Abstract
The corpus callosum (CC), the anterior (AC), and the posterior (PC) commissures are the principal axonal fiber bundle pathways that allow bidirectional communication between the brain hemispheres. Here, we used the Allen mouse brain connectivity atlas and high-resolution diffusion-weighted MRI (DWI) to investigate interhemispheric fiber bundles in C57bl6/J mice, the most commonly used wild-type mouse model in biomedical research. We identified 1) commissural projections from the primary motor area through the AC to the contralateral hemisphere; and 2) intrathalamic interhemispheric fiber bundles from multiple regions in the frontal cortex to the contralateral thalamus. This is the first description of direct interhemispheric corticothalamic connectivity from the orbital cortex. We named these newly identified crossing points thalamic commissures. We also analyzed interhemispheric connectivity in the Balb/c mouse model of dysgenesis of the corpus callosum (CCD). Relative to C57bl6/J, Balb/c presented an atypical and smaller AC and weaker interhemispheric corticothalamic communication. These results redefine our understanding of interhemispheric brain communication. Specifically, they establish the thalamus as a regular hub for interhemispheric connectivity and encourage us to reinterpret brain plasticity in CCD as an altered balance between axonal reinforcement and pruning.
Keywords: aberrant bundles, Balb/c, corpus callosum, diffusion imaging, neuroplasticity
Introduction
Interhemispheric connections are mostly formed by axonal fiber bundles that cross the brain midline at discrete, specific crossing points called commissures, allowing communication between both hemispheres. The main commissures of the mammalian brain are the anterior commissure (AC), the corpus callosum (CC), the hippocampal commissure (HC), and the posterior commissure (PC; Ozdemir 2015).
The AC is evolutionary the oldest commissure (Raybuld 2010). In humans, the AC connects homotopic areas in the inferior temporal gyrus and also in the occipital lobe (Di Virgilio et al. 1999; Patel et al. 2010). Similar to humans, the olfactory bulbs, and the piriform and entorhinal cortices of the mouse brain make homotopic connections via the AC (Oh et al. 2014). Also, several neocortical axons project to the contralateral hemisphere via the AC during the embryonary stages of development (Jacobs et al. 2007). However, most of these projections undergo a strong pruning process and only a few reach adulthood (LaMantia and Rakic 1994).
The CC is the largest and, evolutionarily, the most recent brain commissure, connecting mainly and most of the neocortical regions in placental mammals (Suarez et al. 2014). Developmental malformations (dysgenesis) of the CC (CCD) can produce a series of cognitive deficits associated with several abnormal bundles that may cross the midline via the other commissures. An example is the sigmoid bundle, which fasciculates with the cingulum bundle but still traverses the midline at a CC remnant (Tovar-Moll et al. 2014). The sigmoid bundle was first observed in human CCD subjects (Tovar-Moll et al. 2007) and connects frontal brain regions with contralateral parieto-occipital areas (Paul et al. 2007; Tovar-Moll et al. 2007; Kasprian et al. 2013; Jakab et al. 2015). Recently, our group reported that the Balb/c mouse strain, a spontaneous model of CCD with a full range of CC malformations that vary from complete absence to a fully developed CC (Szczupak et al. 2020), presents a crossed fiber pathway connecting frontal to occipital cortical regions in a similar fashion as the sigmoid bundle. In that study, we used the C57bl6/J mouse strain as healthy controls with normotypical brain anatomy. Interestingly, and to our surprise, we found sigmoidal fibers in C57bl6/J mice connecting frontal cortical regions to contralateral occipital areas and crossing the midline at the CC, raising the possibility that “abnormal” white matter fiber bundles may also be a feature of healthy brains.
In addition to utilizing the main brain commissures, interhemispheric axonal fibers may also cross the midline along alternative crossing points. For example, there are reports of brain regions such as the pre- and infra-limbic cortex (Vertes 2002), the hippocampus (Mathiasen et al. 2019), and the primary motor (MOp) cortex (Hoerder-Suabedissen et al. 2018) projecting to the contralateral hemisphere through the thalamus. However, the specific patterns of these projections, their connectivity, and the prevalence in the healthy brain are still unknown. In the present work, we aimed to further investigate, with histology and high-resolution diffusion MRI, the presence of previously unknown interhemispheric connections in the C57bl6/J mouse strain. We hypothesized that connections that were previously described only in the context of brain malformations could also be present in the healthy brain and that interhemispheric pathways could use the thalamus and the AC as additional crossing points.
Materials and Methods
Histology
We used the Allen Brain Connectivity Atlas (Oh et al. 2014) to analyze specific regions and their fiber pathways in the C57bl6/J mouse strain, the most commonly used wild-type mouse model in biomedical research. The Allen Brain Connectivity Atlas is a collection of histological brain slices injected with AAV9, an intracellular neuronal tracer (labels only one neuron without crossing synapses), in each of the different cortical areas of the mouse brain. The series of coronal slices allows identifying the injection site (IS) and tracing the labeled axons across the entire brain. The atlas also offers a 3D reconstruction of the brain surface and labeled axons in template space. For our work, we used the slice containing the IS to identify the originating brain area and evaluate the precision of the injection. We also used all slices depicting the canonical interhemispheric anterograde white matter pathway(s) coursing away from the IS, and the 3D reconstruction of the fiber bundles in the template space. We used the following experiments of the Allen Brain Connectivity Atlas: 232 311 959 lateral entorhinal cortex (ENTl), 146 857 301 (PIR), 584 903 636 MOp area, and 112 306 316 lateral orbital cortex (ORBl).
MRI
All procedures and experiments were approved by the Animal Care and Use Committee of the National Institute of Neurological Disorders and Stroke. The MRI data used in this paper were previously acquired and processed as thoroughly described (Szczupak et al. 2020). Briefly, we used adult male and female C57bl6/J mice (n = 17) and Balb/c mice (n = 8) in this study. The Balb/c is a well-established spontaneous CCD model (Wahlsten 1989; Whalsten et al. 1992; Ozaki and Wahlsten 1993; Livy and Wahlsten 1997; Szczupak et al. 2020). The cohort of Balb/c mice studied here was a subset of the mice investigated in Szczupak et al. (2020). As shown in our previous report, these mice present highly variable CC malformations that range from a complete absent to a fully developed CC. These malformations are associated with the presence of aberrant white matter bundles, such as the Probst and the sigmoid bundles, which are similar to those found in human CCD patients and which lead to local and global changes in brain structural connectivity (Szczupak et al. 2020).
All mice were humanely euthanized and perfused transcardially with 4% PFA in PBS. Their brains were extracted and immersed in a solution containing 0.2% gadolinium (1 mmol/mL, Gadavist, Bayer) in 1× PBS for 2 weeks to decrease the T1 relaxation time constant and then with 0.2% gadolinium in pure water for 1 day to enhance that of T2, allowing faster acquisition time. The brains were placed in a custom-designed 3D holder and inserted tightly in a tube containing a fluorinated lubricant (Fomblin, Solvay). After degassing with a vacuum pump, the brains were placed in the MRI scanner for diffusion-weighted imaging (DWI).
DWI data for the C57bl6/J mice were acquired in a 16.4 Tesla horizontal magnet with the following parameters: time repetition/time echo (TR/TE) = 400/20 ms, δ/Δ = 2.5/12 ms, field of view = 18.99 × 11.16 × 8 mm, matrix = 190 × 112 × 80, bandwidth = 50 kHz, and 30 direction diffusion-encoding with b-value = 5000 s/mm2 with 2 b0 images yielding an isotropic resolution of 0.1 mm. The Balb/c data were acquired in a 14T vertical magnet at 80 μm with the following parameters: TR/TE = 450/21 ms, δ/Δ = 3/7.5 ms, field of view = 12.80 × 10.24 × 6.40 mm3, matrix = 160 × 128 × 80 yielding an isotropic resolution of 80 μm, number of averages = 2, bandwidth = 300 kHz, 232 diffusion-weighting directions split in 3 shells of 39, 77, and 116 directions, b-values = 1500, 3000, 6000 s/mm2 with 4 b0 images. To assure accurate comparability with the C57bl6/J data, we used only the big shell of 116 directions and processed both strains data with the same pipeline described as follows. The data were processed for eddy current correction in FMRIB Software Library (FSL; Andersson and Sotiropoulos, 2016), denoised in Mrtrix standard pipeline (Tournier et al. 2012), and fitted into a DTI model for FA measurements and colormap characterization in FSL.
Regions of interest (ROIs) were manually drawn at the AC and thalamic (TC) commissures by an experienced researcher at the midline level for standardization. Fractional anisotropy was calculated in FSL and further analyzed with a standard t-test in Prism (GraphPad).
Results
Histological Data Analysis
To study the possible presence of extra-callosal interhemispheric communication, we used histological data from the Allen Brain Institute to fully and accurately characterize the brain connectivity. First, we sought to analyze axonal projections from regions known to typically project interhemispheric fibers via the CC, the MOp cortex (Fig. 1), and the ORBl cortex (Fig. 2). Figure 1A shows the IS in MOp. Figure 1B shows that the vast majority of fibers originating in MOp cross the midline through the CC. However, the Allen Brain Connectivity Atlas data (Oh et al. 2014) revealed that the MOp also sends a small number of interhemispheric fibers through the AC (Fig. 1C) and the thalamus (Fig. 1D). The thalamic fibers from the MOp confirm previous reports of bilateral innervation of the thalamus (Hoerder-Suabedissen et al. 2018), reinforcing the thalamus as a interhemispheric hub of connectivity. It is well known that a few cortical areas such as the lateral entorhinal cortex (ENTl, see Supplementary Fig. 1) or the piriform cortex (PIR, see Supplementary Fig. 2) project interhemispherically via the AC. However, we are not aware of any reports of interhemispheric connectivity from the MOp via the AC in adult mice. The similarity between the interhemispheric fibers passing via the CC, the AC, and the thalamus (Fig. 1) suggests that the thalamus also features a commissural pathway, which we call here the TC. The axons from MOp pass through the caudate/putamen complex and penetrate the thalamic paracentral nuclei (PCN) before crossing the midline through the centromedial (CM) nucleus of the thalamus (Fig. 1D). Both sagittal (Fig. 1E) and axial (Fig. 1F) views show further details of the interhemispheric crossing points through the AC and TC. Figure 1G shows a 3D reconstruction of the axonal connectivity of the MOp with the rest of the brain. Globally, fibers originating in MOp cross the midline via the CC, the AC, and the TC, as indicated by arrows.
Figure 1 .
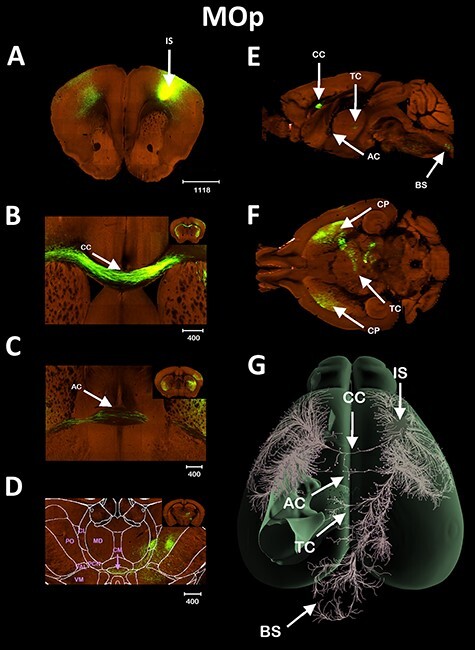
AC interhemispheric connectivity. Tract-tracing of the MOp area, showing the cortical region crossing the midline through the AC and TC. The injection site is shown in (A), CC communication shown in (B), AC communication in (C), TC communication in (D), sagittal (E), and axial slice (F) slice showing the commissural pathways, and 3D reconstruction of the fibers in an axial view (G). Scale bars in μm. The following thalamic nuclei were labeled only in the contralateral hemisphere in order to not obstruct the image. BS = brainstem, CL = centrolateral, VM = ventromedial, VAL = ventro anterior lateral, and PO = posterior complex.
Figure 2 .
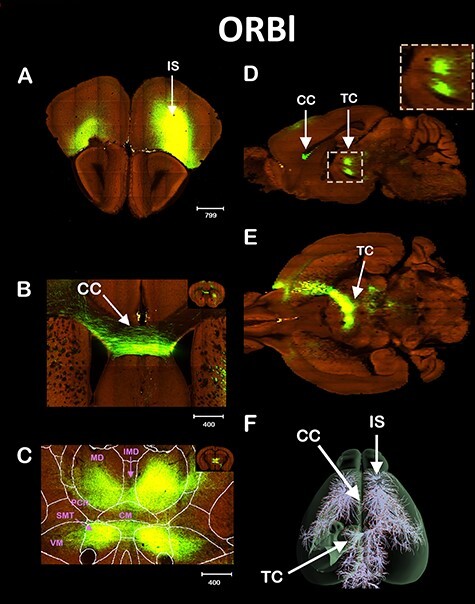
ORBl Interhemispheric thalamic connectivity. Tract tracing of the ORBl cortex. IS shown in (A), Coronal view of the CC crossing (B), and of the TC (C). Sagittal (D) and axial (E) view of the TC, and the 3D reconstruction (F). The following thalamic nuclei were labeled only in the contralateral hemisphere in order to not obstruct the image. VM = ventromedial and SMT = submedial. Scale bars in μm.
We also investigated another anterior cortical region, the ORBl area (Fig. 2A). Similar to MOp, the ORBl also sends interhemispheric projections to the contralateral cortex via the CC (Fig. 2B). However, we found that the ORBl extends most of its interhemispheric projections via the thalamus (Fig. 2C). This interhemispheric thalamic pathway has not been previously described and resembles other reports for thalamic interhemispheric communication from the anterior cingulate cortex and the infra and prelimbic cortices (Vertes 2002). The interhemispheric projections from ORBl pass through the caudate and split into 2 distinct tracts that cross the midline in the thalamus. The superior fiber tract arrives at the thalamus in the mediodorsal (MD) and crosses the midline via the intermediodorsal (IMD) nuclei. The inferior tract crosses the midline through the submedial nuclei (SMT). Both tracts are separated by the centromedial nuclei (CM, Fig. 2C) and can be clearly seen in the sagittal view (Fig. 2D). The crossing of the midline via the thalamus is also shown in the axial view of Figure 2E and in the 3D reconstruction of Figure 2F. Taken together, the examples of direct interhemispheric cortical communication via the thalamus shown in Figures 1 and 2 reveal that the thalamus forms commissures that are regular crossing points for interhemispheric brain pathways.
MRI Data Analysis
We used high-resolution DWI to analyze the mesoscopic white matter structures along the sagittal plane of the C57bl6/J mice and further characterize the interhemispheric thalamic pathways. We were able to clearly visualize 4 distinct white matter patches along the anterior–posterior axis of the thalamus (Fig. 3). We named these commissural patches according to their position along the anteroposterior axis as anterior (aTC), anteromedial (amTC), posteromedial (pmTC), and posterior (pTC) TCs. We were able to identify all 4 patches in a population of 17 C57bl6/J mice (Fig. 4). In a few animals, the relative disposition of the 4 patches varied somewhat. However, the 4 TC patches are a regular feature in the C57bl6/J strain.
Figure 3 .
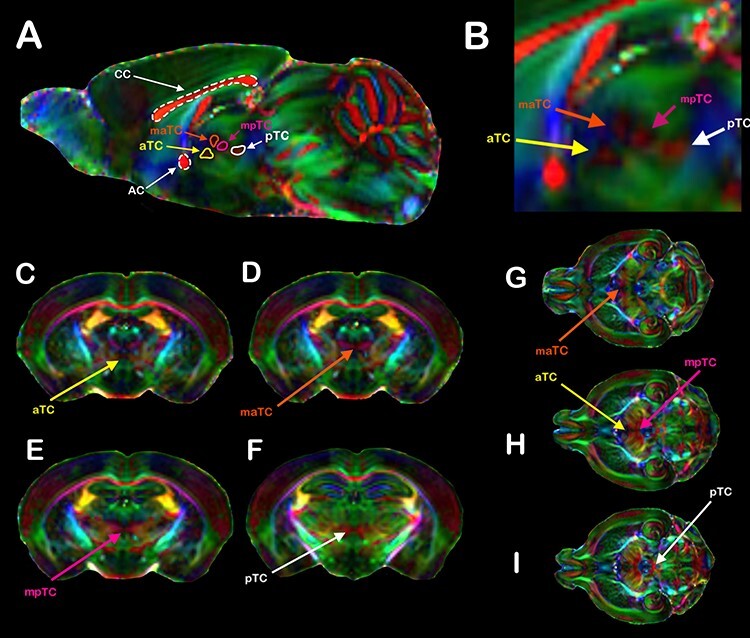
TC revealed by DWI. (A) Direction color encoded (DEC) sagittal DWI maps of a C57bl6/J mouse brain showing the CC, AC, and the TC. In all animals, we were able to distinguish at least 4 distinct TCs, named according to their relative disposition along the anteroposterior axis: the anterior TC (aTC, yellow arrows), the medial anterior TC (maTC, orange arrows), the medial posterior TC (mpTC, magenta arrows), and the posterior TC (pTC, white arrows). (B) Inset showing the TCs in greater detail. Coronal (D–F) and axial (G–I) DWI planes showing the TCs crossing the midline. In the DEC DWI maps, red represents mediolateral (ML) diffusion, green represents anteroposterior (AP) diffusion, and blue represents dorsoventral (DV) diffusion.
Figure 4 .
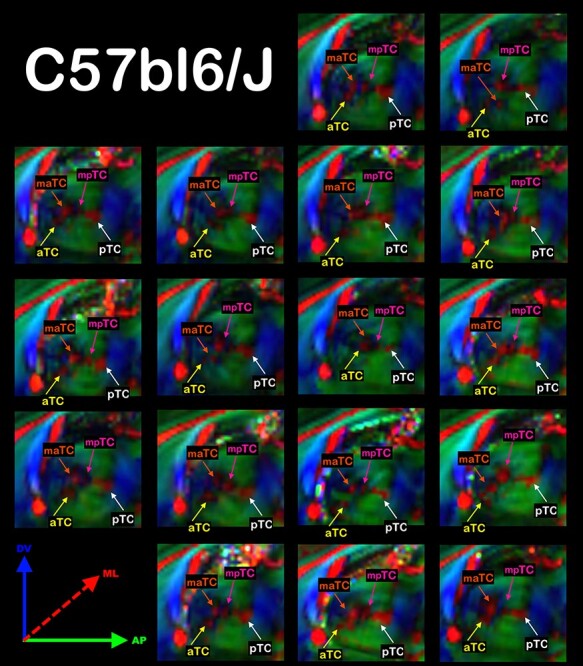
The TC are a Common Feature in a Population of C57bl6/J mice. Schematic anatomical drawing of a mouse brain midsection highlighting the interhemispheric commissures, CC, AC, TC, and the fornix (FX) (A). Direction color encoded (DEC) sagittal DWI maps showing the TC in a population of 17 C57bl6/J mice. In all animals, we were able to distinguish at least 4 distinct TCs, named according to their relative disposition along the anteroposterior axis: the anterior TC (aTC, yellow arrows), the medial anterior TC (maTC, orange arrows), the medial posterior TC (mpTC, magenta arrows), and the posterior TC (pTC, white arrows). In the DEC DWI maps, red represents mediolateral (ML) diffusion, green represents anteroposterior (AP) diffusion and blue represents dorsoventral (DV) diffusion (B).
Finally, we used DWI to investigate the presence of the TC in the Balb/c strain, a spontaneous mouse model of CCD. Compared with the C57bl6/J mice, the Balb/c animals showed a higher variability of the TC patches (Fig. 5). For example, some animals presented more than 4 TC patches with varying anatomical displacement. Additionally, 7 of the 8 Balb/c mice had the AC intersected by a set of anteroposterior (green; Fig. 5) fibers that seemed to originate from the fornix. These structures are highly complex due to the small size and consequential partial volume effects and surrounding crossing fibers of the fornix that impede further analysis with DWI-based tractography. Figure 6 shows the quantification of the fractional anisotropy and cross-sectional sagittal area of the TC and AC in C57bl6/J mice, The TC presented higher FA (Fig. 6A) and larger sagittal area (Fig. 6B) relative to Balb/c mice. On the other hand, the AC FA value does not show any statistical difference (P = 0.33) between strains (Fig. 6C), but Balb/c mice have a smaller AC sagittal area (by almost 50%) than C57bl6/J mice (Fig. 6D). The low FA value of the TC (<0.2) prohibits a thorough study with tractography, as the FA is too low to confidently identify preferential fiber orientation with tract reconstruction finality.
Figure 5 .
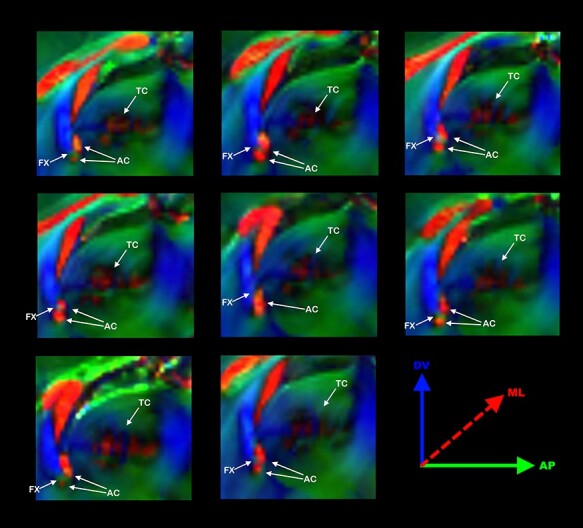
Commissural abnormalities in Balb/c. Schematic anatomical drawing of a mouse brain midsection highlighting the interhemispheric commissures, CC, AC, TC, and the fornix (FX) (A). Direction color encoded (DEC) sagittal DWI map of 8 Balb/C mouse brains showing the anatomical variability of the AC, fornix (FX), and TC. Interestingly, in most (7/8) Balb/c mice, the FX transects the AC (B).
Figure 6 .
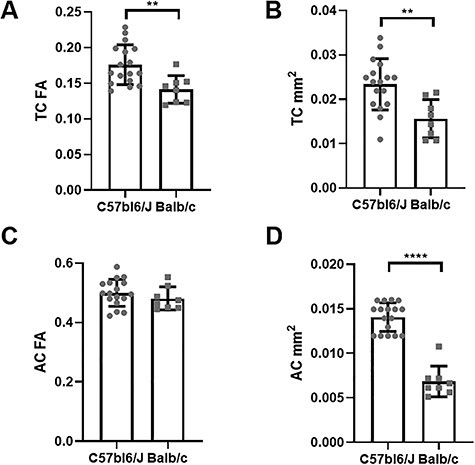
Commissural tissue properties. Comparative quantification of fractional anisotropy (FA) and cross-sectional areas of the thalamic (TC) and anterior (AC) commissures measured in C57bl6/J and Balb/c mice in the midsagittal MRI plane. The TC of Balb/c mice has significantly lower FA (A) and cross-sectional areas (B) than C57bl6/J mice. There was no difference in FA at the AC between the 2 strains (C), but the AC of Balb/c mice is significantly smaller than that of C57bl6/J mice (D). **P < 0.01. ***P < 0.001.
Discussion
In this work, we have found that cortical regions ORBl and MOp that were known only to establish communication across brain hemispheres through the CC also present other, previously unnoticed, interhemispheric pathways that involve the thalamus and the AC, respectively. These connections are in agreement with previous literature that shows other frontal regions projecting through the thalamus to the contralateral cortex (Vertes 2002; Hoerder-Suabedissen et al. 2018; Mathiasen et al. 2019). We identified projections from the MOp area via the AC to the contralateral hemisphere and corticothalamic interhemispheric fiber bundles to the contralateral thalamus. These alternative fiber pathways were present in the brain of adult healthy C57bl6/J mice. We also analyzed interhemispheric connectivity in the Balb/c, a mouse strain recently demonstrated to be a model for CCD. Relative to C57bl6/J, Balb/c presented an atypical and smaller AC and weaker interhemispheric corticothalamic communication. These results redefine our understanding of interhemispheric brain communication. Specifically, they establish the thalamus as a regular hub for interhemispheric connectivity and encourage us to reinterpret brain plasticity in CCD as an altered balance between axonal growth and pruning.
Interhemispheric Fiber Pathways Through the Thalamus
We present evidence of corticothalamic fibers crossing the midline via TCs. We observed TC in both C57bl6/J mice and the Balb/c mouse model of CCD, which shows that the TC is present in the typical brain and not only in those affected by developmental malformations. We attribute our discovery of this novel pathway to the improved spatial resolution of the DWI, which allowed us to resolve fiber bundles smaller than 200 μm. This spatial resolution is significantly superior to that typically employed by human studies. For example, the human connectome project employs a DWI protocol with an isotropic spatial resolution of 1.5 mm. Another factor for revealing the TC is that we investigated fiber bundles with an FA lower than 0.2, the threshold usually employed in tractography. Another major contributing factor to our discovery of the TC was the improvement in viral tract-tracing technology, which currently allows a higher sensitivity as the virus is expressed with equal distribution along the entire axonal pathway and is in agreement with previous reports of interhemispheric cortical projections through the thalamus. The examples of interhemispheric corticothalamic pathways shown in Figures 1 and 2 were extracted from the Allen Brain Connectivity Atlas (Oh et al. 2014). We are confident that this new pathway is generally present in the normal brain because we could identify the TC in 2 entirely independent data sets, in agreement with previous reports describing individual interhemispheric corticothalamic fiber bundles (Vertes 2002; Hoerder-Suabedissen et al. 2018; Mathiasen et al. 2019).
Studies about the formation of cortical connections prioritize understanding corticocortical patterns of connectivity within the central nervous system. Corticofugal tracts are not extensively explored as the former. The thalamus is known for having interhemispheric connections (interthalamic adherence) that connect both thalami (Gonçalves et al. 2011). Corticothalamic connections originate in the deep layers (VI) of the ipsilateral cortex and travel to the thalamus through the internal capsule. Before they get to their target, both corticothalamic and thalamocortical projections use each other to fasciculate and establish a connection to their respective destinations (Cang et al. 2005; Torii and Levitt 2005; Rash and Grove 2006; Rubenstein, 2011). On that account, we were surprised to identify direct projections from both the ORBl cortex and the MOp area to the contralateral thalamus via the TC. While individual fiber bundles connecting the cortex with the contralateral thalamus were described, our dataset allows the visualization not only of individual fiber bundles, but also the TC as a whole. This novel pathway challenges the previous notion that each thalamic hemisphere can be studied as a closed system, and proposes the thalamus as a hub for interhemispheric cortical connectivity. Moreover, future research is necessary to identify the molecular guidance cues and critical period that orient these corticothalamic axons and their differences to the axons that cross the midline through the CC.
The lack of information describing the development of cortical projections to the thalamus from the other hemisphere suggests several possibilities concerning this novel pathway. Since the fluorescent signal is very intense, we infer that there are not just a few projections that cross the midline at the thalamic level. These projections could be from a specific population that establishes a direct connection along this pathway. Also, there could be bifurcations from corticothalamic projections that connect to the ipsilateral thalamus. Either way, this finding raises the possibility that thalamocortical projections might connect in a reciprocal bidirectional pattern to the contralateral thalamus. An additional explanation is that the developing brain employs intrathalamic fibers associated with the TC as a common strategy to use existing axons as a roadmap for other axons, similar to callosal followers fasciculating with the callosal pioneers.
Using DWI, we were able to identify 4 distinct TC patches distributed along the anteroposterior axis of the thalamus. In our C57bl6/J cohort, we found that 15 of 17 animals presented the TC with this split architecture, while in 2 animals, the maTC and the mpTC patches were placed along the same coronal plane. Nevertheless, every animal presented 4 distinct TC patches, suggesting a common motif for crossing the thalamus at the midline. The Balb/c strain shows a larger anatomical variation of the TC patches organization, revealing that not only the size and FA of the TC is altered, but also its anatomical placement is affected. It is possible that with higher spatial resolution, additional TC patches and topography may be identified. We intend to pursue this issue in future work.
Crossed Fiber Pathways Through the AC
The MOp area connects to the contralateral hemisphere primarily via the corpus callosum. We here added to this pattern found that the MOp also sends projections across hemispheres via the AC, a novel interhemispheric cortical pathway. As a counter-example, the lateral entorhinal cortex ENTl sends most of its interhemispheric projections via the AC. We found that the ENTl also projects to the contralateral side via the CC. Unlike the newly discovered TC, the AC is well known to connect the temporal cortex, the olfactory bulb, and the entorhinal cortex across brain hemispheres in mice, humans, and monkeys (Jouandet and Gazzaniga 1979; LaMantia and Rakic 1994; Di Virgilio et al. 1999; Patel et al. 2010). There are also reports of extensive projections from the neocortex to the AC during development (Jacobs et al. 2007), but these axons do not seem to survive adulthood after a strong pruning effect (LaMantia and Rakic 1994). Although our results are surprising, it is reasonable to conclude that the CC, the AC, and the TC can serve as complementary interhemispheric brain communication routes serving multiple cortical regions.
Long-Distance Plasticity
It is well known that developmental malformations may lead to misrouted wiring of the brain by a process known as long-distance plasticity. Abnormal white matter bundles have been described previously both in animal models and humans subjects. The most iconic example is the sigmoid bundle that has already been shown in CCD mouse models (Edwards et al. 2020; Szczupak et al. 2020), extensively described in humans CCD patients (Paul et al. 2007; Tovar-Moll et al. 2007; Jakab et al. 2015) and in other brain malformations, in the form of a hypertrophied dorsal fornix (Arrigoni et al. 2016). Although grossly abnormal bundles seem to only exist in the context of brain malformations, we described the sigmoid bundle in the brain of healthy C57bl6/J mice (Szczupak et al. 2020), suggesting that the sigmoid connectivity is a common feature of typical brain development but may become prominent in developmental malformations. Little is known about the function or target regions of the sigmoid bundle. More studies are necessary to characterize the origin and targets of these projections and the function they subserve.
Previous studies showed that abnormal corticofugal fibers in CCD patients connect to the contralateral hemisphere through the AC (Tovar-Moll et al. 2014). Also, fibers projecting from the lateral cortex and crossing the midline via both the AC and the CC were described in a hamster model (Hedin-Pereira et al. 1992). This result was initially interpreted as misguided fibers that changed their natural course thereafter by removal of the “wrong” AC branch. Here, we offer a new interpretation that cortical intrahemispheric AC connections are part of regular pathways in the healthy brain that have not been fully described to date and which are exacerbated in CCD. There are reports of an enlarged AC in a CCD patient cohort compared with healthy controls and the presence of abnormal cortical bundles that have a topography adjacent to the AC (Tovar-Moll et al. 2014; Siffredi et al. 2019). In our Balb/C animal mouse model of CCD, we found that the area of the AC was significantly smaller than in controls (Fig. 6D) and that its structure has morphological alterations and is crossed by fibers coming from the fornix (Fig. 5). The contrast of an increased size of the AC in CCD patients, against a diminished one in mice is likely due to biological differences between species.
Few studies characterize the AC comprehensively. Both the AC and the CC can be separated into anatomical subregions concerning the timing of development and the brain regions they connect (Jouandet and Hartenstein 1983). The AC development has been shown to be dependent on a variety of signaling molecules as semaphorins, slit, netrin, ephrins, and their receptors (reviewed in Lindwall et al. 2007) that diffuse in the extracellular compartment along gradients. The same molecules play a role in CC development. The overlapping time window in which axons follow shared guidance cues can contribute to the formation of both commissures (Suarez et al. 2014). Therefore, it is possible that specific subpopulations of cells within the cortical regions that cross the midline mainly via one of them, can also traverse through the other. The developmental events are time and spatially regulated. Moreover, depending on which neuronal subpopulation is exposed to the same environment, different phenotypes might develop. The characterization of subpopulations within cortical regions during commissural development is rather obscure. For example, in hamsters, axons first cross the midline at the AC level on day 14 of the embryonic development (E14) while callosal projections start to cross the midline at E16 (Lent et al. 1990; Lent and Guimarães 1991; Pires-Neto and Lent 1993). Evolutionarily, the CC is a more recent structure than the AC (Suárez et al. 2018). It is possible that some remnant corticocortical pathways that use the AC as the main interhemispheric crossing point are still present in the rodent brain. Alternatively, axon exuberance, a longtime known strategy for CC development (Innocenti et al. 1977), creates a greater chance for establishing connections. This process is followed by a pruning period during which redundant or nonfunctional connections are removed (Lent et al. 1990; Hedin-Pereira 1999). The overlap between the CC and AC formation may constitute a way for the brain to secure interhemispheric connectivity via redundancy. This new interhemispheric pathway may offer a complementary explanation to the Sperry paradox where CCD patients can connect the hemispheres in the absence of the CC that was created in the 50s and until now is not fully understood.
The presence of multiple routes for interhemispheric cortical communication forms the basis of normal brain connectivity. We hypothesize that there may be 2 different types of long-distance plasticity in CCD. The first comprises an active process in which axons miss their guidance cues or pathway substrates and fasciculate with neighboring interhemispheric pathways, therefore creating abnormal bundles. An example is the bundle of misguided axons from the prefrontal cortex that find heterotopic interhemispheric connections through the fornix, fasciculate with them and form the sigmoid bundle. The other form of long-distance plasticity is a passive process, in which cortical pathways crossing the midline via multiple commissures undergo a pruning process that eliminates some, but not all of the connections. One example is the cortical communication via the AC, a well-known structure that displays a pronounced pruning (LaMantia and Rakic 1994). Future research should investigate the development of these connections to understand their biological mechanisms.
Conclusion
In summary, we have found that cortical regions thought to establish communication between brain hemispheres only through the CC also display other interhemispheric pathways that involve the AC and the thalamus via TC and proposes the thalamus as a hub of interhemispheric connectivity. These novel pathways redefine our understanding of interhemispheric brain communication and suggest that multiple neuroplasticity processes are at play during the development of the typical brain. Their disturbance leads to abnormal brain connectivity patterns, as observed in Balb/c CCD mouse model, and opens a new research avenue to identifying homologous patterns in humans.
Funding
This work was supported by the PA Department of Health SAP grant 4100083102 to A.C.S.; the Research Support Foundation of the State of Rio de Janeiro (FAPERJ); the National Council for Scientific and Technological Development (CNPq); as well as by intramural grants from D’Or Institute for Research and Education (IDOR). This research was also supported (in part) by the Intramural Research Program of the National Institutes of Health, National Insitute of Neurological Disorders and Stroke (grant ZIANS003041 to A.C.S.).
Notes
We would like to thank Lisa Zhang for technical support. We also thank the members and affiliates of the International Research Consortium for the Corpus Callosum and Cerebral Connectivity (IRC5, https://www.irc5.org) for discussions and input. Conflict of Interest: None declared.
Supplementary Material
Contributor Information
Diego Szczupak, Department of Neurobiology, University of Pittsburgh Brain Institute, University of Pittsburgh, Pittsburgh, PA 15261, USA; Cerebral Microcirculation Section, Laboratory of Functional and Molecular Imaging, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892, USA.
Pamela Meneses Iack, Biomedical Sciences Institute, Federal University of Rio de Janeiro, Rio de Janeiro 21941-590, Brazil.
Cirong Liu, Department of Neurobiology, University of Pittsburgh Brain Institute, University of Pittsburgh, Pittsburgh, PA 15261, USA; Cerebral Microcirculation Section, Laboratory of Functional and Molecular Imaging, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892, USA; Institute of Neuroscience, CAS Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai 200031, China.
IRC5 Consortium, Researchers of the International Research Consortium for the Corpus Callosum and Cerebral Connectivity (IRC5), Pasadena, CA 91125, USA.
Fernanda Tovar-Moll, D’Or Institute of Research and Education, Rio de Janeiro 22281-100, Brazil.
Roberto Lent, Biomedical Sciences Institute, Federal University of Rio de Janeiro, Rio de Janeiro 21941-590, Brazil; D’Or Institute of Research and Education, Rio de Janeiro 22281-100, Brazil.
Afonso C Silva, Department of Neurobiology, University of Pittsburgh Brain Institute, University of Pittsburgh, Pittsburgh, PA 15261, USA; Cerebral Microcirculation Section, Laboratory of Functional and Molecular Imaging, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892, USA.
References
- Andersson JLR, Sotiropoulos SN. 2016. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. 125:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni F, Romaniello R, Peruzzo D, Righini A, Parazzini C, Colombo P, Bassi MT, Triulzi F, Borgatti R. 2016. Aberrant supracallosal longitudinal bundle: MR features, pathogenesis and associated clinical phenotype. Eur Radiol. 26:2587–2596. [DOI] [PubMed] [Google Scholar]
- Cang JLR, Kaneko M, Yamada J, Woods G, Stryker MP, Feldheim DA. 2005. Ephrin-as guide the formation of functional maps in the visual cortex. Neuron. 48(4):577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio G, Clarke S, Pizzolato G, Schaffner T. 1999. Cortical regions contributing to the anterior commissure in man. Exp Brain Res. 124:1–7. [DOI] [PubMed] [Google Scholar]
- Edwards TJ, Fenlon LR, Dean RJ, Bunt J, Sherr EH, Richards LJ. 2020. Altered structural connectivity networks in a mouse model of complete and partial dysgenesis of the corpus callosum. Neuroimage. 217:116868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves ÓF, Carvalho S, Leite J, Pocinho F, Relvas J, Fregni F. 2011. Obsessive compulsive disorder as a functional interhemispheric imbalance at the thalamic level. Med Hypotheses. 77:445–447. [DOI] [PubMed] [Google Scholar]
- Hedin-Pereira C, Uziel D, Lent R. 1992. Bicommissural neurones in the cerebral cortex of developing hamsters. Neuroreport. 3:873–876. [DOI] [PubMed] [Google Scholar]
- Hedin-Pereira C. 1999. Morphogenesis of Callosal Arbors in the parietal cortex of hamsters. Cereb Cortex. 9:50–64. [DOI] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Hayashi S, Upton L, Nolan Z, Casas-Torremocha D, Grant E, Viswanathan S, Kanold PO, Clasca F, Kim Y, et al. 2018. Subset of cortical layer 6b neurons selectively innervates higher order thalamic nuclei in mice. Cereb Cortex. 28:1882–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti GM, Fiore L, Caminiti R. 1977. Exuberant projection into the corpus callosum from the visual cortex of newborn cats. Neurosci Lett. 4:237–242. [DOI] [PubMed] [Google Scholar]
- Jacobs EC, Campagnoni C, Kampf K, Reyes SD, Kalra V, Handley V, Xie Y-Y, Hong-Hu Y, Spreur V, Fisher RS, et al. 2007. Visualization of corticofugal projections during early cortical development in a τ-GFP-transgenic mouse: formation of early cortical circuitry. Eur J Neurosci. 25:17–30. [DOI] [PubMed] [Google Scholar]
- Jakab A, Kasprian G, Schwartz E, Gruber GM, Mitter C, Prayer D, Schöpf V, Langs G. 2015. Disrupted developmental organization of the structural connectome in fetuses with corpus callosum agenesis. Neuroimage. 111:277–288. [DOI] [PubMed] [Google Scholar]
- Jouandet ML, Gazzaniga MS. 1979. Cortical field of origin of the anterior commissure of the rhesus monkey. Exp Neurol. 66:381–397. [DOI] [PubMed] [Google Scholar]
- Jouandet ML, Hartenstein V. 1983. Basal telencephalic origins of the anterior commissure of the rat. Exp Brain Res. 50:183–192. [DOI] [PubMed] [Google Scholar]
- Kasprian G, Brugger PC, Schöpf V, Mitter C, Weber M, Hainfellner JA, Prayer D. 2013. Assessing prenatal white matter connectivity in commissural agenesis. Brain. 136:168–179. [DOI] [PubMed] [Google Scholar]
- LaMantia AS, Rakic P. 1994. Axon overproduction and elimination in the anterior commissure of the developing rhesus monkey. J Comp Neurol. 340:328–336. [DOI] [PubMed] [Google Scholar]
- Lent R, Hedin-Pereira C, Menezes JRL, Jhaveri S. 1990. Neurogenesis and development of callosal and intracortical connections in the hamster. Neuroscience. 38(1):21–37. [DOI] [PubMed] [Google Scholar]
- Lent R, Guimarães RZ. 1991. Development of palecortical projections through the anterior commissure of hamsters adopts progressive, not regressive, strategies. J Neurobiol. 22(5):475–498. [DOI] [PubMed] [Google Scholar]
- Lindwall C, Fothergill T, Richards LJ. 2007. Commissure formation in the mammalian forebrain. Curr Opin Neurobiol. 17(1):3–14. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Wahlsten D. 1997. Retarded formation of the hippocampal commissure in embryos from mouse strains lacking a corpus callosum. Hippocampus. 7:2–14. [DOI] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, et al. 2014. A mesoscale connectome of the mouse brain. Nature. 508:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir N. 2015. The anatomy of the posterior commissure. Turk Neurosurg. 25:837–43. [DOI] [PubMed] [Google Scholar]
- Ozaki HS, Wahlsten D. 1993. Cortical axon trajectories and growth cone morphologies in fetuses of acallosal mouse strains. J Comp Neurol. 336:595–604. [DOI] [PubMed] [Google Scholar]
- Mathiasen ML, Louch RC, Nelson AD, Dillingham CM, Aggleton JP. 2019. Trajectory of hippocampal fibres to the contralateral anterior thalamus and mammillary bodies in rats, mice, and macaque monkeys. Brain Neurosci Adv. 3:239821281987120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MD, Toussaint N, Charles-Edwards GD, Lin J-P, Batchelor PG. 2010. Distribution and fibre field similarity mapping of the human anterior commissure fibres by diffusion tensor imaging. Magn Reson Mater Phy. 23:399–408. [DOI] [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH. 2007. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 8:287–299. [DOI] [PubMed] [Google Scholar]
- Pires-Neto MA, Lent R. 1993. The prenatal development of the anterior commissure in hamsters: pioneer fibers lead the way. Dev Brain Res. 72(1):59–66. [DOI] [PubMed] [Google Scholar]
- Rash BG, Grove EA. 2006. Area and layer patterning in the developing cerebral cortex. Curr Opin Neurobiol. 16(1):25–34. [DOI] [PubMed] [Google Scholar]
- Raybaud C. 2010. The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology. 52:447–477. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL. 2011. Annual research review: development of the cerebral cortex: implications for neurodevelopmental disorders. J Child Psychol Psychiatry. 52(4):339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siffredi V, Wood AG, Leventer RJ, Vaessen M, McIlroy A, Anderson V, Vuilleumier P, Spencer-Smith MM. 2019. Anterior and posterior commissures in agenesis of the corpus callosum: alternative pathways for attention processes? Cortex. 121:454–467. [DOI] [PubMed] [Google Scholar]
- Suarez R, Gobius I, Richards LJ. 2014. Evolution and development of interhemispheric connections in the vertebrate forebrain. Front Hum Neurosci. 8:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez R, Paolino A, Fenlon LR, Morcom LR, Kozulin P, Kurniawan ND, Richards LJ. 2018. A pan-mammalian map of interhemispheric brain connections predates the evolution of the corpus callosum. Proc Natl Acad Sci U S A. 115:9622–9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczupak D, Liu C, Yen CCC, Choi S-H, Meireles F, Victorino C, Richards L, Lent R, Silva AC, Tovar-Moll F. 2020. Long-distance aberrant heterotopic connectivity in a mouse strain with a high incidence of callosal anomalies. Neuroimage. 217:116875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Levitt P. 2005. Dissociation of corticothalamic and thalamocortical axon targeting by an EphA7-mediated mechanism. Neuron. 48(4):563–575. [DOI] [PubMed] [Google Scholar]
- Tournier J-D, Calamante F, Connelly A. 2012. MRtrix: diffusion tractography in crossing fiber regions. Int J Imaging Syst Technol. 22:53–66. [Google Scholar]
- Tovar-Moll F, Moll J, de Oliveira-Souza R, Bramati I, Andreiuolo PA, Lent R. 2007. Neuroplasticity in human Callosal dysgenesis: a diffusion tensor imaging study. Cereb Cortex. 17:531–541. [DOI] [PubMed] [Google Scholar]
- Tovar-Moll F, Monteiro M, Andrade J, Bramati IE, Vianna-Barbosa R, Marins T, Rodrigues E, Dantas N, Behrens TEJ, de Oliveira-Souza R, et al. 2014. Structural and functional brain rewiring clarifies preserved interhemispheric transfer in humans born without the corpus callosum. Proc Natl Acad Sci. 111:7843–7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. 2002. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol. 442:163–187. [DOI] [PubMed] [Google Scholar]
- Wahlsten D. 1989. Genetic and developmental defects of the mouse corpus callosum. Experientia. 45:828–838. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Ozaki HS, Livy D. 1992. Deficient corpus callosum in hybrids between ddN and three other abnormal mouse strains. Neurosci Lett. 136:99–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


