Abstract
Objectives
Postmenopausal women are predisposed to osteoporosis, and those on acidic diets are at a higher risk, because it has been demonstrated that such diets have adverse effects on bone health. In this study, the effect of alkaline drinking water on bone mineral density was evaluated in postmenopausal women with osteoporosis.
Methods
One hundred postmenopausal women with osteoporosis (T-score ≤ −2.5) were equally divided into an intervention group and a control group (n = 50 each). The intervention group received calcium D (daily), alkaline drinking water (1.5 L daily with pH 8.6 ± 0.3), and Osteofos tablet (70 mg weekly), whereas the control group received only calcium D and Osteofos tablet for 3 months. T-scores of the femur and spine bones were obtained using bone densitometry before and 3 months after the intervention.
Results
After the intervention, the mean T-scores of the femur and spine bones significantly increased in both the control and intervention groups (P < 0.05). However, the mean changes in the spine T-score were significantly higher in the intervention group (0.39 ± 0.07) than in the control group (0.08 ± 0.01) (P < 0.05). No significant differences were observed in the mean changes in the femur T-score between the two groups.
Conclusion
Our findings demonstrate that drinking alkaline water improves spine T-scores in postmenopausal women with osteoporosis. Hence, alkaline water can be used to treat osteoporosis due to increased bone density in postmenopausal women. Long-term interventions are necessary to confirm the effects of alkaline water on femur density.
Keywords: Alkaline water, Bone mineral density, Postmenopausal osteoporosis
INTRODUCTION
Osteoporosis is a disease characterized by reduced bone density and loss of bone microstructure, ultimately leading to increased bone fragility and risk of fracture. Today, osteoporosis is one of the public health issues with high physical and financial damage across the world [1,2]. The rate of bone fracture due to the reduction in bone density is higher than the total incidence rate of cancer, stroke, and heart attack [3]. Approximately, one in three women and one in 12 men will suffer from osteoporosis throughout their lifespan [4,5].
The bone regeneration process involves two stages of bone resorption and bone formation. In normal conditions, in healthy and young people, the rates of bone formation and bone resorption are approximately equal [6]. One of the factors that disturb this balance in women is menopause because of physiological and biochemical changes, increased turnover, increased bone resorption [7,8,9]. The main purpose of treatment is to prevent bone loss and increase bone formation [8,10].
Buffer systems Maintain pH in the body including bone, expiration of CO2, and renal acid excretion to increase bone resorption. With increasing pH, the activities of osteoclasts decrease and those of osteoblasts increase in bones [11,12]. Calcium in the form of phosphate or carbonate is the main reserve of the base in the human body. In response to the acid load caused by today's diets, these salts are released into the systemic circulation to maintain pH balance. The amount of calcium lost in the urine due to these diets within 20 years has been estimated to be about 480 g or about half the skeletal calcium [13].
In young people, acidifying diet increases bile excretion by 74% due to increased bone loss [14]. It has been observed in patients with chronic kidney failure that the modification of metabolic acidosis by bicarbonates significantly increases the level of the parathyroid hormone and active vitamin D [15].
Using quantitative computed tomography, it has been shown that chronic metabolic acidosis in rats decreases the total bone mineral density (BMD) [16], and changing the acid-base balance by using diet can affect bone health (acid-ash hypothesis) [17]. The highest proliferation of osteoblast-like cells has been reported at pH 8.0–8.4 [18]. Although several studies have shown that potassium bicarbonate, citrate-potassium, and even bicarbonate-rich mineral water can reduce BMD [19,20,21,22] and stimulate the synthesis of osteoblastic collagen [23], but the effect of alkaline water on T-score and bone density has not yet been investigated. Also, the action mechanism of alkaline pH on bone cell proliferation and differentiation has not yet been sufficiently investigated [17,18]. It has recently been observed that there is a link between an alkaline diet and alkaline water and a lower incidence of osteoporosis., as well as the observation of some positive effects of alkaline diets containing bicarbonate and potassium citrate on bone metabolism [17,24], more studies are needed in this regard [17]. The purpose of this study was the effect of alkaline drinking water on bone density in postmenopausal patients with osteoporosis.
MATERIALS AND METHODS
Participants
In this present experimental study conducted in 2018, the study population consisted of women referred to the Orthopedic Clinic of Shahrekord (southwestern Iran) with their first diagnosis of osteoporosis. The BMD of a person can be compared with the mean BMD in healthy young adults with the same gender and race (T-score) or with the mean value of the BMD of his/her peers of the same race (Z-score, which is measured Dual-Energy X-ray absorptiometry (DXA, previously DEXA) or bone densitometry [25,26,27,28].
The inclusion criteria were postmenopausal women who, according to the World Health Organization (WHO), had at least 12 months of menstruation and osteoporosis with a T-score of ≤ −2.5 in bone densitometry.
According to the WHO definition, osteoporosis in postmenopausal women based on densitometry is a BMD reduction of 2.5 or greater than the standard deviation under the mean value for young adults (T-score ≤ −2.5). In this definition, −2.5 ≤ T-score < −1 is considered osteopenia and T-score ≥ −1 normal [29].
Exclusion criteria were pregnancy, breastfeeding, smoking, drinking alcohol, other hormonal disorders, use hormones or drugs with a negative or positive effect on bone metabolism (such as estrogens and bisphosphates), in the past year, lack of volunteering to participate in the study, allergy to drugs, certain complications such as hallucinations, confusion and numbness, nausea, vomiting, restlessness, seizure, disorders of intestinal absorption, and thyroid, kidney, liver disorders.
Sample size determination
The sample size was calculated at 100 using a sample size formula. Taking into account the potential attrition, 50 individuals were assigned to each group.
| z1−β= 0.84 [d = 0.04] n = 49≈50 |
Ethical consideration
This study was performed under the guidelines shown in the Declaration of Helsinki (2013). It was approved by the Medical Ethics Committee of the Ethics Committee of Shahrekord University of Medical Sciences (No. IR.SKUMS.REC.1396.174). The protocol was approved by the Iranian Registry of Clinical Trials (No. IRCT20170929036477N3).
Study design, intervention, randomization, and blindness
The current study was a randomized double-blind placebo-controlled clinical trial, conducted to evaluate the effects of alkaline drinking water on bone densitometry. Samples were selected by the convenience sampling method. Stratified randomization was performed using the Random Allocation Software (RAS) according to T-scores of the spine bone, age, and to enter the intervention and control group. Study objectives and confidentiality of participants' information were explained to the participants, and then informed consent to participate in the study was obtained from them.
The intervention group received combined calcium and vitamin D (Ca-D3) supplement with a loading dose of 400 International Unit (IU) of cholecalciferol and 500 mg calcium per day once daily, also they consumed 1.5 L/day alkaline drinking water (pH = 8.6 ± 0.3) in a dark bottle [19] and Osteofos tablet (70 mg) once a week, and the control group received Ca-D3 tablet daily and Osteofos tablet (70 mg/kg) once a week also drink 1.5 L/day drinking water that its dark bottle similar to intervention group coded by a person who was not involved in any procedures of the study design. The selected patients used the same drinking water.
All patients and researcher staff in the bone densitometry center were blind to randomization.
For the preparation of alkaline water, samples of acetic alkaline (Neutron Alkaline Stick; Neutron Health Group, Tehran, Iran) were placed in 0.5 mL of water for 1–2 hours. Acetic alkaline makes the water alkaline 10 fold by ionizing calcium and magnesium that will send to water.
The pH of the drinking water of the area is about 6.8–7.2, which increased to 7.8–8.2 by using acetic alkaline. The acidity of each participant was measured before and after the use of steaks. The intake of water by participants was assured by continuous follow-up. Demographic data and bone densitometry results before and after the intervention were recorded in a checklist. After three months, patients were evaluated for osteoporosis improvement.
Bone densitometry assessment
The dual x-ray absorption was used to perform bone densitometry reported as standardized values called T-scores and Z-scores [30,31]. In this procedure, two x-ray sources are sent to the bone to measure its density of the spine, hip, or forearm. Two sources of X-rays are used to assure accurate measurement. In this study, the femur and spine bones were examined. The WHO classification of BMD according t-score: normal (−1, 0 or greater in T-score), osteopenia (−1, 0 or greater in T-score), osteoporosis (−2.5 less in T-score), and severe osteoporosis (−2.5 less in T-score with fracture) [31].
Statistical analysis
Data were analyzed using the Stata software version 13. Qualitative variables were reported as frequency (%) and quantitative variables as mean ± standard deviation and analyzed by paired and independent t test. P < 0.05 was considered a significance level.
RESULTS
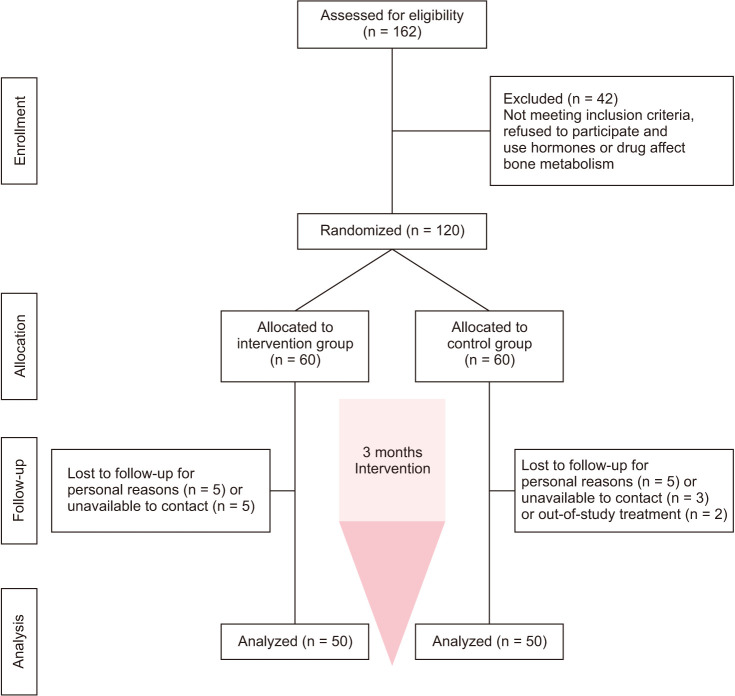
osteoporosis diagnosis were divided into two groups; finally, 100 patients completed the trial (50 participants in the intervention group and 50 participants in the control group) (Fig. 1) to investigate the effect of alkaline drinking water on BMD in postmenopausal women with osteoporosis. In the intervention group, calcium, alkaline drinking water (1.5 L, daily), and Osteofos tablet, and in the control group, calcium D and Osteofos tablet were administered. The mean age of participants was 60.36 ± 7.64 years in the intervention group and 59.12 ± 7.79 years in the control group, without any statistically significant difference (P = 0.97). Other basal characteristics include weight, body mass index (BMI), at the beginning showed no significant differences (P = 0.74, Table 1). The compliance rate of drinking water was more than 90% in the two groups.
Fig. 1. Algorithm of study participants in the two groups.
Table 1. Comparison of basal characteristic in patients of two groups.
| Variable | Control group (n = 50) | Intervention group (n = 50) | P value | |
|---|---|---|---|---|
| Age (y) | 60.36 ± 7.64 | 59.12 ± 7.79 | 0.97a | |
| Weight (kg) | 71.9 ± 12.5 | 73.6 ± 13.3 | 0.33a | |
| Body mass index (kg/m2) | 24.6 ± 4.62 | 24.8 ± 5.9 | 0.74a | |
| Medical history | Diabetes | 4 (8.0) | 3 (6.0) | 0.72b |
| Hypertension | 6 (12.0) | 5 (10.0) | 0.88b | |
| Osteoarthritis | 3 (6.0) | 4 (8.0) | 0.46b | |
| Hyperlipidemia | 8 (16.0) | 7 (14.0) | 0.34b | |
| Rheumatoid arthritis | 4 (8.0) | 3 (6.0) | 0.37b | |
| Thyroid disease | 8 (16.0) | 9 (18.0) | 0.46b | |
Data are presented as mean ± standard deviation or number (%).
aBy independent t test. bBy chi-square test.
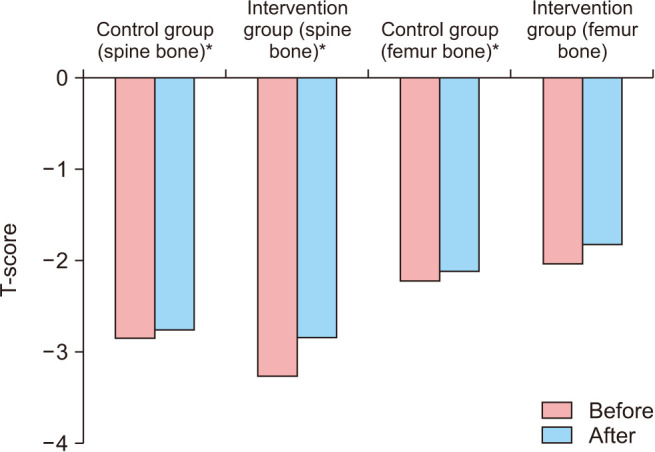
Densitometric results including T-scores of the femur and spine bone in the studied groups before and after the intervention are shown in Table 2 and Fig. 2. The mean T-scores of femur and spine bone improved significantly in both groups after the intervention compared to before the intervention (P < 0.05).
Table 2. Comparison of T-scores of the femur and spine bone before and after intervention.
| Variable | Control group | Intervention group | Total | P valuea | |
|---|---|---|---|---|---|
| T-scores of the spine bone | Before | −2.66 ± 0.69 | −3.05 ± 0.65 | −2.85 ± 0.69 | 0.001* |
| After | −2.57 ± 0.66 | −2.65 ± 0.84 | −2.61 ± 0.75 | ||
| T-scores of the femur bone | Before | −2.08 ± 0.99 | −1.91 ± 0.77 | −1.99 ± 0.88 | 0.001* |
| After | −1.96 ± 1.00 | −1.71 ± 0.80 | −1.84 ± 0.91 | ||
Data are presented as mean ± standard deviation.
*P < 0.01.
aBy independent t test.
Fig. 2. The histogram of T-score of the femur and spine bone between the two groups. *P < 0.01.
The improvement of the mean T-score of the spine bone and femur was 0.24 ± 0.04 and 0.15 ± 0.03, respectively. These values in the two groups are shown in Table 2. Change in the mean T-score of the spine bone after the intervention was 0.39 ± 0.07 in the intervention group and 0.08 ± 0.01 in the control group, with a statistically significant difference (P < 0.05). The improvement of spine bone T-score was higher in the intervention group (0.19 ± 0.05) than in the control group (0.12 ± 0.03), but the difference was not statistically significant (Table 3).
Table 3. Comparison of changes in T-scores of the femur and spine bone between the two groups.
| Variable | Control group | Intervention group | P valuea |
|---|---|---|---|
| T-scores of the spine bone | 0.08 ± 0.01 | 0.39 ± 0.07 | 0.001* |
| T-scores of the femur bone | 0.12 ± 0.03 | 0.19 ± 0.05 | 0.22 |
Data are presented as mean ± standard deviation.
*P < 0.01.
aBy independent t test.
DISCUSSION
It is important to identify modifiable risk factors because osteoporosis can be prevented and treated before the occurrence of fracture [32]. This study investigated the effect of alkaline drinking water on bone density in postmenopausal women with osteoporosis.
After 3 months of intervention, in both groups mean T-scores spine bone was improved compared to before the intervention, whereas changes in the femur T-score were not significantly different between the intervention and control groups.
Generally, the bone is a dynamic tissue with continuous regeneration, which is formed and regenerated as a result of the activity of two opposite types of cells, consisting of osteoblasts that form and modulate the mineral matrix, and osteoclasts that break down the mineral matrix and thin bones. In osteoporosis, bone-breaking or resorption is predominant [33]. According to the acid ash hypothesis, when the diet is unable to maintain acid-base balance, the skeletal structure begins to adjust the concentration of hydrogen ions in the blood and maintain the pH between 7.35 and 7.45 [34]. In vitro studies have also shown that metabolic acidosis results in calcium excretion from the bone, and in a long-term acid diet, pH is adjusted at the cost of the bone [23].
Menopause usually begins at the age of 45–55 [19] and is accompanied by estrogen reduction and, by increasing osteoclastic activity, bone resorption is stimulated [9,23]. At this time, care for and prevention of osteoporosis are more necessary than ever before, and this is why this group was selected in this study.
In study showed the serum calcium level was significantly lower in the post-menopausal group than in the pre-menopausal group, while the serum alkaline phosphatase level was partly higher. Therefore, an increase in bone turnover accelerates bone mass reduction in post-menopausal women [9].
In this study, the density of the spine bone was significantly improved compared to the control group, but there was no significant difference in the femur density improvement between the control and intervention groups, which could be due to higher blood supply to the spine bone and, consequently, to the greater effect of alkaline water on the bone. Given the above-mentioned, it seems that alkaline water prevents the loss of bone mineral content and reduces its density by reducing urinary calcium excretion. The results of our study confirm that alkaline water intake or the consumption of alkali compounds will be associated with maintaining bone density, especially the spine bone, in postmenopausal women. No change in bone density of femur bone seems that the efficacy will be more pronounced after longer periods of follow-up, and alkaline dietary recommendations (reduction of animal protein) maybe have shown significant results. The last study indicated diets with high acidity are related to lower BMD [35]. Supporters of the Alkaline Potassium Diet Hypothesis recommend the paleolithic diet to be ideal to maintain bone integrity over a lifetime [36].
Chen et al. [32] reported low serum bicarbonate levels are related to low lumbar and total BMD in adults of 20 years and over in the general population in the USA. They observed this association as being more pronounced in women, particularly post-menopausal women, than men especially regarding BMD. Jehle et al. [37] randomly divided 161 postmenopausal women aged 58.6 ± 4.8 years with low bone mass (T-scores −1 to −4). One group was treated with potassium citrate and the other with potassium chloride daily. They observed that in women who received potassium citrate chronically, lumbar spine and hip BMD increased; and the rate of urinary calcium excretion and urinary bone resorption marker significantly decreased in the group receiving K citrate [37].
The cited studies are consistent with the present study. However, in previous studies, alkali supplements were used in foods, while the effect of alkaline drinking water was studied in the present study.
However, in one study of supplementation with potassium citrate, no effect on BMD was observed in healthy postmenopausal women [36], but in studies of healthy elderly men and women, consumption of potassium citrate increased lumbar BMD, and consumption of bicarbonate caused desirable effects on bone resorption and calcium excretion [20].
The review article of Burckhardt [19], and the study of Wynn et al. [22] on young women showed that alkaline bicarbonate-rich water reduced bone resorption, parathyroid hormone, and C-telopeptides of type I collagen (CTX) as a marker of bone resorption. However, in these two studies, the effect of alkaline water on the T-score in postmenopausal women was not studied.
In the study of Roux et al. [38], a significant decrease in urinary CTX was observed in people who consumed alkaline water, indicating decreased osteoclast activity and bone fractures. The researchers suggested that consumption of alkaline water improves bone health and prevents osteoporosis.
During the present study, at the end of each month, the LFT (Liver Function Test), creatinine, and blood urea nitrogen were performed to discontinue the use of alkaline water in case of any problem. No problem, however, was observed. In a study on mice, following alkaline water treatment, no pathology was detected in the kidneys, intestine, heart, liver, and brain, and better longevity and survival were observed as compared to the control mice [33].
The limitations of this study were the possibility of using other acidic or alkaline nutrients and diets by participants in the study and not measuring bone markers and acid/base status in patients; therefore, relevant recommendations were provided to them. This, however, was likely to occur in both intervention and control groups. To prevent the unprescribed use of other drugs, patients were given adequate information before the study.
In conclusion, the present study showed that alkaline water in postmenopausal women with osteoporosis significantly improved the T-score of the spine bone as compared to the control group. The rate of femur T-score improvement was not significant, which is probably due to the higher blood supply to the spine bone than that to the femur. Therefore, a longer intervention is necessary to investigate this effect on the femur. According to the results, the consumption of water at alkaline pH, as with alkaline diets, has a positive effect on increasing bone density and can be used to neutralize the adverse effects of acidic diets and prevent osteoporosis in postmenopausal women.
ACKNOWLEDGMENTS
This article was obtained from a research project approved at Shahrekord University of Medical Sciences (code 2551). Hereby, the financial support of the University and the Center for the Development of Clinical Research at Ayatollah Kashani Hospital of Shahrekord is appreciated.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, et al. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001;19:331–337. doi: 10.1007/s007740170001. [DOI] [PubMed] [Google Scholar]
- 2.Akkawi I, Zmerly H. Osteoporosis: current concepts. Joints. 2018;6:122–127. doi: 10.1055/s-0038-1660790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chon SJ, Koh YK, Heo JY, Lee J, Kim MK, Yun BH, et al. Effects of vitamin D deficiency and daily calcium intake on bone mineral density and osteoporosis in Korean postmenopausal woman. Obstet Gynecol Sci. 2017;60:53–62. doi: 10.5468/ogs.2017.60.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura Y, Suzuki T, Kato H. Serum bone alkaline phosphatase is a useful marker to evaluate lumbar bone mineral density in Japanese postmenopausal osteoporotic women during denosumab treatment. Ther Clin Risk Manag. 2017;13:1343–1348. doi: 10.2147/TCRM.S142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guañabens N, Parés A. Osteoporosis in chronic liver disease. Liver Int. 2018;38:776–785. doi: 10.1111/liv.13730. [DOI] [PubMed] [Google Scholar]
- 6.Ikebuchi Y, Aoki S, Honma M, Hayashi M, Sugamori Y, Khan M, et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561:195–200. doi: 10.1038/s41586-018-0482-7. [DOI] [PubMed] [Google Scholar]
- 7.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gossiel F, Altaher H, Reid DM, Roux C, Felsenberg D, Glüer CC, et al. Bone turnover markers after the menopause: T-score approach. Bone. 2018;111:44–48. doi: 10.1016/j.bone.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Bhattarai T, Bhattacharya K, Chaudhuri P, Sengupta P. Correlation of common biochemical markers for bone turnover, serum calcium, and alkaline phosphatase in post-menopausal women. Malays J Med Sci. 2014;21:58–61. [PMC free article] [PubMed] [Google Scholar]
- 10.Altayar O, Al Nofal A, Carranza Leon BG, Prokop LJ, Wang Z, Murad MH. Treatments to prevent bone loss in functional hypothalamic amenorrhea: a systematic review and meta-analysis. J Endocr Soc. 2017;1:500–511. doi: 10.1210/js.2017-00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.New SA. Nutrition Society Medal lecture. The role of the skeleton in acid-base homeostasis. Proc Nutr Soc. 2002;61:151–164. doi: 10.1079/PNS2002159. [DOI] [PubMed] [Google Scholar]
- 12.Arnett TR. Extracellular pH regulates bone cell function. J Nutr. 2008;138:415S–418S. doi: 10.1093/jn/138.2.415S. [DOI] [PubMed] [Google Scholar]
- 13.Fenton TR, Eliasziw M, Lyon AW, Tough SC, Hanley DA. Meta-analysis of the quantity of calcium excretion associated with the net acid excretion of the modern diet under the acid-ash diet hypothesis. Am J Clin Nutr. 2008;88:1159–1166. doi: 10.1093/ajcn/88.4.1159. [DOI] [PubMed] [Google Scholar]
- 14.Buclin T, Cosma M, Appenzeller M, Jacquet AF, Décosterd LA, Biollaz J, et al. Diet acids and alkalis influence calcium retention in bone. Osteoporos Int. 2001;12:493–499. doi: 10.1007/s001980170095. [DOI] [PubMed] [Google Scholar]
- 15.Lu KC, Lin SH, Yu FC, Chyr SH, Shieh SD. Influence of metabolic acidosis on serum 1,25(OH)2D3 levels in chronic renal failure. Miner Electrolyte Metab. 1995;21:398–402. [PubMed] [Google Scholar]
- 16.Gasser JA, Hulter HN, Imboden P, Krapf R. Effect of chronic metabolic acidosis on bone density and bone architecture in vivo in rats. Am J Physiol Renal Physiol. 2014;306:F517–F524. doi: 10.1152/ajprenal.00494.2013. [DOI] [PubMed] [Google Scholar]
- 17.Hanley DA, Whiting SJ. Does a high dietary acid content cause bone loss, and can bone loss be prevented with an alkaline diet? J Clin Densitom. 2013;16:420–425. doi: 10.1016/j.jocd.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Galow AM, Rebl A, Koczan D, Bonk SM, Baumann W, Gimsa J. Increased osteoblast viability at alkaline pH in vitro provides a new perspective on bone regeneration. Biochem Biophys Rep. 2017;10:17–25. doi: 10.1016/j.bbrep.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burckhardt P. The effect of the alkali load of mineral water on bone metabolism: interventional studies. J Nutr. 2008;138:435S–437S. doi: 10.1093/jn/138.2.435S. [DOI] [PubMed] [Google Scholar]
- 20.Dawson-Hughes B, Harris SS, Palermo NJ, Castaneda-Sceppa C, Rasmussen HM, Dallal GE. Treatment with potassium bicarbonate lowers calcium excretion and bone resorption in older men and women. J Clin Endocrinol Metab. 2009;94:96–102. doi: 10.1210/jc.2008-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebastian A, Harris ST, Ottaway JH, Todd KM, Morris RC., Jr Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med. 1994;330:1776–1781. doi: 10.1056/NEJM199406233302502. [DOI] [PubMed] [Google Scholar]
- 22.Wynn E, Krieg MA, Aeschlimann JM, Burckhardt P. Alkaline mineral water lowers bone resorption even in calcium sufficiency: alkaline mineral water and bone metabolism. Bone. 2009;44:120–124. doi: 10.1016/j.bone.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Bushinsky DA. Metabolic alkalosis decreases bone calcium efflux by suppressing osteoclasts and stimulating osteoblasts. Am J Physiol. 1996;271(1 Pt 2):F216–F222. doi: 10.1152/ajprenal.1996.271.1.F216. [DOI] [PubMed] [Google Scholar]
- 24.Lambert H, Frassetto L, Moore JB, Torgerson D, Gannon R, Burckhardt P, et al. The effect of supplementation with alkaline potassium salts on bone metabolism: a meta-analysis. Osteoporos Int. 2015;26:1311–1318. doi: 10.1007/s00198-014-3006-9. [DOI] [PubMed] [Google Scholar]
- 25.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182:1864–1873. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimai HP. Use of dual-energy X-ray absorptiometry (DXA) for diagnosis and fracture risk assessment; WHO-criteria, T- and Z-score, and reference databases. Bone. 2017;104:39–43. doi: 10.1016/j.bone.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Brunader R, Shelton DK. Radiologic bone assessment in the evaluation of osteoporosis. Am Fam Physician. 2002;65:1357–1364. [PubMed] [Google Scholar]
- 29.World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group [meeting held in Rome from 22 to 25 June 1992] Geneva: World Health Organization; 1994. [PubMed] [Google Scholar]
- 30.Salamat MR, Salamat AH, Abedi I, Janghorbani M. Relationship between weight, body mass index, and bone mineral density in men referred for dual-energy X-ray absorptiometry scan in Isfahan, Iran. J Osteoporos. 2013;2013:205963. doi: 10.1155/2013/205963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JE, Kim KM, Kim LK, Kim KY, Oh TJ, Moon JH, et al. Comparisons of TBS and lumbar spine BMD in the associations with vertebral fractures according to the T-scores: a cross-sectional observation. Bone. 2017;105:269–275. doi: 10.1016/j.bone.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Melamed ML, Abramowitz MK. Serum bicarbonate and bone mineral density in US adults. Am J Kidney Dis. 2015;65:240–248. doi: 10.1053/j.ajkd.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magro M, Corain L, Ferro S, Baratella D, Bonaiuto E, Terzo M, et al. Alkaline water and longevity: a murine study. Evid Based Complement Alternat Med. 2016;2016:3084126. doi: 10.1155/2016/3084126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frassetto L, Banerjee T, Powe N, Sebastian A. Acid balance, dietary acid load, and bone effects—a controversial subject. Nutrients. 2018;10:517. doi: 10.3390/nu10040517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mangano KM, Walsh SJ, Kenny AM, Insogna KL, Kerstetter JE. Dietary acid load is associated with lower bone mineral density in men with low intake of dietary calcium. J Bone Miner Res. 2014;29:500–506. doi: 10.1002/jbmr.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frassetto LA, Hardcastle AC, Sebastian A, Aucott L, Fraser WD, Reid DM, et al. No evidence that the skeletal non-response to potassium alkali supplements in healthy postmenopausal women depends on blood pressure or sodium chloride intake. Eur J Clin Nutr. 2012;66:1315–1322. doi: 10.1038/ejcn.2012.151. [DOI] [PubMed] [Google Scholar]
- 37.Jehle S, Zanetti A, Muser J, Hulter HN, Krapf R. Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J Am Soc Nephrol. 2006;17:3213–3222. doi: 10.1681/ASN.2006030233. [DOI] [PubMed] [Google Scholar]
- 38.Roux S, Baudoin C, Boute D, Brazier M, de la Guéronniere V, de Vernejoul MC. Biological effects of drinking-water mineral composition on calcium balance and bone remodeling markers. J Nutr Health Aging. 2004;8:380–384. [PubMed] [Google Scholar]