Abstract
The diagnosis of a potentially lethal cardiovascular disease in a young athlete presents a complex dilemma regarding athlete safety, patient autonomy, team or institutional risk tolerance and medical decision-making. Consensus cardiology recommendations previously supported the ‘blanket’ disqualification of athletes with hypertrophic cardiomyopathy (HCM) from competitive sport. More recently, epidemiological studies examining the relative contribution of HCM as a cause of sudden cardiac death (SCD) in young athletes and reports from small cohorts of older athletes with HCM that continue to exercise have fueled debate whether it is safe to play with HCM. Shared decision-making is endorsed within the sports cardiology community in which athletes can make an informed decision about treatment options and potentially elect to continue competitive sports participation. This review critically examines the available evidence relevant to sports eligibility decisions in young athletes diagnosed with HCM. Histopathologically, HCM presents an unstable myocardial substrate that is vulnerable to ventricular tachyarrhythmias during exercise. Studies support that young age and intense competitive sports are risk factors for SCD in patients with HCM. We provide an estimate of annual mortality based on our understanding of disease prevalence and the incidence of HCM-related SCD in different athlete populations. Adolescent and young adult male athletes and athletes participating in a higher risk sport such as basketball, soccer and American football exhibit a greater risk. This review explores the potential harms and benefits of sports disqualification in athletes with HCM and details the challenges and limitations of shared decision-making when all parties may not agree.
Keywords: cardiovascular, death, heart disease, prevention, sport
‘Medicine is a science of uncertainty and an art of probability’.—William Osler (1849–1919)
Introduction
Sudden cardiac arrest (SCA) is the leading cause of sudden death in young competitive athletes during sports and exercise.1–3 The aetiology of SCA in young athletes (<30 years of age) includes a diverse group of genetic and congenital cardiac diseases such as cardiomyopathies, ion channel disorders and anomalous coronary arteries. Early detection of conditions at elevated risk of SCA through cardiovascular screening allows the potential for risk reduction by disease-specific management and individualised activity restrictions.4 In 2005, both the American Heart Association (AHA) and European Society of Cardiology (ESC) advised the routine disqualification of athletes from competitive sport following the diagnosis of a potentially sinister cardiac disorder capable of causing sudden cardiac death (SCD).5 6 However, new guidelines by the AHA/American College of Cardiology (2015) and the ESC/European Association of Preventive Cardiology (2019) no longer support a simple binary ‘yes/no’ approach to sports eligibility decisions.7 8
Pelliccia et al recently reported results in adult patients with hypertrophic cardiomyopathy (HCM) and the risk of major adverse cardiovascular events with and without continued sports participation.9 In 88 adult athletes (median age 31 years) with a predominantly low-risk HCM phenotype followed for a 7-year period, the study found no difference in the risk of SCA in patients choosing to continue competitive sports versus those who selected out of intensive exercise.9 While the study lacks power to draw definitive conclusions, the results provide important data to inform activity recommendations in middle-aged adult athletes with HCM. However, the authors appropriately caution that these results should not be applied to adolescent and younger adult athletes or those with a more severe HCM phenotype where the risk of SCD with intensive exercise may be higher.
How should these results impact a rapidly evolving paradigm for sports eligibility decisions in athletes identified with conditions at risk for SCA/SCD? This review addresses the principles and limitations of shared decision-making in the context of a potentially lethal cardiovascular disorder, with specific focus on the risks and current controversy of allowing competitive athletes with HCM to return to play. A critical analysis of HCM-related SCD is presented to guide evidence-informed sports eligibility decisions. While not the primary intent of this review, the information presented may be applicable to young persons diagnosed with HCM who wish to engage in recreational sports and exercise.
Shared decision-making in sports cardiology
Shared decision-making is a collaborative process between the patient, other stakeholders (eg, parents) and the physician that combines the principles of informed consent and patient autonomy.10 11 A shared decision-making approach considers the values and preferences of the patient and is especially important when more than one management option is reasonable. Within sports cardiology eligibility decisions, all stakeholders in the outcome are ideally involved in the decision process with consent from the patient (or parents of children), including family members, athletic trainers, physiotherapists, team physicians, cardiologists, coaches and team, school or club officials. A thoughtful discussion of the potential harms and benefits of different treatment options, including continued competitive sports participation, should be explored using the best available evidence. Ideally, the final decision should be acceptable to all parties.
A paradigm shift has evolved regarding a return to sport for competitive athletes diagnosed with cardiovascular disease.10 12 Disorders that once led to universal sports disqualification are now considered for return to play on a case-by-case basis. Shared decision-making in sports cardiology was first highlighted in young individuals with long QT syndrome (LQTS).13 Two observational studies both reported that young athletes with genotype-positive LQTS who underwent appropriate counselling, optimal medical management and remained treatment compliant could return to sport with a low rate of SCA and no increase in mortality.13 14
However, risk mitigation for other cardiovascular diseases that afflict young athletes may not be possible. For instance, compelling data indicate that intense exercise increases the risk of ventricular arrhythmias and disease progression in patients with arrhythmogenic cardiomyopathy.15–18 Indeed, recommendations against competitive sports for patients diagnosed with arrhythmogenic cardiomyopathy remain universally supported among sports cardiology experts. Thus, patient autonomy regarding sports participation is not absolute, and shared decision-making should not be oversimplified as a process where the athlete simply makes the decision.
HCM-related SCD in the young
How should we approach shared decision-making for athletes diagnosed with HCM? Specifically, is there evidence that vigorous exercise or competitive sport increases SCD risk in young athletes with HCM and would exercise restrictions lower their risk? Conversely, is there a subgroup of athletes with HCM who may be able to safely participate in intensive exercise?
Although SCD in sport is relatively uncommon, HCM is frequently diagnosed at autopsy in young athletes, especially in countries without systematic inclusion of a 12-lead ECG in the preparticipation screening programme. Such circumstantial evidence supports that HCM is associated with SCD in young competitive athletes. However, the actual proportion of cases attributable to HCM is variable and may be dependent on the quality of the postmortem examination to differentiate HCM from left ventricular hypertrophy (LVH) commonly found in athletes. In a series of 1306 athletes with SCD in the USA, 842 had an autopsy-confirmed cardiovascular diagnosis. HCM was the leading identified structural disorder representing 36% of confirmed diagnoses and 23% of all cardiovascular cases.3 An analysis of SCD in college athletes from the USA as well as a prospective study of elite adolescent soccer players from the UK both found that HCM represented about one-third of SCD cases (33%–37%).19 20 While other studies have reported a lower proportion of SCD cases due to HCM (6%–16%), HCM remains an important cause of SCD in young athletes.1 2 21 22
Early studies also demonstrated that SCD is more common in younger patients with HCM. A 1982 report examined autopsy and patient records in 78 patients with HCM from the National Heart, Lung, and Blood Institute and found that 71% of SCD cases occurred before age 30% and 63% of these occurred during exertion.23 A 2000 report of 86 patients with HCM-related deaths also found that SCD was more common in younger patients (age 5–25 years) compared with HCM-related stroke and heart failure as the cause of death in older patients.24 A Portuguese registry of 1042 patients with HCM (mean age 53±16 years) also found that heart failure was the most common cause of death in older patients.25
Annual SCD mortality rate in patients with HCM
The risk of SCD in patients with HCM is impacted by several factors including age, symptoms, family history, disease morphology and electrodiagnostics. Early studies reported an annual mortality rate of approximately 4% in children with HCM.26 27 In a 1976 study of 35 children with HCM (age <15 years) followed for 7.4 years, one-third of patients died suddenly (4% mortality per year).26 Similarly, a 1984 study examined 37 children (ages 1–14 years at diagnosis) followed for 9 years and reported an annual mortality rate of 4.3%.27 More recently, a 2011 study from the National Institute of Health followed 131 younger patients (≤20 years of age) for a median of 6.4 years.28 The annual rate of sudden death or life-threatening arrhythmic events was 2%, with a mean age of 20.1 years at the time of the arrhythmic event and a higher risk if septal thickness was ≥20 mm.28
Conventional risk markers for patients with HCM include a prior cardiac arrest, family history of HCM-related sudden death, unexplained recent syncope, multiple episodes of non-sustained ventricular tachycardia (VT), massive LVH (wall thickness ≥30 mm), left ventricular apical aneurysm or an ejection fraction <50%0.29 Other potential risk mediators include extensive late gadolinium enhancement on cardiac MRI, a hypotensive response to exercise, or marked left ventricular outflow tract obstruction at rest. Patients with one or more major risk markers have a primary indication for an implantable cardioverter defibrillator (ICD) and a predicted event rate for VT or ventricular fibrillation (VF) of 4% per year.29
Risk stratification and selective use of ICDs have greatly improved HCM-related mortality.30–33 In 474 younger patients with HCM between 7 and 29 years (mean age 20) with an average follow-up of 7.1 years, 5-year and 10-year survival with application of existing cardiovascular treatment strategies was 97% and 94%, respectively.30 Sixty-three patients experienced sudden death, resuscitated cardiac arrest or appropriate ICD intervention—an annual risk of 1.8% per year including non-fatal HCM events.30 Predictors of HCM mortality and non-fatal aborted events after multivariate analysis included younger age at diagnosis, increased left atrial dimension and greater left ventricular thickness.30
Survival has also improved in older adults with HCM through an enhanced American College of Cardiology/AHA risk stratification model combined with contemporary management strategies. In a cohort of 2094 patients with HCM (mean age 51), 527 received primary prevention ICDs. During a mean follow-up of 4.7 years, 82 (15.6%) patients experienced device terminated VT/VF and no patient with an ICD died from SCD.33 In the 1567 patients without an ICD, 12 (0.8%) had SCA/SCD, of which 7 patients had high-risk indicators but declined an ICD.33 Overall, the annual mortality rate was only 0.1%, supporting that a modern risk stratification and prevention model may avert most SCD in a general population of predominantly older patients with HCM.33
Limited data are available regarding the use of ICDs in competitive athletes with HCM. The ICD sports safety registry studied 328 patients (mean age 33 years) for ICD events over a median follow-up of 31 months and documented no deaths or resuscitated cardiac arrests.34 However, within this cohort, only 60 patients were considered competitive athletes and only 13 had HCM.34 This study provides initial evidence that continued sports participation for patients with HCM and an ICD may be safe, although the small sample size does not allow for definitive conclusions.
Race, sport and HCM-related SCD
There is a potential association of race and HCM-related SCD in young athletes.35 In an autopsy-confirmed series of 842 cases of SCD in athletes (aged 19±6 years) in the USA, the cardiovascular death rate was fivefold higher among African Americans and other minorities than among whites (1:12 778 vs 1:60 746 athlete-years; p<0.001), and HCM accounted for 42% of cases in African Americans and other minorities versus 31% of cases in white athletes (p<0.001).3 Similarly, in a prospective study of SCA/SCD in US competitive athletes including 19 HCM-related cases, HCM represented 30.3% of confirmed diagnoses in male African American athletes compared with only 10.0% of male white athletes (p=0.0186).2 In contrast, a study of 425 older patients with HCM (163 black and 262 white; mean age 52.5 years) from the UK found no difference in the composite primary outcome of cardiovascular death, cardiac arrest or appropriate device therapy over a mean follow-up of 4.3 years (Kaplan-Meier survival analysis, p logrank=0.095).36 These divergent findings regarding the relationship of race and SCD may reflect a difference in risk between young athletes engaging in strenuous exercise compared with a more benign clinical course in older patients with HCM. In addition, the higher prevalence of HCM as a cause of SCD among young black athletes may be in part ascribed to a greater increase in physiological LVH in Afro-Caribbean athletes that can be misinterpreted as HCM at postmortem examination.37 38
HCM also accounts for a higher proportion of cases in male basketball (23.3%) and American football (25%) athletes than in other sports.2 Studies examining US college athletes also have documented higher rates of cardiomyopathy as a cause of SCD in male basketball and American football athletes.1 19 Similarly, in a prospective study of adolescent soccer players (95% male), HCM accounted for 3 of 8 (37%) SCD cases observed over a mean follow-up period of 10.6 years.20 Thus, HCM may represent a disproportionate number of SCA/SCD events in higher risk athlete groups—younger black athletes, male basketball, male soccer and American football—underscoring the need for additional caution when considering a return to competitive sports within these risk groups.
Can we quantify the risk of SCD in athletes with HCM?
SCD from HCM is inherently unpredictable. We have little understanding of the inciting event that suddenly leads to a lethal ventricular tachyarrhythmia in athletes who may have harboured their disease for years. Furthermore, the stresses of the athletic arena are not fully reflected in laboratory testing, highlighting the need for large longitudinal studies. Risk calculators have been developed but are not applicable to young athletes. For example, the ESC HCM Risk-SCD Score estimates the 5-year risk of SCD in patients with HCM but specifically states it should not be used in elite/competitive athletes or paediatric patients<16 years.
While the precise risk of SCD in a young athlete with HCM is challenging to predict, an estimate of risk can be drawn from our current understanding of disease prevalence and the incidence of SCD in competitive athletes (table 1). Based on the annual incidence of SCD in prior studies, the proportion of HCM-related SCD and a disease prevalence from 1:2000 to 1:500 in athletes, we estimate the annual risk of SCD in young athletes with HCM is between 0.1% and 6.6% per year depending on the athlete population.1 2 39 While the risk may exceed the upper limit of what was calculated, the estimated range is consistent with annual mortality rates previously reported in younger patients with HCM.28 30 However, the spectrum of disease phenotypes in athletes is likely less severe than that in the general population, as athletes competing at a high level may not exhibit high-risk phenotypes that could potentially impede athletic performance (ie, greater maximum wall thickness or high left ventricular outflow tract gradients). Phenotypic features of the athletic HCM heart were reported in 106 young athletes (14–35 years) with HCM and compared with 101 sedentary patients with HCM.40 Athletes with HCM had milder LVH (15.8±3.4 mm vs 19.7±6.5 mm, p<0.001) that was frequently confined to the apex (36%), larger left ventricular cavity dimensions (47.8±6.0 mm vs 44.3±7.7 mm, p<0.001) and superior indices of diastolic function (average E/E′ 7.9±2.4 vs 10.7±3.9, p<0.001) compared with sedentary patients with HCM.40
Table 1.
Estimated annual mortality rate from sudden cardiac death in young athletes with HCM
| Modellingassumptions: | |||||
| |||||
| Study and athlete population | Annual incidence of SCD (athlete-years) | Total Number of SCD Cases (in a population of 500000) |
Proportion of SCD from HCM | Number of Cases of SCD from HCM | Annual mortality rate from HCM-related SCD |
| Maron et al 19 | |||||
| College athletes | 1:62 500 | 8 | 32.80% | 2.6 | 0.26%–1.0% |
| Black college athletes | 1:26 315 | 19 | 44.70% | 8.5 | 0.85%–3.4% |
| White college athletes | 1:142 857 | 3.5 | 44.70% | 1.6 | 0.2%–0.6% |
| Harmon et al 1 | |||||
| College athletes | 1:53 703 | 9.3 | 8% | 0.7 | 0.07%–0.3% |
| Black college athletes | 1:21 491 | 23.3 | 8% | 1.9 | 0.2%–0.8% |
| White college athletes | 1:68 354 | 7.3 | 8% | 0.6 | 0.06%–0.2% |
| Division I male basketball | 1:5200 | 96.2 | 8% | 7.7 | 0.8%–3% |
| Maron et al 3 From the US national registry | |||||
| Black/minority athletes | 1:12 778 | 39.1 | 42% | 16.4 | 1.6%–6.6% |
| White athletes | 1:60 746 | 8.2 | 31% | 2.6 | 0.3%–1.0% |
| Harmon et al 22 | |||||
| Male high school athletes | 1:68 742 | 7.3 | 14% | 1 | 0.1%–0.4% |
| Female high school athletes | 1:316 679 | 1.6 | 14% | 0.2 | 0.02%–0.08% |
| Malhotra et al 20 | |||||
| Elite adolescent soccer (95% male)* | 1:14 794 | 33.8 | 37.50% | 12.7 | 1.3%–5.1% |
| Corrado et al 52 | |||||
| Competitive athletes≤35 years old from Veneto region of Italy† | 1:62 500 | 8 | 2% | 0.2 | 0.02%–0.08% |
*Study population underwent cardiovascular screening with ECG and echocardiogram at age 16 years.
†Study population underwent annual cardiovascular screening with ECG.
HCM, hypertrophic cardiomyopathy; SCD, sudden cardiac death.
Our estimates are influenced by the reported incidence of SCD in the athlete population, and higher mortality rates were calculated in black athletes, male athletes and athletes in higher risk sports such as basketball and soccer. Importantly, estimates do not account for resuscitated cardiac arrest in which survival has been reported in up to 48% of athlete cases.41 Thus, the annual risk of any life-threatening ventricular arrhythmia will be higher than the estimates presented.
Is exercise beneficial in patients with HCM?
Recent studies have demonstrated that exercise is beneficial for older patients with HCM. The Randomized Exploratory Study of Exercise Training in Hypertrophic Cardiomyopathy trial established that 16 weeks of moderate-intensity exercise resulted in a small but significant increase in exercise capacity in older adult patients (mean age 50.4 years) with HCM.42 These modest increases in cardiorespiratory fitness may be associated with reductions in cardiovascular mortality.43 Likewise, a retrospective survey of adult patients with HCM (mean age 49 years) suggested that lifetime vigorous exercise correlated with favourable diastolic function and was not associated with ventricular arrhythmias.44 A supervised cardiac rehabilitation exercise programme in symptomatic patients with HCM (mean age 62±13 years) also demonstrated improved functional capacity.45 While these studies inform activity recommendations for older adult patients with HCM, they were not designed to establish safety in adolescent and young adult competitive athletes where the disease course and exercise intensity may be profoundly different.
Events during rest, sleep or exercise?
While the occurrence of SCA/SCD during exercise is well documented in patients with HCM, there is debate if exercise itself presents a higher risk than more sedentary activity. This controversy was recently fueled by a 2019 study examining HCM-related SCD in the general (non-athlete) population (median age 35 years) in Ontario, Canada, that reported 71% of cases occurred during sleep, rest or light activity.46 Similarly, in a study of 196 decedents with HCM (age 43±18 years) reviewed by a specialist cardiac pathology centre in the UK, SCD during exercise occurred in just 46 (23%) individuals but was confined to younger male subjects (age 30±11 years).47 Comorbid obstructive sleep apnea which may be more common in older patients with HCM has been proposed as a risk factor for SCD during sleep, although evidence is limited.48 In younger athletes who engage in intensive training more regularly, SCD during exercise appears to be more common. In a study of 1306 cases of SCD in young athletes including 302 from HCM, 74% of events occurred during practice or competition.3 In a cohort of 79 cases of SCD in college athletes (8% from HCM), 56% occurred with exertion, 22% at rest and 14% during sleep (9% unknown).1 In a prospective series of 179 cases of SCA/SCD in US competitive athletes, 76.0% occurred during or within 1 hour of exercise and 21.8% during rest or sleep (2.2% unknown).2 Additional reports from Sweden and Spain also found cardiomyopathy to be the most common cause of exercise-related SCD in athletes.49 50
Histopathologically, HCM presents an unpredictable arrhythmogenic substrate demonstrating myocardial disarray and interstitial and myocardial scarring as a result of microvascular ischaemia.29 Athletes engaging in intense exercise undergo powerful epinephrine surges, extreme metabolic stresses and dehydration, all of which could provoke a ventricular tachyarrhythmia in the setting of a pathological myocardial substrate. The effect of physiological cardiac adaptations potentially augmenting pathological hypertrophy in athletes with HCM raises additional concern. In a 2019 study of American football players, weight gain and increased systolic blood pressure were associated with development of a pathological cardiovascular phenotype consisting of concentric LVH, arterial stiffening and reduced left ventricular diastolic function.51 The potential adverse consequences of maladaptive cardiac remodelling in athletes with a genetic cardiomyopathy are unknown but potentially troubling. Could exercise-induced substrate changes in patients with HCM make sudden death at any time (during rest or physical exertion) more likely?
The proportion of athletes with SCA/SCD during exercise is further magnified considering vigorous exercise only accounts for one-sixth or less of the total hours in the day even in the most dedicated athletes. Thus, while SCA/SCD can occur at any time in patients with HCM, evidence suggests that intense exercise is a risk factor for potentially lethal ventricular tachyarrhythmias, especially in younger male individuals.
Does disqualification in athletes with HCM decrease SCD?
While limited evidence is available, two prospective studies in young competitive athletes provide important insights into whether stopping competitive sports reduces mortality in athletes diagnosed with HCM. A 1998 study reported on 33 335 screened athletes (mean age 19 years) from the Veneto region of Italy.52 HCM was identified in 22 athletes, all of whom were disqualified from competitive sport and survived over 8 years of follow-up. One athlete with undetected HCM died, compared with 16 HCM-related deaths in the unscreened (non-athlete) general population. While the study was not powered to provide definitive conclusions, early detection of HCM in competitive athletes followed by sports disqualification led to a 73% risk reduction in mortality.52 Similarly, 11 168 elite adolescent soccer players (mean age 16.4 years) from the UK underwent cardiovascular screening and were followed for a mean of 10.6 years.20 Five athletes were diagnosed with HCM through screening; three athletes stopped competitive soccer and survived, while two athletes continued playing against medical advice and died during exercise.20
Potential harms of disqualification
The disqualification of a young athlete from competitive sports is a complex and difficult decision that requires serious considerations. Not surprisingly, athletes diagnosed with potentially lethal cardiac disorders are at risk for significant psychological distress.53 Sports participation establishes healthy lifestyles, builds self-confidence and provides an avenue to educational scholarships or the potential financial rewards of professional sport that may carry different significance depending on the athlete’s socioeconomic background. Athletic dedication and success also are integral to the self-identify of a young athlete, and removal from competitive sports likely will have short-term and possibly long-term consequences on mental and emotional health. In addition, risk tolerance or avoidance is an individual assessment and important for patient autonomy in healthcare decisions.
A 2014 study examined the psychological impact of being diagnosed with a serious or potentially lethal cardiac disease in 25 young competitive athletes, including 3 athletes with HCM that were ultimately disqualified.54 Athletes progressed through four psychological stages following sports disqualification including: (1) immediate challenges to athlete identity, (2) grief/coping, (3) adaptation and (4) acceptance.54 Risk factors for increased psychological morbidity included a higher level of competition, permanent disqualification from sports, persistent reminders of their disease (eg, daily medication, monitoring heart rate during activity) and unanticipated outcomes (eg, failed procedures). Additionally, disqualified athletes were at risk for losing the structure and support afforded by a team environment, and elite or professional athletes may have difficulty shifting to an alternative career. However, early diagnosis also led to new goals such as mentoring or coaching. Remarkably, all athletes diagnosed through advanced cardiovascular screening stated they would repeat the process, suggesting SCD risk reduction was accepted over sports particpation.54
Thus, the potential harms of sports disqualification—loss of self-identity, loss of educational scholarship, loss of potential income and the risk of depression or mental health issues—should be carefully weighed and addressed in the shared decision-making process. Regardless of the outcome, mechanisms to provide emotional and psychological support for athletes diagnosed with a potentially career altering heart condition should be in place. In addition, while difficult to replace the social and psychological benefits of competitive sports, a programme that maintains safe levels of physical activity and cardiorespiratory fitness should be advised for athletes no longer participating in competitive sports. Studies defining the specifics of such programmes are still in the developmental stages.
Limitations of shared decision-making
Other issues warrant consideration in the context of life-critical shared decision-making. Most young athletes do not consider themselves vulnerable to the risks of sport, catastrophic injury or sudden death. In combination with the lure of fame and/or fortune or family pressure for economic gain, this raises an important ethical question: can young athletes truly make an unbiased and informed decision under duress?
Educational institutions, clubs and sports organisations also are impacted by the decision. High school and college administrations have a responsibility to protect the health and safety of their student athletes and potential liability concerns may shape a more conservative risk tolerance. In professional sports, some clubs may be adverse to even the small prospect of a player suffering SCA/SCD during a widely publicised event.
Medical team members, including team physicians and cardiologists, also may view eligibility decisions from different perspectives. Team physicians commonly provide medical ‘clearance’ to play sports. A determination of safety and eligibility is required for every athlete at the time of their preparticipation evaluation. Outside of cardiovascular concerns, team physicians periodically place activity restrictions or even permanent eligibility restrictions in other areas relevant to the health and safety of athletes—such as repetitive concussions, cervical spinal stenosis, or worsening joint degeneration—in which the competitive career of an athlete is stopped prematurely. While the best interest of the athlete is always the top priority, sports medicine professionals also may have an obligation to their club and an ethical responsibility to society to ensure safe athletic participation.
Consequently, in the setting of different perspectives or perhaps even ‘competing interests’, how does the shared decision-making process move forward?
Who ultimately decides?
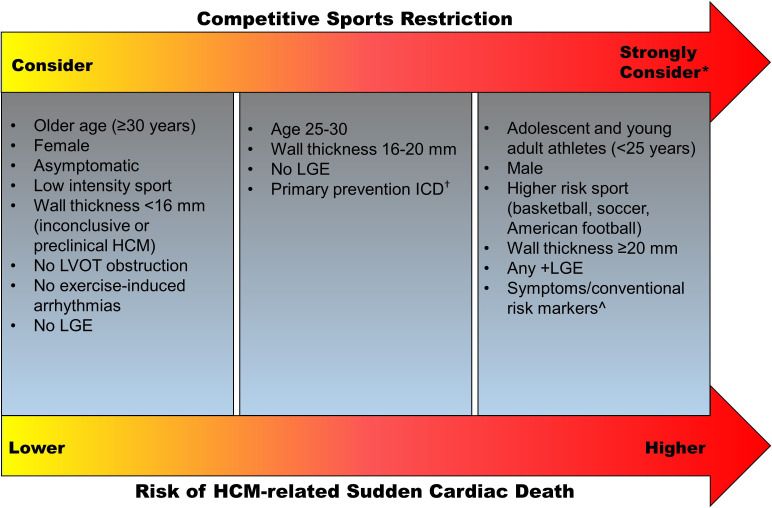
Ideally, an informed athlete will come to the same conclusion about sports participation as their medical team. However, the athlete, team physician, cardiologist, athletic trainer and school or club may have different thresholds to permit a return to competitive sports. For instance, what happens when the cardiologist recognises an athlete with HCM has an elevated risk of SCD but supports the athlete’s right to choose to play and the team physician recommends against it? Or if the team cardiologist/consultant recommends against participation but the athlete’s cardiologist or second opinion endorses a return to play? If all parties do not agree, shared decision-making ultimately comes down to who has the final authority to give the ‘yes’ or ‘no’ regarding eligibility. Allowing the athlete to make the final decision may unintentionally remove everyone else from the shared-decision-making process. Undoubtedly, balancing patient autonomy with respect for the common good in order to prevent catastrophic events in sport is a massive challenge. A proposed framework to consider competitive sports restriction in athletes based on the risk of HCM-related SCD is provided in figure 1.
Figure 1.
Proposed framework for eligibility decisions in athletes with HCM. HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; LGE, late gadolinium enhancement; LVOT left ventricular outflow tract. *Strongly consider competitive sport restriction in individuals with multiple risk factors. †ICDs lower the risk of HCM-related SCD, but recommendations for an ICD are based on conventional high-risk markers and an ICD should not be placed for the sole purpose of allowing sports participation. ˆConventional risk markers include: family history of HCM-related sudden death, unexplained syncope, multiple or repetitive episodes of non-sustained ventricular tachycardia, massive left ventricular hypertrophy (wall thickness ≥30 mm), left ventricular apical aneurysm or an ejection fraction <50%.
In the USA, legal precedent supports that high school students with heart disease have no compelling right to participate in interscholastic sports without medical clearance and that college athletes with life-threatening cardiac conditions can be medically disqualified and eligibility decisions reside with the team physician.55 56 Some professional sports leagues, such as the National Football League (NFL), also explicitly state in the collective bargaining agreement with the NFL Players Association that the head team physician for each club has final decision authority. In some instances, one team may judge an athlete at unacceptable risk, while another, presented with the same evidence, may allow the athlete to participate. In Italy, guidelines for sports eligibility in athletes with heart disease is actually written into law.57 Thus, eligibility decisions may be influenced by the policies and laws dictated by the relevant country or sports governing body.
Conclusion
The diagnosis of serious cardiovascular disease in a competitive athlete presents complex issues regarding safety, patient autonomy and medical decision-making engrossed by scientific and clinical uncertainty. For disorders where effective risk reduction is supported by evidence, continued sports participation in treatment-compliant patients seems reasonable. HCM comprises an electrophysiological substrate that is unpredictable and potentially vulnerable to the stresses of intense exercise even in the absence of conventional risk markers. Epidemiological evidence indicates that HCM is an important cause of SCD in young athletes and sports restriction may prevent SCD. However, the quality of evidence is not sufficient to precisely define risk across all athletes diagnosed with HCM, making eligibility decisions very challenging in the era of shared decision-making. In addition, we lack adequately powered studies that investigate safety with exercise and HCM, particularly in young athletes participating in competitive sport who appear to be at greater risk. While moderate-intensity exercise seems beneficial in older patients with HCM, high-intensity training in young athletes with HCM may trigger life-threatening ventricular tachyarrhythmias. We estimate an annual risk of 0.1%–6.6% for the occurrence of SCD in adolescent or young adult competitive athletes with HCM. Higher risk is exhibited in males and athletes participating in basketball, soccer and American football. Risk stratification tools have been developed for the clinical management of patients with HCM. These were not derived from athletic populations and do not consider some of the unique considerations raised in this review. However, until a suitable alternative is available, these prognostic tools can be used with caution. Shared decision-making implies that the anticipated risk is acceptable to the patient, family, medical team and club/school. Based on this critical analysis, competitive sports participation in young athletes with HCM should be approached with caution, appropriate counselling and careful consideration for sports restriction based on HCM-related SCD risk. Additional research is needed to determine safe levels of exercise and sports participation in young persons with HCM and to develop evidence-based SCD risk stratification algorithms in athletes.
What is already known.
Sudden cardiac death (SCD) is the leading cause of non-traumatic fatalities in young competitive athletes (<30 years old) during sports and exercise.
Evidence supports that hypertrophic cardiomyopathy (HCM) is consistently represented in SCD series in young athletes, deeming it an important cause of SCD in young athletes.
Guidelines for sports participation in athletes with SCD-associated cardiovascular conditions have evolved where the treatment options, potential harms and benefits of continued sports participation and patient values are discussed within a shared decision-making model.
What are the new findings.
A critical review of the evidence confirms that young age is the period of highest risk for unexpected SCD in patients with HCM.
No study to date directly supports the safety of young athletes with HCM participating in competitive sports, while limited data suggest that disqualification from competitive sports may prevent SCD.
Although precisely quantifying the risk of SCD with HCM is challenging, we estimate, based on available evidence, that the risk of HCM-related SCD in young athletes ranges from 0.1% to 6.6% per year, with higher mortality rates in male athletes and athletes in higher risk sports such as basketball, soccer and American football.
Shared decision-making in sports cardiology involves a complex discussion of risk that must balance scientific evidence, clinical uncertainty, patient autonomy and concerns from all parties impacted by a decision to participate in competitive sport.
Footnotes
Twitter: @DreznerJon, @DrAneilMalhotra, @jordanprutkin, @MichaelPapadak2, @DrKimHarmon, @IrfAsif, @SSharmacardio
Contributors: JAD was involved in the conception, design, drafting, critical revision, and approval of the manuscript. AM, KGH, IMA, DSO, and JCM were involved in the conception, design, critical revision, and approval of the manuscript. JMP, MP, and SS were involved in the critical revision and approval of the manuscript. All authors agree to be accountable for all aspects of the work and ensure the integrity of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Harmon KG, Asif IM, Maleszewski JJ, et al. Incidence, cause, and comparative frequency of sudden cardiac death in national collegiate athletic association athletes: a decade in review. Circulation 2015;132:10–19. 10.1161/CIRCULATIONAHA.115.015431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson DF, Siebert DM, Kucera KL, et al. Etiology of sudden cardiac arrest and death in US competitive athletes: a 2-year prospective surveillance study. Clin J Sport Med 2020;30:305–14. 10.1097/JSM.0000000000000598 [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Haas TS, Ahluwalia A, et al. Demographics and epidemiology of sudden deaths in young competitive athletes: from the United States national registry. Am J Med 2016;129:1170–7. 10.1016/j.amjmed.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 4.Drezner JA, O'Connor FG, Harmon KG, et al. AMSSM position statement on cardiovascular Preparticipation screening in athletes: current evidence, knowledge gaps, recommendations and future directions. Br J Sports Med 2017;51:153–67. 10.1136/bjsports-2016-096781 [DOI] [PubMed] [Google Scholar]
- 5.Maron BJ, Zipes DP. 36Th Bethesda conference: eligibility recommendations for competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol 2005;45:1312–77. [DOI] [PubMed] [Google Scholar]
- 6.Pelliccia A, Fagard R, Bjørnstad HH, et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the study group of sports cardiology of the Working group of cardiac rehabilitation and exercise physiology and the Working group of myocardial and pericardial diseases of the European Society of cardiology. Eur Heart J 2005;26:1422–45. 10.1093/eurheartj/ehi325 [DOI] [PubMed] [Google Scholar]
- 7.Maron BJ, Udelson JE, Bonow RO, et al. Eligibility and Disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American heart association and American College of cardiology. Circulation 2015;132:e273–80. 10.1161/CIR.0000000000000239 [DOI] [PubMed] [Google Scholar]
- 8.Pelliccia A, Solberg EE, Papadakis M, et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: position statement of the sport cardiology section of the European association of preventive cardiology (EAPC). Eur Heart J 2019;40:19–33. 10.1093/eurheartj/ehy730 [DOI] [PubMed] [Google Scholar]
- 9.Pelliccia A, Caselli S, Pelliccia M, et al. Clinical outcomes in adult athletes with hypertrophic cardiomyopathy: a 7-year follow-up study. Br J Sports Med 2020;54:1008–12. 10.1136/bjsports-2019-100890 [DOI] [PubMed] [Google Scholar]
- 10.Baggish AL, Ackerman MJ, Lampert R. Competitive sport participation among athletes with heart disease: a call for a paradigm shift in decision making. Circulation 2017;136:1569–71. 10.1161/CIRCULATIONAHA.117.029639 [DOI] [PubMed] [Google Scholar]
- 11.Maron BJ, Nishimura RA, Maron MS. Shared decision-making in HCM. Nat Rev Cardiol 2017;14:125–6. 10.1038/nrcardio.2017.6 [DOI] [PubMed] [Google Scholar]
- 12.Drezner JA. Detect, manage, inform: a paradigm shift in the care of athletes with cardiac disorders? Br J Sports Med 2013;47:4–5. 10.1136/bjsports-2012-091963 [DOI] [PubMed] [Google Scholar]
- 13.Johnson JN, Ackerman MJ. Return to play? Athletes with congenital long QT syndrome. Br J Sports Med 2013;47:28–33. 10.1136/bjsports-2012-091751 [DOI] [PubMed] [Google Scholar]
- 14.Aziz PF, Sweeten T, Vogel RL, et al. Sports participation in genotype positive children with long QT syndrome. JACC Clin Electrophysiol 2015;1:62–70. 10.1016/j.jacep.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawant AC, Bhonsale A, te Riele ASJM, et al. Exercise has a disproportionate role in the pathogenesis of arrhythmogenic right ventricular dysplasia/cardiomyopathy in patients without desmosomal mutations. J Am Heart Assoc 2014;3:e001471. 10.1161/JAHA.114.001471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruwald A-C, Marcus F, Estes NAM, et al. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2015;36:1735–43. 10.1093/eurheartj/ehv110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James CA, Bhonsale A, Tichnell C, et al. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 2013;62:1290–7. 10.1016/j.jacc.2013.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 2017;376:61–72. 10.1056/NEJMra1509267 [DOI] [PubMed] [Google Scholar]
- 19.Maron BJ, Haas TS, Murphy CJ, et al. Incidence and causes of sudden death in U.S. college athletes. J Am Coll Cardiol 2014;63:1636–43. 10.1016/j.jacc.2014.01.041 [DOI] [PubMed] [Google Scholar]
- 20.Malhotra A, Dhutia H, Finocchiaro G, et al. Outcomes of cardiac screening in adolescent soccer players. N Engl J Med 2018;379:524–34. 10.1056/NEJMoa1714719 [DOI] [PubMed] [Google Scholar]
- 21.Finocchiaro G, Papadakis M, Robertus J-L, et al. Etiology of sudden death in sports: insights from a United Kingdom regional registry. J Am Coll Cardiol 2016;67:2108–15. 10.1016/j.jacc.2016.02.062 [DOI] [PubMed] [Google Scholar]
- 22.Harmon KG, Asif IM, Maleszewski JJ, et al. Incidence and etiology of sudden cardiac arrest and death in high school athletes in the United States. Mayo Clin Proc 2016;91:1493–502. 10.1016/j.mayocp.2016.07.021 [DOI] [PubMed] [Google Scholar]
- 23.Maron BJ, Roberts WC, Epstein SE. Sudden death in hypertrophic cardiomyopathy: a profile of 78 patients. Circulation 1982;65:1388–94. 10.1161/01.CIR.65.7.1388 [DOI] [PubMed] [Google Scholar]
- 24.Maron BJ, Olivotto I, Spirito P, et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 2000;102:858–64. 10.1161/01.cir.102.8.858 [DOI] [PubMed] [Google Scholar]
- 25.Cardim N, Brito D, Rocha Lopes L, et al. The Portuguese registry of hypertrophic cardiomyopathy: overall results. Rev Port Cardiol 2018;37:1–10. 10.1016/j.repc.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 26.Maron BJ, Henry WL, Clark CE, et al. Asymetric septal hypertrophy in childhood. Circulation 1976;53:9–19. 10.1161/01.CIR.53.1.9 [DOI] [PubMed] [Google Scholar]
- 27.McKenna WJ, Deanfield JE. Hypertrophic cardiomyopathy: an important cause of sudden death. Arch Dis Child 1984;59:971–5. 10.1136/adc.59.10.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moak JP, Leifer ES, Tripodi D, et al. Long-term follow-up of children and adolescents diagnosed with hypertrophic cardiomyopathy: risk factors for adverse arrhythmic events. Pediatr Cardiol 2011;32:1096–105. 10.1007/s00246-011-9967-y [DOI] [PubMed] [Google Scholar]
- 29.Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med 2018;379:655–68. 10.1056/NEJMra1710575 [DOI] [PubMed] [Google Scholar]
- 30.Maron BJ, Rowin EJ, Casey SA, et al. Hypertrophic cardiomyopathy in children, adolescents, and young adults associated with low cardiovascular mortality with contemporary management strategies. Circulation 2016;133:62–73. 10.1161/CIRCULATIONAHA.115.017633 [DOI] [PubMed] [Google Scholar]
- 31.Maron BJ, Maron MS. Contemporary strategies for risk stratification and prevention of sudden death with the implantable defibrillator in hypertrophic cardiomyopathy. Heart Rhythm 2016;13:1155–65. 10.1016/j.hrthm.2015.12.048 [DOI] [PubMed] [Google Scholar]
- 32.Maron BJ, Rowin EJ, Casey SA, et al. How hypertrophic cardiomyopathy became a contemporary treatable genetic disease with low mortality: shaped by 50 years of clinical research and practice. JAMA Cardiol 2016;1:98–105. 10.1001/jamacardio.2015.0354 [DOI] [PubMed] [Google Scholar]
- 33.Maron MS, Rowin EJ, Wessler BS, et al. Enhanced American College of Cardiology/American heart association strategy for prevention of sudden cardiac death in high-risk patients with hypertrophic cardiomyopathy. JAMA Cardiol 2019;4:644–57. 10.1001/jamacardio.2019.1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lampert R, Olshansky B, Heidbuchel H, et al. Safety of sports for athletes with implantable cardioverter-defibrillators: results of a prospective, multinational registry. Circulation 2013;127:2021–30. 10.1161/CIRCULATIONAHA.112.000447 [DOI] [PubMed] [Google Scholar]
- 35.Maron BJ, Carney KP, Lever HM, et al. Relationship of race to sudden cardiac death in competitive athletes with hypertrophic cardiomyopathy. J Am Coll Cardiol 2003;41:974–80. 10.1016/S0735-1097(02)02976-5 [DOI] [PubMed] [Google Scholar]
- 36.Sheikh N, Papadakis M, Panoulas VF, et al. Comparison of hypertrophic cardiomyopathy in Afro-Caribbean versus white patients in the UK. Heart 2016;102:1797–804. 10.1136/heartjnl-2016-309843 [DOI] [PubMed] [Google Scholar]
- 37.Di Paolo FM, Schmied C, Zerguini YA, et al. The athlete's heart in adolescent Africans: an electrocardiographic and echocardiographic study. J Am Coll Cardiol 2012;59:1029–36. 10.1016/j.jacc.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 38.Sheikh N, Papadakis M, Carre F, et al. Cardiac adaptation to exercise in adolescent athletes of African ethnicity: an emergent elite athletic population. Br J Sports Med 2013;47:585–92. 10.1136/bjsports-2012-091874 [DOI] [PubMed] [Google Scholar]
- 39.Basavarajaiah S, Wilson M, Whyte G, et al. Prevalence of hypertrophic cardiomyopathy in highly trained athletes: relevance to pre-participation screening. J Am Coll Cardiol 2008;51:1033–9. 10.1016/j.jacc.2007.10.055 [DOI] [PubMed] [Google Scholar]
- 40.Sheikh N, Papadakis M, Schnell F, et al. Clinical profile of athletes with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2015;8:e003454. 10.1161/CIRCIMAGING.114.003454 [DOI] [PubMed] [Google Scholar]
- 41.Drezner JA, Peterson DF, Siebert DM, et al. Survival after Exercise-Related sudden cardiac arrest in young athletes: can we do better? Sports Health 2019;11:91–8. 10.1177/1941738118799084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saberi S, Wheeler M, Bragg-Gresham J, et al. Effect of moderate-intensity exercise training on peak oxygen consumption in patients with hypertrophic cardiomyopathy: a randomized clinical trial. JAMA 2017;317:1349–57. 10.1001/jama.2017.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dias KA, Link MS, Levine BD. Exercise Training for Patients With Hypertrophic Cardiomyopathy: JACC Review Topic of the Week. J Am Coll Cardiol 2018;72:1157–65. 10.1016/j.jacc.2018.06.054 [DOI] [PubMed] [Google Scholar]
- 44.Dejgaard LA, Haland TF, Lie OH, et al. Vigorous exercise in patients with hypertrophic cardiomyopathy. Int J Cardiol 2018;250:157–63. 10.1016/j.ijcard.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 45.Klempfner R, Kamerman T, Schwammenthal E, et al. Efficacy of exercise training in symptomatic patients with hypertrophic cardiomyopathy: results of a structured exercise training program in a cardiac rehabilitation center. Eur J Prev Cardiol 2015;22:13–19. 10.1177/2047487313501277 [DOI] [PubMed] [Google Scholar]
- 46.Weissler-Snir A, Allan K, Cunningham K, et al. Hypertrophic cardiomyopathy-related sudden cardiac death in young people in Ontario. Circulation 2019;140:1706–16. 10.1161/CIRCULATIONAHA.119.040271 [DOI] [PubMed] [Google Scholar]
- 47.Finocchiaro G, Papadakis M, Tanzarella G, et al. Sudden death can be the first manifestation of hypertrophic cardiomyopathy: data from a United Kingdom pathology registry. JACC Clin Electrophysiol 2019;5:252–4. 10.1016/j.jacep.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 48.Aggarwal S, Jan MF, Agarwal A, et al. Hypertrophic cardiomyopathy associated with sleep apnea: serious implications and COGENT management strategy. Expert Rev Cardiovasc Ther 2015;13:277–84. 10.1586/14779072.2015.1004314 [DOI] [PubMed] [Google Scholar]
- 49.Morentin B, Suárez-Mier MP, Monzó A, et al. Sports-Related sudden cardiac death due to myocardial diseases on a population from 1-35 years: a multicentre forensic study in Spain. Forensic Sci Res 2019;4:257–66. 10.1080/20961790.2019.1633729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wisten A, Börjesson M, Krantz P, et al. Exercise related sudden cardiac death (SCD) in the young - Pre-mortal characterization of a Swedish nationwide cohort, showing a decline in SCD among athletes. Resuscitation 2019;144:99–105. 10.1016/j.resuscitation.2019.09.022 [DOI] [PubMed] [Google Scholar]
- 51.Kim JH, Hollowed C, Liu C, et al. Weight gain, hypertension, and the emergence of a maladaptive cardiovascular phenotype among US football players. JAMA Cardiol 2019;4:1221. 10.1001/jamacardio.2019.3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corrado D, Basso C, Schiavon M, et al. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med 1998;339:364–9. 10.1056/NEJM199808063390602 [DOI] [PubMed] [Google Scholar]
- 53.Asif IM, Price DE, Ewing A, et al. The impact of diagnosis: measuring the psychological response to being diagnosed with serious or potentially lethal cardiac disease in young competitive athletes. Br J Sports Med 2016;50:163–6. 10.1136/bjsports-2015-095560 [DOI] [PubMed] [Google Scholar]
- 54.Asif IM, Price D, Fisher LA, et al. Stages of psychological impact after diagnosis with serious or potentially lethal cardiac disease in young competitive athletes: a new model. J Electrocardiol 2015;48:298–310. 10.1016/j.jelectrocard.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 55.Paterick TE, Paterick TJ, Fletcher GF, et al. Medical and legal issues in the cardiovascular evaluation of competitive athletes. JAMA 2005;294:3011–8. 10.1001/jama.294.23.3011 [DOI] [PubMed] [Google Scholar]
- 56.Maron BJ, Mitten MJ, Quandt EF, et al. Competitive athletes with cardiovascular disease--the case of Nicholas Knapp. N Engl J Med 1998;339:1632–5. 10.1056/NEJM199811263392211 [DOI] [PubMed] [Google Scholar]
- 57.Biffi A, Delise P, Zeppilli P, et al. Italian cardiological guidelines for sports eligibility in athletes with heart disease: Part 1. J Cardiovasc Med 2013;14:477–99. 10.2459/JCM.0b013e32835f6a21 [DOI] [PubMed] [Google Scholar]