Abstract
Drug‐resistant focal epilepsy with regional neocortical seizure onsets originating from the posterior quadrant can be particularly difficult to treat with resective surgery due to the overlap with eloquent cortex. Published reports indicate that corticothalamic treatment targeting the anterior or centromedian nucleus of the thalamus with direct brain‐responsive stimulation may be an effective approach to treat regional neocortical epilepsy. The pulvinar has remained largely unstudied as a neurostimulation target to treat refractory epilepsy. Because the pulvinar has connections with the posterior quadrant, neurostimulation may be effective if applied to seizures originating in this area. We performed a retrospective chart review of patients with regional neocortical seizure onsets in the posterior quadrant treated with the RNS System. Demographics, epilepsy history, clinical seizure frequencies, and neuropsychological testing results were obtained from the chart. Electrocorticogram (ECoG) records stored by the RNS System were reviewed to evaluate electrographic seizure onset patterns. Our patients were followed for 10, 12.5, and 15 months. All patients were responders (≥50% seizure reduction), and two of the three patients experienced a ≥90% reduction in seizures at the last follow‐up. Pre‐ and postsurgical neuropsychological evaluations were compared for two of the patients, and there was no evidence of cognitive decline found in either patient. Interestingly, mild cognitive improvements were reported. The third patient had only postimplant neuropsychological testing data available. Findings for this patient suggested executive dysfunction that was present prior to the RNS System which did not worsen with surgery. A visual inspection of ECoGs revealed near‐simultaneous seizure onsets in neocortical and pulvinar leads in two patients. Seizure onsets in the third patient were more variable. This is the first published report of brain‐responsive neurostimulation targeting the pulvinar to treat refractory regional onset epilepsy of posterior quadrant origin.
Keywords: pulvinar, refractory epilepsy, responsive neurostimulation, thalamus
1. INTRODUCTION
Focal epilepsy is refractory to medical treatment in approximately one‐third of patients. Posterior quadrant focal epilepsy (PQE) involves seizures arising mainly from regions of the occipital lobe and posterior temporo‐parietal lobes. PQE occurs in approximately 5%–10% of focal epilepsy patients.1 PQE can be particularly challenging to treat surgically because seizure onsets are typically multilobar and in close proximity to visual, language, and somatosensory cortex.2, 3, 4 Neurostimulation offers an alternative approach to treating focal drug‐resistant epilepsy (DRE) originating from the posterior quadrant (PQE‐DRE) that is not amenable to resection or ablation.
There is literature supporting thalamic stimulation for DRE using open‐loop continuous deep brain stimulation (DBS) of the anterior nucleus (ANT). Only a small number (3.5%) of patients with occipital epilepsy received DBS of the ANT during clinical trials.5 The RNS System (NeuroPace, Inc.) is a closed‐loop direct brain‐responsive neurostimulation system that has been proven safe and efficacious in the treatment of focal DRE with one or two seizure foci.6 Similar to the DBS trials of the ANT, only a small number of RNS System trial patients (3%) had occipital lobe onsets. Importantly, the RNS System trial patients did not report visual or other side effects related to stimulation directly over visual cortex.7 The small sample sizes in both of these trials make it difficult to draw conclusions about the efficacy of these neurostimulation approaches in the occipital lobe.
Because PQE is often multilobar in nature, it requires treatment of a broad region of cortex. It has been reported that DRE involving regional neocortical seizure onsets may respond to brain‐responsive neurostimulation by flanking the region of onset either with two leads8 or with corticothalamic treatment.9
The pulvinar is the largest of the thalamic nuclei. The extensive pulvinar connections with the posterior quadrant suggest that the pulvinar may be a potentially effective target for corticothalamic neurostimulation in the treatment of PQE‐DRE. We describe the treatment rationale, surgical targeting approach, electrographic seizures, and clinical response to RNS System corticothalamic treatment in the pulvinar and ipsilateral neocortical structures in three patients with regional neocortical epilepsy of posterior quadrant onset.
2. METHODS
We performed a retrospective chart review on PQE‐DRE patients with regional neocortical seizure onsets treated with the RNS System at Spectrum Health (Grand Rapids, MI). Patients were treated under IRB‐approved study, and an informed consent waiver was obtained. We defined regional neocortical epilepsy as described in the literature: a seizure onset region spanning more than five electrode contacts (>4 cm) during intracranial monitoring for seizure localization.8 All patients received the RNS System with a depth lead placed in the pulvinar nucleus of the thalamus and a depth or cortical strip lead placed over the ipsilateral posterior quadrant cortex. Demographics and epilepsy histories were collected from the patient's chart. Adverse events related to surgery and stimulation side effect data were collected from the patient's chart. Clinically disabling seizure counts were obtained from the patient's chart at preimplant (baseline) and at their last follow‐up visit. The percentage reduction in disabling seizures was calculated as the difference from the pre‐RNS System baseline and the last follow‐up of focal aware (with motor component), focal impaired awareness, or focal to bilateral tonic‐clonic seizures. Neuropsychological testing reports were obtained. All electrocorticograms (ECoG) records stored by the RNS System were reviewed to determine which electrodes (pulvinar or cortex) contained the earliest electrographic seizure onset and propagation patterns.
3. RESULTS
Patient 1 had two RNS Systems placed. The first RNS System was placed with two right‐sided occipital cortical strip leads. The patient experienced improvement in seizure frequency but was still having disabling seizures. Two and a half years after the first RNS System was placed, the patient received a second RNS System with a goal of covering the left‐sided abnormalities noted during surgical evaluation. At this time, the patient received bilateral pulvinar depth leads and a left‐sided depth in the occipital lobe. The right‐sided neurostimulator had a pulvinar depth and occipital strip connected, and the left‐sided neurostimulator had a pulvinar depth and occipital lobe depth lead placed.
Patients 2 and 3 received the RNS System for PQE‐DRE with one depth lead placed in the right pulvinar nucleus and the other lead in the ipsilateral posterior quadrant cortex.
The patient demographics, epilepsy histories, and clinical seizure reductions observed in our patients at their last follow‐up are summarized in Table 1. All three patients had a cryptogenic etiology and underwent intracranial EEG evaluation for seizure localization. Notably, two of the patients (patients 2 and 3) had sEEG monitoring with a depth lead placed in the pulvinar nucleus. In both of these patients, it was possible to see early seizure propagation recorded from the pulvinar during sEEG.
TABLE 1.
Patient epilepsy history
| Pt# | Seizure focus | Age | MRI | Prior epilepsy surgery | Phase II EEG: Ictal onsets | Baseline seizure frequency | AEDs | RNS system leads | Follow‐up (mo) |
|---|---|---|---|---|---|---|---|---|---|
| 1a | Bioccipital | 31 | Postprocedural changes from ablation involving the bilateral frontal periventricular white matter and adjacent to the atrium of the right lateral ventricle |
LITT of right PVNH LITT of left PVNH First RNS System placed with unilateral right‐sided occipital strip leads. |
Grid and Strip study: FAS with regional onsets independently involving right and left lateral and medial parieto‐occipital regions |
FAS–1‐2 cluster days/wk FIAS–1/d FIAS + head deviation–multiple/day prefirst RNS, none since FBTC–4 in entire life, none in months prior to RNS |
Brivaracetam, Clobazam, Lacosamide |
Right pulvinar depth + right occipital cortical strip Left pulvinar depth + left occipital depth |
12.5 |
| 2 | Regional onset–right parietal, frontal and insular | 41 | Postprocedural changes from removal of much of the anterior temporal lobe on the right. Adjacent encephalomalacia in the remaining right temporal lobe. |
Right anterior temporal lobectomy Resection of right superior temporal gyrus remnant |
SEEG study: 1) Right parietal with early pulvinar involvement 2) Right frontal opercular, parietal opercular, posterior insular with simultaneous involvement of pulvinar 3) Posterior insular + parietal + posterior temporal and late pulvinar involvement 4) Anterior insula + posterior cingulate and no appreciable pulvinar involvement |
Mild FAS–20‐25/d Severe FAS–5‐7/wk FIAS–3‐5/mo |
Clonazepam, Lacosamide, Lamotrigine, Levetiracetam | Right pulvinar depth + right parietal depth | 15 |
| 3 | Right temporal–occipital | 65 | Unremarkable | None |
SEEG Study: Inferior right posterior temporo‐occipital region laterally. There was lower voltage reflection of the discharge seen in the right pulvinar contacts at the onset of each seizure. |
FAS–2‐4/wk FIAS–2‐3/mo Clustered FIAS–6‐10/mo FBTC–none since pre‐RNS 2018 EMU evaluation |
Brivaracetam, Clonazepam, Lacosamide | Right pulvinar depth + right occipitotemporal depth | 10 |
Abbreviations: LITT, Laser interstitial thermal therapy; PVNH, Periventricular nodular heterotopia.
The data presented for patient 1 are in relation to the date they were implanted in the bilateral pulvinar nuclei.
A superior parasagittal trajectory was taken to target the pulvinar nucleus in all of our cases. Robotic stereotactic neuronavigation (ROSA robotic stereotactic navigation system) was utilized. The pulvinar was targeted through an extraventricular frontal approach with a 2.5 mm twist drill burr hole in the region of the coronal suture. The pulvinar was defined as the region of the thalamus posterior to the posterior commissure and targeted directly with T1 MR with gadolinium in the geographic center of the nucleus. The ROSA robotic stereotactic navigation system was used for targeting after registration with a bone fiducial head CT utilizing at least four Medtronic fiducials. Registration errors were all less than 1 mm. A 4‐contact, 3.5 mm center‐to‐center spaced RNS System depth lead was placed at the target. A postoperative high‐resolution head CT was done to exclude electrode trajectory deviations and screen for hemorrhage (Figure 1). There were no intra‐operative or postoperative adverse events observed.
FIGURE 1.
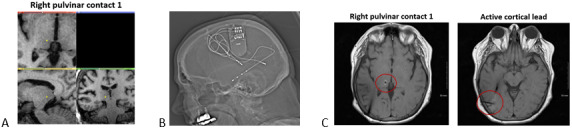
Postoperative Images from Patient 3. (A) Depth lead placement in the pulvinar (B) Post‐implantation CT scan (C) Axial views of right pulvinar contact 1 and right cortical depth lead in the occipitotemporal lobe
Patient 1 had bilateral RNS Systems implanted targeting bilateral pulvinar nuclei and bilateral posterior quadrant cortex. In this patient, the left pulvinar depth lead had the deepest electrode (1) located in the pulvinar, electrode 2 in the pulvinar border, and electrodes 3 and 4 in the ventral lateral nucleus of the thalamus. On the right, this patient had the deepest electrode (1) located in CSF, electrode 2 in the pulvinar border, electrode 3 in the pulvinar, and electrode 4 (most proximal) in the ventral lateral nucleus of the thalamus. Patient 2 had one RNS System placed on the right. The right pulvinar depth lead had the 3 deepest electrodes (1‐3) in the quadrageminal cistern and electrode 4 in the pulvinar. Patient 3 had one RNS System implanted on the right, the pulvinar depth lead had the most distal electrode (1) in the pulvinar, electrode 2 in the pulvinar border, and electrodes 3‐4 in the ventral lateral nucleus of the thalamus.
The patients were followed for 10, 12.5, and 15 months. Antiseizure medications are provided in Table 1. No antiseizure medication changes occurred during the time of follow‐up. All patients were responders (≥50% disabling seizure reduction), and two of the three patients experienced a ≥90% reduction in disabling seizures at the last follow‐up (Figure 2).
FIGURE 2.
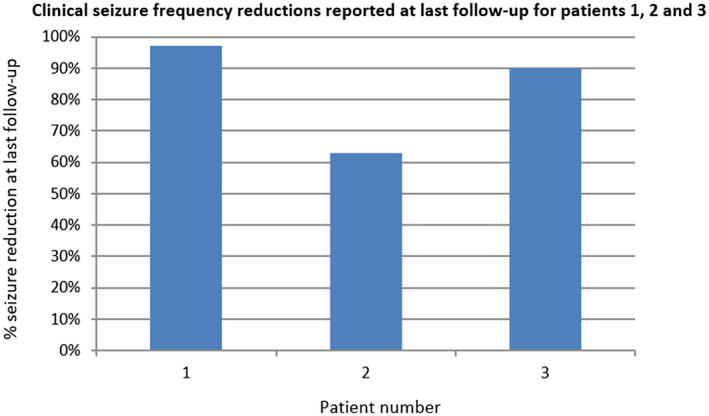
Clinical seizure frequency reductions reported at last follow‐up. Patient 2 had a right pulvinar depth and a right parietal depth, patient 3 a right pulvinar depth and right occipitotemporal depth, and patient 1 had two RNS Systems placed, with bilateral pulvinar depth leads and a right occipital cortical strip and a left occipital depth lead
Pre‐ and postsurgical neuropsychological evaluations were compared for two of our three patients. For patient 1, only postsurgical data were available for review due to the occurrence of multiple daily seizures with postictal psychosis interfering with scheduled presurgical testing. No evidence of cognitive decline was found in the two patients with pre‐ and postsurgical neuropsychological testing data available. In fact, mild improvements were observed. Improvements were primarily noted spanning executive‐based tasks including aspects of working memory and processing speed, reasoning, planning and problem‐solving, and memory recognition. For the patient with only postimplant neuropsychological testing data, findings were suggestive of executive dysfunction; however, the patient's chart indicates that these deficits were long‐standing and no additional decline following surgery was noted.
The programmed stimulation pathways were informed by the electrographic seizure data captured on the pulvinar electrodes and focused on those electrodes with the earliest seizure activity in the pulvinar. Patient 1 had stimulation delivered to both the neocortex (charge density: 1 µC/cm2, frequency: 200 Hz, burst duration: 100 ms) and the pulvinar (charge density: 0.5 µC/cm2, frequency: 125 Hz, burst duration: 2 seconds) with both left and right neurostimulators. Stimulation was initiated solely in the pulvinar in patients 2 and 3 (charge density: 0.5 µC/cm2, frequency: 125 Hz) with a burst duration of 2 seconds [patient 2] or 5 seconds [patient 3]. Because stimulation was effective with pulvinar stimulation in these two patients, cortical stimulation was not initiated. The cortical leads in these two patients were used for detection. During in‐office right pulvinar stimulation testing, patient 1 had peripheral left visual field positive visual phenomena. The patient did not find the side effect troubling; therefore, the settings were programmed. These stimulation‐associated positive visual phenomena have persisted. The other two patients had no perception of stimulation.
A visual inspection of ECoGs containing seizure activity revealed near‐simultaneous (≤0.25 second) seizure onsets in neocortical and pulvinar leads for patients 1 (Figure 3) and 3. Patient 1 had right‐sided electrographic events far more frequently (80%) than left (20%). The vast majority of this patient's electrographic seizures arose independently on either right or left. Onsets for patient 2 were more variable, from near simultaneous to up to a two‐second delay from neocortical onset to the pulvinar.
FIGURE 3.
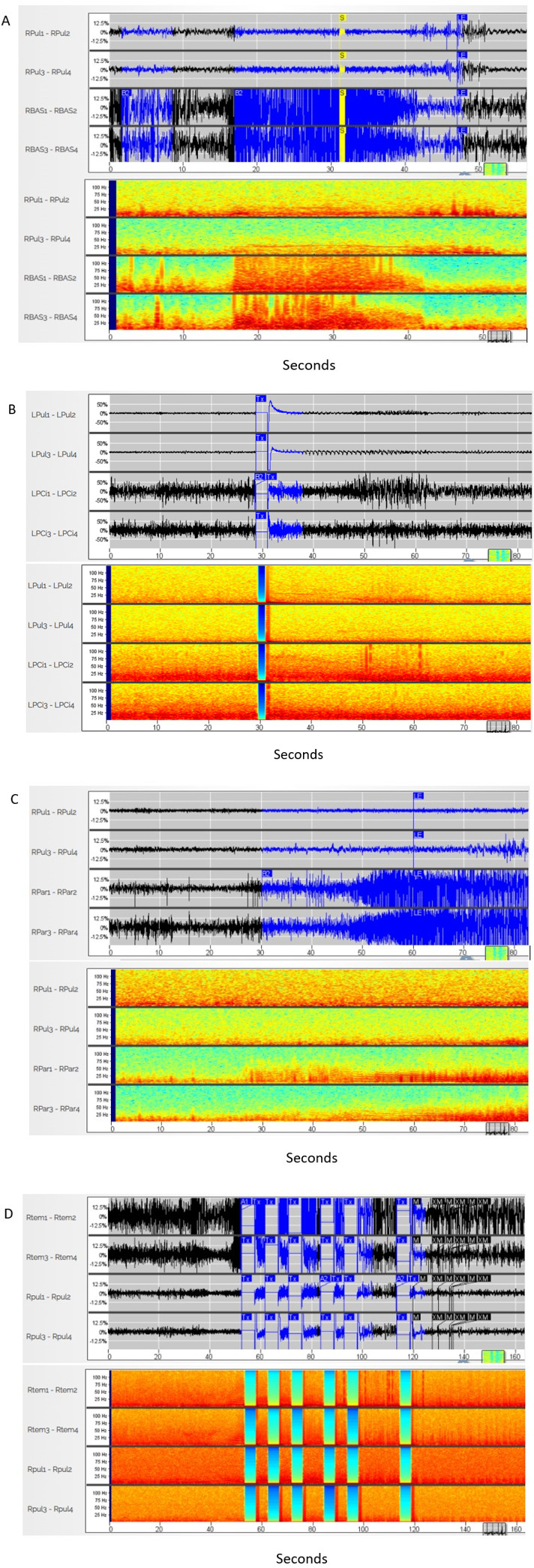
Electrographic seizure example recording in both time and frequency domains for each patient. (A) Patient 1–right pulvinar depth lead displayed on first 2 channels, right occipital cortical strip displayed on bottom 2 channels; (B) patient 1–left pulvinar depth lead displayed on first two channels, occipital depth on lower 2 channels; (C) patient 2–right pulvinar depth displayed on first 2 channels, right parietal depth bottom 2 channels; (D) patient 3–right pulvinar depth displayed on lower two channels and right occipitotemporal depth on upper 2 channels
4. DISCUSSION
To our knowledge, this is the first report of responsive neurostimulation in the pulvinar to treat focal posterior quadrant regional neocortical epilepsy. All three of our patients were responders (≥50% seizure reduction) at approximately one year of follow‐up, and two of those patients had seizure reductions of 90% and greater. Implantation of RNS System depth leads in the pulvinar nucleus was performed without complication in all patients. There were no implant‐related neurocognitive deficits in the two patients with pre‐ and postimplant neuropsychological testing.
The pulvinar is the largest of the thalamic nuclei and has copious connections with neocortical regions particularly those associated with visual processing and memory.10 In our cases, the decision to target the pulvinar was based on data suggesting the pulvinar to be a critical hub in epilepsies arising from posterior brain regions. Extensive connections between the pulvinar and ipsilateral cortical regions provide support as a target for effective neuromodulation in the setting of refractory seizures arising from structures of visual processing.
The emergence of sEEG has facilitated simultaneous neocortical and thalamic recording during standard epilepsy surgery evaluation.11 This approach has demonstrated medial pulvinar reflection of temporal lobe seizures, suggesting that the pulvinar is involved in pathways of seizure propagation.12 Two of the patients in this series were evaluated with a depth lead in the pulvinar during sEEG. In both cases, we were able to record early electrographic seizure propagation patterns in the pulvinar. Data from the RNS System allowed us to capture electrographic seizures from the pulvinar in all three of our patients. Visual review of the electrographic seizures demonstrated the earliest electrographic seizure onsets in the posterior quadrant leads; however, early seizure propagation to the pulvinar was noted. These data support the pulvinar's involvement in seizure networks originating in the posterior quadrant. Whether stimulating the pulvinar is capable of modulating networks outside of the posterior quadrant is unknown.
Our decision to pursue brain‐responsive neurostimulation rather than open‐loop continuous DBS was based upon the prominent role that the medial pulvinar plays in cognitive processing10 and because of the potential value of the intracranial EEG and device data. Open‐loop thalamic stimulation of the anterior nucleus of the thalamus is anticipated to produce disruptive or activating effects on a regional thalamocortical network that can produce adverse effects on sleep function or cognition.13, 14 There were no postimplant cognitive declines in any patient, and pre‐ and postimplant neuropsychological testing in two patients showed improvements in working memory and processing speed, reasoning, planning, problem‐solving, and memory recognition. Our findings are consistent with those from the RNS System clinical trials that demonstrated maintenance of neurocognitive function and, in some cases, improvements. The published neuropsychological data from the trials suggested that the benefits associated with responsive neurostimulation may be due to the disruption of intermittent pathologic brain activity versus an open‐loop approach that delivers stimulation near continuously15
The sample size we present here is small and an obvious limitation of this analysis. Larger, multicenter studies are needed to conclude on the safety and efficacy of RNS System stimulation in the pulvinar nucleus; however, our results in these three patients are informative and encouraging.
5. CONCLUSION
Brain‐responsive neurostimulation of the pulvinar nucleus was feasible, well‐tolerated, and potentially effective in the treatment of focal seizures of posterior quadrant origin in our small cohort of patients. Larger patient populations and longer‐term data are necessary to confirm these findings.
CONFLICT OF INTEREST
Author David E. Burdette has received support from NeuroPace and has served as a paid consultant for NeuroPace, Inc Author Emily A Mirro has equity ownership/stock options with NeuroPace and is an employee of NeuroPace. Author Sanjay E. Patra has received support from and has served as a paid consultant for NeuroPace, Inc Author Michael Lawrence has no conflict of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Burdette D, Mirro EA, Lawrence M, Patra SE. Brain‐responsive corticothalamic stimulation in the pulvinar nucleus for the treatment of regional neocortical epilepsy: A case series. Epilepsia Open. 2021;6:611–617. 10.1002/epi4.12524
REFERENCES
- 1.Harward SC, Chen WC, Rolston JD, Haglund MM, Englot DJ. Seizure outcomes in occipital lobe and posterior quadrant epilepsy surgery: a systematic review and meta‐analysis. Neurosurgery. 2018;82:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salanova V. Parietal lobe epilepsy. Handb Clin Neurol. 2018;151:413–25. [DOI] [PubMed] [Google Scholar]
- 3.Salanova V, Andermann F, Olivier A, Rasmussen T, Quesney LF. Occipital lobe epilepsy: electroclinical manifestations, electrocorticography, cortical stimulation and outcome in 42 patients treated between 1930 and 1991. Surgery of occipital lobe epilepsy. Brain. 1992;115(Pt 6):1655–80. [DOI] [PubMed] [Google Scholar]
- 4.Tandon N, Alexopoulos AV, Warbel A, Najm IM, Bingaman WE. Occipital epilepsy: spatial categorization and surgical management. J Neurosurg. 2009;110:306–18. [DOI] [PubMed] [Google Scholar]
- 5.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. [DOI] [PubMed] [Google Scholar]
- 6.Nair DR, Laxer KD, Weber PB, Murro AM, Park YD, Barkley GL, et al. Nine‐year prospective efficacy and safety of brain‐responsive neurostimulation for focal epilepsy. Neurology. 2020;95:e1244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jobst BC, Skarpaas TL, Morrell MJ. Response: therapeutic brain‐responsive neurostimulation in eloquent cortex can be delivered without symptoms. Epilepsia. 2017;58:1488. [DOI] [PubMed] [Google Scholar]
- 8.Ma BB, Fields MC, Knowlton RC, Chang EF, Szaflarski JP, Marcuse LV, et al. Responsive neurostimulation for regional neocortical epilepsy. Epilepsia. 2020;61:96–106. [DOI] [PubMed] [Google Scholar]
- 9.Burdette DE, Haykal MA, Jarosiewicz B, Fabris RR, Heredia G, Elisevich K, et al. Brain‐responsive corticothalamic stimulation in the centromedian nucleus for the treatment of regional neocortical epilepsy. Epilepsy Behav. 2020;112:e107354. [DOI] [PubMed] [Google Scholar]
- 10.Homman‐Ludiye J, Bourne JA. The medial pulvinar: function, origin and association with neurodevelopmental disorders. J Anat. 2019;235:507–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaitanya G, Toth E, Pizarro D, Irannejad A, Riley K, Pati S. Precision mapping of the epileptogenic network with low‐ and high‐frequency stimulation of anterior nucleus of thalamus. Clin Neurophysiol. 2020;131:2158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg DS, Mauguiere F, Demarquay G, Ryvlin P, Isnard J, Fischer C, et al. Involvement of medial pulvinar thalamic nucleus in human temporal lobe seizures. Epilepsia. 2006;47:98–107. [DOI] [PubMed] [Google Scholar]
- 13.Hartikainen KM, Sun L, Polvivaara M, Brause M, Lehtimaki K, Haapasalo J, et al Immediate effects of deep brain stimulation of anterior thalamic nuclei on executive functions and emotion‐attention interaction in humans. J Clin Exp Neuropsychol. 2014;36:540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voges BR, Schmitt FC, Hamel W, House PM, Kluge C, Moll CK, et al. Deep brain stimulation of anterior nucleus thalami disrupts sleep in epilepsy patients. Epilepsia. 2015;56:e99–103. [DOI] [PubMed] [Google Scholar]
- 15.Loring DW, Kapur R, Meador KJ, Morrell MJ. Differential neuropsychological outcomes following targeted responsive neurostimulation for partial‐onset epilepsy. Epilepsia. 2015;56:1836–44. [DOI] [PubMed] [Google Scholar]


