Abstract
In Gaucher disease (GD), genetic deficiency of acid β-glucosidase leads to accumulation of its substrate glucosylceramide (GlcCer) and glucosylsphingosine (GlcSph). Lipid-laden cells, most prominently seen as macrophages engorged with GlcCer and GlcSph-laden lysosomes, trigger chronic metabolic inflammation and multisystemic phenotypes. Among the pleiotropic effects of inflammatory cascades, the induction of glucosylceramide synthase accentuates the primary metabolic defect. First-line therapies for adults with GD type 1 include Enzyme Replacement Therapy (ERT) and eliglustat Substrate Reduction Therapy (SRT). The ENCORE phase 3 clinical trial of eliglustat demonstrated non-inferiority compared to ERT. It is not known whether switching stable patients from long-term ERT to SRT results in the incremental reversal of the disease phenotype and its surrogate indicators. Herein, we report real-world experience from a single tertiary referral center of 38 adult GD type 1 patients, stable on long-term ERT (mean 13.3 years), who switched to eliglustat SRT (mean 3.1 years). After switch to SRT, there was significant reduction in spleen volume (P = 0.003) while liver volume, which was normal at baseline, remained unchanged. Platelet counts increased significantly (P = 0.026). Concomitantly, there was reduction of three validated biomarkers of Gaucher disease activity: plasma GlcSph decreased from 63.7 ng/ml (95% CI, 37.6-89.8) to 26.1 ng/ml (95% CI, 15.7-36.6) (P < 0.0001); chitotriosidase fell from 1136.6 nmol/ml/h (95% CI, 144.7-2128.6) to 466.9 nmol/ml/h (95% CI, 209.9-723.9) (P = 0.002); and glycoprotein non-metastatic melanoma B (gpNMB) decreased from 59.3 ng/ml (95% CI, 39.7-78.9) to 43.6 ng/ml (95% CI, 30.7-56.6) (P = 0.0006). There were no episodes of avascular necrosis or fractures in patients on SRT. Patients reported favorable experiences of switching from alternate week infusions to oral therapy. Collectively, these results demonstrate that the switch to eliglustat SRT from ERT leads to incremental response, even in stable patients after long-term ERT.
Keywords: Gaucher disease, Substrate reduction therapy, Glucosylsphingosphingosine, Eliglustat, Splenomegaly
1. Introduction
Gaucher disease is caused by biallelic mutations in GBA1 that underlie defective acid β-glucosidase and lysosomal accumulation of glucosylceramide (GlcCer) and its downstream metabolite, glucosylsphingosine (GlcSph) [1]. Lysosomal accumulation of these bioactive immunostimulatory lipids, especially in the macrophages, leads to system-wide metabolic inflammation and a complex phenotype involving the liver, spleen, bones, lungs, and lymph nodes [2]. In addition, there is early onset of neurodegenerative disease in neuronopathic GD types 2 and 3. Recombinant mannose-terminated glucocerebrosidase ERT is targeted to the macrophage mannose receptor and it has been the standard of care since the 1990s [3], [4]. It has led to clinically significant visceral and hematologic responses and reduced risk of osteonecrosis [4]. ERT requires life-long biweekly infusions due to its short tissue half-life and low volume of distribution, as a result of massive uptake of the therapeutic enzyme by hepatic and splenic macrophages, coupled with relatively minor uptake in other organs [5], [6]. Fibrosis occurs frequently in advanced GD, that further hinders delivery of recombinant enzyme to key sites of pathology [7]. There is also significant heterogeneity in Gaucher macrophage populations that can contribute to suboptimal delivery of the enzyme [8]. These considerations have led to sustained efforts to develop small molecule substrate reduction therapy (SRT) via inhibitors of glucosylceramide synthase (GCS) [9].
There are two available SRTs. Miglustat, an iminosugar, exhibits low efficacy and elicits many adverse reactions; therefore, it was approved for rare adults with GD1 in whom ERT is not an option. In contrast, eliglustat, a ceramide analog, is approved as first-line therapy for adults, dosed according to their CYP2D6 metabolizer status. Of the several phase 3 trials of eliglustat, the randomized open label ENCORE clinical trial examined the efficacy of switching stable patients in long-term ERT who had achieved therapeutic goals to eliglustat monotherapy. At one year, the primary end point was met for non-inferiority of eliglustat compared to ERT and long-term results showed continuing stability on eliglustat for up to 4 years [10], [11]. It is not known whether there is an incremental response after switch from ERT to eliglustat SRT. Real-world outcomes of switching from ERT to eliglustat were recently reported from the ICGG Registry (ClinicalTrials.gov: NCT00358943), showing further decrease of splenomegaly and chitotriosidase, an established biomarker of Gaucher disease [12].
Observational Registry data can be confounded by missing data and variability in clinical practice of disease monitoring. Therefore, we assessed real world outcomes of the switch from ERT to eliglustat SRT in a cohort of patients followed longitudinally at a tertiary referral center, where patients undergo a uniform and comprehensive evaluation of GD. The patients had been on long-term ERT with well-documented, detailed outcome data and achieved therapeutic goals, as established by Pastores et al. [13]. This was a single-center study at a tertiary referral center; hence, it followed a uniform protocol for comprehensive evaluation of Gaucher disease, allowing for detailed examination of therapeutic effects and tolerability. Herein, we report that the switch to eliglustat SRT after long-term ERT is well tolerated and results in an incremental response of Gaucher disease activity indicators.
2. Methods
2.1. Patients
We assessed outcomes in 38 patients with GD1 who have been on ERT for a minimum of two years and reached therapeutic goals, before switching to eliglustat SRT. Diagnosis of GD was based on diminished levels (<10%) of acid β-glucosidase activity in peripheral blood leukocytes and GBA1 genotyping. Patients were followed regularly with standard of care evaluations, including MRI to assess organomegaly and marrow infiltration, and laboratory testing [14]. Serum samples were collected at each clinic visit to determine biomarker values and trends. Pre-SRT (before switch) samples were taken at the time of the last clinical visit while the patient was receiving ERT, before switching over to SRT. The study is approved by Yale School of Medicine Human Investigation Committee and all patients provided informed consents. Overall, patients tolerated eliglustat without adverse reactions, except for transient GI reflux which resolved upon taking eliglustat with water. Two patients reverted to ERT, due to persistent reflux in one patient and unexplained fatigue in the other; data on both patients for the duration of SRT was retained in the analysis to assess treatment effect. Post-SRT (after switch) levels were measured at the most recent clinical follow-up after starting eliglustat. Thus, pre-, and post-SRT data were obtained for GlcSph, gpNMB, and chitotriosidase, as well as other indicators of disease activity.
2.2. Chitotriosidase
Serum chitotriosidase activities were determined using a method described previously, with slight modifications [15]. Assay buffer pH was changed briefly from 5.2 to 4.5, and the final concentration of 4-Methylumbelliferyl-beta-D-N,N’,N”-triacetylchitotriose (4MUT) fluorogenic substrate was 10 uM. Pre- and post-SRT samples were assayed at the same time to minimize inter-assay variability. CHIT1 genotyping was performed to normalize serum levels as described previously [16]. There were no patients homozygous null CHIT1 genotype in the study cohort. Patients heterozygous for the null allele had their chitotriosidase doubled to normalize to the wild type homozygous genotype, as described previously [17].
2.3. Glucosylsphingosine
Serum glucosylsphingosine levels were measured, as described previously, via liquid chromatography coupled to tandem mass spectrometry, LC-MS/MS [17].
2.4. Glycoprotein non-metastatic melanoma B
Serum concentrations of gpNMB were determined using the enzyme-linked immunosorbent assay (ELISA) kit (DY2550) from R&D systems (Minneapolis, MN) according to the manufacturer's instructions and as described previously [15].
2.5. Imaging
Patients underwent standard-of-care MRI imaging for liver and spleen volumes and marrow infiltration. DXA bone density was performed annually [14].
2.6. Statistical analysis
Pre- and post-switch data were assessed by the Shapiro-Wilk test to determine normal distribution, and if normal, was followed by mean comparison through paired parametric t-tests, or, when the data did not fit normal distribution, the Wilcoxon Signed Rank test. Values are recorded as: mean with 95% CI. Statistical analyses were performed using Prism 8.0.
3. Results
3.1. Patient demographics
Patients who switched from ERT to eliglustat SRT, “switch patients,” represented a diverse spectrum of genotypes and treatment lengths, with some having been on ERT for several years and others being in their third decade of treatment (Fig. 1). All patients had type 1 Gaucher disease. Thirty six of thirty eight patients had at least one N370S allele. The mean duration of ERT was 171.5 months (ranging 2 to 26 years). At the time of final analysis, switch patients were treated with SRT for a mean of 36.7 months (ranging 5 months to 7 years). We retained the two patients who reverted to ERT in analysis to capture their response prior to switching back. Cumulatively, the cohort represents 543 patient years of ERT experience and, after switch, 116 patient years of SRT experience. The mean age prior to therapy switch was 47 years, (ranging afrom 15 to 80 years). A pediatric patient with severe infusion-related reactions to all ERT preparations was treated with SRT and treatment was well-tolerated. The mean age post-SRT was 51 years (ranging 16 to 84 years). Since the study focused on GD1, the predominant genotype was N370S homozygous. Some individuals were compound heterozygotes, with N370S as the first allele. Two individuals harbored genotypes not typical to GD1 patients: one patient with the genotype R463C/F216Y and the other L444P/G377S. Four patients had undergone prior splenectomy. There was no change in weight on SRT (data not shown). Additional demographic data is provided in Table 1 of the supplementary materials.
Fig. 1.
Patients’ treatment timelines and genotypes. Bars to the left of zero (blue) indicate number of months on enzyme replacement therapy; bars to the right of zero (green) indicate number months on substrate reduction therapy, following switch.
Table 1.
Baseline characteristics of patient cohort.
|
Demographic characteristics | ||
|---|---|---|
| Parameter | Before Switch/ERT | After Switch/SRT |
| No. of patients | 38 | 38 |
| Sex, n (%) | ||
| Male | 23 (61) | |
| Female | 15 (39) | |
| Age at time of visit in years, mean (min-max) |
47 (15-80) | 51 (16-84) |
| N = 38 | N = 38 | |
| Weight at time of visit in kilograms, mean (min-max) |
78.7 (48.2-131.8) | 80.0 (49.9-120.0) |
| N = 36 | N = 36 | |
| No. of splenectomized patients, n (%) | 4 (11) | 4 (11) |
| Length of treatment in months, mean (min-max) |
171.5 (24-312) | 36.7 (5-85) |
| N = 38 | N = 38 | |
| Length of treatment in years, mean (min-max) |
14.3 (2-26) | 3.1 (0.4-7) |
| N = 38 | N = 38 | |
| Patient years of treatment | 543 | 116 |
| N = 38 | N = 38 | |
| CYP2D6-metabolizer phenotype, n (%) | ||
| Ultra-rapid | 1 (3) | |
| Extensive | 26 (68) | |
| Intermediate | 9 (23) | |
| Poor | 2 (6) | |
| Genotype, n (%) | ||
| N370S/N370S | 19 (50) | |
| N370S/Other | 17 (45) | |
| Other/Other | 2 (5) | |
3.2. Hematological and visceral disease indicators
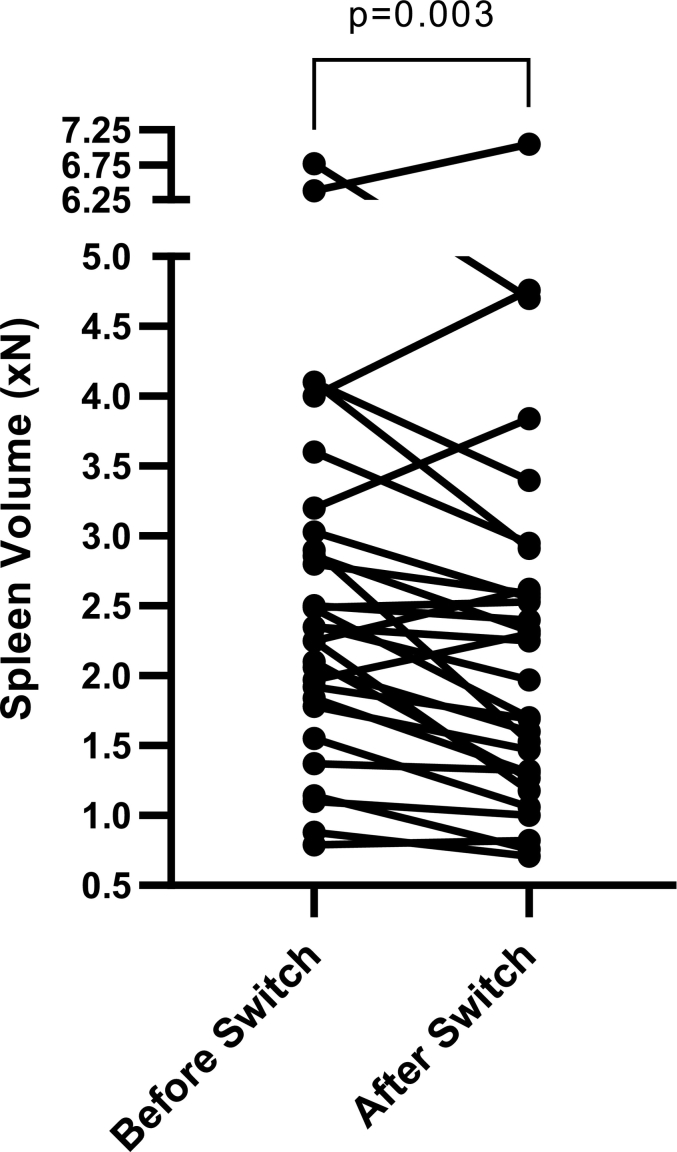
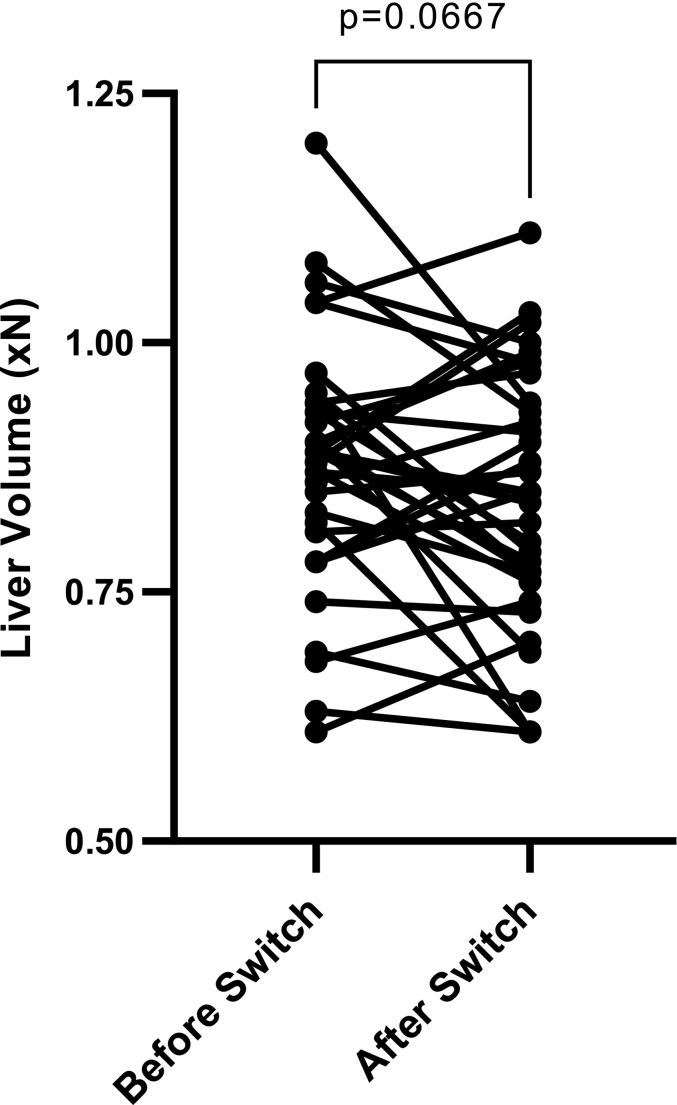
Spleen volume data did not follow a normal distribution while liver volume data showed normal distribution. Therefore, spleen volume data was analyzed using the Wilcoxon-Signed Rank test and liver volume was analyzed through standard paired parametric t-test. After the switch to SRT, spleen volumes decreased significantly, while liver volumes remained stable (Fig. 2). Spleen volumes decreased from 2.6xN (95% CI, 2.1-3.2) to 2.3xN (95% CI, 1.8-2.8), P = 0.003 (xN is the multiple of normal organ volume, i.e. 0.2% body weight for spleen volume and 2.5% body weight for liver volume). In 5 patients, the baseline spleen volume after long-term ERT was ≥4 x N. In 3 of these patients spleen volume decreased. In one patient, spleen volume increased from 6.38 x N to 7.05 x normal. However, this patient discontinued SRT after only few months due to reflux. In another patient, spleen volume increased from 4.0 x N to 4.76 x Normal. Liver volume showed a decreasing trend from 0.88xN (95% CI, 0.84-0.92) to 0.84xN (95% CI, 0.80-0.89), P = 0.067, but it was not statistically significant.
Fig. 2.
A:Spleen volume reported as multiples of normal before and after switch to SRT. Pre-SRT spleen volume: 2.63 xN: 95% CI, 2.11-3.15; post-SRT spleen volume: 2.29 xN: 95% CI, 1.77-2.81; (n = 30, p = 0.0030).
B: Liver volume reported as multiples of normal before and after switch to SRT. Pre-SRT liver volume: 0.88 xN: 95% CI, 0.84-0.92; post-SRT spleen volume: 0.84 xN: 95% CI, 0.80-0.89; (n = 35, p = 0.0667).
Platelets and hemoglobin were normal prior to switch. Using Shapiro-Wilk normality tests, platelet count and hemoglobin data were found to be normally distributed and analyzed with a standard paired parametric t-test. Before the switch to SRT, platelet counts were 195 109/L (95% CI, 176-214). After the switch to SRT, platelet counts increased to 212 109/L (95% CI, 187-236), P = 0.026. Pre-SRT hemoglobin was:14.7 g/dl: 95% CI, 14.3-15.2 and remained stable post-SRT:14.5 g/dl: 95% CI, 14.0-15.1 (P = 0.3281) (Fig. 3). Two patients had decrease in hemoglobin. In one patient, this was related to progression of his myeloma and in the second patient it was due to iron deficiency anemia which normalized after iron supplements.
Fig. 3.
A: Platelet count reported in 109 per liter before and after switch to SRT. Pre-SRT platelet count: 194.87 109/L: 95% CI, 176.29-213.45; post-SRT platelet count: 211.63 109/L: 95% CI, 187.38-235.89; (n = 38, p = 0.0262).
B: Hemoglobin reported in grams per deciliter before and after switch to SRT. Pre-SRT hemoglobin: 14.74 g/dl: 95% CI, 14.32-15.16; post-SRT hemoglobin: 14.53 g/dl: 95% CI, 14.02-15.05; (n = 38, p = 0.3281).
There was no change in ALT or AST (data not shown).
3.3. Serum biomarker response to switch from ERT to SRT
Wilcoxon Signed Rank tests were used to compare the pre- and post-switch measurements for all three GD biomarkers, as the biomarker data did not exhibit normal distribution. SRT plasma glucosylsphingosine was reduced strikingly: from 63.7 ng/ml (95% CI, 37.6-89.8) to 26.1 ng/ml (95% CI, 15.7-36.6), P < 0.0001 (Fig. 5), on SRT. The upper limit of the normal distribution of plasma glucosylsphingosine is 3.3 ng/ml. Notably, 15 patients showed reduction of serum GlcSph to near normal levels. Concomitantly, serum chitotriosidase fell from 1137 nmol/ml/h (95% CI, 145-2129) to 467 nmol/ml/h (95% CI, 209.9-724), P = 0.002 (Fig. 4). Serum soluble gpNMB (also known as osteoactivin) levels at baseline on ERT were 59.3 ng/ml (95% CI, 39.7-78.9) and fell, on SRT, to 43.6 ng/ml (95% CI, 30.7-56.6), P = 0.0006 (Fig. 6). The healthy control gpNMB levels were 19.4 ng/ml (95% CI, 17.2-21.5). Similar to GlcSph, some patients achieved normal levels of gpNMB on SRT. Five patients who achieved normal glucosylsphingosine on SRT, also achieved normal GpNMB.
Fig. 5.
Glucosylsphingosine (GlcSph) levels reported in nanograms per milliliter before and after switch to SRT. Pre-SRT GlcSph: 63.73 ng/ml: 95% CI, 37.62-89.84; post-SRT GlcSph: 26.13 ng/ml: 95% CI, 15.69-36.56; (n = 34, p < 0.0001).
Fig. 4.
Chitotriosidase activity reported in nanomoles per milliliter per hour before and after switch to SRT. Pre-SRT chitotriosidase activity: 1136.61 nmol/ml/h: 95% CI, 144.65-2128.58; post-SRT chitotriosidase activity: 466.91 nmol/ml/h: 95% CI, 209.94-723.94; (n = 31, p = 0.0020).
Fig. 6.
Glycoprotein Non-metastatic Melanoma B (gpNMB) levels reported in nanograms per milliliter before and after switch to SRT. Pre-SRT gpNMB: 59.28 ng/ml: 95% CI, 39.67-78.89; post-SRT gpNMB: 43.62 ng/ml: 95% CI, 30.68-56.56; (n = 24, p = 0.0006).
No patients experienced bone crises, avascular necrosis, or fractures during SRT. Bone density remained unchanged, but the duration of SRT was too short to reliably assess this metric, which is known for lagging behind other parameters in treatment response. Among 6 patients who were in osteopenia range, bone density Z scores at the lumbar spine improved in 4 patients, remained unchanged in one patients and in one patient, it deteriorated but remained within osteopenia range (data not shown). Capture of bone density data was not as complete as for other response indicators analyzed, hence complete analysis of bone density response could not be performed.
About 10% of patients experienced transient reflux, which was managed by patient education on taking pills with water and short-term (2-4 weeks) proton pump inhibitor.
4. Discussion
The standard of care for GD1 has been life-long biweekly intravenous infusions of macrophage targeted ERT. In 2014, an oral first line SRT for adults was approved, in the form of eliglustat tartrate, dosed according to CYP2D6 metabolizer status [18]. ERT has been remarkably effective in reversing hematological, visceral, and some skeletal manifestations [19]. However, there is very high uptake of exogenous enzyme in the liver and the spleen mediated by macrophage mannose receptor (MMR), hindering its delivery to the bone marrow and other organs [5], [6]. This pattern of organ distribution contributes to significant unmet needs in GD1 [20]. In contrast, small molecule SRT (molecular weight 404 vs ERT ~63,000) exhibits a vastly greater volume of distribution, enabling delivery of the therapeutic molecule to organs and cells other than MMR-expressing macrophages that are involved in GD1 pathophysiology. The importance of therapeutic targeting of glucosylceramide synthase in GD is underscored by delineation of inflammatory pathways in Gaucher disease which paradoxically increase GCS, thus amplifying the underlying enzyme deficiency [21]. Eliglustat, a glucosylceramide analog, is a specific and potent inhibitor of glucosylceramide synthase, and its mechanism of action in GD1 is reduction of GlcCer synthesis, alongside its downstream metabolite GlcSph [22]. Clinical trials and real world data suggest that outcomes of eliglustat SRT are similar to those achieved with ERT. In phase 3 ENCORE trial, clinical stability was maintained after switching from ERT to SRT, and there was early indication of incremental bone density response [10], [11].
Herein, we report outcomes in 38 GD1 patients who switched from ERT to SRT. In these stable patients, there was further decrease of splenomegaly, improvement of platelet count, and reduction of key biomarkers of GD activity. The biomarkers: chitotriosidase, GlcSph, and gpNMB, have been shown in numerous studies to reflect disease activity in untreated patients and response to ERT, that correlates well with visceral and hematologic responses. Moreover, once therapeutic goals with respect to hematological and visceral parameters have been achieved, the residual biomarker levels reflect ongoing disease activity in the bones and possibly in other organs [23], [24]. Our patients had achieved therapeutic goals with respect to visceral and hematological parameters, therefore residual biomarker levels are more reflective of disease activity in other organs, such as the bone marrow. Collectively, these results demonstrate the incremental reversal of GD phenotypes, some nearing healthy control levels.
The biomarkers that we examined reflect multidimensional pathologic pathways that underpin the Gaucher disease phenotype [25]. Serum levels of GlcSph fell significantly after the switch from ERT to SRT and, in some patients, were restored to normal levels. Serum levels of chitotriosidase and gpNMB are uniquely produced by Gaucher cells and reflect the body burden of glucosylceramide-laden macrophages. In our cohort, patients had achieved the therapeutic goal on ERT and the residual chitotriosidase level used in this context is likely to reflect disease activity in the marrow compartment. We found significant further reduction of serum chitotriosidase levels after switching from ERT to SRT. Similarly, the soluble fragment of gpNMB (osteoactivin) has been shown to be produced by lipid-laden Gaucher cells and validated as a biomarker of disease burden and response therapy [26]. Serum levels of gpNMB have also been found to correlate with the burden of bone disease. We found that gpNMB serum fell significantly after the switch from ERT to SRT, with some patients achieving normal levels. However, the duration of SRT is considered relatively short for the purpose of examining effects on bone density. Nevertheless, there were no episodes of avascular necrosis or fractures within the total of 116 patient-years of SRT experience in our study.
While ERT is efficacious in reducing GD manifestations, especially visceral and hematologic parameters, and in preventing some skeletal complications after long-term ERT, the response may plateau with residual mild to moderate organomegaly [4]. Here, we report that long-term ERT patients, who have previously reached therapeutic goals, show further significant decrease of spleen volume and a non-significant trend of decreasing liver volume after switching to eliglustat SRT. Determined by magnetic resonance imaging, spleen volume decreased by ~13% following the switch. Similar results were observed in the ENCORE trial after 4 years of SRT treatment and real-world experience reported from the ICGG Registry. Notably, we found that, although platelet counts at baseline on ERT were normal, there was significant increase on SRT. Together, these data bode well for the subset of patients who have had poor platelet response to ERT, in some cases attributed to extensive focal masses of Gaucher cells and fibrosis [27], [28]. These data are consistent with the known efficacy of eliglustat SRT in maintaining long-term ERT outcomes, and, moreover, result in an incremental response.
Chitotriosidase is the most extensively used biomarker for monitoring GD. Many studies have documented a massive elevation of chitotriosidase in untreated GD that falls after ERT. However, chitotriosidase plateaus after two to five years of ERT, reflecting low level chronic macrophage activation. Studies suggest that after reversal of visceral disease, such a residual level of biomarker reflects activity in the bone marrow compartment [24]. In our patient cohort of ERT-to-SRT switch patients, chitotriosidase activity was decreased by ~60% from the baseline residual elevation. We found similar effects of the switch from ERT to SRT in another validated marker for measuring the total burden of Gaucher cells: gpNMB. Unlike chitotriosidase, gpNMB is not subject to common polymorphism-dependent serum levels, demonstrating the consistency of incremental biomarker response after the switch to SRT.
It should be noted that gpNMB as well as chitotriosidase levels represent body burden of GSL-laden macrophages, while LysoGL1 is an indicator of buildup of storage lipids in all cell types beyond macrophage system.
The root cause of macrophage activation, immune dysregulation, and aspects of GD phenotypes have been increasingly attributed to glucosylsphingosine (GlcSph), the downstream metabolite of glucosylceramide [29], [30]. Plasma levels of glucosylsphingosine are validated as a critical diagnostic and prognostic biomarker and it is now an established key biomarker for monitoring treatment response [31]. We have previously shown that GlcSph decreases severalfold after three years of ERT, but that levels plateaued in the years after to 13.4xULN. Using propensity scoring, we found that eliglustat SRT was a significant determinant of lower GlcSph compared to ERT [17]. In the clinic, this plateau remains stable after decades of continued ERT. After switching to eliglustat SRT, GlcSph levels fell to >50% of pre-SRT levels, as shown in Fig. 5. It should be noted that 15 patients achieved near normal levels. These findings are consistent with the response of other markers, as well visceral and hematological responses following switch from ERT.
Overall, patients reported good tolerability with the convenience of oral SRT, obviating the need for two weekly intravenous infusions as reported in clinical trials [21]. In the two patients who reverted to ERT, clinical response to SRT before the switch back was similar to that seen in other patients.
There are some limitations to our study. Namely, it is a single center study. However, an advantage of such a tertiary center study is its uniformity of data collection and fewer missing data points, for example the recent ICGG Registry report. In two patients, hemoglobin decreased significantly. One patient had progression of smoldering myeloma and another patient developed transient iron deficiency anemia. In both patients, all three serum biomarkers improved. These observations underscore the complexity of capturing therapeutic responses in real-world setting, importance of using biomarker set that captures multidimensional pathophysiology of Gaucher disease and utility of tertiary referral center studies where relatively large cohorts of patients are followed longitudinally in a uniform protocol. With respect to clinical relevance of improvements seen in this study, our cohort included patients on long term ERT with significant residual disease indicated by the fact that 7 patients had markedly elevated glucosylsphingosine levels >100 ng/ml despite, many years of ERT. These patients had achieved visceral and hematological therapeutic goals; hence the residual elevation of biomarker levels is emanating from the bone marrow compartment. Such a concept has been validated previously [24]. Hence the effects we have observed are clinically relevant in addressing unmet needs in Gaucher disease.
5. Conclusion
Phase 3 clinical trials of eliglustat SRT have demonstrated non-inferiority compared to ERT. In our single center study with uniform monitoring protocol, patients with Gaucher disease type 1, at therapeutic goals on long-term enzyme replacement therapy, generally tolerated the switch to SRT without major adverse reactions. The switch of a stable patient from ERT to SRT resulted in an incremental response reflected by several indicators of disease activity. Visceral indicators of GD improved, with reduction of spleen volume and increased platelet count. Concomitantly, there was a decrease in three key circulating GD biomarkers: chitotriosidase, glucosylsphingosine, and gpNMB. Collectively, our analysis of therapeutically stable GD1 patients, who switched from long-term ERT to SRT, demonstrates improved clinical outcomes. The three biomarkers we studied have been shown to reflect skeletal disease in the context of stable visceral and hematological disease; therefore, the assessment of long-term skeletal outcomes of patients on SRT will be of interest.
Disclosures
Dr. Pramod Mistry has received research grant support and travel support from Sanofi Genzyme. He has received support from NIH grant R01AR06593.
Bailin Zhang, Lilu Guo, and Katherine Klinger are employees for Sanofi Genzyme who performed glucosylsphingosine analysis.
Acknowledgements
We thank the patients for the privilege to provide their care and their participation in this study.
References
- 1.Grabowski G.A., Antommaria A.H.M., Kolodny E.H., Mistry P.K. Gaucher disease: basic and translational science needs for more complete therapy and management. Mol. Genet. Metab. 2021;132(2):59–75. doi: 10.1016/j.ymgme.2020.12.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grabowski G.A., Petsko G.A., Kolodny E., Valle D., Beaudet A.L., Vogelstein B., Kinzler K.W., Antonarakis S.E., Ballabio A., et al., editors. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw Hill; New York: 2010. [Google Scholar]
- 3.Barton N.W., Brady R.O., Dambrosia J.M., Di Bisceglie A.M., Doppelt S.H., Hill S.C., Mankin H.J., Murray G.J., Parker R.I., Argoff C.E., Grewal R.P., Yu K. Replacement therapy for inherited enzyme deficiency–macrophage-targeted glucocerebrosidase for Gaucher’s disease. N. Engl. J. Med. 1991;324(21):1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- 4.Weinreb N.J., Camelo J.S., Jr., Charrow J., Jr., McClain M.R., Jr., Mistry P., Jr., Belmatoug N., Jr., International Collaborative Gaucher Group (ICGG) Gaucher Registry ( NCT00358943) investigators Gaucher disease type 1 patients from the ICGG Gaucher Registry sustain initial clinical improvements during twenty years of imiglucerase treatment. Mol. Genet. Metab. 2021;132(2):100–111. doi: 10.1016/j.ymgme.2020.12.295. [DOI] [PubMed] [Google Scholar]
- 5.Mistry P.K., Wraight E.P., Cox T.M. Therapeutic delivery of proteins to macrophages: implications for treatment of Gaucher’s disease. Lancet. 1996;348(9041):1555–1559. doi: 10.1016/S0140-6736(96)04451-0. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y.H., Ponce E., Sun Y., Leonova T., Bove K., Witte D., Grabowski G.A. Turnover and distribution of intravenously administered mannose-terminated human acid beta-glucosidase in murine and human tissues. Pediatr. Res. 1996;39(2):313–322. doi: 10.1203/00006450-199602000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Beutler E., Kuhl W., Vaughan L.M. Failure of alglucerase infused into Gaucher disease patients to localize in marrow macrophages. Mol. Med. 1995;1:320–324. doi: 10.1007/BF03401556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boven L.A., van Meurs M., Boot R.G., Mehta A., Boon L., Aerts J.M., Laman J.D. Gaucher cells demonstrate a distinct macrophage phenotype and resemble alternatively activated macrophages. Am. J. Clin. Pathol. 2004;122(3):359–369. doi: 10.1309/BG5V-A8JR-DQH1-M7HN. [DOI] [PubMed] [Google Scholar]
- 9.Platt F.M., d’Azzo A., Davidson B.L., Neufeld E.F., Tifft C.J. Lysosomal storage diseases. Nat. Rev. Dis. Primers. 2018;4(1):27. doi: 10.1038/s41572-018-0025-4. [DOI] [PubMed] [Google Scholar]
- 10.Cox T.M., Drelichman G., Cravo R., Balwani M., Burrow T.A., Martins A.M., Lukina E., Rosenbloom B., Ross L., Angell J., Puga A.C. Eliglustat compared with imiglucerase in patients with Gaucher’s disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomised, open-label, non-inferiority trial. Lancet. 2015;385(9985):2355–2362. doi: 10.1016/S0140-6736(14)61841-9. [DOI] [PubMed] [Google Scholar]
- 11.Cox T.M., Drelichman G., Cravo R., Balwani M., Burrow T.A., Martins A.M., Lukina E., Rosenbloom B., Goker-Alpan O., Watman N., El-Beshlawy A., Kishnani P.S., Pedroso M.L., Gaemers S.J.M., Tayag R., Peterschmitt M.J. Eliglustat maintains long-term clinical stability in patients with gaucher disease type 1 stabilized on enzyme therapy. Blood. 2017;129(17):2375–2383. doi: 10.1182/blood-2016-12-758409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mistry P.K., Balwani M., Charrow J., Kishnani P., Niederau C., Underhill L.H., McClain M.R. Real-world effectiveness of eliglustat in treatment-naïve and switch patients enrolled in the international collaborative Gaucher group Gaucher registry. Am. J. Hematol. 2020;95(9):1038–1046. doi: 10.1002/ajh.25875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pastores G.M., Weinreb N.J., Aerts H., Andria G., Cox T.M., Giralt M., Grabowski G.A., Mistry P.K., Tylki-Szymanska A. Therapeutic goals in the treatment of Gaucher disease. Semin. Hematol. 2004;41(4 Suppl 5):4–14. doi: 10.1053/j.seminhematol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Charrow J., Esplin J.A., Gribble T.J., Kaplan P., Kolodny E.H., Pastores G.M., Scott C.R., Wappner R.S., Weinreb N.J., Wisch J.S. Gaucher disease: recommendations on diagnosis, evaluation, and monitoring. Arch. Intern. Med. 1998;158(16):1754–1760. doi: 10.1001/archinte.158.16.1754. [DOI] [PubMed] [Google Scholar]
- 15.Murugesan V., Liu J., Yang R., Lin H., Lischuk A., Pastores G., Zhang X., Chuang W.L., Mistry P.K. Validating glycoprotein non-metastatic melanoma B (gpNMB, osteoactivin), a new biomarker of Gaucher disease. Blood Cells Mol. Dis. 2018;68:47–53. doi: 10.1016/j.bcmd.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boot R.G., Renkema G.H., Verhoek M., Strijland A., Bliek J., de Meulemeester T.M., Mannens M.M., Aerts J.M. The human chitotriosidase gene: nature of inherited enzyme deficiency. J. Biol. Chem. 1998;273(40):25680–25685. doi: 10.1074/jbc.273.40.25680. [DOI] [PubMed] [Google Scholar]
- 17.Murugesan V., Chuang W.L., Liu J., Lischuk A., Kacena K., Lin H., Pastores G.M., Yang R., Keutzer J., Zhang K., Mistry P.K. Glucosylsphingosine is a key biomarker of Gaucher disease. Am. J. Hematol. 2016;91(11):1082–1089. doi: 10.1002/ajh.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes D.A., Pastores G.M. Eliglustat for Gaucher’s disease: trippingly on the tongue. Lancet. 2015;385(9985):2328–2330. doi: 10.1016/S0140-6736(15)60206-9. [DOI] [PubMed] [Google Scholar]
- 19.Weinreb N.J., Charrow J., Andersson H.C., Kaplan P., Kolodny E.H., Mistry P., Pastores G., Rosenbloom B.E., Scott C.R., Wappner R.S., Zimran A. Effectiveness of enzyme replacement therapy in 1028 patients with type 1 Gaucher disease after 2 to 5 years of treatment: a report from the Gaucher registry. Am. J. Med. 2002;113(2):112–119. doi: 10.1016/s0002-9343(02)01150-6. [DOI] [PubMed] [Google Scholar]
- 20.Mehta A. Gaucher disease: unmet treatment needs. Acta Paediatr. 2008;97(457):83–87. doi: 10.1111/j.1651-2227.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- 21.Peterschmitt M.J., Freisens S., Underhill L.H., Foster M.C., Lewis G., Gaemers S.J.M. Long-term adverse event profile from four completed trials of oral eliglustat in adults with Gaucher disease type 1. Orphanet J. Rare Dis. 2019;14(1):128. doi: 10.1186/s13023-019-1085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox T.M. Eliglustat tartrate, an orally active glucocerebroside synthase inhibitor for the potential treatment of gaucher disease and other lysosomal storage diseases. Curr. Opin. Investig. Drugs. 2010;11(10):1169–1181. [PubMed] [Google Scholar]
- 23.Poll L.W., Koch J.A., Willers R., Aerts H., Scherer A., Häussinger D., Mödder U., vom Dahl S. Correlation of bone marrow response with hematological, biochemical, and visceral responses to enzyme replacement therapy of nonneuronopathic (type 1) Gaucher disease in 30 adult patients. Blood Cells Mol. Dis. 2002;28(2):209–220. doi: 10.1006/bcmd.2002.0511. [DOI] [PubMed] [Google Scholar]
- 24.de Fost M., Hollak C.E., Groener J.E., Aerts J.M., Maas M., Poll L.W., Wiersma M.G., Häussinger D., Brett S., Brill N. Superior effects of high-dose enzyme replacement therapy in type 1 Gaucher disease on bone marrow involvement and chitotriosidase levels: a 2-center retrospective analysis. Blood. 2006;108(3):830–835. doi: 10.1182/blood-2005-12-5072. [DOI] [PubMed] [Google Scholar]
- 25.Ferraz M.J., Kallemeijn W.W., Mirzaian M., Moro D.Herrera, Marques A., Wisse P., Boot R.G., Willems L.I., Overkleeft H.S., Aerts J.M. Gaucher disease and Fabry disease: new markers and insights in pathophysiology for two distinct glycosphingolipidoses. Biochim. Biophys. Acta. 2014;1841(5):811–825. doi: 10.1016/j.bbalip.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Kramer G., Wegdam W., Donker-Koopman W., Ottenhoff R., Gaspar P., Verhoek M., Nelson J., Gabriel T., Kallemeijn W., Boot R.G., Laman J.D., Vissers J.P., Cox T., Pavlova E., Moran M.T., Aerts J.M., van Eijk M. Elevation of glycoprotein nonmetastatic melanoma protein B in type 1 gaucher disease patients and mouse models. FEBS Open Bio. 2016;6(9):902–913. doi: 10.1002/2211-5463.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein P., Malhotra A., Haims A., Pastores G.M., Mistry P.K. Focal splenic lesions in type I Gaucher disease are associated with poor platelet and splenic response to macrophage-targeted enzyme replacement therapy. J. Inherit. Metab. Dis. 2010;33(6):769–774. doi: 10.1007/s10545-010-9175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollak C.E., Belmatoug N., Cole J.A., Vom Dahl S., Deegan P.B., Goldblatt J., Rosenbloom B., van Dussen L., Tylki-Szymanska A., Weinreb N.J., Zimran A., Cappellini M.D. Characteristics of type I Gaucher disease associated with persistent thrombocytopenia after treatment with imiglucerase for 4–5 years. Br. J. Haematol. 2012;158(4):528–538. doi: 10.1111/j.1365-2141.2012.09175.x. [DOI] [PubMed] [Google Scholar]
- 29.Mistry P.K., Liu J., Yang M., Nottoli T., McGrath J., Jain D., Zhang K., Keutzer J., Chuang W.L., Mehal W.Z., Zhao H., Lin A., Mane S., Liu X., Peng Y.Z., Li J.H., Agrawal M., Zhu L.L., Blair H.C., Robinson L.J., Iqbal J., Sun L., Zaidi M. Glucocerebrosidase gene-deficient mouse recapitulates Gaucher disease displaying cellular and molecular dysregulation beyond the macrophage. Proc. Natl. Acad. Sci. U. S. A. 2010;107(45):19473–19478. doi: 10.1073/pnas.1003308107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair S., Boddupalli C.S., Verma R., Liu J., Yang R., Pastores G.M., Mistry P.K., Dhodapkar M.V. Type II NKT-TFH cells against gaucher lipids regulate B-cell immunity and inflammation. Blood. 2015;125(8):1256–1271. doi: 10.1182/blood-2014-09-600270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dekker N., van Dussen L., Hollak C.E., Overkleeft H., Scheij S., Ghauharali K., van Breemen M.J., Ferraz M.J., Groener J.E., Maas M., Wijburg F.A., Speijer D., Tylki-Szymanska A., Mistry P.K., Boot R.G., Aerts J.M. Elevated plasma glucosylsphingosine in gaucher disease: relation to phenotype, storage cell markers, and therapeutic response. Blood. 2011;118(16):e118–e127. doi: 10.1182/blood-2011-05-352971. [DOI] [PMC free article] [PubMed] [Google Scholar]