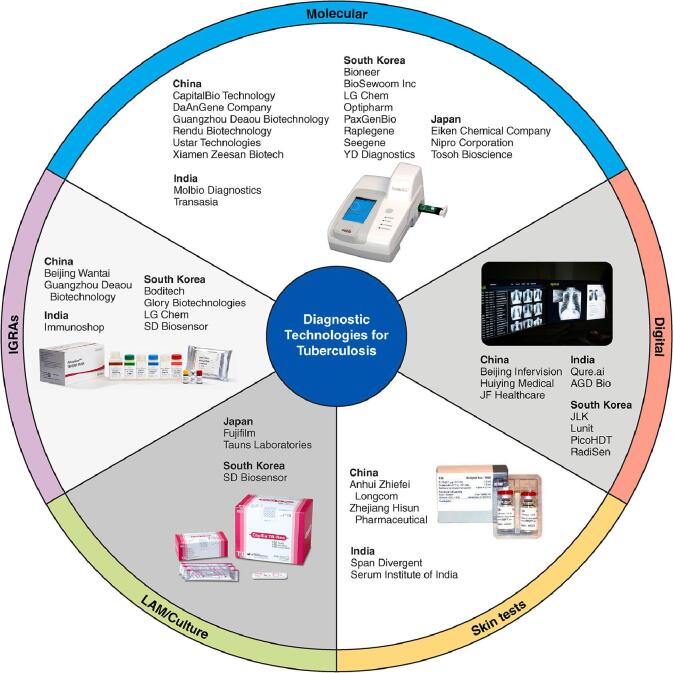
The diagnostic technology landscape for tuberculosis (TB) has never been more varied. Eight products or classes of technologies are recommended by the World Health Organization (WHO) [1], a further eighteen products are under evaluation by the WHO [2] and dozens more are on the market—a recent landscape analysis found 20 tests for TB infection alone [3].
However, actual use and procurement remains concentrated to a handful of tests. Further, the list of WHO-endorsed products is dominated by companies from Europe and North America. Many of these technologies remain expensive, despite advocacy campaigns for price reductions [4], or not ideal for low-resource settings, meaning there have been problems scaling up their use in low- and middle-income countries (LMICs) where the TB burden is highest [5], [6], [7], [8].
In general, the field of global health has a problem—an excessive reliance on product and innovations being developed in the Global North that then slowly trickle down to the Global South, where the biggest needs are, and where technologies often have the greatest impact [9]. However, there is capacity in other parts of the world to develop and commercialize diagnostics technologies that should be part of the solution to address global health challenges. This issue has received a lot of attention during the ongoing Covid-19 pandemic, with calls to expand vaccine manufacturing to Africa and other regions, along with TRIPS waiver and technology transfer.
In 2019, Indian company Molbio Diagnostics became the first company from an LMIC to receive WHO approval for its TB diagnostic technology [10]. Along with Eiken and Nipro from Japan, it is the only Asian company with a WHO-recommended molecular TB diagnostic. All these products are available for countries to procure via the Global Drug Facility (GDF) [11], housed at the Stop TB Partnership. By contrast, the WHO’s emergency use listing for SARS‐CoV‐2 in-vitro diagnostics (IVDs) includes several companies from Asia, particularly China, showing that the capacity for diagnostic innovation and manufacturing on the continent is strong [12].
In fact, Asia is also a hotbed of innovation for TB diagnostics, with products on the market which span the complete spectrum of TB diagnosis, from laboratory-based molecular diagnostics to artificial intelligence (AI) for chest x-ray (CXR) interpretation. In 2021, analysis of the results of studies of Qure.ai from India and Lunit from South Korea contributed to the WHO’s recommendation for computer-aided detection (CAD) of CXRs [13]. As seen in the current pandemic, having a broad global manufacturing base for diagnostic technologies is highly advantageous.
We recently conducted a landscape analysis of the diagnostic technologies for TB produced by companies in four Asian countries—China, India, South Korea, and Japan. Diagnostic technologies were identified through a review of the literature, internet searches and contacts in Asian countries. Where possible, specific contacts within each company were identified, with help from contacts who spoke Chinese and Korean, and emailed questions in English concerning product technical details and national and international regulatory approval. Where specific contacts were unavailable, companies were contacted using generic email addresses or through contact forms on their websites. Data on companies which could not be contacted were compiled using information in the public domain. Data were collected on company and product names, diagnostic method, use case and regulatory approval. Product variants which identify different combinations of drug resistance and are marketed separately were classified as distinct products. Product use cases are as described by the companies.
In total, 82 TB diagnostic products were identified from 39 companies [Appendix]. Twelve companies are based in China, seven in India, five in Japan and fifteen in South Korea. Fig. 1 displays company names and diagnostic categories. Most identified products were molecular diagnostics, with a wide range of diagnostic methods and drug resistance detection options available. Diagnostics currently endorsed by the WHO include three chip-based real-time PCR assays by Molbio (Truenat MTB, MTB Plus and MTB-RIF Dx), two line-probe assays (LPAs) by Nipro for detection of resistance to rifampicin and isoniazid (Genoscholar NTM + MDRTB II) and pyrazinamide (Genoscholar PZA-TB II), a loop-mediated isothermal amplification (LAMP) assay by Eiken (Loopamp MTBC Detection Kit), a rapid species identification test from culture by Tauns Laboratories (Capilia TB-Neo) and CAD technologies such as Qure.ai (qXR v2) and Lunit (Lunit Insight CXR).
Fig. 1.
TB diagnostic products were identified from 39 companies in Asia.
A number of Asian companies make diagnostics for multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB. Xiamen Zeesan Biotech makes the MeltPro real-time PCR assays which are approved by the Chinese regulator and can detect resistance to rifampicin, streptomycin, isoniazid, ethambutol, fluroquinolones and second-line injectables. Bioneer makes the Accupower real-time PCR assays, including for MDR and XDR TB; both Optipharm (OPTIMYGENE) and YD diagnostics (MolecuTech) make reverse blot hybridization assays for MDR and XDR TB; and Seegene makes the AllPlex and Anyplex II PCR-based assays for MDR and XDR TB. Other diagnostic methods include isothermal target and probe amplification (RapiDx MTB test by Raplegene), transcription-reverse transcription concerted reaction (TRCRapid-160 M.TB by Tosoh Bioscience), isothermal amplification lateral flow (EasyNAT Diagnostic Kit by Ustar Technologies), simultaneous amplification and testing (SAT-TB by Rendu Biotechnology) and DNA microarray assays (CapitalBio M. Tuberculosis Drug Resistance Detection Array Kit).
Interferon-gamma release assays (IGRAs) are made by seven companies (Beijing Wantai, Guangzhou Deauou, Immunoshop, Boditech, Glory Biotechnologies, LG Chem and SD Biosensor). The Beijing Wantai and SD Biosensor IGRAs are approved for procurement by the Global Fund’s Expert Review Panel for Diagnostics [14]. Four companies manufacture skin tests (Anhui Zhifei Longcom, Zhejiang Hisum Pharmaceutical, Span Divergent and Serum Institute of India). Two make culture-based diagnostics for rapid species identification (Tauns Laboratories and SD Biosensor), with the Tauns Laboratories test recommended by the WHO. Fujifilm makes a urine lipoarabinomannan assay identifying M. tb in people who are HIV positive.
Seven companies make AI-based technologies for reading CXRs (Beijing Infervision Technology, Huiying Medical Technology, JF Healthcare, Qure.ai, JLK, Lunit and Radisen) and PicoHDT makes both wireless and wired versions of the Mine-2 portable x-ray machine, which incorporates diagnostic AI technology. AGD Bio makes the Mycovision smart microscope which uses AI to detect acid-fast bacilli under Ziehl-Neelsen stained slides.
Given the wide array of promising products identified, it is surprising that only a few Asian technologies are included in the WHO-recommended product list. To enter the global health market, technologies have needed to undergo independent, international evaluation studies for policy review and WHO endorsement. Such policy review has not happened with most Asian TB technologies. CE-IVD marking is currently not sufficient to meet these quality assurance criteria. Also, to be internationally competitive, companies must have the ability to provide service and maintenance at the global level.
The process for WHO endorsement of TB IVDs is currently changing. The WHO Global Tuberculosis Programme will now focus on the evaluation of classes of TB diagnostic technologies for WHO recommendation, while WHO prequalification will evaluate specific product brands for quality, safety and performance [15]. Companies may also need to meet quality assurance criteria of major donors and procurers such as the Global Fund [16]. An additional recent opportunity for manufacturers of TB diagnostics to get their products into the global marketplace is via the Global Fund’s Expert Review Panel for Diagnostics, the approval of which allows for countries to use Global Fund support (the largest international funding source for TB) to procure products that are on the pathway to becoming WHO-approved.
We hope more Asian companies can learn from the experience of innovators such as Eiken, Nipro, Molbio, Tauns, Qure.ai and Lunit, and find ways to validate promising technologies further and have them considered for international policy guidance [17]. For WHO endorsement, the current requirements are clearly defined [18] and involve demonstrating analytical and clinical validity, clinical and epidemiologic utility, economic outcomes and operational and qualitative aspects. The expectations for validation data and study design considerations for evaluating different types of TB tests are also published [18], [19], [20], [21], [22], along with target product profiles [23], [24]. Partnerships with international organizations such as the Foundation for Innovative New Diagnostics (FIND), and initiatives such as the Stop TB Partnership’s Accelerator for Impact (a4i) [25] and the recently launched NIH-funded FEND-TB [26] and R2D2 TB Network [27] initiatives, might help Asian innovators better navigate the global policy process.
Beyond validation and inclusion in global policy, there is a need to also address other barriers to product uptake and scale, including pricing that is suitable for LMICs, capacity for service and maintenance, donor support for scale-up, and better guidance on when or how to switch from one product to another.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix.
Molecular diagnostics
| Company | Product | Diagnostic method | Use | Regulatory approval |
|---|---|---|---|---|
| China | ||||
| CapitalBio | CapitalBio Mycobacteria Species Identification Array Kit | DNA microarray assay | Identifies M. tb complex and 16 other Mycobacterial species in sputum | CE marking |
| CapitalBio M. Tuberculosis Drug Resistance Detection Array Kit | Identifies M. tb complex Detects resistance to rifampicin and isoniazid |
CE marking | ||
| DaAN GeneCompany | Mycobacterium Tuberculosis (TB) PCR Kit | PCR | Identifies M. tb complex in sputum and bronchoalveolar lavage fluid specimens | No information |
| Rendu Biotechnology | SAT-TB | Simultaneous amplification and testing | Identifies M. tb | No information |
| Guangzhou Deaou Biotechnology | DeFast.TB Mycobacterium Tuberculosis Complex (MTC) Nucleic Acid Detection Solution | No information | Identifies M. tb in bronchoalveolar lavage fluid, pleural effusion, abdominal effusion, cerebrospinal fluid, joint effusion, urine and other specimen types. | NMDA (China) approved |
| DeFine.TB Mycobacterium Tuberculosis Specific Cellular Immunoreaction Detection Kit | Double-antibody sandwich ELISA | No information | No information | |
| Ustar Technologies | EasyNAT Diagnostic Kit for Mycobacterium Tuberculosis (TB) DNA | Isothermal Amplification Lateral Flow | Identifies M. tb complex | CE marked |
| EasyNAT Diagnostic Kit for Mycobacterium Tuberculosis Complex DNA | Isothermal Amplification Real Time Florescence Assay | Identifies M. tb complex in sputum | CE marked | |
| Xiamen Zeesan Biotech | MeltPro Mycobacterium Tuberculosis test kit | Real-time PCR | Identifies M. tb complex | NMDA (China) approved |
| MeltPro Mycobacteria identification kit | Identifies 19 common Mycobacterial species | CE marked | ||
| MeltPro MTB/RIF; MTB/STR; MTB/INH; MTB/EMB; MTB/FQ; MTB/SL | Detects resistance to rifampicin; streptomycin; isoniazid; ethambutol; fluoroquinolones; second-line injectables | All NMDA (China) approved, except MTB/SL. All CE marked | ||
| India | ||||
| Molbio | Truenat MTB | Chip-based Real-time PCR | Quantitative detection of M.tb in human pulmonary and EPTB specimens | WHO endorsed CDSCO (India) approved CE marked |
| Truenat MTB Plus | Semiquantitative detection of M.tb in human pulmonary and EPTB specimens | WHO endorsed CDSCO (India) approved CE marked |
||
| Truenat MTB-RIF Dx | Detects resistance to rifampicin. “Follow on test, to be performed only on the extracted DNA from Truenat MTB/MTB Plus positive sample” | WHO endorsed CDSCO (India) approved CE marked |
||
| Transasia/Erba Molecular | MX16 | No information | No information | No information |
| Japan | ||||
| Eiken Chemical Company | Loopamp MTBC Detection Kit | Loop-mediated isothermal amplification (LAMP) | Identifies M. tb extracted from sputum | WHO endorsed CE marked |
| Nipro Corporation | Genoscholar PZA TB II | Line probe assay | Identifies M. tb complex in sputum Detects resistance to pyrazinamide |
CE marked |
| Genoscholar NTM + MDRTB II | Identifies M. tb complex, M. avium, M. intracellulare and M. kansasii Detects resistance to rifampicin and isoniazid in M. tb |
WHO endorsed CE marked |
||
| Genoscholar FQ + KM-TB II | Detects resistance to fluoroquinolones and kanamycin in M. tb For research use only |
CE marked | ||
| Tosoh Bioscience | TRCRapid-160 M.TB | Transcription-reverse transcription concerted reaction | Detection of M.tb complex in a clinical specimen or suspension of cultured cells | No information |
| TRCReady-80 M.TB | Detection of M.tb complex in a clinical specimen or suspension of cultured cells | No information | ||
| South Korea | ||||
| Bioneer | Accupower MTB | Real-time PCR | Identifies M. tb in sputum, bronchoalveolar lavage and urine | No information |
| Accupower MTB & NTM | Identifies M. tb and non-tuberculous mycobacteria | No information | ||
| Accupower TB & MDR | Identifies M. tb; detects resistance to rifampicin and isoniazid | No information | ||
| Accupower XDR-TB | Identifies M. tb; detects resistance to fluoroquinolones, aminoglycosides, ethambutol and streptomycin | No information | ||
| Biosewoom | Real-Q M. tuberculosis kit | Line probe assay | Identifies M. tb and non-tuberculous mycobacteria | No information |
| LG Chem | AdvanSure TB/NTM assay | Real-time PCR | Identifies M. tb and non-tuberculous mycobacteria in sputum, bronchial washing fluid, cerebrospinal fluid, urine, body fluid, EDTA-whole blood and tissues | No information |
| AdvanSure MDR-TB GenoBlot assay | Reverse blot hybridization assay | Detects resistance to rifampicin and isoniazid in M. tb | No information | |
| Optipharm | OPTIMYGENE Real TB – Tag Kit | Real-time quantitative PCR | Identifies M. tb | No information |
| OPTIMYGENE Real MTB – ID Kit | Identifies M. tb and non-tuberculous mycobacteria | No information | ||
| OPTIMYGENE Inf – TB Kit | No information | Differentiation of active TB and latent TB infection by measuring interferon-gamma expression by RT-qPCR | No information | |
| OPTIMYGENE REBA MTB – MDR Kit | Reverse blot hybridization assay | Detects rifampin and isoniazid resistance | No information | |
| OPTIMYGENE REBA MTB – XDR Kit | Detects fluoroquinolone, kanamycin and streptomycin resistance | No information | ||
| QMAP Dual-ID (Disk-based PCR) | Disk-based PCR | Identifies M. tb and NTM; detects rifampin resistance | No information | |
| PaxGenBio | PaxView TB/NTM MPCR-UFLA kit | Multiplex PCR and universal lateral flow assay | Identifies M. tb and NTM in sputum or brochoalveolar lavage | CE marked South Korean FDA approved |
| Raplegene | RapiDx MTB test | Isothermal target and probe amplification | Identifies M. tb | No information |
| Seegene | AllPlex MTB/MDRe; MTB/MDR/XDRe; MTB/XDRe | PCR | Identifies M. tb and detects resistance to rifampicin and isoniazid; rifampicin and isoniazid, fluoroquinolones and injectable drugs; fluoroquinolones and injectable drugs | All CE marked |
| Anyplex II MTB/MDR; MTB/MDR/XDR; MTB/XDR; MTB/NTM | Identifies M. tb and detects resistance to rifampicin and isoniazid; rifampicin and isoniazid, fluoroquinolones and injectable drugs; fluoroquinolones and injectable drugs; Identifies M. tb and NTB and detects resistance to rifampicin and isoniazid | All CE marked | ||
| YD (Youngdong) Diagnostics | MolecuTech TB-Tag Two | PCR | Identification of M. tb | No information |
| MolecuTech MTB-ID V3 | Identification of M. tb and non-tuberculous mycobacteria | No information | ||
| MolecuTech Real TB-Tag | Real-time PCR | Identification of M. tb | No information | |
| MolecuTech Real MTB-ID | Identification of M. tb and non-tuberculous mycobacteria | No information | ||
| MolecuTech REBA Myco-ID | Reverse blot hybridization assay | Identification of M. tb and non-tuberculous mycobacteria | No information | |
| MolecuTech REBA MTB-MDR | Detection of resistance to rifampin and isoniazid | No information | ||
| MolecuTech REBA MTB-XDR | Detection of resistance to fluoroquinolones, kanamycin and streptomycin | No information |
Lipoarabinomannan (LAM) assays
| Company | Product | Diagnostic method | Use | Regulatory approval |
|---|---|---|---|---|
| Japan | ||||
| Fujifilm | SILVAMP TB LAM (FujiLAM) | Urine lipoarabinomannan assay | Identifies M.tb in urine of people who are HIV positive | No information |
Rapid species identification of M. tuberculosis from culture
| Company | Product | Diagnostic method | Use | Regulatory approval |
|---|---|---|---|---|
| Japan | ||||
| Tauns Laboratories | Capilia TB-Neo | Immuno-chromatographic assay detecting MPB64 antigen | Identifies M. tb complex isolates from solid and liquid cultures | WHO endorsed CE marked |
| South Korea | ||||
| SD Biosensor | STANDARD Q TB MPT64 Ag | Immuno-chromatographic assay detecting MPT64 antigen | Identifies M. tb in solid or liquid culture | No information |
Interferon-gamma release assays (IGRAs)
| Company | Product | Diagnostic method | Use | Regulatory approval |
|---|---|---|---|---|
| China | ||||
| Beijing Wantai | TB-IGRA | IGRA ELISA | Identifies M. tb infection in whole blood | GF ERPD recommended CE marked |
| Guangzhou Deaou Biotechnology | SPOTestTM Mycobacterium Tuberculosis Specific Cellular Immunoreaction Detection Kit | IGRA ELISPOT | Identifies M. tb infection | No information |
| India | ||||
| Immunoshop | TB Platinum | IGRA | Identifies M. tb infection in whole blood | No information |
| South Korea | ||||
| Boditech Inc | Ichroma IGRA-TB | ESAT-6 and CFP-10 IGRA lateral flow assay | Diagnosis of infection with M. tb in whole blood | CE marked |
| Glory Biotechnologies Group | GBTsol Latent TB Test Kit | ESAT-6 and CFP-10 IGRA | Identifies M. tb infection | No information |
| LG Chem | Advansure I3 TB-IGRA | ESAT-6 and CFP-10 chemo-luminescence IGRA | Diagnosis of M.tb infection in whole blood | No information |
| Advansure TB IGRA | ESAT-6 and CFP-10 IGRA ELISA | Diagnosis of M.tb infection in whole blood | CE marked | |
| SD Biosensor | STANDARD E TB-Feron ELISA | ESAT-6, CFP-10 and TB7.7 IGRA ELISA | Diagnosis of infection with M. tb in whole blood | GF ERPD recommended CE marked |
| STANDARD F TB-Feron FIA (IFN-gamma) | ESAT-6, CFP-10 and TB7.7 IGRA lateral flow assay | Diagnosis of infection with M. tb | CE marked |
Further details on tests for tuberculosis infection are available in Hamada Y, Cirillo DM, Matteelli A, et al. Tests for tuberculosis infection: landscape analysis. Eur Respir J 2021; in press (https://doi.org/10.1183/13993003.00167–2021).
Skin tests
| Company | Product | Diagnostic method | Use | Regulatory approval |
|---|---|---|---|---|
| China | ||||
| Anhui Zhifei Longcom Biopharmaceutical Co., Ltd | EC-Test | ESAT6 and CFP10 skin test | Identifies M. tb infection | NMPA (China) approved |
| Zhejiang Hisun Pharmaceutical Co., Ltd | Identification Allergen | ESAT6 and CFP10 skin test | Identifies M. tb infection | No information |
| India | ||||
| Span Divergent/Arkray Japan | Tuberculin PPD | Skin test | Identifies M. tb infection | No information |
| Serum Institute of India/Statens Serum Institute | C-Tb | ESAT6 and CFP10 skin test | Identifies M. tb infection | No information |
Further details on tests for tuberculosis infection are available in Hamada Y, Cirillo DM, Matteelli A, et al. Tests for tuberculosis infection: landscape analysis. Eur Respir J 2021; in press (https://doi.org/10.1183/13993003.00167–2021).
Digital CXR and CAD technologies
| Company | Product | Diagnostic method | Use | Regulatory approval |
|---|---|---|---|---|
| China | ||||
| Beijing Infervision Technology Company | InferRead DR Chest | Artificial intelligence | AI-aided screening of CXR, including for TBAI-aided detection of TB on CXR | CE marked |
| Huiying Medical Technology | DR Chest | Artificial intelligence | AI-aided preliminary screening of CXR, including for TB | No information |
| JF Healthcare | JF CXR-1 | Artificial intelligence | AI-aided detection of TB on CXR | NMPA (China) Tier 2 approved |
| JF CXR-2 | Artificial intelligence | AI-aided multi-thorax disease model | No information | |
| India | ||||
| Qure.ai | qXR TB | Automated chest x-ray interpretation | Detects signs of pulmonary, hilar, and pleural tuberculosis on CXR | CE marked |
| South Korea | ||||
| JLK | JLD-02 K (JVIEWER-X) | Automated chest x-ray interpretation | Detects several pulmonary abnormalities, including signs of pulmonary tuberculosis | CE marked South Korean FDA approved Australian FDA approved |
| Lunit | Lunit INSIGHT CXR | Artificial intelligence-assisted x-ray reading | Acts as second reader for physicians reading CXRs | CE marked |
| PicoHDT | Mine-2 system (wired type) | Portable x-ray machine with diagnostic AI technology | Produces x-ray images; interprets x-rays | CE marked |
| Mine-2 system (wireless type) | Produces x-ray images; interprets x-rays | CE marked | ||
| RadiSen | AXIR TB | Artificial intelligence | Detects signs of pulmonary tuberculosis on CXR | CE marked |
Further details on digital technologies are available:
- (i).
-
(ii).
In FIND (2021). Digital chest radiography and computer-aided detection (CAD) solutions for tuberculosis diagnostics: technology landscape analysis. (https://www.finddx.org/wp-content/uploads/2021/04/FIND-CXR-CAD-solutions-for-TB-diagnosis-7Apr2021.pdf)
-
(iii).
In Stop TB Partnership (2021) Screening and Triage for TB using Computer-Aided Detection (CAD) Technology and Ultra-portable X-Ray Systems: A Practical Guide. (http://stoptb.org/dhthub/practicalguide.asp)
Smart microscope
| Company | Product | Diagnostic method | Use | Regulatory approval |
|---|---|---|---|---|
| India | ||||
| AGD Bio | Mycovision | Smart microscope | Artificial intelligence-based automated microscope for detection of acid fast bacilli under Ziehl-Neelsen stained slides | No information |
References
- 1.World Health Organization, WHO consolidated guidelines on tuberculosis. Module 3: diagnosis - rapid diagnostics for tuberculosis detection, 2021 update. 2021: Geneva.
- 2.World Health Organization . World Health Organization; Geneva: 2020. Global tuberculosis report 2020. [Google Scholar]
- 3.Hamada, Y., et al., Tests for tuberculosis infection: landscape analysis. Eur Respir J, 2021. [DOI] [PubMed]
- 4.MSF Access Campaign . Médecins Sans Frontières; Geneva: 2019. Time for $5: GeneXpert diagnostic tests. [Google Scholar]
- 5.Pai, M. and J. Furin, Tuberculosis innovations mean little if they cannot save lives. eLife, 2017. 6: p. e25956. [DOI] [PMC free article] [PubMed]
- 6.Albert H., Nathavitharana R.R., Isaacs C., Pai M., Denkinger C.M., Boehme C.C. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur Respir J. 2016;48(2):516–525. doi: 10.1183/13993003.00543-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singhroy D.N., MacLean E., Kohli M., Lessem E., Branigan D., England K., et al. Adoption and uptake of the lateral flow urine LAM test in countries with high tuberculosis and HIV/AIDS burden: current landscape and barriers [version 2; peer review: 2 approved] Gates Open Research. 2020;4:24. doi: 10.12688/gatesopenres10.12688/gatesopenres.13112.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cazabon D., Pande T., Kik S., Van Gemert W., Sohn H., Denkinger C., et al. Market penetration of Xpert MTB/RIF in high tuberculosis burden countries: A trend analysis from 2014–2016 [version 2; peer review: 4 approved] Gates Open Research. 2018;2:35. doi: 10.12688/gatesopenres10.12688/gatesopenres.12842.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pai M. 2020. Global Health Technologies: Time To Re-Think The ‘Trickle Down’ Model. [Google Scholar]
- 10.World Health Organization . World Health Organization; Geneva: 2019. Global tuberculosis report 2019. [Google Scholar]
- 11.Stop TB Partnership/Global Drug Facility, June 2021 Diagnostics Catalog. 2021: Geneva.
- 12.World Health Organization, WHO Emergency Use Listing for In vitro diagnostics (IVDs) Detecting SARS‐CoV‐2. 2021.
- 13.World Health Organization, WHO consolidated guidelines on tuberculosis. Module 2: screening – systematic screening for tuberculosis disease. 2021: Geneva. [PubMed]
- 14.The Global Fund. List of TB Diagnostic test kits and equipments classified according to the Global Fund Quality Assurance Policy, version 6. 2021 2021/08/05]; Available from: https://www.theglobalfund.org/media/9461/psm_productsdiagnosticstb_list_en.pdf.
- 15.World Health Organization. Public announcement to TB in vitro diagnostics manufacturers, procurement agencies and national TB programmes on inclusion of WHO Prequalification for TB in vitro diagnostics. 2021 2021/08/13]; Available from: https://www.who.int/publications/m/item/public-announcement-to-tb-in-vitro-diagnostics-manufacturers.
- 16.The Global Fund, Global Fund quality assurance policy for diagnostics products. 2017.
- 17.Prakash. K; Brizzolara, G., How China can reshape the mechanics of global healthcare innovation by taking on Tuberculosis: An interview with Professor Madhukar Pai. 2019.
- 18.Denkinger C.M., et al. Guidance for the Evaluation of Tuberculosis Diagnostics That Meet the World Health Organization (WHO) Target Product Profiles: An Introduction to WHO Process and Study Design Principles. J Infect Dis. 2019;220(Supplement_3):S91–S98. doi: 10.1093/infdis/jiz097. [DOI] [PubMed] [Google Scholar]
- 19.Schumacher S.G., et al. Guidance for Studies Evaluating the Accuracy of Sputum-Based Tests to Diagnose Tuberculosis. J Infect Dis. 2019;220(Supplement_3):S99–S107. doi: 10.1093/infdis/jiz258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drain P.K., et al. Guidance for Studies Evaluating the Accuracy of Biomarker-Based Nonsputum Tests to Diagnose Tuberculosis. J Infect Dis. 2019;220(Supplement_3):S108–S115. doi: 10.1093/infdis/jiz356. [DOI] [PubMed] [Google Scholar]
- 21.Nathavitharana R.R., et al. Guidance for Studies Evaluating the Accuracy of Tuberculosis Triage Tests. J Infect Dis. 2019;220(Supplement_3):S116–S125. doi: 10.1093/infdis/jiz243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georghiou S.B., et al. Guidance for Studies Evaluating the Accuracy of Rapid Tuberculosis Drug-Susceptibility Tests. J Infect Dis. 2019;220(Supplement_3):S126–S135. doi: 10.1093/infdis/jiz106. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization, High-priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting. 2014: Geneva.
- 24.World Health Organization . World Health Organization; Geneva: 2021. Target product profile for next-generation drug-susceptibility testing at peripheral centres. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stop TB Partnership. Accelerator for Impact (a4i). 2021 2021/08/13]; Available from: http://stoptb.org/siif/a4i/.
- 26.FEND-TB. Who We Are. 2020; Available from: https://www.fend-tb.org/who-we-are.
- 27.R2D2 TB Network. What We Do. 2021 2021/08/13]; Available from: https://www.r2d2tbnetwork.org/what-we-do.