Abstract
The negative regulation of T- or B-cell antigen receptor signaling by CD5 was proposed based on studies of thymocytes and peritoneal B-1a cells from CD5-deficient mice. Here, we show that CD5 is constitutively associated with phosphotyrosine phosphatase activity in Jurkat T cells. CD5 was found associated with the Src homology 2 (SH2) domain containing hematopoietic phosphotyrosine phosphatase SHP-1 in both Jurkat cells and normal phytohemagglutinin-expanded T lymphoblasts. This interaction was increased upon T-cell receptor (TCR)-CD3 cell stimulation. CD5 co-cross-linking with the TCR-CD3 complex down-regulated the TCR-CD3-increased Ca2+ mobilization in Jurkat cells. In addition, stimulation of Jurkat cells or normal phytohemagglutinin-expanded T lymphoblasts through TCR-CD3 induced rapid tyrosine phosphorylation of several protein substrates, which was substantially diminished after CD5 cross-linking. The CD5-regulated substrates included CD3ζ, ZAP-70, Syk, and phospholipase Cγl but not the Src family tyrosine kinase p56lck. By mutation of all four CD5 intracellular tyrosine residues to phenylalanine, we found the membrane-proximal tyrosine at position 378, which is located in an immunoreceptor tyrosine-based inhibitory (ITIM)-like motif, crucial for SHP-1 association. The F378 point mutation ablated both SHP-1 binding and the down-regulating activity of CD5 during TCR-CD3 stimulation. These results suggest a critical role of the CD5 ITIM-like motif, which by binding to SHP-1 mediates the down-regulatory activity of this receptor.
CD5 is a 67-kDa cell surface glycoprotein expressed on thymocytes, mature peripheral T cells, and a subpopulation of peritoneal B cells (B-1a cells) which are increased in some autoimmune diseases and are associated with the production of autoantibodies (7). Molecular cloning of mouse and human CD5 (mCD5 and hCD5) (16, 17) revealed that it belongs to the scavenger receptor cysteine-rich (SRCR) family group B, which comprises a group of leukocyte membrane or soluble proteins with one or more domains homologous to the amino-terminal domain of type I macrophage SRCR domain (21). Thus far, 10 members of this group of proteins have been identified: CD5, CD6, WC1, M130, Spα, Pema-SREG, Ebnerin, CPR-ductin, hensin, and gallbladder mucin (2).
Biochemical studies suggest that CD5 is associated with CD3ζ in the T-cell receptor (TCR)-CD3 complex and with the B-cell receptor (BCR) complex (6, 24, 32). Two different ligands for CD5 have been reported: CD72, a 42-kDa type II constitutively expressed glycoprotein on B cells (28, 51); and CD5L, an activation antigen expressed on splenocytes (3). The physiologic roles of CD5-CD72 and CD5-CD5L interactions are not known but would be consistent with a potential T-cell–B-cell cooperation during antibody-mediated immune responses (8).
Early in vitro studies of T lymphocytes and thymocytes demonstrated that monoclonal antibodies (MAbs) to CD5 were costimulatory for T-cell proliferation (9, 18, 42). However, in vivo studies showed that CD5 down-modulation by specific MAbs induced T-cell unresponsiveness and prevented experimental autoimmune encephalomyelitis in rats (44), and the MAbs were efficacious in the treatment of collagen type II-induced arthritis in DBA/1 mice (36). In addition, studies of CD5-deficient mice revealed that CD5−/− thymocytes are hyperresponsive to TCR-CD3 stimulation, showing enhanced proliferation, increased cytoplasmic free Ca2+ concentration, and enhanced phospholipase Cγ1 (PLCγ1), CD3ζ, pp36 (LAT) and Vav tyrosine phosphorylation following ligation of the TCR-CD3 complex (45). In comparison to normal B-1a cells, immunoglobulin M (IgM) cross-linking on CD5-deficient B-1a cells induces increased Ca2+ mobilization, proliferation, and resistance to apoptosis of cells entering the cell cycle (4). Taken together, these studies support the idea that under certain circumstances, CD5 acts as a negative regulator of cellular activation.
Structurally, CD5 contains three extracellular SRCR domains followed by a hydrophobic transmembrane region and a large cytoplasmic domain. The CD5 cytoplasmic domain has four tyrosine residues (at positions 378, 429, 441, and 463) and several putative sites for serine/threonine phosphorylation (6, 13). Tyrosines 429 and 441 are embedded in an imperfect immunoreceptor tyrosine-based activating motif (ITAM). Upon tyrosine phosphorylation, this ITAM forms a docking site for the Src homology 2 (SH2) domain containing protein kinases p56lck and p59fyn and for phosphatidylinositol 3-kinase (PI3-K) (6, 14, 37). However, tyrosine 378 is contained within an immunoreceptor tyrosine-based inhibitory motif (ITIM)-like sequence (50), which suggests that CD5 may also interact with intermediate proteins involved in down-regulatory function.
SH2-containing tyrosine phosphatase 1 (SHP-1 or protein phosphotyrosine phosphatase 1C [PTP-1C]), a down-regulating intracellular PTP expressed by hematopoietic cells, has been shown to interact with ITIMs via SH2 domains and plays a critical role in regulating differentiation and function by modulating a multiplicity of intracellular signaling pathways (10–12, 15, 20, 27, 29, 34, 35, 46, 52). Mutations within the SHP-1 gene induce the phenotypes observed in motheaten (me) and viable motheaten (mev) mice (41, 47). Mice homozygous for these mutations develop severe defects in hematopoiesis, thymocyte hyperresponsiveness, abnormal expansion of the B-1 subset of B cells, elevated levels of serum IgM, and a lower threshold for membrane immunoglobulin (mIg) signaling (22, 27, 41). Biochemical data have shown that SHP-1 associates with several membrane receptors, regulating their function, and several substrates which are potentially tyrosine dephosphorylated by SHP-1, including CD3ζ, CD19, CD22, PIR B/p91A, BIT, PLCγ1, and the intracellular protein tyrosine kinases p56lck, p59fyn, CD3ζ-associated protein 70 (ZAP-70), and Syk (27, 30).
We report here that CD5 is associated with tyrosine phosphatase activity that is at least partially attributed to its interaction with SHP-1. SHP-1 is constitutively associated with the cytoplasmic domain of CD5 in Jurkat cells or normal phytohemagglutinin (PHA)-expanded T lymphoblasts (PHA T lymphoblasts), and the level of association is increased upon anti-TCR-CD3 stimulation. The sequence involved in SHP-1 association was mapped to tyrosine 378 in an ITIM-like sequence in the cytoplasmic domain of CD5. We also demonstrate negative regulation by CD5 of TCR-CD3-increased Ca2+ mobilization, most likely by affecting the tyrosine phosphorylation level of several protein substrates in the TCR-CD3 signal transduction pathway.
MATERIALS AND METHODS
Antibodies and FACS analysis.
Anti-human CD3 MAb G19-4, anti-human CD5 MAb 10.2, and anti-human CD6 MAb G3-6 were previously described (25, 26). The rat anti-mouse CD6 MAbs, M6-1A.1 and M6-3E.124, and the rat anti-mouse CD40 MAb, 40-4.8E1, were previously described (43). Secondary antibodies (fluorescein isothiocyanate [FITC]-conjugated anti-rat IgG and FITC-conjugated anti-mouse IgG) were purchased from Biosource International (Camarillo, Calif.). Fluorescence-activated cell sorting (FACS) analysis was performed with a FACScan (Becton Dickinson, Los Angeles, Calif.). Biotinylation of purified MAbs was performed with sulfosuccinimidobiotin (Pierce, Rockford, Ill.) as specified by the manufacturer. Rabbit antisera to human CD3ζ, ZAP-70, Syk, and p56lck were kindly provided by J. Fargnoli (Bristol-Myers Squibb, Princeton, N.J.). The rabbit antiserum to human PLCγ1 was previously described (19).
Cell lines and cell culture.
The wild-type lymphoma T-cell line Jurkat, used for stable transfection and the generation of clones, was cultured in RPMI 1640 containing 10% heat-inactivated fetal calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). Jurkat cells stably transfected with mCD6-hCD5 constructs were cultured in the same medium supplemented with G418 (Genetran; 1 mg/ml; G418 Gibco BRL, Gaithersburg, Md.). The CD3-Jurkat cell clone 4.2, kindly provided by Miguel Lopez-Botet (Hospital de La Princesa, Madrid, Spain), is described elsewhere (38). For generation of PHA T lymphoblasts, human peripheral blood cells were obtained from healthy volunteers, and mononuclear cell suspensions were prepared by Ficoll-Hypaque density gradient centrifugation. T lymphocytes were isolated by 2-aminoethylisothiouronium bromide-treated sheep erythrocyte rosetting. The sheep erythrocytes were lysed according to standard procedures. The remaining cell preparations contained more than 98% T lymphocytes as assessed by flow cytometric analysis after staining with an anti-CD3 MAb (Becton Dickinson, Mountain View, Calif.). After isolations, T lymphocytes were cultured in RPMI 1640 (Gibco) containing 10% fetal calf serum and 1 μg of PHA per ml for 7 days.
Phosphatase activity assay.
For analysis of phosphatase activity, immunoprecipitates were prepared as follows. Jurkat cells (8 × 107), unstimulated or stimulated with biotinylated anti-CD3 MAb G19-4 at 10 μg/ml plus avidin at 50 μg/ml for 5 min at 37°C, were lysed into 600 μl of lysis buffer (50 mM Tris HCl, 150 mM NaCl, 1% Nonidet P-40 [NP-40] [pH 7.5]) plus Complete protease inhibitor mixture (Boehringer Mannheim, Indianapolis, Ind.). Nuclei and unlysed cells were removed by centrifugation at 4°C for 10 min at 14,000 rpm. Lysates were precleared by incubation for 1 h at 4°C with 100 μl of mouse IgG coupled to CNBr-activated Sepharose beads (Pharmacia, Uppsala, Sweden) and subjected to immunoprecipitation with an MAb to hCD5 (10.2) or mCD6 (M6-3E.124) or with mouse IgG coupled to CNBr-activated Sepharose beads. Immunoprecipitates were incubated at 37°C for 90 min with 1 mM phosphopeptide RRLIEDAEY-pAARG (Upstate Biotechnology, Lake Placid, N.Y.) in 20 mM Tris HCl–150 mM NaCl (pH 7.5) phosphatase buffer. Free phosphate detection was carried out as specified by the manufacturer.
Chimeric m CD6-CD5 construct generation.
The chimeric CD6 constructs, consisting of the extracellular portion of mCD6 and different cytoplasmic portions, or tyrosine-to-phenylalanine point mutations derived from hCD5, were generated by PCR, using an EcoRI restriction site located in the transmembrane coding region of mCD6 and creating an in-frame EcoRI site in CD5 by PCR. The chimeric gene was constructed and cloned into the expression vector pcDNA3 (Invitrogen, San Diego, Calif.) as described previously (5). The plasmid construct encoding the entire cytoplasmic domain of hCD5 served as a template for generation of the truncated and point mutant forms of hCD5. Plasmids encoding the cytoplasmic truncated forms of hCD5 were produced by ligating the PCR products between the EcoRI and NotI sites of plasmid pcDNA3. As a forward primer for cloning of the truncated cytoplasmic regions, the following oligonucleotide, which included an EcoRI site (in boldface), was used: GTG GCA AGC ATC ATC CTG GGA ATT CTG CTG GTG GTG CTG. The reverse oligonucleotides for cloning of the truncated chimeras encoding a NotI restriction site (in boldface) and a stop codon (underlined; truncation is denoted by * in designations) to terminate translation were CD5-428* (GCG GCC CTA TTC GTT ATC CAC GTC GGA GGC) and CD5-462* (GCG GCC CTA GTC ACT GTC GGA GGA GTT GTC). Numbering of the CD5 truncated forms refers to the last amino acid encoded by the construct and is based on the amino acid numbering for full-length CD5 (hCD5-fl): Met 1 to Leu 471. The sequence of the constructs was confirmed by DNA sequencing.
Construction of the tyrosine mutants of CD5.
The desired mutations of the four tyrosine residues at positions 378, 429, 441, and 463 were introduced by overlapping extension PCR using the pcDNA3-hCD5-fl plasmid DNA as the template. The 5′ oligonucleotide contained the same EcoRI restriction site as described above, and the 3′ oligonucleotide contained a NotI restriction site. The resulting mutated PCR products were cut with the restriction enzymes EcoRI and NotI and reinserted into pcDNA3 vector cut with the same restriction enzymes. Each mutation was verified by DNA sequencing.
Stable expression of chimeric CD5 proteins in Jurkat cells.
Jurkat cells were transfected with recombinant plasmids described above to generate stable expressing cell lines. Transfection of plasmids was performed with the lipid transfectant DMRIE-C (Gibco BRL). G418 (Gibco BRL) at a concentration of 1 mg/ml served as the selection agent for transfected cells. Three to four weeks after transfection, colonies at the bottom of the plate were expanded, tested for surface expression of mCD6 by FACS analysis, and cloned by limiting dilution.
Cell stimulation, immunoprecipitation, and immunoblotting analysis.
Wild-type or stably transfected Jurkat cells expressing the different chimeric mCD6-hCD5 proteins were washed and incubated at 4°C. For TCR-CD3 stimulation, biotinylated anti-CD3 MAb G19-4 was added to 10 μg/ml for 2 min at 4°C, and cells were washed to remove unbound MAb and incubated with avidin at 50 μg/ml at 37°C for various times. For pervanadate stimulation, cells were incubated with 0.03% H2O2 and 100 μM orthovanadate (pervanadate) for 5 min at 37°C. For CD5 regulation of the TCR-CD3 signal transduction pathway, Jurkat cells were preincubated with 10 μg of biotinylated anti-CD3 MAb G19-4 per ml in combination with biotinylated anti-hCD5 MAb 10.2 or anti-mCD6 MAb M6-1A.1 for 2 min at 4°C. After unbound MAbs were washed away, the cells were incubated with 50 μg of avidin per ml for various times at 37°C. After stimulation, cells were lysed in 1 ml of lysis buffer containing (50 mM Tris [pH 7.5], 1% NP-40, 150 mM NaCl, 2 mM EGTA, 1 mM sodium orthovanadate) plus Complete protease inhibitor mixture (Boehringer Mannheim). Samples were centrifuged at 14,000 rpm for 2 min (to remove nuclei); lysates were precleared twice with 50 μl of rat or mouse Ig-coupled CNBr-activated Sepharose beads (Pharmacia) or protein A-Sepharose (Pharmacia) for 60 min at 4°C and subjected to immunoprecipitation with antibody to mCD6 (M6-3E.124) covalently coupled to CNBr-activated Sepharose or with immune rabbit antisera to specific proteins. The immunoprecipitates were analyzed under reducing conditions, except for p56lck (nonreducing conditions), by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on gradient 4 to 20% gels and subsequently transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Marlborough, Mass.). For mCD6 detection, membranes were blocked in 5% bovine serum albumin and treated with biotinylated anti-mCD6 MAb M6-1A.1 as the primary reagent and horseradish peroxidase (HRP)-streptavidin (Vector Laboratories, Burlingame, Calif.) as the secondary reagent. For phosphotyrosine analysis, blots were treated with biotinylated antiphosphotyrosine antibody 4G10 (Upstate Biotechnology) at 0.5 μg/ml plus HRP-streptavidin. For SHP-1 blots, membranes were incubated with a 1/500 final dilution of anti-SHP-1 MAb (Transduction Laboratories, Lexington, Ky.) or anti-SHP-1 rabbit antiserum (10 μg/ml; Upstate Biotechnology). For CD3ζ, ZAP-70, Syk, PLCγ1, and p56lck detection, blots were incubated with a 1/500 final dilution of antisera specific to these proteins, followed by incubation with anti-rabbit-HRP antiserum. The binding of HRP was detected by enhanced chemiluminescence (Amersham, Buckinghamshire, England) and exposure to film. Blots were stripped by incubating membranes in 62.5 mM Tris HCl (pH 6.8)–2% SDS–50 mM β-mercaptoethanol at room temperature for 60 min.
Measurement of cytosolic calcium in Jurkat cells.
Jurkat cells were loaded with Indo 1 (Sigma Chemical Co., St. Louis, Mo.) at 1 μg/ml in 2 ml of RPMI 1640 plus 10% fetal bovine serum for 45 min at 37°C. Cells were incubated at 37°C with 0.3 μg of anti-CD3 MAb G19-4 per ml alone or together with 2 μg of anti-CD5 MAb 10.2 or anti-mCD6 MAb M6-3E.124 per ml. Where indicated, rabbit anti-rat F(ab’)2 (10 μg/ml) was added as cross-linker. Cells were analyzed on a flow cytofluorimeter (EPICS; Becton Dickinson) to detect calcium mobilization. Only live (based on forward scatter criteria) and Indo 1-loaded cells (based on 405 nM versus 525 nM emission spectra) were included in the analysis. Intracellular calcium concentrations ([Ca2+]i) were calculated as described elsewhere (19, 25).
RESULTS
CD5-associated tyrosine phosphatase activity in Jurkat cells: interaction with SHP-1.
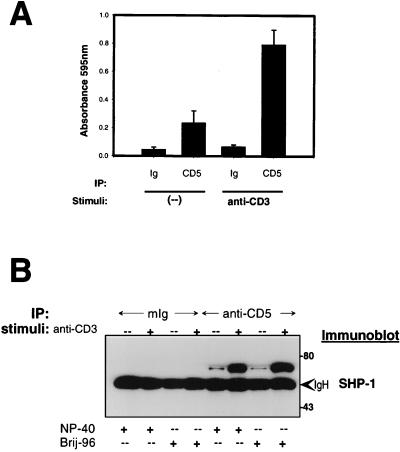
Initial observations in CD5-deficient mice, suggesting that CD5 acts as a negative regulator of B-1a cells or thymocytes (4, 45), raised the possibility that in human peripheral T cells, CD5 could be physically and/or functionally associated with intermediate proteins involved in down-regulating intracellular signaling pathways. Previous observations suggested that CD5 associated with SHP-1 in thymocytes (34). We therefore analyzed the phosphatase activity of CD5 immunoprecipitates from the human T-cell lymphoma cell line Jurkat. As shown in Fig. 1A, hCD5 immunoprecipitates displayed a moderate capacity to dephosphorylate the phosphopeptide RRLIEDAEY-pAARG, an activity which substantially increased upon anti-TCR-CD3 stimulation. As a control, mouse IgG immunoprecipitates did not show this activity. Since the CD5 cytoplasmic domain possesses no intrinsic phosphatase activity, this finding suggested the coprecipitation of a catalytically active tyrosine phosphatase with hCD5 in Jurkat cells.
FIG. 1.
Associated phosphatase activity in hCD5 immunoprecipitates: interaction with SHP-1. (A) Unstimulated or TCR-CD3-stimulated Jurkat cells (5 × 107) were lysed in 1% NP-40-containing lysis buffer and immunoprecipitated (IP) with mIgG or anti-hCD5 MAb 10.2 coupled to CNBr-activated Sepharose beads. Precipitated proteins were incubated for 90 min at 37°C with 1 mM phosphopeptide RRLIEDAEY-pAARG in phosphatase buffer. Data represent means ± standard errors of triplicate cultures from two independent experiments. (B) Jurkat cells (5 × 107) were unstimulated or stimulated with TCR-CD3 (5 min), lysed in 1% NP-40- or Brij 96-containing lysis buffer, and subjected to immunoprecipitation with mIg or anti-hCD5 MAb 10.2 coupled to CNBr-activated Sepharose beads; precipitated proteins were analyzed by SDS-PAGE and immunoblotted with anti-human SHP-1 MAb. Numbers at the right represent molecular masses of proteins in kilodaltons.
To elucidate the molecular basis of this tyrosine phosphatase activity in hCD5 immunoprecipitates, we analyzed the physical association of several intracellular signal transduction proteins. Immunoblotting analysis of hCD5 immunoprecipitates from wild-type Jurkat cell lysates revealed its constitutive association, in both Brij 96- and NP-40-containing lysis buffers, with the hematopoietic SH2 domain-containing protein tyrosine phosphatase SHP-1/PTP-1C. This association was increased upon TCR-CD3 cell stimulation (Fig. 1B). We also detected constitutive association between CD5 and SHP-1 in normal human PHA T lymphoblasts (data not shown).
Since it has been shown that SHP-2/PTP-1D binds sequences similar to those recognized by SHP-1 (30, 50), we analyzed whether SHP-2 also associates with CD5. We found no association between CD5 and SHP-2 in either resting or TCR-CD3-stimulated Jurkat cells (data not shown).
CD5 has been reported to be physically associated with the TCR-CD3 complex through the CD3ζ chain on T cells (6, 32). To determine if SHP-1 indirectly associates with CD5 through this complex, we analyzed a CD3-negative Jurkat cell line variant and still detected SHP-1–CD5 association (data not shown).
Generation of Jurkat cell lines stably expressing mCD6-hCD5 mutants, tyrosine phosphorylation pattern, and role of tyrosine 378 in SHP-1 association.
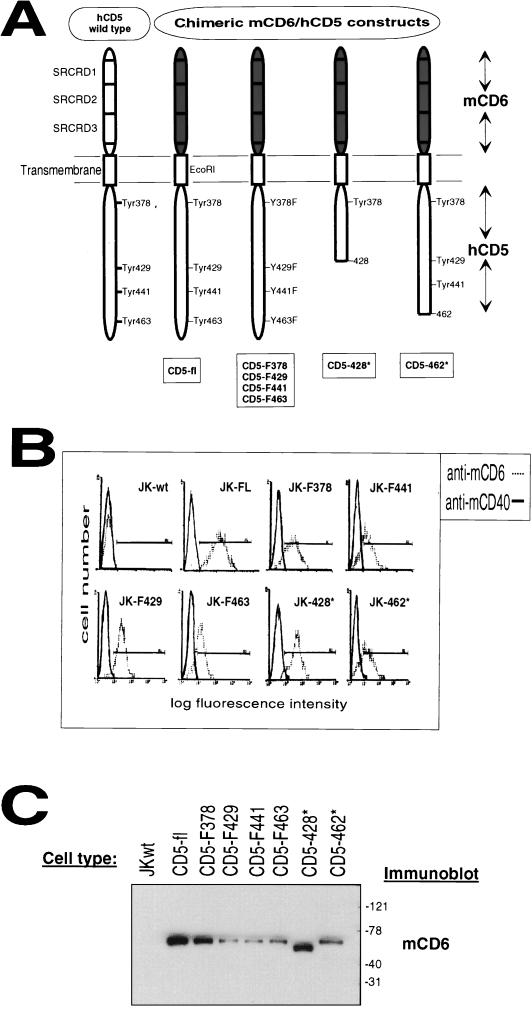
The finding that SHP-1 association with hCD5 was increased upon TCR-CD3 ligation or pervanadate treatment (data not shown) suggested that one or more tyrosine residues in the cytoplasmic domain of hCD5 may be involved in this association. hCD5 has a relatively large cytoplasmic domain (94 residues) with four tyrosines located at positions 378, 429, 441, and 463 and several consensus sites for serine/threonine phosphorylation (17). We generated a panel of different chimeric constructs consisting of the extracellular portion of mCD6 fused to full-length, tyrosine-to-phenylalanine point-mutated or truncated forms of the cytoplasmic domain of hCD5 (Fig. 2A). These chimeric constructs were stably expressed in wild-type Jurkat cells. The surface expression levels were measured by FACS analysis using an anti-mCD6 MAb (Fig. 2B). As expected, only stably transfected cell lines expressing the chimeric mCD6-hCD5 proteins were stained by the anti-mCD6 MAb. All clones expressed similar levels of endogenous hCD5 (not shown). The biochemical characterization of different chimeric mCD6-hCD5 molecules expressed by stably transfected Jurkat cell clones and subsequent Western blot analysis for mCD6 are shown (Fig. 2C). The relative molecular masses (∼70 kDa) of the different chimeric tyrosine-to-phenylalanine point mutants (CD5-F378, CD5-F429, CD5-F441, and CD5-F463) corresponded to the chimeric mCD6–hCD5-fl polypeptide which contained the complete cytoplasmic portion of hCD5. The relative molecular masses of truncated forms CD5-428* and CD5-462* were 65 and 69 kDa, corresponding to the deletion of the last 43 and 9 amino acids, respectively, of the full-length cytoplasmic domain.
FIG. 2.
Schematic representation, FACS analysis, and biochemical characterization of the chimeric mCD6-hCD5 constructs. (A) As a reference, wild-type hCD5 is shown on the left. Shown on the right side are mCD6-hCD5 chimeric constructs, consisting of the extracellular domain of mCD6 and intracellular domain of hCD5 (CD5-fl), tyrosine-to-phenylalanine point mutants (CD5-F378, CD5-F429, CD5-F441, and CD5-F463), and truncated forms (CD5-428* and CD5-462*; numbered according to the last amino acid encoded in the construct with respect to hCD5). (B) Wild-type Jurkat (JK-wt) cells or stably transfected Jurkat cell clones (CD5-fl, CD5-F378, CD5-F429, CD5-F441, CD5-F463, CD5-428*, and CD5-462*) were stained with the isotype-matched control rat anti-mCD40 MAb 40 4.8E1 (solid line) or rat anti-mCD6 MAb M6-3E.124 (dotted line) plus goat anti-rat-FITC and analyzed by FACScan. (C) The stably mCD6-hCD5 transfected or wild-type Jurkat (JKwt) cell clones were lysed in 1% NP-40-containing lysis buffer, immunoprecipitated with anti-mCD6 MAb M6-3E.124, and subsequently analyzed by SDS-PAGE on 14% gels. Immunoblotting detection was performed with anti-mCD6 MAb M6-1A.1. Numbers on the right represent molecular masses of proteins in kilodaltons.
The hCD5 cytoplasmic domain possesses no obvious intrinsic enzymatic activity; however, several studies have shown that hCD5 is phosphorylated on tyrosine, serine, and threonine residues after TCR-CD3 stimulation (1, 6, 13). Jurkat cell clones expressing the different chimeric mCD6-hCD5 proteins were analyzed for tyrosine phosphorylation pattern in resting cells and upon TCR-CD3 stimulation. Low-level tyrosine phosphorylation in chimeric mCD6-hCD5 proteins was constitutive; however, all mutants became strongly tyrosine phosphorylated after TCR-CD3 ligation (Fig. 3A, upper panels), suggesting that more than one tyrosine residue was phosphorylated. We observed the same result upon pervanadate cell stimulation (not shown). It is important to note that the CD5-428* mutant, which possesses only tyrosine 378, was constitutively tyrosine phosphorylated and became significantly more phosphorylated after TCR-CD3 stimulation. This result supports the idea that the ITIM-like motif, in which tyrosine 378 is embedded, could be functional and associates with intracellular signaling proteins.
FIG. 3.
Tyrosine phosphorylation pattern of chimeric mCD6-hCD5 proteins expressed in Jurkat cells: SHP-1 coprecipitation with different chimeric mCD6-hCD5 proteins. (A) Wild-type (JKwt) or stably mCD6-hCD5 transfected Jurkat cells, untreated or TCR-CD3 stimulated (2 × 107/lane), were lysed in 1% NP-40-containing lysis buffer, subjected to immunoprecipitation (IP) with anti-mCD6 MAb M6-3E.124, and analyzed for phosphotyrosine (pTyr) content by immunoblotting (upper panels). The middle panels represent the upper panels stripped and reprobed with the mouse anti-human SHP-1 MAb; the lower panels represent the middle panels stripped and reprobed with the anti-mCD6 MAb M6-1A.1. Numbers at the right represent molecular masses of proteins in kilodaltons. (B) Pervanadate-stimulated Jurkat cells, wild type or stably transfected with chimeric mCD6-hCD5 constructs (5 × 107/lane), were lysed in 1% Brij 96-containing lysis buffer, immunoprecipitated with rabbit anti-human SHP-1 antiserum, and then immunoblotted with anti-mCD6 (upper panel); the blot was then stripped and subjected to SHP-1 immunoblot detection (lower panel). Numbers on the right represent molecular masses of proteins in kilodaltons. (C) TCR-CD3-stimulated stably transfected JK-CD5fl or JK-F378 cell clones (5 × 107) were lysed in 1% NP-40-containing lysis buffer and immunoprecipitated with mIgG, anti-hCD5 MAb 10.2, or anti-mCD6 MAb M6-3E.124 coupled to CNBr-activated Sepharose beads. Precipitated proteins were incubated for 90 min at 37°C with 1 mM tyrosine phosphopeptide RRLIEDAEY-pAARG in phosphatase buffer. Data represent means ± standard errors of triplicate cultures from two independent experiments.
To determine the intracellular sequence in hCD5 involved in SHP-1 association, we used the same Jurkat cell panel expressing the different chimeric mCD6-hCD5 mutants. Since TCR-CD3 or pervanadate treatment increased SHP-1 association with CD5, we also used these treatments to analyze the target sequence in CD5 involved in SHP-1 association. Chimeric mCD6-hCD5 receptors were immunoprecipitated from resting or TCR-CD3-stimulated cells, and subsequent immunoblotting with anti-SHP-1 MAb showed that all chimeric mCD6-hCD5 mutants except CD5-F378 associated with SHP-1 (Fig. 3A, middle panels). Similar levels of chimeric mCD6-hCD5 were immunoprecipitated from each of the clones, as shown by reprobing of the membrane with anti-mCD6 MAb (Fig. 3A, lower panels). Reciprocally, SHP-1 immunoprecipitation from pervanadate-activated Jurkat clones and subsequent mCD6 immunoblotting revealed that SHP-1 associated with all mCD6-hCD5 chimeras except CD5-F378 (Fig. 3B, upper panel), again demonstrating that tyrosine 378 is critical for SHP-1 association. To demonstrate similar levels of SHP-1 immunoprecipitation, membranes were reprobed with anti-SHP-1 antiserum (Fig. 3B, lower panel). We therefore analyzed in CD5-fl and CD5-F378 Jurkat cell clones the tyrosine phosphatase activity associated with mCD6-hCD5 chimeric proteins after TCR-CD3 cell stimulation. As expected, mCD6–hCD5-fl but not mCD6–hCD5-F378 showed tyrosine phosphatase activity when assayed with phosphopeptides as the substrates (Fig. 3C). As a control, endogenous CD5 was immunoprecipitated from both stable transfectants showing comparable tyrosine phosphatase activity (Fig. 3C).
CD5 costimulation down-modulates the TCR-CD3 signal transduction pathway.
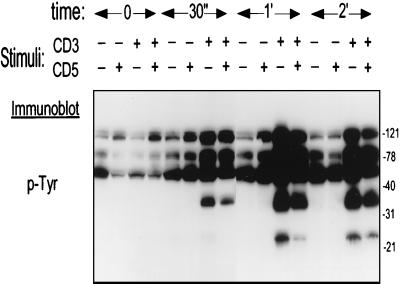
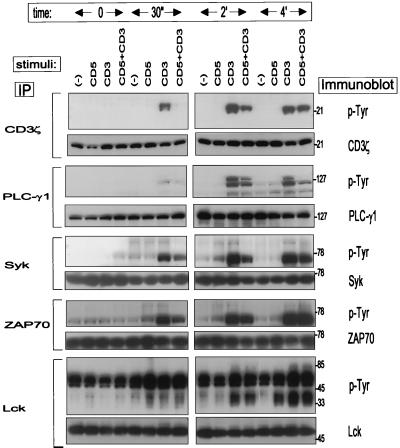
To elucidate the basis for CD5 negative regulation of the TCR-CD3-initiated T-cell activation, Jurkat cells were stimulated with biotinylated anti-CD3 MAb alone or in combination with biotinylated anti-CD5 MAb and subsequently cross-linked with avidin for different times at 37°C. As demonstrated by antiphosphotyrosine immunoblotting analysis, anti-CD3 MAb treatment resulted in the rapid (starting earlier than 30 s) tyrosine phosphorylation of several substrates (Fig. 4). However, CD3-CD5 co-cross-linking resulted in decreased levels of tyrosine phosphorylation of several proteins at relative molecular masses of ∼23, 36, and 120 kDa. Soluble anti-CD5 MAbs without co-cross-linking did not induce this effect (data not shown). To identify some of these substrates under the same conditions, we immunoprecipitated different proteins by specific antisera (CD3ζ, ZAP-70, Syk, PLCγ1, and p56lck) and analyzed their tyrosine phosphorylation pattern. As shown in Fig. 5, CD3ζ, ZAP-70, Syk, and PLCγ1 were phosphorylated following TCR-CD3 stimulation, but upon CD5 coligation the tyrosine phosphorylation levels of these proteins were substantially diminished. We analyzed the tyrosine phosphorylation pattern of the Src family tyrosine kinase p56lck under the same conditions but found no significant differences between TCR-CD3 and CD3-CD5 co-cross-linking (Fig. 5). Since p56lck mediates its own tyrosine phosphorylation upon activation, tyrosine phosphorylation levels in p56lck may not represent a measurement of activity regulation. In addition, Lorenz et al. (27) reported that CD4-associated p56lck was selectively enhanced in thymocytes from SHP-1-deficient (me/me) mice. We therefore studied the p56lck kinase activity after CD3 or CD3-CD5 costimulation in both the p56lck intracellular pool and CD4-associated p56lck. We found no differences in p56lck autophosphorylation status after CD3 ligation or CD3-CD5 costimulation (data not shown). Further, levels of tyrosine phosphorylation of recombinant CD3ζ in the same kinase reactions with p56lck immunoprecipitates were similar after TCR-CD3 or TCR-CD3-plus-CD5 stimulation (data not shown).
FIG. 4.
Regulation of tyrosine phosphorylation pattern by CD5. Jurkat cells (2 × 107/lane) were incubated for 0, 0.5, 1, or 2 min at 37°C with the indicated cross-linked MAbs at 10 μg/ml and lysed in 500 μl of 1% NP-40 lysis buffer. Tyrosine phosphorylation (p-Tyr) pattern of equivalent amounts (10 μl per lane) of whole-cell lysates was analyzed by immunoblotting. Numbers on the right represent molecular masses of proteins in kilodaltons.
FIG. 5.
CD5 costimulation down-modulates the TCR-CD3-induced tyrosine phosphorylation of CD3ζ, ZAP-70, Syk, and PLCγ1 but not p56lck. Jurkat cells (108) were incubated for 0, 0.5, 2, or 4 min at 37°C with indicated cross-linked MAbs at 10 μg/ml and lysed in 1 ml of 1% NP-40 lysis buffer. Proteins resolved from CD3ζ, PLCγ1, Syk, ZAP-70, and p56lck immunoprecipitates (IP) were probed with antiphosphotyrosine (p-Tyr) MAb (top panels) or anti-CD3ζ, -PLCγ1, -Syk, -ZAP-70, or -p56lck immune serum (bottom panels). Results are representative of three different experiments. Numbers on the right represent molecular masses of proteins in kilodaltons.
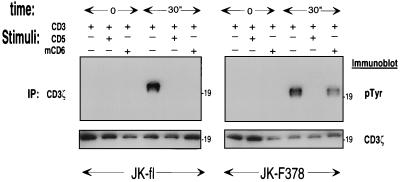
To further investigate the involvement of SHP-1 in hCD5-associated tyrosine dephosphorylation, we studied the ability of the mCD6-hCD5 chimeras in transfected Jurkat cell clones to down-regulate tyrosine phosphorylation. As shown in Fig. 6, in Jurkat CD5-F378 cells, cross-linking of mCD6 to CD3 resulted in no detectable alteration of the TCR-CD3-induced phosphotyrosine content of CD3ζ. However, in Jurkat CD5-fl cells, cross-linking of mCD6 resulted in the down-regulation of the TCR-CD3-induced tyrosine phosphorylation of CD3ζ. As expected, co-cross-linking of endogenous hCD5 down-regulated in both cell types the TCR-CD3-induced tyrosine phosphorylation of CD3ζ. Taken together, these data show that tyrosine 378 in the hCD5 cytoplasmic domain is involved in SHP-1 association, and therefore we propose that SHP-1 is responsible at least in part for the hCD5-induced down-regulation of signals through the TCR-CD3 complex.
FIG. 6.
Tyrosine phosphorylation regulation by chimeric mCD6-hCD5 mutants. CD5-fl or CD5-F378 stably transfected Jurkat cells (3 × 107) were incubated at 37°C with the indicated cross-linked MAbs at 10 μg/ml for 0 or 30 s and lysed in 500 μl of 1% NP-40 buffer. CD3ζ immunoprecipitates (IP) were analyzed for phosphotyrosine (pTyr) content by immunoblotting with antiphosphotyrosine MAb. The lower panels represent the upper panels stripped and reprobed with anti-CD3ζ rabbit antiserum. Numbers on the right represent molecular masses of proteins in kilodaltons.
CD5 down-modulates Ca2+ mobilization triggered via TCR-CD3 in Jurkat cells.
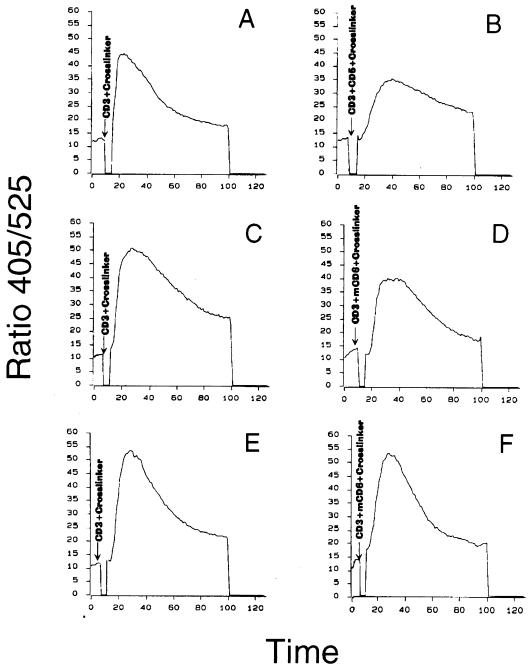
To explore the regulatory role of CD5 in T-cell activation, we tested whether CD5 could inhibit Ca2+ mobilization triggered via TCR-CD3. Co-cross-linking of CD5 together with TCR-CD3 induced a moderate down-modulation of the increased [Ca2+]i triggered through cell stimulation via TCR-CD3 in Jurkat cells (Fig. 7A and B). In addition, CD5 costimulation delayed the kinetics of induced calcium fluxing compared with that increased through TCR-CD3 in Jurkat cells. The change in amplitude was 34%, and the delay to maximal peak response was approximately 2 min. We also analyzed the TCR-CD3-induced Ca2+ mobilization in both CD5-fl and CD5-F378 cells and the regulation through mCD6-hCD5 chimeric proteins. As shown in Fig. 7E and F, in Jurkat CD5-F378 cells, cross-linking of mCD6 to CD3 resulted in no detectable alteration of the TCR-CD3-increased [Ca2+]i. However, in Jurkat CD5-fl cells (Fig. 7C and D), cross-linking of mCD6 resulted in the down-regulation of the TCR-CD3-increased [Ca2+]i by 20%. The difference in the magnitude of down-regulation between the endogenous CD5 (Fig. 7B) and the chimeric CD5-fl (Fig. 7D) may be due to the 10-fold-higher level of endogenous CD5 relative to the chimera (data not shown). These results demonstrate that CD5 down-modulated signaling in T cells; however, the ultimate physiological function regulated by CD5 in this cell type has yet to be defined.
FIG. 7.
Intracellular Ca2+ mobilization induced by anti-TCR-CD3 antibodies is down-regulated by CD5 in Jurkat cells. Indo 1-loaded wild-type Jurkat cells (A and B) or CD5-fl (C and D) or CD5-F378 (E and F) stably transfected cells were incubated with 0.3 μg of anti-CD3 MAb G19-4 per ml alone (A, C, and E) or together with 2 μg of anti-CD5 MAb 10.2 (B) or anti-mCD6 MAb M6-3E.124 (D and F) per ml, followed by surface receptor cross-linking with rabbit anti-rat antiserum (10 μg/ml). In all cases, the baseline signal was recorded for 1 min. On the abscissa, 10 arbitrary units represent 1 min.
DISCUSSION
Lymphocyte function involves stimulation through specific receptors, as well as combined and coordinate action of different coreceptors, protein tyrosine kinases, and PTPs. Early studies indicated that CD5 functions in both mice and humans by delivering costimulatory signals in T cells (9, 18, 42). However, recent observations in thymocytes or B-1a cells from CD5-deficient mice show that CD5 negatively regulates TCR- or BCR-induced activation (4, 45). TCR-CD3-induced activation promotes rapid CD5 tyrosine and serine/threonine phosphorylation (1, 6, 13). The cytoplasmic tail of hCD5 does not possess enzymatic activity but has four tyrosine residues, at positions 378, 429, 441 and 463, which may be involved in the CD5 signal transduction pathway. Tyrosines 429 and 441 are contained in an ITAM-like motif (Yx11YxxL; in single-letter amino acid code, where x represents any amino acid), which after tyrosine phosphorylation forms a docking site for SH2-containing kinases such as p56lck, p59fyn, and PI3-K (6, 14, 37). The tyrosine kinase p56lck appears to be responsible for CD5 phosphorylation. In the present study, we show that hCD5 associates with the SH2-containing tyrosine phosphatase SHP-1 in human T cells, analyzing in detail the hCD5 sequence important for SHP-1 association, and we demonstrate TCR-CD3 signal transduction regulation through hCD5.
Here we report that hCD5 immunoprecipitates from the human T-cell line Jurkat displayed moderate constitutive associated tyrosine phosphatase activity. Since CD5 has been proposed as a down-regulating receptor, this phosphatase activity could contribute to this phenomenon. We carried out different experiments in order to identify putative proteins interacting with the cytoplasmic tail of hCD5. In normal PHA T lymphoblasts and Jurkat T cells, we found constitutive coprecipitation of hCD5 together with the SH2-containing tyrosine phosphatase SHP-1. CD5 may be associated with CD3ζ chain in the CD3 complex (6, 32); thus, we analyzed the hCD5–SHP-1 interaction in a CD3-defective variant Jurkat cell line. We noticed a similar association, suggesting that hCD5–SHP-1 association was not mediated through the TCR-CD3 complex. This interaction was increased upon TCR-CD3 or pervanadate stimulation. Both TCR-CD3 ligation and pervanadate treatment are potent tyrosine phosphorylation stimulators (40); we hypothesized that one or more tyrosine residues in the hCD5 cytoplasmic domain could be involved in this association. To resolve this question, we generated a battery of different chimeric mCD6-hCD5 constructs. Tyrosine phosphorylation analysis upon TCR-CD3 stimulation showed that all mutants, even the CD5-428* truncated form, which contains only tyrosine 378, were tyrosine phosphorylated under these conditions. In addition, this mutant displayed a constitutive tyrosine phosphorylation pattern. This result, together with the fact that tyrosine Y378 is located within the LAY378KKL (in single-letter amino acid code) motif, similar to the consensus ITIM sequence (I/V)xYxxL, suggested the possibility that tyrosine Y378 resulted in a functional ITIM-like sequence. We therefore tested the complete battery of chimeric mCD6-hCD5 proteins by immunoprecipitation with either an anti-mCD6 or anti-SHP-1 MAb. We observed that various chimeras, but not CD5-F378, were found in association with SHP-1, suggesting that tyrosine Y378 plays a critical role in SHP-1 binding. The tyrosine phosphatase activity of mCD6-hCD5 chimeric proteins in CD5-fl and CD5-F378 transfectants supports this result.
It has been reported that SHP-1 is detectable in TCR-CD3 immunoprecipitates from thymocytes; similarly, SHP-1 was coprecipitated with CD3-ɛ from both resting and TCR-CD3-stimulated thymocytes (34). However, the structural basis for the association between SHP-1 and TCR-CD3 remains unknown. Since this association does not appear to be modulated by TCR-CD3 stimulation, it is unlikely to be directly mediated by SH2 domain binding to TCR-CD3 phosphotyrosine residues (34). In contrast to CD3ζ or CD3ɛ, SHP-1 association with CD5 was somewhat stimulated upon thymocyte activation, likely involving CD5 phosphotyrosine residues (34). SHP-1 association with hCD5 and phosphotyrosine immunoblotting from resting and activated Jurkat T cells (data not shown) confirmed that hCD5 is constitutively tyrosine phosphorylated and becomes hyperphosphorylated upon cellular activation. This result is consistent with a constitutive association with SHP-1, which is increased upon activation.
The TCR-CD3-initiated activating signals involve a proximal protein tyrosine kinase cascade, including interactions with Src family (i.e., p56lck and p59fyn) and Syk family (i.e., Syk and ZAP-70) tyrosine kinases. As demonstrated by antiphosphotyrosine immunoblotting analysis, cell stimulation through the TCR-CD3 antigen receptor complex induced rapid tyrosine phosphorylation and subsequent activation of different substrates. However, CD5 costimulation specifically down-modulated the CD3-induced tyrosine phosphorylation of CD3ζ, ZAP-70, Syk, and PLCγ1 but not p56lck. In each case, the inhibition was partial and time dependent, as we detected CD5-induced tyrosine dephosphorylation early after TCR-CD3 stimulation (between 30 s and 4 min). No significant difference in the tyrosine phosphorylation level or kinase activity of the Src family tyrosine kinase p56lck was observed. We did not notice changes in in vitro autophosphorylation of p56lck or CD3ζ-induced tyrosine phosphorylation by p56lck. These results suggest that p56lck activity is not primarily or directly regulated by CD5 costimulation. However, we did observe tyrosine phosphorylation down-regulation in CD3ζ, ZAP-70, Syk, and PLCγ1 upon CD5 costimulation. Since one of the most immediate events upon TCR-CD3 activation is up-regulation of p56lck kinase activity and subsequently CD3ζ tyrosine phosphorylation, then if CD3ζ is a direct target for CD5 costimulation, anything downstream may be affected indirectly. This possibility is consistent with a specific down-regulating role for CD5, rather than a general inhibition of cellular activation, and may explain some of the costimulatory activity attributed to CD5. It seems likely that the intracellular mediator for this down-regulating role of hCD5 is the tyrosine phosphatase SHP-1; this affirmation is supported by data for the CD5-F378 chimeric protein, which is unable to mediate inhibitory function (tyrosine phosphorylation or intracellular Ca2+ fluxing down-regulation) and does not associate with SHP-1.
In earlier studies, CD22 was also proposed to be a positive regulator of signaling (48, 49); however, additional studies found that B cells from CD22-deficient mice display enhanced Ca2+ response to BCR ligation and other characteristics that could be explained by a negative regulatory function of CD22 (31, 33, 39). Following CD22 tyrosine phosphorylation induced by mIg, CD22 down-regulates signaling by recruiting the inhibitory tyrosine phosphatase SHP-1 (11, 15). Other similar models are the regulation of NK cell-mediated killing by SHP-1. The killer inhibitory receptors and CD94/NKG2 receptors inhibit natural killing and antibody-dependent cellular cytotoxicity in NK and T cells (23).
Here, we analyzed how hCD5 down-regulates the TCR-CD3 signal transduction pathway. Thus far, two different hypotheses have prevailed: first, Burgess and colleagues (6) proposed that CD5 tyrosine phosphorylation negatively regulated Src family kinase (p56lck and p59fyn) activity by competing with the ability of the kinases to autophosphorylate; second, TCR-CD3-induced CD5 phosphorylation could recruit SH2-containing phosphatases, resulting in their activation and tyrosine dephosphorylation of different substrates. Our data clearly support this last hypothesis and are in accordance with observations that in CD5-deficient mice, the absence of CD5 rendered thymocytes hyperresponsive to stimulation through TCR-CD3 (45). In CD5-deficient thymocytes, cross-linking of TCR-CD3 receptor results in (i) induction of the hyperphosphorylated pp23 isoform of the CD3ζ chain and (ii) increased tyrosine phosphorylation of PLCγ1 and Vav proteins (45).
In addition, thymocytes or peripheral T cells from SHP-1-deficient mice (me or mev) exhibit increased proliferative responses to TCR-CD3 stimulation compared to normal cells (27, 34). Compared to normal thymocytes, SHP-1-deficient thymocytes showed increased constitutive tyrosine phosphorylation of the TCR-CD3 complex, increased interleukin-2 production upon TCR-CD3 stimulation, enhanced and prolonged TCR-CD3-induced tyrosine phosphorylation of different substrates, and increased activation of the Src family kinases p56lck and p59fyn. In this context, the SHP-1–hCD5 interaction is of particular interest since the SHP-1 pool associated with CD5 might be important in regulating both constitutive and TCR-CD3-induced tyrosine phosphorylation of the TCR-CD3 components and subsequent activation of different kinases leading to changes in the activation or maturation state. Our results strongly implicate SHP-1 tyrosine phosphatase activity, activated through CD5, in down-regulation of the TCR-CD3-induced activation pathway.
ACKNOWLEDGMENTS
This study was supported by the Bristol-Myers Squibb Pharmaceutical Research Institute and grant FP 08986319 from the Spanish Ministry of Education and Culture to J.J.P.-V.
We thank Deryk T. Loo and Robert S. Mittler for helpful discussions and critical review of the manuscript. We also thank Tai-an Lin for helpful advice on the kinase experiments and Hernado de Fex for help with interleukin-2 production experiments.
REFERENCES
- 1.Alberola-Ila J, Places L, Lozano F, Vives J. Association of an activation inducible serine kinase activity with CD5. J Immunol. 1993;151:4423–4430. [PubMed] [Google Scholar]
- 2.Aruffo A, Bowen M A, Patel D D, Haynes B F, Starling G C, Gebe J A, Bajorath J. CD6-ligand interactions: a paradigm for SRCR domain function? Immunol Today. 1997;18:498–504. doi: 10.1016/s0167-5699(97)01130-4. [DOI] [PubMed] [Google Scholar]
- 3.Biaconte L, Bowen M A, Lim A, Aruffo A, Andres G, Stamenkovic I. Identification of a novel inducible cell-surface ligand of CD5 on activated lymphocytes. J Exp Med. 1996;184:811–819. doi: 10.1084/jem.184.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bikah G, Carey J, Ciallella J R, Tarakhovsky A, Bondada S. CD5-mediated negative regulation of antigen receptor-induced growth signal in B-1 cells. Science. 1996;274:1906–1909. doi: 10.1126/science.274.5294.1906. [DOI] [PubMed] [Google Scholar]
- 5.Bowen M A, Whitney G S, Neubauer M, Starling G C, Palmer D, Zhang J, Nowak N J, Shows T B, Aruffo A. Structural and chromosomal location of the human CD6 gene: detection of five human isoforms. J Immunol. 1996;158:1149–1156. [PubMed] [Google Scholar]
- 6.Burgess K E, Yamamoto M, Prasad K V S, Rudd C E. CD5 acts as a tyrosine kinase substrate within a receptor complex comprising T-cell receptor ζ chain/CD3 and protein-tyrosine kinases p56lck and p59fyn. Proc Natl Acad Sci USA. 1992;89:9311–9315. doi: 10.1073/pnas.89.19.9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casali P, Burastero S E, Nakamura M, Inghirami G, Notkins A L. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to the Leu-1+B cell subset. Science. 1987;236:77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- 8.Cerutti A, Trentin L, Zambello R, Sancetta R, Milani A, Tassinari C, Adami F, Agostini C, Semenzato G. The CD5/CD72 receptor system is coexpressed with several functionally relevant counterstructures on human B cells and delivers a critical signaling activity. J Immunol. 1996;157:1854–1862. [PubMed] [Google Scholar]
- 9.Ceuppens J L, Baroja M L. Monoclonal antibodies to the CD5 antigen can provide the necessary second signal for activation of isolated resting T cells by solid-phase bound OKT3. J Immunol. 1986;137:1816–1821. [PubMed] [Google Scholar]
- 10.Cyster J G, Goodenow C C. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity. 1995;2:13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 11.Cornall R J, Cyster J C, Hibbs M L, Dunn A R, Otipoby K L, Clark E A, Goodenow C C. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 12.D’Ambrosio D, Hippen K L, Minskoff S A, Mellman I, Pani G, Siminovitch K A, Cambier J C. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by Fc gammaRIIB1. Science. 1995;268:293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- 13.Davies A A, Ley S C, Crumpton M J. CD5 is phosphorylated on tyrosine after stimulation of the T-cell antigen receptor complex. Proc Natl Acad Sci USA. 1992;89:6368–6372. doi: 10.1073/pnas.89.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennehy K M, Broszeit R, Garnett D, Durrheim G A, Spruyt L L, Beyers A D. Thymocyte activation induces the association of phosphatidylinositol 3-kinase and pp120 with CD5. Eur J Immunol. 1997;27:679–686. doi: 10.1002/eji.1830270316. [DOI] [PubMed] [Google Scholar]
- 15.Doody G M, Justement L B, Delibrias C C, Matthews R J, Lin J, Thomas M L, Fearon D T. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242–244. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 16.Huang H J S, Jones N H, Strominger J L, Herzenberg L A. Molecular cloning of Ly-1, a membrane glycoprotein of mouse T lymphocytes and a subset of B cells: molecular homology to the human counterpart Leu-1/T1 (CD5) Proc Natl Acad Sci USA. 1987;84:204–208. doi: 10.1073/pnas.84.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones N H, Clabby M L, Dialynas D P, Huang H J S, Herzenberg L A, Strominger J L. Isolation of complementary DNA clones encoding the human lymphocyte glycoprotein T1/Leu-1. Nature. 1986;323:346–349. doi: 10.1038/323346a0. [DOI] [PubMed] [Google Scholar]
- 18.June C H, Ravinovitch P S, Ledbetter J A. CD5 antibodies increase intracellular ionized calcium concentration in T cells. J Immunol. 1987;138:2782–2792. [PubMed] [Google Scholar]
- 19.Kanner S B, Damle N K, Blake J, Aruffo A A, Ledbetter J A. CD2/LFA3 ligation induces phospholipase-Cγ1 tyrosine phosphorylation and regulates CD3 signaling. J Immunol. 1992;148:2023–2029. [PubMed] [Google Scholar]
- 20.Klingmuller U, Lorenz U, Cantley L C, Neel B G, Lodish H F. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of Jak2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 21.Kodama T, Freeman L, Rohrer J, Zabrecky J, Matsudaira P, Krieger M. Type I macrophage scavenger receptor contains alpha helical and collagen-like coiled coils. Nature. 1990;343:531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- 22.Kozlowski M, Mlinaric-Rascan I, Feng G S, Shen R, Pawson T, Siminovitch K A. Expression and catalytic activity of the tyrosine phosphatase PTP1C is severely impaired in motheaten and viable motheaten mice. J Exp Med. 1993;178:2157–2163. doi: 10.1084/jem.178.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanier L L. Natural killer receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 24.Lankester A C, van Schijndel G M, Cordell J L, van Noesel C J, van Lier R A. CD5 is associated with the human B cell antigen receptor complex. Eur J Immunol. 1994;24:812–816. doi: 10.1002/eji.1830240406. [DOI] [PubMed] [Google Scholar]
- 25.Ledbetter J A, June C H, Grosmaire L S, Rabinovitch P S. Crosslinking of surface antigens causes mobilization of intracellular ionized calcium in T lymphocytes. Proc Natl Acad Sci USA. 1987;84:1384–1388. doi: 10.1073/pnas.84.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ledbetter J A, June C H, Martin P J, Spooner C E, Hansen J A, Meier K E. Valency of CD3 binding and internalization of the CD3 cell-surface complex control T cell responses to second signals: distinction between effects on protein kinase C, cytoplasmic free calcium, and proliferation. J Immunol. 1986;136:3945–3952. [PubMed] [Google Scholar]
- 27.Lorenz U, Ravichandran K S, Burakoff S F, Neel B J. Lack of SHPTP1 results in src-family kinase hyperactivation and thymocyte hyperresponsiveness. Proc Natl Acad Sci USA. 1996;93:9624–9629. doi: 10.1073/pnas.93.18.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo W, van de Velde H, von Hoegen I, Parnes J R, Thielemans K. Ly-1 (CD5), a membrane glycoprotein of mouse T lymphocytes and a subset of B cells, is a natural ligand of the B cell surface protein Lyb-2 (CD72) J Immunol. 1992;148:1630–1634. [PubMed] [Google Scholar]
- 29.Migone T S, Cacalano N A, Taylor N, Yi T, Waldmann T A, Johnston J A. Recruitment of SH2-containing protein tyrosine phosphatase SHP-1 to the interleukin 2 receptor; loss of SHP-1 expression in human T-lymphotropic virus type I-transformed cells. Proc Natl Acad Sci USA. 1998;95:3845–3850. doi: 10.1073/pnas.95.7.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neel B G. Role of phosphatases in lymphocyte activation. Curr Opin Immunol. 1997;9:405–420. doi: 10.1016/s0952-7915(97)80088-x. [DOI] [PubMed] [Google Scholar]
- 31.O’Keefe T L, Williams G T, Davies S L, Neuberger M S. Hyperresponsive B cells in CD22-deficient mice. Science. 1996;275:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 32.Osman N, Ley S C, Crumpton M J. Evidence for an association between the T cell receptor/CD3 antigen complex and the CD5 antigen in human T lymphocytes. Eur J Immunol. 1992;22:2995–3000. doi: 10.1002/eji.1830221135. [DOI] [PubMed] [Google Scholar]
- 33.Otipoby K L, Andersson K B, Draves K E, Klaus S J, Farr A G, Kenner J D, Perlmutter R M, Law C-L, Clark E A. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 1996;384:634–637. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 34.Pani G, Fischer K-D, Mlinaric-Rascan I, Siminovitch K A. Signaling capacity of the T cell antigen receptor is negatively regulated by the PTP1C tyrosine phosphatase. J Exp Med. 1996;184:839–852. doi: 10.1084/jem.184.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plas D R, Johnson R, Pingel J T, Matthews R J, Dalton M, Roy G, Chan A C, Thomas M L. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 1996;272:1173–1176. doi: 10.1126/science.272.5265.1173. [DOI] [PubMed] [Google Scholar]
- 36.Plater-Zyberk C, Taylor P C, Blaylock M G, Maini R N. Anti-CD5 therapy decreases severity of established disease in collagen type II-induced arthritis in DBA/1 mice. Clin Exp Immunol. 1994;98:442–447. doi: 10.1111/j.1365-2249.1994.tb05510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raab M, Yamamoto M, Rudd C E. The T-cell antigen CD5 acts as a receptor and substrate for the protein-tyrosine kinase p56lck. Mol Cell Biol. 1994;14:2862–2870. doi: 10.1128/mcb.14.5.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rincon M, Cebrian M, Sanchez-Madrid F, Lopez-Botet M. Induction of T cell function via the gp33/27 activation inducer molecule (AIM) requires co-expression of the CD3/TcR complex. Eur J Immunol. 1989;19:959–962. doi: 10.1002/eji.1830190528. [DOI] [PubMed] [Google Scholar]
- 39.Sato S, Miller A S, Inaoki M, Bock C B, Jansen P J, Tang M L, Tedder T F. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling on CD22-deficient mice. Immunity. 1996;5:551–562. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- 40.Secrist J P, Burns L A, Karnitz L, Koretzky G A, Abraham R T. Stimulatory effect of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J Biol Chem. 1993;268:5886–5893. [PubMed] [Google Scholar]
- 41.Shultz L D, Schweitzer P A, Rajan T V, Yi T, Ihle J N, Matthews R J, Thomas M L, Beier D R. Mutations at the murine motheaten locus are within the haematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 42.Spertini F, Stohl W, Ramesh N, Moody C, Geha R S. Induction of human T cell proliferation by a monoclonal antibody to CD5. J Immunol. 1991;146:47–52. [PubMed] [Google Scholar]
- 43.Starling G C, Whitney G S, Siadak A W, Llewellyn M C, Bowen M A, Farr A G, Aruffo A. Characterization of mouse CD6 with novel monoclonal antibodies which enhance the allogeneic mixed leukocyte reaction. Eur J Immunol. 1996;26:738–746. doi: 10.1002/eji.1830260403. [DOI] [PubMed] [Google Scholar]
- 44.Sun D, Branum K, Sun Q. Prevention of experimental autoimmune encephalomyelitis in Lewis rats by treatment with an anti-rat CD5 antibody (OX19) Cell Immunol. 1992;145:263–271. doi: 10.1016/0008-8749(92)90330-r. [DOI] [PubMed] [Google Scholar]
- 45.Tarakhovsky A, Kanner S B, Hombach J, Ledbetter J A, Muller W, Killeen N, Rajewsky K. A role for CD5 in TcR-mediated signal transduction and thymocyte selection. Science. 1995;269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 46.Timms J F, Carlberg D, Gu H, Chen H, Kamatkar S, Nadler M J, Rohrscheider L R, Neel B G. Identification of major binding proteins and substrates for the SH2-containing protein tyrosine phosphatase SHP-1 in macrophages. Mol Cell Biol. 1998;18:3838–3850. doi: 10.1128/mcb.18.7.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsui H W, Siminovitch K A, de Souza L, Tsui F W. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 48.Tuskano J, Engel P, Tedder T F, Kehrl J H. Engagement of the adhesion receptor CD22 triggers a potent stimulatory signal for B cells and blocking CD22/CD22L interactions impairs T-cell proliferation. Blood. 1996;87:4723–4730. [PubMed] [Google Scholar]
- 49.Tuskano J M, Engel P, Tedder T F, Argarwa A, Kehrl J H. Involvement of p72 syk kinase, p53/56 lyn kinase and phosphatidyl inositol-3 kinase in signal transduction via the human B lymphocyte antigen CD22. Eur J Immunol. 1996;26:1246–1252. doi: 10.1002/eji.1830260610. [DOI] [PubMed] [Google Scholar]
- 50.Unkeless J C, Lin J. Inhibitory receptors, ITIM sequences and phosphatases. Curr Top Immunol. 1997;9:338–343. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 51.Van de Velde H, von Hoegen I, Luo W, Parnes J R, Thielemans K. The B-cell surface protein CD72/Lyb-2 is the ligand for CD5. Nature. 1991;351:662–665. doi: 10.1038/351662a0. [DOI] [PubMed] [Google Scholar]
- 52.Yi T, Mui A L, Krystal G, Ihle J N. Hemopoietic cell phosphatase associates with the interleukin (IL-3) receptor B chain and down-regulates IL-3-induced tyrosine phosphorylation and mitogenesis. Mol Cell Biol. 1993;13:7577–7586. doi: 10.1128/mcb.13.12.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]