soft capsule was standardized based on 70 to 140 mg of γ-linoleic acid) and the placebo. Each placebo capsule was similar to evening primrose oil containing 1,000 mg of paraffin oil, corn oil and sunflower oil. Therefore, the participants randomly selected and used one drug box at the time of referral.
Upon approval by the Ethics Committee of Shahrekord University of Medical Sciences, Shahrekord, Iran, the researcher referred to the research context, introduced herself to the center's authorities, completely justified the relevant personnel, obtained written informed consent from the participants, explained the goals of the study, and finally, handed over the demographic questionnaire and the checklist of pearl use as well as the Menopause Rating Scale (MRS) to the participants. Then, she asked them to complete and return the first questionnaire immediately. Next, the participants were asked to select one of the one-hundred boxes randomly. Each box contained 30 pearls to be taken within 15 days. When delivering the drug and explaining the way to use it (one pearl to be taken once in the morning and one more at night with a glass of water), they were given some explanation on the drug side effects and the researcher's cell phone number so that they can contact with the researcher in case of any problem while taking the pearl. During these 15 days, the researcher ensured the appropriate use of drugs through phone call and the weekly checklist. The second questionnaire was completed after the first 15-day period of drug consumption, and the second box containing drug or placebo with the same previous codes was handed over to the units under research. The same procedure of phone call checking and consumption checklist completion was performed. The third questionnaire was completed on the 30th day after the second period of drug/placebo consumption.
The variables such as age of menopause women, time lapsed after the last menstruation (month), number of pregnancies, deliveries of number, and education level, were used for assessing the balancing of groups.
To determine the menopause-related psychological symptoms, we used the psychological section in the MRS. This instrument, which was first developed by the specialized committee of German-speaking countries in 1996, includes three subscales. The reliability and validity of the instrument were confirmed in different countries (Iran, Germany, England, France, Sweden, etc.) through calculation of the Cronbach's alpha in a great number of populations [16,28]. In the present research, the Persian version of the instrument translated by Taavoni et al. [28] in 2012 from its English version was used. The validity of this version was confirmed and its Cronbach's alpha was obtained as 0.79, which showing its good reliability. The instrument assessed the severity of menopause-related psychological symptoms including the four symptoms of (1) depressed mood (sense of frustration, feeling of inclination to cry, and lack of motivation), (2) irritability (sense of nervousness, internal tension and aggression), (3) anxiety (concern and feeling of fear), and (4) mental exhaustion (inability to do something, weak memory, forgetfulness, and lack of concentration). To use this instrument, first, the severity of each symptom was scored based on the degree reported by the participants (0 for no symptoms, 1 for light symptoms, 2 for mediate symptoms, 3 for severe symptoms, and 4 for very severe symptoms). The total of these four scores represented the menopause-related psychological symptoms that was varied from 0 to 16. It is to be mentioned that the data collection method in both questionnaires was self-report.
Data were presented as mean ± standard deviation for normally distributed variables, and median (interquartile range) for non-normal variables. Comparison of baseline variables between the two groups was done using independent t-test. Because the psychological symptoms had been measured based on ordinal scale, nonparametric tests were used for analysis. Comparison of psychological symptoms between the two groups was done using Mann–Whitney's test. Comparison of psychological symptoms during the study was done using Friedman's test. Statistical analysis was performed by the IBM SPSS Statistics (ver. 23; IBM, Armonk, NY, USA), and P values < 0.05 were statistically significant.
RESULTS
The study flowchart is shown in Figure 1. From 100 individuals agreed to participate in the study, 11 women were dropped out or excluded from the study because of reluctance, weight increase probability, and drug effect. Totally, 89 women participated in the study, of whom 46 women received the drug and 43 women received the placebo.
Fig. 1. CONSORT (CONsolidated Standards Of Reporting Trials) flowchart of the study population.
The age of participants was in the range of 46–63 years with mean of 54.7 ± 4.1 years. The time lapsed from their last menstruation was in the range of 13–26 months with the mean of 18.5 ± 3.6 months. As observed in Table 1, participants of two groups were balanced in term of demographic characteristics such as age, mean time after the last menstruation, mean age at menarche, number of pregnancies, mean delivery number, education status, exercise, smoking, marital status, and body mass index.
Table 1. Some characteristics of the participants in the two groups of drug and placebo.
| Characteristic | Drug | Placebo | P value | |
|---|---|---|---|---|
| Age (y) | 54.6 ± 3.8 | 54.7 ± 4.6 | 0.09 | |
| Mean time after the last menstruation (mo) | 18.8 ± 3.3 | 18.8 ± 3.9 | 0.42 | |
| Age at menarche (y) | 12.4 ± 1.5 | 12.1 ± 1.5 | 0.94 | |
| No. of pregnancies | 5.8 ± 1.4 | 5.5 ± 1.5 | 0.26 | |
| No. of deliveries | 6.0 ± 1.5 | 5.9 ± 1.6 | 0.98 | |
| Education | Elementary | 24 (48.0) | 23 (46.0) | 0.5 |
| Up to diploma | 26 (52.0) | 27 (54.0) | ||
| Exercise | Yes | 32 (64.0) | 33 (66.0) | 0.5 |
| No | 18 (36.0) | 17 (34.0) | ||
| Smoking | Yes | 41 (82.0) | 41 (82.0) | 0.6 |
| No | 9 (18.0) | 9 (18.0) | ||
| Marital status | Single | 14 (28.0) | 14 (28.0) | 0.97 |
| Married | 23 (46.0) | 22 (44.0) | ||
| Divorced | 13 (26.0) | 14 (28.0) | ||
| Body mass index | Thin (< 20 kg/m2) | 0 | 0 | 0.98 |
| Normal (20–24.9 kg/m2) | 14 (28.0) | 14 (28.0) | ||
| Overweight (25–29.9 kg/m2) | 23 (46.0) | 23 (46.0) | ||
| Obese (≥ 30 kg/m2) | 13 (26.0) | 13 (26.0) | ||
Data are presented as mean ± standard deviation or number (%).
Table 2 presents the results of different symptoms of the psychological subscale of MRS. Based on these results, before the intervention, the two groups of drug and placebo did not have any significant difference in terms of symptoms of depressed mood, anxiety and mental exhaustion as well as total MRS. However, after the intervention in the all of these symptoms and total MRS, the severity of symptoms was significantly lower in the drug group as compared to the placebo group (P < 0.001).
Table 2. Comparison of the median severity of postmenopausal psychological symptoms before and during the intervention in the two groups.
| Psychological disorder | Drug | Placebo |
P value (between group) |
|
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | |||
| Depressed mood | Before intervention | 3 (2, 3) | 3 (2, 3) | 0.609 |
| Two weeks later | 2 (1, 2) | 3 (2, 3) | < 0.001 | |
| Four weeks later | 0 (0, 1) | 3 (3, 3) | < 0.001 | |
| P value (within group) | < 0.001 | < 0.001 | - | |
| Difference during the study | 2 (2, 3) | 0 (0, 0) | < 0.001 | |
| Irritability | Before intervention | 3 (2, 3) | 2 (2, 3) | 0.001 |
| Two weeks later | 2 (2, 2) | 2 (2, 2) | 0.015 | |
| Four weeks later | 1 (1, 1) | 2 (2, 3) | < 0.001 | |
| P value (within group) | < 0.001 | 0.007 | - | |
| Difference during the study | 2 (1, 2) | 0 (−1, 0) | < 0.001 | |
| Anxiety | Before intervention | 2 (2, 2) | 2 (1, 2) | 0.073 |
| Two weeks later | 1 (1, 1) | 2 (2, 2) | < 0.001 | |
| Four weeks later | 1 (1, 1) | 2 (2, 3) | < 0.001 | |
| P value (within group) | < 0.001 | 0.001 | - | |
| Difference during the study | 1 (1, 1) | −1 (−1, 0) | < 0.001 | |
| Mental exhaustion | Before intervention | 3 (2, 4) | 3 (3, 4) | 0.361 |
| Two weeks later | 1 (1, 2) | 3 (3, 4) | < 0.001 | |
| Four weeks later | 1 (0, 1) | 4 (3, 4) | < 0.001 | |
| P value (within group) | < 0.001 | 0.028 | - | |
| Difference during the study | 3 (2, 3) | 0 (−1, 0) | < 0.001 | |
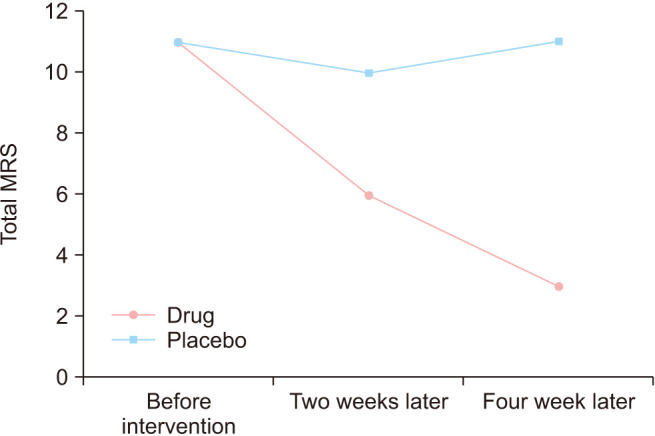
| Total MRS | Before intervention | 11 (10, 12) | 11 (9, 11) | 0.085 |
| Two weeks later | 6 (5, 7) | 10 (9, 11) | < 0.001 | |
| Four weeks later | 3 (2, 4) | 11 (10, 12) | < 0.001 | |
| P value (within group) | < 0.001 | < 0.001 | - | |
| Difference during the study | 8 (7, 9) | −1 (−2, 0) | < 0.001 | |
IQR: interquartile range, MRS: Menopause Rating Scale.
Regarding the symptom of irritability, its severity before the intervention was significantly higher in the drug group; however, two and four weeks after the intervention, the severity of this symptom was significantly lower in the drug group in comparison with the placebo group (P < 0.001). Also, the severity reduction of irritability was higher in the drug group as compared to the placebo group during the study (P < 0.001).
The Friedman test results showed that the severity of all symptoms was changed during the study in both groups. However, the amount of severity reduction was significantly higher in the drug group as compared to the placebo group for all symptoms and total MRS (P < 0.001).
The median of total severity of psychological disorders in the menopause women based on MRS during the study is illustrate in Figure 2. As shown, the MRS of the placebo group did not change during the intervention, but the MRS in the drug group decreased during the intervention.
Fig. 2. The median severity of psychological disorders in the menopause women during the intervention. MRS: Menopause Rating Scale.
DISCUSSION
This study investigated the influence of evening primrose oil pearls on menopausal psychological symptoms. The results showed, improved mood and decreased psychological disorders upon a one-month consumption of the mentioned plant oil. Although there are many studies conducted on the influence of supplements on menopausal physical symptoms, there are limited reports in the literature on the influence of medicinal herbs on the improvement of psychological symptoms in menopause women. For example, Taavoni et al. [16,21] investigated the influence of red clover and aromatherapy massage on menopausal psychological symptoms in two separate studies, in both of which the severity of anxiety and psychological symptoms in menopause women decreased after two intervention. The results of these studies are compatible with ours. In addition, Tode et al. [22] assessed the influence of Ginseng on menopausal psychological symptoms. In another study, consumption of fenugreek seeds improved the depression in menopause women [29]. The results of the two latter studies are also in line with the results of the present study. However, the instrument used in the present study and in Taavoni's study [21] was MRS, but Tode et al. [22] employed State-Trait Anxiety Inventory, and the instrument used by Akbari et al. [29] was Beck's questionnaire.
According to Rombaldi Bernardi et al. [30], increase of one standard deviation in serum arachidonic acid (AA) and adrenic acid (AdA) levels-two omega-6 fattyacids-was associated with a greater likelihood of suicide risk and major depressive episodes in early pregnancy, independent of confounding variables. The net overlapping effects of at least three mood stabilizers are a decreased turnover of AA, as well as decreased brain cyclo-oxygenase-2 and prostaglandin E2. Two randomized clinical trials have reported that inhibition of cyclo-oxygenase 2 with Celebrex significantly reduced symptoms of depression. Another putative link between diet, serum levels of n-6 long chain polyunsaturated fatty acid (LCPUFA) and mood disorders is the endocanobinnoid system of the CNS, which is involved in regulating the physiological and pathophysiological aspects of mood, in addition to other functions. The two principal endocannabinoids-anandamide (AEA) and 2-arachidonoylglycerol (2-AG)—are structurally derived from essential n-6fatty acids, mainly AA, and have cellular signaling functions in the modulation of neurotransmitter release that have been implicated in the pathophysiology of suicide and depression [31].
The present study investigated four symptoms in psychological disorders including depressed mood (sense of frustration, feeling of sorrow inclination to cry, and lack of motivation), irritability (sense of nervousness, internal tension, and aggression), anxiety (concern and feeling of fear), and mental exhaustion (inability to do something, weak memory, forgetfulness, and lack of concentration). In all of these four dimensions, the participants showed a high degree of disorders prior to the intervention, and the influence of drug as compared to placebo was very significant after the intervention. One probable reason for the decrease of psychological symptoms could be high volumes of unsaturated fatty acids, especially gamma linoleic acid in evening primrose oil [24]. Gamma linoleic acid is a member of the n-6 family of essential polyunsaturated fatty acids [27]. According to the available evidence, depression decreases by the use of three LCPUFAs. Based on epidemiologic studies, depression is less prevalent in populations using three LCPUFAs than in people not using these compounds [26,30,32]. Accumulating evidence supports the hypothesis that occurrence of depression is associated with a low LCPUFA status. The latter study showed the influence of evening primrose oil on menopausal psychological symptoms. A double blind study with control group confirmed the great influence of the evening primrose oil on the treatment of premenstrual syndrome, irritability, breast pain, sensitivity, water retention, and depression when other therapies did not work [33]. Finally, however, to support the hypothesis of the role of gamma linoleic acid in the decrease of psychological symptoms in menopause women, intervention studies with appropriate sample sizes are recommended.
This study revealed the positive impact of primrose plant on the psychological symptoms of menopause. In addition, no specific complications were observed during the study period. Hence, this plant can be used to treat postmenopausal women; however, to prove this claim, more intervention studies with large sample sizes are needed.
Among the limitations of the present study were variables such as pain, some background diseases and the degree of such diseases that definitely influence the severity of psychological symptoms, and were not under the control of the researcher. Another limitation was the fact that the participants underwent no other follow-up study to find out the duration of the drug effect. It is suggested that follow-up studies be done in the future studies. Third, in this study we did not obtain information about the patient's daily diet that can alter fatt., and it is suggested that the patient's diet be monitored in future studies.
ACKNOWLEDGMENTS
This research protocol was funded by the Shahrekod University of Medical Sciences, Yazd, Iran.
The authors would like to thank Shahrekord University of Medical Sciences for supporting this research and Shahrekord Brench Islamic Azad University.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Sarmento M. A “Mental Health Profile” of higher education students. Proc-Soc Behav Sci. 2015;191:12–20. [Google Scholar]
- 2.Prang KH, Bohensky M, Smith P, Collie A. Return to work outcomes for workers with mental health conditions: a retrospective cohort study. Injury. 2016;47:257–265. doi: 10.1016/j.injury.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Sivris KC, Leka S. Examples of holistic good practices in promoting and protecting mental health in the workplace: current and future challenges. Saf Health Work. 2015;6:295–304. doi: 10.1016/j.shaw.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalia M. Assessing the economic impact of stress--the modern day hidden epidemic. Metabolism. 2002;51(6 Suppl 1):49–53. doi: 10.1053/meta.2002.33193. [DOI] [PubMed] [Google Scholar]
- 5.Bromberger JT, Matthews KA, Schott LL, Brockwell S, Avis NE, Kravitz HM, et al. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN) J Affect Disord. 2007;103:267–272. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Research on the menopause in the 1990s: report of a WHO scientific group. Geneva: World Health Organization; 1996. [PubMed] [Google Scholar]
- 7.Palacios S, Henderson VW, Siseles N, Tan D, Villaseca P. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric. 2010;13:419–428. doi: 10.3109/13697137.2010.507886. [DOI] [PubMed] [Google Scholar]
- 8.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 9.Whelan TJ, Goss PE, Ingle JN, Pater JL, Tu D, Pritchard K, et al. Assessment of quality of life in MA.17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol. 2005;23:6931–6940. doi: 10.1200/JCO.2005.11.181. [DOI] [PubMed] [Google Scholar]
- 10.US Census Bureau. Global aging into the 21st century-the wall chart. Suitland: US Census Bureau; 1996. [Google Scholar]
- 11.Abbaspour Z, Mohammadi Nik F, Zand Moghadam A, Saadati N, Latifi SM. The effect of dietary soy protein isolate (SPI) on hot flushes in postmenopausal women. Jundishapur Sci Med J. 2003;(36):18–25. [Google Scholar]
- 12.Cray LA, Woods NF, Mitchell ES. Identifying symptom clusters during the menopausal transition: observations from the Seattle Midlife Women's Health Study. Climacteric. 2013;16:539–549. doi: 10.3109/13697137.2012.746657. [DOI] [PubMed] [Google Scholar]
- 13.Dennerstein L, Burrows GD. A review of studies of the psychological symptoms found at the menopause. Maturitas. 1978;1:55–64. doi: 10.1016/0378-5122(78)90010-5. [DOI] [PubMed] [Google Scholar]
- 14.Baksu A, Ayas B, Citak S, Kalan A, Baksu B, Goker N. Efficacy of tibolone and transdermal estrogen therapy on psychological symptoms in women following surgical menopause. Int J Gynaecol Obstet. 2005;91:58–62. doi: 10.1016/j.ijgo.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- 16.Taavoni S, Darsareh F, Joolaee S, Haghani H. The effect of aromatherapy massage on the psychological symptoms of postmenopausal Iranian women. Complement Ther Med. 2013;21:158–163. doi: 10.1016/j.ctim.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Berek JS, Novak E. Berek & Novak's gynecology. 15th ed. Philadelphia: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 18.Sandberg M, Wijma K, Wyon Y, Nedstrand E, Hammar M. Effects of electro-acupuncture on psychological distress in postmenopausal women. Complement Ther Med. 2002;10:161–169. doi: 10.1016/s0965229902000547. [DOI] [PubMed] [Google Scholar]
- 19.Innes KE, Selfe TK, Vishnu A. Mind-body therapies for menopausal symptoms: a systematic review. Maturitas. 2010;66:135–149. doi: 10.1016/j.maturitas.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwee SH, Tan HH, Marsman A, Wauters C. The effect of Chinese herbal medicines (CHM) on menopausal symptoms compared to hormone replacement therapy (HRT) and placebo. Maturitas. 2007;58:83–90. doi: 10.1016/j.maturitas.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Taavoni S, Sahkeri F, Goshegir S, Haghani H. Effect of Red Clover on postmenopausal psychological symptoms, a triple blind randomized placebo control clinical trial. European Psychiatry. 2015;30 Suppl 1:1829. [Google Scholar]
- 22.Tode T, Kikuchi Y, Hirata J, Kita T, Nakata H, Nagata I. Effect of Korean red ginseng on psychological functions in patients with severe climacteric syndromes. Int J Gynaecol Obstet. 1999;67:169–174. doi: 10.1016/s0020-7292(99)00168-x. [DOI] [PubMed] [Google Scholar]
- 23.Rees M. Alternative treatments for the menopause. Best Pract Res Clin Obstet Gynaecol. 2009;23:151–161. doi: 10.1016/j.bpobgyn.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Montserrat-de la Paz S, Fernández-Arche MA, Ángel-Martín M, García-Giménez MD. Phytochemical characterization of potential nutraceutical ingredients from Evening Primrose oil (Oenothera biennis L.) Phytochem Lett. 2014;8:158–162. [Google Scholar]
- 25.Mahboubi M. Evening primrose (Oenothera biennis) oil in management of female ailments. J Menopausal Med. 2019;25:74–82. doi: 10.6118/jmm.18190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peet M. International variations in the outcome of schizophrenia and the prevalence of depression in relation to national dietary practices: an ecological analysis. Br J Psychiatry. 2004;184:404–408. doi: 10.1192/bjp.184.5.404. [DOI] [PubMed] [Google Scholar]
- 27.Kenny FS, Pinder SE, Ellis IO, Gee JM, Nicholson RI, Bryce RP, et al. Gamma linolenic acid with tamoxifen as primary therapy in breast cancer. Int J Cancer. 2000;85:643–648. doi: 10.1002/(sici)1097-0215(20000301)85:5<643::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 28.Taavoni S, Shakeri F, Haghani H, Gooshegir SA. Effect of red clover on vasomotor symptoms and heart discomfort of menopausal women. Complement Med J Fac Nurs Midwifery. 2012;2:32–40. [Google Scholar]
- 29.Akbari N, Atarha M, Heidari T, Farahani L, Roozbehani N. Effect of fenugreek seed on menopausal depression: a randomized double-blind clinical trial. J Arak Uni Med Sci. 2013;16:1–7. [Google Scholar]
- 30.Rombaldi Bernardi J, de Souza Escobar R, Ferreira CF, Pelufo Silveira P. Fetal and neonatal levels of omega-3: effects on neurodevelopment, nutrition, and growth. ScientificWorldJournal. 2012;2012:202473. doi: 10.1100/2012/202473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaz JS, Kac G, Nardi AE, Hibbeln JR. Omega-6 fatty acids and greater likelihood of suicide risk and major depression in early pregnancy. J Affect Disord. 2014;152-154:76–82. doi: 10.1016/j.jad.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raeder MB, Steen VM, Vollset SE, Bjelland I. Associations between cod liver oil use and symptoms of depression: the Hordaland Health Study. J Affect Disord. 2007;101:245–249. doi: 10.1016/j.jad.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Horrobin DF. The role of essential fatty acids and prostaglandins in the premenstrual syndrome. J Reprod Med. 1983;28:465–468. [PubMed] [Google Scholar]