Abstract
Streptococcus pneumoniae remains an important bacterial pathogen, particularly for young children in low- and middle-income countries. A systematic review was conducted of peer-reviewed literature from PubMed published as of May 13, 2020, to identify articles relevant to invasive pneumococcal disease, pneumonia, otitis media (OM), nasopharyngeal carriage (NPC), antimicrobial resistance (AMR), and vaccination coverage in Egypt, with particular focus on children ≤ 18 years of age. A total of 16 relevant articles spanning three decades were included in this review. Among studies reviewed, S. pneumoniae was the causative agent of meningitis in 21–30% of cases among hospitalized children between 1983 and 2003. One study showed that serotypes 6A and 6B predominated among meningitis cases of pediatric patients aged < 5 years. This review also revealed that S. pneumoniae was the most commonly identified bacterial pathogen of acute mastoiditis, a severe complication of acute OM, among children aged 9 months to 11 years. NPC studies showed that approximately 30% of Egyptian children were carriers of S. pneumoniae. AMR, especially to penicillin, continues to be a growing concern in low- and middle-income countries, including among Egyptian children. Several predominant serotypes were identified to be associated with penicillin resistance, such as 6B, 1, 19A, 23F, and 6A. Currently available pneumococcal vaccines (PCVs) such as PCV10 and PCV13 may provide coverage against the most prevalent circulating serotypes among Egyptian children. Comprehensive disease surveillance and immunization programs are needed to ensure that this vulnerable population is sufficiently protected against pneumococcal disease.
Keywords: Antimicrobial resistance, Carriage, Children, Disease, Egypt, Meningitis, Otitis media, Pneumococcal vaccine, Streptococcus pneumoniae, Systematic review
Key Summary Points
| Streptococcus pneumoniae remains an important bacterial pathogen, particularly for young children in low- and middle-income countries. |
| This systematic literature review summarizes published data regarding invasive pneumococcal disease, pneumonia, otitis media, pneumococcal carriage, antimicrobial resistance, and pneumococcal vaccination in Egyptian children aged ≤ 18 years. |
| Approximately 30% of Egyptian children are carriers of S. pneumoniae; the pathogen is a predominant cause of bacterial meningitis and acute otitis media in children. |
| There is a need for comprehensive pneumococcal disease surveillance and immunization programs to ensure that Egyptian children are sufficiently protected against pneumococcal diseases. |
Introduction
Streptococcus pneumoniae is an opportunistic human pathogen that poses significant worldwide public health concern [1, 2]. The bacterium is a leading cause of serious, invasive pneumococcal diseases (IPDs) including pneumonia, meningitis, and bacteremia, as well as less serious ailments such as sinusitis and otitis media (OM) [1, 2]. Pneumococcal disease is preceded by bacterial colonization of the nasopharynx, a primary step in disease pathogenesis [1, 3]. Once colonized, the bacteria can then spread contiguously from the nasopharynx to cause OM, a commonly diagnosed childhood disease, and/or enter the bloodstream, typically leading to IPD [1, 4]. Children and infants represent the main reservoirs for the pathogen, with nasopharyngeal (NP) carriage (NPC) rates estimated to range between 27 and 85% in this age group [1]. As such, comprehensive data on NPC and pneumococcal disease are prerequisites for understanding the true burden of disease, which can in turn inform public health decisions surrounding strategies to combat the potentially devastating bacterial pathogen. S. pneumoniae is encapsulated, and the polysaccharide capsule is crucial for bacterial virulence [1]. Based on differences in polysaccharide capsule composition, > 90 distinct serotypes of S. pneumoniae have been identified to date [1]. However, only a few serotypes cause disease, and their distribution can vary over time, by age group, and by geographic region [1]. Morbidity and mortality rates resulting from pneumococcal diseases remain high, particularly among young children aged 1 month to < 5 years, with the pathogen responsible for an estimated 11% of all deaths worldwide among this population in 2015 [5]. Notably, the risk and burden of disease are heightened in certain regions of the world, such as countries in Africa and Asia [1]. In Africa specifically, the incidence of pneumococcal pneumonia in 2015 was 1504 per 100,000 children aged < 5 years, resulting in approximately 137,000 deaths [5]. In Egypt, S. pneumoniae is the leading cause of bacterial meningitis, and pneumonia accounts for 19% of all deaths in children aged < 5 years [6, 7].
Pneumococcal disease is vaccine preventable, with available vaccines including the 10- and 13-valent pneumococcal conjugate vaccines (PCVs; PCV10 and PCV13, respectively) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23) [1, 8]. As a result of global PCV immunization programs, large decreases in IPD-associated deaths in children aged < 5 years have been observed: between 2000 and 2015, global IPD-related deaths decreased from 735,000 to 294,000 in this age group [5]. However, pneumococcal disease burden remains high worldwide [5], especially in low- and middle-income countries [1], and the emergence of antibiotic-resistant strains is a growing global concern, making the treatment of pneumococcal disease more challenging [8]. Thus, the implementation of PCVs into national immunization programs represents an important step toward reducing pneumococcal disease burden and associated deaths. To that end, continuous surveillance for epidemiologic shifts in serotype distribution and antibiotic resistance is crucial to inform ongoing and future regional vaccination policies [1, 9].
In Egypt, pneumococcal vaccines are available but, as of November 2020, are not included in the national immunization program [10, 11]. The purpose of this systematic literature review is to summarize available data regarding IPD, pneumonia, OM, pneumococcal carriage, antimicrobial resistance (AMR), and pneumococcal vaccination in Egypt, with particular focus on children aged ≤ 18 years.
Methods
Protocol and Registration
The protocol for this systematic review was registered at PROSPERO (#CRD42020168244; https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=168244). For this review, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Eligibility Criteria
This review focused on studies that contained epidemiologic data in healthy Egyptian children aged ≤ 18 years on either pneumococcal (1) invasive disease infections, (2) pneumonia burden, (3) OM burden, (4) NPC, (5) AMR, or (6) vaccination coverage. For the purposes of this review, healthy children were defined as those without underlying or compromising medical conditions. The following definitions of the above topics were applied: IPD was considered isolation of S. pneumoniae from sterile sites [i.e., cerebrospinal fluid (CSF) or blood]; pneumococcal pneumonia was defined as pneumonia caused by S. pneumoniae, with pneumonia categorized as a clinical pulmonary infection that had a minimum criteria of the presence of a new pulmonary infiltrate on chest radiograph; pneumococcal OM was defined as isolation of S. pneumoniae from middle ear fluid; pneumococcal NPC was defined as colonization of S. pneumoniae in the upper respiratory tract; and pneumococcal AMR was defined as resistance of pneumococcal carriage or infections as determined by either laboratory- or clinic-based methods. For pneumococcal vaccine coverage, we considered the PPSV23 and PCVs, including the 7-valent PCV (PCV7), PCV10, and PCV13.
Observational studies [i.e., cross-sectional, cohort, case–control, or surveillance (prospective and retrospective)] and randomized controlled trials (RCTs) that contained data specific to Egypt; healthy children (aged ≤ 18 years), or S. pneumoniae were included, while narrative reviews and opinion articles were excluded. In relation to specific topics, we also excluded articles without data specific to pneumococcal NPC or OM for searches on carriage or OM, respectively; for invasive pneumococcal infections, data not obtained from a sterile site were also excluded.
Information Sources
We identified published literature using PubMed to search MEDLINE on May 13, 2020; there were no restrictions on language or year of publication.
Search
Six separate searches related to individual topics encompassing pneumococcal epidemiology in healthy Egyptian children were conducted, including invasive infections, pneumonia, OM, NPC, AMR, and vaccination coverage. The search strings used for each topic are outlined in Table 1.
Table 1.
Search strings
| Searcha | Search string |
|---|---|
| Invasive pneumococcal infections | ("pneumococcal" OR "Streptococcus pneumoniae" OR "S pneumoniae") AND (“Invasive” OR “Invasive pneumococcal disease” OR “IPD” OR “Invasive pneumococcal infection” OR “Septicemia” OR “Septicaemia” OR “Meningitis” OR “Bacteremia” OR “Bacteraemia”) AND ("epidemiology" OR "burden" OR "incidence" OR "hospitalization" OR “hospitalisation” OR “morbidity” OR “mortality” OR “deaths” OR “fatality” OR “case fatality” OR “case fatality rate” OR “CFR” OR “prevalence” OR “cases” OR “notification” OR “notification rate”) AND (“Egypt” OR “Egyptian”) AND ("children" OR "pediatrics" OR "paediatrics" OR "infants" OR "toddlers" OR “adolescents” OR “teenagers”) |
| Pneumococcal pneumonia burden | ("pneumococcal" OR "Streptococcus pneumoniae" OR "S pneumoniae") AND (“pneumonia” OR “community acquired pneumonia” OR “CAP” OR "epidemiology" OR "burden" OR "incidence" OR "hospitalization" OR “hospitalisation” OR “morbidity” OR “mortality” OR “deaths” OR “fatality” OR “case fatality” OR “case fatality rate” OR “CFR” OR “prevalence” OR “cases” OR “notification” OR “notification rate”) AND (“Egypt” OR “Egyptian”) AND ("children" OR "pediatrics" OR "paediatrics" OR "infants" OR "toddlers" OR “adolescents” OR “teenagers”) |
| Pneumococcal otitis media burden | ("pneumococcal" OR "Streptococcus pneumoniae" OR "S pneumoniae") AND ("AOM" OR "OM" OR "otitis media" OR "acute otitis media") AND (“Egypt” OR “Egyptian”) AND ("children" OR "pediatrics" OR "paediatrics" OR "infants" OR "toddlers" OR “adolescents” OR “teenagers”) |
| Pneumococcal nasopharyngeal carriage | ("pneumococcal" OR "Streptococcus pneumoniae" OR "S pneumoniae") AND ("Carriage" OR "Colonization" OR “Colonisation” OR "NPC" OR “Nasopharyngeal carriage” OR "Acquisition") AND (“Egypt” OR “Egyptian”) AND ("children" OR "pediatrics" OR "paediatrics" OR "infants" OR "toddlers" OR “adolescents” OR “teenagers”) |
| Pneumococcal antimicrobial resistance | ("pneumococcal" OR "Streptococcus pneumoniae" OR "S pneumoniae" OR “otitis media” OR “acute otitis media” OR “OM” OR “AOM” OR “invasive pneumococcal disease” OR “pneumococcal disease” OR “pneumonia” OR “pneumococcal pneumonia”) AND (“Antibiotic resistance” OR “Antimicrobial resistance” OR “Resistance” OR “Antibiotic” OR “Antimicrobial” OR “AMR”) AND (“Egypt” OR “Egyptian”) AND ("children" OR "pediatrics" OR "paediatrics" OR "infants" OR "toddlers" OR “adolescents” OR “teenagers”) |
| Pneumococcal vaccination coverage | ("pneumococcal" OR "Streptococcus pneumoniae" OR "S pneumoniae" OR “otitis media” OR “acute otitis media” OR “OM” OR “AOM” OR “invasive pneumococcal disease” OR “pneumococcal disease” OR “pneumonia” OR “pneumococcal pneumonia”) AND (“Vaccination” OR “Vaccine” OR “PCV” OR “PCV7” OR “PCV9” OR “PCV10” OR “PCV13” OR “PPSV23” OR “Pneumococcal Conjugate Vaccine” OR “PPV23” OR “Prevenar” OR “Prevnar” OR “Coverage” OR “Uptake” OR “Vaccination rate”) AND (“Egypt” OR “Egyptian”) AND ("children" OR "pediatrics" OR "paediatrics" OR "infants" OR "toddlers" OR “adolescents” OR “teenagers” |
aPubMed search conducted on May 13, 2020
Study Selection
Titles and abstracts of articles retrieved from PubMed were screened for eligibility by two researchers. The first reviewer screened records for inclusion, and the second reviewer checked these decisions; reviewers were not blinded to the other’s decisions. Cases of disagreement were resolved by discussion between the reviewers or with the assistance of a third reviewer. If an abstract or title did not contain sufficient information to determine eligibility, the full text of an article was reviewed.
Data Extraction
Data were separately extracted for each of the six searches into an Excel spreadsheet. Studies that met eligibility criteria had data extracted by one reviewer while a second reviewer checked these decisions. Missing data were not included and investigators were not contacted to obtain missing data. All included articles had basic information collected on article type, study location, study population (age and size), and study observation period. Included articles on invasive pneumococcal infections had data extracted for sampling site and methodologies for characterization and serotyping. For pneumococcal pneumonia or OM burden, information on diagnostic criteria and serotyping methodology was also extracted. For pneumococcal NPC articles, we also extracted data on the sampling site and methodologies for sampling, characterization, and serotyping. For antimicrobial resistance, information on the assessment methodology, resistance definitions, and investigated antimicrobial agent were obtained. Observational data in the included studies were assessed for risk of bias or quality based on the criteria developed by Hayden and colleagues [12], which included consideration of potential bias in study sample characteristics, assessments of study outcomes, and statistical analyses; additional considerations for risk of bias included determining that potential confounds were identified and that eligibility criteria or measurements were appropriately applied in the included studies.
Data Synthesis
Extracted data were tabulated with available evidence described narratively. No quantitative analyses were performed due to the variation in the available data.
Results
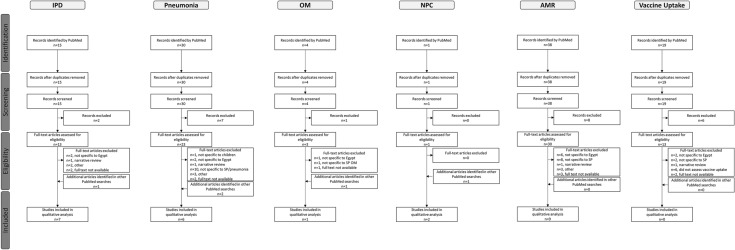
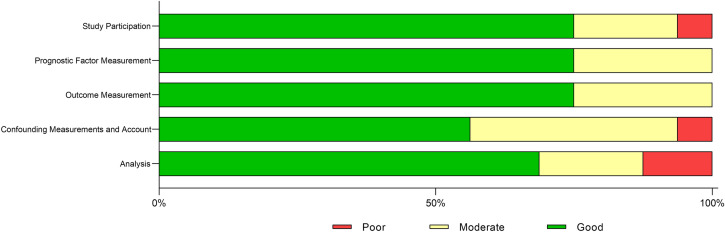
The number of articles that were identified, screened, eligible, and included in this review are summarized in Fig. 1 for each of the six searches. A total of 107 records were identified using PubMed and screened, and 25 records were included in the qualitative analyses. The search on vaccination uptake identified 19 records, none of which were eligible for inclusion. A summary of the risk of bias assessment is shown in Fig. 2. Across the five areas of potential bias, 56.3–75.0% of studies were classified as “good” and 18.8–37.5% as “moderate.” No studies were classified as “poor” for prognostic factor measurement and outcome management, whereas 6.3–12.5% were classified as “poor” across the remaining factors. Across the six searches, a total of 16 studies were included. The majority of studies (9/16, 56.3%) were conducted in Cairo; two studies (12.5%) were conducted in Alexandria and two (12.5%) were conducted at multiple sites across Egypt. Study sample size ranged from 19 (OM) to 22,018 (IPD, pneumonia, and AMR). Laboratory methods varied by pneumococcal epidemiology topic.
Fig. 1.
Flow chart of articles identified and included in the review. Articles were identified by PubMed through six different search strategies on IPD infections and pneumococcal pneumonia burden, OM burden, NPC, AMR, or vaccination coverage. Articles were screened based on title/abstract, and articles meeting selection criteria were included in the review. AMR antimicrobial resistance, IPD invasive pneumococcal disease, NPC nasopharyngeal carriage, OM otitis media, SP Streptococcus pneumoniae
Fig. 2.
Summary of risk of bias qualitative assessment of articles identified and included in the review. Risk of bias and quality were assessed based on the criteria developed by Hayden and colleagues [12]
IPD Infections
Seven articles on IPD in Egyptian children are analyzed in this review. Two RCTs conducted during the 1980s at the Abbassia Fever Hospital in Cairo evaluated meningitis treatment strategies (Table 2) [13, 14]. The first study compared the efficacy of ceftriaxone with ampicillin plus chloramphenicol in 70 children and 30 adults (age range 5 months to 28 years) with culture-confirmed meningitis, including 48 patients with Neisseria meningitidis, 35 with S. pneumoniae, and 17 with Haemophilus influenzae [13]. In total, 15 pediatric patients (21.4%) died, 7 (46.7%) of whom had pneumococcal meningitis; no significant differences in mortality rates between the two antibiotic treatment groups were determined [13]. The second, larger study compared the outcomes of patients with meningitis treated with antibiotics plus dexamethasone or with antibiotics alone [14]. In total, 429 patients with meningitis (age range 3 months to 60 years) were included in the final analysis, 282 of whom were aged ≤ 12 years. Among these pediatric patients, 67 (23.8%) were diagnosed with S. pneumoniae infection, with a case fatality rate (CFR) of 22.4% (15/67). Among the patients of all ages with pneumococcal meningitis (n = 106), the CFR was significantly lower in the antibiotics with dexamethasone group than in the antibiotics-alone group (13.5% vs. 40.7%, P < 0.002) [14].
Table 2.
Summary of studies on IPD in generally healthy Egyptian children
| Reference | Study type | Location | Study date | Population | Methodology | Outcomes relevant to IPD |
|---|---|---|---|---|---|---|
| Draz et al. [18] | Surveillance, prospective | Cairo University Specialized Pediatric Hospital | Jan 2008–Dec 2011 | Children aged < 5 years admitted with fever; a possible clinical diagnosis of meningitis, pneumonia, or septicemia; and for whom culture was performed (n = 22,018) |
Blood culture samples and sterile site specimens collected S. pneumoniae identified by Gram stain, colony morphology, optochin disk inhibition, and bile solubility test |
Epidemiology |
| Of 22,018 patients evaluated, 4 were diagnosed with IPD (meningitis, n = 1; septicemia, n = 1; pleural effusion, n = 2) | ||||||
| All patients diagnosed with IPD were aged < 1 year | ||||||
| Of the 4 patients with IPD, 3 had underlying congenital heart disease and/or immunodeficiency | ||||||
| Estimated annual detection rate of IPD: 18.2 per 100,000 | ||||||
| Morbidity and mortality | ||||||
| CFR of IPD: 75% | ||||||
| Farag et al. [17] | Surveillance, prospective | Alexandria Fever Hospital | “During the seasons of” 2002 and 2003 | Children aged 3 months–15 years admitted to the hospital with a confirmed diagnosis of meningitis/meningoencephalitis (n = 310) |
CSF sample analysis included cell count, glucose, Gram stain, culture, ELISA Collection and analysis of blood samples not described |
Epidemiology |
| ABM was confirmed in 202/310 patients (65.2%) with confirmed meningitis | ||||||
| H. influenzae: 65/310 | ||||||
| N. meningitidis: 44/310 | ||||||
| S. pneumoniae: 43/310 | ||||||
| Other bacteria: 50/310 | ||||||
| Aseptic meningitis was diagnosed in 108/310 patients (34.8%) | ||||||
| Hospitalization | ||||||
| Of 43 patients with meningitis due to S. pneumoniae | ||||||
| 28/43 (65.1%): WHO prognostic score < 9 | ||||||
| 15/43 (34.9%): WHO score ≥ 9 | ||||||
| For all ABM types, CSF glucose was significantly lower in the WHO score ≥ 9 group compared with the < 9 group (P < 0.001) | ||||||
| Compared with H. influenzae and aseptic meningitis, S. pneumoniae, N. meningitidis, and Other bacteria had significantly higher proportions of cases with WHO scores ≥ 9 (P < 0.05) | ||||||
| Morbidity and mortality | ||||||
| CFRs: 13.9% for ABM and 3.4% for aseptic meningitis | ||||||
| For both types, death occurred only in patients with a WHO score ≥ 9 | ||||||
| Logistic regression analysis showed that risk of poor prognosis (death and epilepsy) increased significantly with WHO score ≥ 9 (OR, 22.7; 95% CI 18.3, 69.2) and CSF glucose level < 10 mg/dL (OR, 13.3; 95% CI 9.01, 51.1) | ||||||
| Girgis et al. [13] | RCT | US Naval Medical Research Unit 3/Abbassia Fever Hospital, Cairo | Not stated | Children and adults presenting with signs and symptoms of meningitis who had organisms isolated from CSF (70 children and 30 adults aged 5 months–28 years) |
CSF sample analysis included leukocyte count, glucose and protein content, Gram stain, and culture Blood sample analysis included CBC, culture, glucose, blood urea nitrogen, aspartate and alanine aminotransferase, and creatinine content |
Epidemiology |
| Diagnosed causal agents of meningitis in the 100 patients | ||||||
| N. meningitidis: 48/100 | ||||||
| S. pneumoniae: 35/100 | ||||||
| H. influenzae: 17/100 | ||||||
| Morbidity and mortality | ||||||
| No significant difference in mortality between treatment groups | ||||||
| 15/70 children died | ||||||
| 7/15 with S. pneumoniae | ||||||
| Girgis et al. [14] | RCT | US Naval Medical Research Unit 3/Abbassia Fever Hospital, Cairo | Study commenced in 1983 | Children and adults presenting to the hospital with signs and symptoms of meningitis and bacteria identified in CSF samples (429 patients aged 3 months–60 years were analyzed, 282 of whom were aged ≤ 12 years) |
CSF sample analysis included leukocyte count, glucose and protein content, Gram stain, and culture Blood sample analysis included CBC, culture, glucose, blood urea nitrogen, aspartate and alanine aminotransferase, and creatinine content |
Epidemiology |
| Of 282 patients aged ≤ 12 years | ||||||
| N. meningitidis: 159/282 (56.4%) | ||||||
| S. pneumoniae: 67/282 (23.8%) | ||||||
| H. influenzae: 56/282 (19.9%) | ||||||
| 106 patients were diagnosed with pneumococcal meningitis (67 aged ≤ 12 years) | ||||||
| Morbidity and mortality | ||||||
| CFR among patients aged ≤ 12 years | ||||||
| S. pneumoniae: 15/67 (22.4%) | ||||||
| Among all patients with pneumococcal meningitis, CFR was significantly lower in the dexamethasone/antibiotic group (13.5% vs 40.7%, P < 0.002) | ||||||
| Among all pneumococcal meningitis survivors, the rate of hearing loss was significantly lower in the dexamethasone/antibiotic group (0% vs 12.5%, P < 0.05) | ||||||
| Sadek et al. [19] | Surveillance, prospective | Sohag University Hospital, Sohag | Dec 2012–Nov 2013 | Infants and children aged 1 month–12 years presenting with fever and convulsions (85 patients enrolled, 69 of whom were 1 month–2 years) |
CSF samples were collected by lumbar puncture and examined for physical, chemical, and cytologic aspects Abnormal WBC was defined as ≥ 5/HPF CSF culture was only performed for samples with WBC counts suggestive of acute bacterial infection (ie, 1000–10,000/HPF) CT brain scans were performed for patients in whom neurologic examination findings suggested intracranial pathology |
Epidemiology |
| Of 85 patients enrolled, 65 (76.5%) had normal CSF WBC counts and 20 (23.5%) had elevated CSF WBC counts | ||||||
| 3/20 patients had WBC counts suggestive of acute bacterial infection; S. pneumoniae were isolated from all 3 patients | ||||||
| CT brain scans were performed in 36 patients (25 with normal CSF WBC counts and 11 with elevated counts) | ||||||
| 3/25 patients (12%) with normal CSF WBC counts had abnormal CT findings | ||||||
| 7/11 patients (63.6%) with elevated CSF WBC counts had abnormal CT findings | ||||||
| Wasfy et al. [16] | Surveillance, prospective | 13 infectious disease hospitals across Egypt | 1998–2003 | Patients of all ages hospitalized with meningitis; specific age groups were defined, including those aged < 2, 2–5, and 6–17 years (number of patients included in the study was not stated) |
All patients with suspected meningitis had CSF samples collected S. pneumoniae were identified based on colony morphology, Gram stain reaction, optochin susceptibility, and bile solubility Isolates serotyped by latex agglutination and confirmatory Quellung reaction |
Epidemiology |
| 205 pneumococcal isolates were recovered from patients with acute bacterial meningitis | ||||||
| 25% from patients aged < 2 years | ||||||
| 7% from patients aged 2–5 years | ||||||
| 29% from patients aged 6–17 years | ||||||
| 39% from patients aged ≥ 18 years | ||||||
| In patients aged < 2 years, serotypes 6B, 6A, 14, 5, and 23F predominated | ||||||
| In patients aged 2–5 years, serotypes 6A, 6B, and 19F predominated | ||||||
| In patients aged 6–17 years, serotypes 1, 6A, 6B, and 19A predominated | ||||||
| Serogroup 6 (6A and 6B) comprised the most common isolates in patients aged < 2 (22%) and 2–5 years (31%) | ||||||
| Predicted strain coverage of PCV7 and PCV11 vaccines | ||||||
| Among patients < 2 years, 49% and 69% of isolates were covered, respectively | ||||||
| Among patients 2–5 years, 61% and 69% of isolates were covered, respectively | ||||||
| Youssef et al. [15] | Surveillance, prospective | 12 referral hospitals in Egypt for patients with communicable disease and public sector patients | May 1998–Dec 2000 | Patients aged < 6 years presenting to hospitals with suspected meningitis (n = 2047) |
All patients with suspected meningitis had CSF samples collected CSF samples were processed by standard methods, including Gram stain, cell count, and culture Purulent meningitis was defined as CSF WBC count > 100/mm3 (or turbid appearance in absence of a WBC count) |
Epidemiology |
| 647/2047 patients (32%) had CSF WBC count > 100/mm3 and 228 (11%) had culture-confirmed meningitis | ||||||
| Most frequently diagnosed causes of meningitis | ||||||
| H. influenzae: 89/228 (39%) | ||||||
| S. pneumoniae: 68/228 (30%) | ||||||
| N. meningitidis: 30/228 (13%) | ||||||
| M. tuberculosis: 18/228 (8%) | ||||||
| S. pneumoniae was the most common cause of meningitis in children aged ≥ 12 months: 23/76 (30.3%) | ||||||
| Morbidity and mortality | ||||||
| Pneumococcal meningitis CFR: 15% | ||||||
| Mean days from onset of illness to hospital admission in patients with pneumococcal meningitis: 4.5 |
ABM acute bacterial meningitis, CBC complete blood count, CFR case fatality rate, CSF cerebrospinal fluid, CT computed tomography, ELISA enzyme-linked immunosorbent assay, H. influenzae Haemophilus influenza, HPF high power field, IPD invasive pneumococcal disease, M. tuberculosis Mycobacterium tuberculosis, N. meningitidis Neisseria meningitides, OR odds ratio, PCV7 7-valent pneumococcal conjugate vaccine, PCV11 11-valent pneumococcal conjugate vaccine, RCT randomized controlled trial, S. pneumoniae Streptococcus pneumoniae, WBC white blood cell, WHO World Health Organization
Three articles reported hospital-based prospective surveillance studies conducted in patients with pediatric meningitis between 1998 and 2003 (Table 2) [15–17]. The first study was conducted in 12 referral hospitals in Egypt from May 1998 to December 2000, and assessed CSF samples from 2047 patients aged < 6 years presenting with suspected meningitis [15]. In total, 647/2047 patients (32%) had purulent meningitis, and 228 patients (11%) had culture-confirmed meningitis. S. pneumoniae was among the most frequently cultured pathogens, accounting for 30% of cases among all children aged < 6 years, while in children aged ≥ 12 months, S. pneumoniae was most common (23/76, 30%). At 15%, the CFR for pneumococcal meningitis in children aged < 6 years was lower than that of other common pathogens, including H influenzae (27%), N meningitidis (23%), and Mycobacterium tuberculosis (56%) [15].
The second study, which was also conducted in multiple hospitals across Egypt, characterized S. pneumoniae isolates collected from patients of all ages hospitalized with acute bacterial meningitis between 1998 and 2003 [16]. Of 205 pneumococcal isolates, 25% were isolated from patients aged < 2 years, 7% from patients aged 2–5 years, 29% from patients aged 6–17 years, and 39% from patients aged ≥ 18 years. Isolate serotyping showed that the predominant serotypes varied somewhat with patient age, although serotypes 6A and 6B were prominent in all three pediatric age groups, and were the most common isolates in patients aged < 2 years (22% of isolates) and 2–5 years (31% of isolates). Based on serotyping results, the predicted serotype coverages of PCV7 and an experimental 11-valent PCV (containing all PCV7 serotypes and serotypes 1, 5, 3, and 7F) were 49% and 69%, respectively, in patients aged < 2 years and 61% and 69% in patients aged 2–5 years [16].
The third study investigated potential predictors of meningitis outcome in children admitted to Alexandria Fever Hospital during the seasons of 2002 and 2003 [17]. Meningitis was diagnosed based on clinical criteria and CSF analysis/culture. A total of 310 patients aged 3 months to 15 years were included in the study, with 202 patients (65.2%) with confirmed acute bacterial meningitis (S. pneumoniae, n = 43) and 108 patients (34.8%) with aseptic meningitis. Clinical signs were assessed using the World Health Organization (WHO) meningitis prognostic scoring system; subsequent logistic regression analysis showed that the risk of poor prognosis (death and epilepsy) in the studied patients increased significantly with WHO score ≥ 9 [odds ratio (OR) = 22.7; 95% CI 18.3, 69.2]. Notably, of the 43 patients with pneumococcal meningitis, 15 (34.9%) had a WHO prognostic score ≥ 9 [17].
The two most recently published articles also reported hospital-based surveillance studies [18, 19]. Draz et al. [18] investigated the incidence of IPD in children aged < 5 years admitted to hospitals during 2008–2011 with fever and a potential clinical diagnosis of meningitis, pneumonia, or septicemia. Among 22,018 patients admitted with these signs, and for whom culture was performed, IPD was confirmed in four patients all aged < 1 year: two patients with pleural effusion, one patient with meningitis, and one with septicemia. The IPD CFR was 75%. It was noted that three of the four patients with IPD had comorbid congenital heart disease and/or immunodeficiency, illustrating the heightened vulnerability of children with underlying conditions. Based on the study results, the annual IPD detection rate was estimated to be 18.2 per 100,000 hospitalized children aged < 5 years. Sadek et al. [19] evaluated children aged ≤ 12 years who presented to hospitals with fever and convulsions from December 2012 to November 2013. Of 85 patients enrolled in the study, 69 were aged ≤ 2 years. CSF samples were collected from all patients for physical, chemical, and cytologic analyses. Samples from 23.5% of patients had elevated white blood cell counts, of which 3.5% of the samples had counts indicative of acute bacterial infection, all of which were positive for S. pneumoniae.
Pneumococcal Pneumonia
Six articles on pneumonia are included in this qualitative analysis. Of the six included studies, three studies evaluated pediatric patients presenting to hospitals with CAP [18, 20, 21], two studies assessed pneumonia in pediatric intensive care patients undergoing mechanical ventilation (MV) [22, 23], and one study investigated congenital pneumonia in full-term neonates (Table 3) [24].
Table 3.
Summary of studies on pneumococcal pneumonia in generally healthy Egyptian children
| Reference | Study type | Location | Study date | Population | Methodology | Outcomes relevant to Pneumonia |
|---|---|---|---|---|---|---|
| Badr et al. [22] | Surveillance, prospective | NICU of Zagazig University Hospital | Jan 2010–Oct 2010 | Critically ill newborn infants aged 6–18 days admitted to NICU with different illnesses who received MV for > 48 h (n = 56) |
Neonates with pneumonia at initiation of MV were excluded from the study Physical examination, routine laboratory assessments and arterial blood gas monitoring, chest X-ray at baseline and 48 h after commencing MV, and nonbronchoscopic bronchoalveolar lavage (NB-BAL) culture performed |
Epidemiology |
| Organisms isolated from NB-BAL fluid culture of VAP group neonates | ||||||
| Klebsiella species: 11/32 (34.3%) | ||||||
| Pseudomonas species: 7/32 (21.8%) | ||||||
| S. aureus: 5/32 (15.6%) | ||||||
| E coli: 4/32 (12.5%) | ||||||
| Candida species: 3/32 (9.3%) | ||||||
| S. pneumoniae: 2/32 (6.2%) | ||||||
| Hospitalization | ||||||
| VAP group included 32 neonates with a clinical diagnosis of VAP; the non-VAP group included 24 neonates | ||||||
| The VAP group had significantly longer duration on MV compared with the non-VAP group (P = 0.001) | ||||||
| Draz et al. [18] | Surveillance, prospective | Cairo University Specialized Pediatric Hospital | Jan 2008–Dec 2011 | Children aged < 5 years admitted with fever; a possible clinical diagnosis of meningitis, pneumonia, or septicemia; and for whom culture was performed (n = 22,018) | Sputum and blood samples cultured; S. pneumoniae identified by Gram stain, colony morphology, optochin disk inhibition, bile solubility test | Epidemiology |
| 8/22,018 patients evaluated were diagnosed with non-IPD pneumococcal infections, 7/8 of whom were diagnosed with pneumococcal pneumonia | ||||||
| Of 8 patients diagnosed with non-IPD pneumococcal infection, 3 had underlying congenital heart disease and/or immunodeficiency | ||||||
| Estimated annual detection rate of non-IPD pneumococcal disease was 36.4 per 100,000 | ||||||
| Annual detection rate in infants aged < 1 year was significantly higher than that in children aged 1–5 years (61.4 vs 21.6, P < 0.001) | ||||||
| Morbidity and mortality | ||||||
| CFR of non-IPD pneumococcal disease: 12.5% | ||||||
| El Seify et al. [20] | Surveillance, prospective | Ain Shams University Hospitals, Cairo | Feb 2012–Mar 2013 | Infants and children aged 1–72 months consecutively admitted to the Children’s Unit with clinical signs of CAP; n = 90) |
Blood and respiratory specimens were cultured and bacterial isolates identified using standard microbiologic techniques Atypical bacteria and viruses were identified using serologic techniques |
Epidemiology |
| Etiologic agent identified in 59/90 (65.5%) of pediatric patients with CAP | ||||||
| 43 bacterial isolates and 23 viral isolates were identified (7 patients were coinfected with 2 agents) | ||||||
| Most commonly identified bacterial agents in respiratory specimens | ||||||
| S. aureus (12/90; 13.3%) | ||||||
| M. pneumoniae (10/90; 11.1%) | ||||||
| S. pneumoniae (7/90; 7.8%) | ||||||
| K. pneumoniae (7/90; 7.8%) | ||||||
| Bacteria were isolated from 3/89 blood cultures | ||||||
| S aureus, 2/89 | ||||||
| K. pneumoniae, 1/89 | ||||||
| El-Nawawy et al. [23] | Cohort, prospective | The PICU of a university-affiliated hospital, Egypt | May 2015–Mar 2018 | Patients aged 1 months–12 years admitted to PICU with severe pneumonia requiring MV and patients in whom VAP developed during their PICU stay (n = 85) |
Patients on MV were classified based on origin of infection as CAP, HAP, or VAP NB-BAL specimens collected at diagnosis and before patients started empiric antibiotics were cultured and Gram stained |
Hospitalization outcomes |
| Of 642 patients admitted to PICU during the study period, 85 patients with 96 episodes of pneumonia required MV | ||||||
| 43 episodes of CAP | ||||||
| 12 episodes of HAP | ||||||
| 41 episodes of VAP | ||||||
| Epidemiology | ||||||
| S. pneumoniae was isolated from only 1 pneumonia episode (CAP) | ||||||
| Gad et al. [24] | Case–control | NICU, Faculty of Medicine, Ain Shams University, Cairo | Not stated | Full-term neonates with history of premature membrane rupture who were diagnosed with congenital pneumonia within 72 h of birth and who had a positive blood culture (30 cases and 30 healthy neonates as controls) | Blood cultures were performed using standard methods | Epidemiology |
| Of 30 included cases of congenital pneumonia, isolates from blood culture were: | ||||||
| S. aureus, 12/30 (40%) | ||||||
| E coli, 7/30 (23.3%) | ||||||
| S. pneumoniae, 6/30 (20%) | ||||||
| K. pneumoniae, 3/30 (10%) | ||||||
| Pseudomonas species, 2/30 (6.7%) | ||||||
| Hospitalization | ||||||
| 10 cases required MV and 20 required nasal continuous positive airway pressure ventilation | ||||||
| Ostroff et al. [21] | Surveillance, prospective | Abbassia Fever Hospital and Embaba Fever Hospital, Cairo | Nov 1991–Apr 1993 | Children aged 2–60 months who presented to the 2 hospitals and met WHO clinical criteria for pneumonia (maximum of 5 patients enrolled daily per site; total n = 1635) | NP and blood culture performed using standard methods | Epidemiology |
| S. pneumoniae and/or H. influenzae were isolated from the NP swabs of 73% (1199/1635) of patients | ||||||
| 1845 isolates of S. pneumoniae and H influenzae were cultured from 1199 swabs | ||||||
| S. pneumoniae: 961/1635 (59%) | ||||||
| S. pneumoniae were isolated from 61 blood cultures | ||||||
| S. pneumoniae: 52/1635 (3.2%) | ||||||
| Morbidity and mortality | ||||||
| Of 1635 patients, 47% were diagnosed with uncomplicated pneumonia, 45% with severe pneumonia, and 8% with very severe disease (morbidity specific to S. pneumoniae was not reported) |
CAP community-acquired pneumonia, CFR case fatality rate, E coli Escherichia coli, HAP hospital-acquired pneumonia, H. influenza Haemophilus influenza, IPD invasive pneumococcal disease, K. pneumoniae Klebsiella pneumoniae, MV mechanical ventilation, NB-BAL nonbronchoscopic bronchoalveolar lavage, NICU neonatal intensive care unit, NP nasopharyngeal, PICU pediatric intensive care unit, S. aureus Staphylococcus aureus, S. pneumoniae Streptococcus pneumoniae, VAP ventilator-associated pneumonia, WHO World Health Organization
A large, prospective surveillance survey conducted in two hospitals in Cairo from November 1991 to April 1993 enrolled 1635 children aged 2–60 months who presented with clinical signs of pneumonia that met published WHO criteria [21]. All enrolled children were hospitalized; NP swabs and blood samples were collected from all patients, and pneumonia severity was categorized according to WHO guidelines. Overall, 1199/1635 patients (73%) had S. pneumoniae and/or H influenzae isolated from NP swab specimens: 961/1635 (59%) had S. pneumoniae and 884/1635 (54%) had H influenzae (646 patients had both bacteria isolated). Of the 1635 blood cultures undertaken, S. pneumoniae was isolated from 52 (3.2%) cultures. Based on the assessed clinical criteria, 47% of patients were diagnosed with uncomplicated pneumonia, 45% with severe pneumonia, and 7% with very severe disease; children with very severe disease were more likely to have a positive blood culture result than children with severe or uncomplicated pneumonia (6.4% vs. 3.5%, P = 0.08) [21].
Two more recent surveillance studies also assessed S. pneumoniae infections among children hospitalized with pneumonia in Cairo [18, 20]. The first study was conducted at a large referral pediatric hospital and aimed to characterize the detection rate and outcome of pneumococcal disease in children younger than 5 years [18]. During the study period from January 2008 to December 2011, 22,018 children were admitted with fever and/or clinical signs suggestive of pneumonia, meningitis, or septicemia and had ≥ 1 culture performed. Of those admitted, eight had culture-confirmed non-IPD (aged ≤ 1 year, n = 5; aged 1–5 years, n = 3), seven of whom were diagnosed with pneumococcal pneumonia. (Of note, three of the eight patients with non-IPD also had comorbidities such as congenital heart disease and/or immune deficiency.) Overall, seven of the patients with non-IPD survived and one died. Based on these findings, the CFR of non-IPD was 12.5%, and the annual detection rate was estimated to be 36.4/100,000. Additionally, the annual detection rate of non-IPD in infants aged < 1 year was significantly higher than in children aged 1–5 years (61.4 vs. 21.6 per 100,000; P < 0.001), illustrating the vulnerability of younger children in Egypt to pneumococcal infection [18].
El Seify et al. [20] reported findings from a prospective study in children aged 1–72 months who were consecutively admitted with CAP to the pediatric unit of a university hospital in Cairo between February 2012 and March 2013. A total of 90 children were included in the study, all of whom exhibited clinical and radiologic signs of CAP. Based on cultures of collected respiratory specimens and serologic analysis, an etiologic agent (bacterial, viral, or mixed) was identified in 59/90 patients (65.5%). S. pneumoniae was among the five most commonly identified pneumonia pathogens (7.8%) [20].
Two studies evaluated pneumonia in pediatric patients receiving MV. The first study was conducted during 2010 and assessed the cause of ventilator-associated pneumonia (VAP) in critically ill neonates aged 6–18 days [22]. Among 32 patients diagnosed with VAP, nonbronchoscopic bronchoalveolar lavage (NB-BAL) culture identified S. pneumoniae in two patients (6.2% of patients overall). The second study assessed patients aged 1 month to 12 years who were either admitted to pediatric intensive care unit (ICU) with severe pneumonia requiring MV or who acquired VAP during their stay in the unit [23]. From May 2015 to March 2018, 642 patients were admitted to the ICU, of whom 85 patients required MV for 96 episodes of pneumonia. Based on the timing of infection relative to the commencement of MV, pneumonia was classified as CAP (44.8%), hospital-acquired pneumonia (HAP; 12.5%), or VAP (42.7%). S. pneumoniae were isolated from the NB-BAL fluid of only one CAP episode (2.3% of CAP overall) [23].
A case–control study was performed in full-term neonates to assess the diagnostic utility of an antimicrobial peptide (cathelicidin) in congenital pneumonia [24]. Conducted in the neonatal ICU of a Cairo hospital, the study compared 30 neonates with congenital pneumonia (cases) and 30 healthy neonates (controls). The diagnosis of congenital pneumonia was based on defined clinical signs and associated radiologic changes; only full-term neonates with a history of premature membrane rupture, a diagnosis of congenital pneumonia within 72 h of birth, and a positive blood culture result were included as cases. Of the 30 included neonates with congenital pneumonia, S. pneumoniae was identified from 6 (20%) blood cultures [24].
Pneumococcal OM
The sole article included in the OM qualitative analysis described a study conducted in children aged 9 months to 11 years with acute mastoiditis who presented to the otolaryngology unit of a pediatric hospital in Cairo during 2007 [25]. All 19 patients included in the study had acute mastoiditis develop as a complication of acute OM (Table 4). On admission, 12/19 patients who had not commenced antibiotic therapy had samples of ear discharge submitted for culture. In these 12 patients, S. pneumoniae were the most commonly identified bacteria (5/12), followed by Streptococcus pyogenes (3/12) and Staphylococcus aureus (1/12). All isolates were sensitive to ceftriaxone. Treatment outcomes over a follow-up period of ≥ 1 year were reported across all 19 patients: 5 patients recovered after antibiotic therapy alone; 7 patients were treated with myringotomy in addition to antibiotics, 4 of whom recovered without recurrence and 3 subsequently required mastoidectomy; and 10 patients were treated with cortical mastoidectomy with myringotomy and antibiotic therapy, all of whom recovered without recurrence [25]. No associations between pathogen type and severity of mastoiditis or treatment outcomes were reported.
Table 4.
Summary of studies on pneumococcal OM in generally healthy Egyptian children
| Reference | Study type | Location | Study date | Population | Methodology | Outcomes relevant to OM |
|---|---|---|---|---|---|---|
| Abdel-Aziz et al. [25] | Surveillance, prospective | Pediatric Hospital of Cairo University | Jan 2007–Dec 2007 | Children aged 9 months–11 years presenting to the otolaryngology unit with acute mastoiditis as a complication of acute OM (n = 19) |
Ear discharge culture of patients not already receiving antibiotic therapy at admission All patients had CT of the temporal bone treated on admission with intravenous ceftriaxone for ≥ 1 week (unless culture results prompted change) Myringotomy was considered for patients who did not respond to medical treatment after 48 h Cortical mastoidectomy with myringotomy was performed in patients who presented with subperiosteal abscess and in patients who showed no response to myringotomy alone after 48 h Patients were followed up for ≥ 1 year after discharge from hospital |
Prevalence |
| Ear discharge samples from 12 patients were submitted for culture | ||||||
| S. pneumoniae: 5/12 | ||||||
| S. pyogenes: 3/12 | ||||||
| S. aureus: 1/12 | ||||||
| No growth: 3/12 | ||||||
| Disease outcome | ||||||
| CT of the temporal bone | ||||||
| Subperiosteal abscess and destruction of cortex were observed in 7/19 patients | ||||||
| Treatment outcomes | ||||||
| 5/19 patients recovered with antibiotic therapy alone | ||||||
| 5/7 patients treated with myringotomy improved (3 of the 7 patients with myringotomy subsequently underwent cortical mastoidectomy) | ||||||
| 10/10 patients treated with cortical mastoidectomy improved |
CT computed tomography, OM otitis media, S. aureus Staphylococcus aureus, S. pneumoniae Streptococcus pneumoniae, S pyogenes Streptococcus pyogenes
Pneumococcal NPC
Two studies on pneumococcal NPC were included in the review, both performed in children aged ≤ 5 years and together spanning the period of late 2012 to early 2014 [10, 26]. One study was based in Cairo and evaluated NPC in 200 children aged 6 months to 5 years (healthy or with noninfectious disease) attending outpatient clinics [10], and the other assessed carriage in 600 healthy children aged 2–5 years attending a hospital in Alexandria (Table 5) [26]. The prevalence of S. pneumoniae carriage was comparable between the studies when measured by bacterial culture, with 31% of all the Cairo subjects [10] and 29.2% of all the Alexandria subjects [26] identified as carriers of S. pneumoniae; among the Alexandria subjects, higher S. pneumoniae carriage was observed in children aged < 2 years ranging between 37.7% and 39.4%. Of note, when NPC samples from the Cairo subjects were assessed by real-time polymerase chain reaction (PCR), S. pneumoniae carriage prevalence increased to 56.5% [10]. Differences in serotyping methodology between studies limited the extent of direct comparisons, although there was a clear predominance of 19F isolates in Cairo and Alexandria carriers (18.8% and 24.6%, respectively). Serotypes 6A/6B/(6C/6D) were also commonly carried (20.8% and 25.1%, respectively), whereas serotype 1 was noticeably more frequent in Cairo carriers (10.9% vs. 0%) [10, 26]. Serotype 19A was carried by 5.9% and 1.7% of Cairo and Alexandria subjects, respectively. Based on the serotypes observed in the Cairo study, it was estimated that the PCV13 and PCV10 vaccines covered 72.4% and 65.5% of the carried NP isolates, respectively [10].
Table 5.
Summary of studies on pneumococcal NPC in generally healthy Egyptian children
| Reference | Study type | Location | Study date | Population | Methodology | Outcomes relevant to NPC |
|---|---|---|---|---|---|---|
| Badawy et al., [10] | Consecutive cross-sectional | Cairo University Children Hospital |
Dec 2012–Feb 2013; Dec 2013–Feb 2014 |
Children aged 6 months–5 years, healthy or with noninfectious disease, attending outpatient clinics (n = 200 screened, 77 healthy and 123 with noninfectious disease) |
NP swabs cultured on blood agar; S. pneumoniae identified by optochin susceptibility, bile-solubility, and real-time PCR S. pneumoniae serotype determined using CDC sequential multiplex PCR protocol |
Carriage prevalence |
| S. pneumoniae cultured from 62/200 subjects | ||||||
| 113/200 subjects (56.5%) were positive for pneumococci by PCR | ||||||
| PCR-positive cases significantly higher (P = 0.024) among infants (70.3%) compared with toddlers (59.4%) and preschoolers (43.5%) | ||||||
| Serotype prevalence | ||||||
| 6A/6B/6C/6D: n = 21 (20.8%) | ||||||
| 19F: n = 19 (18.8%) | ||||||
| 1: n = 11 (10.9%) | ||||||
| 34: n = 8 (7.9%) | ||||||
| 19A: n = 6 (5.9%) | ||||||
| Other serotypes: n < 4 | ||||||
| PCV serotype coverage | ||||||
| PCV13: 72.4% | ||||||
| PCV10: 65.5% | ||||||
| El-Nawawy et al. [26] | Cross-sectional | Alexandria University Children's Hospital, El-Shatby | Jan 2013–Jan 2014 | Healthy, asymptomatic children aged 2–60 months attending the hospital (n = 600 screened) |
NP swabs cultured on blood agar; S. pneumoniae identified by standard laboratory methods (CDC protocol) Serotype determined by Quellung reaction using antisera to PCV13 serotypes |
Carriage prevalence |
| 175/600 (29.2%) subjects were S. pneumoniae carriers | ||||||
| Median age of carriers was 15 months vs noncarriers 14 months | ||||||
| Carriage prevalence highest in autumn/winter vs spring/summer (P = 0.001) | ||||||
| Serotype prevalence | ||||||
| 19F: 43/175 (24.6%) | ||||||
| 6B: 25/175 (14.3%) | ||||||
| 6A: 19/175 (10.9%) | ||||||
| 14: 8/175 (4.6%) | ||||||
| 18C: 8/175 (4.6%) | ||||||
| 9V: 6/175 (3.4%) | ||||||
| 7A: 4/175 (2.3%) | ||||||
| 19A: 3/175 (1.7%) | ||||||
| 23F: 2/175 (1.1%) | ||||||
| PCV serotype coverage | ||||||
| PCV13: 118/175 (67.4%) | ||||||
| Non-PCV13: 57/175 (32.6%) |
CDC Centers for Disease Control and Prevention, NPC nasopharyngeal carriage, PCR polymerase chain reaction, PCV pneumococcal conjugate vaccine, PCV10 10-valent pneumococcal conjugate vaccine, PCV13 13-valent pneumococcal conjugate vaccine, S. pneumoniae Streptococcus pneumoniae
Antimicrobial Resistance
Of nine included studies, AMR was investigated in one study among children with conjunctivitis [27], two that characterized pneumococcal NPC in children [10, 26], one assessing treatments in children with OM and acute mastoiditis [25], two in children with pneumonia [21, 23], one including children with either invasive or noninvasive pneumococcal disease [18], and two that investigated children with meningitis (Table 6) [15, 16].
Table 6.
Summary of studies on S. pneumoniae AMR in generally healthy Egyptian children
| Reference | Study type | Location | Study date | Population | Methodology | Outcomes relevant to S. pneumoniae AMR |
|---|---|---|---|---|---|---|
| Abdel-Aziz et al. [25] | Surveillance, prospective | Pediatric Hospital of Cairo University | Jan 2007–Dec 2007 | Children aged 9 months–11 years presenting to the otolaryngology unit with acute mastoiditis as a complication of acute otitis media (n = 19) | Ear discharge culture on patients not already receiving antibiotic therapy at admission | Resistance rates |
| All cultured isolates showed sensitivity to ceftriaxone | ||||||
| All patients treated on admission with intravenous ceftriaxone for ≥ 1 week unless culture results indicated change needed | Treatment outcomes | |||||
| 5/19 patients recovered with antibiotic therapy alone | ||||||
| Badawy et al. [10] | Consecutive cross-sectional | Cairo University Children Hospital |
Dec 2012–Feb 2013; Dec 2013–Feb 2014 |
Children aged 6 months–5 years, healthy or with noninfectious disease, attending outpatient clinics (n = 200 screened, 77 healthy and 123 with noninfectious disease) | S. pneumoniae isolates tested for AMR using disk diffusion (oxacillin, vancomycin, tetracycline, doxycycline, clindamycin, erythromycin, levofloxacin, ofloxacin, sulfamethoxazole-trimethoprim) and E-test for MICs (penicillin, vancomycin, ceftriaxone), in accordance with CLSI guidelines | Carriage prevalence |
| S. pneumoniae cultured from 62/200 subjects | ||||||
| PCR showed 113/200 subjects (56.5%) were positive for pneumococci | ||||||
| Serotype identification | ||||||
| Most frequently isolated serotypes were 6A/6B/6C/6D (20.8%), 19F (18.8%), 1 (10.9%), 34 (7.9%), and 19A (5.9%) | ||||||
| Resistance rates | ||||||
| Majority showed resistance to multiple antibiotics | ||||||
| Frequency of resistant and intermediate isolates was highest for sulfamethoxazole-trimethoprim (96.7% and 0%), doxycycline (77.4% and 8.1%), tetracycline (72.6% and 11.3%), and oxacillin (72.6% and 0%) | ||||||
| Sensitivity was highest to vancomycin (100%), levofloxacin (100%), ofloxacin (72.5%), clindamycin (64.5%), and erythromycin (50%) | ||||||
| MICs (by E-test): penicillin (3.2% resistant and 54.8% intermediate), ceftriaxone (0% and 1.6%), vancomycin (both 0%) | ||||||
| Draz et al. [18] | Surveillance, prospective |
Cairo University Specialized Pediatric Hospital |
Jan 2008–Dec 2011 | Children aged < 5 years admitted with fever; a possible clinical diagnosis of meningitis, pneumonia, or septicemia; and for whom culture was performed (n = 22,018) | S. pneumoniae isolates were tested for AMR using disk diffusion method, in accordance with CLSI guidelines; benzyl penicillin MIC determined by E-test (antibiotics tested included sulfamethoxazole-trimethoprim, penicillin, vancomycin, ceftriaxone, oxacillin, erythromycin, tetracycline, ciprofloxacin, clindamycin, doxycycline) | Resistance rates (IPD isolates) |
| All 4 IPD isolates were sensitive to oxacillin, vancomycin, sulfamethoxazole-trimethoprim, doxycycline, clindamycin, ceftriaxone, and tetracycline | ||||||
| 75% were resistant to ciprofloxacin, 50% to benzyl penicillin, and 25% to erythromycin | ||||||
| None were MDR | ||||||
| Resistance rates (non-IPD isolates) | ||||||
| All 8 isolates were sensitive to vancomycin, ceftriaxone, and tetracycline | ||||||
| MDR was defined as nonsusceptibility to ≥ 3 antibiotic groups | 37.5% were resistant to sulfamethoxazole-trimethoprim, 37.5% to erythromycin, 25% to benzyl penicillin, and 25% to oxacillin | |||||
| 37.5% were MDR | ||||||
| El-Nawawy et al. [26] | Cross-sectional | Alexandria University Children's Hospital, El-Shatby | Jan 2013–Jan 2014 | Healthy, asymptomatic children aged 2–60 months attending the hospital (n = 600 screened) |
Antibiotic susceptibility testing was performed on 100 S. pneumoniae isolates via standard disc diffusion, disc approximation (for clindamycin), and broth microdilution (for MICs) MDR was defined as resistance to ≥ 3 antibiotic classes included in the study |
Resistance rates |
| 90% were resistant to ≥ 1 antibiotic | ||||||
| MDR was observed in 41% (isolates resistant to 3, 4, and 5 antibiotic groups were 15%, 14%, and 12%, respectively) | ||||||
| Highest resistance was to penicillin (55% by meningitis MIC break points), sulfamethoxazole–trimethoprim (55%), tetracyclines (49%), erythromycin (40%), clindamycin (25%) | ||||||
| Of the 70 oxacillin-resistant isolates (by disk diffusion) that were tested for MIC | ||||||
| Using meningitis break points, 15/70 (21.4%) were sensitive to penicillin and 55/70 (78.6%) were resistant | ||||||
| Using nonmeningitis break points, 55/70 (78.6%) were sensitive to penicillin, 13/70 (18.6%) were resistant, and 2/70 (2.8%) were intermediate | ||||||
| Serotype identification | ||||||
| Serotypes most associated with MDR: 19F (41.4%) and 6 (26.8%) | ||||||
| Serotype 9V showed the highest penicillin resistance rate (100% resistance for meningitis break points and 25% resistance for nonmeningitis break points), followed by serotype 19F (74% and 22%, respectively), whereas the non-PCV13 serotype group showed the lowest resistance rate (31% and 9%, respectively) | ||||||
| El-Nawawy et al. [23] | Cohort, prospective | The PICU of a university-affiliated hospital, Egypt | May 2015–Mar 2018 | Patients aged 1 months–12 years admitted to PICU with severe pneumonia requiring MV and patients in whom VAP developed during their PICU stay (n = 85) | Antibiotic susceptibility testing was performed by disk diffusion using CLSI guidelines | Resistance rates |
| S. pneumoniae were isolated from only 1 pneumonia episode (CAP); this isolate was classified as antibiotic susceptible | ||||||
| Ostroff et al. [21] | Surveillance, prospective | Abbassia Fever Hospital and Embaba Fever Hospital, Cairo | Nov 1991–Apr 1993 | Children aged 2–60 months who presented to the 2 hospitals and met WHO clinical criteria for pneumonia (maximum of 5 patients enrolled daily per site; total n = 1635) |
All S. pneumoniae isolates were screened by disk diffusion for susceptibility to oxacillin, co-trimoxazole, erythromycin, and chloramphenicol MICs were determined by agar dilution test (penicillin substituted for oxacillin) for most samples NP S. pneumoniae isolates that did not have MIC determined directly had MIC estimated based on tested strains with matching inhibition zones |
Resistance rates |
| AMR by MICs for S. pneumoniae isolates from NP samples | ||||||
| Penicillin: Susceptible, 70.9%; Intermediate, 29.1%; Resistant, 0% | ||||||
| Co-trimoxazole: S, 73.0%; I, 26.4%; R, 0.6% | ||||||
| Erythromycin: S, 93.9%; I, 3.6%; R, 2.5% | ||||||
| Chloramphenicol: S, 78.5%; R, 21.5% | ||||||
| AMR by MICs for S. pneumoniae isolates from blood samples | ||||||
| Penicillin: S, 77.6%; I, 22.4%; R, 0%; | ||||||
| Co-trimoxazole: S, 75.0%; I, 25.0%; R, 0%; | ||||||
| Erythromycin: S, 100%; | ||||||
| Chloramphenicol: S, 70.2%; R, 29.7% | ||||||
| Of S. pneumoniae blood isolates with intermediate susceptibility to penicillin, 36% were intermediately susceptible to co-trimoxazole, and 27% were resistant to chloramphenicol | ||||||
| van Bijsterveld et al. [27] | RCT | Pediatric clinic at Kasr El-Aini Hospital, Cairo University | Jul 1983 | Children aged ≤ 15 years consecutively admitted during July with an external eye infection (total n = 248; < 2 years, n = 165; 2–6 years , n = 62; 7–15 years, n = 21) | Fusidic acid eye viscous drops or chloramphenicol eye drops were assigned using an open, randomized protocol; every fifth child receiving chloramphenicol | Resistance rates |
| Of 248 patients with conjunctivitis, 206 received fusidic acid viscous eye drops and 42 received chloramphenicol eye drops | ||||||
| S. pneumoniae, n = 20/159 (13%) of bacterial isolates | ||||||
| 16/18 treated with fusidic acid were clinically classified as “success” and were sensitive to fusidic acid by in vitro testing | ||||||
| Isolates were identified and tested for susceptibility against fusidic acid and chloramphenicol using disk diffusion | 2/18 treated with fusidic acid were clinically classified as “improvement” and were resistant to fusidic acid by in vitro testing | |||||
| 2/2 treated with chloramphenicol were clinically classified as “success” and were sensitive to chloramphenicol by in vitro testing | ||||||
| Wasfy et al. [16] | Surveillance, prospective | 13 infectious disease hospitals across Egypt | 1998–2003 | Patients of all ages hospitalized with meningitis; specific age groups were defined, including those aged < 2, 2–5, and 6–17 years (number of patients included in the study was not stated) | AMR testing by disk diffusion and E-test was performed and interpreted in accordance with the NCCLS | Resistance rates |
| 100/205 (49%) isolates were nonsusceptible (intermediate and resistant) to penicillin, including all 6B and 19A isolates | ||||||
| 13/205 (6%) isolates were nonsusceptible (intermediate and resistant) to ceftriaxone | ||||||
| Resistance to tetracycline was 52%, to sulfamethoxazole-trimethoprim 59%, erythromycin 11%, and chloramphenicol 9% | ||||||
| Overall, 32/205 isolates (15.6%) were MDR | ||||||
| In children aged < 2 years, 51% of isolates were nonsusceptible to penicillin and 9% were nonsusceptible to ceftriaxone | ||||||
| Antibiotics evaluated included chloramphenicol, tetracycline, erythromycin, sulfa-trimethoprim, penicillin (E-test), ceftriaxone (E-test) | Serotype identification | |||||
| 50% of the MDR isolates were serotypes 6A (n = 5), 6B (n = 4), and 23F (n = 7) | ||||||
| Predominant serotypes (6B, 1, 19A, 23F, and 6A) were significantly more likely to be nonsusceptible to penicillin (85% vs 25% for other serotypes, P < 0.001) | ||||||
| Pneumococcal isolates with resistance to ≥ 3 different classes of antibiotics were defined as MDR | ||||||
| Youssef et al. [15] | Surveillance, prospective | 12 referral hospitals in Egypt for patients with communicable disease and public sector patients | May 1998–Dec 2000 | Patients aged < 6 years presenting to hospitals with suspected meningitis (n = 2047) | AMR of bacterial isolates was tested by disk diffusion, using NCCLS to define cut-off points (antibiotics tested included oxacillin, chloramphenicol, ceftriaxone, tetracycline, sulfamethoxazole-trimethoprim) | Resistance rates |
| For 29 S. pneumoniae isolates from CSF samples | ||||||
| Penicillin (E-test): susceptible, 48%; intermediate, 52%; resistant, 0% | ||||||
| Oxacillin: susceptible, 38%; intermediate, 0%; resistant, 62% | ||||||
| Chloramphenicol: susceptible, 79%; intermediate, 0%; resistant, 21% | ||||||
| Ceftriaxone: susceptible, 100% | ||||||
| E-test was used to identify penicillin-resistant S. pneumoniae; strains were classified as susceptible with E-test < 0.06 µg/mL, intermediate with 0.12–1 µg/mL, and resistant with > 2 μg/mL | Tetracycline: susceptible, 38%; intermediate, 21%; resistant, 41%; | |||||
| Sulfamethoxazole-trimethoprim: susceptible, 61%; intermediate, 14%; resistant, 25% | ||||||
| 1 isolate was a “high-level resistant strain” |
AMR antimicrobial resistance, CAP community-acquired pneumonia, CLSI Clinical and Laboratory Standards Institute, CSF cerebrospinal fluid, IPD invasive pneumococcal disease, MDR multidrug resistant, MIC minimum inhibitory concentration, MV mechanical ventilation, NCCLS National Committee for Clinical Laboratory Standards, NP nasopharyngeal, PCR polymerase chain reaction, PCV13 13-valent pneumococcal conjugate vaccine, PICU pediatric intensive care unit, RCT randomized controlled trial, S. pneumoniae Streptococcus pneumoniae, VAP ventilator-associated pneumonia, WHO World Health Organization
Two cross-sectional studies investigated AMR of S. pneumoniae isolates from NPC in Egyptian children [10, 26]. The first study was conducted in healthy children aged 2–60 months who were sampled between January 2013 and January 2014 [26]. Antimicrobial susceptibility testing results of 100 isolates showed high levels of resistance to penicillin, sulfamethoxazole-trimethoprim, tetracyclines, erythromycin, and clindamycin. Among the identified serotypes, the highest frequency of penicillin resistance was observed in serotypes 9V (100% using meningitis breakpoints and 25% using nonmeningitis breakpoints) and 19F (74% and 22%). Multidrug-resistance (MDR), which was defined as resistance to ≥ 3 antimicrobial groups, was identified in 41% of isolates; serotypes 19F and six predominated among MDR isolates (41.4% and 26.8%, respectively) [26]. The second NPC study was performed in Alexandria during the winter seasons of 2012–2013 and 2013–2014 and sampled 200 children aged 6 months to 5 years [10]. S. pneumoniae was cultured from the swabs of 62/200 subjects; all isolates were tested for AMR, with the majority showing resistance to multiple antibiotics. By disk diffusion, the frequencies of resistant and intermediate isolates were highest for sulfamethoxazole-trimethoprim (96.7%), doxycycline (85.5%), tetracycline (83.9%), and oxacillin (72.6%) and lowest for erythromycin (50.0%), clindamycin (35.5%), ofloxacin (27.4%), vancomycin (0%), and levofloxacin (0%). Serotype-specific antibiotic susceptibilities were not reported in this article [10].
A study conducted during 2007 evaluated the treatment of acute mastoiditis in 19 children presenting to a pediatric hospital in Cairo [25]. Acute mastoiditis is considered a serious complication of acute OM that can in turn lead to further severe complications. All patients included in the study received intravenous ceftriaxone (20–50 mg/kg body weight) on admission; this dosage was continued for ≥ 1 week. Culture of ear discharge was performed for 12 patients; S. pneumoniae was isolated in 5/12 (42%), and all isolates were sensitive to ceftriaxone [25].
A prospective surveillance study conducted in two hospitals in Cairo during the early 1990s enrolled 1635 children aged 2–60 months who presented with clinical signs of pneumonia [21]. Overall, S. pneumoniae was isolated from 961/1635 NP swabs and 52/1635 blood samples collected from these patients. Susceptibility patterns by minimum inhibitory concentration for the tested antibiotics were found to be similar for S. pneumoniae isolates from NP samples and blood. Susceptibility rates to penicillin of NP isolates and blood isolates were 70.9% and 77.6%, respectively; to co-trimoxazole, 73.0% and 75.0%; to erythromycin, 93.9% and 100%; and to chloramphenicol, 78.5% and 70.2% [21]. Another study characterized the pathogens associated with severe pneumonia in hospitalized Egyptian children [23]. Among 85 hospitalized pediatric patients aged 1 month to 12 years with pneumonia requiring MV or in whom VAP developed during hospitalization (96 pneumonia episodes in total), S. pneumoniae was isolated from one NB-BAL specimen. This isolate was described as antibiotic susceptible [23].
A surveillance study conducted in a Cairo pediatric hospital to detect cases of invasive and noninvasive pneumococcal disease in patients aged < 5 years also investigated antimicrobial susceptibility of obtained isolates [18]. During the 4-year study period (2008–2011), four cases of IPD and eight cases of non-IPD were confirmed by culture. Of the S. pneumoniae isolates from IPD, all were sensitive to oxacillin, vancomycin, sulfamethoxazole-trimethoprim, doxycycline, and clindamycin, while 50% were resistant to benzyl penicillin, 75% to ciprofloxacin, and 25% to erythromycin. Of the isolates from non-IPD, all were sensitive to vancomycin, ceftriaxone, and tetracycline; 37.5% were resistant to sulfamethoxazole-trimethoprim, 37.5% were resistant to erythromycin, 25% were resistant to benzyl penicillin, and 25% were resistant to oxacillin. None of the IPD isolates were MDR (defined by the study as nonsusceptibility to ≥ 3 antibiotic groups), whereas 37.5% of non-IPD isolates were MDR. Although isolate numbers were small, there appeared to be increased frequency of resistance in the final year of the study, particularly in the non-IPD isolates (all three MDR isolates were cultured in 2011) [18].
Two studies characterized AMR in S. pneumoniae isolates from pediatric patients with meningitis treated in infectious disease hospitals participating in sentinel meningitis surveillance across Egypt [15, 16]. Youssef et al. [15] investigated the cause of meningitis in patients aged < 6 years who presented with suspected meningitis between May 1998 and December 2000; in total, 228/2047 patients had culture-confirmed meningitis, 68 (30%) of whom had S. pneumoniae isolated. Results from AMR testing conducted on 29 of these S. pneumoniae isolates showed significant levels of resistance against oxacillin (62% resistant and 0% intermediate), tetracycline (41% and 21%, respectively), sulfamethoxazole-trimethoprim (25% and 14%), chloramphenicol (21% and 0%), and penicillin (E-test; 0% resistant and 52% intermediate), whereas all isolates were sensitive to ceftriaxone [15]. Wasfy et al. [16] characterized 205 S. pneumoniae isolates recovered from patients with meningitis of all ages between 1998 and 2003; 61% of the studied patients were aged < 18 years. Although AMR rates were mostly reported across all age groups, it was noted that, in children aged < 2 years, 51% of isolates were nonsusceptible to penicillin and 9% were nonsusceptible to ceftriaxone. Additionally, of the 32 MDR isolates identified, 50% belonged to three of the serotypes that were prominent in pediatric patients (6A, 6B, and 23F) [16].
Discussion
A comprehensive understanding of S. pneumoniae, the illnesses it can cause, and the measures to prevent disease pathogenesis among young children in low- and middle-income countries is critical toward informing public health strategies. Our review summarizes the limited available evidence on multiple aspects of S. pneumoniae in Egyptian children, including invasive disease, pneumonia, OM, NPC, AMR, and vaccination. A total of 16 relevant articles spanning three decades of research were included in our review, which together highlight the concerning role the pneumococcal pathogen has among healthy Egyptian children, as well as the necessity for comprehensive, contemporary surveillance measures to fully capture the extent of disease burden among this population, including prevalent serotypes and disease dynamics.
Several studies investigating the epidemiology of invasive disease among Egyptian children identified S. pneumoniae as a predominant cause of illness. Findings were primarily derived from hospital-based surveillance studies that assessed pediatric meningitis. The majority of studies showed that S. pneumoniae remains a prevalent cause of meningitis among Egyptian children, causing 24% of meningitis cases at a Cairo hospital in 1983 [13], 30% of cases across 12 referral hospitals from 1998 to 2000 [15], and 21% of bacterial meningitis cases among children admitted to a single hospital during 2002–2003 [17]. Similarly, studies from neighboring countries, such as Jordan, Kuwait, and Saudi Arabia, conducted in the 1980s and 1990s showed that S. pneumoniae was the etiologic agent in approximately 9–35% of bacterial meningitis cases in neonates and children aged < 12 years [28–30]. However, contemporary data on the epidemiology of IPD are needed, especially regarding nationwide surveillance studies that comprehensively evaluate disease incidence, prevalence, and mortality. Further, our review showed that available information on prevalent pneumococcal serotypes underlying IPD in Egyptian children is incredibly limited. Only one identified study performed isolate serotyping, which determined that serotypes 6A and 6B predominated among meningitis cases of pediatric patients aged < 5 years [16]. In contrast, a study from Israel showed that serotypes 5 and 1 were the most frequent in children aged between 15 days and 13 years [31]. Importantly, the predicted serotype coverage rates of PCV7 and an experimental 11-valent PCV were 61% and 69%, respectively, among Egyptian children aged 2–5 years [16]. Together, these findings indicate that S. pneumoniae is an important underlying cause of invasive disease, primarily when manifesting as meningitis, with currently available pneumococcal vaccines such as PCV10 and PCV13 potentially providing coverage against the most prevalent circulating serotypes.
Our literature review also highlights that S. pneumoniae is an important cause of pneumonia and OM among healthy Egyptian children. The prevalence of S. pneumoniae as a cause of pneumonia was variable, which might reflect the largely single-unit nature of the studies and the different types of pneumonia investigated (i.e., CAP, HAP, and VAP) [18, 20–24]. Regardless, S. pneumoniae was found to be a relatively common etiologic agent for pneumonia among Egyptian children. For instance, a large study in the early 1990s identified S. pneumoniae in NP swabs of 59% of children aged 2–60 months presenting with pneumonia [21]. More recently, the pneumococcus generally remained a frequent cause of pneumonia, causing ≤ 8% of CAP episodes in children aged 1–72 months in a report from 2016 [20, 24]. Further, a study from 2015 estimated the CFR of non-IPD (which predominantly consisted of pneumococcal pneumonia cases) as 12.5%, with an annual detection rate of 61.4 and 21.6 per 100,000 among Egyptian children aged < 1 and 1–5 years, respectively [18]. However, the generalizability of these findings could be limited by the small number of patients with non-IPD involved in the study (n = 8), which included children with underlying medical conditions. Additionally, pneumococcal disease detection methods are not standardized across Egypt, which may lead to variability in estimated incidence rates [32]. Comparatively, a 2001–2005 study in children aged < 5 years in Abu Dhabi, United Arab Emirates, reported the incidence of non-IPD to be 172.5 per 100,000 individuals [33]. These findings are of major interest, as pneumonia is a significant public health concern among Egyptian children aged < 5 years, with 680,000 pneumonia episodes among this population estimated to have occurred in 2010, causing approximately 5000 annual deaths [34]. Although only one article on OM was included in our review, the pneumococcus was the most commonly identified bacterial pathogen of acute mastoiditis, a severe complication of acute OM, among children aged 9 months to 11 years [25]. This result remains important, as OM is one of the most common diseases affecting children. Collectively, these data emphasize the burden of pneumonia and pneumococcal OM among Egyptian children but also illustrate the need for additional studies that include national surveillance data to comprehensively assess the epidemiology of these diseases among this vulnerable population.
While only two articles on pneumococcal carriage in Egyptian children were identified, both were relatively recently published articles that showed approximately 30% of children were carriers of the pathogen [10, 26]. Using sensitive molecular versus culture techniques, carriage rates were higher, reaching 57% among younger Egyptian children [10]. The observed carriage rates of approximately 30% are slightly lower than those reported in other countries in the region: in Ethiopia, Ghana, and South Africa, between 40 and 51% of children are reported to be carriers of S. pneumoniae [35–37]. It is understood that these rates are of utmost importance, as pneumococcal colonization of the nasopharynx is an obligatory step in the progression and development of disease, including OM and invasive disease, and also serves as a basis for disease transmission [1, 3]. Further, vaccination strategies also aim to protect against disease by preventing colonization of pneumococcal serotypes responsible for disease [3]. Although pneumococcal vaccines are not currently included in the Egyptian immunization program, the studies included in our review estimated that PCV10 and/or PCV13 would theoretically cover the majority of colonizing pneumococcal serotypes (PCV10 coverage, 65.5%; PCV13 coverage, 72.4%) [10, 26].
Our review also summarizes important findings regarding the resistance of pneumococci to commonly used antimicrobial agents. Antibiotic resistance of bacterial pathogens is a growing concern among the global medical community, particularly among low- and middle-income countries, where limited surveillance and general misuse of antibiotic agents compound their effectiveness [38, 39]. More recently, WHO designated penicillin-nonsusceptible pneumococci as a global priority pathogen [40, 41]. In our review, two studies investigating AMR of pneumococcal meningitis isolates among Egyptian children identified heightened resistance rates to penicillin [15, 16]. Predominant serotypes associated with penicillin resistance were identified as 6B, 1, 19A, 23F, and 6A, all of which would be covered by PCV13 [16]. Similarly, high levels of penicillin resistance among isolates from children aged < 15 years have also been reported in Saudi Arabia, where penicillin resistance was especially high for serotypes 23F, 6B, and 19F, all of which are included in PCV13 [42]. For pneumococcal NP isolates from young children from 2013 to 2014, 55% showed resistance to penicillin, with serotypes 9V and 19F most frequently associated with resistance and non-PCV13 serotypes showing lowest resistance [26]. Additionally concerning are MDR pneumococcal isolates, with the above study showing 90% of NP isolates were resistant to ≥ 1 antibiotic and 12% were resistant to 5 different antibiotic classes; serotype 19F was most commonly associated with MDR [26]. Of note, antibiotic use for pneumococcal disease is not standardized across Egypt [7]. Taken together, reported pneumococcal antibiotic resistance rates might present a challenge for the Egyptian medical community, with further knowledge to be gained from national surveillance studies. However, it remains encouraging that current serotypes associated with AMR are those covered by currently available PCVs, suggesting that a preemptive vaccination strategy might prove a useful therapeutic alternative to antibiotic use for pneumococcal disease among Egyptian children.
Although pneumococcal vaccines are available in Egypt, these prophylactic agents are not currently included in the national immunization program [10, 11]. According to 2018 WHO and United Nations Children’s Fund estimates, approximately 74.0 million infants (55%) worldwide were unvaccinated against pneumococcal disease, which was largely due to PCV accessibility issues (i.e., the country had not yet introduced PCVs or patients could not access routine immunization services) [43]. Notably, as of June 2020, PCVs had been included in the national programs of 146 different countries, including 60 Global Alliance (formerly the Global Alliance for Vaccines and Immunizations) countries and several countries that neighbor Egypt [43]. Additionally, a study from 2013 reported that the introduction of PCV13 into the Egyptian national immunization program would be cost-effective compared with no vaccination, and could potentially prevent approximately 42% of pneumococcal-related deaths in the country [44]. Further, for greater protection against pneumococcal disease in children aged ≥ 2 years with comorbidities, WHO recommends PPSV23 after a primary PCV vaccination [8, 45]. However, the lack of comprehensive data in Egyptian children may stifle public health decisions regarding immunization. A limitation of this review is the limited number of studies available, particularly for pneumococcal OM and NPC. Additionally, diagnostic methods were not consistent across the included studies, and the sensitivity of each method may vary [46]. Thus, our review illustrates the critical need for improved disease surveillance and contemporary approaches toward elucidating the serotypes causing disease among children throughout Egypt. This extensive understanding can help guide the implementation of appropriate health strategies specifically targeted toward the prevention and treatment of pneumococcal disease among Egyptian children.
Conclusion
Pneumococcal disease represents a significant public health concern to children in Egypt. Additional studies that use contemporaneous and inclusive approaches toward data collection should provide further clarity regarding the multifaceted aspects of the disease among this population. A national strategy toward reducing disease burden among Egyptian children may ensure that this vulnerable population is sufficiently protected against the potentially devastating disease.
Acknowledgements
Funding
This study and the article processing charges, including the journal’s Rapid Service Fee, were sponsored by Pfizer Inc.
Medical Writing and Editorial Assistance
Editorial/medical writing support was provided by Emily Stackpole, PhD, Philippa Jack, PhD, and Srividya Ramachandran, PhD, of ICON plc (North Wales, PA, USA), and was funded by Pfizer Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to study conception, design, data analysis, and interpretation. All authors also contributed substantially to the preparation and editing of the manuscript, and approved the final manuscript.
Disclosures
Nader Fasseeh and Moustafa El-Saied have no conflicts of interest to report. Ahmed El-Beleidy has received fees for giving presentations to pharmaceutical companies, including Pfizer Inc. Rehab Z El Saie and Hammam Haridy are employees of Pfizer Inc and may hold stock or stock options.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ahmed El-Beleidy, Email: drbeleidy@hotmail.com.
Moustafa El-Saied, Email: moustafa13@yahoo.com.
Nader Fasseeh, Email: nader.fasih@alexmed.edu.eg.
Rehab Z. El Saie, Email: rehab.z.elsaie@pfizer.com
Hammam Haridy, Email: hammam.haridy@pfizer.com.
References
- 1.World Health Organization. Pneumococcal Conjugate Vaccines in Infants and Children Under 5 Years of Age: WHO Position Paper - February 2019. Available at: https://apps.who.int/iris/bitstream/handle/10665/310968/WER9408.pdf?ua=1. Accessed September 23, 2020.
- 2.Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018;16:355–367. doi: 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 4.Faden H, Duffy L, Wasielewski R, et al. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. J Infect Dis. 1997;175:1440–1445. doi: 10.1086/516477. [DOI] [PubMed] [Google Scholar]
- 5.Wahl B, O'Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type B disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6:e744–e757. doi: 10.1016/S2214-109X(18)30247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fadl N, Ashour A, Muhammed Y. Medical management of pneumonia in children aged under 5 years in Alexandria, Egypt: mothers' perspective. East Mediterr Health J. 2020;26:1042–1051. doi: 10.26719/emhj.20.013. [DOI] [PubMed] [Google Scholar]
- 7.Shaban L, Siam R. Prevalence and antimicrobial resistance pattern of bacterial meningitis in Egypt. Ann Clin Microbiol Antimicrob. 2009;8:26. doi: 10.1186/1476-0711-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Pneumococcal vaccines WHO position paper–2012. Wkly Epidemiol Rec. 2012;87:129–144. [PubMed] [Google Scholar]
- 9.Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLoS ONE. 2017;12:e0177113. doi: 10.1371/journal.pone.0177113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badawy M, El Kholy A, Sherif MM, et al. Serotypes of Streptococcus pneumoniae in Egyptian children: are they covered by pneumococcal conjugate vaccines? Eur J Clin Microbiol Infect Dis. 2017;36:2385–2389. doi: 10.1007/s10096-017-3071-z. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2020 global summary: Egypt. Available at: https://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=EGY. Accessed August 11, 2020.
- 12.Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- 13.Girgis NI, Abu El-Ella AH, Farid Z, Haberberger RL, Woody JN. Ceftriaxone alone compared to ampicillin and chloramphenicol in the treatment of bacterial meningitis. Chemotherapy. 1988;34(suppl 1):16–20. doi: 10.1159/000238642. [DOI] [PubMed] [Google Scholar]
- 14.Girgis NI, Farid Z, Mikhail IA, et al. Dexamethasone treatment for bacterial meningitis in children and adults. Pediatr Infect Dis J. 1989;8:848–851. doi: 10.1097/00006454-198912000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Youssef FG, El-Sakka H, Azab A, et al. Etiology, antimicrobial susceptibility profiles, and mortality associated with bacterial meningitis among children in Egypt. Ann Epidemiol. 2004;14:44–48. doi: 10.1016/S1047-2797(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 16.Wasfy MO, Pimentel G, Abdel-Maksoud M, et al. Antimicrobial susceptibility and serotype distribution of Streptococcus pneumoniae causing meningitis in Egypt, 1998–2003. J Antimicrob Chemother. 2005;55:958–964. doi: 10.1093/jac/dki101. [DOI] [PubMed] [Google Scholar]
- 17.Farag HF, Abdel-Fattah MM, Youssri AM. Epidemiological, clinical and prognostic profile of acute bacterial meningitis among children in Alexandria,Egypt. Indian J Med Microbiol. 2005;23:95–101. doi: 10.1016/S0255-0857(21)02647-5. [DOI] [PubMed] [Google Scholar]
- 18.Draz IH, Halawa EF, Wahby G, Ismail DK, Meligy BS. Pneumococcal infection among hospitalized Egyptian children. J Egypt Public Health Assoc. 2015;90:52–57. doi: 10.1097/01.EPX.0000465234.31794.b1. [DOI] [PubMed] [Google Scholar]
- 19.Sadek AA, Mohamad MA, Ali SH, Hassan IA, Hussein MF. Diagnostic value of lumbar puncture among infants and children presenting with fever and convulsions. Electron Physician. 2016;8:2255–2262. doi: 10.19082/2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Seify MY, Fouda EM, Ibrahim HM, et al. Microbial etiology of community-acquired pneumonia among infants and children admitted to the pediatric hospital, Ain Shams University. Eur J Microbiol Immunol (Bp) 2016;6:206–214. doi: 10.1556/1886.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostroff SM, Harrison LH, Khallaf N, et al. Resistance patterns of Streptococcus pneumoniae and Haemophilus influenzae isolates recovered in Egypt from children with pneumonia. The Antimicrobial Resistance Surveillance Study Group. Clin Infect Dis. 1996;23:1069–1074. doi: 10.1093/clinids/23.5.1069. [DOI] [PubMed] [Google Scholar]
- 22.Badr MA, Ali YF, Albanna EA, Beshir MR, Amr GE. Ventilator associated pneumonia in critically-ill neonates admitted to neonatal intensive care unit Zagazig University hospitals. Iran J Pediatr. 2011;21:418–424. [PMC free article] [PubMed] [Google Scholar]
- 23.El-Nawawy A, Ramadan MA, Antonios MA, Arafa SA, Hamza E. Bacteriologic profile and susceptibility pattern of mechanically ventilated paediatric patients with pneumonia. J Glob Antimicrob Resist. 2019;18:88–94. doi: 10.1016/j.jgar.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Gad GI, Abushady NM, Fathi MS, Elsaadany W. Diagnostic value of anti-microbial peptide, cathelicidin in congenital pneumonia. J Matern Fetal Neonatal Med. 2015;28:2197–2200. doi: 10.3109/14767058.2014.981806. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Aziz M, El-Hoshy H. Acute mastoiditis: a one year study in the pediatric hospital of Cairo University. BMC Ear Nose Throat Disord. 2010;10:1. doi: 10.1186/1472-6815-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Nawawy AA, Hafez SF, Meheissen MA, Shahtout NM, Mohammed EE. Nasopharyngeal carriage, capsular and molecular serotyping and antimicrobial susceptibility of Streptococcus pneumoniae among asymptomatic healthy children in Egypt. J Trop Pediatr. 2015;61:455–463. doi: 10.1093/tropej/fmv060. [DOI] [PubMed] [Google Scholar]
- 27.van Bijsterveld OP, el Batawi Y, Sobhi FS, Nassar MW. Fusidic acid in infections of the external eye. Infection. 1987;15:16–19. doi: 10.1007/BF01646112. [DOI] [PubMed] [Google Scholar]
- 28.Zaki M, Daoud AS, Al Saleh Q, Abd Al Rasool MM. Bacterial meningitis in the newborn: a Kuwaiti experience. J Trop Pediatr. 1990;36:63–65. doi: 10.1093/tropej/36.2.63. [DOI] [PubMed] [Google Scholar]
- 29.Daoud AS, Al-Sheyyab M, Batchoun RG, et al. Bacterial meningitis: still a cause of high mortality and severe neurological morbidity in childhood. J Trop Pediatr. 1995;41:308–310. doi: 10.1093/tropej/41.5.308. [DOI] [PubMed] [Google Scholar]
- 30.Al-Jurayyan NA, Al Mazyad AS, Al-Nasser MN, et al. Childhood bacterial meningitis in Al-Baha province, Saudi Arabia. J Trop Med Hyg. 1992;95:180–185. [PubMed] [Google Scholar]
- 31.Rahav G, Toledano Y, Engelhard D, et al. Invasive pneumococcal infections. A comparison between adults and children. Medicine (Baltimore) 1997;76:295–303. doi: 10.1097/00005792-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Knoll MD, Moisi JC, Muhib FB, et al. Standardizing surveillance of pneumococcal disease. Clin Infect Dis. 2009;48(Suppl 2):S37–48. doi: 10.1086/596480. [DOI] [PubMed] [Google Scholar]
- 33.Howidi M, Muhsin H, Rajah J. The burden of pneumococcal disease in children less than 5 years of age in Abu Dhabi, United Arab Emirates. Ann Saudi Med. 2011;31:356–359. doi: 10.4103/0256-4947.83214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudan I, O'Brien KL, Nair H, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 2013;3:010401. doi: 10.7189/jogh.03.010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Assefa A, Gelaw B, Shiferaw Y, Tigabu Z. Nasopharyngeal carriage and antimicrobial susceptibility pattern of Streptococcus pneumoniae among pediatric outpatients at Gondar University Hospital North West Ethiopia. Pediatr Neonatol. 2013;54:315–321. doi: 10.1016/j.pedneo.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Huebner RE, Wasas AD, Klugman KP, Paediatric Study Group Prevalence of nasopharyngeal antibiotic-resistant pneumococcal carriage in children attending private paediatric practices in Johannesburg. S Afr Med J. 2000;90:1116–1121. [PubMed] [Google Scholar]
- 37.Denno DM, Frimpong E, Gregory M, Steele RW. Nasopharyngeal carriage and susceptibility patterns of Streptococcus pneumoniae in Kumasi Ghana. West Afr J Med. 2002;21:233–236. [PubMed] [Google Scholar]
- 38.Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control. 2017;6:47. doi: 10.1186/s13756-017-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hay SI, Rao PC, Dolecek C, et al. Measuring and mapping the global burden of antimicrobial resistance. BMC Med. 2018;16:78. doi: 10.1186/s12916-018-1073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Available at: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1. Accessed October 19, 2020.
- 41.World Health Organization. Pneumococcus: vaccine-preventable diseases surveillance standards. Available at: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/WHO_SurveillanceVaccinePreventable_17_Pneumococcus_R2.pdf?ua=1. Accessed September 15, 2020.
- 42.Al-Sheikh YA, Gowda LK, Mohammed Ali MM, et al. Distribution of serotypes and antibiotic susceptibility patterns among invasive pneumococcal diseases in Saudi Arabia. Ann Lab Med. 2014;34:210–215. doi: 10.3343/alm.2014.34.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johns Hopkins Bloomberg School of Public Health International Vaccine Access Center (IVAC). VIEW-hub report: global vaccine introduction and implementation. Available at: https://view-hub.org/sites/default/files/2020-05/VIEW-hub_Report_Mar2020.pdf. Accessed October 19, 2020.
- 44.Sibak M, Moussa I, El-Tantawy N, et al. Cost-effectiveness analysis of the introduction of the pneumococcal conjugate vaccine (PCV-13) in the Egyptian national immunization program, 2013. Vaccine. 2015;33(suppl 1):A182–A191. doi: 10.1016/j.vaccine.2014.12.044. [DOI] [PubMed] [Google Scholar]
- 45.Pneumovax® 23 (pneumococcal vaccine polyvalent). Full Prescribing Information, Merck & Co., Inc., Whitehouse Station, NJ, 2015.
- 46.Murdoch DR, O'Brien KL, Driscoll AJ, et al. Laboratory methods for determining pneumonia etiology in children. Clin Infect Dis. 2012;54(Suppl 2):S146–S152. doi: 10.1093/cid/cir1073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.