Abstract
West Nile Virus (WNV) has recently emerged as a major public health concern in Europe; its recent expansion also coincided with some remarkable socio-economic and environmental changes, including an economic crisis and some of the warmest temperatures on record. Here we empirically investigate the drivers of this phenomenon at a European wide scale by constructing and analyzing a unique spatial–temporal data-set, that includes data on climate, land-use, the economy, and government spending on environmental related sectors. Drivers and risk factors of WNV were identified by building a conceptual framework, and relationships were tested using a Generalized Additive Model (GAM), which could capture complex non-linear relationships and also account for spatial and temporal auto-correlation. Some of the key risk factors identified in our conceptual framework, such as a higher percentage of wetlands and arable land, climate factors (higher summer rainfall and higher summer temperatures) were positive predictors of WNV infections. Interestingly, winter temperatures of between 2 °C and 6 °C were among some of the strongest predictors of annual WNV infections; one possible explanation for this result is that successful overwintering of infected adult mosquitoes (likely Culex pipiens) is key to the intensity of outbreaks for a given year. Furthermore, lower surface water extent over the summer is also associated with more intense outbreaks, suggesting that drought, which is known to induce positive changes in WNV prevalence in mosquitoes, is also contributing to the upward trend in WNV cases in affected regions. Our indicators representing the economic crisis were also strong predictors of WNV infections, suggesting there is an association between austerity and cuts to key sectors, which could have benefited vector species and the virus during this crucial period. These results, taken in the context of recent winter warming due to climate change, and more frequent droughts, may offer an explanation of why the virus has become so prevalent in Europe.
Keywords: West-Nile-virus, Climate-change, Economic-crisis, Mosquito, Austerity, Vector-borne-disease, Drought
1. Introduction
Over the past few decades, new health risks have been emerging in Europe, particularly with the recent appearance of vector borne diseases (VBDs) such as Chikungunya, West Nile Virus (WNV), Dengue (DENV-1) and Crimean-Congo haemorrhagic fever [1], [2], [3]. Rising temperatures are likely increasing the transmission potential of tropical VBDs in Europe, by affecting the geographic spread, abundance, survival and feeding activity of vector species and benefiting pathogen development in infected vectors [4], [5], [6], [7], [8], [9]. This, combined with other factors such as human population growth, intensive animal rearing, global commerce, air travel, urbanization and land-use changes, is increasing the chances of novel diseases to enter and emerge in Europe [10], [11], [12], [13].
In this study, we focus on WNV, a single-stranded RNA Flavivirus closely related to other Flaviviridae pathogens such as dengue, Japanese encephalitis and yellow fever viruses [14]. Although WNV is a zoonotic pathogen, infecting mammals, particularly humans and horses, the transmission cycle is believed to be driven mainly by mosquitoes and birds [15], although some wild mammals may serve as intermediate hosts for West Nile virus [16].
West Nile Virus (WNV) has recently emerged as a major public health concern in Europe, its recent expansion also coincided with some remarkable socio-economic and environmental changes, including an economic crisis and some of the warmest temperatures on record. To date, there has been very little research investigating this phenomenon at a European wide scale and more work is required to reveal the key drivers of the disease. A better understanding of this phenomenon can help public health officials design health prevention measures and develop better predictive models for public health risk management. More specifically, little work has been done to explore the association between the rise of WNV in Europe and the economic crisis unfolding from 2008 on-wards. Although the study of physical factors is key in understanding disease transmission and distribution, few articles have considered links among societal factors, like changes in the economy and in policy making [17], [18], which we know can have wide and unintended effects on natural ecosystems and, eventually, on disease [19]. Although examining such factors presents certain challenges and uncertainties, given the scales involved and lack of data, the continuation of abrupt socio-economic changes (brought by the COVID-19 pandemic) and climate change impacts indicate an urgent need to examine such statistical relationships more closely, at the very least to open up scholarly debate and instigate further research on the topic.
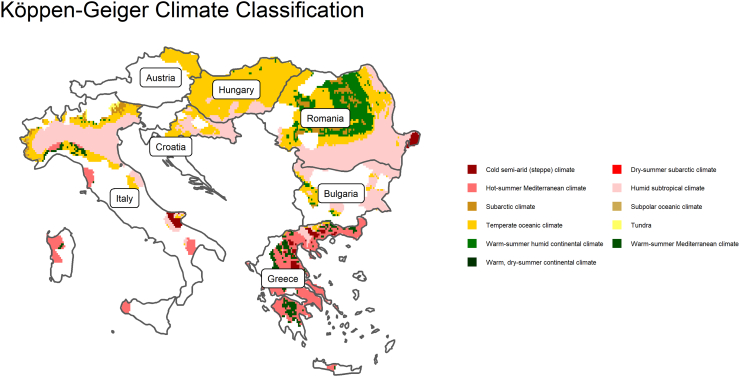
Since 2010, WNV has been reported in 14 EU countries including Austria, Bulgaria, Croatia, Cyprus, Czechia, France, Greece, Hungary, Italy, the Netherlands, Portugal, Romania, Slovenia and Spain; and has also been reported in five neighboring EU candidate countries including Albania, Montenegro, Serbia, Turkey and Kosovo [20], [21]. In 2010, major outbreaks hit Greece, Hungary, Romania, and Turkey. Since then, outbreaks have occurred annually in multiple regions, including more northerly regions that had not previously reported cases, like Germany and the Czech Republic (see Fig. 1). This culminated in another major outbreak in 2018, that affected more regions than had been recorded in previous years.
Fig. 1.
Koppen–Geiger (CG) Climate Classification in study regions. Colored areas correspond to the overlap between the known WNV distribution and the CG classification in those areas. Areas highlighted in white represent places where human WNV infections have not been reported. (Data source: koeppen-geiger.vu-wien.ac.at). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Generally, WNV distribution is determined by the presence of suitable mosquito vectors and avian hosts, such as terrestrial and wetland birds. The spring migration of birds from infected regions of Sub-Saharan Africa to temperate regions of Europe is considered to be one of the main drivers of the disease in Europe [22], [15]. In Europe, the main vectors are Culex pipiens, Culex modestus, and Coquillettidia richiardii, although Aedes species can also transmit the disease [15] (see [23], [24], [25] for vector distribution maps).
Although WNV infections are more common of late, sporadic outbreaks have occurred in humans and equines in southern and eastern European countries over the last century; the majority of which have occurred in wetland areas and densely inhabited urban areas [15].
To examine the recent rise of WNV infections in Europe in more depth, we empirically investigate the combined effect of three sets of factors: (1) Climate/environmental factors including temperature, rainfall and surface water; (2) land-use factors including continuous/discontinuous urban fabric which represents physical characteristics of urban areas e.g. densely populated areas like cities or less dense areas like villages, regional coverage of wetlands and arable land; (3) socio-economic factors that capture the associations of the economic crisis and are proxied by GDP growth, central government spending on areas of the environment including agriculture, forestry and fisheries and waste water management. Our analysis focuses on regions in the 7 European countries where WNV has been regularly reported – Austria, Bulgaria, Croatia, Greece, Hungary Italy, and Romania. The time series data set captures the time period before and after the economic crisis (2007–2019).
1.1. Conceptual framework
WNV transmission requires the presence of competent vectors, a suitable climate and a susceptible host population. Studying WNV transmission at a macro scale presents significant challenges, since key data on the seasonal and annual abundance of competent mosquitoes and birds are not available; this is further complicated given the hundreds of potential host bird species in Europe [26]. To explain human infections, we therefore use environmental risk factors known to attract vector, and host. Furthermore, vector abundance for a given season is modulated by physical and environmental factors, such as temperature, rainfall and water resource availability [15], [27], [14]. We therefore use proxies that can predict mosquito abundance.
1.1.1. Modulating factors
Typically, with most tropical and temperate mosquito species, elevated temperatures allow vector populations to increase their growth and reproduction rates, which in turn decreases blood meals intervals, accelerating transmission and virus evolution rates [28]. Furthermore, increasingly warmer winters allow mosquito vectors to expand their breeding seasons and survive during winter, either as eggs or as overwintering female mosquitoes. Weather conditions and climatic factors can also affect vector competence [14]. Viral replication rates and transmission of WNV are modulated by ambient temperature, affecting the length of the extrinsic incubation period (EIP), seasonal phenology of mosquito host populations and also times at which humans and mosquitoes come into contact [29]. Generally, higher rainfall in warmer weather can lead to higher mosquito abundance and disease transmission by increasing the potential habitat suitable for mosquito reproduction, e.g. standing water [30], [15], [31]. Conversely, sometimes drought and shrinking water resources can also bring some species into closer contact, facilitating transmission and amplification of WNV within these locations [14]. To represent these points in our data-set/model, we selected mean winter temperature, mean summer temperature, number of days of rainfall in summer and summer surface water extent (a count of the number of satellite surface water observations per region represented as pixels in a geographic raster layer).
1.1.2. Risk factors
West Nile virus circulation in Europe is usually confined to two different cycles and ecosystems: the sylvatic and the urban synanthropic cycle. Rural locations, including river deltas and floodplain areas, help create a sylvatic cycle, where wild, usually nesting wetland birds and ornithophilic mosquitoes Culex pipiens, Culex modestus, Coquillettidia richiardii) create the conditions for maintaining WNV transmission. In urban synanthropic cycles, mosquitoes, such as Culex pipiens or Culex modestus, feed on domestic birds and humans. However, these two cycles can overlap, so areas with wetlands close to human populations can be particularly vulnerable to the disease [15]. Irrigation from agriculture is also heavily linked to a greater incidence of human and veterinary WNV infections [32]. In order to represent these factors in our data set and final models, we selected land use variables (% cover) representing urban areas (metro areas), semi urban areas (lower density human settlements), wetlands and arable land.
1.1.3. Economic crisis
We would expect the repercussions of an economic crisis to affect WNV transmission in several ways, at the individual level (bottom-up) and government level (top-down). Previous studies have shown that socio-economic factors tend to influence the distribution and intensity of mosquito-borne diseases both pre-infection and post-infection [33]. Poorer communities are less likely to have air-conditioned homes, tap water and adequate drainage, and therefore may be more exposed to biting mosquitoes. Several studies have demonstrated the link between WNV infections and a range of local-level socio-economic and demographic factors such as income, sanitation, and population density [22], [34], [35]. In general, we would expect to see a drop in living standards in regions experiencing an economic shock followed by sluggish economic growth. Those people most affected would find it more difficult to prevent mosquito infections through direct measures i.e. sprays and repellents and less likely to pay for things that indirectly influence WNV transmission, like the upkeep of homes and use of air conditioning. Factors associated with higher economic status can also bring humans into closer contact with mosquitoes, for example, home owners with gardens and potted plants, swimming pools and ponds or having good access to recreational space where mosquitoes can breed [36], [37]. However, neglect of such things through economic decline can have further unintended effects, even in wealthy neighborhoods [38], [39], [40].
Mosquito control (mosquito abatement) is regarded as an effective way to reduce the incidence of WNV in humans [14]. It is well documented, for example, that during the European debt crisis, the Greek government cut mosquito abatement budgets, which may have led to a rise in vector borne disease outbreaks such as malaria and WNV [41]. WNV transmission is most likely to occur in places that favor the larval development of Culex pipiens, such as poorly drained low-lying areas, urban storm-water catch basins and manhole chambers, roadside ditches, sewage treatment lagoons, and man-made containers around houses, or other aquatic environments where mosquitoes deposit their eggs [14]. Additionally, during periods of austerity, governments can neglect hazard prevention efforts, such as spending on flood defenses, as well as essential works like sanitation and up-keep and improvement of infrastructure [42]. Such degradation can lead to the creation of mosquito habitats [14], [15], [42]. Another critical component of preventing disease transmission is through public education programs and health promotion, educating the public on measures which can prevent being bitten can reduce risk of exposure. In general, we would expect to see a general deterioration in a government ability to run such programs during crisis and austerity. Other consequences of austerity can be expected in decreased disease detection because of cuts in public health services, prolonged periods between initial infection and treatment seeking due to dysfunctional healthcare systems, and reduced treatment of disease, all of which can lead to more intense outbreaks [43].
In order to represent the economic crisis in our model, we selected regional GDP and central government spending on healthcare; agriculture, forest and fisheries management; and wastewater management. Rather than using actual annual values, or year on year growth, we look at increases or decreases in growth using 2007 baseline levels, just before the crisis hit Europe. As a priori, we would expect WNV incidence to be associated with negative growth or very low growth in these sectors.
2. Materials and methods
In this study, we compiled a unique spatial–temporal data-set that captures the main drivers and risk factors of WNV infections in Europe, based on findings from the conceptual framework. Since WNV infection data is only available at the European NUTS 3 level (aggregated areal health data), our empirical strategy relies on aggregated areal health data. We selected regions for the study where autochthonous virus transmission had occurred at least once over the reporting period in the selected countries. By applying this criteria, we were left with 166 regions in total for the analysis. We assumed that all regions included in the study could be influenced by migratory birds that form part of the African and European flyways [44].
2.1. Data sources
The following subsection describes data sources for the study. For an extended description of data extraction and processing techniques, see Supplementary File 1.
All data were aggregated annually at the European NUTS 3 country subdivision level (apart from central government spending data which was sourced at the country level), to produce a yearly panel data-set. The NUTS 3 classification represents small regions with a population ranging from 150,000 to 800,000 and is part of the Nomenclature of Territorial Units for Statistics (NUTS) classification system, used to divide economic territories of the EU into three hierarchical sub categories for the purpose of data collection and statistical analysis (see [45] for further information).
WNV case data were provided on request by the European Centre for Disease Prevention and Control (http://www.ecdc.europa.eu). Case data are collected weekly by EU member states and affiliates. Positive cases were confirmed by at least one of the following techniques: (1) isolating WNV or WNV nucleic acid from blood or cerebrospinal fluid (CSF); (2) inducing a WNV-specific antibody response (either IgG/IgM) in a serological test. We should also bear in mind that the actual number of cases in Europe is likely to be much higher than reported, since most people infected with WNV will not develop symptoms (are asymptomatic). Around 20% of those infected with WNV will develop West Nile fever, a flu like illness, or severe West Nile disease [14]. All cases were aggregated yearly to create the annual panel data-set.
Economic data were extracted from the Eurostat database (https://ec.europa.eu/eurostat/data/database), which provides comparable statistics and indicators and is presented in yearly time series. To capture factors determining the economic crisis, austerity and cuts to public spending, we selected regional Gross Domestic Product (GDP); country level agriculture, forestry, fisheries spending; country level waste water spending, and country level health spending. The “Agriculture, forestry, fisheries spending” variable captures spending in rural areas that help to improve the environment and agricultural development, that can benefit agricultural workers and/or mechanize production [46]. In order to represent spending before and after the economic crisis, we created a baseline index for each variable set at 2007 levels, which represented negative or positive growth from the point just before the economic crisis hit Europe. Population count data to predict the number of people at risk in a region were sourced from the Socio-economic Data and Applications Center's Gridded Population of the World data set [47]. This data-set estimates the population count, consistent with national censuses and population registers.
Climate data were sourced from the E-OBS Gridded Data-set [48]. This data-set was created using a series of daily temperature and rainfall observations at meteorological stations throughout Europe.
Land use statistics i.e., “Continuous urban fabric”, “Discontinuous urban fabric’, “Wetlands (fresh water)” and “Arable land” were captured sourced from the CORINE Land Cover (CLC) database [49], which provides data on the biophysical characteristics of the Earth's surface. Regional surface water data was sourced using the JRC Monthly Water History, v1.2 data set [50]. This data set contains maps of the location and temporal distribution of surface water from 1984 to 2019 and provides statistics on the extent and change of water surfaces.
2.2. Final data-set
Table 1 provides descriptive statistics of the final data-set. We did not include “Mean temp spring (°C)” in our final data set as it was correlated with winter and summer temperature variables; we concluded that we would capture more variation using the winter and summer variables which were not highly correlated. Healthcare spending was also not included in the final analysis as it was highly correlated with GDP (see Additional File 1 for data and model diagnostics).
Table 1.
Summary statistics of variables selected for statistical analysis - 2007–2019.
| Statistic | Min | Max | Mean | St. Dev. |
|---|---|---|---|---|
| WNV cases | 0 | 100 | 1.649 | 6.012 |
| Human population | 6254 | 10,534,640 | 443,384 | 513,000 |
| Mean temp winter (C) | −6.072 | 14.564 | 3.190 | 3.623 |
| Mean temp spring (C) | 4.092 | 18.684 | 12.433 | 2.157 |
| Mean temp summer (C) | 13.109 | 28.011 | 22.465 | 2.285 |
| Days of rain in winter | 0 | 68 | 30.156 | 12.546 |
| Days of rain in spring | 0 | 71 | 31.723 | 12.918 |
| Days of rain in summer | 0 | 65 | 26.021 | 14.374 |
| Spring surface water extent Z-score (30 m2) | −2.876 | 2.404 | 0.000 | 0.958 |
| Summer surface water extent Z-score (30 m2) | −3.301 | 3.080 | −0.000 | 0.958 |
| Continuous urban fabric % cover | 0.000 | 45.056 | 1.336 | 6.754 |
| Discontinuous urban_fabric (% cover) | 0.534 | 60.457 | 5.511 | 7.044 |
| Wetlands (% cover) | 0.000 | 25.460 | 0.569 | 2.026 |
| Arable land (% cover) | 0.000 | 86.307 | 33.893 | 22.172 |
| Regional GDP growth (2007 = 100%) | 57 | 217 | 106.752 | 24.587 |
| Agri, forest + fish spending (2007 = 100%) | 27 | 251 | 80.202 | 35.165 |
| Waste water mngmnt spending (2007 = 100%) | 5 | 352 | 93.476 | 53.933 |
| Health spending (2007 = 100%) | 60 | 212 | 114.802 | 33.307 |
2.3. Statistical methods
The relationship between the incidence of WNV infections (per 100,000) and the climate, land-use, and economic factors was modeled via a Generalized Additive Model (GAM), which also accounted for the spatial and temporal auto-correlation. One of the main issues with our data-set is that it does not meet some basic assumptions for statistical inference, and specifically the data are not independent and identically distributed random variables (iid). More specifically, the data-set captured repeated measurements over the same regions, and observations were not independent because of spill over effects from neighboring regions. Therefore, spatial auto-correlation in the GAM model was approximated by a Markov random field (MRF) smoother, defined by the geographic areas and their neighborhood structure. We used R's Spdep package [51] to create a queen neighbors list (adjacency matrix) based on regions with contiguous boundaries i.e. those sharing one or more boundary point. We used a full rank MRF, which represented roughly one coefficient for each area. The local Markov property assumes that a region is conditionally independent of all other regions unless regions share a boundary. This feature allowed us to model the correlation between geographical neighbors and smooth over contiguous spatial areas, summarizing the trend of the response variable as a function of the predictors (see Section 5.4.2 of [52]. In order to account for variation in the response variable over time, not attributed to the other explanatory variables in our model, we used a saturated time effect for years, where a separate effect per time point is estimated.
Since not all regions report cases every year, we fit the our main model using the Tweedie distribution, which can handle excess zeros [53]. This distribution also allowed us to model the non-negative, right-skewed integer case data as the incident rate per 100,000.
The empirical model can then be written as:
where the f(.) stands for smooth functions; E(Y)it is equal to the WNV infection incidence per 100,000 in region i at time t, which we assume to be Tweedie distributed; Xit is a vector of economic, demographic, environmental and climate variables. Yeart is a function of the time intercept and Regioni represents neighborhood structure of region.
We built the statistical model in a step-wise fashion using the lowest Akaike Information Criterion (AIC) to help us assess the different specifications. The AIC allows us to measure model performance accounting for model complexity and reflects how well the model fits the data.
We selected relevant variables in each specification according to their category, i.e. climate, land-use and economic. All variables were included in the final specification to ascertain the contribution of each driver, all else equal.
3. Results
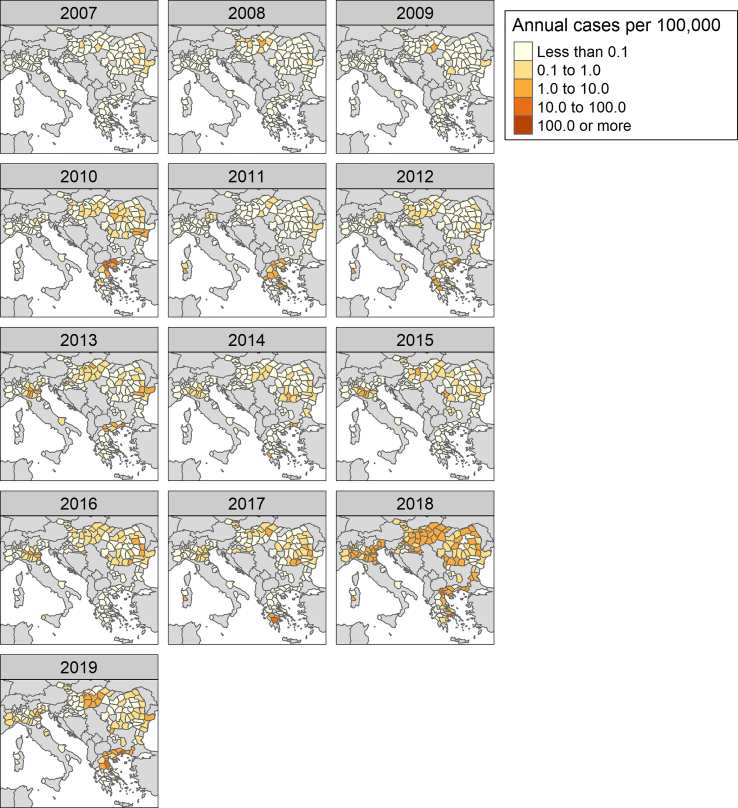
Fig. 1 characterizes the climate in the study regions. As we can observe, WNV infections occur in regions with climates that can be described as “Hot-summer Mediterranean”, “Humid subtropical”, “Temperature oceanic” and “Warm-summer humid continental” or “Temperate oceanic”. Fig. 2 shows the WNV infection incidence rates over the study period. From 2007 to 2009, very few regions were affected by WNV, however, 2010 saw an outbreak that spread far and wide. Since 2010, the number of regions that have been affected by WNV increased. In 2018, a massive outbreak affected almost all of the regions in our study.
Fig. 2.
Distribution of regional West Nile virus infections per 100,000 in humans from 2006 to 2019: (Data source: ECDC).
Table 2 shows the results of our statistical analysis and summarizes the relevant statistics (AIC, BIC and Deviance explained and so on) to compare the different specifications. We find that our final model (Full model) has the best fit in terms of the AIC, followed by the economic model, the climate model and land use model, as shown in Table 2. Note that as we are not estimating a standard regression model, the figures reported should not be read as coefficients, but degrees of freedom of the smooth terms. Given that we cannot interpret the coefficients to infer the sign and magnitude of the relationship, we visualize it by plot. Fig. 3, Fig. 4, Fig. 5 plot the partial effects—the relationship between a change in each of the covariates and a change in the fitted values in the full model.
Table 2.
Generalized additive regression model for assessing associations between climate, land use and socio-economic factors on regional WNV incidence per 100,000 people.
| Clim model | Land-use model | Econ model | Full model | |
|---|---|---|---|---|
| Intercept | −2.21*** | −2.13*** | −2.25*** | −2.35*** |
| (0.47) | (0.45) | (0.33) | (0.40) | |
| Mean temp summer (C) | 1.00*** | 1.00* | ||
| (1.00) | (1.00) | |||
| Mean temp winter (C) | 1.96*** | 1.94*** | ||
| (1.99) | (1.99) | |||
| Days of rain in summer | 1.00** | 1.00** | ||
| (1.00) | (1.00) | |||
| Summer surface water extent (30 m2) | 1.60** | 1.02*** | ||
| (1.84) | (1.03) | |||
| Continuous urban fabric % | 1.00 | 1.00 | ||
| (1.00) | (1.00) | |||
| Discontinuous urban fabric % | 1.00 | 1.00 | ||
| (1.00) | (1.00) | |||
| Wetlands % | 1.00** | 1.00 | ||
| (1.00) | (1.00) | |||
| Arable land % | 1.81*** | 1.74** | ||
| (1.89) | (1.84) | |||
| Regional GDP index (2007 = 100%) | 1.00* | 1.00 | ||
| (1.00) | (1.00) | |||
| Agri, forest + fish spending (2007 = 100%) | 1.95*** | 1.93*** | ||
| (1.99) | (1.99) | |||
| Waste water management spending (2007 = 100%) | 1.60*** | 1.10*** | ||
| (1.83) | (1.19) | |||
| Spatial lag | 78.44*** | 80.54*** | 88.90*** | 76.19*** |
| (109.60) | (111.42) | (121.08) | (106.52) | |
| Year | 11.73*** | 11.75*** | 11.48*** | 11.56*** |
| (12.00) | (12.00) | (12.00) | (12.00) | |
| AIC | 3952.99 | 3992.59 | 3931.25 | 3907.56 |
| BIC | 4526.68 | 4569.26 | 4560.01 | 4538.85 |
| Log Likelihood | −1875.44 | −1894.71 | −1854.87 | −1842.57 |
| Deviance | 3747.15 | 3895.52 | 3592.25 | 3520.85 |
| Deviance explained | 0.63 | 0.62 | 0.64 | 0.65 |
| Dispersion | 2.85 | 2.95 | 2.76 | 2.73 |
| R^2 | 0.26 | 0.36 | 0.32 | 0.25 |
| GCV score | 1900.89 | 1927.27 | 1889.38 | 1871.90 |
| Num. obs. | 2158 | 2158 | 2158 | 2158 |
| Num. smooth terms | 6 | 6 | 5 | 13 |
p < 0.1.
p < 0.05.
p < 0.01.
****p < 0.001.
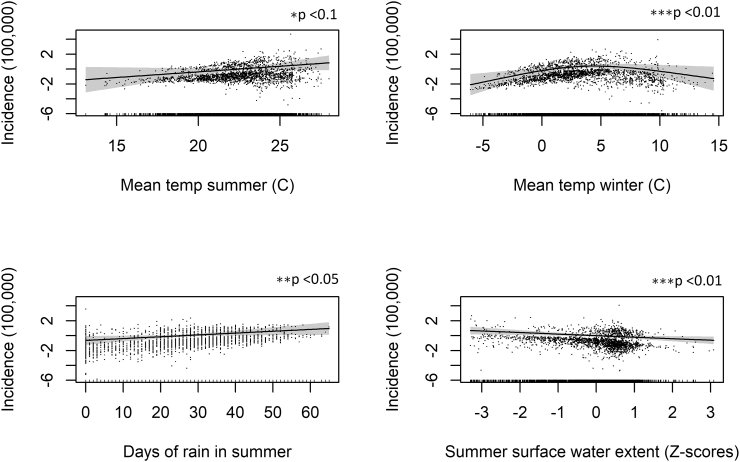
Fig. 3.
Generalized additive model (GAM) plots showing the partial effects of the explanatory variables on the incidence of WNV per 100,000. The tick marks on the x-axis are observed data points. The y-axis represents the partial effect of each variable. The dots represent partial residuals. The shaded areas indicate the 95% confidence intervals.
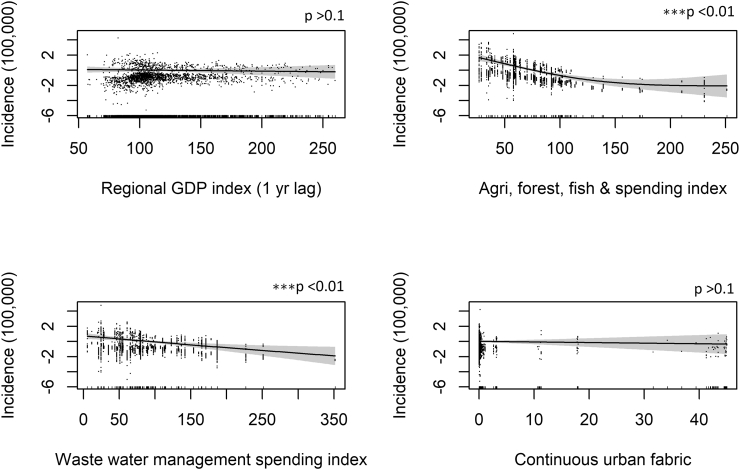
Fig. 4.
Generalized additive model (GAM) plots showing the partial effects of the explanatory variables on the incidence of WNV per 100,000. The tick marks on the x-axis are observed data points. The y-axis represents the partial effect of each variable. The dots represent partial residuals. The shaded areas indicate the 95% confidence intervals.
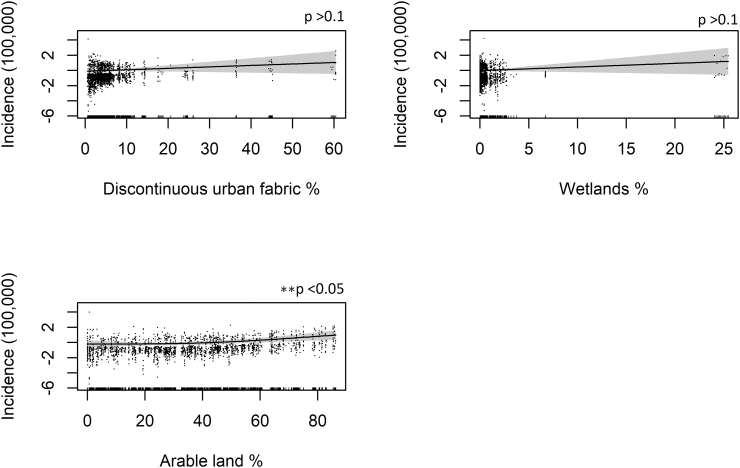
Fig. 5.
Generalized additive model (GAM) plots showing the partial effects of the explanatory variables on the incidence of WNV per 100,000. The tick marks on the x-axis are observed data points. The y-axis represents the partial effect of each variable. The dots represent partial residuals. The shaded areas indicate the 95% confidence intervals.
All climate variables in both model specifications are statistically significant (“Clim model” & “Full model”). Mean summer temperatures of above 22 °C are (Fig. 3, top left) positively associated with WNV infections; the relationship is linear and strong in the first specification, however, becomes less significant in the “Full model” specification after controlling for all other variables.
Mean winter temperature has a quadratic relationship with WNV. Temperatures of between 2 °C and 6 °C have a positive association with WNV infections, whereas colder and warmer temperatures outside of this range are negatively associated with WNV infections. The number of rain days per summer is also a strong predictor of WNV infections and has a linear positive relationship. Higher surface water in the summer is negatively correlated with WNV infections. The relationship is fairly strong considering its complexity i.e., the variable complexity has been reduced to standard deviation scores to standardize it across regions and seasons.
As for the land use variables, the percentage of arable land and wetlands in a region (“Land-use model”) is positively correlated with the incidence of WNV and highly significant. However, the wetlands variable loses significance in the final model. The percentage of “Continuous” and “Discontinuous urban fabric” variables, which represent metropolitan and built up areas (residential suburbs, villages), are not statistically significant in any of the specifications. Although the partial effect plot for “Continuous urban fabric” reveals that it has a slightly negative relationship with WNV infections and “Discontinuous urban fabric” has a positive association with WNV infections.
The economic indicators are negatively correlated with WNV infections. These variables represent 2007 baseline regional GDP and central government spending growth. Regional GDP has a gentle negative association, but statistically significant relationship with WNV infections in the “Econ model specification”, but loses significance in the final model. The two indicators that directly represent central government spending on areas of the environment, such as agriculture, forest & fisheries spending; and waste water management, have highly statistically significant negative associations with WNV infections.
4. Discussion
To investigate the rise in WNV infections in Europe over the last 13 years (2007–2019), we compiled a unique spatial–temporal data-set including variables identified in the conceptual framework, following a thorough review of the literature. By taking this approach, we were able to carefully evaluate and adjust for environmental and economic factors that may have contributed to the recent rise in infections over the past decade or so. This study focuses on geographical factors which tend to influence the spread of the disease at the regional level, rather than trying to infer the determinants of the disease at the individual level.
4.1. Meteorological factors
Over the past 70 years, the countries analyzed in this study have been experiencing increasingly warmer temperatures throughout the year and according to our initial analysis, the last decade has been the warmest (see Additional File 1: Figs. S1–4). The results of our final model (Fig. 3, Fig. 4, Fig. 5) show that average summer temperatures above 22 °C are positively associated with an increase in WNV incidence. This finding is consistent with the literature, according to which warmer temperatures influence the hatching rate and development time of mosquitoes, and shorten the extrinsic incubation period (EIP) of WNV and related viruses, therefore representing a key driver of WNV transmission (especially in summer months) [54], [55], [56], [57], [58], [29]. However, this variable lost significance in our final model specification, suggesting it is not one of the main drivers associated with transmission in our study locations and that other factors may be at play. In particular, average summer temperatures lose their explanatory power once economic and land use factors are taken into account.
Our analysis of the mean winter temperature (Dec–Feb) reveals a quadratic relationship with WNV and is one of the main predictors of annual WNV infections in the model. Temperatures below 2 °C and above 6 °C have a negative association with WNV infections. These results are consistent with findings by Koenraadt et al., 2019 [59], who found that diapausing Culex pipiens mosquitoes do not necessarily do better under warmer conditions and there is a temperature range in which they can successfully diapause. Furthermore, other authors suggest [60], [61], [20] that outbreaks may be more intense following winters with optimal temperatures for diapausing mosquitoes. Since a larger number of mosquitoes can successfully survive the winter, and those that are infected with WNV can transmit it earlier on in the year, leading to increased disease prevalence in mosquitoes and reservoir bird species than in years when winter conditions are not optimal. It is also important to note that it is not currently clear at what temperatures Culex modestus and Coquillettidia richiardii overwinter as adults since our literature search did not yield any findings.
The number of rain days per summer is also positively correlated with WNV infections. This result is consistent with the literature, i.e., a steady flow of aquatic resources for mosquitoes has a positive association with their abundance, and therefore an increase in disease transmission [31], [30]. Rainfall patterns have also been shifting since the 1950s (see Additional File 1: Figs. S1–4) although unlike climate, there is no a clear trend and results are more difficult to interpret. In general, Austria, Croatia, and Italy are seeing less intense rainfall in the summer months, but higher in autumn, whereas Bulgaria, Greece, Hungary and Romania are receiving more rainfall in summer months.
Our results also show that higher regional summer surface water extent for a given year, is negatively associated with WNV and is one of the strongest predictors of WNV incidence. This was not an expected finding, since we would expected higher levels of surface water to be positively correlated with WNV incidence because of the extra water resources available to mosquitoes. However, it may be explained by the fact that sometimes desiccation of water resources can bring mosquito and bird hosts closer together, increasing transmission potential and therefore the prevalence of the virus [15], [14]. This was also a major finding in a recent study by Paull et al., 2017 [62], who reported that drought was closely linked to the intensity of outbreaks for a given year in the United States. Another explanation for this result is that with higher surface water extent, there may be more flooding and fast water movement, which may wash away mosquito eggs and larvae [63]. This, along with increases in surface water may also inhibit contact between birds, mosquitoes and humans [15], [14]. It is important to note that this variable probably does not capture the creation of short-term water resources created by rainfall (e.g. pools, puddles), which can be used as breeding habitat by mosquitoes. It rather captures long term and large water surface such as deltas, lakes, and flood plains.
4.1.1. Land-use
As for the land-use variables, as expected, regions with a larger proportion of arable land and wetlands are associated with higher WNV incidence. This is consistent with other literature, according to which humans are particularly at risk in areas close to rice paddies, irrigated agriculture and wetlands, since these areas tend to attract susceptible mosquitoes and birds [15], [32]. The percentage of discontinuous urban fabric, that represents populated areas of low to medium density that tend to have gardens, parks, ponds, such as residential suburbs and villages [64], is not statistically significant in our model, although it is often cited as a driver of WNV infections in humans.
4.1.2. Economic-factors
In terms of economic factors associated with WNV infections, higher GDP growth, higher spending, growth on environmental factors - such as agriculture, forest, fisheries - and waste water management are negatively associated with WNV incidence, consistently with concepts laid out in the conceptual framework. In other words, populations living in locations harder hit by economic slowdown and austerity could have been more exposed to mosquitoes, for instance drops in income make it difficult to afford mosquito repellents, air conditioning and upkeep of homes leading to the creation of mosquito habitats. General cuts to waste water management and hazard prevention efforts, such as spending on flood defenses, essential works like sanitation and upkeep of infrastructure, could have also led to the creation of mosquito breeding habitats, e.g. potholes, blocked drains [14], [15], [42]. Furthermore, many studies report strong associations between agriculture [65], [32], [66], [67] and WNV incidence. In general, cuts and lower spending in this sector, may have led to degradation on farms and the wider environment which may have benefited mosquitoes through the creation of habitat or lack of measures to control their abundance. The literature is scarce on this topic which makes it very difficult to compare our findings with other sources of information, so our interpretations of such results can only be speculative.
4.1.3. Limitations
Some of the limitations of the study are as follows. Since we were limited to using aggregated data at the NUTS-3 regional level, we cannot make inference about individual-level associations and could not adjust for individual-level risk factors e.g. age, gender, race, and occupation. However, that would be outside the scope of this study, since we were interested in macro ecological and socio-economic trends and drivers. Additionally, we cannot draw causal inference as the methodology we applied only reveals adjusted correlations. Indeed, we would have also liked to include further explanatory variables on avian host and mosquito abundance but were restricted by the availability of data. It is also important to note that data quality issues arise owing to the under-reporting of cases through under-diagnosis, lack of diagnostic tests and a lack of resources/time to carry out and implement mass testing. Another factor we did not consider is bird immunity, which may influence WNV incidence following a major outbreak, although this was not considered an important factor in explaining the rise in WNV infections in Europe, but may have influenced the results. Furthermore, a growing body of literature reports that mammals can serve as intermediate hosts for West Nile virus [16] and more research needs to be done to determine if wild mammals act as reservoirs and contribute significantly to the transmission cycle. We also realize that the economic analysis is limited, in part because of a lack of refined data and in part because of scale issues, i.e., the amount of work required to look at individual local level policies and spending was not feasible for 166 regions.
4.1.4. Conclusions
In this study, we set out to investigate why WNV outbreaks have become so frequent in Europe over the past decade. If we consider the findings of this work, together with other important research in this area, we can start to build a picture of why the virus has become so prevalent in Europe. We hypothesize that:
-
(1)
Rising winter temperatures, or rather the creation of optimal temperature conditions allowed the virus to overwinter with Culex pipiens. Given current trends, we can also expect to see regions that have previously been too cold for Culex pipiens to survive over winter become viable locations and cause further havoc in regions that are currently experiencing just a few annual cases. On the contrary, regions which currently have optimal conditions for overwintering mosquitoes may become too warm in the future.
-
(2)
Warmer summer temperatures are benefiting mosquitoes, influencing their hatching rate and development time, and shortening the extrinsic incubation period (EIP) of WNV.
-
(3)
Shrinking water resources are increasing WNV prevalence in birds and mosquitoes during some seasons. It may be the case that this phenomenon is also acting at a macro-scale in Europe and is a significant driver of recent outbreaks, especially given that meteorological and hydrological droughts are becoming more frequent and extreme [68]. These changes also occurred during an economic crisis and subsequent austerity, where government institutions were severely weakened and had to limit spending on key sectors, and segments of the human population were exposed to increased financial hardship.
We hope this study will spur further research into this topic, especially in areas less explored, such as the impacts of the European debt crisis on health, and the long-term trade-offs and unintended consequences austerity can have on the environment and human health. This is an especially important topic when considering we are facing multiple threats brought about by global warming and other anthropogenic induced changes that can benefit emerging diseases, i.e., global trade in wild animals, intensive agriculture/animal rearing and land use conversion.
Data availability
An R project containing all data that supports the findings of this study are available in.Rdata format from https://doi.org/10.5281/zenodo.4656902.
Code availability
An R project containing all code used to set-up the models is available here is available from 10.5281/zenodo.4656902.
Authors’ contribution
MW led the work and was responsible for the conceptualization of the project, data collection, data processing, formulation of the methodology, statistical analysis, modeling, analysis and writing the original draft and interpreting the results. All authors made contributions to developing the original paper outline. PK gave critical advice throughout the project. All contributors revised the manuscript and copy-edited the final submission version. All authors were also involved in revising the manuscript critically for important intellectual content. All authors read and approved the final manuscript. PK, PGM VSM supervised the project and are responsible for formulating ideas for the umbrella project Impacts of Climate Change (CC) on Human Health (HH) at ICTA-UAB: Integrating socio-economic and policy studies with natural science studies to enhance consilience of climate policy science.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
This research was funded by ICTA's Maria de Maeztu Unit of Excellence, awarded by the Spanish Ministry of Economy and Competitiveness. Thanks also to Patrizia Ziveri and Pedro Manuel Gonzalez Hernandez for supporting the project. Thanks also to Cesira Urzi Brancati for statistical help and advice.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2021.100315.
Appendix A. Supplementary data
Supplementary material
References
- 1.Hotez P.J. Southern Europe's coming plagues: vector-borne neglected tropical diseases. PLoS Negl. Trop. Dis. 2016;10(6):e0004243. doi: 10.1371/journal.pntd.0004243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olesen O., Ackermann M. Increasing European support for neglected infectious disease research. Comput. Struct. Biotechnol. J. 2017;15:180–184. doi: 10.1016/j.csbj.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calleri G., Angheben A., Albonico M. Neglected tropical diseases in Europe: rare diseases and orphan drugs? Infection. 2018;47(1):3–5. doi: 10.1007/s15010-018-1241-2. [DOI] [PubMed] [Google Scholar]
- 4.Patz J.A., Epstein P.R., Burke T.A., Balbus J.M. Global climate change and emerging infectious diseases. JAMA. 1996;275 [PubMed] [Google Scholar]
- 5.Bouzid M., Colón-González F.J., Lung T., Lake I.R., Hunter P.R. Climate change and the emergence of vector-borne diseases in Europe: case study of dengue fever. BMC Public Health. 2014;14(1):781. doi: 10.1186/1471-2458-14-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabachnick W.J. Climate change and the arboviruses: lessons from the evolution of the dengue and yellow fever viruses. Annu. Rev. Virol. 2016 doi: 10.1146/annurev-virology-110615-035630. [DOI] [PubMed] [Google Scholar]
- 7.Mweya C.N., Kimera S.I., Stanley G., Misinzo G., Mboera L.E.G. Climate change influences potential distribution of infected Aedes aegypti co-occurrence with dengue epidemics risk areas in Tanzania. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0162649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shope R. Global climate change and infectious diseases. Environ. Health Perspect. 1991;96 doi: 10.1289/ehp.9196171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison A., Astete H., Rocha C., Lopez V., Olson J., Kochel T., Sihuincha M., Stancil J. Impact of the dengue vector control system (DVCS) on Aedes aegypti populations in Iquitos, Peru 2004–2005. Am. J. Trop. Med. Hyg. 2005;73(6):326. [Google Scholar]
- 10.Patz J.A., Martens W.J., Focks D.A., Jetten T.H. Dengue fever epidemic potential as projected by general circulation models of global climate change. Environ. Health Perspect. 1998;106 doi: 10.1289/ehp.98106147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naish S., Dale P., Mackenzie J.S., McBride J., Mengersen K., Tong S.L. Climate change and dengue: a critical and systematic review of quantitative modelling approaches. BMC Infect. Dis. 2014;14 doi: 10.1186/1471-2334-14-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gubler D.J. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop. Med. Health. 2011;39(Suppl. 4):3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magori K., Drake J.M. 2013. The Population Dynamics of Vector-Borne Diseases. [Google Scholar]
- 14.Paz S., Semenza J.C. Environmental drivers of West Nile fever epidemiology in Europe and Western Asia – a review. Int. J. Environ. Res. Public Health. 2013;10(8):3543–3562. doi: 10.3390/ijerph10083543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubálek Z., Halouzka J. West Nile fever a reemerging mosquito borne viral disease in Europe. Emerg. Infect. Dis. 1999;5(5):643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Root J., Bosco-Lauth A. West Nile virus associations in wild mammals: an update. Viruses. 2019;11(5):459. doi: 10.3390/v11050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karanikolos M., Mladovsky P., Cylus J., Thomson S., Basu S., Stuckler D., Mackenbach J.P., McKee M. Financial crisis, austerity, and health in Europe. Lancet. 2013;381(9874):1323–1331. doi: 10.1016/s0140-6736(13)60102-6. [DOI] [PubMed] [Google Scholar]
- 18.Baumbach A., Gulis G. Impact of financial crisis on selected health outcomes in Europe. Eur. J. Public Health. 2014;24(3):399–403. doi: 10.1093/eurpub/cku042. [DOI] [PubMed] [Google Scholar]
- 19.Quinn S., Kumar S. Health inequalities and infectious disease epidemics: a challenge for global health security. Biosecur. Bioterrorism. 2014;12(5):263–273. doi: 10.1089/bsp.2014.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young J., Haussig J., Aberle S., Pervanidou D., Riccardo F., Sekulić N., Bakonyi T., Gossner C. Epidemiology of human West Nile virus infections in the European Union and European Union enlargement countries, 2010 to 2018. Eurosurveillance. 2021;26(19 (05)) doi: 10.2807/1560-7917.es.2021.26.19.2001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sikkema R.S., Schrama M., van den Berg T., Morren J., Munger E., Krol L., van der Beek J.G., Blom R., Chestakova I., van der Linden A., Boter M., van Mastrigt T., Molenkamp R., Koenraadt C.J., van den Brand J.M., Oude Munnink B.B., Koopmans M.P., van der Jeugd H. Detection of west Nile virus in a common whitethroat (Curruca communis) and culex mosquitoes in the Netherlands, 2020. Eurosurveillance. 2020;25(40) doi: 10.2807/1560-7917.ES.2020.25.40.2001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landesman W.J., Allan B.F., Langerhans R.B., Knight T.M., Chase J.M. Inter-annual associations between precipitation and human incidence of west Nile virus in the United States. Vector Borne Zoonot. Dis. 2007;7(3):337–343. doi: 10.1089/vbz.2006.0590. [DOI] [PubMed] [Google Scholar]
- 23.ECDC, Confirmed Culex modestus distribution, 2021.
- 24.ECDC, Confirmed Culex pipiens distribution, 2021.
- 25.ECDC, Confirmed Coquillettidia richiardii distribution, 2021.
- 26.Durand B., Tran A., Balança G., Chevalier V. Geographic variations of the bird-borne structural risk of west Nile virus circulation in Europe. PLoS One. 2017;12(10):e0185962. doi: 10.1371/journal.pone.0185962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ECDC, West Nile virus infection, 2020.
- 28.Meyer R.P., Hardy J.L., Reisen W.K. Diel changes in adult mosquito microhabitat temperatures and their relationship to the extrinsic incubation of arboviruses in mosquitoes in Kern county, California. J. Med. Entomol. 1990;27(4):607–614. doi: 10.1093/jmedent/27.4.607. [DOI] [PubMed] [Google Scholar]
- 29.Reisen W.K., Fang Y., Martinez V.M. Effects of temperature on the transmission of west Nile virus by Culex tarsalis (diptera: Culicidae) J. Med. Entomol. 2006;43(2):309–317. doi: 10.1603/0022-2585(2006)043[0309:Eotott]2.0.Co;2. [DOI] [PubMed] [Google Scholar]
- 30.Soverow J.E., Wellenius G.A., Fisman D.N., Mittleman M.A. Infectious disease in a warming world: how weather influenced west Nile virus in the United States (2001-2005) Environ. Health Perspect. 2009;117(7):1049–1052. doi: 10.1289/ehp.0800487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda T., Whitehouse C.A., Brewer M., Gettman A.D., Mather T.N. Arbovirus surveillance in Rhode island: assessing potential ecologic and climatic correlates. J. Am. Mosq. Control Assoc. 2003;19(3):179–189. [PubMed] [Google Scholar]
- 32.Gates M.C., Boston R.C. Irrigation linked to a greater incidence of human and veterinary west Nile virus cases in the United States from 2004 to 2006. Prev. Vet. Med. 2009;89(1-2):134–137. doi: 10.1016/j.prevetmed.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Brunkard J.M., Lopez J.L.R., Ramirez J., Cifuentes E., Rothenberg S.J., Hunsperger E.A., Moore C.G., Brussolo R.M., Villarreal N.A., Haddad B.M. Dengue fever seroprevalence and risk factors, Texas-Mexico border, 2004. Emerg. Infect. Dis. 2007;13(10):1477–1483. doi: 10.3201/eid1310.061586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeGroote J.P., Sugumaran R. National and regional associations between human west Nile virus incidence and demographic, landscape, and land use conditions in the coterminous United States. Vector Borne Zoonot. Dis. 2012;12(8):657–665. doi: 10.1089/vbz.2011.0786. [DOI] [PubMed] [Google Scholar]
- 35.Tackett J., Charnigo R., Caldwell G. Relating west Nile virus case fatality rates to demographic and surveillance variables. Public Health Rep. 2006;121(6):666–673. doi: 10.1177/003335490612100606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dowling Z., Ladeau S.L., Armbruster P., Biehler D., Leisnham P.T. Socioeconomic status affects mosquito (diptera: Culicidae) larval habitat type availability and infestation level. J. Med. Entomol. 2013;50(4):764–772. doi: 10.1603/me12250. [DOI] [PubMed] [Google Scholar]
- 37.Unlu I., Farajollahi A., Strickman D., Fonseca D.M. Crouching tiger, hidden trouble: urban sources of Aedes albopictus (diptera: Culicidae) refractory to source-reduction. PLoS One. 2013;8(10):e77999. doi: 10.1371/journal.pone.0077999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reisen W., Takahashi R., Carroll B., Quiring R. Delinquent mortgages, neglected swimming pools, and west Nile virus, California. Emerg. Infect. Dis. 2008;14(11):1747–1749. doi: 10.3201/eid1411.080719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim M., Holt J., Eisen R., Padgett K., Reisen W., Croft J. Detection of swimming pools by geographic object-based image analysis to support west Nile virus control efforts. Photogramm. Eng. Remote Sens. 2011;77(11):1169–1179. doi: 10.14358/PERS.77.11.1169. [DOI] [Google Scholar]
- 40.Reisen W., Carroll B., Takahashi R., Fang Y., Garcia S., Martinez V., Quiring R. Repeated west Nile virus epidemic transmission in Kern county, California, 2004–2007. J. Med. Entomol. 2009;46(1):139–157. doi: 10.1603/033.046.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kondilis E., Giannakopoulos S., Gavana M., Ierodiakonou I., Waitzkin H., Benos A. Economic crisis, restrictive policies, and the population's health and health care: the Greek case. Am. J. Public Health. 2013;103(6):973–979. doi: 10.2105/AJPH.2012.301126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallace R., Chaves L.F., Bergmann L.R., Ayres C., Hogerwerf L., Kock R., Wallace R.G. Springer; 2018. Clear-Cutting Disease Control: Capital-Led Deforestation, Public Health Austerity, and Vector-Borne Infection. [Google Scholar]
- 43.Steele L., Orefuwa E., Dickmann P. Drivers of earlier infectious disease outbreak detection: a systematic literature review. Int. J. Infect. Dis. 2016;53:15–20. doi: 10.1016/j.ijid.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 44.UNEP, A review of migratory bird flyways and priorities for management, CMS Technical Series (2014) 665. https://www.cms.int/sites/default/files/publication/CMS_Flyways_Reviews_Web.pdf.
- 45.Eurostat, Nuts – nomenclature of territorial units for statistics, 2020.
- 46.Eurostat, Agriculture, forestry and fishery statistics, Report, Eurostat (2019). doi:10.2785/798761. https://ec.europa.eu/eurostat/web/products-statistical-books/-/KS-FK-19-001.
- 47.SEDAC, Gridded population of the world, version 4 (gpwv4): population count, revision 11, 2018.
- 48.Cornes R.C., van der Schrier G., van den Besselaar E.J.M., Jones P.D. An ensemble version of the E-OBS temperature and precipitation data sets. J. Geophys. Res. Atmos. 2018;123(17):9391–9409. doi: 10.1029/2017JD028200. [DOI] [Google Scholar]
- 49.EU, Copernicus land monitoring service 2018, 2018.
- 50.Pekel J.-F., Cottam A., Gorelick N., Belward A.S. High-resolution mapping of global surface water and its long-term changes. Nature. 2016;540(7633):418–422. doi: 10.1038/nature20584. [DOI] [PubMed] [Google Scholar]
- 51.Bivand R.S., Pebesma E., Gomez-Rubio V. second ed. Springer; NY: 2013. Applied Spatial Data Analysis With R.https://www.asdar-book.org/ [Google Scholar]
- 52.Wood S.N. CRC Press; 2017. Generalized Additive Models: An Introduction With R. [Google Scholar]
- 53.Kurz C. Tweedie distributions for fitting semicontinuous health care utilization cost data. BMC Med. Res. Methodol. 2017;17(1 (12)) doi: 10.1186/s12874-017-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen S., Blanford J., Fleischer S., Hutchinson M., Saunders M., Thomas M. Estimating west Nile virus transmission period in Pennsylvania using an optimized degree-day model. Vector Borne Zoonot. Dis. 2013;13(7):489–497. doi: 10.1089/vbz.2012.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamiya T., Greischar M., Wadhawan K., Gilbert B., Paaijmans K., Mideo N. Temperature-dependent variation in the extrinsic incubation period elevates the risk of vector-borne disease emergence. Epidemics. 2020;30 doi: 10.1016/j.epidem.2019.100382. [DOI] [PubMed] [Google Scholar]
- 56.Mohammed A., Chadee D.D. Effects of different temperature regimens on the development of Aedes aegypti (l.) (diptera: Culicidae) mosquitoes. Acta Trop. 2011;119(1):38–43. doi: 10.1016/j.actatropica.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Tun-Lin W., Lenhart A., Nam V.S., Rebollar-Tellez E., Morrison A.C., Barbazan P., Cote M., Midega J., Sanchez F., Manrique-Saide P., Kroeger A., Nathan M.B., Meheus F., Petzold M. Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: a multi-country non-inferiority cluster randomized trial. Trop. Med. Int. Health. 2009;14(9):1143–1153. doi: 10.1111/j.1365-3156.2009.02341.x. [DOI] [PubMed] [Google Scholar]
- 58.Watts D.M., Burke D.S., Harrison B.A., Whitmire R.E., Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am. J. Trop. Med. Hyg. 1987;36(1):143–152. doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- 59.Koenraadt C.J.M., Mohlmann T.W.R., Verhulst N.O., Spitzen J., Vogels C.B.F. Effect of overwintering on survival and vector competence of the west Nile virus vector Culex pipiens. Parasites Vectors. 2019;12(1):147. doi: 10.1186/s13071-019-3400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riccardo F., Monaco F., Bella A., Savini G., Russo F., Cagarelli R., Dottori M., Rizzo C., Venturi G., Di Luca M., Pupella S., Lombardini L., Pezzotti P., Parodi P., Maraglino F., Costa A.N., Liumbruno G.M., Rezza G. An early start of west nile virus seasonal transmission: the added value of one heath surveillance in detecting early circulation and triggering timely response in Italy, June to July 2018. Eurosurveillance. 2018;23(32) doi: 10.2807/1560-7917.ES.2018.23.32.1800427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rudolf I., Betasova L., Blazejova H., Venclíkova K., Strakova P., vebesta O., Mendel J., Bakonyi T., Schaffner F., Nowotny N., Hubalek Z. West Nile virus in overwintering mosquitoes, Central Europe. Parasites Vectors. 2017;10 doi: 10.1186/s13071-017-2399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paull S., Horton D., Ashfaq M., Rastogi D., Kramer L., Diffenbaugh N., Kilpatrick A. Drought and immunity determine the intensity of west Nile virus epidemics and climate change impacts. Proc. R. Soc. B: Biol. Sci. 2017;284(1848):20162078. doi: 10.1098/rspb.2016.2017.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koenraadt C.J.M., Harrington L. Flushing effect of rain on container-inhabiting mosquitoes Aedes aegypti and Culex pipiens (diptera: Culicidae) J. Med. Entomol. 2008;45(1):28–35. doi: 10.1603/0022-2585(2008)45[28:feoroc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 64.ETC/ULS . European Environment Agency; 2019. Updated CLC Illustrated Nomenclature Guidelines, Report.https://land.copernicus.eu/user-corner/technical-library/corine-land-cover-nomenclature-guidelines/html/index-clc-112.html [Google Scholar]
- 65.Crowder D., Dykstra E., Brauner J., Duffy A., Reed C., Martin E., Peterson W., Carrière Y., Dutilleul P., Owen J. West Nile virus prevalence across landscapes is mediated by local effects of agriculture on vector and host communities. PLoS One. 2013;8(1):e55006. doi: 10.1371/journal.pone.0055006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eisen L., Barker C., Moore C., Pape W., Winters A., Cheronis N. Irrigated agriculture is an important risk factor for west Nile virus disease in the hyperendemic larimer-boulder-weld area of north central colorado. J. Med. Entomol. 2010;47(5):939–951. doi: 10.1093/jmedent/47.5.939. [DOI] [PubMed] [Google Scholar]
- 67.Miramontes R., Lafferty W., Lind B., Oberle M. Is agricultural activity linked to the incidence of human west Nile virus? Am. J. Prev. Med. 2006;30(2):160–163. doi: 10.1016/j.amepre.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Hanel M., Rakovec O., Markonis Y., Máca P., Samaniego L., Kyselý J., Kumar R. Revisiting the recent European droughts from a long-term perspective. Sci. Rep. 2018;8(1 (06)) doi: 10.1038/s41598-018-27464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
An R project containing all data that supports the findings of this study are available in.Rdata format from https://doi.org/10.5281/zenodo.4656902.