Abstract
p70 S6 kinase (p70S6K) is an important regulator of cell proliferation. Its activation by growth factor requires phosphorylation by various inputs on multiple sites. Data accumulated thus far support a model whereby p70S6K activation requires sequential phosphorylations at proline-directed residues in the putative autoinhibitory pseudosubstrate domain, as well as threonine 389. Threonine 229, a site in the catalytic loop is phosphorylated by phosphoinositide-dependent kinase 1 (PDK-1). Experimental evidence suggests that p70S6K activation requires a phosphoinositide 3-kinase (PI3-K)-dependent signal(s). However, the intermediates between PI3-K and p70S6K remain unclear. Here, we have identified PI3-K-regulated atypical protein kinase C (PKC) isoform PKCζ as an upstream regulator of p70S6K. In coexpression experiments, we found that a kinase-inactive PKCζ mutant antagonized activation of p70S6K by epidermal growth factor, PDK-1, and activated Cdc42 and PI3-K. While overexpression of a constitutively active PKCζ mutant (myristoylated PKCζ [myr-PKCζ]) only modestly activated p70S6K, this mutant cooperated with PDK-1 activation of p70S6K. PDK-1-induced activation of a C-terminal truncation mutant of p70S6K was also enhanced by myr-PKCζ. Moreover, we have found that p70S6K can associate with both PDK-1 and PKCζ in vivo in a growth factor-independent manner, while PDK-1 and PKCζ can also associate with each other, suggesting the existence of a multimeric PI3-K signalling complex. This work provides evidence for a link between a phorbol ester-insensitive PKC isoform and p70S6K. The existence of a PI3-K-dependent signalling complex may enable efficient activation of p70S6K in cells.
p70 S6 kinase (p70S6K) has emerged as an important regulator of cell growth, playing a positive role during progression through the G1 phase of the cell cycle (12). Earlier studies on p70S6K regulation using pharmacological inhibitors and platelet-derived growth factor receptor mutants, as well as cotransfection studies with a constitutively active form of phosphoinositide 3-kinase (PI3-K), have revealed that p70S6K activation depends, to a large extent, on PI3-K (9, 14, 38). The regulation of p70S6K is complex in that phosphorylation at multiple sites is required for full activation of the kinase. Several proline-directed sites have been identified within the C-terminal autoinhibitory domain of p70S6K. In vitro and in vivo studies suggest that these sites are phosphorylated by members of the mitogen-activated protein kinase (MAPK) family, p38, and extracellular signal-related kinases (28, 33). Phosphorylation of these sites is thought to induce a conformational change in p70S6K, relieving an inhibitory intramolecular interaction between the autoinhibitory and catalytic domains. This allows the kinase to be phosphorylated at other critical sites, Thr-229, Thr-389, and a newly identified site, Ser-371 (21, 27, 30; reviewed in reference 32).
Thr-229 is located in the catalytic loop of p70S6K and must be phosphorylated for full kinase activity. Recently, PDK-1 (phosphoinositide-dependent kinase 1) (2, 31) has been identified as the kinase responsible for phosphorylation of this site. Mutation of this site to an alanine or even an acidic residue intended to mimic phosphorylation abolishes kinase activity. Phosphorylation of Thr-229 has been reported to be wortmannin sensitive (21), suggesting a PI3-K requirement. PI3-K-dependent regulation of p70S6K phosphorylation at other sites may promote phosphorylation at Thr-229 by a constitutively active kinase such as PDK-1 (31). Furthermore, it has been suggested that Thr-389 is phosphorylated by FRAP/RAFT/mTOR (mammalian target of rapamycin) (8). However, the mechanism by which mTOR regulates p70S6K remains unclear, as an amino- and carboxy-terminal deletion mutant of p70S6K which contains Thr-389 and retains mitogen responsiveness is wortmannin sensitive but rapamycin insensitive (10, 39). Ser-371 is also a mitogen-regulated site, and interestingly, its phosphorylation has been reported to be rapamycin insensitive. The wortmannin sensitivity of this site and the kinase(s) which regulates Ser-371 remain unknown.
The protein kinase Akt/PKB, the first identified substrate of PDK-1, also requires PI3-K for its activation (1, 16, 36; reviewed in reference 19) and has been identified as an upstream regulator of p70S6K (7). Akt does not appear to directly phosphorylate p70S6K (2), and the intermediates between Akt and p70S6K are not known. Furthermore, it has not been shown that a dominant negative mutant of Akt can inhibit activation of p70S6K (7). The Rho family GTPases Rac1 and Cdc42 have also been shown to regulate p70S6K (11). Moreover, the activation of p70S6K by Cdc42 or Rac1 requires membrane targeting of these G proteins and is sensitive to wortmannin, which is consistent with the notion that multiple PI3-K-dependent pathways are required for the phosphorylation and activation of p70S6K.
Atypical protein kinase Cζ (PKCζ) has been identified as a downstream target of PI3-K. This isoform differs from the conventional and novel classes of PKCs in that it does not require diacylglycerol or calcium for its activation. In vitro studies have shown that the PI3-K lipid product phosphatidylinositol-3,4,5-triphosphate activates PKCζ (22, 29). In vivo, growth factors and a constitutively activated mutant of PI3-K activate PKCζ in a wortmannin-sensitive fashion (4, 13, 25, 26, 35). Moreover, PKCζ has been implicated in several growth-related processes such as extracellular signal-related kinase activation, Xenopus oocyte maturation, and fibroblast proliferation (5, 6, 18, 34). More recently, it has been reported that PDK-1 phosphorylates and activates PKCζ (13, 24).
Since it is clear that the PI3-K signalling pathway is important for p70S6K activation and that Akt is probably not the sole effector of PI3-K in p70S6K activation, we sought to identify other PI3-K-regulated kinases as mediators of p70S6K activation. Here we have investigated the possibility that PKCζ is an upstream regulator of p70S6K. We have used constitutively activated and kinase-inactive mutants of PKCζ to show that this PKC isoform participates in p70S6K activation. We also found that p70S6K can associate with both PDK-1 and PKCζ in vivo and that PDK-1 and PKCζ also associate with each other, suggesting that a multimeric PI3-K-regulated signalling complex exists in cells. Our study provides direct evidence that a specific PI3-K regulated PKC isoform (PKCζ) regulates p70S6K, providing a further link between PI3-K and p70S6K.
MATERIALS AND METHODS
Cell culture and transfections.
293 cells were maintained in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal calf serum. For p70S6K transient-cotransfection studies, 293 cells were transfected by the calcium phosphate method. Cells were seeded at a density of 106 per 60-mm-diameter dish 16 h prior to transfection. Hemagglutinin (HA)-p70S6K (0.25 to 1 μg) was cotransfected with FLAG-PKCζ, myc-p110*, myc-PDK-1, glutathione S-transferase (GST)–Cdc42V12, FLAG-p38 plasmid DNA and/or an empty vector, as indicated in the figure legends, for a total of 12 μg of DNA. Cells were incubated with the calcium phosphate-DNA mixture for 5 h, washed twice with phosphate-buffered saline supplemented with 0.8 CaCl2 mM and 1 mM MgCl2, and then recovered by incubation for 16 h in DMEM containing 10% fetal calf serum. For PKCζ activation studies, cells were transfected by the Lipofectamine method with 400 to 600 ng of PKCζ DNA and an empty vector for a total of 2 μg of plasmid DNA. Cells were starved in serum-free DMEM for 24 h prior to lysis, and lysates were prepared at 48 h posttransfection.
Cell extract preparation.
Cells were stimulated with epidermal growth factor (EGF) for 10 min for PKCζ activity measurements or for 30 min for coimmunoprecipitations and p70S6K activity measurements. Following stimulation, cells were lysed in 300 μl of lysis buffer (10 mM KPO4, 1 mM EDTA, 10 mM MgCl2, 50 mM β-glycerophosphate, 5 mM EGTA, 0.5% Nonidet P-40 [NP-40], 0.1% Brij 35, 0.1% sodium deoxycholate, 1 mM sodium orthovanadate, 40-mg/ml phenylmethylsulfonyl fluoride, 10-μg/ml leupeptin, 5-μg/ml pepstatin, pH 7.28) at 4°C. Lysates were cleared of debris by centrifugation at 15,000 × g.
Immunoblots.
Whole-cell lysate (10% of total cell extract) or washed immunoprecipitates were resolved by sodium dodecyl sulfate (SDS)–7.5 or 12% polyacrylamide gel electrophoresis (PAGE). Proteins were transferred to a nitrocellulose membrane and blotted with the appropriate antibody. All immunoblots were detected by enhanced chemiluminescence. Anti-FLAG monoclonal antibody M2 was purchased from Eastman Kodak Company, New Haven, Conn. Anti-PKCζ antibody was purchased from Santa Cruz Biotechnology Inc., Santa Cruz, Calif. Anti-GST antibody was a generous gift from T. Rapaport. Anti-p70S6K antibody was raised against a C-terminal peptide of the protein.
Immunoprecipitations and immune complex kinase assays.
For coimmunoprecipitation studies, precleared lysates (33% of the total cell extract) were immunoprecipitated with an anti-FLAG or anti-myc antibody and washed twice with phosphate-buffered saline and 1% NP-40 and once in TNE (10 mM Tris, 100 mM NaCl, 1 mM EDTA, pH 7.4). Proteins were resolved and immunoblotted as described above. For S6 kinase assays, lysates prepared as described above were immunoprecipitated with an anti-HA antibody. For cotransfection studies with the kinase-inactive mutant of PKCζ (PKCζ K/W), lysates were normalized for amounts of HA-p70S6K protein expressed (quantitated from Western blots by using a Bio-Rad Fluor-S MultiImager) prior to immunoprecipitation; otherwise, 33% of the total lysate was used for immunoprecipitations for S6 kinase assays. Immunoprecipitates were stringently washed once in 1 ml each of buffers A (10 mM Tris, 1% NP-40, 0.5% sodium deoxycholate, 100 mM NaCl, 1 mM EDTA, 1 mM sodium orthovanadate, 2 mM dithiothreitol, 40-mg/ml phenylmethylsulfonyl fluoride, 10-μg/ml leupeptin, 5-μg/ml pepstatin, pH 7.2), B (same as buffer A, except for 0.1% NP-40 and 1 M NaCl), and ST (50 mM Tris-HCl, 5 mM Tris base, 150 mM NaCl). Kinase assays were then carried out by using a GST fusion of the last 32 amino acids of 40S ribosomal protein S6 as a substrate at 30°C for 10 min as described previously (15). For Akt immune complex assays, anti-HA immunoprecipitates from lysates which were normalized for expression of HA-Akt were washed as described above for S6 kinase assays. Reactions were carried out by using recombinant GST-BAD as a substrate (17) with 20 mM HEPES–10 mM MgCl2–5 μCi of [γ-32P]ATP (50 μM; New England Nuclear) for 10 min at 30°C. Reactions were resolved by SDS–12% PAGE. S6 and Akt kinase activities were quantitated on a PhosphorImager and expressed as PhosphorImager Units. PKCζ activity was assayed as previously described, by using myelin basic protein as the substrate (13).
cDNA constructs.
The HA-p70S6K wild type (WT) and a C-terminal deletion mutant form (ΔCT) were generated as previously described (10) and subcloned into mammalian expression vector pRK7. FLAG-PKCζ WT and the myristoylated (myr)-PKCζ-FLAG constructs have been previously described (13). FLAG-tagged PKCζ K281W (FLAG-PKCζ K/W) was generated by addition of the FLAG epitope (MDYDDDDK) to the N terminus of PKCζ K/W (provided by S. Ohno). The myr-PKCζ K/W-FLAG mutant was generated by site-directed PCR mutagenesis by mutating lysine 281 to a tryptophan residue using myr-PKCζ-FLAG as the template. Cdc42V12, myc-PDK-1, and myc-p110* were previously described (11, 13, 23). All of the mammalian expression vectors used in this study are driven by the cytomegalovirus promoter.
RESULTS
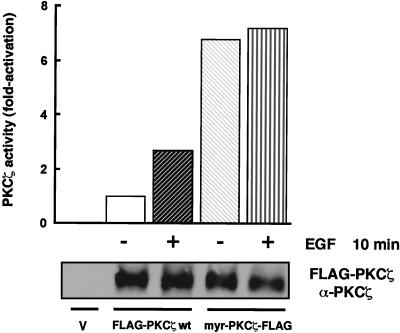
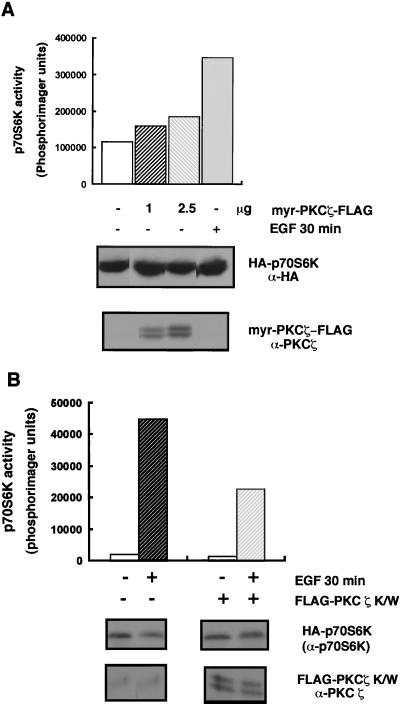
PKCζ is regulated by EGF in 293 cells and participates in the activation of p70S6K.
It has been previously reported that PKCζ can be activated by growth factors in different cell types (4, 13, 25, 26, 35). We wanted to verify this in the cell line used in this study, under our experimental conditions. Indeed, EGF activated FLAG-tagged WT PKCζ (FLAG-PKCζ wt) 2.7-fold following a 10-min stimulation (Fig. 1). Myristoylation of PKCζ targets it to the membrane, resulting in a constitutively active kinase (13). EGF did not further activate the constitutively active PKCζ mutant (myr-PKCζ-FLAG), whose basal activity was six- to sevenfold higher than that of WT PKCζ. We used this activated PKCζ mutant to test whether PKCζ is an upstream activator of p70S6K. In the absence of growth factor, myr-PKCζ expression induced variable and modest activations of p70S6K, ranging from 1.5- to 2-fold (Fig. 2A). In the same experiment, EGF led to threefold activation of HA-p70S6K. The variability that we observed in these experiments may have been due to differences in the expression levels of activated PKCζ, as well as differences in the basal phosphorylations of other sites on p70S6K which may be required for activation of the kinase by myr-PKCζ. Since multiple signals are required for p70S6K activation, it is possible that PKCζ alone is not sufficient for full p70S6K activation but participates in growth factor-dependent activation of p70S6K. To test this hypothesis, we coexpressed HA-p70S6K with a kinase-inactive PKCζ mutant (FLAG-PKCζ K/W) which contains a mutation at the conserved lysine residue in the ATP-binding domain. PKCζ K/W inhibited EGF-dependent activation of p70S6K by 50% (Fig. 2B). The partial inhibition is consistent with the existence of multiple inputs signalling to p70S6K. In addition to PI3-K, EGF recruits and activates phospholipase Cγ, which activates p70S6K in a wortmannin-independent but phorbol ester-sensitive manner (14). In agreement with this, we found that PKCζ K/W did not affect PMA (phorbol 12-myristate 13-acetate)-induced p70S6K activation (data not shown).
FIG. 1.
PKCζ is regulated by EGF in 293 cells. 293 cells were transfected with 400 to 600 ng of FLAG-PKCζ wt or myr-PKCζ-FLAG cDNA along with an empty vector (V [pCMV5]) or with an empty vector alone. Cells were lysed following 24 h of starvation in serum-free medium and 10 min of stimulation with EGF (50 ng/ml), as indicated. PKCζ activity was measured as described in Materials and Methods and is expressed as fold activation over the basal level. Whole-cell lysates were analyzed for expression of PKCζ by immunoblotting with a PKCζ-specific antibody (lower panel).
FIG. 2.
PKCζ is not sufficient for p70S6K activation but participates in the activation of p70S6K by EGF. (A) HA-p70S6K (0.5 μg) was cotransfected with an empty vector (pCMV5) alone or with the indicated amounts of myr-PKCζ-FLAG. Cells were starved for 24 h in serum-free medium and stimulated with EGF (50 ng/ml) or not stimulated prior to lysing. Whole-cell lysates were resolved by SDS–7.5% PAGE, and the anti-HA and anti-PKCζ immunoblots (lower panels) indicate the expression of HA-p70S6K and myr-PKCζ-FLAG, respectively. HA-p70S6K activity was measured as described in Materials and Methods. (B) 293 cells were cotransfected with 0.25 μg of HA-p70S6K and 8 μg of FLAG-PKCζ K/W or with HA-p70S6K and an empty vector (pCMV5). Cells were starved in serum-free medium for 24 h and then stimulated with EGF (50 ng/ml) for 30 min where indicated. HA-p70S6K immunoprecipitated for S6 kinase activity assays was resolved by SDS–12% PAGE and analyzed by immunoblotting using a C terminus-specific p70S6K antibody. Whole-cell lysates were evaluated for expression of PKCζ as described in the legend to Fig. 1. S6 kinase assays were performed as described in Materials and Methods, and activity was quantitated by using a PhosphorImager and is represented as a bar graph. These results are representative of at least three independent experiments.
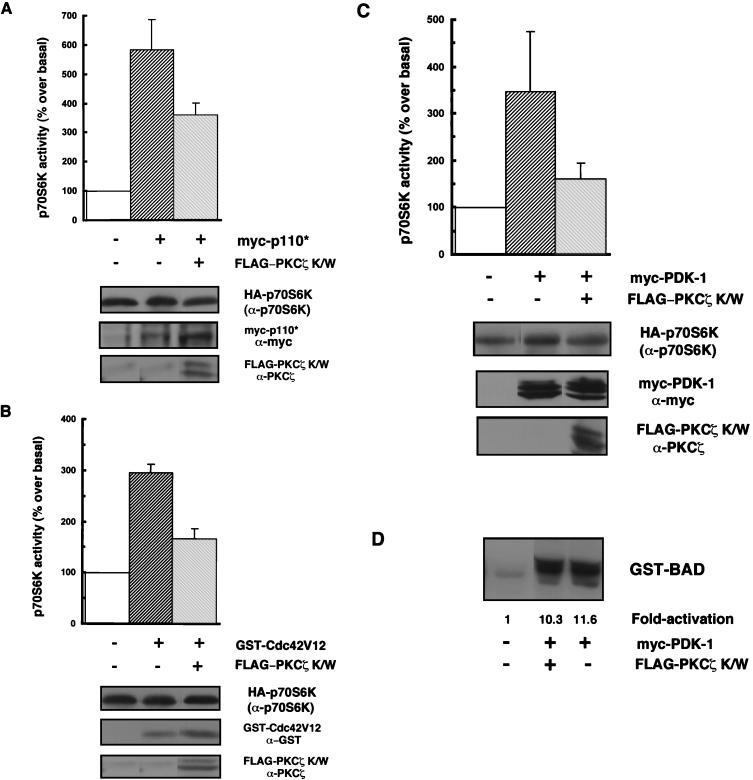
Consistent with the model in which PKCζ is downstream of PI3-K and may mediate PI3-K-dependent signalling to p70S6K, we found that PKCζ K/W partially (42%) inhibited the activation of p70S6K by a constitutively activated PI3-K, p110* (Fig. 3A). Again, it is not surprising to find a partial inhibitory effect, since Akt, another effector of PI3-K, has been identified as an upstream activator of p70S6K (19) and could account for the residual activation of p70S6K.
FIG. 3.
Kinase-inactive PKCζ antagonizes p70S6K activation by various stimuli. (A, B, and C) 293 cells were cotransfected with HA-p70S6K (0.25 to 5 μg) along with 2 μg of myc-p110* (A) or myc-PDK-1 (C) or with 3 μg of GST-Cdc42V12 (B) in the presence or absence of 8 μg of PKCζ K/W, or HA-p70S6K was cotransfected only with an empty vector (pCMV5). Cells were serum starved for 24 h prior to harvesting. myc-p110*, myc-PDK-1, GST-Cdc42V12, and FLAG-PKCζ protein expression levels were monitored in whole-cell lysates by immunoblotting using anti-myc, anti-GST, or anti-PKCζ antibodies, respectively (lower panels). HA-p70S6K protein expression (top of three lower panels) and activity were monitored and quantitated as described in the legend to Fig. 2B. The bar graphs represent p70S6K activity expressed as a percentage of basal p70S6K activity. Results are expressed as the mean percentage over the basal level ± the standard error of the mean (n = 3–6). Blots are representative of independent experiments. (D) Equal amounts of HA-Akt were immunoprecipitated from lysates from 293 cells which were cotransfected with HA-Akt (1 μg) and PDK-1 (2 μg) with or without FLAG-PKCζ K/W (8 μg) or with HA-Akt and an empty vector, and HA-Akt activity was assayed by in vitro phosphorylation of GST-BAD as described in Materials and Methods and is expressed as fold activation over the basal level.
Recent studies have shown that activation of p70S6K by an activated allele of Cdc42 or Rac1 is wortmannin sensitive (11). We have found that activation of p70S6K by PDK-1 is also wortmannin sensitive (data not shown). These results are consistent with the existence of additional PI3-K-regulated p70S6K-activating signals. We therefore asked if these signals might be mediated by PKCζ. To address this possibility, we tested the effect of kinase-inactive PKCζ on p70S6K activation by Cdc42V12 or PDK-1. PKCζ K/W antagonized the increase in p70S6K activity induced by both PDK-1 and Cdc42V12. The positive effects of both Cdc42V12 and PDK-1 on p70S6K activity were partially inhibited, by 44 and 54% (respectively), by PKCζ K/W (Fig. 3B and C). These results suggest an important role for PKCζ in p70S6K activation by various stimuli. Since it has been reported that PDK-1 can directly phosphorylate and activate PKCζ (13, 24), we wanted to verify that PKCζ does not merely act through sequestration of PDK-1. We therefore tested whether PKCζ K/W would interfere with the activation of Akt by PDK-1. In a cotransfection experiment with myc-PDK-1 and HA-Akt, we found that PDK-1 induced an 11-fold increase in HA-Akt activity toward GST-BAD (17) (Fig. 3D). Unlike the activation of p70S6K by PDK-1, the activation of Akt was not affected by PKCζ K/W (Fig. 3D).
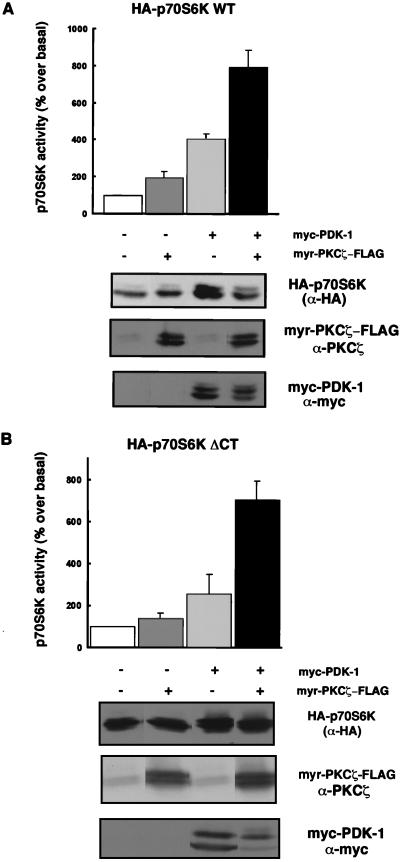
PDK-1 cooperates with PKCζ in the activation of p70S6K.
Since PKCζ was not sufficient for full p70S6K activation, we hypothesized that it may cooperate with another upstream regulator, such as PDK-1, for p70S6K activation. We therefore cotransfected myr-PKCζ with PDK-1 and found that myr-PKCζ cooperated with PDK-1 in p70S6K activation. Coexpression of myr-PKCζ with PDK-1 increased p70S6K activity eightfold (Fig. 4A), whereas PDK-1 alone stimulated p70S6K activity fourfold in the absence of a growth factor. myr-PKCζ alone induced a twofold increase in p70S6K activity. At higher levels of expression, wild-type FLAG-PKCζ also cooperated with PDK-1 (data not shown). The enhancement of p70S6K activity was not due to increased expression of myc-PDK-1 or HA-p70S6K, as comparable levels of protein were expressed in the absence or presence of myr-PKCζ-FLAG (Fig. 4A). Furthermore, cooperativity was not due to activation of myr-PKCζ by PDK-1, as constitutively active myr-PKCζ is not further activated by PDK-1 (13). These results suggest that the inhibitory effects of kinase-inactive PKCζ are due to a lack of PKCζ function as opposed to sequestration of an upstream effector.
FIG. 4.
PDK-1 cooperates with PKCζ in the activation of WT p70S6K and a carboxy-terminal truncation mutant (p70S6K ΔCT). (A and B) 293 cells were cotransfected with 0.5 or 1 μg of WT (A) or ΔCT (B) HA-p70S6K either with 0.5 μg of myc-PDK-1, with 1 μg of myr-PKCζ-FLAG, or with both myc-PDK-1 and myr-PKCζ-FLAG, as indicated. Cells were serum starved for 24 h prior to harvesting. Protein expression levels were evaluated in whole-cell lysates by immunoblotting as described in the legend to Fig. 3. Blots are representative of independent experiments. p70S6K activity results are expressed as the mean percentage over basal activity ± the standard error of the mean (n = 6 for A, n = 3 for B).
PKCζ regulates a carboxy-terminal deletion mutant of p70S6K.
Since PKCζ has been reported to be an upstream activator of MAPK (6, 34, 37) and MAPK has been postulated to phosphorylate several residues in the C-terminal pseudosubstrate domain of p70S6K, we considered the possibility that PKCζ regulates p70S6K through its carboxy-terminal domain. We therefore tested the ability of myr-PKCζ to cooperate with PDK-1 in the activation of a p70S6K C-terminal deletion mutant (HA-p70S6K ΔCT). This mutant lacks the last 104 amino acids of p70S6K, including the proline-directed sites which are likely targets of MAPK (and/or other proline-directed kinases). We found that myr-PKCζ alone activated HA-p70S6K ΔCT almost twofold in the absence of EGF. HA-p70S6K ΔCT was activated by PDK-1 in the absence of a growth factor, as previously reported (2), and this activation was further enhanced by myr-PKCζ (Fig. 4B). A 7-fold activation of basal ΔCT activity was detected with myr-PKCζ and PDK-1 versus the 2.5-fold activation obtained with PDK-1 alone (Fig. 4B). These observations are consistent with a role for PKCζ in p70S6K regulation which is independent of its C-terminal regulatory sites.
PKCζ, PDK-1, and p70S6K associate in vivo.
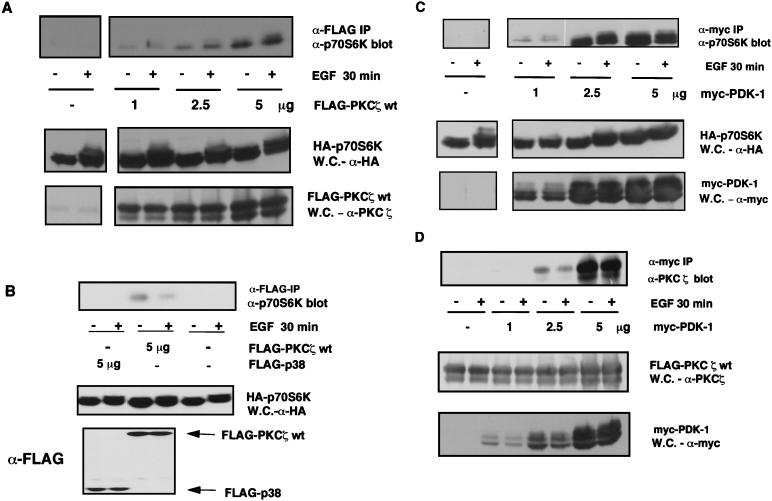
Since PKCζ and several other PI3-K-regulated molecules participate in the rapid growth factor-dependent activation of p70S6K, we hypothesized that p70S6K-activating enzymes could rapidly and efficiently converge on their targets if multiple PI3-K-regulated signalling molecules existed in a complex. We therefore first addressed whether PKCζ was associated with p70S6K. We found that p70S6K coimmunoprecipitated with exogenously expressed WT-PKCζ in a dose-dependent manner and that this association was growth factor independent (Fig. 5A). As a control, we showed that p70S6K did not associate with FLAG-p38-MAPK (Fig. 5B), suggesting that the coimmunoprecipitation of p70S6K with PKCζ is not due to nonspecific interactions. We then asked if p70S6K could also associate with PDK-1. Again, a stable, dose-dependent interaction was detected that was growth factor independent (Fig. 5C). Finally (Fig. 5D), we showed that PKCζ and PDK-1 could also be coimmunoprecipitated.
FIG. 5.
PKCζ, PDK-1, and p70S6K coimmunoprecipitate. (A, B, and C) 293 cells were cotransfected with HA-p70S6K (0.5 μg) and the indicated amounts of FLAG-PKCζ wt, FLAG-p38-MAPK or myc-PDK-1 or with an empty vector (pCMV5), as indicated. Cells were starved in serum-free medium for 24 h and stimulated with EGF for 30 min or not stimulated. FLAG-PKCζ wt, FLAG-p38, or myc-PDK-1 was immunoprecipitated (IP) from 33% of the total cell extract by using an anti-FLAG or anti-myc antibody, respectively. Coimmunoprecipitating HA-p70S6K was detected by immunoblotting using a C-terminal p70S6K-specific antibody. Whole-cell (W.C.) lysate was analyzed for expression of HA-p70S6K, FLAG-PKCζ wt, FLAG-p38, and myc-PDK-1. (D) 293 cells were cotransfected with FLAG-PKCζ wt (2.5 μg) and the indicated amounts of myc-PDK-1 or with an empty vector (pCMV5). myc-PDK-1 was immunoprecipitated as described above. Coimmunoprecipitating FLAG-PKCζ was detected by immunoblotting using a PKCζ-specific antibody. Whole-cell extract was also analyzed for expression of FLAG-PKCζ and myc-PDK-1. These data are representative of at least three independent transfections.
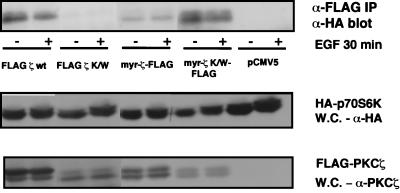
We also wanted to determine whether PKCζ activity is required for complex formation with p70S6K. To do this, we tested the ability of FLAG-PKCζ K/W, as well as myr-PKCζ K/W, to coimmunoprecipitate with HA-p70S6K. We were not able to detect any p70S6K coimmunoprecipitating with PKCζ K/W, which may be due to poor expression of this construct compared to WT PKCζ (Fig. 6). However, the myr-PKCζ K/W mutant, which was expressed to levels comparable to those of PKCζ K/W, seems to form a more stable association with p70S6K (Fig. 6). This increased stability may be due to the added contribution of other signalling molecules such as Rho family G proteins, PDK-1, and possibly yet-to-be-identified adapter molecules which may contribute to the stable formation of a signalling complex at the membrane. From these observations, we can conclude that PKCζ activity is not required for interaction with p70S6K, at least when PKCζ is targeted to the membrane. Interestingly, the activated myr-PKCζ formed a less stable complex with p70S6K than did the WT kinase and myr-PKCζ K/W (Fig. 6). One possible explanation for this is that myr-PKCζ (which is constitutively active and more active than growth factor-activated WT PKCζ (Fig. 1), participates in p70S6K activation (Fig. 3A) and thus promotes its release from a signalling complex.
FIG. 6.
PKCζ activity is not required for association with p70S6K. 293 cells were cotransfected with 0.5 μg of HA-p70S6K and 5 μg of FLAG-PKCζ wt or myr-PKCζ-FLAG, with 8 μg of either FLAG-PKCζ K/W or myr-PKCζ K/W-FLAG, or with pCMV5. FLAG-PKCs were immunoprecipitated (IP) with an anti-FLAG antibody, and coimmunoprecipitating HA-p70S6K was detected by immunoblotting with an anti-HA antibody. Whole-cell (W.C.) lysates were analyzed for expression of HA-p70S6K and the different PKC mutants. These results are representative of three independent experiments.
DISCUSSION
Our data reveal an important role for PKCζ in the regulation of p70S6K. Although we observed only modest activation of p70S6K by an activated PKCζ mutant (myr-PKCζ) (Fig. 2A), a requirement for PKCζ activity in mitogen-induced p70S6K activation was demonstrated when a kinase-inactive mutant of this PKC isoform was used. PKCζ K/W diminished activation of p70S6K by EGF (Fig. 2B), as well as by PI3-K, PDK-1, and Cdc42V12 (Fig. 3A, B, and C). However, expression of PKCζ K/W did not affect activation of Akt by PDK-1 (Fig. 3D). In addition, we observed cooperation between myr-PKCζ and PDK-1 in activating p70S6K (Fig. 4A). Taken together, these data indicate that PKCζ positively regulates p70S6K through a concerted effect with other p70S6K regulators and that the inhibitory effects of PKCζ K/W are due to a lack of positive function rather than to sequestration of upstream p70S6K activators. Recently, it was reported that a kinase-inactive mutant of PKCζ containing a mutation in its activation loop (threonine 410 mutated to alanine) can antagonize PKCɛ and PKCα activities due to a common activation mechanism (20). We found that PKCζ K/W did not affect activation of p70S6K by PMA (data not shown), which activates conventional and/or novel PKCs but not atypical isoforms, such as PKCζ and -λ. This is supportive of a specific effect of PKCζ K/W in the PI3-K-dependent activation of p70S6K rather than a nonspecific role through the inhibition of other PKCs. The latter observation also argues against sequestration of PDK-1 as a mode of action for PKCζ K/W, since such an effect would lead to decreased basal phosphorylation of Thr-229, which is likely to be required for activation of p70S6K by PMA.
This work provides evidence that PI3-kinase-regulated, phorbol ester-insensitive, atypical PKCζ participates in the regulation of p70S6K. We do not, however, rule out the possibility that other PKC isoforms can contribute to the activation of p70S6K. Although the precise mechanism of the activation of p70S6K by PMA is not known, it is likely that conventional and/or novel PKC isoforms are involved. The specific role of PKCζ in the regulation of p70S6K remains to be determined. Our data raise two possibilities that are not mutually exclusive, i.e., (i) that PKCζ participates in the regulation of p70S6K by allowing proper subcellular localization and (ii) that PKCζ regulates p70S6K through phosphorylation, either directly or through another p70S6K kinase. Although PDK-1 has been reported to be constitutively activated in cells, it is hypothesized that upon stimulation of cells by growth factors, PDK-1 is recruited to the membrane due to binding of phosphatidylinositol-3,4,5-triphosphate to its PH domain (3). PKCζ is a lipid-regulated kinase which needs to localize to the membrane in order to be activated. p70S6K does not have a lipid-binding domain and therefore may require interacting proteins such as PKCζ, and Cdc42/Rac1 to efficiently recruit it to the membrane, where it is in close proximity to its activating kinases, such as PDK-1 (2, 31). We considered the possibility that myr-PKCζ, which is membrane targeted, regulates p70S6K by recruiting it to the plasma membrane, where it could be activated by PDK-1 and perhaps other kinases. Indeed we found that myr-PKCζ K/W can associate with p70S6K (Fig. 6). However, this mutant did not cooperate with PDK-1 for the activation of p70S6K and inhibited p70S6K activation by EGF (data not shown). Our results therefore indicate that membrane targeting of PKCζ is not sufficient for a positive effect on p70S6K.
It is possible that PKCζ directly phosphorylates p70S6K. Multiple phosphorylation events are required for maximal activation of p70S6K, and prior inputs may be required before a PKCζ-regulated site is revealed. It has been suggested, based on a variety of mutagenesis studies, that the carboxy-terminal proline-directed sites must be phosphorylated to expose other p70S6K phosphorylation sites required for full activation (32). Furthermore, one report has suggested that PDK-1 will not efficiently phosphorylate and activate WT p70S6K unless Thr-389 is phosphorylated (31). In contrast, we and others (2) have found that PDK-1 can activate full-length WT p70S6K to some extent. These contrasting data may be due to experimental differences such as cell culture conditions. It remains to be determined which p70S6K site(s) PKCζ regulates, although it is likely to be a wortmannin-sensitive site, given that activation of PKCζ is wortmannin sensitive. The activation of HA-p70S6K ΔCT by myr-PKCζ and the cooperativity observed with PDK-1 (Fig. 4B) suggest that PKCζ does not regulate p70S6K via its C-terminal domain. A candidate site is Ser-371, a critical p70S6K phosphorylation site whose regulation by PI3-K has not been determined. Moreover, this site is similar to a PKC autophosphorylation site.
The association of both PDK-1 and PKCζ with p70S6K, as well as with each other (Fig. 5A, B, and C), is an intriguing observation and suggests that efficient PI3-K-mediated signalling is accomplished by the existence of effectors and their downstream targets such as p70S6K in preformed complexes. It is interesting and relevant that p38-MAPK was found not to associate with p70S6K, as this member of the MAPK family can directly phosphorylate and possibly regulate p70S6K (28, 33). This observation emphasizes the specificity of a putative PI3-K signalling complex. The formation of such a PI3-K signalling complex could be mediated by a scaffolding protein that would allow protein kinases in the PI3-K signalling pathway to be in close proximity, facilitating phosphorylation of substrates. Such signalling complexes could also be important for ensuring specificity to signals emanating from different receptors in distinct cell types which may employ common effector molecules but diverge at different levels in the signalling cascade.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants GM51405 (J.B.) and CA75134 (A.T.). A.R. is a recipient of a postdoctoral fellowship award from the Juvenile Diabetes Foundation International.
We thank Shigeo Ohno for providing the PKCζ K/W construct. We also thank members of the Blenis lab for helpful discussions and for critical reading of the manuscript.
ADDENDUM IN PROOF
Following submission of this paper, an article showing that atypical protein kinase Cλ binds and regulates p70 S6 kinase was published (Akimoto et al., Biochem. J. 338:417–424, 1998).
REFERENCES
- 1.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 2.Alessi D R, Kozlowski M T, Weng Q P, Morrice N, Avruch J. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr Biol. 1998;8:69–81. doi: 10.1016/s0960-9822(98)70037-5. [DOI] [PubMed] [Google Scholar]
- 3.Anderson K E, Coadwell J, Stephens L R, Hawkins P T. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr Biol. 1998;8:684–691. doi: 10.1016/s0960-9822(98)70274-x. [DOI] [PubMed] [Google Scholar]
- 4.Bandyopadhyay G, Standaert M L, Zhao L, Yu B, Avignon A, Galloway L, Karnam P, Moscat J, Farese R V. Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J Biol Chem. 1997;272:2551–2558. doi: 10.1074/jbc.272.4.2551. [DOI] [PubMed] [Google Scholar]
- 5.Berra E, Diaz-Meco M T, Dominguez I, Municio M M, Sanz L, Lozano J, Chapkin R S, Moscat J. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell. 1993;74:555–563. doi: 10.1016/0092-8674(93)80056-k. [DOI] [PubMed] [Google Scholar]
- 6.Berra E, Diaz-Meco M T, Lozano J, Frutos S, Municio M M, Sanchez P, Sanz L, Moscat J. Evidence for a role of MEK and MAPK during signal transduction by protein kinase C zeta. EMBO J. 1995;14:6157–6163. doi: 10.1002/j.1460-2075.1995.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgering B M T, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 8.Burnett P E, Barrow R K, Cohen N A, Snyder S H, Sabatini D M. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheatham B, Vlahos C J, Cheatham L, Wang L, Blenis J, Kahn C R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheatham L, Monfar M, Chou M M, Blenis J. Structural and functional analysis of p70 S6 kinase. Proc Natl Acad Sci USA. 1995;92:11696–11700. doi: 10.1073/pnas.92.25.11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou M M, Blenis J. The 70kD S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell. 1996;85:573–583. doi: 10.1016/s0092-8674(00)81257-x. [DOI] [PubMed] [Google Scholar]
- 12.Chou M M, Blenis J. The 70kD S6 kinase: regulation of a kinase with multiple roles in mitogenic signalling. Curr Opin Cell Biol. 1995;7:806–814. doi: 10.1016/0955-0674(95)80064-6. [DOI] [PubMed] [Google Scholar]
- 13.Chou M M, Hou W, Johnson J, Graham L K, Lee M H, Chen C-S, Newton A C, Schaffhausen B S, Toker A. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 14.Chung J, Grammer T, Lemon K, Kazlauskas A, Blenis J. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994;370:71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- 15.Chung J, Kuo C J, Crabtree G R, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signalling by the 70kD S6 protein kinases. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- 16.Dahl J, Freund R, Blenis J, Benjamin T L. Studies of partially transforming polyomavirus mutants establish a role for phosphatidylinositol 3-kinase in activation of pp70 S6 kinase. Mol Cell Biol. 1996;16:2728–2735. doi: 10.1128/mcb.16.6.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 18.Dominguez I, Diaz-Meco M T, Municio M M, Berra E, García de Herreros A, Cornet M E, Sanz L, Moscat J. Evidence for a role of protein kinase C ζ subspecies in maturation of Xenopus laevis oocytes. Mol Cell Biol. 1992;12:3776–3783. doi: 10.1128/mcb.12.9.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Paramio P, Cabrerizo Y, Bornancin F, Parker P J. The broad specificity of dominant inhibitory protein kinase C mutants infers a common step in phosphorylation. Biochem J. 1998;333:631–636. doi: 10.1042/bj3330631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han J W, Pearson R B, Dennis P B, Thomas G. Rapamycin, wortmannin, and the methylxanthine SQ20006 inactivate p70S6k by inducing dephosphorylation of the same subset of sites. J Biol Chem. 1995;270:21396–21403. doi: 10.1074/jbc.270.36.21396. [DOI] [PubMed] [Google Scholar]
- 22.Herrera-Velit P, Knutson K L, Reiner N E. Phosphatidylinositol 3-kinase-dependent activation of protein kinase C-zeta in bacterial lipopolysaccharide-treated human monocytes. J Biol Chem. 1997;272:16445–16452. doi: 10.1074/jbc.272.26.16445. [DOI] [PubMed] [Google Scholar]
- 23.Hu Q, Klippel A, Muslin A J, Fantl W J, Williams L T. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science. 1995;268:100–102. doi: 10.1126/science.7701328. [DOI] [PubMed] [Google Scholar]
- 24.Le Good J A, Ziegler W H, Parekh D B, Alessi D R, Cohen P, Parker P J. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 25.Liu Q, Ning W, Dantzer R, Freund G G, Kelley K W. Activation of protein kinase C-zeta and phosphatidylinositol 3′-kinase and promotion of macrophage differentiation by insulin-like growth factor-I. J Immunol. 1998;160:1393–1401. [PubMed] [Google Scholar]
- 26.Mendez R, Kollmorgen G, White M F, Rhoads R E. Requirement of protein kinase Cζ for stimulation of protein synthesis by insulin. Mol Cell Biol. 1997;17:5184–5192. doi: 10.1128/mcb.17.9.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moser B A, Dennis P B, Pullen N, Pearson R B, Williamson N A, Wettenhall R E, Kozma S C, Thomas G. Dual requirement for a newly identified phosphorylation site in p70s6k. Mol Cell Biol. 1997;17:5648–5655. doi: 10.1128/mcb.17.9.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukhopadhyay N K, Price D J, Kyriakis J M, Pelech S, Sanghera J, Avruch J. An array of insulin-activated, proline-directed serine/threonine protein kinases phosphorylate the p70 S6 kinase. J Biol Chem. 1992;267:3325–3335. [PubMed] [Google Scholar]
- 29.Nakanishi H, Brewer K A, Exton J H. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993;268:13–16. [PubMed] [Google Scholar]
- 30.Pearson R B, Dennis P B, Han J W, Williamson N A, Kozma S C, Wettenhall R E, Thomas G. The principal target of rapamycin-induced p70S6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J. 1995;14:5279–5287. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pullen N, Dennis P B, Andjelkovic M, Dufner A, Kozma S C, Hemmings B A, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- 32.Pullen N, Thomas G. The modular phosphorylation and activation of p70S6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- 33.Romanelli, A., H. S. Poon, and J. Blenis. Unpublished observations.
- 34.Schonwasser D C, Marais R M, Marshall C J, Parker P J. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Standaert M L, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese R V. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 36.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter G F, Holmes A B, Gaffney P R, Reese C B, McCormick F, Tempst P, Coadwell J, Hawkins P T. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 37.van Dijk M C, Hilkmann H, van Blitterswijk W J. Platelet-derived growth factor activation of mitogen-activated protein kinase depends on the sequential activation of phosphatidylcholine-specific phospholipase C, protein kinase C-zeta and Raf-1. Biochem J. 1997;325:303–307. doi: 10.1042/bj3250303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weng Q, Andrabi K, Klippel A, Kozlowski M T, Williams L T, Avruch J. Phosphatidylinositol 3-kinase signals activation of p70 S6 kinase in situ through site-specific p70 phosphorylation. Proc Natl Acad Sci USA. 1995;92:5744–5748. doi: 10.1073/pnas.92.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weng Q, Andrabi K, Kozlowski M T, Grove J R, Avruch J. Multiple independent inputs are required for activation of the p70 S6 kinase. Mol Cell Biol. 1995;15:2333–2340. doi: 10.1128/mcb.15.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]